Translate this page into:
Application of BaTiO3-based catalysts for piezocatalytic, photocatalytic and piezo-photocatalytic degradation of organic pollutants and bacterial disinfection in wastewater: A comprehensive review
⁎Corresponding author. nmabuba@uj.ac.za (Nonhlangabezo Mabuba)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
The coupling of piezocatalysis and photocatalysis known as piezo-photocatalysis has attracted a lot of attention as one of the most effective advanced oxidation process (AOPs) for wastewater treatment, especially for the degradation of organic pollutants and disinfection of microbes. To advance this technology, there’s a need to develop lead free piezoelectric materials to drive both piezocatalytic and photocatalytic process to prevent secondary pollution due to lead toxicity. Hence, barium titanate (BaTiO3) has been widely used as lead free piezoelectric material for several applications including water splitting, bacterial disinfection, and wastewater treatment due to its exceptional optical and piezoelectric properties. This work presents a comprehensive review on the application of BaTiO3 as a promising lead-free piezo-photocatalyst for the catalytic degradation of organic pollutants and bacterial disinfection from aqueous solution. This review article details the optical and piezoelectric properties, modification strategies, and synthetic methods of BaTiO3. Furthermore, the application of BaTiO3 as a preferred piezo-photocatalyst for wastewater treatment and a future perspective is presented.
Keywords
Barium titanate
Piezo-photocatalyst
Organic pollutants
Bacterial disinfection
Wastewater treatment
1 Introduction
Water still remains an essence of life, however with continuous discharge of waste into water bodies, access to clean and potable water has continued to dwindle. The detection of various waste organic contaminants such as pharmaceutical, dyes, pesticides, personal care products in surface, ground and drinking water is of major challenge globally due to their detrimental effects (Du and Zhou, 2021). For example, the consumption of water containing organic dyes like methylene blue (MB), rhodamine B (RhB) and methyl orange (MO) can cause eye irritation, bladder cancer and respiratory problems (Fernández et al., 2010; Rai et al., 2005; Tan et al., 2015). With the rapid growth of textile industries, one of the major industrial sources of organic dyes owing to>100 000 tons of production of dyes per year, thus it is vital to monitor and treat these industrial waste dyes before reaching the environment (Abdi et al., 2017; Gupta et al., 2013; Gupta and Suhas, 2009; Holkar et al., 2016; Katheresan et al., 2018). Of major concern, are pharmaceutical products that have been widely used in various fields including households, agriculture and medicine. In medicine, products such as penicillin, ciprofloxacin, tetracycline and sulfamethoxazole are used as antibiotics to treat bacterial infections (Huang et al., 2021; Mo et al., 2017). The production of antibiotics increases daily due to their high demand for bacterial infection prevention or to cure diseases. The production of penicillin per year was reported to be approximately 28,000 tons, thus making it 68 % of the global consumption of antibiotics (An et al., 2015; Du and Liu, 2012). The presence of some of these antibiotics are of great concern due to their serious health effects such as vomiting, nausea, acute renal failure and diarrhea (Orimolade et al., 2020).
Other water contaminants which have been detected in different water bodies include pathogenic bacteria such as escherichia coli (E.coli), staphylococcus aureus (S. aureus), pseudomonas aeruginosa (P.aeruginosa), enterococcus faecalis (E.facelis) and other microbes. Inappropriate disposal of sewage and animal waste are the most common sources of faecal matter in the environment. The discharge of these waste materials from the environment into different water bodies such as rivers, lakes, oceans and streams does not affect only chemical oxygen demand (COD), biological oxygen demand (BOD) and turbidity of the surface water but also increases the number of various pathogenic pollutants (viruses and bacteria) existing in them (Pandey et al., 2014). In 2020, approximately>12 % of the global population was reported to be drinking water containing a substantial amount of unsafe pathogens. Drinking water containing these harmful pathogens can be lethal and cause some serious health issues and waterborne diseases such as diarrhoea, polio, typhoid and malaria etc (Pandey et al., 2014). According to the World Health Organisation (WHO) and standards, the allowed recommendable limitation concentration of organic dyes and bacteria should be below 1 ppm and 0 CFU/100 ml in drinking water, respectively (Katheresan et al., 2018; Masekela et al., 2020; Mahlaule-Glory et al., 2019). Thus, it is crucial to maintain the level of organic dyes, pharmaceutical and pathogenic bacterial within permissible limit so as to provide clean drinking water to humans and protect the environment.
Several water technologies and bacterial disinfection techniques including chlorination, chlorine dioxide, ozonation, ultraviolet light (UV), adsorption, membrane filtration and coagulation have been developed to maintain the level of waste water pollutants (organic dyes, bacteria and pharmaceuticals) within a safe level (Hassan et al., 2012; Masekela et al., 2022b; Saucier et al., 2017; Sirés et al., 2014; Sirés and Brillas, 2012a). However, these methods suffer from several limitations including generation of secondary toxic waste, high cost maintenance, incomplete removal of wastewater pollutants, poor recyclability and the use of toxic chemicals. Chlorination is one of the most popular and inexpensive bacterial disinfection techniques for the removal of all micro-organisms present in drinking water. Even though this technique is relatively less expensive, it produces harmful toxic by products such as trihalomethanes (THMs), haloacetonitriles (HANs), haloacetic acids (HAAs) etc (Xiang et al., 2018). These disinfection by products (DBPs) have negative impacts on human health as they can cause intestinal cancer. Additionally, chlorination with other methods like adsorption and filtration partially removes pharmaceuticals from wastewater, since 60 % of pharmaceutical residues remain even after treatment (Orimolade and Arotiba, 2020; Sirés and Brillas, 2012b; Xiao et al., 2015). Furthermore, adsorption and membrane filtration generate secondary toxic waste pollutants, thus require additional treatment which is very expensive (Gupta et al., 2012). Therefore, it is very important implement methods which are highly effective, economical and can completely degrade a majority of the wastewater pollutants into less harmful by products.
Advanced oxidation processes (AOPs) such photocatalysis and piezocatalysis have been used as effective methods for complete destruction of organic waste pollutants into less harmful by products. Photocatalysis and piezocatalysis uses generated strong oxidants such hydroxyl radicals (•OH) and superoxide anion (•O2–) to completely decompose organic pollutants under the influence of visible light and ultrasonic vibration, respectively (Chen et al., 2020; Koe et al., 2020; Li et al., 2019; Liang et al., 2018; Wu et al., 2018a). Unlike other conventional methods, AOPs completely oxidise organic waste pollutants into less harmful by products such as carbon dioxide (CO2) and water (H2O). In the photocatalytic degradation process, one main disadvantage is the fast recombination of electrons and holes (X. Liu et al., 2020). Over the past years, several methods such as metallic or non-metallic doping, formation of heterojunction and composites have been employed to enhance their photocatalytic activity, however effective electron-holes separation still remains a problem (Alex et al., 2019; Kanhere et al., 2014; Qi et al., 2017; Wang et al., 2017; Yong Zhang et al., 2019). Consequently, a piezo-electric field that is built within semiconductors has been shown to effectively separate charge carriers (electron and holes) to prevent recombination reactions. Recently, piezoelectric perovskites (ABO3) structure materials have been employed as an alternative way for better separation of charge carriers (e- and h+) (Y. Feng et al., 2018; Fu et al., 2021; X. Li et al., 2021, 2021; Liu et al., 2021; Y. Liu et al., 2020; J. Wu et al., 2020; J. Zhang et al., 2019).
Piezoelectric materials are known as smart materials which produce electric charges under the influence of applied mechanical vibration. These smart materials also tend to exhibit inverse piezoelectric effect, like the generation of mechanical stress under the influence of applied electric field (Xu et al., 2018). The generated electric charges on the opposite site of piezoelectric materials tends to form an electric field across the material. The built in electric field significantly separates the charge carrier (e- and h+) which further reacts with dissolved water and oxygen molecules to generate reactive oxygen species (hydroxyl and superoxide radicals), which are responsible for the breakdown of organic waste water pollutants (Y. Feng et al., 2018; Mushtaq et al., 2018; J. Wu et al., 2020).
Among the numerous piezoelectric materials which have been used as piezocatalysts for catalytic degradation of organic waste pollutants present in wastewater, (BaTiO3) has grabbed more attention as a piezocatalyst due to its excellent piezoelectric properties and biocompatibility (Kumar et al., 2019a; Ray et al., 2021). Besides that, it is a lead-free piezoelectric material thus making it more appropriate to be applied in environmental applications. Previously, BaTiO3 as a lead free piezocatalyst has been widely used in sensors. However, recently piezo-photocatalytic applications of BaTiO3 as a piezo-photocatalyst has attracted more attention in environmental wastewater treatment (Aksel and Jones, 2010; Ray et al., 2021). Therefore, this review article gives an overview of the recent applications of BaTiO3 as a piezo-photocatalyst for the catalytic breakdown of organic dyes, bacteria and pharmaceutical pollutants. Moreover, the concept of piezocatalysis, photocatalysis, different fabrication methods, relevant piezoelectric properties and modification methods of BaTiO3 are discussed in detail.
2 Basic principle of piezocatalysis, photocatalysis and piezo-photocatalysis
Photocatalysis and piezocatalysis processes are regarded as advanced oxidation processes. These two processes have been widely used in many applications including water splitting, bacterial disinfection, degradation and wastewater treatment (Mengying et al., 2017). The concept of piezocatalysis is similar to that of photocatalysis, the only difference lies on the triggering source to generate reactive oxygen species (ROS) which participate in redox reactions to degrade organic pollutants. In photocatalysis, a light source is usually utilized in the presence of a semiconductor (photocatalyst) to generate electron-holes pairs. The semiconductor absorbs the irradiated UV light with high energy thus resulting in electron excitation from valence band (VB) to conduction band (CB) leaving holes behind as displayed in Fig. 1.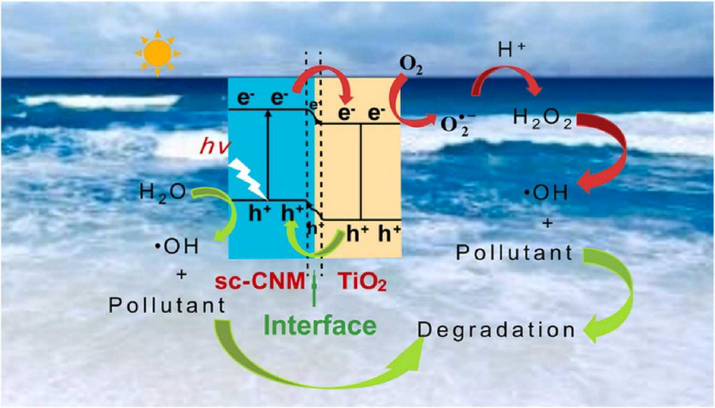
Photocatalytic degradation mechanism (Zhang et al., 2022).
As shown in Fig. 1, the photo-generated electron-hole pairs move on separate active sites of the semi-conductor to initiate redox-reactions which generates reactive oxygen species (ROS) responsible for the decomposition of organic waste pollutants. Unfortunately, the rate of electron-holes recombination is very fast which limits the application of semiconductors for photocatalysis. However, in piezo-photocatalysis, an internal voltage is generated under ultrasonic vibration with a built-in-electric field within the semiconductor. The in-built electric field piezo-semiconductors assists in the separation of photo-generated charge carries thus improving the photoactivity of the semiconductor. As shown in Fig. 2(a) and (b), the separated charge carries due to piezoelectric effect at the opposite surfaces generates free radicals through redox reactions.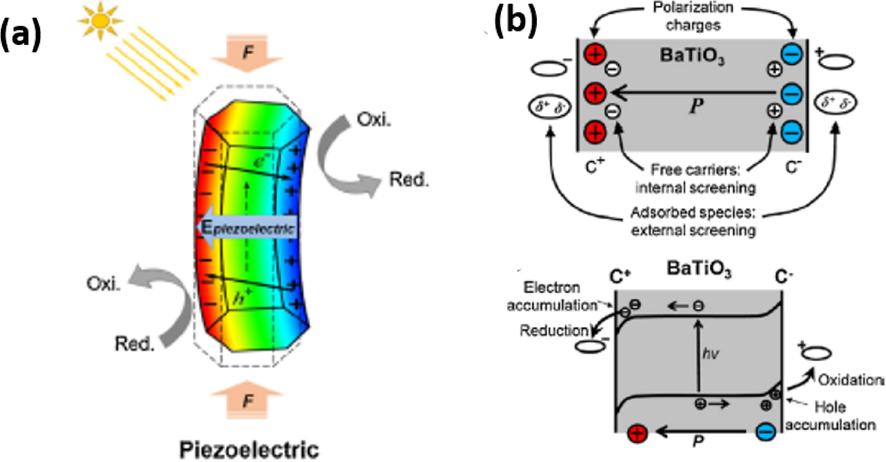
Piezocatalytic degradation principle (Liang et al., 2019).
Piezo semiconductor materials under the influence of applied pressure have been shown to behave like electrocatalytic reactors. The free electric charges (electrons and holes) at opposite sides of these materials tend to act as anode and cathode (Liang et al., 2018). The reaction (1) and (3) shows the formation of reactive oxygen species from free electric charges on the opposite sites of piezoelectric semiconductor materials. As shown in equation (2) and (3), the free positive charges react with water to form hydroxyl radicals (•OH), whereas negative charges react with free oxygen molecules to form superoxide radicals (•O2–). These reactive oxygen species (•O2– and •OH) are regarded as strong oxidants and are responsible for the degradation of organic dyes and bacterial disinfection.
Negatively charged surface of piezoelectric material
Positively charged surface of piezoelectric material
Unlike photoelectrocatalysis which is another type of electrochemical advanced oxidation for wastewater treatment, this process requires an external high voltage to reduce the rate of electron-holes recombination. Instead of using an external voltage, piezoelectrics materials are used to produce an internal voltage under mechanical vibration. The most extensively used are lead based piezoelectric materials such as lead zirconate (PZT). However, PZT contains about 80 % of the lead (Pb) content thus limiting their use in various applications (Panda and Sahoo, 2015). Due to lead being toxic, it is very important to develop piezoelectric materials which are lead free. Over the past few years, barium titanate (BaTiO3) has been given more attention as a lead free piezoelectric material for the production of piezoelectricity under mechanical vibration. Furthermore, recently BaTiO3 has been widely used as one of the piezo semiconductors in piezo-photocatalytic wastewater treatment applications.
2.1 Piezo-photocatalytic experiment based on suspended powder catalyst
Piezo-photocatalytic experiments using powder catalyst involves dispersing a certain amount of the catalyst into a contaminated solution. Since powder catalyst offers high surface to volume ratio, the solution mixture consisting of the catalyst is stirred for a certain time, normally for 30 min to reach an adsorption–desorption equilibrium in the dark. Thereafter, the solution mixture gets exposed to a light source. Some of the important parameters which need to be considered during conducting piezo-photocatalytic experiments in suspension systems includes the type of the light source (solar or UV light), UV light power, Ultrasonic power, UV intensity, the amount of the material used (dosage), reaction time and pH of the solution. Recently, a majority of the piezoelectric semiconductors such as BaTiO3 have been modified to convert their absorption from UV region to visible region (reduce their band gap) to utilize the visible light as a source of light, which constitutes of 43 % of the solar energy. For instance, the activity of piezoelectric semiconductors like ZnO and BaTiO3 were tested under different light sources such as sunlight and artificial visible light (Xenon lamp 1000 W, which emits visible light in the wavelength between 400 and 800 nm). Under solar light irradiation, the total organic carbon (TOC) results showed complete mineralization of phenol at lower concentrations as compared to artificial visible light irradiation (Pardeshi and Patil, 2008).
The type of the vibration normally employed in piezo-photocatalysis process is ultrasonic vibration. Ultrasonic excitation, can be used to induce piezoelectric materials to produce piezoelectric potential, which can effectively encourage the deterioration of organic dyes. However, under stress the generated free carriers will move in a specific direction to their end two poles and shield the piezo-potential, reducing the driving force. As a result, to maintain the electric field during the piezocatalysis process, continual oscillation is necessary. The ultrasound has the capacity to deliver continuous stress as a physical expression of mechanical energy (Lu et al., 2022). It is important to note that prolonged ultrasonic vibration will have both sonochemical and piezoelectric effects on materials that are made of piezoelectric components (Torres et al., 2008). The sonochemical effect can also help in the degradation of organic or inorganic wastewater pollutants.
2.2 Piezo-photocatalytic experiment based on thin film electrode catalyst.
The issues associated with powder piezo-photocatalyst such as low separation efficiency, poor recovery and regeneration ability, could be resolved by fabricating piezo-photocatalysts supported on the substrate to produce thin film electrodes. Typically, powder catalysts are separated from aqueous solution via filtration and centrifugation process, thus time consuming and some of the catalyst residue might remain in the solution and lead to secondary pollution. Piezo-photocatalyst thin film electrodes offer a good recoverability and recyclability, unlike powder catalysts. However, thin film electrodes during degradation process do not offer the full contact with the solution as compared to powder catalyst. Due their limited contact with the solution or low surface area, thin films exhibit slow degradation rate and low degradation efficiency. Besides that, growing interest is being shown in thin films with nanostructures that are directly formed on the surface of the substrate and are particularly susceptible to exposure to the dye solution. The degradation of organic pollutants via piezo-photocatalytic processes can be illustrated in Fig. 3. As shown in the Fig. 3 experiment, the prepared piezo-photocatalyst thin film is dispersed into a solution containing organic pollutants, thereafter exposed to light and ultrasonic irradiation. Just like piezo-photocatalytic experiment in suspension system, the parameters such as; the distance between the thin film electrode and light source, distance between thin film electrode and ultrasonic probe, ultrasonic power and light source need to be considered. Recently, floatable thin films are designed, which freely moves atop the water offering better utilization of sunlight. Unlike, steady thin film which requires photoreactor and external light source. Furthermore steady thin film requires a specific platform to control the distance between the light source and thin film electrode, which obviously raises the cost of scalable water purification (Yaozhong Zhang et al., 2019).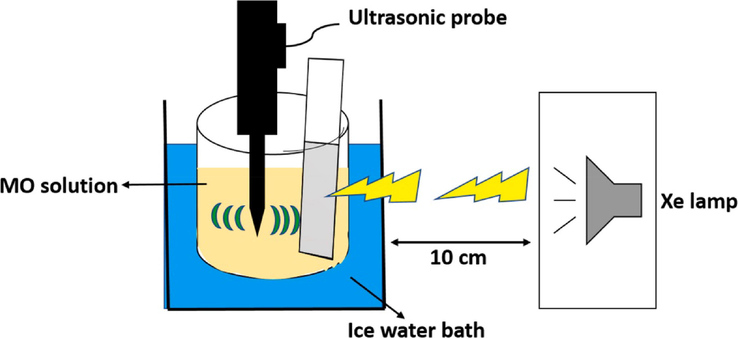
Piezo-photocatalytic degradation experiment based on thin film (Masekela et al., 2022a).
Another form of using thin film electrode is via sono (piezo)-photoelectrocatalytic degradation processes. Sono(piezo)-photoelectrocatalytic processes is a combination of sonocatalysis/piezocatalysis, photocatalysis and electrocatalysis. These processes have not yet been extensively investigated. In this experiment, light irradiation, ultrasonic vibration and bias voltage is applied on the surface of the thin film electrode. The degradation experiment is conducted using potentiostat/galvanostat, the prepared piezo-photocatalyst thin film is employed as a working electrode in the presence of a reference (Ag/AgCl) and counter electrode (platinum wire). Generally, the fabricated thin film electrode is positioned vertically opposite to the ultrasonic probe and light source (Fig. 4).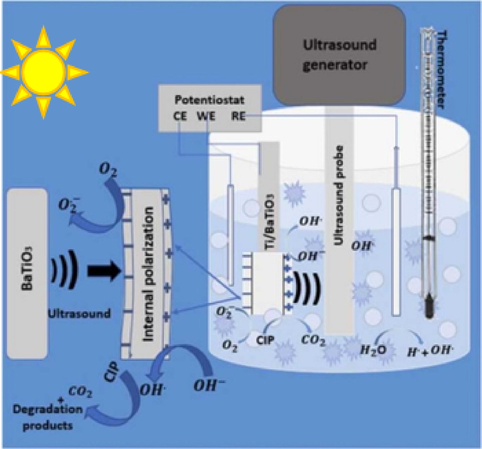
Photo assisted sonoelectrochemical degradation experiment (Ojo et al., 2022).
3 Structural, piezoelectric, and optical properties of BaTiO3
BaTiO3 is one of the highly applied ferroelectric materials which exhibit piezoelectricity under any form of mechanical vibration. It belongs to the perovskites family (ABO3), whereby A denotes a Barium (Ba) atom and B is a Titanium (Ti) atom. The crystal structure of BaTiO3 consists of Ti4+ atoms co-ordinated to six oxygen atoms to produce octahedral cluster’s (TiO6) and Ba2+ co-ordinated to twelve oxygen atoms to form (BaO12) clusters (Fig. 5). As shown in Fig. 5, Ba atoms are situated at every corner position, O atoms at face centred positions and Ti atoms at the centre.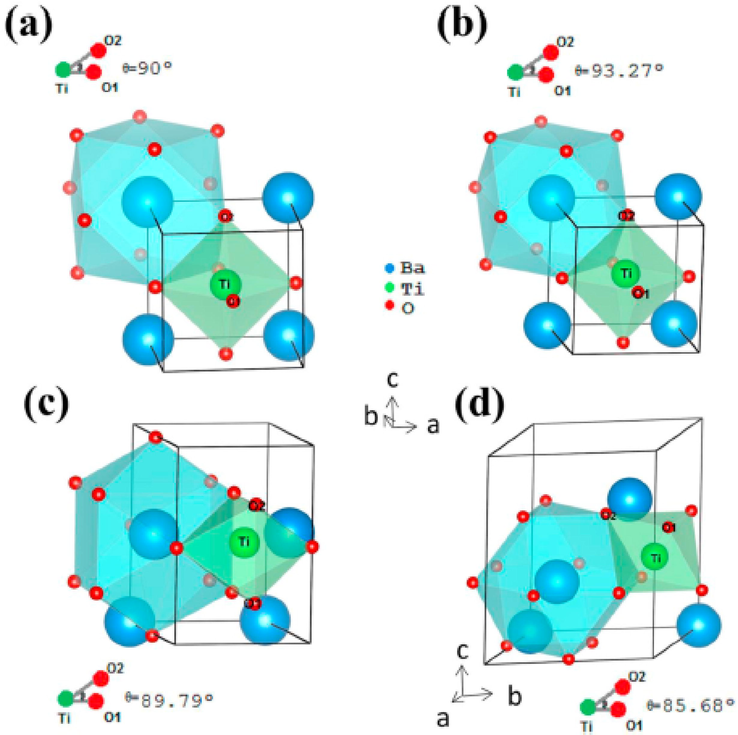
Schematic illustrations of BaTiO3 crystal structures for (a) cubic (b) tetragonal (c)orthorhombic and (e) rhombohedral (Oliveira et al., 2020).
Barium titanate can exist in different crystal structures such as cubic, tetragonal, orthorhombic and rhombohedral depending on the theta angles and phase transition temperature. The major distinction between cubic and tetragonal phases of BaTiO3 lies on the slight shift of theta angles of octahedral (TiO6) clusters from 90° to ≈ 93.3°, whereas the orthorhombic and rhombohedral phase occurs in the theta angles from approximately 89.9° to ∼ 85.7°(Itoh et al., 1985). The BaTiO3 crystal structures undergoes three different phase transitions under different temperatures. At temperature between 26.85 °C and 46.85 °C, cubic crystal structures transform into tetragonal structures, and to orthorhombic at approximately –23.15 °C to 6.85 °C, then ultimately to rhombohedral at temperatures around −73.15° C and –33.15 °C (Acosta et al., 2017; Oliveira et al., 2020). The band energy gap of each crystal structure of BaTiO3 plays a significant role in the photocatalysis process. The cubic crystal structure of BaTiO3 has a theoretical direct band energy gap of 4.68 eV, while orthorhombic, tetragonal and rhombohedral exhibit an indirect band energy gap of 5.06, 4.73 and 5.06 eV, respectively (Oliveira et al., 2020; Piskunov et al., 2004). Amongst these crystal structures, tetragonal-BaTiO3 (t-BaTiO3) has the lowest band energy gap than other phases (orthorhombic and rhombohedral). Owing to its low energy band gap (t-BaTiO3) when compared to other phases, this makes it a suitable piezo semiconductor for photocatalytic degradation of organic waste pollutants present in wastewater. Moreover, due to its well-positioned valence band, it also an important material in water splitting for hydrogen production.
Furthermore, BaTiO3 has a wide band energy gap just like other metal oxides such as TiO2, ZnO, SnO2, WO3 and BiVO4 etc., and its photoactivity is limited by the recombination of photogenerated charge carriers (e- and h+), which occurs rapidly (Demircivi and Simsek, 2019). However, unlike normal semiconductors, BaTiO3 is considered also as a piezo semiconductor which produces an internal piezo electric field under mechanical vibration. The induced built-in piezoelectric field separates the photogenerated charge carries, thus reducing their rate of recombination (X. Liu et al., 2020).
Several methods have been proposed to improve the photocatalytic performance of other metal oxide semiconductors such as metal/non-metal doping, formation of several metal oxide based composites, synthesis tailoring to attain certain morphology with improved photocatalytic activity and heterojunction formation with other semiconductors (Ray et al., 2021). These modification methods have been reported to help spatial charge separation and mitigate against fast recombination of photogenerated holes. However, to achieve effective degradation performance, it is proposed that the surface of the semiconductor must be loaded with a reduction cocatalyst and oxidation cocatalyst to achieve long live charge separation and speed up photogenerated hole transfer (G. C. Zhang et al., 2019). The next section, hence discusses the method of preparation and other modification strategies researchers have adopted to improve the performance of BaTiO3.
4 Preparation methods for powdered BaTiO3 and their thin films
Since the discovery of BaTiO3 during World War II (1941–1944) (Bouzidi et al., 2019), there has been a progressive development of BaTiO3 using different preparation approaches including sol–gel, hydrothermal/solvothermal, co-precipitation, mechanochemical and solid-state method. These synthesis methods have an impact on the physical and chemical characteristics of BaTiO3. Thus, it is critical to select appropriate preparation methods, since piezo-photocatalytic activity greatly depends on the physical and chemical properties of BaTiO3.
