Translate this page into:
Aptamer functionalized fluorescent probe for detection of thrombin in human serum via static quenching
⁎Corresponding author. shuchang@cpu.edu.cn (Chang Shu)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Thrombin plays a pivotal role in blood coagulation, wound healing, and tumor metastasis. In this study, we developed a fluorescent probe (Apt15@QDs) by functionalizing quantum dots (QDs) with an aptamer 15 to specifically recognize thrombin. The aptamer Apt15 binds specifically to thrombin by adopting a G-quadruplex conformation, resulting in the formation of a binary complex that quenches the fluorescence of the probe. Analysis using the classical fluorescence quenching equations Stem-Volmer and Lineweaver-Burk revealed that the mechanism of quenching is static in nature. The established method was successfully applied for detecting thrombin in human serum samples. As shown from the results, the established method exhibited high sensitivity, with a limit of detection (LOD) of 8 nM, and possessed a wide linear range within the concentration span of 20 to 200 nM. Additionally, this method circumvented the need for intricate experimental procedures. Furthermore, it offered a short analysis time, obviated the requirement for additional reagents, and proved suitable for high-throughput analysis of biological samples. This study presents a sensitive, straightforward, and rapid approach for detecting thrombin in complex biological matrices.
Keywords
Thrombin
Quantum dots
Static quenching
Aptamer
Human serum samples
1 Introduction
Thrombin, a trypsin-like serine protease generated through prothrombin activation, plays a crucial role in converting fibrinogen into fibrin to effectively control capillary and venous bleeding (Danckwardt et al., 2013; Posma et al., 2016). Clinically employed as a local hemostatic agent during surgical procedures, thrombin faces numerous challenges when analyzed and detected alongside its protein-like peptides within complex biological samples due to their low concentration and susceptibility to degradation and inactivation during sample pretreatment (Cheng et al., 2009). Currently, commonly employed analytical methods include colorimetric assays, electrochemiluminescence (ECL) techniques, and enzyme-linked immunosorbent assays (ELISAs), etc. However, colorimetric methods (Wu and Li, 2022) suffer from low sensitivity and poor reproducibility due to their reliance on thrombin activity for chromogenic substrate production. Although the ECL method offers high sensitivity, it is associated with high equipment costs (Liu et al., 2020). ELISA is widely used for biomacromolecule analysis but its operation is cumbersome and time-consuming, leading to non-specific adsorption of microplates resulting in false positive results, reduced accuracy, and poor reproducibility (White et al., 2021). Therefore, there is an urgent need to establish a rapid, accurate, and sensitive analytical detection technique for thrombin detection in complex biological samples.
Quantum dots (QDs) have gradually replaced traditional organic dyes as powerful tools for biological markers due to their unique optical properties, including high fluorescence efficiency, photostability, and reaction kinetics (He et al., 2016; Liu et al., 2013; Zhang et al., 2021). QDs hold broad application prospects in the fields of biological imaging (Gu et al., 2012), biosensing (Ncapayi et al., 2021), and photoelectronics (Wang et al., 2016). Hydrothermally synthesized QDs exhibit excellent biocompatibility and can be bioanalyzed using surface-modified antibodies (Ehzari and Safari, 2022), aptamers (Lao et al., 2016), small molecules (Kateshiya et al., 2022), and other substances. An aptamer is an oligonucleotide sequence obtained through systematic evolution of ligands by exponential enrichment (SELEX) that exhibits specific recognition towards a target molecule (Wu et al., 2021). Under appropriate conditions, aptamers can adopt a distinct spatial conformation facilitating precise binding to the target (Ali and Omer, 2022a, Ali and Omer, 2022b, Ali and Omer, 2022c). In comparison to antibodies (Ni et al., 2021), aptamers possess simpler chemical structures and enhanced chemical stability. Furthermore, they can be synthesized chemically in a highly automated manner at significantly reduced costs compared to antibodies. Recently, numerous biomacromolecule analysis methods utilizing aptamers as identification tools have been reported (He et al., 2022; Liang et al., 2023; Tian et al., 2021). Dynamic and static quenching are significant mechanisms of fluorophore quenching. In brief, static quenching refers to the formation of weakly fluorescent or non-fluorescent complexes due to interactions between the ground state fluorescence molecule and the quenching agent, resulting in a reduction in fluorescence intensity (Zhang et al., 2013, Liu et al., 2018, Saa et al., 2019, Sun et al., 2022). Unlike static quenching, dynamic quenching typically occurs during collisions between the excited state fluorophore and the quencher, involving processes such as energy transfer or charge transfer (Mahanwar et al., 2019). Indeed, the occurrence and recovery of dynamic or static quenching are often utilized for reflecting analyte concentration when designing fluorescent probes (Bai et al., 2021; Zhong et al., 2022). In this experiment, we employed the property of static quenching between thrombin and QDs to detect thrombin.
