Translate this page into:
Applications of Fenton oxidation processes for decontamination of palm oil mill effluent: A review
⁎Corresponding author. r_adnan@usm.my (Rohana Adnan)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
Oil palm agro-industry is a major revenue earner for Malaysia with the country being one of the major producers of crude palm oil (CPO) and oil palm products. Its growth has, however, led to massive water consumption and high generation of highly polluting palm oil mill effluent (POME). The inadequacies of ponding system adopted by most palm oil mills (POMs) for the treatment of POME to alleviate environmental and public health concerns are quite alarming. Fenton advanced oxidation technologies are a current research area providing viable alternatives for POME treatment, recovery and managing high demand for water. Its major setback is the generation of a large amount of unwanted sludge of iron (III) complexes thus increasing the costs of sludge management, treatment, and disposal. The salient and promising features of this technique for industrial applications motivate researchers to find ways to overcome its inherent drawbacks. This brief review aimed at discussing and evaluating the performances of the various Fenton oxidation processes, including homogeneous, heterogeneous, photo-Fenton, electro-Fenton, sono-Fenton etc., for POME treatment. Discussions on the future direction of these Fenton processes points towards the utilization of abundant magnetically separable heterogeneous composites as catalysts with high stability, activity, recyclability, and cost-effectiveness for decontamination of POME and other agro-industrial effluents from recalcitrant organic pollutants. The low deployment of such composite catalyst coupled with scarce literature on POME treatment in this regard offers a vast opportunity for research exploration.
Keywords
Agro-industrial effluent
Fenton oxidation
Heterogeneous catalyst
Homogenous catalyst
Palm oil mill effluent
Water pollution
1 Introduction
The palm genus Elaeis (Greek for ‘oil’) consists of two different species: Elaeis guineensis, originally from West Africa, and Elaeis oleifera (‘oil-producing’), originally from Central and South America (Abu Bakar et al., 2018). Both palm species are well known as highly efficient oil-producing plants, similar to rapeseed, sunflower, cottonseed and soybean plants (Alliance, 2016). Oil palm represents one of the most versatile crops in tropical regions, especially Malaysia and Indonesia. In Malaysia, the palm oil industry is one of the leading agricultural industries, with average crude palm oil (CPO) production growing significantly from less than 100,000 tonnes in 1960 to about 17.32 million tonnes in 2016 (Nambiappan et al., 2018). This massive production, however, has consequently produced an even larger volume of palm oil mill effluent (POME). Despite its contribution to Malaysia's economy, the palm oil industry is one of the agricultural industries that discharge the highest pollution loads into rivers in the country (Bala et al., 2015). POME contains soluble materials that are harmful to the environment, either in the form of soluble gases such as methane (CH4), sulphur dioxide (SO2) and ammonia (NH3), soluble liquids or solids, with concentrations above the threshold limit values (Igwe and Onyegbado, 2007). Consequently, it has become necessary to treat the effluent to the highest degree possible before it is discharged into the environment. Various types of treatment have been proposed to curb pollution by POME, including evaporation, simple skimming, coagulation, adsorption and anaerobic and aerobic biodegradation (Zhang et al., 2008). Only a few of these treatments have been used in full-scale operations because of their unsatisfactory performance, high capital investment and high maintenance costs (Zhang et al., 2008).
In the past two decades, advanced oxidation processes (AOPs) have received great attention as alternative methods for the reduction of the organic load in different kinds of wastewaters generated from different processing agro-industries such as olive oil mill (Alver et al., 2015; Amor et al., 2019a; Michael et al., 2014), winery (Amor et al., 2019b; Souza et al., 2013) and POMs (Aris et al., 2008; Bashir et al., 2017; Bello and Abdul Raman, 2017). AOPs can transform non-biodegradable pollutants into nontoxic biodegradable substances by oxidizing a broad range of organic pollutants quickly and non-selectively through the generation of very reactive species such as hydroxyl radical (•OH) (Mohajeri et al., 2010; Salari et al., 2009). Oxidation by H2O2 alone has been found ineffective for high concentrations of certain refractory contaminants, because of low rates of reaction at reasonable H2O2 concentrations. Utilization of ozonation AOPs, for example, could have the disadvantage of forming bromate ion species, a strong carcinogen, as bye-product during the treatment of bromide-containing effluent (Baus et al., 2005). Transition metal salts (e.g. iron salts), ozone and UV-light can activate H2O2 to form •OH (Shu et al., 2006). The homogeneous Fenton process has attracted great interest given its high capacity to generate •OH through the decomposition of H2O2 by Fe (II) in acidic conditions (Kakavandi et al., 2019). Moreover, it can be applied to a wide range of organic pollutants, because of its simplicity and ease of implementation; operation under mild conditions of temperature and pressure (Kakavandi et al., 2019). The recent shift to heterogeneous Fenton processes could be due to their ability for catalyst recovery and recyclability post-treatment using, for example, a magnetically removable magnetite nanoparticles/activated carbon composite in the treatment of recalcitrant pollutants in wastewater (Kakavandi and Ahmadi, 2019; Kakavandi and Babaei, 2016; Khaghani et al., 2019).
1.1 Palm oil industry and production at a glance
In Malaysia, the palm oil industry contributes enormously to the income of the country, since oil palm plantations account for 77% of agricultural land and approximately 15% of the total land area (Alliance, 2016). As reported by the Department of Statistics Malaysia (2016), palm oil production increased by as much as 338,200 tons (0.40%) from 2013/2014 to 2014/2015. Based on Fig. 1, the production of palm oil superseded soybean oil from just 13% in 1990 to 31% of total oil and fats production in 2015. This is because oil palm has higher annual oil yield per hectare compared to other oilseeds crops including soybean (Chin et al., 2013). Other countries that contribute to palm oil production include Thailand, Columbia, Nigeria, and Indonesia. Indonesia and Malaysia are the two top palm oil producers and together produce 85% of the world's palm oil (Sheil et al., 2009). Malaysia is currently the second-largest producer of crude palm oil in the world with production standing at 39% (19.92 million tonnes) of the total global production in 2017. It is also the largest exporter of refined palm oil in the world with export value standing at 16.56 million tonnes of palm oil in 2017, or 44% of global total exportation (Cheng et al., 2017; Government of Malaysia, 2018). Fig. 1 compares the global production of oils and fats in 1990 and 2015.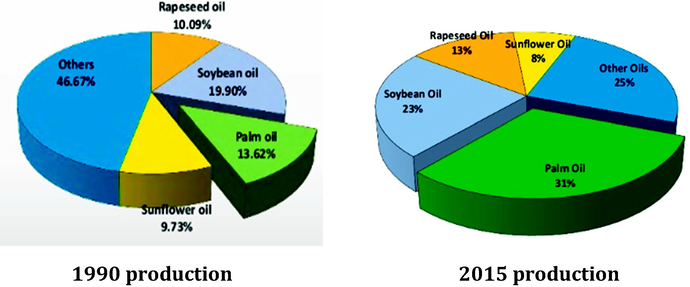
World oil and fat production in 1990 and 2015 (Government of Malaysia, 2016; Ahmad et al., 2003).
While crude palm oil production increased by 11.97% to 19.39 million tonnes in 2017, foreign exchange earnings from the export of palm oil have increased by 15% to RM 77.85 billion in the same year compared to the previous year records (Malaysian Palm Oil Council, 2017). Oil palm plantation acreage has also expanded to 5.81 million hectares in 2017 in contrast to 5.47 million hectares recorded in the previous year (Malaysian Palm Oil Council, 2017). Tables 1 and 2 summarise the performances and future projections of the Malaysian palm oil industry. Despite the immense contributions to the Malaysian economy and improved living standards among citizens of these countries, the oil palm industry has also been recognised as a major contributor source of enormous pollution loadings into local canals and rivers (Wu et al., 2009; Zainuri et al., 2018b). *PA: planted acreage; CPOP: crude palm oil production (Million tonnes); POE: palm oil exports (Million tonnes); OPPTE: oil palm products total exports (Million tonnes); POER: palm oil exports revenue (Million tonnes); OPPER: oil palm products export revenue (Billion RM).
Marketing Year
Palm oil production performance indices
Reference
PA
CPOP
POE
OPPTE
POER
OPPER
2009
4.69
17.56
15.88
22.43
36.95
49.66
Government of Malaysia (2010)
2010
4.85
16.99
16.66
23.06
44.86
59.77
Government of Malaysia (2011)
2011
5.00
18.91
17.99
24.27
60.47
80.41
Government of Malaysia (2012)
2012
5.08
18.79
17.56
24.56
52.96
71.40
Government of Malaysia (2013)
2013
5.23
19.22
18.15
25.70
45.27
61.36
Government of Malaysia (2014)
2014
5.39
19.67
17.31
25.07
44.50
63.62
Government of Malaysia (2015)
2015
5.64
19.96
17.45
25.37
41.26
60.17
Government of Malaysia (2016)
2016
5.74
17.32
16.05
23.29
43.34
64.59
Government of Malaysia (2017)
2017
5.81
19.92
10.56
23.97
46.12
77.85
Government of Malaysia (2018)
Marketing year
Global vegetable oils production (million tonnes)
The proportion of palm oil from vegetable oil production
Average price (USD/tonne)
Production (million tonnes)
Production (%)
2015–2017
190
65.1
34.3
784
2018
202
71.1
35.2
829
2019
206
72.6
35.3
829
2020
210
74.2
35.4
830
2021
214
75.8
35.5
834
2022
217
77.1
35.5
843
2023
221
78.4
35.5
853
2024
224
79.6
35.5
863
2025
228
80.8
35.5
875
2026
231
82.0
35.5
883
2027
235
83.2
35.5
892
Oils that fall into the category of vegetable oil comprises of soybeans oils/other oilseeds oils (≈55% global production), palm oil (≈35% of global production), coconut oil, cottonseed oil and palm kernel oil (OECD/FAO, 2018).
Discharge of untreated POME effluents into receiving water bodies could cause significant environmental problems due to its very high biochemical oxygen demand (BOD), chemical oxygen demand (COD) (Santana et al., 2019), oil and grease, total solids (TS) and total suspended solids (TSS) (Parthasarathy et al., 2016; Wu et al., 2009). While water pollution originating from this practice has continued to be a subject of public complaints, reports on histopathological changes in fish organs exposed to POME attested to the probable toxicity of the effluent to humans (Bello et al., 2013; Gamaralalage et al., 2019).
1.2 Palm oil extraction as a source of water pollution
There are several stages involved in crude palm oil (CPO) extraction from fresh fruit bunches (FFBs). The first one is sterilization where FFBs just brought in from the mill are subjected to high-pressure steam (120–140 °C at 40 psi) (Liew et al., 2015). The next stage is stripping in which the sterilized fruits are separated from the bunch stalks via a rotary drum thresher (Wu et al., 2010). The detached fruits then undergo the stripper’s bar screen. Then, a bucket conveyor collects the screened fruits and channels them into a digester with a central rotating shaft equipped with some mechanical arms fitted to a steam-heated cylindrical vessel that softens and mashes the fruits. The rotation of the shaft coupled with temperatures of 80–90 °C loosens the nut’s outer covering (mesocarp). The CPO in the nuts is squeezed out using a mechanical press machine (Igwe and Onyegbado, 2007; Wu et al., 2010). The press machine gives out a mix of highly viscous fluids (fibrous material, debris, insoluble solids, palm oil, and water) that must be clarified. Therefore, the oil emulsion is first broken into two phases – lighter oil droplets flow through the watery mixture to the top while the emulsion settles to the bottom of the clarifier – by adding hot water as a barrier. The bottom phase in the clarifier is the sludge and POME that is drained off and purified before being discharged (Rupani et al., 2010). Significant waste is also generated as a result of the process of extracting the oil, as shown in Fig. 2.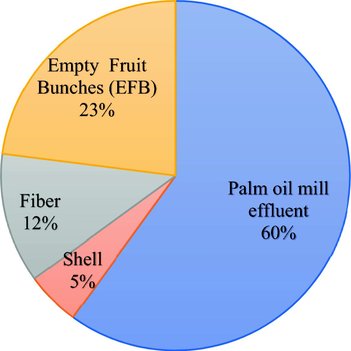
Waste produced from the palm oil mill (Bala et al., 2014a).
For every tonne of FFBs in the mill, the waste can be divided into 23% EFBs, 12% mesocarp fibre, 5% shell and 60% POME (Baharuddin et al., 2010; Bala et al., 2014b, 2014a). Based on these values, POME represents most of the waste from the palm oil industry. During the wet extraction of palm oil, copious quantities (of about 1.5 m3) of water are consumed per tonne of FFBs processed (Azmi et al., 2012). Of an estimated 5.0–7.5 tonnes of water consumed during the wet extraction process of FFBs per tonne of crude palm oil produced, >2.5–3.75 tonnes are converted into POME, a major drawback to the industry (Bello et al., 2013; Gamaralalage et al., 2019; Parthasarathy et al., 2016; Wu et al., 2009). Lorestani (2006) found that FFBS sterilization, CPO extraction and clarification, and kernel and shell separation of cracked mixtures in the hydrocyclone were the processes that generated the most POME at 36%, 60%, and 4%, respectively. A rapid increase in the number of POMs from 355 to 426 mills, further exacerbated this problem (Chin et al., 2013). Fig. 3 shows a diagram that simplifies the palm oil production process.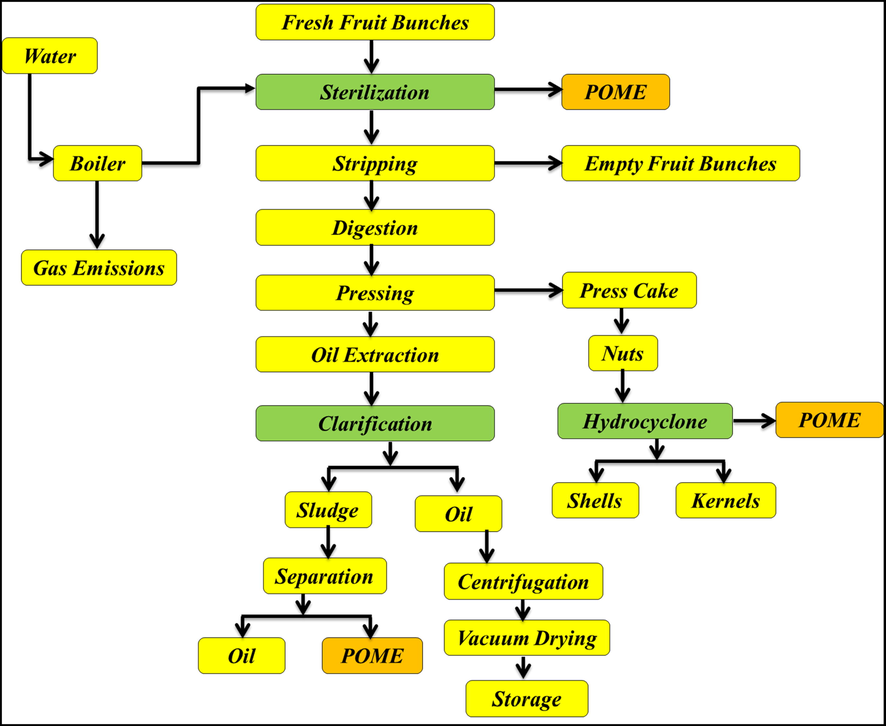
Diagram of the palm oil mill processes (Igwe and Onyegbado, 2007; Rupani et al., 2010).