4.1 Methods to prepare powdered BaTiO3
4.1.1 Hydrothermal method
Hydrothermal synthesis is one of the popular methods for the fabrication of powdered BaTiO3 since it is not expensive and can form stable and pure materials. This method involves a reaction between Barium (Ba) and Titanium (Ti). During their synthesis the most widely used precursors include barium chloride (BaCl2), barium hydroxide (Ba(OH)2), TiCl4 and TiO2 materials. The hydrothermal reactions take place in an autoclave at temperatures above 100 °C. Several parameters including reaction time, temperature, solvents and solution pH can influence the morphology, particle size and crystal structure of BaTiO3. Xia et al. prepared BaTiO3 nano/microcrystals using commercial titanium dioxide (TiO2) and Ti(OH)4 as a titanium (Ti) precursor mixed with Ba(OH)4 as barium (Ba) precursor (Xia et al., 1996). A very well crystalline and dispersed BaTiO3 with a crystallite size < 100 nm was formed when Ti(OH)4 gel and Ba(OH)2 solution were used as precursors. The results showed that the starting precursors also had a strong impact on the morphology and crystallite size of the prepared BaTiO3. Furthermore, the hydrothermal reaction temperature had a strong influence on the crystal structure. As shown in Table 1, the lattice constant “a” slightly decreased with an increase in reaction temperature. According of the study conducted by Wen et al., it was found that lattice parameter a can affect photocatalytic activity of the semiconductor (Wen et al., n.d.). The photocatalyst (TiO2) anatase material with same composition, morphology, phase, and surface states but different lattice parameter ‘’a’’ were employed for photocatalytic degradation and photo-reduction of toluene and Cr(VI), respectively. However, greater catalytic activity was achieved by TiO2 with the extended lattice parameter than standard TiO2. Increasing in the length of the lattice parameter ‘’a’’ caused the bottom of the TiO2 conduction band to move higher, thus improving its photocatalytic activity.
Temperature (°C)
Lattice constant “a” (Å)
Unit cell volume Vc (Å3)
75
4.031
65.50
100
4.025
65.21
150
4.016
64.77
250
4.012
64.58
300
4.008
64.38
400
4.003
64.14
Moreover, Habib et al. showed that the structural morphology of the powdered BaTiO3 was temperature dependent (Habib et al., 2008). According to their result, the hydrothermal BaTiO3 obtained at low temperature (90 °C) had less pores compared to those attained at 120 and 150 °C. The study involving the relationship between photocatalytic activity of BaTiO3 thin film with porosity and surface area was conducted by Augurio et al.(Augurio et al., 2022). The porous BaTiO3 thin films exhibited higher photocurrent response than non-porous BaTiO3 thin film, indicating that porosity is beneficial in photocatalysis. This suggested that porous BaTiO3 can enhance interfacial charge transfer whilst lowering the charge carrier recombination rates, thus improving the photocatalytic activity. In another study, Zhan et al. controlled the hydrothermal reaction time (from 15 min to 480 h) to obtain BaTiO3 nanoparticles (Zhan et al., 2012). The XRD results showed no diffraction peaks after 15 min of hydrothermal reaction, demonstrating that the material lacked crystalline phases. However, longer hydrothermal reaction times (20 min to 48 h) led to the appearance of diffraction peaks in the XRD patterns that were attributed to the cubic BaTiO3. A continuous increase in the intensity of the diffraction peaks was observed with an increase in reaction time, demonstrating a persistent rise in the crystallinity and size of the crystals. Surmenev et al. produced BaTiO3 nano and micro rods via the hydrothermal method. The BaTiO3 nano- and micro rods were obtained at a temperature of 160–210 °C, using 0.02 and 0.15 M (NaOH) concentration and within 45–90 min (Surmenev et al., 2021). The XRD results showed that BaTiO3 purity drastically increased as NaOH concentration increased from 0.025 to 0.15 M. Furthermore, BaTiO3 tetragonal phase was clearly visible after 6 hrs of hydrothermal synthesis at 210 °C and varied alkalinities (from 0.025 to 0.15 M), whereas 45 and 90 min produced a combination of cubic or tetragonal phases. The results showed that the hydrothermal reaction conditions such as temperature, alkalinity and time, have a great impact on the formation of BaTiO3 structures with different morphologies. Wei et al. controlled the size of BaTiO3 nanoparticles via hydrothermal approach with Fe doping and ethylenediamine (en) addition (Wei et al., 2008). The crystal size of the synthesized BaTiO3 were investigated by X-ray powder diffraction (XRD), transmission electron microscopy (TEM), scanning electron microscope (SEM) and high-resolution transmission electron microscopy (HR-TEM). The results showed that BaTiO3 crystal size decreased as it was doped with Fe, indicating that Fe-doping suppress the crystal growth. It was further noticed that as Fe doping concentration increases, the average particle size also decreases (Fig. 6). Additionally, the addition of en, which served as both a solvent and a capping agent, may inhibit particle growth and cause a contained effect that changed the shape of the particles from spherical to cubic. It has been reported that semiconductors with smaller particle sizes have excellent photocatalytic activity as compared to those with large particles.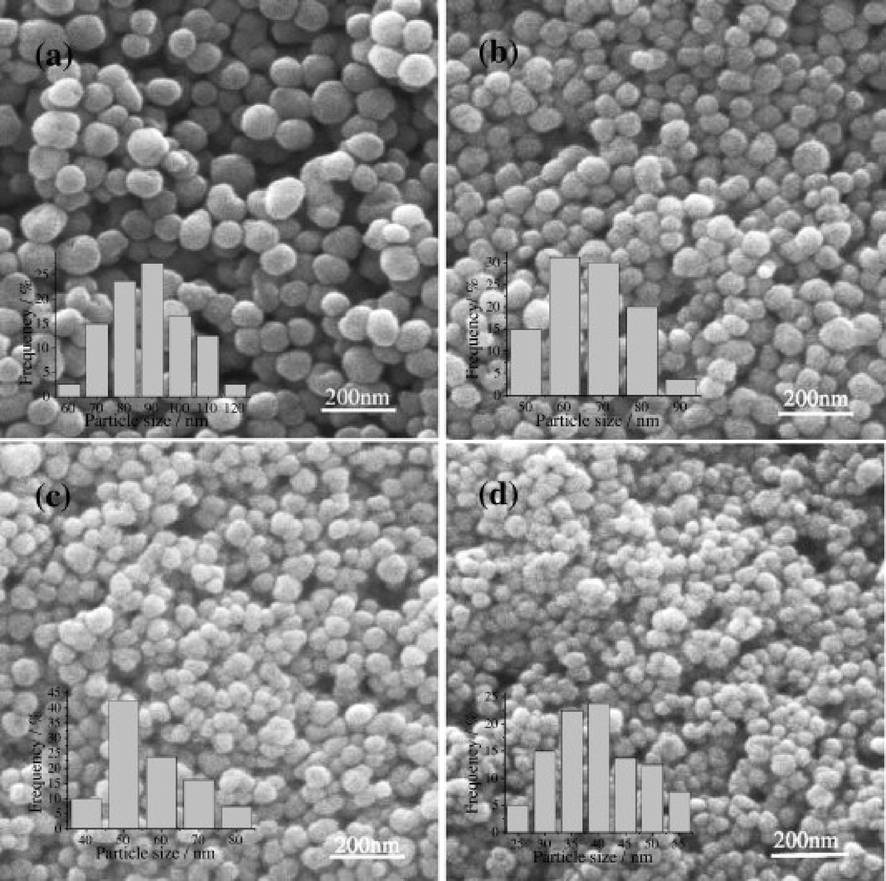
SEM images of BaTiO3 doped with different concentrations (a)Fe0%, (b) Fe2%, (c) Fe4%, and (d) Fe8% (Wei et al., 2008).
BaTiO3 heterostructures are easily fabricated using the hydrothermal method. Li et al. prepared BaTiO3/TiO2 heterostructure nanotube arrays using a straight forward hydrothermal process, the hydrothermal reaction was carried out at different reaction times, temperature and concentration of Ba(NO3)2 (R. Li et al., 2013). Zhao et al. and Kappadan et al. demonstrated the preparation of Ag2O/BaTiO3 and BaTiO3/ZnO heterostructures, respectively, using hydrothermal method (Zhao et al., 2020)(Kappadan et al., 2020a). Based on their experimental results, BaTiO3 nanoparticles were anchored on hexagonal rod-shaped ZnO (Kappadan et al., 2020a). The combination of hydrothermal and microwave method could be used to fabricate BaTiO3 (Sun et al., 2006). For example, Amaechi et al. prepared Fe-doped BaTiO3 via ultrafast microwave-assisted hydrothermal method (Amaechi et al., 2021). Furthermore, the hydrothermal approach could be used with the electrospinning method. By combining an electrospinning and a hydrothermal technique, Ren et al. developed ZnO/BaTiO3 nanofiber heterostructures (Ren et al., 2012). As seen in Fig. 7(a), BaTiO3 nanofibers had a rather smooth surface and formed a network topology. BaTiO3 nanofibers ranged from 300 to 400 nm in diameter and up to several micrometers in length. The ZnO nanoparticles were uniformly dispersed on the rough surface of BaTiO3 nanofibers (Fig. 7(b)). The elemental composition of the pure BaTiO3 nanofibers and ZnO/BaTiO3 nanofiber heterostructures showed the presence of Ba, Ti, O and Zn. The detected Al element was from aluminium foil.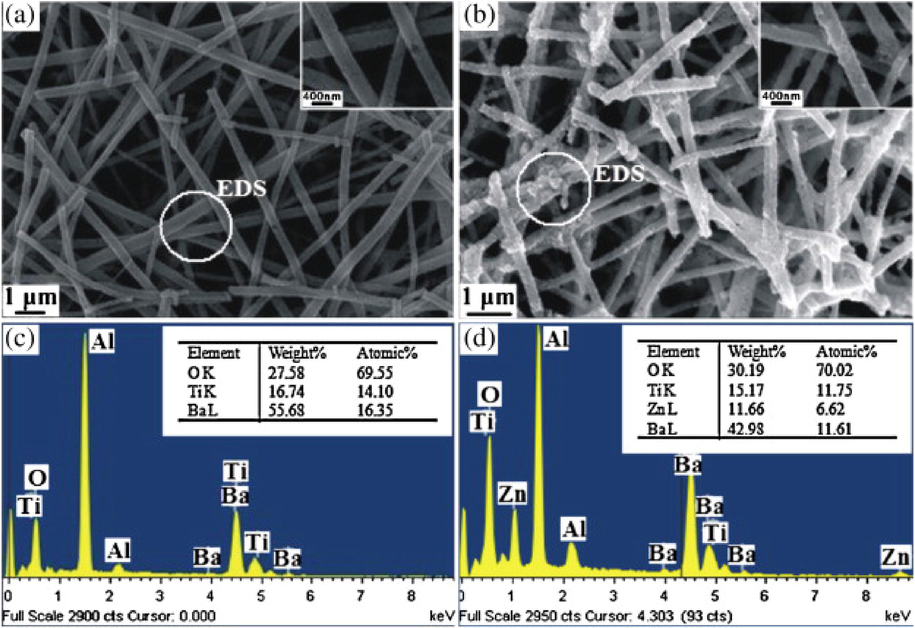
SEM images of (a) pure BaTiO3 nanofibers, (b) ZnO/BaTiO3 heterostructures, (c) EDS of BaTiO3 nanofibers and (d) EDS of ZnO/BaTiO3 heterostructures (Ren et al., 2012).
4.1.2 Sol-gel method
The sol–gel method is another simple method that is used to prepare barium titanates such as BaTiO3, BaTi4O9, Ba2TiO4 and BaTi2O5. Normally, barium acetate (Ba(OAc)2 and titanium (VI) isopropoxide (C12H28O4Ti) are used as barium and titanium precursors, respectively. The mixture of titanium (VI) isopropoxide and barium acetate solution tends to form BaTiO3-gel which is further dried and calcined at high temperatures (400–1200 °C) (Kavian and Saidi, 2009). The synergic sol gel and template method was used to fabricate BaTiO3 nanotubes (Cao et al., 2006). The formation of BaTiO3-gel was attained via mixing (Ba(OAc)2 and titanium isopropoxide, thereafter the nanostructured BaTiO3-gel grew on the porous alumina membrane (pore size 200 nm). The resultant alumina template covered with BaTiO3-gel was calcined at 700 °C to form BaTiO3 nanotubes with 50 µm length. The calcination temperature had a significant impact on the BET surface area of the BaTiO3. Pffaf (Pfaff, 1992) indicated that the BET surface area of BaTiO3 prepared via sol–gel method decreased as the calcination temperature increased (Table 2). The high specific surface was obtained when BaTiO3-gel was calcined at low temperature (200 °C). It has been reported that at elevated temperatures, nanoparticles tend to agglomerate extensively thus resulting in a significant reduction in BET surface area and pore diameter (Zhang et al., 2015).
Calcination temperature (°C)
Specific surface area (m2/g)
Average particle diameter (µm)
200
125
0.01
400
105
0.01
500
80
0.01
600
55
0.02
700
37
0.03
800
23
0.04
900
14
0.07
However, XRD diffraction showed highly pure crystalline BaTiO3 obtained at higher temperature. At high calcination temperatures above 800 °C, the crystal structure of BaTiO3 transformed from cubic to tetragonal structure. This crystal structure transformation (cubic to tetragonal) was depicted by XRD peak splitting at 2θ value approximately 45° (Fig. 8) [70]. From this study, it can be noted that crystallinity did have an influence on the optical properties of the semiconductors. According to literature, the optical band energy gap of the semiconductors decreases with an increase in crystallinity. Nishioka and Maeda (Nishioka and Maeda, 2015) studied the influence of the post heating of the hydrothermally synthesized Rhodium-doped barium titanate (BaTiO3:Rh) which could increase crystallinity and further improve photocatalytic activity. The XRD patterns were stronger and narrower after post heating 900 °C, thus confirming crystallization. However, the specific surface area was reduced from 8 to 4 m2 g−1. UV–vis diffuse reflectance spectroscopy (DRS) was employed to investigate the optical properties of BaTiO3:Rh. Upon post heating, DRS exhibited a series of changes as the temperature increased (with exception of the sample at 1150 °C). At higher temperatures, Rh4+ species induced greater absorption at longer wavelengths. This would make sense because at a high-temperature heat treatment increased the oxidation of Rh3+ to Rh4+ in BaTiO3:Rh. In terms of photocatalytic activity, unheated samples tend to show low activity, while on the other hand the activity increased with an increase in post heating temperature until 1000 °C. At elevated temperature above 1000 °C, the photocatalytic activity of BaTiO3:Rh decreased drastically.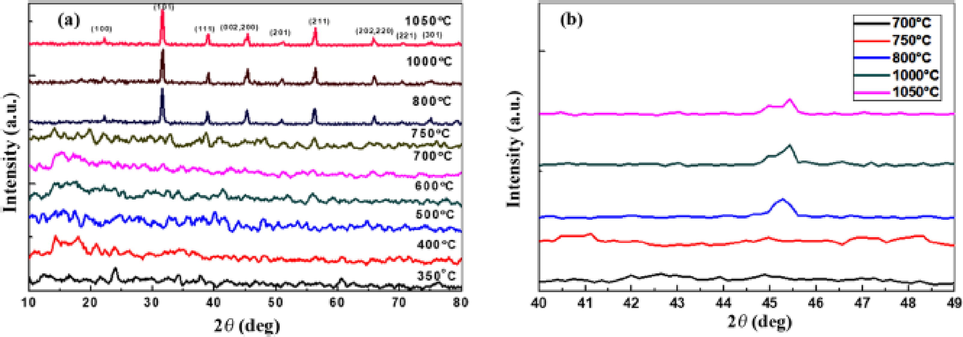
(a) and (b) XRD patterns for BaTiO3 (El-Sayed et al., 2020).
Sol gel was combined with low temperature hydrothermal reaction procedure to prepare BaTiO3 nanopowder (Wang et al., 2013). In the study reported by Wang et al., it was found that experimental conditions such as potassium hydroxide concentration (KOH), reaction temperature and time had a significant role on the crystallinity and morphology of BaTiO3 powder (Wang et al., 2013). A highly crystalline pure BaTiO3 with a cubic structure was obtained at 120 °C (after 2 h of reaction time) with KOH concentration over 1.0 M. The hydrothermal and reaction time showed less effect on the crystallinity and morphology, whereas KOH concentration showed a significant impact on the crystallinity and morphology. It was observed that, when the KOH concentration rised from 1.0 M to 8.0 M, the average size of the BaTiO3 particles decreased from 370 nm to 100 nm.
4.1.3 Solid state-method
Solid state synthesis is a common method which is usually employed to produce polycrystalline materials such as barium titanate (BaTiO3). This method requires a very high temperature, however its benefits include simplicity and high yield production. The main factors which affect solid state reaction include reaction temperature, pressure, chemical and morphological properties of the starting reagents/materials. The solid state synthesis of BaTiO3 nanoparticles was reported by Qi et al. (Qi et al., 2020). In their studies, different molar ratios of Barium nitrate (Ba(NO3)2) and Ti powder (Ba/Ti) as starting materials were varied and calcined at different temperatures (500, 550 and 600 °C). The calcination temperature played a crucial role in the formation of BaTiO3. This was confirmed by the XRD pattern which showed that there was no formation of BaTiO3 at temperatures below 500 °C, since only XRD peaks for starting materials (Ba(NO3)2 and Ti) were revealed (Fig. 9). At high temperature (600 °C), the peaks were almost indexed to BaTiO3 material, thus now confirming the effect of calcination temperature on these materials. Other studies reported the thermal decomposition reaction of barium carbonate (BaCO3) and titanium dioxide (TiO2) for the formation of BaTiO3 (Pithan et al., 2005). Since the rate of reaction is controlled by the diffusion rate of Ba ions into Titanium dioxide (TiO2) lattice, the shape and size of the BaTiO3 produced was more influenced by the TiO2 morphology. The formation of titanates were explained in detail in the literature (Beauger et al., 1983). Trzebiatowski et al. reported that the formation of barium titanate (BaTiO3) and barium orthotitanate (Ba2TiO4) occurs simultaneously via the below chemical reaction (Brdi et al., 1950);
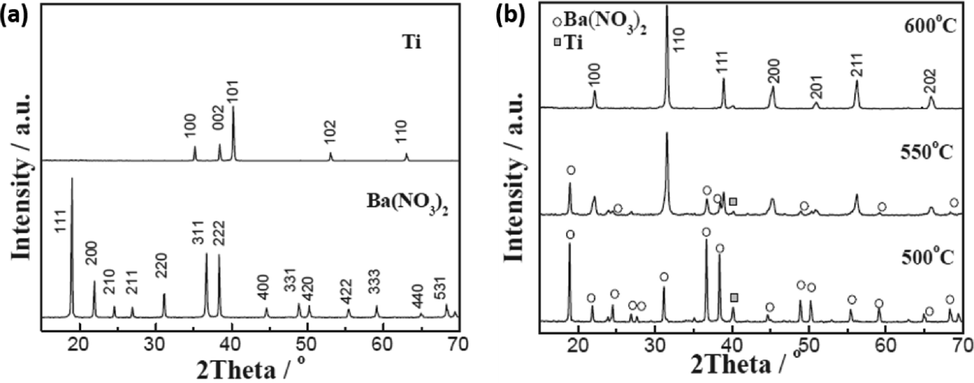
(a) and (b) XRD patterns for BaTiO3 (Qi et al., 2020).
In other studies they have reported that Ba2TiO4 forms when BaTiO3 reacts with TiO2 as shown in equation (4), thereafter the formed Ba2TiO4 reacts with the remaining TiO2 to produce meta titanate as shown in equation (5) (Beauger et al., 1983).
Solid state reaction method could be combined with sol–gel method. Mi et al. prepared nano BaTiO3 ceramics using TiO2 precursor gel and BaCO3 as starting raw materials (Mi et al., 2020). The XRD results showed the initial formation of BaTiO3 at calcination temperatures of 600 °C. A cubic BaTiO3 structure was formed when the calcination temperature reached 800 °C. At 900 °C calcination temperature, the diffraction peak of (2 0 0) separated into peaks of (0 0 2) and (0 0 3), thus suggesting phase transition from cubic to tetragonal phase. In another experiment, Ren et al. used a solid state method to fabricate Bi2O3/BaTiO3 heterostructure (Ren et al., 2013). Firstly, BaTiO3 was prepared from Ba(CH3COO)2 and TiCl4 via the hydrothermal treatment process. Thereafter, Bi2O3/BaTiO3 heterostructure were prepared through ball milling and calcination process using the prepared BaTiO3 and commercial Bi2O3 (mass ratio BaTiO3: Bi2O3 = 4:1). After the calcination procedure, it was discovered that Bi3+ had dissolved in the BaTiO3 lattice and that a chemical connection had been created at the interface between Bi2O3 and BaTiO3.
4.1.4 Ultrasound assisted
Recently sound energy has been utilized to prepare different metal oxide semiconductors such as BaTiO3 for different applications. In contrast to basic reactions, ultrasound-assisted reactions actually have a lot of advantages. High pressure, low pressure, and localized boiling zones are all produced by ultrasound in the reaction mixture. This shortens the reaction period and makes room-temperature synthesis possible. It has been noted that ultrasonography facilitates the uniform dispersion of reactants in a reaction mixture. Dang et al. reported sonochemically synthesized BaTiO3 nanoparticles (Dang et al., 2011). In their study, mixtures of ethanol and distilled water were prepared with different volume ratios. Thereafter, BaCl2 and TiCl4 (molar ratio Ba:Ti = 1:1 were added to the above solution mixture, followed by addition of NaOH. The solution suspension was exposed to ultrasonic irradiation for 40 min at low temperature (50 °C). The applied ultrasonic energy was 150 W/cm2. Following synthesis, the precipitate was centrifugally separated, twice washed with deionized water, and then dried for two hours in a vacuum at 100 °C. In another study, BaTiO3 submicronic particles were prepared following multiple procedures such ultrasonication, microwave drying and thermal treatment (Rotaru et al., 2017). Mixture of BaCO3 and TiO2 as raw materials were ultrasonicated (ultrasonic frequency: 20 kHz, 750 W nominal electric power) in milli-Q ultrapure water. After 30 and 60 min of ultrasonication, the prepared samples were dried in the microwave furnace for 10 min. The last procedure was thermal treatment of the samples at different temperatures (780–1300 °C) for 3 hrs. Ashiri et al. reported similar approach to obtain BaTiO3 nanocrystals via rapid ultrasound-assisted wet chemical method (Ashiri et al., 2015). Utara and Hunpratub synthesized cubic structure of BaTiO3 nanoparticles using ultrasonic method at room temperature without thermal treatment step (Utara and Hunpratub, 2018a). The starting precursors were barium hydroxide (BaOH)2 and diisopropoxytitanium bis(acetylacetonate) (C12H28O6Ti). The effect of ultrasonic reaction time on the morphology of BaTiO3 nanoparticles (NPs) was investigated using TEM micrographs. It was found that the particle sizes of the BaTiO3 NPs decreased with increase in ultrasonic reaction time. The average particle size reduced from 56.69 ± 30.14 nm (30 min of ultrasonic irradiation) to 32.72 ± 11.83 nm (4 hr of ultrasonic irradiation). Similar observations were reported by Moghtada and Ashiri (Moghtada and Ashiri, 2016). It was concluded that smaller particles were produced as a result of ultrasonic irradiation at 50 °C.
4.1.5 Co-precipitation
Co-precipitation method is the most frequently utilized synthesis approach for metal oxides (Rao et al., 2017). This method involves dissolving of metals salts in an appropriate solvent, followed by the addition of a precipitating agent. The most widely used precipitating agents include sodium hydroxide (NaOH), ammonium hydroxide (NH4OH) and potassium hydroxide (KOH). In case of preparing BaTiO3 using oxalate co-precipitation, it is challenging to obtain optimal conditions where both Barium (Ba) and Titanium (Ti) precipitates at the same time. Since Titanium (Ti) precipitates as titanly oxalate in the presence of alcohol at pH
2 whereas Barium (Ba) precipitates as BaC2O4 at pH
4. Titanium generates soluble anionic species such as TiO(C2O4)22– in the pH between 2 and 4, thus influencing the stoichiometry ratio of Ba/Ti simultaneously (Geetha et al., 2016). It has been reported that through manipulation of several chemical conditions such as pH, reactants, and reaction medium, it is possible to make Ba and Ti to precipitate at the same time. Prasadarao et al. investigated the influence of pH (range 2–10) on the synthesis of BaTiO3 from barium chloride (BaCl2) and potassium titanyl oxalate (KTO) (Prasadarao et al., 2001). The formation of barium titanyl oxalate was obtained at pH 2.5 and an increase in pH to 5 led to the formation of barium titanyl hydroxy oxalate. At higher pH values (7–9), precipitation reactions yielded a mixture of titanium dioxide (TiO2) and barium oxalate (BaC2O4). He et al. also prepared BaTiO3 powder via the co-precipiation of BaCl2 and TiOCl2 in an highly-alkaline environment (He et al., 2014). The pH solution and concentration of the starting precursors (BaCl2 and TiOCl2) had an effect on the particle grain size and homogeneity of the BaTiO3 powder. An average particle size of approximately 80 nm was obtained at pH 14 and reaction temperature of 80 °C. In another study, Zhang et al. used BaCl2, TiCl4 as starting raw materials and tartaric acid as a precipitant agent for the preparation of tetragonal BaTiO3 nano-powder (X. Zhang et al., 2021). The white precipitated were formed by adding slowly a solution of ammonium hydroxide solution. Followed by thermal treatment at different calcination temperatures (750–1050 °C) for 4 hrs. The microwave assisted co-precipitation was reported to produce BaTiO3@rGO nanocomposite (Khan et al., 2021a). BaTiO3 and GO as starting materials were prepared separately via the sol–gel method and modified Hummers method, respectively. Thereafter, a certain amount of BaTiO3 and rGO were added to 50 ml of deionised water and stirred for 1 hr at room temperature. Then, NaOH solution was slowly added to the above mixture solution, and heated for 1 hr in a microwave oven. The reduction of GO into rGO was confirmed by color change of the solution from brown to black. The co-precipitated nanocomposite was washed with mixture of ethanol/water and dried at 60 °C in an oven for 12 hr. The TEM images of pure BaTiO3 and BaTiO3@rGO are shown in Fig. 10 (a-b). As shown in Fig. 10(a), pure BaTiO3 exhibits spherical nanoparticles with a size distribution of 10–30 nm, whereas Fig. 10(b) shows spherical BaTiO3 nanoparticles with an average particle size range of 15–34 nm, which are uniformly distributed on the surface of rGO sheet. Table 3 highlights the summary of some of the synthetic methods for BaTiO3 powder.