In this study, we developed a fluorescent probe (Apt15@QDs) for the simple, rapid, and sensitive detection of thrombin (Fig. 1). The probe utilized CdTe@ZnS QDs as the fluorophore, with covalently modified aptamer Apt15 specifically binding to thrombin on the surface of CdTe@ZnS QDs as a recognition probe. We employed the classical Stem-Volmer equation and Lineweaver-Burk equation to elucidate the quenching mechanism between QDs and thrombin. The developed method was successfully applied to detect thrombin in human serum samples. Compared to traditional methods for thrombin detection, our approach eliminates the need for additional reagents, simplifies experimental procedures, and significantly reduces sample analysis time, making it suitable for high-throughput biological sample analysis. This method offers a sensitive, simple, and rapid solution for detecting thrombin in complex biological matrices.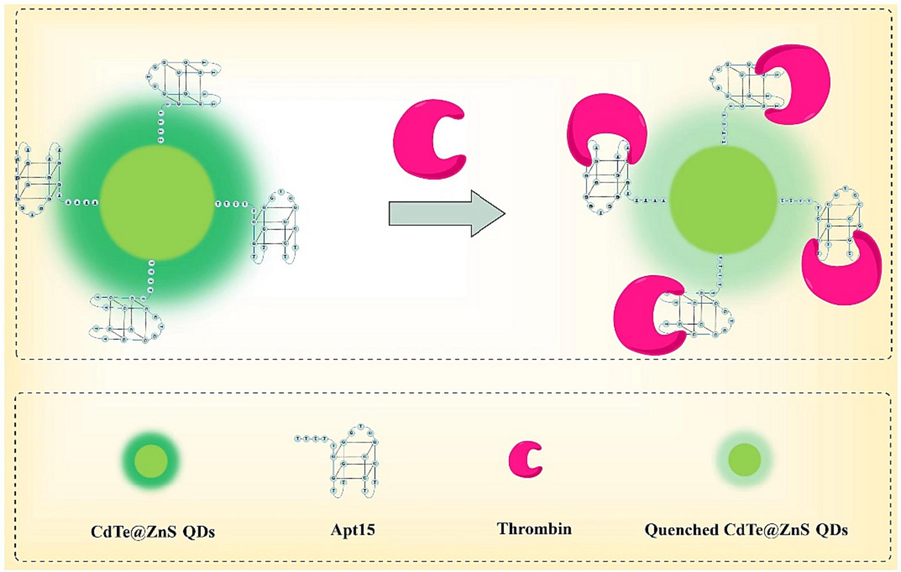
Schematic illustration of aptamer functionalized fluorescent probe for thrombin detection in human serum via static quenching.