Raw or fresh POME is a mixture of 95–96% water, 4–5% total solids and 0.6–0.7% oil/grease. About half to four-fifths of the total solids are suspended solids, constituting mostly of palm fruit mesocarp debris generated by sterilizer condensate, separator sludge and hydrocyclone process (Bello et al., 2013; Parthasarathy et al., 2016; Wu et al., 2009).
POME is a thick brownish liquid with high COD and BOD (Poh et al., 2010). Moreover, as POME has high acidity and solids, it cannot be directly discharged into the waterway. Liew et al. (2015) highlighted that the massive quantities of POME generated from most of the water output of crude palm oil processing, if not properly managed, will pollute the waterways near the POMs. Table 3 lists the typical characteristics of raw POME. Raw POME has high temperatures (80–90 °C) and the effluent is rich in organic contaminants such as lignin (4700 ppm), phenolics (5800 ppm), pectin (3400 ppm) and carotene (8 ppm) (Bello et al., 2013; Zainuri et al., 2018b). The industry has been generating a large volume of wastes annually which kept threatening the sustainability of the ecological environment (Zainuri et al., 2018a).
Parameter
Range (mg/L)
Reference
BOD
25,000–65,714
Alhaji et al. (2016); Kamyab et al. (2015)
COD
44,300–102,696
Abdulrahman et al. (2016); Wang et al. (2015c)
Total solids
40,500–72,058
Tee et al. (2016)
Suspended solids
18,000–46,011
Ahmad et al. (2016); Liew et al. (2015)
Oil and grease
4000–9341
Abu Bakar et al. (2018)
NH3-N
35–103
Chin et al. (2013); Wu et al. (2007)
Total nitrogen
750–770
Chin et al. (2013); Zhang et al. (2008)
pH
4–5
Chin et al. (2013)
1.3 Regulatory frameworks for effluent discharge limits
Several regulatory and legislative frameworks have been enforced to ensure environmental protection and safety against the ills of untreated POME and other agro-industrial wastewaters discharge into the environment. For example, the Environmental Quality Order of 1977 and Environmental Quality Regulations of 1977, with the Environmental Quality Act (EQA) of 1974 which granted legal jurisdiction to the Malaysian Department of Environment (DOE) for enforcement and compliance (Azmi et al., 2012; Zainuri et al., 2018a). The DOE sets the standard characteristics and discharge limits of POME as illustrated in Table 4 along with the standards provided by the World Health Organization (WHO) and the United States Environmental Protection Agency (USEPA). Therefore, strict compliance with these legislations is necessary to ensure environmental sustainability through wastewater reclamation and recycling for domestic and industrial uses (Azmi et al., 2012).
Parameters
POME discharge limits
DOEa
EQA
USEPA standard
WHO standarde
BOD5
50b
100
–
–
COD (mg/L)
1000
1000c
NRd
Total solids (mg/L)
1500
1500c
–
500–1500
Suspended solids (mg/L)
400
400
–
–
Oil and grease (mg/L)
50
50
0.3
–
Ammoniacal nitrogen (mg/L)
100
150c
NR
–
Total nitrogen (mg/L)
200
200c
–
–
pH
5.0
5.0–9.0
6.5–8.5
6.5–9.2
Temperature (°C)
45
45
–
–
1.4 Conventional treatment methods
More than 85% of POMs in Malaysia have adopted the ponding system for POME treatment while the rest opted for open digestion tank, with anaerobic digestion as their primary treatment technique (Poh and Chong, 2009). Laboratory-scale high-rate anaerobic bioreactors developed for POME treatment include up-flow anaerobic sludge blanket (UASB) reactor (Fang et al., 2011), up-flow anaerobic filtration (Borja and Banks, 1994), up-flow anaerobic sludge blanket-hollow centred packed bed (UASB-HCPB) reactor (Poh and Chong, 2014), fluidized bed reactor (Borja and Banks, 1995) and up-flow anaerobic sludge fixed-film (UASFF) reactor (Zinatizadeh et al., 2007), anaerobic contact digester and continuously stirred tank reactor (CSTR) (Choorit and Wisarnwan, 2007) have also been studied for the treatment of POME. POME has also been treated using membrane technology, coagulation-flocculation method, aerobic activated sludge reactor (Vijayaraghavan et al., 2007) and evaporation method (Hassan et al., 2002).
The pale-yellow colour (λ = 400–700 nm) of POME for discharge – which is a shade of the initial brownish colour – that remains even after the conventional ponding, biological, coagulation-flocculation treatments signifies the presence of recalcitrant pollutants (Bello et al., 2013; Soleimaninanadegani and Manshad, 2014; Zainuri et al., 2018a). Continuous colour accumulation in the receiving water shields sunlight penetration which consequently paralyses photosynthetic activities in the aquatic environment (Kant, 2012). Chelation of metal ions by some high-affinity chemical pollutants in POME may occur, exposing aquatic biota to the toxicity of the resulting hazardous complex products. Pollutant and pigments in POME are non-biodegradable, making conventional treatments insufficient and inefficient for POME degradation and mineralisation (Bello et al., 2013; Wu et al., 2009). Detailed cost analysis revealed that the conventional POME treatment systems, such as ponding, are not only prone to further environmental pollution but also have the lowest level for the utilization of renewable resources without any significant profits (Wu et al., 2009). The utilization of these conventional techniques is disadvantaged for having long retention time, slow start-up reactors, large land area requirement for digesters, high energy consumption, low rate of pathogen inactivation, membrane short-life/fouling and high operational costs (Poh and Chong, 2009). Therefore, other alternative treatment technique is necessary to replace or reinforce the existing conventional ones to ensure more effective degradation and mineralization of POME pollutants (Bello et al., 2013).
As a result, multiple research efforts are currently in progress to develop inexpensive and more effective methods for the treatment of raw POME to ensure complete compliance with the existing stringent environmental regulations (Gamaralalage et al., 2019).
Among the potential methods available, advanced oxidation process (AOPs) have gained attention as an interesting and economically viable technique for the development of wastewater treatment method. Wastewater treatment using AOPs is based on the generation of highly reactive and non-selective radicals as powerful oxidants (Huang et al., 1993). The term referred to oxidation processes capable of rendering water treatment at ambient temperature and pressure through the generation of an adequate amount of •OH as originally defined by Glaze et al. (1987). AOPs have currently evolved to include, among other methods i.e photocatalysis (Cheng et al., 2017; Ng et al., 2016b, 2016a; Ng and Cheng, 2017), ozonation/UV at elevated pH’s (Wu et al., 2018), ozonation/hydrogen peroxide (Qiang et al., 2010), ozonation/catalysts (Dai et al., 2014), ozonation/activated carbon (Wang et al., 2019), ultrasound-assisted oxidation (Kakavandi and Ahmadi, 2019; Manickam et al., 2014), microwave-assisted oxidation (Garcia-Costa et al., 2019), wet air oxidation (Palas et al., 2018), iron (II)-activated persulphate oxidation (Vicente et al., 2011), and Fenton oxidation processes (Saleh and Taufik, 2019).
AOPs have been extensively used for overall organic COD reduction, destruction of a specific pollutant, sludge treatment, increase of bioavailability of recalcitrant organics, destruction of micropollutants, colour and odour removal. AOPs have the potential for generating a relatively higher amount of radicals and are found to be more attractive in the treatment of high-strength industrial effluent (Rahim Pouran et al., 2015). Table 5 summarizes some recent technologies used for the treatment of POME.
Technology
Process description
Removal
Reference
Adsorption techniques
Steam activated bio-adsorbent from oil palm biomass
At first, under slight oxygen atmosphere oil palm mesocarp fibre (OPMF) was carbonised at 600 °C for 30 min. In the second step, OPMF was activated via steam at 105 °C and 120 mL/min flow rate (2 bar) with a furnace at 600 °C (30 min). Finally, the activated sample was washed, dried, and then sieved to the size of 150–350 µm.
COD (70%) SS (88%)
Ibrahim et al. (2017)
Coconut shell-based activated carbon absorbent
Batch adsorption uptake was studied at various contact times (0.5–48 h) and POME initial concentrations (10–50%).
COD, TSS & Colour (̴70%)
Kaman et al. (2017)
Electrocoagulation and coagulation processes
Electrocoagulation on polialuminum chloride (PAC) coagulant with hydrogen peroxide as an oxidizing agent
First phase: Two monopolar electrodes – the anode and other one cathode – of the same dimensions and separated by a 10-mm horizontal spacing were set up in the electrocoagulation cell of the mixture. Second phase: A stirrer was used to stir the mixture to maintain composition homogeneity and avoid the association of flocs. Final phase: PAC and hydrogen peroxide (H2O2) were then added to the system as a coagulant and an oxidant, respectively.
COD (86.7%)
Nasrullah et al. (2017)
Coagulation on chitosan or in combination with FeSO4, H2O2 or Fenton reagent
Step 1: use of coagulation by chitosan. Then, the addition of ferrous sulphate (FeSO4). Next: chitosan with hydrogen peroxide (H2O2). Final: the use of chitosan with Fenton oxidation
COD (60.2–84.5%)
TSS (85.5–100%)Parthasarathy et al. (2016)
Coagulation with alum, PAC and FeCl3 as coagulants
Jar test was carried out in the presence of alum, PAC and FeCl3 as coagulants.
COD (98.1%)
SS (99.1%)Othman et al. (2014)
Coagulation–flocculation with alum and unmodified Cassia obtusifolia seed gum as coagulants
The procedure was carried out using the batch jar test employing Cassia obtusifolia seed gum and alum as coagulants.
COD (48.2%)
TSS (81.6)Shak & Wu (2015)
Advanced oxidation processes
Fenton oxidation
Batch Fenton oxidation modelled experiments were performed on diluted POME (non-filtered) in a pair set involving an open system for liquid samples analyses and a closed flask system for gaseous samples analyses.
NH3-N (73%),
TOC (91%),
TN (11%),
TP (99.9%).Gamaralalage et al. (2019)
UV/TiO2 and UV/ZnO photocatalytic systems
Both TiO2 and ZnO photocatalysts were calcined at 573 K and stored in amber glass sampling bottles. After photoreaction, photocatalysts were filtered, washed multiple times with water and ethanol, and then recycled.
COD (74.1% on ZnO; 80% on TiO2)
Ng et al. (2017)
Filtration technique
Calcined limestone horizontal roughing filter
First Phase: The limestone was crushed into 3 different sizes (mm), washed and then dried. Second phase: The limestone was calcined at 800 °C and finally used in column studies as horizontal roughing filters with 3 different flowrates.
COD (51%)
Dashti et al. (2019)
1.5 Homogeneous Fenton chemistry and processes
In 1894, approximately 126 years ago, Henry John Horstman Fenton reported the exceptional ability of iron (II) ions to activate the hydrogen peroxide oxidation of tartaric acid (Fenton, 1894). Later, Haber and Weiss (1934) ruled that •OH was the reactive chemical species responsible for the tartaric acid oxidation, and it was the product of iron-(II)-catalysed cleavage of hydrogen peroxide in solution. The term “Fenton reagent” refers to the mixture of iron (II) salt and hydrogen peroxide known for its high effectiveness in oxidizing many organic substances (Hartmann et al., 2010). Nowadays, Fenton oxidation process has evolved from being a classical oxidation technique for the treatment of simulated wastewater samples containing pure organic compounds into a series of techniques integrating various sources of energy and other phenomena (such as solar, sound, visible, ultraviolet, etc.).
Among advanced oxidation processes (AOPs), the photo-Fenton process using a homogeneous or heterogeneous catalyst has been investigated to be a cheaper and more effective alternative for the treatment of palm oil industrial effluent resulting from enhanced degradability of recalcitrant contaminants leading to a significant increase in COD removal, reduced chemical consumption, lower volume of sludge in addition to improved catalyst recovery and recyclability for heterogeneous setup (Buthiyappan and Abdul Raman, 2019; Kanakaraju et al., 2017).
The general Fenton oxidation process reaction mechanism is represented by Eqs. (1)–(4).
1.6 Conventional Fenton oxidation process
Conventional, ambient, or classical Fenton oxidation process utilises the ability of hydrogen peroxide to generate •OH and other species in the presence of a homogeneous ferrous iron catalyst. It is almost 90 years now since Haber and Weiss (1932) published their work on the decomposition of H2O2 into •OH in the presence of iron (II). The mechanism through which organic pollutants are oxidized in conventional Fenton treatment has been established to include over 20 well-studied and documented chemical reactions (Pliego et al., 2015; Zhang et al., 2020a, 2020b). The transfer of an electron between hydrogen peroxide and ferrous ion (shown in Eq. (1)) is the overwhelmingly accepted key reaction that produces the •OH during the Fenton treatment process with the recycling of iron catalyst between the Fe2+ and Fe3+ oxidation states (Pliego et al., 2015; Rahim Pouran et al., 2015; Zhang et al., 2019) while Eq. (5) depicts the mechanism of pollutants degradation by the •OH species from the catalytic decomposition of H2O2 (Machulek et al., 2012).