TEM images for (a) pure BaTiO3 and (b) BaTiO3@rGO (Khan et al., 2021a).
Materials
Synthesis method
Starting materials
Morphology
Ref
BaTiO3
Hydrothermal
Ba(OH)2·8H2O, TiO2
Cubic phase
(Zhan et al., 2012)
BaTiO3
Hydrothermal
Ba(OH)2·8H2O, Ti(OBu)4
Cubic, Tetragonal
(Ji et al., 2022)
BaTiO3
Sol-gel
C₄H₆BaO₄, C12H28O4Ti
Tetragonal phase
(Kavian and Saidi, 2009)
BaTiO3
Sol-gel template
Ba(OAc)2, Ti(OPri)4
Nanotubes
(Cao et al., 2006)
BaTiO3
Hydrothermal
(Ba(NO3)2, TiO2
Spherical like, Tetragonal
(Li et al., 2020)
BaTiO3
Sol-gel and solid state
BaCO3, TiO2
Whisker like, Cubic, Tetragonal
(Mi et al., 2020)
BaTiO3/TiO2
Hydrothermal
(Ba(NO3)2, TiO2
Nanotube arrays
(R. Li et al., 2013)
BaTiO3
Hydrothermal
Ba(OH)2·8H2O, Na2Ti3O7, TiO2
Nanoparticles, Nanowires, Nanosheets
(Yu et al., 2021)
BaTiO3@rGO
Microwave assisted- co-precipitation
C₄H₆BaO₄, C12H28O4Ti
Spherical
(Khan et al., 2021a)
BaTiO3
Sonochemical
Ba(OH)2, C12H28O6Ti
Irregular Bowl-like structure
(Utara and Hunpratub, 2018b)
BaTiO3
Microwave-hydrothermal
Ba(OH)2·8H2O, (Ba(NO3)2 BaCl2, Ti(OBu)₄
Nanocuboid
(Chen et al., 2016)
These techniques are mostly applied to prepare powdered BaTiO3, however powdered piezo-photocatalyst are difficult to be recycled in practical applications. For instance, after the degradation process, some parts of the powdered catalyst may persist in the aqueous solution, thus leading to secondary pollution. Therefore, recently piezo-photocatalyst based thin films are being developed for better recoverability, thus in the next section some common techniques that are used to produce BaTiO3 based thin films will be highlighted.
4.2 Preparation BaTiO3 based thin films
There are various methods implemented for the preparation of BaTiO3 thin films, these include physical and chemical techniques. The physical methods include sputtering deposition, pulsed laser deposition (PLD), spin coating, dip coating and the Dr Blade method (Asadzadeh et al., 2021; Cernea, 2004). Chemical methods include chemical vapour deposition (CVD), sol–gel method and hydrothermal method. All of these have their own advantages and disadvantages. Cernea et al. explained most of these methods basic principle and their own benefits (Cernea, 2004). In this review, a few common physical and chemical methods are discussed below.
4.2.1 Dip coating
Dip coating is one of the most popular liquid-phase deposition methods for the fabrication of thin-films. This method involves dipping a substrate in the solution containing a starting material/ceramic powder, binder, solvent and dispersant. Once the material of interest has been deposited, the substrate is removed slowly from the solution and dried at ambient temperature. Several parameters such as immersion period, withdrawal rate, number of immersion cycles, solution composition, concentration and temperature tends to affect the film characteristics, smoothness and thickness (Schneller et al., 2013). This method has been used for the production of numerous piezoelectric thin-films including Pb (Zr, Ti)O3, CaBi4Ti4O15, ZnO, PVDF and BaTiO3. Ashiri et al. reported a crack-free nanostructured BaTiO3 produced from a modified sol–gel dip coating method (Ashiri et al., 2014). The silica substrate was immersed into a sol prepared from barium acetate, glacial acetic acid, titanium tetraisopropyl alkoxide and 2-propanol. After deposition, the substrate with coated BaTiO3 was taken out from the sol–gel solution with a withdrawal rate of 1 cm/min and dried at 100 °C. The resultant substrate coated with BaTiO3 was further calcined at 800 °C for 1 hr (heating rate 5 °C/min) to produce a thin film with a thickness of approximately 2 nm.
4.2.2 Spin coating
In the spin coating process, the coating material is firstly dissolved in an appropriate solvent and the solution is dropped at the centre of the solid substrate surface. The solid substrate is then spun at controlled high speed. During this process, the solid substrate is rotated around an axis which is perpendicular to the coated region. The thickness and other properties of the final thin film depends greatly on the spinning rate of the substrate, viscosity of the solution, solvent evaporation rate, spinning time and surface wettability. This method is suitable and can be used for fabrication of several ceramics, including barium titanates such as BaTiO3 (Aminirastabi et al., 2020).
4.2.3 Chemical vapour deposition (CVD)
Chemical vapour deposition is a widely used method to produce 2D nanomaterials and thin films. In this process, a solid material is deposited from the vapour by a chemical reaction occurring on or in the vicinity of a typically heated substrate (Mittal et al., 2021). The thin film nanostructures and thickness can be tuned by controlling the deposition conditions and the CVD system key factors. These include the substrate material and precursors, composition of reaction gas mixture, total pressure gas flows and temperature. Suzuki and Kijima (Suzuki and Kijima, 2006) prepared nanostructured BaTiO3 thin film from bis-dipivaloylmethanate barium (Ba(DPM)2) and titanium (IV) isopropoxide (Ti(OiPr)4 deposited on platinum/alumina/silica/silicon (Pt/Al2O3/SiO2/Si) substrate using the CVD technique assisted with Inductively Coupled Plasma (ICP). The size of the resultant nanostructured BaTiO3 thin film was greatly influenced by substrate temperature. The single phase BaTiO3 structure and particles sizes of approximately 30 nm were successfully obtained at temperature of 500 °C (Fig. 11(a)). The deposited spherical BaTiO3 nanoparticles on the surface of the substrate were more agglomerated with less pores. Fig. 11(b) shows a cross section image of the deposited dense nanoparticles on the substrate surface, however the thickness of the thin film was not determined. At substrate temperatures above 600 °C, the deposited BaTiO3 nanoparticles fused into a columnar form as shown in Fig. 11(c)-(d). The bottom of the thin films formed, exhibited a columnar structure, whereas the structure surrounding the surface was made of nanoparticles.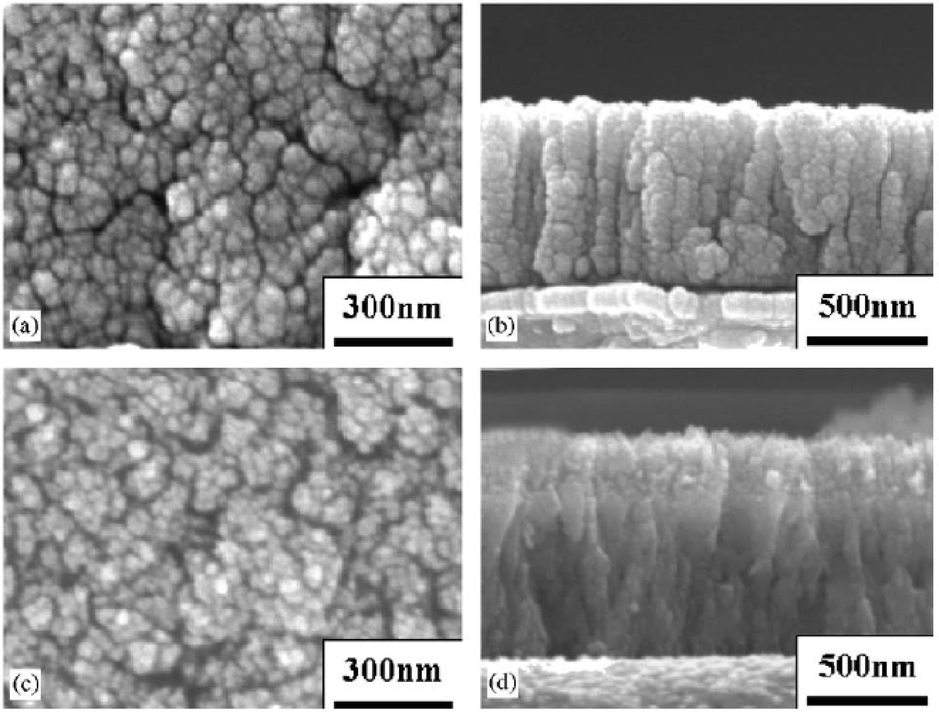
Surface SEM images and cross section images of the films deposited at different substrate temperatures; (a) surface image at 500 °C, (b) cross section at 500 °C, (c) surface at 600 °C and (d) cross section at 600 °C (Suzuki and Kijima, 2006).
4.2.4 Dr Blade /Tape casting method
Tape casting known as the Dr.Blade method has been widely used for the production of ceramic thin films. This technique is usually used to obtain thin films with a thickness ranging from 10 to 1000 μm. In this method, the powdered starting materials are mixed with appropriate solvents and binders to form a homogeneous mixture which is tape casted on the solid substrate (Asadzadeh et al., 2021). Thereafter, the tape casted substrate is dried at certain temperatures. The drying rate and temperature tend to be the most important factors which control the crack free of the thin film. Other factors which can affect the thin film properties and thickness include relative content of ceramic powder (starting materials), solvent and binder. Lilge et al. hydrothermally synthesized BaTiO3 powder from BaCl2.8H₂O and Ti [OCH(CH3)₂]₄ (Lilge et al., 2020). The hydrothermal reaction place was performed in a microwave for 120 min at 140 °C. The resultant BaTiO3 powder was further used to prepare a photoanode electrode using the Dr Blade method. For the preparation of the photoanode electrode, the powdered BaTiO3 was mixed with ethylene glycol Triton X-100 and ethanol. The slurry mixture was then taped casted on the FTO substrate (area of 1 cm2) to form a thin film. The BaTiO3 pasted on the surface of FTO appeared to be spherical in shape and agglomerated (Fig. 12).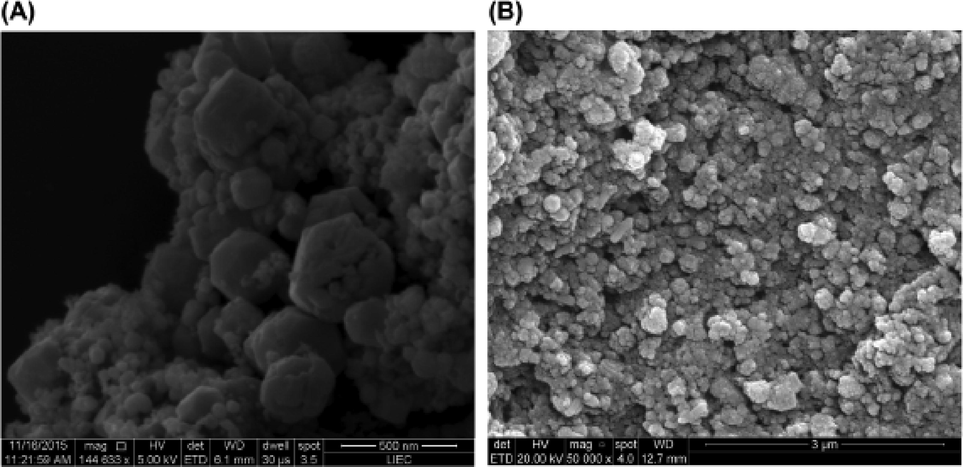
(a) and (b) Surface SEM images of FTO/BaTiO3 (Lilge et al., 2020).
5 Modification of BaTiO3 for enhanced piezo-photocatalytic efficiency
Despite the fact that BaTiO3 as a semiconductor has received a lot of attention for piezo-photocatalytic applications due to its incredible ferroelectric/piezoelectric properties and accessibility in a wide assortment of sizes and morphologies, it has significant limitations, most which are linked to its photocatalytic activity (X. Liu et al., 2020; Ray et al., 2021). Owing to its wide energy band gap of approximately 3.2 and 3.4 eV, it is associated with rapid recombination of photogenerated electron-holes which reduces its photocatalytic activity. Recently, various strategies have been explored including tailoring the morphology and particle sizes, doping and fabrication of heterojunction/composite photocatalyst to prevent some of these limitations (Scheme 1).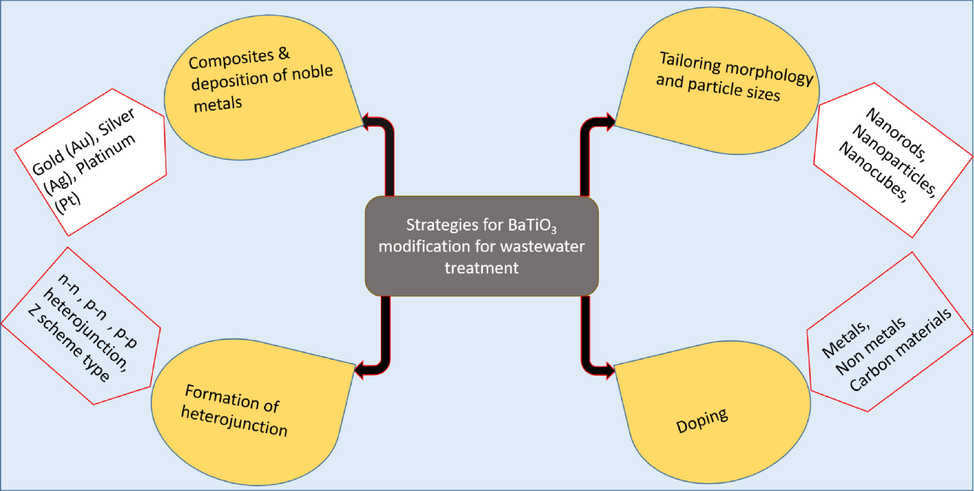
Illustrations of BaTiO3 modifications for water and wastewater treatment.
5.1 Tailoring BaTiO3 morphology and particle size
The surface morphology and particles size of the semiconductor photocatalyst/piezocatalyst plays an important role in the catalytic degradation of organic waste pollutants. It has been reported that BaTiO3 with different morphological structures including nanowires, nanofibers, nanorods, nanotubes, nanocubes and nanoparticles exhibits different piezoelectricity. For example, 1-D fiber/wire piezoelectric materials show a superior piezocatalytic response as compared to spherical particles. Whereas, thin sheet-like 2-D structures also generate more piezoelectricity under mechanical vibration (Mondal et al., 2022). As piezocatalyst, Liu et al. explored different nanostructures (nanocubes (NCs), nanoparticles (NPs) and nanofibers (NFs)) of BaTiO3 for piezocatalytic degradation of Rhodamine B (Rh B) (D. Liu et al., 2020). BaTiO3 nanofibres showed greater piezocatalytic performance as compared to nanocubes (NCs) and nanoparticles (NPs) due to a higher surface area, easy deformation structure and fine crystal size. Moreover, Jiao et al. prepared different BaTiO3 nanostructures via the hydrothermal route at different reaction times (starting from 4 to 16 hr) (Jiao et al., 2017). Spherical BaTiO3 nanoparticles formed at 4–8 hrs were more effective for photocatalytic degradation of Rh B dye than other morphological nanostructures such as bowl like and agglomerated spherical particles. To further understand the enhancement in photocatalytic activity due to morphological-tuning of the semiconductor photocatalyst, different analyses including BET surface area, photon energy, electrochemical Impedance Spectroscopy (EIS), photoluminescence (PL) and photocurrent also need to be conducted. Since the method of preparation has an impact on the final morphological product, various authors have also synthesized these piezomaterials using varying methods Xiong et al. fabricated BaTiO3 nanocubes, since cubic structures are known to have the best ability to reduce crystal defects and increase the surface-to-volume ratio (Xiong et al., 2015). The effect of reaction time (24, 48, 74 hr) using the hydrothermal method was employed to produce the cubic like BaTiO3 structure (Fig. 13(a)-(f)). The particle size increased with an increase in hydrothermal synthesis duration. Furthermore, the edges of the cube got sharper as the reaction time increased, showing an increase in the cubic phase's crystallinity. The BaTiO3 nanocubes formed over period of 48 hrs exhibited impressive photocatalytic performance under light irradiation. This better performance was due to more uniform morphological distribution, higher crystallinity, small particle size and higher surface area which lead to more active sites, reduction in migration path of charge carriers, narrowing the energy band gap and reducing the rate of charge carrier’s recombination.![(a)-(f) TEM images of cubic like structure of BaTiO3 synthesized at various temperatures [90].](/content/184/2023/16/2/img/10.1016_j.arabjc.2022.104473-fig14.png)
(a)-(f) TEM images of cubic like structure of BaTiO3 synthesized at various temperatures [90].
The optical properties of the BaTiO3 nanocubes calcined at different temperature were investigated by photoluminescence (PL) and UV vis spectrophotometer. The calcination temperature had an effect on the optical properties of the hydrothermally synthesized BaTiO3 reported by Hasbullah et al.(Hasbullah et al., 2019). The energy band gap calculated from tauc’s plot (Fig. 14) for BaTiO3 calcined at 500, 600, 700 and 1000 °C were 3.18, 2.87, 2.83 and 2.74 eV, respectively. Upon increasing the calcination temperature, the energy band gaps of BaTiO3 were expected to increase due to their high crystallinity. However, BaTiO3 resulted in lower energy band gap than expected. This could be due to inadequate oxygen delivery during the calcination process in ambient air resulted in oxygen deficiency in BaTiO3 structures (Orhan et al., 2005). As a result, the calcined BaTiO3 had a greater density of oxygen vacancy or non-bridging oxygen. The oxygen vacancy has the potential to change the BaTiO3 structure and cause localized electronic states. Therefore, resulting in reduction of energy band gaps for extremely crystalline BaTiO3 structures.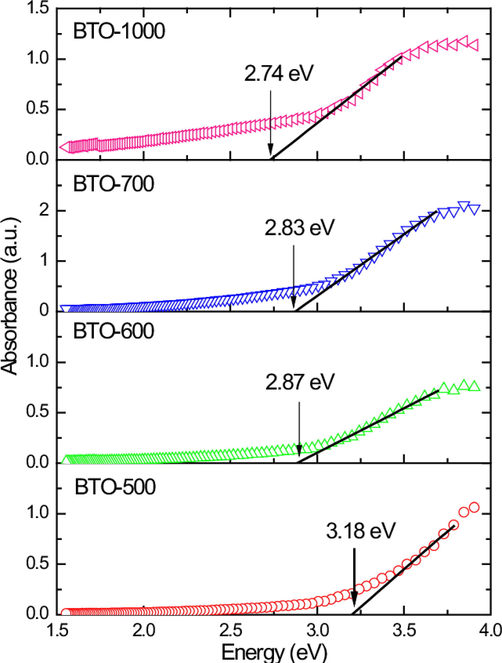
Tauc’s plot for BaTiO3 calcined at different temperatures (Hasbullah et al., 2019).
Moreover, photoluminescence (PL) was employed to study the rate of photogenerated electrons and holes recombination. As seen in Fig. 15(a)-(f), the PL intensity was expected to decrease with increase in crystallinity. However, in this study the PL intensity reached its highest peak as the calcination temperature was elevated to 1000 °C. It was hypothesized that the increase in photoluminescence intensities is due to the presence of a localized state within BaTiO3 structures. With sufficient stimulation, the localized state effectively lowers the band gap of BaTiO3 structures, hence resulting in strong photoluminescence intensity.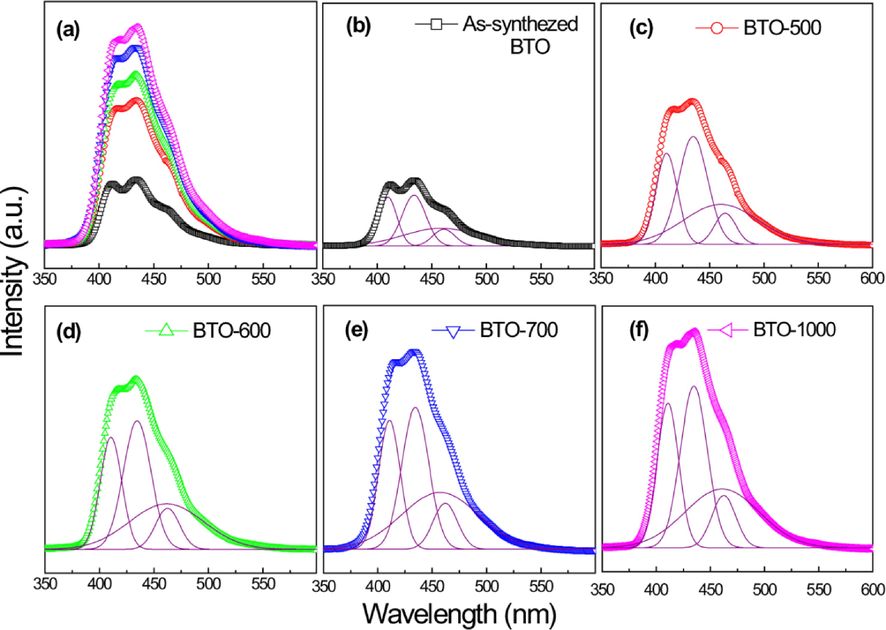
(a)-(f) Photoluminescence emission and Gaussian deconvolution plots for BaTiO3 calcined at different temperatures (Hasbullah et al., 2019).
5.2 Doping
Metal or non-metal doping is one of the most popular methods used to modify semiconductor photocatalysts to improve their optical properties such as a reduction of band gap and photogenerated charge carriers (electron-holes), increase in photocurrent response and interfacial charge carries. The improvement of these properties tends to enhance the photocatalytic and piezo-photocatalytic degradation of organic wastewater pollutants. It has been reported that the modification of photocatalyst semiconductors such as TiO2 (Khairy and Zakaria, 2014), ZnO (Kaur and Singhal, 2014), WO3 (Peleyeju and Viljoen, 2021), BiVO4 (Orimolade and Arotiba, 2020)and BaTiO3 (Ray et al., 2021) by metal ion doping can successfully shift their optical absorption to the visible light region, thus narrowing their band gaps. Recently, a lot of research has shifted towards metal doping rather than no-metal doping since metal doping synthesis is easily achievable. To date, many transition metals including copper (Cu), Iron (Fe), Manganese (Mn), Tungsten (W) and Cerium (Ce) to mention a few have been explored as BaTiO3 photocatalyst dopants for their improved break down of several organic contaminates like methylene blue (MB), tetraclycline (TC), methyl orange (MO), and atrazine. Among these transition metal dopants, Cu has been shown to be the most efficient BaTiO3 dopants due to the fact it has shown greater improvement in degradation of organic pollutants as compared to Mn-, Fe-, Ce-, W- and Cr doped BaTiO3 (I. C. Amaechi et al., 2019; Ifeanyichukwu C. Amaechi et al., 2019; Basaleh and Mohamed, 2020; Nageri and Kumar, 2018; Senthilkumar et al., 2019). Basaleh and Mohamed (Basaleh and Mohamed, 2020) investigated the degradation activity of undoped and cu-doped BaTiO3 for the removal atrazine from wastewater. According to their outcomes, 5 wt% Cu/BaTiO3 showed the highest degradation efficiency of 100 % after 60 min, which was 33 times better compared to the undoped BaTiO3. The addition of Cu to the BaTiO3 surface reduced the band gap of undopoed BaTiO3 sample from 3.28 to 2.77 eV, thus improving the photocatalytic activity of the Cu-doped BaTiO3 sample.
Noble metals including gold (Au), silver (Ag), platinum (Pt) and palladium (Pd) have been shown to improve the BaTiO3 piezo-photocatalyst sensitivity either under visible light or ultrasonic vibration. These noble metals are receiving more attention from researchers because of their superb utilisation of the solar spectrum, from visible to infrared, through the SPR effect (Chao et al., 2020; Cui et al., 2013). The BaTiO3 plasmonic photocatalyst have been fabricated from doping these noble metals with pure BaTiO3 sample. Under solar irradiation, the plasmonic photocatalyst generates an internal electric field which causes the photogenerated charge carriers to move in opposite directions. According to the charge transfer process in plasmonic photocatalysts, electrons from noble metal NPs can travel to the photocatalyst's CB and vice versa. Therefore, resulting in improved separation of charge carriers of plasmonic photocatalyst for better photocatalytic performance. In a study conducted by Xu et al., plasmonic piezo-photocatalyst (Ag/BaTiO3) compared to pure BaTiO3 showed an improved absorption under simulated solar irradiation (Xu et al., 2019). Due to this improvement, they discovered that Ag/BaTiO3 had a greater photocatalytic effectiveness than pure BaTiO3. The SPR of silver (Ag) nanoparticles resulting from internal band transitions from the 5d band to and within the 6sp band of the noble metal resulted in an increase in the piezo-photocatalytic activity of the modified BaTiO3.