The procedure for the Fenton treatment involves (i) agitating raw wastewater samples mechanically to ensure homogenization (ii) addition of appropriate reagent for the adjustment of the pH to its optimum value of around 3 and (iii) transferring the consequent feed mixture into a reactor. To initiate the reaction, known aliquots dosages of an iron (II) salt and hydrogen peroxide solutions are directly transferred into the reactor once the temperature rises to a required value of between 20 and 50 °C (Munoz et al., 2015; Santana et al., 2019). The reaction is then allowed to proceed for a predetermined period, after which it is stopped by the addition of sodium hydroxide to neutralize the acidic medium necessary for the Fenton reagent to degrade the pollutants in the mixture (Riadi et al., 2019). Finally, finely aggregated particulates formed at the end of the treatment process must be removed by filtration through 0.45 μm membrane to ensure the treated wastewater meeting the regulatory limit for an effluent recycle, reuse or discharge into the adjacent water bodies (Munoz et al., 2015; Santana et al., 2019). Several factors that influence the degradation and mineralization of pollutant by the conventional Fenton treatment include pH value, Fe (II)/H2O2 molar ratio, the concentration of the pollutants and the amount of iron salt (Muruganandham et al., 2014; Rasalingam et al., 2014).
Fenton oxidation technique has been successfully used to treat highly polluted wastewaters including those from agro-industries such as POMs, olive oil mills, breweries, tanneries, pulp and paper mills and sawmills (Munoz et al., 2015). For example, Gamaralalage et al. (2019) have reported high TOC removal (91%) from POME under the optimum parameters of pH (3), time (180 min), stirring speed (500 rpm), temperature (25 °C), TOC:H2O2:Fe2+ molar ratio (1:3.7:0.6) and initial COD (0.5 g/L). This shows substantial mineralisation of the pollutants in POME even though the formation of a reasonable amount of sludge-like material suspected to have resulted from the oligomerization of organic pollutants was reported.
Nithyanandam et al. (2015) achieved COD removal of 63.9% and BOD removal of 61.3% at the pH of 3 within 30 min of the reaction time at room temperature (25 °C) and pressure of 1 atm; Fe2+:H2O2 molar ratio (1:2); H2O2 concentration (0.1–0.5 M); initial COD (1408 mg/L); and initial BOD (1359 mg/L). The high COD removal suggests reasonable degradation of the high content of recalcitrant substances in the POME. In addition to that, the BOD removal value suggests the high content of substances amenable to biological degradation in the original sample remaining in the treated samples. Similarly, Ahmed et al. (2011) reported COD and colour removals of 75% and 65%, respectively.
An integrated ambient Fenton/chitosan system was investigated for the treatment of POME under the parameters of chitosan dosage (500–12,500 mg/L), FeSO4 dosage (500–12,500 mg/L), mixing time (15–60 min), sedimentation time (1–4 h), pH (2–9) and H2O2 concentration (500–7500 mg/L). Despite the role of chitosan as a stabiliser of coagulants produced during the process, the integrated ambient system was found to have lower COD removal efficiency (73.1%) than chitosan/H2O2 system (82.8%) but higher efficiency for TSS removal (100%) than chitosan/H2O2 system (89.9%). The results indicated that the integrated ambient Fenton/chitosan system was less effective in the degradation of recalcitrant pollutant but was more effective in the removal of TSS in POME than the chitosan/H2O2 system (Parthasarathy et al., 2016).
Lim et al. (2017) had reported a lower COD removal efficiency of 48% implying lower degradation of the recalcitrant compounds in the treated POME samples despite a relatively longer reaction time of 4 h. On the contrary, TOC and COD removal of as much as 92.1% and 85.1%, respectively were reported by Saeed et al. (2015) under the optimum parameters of pH (4.57), H2O2 concentration (3.50 g/L), Fe2+ concentration (1.88 g/L), reaction time (30 min) and agitation rate (120 rpm). The high TOC and COD removals attained show excellent efficiency in both degradation and mineralization of the recalcitrant pollutant in POME within a relatively short time. Despite the slow rate of iron (II) ion regeneration, small dosages of iron (II) salt are required for the process. Usually, the stoichiometric dosage of the oxidant serves as the optimum amount for complete mineralization of pollutants in the effluent being treated (Munoz et al., 2015). Table 6 summarizes some recent studies on the homogeneous ambient Fenton treatment of POME and other agro-industrial wastewater along with their operational and performance parameters.
Wastewater type
Fenton process
Operational parameters
Removal efficiency
Reference
POME
Fe2+/H2O2
pH (2–5); Time (90–180 min); stirring (500 rpm); Temperature (25 °C); TOC:H2O2:Fe2+ molar ratio (1:3.7:0.6); Initial COD (0.5 g/L)
TOC: 91%
Gamaralalage et al. (2019)
POME
Fe2+/H2O2
pH (3); Time (30 min); Temperature (25 °C); pressure (101.325 kPa); Fe2+:H2O2 molar ratio (1:2); H2O2 (0.1–0.5 M); Initial COD (1408 mg/L); Initial BOD (1359 mg/L)
COD: 63.6%
BOD: 61.3%Rajesh Nithyanandam et al. (2015)
POME
Fe2+/H2O2
retention time (4 h); H2O2 concentration (0.05 M); FeSO4 concentrations (0.10 M)
COD: 48%
Lim et al. (2017)
POME
Fe2+/H2O2
pH (3.0–5.0); H2O2 concentration (4.57 g/L); Fe2+ concentration (1.88 g/L); reaction time (30 min); agitation rate (120 rpm)
COD: 85.1%
TOC: 92.1%Saeed et al. (2015)
OOMW
Fe2+/H2O21
H2O2 concentration (10 g/L); [Fe2+] (240 mg/L); pH (3.5); Temperature (26 °C); Initial COD (2000 mg O2 /L)
COD: 75%
Colour: 65%Ahmed et al. (2011)
OOMW
Fe3+/H2O2
H2O2 dose (20,000–45,000 mg/L); FeCl3 dose (0–400 mg/L); Temperature (25 °C); pH (3–7); FeCl3/H2O2 (0.058 w/w); resident time (4 h); Initial COD (4017 mg/L)
COD: 66–97%
Hodaifa et al. (2013)
OOMW
Fe2+/H2O2
H2O2 dose (5,000 mg/L); Fe2+ dose (5–10 mM); Temperature (25–65 °C); pH (2.8); irradiation time (40–280 h)
COD: 72–74%
Gernjak et al. (2004)
OOMW
Fe3+/H2O2
FeCl3 dose (2,500 mg/L); H2O2 dose (3,000 mg/L); pH (3); Temperature (20 °C); reaction time (4 h)
COD: 90%
Kiril Mert et al. (2010)
OOMW
Fe2+/H2O2
FeSO4 dose (3000 mg/L); H2O2 dose (3,500 mg/L); pH (3); Temperature (20 °C); reaction time (4 h)
COD: 93%
Kiril Mert et al. (2010)
OOMW
Fe2+/H2O2
H2O2 concentration (10 g /L); [Fe2+] (240 mg/L); pH (3.5); Temperature (26 °C); Initial COD (2000 mg O2 /L)
COD: 75%
Colour: 65%Ahmed et al. (2011)
Winery wastewater
H2O2/Fe2+
Initial COD (1700–2280 mg/L); Initial BOD5 (597–1092 mg/L); pH (3); stirring speed (200 rpm); reaction time (1 h); Fe2+ dose (0.03 mol/L); H2O2 dose (0.206 mol/L)
COD: 52%
Ferreira et al. (2018)
Winery wastewater
H2O2/Fe2+
Initial COD (4,500–6,000 mg/L); Initial BOD5 (1,800–2,600 mg/L); initial colour (300 C.U.); FeSO4 dose (50–600 mg/L); H2O2 dose (150–1000 mg/L); reaction time (30 min); pH (3.5)
COD: 94.4–95.8% BOD: 96.7–97.7%; Colour: 91.7%
Yang et al. (2008)
Winery wastewater
Yeast isolates + Fe2+/H2O2
H2O2/Fe2+ (15:1); H2O2 dose (0.98–39.2 mM); initial COD (3820 mg/L); initial TOC (1628 mg/L); pH (3.5); Temperature (20 °C); reaction time (0–24 h)
COD: 97.5%
TOC: 81%Santos et al. (2014)
POME
Chitosan/FeSO4/H2O2
Chitosan: FeSO4: H2O2 dosage (12,500:500 mg/L); mixing time (15–60 min); sedimentation time (1–4 h); pH (2–9); (500–7500 mg/L)
COD: 73.08%
TSS: 100%Parthasarathy et al. (2016)
Although Zazo et al. (2011) demonstrated that increasing the temperature of up to 120 °C improved the rates of both degradation and mineralization of the pollutants, there was also evidence that shows decreased inefficiency with the increase in temperature due to the increase of the decomposition of the oxidant into water and oxygen (Munoz et al., 2015).
The advantages of the Fenton oxidation technique over other AOPs lies in its use of simple equipment, mild operating conditions and relatively benign Fenton reagents (Munoz et al., 2015). Both the reagents ie. the iron (II) salt and hydrogen peroxide can be handled safely and are relatively friendly to the environment. While the iron (II) salt is relatively cheap, hydrogen peroxide easily decomposes into the water to liberate gaseous oxygen (Munoz et al., 2015). However, the major setback of homogeneous Fenton technique is the generation of a large amount of unwanted sludge of iron (III) complexes coupled with its associated costs of treatment and disposal which have been estimated to be at 10–50% of the total operational cost (Munoz et al., 2015).
Several approaches for either the reduction or regeneration of the volume of the sludge produced during homogeneous Fenton treatments have been proposed. Yoo et al. (2001) demonstrated that recycling the sludge as a coagulant in a coagulation pre-treatment step before Fenton treatment could significantly reduce the sludge volume for disposal by up to 50% and improved the COD removal rate by 9%. Rossi et al. (2013) employed iron-containing sludge calcined at various temperatures (300–1000 °C) as a catalyst for Fenton treatment of pollutants in a simulated olive oil mill effluent and reported significant removals of total phenols (̴ 100%), COD (>50%) and TOC (̴ 45%) but with adversely very high iron leaching (172 mg/L). Iron from the sludge could be recycled using an electrochemical setup leading to 100% iron recovery but with an additional cost for chemical reagents and electricity (Kishimoto et al., 2013).
To ensure a substantial reduction in the amount of sludge deposited and avoid persistent depletion of the catalyst during the homogeneous Fenton treatment, it is pertinent to improve the process by incorporating catalyst regeneration technique or by utilizing iron-bearing condensed phase heterogeneous catalysts (Munoz et al., 2015).
Fenton and modified Fenton processes generally have complex mechanisms involving several consecutive and simultaneous reactions steps and reaction pathways which are influenced by several factors (Rahim Pouran et al., 2015). Some modifications that have been successfully introduced to improve the efficiency of the Fenton treatment include the addition of energy sources such as UV radiation (Ahmed et al., 2011; Kongnoo et al., 2012; Leong and Bashah, 2012), solar radiation (Aris et al., 2008; Kanakaraju et al., 2017), electrical energy (Babu et al., 2010; Chairunnisak et al., 2018; Lim et al., 2017) and sound energy (Taha et al., 2014).
1.7 Homogeneous photo-Fenton process
In photo-Fenton configuration, decreased generation of the unwanted sludge during the treatment process to decontaminate recalcitrant organic pollutants from the agro-industrial wastewater is substantially achieved. Integrating the ambient Fenton process with light radiations by focussing an incident beam of UV light, simulated visible light or solar radiation onto the reactor ensures the enhancement of the catalytic activity and efficiency in the degradation and mineralization of the organic pollutant. The light radiations serve as an energy source to activate the reductive conversion of Fe (III) ions to Fe (II) ions acting as a source of sludge deposits in the ambient homogeneous Fenton process (Babuponnusami and Muthukumar, 2014; Kalal et al., 2014; Lee et al., 2014). Fe (III) ions which exist predominantly as the [Fe(OH)]2+ complex within the pH range of 2.8–3.5 exhibit charge transfer excitation to produce the more active species of Fe (II) ions when irradiated with photons of light with the required threshold energy (Eqs. (6)-(7)) (Ahmed et al., 2011; Simunovic et al., 2011; Sirés and Brillas, 2012). Besides, direct photolysis of H2O2 also produces •OH which can be used for the degradation of organic pollutants (Li et al., 2013; Wang et al., 2019). The resulting regenerated species of Fe (II) ions subsequently act as the catalyst to decompose H2O2 to furnish more •OH towards an efficient degradation and mineralization of the persistent organic pollutant molecules (Eq. (8)) (Avetta et al., 2015). Consequently, the efficiency of photo-Fenton oxidation of the pollutants by the catalysts is enhanced by the production of more •OH due to the synergy between Fe (III) ions and light radiations. The following equations (Eqs. (6)–(8)) enumerate the reaction mechanism of the photo-Fenton oxidation:
The mechanism of photo-Fenton degradation of the pollutants is best expressed by a chemical reaction in Eq. (9):
UV-integrated photo-Fenton treatment has been carried out by Leong and Bashah (2012) for degradation of POME. COD removal efficiencies within the range of 50–80% were attained, indicating substantial degradation of the pollutants in the effluent. Kongnoo et al. (2012) adopted similar process under the influence of the optimized reaction time (30–60 min), H2O2 concentration (50 mM), Fe2+ concentration (1.0 mM) and pH (0.2–3.0) and reported COD and colour removal efficiencies of 82% and > 90%, respectively.
Ahmed et al. (2011) have compared the COD removal efficiencies of ambient Fenton, H2O2 UV-assisted photolysis and UV-assisted photo-Fenton treatment for degradation of pollutants in OOMW under the reaction conditions of temperature (26 °C), reaction time (3 h) and initial COD (2000 mg/L). The COD removals were found to increase in the order: ambient Fenton (75%) > H2O2 UV-assisted photolysis (83%) > UV-assisted photo-Fenton (95%). The dependence of the three processes on the concentrations of H2O2 and Fe2+ increased in the order: UV-assisted H2O2 photolysis (0 mg/L) < UV-assisted photo-Fenton (30 mg/L) < ambient Fenton (240 mg/L) and UV-assisted H2O2 photolysis (0 mg/L) < UV-assisted photo-Fenton (30 mg/L) < ambient Fenton (240 mg/L), respectively. The synergetic effect of integrating ambient Fenton with ultraviolet radiation enhanced the COD removal efficiency of the UV-assisted photo-Fenton treatment compared to the two other treatment methods. Moreover, the COD removal efficiency of UV-assisted H2O2 photolysis (pH: 5.8) was achieved at elevated pH value compared to UV-assisted photo-Fenton (pH: 3.0) and ambient Fenton (pH: 3.5) treatments.