Another way of adjusting the band gap and improving the photocatalytic performance of the semiconductor is via non-metal doping. Unlike noble metals which are very expensive, non-metal materials are less expensive and can be applied as dopants for several photocatalyst to be used in wastewater treatment. Carbon based materials have been widely used as non-metal dopants to improve piezo-photocatalytic performance of BaTiO3 since they can improve the rate of electron transfer and also reduce the electron-hole recombination rate. Some of the widely used carbon-based materials include carbon nanotubes (CNTs), activated carbon nanofibres (ACFs), graphene oxide (GO), Biochar, and Carbon nanodots (CNDs) to mention a few (Orimolade et al., 2021a). These distinct carbonaceous materials have different morphologies (surface area and pore size) and surface chemical characteristics (functional groups, hydrophobicity, and hydrophilicity) which all have a significant role in photocatalytic degradation of waste pollutants. Over past years, several few types of these carbonaceous materials have been employed to modify BaTiO3 structure. However, the utilization of graphene oxide (GO) has been shown to be the most effective strategy. Unlike other carbons, GO offers a variety of benefits including high UV–visible light transmittance, quick electrical and thermal conductivity, superior mechanical and tribological characteristics, and corrosion resistance. In addition, the delocalization of pi (π) network of the layers effectively suppresses electron-hole recombination thus resulting in improved photocatalytic performance (Zou et al., 2019). For instance, Zhao et al. showed an improved photocatalytic performance of BaTiO3 after loading it with different mass ratios of graphene oxide (Zhao et al., 2018). Firstly, the graphene oxide was prepared from the oxidation of graphite powder using the Hummer’s method and, later the freeze drying method was employed for the preparation of graphene oxide-BaTiO3 hybrid photocatalyst (Fig. 16(a)). Under light exposure, the hybrid material had superior photocatalytic performance than the unmodified BaTiO3, as shown in Fig. 16(b-c). Similar observations were reported by Rastogi et al. and Wang et al., whereby the introduction of graphene oxide (GO) into BaTiO3 lattice structure resulted in a higher photoresponse under the ultraviolet region or in the visible region than BaTiO3 pristine (Rastogi et al., 2016b) (Wang et al., 2015).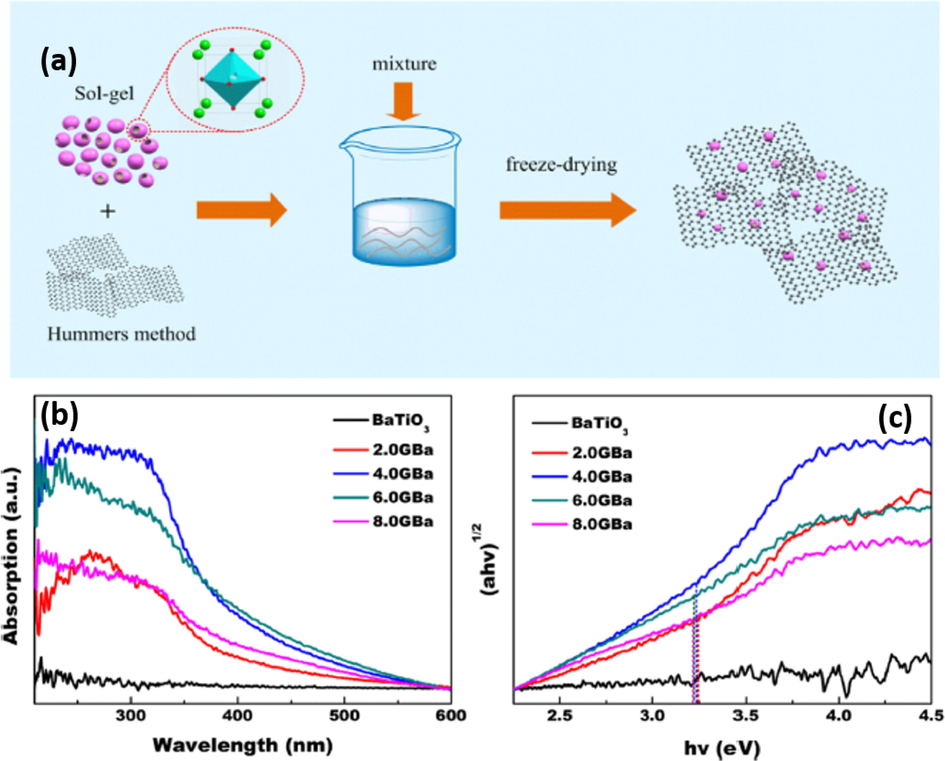
(a) schematic illustrations of BaTiO3/GO synthesis, (b) UV–vis absorption spectra and (c) Tauc’s plot (Photon energy curves) for composites (Zhao et al., 2018).
5.3 Formation of semiconductor heterojunction photocatalyst
The combination of BaTiO3 with other several semiconductors to form BaTiO3 based heterojunction photocatalyst is another approach of improving piezo-photocatalytic efficiency of the BaTiO3 pristine. Mostly metal oxide semiconductors such as TiO2, ZnO, SnO2, Bi2O3, Bi2WO6 and Cu2O which have an unequal band gap as BaTiO3 are used to form heterojunctions (Mengying et al., 2017; Ray et al., 2021; Sharma et al., 2016; Wang et al., 2021). The formation of a heterojunction results in a band alignment which promotes the extension lifetime of the photoexcited holes and electrons within the heterostructured catalyst, thus reducing the rate of electron and holes recombination. Heterojunctions such as p-n (between a p-type semiconductor and an n-type semiconductor), n-n (between two n-type semiconductors), and p-p (between two p-type semiconductors) can be created depending on the kind of semiconductors that are combined. Furthermore, the band alignment of the heterostructured catalyst can be classified as Type I (straddling), Type II (staggered), and Type III (broken). In semiconductors, type II (including Z scheme) have been reported to efficiently improve electrons and holes separation (Orimolade and Arotiba, 2020). The charge transfer mechanism of type II was explained more in detail by Orimolade et al., Zhang et al. and Peleyeju et al. (Orimolade and Arotiba, 2020)(Zhang and Jaroniec, 2018)(Peleyeju and Arotiba, 2018). As shown in Fig. 17, electron transfer within a heterojunction interface commonly follows a two-step pathway depending on the Femi energy level of the coupled semiconductors. On the first pathway mechanisms (Fig. 17(a)), when the Fermi energy level of SC-1 (p-type semiconductor) is smaller than that of SC-2 (n-type semiconductor), electrons (e-) migrate from SC-1 conduction band (CB) to SC-2 conduction band (CB), while holes (h+) migrate from SC-2′s valence band (VB) to SC-1′s valence band (VB). However, when the Fermi energy level of SC-1 is greater than that of SC-2, electrons (e-) from SC-2 merge with the holes (h+) from SC-1 following band alignment in the heterojunction, thus resulting in electrons and holes separation from SC-1 and SC-2 in Fig. 17(b). The accessible separated holes (h+) in SC-2 and electrons (e-) in SC-1 are responsible for piezo-photocatalytic breakdown of organic waste pollutants. This type mechanism pathway of electrons and holes separation is known also as Z-scheme (Fig. 17(b)).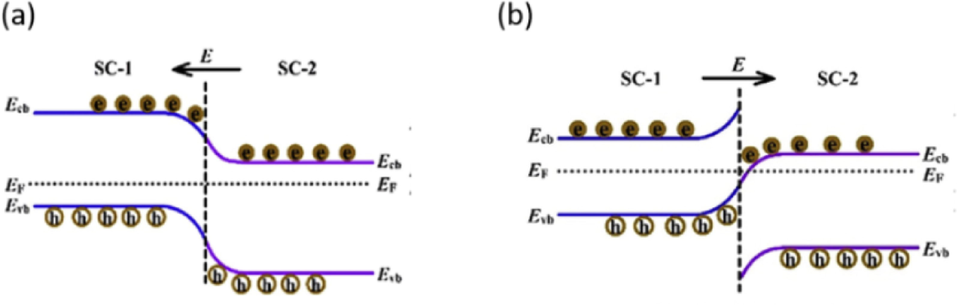
(a) Electrons and holes separation pathway in type II heterojunction and (b) Z-scheme heterojunction charge separation pathway (Zhang and Jaroniec, 2018).
Several BaTiO3 based heterojunctions have been fabricated using different synthetic methods such as hydrothermal, sol–gel, solid state method and co-precipitation method for various applications, including wastewater treatment. In water and wastewater treatment, BaTiO3 have been coupled with several metal oxide semiconductors including ZnO, SnO2, TiO2, Bi2O3, Fe2O3 and MnO2 for better piezocatalytic/photocatalytic removal performance. Other non-metal oxides including g-C3N4, Ag3PO4 and AgBr have also been coupled with BaTiO3 for improved photocatalytic/piezocatalytic activity (Mengying et al., 2017; Ray et al., 2021). Feng et al. synthesized BaTiO3/SnO2 hybrid heterostructured catalyst using the hydrothermal method for piezocatalytic degradation of organic contaminates (Feng et al., 2020). The effect of SnO2 loading on BaTiO3 had a huge impact of piezo-current response, as shown in Fig. 18(a), BaTiO3 loaded with SnO2 and SnO2-Sb generated greater piezoelectrochemical current response than pure BaTiO3. Furthermore, electrochemical impedance measurements showed the evidence of improved electron mobility via reduction in charge transfer resistance (Rct) of the composites (BaTiO3/SnO2 and BaTiO3/SnO-Sb) (Fig. 18(b)).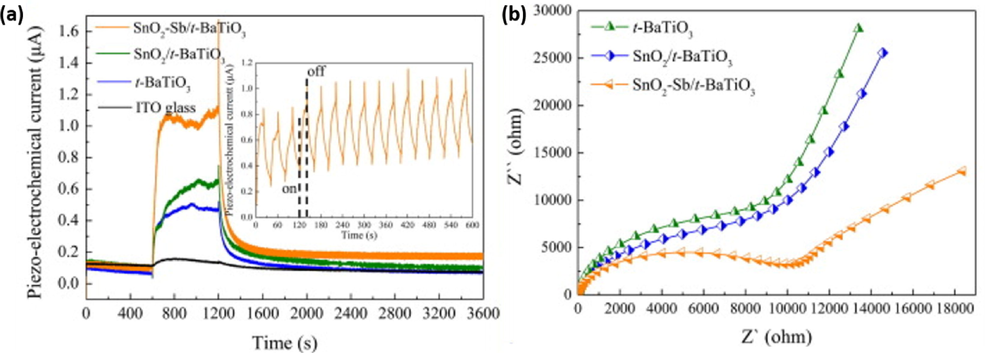
(a) Piezoelectrochemical current response and (b) electrochemical impedance (EIS) for BaTiO3, BaTiO3/SnO2 and BaTiO3/SnO-Sb (Feng et al., 2020).
6 Piezocatalytic, photocatalytic and piezo-photocatalytic removal of organic waste pollutants and bacteria
Over the past decades, several wastewater technologies including non-destructive and destructive methods as shown in Fig. 19, have been employed to remove toxic organic contaminates and pathogenic bacteria. Among them, advanced oxidation methods have been extensively applied as the most effective methods that accelerates the oxidation and degradation of a wide range of organic and inorganic chemicals that are resistant to traditional treatment methods. Piezocatalaysis is one of the emerging AOPs which uses energy harvesting materials called piezoelectric materials to convert mechanical energy into electrical energy. Recently, pieozocatalysis has gained much attention in several electrochemical applications including bacterial disinfection (Kumar et al., 2019a), hydrogen production (Hong et al., 2010), wastewater treatment and degradation of water pollutants (Mengying et al., 2017). In bacterial disinfection and degradation of pollutants, the piezoelectric materials (piezocatalyst) generate negative and positive electric charges under the influence of mechanical vibration at opposite surfaces. These free electric charges are responsible for redox reactions resulting in highly reactive species (ROS) such as •O2– and •OH. These strong reactive oxygen species (ROS) are capable of breaking down toxic organic compounds into less toxic compounds, hydroxyl radicals (•OH) have been recognized as secondary oxidants (after the strongest fluorine) due to their high standard reduction potential (Eo •OH/H2O) of roughly 2.8 V versus SHE. Coupling piezocatalysis with photocatalysis can enhance the degradation performance of piezo-photocatalyst and supress the rate of electron and holes recombination in photocatalytic degradation processes. Therefore, this article intends to review enhanced piezo-photocatalytic degradation of organic dyes (section 6.1), pharmaceuticals (section 6.2) and bacteria (section 6.3) using BaTiO3 based catalysts.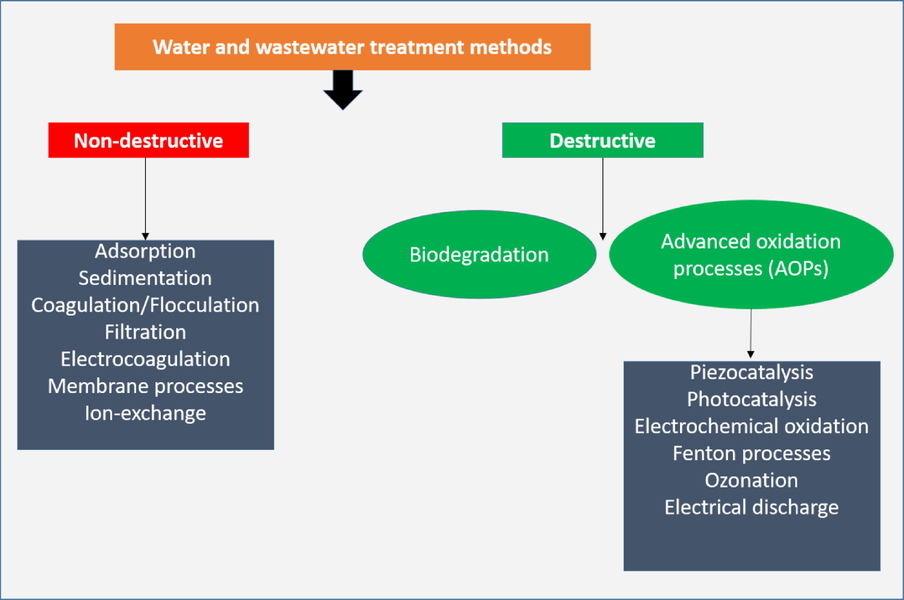
Schematic diagram of non-destructive and destructive water and wastewater treatment methods.
6.1 Decomposition organic dyes
In the past, BaTiO3 based catalysts have been extensively investigated for their piezocatalytic and piezo-photocatalytic removal ability of several wastewater pollutants including organic dyes. For example, Wu et al. synthesized BaTiO3 nanoparticles and nanowires using a two-step hydrothermal method for piezocatalytic removal of methyl orange (MO) from wastewater (Wu et al., 2018b). Under ultrasonic vibration, BaTiO3 nanowires (NWs) were easily deformed therefore showed better piezocatalytic performance as compared to BaTiO3 nanoparticles (NPs) with poor deformability. The highest piezocatalytic efficiency obtained by BaTiO3 NWs under ultrasonic vibration (power 80 W) was about 92 % within 160 min, with reaction processes following pseudo-first order kinetics model (Fig. 20(a)). Scavenger studies were conducted to investigate the reactive oxygen species that were more effective in breaking down of MO into CO2 and H2O. Various trapping agents such as tert-butyl alcohol (TBA), benzoquinone (BQ) and disodium ethylene diamine tetra-acetate dehydrates (EDTA-2Na) were used to supress hydroxyl radicals (•OH), superoxide (•O2–) and holes (h+), respectively. As shown in Fig. 20(b), upon the addition of TBA, the degradation efficiency reduced dramatically thus confirming that hydroxyl radicals (•OH) were the most effective ROS species for MO break down, followed by superoxide radicals ((•O2–). Another study was conducted by Hong et al., were BaTiO3 as a piezocatalyst generated more hydroxyl radicals (•OH) and superoxide radicals ((•O2–) to break down Acid orange 7 (AO7) dye into CO2 and H2O (Hong et al., 2012). Under piezoelectric effect, the strained BaTiO3 dendrites decomposed about 80 % of the AO7 dye after 90 min. Several factors including influence of pH, catalyst dose and initial concentration which can affect the piezocatalytic process were investigated. In case of catalyst loading, the piezocatalytic efficiency increased with an increase in catalyst dosage until reaching a plateau region with 0.025 g of BaTiO3 catalyst. This was due to more available strained induced charges on the surface of BaTiO3 as its total surface-active sites increased with an increase in the amount of catalyst dosage. Therefore, resulting in enhancement of piezocatalytic degradation efficiency. The pH solution and initial AO7 concentration significantly influenced piezocatalytic processes, the piezocatalytic efficiency decreased with an increase in intial AO7 concentration. It was speculated that as initial concentration increases, more AO7 molecules increases which cover less active surface sites of the catalyst thus leading to a decrease in piezocatalytic efficiency. The highest piezocatalytic efficiency was slightly reduced in alkaline media and enhanced in acidic media. In acidic conditions, the surface of the BaTiO3 dendrites is protonated (positively charged) thus enlarges electrostatic interaction between anionic AO7 dye (negatively charged) and positively charged BaTiO3 surface, and resulting in higher piezocatalytic removal of AO7.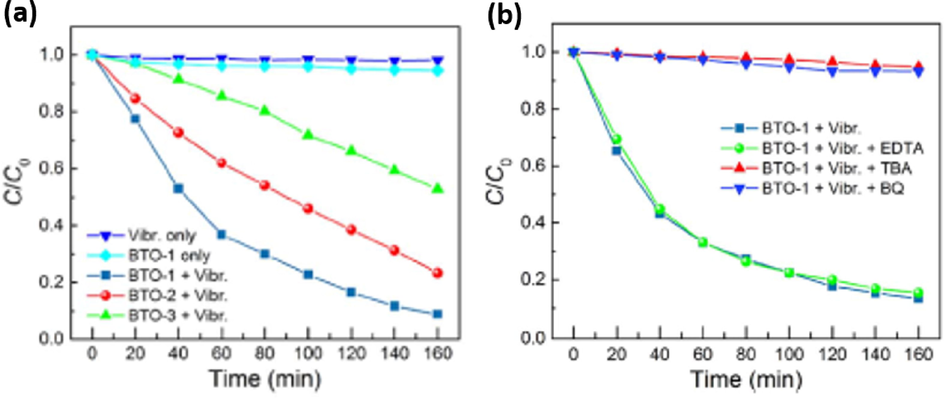
(a) Piezocatalytic degradation of MO by BaTiO3 NWs and (b) Scavenger studies (Wu et al., 2018b).
To improve the photocatalytic activity of BaTiO3, Li et al. fabricated new hybrid composites (Ag2O-BaTiO3) by combining BaTiO3 ferroelectric with Ag2O semiconductor. Under ultrasonic vibration, an internal electric field was generated by ferroelectric BaTiO3 nanocrystal to reduce the rate of electrons and holes recombination thus enhancing the photocatalytic performance of the hybrid composite (Ag2O-BaTiO3) (Li et al., 2015). Fig. 21 shows the effect of ultrasonic vibration on the photocatalytic degradation of Rh B dye using hybrid Ag2O-BaTiO3 photocatalyst. Four photocatalyst materials such as commercial P25 nanoparticles, Ag2O, BaTiO3, mixture of BaTiO3 and Ag2O were used for photocatalytic degradation comparison study. As depicted in Fig. 21(a), P25, BaTiO3 nanocubes, or Ag2O nanoparticles were not effective in the degradation of Rh B under ultrasonic irradiation only. However, the physical mixture of Ag2O and BaTiO3 as well as the Ag2O-BaTiO3 hybrid composite showed a slight deterioration of Rh B. These results shows that the combination of ferrolectric BaTiO3 nanoctrystal and Ag2O semiconductor can improve piezocatalysis/sonocatalysis performance of the Ag2O-BaTiO3 hybrid piezocatalyst. Fig. 21(b) illustrates the photocatalytic degradation of Rh B with all four samples in the absence of an ultrasonic irradiation. The synthesized BaTiO3 showed no photocatalytic degradation towards Rh B, whereas Ag2O, Ag2O-BaTiO3 and their physical mixtures showed higher photocatalytic degradation performance towards the removal of Rh B. Under both UV light and ultrasonic irradiation, Ag2O-BaTiO3 hybrid photocatalyst completely degraded all Rh B within a short space of time (1.5 h) (Fig. 21(c-d)). Their piezo-photocatalytic or sono-photocatalytic mechanisms were explained as follows: under UV light irradiation, the Ag2O surface generates electrons (e-) and holes (h+), these charge carriers (e-, h+) are required to be separated in order to produce reactive oxygen species (ROS) such as hydroxyl and superoxide radicals for deterioration of Rh B. Under ultrasonic vibration, the ferroelectric BaTiO3 nanocrystal in the Ag2O-BaTiO3 hybrid composite generated an internal piezo-electric field which acted as a driving force for the separation of charge carrier’s (electrons and holes) (Fig. 21(e-f)). The suppression of rapid electrons and holes recombination separation led to an improved photocatalytic activity of the hybrid composite (Ag2O-BaTiO3).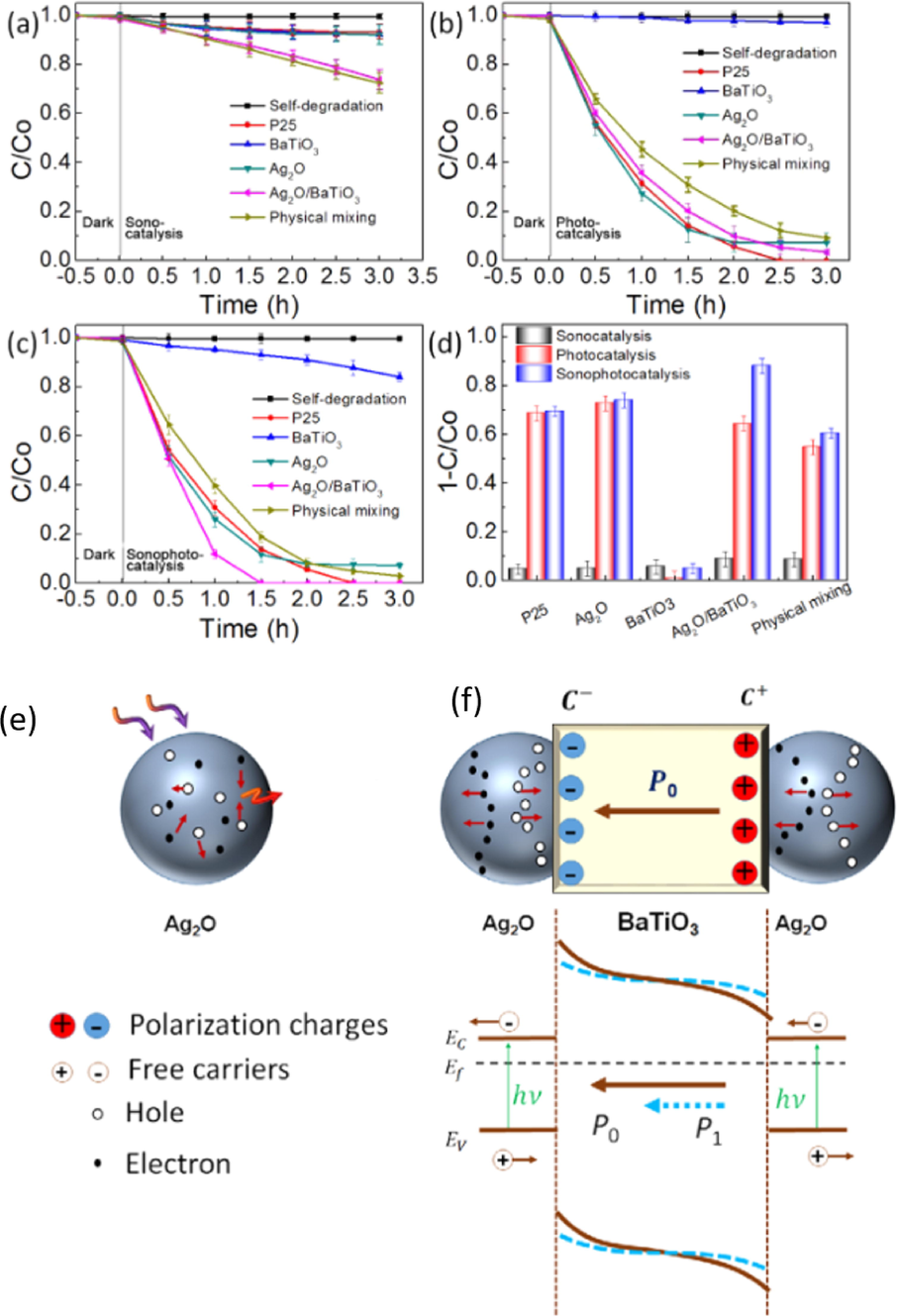
(a) sonocatalytic, (b) photocatalytic, (c) sono-photocatalytic degradation of Rh B (d) sono-photocatalytic kinetics and (e-f) sono-photocatalytic mechanism (Li et al., 2015).
In addition, BaTiO3 photocatalytic performance was further improved by modifying it with several co-catalysts such as ZnO, SnO2, Fe2O3, TiO2 and Bi2O3. Heterostructured BaTiO3/ZnO composites were prepared using the sol–gel and hydrothermal methods for the degradation of MB, MO, and RhB (Kappadan et al., 2020b; Karunakaran et al., 2014; L. Wang et al., 2019). In the study conducted by Kappadan et al., hydrothermal method was employed to prepare n-n heterojunction photocatalyst of BaTiO3/ZnO using Barium acetate, titanium tetra isopropoxide and Zinc nitrate hexahydrate as precursors (Kappadan et al., 2020b). The scanning electron microscopic images of the composite (BaTiO3/ZnO) clearly showed spherical BaTiO3 nanoparticles anchored on hexagonal rod-shaped ZnO. The formation of n-n heterojunction resulted in improved photocatalytic performance, as evidenced by the reduction of band gap energy from 3.1 to 2.97 eV and charge transfer resistance from 871 to 745 Ω. The mechanisms, as shown in Fig. 22(a), revealed that an improved charge carriers separation was achieved by forming a typical type II band alignment within the BaTiO3/ZnO heterojunction interface, which allowed BaTiO3 photogenerated electrons to migrate into the conduction band (CB) of ZnO, whereas ZnO photogenerated holes were transported into the valence band (VB) of BaTiO3. The prepared heterostructured BaTiO3/ZnO (BTZ) photocatalyst showed good stability since even after the 3rd cycle the degradation efficiency was above 91 % for methylene blue (MB) (Fig. 22(b)). However, after the 4th cycle the photocatalytic degradation efficiency slightly reduced from 91 to 86 %. The total mineralisation of MB dye was calculated using TOC, under UV light irradiation, the BTZ heterostructure recorded a TOC removal of 87.1 % after 60 min. A recent report by Liu et al. modified BaTiO3 with TiO2 via hydrothermal process to form BaTiO3-TiO2 core–shell heterostructures (Liu et al., 2019). The heterostructured photocatalyst was applied for photocatalytic deterioration of Rh B dye from wastewater. The ratio of BaTiO3:TiO2 played a significant role in the photocatalytic degradation of Rh B. All BaTiO3-TiO2 core–shell heterostructures with different molar ratios exhibited better photocatalytic removal towards Rh B as compared to pure BaTiO3. The BaTiO3-TiO2 core–shell heterostructures (1.2:1) showed the greatest photocatalytic performance compared to other samples, its performance was 1.8 times greater than pure TiO2. The improved photodegradation activity was due to the fact that BaTiO3-TiO2 core–shell heterostructures (1.2:1) exhibited the lowest photoluminiscent (PL) intensity thus lowering the rate of electrons and holes recombination. Wu et al. boosted the photocatalytic activity of heterostructured BaTiO3/TiO2 nanocomposites with piezotronic effect under ultrasonic vibration (J. Wu et al., 2020). Under ultrasonic activation, the built-in electric field exhibited by ferroelectric BaTiO3 facilitated the charge transfer and separation within BaTiO3/TiO2, thus improving its photocatalytic activity. Comparing with other BaTiO3 based metal oxides (Alex et al., 2019; Cui et al., 2017; Fan et al., 2012; Karunakaran et al., 2014; Lin et al., 2007; Liu et al., 2019; Selvarajan et al., 2017; Zhou et al., 2019), BaTiO3/TiO2 composite outperformed them in terms of photocatalytic performance due to excellent suppression of electrons and holes recombination.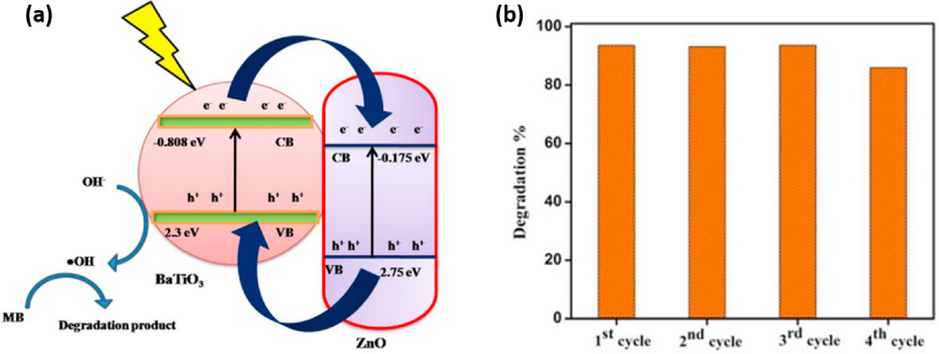
(a) Photocatalytic degradation mechanism of MB using BaTiO3/ZnO and (b) re-usability of BaTiO3/ZnO (Kappadan et al., 2020b).
Plasmonic photocatalysts have also attracted a lot of attention in the photocatalytic removal of organic pollutants in wastewater. Several noble metals including platinum (Pt), gold (Au), rhodium (Rh) and silver (Ag) have been doped with BaTiO3 to promote chemical redox reaction under UV light and ultrasonic irradiation. For example, chao et al. fabricated a heterostructured Au@BaTiO3 photocatalyst using the hydrothermal method for the breakdown of Rh B under UV light exposure (Chao et al., 2020). The plasmonic heterostructured photocatalyst showed an enhanced photocatalytic activity towards the removal of Rh B. The heterostructured composite (Au@BaTiO3) nearly degraded about 100 % of Rh B after 36 min and the reaction process followed Langmuir-Hinshelwood model. The degradation rate constant (k) obtained from Langmuir-Hinshelwood model were 0.05446 and 0.01118 min−1 for Au@BaTiO3 and pure BaTiO3, respectively. The apparent rate constant (k) for Au@BaTiO3 was 4.9 times than that of pristine BaTiO3. These results confirmed an enhancement in the photocatalytic properties of heterostructured Au@BaTiO3 photocatalyst. Several studies investigated the photocatalytic performance of silver (Ag) doped BaTiO3 photocatalyst for the catalytic degradation of hazardous organic dyes (Rh B and MO) (Cui et al., 2013; Lin et al., 2021; Nithya and Devi, 2019; Xu et al., 2019). For instance, Khan et al. reported Ag-doped BaTiO3 prepared via sol–gel method for photodegradation of Rh B (Khan et al., 2021b). The XRD and HR-TEM results confirmed their tetragonal phase and crystallite size range of 46–54 nm, respectively. The incorporation of silver (Ag) dopants into BaTiO3 reduced its band gap from 3.87 to 3.47 eV. Furthermore, they observed a reduction in photoluminescent peak intensity upon addition of silver ions into tetragonal BaTiO3, confirming restrain in electrons and holes recombination. The BET surface of Ag (5 %)-doped BaTiO3 had a higher surface area of 20.1 m2.g−1 as compared to pure BaTiO3 (15.4 m2.g−1). Therefore, due to Ag-doped BaTiO3 having a higher surface area and lower band gap, their photocatalytic performance was improved. Under light irradiation (400 W sodium lamp), 1 %Ag@ BaTiO3, 3 %Ag@BaTiO3 and 5 %Ag@BaTiO3 showed better photocatalytic performance than pure BaTiO3. The improved photocatalytic activity of Ag-doped BaTiO3 could be due to the reduced rate of electrons and holes recombination, surface area increase and reduction in band gap energy after Ag-deposition. The highest photocatalytic removal for Rh B was 79, 58, 46 and 51 % when 5 %Ag@BaTiO3, 3 %Ag@ BaTiO3, 1 %Ag@ BaTiO3 and BaTiO3 were applied as photocatalysts, respectively. Niu and Xu observed the same photocatalytic enhancement when Ag-BaTiO3 was further co-doped with other metal dopants such as Ni, Pd and Pd–Sn–Ni through a one-step ball milling process (Niu and Xu, 2019). The co-doped Ag@BaTiO3 rate constant was 4.5 times greater than undoped BaTiO3. Table 4 summarizes an overview application of BaTiO3-based piezo/photocatalyst for catalytic degradation of organic dyes in water and wastewater.
Catalyst
Organic dyes
Reaction conditions
% Removal & reaction time
Refs
BaTiO3
MO
80 W Ultrasonic
92 %, 160 min
(Wu et al., 2018b)
AO7
40 kHz Ultrasonic
80 %, 90 min
(Hong et al., 2012)
Ag2O-BaTiO3
Rh B
50 W ultrasonic + UV light
100 %, 90 min
(Li et al., 2015)
BaTiO3/ZnO
MB
250 W UV lamp
93.6 %, 60 min
(Kappadan et al., 2020b)
Rh B
Mercury vapor lamp (8 W)
100 %, 60 min
(Karunakaran et al., 2014)
Rh B
120 W Ultrasonic + Xe lamp (500 W)
97 %, 30 min
(Zhou et al., 2019b)
MO
50 W high-presure mercury lamp
NA
(Ren et al., 2012)
BaTiO3-TiO2
Rh B
mercury-xenon lamp (200 W)
>70 %, 120 min
(Liu et al., 2019)
Rh B
300 W Ultrasonic + mercury lamp(250 W)
∼100 %, 60 min
(J. Wu et al., 2020)
Rh B, MB & IC
Ultrasonic (45 kHz, 200 W) + Xe lamp (300 W)
99.5, 99.8 & 99. 7 %
(Liu et al., 2022)
BaTiO3/BiOI
MO
Ultrasonic (40 kHz, 300 W) + 300 W Xe lamp
95.4 %, 90 min
(Liu et al., 2022)
BiFeO3 − BaTiO3
Rh B, MB & MO
Ultrasonic (100 W, 40 kHz)
99.5, 90.1 & 84.5 %
(Sun et al., 2021)
CoFe2O4/BaTiO3
MB
Osram UV lamp (250 W)
99.3 %, 5 hr
(Dang et al., 2018)
Ag– BaTiO3/TiO2
MB
Xenon lamp (300 W)
95 %, 180 min
(Q. Li et al., 2013)
Bi2O3/BaTiO3
MO
500 W high-pressure mercury lamp
98.9 %, 50 min
(Lin et al., 2007)
Rh B
500 W mercury lamp
100 %
(Fan et al., 2012)
MO
50 W high-pressure mercury lamp
NA, 140 min
(Ren et al., 2013)
BaTiO3/SnO2
MB
300 W Xenon lamp
83.67 %, 180 min
(Selvarajan et al., 2017)
MB
300 W Tungsten lamp
>85 %, 300 min
(Nageri et al., 2017)
Ag-BaTiO3
MO
350 W high-pressure mercury lamP
98.53 %, 60 min
(Liu et al., 2012)
Rh B
solar simulator (100 mW cm − 2)
∼100 %, 60 min
(Cui et al., 2013)
Rh B
solar light, visible light and UV
99 %, 60 min
(Cui et al., 2015)
MO
ultrasonic cleaner (120 W)
81 %, 120 min
(Lin et al., 2018)
Rh B
ultrasonic (70 W)
∼94 %, 150 min
(Z. He et al., 2021)
Au-BaTiO3
Rh B
UV light
100 %, 36 min
(Chao et al., 2020)
Aux/BaTiO3 film
Rh B
500 W Xe lamp film
45 %, 300 min
(Zhang et al., 2016)
Mn-BaTiO3
MB
300 W Tungsten lamp
97.04 %, 360 min
(Nageri and Kumar, 2018)
Ce-BaTiO3
MB, CR & MV
300 W Tungsten lamp
90, 78.5 & 82.4 %
(Senthilkumar et al., 2019)
I-BaTiO3
CR
125 W mercury vapor lamp
89.33 %, 150 min
(P.M and Devi, 2020)
BaTiO3/AgI
Rh B
Visible light
92.2 %, 15 min
(C. He et al., 2021)
OA-BaTiO3
Rh B
Ultrasonic bath (40 kHz, 100 W)
>87 %, 30 min
(Gao et al., 2021)
n-BaTiO3/Ag/p-AgBr
Rh B
200-W xenon lamp
99.3 %, 12 min
(Y. Wang et al., 2020)
Ag/BaTiO3/MnOx
Rh B
300 W xenon lamp
98.10 %, 120 min
(Cui et al., 2020)
Ag-Ag2S)/BaTiO3
MO
UV light + Ultrasonic vibration
90 %, 30 min
(Y. Wang et al., 2020)
BaTiO3/GO
MB
Xe lamp (GX-500)
69.5 %, 8 hr
(Wang et al., 2015)
MB
irradiation of Xe light
80 %, 150 min
(Zhao et al., 2018)
Rh B
500 W halogen lamp
>80 %, 90 min
(Rastogi et al., 2016b)
Rh B & MO
Ultrasonic (35 kHz, 200 W) + 150 W halogen lamp
97.3 & 81.9 %
(Rastogi et al., 2016a)
MB
Ultrasonic cleaner (100 W, 40 kHz)
98 %, 120 min
(J. Wang et al., 2020)
BaTiO3/MoO3
Rh B
150 W halogen lamp
86 %, 60 min
(Alex et al., 2019)
BaTiO3/g-C3N4
Rh B
vibration source (40 kHz)
82 %, 200 min
(Zheng et al., 2020)
MO
200 W xenon lamp
76 %, 6 hr
(Xian et al., 2015)
MB
Sun light
91 %, 60 min
(Kappadan et al., 2021)
MB
Visible light (75 W–220 V lamp)
98.72 %, 7 hr
(Nguyen et al., 2021)
BaTiO3/CuO
MO
Ultrasonic cleaner + 200 W xenon lamp
∼100 %, 90 min
(Yu et al., 2022)
BaTiO3/KNbO3
DLB 5B
300 W xenon lamp + ultrasonic cleaner (45 KHz)
93.3 %, 180 min
(Y. Zhang et al., 2021)
BaTiO3/Au/g-C3N4
Rh B
300 W xenon lamp
100 %, 20 min
(M. Wu et al., 2020)
BaTiO3/In2S3
MO
350 W-xenon lamp
93 %, 90 min
(Wei et al., 2019)
SnS2/BaTiO3
MO
350 W-xenon
98 %, 20 min
(Zhang et al., 2016)
6.2 Pharmaceuticals
In recent decades, antibiotics have been identified as emerging contaminants owing to their endurance in aquatic ecosystems. They are widely used for treating numerous bacterial infections. However, they find their way into several waterbodies through incorrect disposal of unused or expired medications and human excretion. In ground and surface water, antibiotics are found in lower concentrations ranging from μg/L to mg/L (Cabeza et al., 2012; Orimolade et al., 2021b; Wang and Wang, 2016). Unfortunately, the detection of these pharmaceuticals in aquatic environment can lead to several harmful biological and economic impacts. For example, continuous ingestion of drinking water contaminated with pharmaceuticals can result in the formation of drug-resistant bacterium strains to humans and animals. The elimination of several emerging pharmaceuticals by BaTiO3 based ferroelectric/piezo-photocatalyst system have attracted so much attention recently. Kurniawan et al. investigated the photocatalytic performance of BaTiO3 and BaTiO3/TiO2 composites for the removal of acetaminophen (Ace) from distilled water under UV–vis irradiation (Kurniawan et al., 2018). The XRD patterns confirmed the cubic phase structure of BaTiO3 nanoparticles. The BaTiO3/TiO2 composites as compared to individual pristine (BaTiO3 and TiO2) performed better, thus confirming that the synergetic effect of the two semiconductors can improve visible light absorption and efficient charge separation. Furthermore, it was found that the photocatalytic performance of the composites (BaTiO3/TiO2) can be enhanced by varying the molar weight ratios (w/w) of BaTiO3 and TiO2. Under optimal conditions, dosage of 1 g/L, pH 7, 4 hrs reaction time and initial Ace concentration of 5 mg/L, BaTiO3, TiO2 and BaTiO3/TiO2 photocatalytically degraded about 18, 33 and 95 % of Ace respectively. As shown in Fig. 23, initial degradation pathways for Ace were through hydroxylation and photolysis. The intermediates formed during this route included hydroquinone and 1,4-benzoquinone, which were consistent with the results obtained by Zhang et al. and Aguilar et al. (Zhang et al., 2008) (Aguilar et al., 2011). The hydroxyl radicals (•OH) further attacked these intermediates to form hydroxylation products. The first detected intermediate was quinoneimine which was effortlessly hydrolized to 1,4-benzoquinone. The intermediates were then oxidized further into carboxylate acid and carbon dioxide by destroying their aromatic structures. The total mineralization via photocatalysis process generally does not occur quickly, however after some few hours these organics can be totally mineralized. Demircivi and Simsek reported tungsten-doped BaTiO3 (W-BaTiO3) for enchanted photocatalytic removal of tetracycline under and visible light-driven and UV-A light irradiation (Demircivi and Simsek, 2019). The composite was prepared through a simple hydrothermal method using a Teflon-lined stainless-steel autoclave at 200 °C, followed by washing, drying and calcination for 2 hrs at 700 °C. The effect of tungsten (W) loading on BaTiO3 was investigated for tetracycline degradation. It was found that BaTiO3 doped with low amounts of tungsten (W) exhibited higher photocatalytic activity than pure BaTiO3 and BaTiO3 doped with higher amount of tungsten (W). According to this study, pH played a significant role in the photocatalytic degradation of tetracycline. The photocatalytic degradation removal percentage increased with an increase in pH solution. The degradation efficiency was recorded to be 3, 80 and 90 % at pH 3, 5.60 and 10, respectively. In addition, tungsten-doped BaTiO3 (W-BaTiO3) was used to treat spiked real water samples.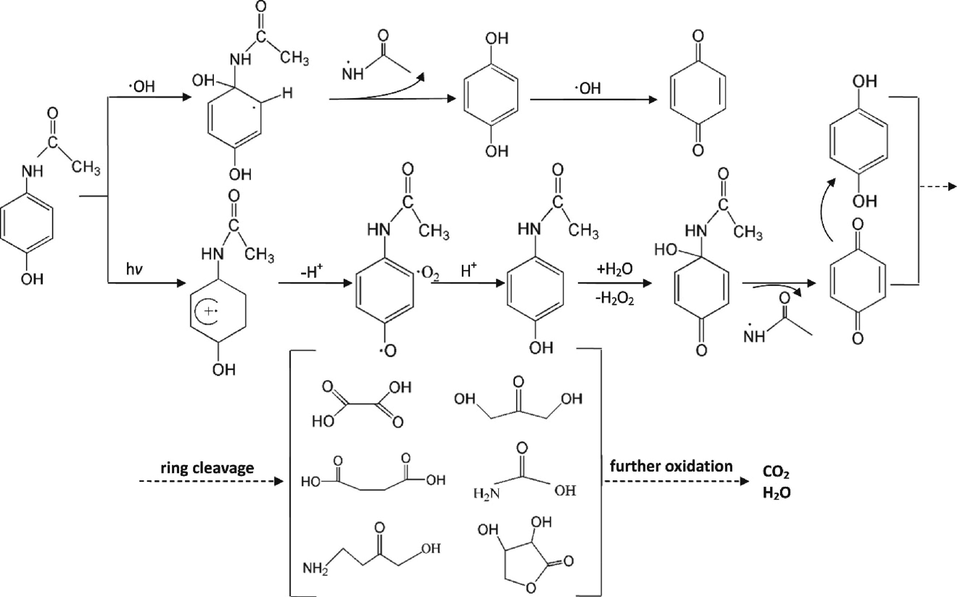
Photocatalytic degradation pathway for acetaminophen (Ace) (Kurniawan et al., 2018).
(tap and drinking water). Under light irradiation (after 3 hrs), the composite achieved a degradation efficiency of 74 and 76 % in drinking and tap water, respectively. The decrease in the degradation efficiency from tap and drinking water was due to other various pollutants or ions detected in the water matrices, which has some negative effect on the photocatalytic process (Bilgin Simsek, 2017). In a study conducted by Demircivi et al., decorated BaTiO3 with carbon fibers (CFs) were synthesized for enhanced photocatalytic degradation of tetracycline (Demircivi et al., 2020). The incorporation of CFs to BaTiO3 reduced the band gap of the composite and showed enhancement in photocatalytic activity of BaTiO3/CF. Under UV and visible light irradiation, BaTiO3/CF showed the highest degradation efficiency of 96 % as compared to BaTiO3 (W-BaTiO3). According to re-usability studies, the composite showed the highest stability within 5 cycles. However, after 6 cycles, a sharp decline in degradation efficiency was observed from 96 to 77 %. This could be due to the loss of tiny photocatalyst powder with an increase in recyclability. Almost a 100 % degradation efficiency was reported when Ti32-oxo-cluster/BaTiO3/CuS p-n heterojunction was employed for wastewater treatment under both visible light irradiation and mechanical vibration (Piezo-photocatalysis) (Zhou et al., 2021).
The piezoelectric/ferroelectric materials including ZnSnO3, MoS2, NaNbO3, BiFeO3, KNbO3, BiOCl, Bi4 Ti3O12 and BaTiO3 nanoparticles in powder forms have been utilized as promising piezocatalysts for several applications. However their applications are limited in cleaning water due to the inability of being recovered from aqueous solution (Lin et al., 2020). Sharma et al. investigated the piezocatalytic activity of cement-based BaTiO3 composites for the removal of several pollutants in water such as pharmaceutical (paracetamol) and dyes ((Rh B), (MO) and (MB)) (Sharma et al., 2020). Sharma and co-workers, combined powdered BaTiO3 nanoparticles with cement to form cement-ferroelectric composites which can be easily recovered from aqueous solution after wastewater treatment. Under ultrasonic vibration, the poled BaTiO3 cement composites showed significant piezocatalytic removal of all organic pollutants. The poled composite exhibited the highest piezocatalytic degradation of approximately > 90, 86, 85, and 79 % for Rh B, MB, MO and paracetamol, respectively. The composites could be reused up to the 5th cycle of piezocatalysis under ultrasonic vibration. Another antibiotic drug which is in the class of fluoroquinolone antibiotics is Norfloxacin (NFX). This antibiotic drug is not easily degradable and can further contribute to antibiotic resistance when used. Therefore, the double Z-scheme of BiFeO3/CuBi2O4/BaTiO3 was fabricated by Zhang et al., and employed for photocatalytic degradation of Norfloxacin (NFX) under solar-light irradiation (Zhang et al., 2020). The combination of BiFeO3, CuBi2O4 and BaTiO3 to form the composites, extended the light absorption to UV–visible and near-infrared (NIR) light to allow efficient use of the whole solar light spectrum. As shown in Fig. 24(a), the nanocomposites Z-scheme of BiFeO3/CuBi2O4/BaTiO3 strongly absorbed in the 200–800 nm range. The calculated optical band energy gap from the tauc’s plot were approximately 3.30, 1.76, 2.29 and 2.20 eV for BaTiO3, CuBi2O4, BiFeO3 and BiFeO3/CuBi2O4/BaTiO3, respectively (Fig. 24(b)). The effect of irradiation time, catalyst amount and initial NFX concentration were investigated on the photocatalytic performance of the Z-scheme composites. It was noticed that as irradiation time and catalyst dosage increased, the degradation efficiency also increased (Fig. 24(c-d)). The degradation rate increased with an increase in initial NFX concentration within a particular concentration range (Fig. 24(e)), this was due to the fact that more NFX molecules remained in aqueous solution at higher concentrations. Under the same optimal parameters (dosage of 1.0 g/L, within 60 min, and NFX concentration of 2.5 mg/L), BiFeO3/CuBi2O4/BaTiO3 nanocomposites had a better photocatalytic activity than individual samples of BaTiO3, CuBi2O4, BiFeO3. The composite reached its highest degradation of 93.5 % which could be attributed to a better separation of electrons (e-) and holes (h+) to improve the redox ability thus enhancing the photocatalytic activity of the catalyst. The electrons and holes separation were confirmed by photoluminescence (PL) spectrum (Fig. 24(f)). Generally, a low PL intensity reveals greater electrons (e-) and holes (h+) separation efficiency. As shown in Fig. 24(f), BiFeO3/CuBi2O4/BaTiO3 had the lowest PL intensity than BaTiO3, CuBi2O4 and BiFeO3, thus indicating better enhancement in photocatalytic activity of the composite. The extent of mineralization of NFX (10.0 mg/L) obtained from TOC was 3.9, 6.8, 40.9 and 60.3 % for BaTiO3, CuBi2O4, BiFeO3 and BiFeO3/CuBi2O4/BaTiO3, respectively. However, the extent of mineralization for NFX (2.5 mg/L) reached up to 93.5 % within 1 hr. According to scavenger experiments conducted, it was found that hydroxyl radicals (•OH) and holes (h+) played an important role in the deterioration of NFX than superoxide radicals (•O2–).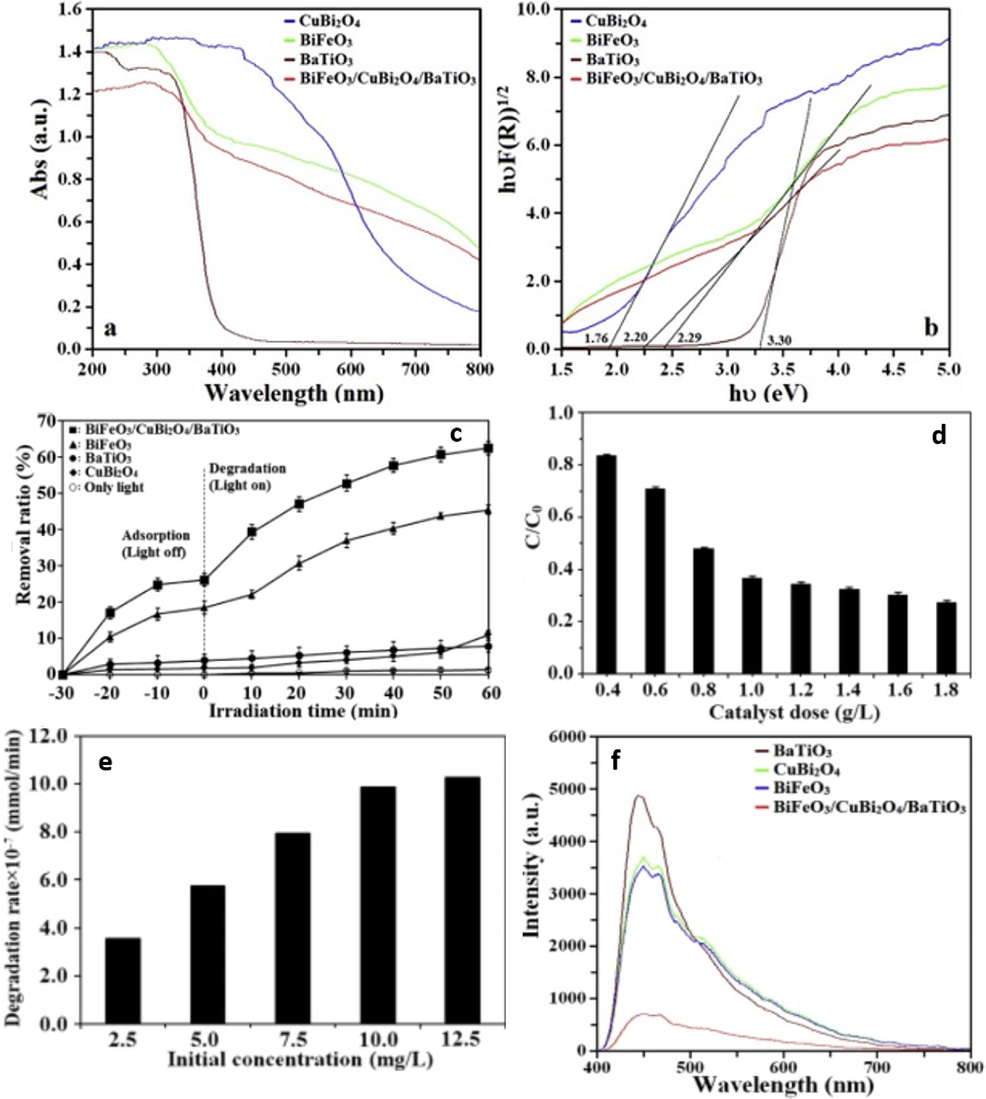
(a) UV–vis DRS spectra and (b) Tauc’s plot curves for BiFeO3, CuBi2O4, BaTiO3 and BiFeO3/CuBi2O4/BaTiO3, (c) effect of irradiation time (d) effect of catalyst dosage (e) effect of initial concentration o photocatalytic degradation and (f) (PL) spectra’s of materials (Zhang et al., 2020).