Previously, Kanakaraju et al. (2017) carried out the degradation of POME pollutants with COD removal efficiency of 89% using solar radiation-assisted photo-Fenton treatment under the conditions of Fe2+: H2O2 molar ratio (1:30); pH (∼2.8); exposure time (3 h). Similar work was carried out by Aris et al. (2008) under the optimum conditions of Fe2+ dosage (200 mg/L), H2O2 dosage (1150 mg/L), reaction time (10–60 min) with better Fe2+: H2O2 molar ratio (1:5.75) using 173 times less amount of H2O2. While the TOC removal efficiency achieved was within the range of 87.6–95.6%, the COD removal efficiency attained was 82.4%. Table 7 reports examples from the literature on homogeneous photo-Fenton treatment of POME and other agro-industrial wastewaters.
Wastewater type
Fenton process
Operational parameters
Removal efficiency
Reference
POME
Fe2+/H2O2/solar radiation
Fe2+: H2O2 (1:30); pH (∼2.8); solar exposure time (3 h)
COD: 89%
Kanakaraju et al. (2017)
POME
Fe2+/H2O2/electrical energy
Voltage (15.78 V); electrolyte concentration (0.06 M); H2O2 volume (14.79 mL); time (35.92 min)
COD: 99.56%
Chairunnisak et al. (2018)
POME
Fe2+/H2O2/electrical energy
H2O2 dosage (800–1500 mg/L); Fe2+ dosage (50–400 mg/L)
COD: 75.2%
TOC: 87.6–95.6%
Colour: 92.4%Aris et al. (2008a)
POME
Fe2+/H2O2/solar radiation
Fe2+ dosage and of 200 mg/L and H2O2 dosage (1150 mg/L); reaction time (10–60 min)
COD: 82.4%
TOC: 87.6–95.6%
Colour: 95.1%Aris et al. (2008a)
POME
Fe2+/H2O2/electrolysis
Retention time (4 h); H2O2 concentration (0.05 M); FeSO4 concentrations (0.10 M); power input (1.5 V); electrolyte (0.25 M Na2SO4); anode (stainless-steel rod); cathode (graphite rod)
COD: 94%
Lim et al. (2017)
POME
Fe2+/H2O2/UV irradiation
Reaction temperature (24–40 °C); time (5–30 min); H2O2 dosage (2.125 g); H2O2:FeSO4⋅7H2O (15:1); incubator agitation speed (250 rpm)
COD: 50–80%
Leong & Bashah (2012)
POME
Fe2+/H2O2/electrolysis
Retention time (2 h); H2O2 concentration (0.05 M); FeSO4 concentration (3 mM); current density (0.05 A/cm2); anode (Ti/RuO2); cathode (stainless-steel rod)
COD: 48.4%
Babu et al. (2010)
POME
Fe2+/H2O2/UV irradiation
Optimized reaction time (½ − 1 h); H2O2 concentration (50 mM); [Fe2+] (1.0 mM); pH (3.0–0.2)
COD: 82%
Colour: >90%Kongnoo et al. (2012)
OOMW
H2O2/UV irradiation1
H2O2 concentration (6.0 g/L); [Fe2+] (0 mg/L); pH (5.8); Temperature (26 °C); UV irradiation time (3 h); Initial COD (2000 mg O2 /L)
COD: 83%
Colour: 71%Ahmed et al. (2011)
OOMW
Fe2+/H2O2/UV irradiation2
H2O2 concentration (3.0 g/L); [Fe2+] (30 mg/L); pH (3.0); Temperature (26 °C); UV irradiation time (3 h); Initial COD (2000 mg O2 /L)
COD: 95%
Colour: 82%Ahmed et al. (2011)
Winery Wastewater
Fe2+/H2O2/solar irradiation
Initial COD (10290 mg/L); Fe2+ (20 mg/L); H2O2 dose (1000 mg/L); lamp power (1000 W); pH (2.9); Temperature (25 °C)
COD: 75%
Ioannou et al. (2013)
OOMW
Fe2+/H2O2/solar irradiation
H2O2 dose (5,000–20,000 mg/L); Fe2+ dose (1–5 mM); Temperature (25–65 °C); pH (2.8); irradiation time (3.7–42 h)
COD: 41–89%
Gernjak et al. (2004)
OOMW
H2O2/UV irradiation
H2O2 concentration (6.0 g/L); [Fe2+] (0 mg/L); pH (5.8); Temperature (26 °C); UV irradiation time (3 h); Initial COD (2000 mg O2 per L)
COD: 83%
Colour: 71%Ahmed et al. (2011)
OOMW
Fe2+/H2O2/UV irradiation
H2O2 dose (3 g/L); [Fe2+] (30 mg/L); pH (3); Temperature (26 °C); irradiation time (3 h); Initial COD (2000 mg O2 /L)
COD: 95%
TOC: >80%
Colour: 86%Ahmed et al. (2011)
During homogeneous photo-Fenton treatment, light radiation energy sources (e.g. solar, ultraviolet and visible light sources) furnish the light beam with the threshold energy required by the reacting system to speed up the conversion of the sludge bound Fe (III) species into the Fenton-active Fe (II). Thus, the amount of unwanted sludge generated is drastically mitigated by the recovery of Fe (II) species; the oxidation ability of the photo-Fenton catalysis system is enhanced, and the efficiencies for the degradation as well as the mineralization of organic compounds in POME improved. On the contrary, the demerits against the large-scale implementation of homogeneous photo-Fenton treatment technique include high costs of photoreactor design or operation and poor utilization of photons energy (Pliego et al., 2015; Wang et al., 2016; Zhang et al., 2019).
1.8 Homogeneous electro-Fenton process
To improve on the inherent limitations of the classical Fenton treatment processes, electro-Fenton treatment technique came into existence through the integration of conventional Fenton process with electrochemical phenomena. It has been applauded for having higher efficacy for energy, being more amenable to automation, versatile and friendlier to the environment (He and Zhou, 2017). The two important features of electro-Fenton oxidation are the onsite generation of hydrogen peroxide via cathodic reduction of oxygen which eliminates costs due to transporting, handling and storing H2O2 (Gao et al., 2015; Jiang et al., 2018; Zhang et al., 2019); and the recovery of Fe (II) species from the spent Fe (III) species via cathodic reduction resulting in the decrease of the amount of sludge generated during the process (Trellu et al., 2018; Zhang et al., 2020a, 2020b).
Eqs. (10)–(12), describe the important steps in the reaction mechanisms of electro-Fenton processes (Annabi et al., 2016; He et al., 2014):
The mechanism of electro-Fenton degradation of the pollutants can be best depicted by a chemical reaction in Eq. (13) (Guivarch et al., 2003):
Studies on homogeneous electro-Fenton treatment of POME and other wastewaters are scanty in the literature. Babu et al. (2010) studied the influence of combining biological oxidation using Aspergillus niger and Pseudomonas putida under an anaerobic condition with electro-Fenton process for the degradation of recalcitrant substances in POME. While the electro-Fenton process alone achieved COD removal of 48.4%, the subsequent biological oxidation raised the COD and BOD removals to 86.1% and 85.2%, respectively (Babu et al., 2010).
In addition to that, Chairunnisak et al. (2018) have compared the COD removal efficiencies of electrocoagulation and electro-Fenton processes in the treatment of POME. The COD removal efficiency of electro-Fenton treatment of 99.6% was achieved at the optimized operating conditions of voltage (15.78 V), electrolyte concentration (0.06 M), H2O2 volume (14.79 mL) and reaction time (35.92 min) higher than that achieved via electrocoagulation method (94.5%) at optimized operating conditions of reaction time (39.28 min) and voltage (20 V) (Chairunnisak et al., 2018).
Aris et al. (2008) compared the COD and colour removal efficiencies of ambient Fenton and solar Fenton processes for degradation of POME samples. The latter treatment process recorded better COD (82.4%) and colour (95.1%) removal efficiencies than the former with slightly lower COD (75.2%) and colour (92.4%) removal efficiencies. Similarly, Lim et al. (2017) made a comparison between the COD removal efficiencies of electro-Fenton and ambient Fenton processes for the degradation of recalcitrant chemical pollutants in POME. The former treatment achieved better COD removal efficiency (94%) than the latter (48%), and thus indicating the electro-Fenton is a more effective method for degradation of recalcitrant pollutants in POME. Table 7 summarizes some recent literature on the application of homogeneous electro-Fenton on POME and other agro-industrial wastewaters.
Mohajeri et al. (2010) have demonstrated the effectiveness of the electro-Fenton process in the treatment of recalcitrant organic pollutants from landfill leachate using response surface methodology (RSM) under optimized operational parameters (pH 3, reaction time of 43 min, H2O2/Fe2+ molar ratio of 1:1, and current density of 49 mA/cm2). The authors reported substantial COD (94%) and colour removal (96%). However, the major disadvantages of homogeneous electro-Fenton treatment include inadequate yield of H2O2, impeded current density, high resistivity, low efficiency per unit electrochemical cell (Martínez-Huitle et al., 2009; Sirés et al., 2014; Yuan et al., 2011). To improve the performance of homogeneous electro-Fenton treatment of POME and other agro-industrial wastewaters, the foregoing inherent drawbacks should be addressed.
2 Heterogeneous Fenton catalytic configurations
Heterogeneous Fenton processes ensure the replacement of the iron (II) ion species in the homogeneous Fenton system with a solid catalyst containing the active catalytic components. The used of solid catalysts in heterogeneous Fenton reactions have been reported to prevent or minimize the leaching of iron (II) ions into the effluent, extend the operational pH range and minimize the iron sludge deposits generation (Domingues et al., 2018; Nidheesh, 2015).
The leaching of iron (II) ions during the heterogeneous Fenton treatments was shown to be extremely low with concentrations far lower than the legally allowed limit of 2 mg/L imposed by the EU directives (Yao et al., 2017). The protective effects of the support such as zeolite, clay, graphene and activated carbon prevent the iron species from leaching into the effluent thereby reducing the catalyst losses and additional costs (Matavos-aramyan et al., 2017; Zheng et al., 2015).
The development of heterogeneous Fenton catalysts with long-term stability, high catalytic activity, and efficiency, usable at a wider pH range and easily separable from the reaction medium without additional energy/resource input is the key to the application of heterogeneous Fenton processes for effluents treatment. Their surface composition and facilitate the efficient inter-conversion between Fe (III) and Fe (II) through faster electron transfer (Javaid and Qazi, 2019; Nidheesh, 2015).
A sono-Fenton treatment of POME pollutants utilizing nano zero-valent iron (nZVI) as heterogeneous catalyst (Fe0/H2O2/ultrasound radiation) under the conditions of sonication time (2 h), initial COD (1160 mg/L), pH (2–4) and sonication intensity (20–40%), H2O2 dosage (0.4 mL of 30% solution) and nZVI dosage (0.6 g) was reported by Taha and Ibrahim (2014a). The process has been able to achieve a COD removal of 80%. Taha et al. (2014) also reported similar studies under the conditions of nZVI dosage (3.91 g/L), H2O2 dosage (1.84 g/L), aeration flow rate (23.84 L/h), reaction time (240 min) and pH. The COD removal efficiency of 75.5% under the heterogeneous catalyst dispersion effect of aeration was attained. Vilardi et al. (2018a, 2018b) have developed olive-stones/nZVI/MNPs composite for the removal of organic matter and total phenols from wastewater, optimized in batch reactors and scaled-up in serial fixed-bed columns. Significant removal efficiencies for COD (58.4%) and total phenols (59.2%) at H2O2/COD (w/w) ratio of 0.875 that showed no decrease in the efficiency of the composite within 5 re-use cycles were achieved. Similarly, Vilardi et al. (2018a, 2018b) have reported a comparative study on pollutant removal efficiencies from tannery wastewater between conventional Fenton oxidation under optimal operational parameters (catalyst/oxidant (w/w) ratio 0.2; pH 2.5) and heterogeneous Fenton oxidation using fabricated nZVI solid catalyst at optimal conditions (catalyst/oxidant (w/w) ratio 0.5; pH 2.5) both in batch mode (for 2 h) followed by continuous mode (for 8 h). The latter has been demonstrated to have better performance with higher removal efficiencies for COD (76.6%) and total polyphenols (85.5%) compared to the former. It also generated a lower amount of iron sludge (18%) compared to the larger amounts generated by the earlier process (22%). A simplified kinetic model based on a non-linear regression method proposed to evaluate the kinetics of the heterogeneous treatment procedure fitted the data very well (R2: 99.1–99.3%) and the kinetic rate constants were estimated to range from 1.93 × 102 to 5.74 × 106 per M s.
Qin et al. (2015) have achieved for the first time, an in situ organic-acid-directed (citric and tartaric acids) growth of iron-carbon oxide nanoparticles (NPs) precursor assembly into the orderly cavities of coordinatively unsaturated chromium sites on MIL-101(Cr) metal–organic frameworks (MOFs) without any agglomeration on external surfaces of the MOFs. The morphological structure, crystallinity, and porosity of the supported MIL-101(Cr) remained unaltered. Experimental results showed that Fe-C oxides immobilized onto MIL-101(Cr) in the presence of citric acid (or tartaric acid) had a remarkable catalytic activity for visible-light assisted Fenton reaction at neutral pH and possessed excellent tolerance to poisoning by recalcitrant organic pollutant. The catalyst was both stable and durable having achieved significant removals for colour (>94%) and TOC (>60%) in 120 min for 5 runs without any significant reduction in the efficiency with marginally low leaching of iron (0.08 mg/L) and chromium (0.01 mg/L) contents of the catalyst during treatment.
One-pot synthesis of a magnetic core–shell Fe3O4/CuO/carbon hollow sphere was achieved by Qin et al. (2020) via subjecting MOFs/sodium alginate hydrogel to freeze-drying in n-hexane, ferrocene CVD and pyrolysis. The photocatalytic activity of the prepared core–shell hollow sphere was investigated by photo-Fenton oxidation of toxic and recalcitrant contaminants under visible-light irradiation. The degradation rate of organic pollutants by the catalyst under visible light irradiation remained very high (>90%) within three repeated reuse runs and very low Fe (III) ions leaching (less than0.25 mg/L) was observed.
Li et al. (2019a, 2019b) have demonstrated that a heterogeneous Fenton oxidation pre-treatment can efficiently decompose most non-biodegradable organic pollutants, such as grease and aromatic hydrocarbons, with a substantial colour reduction in seafood processing wastewater. It was able to reduce the average COD of the wastewater (3408 mg/L) at the pre-treatment stage by 51% which on further treatment with polyvinylidene fluoride (PVDF) and graphene oxide-polyvinylidene fluoride (GO-PVDF) membranes led to overall COD removals of 96.8% and 97.5%, respectively. The data from organic matter degradation during this process fitted the pseudo-first-order model with high regression coefficient (R2 = 0.9575) and the kinetic rate constant was estimated at 0.0086 per min. Similarly, Li et al. (2019a, 2019b) reported a facile one-step phase inversion method on the preparation of transition-metal ions-(Mn, Fe, and Co)-amidoximated polyacrylonitrile (AOPAN) polymeric beads complex as low-cost reusable catalysts for efficient and stable heterogeneous electro-Fenton oxidation of pollutants in wastewater. During the electro-Fenton process, Mn − AOPAN was found to exhibit a higher removal efficiency for acid red 73 (AR-73) in terms of colour (96%) and TOC (71%) and turnover frequency (TOF) (1.49 per h) than Fe − AOPAN and Co − AOPAN. The higher removal observed for Mn − AOPAN catalyst could be due to the electron availability provided by the higher number of valency states in manganese as well as the largest standard reduction potential of Mn (III) ion (1.50 V NHE) that made it readily accept electron compared to Fe (III) (0.771 V NHE) and Co (III) (0.17 V NHE) (Li et al., 2019a, 2019b). The composite catalysts exhibited remarkably high activity and stability in electro-Fenton oxidation over a wide pH range (3–10) without any addition of H2O2. The grafted amidoxime group was believed to have extremely improved metal loading, which in turn accelerated electron transfer excitation from the active sites on the composite and enhanced the autocatalytic generation of hydroxyl radicals from H2O2 decomposition.
Recently, Xu et al. (2019) developed a strategy based on chelating and ion exchange capacity of resins aided by some functional groups (–NH2, –COOH and -SO3H) for application in the fabrication of a large scale in-situ assembly of manganese metal ion-organic composites. The composites exhibited high activity during electro-Fenton treatment of methylene blue (MB) for decolouration (98%) and TOC removal (55%) under a wide pH range (2–9), low current density (7.53 mA/cm) and dosage (3 g/L) of the composite catalyst in 2.5 h. The synergetic effect between Mn (II) ion and organic supports in electrochemical oxidation was attributed to intramolecular electron transfer that greatly accelerated Mn (II)/Mn (III) autocatalysis, leading to a better kinetic rate constant (0.037 per min) and TOF (0.23 per hour) than some supported catalysts.
Table 8 summarizes some literature on heterogeneous and modified heterogeneous Fenton processes for POME and other agro-industrial wastewater treatment.
Wastewater type
Fenton process
Operational parameters
Removal efficiency
Reference
POME
nZVI(Fe0)/H2O2/ultrasound radiation
Sonication time (2 hr); Initial COD (1160 mg/L); pH (2–4); Sonication intensity (20–40%)
COD: 80%
Taha et al. (2014)
POME
nZVI(Fe0)/H2O2/aeration/ultrasound radiation
Power intensity (149.98 W); Ultrasound working cycle (70%); Reaction time (155.83 min); Flow rate (10 mL/sec); H2O2/COD (1.721: 0.81 w/w); nZVI/H2O2 (2.125:1 w/w)
COD: 93.7%
Ibrahim et al. (2015)
POME
nZVI(Fe0)/H2O2/aerated
Dosage of nZVI (3.91 g/L); H2O2 dosage (1.84 g/L); aeration (23.84 L/h); reaction time (240 min)
COD: 75.5%
Taha and Ibrahim (2014b)
Winery wastewater
Raw natural clay/H2O2 /UV irradiation
Initial COD (5000–10000 mg/L; Initial TOC (1500–3000 mg/L); pH (3–5); H2O2 dose (3–300 mM); clay dose (100–2000 mg/L); particle size (80–500 μm); light intensity (500 W/m2); temperature (35 °C); reaction time (15–60 min);
TOC: 53.2% (WW)
TOC: 55.1% (WG)Mosteo et al. (2006)
OOMW
O3/BiFeO3/S2O82–/H2O2
pH (4.6–12); H2O2 or S2O82– dose (10 mL); catalyst dose (0.15–1.2 g); temperature (20–60 °C); reaction time (1–40 min)
COD: 98.0% (four cycles, S2O82–) Phenols: 82.9%
Iboukhoulef et al. (2019)
OOMW
Red mud/H2O2
H2O2 dose (100 mg/L); catalyst dose (1 g/L); optimal reaction time (60 min)
Phenols: 100% TOC: 25%
Domingues et al. (2019)
3 Future direction of research
Integrations of the classical Fenton treatments with UV lights, solar radiations, ultrasound and electrochemical processes during the photo-Fenton, sono-Fenton and electro-Fenton treatments of POME and other agro-industrial wastewaters, respectively have shown significant improvements in overcoming the inherent setbacks associated with homogeneous Fenton proceses which are the deposition of unwanted sludge, the need for pH adjustment before or after reaction and the lost of the catalyst. To a certain extend these drawbacks make the treatment processes generally cost-ineffective relative to their heterogeneous counterparts because they hamper catalysts recyclability, limit their extended applications and deter drastic reduction in the cost of catalysts in the treatment of POME and related effluents. The evolution of Fenton oxidation processes over time in other words from the utilization of homogeneous catalysts in ambient mode to the use of solid catalysts either solely by itself or immobilized on appropriate support in the heterogeneous mode show the many efforts made to overcome these problems (Gutierrez-Mata et al., 2017; Matafonova and Batoev, 2018).
Heterogeneous Fenton process employs catalysts either in their pure or supported forms. The supports enhance the dispersion of the active phase metallic species by increasing the surface area, improving the active phase’s resistance to sintering and elevating the stability of the catalysts both chemically and thermally (Ahmed et al., 2011; Gutierrez-Mata et al., 2017; Rojas-Cervantes and Castillejos, 2019).
These attributes have immensely stimulated more interest in the scientific community on the utilization of supported catalysts for Fenton treatment of agro-wastewaters. The common materials used as catalysts supports include pillared clay (Catrinescu et al., 2012; Luo et al., 2009; Pariente et al., 2008; Yang et al., 2017), zeolites (Aleksić et al., 2010; Wang et al., 2010), silica (Martínez et al., 2007; Tušar et al., 2012; Wang et al., 2015a), activated carbons (Lan et al., 2015; Zhao et al., 2012) and alumina (Rodríguez et al., 2010). Many heterogeneous Fenton catalysts have been recently considered ranging from naturally occurring minerals (Garrido-Ramírez et al., 2010), artificially synthesized composites (Martinez et al., 2020), nanomaterials (Zhang et al., 2020), to waste materials from industries (He et al., 2020; Li et al., 2020). However, oxides and minerals of iron have received little attention. Although many metals such as Cu, Mn, Ni, Co, and Au have been considered, iron species are the most frequently used active phase during the heterogeneous Fenton oxidation treatments. Most solid catalysts studied have been characterized by low stability (Wang et al., 2015b) and poor to moderate activity resulting from catalyst poisoning (Han et al., 2011), surface area diminution and leaching of the iron catalysts (Ma et al., 2015). Thus, the fabrication of catalysts with enhanced activity and stability for effective treatment of agro-industrial wastewaters using heterogeneous Fenton process remains a crucial challenge among the researchers. The recent treatment of industrial wastewater and phenolic compounds using alumina supported iron oxides (FexOy/γ-Al2O3) revealed high stability and activity of the catalyst in a 100-hour continuous batch experiment, recording catalyst leaching of less than 3%. A mineral clay supported iron material (Clay/Fe) calcined at 500–700 °C has been demonstrated to exhibit high stability and activity within five consecutive cycles of batch treatment (Annan et al., 2018; Herney-Ramírez and Madeira, 2010; Zhang et al., 2016).
Two other important factors affecting the application of heterogeneous Fenton process for the treatment of agro-industrial wastewaters are catalysts recovery and reusability. During treatment, solid catalysts are employed in dispersed fine powdered form which needs to be separated from the reaction mixture after the process. The available methods of separating such fine particles are often inefficient and time-consuming. Advancement of magnetic catalysts and supports, however, provides easier, faster, and cheaper means to separate the catalyst thus making catalysts recovery and reusability more practical and realistic (Zhang et al., 2019).
The three categories of magnetic materials (Fig. 4) explored as catalysts for wastewater treatments are natural materials, supported magnetite and ferromagnetic nanoparticles (MNPs). Application of magnetite MNPs in the degradation of phenol by Zhang et al. (2019) was the first reported attempt for heterogeneous Fenton treatment with promising higher catalytic activity, stability, recovery, and reusability. Moreover, only nano-zero valent iron (nZVI) and raw clay mineral were so far explored for heterogeneous Fenton treatment of POME and other agro-industrial wastewaters. Although treatments of POME and other agro-industrial wastewaters have not taken full advantages of such processes, the direction of the research seem to meander towards the utilization of magnetic magnetite-based Fenton catalysts for their effective treatment.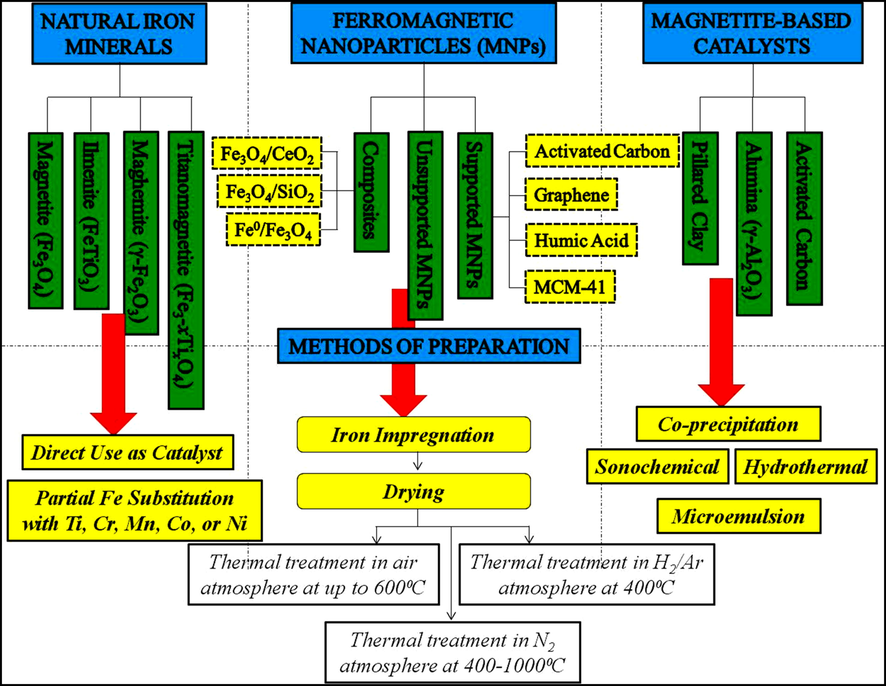
Magnetite-based magnetic materials utilized as heterogeneous Fenton catalysts (Zhang et al., 2019).
The demonstration of the ability of magnetically separable and recyclable composites based on MOFs (Fe3O4/Fe-MOFs) as excellent photo-Fenton catalysts for the degradation of aqueous RhB and MB concentrations is widespread (Jin et al., 2016; Zhang et al., 2015, 2013; Zhao et al., 2015). Solvothermal approach for the synthesis of magnetite nanospheres-decorated MIL-53(Fe) composite (Fe3O4/MIL-53(Fe)) as a porous, crystalline, magnetically separable catalyst has been developed by Zhang et al. (2015). The catalyst has been found to possess high efficiency for visible light-mediated photo-Fenton oxidation with enhanced apparent rate constants for the degradation of Rhodamine B (RhB) and p-nitrophenol (PNP). The composite, Fe3O4/MIL-53(Fe), was able to degrade about 99% of RhB and 60% of PNP from their 10 mg/L initial concentration within 70 min and 150 min of visible-light irradiation, respectively. Lower degradation of the effluents was recorded by Fe2O3 (25%) and Fe3O4 (21%) under the same condition. The apparent reaction rate constants for the photo-Fenton catalytic process of the Fe3O4/MIL-53(Fe) (0.0513 per min) indicated that degradation was about 5–18 times faster than by photocatalysis using the catalyst (0.0102 per min), Fe2O3 (0.0053 per min), Fe3O4 (0.0035 per min) and P25 (0.0028 per min) (Zhang et al., 2015). Similar works were reported on the development of Fe3O4/MIL-88B(Fe) (Jin et al., 2016) and Fe3O4/MIL-100(Fe) (Zhang et al., 2013; Zhao et al., 2015) as recyclable photo-Fenton magnetic catalysts for the treatment of the effluents. The outcomes confirmed the high efficiency and enhanced photo-Fenton catalytic performance of the Fe3O4/MIL-53(Fe) system under visible light irradiation as a magnetically separable photo-Fenton catalyst based on MOFs. Moreover, the synergistic influence of UV/visible light irradiation and the immobilized Fe-O clusters, Fe3O4 nanoparticles has been proven to enhance the generation of large number of hydroxyl radicals from the induced decomposition of H2O2 which immensely boost the efficiency of the heterogeneous photo-Fenton system.
4 Conclusion
Over the years, oil palm agro-industry in Malaysia has witnessed a reasonable expansion in commercial activities as evidenced by the rise of the rate of crude palm oil production and the number of POMs. However, the industry is facing the challenges of shifting technologies for effective management and treatment of the increased waste generation. Navigating away from the existing conventional methods to the more refined advanced oxidation technologies is required. The discussion and evaluation of the performances of the various Fenton advanced oxidation processes on the treatment of related effluents have been carefully considered with the focus on the abatement of pollution due to the discharge of untreated or partially treated POME into the environment. Based on the various information gathered from the literature on the subject of this review, the following conclusions have been drawn:
-
Oil palm agro-industry continues to expand over the years with increased acreage of oil palm cultivated farmlands, improved oil palm yields, increased number of POMs, increased production of palm oil and oil palm products, and improved annual revenues.
-
Crude palm oil extraction processes in POMs continue to record increased consumption of water and larger volumes of highly polluting POME being released into the environment.
-
Conventional technologies (e.g. ponding, biological, membrane, evaporation, etc.) utilized in the treatment of POME and related effluents are inadequate for decontamination of its recalcitrant pollutants.
-
Application of both ambient and integrated homogeneous Fenton oxidation processes in the treatment of POME and related effluents has received considerable attention with literature surveyed on POME treatment evenly distributed between ambient Fenton (33.3%) and electro-Fenton (33.3%) approaches as well as between UV-Fenton (16.7%) and solar-Fenton (16.7%) configurations.
-
Homogeneous Fenton advanced oxidation, both in ambient and integrated forms, exhibited good performances in the degradation of recalcitrant organic pollutants from POME and related effluents with high efficiencies in terms of degradation (48–100%), mineralization (88–96%) and colour removal (90–95%).