Pharmaceuticals used in aquaculture are also a source of pollution that is delivered directly into surface water. For example, atrazine is a well-known herbicide that is used to control broadleaf and grassy weeds in water. It was banned in most countries because of its negative impact on aquaculture and humans (Cavas, 2011). Due to that, Basaleh and Mohamed (Basaleh and Mohamed, n.d.) developed Copper (Cu)-doped BaTiO3 photocatalyst for the removal of this toxic herbicide (atrazine) from wastewater so as to provide clean water to the environment. The photocatalyst (Cu-BaTiO3) was prepared through the hydrothermal and photo-assisted deposition method. The prepared photocatalyst was added into 300 ml atrazine solution (50 ppm) and irradiated with Xenon lamp. The photocatalytic removal for atrazine using 0.5 Cu/BaTiO3, 1.0 Cu/BaTiO3, 3.0 Cu/BaTiO3 and 5.0 Cu/BaTiO3 were recorded to be 45, 65, 100 and 100 %, respectively. As for pure BaTiO3, the removal efficiency was low as 3 % due to the high electron and holes recombination. These results confirmed that doping BaTiO3 with Cu can suppress the rate of electrons and holes recombination, thus increasing photocatalytic performance of Cu-BaTiO3. The suppression of electrons and holes recombination was confirmed by photocurrent response and photoluminescence (PL) spectrum. The Cu/BaTiO3 had a greater photocurrent response of 12.8 mA cm−2 than pure BaTiO3 (2.8 mA cm−2). In another study, BaTiO3 was co-loaded with two electrocatalyst (Pt and RuO2) to promote sufficient redox ability for piezocatalytic degradation of tricyclazole under mechanical vibration (Feng et al., 2019). The platinum (Pt) was selected since it has been considered as the best catalyst for oxygen reduction, whereas RuO2 can produce large amounts of hydroxyl radicals and facilitate protons transport during electrocatalytic reaction. The loading of co-catalysts to BaTiO3 resulted in surface area increment from 25.5 to 28.8 m2g−1. Specific surface area of the materials is another factor which can influence the photocatalytic performance of the photocatalyst. Under ultrasonic vibration (40 kHz, 110 W), the composite achieved the highest removal percentage of 86 % which was higher than the values obtained using RuO2/t-BaTiO3 (51.0 %) and Pt/t-BaTiO3 (75.9 %). According to apparent rate constant values (k) obtained from pseudo-first order kinetics, the piezocatalytic reaction rate for the composites (k = 0.0320 min−1) was 3.11 times greater than pure BaTiO3 (k = 0.0103 min−1) and the sum of k values of Pt/t-BaTiO3 (0.0125 min−1) and RuO2/t-BaTiO3 (0.0124 min−1). The outstanding performance of the composite confirmed that coupling ferroelectric materials with a good catalyst can yield a synergistic enhancement effect on the piezocatalytic degradation. Another BaTiO3 based heterostructured catalyst which has been employed for piezocatalytic/photocatalytic and piezo-photocatalytic removal of pharmaceutical pollutants is BaTiO3/La2Ti2O7 heterojunction. Li et al. prepared BaTiO3/La2Ti2O7 composites via a two-step hydrothermal and microwave hydrothermal synthesis for piezo-photocatalytic degradation of ciprofloxacin (Y. Li et al., 2021). After 90 min of photocatalytic degradation, BaTiO3/La2Ti2O7 recorded a degradation efficiency of 16.5 and 22.7 % greater than that of BaTiO3 and La2Ti2O7, respectively, thus indicating that the formation of a heterojunction improved the photocatalytic degradation process. Under ultrasonic vibration (piezocatalysis), the degrading efficiency of CIP over BaTiO3/La2Ti2O7 (37.7 %) was roughly 19 % greater than that of BaTiO3 and La2Ti2O7. The degradation rate constant (k value) obtained from pseudo first-order kinetics model was 0.00593 min−1 for BaTiO3/La2Ti2O7, which is roughly 2.8 and 2.3 times greater than BaTiO3 and La2Ti2O7, respectively. The higher degradation efficiency of 50.2 % was achieved when piezocatalysis and photocatalysis were merged. For all samples, the performance of piezo-photocatalysis was superior to that of photocatalysis or piezocatalysis. This might be due to photocatalysis's low visible-light absorption and low carrier separation efficiency. The catalytic performance was likewise low during the piezocatalytic procedure due to the restricted amount of free charge carriers created. Photogenerated charge carriers were efficiently separated under the combined action of visible light and ultrasound, and CIP degradation efficiency was greatly increased when compared to sole-ultrasound and sole-visible-light irradiation. The composites showed higher catalytic activity than individual samples, thus indicating that the BaTiO3/La2Ti2O7 heterojunctions boost the catalytic process. The photocatalytic degradation of tetracycline and Rh B was reported by Zheng et al., using a Z-type BaTiO3/γ-Bi2O3 heterojunction which was prepared via hydrothermal, co-precipitation, and calcination (Zheng et al., 2022). The morphological structure of the prepared samples appeared to be of a tetrahedron shape, irregular nano-particles and a mixture of both shapes (tetrahedron and nanoparticles) for γ-Bi2O3, BaTiO3 and BaTiO3/γ-Bi2O3 heterojunction, respectively (Fig. 25(a-c)). It was found that the calcination temperature had no effect on the morphology, the obtained average particles for γ-Bi2O3, BaTiO3 and BaTiO3/γ-Bi2O3 (HS3) were roughly around 5.9 μm, 425.9 nm, and 1.9 μm, respectively. The effect of catalyst dose, pH of the solution and different water bodies on photodegradation of tetracycline was explored, however these parameters had a little impact on the photocatalytic degradation process (Fig. 25(d-g)). The degradation efficiency for both pristine (γ-Bi2O3 and BaTiO3) were below 67 %, for γ-Bi2O3 and BaTiO3 were found to be 59.65 and 66.28 %, respectively. However, for all Z-type BaTiO3/γ-Bi2O3 (HS) heterojunctions with different molar ratio, reaction time and calcination temperature, the degradation removal percentages were above 93 %, with HS3 exhibiting the highest degradation efficiency of 97.95 % for tetracycline. Even for Rh B dye, the degradation efficiency for single γ-Bi2O3 and BaTiO3 were below 67 %. It was found that γ-Bi2O3 and BaTiO3 degraded about 63.34 and 45.35 %, respectively. The photocatalytic degradation efficiencies for all HS heterojunction samples were above 73 %, with HS3, HS4, and HS5 breaking down the Rh B molecule entirely. The enhanced photocatalytic activity was due to electrons transferred via Z-type from Bi2O3 conductor band (CB) to BaTiO3 valence band (VB) by work function and charge density difference which resulted in charge separation.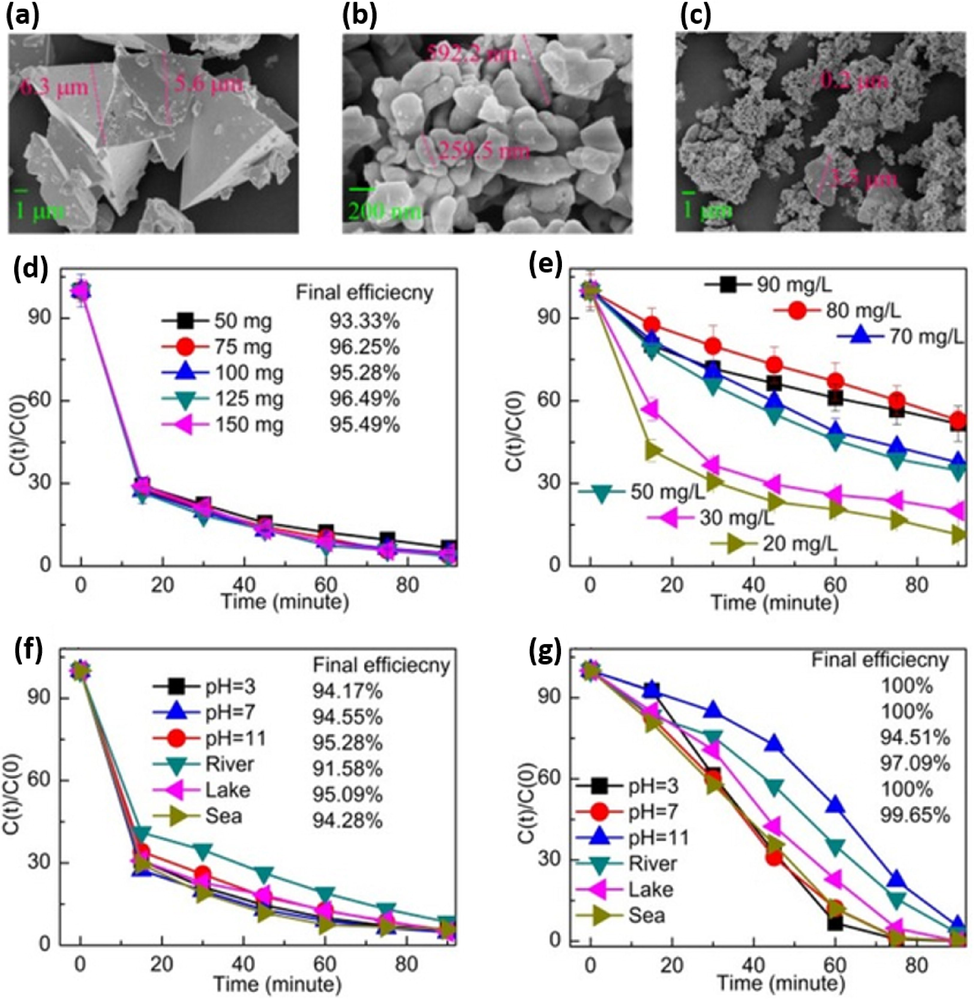
(a) Effect of catalyst dosage, (b) initial concentration on degradation of tetracycline, as well as effect of pH and water bodies on the degradation of (f) tetracycline and (g) Rh B (Zheng et al., 2022).
Another type of hydrothermally synthesized Z scheme heterojunction of La(OH)3@BaTiO3 (LB) composite was investigated for deterioration of an A-ring of tetracycline (Zheng et al., 2022). The reactive oxygen species (ROS) such as hydroxyl radicals (OH), superoxide (O2–), holes and electrons completely degraded 100 % of tetracycline. The scavenger studies confirmed that four active species played a role in their photocatalytic degradation, as follows: h+ = •O2− >•OH > e- (before 20 min reaction) and h+ > •OH >•O2– > e- (after 20 min reaction). According to these results, it means that holes (h+) played a major role in the photocatalytic degradation whereas electrons (e-) showed the least contribution during the photocatalytic degradation of tetracycline. The application of BaTiO3-based catalyst for removal of pharmaceuticals is summarized in Table 5.
Catalyst
Pharmaceuticals
Reaction conditions
% Removal & reaction time
Refs
BaTiO3/TiO2
Ace
500 W xenon lamp
95 %, 4 hr
(Kurniawan et al., 2018)
W-BaTiO3
TC
18 UV lamps
80 %, 180 min
(Demircivi and Simsek, 2019)
BaTiO3/CF
TC
UV-A light & visible light
96 %, 180 min
(Demircivi et al., 2020)
BaTiO3 –cement
PCT
Ultrasonic (40 kHz, 70 W)
>79 %, 240 min
(Sharma et al., 2020)
Cu-BaTiO3
ATZ
Xenon lamp (cut-off filter, 420 nm)
100 %, 60 min
(Basaleh and Mohamed, n.d.)
RuO2/t-BaTiO3/Pt
TCZ
Ultrasonic Cleaner (40 kHz, 110 W)
86 %, 60 min
(Feng et al., 2019)
BiFeO3/CuBi2O4/BaTiO3
NFX
500 W xenon lamp
93.5 %, 60 min
(Zhang et al., 2020)
BaTiO3/γ-Bi2O3
TC
250 W mercury lamp irradiation
98 %, 90 min
(Zheng et al., 2022)
La(OH)3@BaTiO3
TC
350 W Xe lamp
100 %, 120 min
(B. Wang et al., 2019)
BaTiO3/La2Ti2O7
CIP
Ultrasonic (40 kHz, 210 W) + 300 W Xe lamp
50.2 %, 90 min
(Y. Li et al., 2021)
Despite using BaTiO3-based catalyst for piezo-photocatalytic degradation of organic dyes and pharmaceuticals, there are some factors which affects the piezo-photocatalytic degradation process negatively. Some of these factors include nature of the semiconductor, amount of the catalyst, solution pH, reaction time, light intensity, dissolved reactive oxygen species and temperature. For examples, some organic pollutants showed maximum adsorption removal and piezo-photodegradation at lower (acidic media) or higher pH (basic media) due their complex structure. Therefore, limiting their applications in real wastewater treatment because it means prior to degradation processes, the pH of the real wastewater samples needs to be adjusted. Another critical factor is the surface area of the piezo-photocatalyst, since the piezo-photodegradation efficiency increases with an increase in surface area of the catalyst. It is very important to select piezo-photocatalyst with very high surface area because of more active sites, which assist in the enhancement of piezo-photodegradation. Furthermore, this processes requires reactive high amount of reactive oxygen species (ROS) such as hydroxyl radicals and superoxide to completely oxidize organic dyes and pharmaceutical into less harmful by-products such as carbon dioxide (CO2) and water (H2O). Therefore, lower levels of reactive oxygen species would result in incomplete oxidation of organic pollutants.
6.3 Pathogenic bacteria
Piezocatalytic, photocatalytic and piezo-photocatalytic disinfection has attracted more attention in elimination of pathogenic bacteria. These processes usually use reactive oxygen species (ROS) such as hydroxyl radicals, superoxide, hydrogen peroxide and h+, e- generated when piezo-photocatalyst is exposed under light irradiation or mechanical vibration to kill bacteria (J. He et al., 2021). Generally, the idea of photocatalytic disinfection is to first remove each bacterium's cell wall, removing its protection, and then to damage its cytoplasmic membrane, causing the cellular material inside the newly torn cell envelope to degrade (Fig. 26). Ferroelectric based catalysts such as barium titanate (BaTiO3) have been used as piezocatalyst/photocatalysts for bacterial disinfection due to its exceptional optical and piezoelectric/ferroelectric properties. For example, Zhao et al. fabricated a novel p-n type Cu2MgSnS4/BaTiO3 (CMTS@BaTiO3) heterojunction for the degradation of organic dyes and bacterial disinfection (Ali et al., 2021). In terms of bacterial disinfection, CMTS@BaTiO3 obtained 72–76 % and 84–90 % inhabitation for E.coli and S.aureus, respectively, which was three times greater than pure CMTS and BaTiO3. Furthermore, CMTS@BaTiO3 heterojunction was tested for inactivation of E.coli and S.aureus in the presence of wastewater containing dyes (MG and MB). When compared to as-grown bacteria, inactivated levels of bacterial percentages employing CMTS@BaTiO3 composites were around 94.22–101.24 % with MB and MG, respectively, against E.coli and 97.59 to 87.96 % for S.aureus. In comparison to CMTS@BaTiO3, Kumar et al. demonstrated piezocatalytic, photocatalytic and piezo-photocatalytic disinfection of E.coli using unpoled and poled BaTiO3 ceramic under UV light and ultrasonic vibration (Kumar et al., 2019a). During the piezocatalytic process (under ultrasonic vibration), unpoled BaTiO3 showed a 56 % of bacterial disinfection within 30 min. Under light exposure and ultrasonic vibration, piezo-photocatalysis improved the bacterial degradation rate and the piezo-photocatalytic degradation of E.coli increased from 56 to 70 % within the same reaction time. When the poled BaTiO3 ceramic were employed for piezocatalytic degradation (under ultrasonic vibration), it was found that about 97 % of bacteria were killed. Under the piezo-photocatalysis process (ultrasonic vibration and light exposure), poled BaTiO3 ceramic showed some catalytic enhancement towards catalytic degradation of bacteria (99.99 % recorded within 20 min). From these results, it can be concluded that poled BaTiO3 ceramic have better photocatalytic activity than unpoled BaTiO3 ceramic and coupling piezocatalysis with photocatalysis further enhanced the catalytic degradation activity of BaTiO3.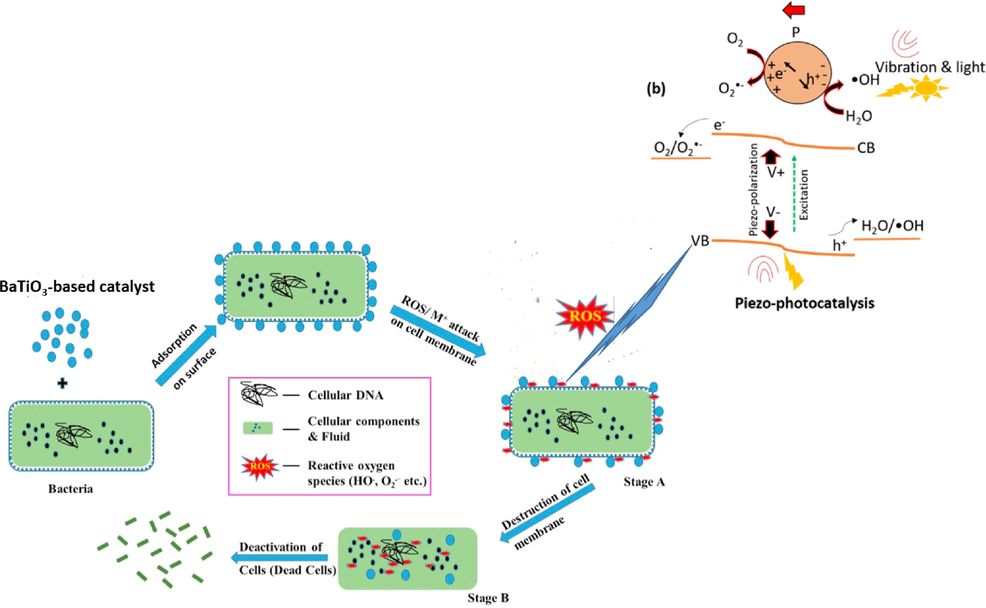
Possible photocatalytic bacterial disinfection mechanism (Laxma Reddy et al., 2017).
Kumar et al. investigated the photocatalytic and antibacterial activity of poled BaTiO3 prepared via a solid state method (Kumar et al., 2019b). From the FESEM analysis, it was found that BaTiO3 consisted of large dense grains with clear grain boundaries (Fig. 27(a)). The calculated average grain size of BaTiO3 sample ranged from 40 to 60 μm (Fig. 27(b)). Both unpoled and poled BaTiO3 were shown to respond under UV irradiation. The poled BaTiO3 exhibited the highest photocurrent response of about 0.006 μA, which was>100 times greater than that of unpoled BaTiO3. Thus, indicating that poled BaTiO3 had a higher photocatalytic activity (Fig. 27(c-d)). This was due to its remnant polarization which inhibited recombination of photogenerated charges. However, under dark conditions both samples (poled and unpoled BaTiO3) showed no response to UV irradiation.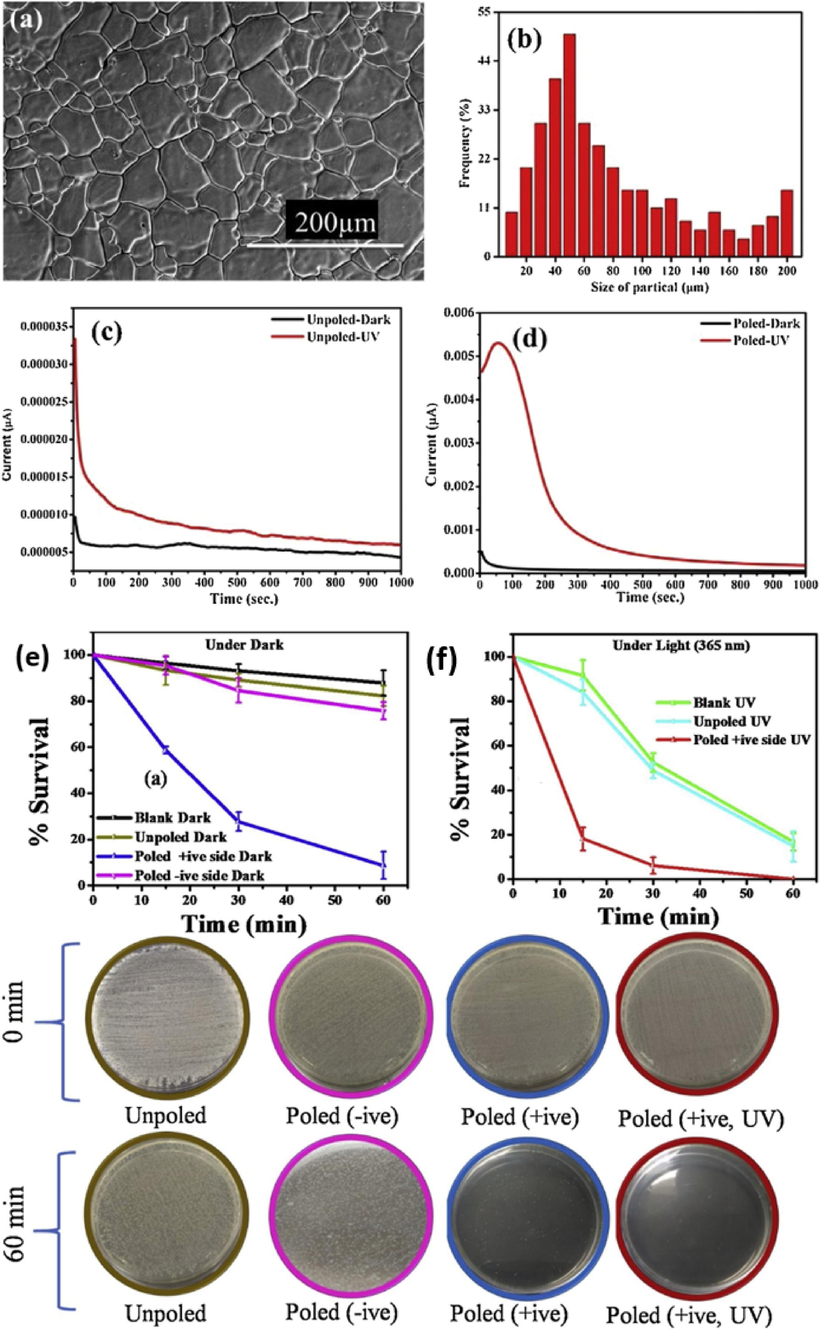
(a-b) SEM images and particle size of BaTiO3, (c-d) photocurrent response under dark and UV light, catalytic degradation of bacteria under (e) dark and (f) UV light (Kumar et al., 2019b).
The colony forming unit (CFU) method was employed to study the effect of poled BaTiO3 samples on absolute bacterial mortality. As shown in Fig. 27(e), the results display that there was no substantial antibacterial activity with unpoled BaTiO3 and negative side of poled samples in the dark. However, the positive side of poled BaTiO3 exhibited strong antibacterial activity with about 90 % bacterial destruction after 60 min when UV light was irradiated (Fig. 27(f)). In the absence of a catalyst (BaTiO3 poled or unpoled), the UV light alone showed antibacterial activity since it can prevent bacterial growth by destroying their structural DNA. Kushwana and co-workers reported (Kushwaha et al., 2015) Li-Doped Bi0.5Na0.45 K0.5TiO3–BaTiO3 (BNKLBT) for antibacterial activity against E.coli and A.flavus using standard disc diffusion method. This method involves bacterial growth using Luria–Bertani (LB) broth–agar in a petridish disc. Three different concentrations (10, 50 and 100 μg) of BNKLBT were tested against bacteria and compared with commercial antibiotics (kanamycin (k30)). Based on their results, the zone of inhibition increased as BNKLBT concentration increased on the disc. The reason for this trend was not explained in this work. In another report, BaTiO3 nanoparticles were tested for antibacterial efficacy against S.aureus and P. aeruginosa by Shah et al.(Shah et al., 2018). The diffusion method was employed to investigate the antimicrobial activity of BaTiO3 nanoparticles. The optimal concentration of 100 μg/ml (BaTiO3) achieved bacterial inhibition of 85 ± 3.5 % and 80 ± 3 % against S.aureus and P.aeruginosa biofilms, respectively.
Shuai et al. prepared PVDF/xAg-pBT composites via laser sintering method for bacterial disinfection (Shuai et al., 2020a). According to their studis, PVDF/xAg-pBT composites generated piezoelectric potential/voltage. Furthmore, it was found that with an increase in Ag concentration in the PVDF/pBT composites, the piezoelectrical current and voltage firstly rose then dropped. Babu et al. and Parl et al. observed comparable enhancement after adding conductive fillers to polymer-ceramic composites (Babu and de With, 2014) (Park et al., 2012). Their assumptions on the output voltage and current improvement were based on the conductivity enhancement. The antibacterial activity of the prepared scaffolds (PVDF/4Ag-pBT and PVDF/pBT) were evaluated using zone of inhibition method. Fig. 28(a-b) shows the bacterial inhibition zones of the scaffolds. PVDF/4Ag-pBT was shown to be more successful in inhibiting the growth of E.coli as compared to PVDF/pBT scaffold. Further test including Turbidimetric test were conducted to verify antibacterial activity of the scaffolds. The transparent vials including and excluding E.coli suspensions were labelled as control and blank group, respectively. The turbidity was the same for vials containing PVDF/4Ag-pBT and control. Surprisingly, the vial containing E.coli solution incubated with PVDF/4Ag-pBT was clear as a blank group, thus indicating that the scaffold inhibited bacterial growth of E.coli (Fig. 28(c)). The SEM images also confirmed that PVDF/4Ag-pBT scaffold raptured and destroyed the whole rod-shape structure of E.coli, whereas PVDF/pBT scaffold had minimal impact on the smooth rod-shape of E.coli (Fig. 28(d-e)). Fig. 28(f-g) displays bacterial inhibition rate of scaffolds and cumulative or non-cumulative of silver ion concentration released by PVDF/4Ag-pBT. The PVDF/4Ag-pBT scaffold inhibited bacteria at a rate of over 81 %, while the PVDF/pBT scaffold had no antibacterial activity.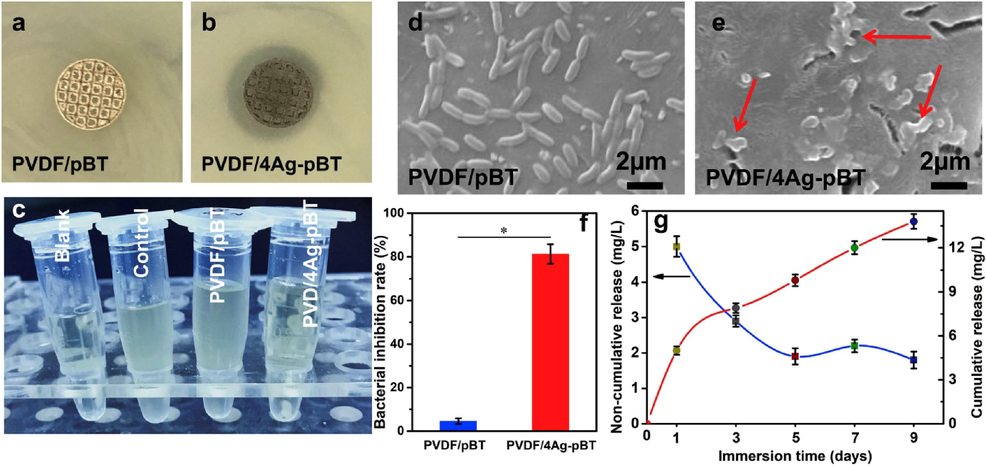
(a-b) Photographs of zone of inhibition, (c) turbidity test, (d-e) SEM images of bacteria, (f) bacterial zone of inhibition rate and (g) cumulative or non-cumulative of silver ion concentration released by PVDF/4Ag-pBT (Shuai et al., 2020a).
Overall, BaTiO3-based catalyst have been shown to be appropriate for applications involving the combination of photocatalysis, piezocatalysis and other catalysis processes. An overview of the previous studies addressing the use of BaTiO3-based catalyst for water disinfection is summarized in Table 6. The use of piezo-photocatalyst based materials for bacterial disinfection is still in its infancy. The process of piezo-photocatalysis has been widely applied including hydrogen production (H2), degradation of organic pollutants in wastewater, carbon dioxide (CO2) conversion and hydrogen peroxide (H2O2) production. In piezo-photocatalytic disinfection, it is challenging to develop a general mechanism for bacteria inactivation because of a wide range of pathogens and their cell complexity. Therefore, deeper understanding of the mechanisms the piezo-photocatalytic process is still required since suggested mechanisms highly rely on the generated reactive oxygen species during redox reactions. It is still debatable whether polarized charge carriers participate in the redox catalytic process. According to certain studies, photogenerated electrons and holes play a crucial part in redox processes, and the polarization potential of piezoelectric materials merely helps to separate photogenerated charge carriers (Fu et al., 2022).
Catalyst
Bacteria
Reaction conditions
% Removal & reaction time
Refs
Cu2MgSnS4/BaTiO3
E. coli and S. aureus
Ultrasonic (40 kHz)
>90 %, 3 hr
(Ali et al., 2021)
BaTiO3
E.coli
Vibration (8 Hz) + light irradiation (375 nm)
99.99 %, 20 min
(Kumar et al., 2019a)
E.coli
UV light (365 nm)
90 %, 60 min
(Kumar et al., 2019b)
P.aeruginosa & S.aureus
NA
85 ± 3.5 % & 80 ± 3 %
(Shah et al., 2018)
PVDF/xAg-pBT
E.coli
Hammer
81 %
(Shuai et al., 2020b)
TiO2 @BaTiO3–V(P)
S. aureus
NA
100 %, 24 hr
(Wu et al., 2021)
Ag/t-BaTiO3
E.coli
Ultrasonic Cleaner (40 kHz, 110 W)
>95 %, 30 min
(J. Feng et al., 2018)
7 Conclusions, limitations and future perspectives
Barium titanate (BaTiO3) offers a wide range of applications in the energy and environmental fields. In comparison to certain applications like water hydrogen production, its usage in the piezo-photocalaytic wastewater treatment and bacterial disinfection is limited and recent. This review article has presented a complete summary of current developments in the use of barium titanate-based catalysts in photocatalytic, piezocatalytic and piezo-photocatalytic decomposition of organics and bacterial disinfection in water and wastewater. The selection of BaTiO3 as a suitable piezo-photocatalyst was due to its outstanding dielectric/ferroelectric/piezoelectric characteristics, low toxicity, low cost, environmental friendliness, existence in broad range of sizes and morphologies, multiple crystal structures and good stability. These characteristics are undoubtedly being used in the piezo-photocatalytic elimination of a variety of organic contaminants. Since, BaTiO3 as a photocatalyst suffers from poor conductivity and rapid recombination of photogenerated charge carriers. In this review, we have covered several strategies to circumvent these restrictions including morphology control, doping, metal loading, and heterojunction construction with appropriate semiconductors. Another way of improving electrons and holes separation is through coupling photocatalysis with other catalysis processes such as piezocatalysis and electrocatalysis. These strategies have achieved excellent results while conserving energy. The effectiveness of BaTiO3-based catalyst for piezo-photocatalytic degradation of organic pollutants depends mainly on several factors such as pH, reaction time, amount of catalyst, ultrasonic and light power. The fundamental benefit of heterogeneous photocatalysis is its capacity to use solar energy in the form of solar photons, which gives the degradation process a large boost in environmental value. Particularly for large-scale aqueous-phase applications, solar light-assisted photodegradation of wastewater contaminants can make it an economically viable method.
Even though several reports have shown some strategic ways to improve photocatalytic activity of barium titanate, however some few other limitations and challenges needs to be highlighted for its success in wastewater treatment.
-
There is limited research on the structure of the barium titanate after being exposed to ultrasonic vibration and light irradiation. It is very important to investigate the stability of the barium titanate structure using TEM, SEM and XRD before and after wastewater treatment.
-
Even though doping barium titanate with plasmonic metals have shown significant improvement in photocatalytic activity, the practical applicability of plasmonic BaTiO3 piezo-photocatalyst is limited due to the high cost and photo-corrosion associated with noble metal nanoparticles.
-
Since the size and morphology of the doped noble metals can affect the photocatalytic activity of the BaTiO3, there are no reports in the literature on the size and morphology of the noble metals nanoparticles doped on the surface of BaTiO3 piezo-photocatalyst. To establish the ideal condition of noble metal nanoparticles needed for photocatalytic enhancement, it is therefore necessary to assess the surface plasmon resonance (SPR) impact from different sizes and shapes.
-
From our analysis, there’s limited understanding of piezo-photocatalytic mechanism for BaTiO3-heterojunction piezo-photocatalyst. Therefore, for future studies it is very important to study reaction mechanisms for piezo-photocatalytic degradation of organic pollutants in detail.
-
Furthermore, many reports in literature have given limited information regarding how synthetic methods and structural properties of BaTiO3 piezo-photocatalyst affects the efficiency of the process. As a result, future research should pay close attention to how these factors impact piezo-photocatalyst effectiveness.
-
The amount of organic pollutant degradation is determined by the photocatalysts' mineralization capacity. However, the majority of earlier studies on BaTiO3-based materials did not identify the total organic carbon (TOC) in the mineralization process, which should be taken into account for an accurate assessment of the whole degradation of organic contaminants.
Overall, the use of BaTiO3-based piezo-photocatalysts for real-world remediation of pharmaceutical, organic dyes and microbes from wastewater has a bright future. Therefore, it is highly recommended that advanced oxidation processes such as photocatalysis and piezocatalysis should be used for wastewater treatment in the future instead of traditional techniques.
Acknowledgements
This work was supported by GES 4.0 (Global Excellence Structure 4.0), the Centre for Nanomaterials Science Research (CNSR) University of Johannesburg), National Research Foundation of South Africa (SRUG200326510622), National Research Foundation of South Africa (NRFTTK117999) and the Faculty of Science University of Johannesburg (UJ), South Africa for financial support.
References
- Synthesis of metal-organic framework hybrid nanocomposites based on GO and CNT with high adsorption capacity for dye removal. Chem. Eng. J.. 2017;326:1145-1158.
- [CrossRef] [Google Scholar]
- BaTiO3-based piezoelectrics: Fundamentals, current status, and perspectives. Appl. Phys. Rev.. 2017;4
- [CrossRef] [Google Scholar]
- Photocatalytic degradation of acetaminophen. Int. J. Environ. Res.. 2011;5:1071-1078.
- [Google Scholar]
- Advances in lead-free piezoelectric materials for sensors and actuators. Sensors. 2010;10:1935-1954.
- [CrossRef] [Google Scholar]
- Charge Coupling Enhanced Photocatalytic Activity of BaTiO3/MoO3 Heterostructures. ACS Appl. Mater. Interfaces. 2019;11:40114-40124.
- [CrossRef] [Google Scholar]
- Stimulated piezotronical decontamination using Cu2MgSnS4 modified BaTiO3. Mater. Today Energy. 2021;21
- [CrossRef] [Google Scholar]
- Ferroelectric Fe-Cr Codoped BaTiO3 Nanoparticles for the Photocatalytic Oxidation of Azo Dyes. ACS Appl. Nano Mater.. 2019;2:2890-2901.
- [CrossRef] [Google Scholar]
- B-site modified photoferroic Cr3+-doped barium titanate nanoparticles: microwave-assisted hydrothermal synthesis, photocatalytic and electrochemical properties. RSC Adv.. 2019;9:20806-20817.
- [CrossRef] [Google Scholar]
- Ultrafast microwave-assisted hydrothermal synthesis and photocatalytic behaviour of ferroelectric Fe3+-doped BaTiO3 nanoparticles under simulated sunlight. Catal. Today. 2021;360:90-98.
- [CrossRef] [Google Scholar]
- Novel fractal analysis of nanograin growth in BaTiO3 thin film. Mater. Chem. Phys.. 2020;239
- [CrossRef] [Google Scholar]
- Antibiotic contamination in animal manure, soil, and sewage sludge in Shenyang, northeast China. Environ. Earth Sci.. 2015;74:5077-5086.
- [CrossRef] [Google Scholar]
- Facile deposition of porous fluorine doped tin oxide by Dr. Blade method for capacitive applications. Ceram. Int.. 2021;47:5487-5494.
- [CrossRef] [Google Scholar]
- Crack-free nanostructured BaTiO3 thin films prepared by sol-gel dip-coating technique. Ceram. Int.. 2014;40:8613-8619.
- [CrossRef] [Google Scholar]
- Processing and Characterization of Carbonate-Free BaTiO3 Nanoscale Particles Obtained by a Rapid Ultrasound-Assisted Wet Chemical Approach. Metall. Mater. Trans. B Process Metall. Mater. Process. Sci.. 2015;46:1912-1923.
- [CrossRef] [Google Scholar]
- Controlled Porosity in Ferroelectric BaTiO3Photoanodes. ACS Appl. Mater. Interfaces. 2022;14:13147-13157.
- [CrossRef] [Google Scholar]
- Enhanced electromechanical properties of piezoelectric thin flexible films. Compos. Sci. Technol.. 2014;104:74-80.
- [CrossRef] [Google Scholar]
- Basaleh, A.S., Mohamed, R.M., n.d. Synthesis and characterization of Cu-BaTiO 3 nanocomposite for atrazine remediation under visible-light radiation from wastewater. Integr. Med. Res. 9, 9550–9558. https://doi.org/10.1016/j.jmrt.2020.06.081.
- Synthesis and characterization of Cu-BaTiO3 nanocomposite for atrazine remediation under visible-light radiation from wastewater. J. Mater. Res. Technol.. 2020;9:9550-9558.
- [CrossRef] [Google Scholar]
- Synthesis reaction of metatitanate BaTiO3 - Part 1 Effect of the gaseous atmosphere upon the thermal evolution of the system BaCO3-TiO2. J. Mater. Sci.. 1983;18:3041-3046.
- [CrossRef] [Google Scholar]
- Solvothermal synthesized boron doped TiO2 catalysts: Photocatalytic degradation of endocrine disrupting compounds and pharmaceuticals under visible light irradiation. Appl. Catal. B Environ.. 2017;200:309-322.
- [CrossRef] [Google Scholar]
- Structural and dielectric properties of BaTi0.5 (Co0.33 Mo0.17) O3 perovskite ceramic. J. Alloys Compd.. 2019;781:936-944.
- [CrossRef] [Google Scholar]
- Brdi, R., Mann, A.-, Ii, C., 1950. (ScH%VARZENBACH’S 77, 1947–1948.
- Monitoring the occurrence of emerging contaminants in treated wastewater and groundwater between 2008 and 2010. The Baix Llobregat (Barcelona, Spain) J. Hazard. Mater.. 2012;239–240:32-39.
- [CrossRef] [Google Scholar]
- Sol-Gel Template Synthesis and Photoluminescence of n- and p-Type Semiconductor Oxide Nanowires. ChemPhysChem. 2006;7:497-501.
- [CrossRef] [Google Scholar]
- In vivo genotoxicity evaluation of atrazine and atrazine-based herbicide on fish Carassius auratus using the micronucleus test and the comet assay. Food Chem. Toxicol.. 2011;49:1431-1435.
- [CrossRef] [Google Scholar]
- Methods for preparation of BaTiO3 thin films. J. Optoelectron. Adv. Mater.. 2004;6:1349-1356.
- [Google Scholar]
- Ferroelectric polarization-enhanced photocatalytic properties and photo-induced charge carrier behavior of Au/BaTiO3. J. Alloys Compd.. 2020;825:154060
- [CrossRef] [Google Scholar]
- Photocatalytic degradation of organic pollutants using TiO2-based photocatalysts: A review. J. Clean. Prod.. 2020;268:121725
- [CrossRef] [Google Scholar]
- Facile microwave synthesis and photocatalytic activity of monodispersed BaTiO3 nanocuboids. Mater. Charact.. 2016;114:243-253.
- [CrossRef] [Google Scholar]
- Effect of ferroelectricity on solar-light-driven photocatalytic activity of BaTiO3 - Influence on the carrier separation and stern layer formation. Chem. Mater.. 2013;25:4215-4223.
- [CrossRef] [Google Scholar]
- Photodegradation of Rhodamine B over Ag modified ferroelectric BaTiO3 under simulated solar light: Pathways and mechanism. RSC Adv.. 2015;5:30372-30379.
- [CrossRef] [Google Scholar]
- Enhanced Photocatalytic Activity of Heterostructured Ferroelectric BaTiO3/α-Fe2O3 and the Significance of Interface Morphology Control. ACS Appl. Mater. Interfaces. 2017;9:24518-24526.
- [CrossRef] [Google Scholar]
- Effect of Dual-Cocatalyst Surface Modification on Photodegradation Activity, Pathway, and Mechanisms with Highly Efficient Ag/BaTiO3/MnO x. Langmuir. 2020;36:498-509.
- [CrossRef] [Google Scholar]
- Growth of BaTiO3 nanoparticles in ethanol-water mixture solvent under an ultrasound-assisted synthesis. Chem. Eng. J.. 2011;170:333-337.
- [CrossRef] [Google Scholar]
- Enhanced Photocatalytic Activity for Degradation of Organic Dyes Using Magnetite CoFe 2 O 4 /BaTiO 3 Composite. J. Nanosci. Nanotechnol.. 2018;18:7850-7857.
- [CrossRef] [Google Scholar]
- Enhanced photocatalytic degradation of tetracycline using hydrothermally synthesized carbon fiber decorated BaTiO3. Mater. Chem. Phys.. 2020;241:122236
- [CrossRef] [Google Scholar]
- Visible-light-enhanced photoactivity of perovskite-type W-doped BaTiO3 photocatalyst for photodegradation of tetracycline. J. Alloys Compd.. 2019;774:795-802.
- [CrossRef] [Google Scholar]
- Occurrence, fate, and ecotoxicity of antibiotics in agro-ecosystems. A review. Agron. Sustain. Dev.. 2012;32:309-327.
- [CrossRef] [Google Scholar]
- Strategies to enhance catalytic performance of metal–organic frameworks in sulfate radical-based advanced oxidation processes for organic pollutants removal. Chem. Eng. J.. 2021;403:126346
- [CrossRef] [Google Scholar]
- Calcination temperature effect on dielectric, structural and morphology properties of BaTiO3 nano-structure prepared by modified sol - Gel technique. Adv. Nat. Sci. Nanosci. Nanotechnol.. 2020;11
- [CrossRef] [Google Scholar]
- Photoinduced charge transfer properties and photocatalytic activity in Bi 2O 3/BaTiO 3 composite photocatalyst. ACS Appl. Mater. Interfaces. 2012;4:4853-4857.
- [CrossRef] [Google Scholar]
- Feng, Y., Li, Hao, Ling, L., Yan, S., Pan, D., Ge, H., Li, Hexing, 2018. Enhanced Photocatalytic Degradation Performance by Fluid-induced Piezoelectric Field. https://doi.org/10.1021/acs.est.8b00946.
- Significant Improvement and Mechanism of Ultrasonic Inactivation to Escherichia coli with Piezoelectric Effect of Hydrothermally Synthesized t-BaTiO3. ACS Sustain. Chem. Eng.. 2018;6:6032-6041.
- [CrossRef] [Google Scholar]
- Enhancement and mechanism of nano-BaTiO3 piezocatalytic degradation of tricyclazole by co-loading Pt and RuO2. Environ. Sci. Nano. 2019;6:2241-2252.
- [CrossRef] [Google Scholar]
- Coupling effect of piezomaterial and DSA catalyst for degradation of metronidazole: Finding of induction electrocatalysis from remnant piezoelectric filed. J. Catal.. 2020;381:530-539.
- [CrossRef] [Google Scholar]
- An analytical overview of processes for removing organic dyes from wastewater effluents. TrAC - Trends Anal. Chem.. 2010;29:1202-1211.
- [CrossRef] [Google Scholar]
- Modulation of electric dipoles inside electrospun BaTiO3@TiO2 core-shell nanofibers for enhanced piezo-photocatalytic degradation of organic pollutants. Nano Energy. 2022;93
- [CrossRef] [Google Scholar]
- Improved Capture and Removal Efficiency of Gaseous Acetaldehyde by a Self-Powered Photocatalytic System with an External Electric Field. ACS Nano. 2021;15:10577-10586.
- [CrossRef] [Google Scholar]
- Oxalic acid functionalization of BaTiO3 nanobelts for promoting their piezo-degradation organic contaminants. J. Mater.. 2021;7:1275-1283.
- [CrossRef] [Google Scholar]
- Synthesis, Structure, Properties and Applications of Barium Titanate Nanoparticles. Int. J. Adv. Technol. Eng. Sci.. 2016;4:178-187.
- [Google Scholar]
- Chemical treatment technologies for waste-water recycling - An overview. RSC Adv.. 2012;2:6380-6388.
- [CrossRef] [Google Scholar]
- Adsorptive removal of dyes from aqueous solution onto carbon nanotubes: A review. Adv. Colloid Interface Sci.. 2013;193–194:24-34.
- [CrossRef] [Google Scholar]
- Application of low-cost adsorbents for dye removal - A review. J. Environ. Manage.. 2009;90:2313-2342.
- [CrossRef] [Google Scholar]
- Effect of temperature, time and particle size of Ti precursor on hydrothermal synthesis of barium titanate. Mater. Sci. Eng. B Solid-State Mater. Adv. Technol.. 2008;152:60-65.
- [CrossRef] [Google Scholar]
- Photoluminescence activity of BaTiO 3 nanocubes via facile hydrothermal synthesis. J. Mater. Sci. Mater. Electron.. 2019;30:5149-5157.
- [CrossRef] [Google Scholar]
- Hassan, B.A.R., Yusoff, Z.B.M., Othman, M.A.H., Bin, S., 2012. information is available at the end of the Chapter, A., Http://dx.doi.org/10.5772/55358, We are IntechOpen , the world ’ s leading publisher of Open Access books Built by scientists , for scientists TOP 1 %. Intech 13.
- A novel hierarchical BaTiO3/AgI heterojunction with boosting spatial charge kinetics for photocatalytic degradation of organic pollutant. Ceram. Int.. 2021;47:33426-33434.
- [CrossRef] [Google Scholar]
- Critical review of photocatalytic disinfection of bacteria: from noble metals- and carbon nanomaterials-TiO2 composites to challenges of water characteristics and strategic solutions. Sci. Total Environ.. 2021;758:143953
- [CrossRef] [Google Scholar]
- Effects of the processing parameters in the synthesis of BaTiO3 nanoparticle by using the co-precipitation method. J. Korean Phys. Soc.. 2014;65:404-407.
- [CrossRef] [Google Scholar]
- Surface plasmon resonance-induced Ag-BaTiO3 composites for catalytic performance. J. Am. Ceram. Soc.. 2021;104:4389-4397.
- [CrossRef] [Google Scholar]
- A critical review on textile wastewater treatments: Possible approaches. J. Environ. Manage.. 2016;182:351-366.
- [CrossRef] [Google Scholar]
- Hong, K., Xu, H., Konishi, H., Li, X., 2010. Direct Water Splitting Through Vibrating Piezoelectric Microfibers in Water 997–1002. https://doi.org/10.1021/jz100027t.
- Piezoelectrochemical effect: A new mechanism for azo dye decolorization in aqueous solution through vibrating piezoelectric microfibers. J. Phys. Chem. C. 2012;116:13045-13051.
- [CrossRef] [Google Scholar]
- A review of processes for removing antibiotics from breeding wastewater. Int. J. Environ. Res. Public Health. 2021;18
- [CrossRef] [Google Scholar]
- Crystal Structure of BaTiO3in the Cubic Phase. Ferroelectrics. 1985;63:29-37.
- [CrossRef] [Google Scholar]
- Hydroxylation mechanism of phase regulation of nanocrystal BaTiO3 synthesized by a hydrothermal method. Ceram. Int.. 2022;48:2281-2288.
- [CrossRef] [Google Scholar]
- A simple one-step hydrothermal synthesis and photocatalysis of bowl-like BaTiO3 nanoparticles. Inorg. Nano-Metal Chem.. 2017;47:647-654.
- [CrossRef] [Google Scholar]
- Mono- and co-doped NaTaO3 for visible light photocatalysis. Phys. Chem. Chem. Phys.. 2014;16:16085-16094.
- [CrossRef] [Google Scholar]
- BaTiO3/ZnO heterostructured photocatalyst with improved efficiency in dye degradation. Mater. Chem. Phys.. 2020;255:123583
- [CrossRef] [Google Scholar]
- Enhanced photocatalytic performance of BaTiO3/g-C3N4 heterojunction for the degradation of organic pollutants. Chem. Phys. Lett.. 2021;771:138513
- [CrossRef] [Google Scholar]
- Optical, electrical, and photocatalytic properties of polyethylene glycol-assisted sol-gel synthesized BaTiO3@ZnO core-shell nanoparticles. Powder Technol.. 2014;254:480-487.
- [CrossRef] [Google Scholar]
- Efficiency of various recent wastewater dye removal methods: A review. J. Environ. Chem. Eng.. 2018;6:4676-4697.
- [CrossRef] [Google Scholar]
- Facile synthesis of ZnO and transition metal doped ZnO nanoparticles for the photocatalytic degradation of Methyl Orange. Ceram. Int.. 2014;40:7417-7424.
- [CrossRef] [Google Scholar]
- Effect of metal-doping of TiO2 nanoparticles on their photocatalytic activities toward removal of organic dyes. Egypt. J. Pet.. 2014;23:419-426.
- [CrossRef] [Google Scholar]
- BaTiO3@rGO nanocomposite: enhanced photocatalytic activity as well as improved electrode performance. J. Mater. Sci. Mater. Electron.. 2021;32:12911-12921.
- [CrossRef] [Google Scholar]
- Influence of silver doping on the structure, optical and photocatalytic properties of Ag-doped BaTiO3 ceramics. Mater. Chem. Phys.. 2021;259:124058
- [CrossRef] [Google Scholar]
- An overview of photocatalytic degradation: photocatalysts, mechanisms, and development of photocatalytic membrane. Environ. Sci. Pollut. Res.. 2020;27:2522-2565.
- [CrossRef] [Google Scholar]
- Rapid bacterial disinfection using low frequency piezocatalysis effect. J. Ind. Eng. Chem.. 2019;77:355-364.
- [CrossRef] [Google Scholar]
- Impact of remnant surface polarization on photocatalytic and antibacterial performance of BaTiO 3. J. Eur. Ceram. Soc.. 2019;39:2915-2922.
- [CrossRef] [Google Scholar]
- BaTiO3/TiO2 composite-assisted photocatalytic degradation for removal of acetaminophen from synthetic wastewater under UV–vis irradiation. Mater. Sci. Semicond. Process.. 2018;73:42-50.
- [CrossRef] [Google Scholar]
- Visible Light-Induced Photocatalytic and Antibacterial Activity of Li-Doped Bi0.5Na0.45K0.5TiO3–BaTiO3 Ferroelectric Ceramics. J. Electron. Mater.. 2015;44:4334-4342.
- [CrossRef] [Google Scholar]
- TiO2-based photocatalytic disinfection of microbes in aqueous media: A review. Environ. Res.. 2017;154:296-303.
- [CrossRef] [Google Scholar]
- Tio2-seeded hydrothermal growth of spherical batio3 nanocrystals for capacitor energy-storage application. Crystals. 2020;10
- [CrossRef] [Google Scholar]
- Enhanced photocatalytic degradation and H2/H2O2 production performance of S-pCN/WO2.72 S-scheme heterojunction with appropriate surface oxygen vacancies. Nano Energy. 2021;81:105671
- [CrossRef] [Google Scholar]
- Photoelectrochemical and photocatalytic properties of Ag-loaded BaTiO 3/TiO2 heterostructure nanotube arrays. Int. J. Hydrogen Energy. 2013;38:12977-12983.
- [CrossRef] [Google Scholar]
- BaTiO3/TiO2 heterostructure nanotube arrays for improved photoelectrochemical and photocatalytic activity. Electrochim. Acta. 2013;91:30-35.
- [CrossRef] [Google Scholar]
- Improved photocatalytic activity of BaTiO3/La2Ti2O7 heterojunction composites via piezoelectric-enhanced charge transfer. Appl. Surf. Sci.. 2021;570:151146
- [CrossRef] [Google Scholar]
- Enhanced Ferroelectric-Nanocrystal-Based Hybrid Photocatalysis by Ultrasonic-Wave-Generated Piezophototronic Effect. Nano Lett.. 2015;15:2372-2379.