-
Electro-Fenton process recorded the marginally highest performance, followed by solar photo-Fenton, ambient-Fenton, and UV photo-Fenton. The most important advantages of POME treatment using electro-Fenton processes are in situ generation of H2O2 and the regeneration of the metal catalyst via cathodic reduction.
-
Integration of simulated UV and solar energy sources ensures some significant improvement in the performances of the treatment procedures in terms of reduced operational costs and cost-effectiveness. In photo-Fenton processes, the photochemical process constituted the main mechanism for reduction of the metal catalysts.
-
Deposition of unwanted ferric sludge as a secondary pollutant, electrode inactivation via continued layered deposition of pollutants and intermediates, the needs for pH adjustment before and after treatment, resource wastage, catalyst leaching, difficulties in separation, high cost for regeneration and recovery etc. are some of the inherent disadvantages that often hamper the utilization of homogeneous Fenton oxidation configurations for the treatment of POME and related effluents.
-
Currently, the utilization of heterogeneous Fenton advanced oxidation configurations in the treatment of POME and related effluent is very low and scarce. Nano zero valent iron (nZVI) is the only heterogeneous catalyst whose application for the treatment of pollutants in POME has been reported so far with COD removal efficiencies ranging from 76 to 93%.
-
The future direction of research on the treatment of POME and related effluents points towards the utilization of ternary nanocomposites based on magnetite and MOFs (Fe3O4/Fe-MOFs) or based on metal oxide semiconductors (MxOy) composites with mesoporous activated carbon and magnetite (Fe3O4/C/MxOy). Such composites are highly stable, magnetically recoverable, recyclable, and possess high catalytic activities for POME and related effluent.
-
The operating costs of Fenton treatment processes depend on the cost of the starting materials used to prepare the catalyst, the utility cost (such as electricity) and the costs of other chemical reagents. The costs for POME treatment may vary depending on the prevailing inflation rate, labour, maintenance and other fixed costs, the nature of the Fenton process as well as the nature of effluent under treatment.
Acknowledgments
The authors gratefully acknowledge the financial support provided by Universiti Sains Malaysia under the RUI Grant No. 1001/PKIMIA/8011117.
Declaration of Competing Interest
The authors declare no competing of interest.
References
- Abdulrahman, A., Latiff, A.A.A.A., Daud, Z., Ridzuan, M.B., Jagaba, A.. H., D, N.F.M., Jagaba, A.. H., 2016. Preparation and characterization of activated cow bone powder for the adsorption of cadmium from palm oil mill effluent. IOP Conf. Ser.: Mater. Sci. Eng., 136, 012045. Doi: 10.1088/1757-899X/136/1/012045.
- A review of moving-bed biofilm reactor technology for palm oil mill effluent treatment. J. Cleaner Prod.. 2018;171:1532-1545.
- [CrossRef] [Google Scholar]
- Drinking water reclamation from palm oil mill effluent (POME) using membrane technology. Desalination. 2006;191:35-44.
- [CrossRef] [Google Scholar]
- Ahmad Shahrifun, N.S., Ab’lah, N.N., Hussain, H., Aris, A., Omar, Q., Ahmad, N., 2015. Reusability of Fenton sludge to reduce COD and color on palm oil mill secondary effluent (POMSE). Adv. Mater. Res., vol. 1113, 486–491. Doi: 10.4028/www.scientific.net/AMR.1113.486.
- Photo-Fenton treatment of actual agro-industrial wastewaters. Ind. Eng. Chem. Res.. 2011;50:6673-6680.
- [CrossRef] [Google Scholar]
- Heterogeneous Fenton type processes for the degradation of organic dye pollutant in water - The application of zeolite assisted AOPs. Desalination. 2010;257:22-29.
- [CrossRef] [Google Scholar]
- Photocatalytic treatment technology for palm oil mill effluent (POME) - A review. Process Saf. Environ. Prot.. 2016;102:673-686.
- [CrossRef] [Google Scholar]
- Alliance, E.P.O., 2016. The palm oil story: Fact and figures [WWW Document].
- Use of advance oxidation process to improve the biodegradability of olive oil mill effluents. Process Saf. Environ. Prot.. 2015;98:319-324.
- [CrossRef] [Google Scholar]
- Application of advanced oxidation processes for the treatment of recalcitrant agro-industrial wastewater: A review. Water. 2019;11:205.
- [CrossRef] [Google Scholar]
- Winery wastewater treatment by sulphate radical based-advanced oxidation processes (SR-AOP): Thermally vs UV-assisted persulphate activation. Process Saf. Environ. Prot.. 2019;122:94-101.
- [CrossRef] [Google Scholar]
- Degradation of enoxacin antibiotic by the electro-Fenton process: Optimization, biodegradability improvement and degradation mechanism. J. Environ. Manage.. 2016;165:96-105.
- [CrossRef] [Google Scholar]
- Application of clay ceramics and nanotechnology in water treatment: A review. Cogent Eng.. 2018;5:1-35.
- [CrossRef] [Google Scholar]
- Tertiary treatment of palm oil mill effluent using Fenton oxidation. Malays. J. Civil Eng.. 2008;20:12-25.
- [Google Scholar]
- Activation of persulfate by irradiated magnetite: Implications for the degradation of phenol under heterogeneous photo-Fenton-like conditions. Environ. Sci. Technol.. 2015;49:1043-1050.
- [CrossRef] [Google Scholar]
- Application of sandwich membrane for the treatment of palm oil mill effluent (POME) for water reuse. Proc. Eng.. 2012;44:1980-1981.
- [CrossRef] [Google Scholar]
- Removal of fatty acids from palm oil effluent by combined electro-Fenton and biological oxidation process. Water Air Soil Pollut.. 2010;211:203-210.
- [CrossRef] [Google Scholar]
- A review on Fenton and improvements to the Fenton process for wastewater treatment. J. Environ. Chem. Eng.. 2014;2:557-572.
- [CrossRef] [Google Scholar]
- Effects of palm oil mill effluent (POME) anaerobic sludge from 500 m3 of closed anaerobic methane digested tank on pressed-shredded empty fruit bunch (EFB) composting process. Afr. J. Biotechnol.. 2010;9:2427-2436.
- [CrossRef] [Google Scholar]
- Studies on the reduction of organic load from palm oil mill effluent (POME) by bacterial strains. Int. J. Recycl. Organ. Waste in Agric.. 2015;4:1-10.
- [CrossRef] [Google Scholar]
- Palm oil mill effluent (POME) Treatment “Microbial communities in an anaerobic digester”: A review. Int. J. Scient. Res. Publ.. 2014;4:1-24.
- [Google Scholar]
- Biodegradation of palm oil mill effluent (POME) by bacterial. Int. J. Scient. Res. Publ.. 2014;4:2250-3153.
- [Google Scholar]
- Electro persulphate oxidation for polishing of biologically treated palm oil mill effluent (POME) J. Environ. Manage.. 2017;193:458-469.
- [CrossRef] [Google Scholar]
- Efficiency of ozonation and AOP for methyl-tert-butylether (MTBE) removal in waterworks. Ozone Sci. Eng.. 2005;27:27-35.
- [CrossRef] [Google Scholar]
- Trend and current practices of palm oil mill effluent polishing: Application of advanced oxidation processes and their future perspectives. J. Environ. Manage.. 2017;198:170-182.
- [CrossRef] [Google Scholar]
- POME is treated for removal of color from biologically treated POME in fixed bed column: Applying wavelet neural network (WNN) J. Hazard. Mater.. 2013;262:106-113.
- [CrossRef] [Google Scholar]
- Response of an anaerobic fluidized bed reactor treating ice-cream wastewater to organic, hydraulic, temperature and pH shocks. J. Biotechnol.. 1995;39:251-259.
- [CrossRef] [Google Scholar]
- Treatment of palm oil mill effluent by upflow anaerobic filtration. J. Chem. Technol. Biotechnol.. 1994;61:103-109.
- [CrossRef] [Google Scholar]
- Energy intensified integrated advanced oxidation technology for the treatment of recalcitrant industrial wastewater. J. Cleaner Prod.. 2019;206:1025-1040.
- [CrossRef] [Google Scholar]
- Degradation of 4-chlorophenol from wastewater through heterogeneous Fenton and photo-Fenton process, catalyzed by Al-Fe PILC. Appl. Clay Sci.. 2012;58:96-101.
- [CrossRef] [Google Scholar]
- Chairunnisak, A., Arifin, B., Sofyan, H., Lubis, M.R., Darmadi, 2018. Comparative study on the removal of COD from POME by electrocoagulation and electro-Fenton methods: Process optimization. IOP Conf. Ser.: Mater. Sci. Eng., vol. 334, 12026. Doi: 10.1088/1757-899X/334/1/012026.
- Photocatalytic restoration of liquid effluent from oil palm agroindustry in Malaysia using tungsten oxides catalyst. J. Cleaner Prod.. 2017;162:205-219.
- [CrossRef] [Google Scholar]
- Biogas from palm oil mill effluent (POME): Opportunities and challenges from Malaysia’s perspective. Renew. Sustain. Energy Rev.. 2013;26:717-726.
- [CrossRef] [Google Scholar]
- Effect of temperature on the anaerobic digestion of palm oil mill effluent. Electron. J. Biotechnol.. 2007;10:376-385.
- [CrossRef] [Google Scholar]
- Catalytic ozonation for the degradation of acetylsalicylic acid in aqueous solution by magnetic CeO2 nanometer catalyst particles. Appl. Catal. B. 2014;144:686-693.
- [CrossRef] [Google Scholar]
- Calcined limestone horizontal roughing filter for treatment of palm oil mill effluent polishing pond. Int. J. Environ. Sci. Technol.. 2019;16:6419-6430.
- [CrossRef] [Google Scholar]
- Department of Environment, 2009. Environmental quality (industrial effluent) regulations 2009. Environmental Quality Act 1974.
- Department of Statistics Malaysia, 2016. Press release: Selected agricultural indicators, Malaysia, 2016. Compendium of Environment Statistics 5–9. Doi: 10.1017/CBO9781107415324.004.
- Catalytic efficiency of red mud for the degradation of olive mill wastewater through heterogeneous Fenton’s process. Water. 2019;11:1183.
- [CrossRef] [Google Scholar]
- Detoxification of olive mill wastewaters by Fenton’s process. Catalysts. 2018;8:662.
- [CrossRef] [Google Scholar]
- Comparison of UASB and EGSB reactors performance, for treatment of raw and deoiled palm oil mill effluent (POME) J. Hazard. Mater.. 2011;189:229-234.
- [CrossRef] [Google Scholar]
- Oxidation of tartaric acid in presence of iron. J. chem. Soc. Trans.. 1894;65:899-910.
- [CrossRef] [Google Scholar]
- Winery wastewater treatment by integrating Fenton’s process with biofiltration by Corbicula fluminea. J. Chem. Technol. Biotechnol.. 2018;93:333-339.
- [CrossRef] [Google Scholar]
- Degradation behavior of palm oil mill effluent in Fenton oxidation. J. Hazard. Mater.. 2019;364:791-799.
- [CrossRef] [Google Scholar]
- Carbon nanotube membrane stack for flow-through sequential regenerative electro-Fenton. Environ. Sci. Technol.. 2015;49:2375-2383.
- [CrossRef] [Google Scholar]
- Microwave-assisted catalytic wet peroxide oxidation: Energy optimization. Sep. Purif. Technol.. 2019;215:62-69.
- [CrossRef] [Google Scholar]
- Clays and oxide minerals as catalysts and nanocatalysts in Fenton-like reactions - A review. Appl. Clay Sci.. 2010;47:182-192.
- [CrossRef] [Google Scholar]
- Pilot-plant treatment of olive mill wastewater (OMW) by solar TiO2 photocatalysis and solar photo-Fenton. Sol. Energy. 2004;77:567-572.
- [CrossRef] [Google Scholar]
- Government of Malaysia, M.P.O.B., 2018. Overview of the Malaysian Oil Palm Industry 2017. Malaysian Palm Oil Board 1–6.
- Government of Malaysia, M.P.O.B., 2017. Overview of the Malaysian Oil Palm Industry 2016. Malaysian Palm Oil Board 1–6.
- Government of Malaysia, M.P.O.B., 2016. Overview of the Malaysian Oil Palm Industry 2015. Malaysian Palm Oil Board 1–7.
- Government of Malaysia, M.P.O.B., 2015. Overview of the Malaysian Oil Palm Industry 2014. Malaysian Palm Oil Board 1–6.
- Government of Malaysia, M.P.O.B., 2014. Overview of the Malaysian Oil Palm Industry 2013. Malaysian Palm Oil Board 1–6.
- Government of Malaysia, M.P.O.B., 2013. Overview of the Malaysian Oil Palm Industry 2012. Malaysian Palm Oil Board 1–5.
- Government of Malaysia, M.P.O.B., 2012. Overview of the Malaysian Oil Palm Industry 2011. Malaysian Palm Oil Board 1–4.
- Government of Malaysia, M.P.O.B., 2011. Overview of the Malaysian Oil Palm Industry 2010. Malaysian Palm Oil Board 1–4.
- Government of Malaysia, M.P.O.B., 2010. Overview of the Malaysian Oil Palm Industry 2009. Malaysian Palm Oil Board 1–4.
- Degradation of azo dyes in water by electro-Fenton process. Environ. Chem. Lett.. 2003;1:38-44.
- [CrossRef] [Google Scholar]
- Recent overview of solar photocatalysis and solar photo-Fenton processes for wastewater treatment. Int. J. Photoenergy. 2017;2017:1-27.
- [CrossRef] [Google Scholar]
- Haber, F., Weiss, J., 1934. The catalytic decomposition of hydrogen peroxide by iron salts. Proc. Royal Soc. London. Series A – Math. Phys. Sci., vol. 147, 332–351. Doi: 10.1098/rspa.1934.0221.
- Über die katalyse des hydroperoxydes. Die Naturwissenschaften. 1932;20:948-950.
- [CrossRef] [Google Scholar]
- Copper–iron bimetal modified PAN fiber complexes as novel heterogeneous Fenton catalysts for degradation of organic dye under visible light irradiation. J. Hazard. Mater.. 2011;189:241-248.
- [CrossRef] [Google Scholar]
- Wastewater treatment with heterogeneous Fenton-type catalysts based on porous materials. J. Mater. Chem.. 2010;20:9002-9017.
- [CrossRef] [Google Scholar]
- A proposal for zero emission from palm oil industry incorporating the production of polyhydroxyalkanoates from palm oil mill effluent. J. Chem. Eng. Jpn.. 2002;35:9-14.