- [CrossRef] [Google Scholar]
- Few-layer transition metal dichalcogenides (MoS2, WS2, and WSe2) for water splitting and degradation of organic pollutants: Understanding the piezocatalytic effect. Nano Energy. 2019;66
- [CrossRef] [Google Scholar]
- Piezoelectric materials for catalytic / photocatalytic removal of pollutants : Recent advances and outlook Applied Catalysis B : Environmental Piezoelectric materials for catalytic / photocatalytic removal of pollutants : Recent advances and outlook. Appl. Catal. B Environ.. 2018;241:256-269.
- [CrossRef] [Google Scholar]
- Piezoelectric materials for catalytic/photocatalytic removal of pollutants: Recent advances and outlook. Appl. Catal. B Environ.. 2019;241:256-269.
- [CrossRef] [Google Scholar]
- Lilge, T.S., Ramires das Neves Stigger, A., Fernandes, C.D., Gularte, L.T., Raubach, C.W., da Silva Cava, S., Gomes Jardim, P.L., Giroldo Valerio, M.E., Moreira, M.L., 2020. Increase of Voc using heterojunctions of BaTiO3 without sensitization. Ceram. Int. 46, 4907–4913. https://doi.org/10.1016/j.ceramint.2019.10.227.
- Silver modified barium titanate as a highly efficient piezocatalyst. Catal. Sci. Technol.. 2018;8:4788-4796.
- [CrossRef] [Google Scholar]
- BaTiO3 Nanosheets and Caps Grown on TiO2 Nanorod Arrays as Thin-Film Catalysts for Piezocatalytic Applications. ACS Appl. Mater. Interfaces. 2020;12:14005-14015.
- [CrossRef] [Google Scholar]
- BaTiO3 nanocubes/cuboids with selectively deposited Ag nanoparticles: Efficient piezocatalytic degradation and mechanism. Appl. Catal. B Environ.. 2021;285:119823
- [CrossRef] [Google Scholar]
- Photocatalytic activities of heterojunction semiconductors Bi 2O3/BaTiO3: A strategy for the design of efficient combined photocatalysts. J. Phys. Chem. C. 2007;111:18288-18293.
- [CrossRef] [Google Scholar]
- Synthesizing BaTiO3 Nanostructures to Explore Morphological Influence, Kinetics, and Mechanism of Piezocatalytic Dye Degradation. ACS Appl. Mater. Interfaces. 2020;12:17443-17451.
- [CrossRef] [Google Scholar]
- Ferroelectric polarization-enhanced photocatalysis in BaTiO3-TiO2 core-shell heterostructures. Nanomaterials. 2019;9
- [CrossRef] [Google Scholar]
- Enhancement of Triboelectric Charge Density by Chemical Functionalization. Adv. Funct. Mater.. 2020;30:1-33.
- [CrossRef] [Google Scholar]
- Piezotronic-enhanced photocatalytic performance of heterostructured BaTiO3/SrTiO3 nanofibers. Nano Energy. 2021;89:1-9.
- [CrossRef] [Google Scholar]
- Ag loaded flower-like BaTiO 3 nanotube arrays: Fabrication and enhanced photocatalytic property. CrystEngComm. 2012;14:1473-1478.
- [CrossRef] [Google Scholar]
- Significantly enhanced piezo-photocatalytic capability in BaTiO3 nanowires for degrading organic dye. J. Mater.. 2020;6:256-262.
- [CrossRef] [Google Scholar]
- Piezo-photoelectronic coupling effect of BaTiO3@TiO2 nanowires for highly concentrated dye degradation. Nano Energy. 2022;92
- [CrossRef] [Google Scholar]
- Highly efficient sono-piezo-photo synergistic catalysis in bismuth layered ferroelectrics via finely distinguishing sonochemical and electromechanochemical processes. J. Mater.. 2022;8:47-58.
- [CrossRef] [Google Scholar]
- Biological therapeutics of AgO nanoparticles against pathogenic bacteria and A549 lung cancer cells Biological therapeutics of AgO nanoparticles against pathogenic bacteria and A549 lung cancer cells. Mater. Res. Express. 2019;6:105402
- [CrossRef] [Google Scholar]
- Diethylamine functionalised Moringa oleifera leaves for the removal of chromium(VI) and bacteria from wastewater. Int. J. Environ. Anal. Chem.. 2020;00:1-21.
- [CrossRef] [Google Scholar]
- Application of a piezo-photocatalytic thin film (FTO / BaTiO 3 / SnO 2) for enhanced degradation of organic pollutants and disinfection of wastewater. Ceram. Int. 2022
- [CrossRef] [Google Scholar]
- Low Cost, Recyclable and Magnetic Moringa Oleifera Leaves for Chromium(VI) Removal From Water. Front. Water. 2022;4:1-13.
- [CrossRef] [Google Scholar]
- As featured. In 2017
- [CrossRef]
- Synthesis of BaTiO3 nanoparticles by sol-gel assisted solid phase method and its formation mechanism and photocatalytic activity. Ceram. Int.. 2020;46:10619-10633.
- [CrossRef] [Google Scholar]
- Mittal, M., Sardar, S., Jana, A., 2021. Nanofabrication techniques for semiconductor chemical sensors, Handbook of Nanomaterials for Sensing Applications. Elsevier Inc. https://doi.org/10.1016/b978-0-12-820783-3.00023-3.
- Application of veterinary antibiotics in China’s aquaculture industry and their potential human health risks. Environ. Sci. Pollut. Res.. 2017;24:8978-8989.
- [CrossRef] [Google Scholar]
- Enhancing the formation of tetragonal phase in perovskite nanocrystals using an ultrasound assisted wet chemical method. Ultrason. Sonochem.. 2016;33:141-149.
- [CrossRef] [Google Scholar]
- Recent advances in piezocatalytic polymer nanocomposites for wastewater remediation. Dalt. Trans.. 2022;51:451-462.
- [CrossRef] [Google Scholar]
- Piezoelectrically Enhanced Photocatalysis with BiFeO3 Nanostructures for Efficient Water Remediation.. 2018;iScience 4:236-246.
- [CrossRef]
- Manganese-doped BaTiO3 nanotube arrays for enhanced visible light photocatalytic applications. Mater. Chem. Phys.. 2018;213:400-405.
- [CrossRef] [Google Scholar]
- SnO2-loaded BaTiO3 nanotube arrays: fabrication and visible-light photocatalytic application. J. Mater. Sci. Mater. Electron.. 2017;28:9770-9776.
- [CrossRef] [Google Scholar]
- A facile synthesis of g-C3N4/BaTiO3 photocatalyst with enhanced activity for degradation of methylene blue under visible light. Bull. Mater. Sci.. 2021;44
- [CrossRef] [Google Scholar]
- Hydrothermal synthesis of rhodium-doped barium titanate nanocrystals for enhanced photocatalytic hydrogen evolution under visible light. RSC Adv.. 2015;5:100123-100128.
- [CrossRef] [Google Scholar]
- Effect of surface Ag metallization on the photocatalytic properties of BaTiO 3: Surface plasmon effect and variation in the Schottky barrier height. Surfaces and Interfaces. 2019;15:205-215.
- [CrossRef] [Google Scholar]
- Innovating e-waste recycling: From waste multi-layer ceramic capacitors to Nb–Pb codoped and ag-Pd-Sn-Ni loaded BaTiO 3 nano-photocatalyst through one-step ball milling process. Sustain. Mater. Technol.. 2019;21:e00101.
- [Google Scholar]
- Sonoelectrochemical degradation of ciprofloxacin in water on a Ti/BaTiO3 electrode. J. Environ. Chem. Eng.. 2022;10:107224
- [CrossRef] [Google Scholar]
- Temperature dependence on phase evolution in the BaTiO3 polytypes studied using ab initio calculations. Int. J. Quantum Chem.. 2020;120:1-10.
- [CrossRef] [Google Scholar]
- Room-temperature photoluminescence of BaTiO 3: Joint experimental and theoretical study. Phys. Rev. B - Condens. Matter Mater. Phys.. 2005;71:1-7.
- [CrossRef] [Google Scholar]
- Bismuth vanadate in photoelectrocatalytic water treatment systems for the degradation of organics: A review on recent trends. J. Electroanal. Chem.. 2020;878:114724
- [CrossRef] [Google Scholar]
- Solar photoelectrocatalytic degradation of ciprofloxacin at a FTO/BiVO4/MnO2 anode: Kinetics, intermediate products and degradation pathway studies. J. Environ. Chem. Eng.. 2020;8:103607
- [CrossRef] [Google Scholar]
- Recent advances in degradation of pharmaceuticals using Bi2WO6 mediated photocatalysis – A comprehensive review. Environ. Pollut.. 2021;289
- [CrossRef] [Google Scholar]
- Recent advances in degradation of pharmaceuticals using Bi2WO6 mediated photocatalysis – A comprehensive review. Environ. Pollut.. 2021;289:117891
- [CrossRef] [Google Scholar]
- P.M, N., Devi, L.G., 2020. Heavy atom perturbation by the incorporation of iodine ion into BaTiO3 lattice: Reduction of fluorescence and enhancement of rate of interfacial charge transfer process under the visible light irradiation. Surfaces and Interfaces 18, 100411. https://doi.org/10.1016/j.surfin.2019.100411.
- Panda, P.K., Sahoo, B., 2015. P[1] P. K. Panda and B. Sahoo, “PZT to lead free piezo ceramics: A review,” Ferroelectrics, vol. 474, no. 1, pp. 128–143, 2015.ZT to lead free piezo ceramics: A review. Ferroelectrics 474, 128–143. https://doi.org/10.1080/00150193.2015.997146.
- Contamination of water resources by pathogenic bacteria. AMB Express. 2014;4:1-16.
- [CrossRef] [Google Scholar]
- A simple route for photocatalytic degradation of phenol in aqueous zinc oxide suspension using solar energy. Sol. Energy. 2008;82:700-705.
- [CrossRef] [Google Scholar]
- Flexible nanocomposite generator made of BaTiO 3 nanoparticles and graphitic carbons. Adv. Mater.. 2012;24:2999-3004.
- [CrossRef] [Google Scholar]
- Recent trend in visible-light photoelectrocatalytic systems for degradation of organic contaminants in water/wastewater. Environ. Sci. Water Res. Technol.. 2018;4:1389-1411.
- [CrossRef] [Google Scholar]
- WO3-based catalysts for photocatalytic and photoelectrocatalytic removal of organic pollutants from water – A review. J. Water Process Eng.. 2021;40:101930
- [CrossRef] [Google Scholar]
- Sol-gel synthesis of barium titanate powders of various compositions. J. Mater. Chem.. 1992;2:591-594.
- [CrossRef] [Google Scholar]
- Bulk properties and electronic structure of SrTiO3, BaTiO 3, PbTiO3 perovskites: An ab initio HF/DFT study. Comput. Mater. Sci.. 2004;29:165-178.
- [CrossRef] [Google Scholar]
- Progress in the synthesis of nanocrystalline BaTiO3 powders for MLCC. Int. J. Appl. Ceram. Technol.. 2005;2:1-14.
- [CrossRef] [Google Scholar]
- PH dependent coprecipitated oxalate precursors - A thermal study of lead titanate. J. Mater. Sci.. 2001;36:767-770.
- [CrossRef] [Google Scholar]
- Review on the improvement of the photocatalytic and antibacterial activities of ZnO. J. Alloys Compd.. 2017;727:792-820.
- [CrossRef] [Google Scholar]
- Solid-state synthesis semiconducting BaTiO3 nanoparticles at low temperature. Mater. Chem. Phys.. 2020;242:122496
- [CrossRef] [Google Scholar]
- Removal of dyes from the effluent of textile and dyestuff manufacturing industry: A review of emerging techniques with reference to biological treatment. Crit. Rev. Environ. Sci. Technol.. 2005;35:219-238.
- [CrossRef] [Google Scholar]
- Rao, B.G., Mukherjee, D., Reddy, B.M., 2017. Novel approaches for preparation of nanoparticles, Nanostructures for Novel Therapy: Synthesis, Characterization and Applications. Elsevier Inc. https://doi.org/10.1016/B978-0-323-46142-9.00001-3.
- First principles insights into improved catalytic performance of BaTiO3- graphene nanocomposites in conjugation with experimental investigations. Mater. Sci. Semicond. Process.. 2016;51:33-41.
- [CrossRef] [Google Scholar]
- Highly efficient visible light mediated azo dye degradation through barium titanate decorated reduced graphene oxide sheets. Electron. Mater. Lett.. 2016;12:281-289.
- [CrossRef] [Google Scholar]
- A critical review on strategies for improving efficiency of BaTiO3-based photocatalysts for wastewater treatment. J. Environ. Manage.. 2021;290:112679
- [CrossRef] [Google Scholar]
- Electrospun nanofibers of ZnO/BaTiO 3 heterostructures with enhanced photocatalytic activity. Catal. Commun.. 2012;25:32-35.
- [CrossRef] [Google Scholar]
- Solid-state synthesis of Bi2O3/BaTiO3 heterostructure: Preparation and photocatalytic degradation of methyl orange. Appl. Phys. A Mater. Sci. Process.. 2013;111:1139-1145.
- [CrossRef] [Google Scholar]
- Preparation of ferroelectric barium titanate through an energy effective solid state ultrasound assisted method. J. Am. Ceram. Soc.. 2017;100:4511-4518.
- [CrossRef] [Google Scholar]
- Efficient removal of amoxicillin and paracetamol from aqueous solutions using magnetic activated carbon. Environ. Sci. Pollut. Res.. 2017;24:5918-5932.
- [CrossRef] [Google Scholar]
- Schneller, T., Waser, R., Kosec, M., Payne, D., 2013. Theodor Schneller · Rainer Waser Marija Kosec · David Payne.
- Fabrication of mesoporous BaTiO3/SnO2 nanorods with highly enhanced photocatalytic degradation of organic pollutants. J. Ind. Eng. Chem.. 2017;53:201-212.
- [CrossRef] [Google Scholar]
- Built-in Electric Field Assisted Photocatalytic Dye Degradation and Photoelectrochemical Water Splitting of Ferroelectric Ce Doped BaTiO3 Nanoassemblies. ACS Sustain. Chem. Eng.. 2019;7:12032-12043.
- [CrossRef] [Google Scholar]
- Antibacterial and Antibiofilm Activity of Barium Titanate Nanoparticles. Mater. Lett.. 2018;229:130-133.
- [CrossRef] [Google Scholar]
- Nanostructured BaTiO3/Cu2O heterojunction with improved photoelectrochemical activity for H2 evolution: Experimental and first-principles analysis. Appl. Catal. B Environ.. 2016;189:75-85.
- [CrossRef] [Google Scholar]
- Effect of poling condition on piezocatalysis activity of BaTiO 3 -cement composites. Mater. Lett.. 2020;280:128583
- [CrossRef] [Google Scholar]
- A strawberry-like Ag-decorated barium titanate enhances piezoelectric and antibacterial activities of polymer scaffold. Nano Energy. 2020;74:104825
- [CrossRef] [Google Scholar]
- Remediation of water pollution caused by pharmaceutical residues based on electrochemical separation and degradation technologies: A review. Environ. Int.. 2012;40:212-229.
- [CrossRef] [Google Scholar]
- Remediation of water pollution caused by pharmaceutical residues based on electrochemical separation and degradation technologies : A review. Environ. Int.. 2012;40:212-229.
- [CrossRef] [Google Scholar]
- Electrochemical advanced oxidation processes: Today and tomorrow. A review. Environ. Sci. Pollut. Res.. 2014;21:8336-8367.
- [CrossRef] [Google Scholar]
- Microwave-hydrothermal synthesis of tetragonal BaTiO3 under various conditions. Mater. Chem. Phys.. 2006;97:481-487.
- [CrossRef] [Google Scholar]
- Hydrogen Generation and Degradation of Organic Dyes by New Piezocatalytic 0.7BiFeO3-0.3BaTiO3Nanoparticles with Proper Band Alignment. ACS Appl. Mater. Interfaces. 2021;13:11050-11057.
- [CrossRef] [Google Scholar]
- Hydrothermal synthesis of barium titanate nano/microrods and particle agglomerates using a sodium titanate precursor. Ceram. Int.. 2021;47:8904-8914.
- [CrossRef] [Google Scholar]
- Preparation and dielectric properties of polycrystalline films with dense nano-structured BaTiO3 by chemical vapor deposition using inductively coupled plasma. Vacuum. 2006;80:519-529.
- [CrossRef] [Google Scholar]
- Adsorption of dyes by nanomaterials: Recent developments and adsorption mechanisms. Sep. Purif. Technol.. 2015;150:229-242.
- [CrossRef] [Google Scholar]
- Ultrasonic cavitation applied to the treatment of bisphenol A. Effect of sonochemical parameters and analysis of BPA by-products. Ultrason. Sonochem.. 2008;15:605-611.
- [CrossRef] [Google Scholar]
- Ultrasonic assisted synthesis of BaTiO3 nanoparticles at 25 °C and atmospheric pressure. Ultrason. Sonochem.. 2018;41:441-448.
- [CrossRef] [Google Scholar]
- Low-temperature synthesis of BaTiO3 powders by the sol-gel-hydrothermal method. Ceram. Int.. 2013;39:7127-7134.
- [CrossRef] [Google Scholar]
- Ferroelectric BaTiO3@ZnO heterostructure nanofibers with enhanced pyroelectrically-driven-catalysis. Ceram. Int.. 2019;45:90-95.
- [CrossRef] [Google Scholar]
- Harvesting the Vibration Energy with BaTiO3@Graphene for the Piezocatalytic Degradation of Methylene Blue. J. Environ. Sci. Eng. Technol.. 2020;8:84-91.
- [Google Scholar]
- Recent progress in carbon quantum dots: synthesis, properties and applications in photocatalysis. J. Mater. Chem. A. 2017;5:3717-3734.
- [CrossRef] [Google Scholar]
- Photocatalytic purification of simulated dye wastewater in different pH environments by using BaTiO3/Bi2WO6 heterojunction photocatalysts. Opt. Mater. (Amst).. 2021;113:110853
- [CrossRef] [Google Scholar]
- Removal of pharmaceuticals and personal care products (PPCPs) from wastewater: A review. J. Environ. Manage.. 2016;182:620-640.
- [CrossRef] [Google Scholar]
- Effects of active species on degrading A-ring of tetracycline in the Z-scheme heterostructured core-shell La(OH)3@BaTiO3 composition. J. Alloys Compd.. 2019;804:100-110.
- [CrossRef] [Google Scholar]
- Preparation and photocatalytic application of ternary n-BaTiO3/Ag/p-AgBr heterostructured photocatalysts for dye degradation. Mater. Res. Bull.. 2020;124:110754
- [CrossRef] [Google Scholar]
- BaTiO3-graphene nanocomposites: Synthesis and visible light photocatalytic activity. New J. Chem.. 2015;39:4407-4413.
- [CrossRef] [Google Scholar]
- Photocatalytic properties of a new Z-scheme system BaTiO3/In2S3 with a core-shell structure. RSC Adv.. 2019;9:11377-11384.
- [CrossRef] [Google Scholar]
- Size-controlled synthesis of BaTiO3 nanocrystals via a hydrothermal route. Mater. Lett.. 2008;62:3666-3669.
- [CrossRef] [Google Scholar]
- Wen, W., Hai, J., Yao, J., Gu, Y., Kobayashi, H., n.d. Supporting Information Univariate Lattice Parameter Modulation of Single-Crystal-like Anatase TiO 2 Hierarchical Nanowire Arrays to Improve.
- Rational construction of plasmon Au assisted ferroelectric-BaTiO3/Au/g-C3N4 Z-scheme system for efficient photocatalysis. Catal. Today. 2020;355:311-318.
- [CrossRef] [Google Scholar]
- Effective enhancement of piezocatalytic activity of BaTiO3 nanowires under ultrasonic vibration. Nano Energy. 2018;45:44-51.
- [CrossRef] [Google Scholar]
- Piezotronic effect boosted photocatalytic performance of heterostructured BaTiO3/TiO2 nanofibers for degradation of organic pollutants. Nano Energy. 2020;77:105122
- [CrossRef] [Google Scholar]
- Novel dam-like effect based on piezoelectric energy conversion for drug sustained release of drug-loaded TiO2 @ BaTiO3 coaxial nanotube coating. Ceram. Int.. 2021;47:17550-17559.
- [CrossRef] [Google Scholar]
- Xia, C., Shi, E., Zhong, W., Guo, J., 1996. Hydrothermal synthesis of BaTiO 3 nano / microcrystals 166, 961–966.
- Enhanced photocatalytic activity of BaTiO3@g-C3N4 for the degradation of methyl orange under simulated sunlight irradiation. J. Alloys Compd.. 2015;622:1098-1104.
- [CrossRef] [Google Scholar]
- Degradation of diuron by chlorination and UV/chlorine process: Degradation kinetics and the formation of disinfection by-products. Sep. Purif. Technol.. 2018;202:365-372.
- [CrossRef] [Google Scholar]
- Enhanced mineralization of antibiotic berberine by the photoelectrochemical process in presence of chlorides and its optimization by response surface methodology. Environ. Earth Sci.. 2015;4947–4955
- [CrossRef] [Google Scholar]
- Formation and photocatalytic activity of BaTiO3 nanocubes via hydrothermal process. J. Nanomater.. 2015;2015:12-15.
- [CrossRef] [Google Scholar]
- Application of piezoelectric transducer in energy harvesting in pavement. Int. J. Pavement Res. Technol.. 2018;11:388-395.
- [CrossRef] [Google Scholar]
- Piezotronics enhanced photocatalytic activities of Ag-BaTiO3 plasmonic photocatalysts. J. Alloys Compd.. 2019;801:483-488.
- [CrossRef] [Google Scholar]
- Ultrahigh piezocatalytic capability in eco-friendly BaTiO3 nanosheets promoted by 2D morphology engineering. J. Colloid Interface Sci.. 2021;596:288-296.
- [CrossRef] [Google Scholar]
- Remarkably enhanced piezo-photocatalytic performance in BaTiO3/CuO heterostructures for organic pollutant degradation. J. Adv. Ceram.. 2022;11:414-426.
- [CrossRef] [Google Scholar]
- Multiple nucleation and crystal growth of barium titanate. Cryst. Growth Des.. 2012;12:1247-1253.
- [CrossRef] [Google Scholar]
- Boosting photocatalytic water splitting by tuning built-in electric field at phase junction. J. Mater. Chem. A. 2019;7:10264-10272.
- [CrossRef] [Google Scholar]
- A Floatable Piezo - Photocatalytic Platform Based on Semi - Embedded ZnO Nanowire Array for High - Performance Water Decontamination. Nano-Micro Lett.. 2019;11:1-14.
- [CrossRef] [Google Scholar]
- Toward designing semiconductor-semiconductor heterojunctions for photocatalytic applications. Appl. Surf. Sci.. 2018;430:2-17.
- [CrossRef] [Google Scholar]
- Modulating Photovoltaic Conversion Efficiency of BiFeO3-Based Ferroelectric Films by the Introduction of Electron Transport Layers. ACS Appl. Energy Mater.. 2019;2:5540-5546.
- [CrossRef] [Google Scholar]
- The effect of piezo-photocatalysis on enhancing the charge carrier separation in BaTiO3/KNbO3 heterostructure photocatalyst. Appl. Surf. Sci.. 2021;562:150164
- [CrossRef] [Google Scholar]
- Carbonaceous nanomaterial-TiO2 heterojunctions for visible-light-driven photocatalytic degradation of aqueous organic pollutants. Appl. Catal. A Gen.. 2022;630:118460
- [CrossRef] [Google Scholar]
- Photodegradation of acetaminophen in TiO2 suspended solution. J. Hazard. Mater.. 2008;157:300-307.
- [CrossRef] [Google Scholar]
- Applied Catalysis B : Environmental Construction of novel symmetric double Z-scheme BiFeO 3 / CuBi 2 O 4 / BaTiO 3 photocatalyst with enhanced solar-light-driven photocatalytic performance for degradation of nor fl oxacin. Appl. Catal. B Environ.. 2020;272:119017
- [CrossRef] [Google Scholar]
- Synthesis of tetragonal BaTiO3 nano-particle via a novel tartaric acid co-precipitation process. Ceram. Int.. 2021;47:7263-7267.
- [CrossRef] [Google Scholar]
- Effect of calcination temperature on the activity and structure of MnOx/TiO2 adsorbent for Hg0 removal. Fuel Process. Technol.. 2015;135:25-33.
- [CrossRef] [Google Scholar]
- SPR enhanced photocatalytic properties of Au-dispersed amorphous BaTiO3 nanocomposite thin films. J. Alloys Compd.. 2016;654:112-119.
- [CrossRef] [Google Scholar]
- Ternary BiVO4/NiS/Au nanocomposites with efficient charge separations for enhanced visible light photocatalytic performance. Chem. Eng. J.. 2019;375
- [CrossRef] [Google Scholar]
- Graphene oxide modified nano-sized BaTiO3 as photocatalyst. Ceram. Int.. 2018;44:15929-15934.
- [CrossRef] [Google Scholar]
- Zhao, W., Zhang, Q., Wang, H., Rong, J., E, L., Dai, Y., 2020. Enhanced catalytic performance of Ag2O/BaTiO3 heterostructure microspheres by the piezo/pyro-phototronic synergistic effect. Nano Energy 73, 104783. https://doi.org/10.1016/j.nanoen.2020.104783.
- High efficiency degradation of tetracycline and rhodamine B using Z-type BaTiO3/γ-Bi2O3 heterojunction. Sep. Purif. Technol.. 2022;278:119666
- [CrossRef] [Google Scholar]
- Enhanced piezo-electro-chemical coupling of BaTiO3/g-C3N4 nanocomposite for vibration-catalysis. J. Mater. Sci.. 2020;55:14787-14797.
- [CrossRef] [Google Scholar]
- Piezoelectric effect synergistically enhances the performance of Ti32-oxo-cluster/BaTiO3/CuS p-n heterojunction photocatalytic degradation of pollutants. Appl. Catal. B Environ.. 2021;291:120019
- [CrossRef] [Google Scholar]
- Piezophototronic effect in enhancing charge carrier separation and transfer in ZnO/BaTiO3 heterostructures for high-efficiency catalytic oxidation. Nano Energy. 2019;66:104127
- [CrossRef] [Google Scholar]
- Integrated adsorption and photocatalytic degradation of volatile organic compounds (VOCs) using carbon-based nanocomposites: A critical review. Chemosphere. 2019;218:845-859.
- [CrossRef] [Google Scholar]