- [CrossRef] [Google Scholar]
- Electro-Fenton process for water and wastewater treatment. Crit. Rev. Environ. Sci. Technol.. 2017;47:2100-2131.
- [CrossRef] [Google Scholar]
- Fabrication and environmental assessment of photo-assisted Fenton-like Fe/FBC catalyst utilizing mealworm frass waste. J. Cleaner Prod.. 2020;256:120259
- [CrossRef] [Google Scholar]
- Electro-Fenton process catalyzed by Fe3O4 magnetic nanoparticles for degradation of C.I. reactive blue 19 in aqueous solution: Operating conditions, influence, and mechanism. Ind. Eng. Chem. Res.. 2014;53:3435-3447.
- [CrossRef] [Google Scholar]
- Use of pillared clay-based catalysts for wastewater treatment through Fenton-like processes. In: Pillared clays and related catalysts. New York, New York, NY: Springer; 2010. p. :129-165.
- [CrossRef] [Google Scholar]
- Optimization of continuous reactor at pilot scale for olive-oil mill wastewater treatment by Fenton-like process. Chem. Eng. J.. 2013;220:117-124.
- [CrossRef] [Google Scholar]
- Advanced chemical oxidation: Its present role and potential future in hazardous waste treatment. Waste Manage.. 1993;13:361-377.
- [CrossRef] [Google Scholar]
- Heterogeneous Fenton like degradation of olive mill wastewater using ozone in the presence of BiFeO3 photocatalyst. J. Photochem. Photobiol., A. 2019;383:112012
- [CrossRef] [Google Scholar]
- Removal of COD from palm oil mill effluent (POME) via advanced Fenton process: Optimization study. Adv. Environ. Biol.. 2015;9:1-10.
- [Google Scholar]
- Reduction of residual pollutants from biologically treated palm oil mill effluent final discharge by steam activated bioadsorbent from oil palm biomass. J. Cleaner Prod.. 2017;141:122-127.
- [CrossRef] [Google Scholar]
- A review of palm oil mill effluent (POME) water treatment. Global J. Environ. Res.. 2007;1:54-62.
- [CrossRef] [Google Scholar]
- Winery wastewater purification by reverse osmosis and oxidation of the concentrate by solar photo-Fenton. Sep. Purif. Technol.. 2013;118:659-669.
- [CrossRef] [Google Scholar]
- Catalytic oxidation process for the degradation of synthetic dyes: An overview. Int. J. Environ. Res. Public Health. 2019;16:2066.
- [CrossRef] [Google Scholar]
- Graphene modified electro-fenton catalytic membrane for in situ degradation of antibiotic florfenicol. Environ. Sci. Technol.. 2018;52:9972-9982.
- [CrossRef] [Google Scholar]
- One-pot preparation of hierarchical nanosheet-constructed Fe3O4/MIL-88B(Fe) magnetic microspheres with high efficiency photocatalytic degradation of dye. ChemCatChem. 2016;8:3510-3517.
- [CrossRef] [Google Scholar]
- Efficient treatment of saline recalcitrant petrochemical wastewater using heterogeneous UV-assisted sono-Fenton process. Ultrason. Sonochem.. 2019;56:25-36.
- [CrossRef] [Google Scholar]
- Heterogeneous Fenton-like oxidation of petrochemical wastewater using a magnetically separable catalyst (MNPs@C): Process optimization, reaction kinetics and degradation mechanisms. RSC Adv.. 2016;6:84999-85011.
- [CrossRef] [Google Scholar]
- Heterogeneous catalytic degradation of organic compounds using nanoscale zero-valent iron supported on kaolinite: Mechanism, kinetic and feasibility studies. J. Taiwan Inst. Chem. Eng.. 2019;96:329-340.
- [CrossRef] [Google Scholar]
- Role of copper pyrovanadate as heterogeneous photo-Fenton like catalyst for the degradation of neutral red and azure-B: An eco-friendly approach. Korean J. Chem. Eng.. 2014;31:2183-2191.
- [CrossRef] [Google Scholar]
- Palm oil mill effluent treatment using coconut shell-based activated carbon: Adsorption equilibrium and isotherm. MATEC Web Conf.. 2017;87:03009.
- [CrossRef] [Google Scholar]
- Efficiency of microalgae Chlamydomonas on the removal of pollutants from palm oil mill effluent (POME) Energy Proc.. 2015;75:2400-2408.
- [CrossRef] [Google Scholar]
- Performance of solar photocatalysis and photo-Fenton degradation of palm oil mill effluent. Malays. J. Anal. Sci.. 2017;21:996-1007.
- [CrossRef] [Google Scholar]
- Textile dyeing industry an environmental hazard. Nat. Sci.. 2012;04:22-26.
- [CrossRef] [Google Scholar]
- Preparation, characterization and catalytic potential of Γ-Fe2O3@AC mesoporous heterojunction for activation of peroxymonosulfate into degradation of cyfluthrin insecticide. Microporous Mesoporous Mater.. 2019;284:111-121.
- [CrossRef] [Google Scholar]
- Pre-treatment studies on olive oil mill effluent using physicochemical, Fenton and Fenton-like oxidations processes. J. Hazard. Mater.. 2010;174:122-128.
- [CrossRef] [Google Scholar]
- Reusability of iron sludge as an iron source for the electrochemical Fenton-type process using Fe2+/HOCl system. Water Res.. 2013;47:1919-1927.
- [CrossRef] [Google Scholar]
- Decolorization and organic removal from palm oil mill effluent by Fenton’s process. Environ. Eng. Sci.. 2012;29:855-859.
- [CrossRef] [Google Scholar]
- Heterogeneous photo-Fenton degradation of acid red B over Fe2O3 supported on activated carbon fiber. J. Hazard. Mater.. 2015;285:167-172.
- [CrossRef] [Google Scholar]
- Water recycling from palm oil mill effluent (POME) using membrane technology. Desalination. 2003;157:87-95.
- [CrossRef] [Google Scholar]
- Degradation of diclofenac and carbamazepine by the copper(II)-catalyzed dark and photo-assisted Fenton-like systems. Chem. Eng. J.. 2014;245:258-264.
- [CrossRef] [Google Scholar]
- Kinetic Study on COD removal of palm oil refinery effluent by UV-Fenton. APCBEE Proc.. 2012;3:6-10.
- [CrossRef] [Google Scholar]
- Membrane photo-bioreactor coupled with heterogeneous Fenton fluidized bed for high salinity wastewater treatment: Pollutant removal, photosynthetic bacteria harvest and membrane anti-fouling analysis. Sci. Total Environ.. 2019;696:1-11.
- [CrossRef] [Google Scholar]
- Facile and green synthesis of highly dispersed tar-based heterogeneous Fenton catalytic nanoparticles for the degradation of methylene blue. J. Cleaner Prod.. 2020;246
- [CrossRef] [Google Scholar]
- Degradation of orange II by UV-assisted advanced Fenton process: Response surface approach, degradation pathway, and biodegradability. Ind. Eng. Chem. Res.. 2013;52:15560-15567.
- [CrossRef] [Google Scholar]
- Self-assembly of Mn(II)-amidoximated PAN polymeric beads complex as reusable catalysts for efficient and stable heterogeneous electro-Fenton oxidation. ACS Appl. Mater. Interfaces. 2019;11:3925-3936.
- [CrossRef] [Google Scholar]
- Conventional methods and emerging wastewater polishing technologies for palm oil mill effluent treatment: A review. J. Environ. Manage.. 2015;149:222-235.
- [CrossRef] [Google Scholar]
- Optimization of hydroxyl radical production using electro-Fenton method for chemical oxygen demand reduction in diluted palm oil mill effluent. Water Environ. J.. 2017;31:578-583.
- [CrossRef] [Google Scholar]
- Lorestani, A.A.Z., 2006. Biological treatment of palm oil mill effluent (POME) using an up-flow anaerobic sludge fixed film (UASFF) bioreactor [TD899. P4 L869 2006 frb]. Universiti Sains Malaysia.
- Catalytic property of Fe-Al pillared clay for Fenton oxidation of phenol by H2O2. Appl. Catal. B. 2009;85:201-206.
- [CrossRef] [Google Scholar]
- Novel magnetic porous carbon spheres derived from chelating resin as a heterogeneous Fenton catalyst for the removal of methylene blue from aqueous solution. J. Colloid Interface Sci.. 2015;446:298-306.
- [CrossRef] [Google Scholar]
- Machulek, A., H., F., Gozzi, F., O., V., C., L., F. Moraes, J.E., 2012. Fundamental mechanistic studies of the photo-Fenton reaction for the degradation of organic pollutants. Organic pollutants ten years after the Stockholm Convention - Environmental and Analytical Update. Doi: 10.5772/30995
- Palm oil mill effluent (POME) from Malaysia palm oil mills: Waste or resource. Int. J. Sci., Environ. Technol.. 2013;2:1138-1155.
- [Google Scholar]
- Malaysian Palm Oil Council, M.P.O.C., 2017. Annual Report 2017 1–30.
- Manickam, S., Zainal Abidin, N. binti, Parthasarathy, S., Alzorqi, I., Ng, E.H., Tiong, T.J., Gomes, R.L., Ali, A., 2014. Role of H2O2 in the fluctuating patterns of COD (chemical oxygen demand) during the treatment of palm oil mill effluent (POME) using pilot scale triple frequency ultrasound cavitation reactor. Ultrason. Sonochem., vol. 21, 1519–1526. Doi: 10.1016/J.ULTSONCH.2014.01.002.
- Decontamination of wastewaters containing synthetic organic dyes by electrochemical methods: A general review. Appl. Catal. B. 2009;87:105-145.
- [CrossRef] [Google Scholar]
- Preparation, characterization, and application of nano-FeOx/Al2O3/cordierite monolithic catalysts for heterogeneous dark-Fenton reaction. J. Chem. Technol. Biotechnol. jctb.6446. 2020
- [CrossRef] [Google Scholar]
- Iron species incorporated over different silica supports for the heterogeneous photo-Fenton oxidation of phenol. Appl. Catal. B. 2007;70:452-460.
- [CrossRef] [Google Scholar]
- Recent advances in application of UV light-emitting diodes for degrading organic pollutants in water through advanced oxidation processes: A review. Water Res.. 2018;132:177-189.
- [CrossRef] [Google Scholar]
- Advances in Fenton and Fenton based oxidation processes for industrial effluent contaminants control-A review. Int. J. Environ. Sci. Nat. Resources. 2017;2
- [CrossRef] [Google Scholar]
- Utilizing solar energy for the purification of olive mill wastewater using a pilot-scale photocatalytic reactor after coagulation-flocculation. Water Res.. 2014;60:28-40.
- [CrossRef] [Google Scholar]
- Statistical optimization of process parameters for landfill leachate treatment using electro-Fenton technique. J. Hazard. Mater.. 2010;176:749-758.
- [CrossRef] [Google Scholar]
- Factorial experimental design of winery wastewaters treatment by heterogeneous photo-Fenton process. Water Res.. 2006;40:1561-1568.
- [CrossRef] [Google Scholar]
- Preparation of magnetite-based catalysts and their application in heterogeneous Fenton oxidation – A review. Appl. Catal. B. 2015;176–177:249-265.
- [CrossRef] [Google Scholar]
- Recent developments in homogeneous advanced oxidation processes for water and wastewater treatment. Int. J. Photoenergy. 2014;2014:1-21.
- [CrossRef] [Google Scholar]
- Malaysia: 100 years of resilient palm oil economic performance. J. Oil Palm Res.. 2018;30:13-25.
- [CrossRef] [Google Scholar]
- Treatment of palm oil mill effluent by electrocoagulation with presence of hydrogen peroxide as oxidizing agent and polialuminum chloride as coagulant-aid. Water Resour. Ind.. 2017;17:7-10.
- [CrossRef] [Google Scholar]
- Photocatalytic degradation of palm oil mill effluent over ultraviolet-responsive titania: Successive assessments of significance factors and process optimization. J. Cleaner Prod.. 2017;142:2073-2083.
- [CrossRef] [Google Scholar]
- Optimization of photocatalytic degradation of palm oil mill effluent in UV/ZnO system based on response surface methodology. J. Environ. Manage.. 2016;184:487-493.
- [CrossRef] [Google Scholar]
- Restoration of liquid effluent from oil palm agroindustry in Malaysia using UV/TiO2 and UV/ZnO photocatalytic systems: A comparative study. J. Environ. Manage.. 2017;196:674-680.
- [CrossRef] [Google Scholar]
- Photocatalytic degradation of recalcitrant POME waste by using silver doped titania: Photokinetics and scavenging studies. Chem. Eng. J.. 2016;286:282-290.
- [CrossRef] [Google Scholar]
- Heterogeneous Fenton catalysts for the abatement of organic pollutants from aqueous solution: A review. RSC Adv.. 2015;5:40552-40577.
- [CrossRef] [Google Scholar]
- OECD/FAO, 2018. OECD FAO Agricultural outlook 2018 2027 oilseeds and oilseed products 1–127.
- Treatment of effluents from palm oil mill process to achieve river water quality for reuse as recycled water in a zero emission system. J. Cleaner Prod.. 2014;67:58-61.
- [CrossRef] [Google Scholar]
- Catalytic wet air oxidation of Reactive Black 5 in the presence of LaNiO3 perovskite catalyst as a green process for azo dye removal. Chemosphere. 2018;209:823-830.
- [CrossRef] [Google Scholar]
- Heterogeneous photo-Fenton oxidation of benzoic acid in water: Effect of operating conditions, reaction by-products and coupling with biological treatment. Appl. Catal. B. 2008;85:24-32.
- [CrossRef] [Google Scholar]
- Process intensification of anaerobically digested palm oil mill effluent (AAD-POME) treatment using combined chitosan coagulation, hydrogen peroxide (H2O2) and Fenton’s oxidation. Clean Technol. Environ. Policy. 2016;18:219-230.
- [CrossRef] [Google Scholar]
- Trends in the intensification of the Fenton process for wastewater treatment: An overview. Crit. Rev. Environ. Sci. Technol.. 2015;45:2611-2692.
- [CrossRef] [Google Scholar]
- Development of anaerobic digestion methods for palm oil mill effluent (POME) treatment. Bioresour. Technol.. 2009;100:1-9.
- [CrossRef] [Google Scholar]
- Palm oil mill effluent (POME) characteristic in high crop season and the applicability of high-rate anaerobic bioreactors for the treatment of pome. Ind. Eng. Chem. Res.. 2010;49:11732-11740.
- [CrossRef] [Google Scholar]
- Upflow anaerobic sludge blanket-hollow centered packed bed (UASB-HCPB) reactor for thermophilic palm oil mill effluent (POME) treatment. Biomass Bioenergy. 2014;67:231-242.
- [CrossRef] [Google Scholar]
- Degradation mechanism of alachlor during direct ozonation and O3/H2O2 advanced oxidation process. Chemosphere. 2010;78:517-526.
- [CrossRef] [Google Scholar]
- Organic-acid-directed assembly of iron–carbon oxides nanoparticles on coordinatively unsaturated metal sites of MIL-101 for green photochemical oxidation. Appl. Catal. B. 2015;179:500-508.
- [CrossRef] [Google Scholar]
- Assembly of MOFs/polymer hydrogel derived Fe3O4-CuO@hollow carbon spheres for photochemical oxidation: Freezing replacement for structural adjustment. Appl. Catal. B. 2020;269:118754
- [CrossRef] [Google Scholar]
- Review on the main advances in photo-Fenton oxidation system for recalcitrant wastewaters. J. Ind. Eng. Chem.. 2015;21:53-69.
- [CrossRef] [Google Scholar]
- Treatment of palm oil refinery effluent using advanced oxidation process. J. Eng. Sci. Technol.. 2015;10:26-34.
- [Google Scholar]
- Removal of hazardous pollutants from wastewaters: Applications of TiO2-SiO2 mixed oxide materials. J. Nanomater.. 2014;2014:1-42.
- [CrossRef] [Google Scholar]
- Fenton degradation of lignin wastewater in a batch process for pulp and paper industry : Kinetic and economic aspect. Int. J. Eng. Technol.. 2019;11:1102-1107.
- [CrossRef] [Google Scholar]
- Heterogeneous Fenton catalyst supports screening for mono azo dye degradation in contaminated wastewaters. Ind. Eng. Chem. Res.. 2010;49:498-505.
- [CrossRef] [Google Scholar]
- Perovskites as catalysts in advanced oxidation processes for wastewater treatment. Catalysts. 2019;9:230.
- [CrossRef] [Google Scholar]
- Reuse of homogeneous Fenton’s sludge from detergent industry as Fenton’s catalyst. J. Adv. Oxid. Technol.. 2013;16:298-305.
- [CrossRef] [Google Scholar]
- Review of current palm oil mill effluent (POME) treatment methods: Vermicomposting as a sustainable practice. World Appl. Sci. J.. 2010;10:1190-1201.
- [CrossRef] [Google Scholar]
- Application of CCD in RSM to obtain optimize treatment of POME using Fenton oxidation process. J. Water Process Eng.. 2015;8:e7-e16.
- [CrossRef] [Google Scholar]
- Electrochemical treatment of dye solution containing C.I. Basic Yellow 2 by the peroxi-coagulation method and modeling of experimental results by artificial neural networks. J. Electroanal. Chem.. 2009;629:117-125.
- [CrossRef] [Google Scholar]
- Degradation of methylene blue and congo-red dyes using Fenton, photo-Fenton, sono-Fenton, and sonophoto-Fenton methods in the presence of iron(II, III) oxide/zinc oxide/graphene (Fe3O4/ZnO/graphene) composites. Sep. Purif. Technol.. 2019;210:563-573.
- [CrossRef] [Google Scholar]
- Kinetic evaluation of dye decolorization by Fenton processes in the presence of 3-hydroxyanthranilic acid. Int. J. Environ. Res. Public Health. 2019;16:1602.
- [CrossRef] [Google Scholar]
- Winery wastewater treatment by combination of Cryptococcus laurentii and Fenton’s reagent. Chemosphere. 2014;117:53-58.
- [CrossRef] [Google Scholar]
- Optimized use of alum together with unmodified Cassia obtusifolia seed gum as a coagulant aid in treatment of palm oil mill effluent under natural pH of wastewater. Ind. Crops Prod.. 2015;76:1169-1178.
- [CrossRef] [Google Scholar]
- Sheil, D., Casson, A., Meijaard, E., Noordwjik, M. van, Gaskell, J., Sunderland-Groves, J., K., W., M., K., 2009. The impacts and opportunities of oil palm in Southeast Asia: What do we know and what do we need to know?, Occasional paper no. 51. CIFOR. Center for International Forestry Research (CIFOR), Bogor, Indonesia. Doi: 10.17528/cifor/002792.
- Treatment of MSW landfill leachate by a thin gap annular UV/H2O2 photoreactor with multi-UV lamps. J. Hazard. Mater.. 2006;129:73-79.
- [CrossRef] [Google Scholar]
- Treatment of simulated industrial wastewater by photo-Fenton process: Part II. The development of mechanistic model. Chem. Eng. J.. 2011;173:280-289.
- [CrossRef] [Google Scholar]
- Remediation of water pollution caused by pharmaceutical residues based on electrochemical separation and degradation technologies: A review. Environ. Int.. 2012;40:212-229.
- [CrossRef] [Google Scholar]
- Electrochemical advanced oxidation processes: Today and tomorrow. A review. Environ. Sci. Pollut. Res.. 2014;21:8336-8367.
- [CrossRef] [Google Scholar]
- Enhancement of Biodegradation of palm oil mill effluents by local isolated microorganisms. Int. Scholar. Res. Notices. 2014;2014:1-8.
- [CrossRef] [Google Scholar]
- Application of biological oxidation and solar driven advanced oxidation processes to remediation of winery wastewater. Catal. Today. 2013;209:201-208.
- [CrossRef] [Google Scholar]
- Characterization of nano zero-valent iron (nZVI) and its application in sono-Fenton process to remove COD in palm oil mill effluent. J. Environ. Chem. Eng.. 2014;2:1-8.
- [CrossRef] [Google Scholar]
- Applicability of nano-zero valent iron (nZVI) in sono-Fenton process. J. Phys. Conf. Ser.. 2014;495
- [CrossRef] [Google Scholar]
- COD removal from anaerobically treated palm oil mill effluent (AT-POME) via aerated heterogeneous Fenton process: Optimization study. J. Water Process Eng.. 2014;1:8-16.
- [CrossRef] [Google Scholar]
- Performance evaluation of a hybrid system for efficient palm oil mill effluent treatment via an air-cathode, tubular upflow microbial fuel cell coupled with a granular activated carbon adsorption. Bioresour. Technol.. 2016;216:478-485.
- [CrossRef] [Google Scholar]
- Regeneration of activated carbon fiber by the electro-Fenton process. Environ. Sci. Technol.. 2018;52:7450-7457.
- [CrossRef] [Google Scholar]
- Manganese functionalized silicate nanoparticles as a Fenton-type catalyst for water purification by advanced oxidation processes (AOP) Adv. Funct. Mater.. 2012;22:820-826.
- [CrossRef] [Google Scholar]
- United States Environmental Protection Agency: USEPA, 2000. EPA drinking water standards and health advisories. Office of Water. Washington, D.C. 1–30.
- Kinetic study of diuron oxidation and mineralization by persulphate: Effects of temperature, oxidant concentration and iron dosage method. Chem. Eng. J.. 2011;170:127-135.
- [CrossRef] [Google Scholar]
- Aerobic treatment of palm oil mill effluent. J. Environ. Manage.. 2007;82:24-31.
- [CrossRef] [Google Scholar]
- Fenton oxidation and chromium recovery from tannery wastewater by means of iron-based coated biomass as heterogeneous catalyst in fixed-bed columns. Chem. Eng. J.. 2018;351:1-11.
- [CrossRef] [Google Scholar]
- Large laboratory-plant application for the treatment of a tannery wastewater by Fenton oxidation: Fe(II) and nZVI catalysts comparison and kinetic modelling. Process Saf. Environ. Prot.. 2018;117:629-638.
- [CrossRef] [Google Scholar]
- Iron-copper bimetallic nanoparticles supported on hollow mesoporous silica spheres: An effective heterogeneous Fenton catalyst for orange II degradation. RSC Adv.. 2015;5:69593-69605.
- [CrossRef] [Google Scholar]
- Zero discharge performance of an industrial pilot-scale plant treating palm oil mill effluent. Biomed Res. Int.. 2015;2015:617861
- [CrossRef] [Google Scholar]
- A review on Fenton-like processes for organic wastewater treatment. J. Environ. Chem. Eng.. 2016;4:762-787.
- [CrossRef] [Google Scholar]
- Advanced treatment of bio-treated dyeing and finishing wastewater using ozone-biological activated carbon: A study on the synergistic effects. Chem. Eng. J.. 2019;359:168-175.
- [CrossRef] [Google Scholar]
- Novel NaY zeolite-supported nanoscale zero-valent iron as an efficient heterogeneous Fenton catalyst. Catal. Commun.. 2010;11:937-941.
- [CrossRef] [Google Scholar]
- Iron-copper bimetallic nanoparticles embedded within ordered mesoporous carbon as effective and stable heterogeneous Fenton catalyst for the degradation of organic contaminants. Appl. Catal. B. 2015;164:396-406.
- [CrossRef] [Google Scholar]
- World Health Organization: WHO, 1971. International standards for drinking-water, 3rd ed, International standards for drinking-water. Elsevier, Geneva.
- Efficient PFOA degradation by persulfate-assisted photocatalytic ozonation. Sep. Purif. Technol.. 2018;207:255-261.
- [CrossRef] [Google Scholar]
- Pollution control technologies for the treatment of palm oil mill effluent (POME) through end-of-pipe processes. J. Environ. Manage.. 2010;91:1467-1490.
- [CrossRef] [Google Scholar]
- A holistic approach to managing palm oil mill effluent (POME): Biotechnological advances in the sustainable reuse of POME. Biotechnol. Adv.. 2009;27:40-52.
- [CrossRef] [Google Scholar]
- Palm oil mill effluent (POME) treatment and bioresources recovery using ultrafiltration membrane: Effect of pressure on membrane fouling. Biochem. Eng. J.. 2007;35:309-317.
- [CrossRef] [Google Scholar]
- In-situ green assembly of spherical Mn-based metal-organic composites by ion exchange for efficient electrochemical oxidation of organic pollutant. J. Hazard. Mater.. 2019;369:299-308.
- [CrossRef] [Google Scholar]
- Degradation of recalcitrant organics from winery wastewater by Fenton’s reaction. Environ. Eng. Sci.. 2008;25:1229-1234.
- [CrossRef] [Google Scholar]
- Regeneration of iron-montmorillonite adsorbent as an efficient heterogeneous Fenton catalytic for degradation of bisphenol A: Structure, performance and mechanism. Chem. Eng. J.. 2017;328:737-747.
- [CrossRef] [Google Scholar]
- Controllable preparation and catalytic performance of heterogeneous Fenton-like α-Fe2O3/crystalline glass microsphere catalysts. Ind. Eng. Chem. Res.. 2017;56:13751-13759.
- [CrossRef] [Google Scholar]
- Modification of coagulation and Fenton oxidation processes for cost-effective leachate treatment. J. Environ. Sci. Health - Part A Toxic/Hazard. Substan. Environ. Eng.. 2001;36:39-48.
- [CrossRef] [Google Scholar]
- Pd-catalytic in situ generation of H2O2 from H2 and O2 produced by water electrolysis for the efficient electro-Fenton degradation of Rhodamine B. Environ. Sci. Technol.. 2011;45:8514-8520.
- [CrossRef] [Google Scholar]
- Palm oil mill secondary effluent (POMSE) treatment via photocatalysis process in presence of ZnO-PEG nanoparticles. J. Water Process Eng.. 2018;26:10-16.
- [CrossRef] [Google Scholar]
- Reusability performance of zinc oxide nanoparticles for photocatalytic degradation of POME. E3S Web Conf.. 2018;34:02013.
- [CrossRef] [Google Scholar]
- Intensification of the Fenton process by increasing the temperature. Ind. Eng. Chem. Res.. 2011;50:866-870.
- [CrossRef] [Google Scholar]
- Solvothermal synthesis of MIL-53(Fe) hybrid magnetic composites for photoelectrochemical water oxidation and organic pollutant photodegradation under visible light. J. Mater. Chem. A. 2015;3:3074-3081.
- [CrossRef] [Google Scholar]
- A novel magnetic recyclable photocatalyst based on a core-shell metal-organic framework Fe3O4@MIL-100(Fe) for the decolorization of methylene blue dye. J. Mater. Chem. A. 2013;1:14329-14334.
- [CrossRef] [Google Scholar]
- Zhang, M. hui, Dong, H., Zhao, L., Wang, D. xi, Meng, D., 2019. A review on Fenton process for organic wastewater treatment based on optimization perspective. Sci. Total Environ. 670, 110–121. Doi: 10.1016/j.scitotenv.2019.03.180
- Fenton’s oxidation kinetics, pathway, and toxicity evaluation of diethyl phthalate in aqueous solution. J. Adv. Oxid. Technol.. 2016;19:125-133.
- [CrossRef] [Google Scholar]
- A novel reduced graphene oxide-attapulgite (RGO-ATP) supported Fe2O3 catalyst for heterogeneous Fenton -like oxidation of ciprofloxacin: Degradation mechanism and pathway. Catalysts. 2020;10:1-16.
- [CrossRef] [Google Scholar]
- Facile synthesis of mesoporous anatase/rutile/hematite triple heterojunctions for superior heterogeneous photo-Fenton catalysis. Appl. Catal. B. 2020;263:118335
- [CrossRef] [Google Scholar]
- Startup and operation of anaerobic EGSB reactor treating palm oil mill effluent. J. Environ. Sci.. 2008;20:658-663.
- [CrossRef] [Google Scholar]
- Introduction of a Fe3O4 core enhances the photocatalytic activity of MIL-100(Fe) with tunable shell thickness in the presence of H2O2. ChemCatChem. 2015;7:4148-4155.
- [CrossRef] [Google Scholar]
- Electro-Fenton oxidation of pesticides with a novel Fe3O4@Fe2O3/activated carbon aerogel cathode: High activity, wide pH range and catalytic mechanism. Appl. Catal. B. 2012;125:120-127.
- [CrossRef] [Google Scholar]
- Hydrophilic modification of ordered mesoporous carbon supported Fe nanoparticles with enhanced adsorption and heterogeneous Fenton-like oxidation performance. RSC Adv. 2015
- [CrossRef] [Google Scholar]
- Optimization of pre-treated palm oil mill effluent digestion in an up-flow anaerobic sludge fixed film bioreactor: A comparative study. Biochem. Eng. J.. 2007;35:226-237.
- [CrossRef] [Google Scholar]