2 Experiment
2.1 Reagents
Cadmium chloride (CdCl2•2.5 H2O), reduced Glutathione and Tellurium (Te) powder were all purchased from Aladdin Chemistry Co., Ltd. (Shanghai, China). Sodium metaborate (NaBH4) was obtained from Nanjing Chemical Reagent Co., Ltd. (Nanjing, China). Mercaptopropionic acid (MPA) and Rhodamine 6G were purchased from Shanghai Macklin Biochemical Co., Ltd. (Shanghai, China). 5′-TTTTTGGTTGGTGTGGTTGG-3′ was synthesized by Sangon Biotechnology Co., Ltd. (Shanghai, China). Thrombin was purchased from Beyotime Biotechnology (Shanghai, China). Bovine albumin (BSA) was purchased from Shanghai Yuanye Bio-Technology Co., Ltd. (Shanghai, China). Alkaline phosphatase (ALP) and Proteinase K(Pro-K) were both purchased from Sigma-Aldrich (Saint Louis, U.S.A.). Pancreatin and Pepsin were obtained from Nanjing KeyGen Biotech. Co., Ltd. (Nanjing, China). Human serum was purchased from Shanghai Xinfan Biotechnology Co., Ltd. (Shanghai, China). Ultrapure deionized water was obtained using a Milli-Q water system (Millipore, U.S.A.).
2.2 Preparation of CdTe@ZnS QDs
Firstly, the Cd2+ precursor solution was prepared by mixing CdCl2•2.5 H2O (0.8 mmol), reduced glutathione (1.6 mmol) and ultrapure water (150 mL) in a 250 mL three-necked flask, and then adjusted the pH of the mixture to 10.0 with NaOH solution (1 M). Subsequently, Te powder (0.4 mmol), NaBH4 (2.4 mmol), newly boiled and cooled ultrapure water(4 mL) were added to a 25 mL three-necked flask, and then the mixture was stirred at 40 °C for 45 min under the N2 protection to obtain NaHTe solution. Afterwards, the freshly prepared NaHTe solution was placed in an ice bath for 10 min before adding H2SO4 solution (0.05 M) dropwise until the solution turned brown-yellow. The H2Te generated from this process entered into the Cd2+ precursor solution while ventilation stopped when it changed from colorless to brown-yellow. Finally, the solution was refluxed for 5 min and then cooled to room temperature in ice bath to obtain the CdTe QDs solution.
The mixture of 50 mL CdTe QDs solution and 50 mL of ZnSO4 solution (2.5 mM) were taken in a 250 mL three-necked flask and vigorously stirred for 10 min. Subsequently, 50 mL MPA solution (2.5 mM) and NaOH solution (1 M) were added to adjust the pH of the mixture to 10.0, followed by continued stirring for an addition 30 min. The resulting solution was then refluxed for 5 min and cooled to room temperature, yielding the CdTe@ZnS QDs solution. Finally, this solution was concentrated using a rotary evaporator to 1/3 of the original volume. Subsequently, 3 times the amount of absolute ethyl alcohol was added, and centrifugation at 2500g for 30 min allowed collection of the precipitate. The precipitation was underwent thrice washing with absolute ethyl alcohol before being vacuum dried overnight at 50 °C to obtain CdTe@ZnS QDs.
2.3 Preparation of Apt15@QDs
Firstly, the CdTe@ZnS QDs (8.36 mg/mL) was purified 3 times by using Amicon Ultra-0.5 ultrafiltration centrifuge tubes (MWCO, 30000 Da), each time using 10 mM PBS buffer (pH 7.38, 137 mM NaCl, 3 mM KCl) as washing solution. Additionally, a mixture of EDC/NHS (20 mM/10 mM) was prepared by adding 100 μL of purified CdTe@ZnS QDs solution followed by incubation for 15 min. Subsequently, Apt15 solution (100 μM) was then added and the mixture was stirred for 2 h. To complete the reaction, ethanolamine solution (0.1% wt) was introduced into the mixture and stirred for an additional period lasting 1.5 h. Finally, 10 mM PBS buffer (pH 8.0, 137 mM NaCl, 3 mM KCl) was added to make a total volume of 0.5 mL. All aforementioned experimental procedures were conducted under room temperature conditions. In order to remove the excess Apt15 and catalyst, the Apt15@QDs was centrifuged three times by using ultrafiltration centrifuge tubes (MWCO, 30000 Da), redispersed in 0.5 mL PBS buffer (10 mM, pH 8.0, 137 mM NaCl, 3 mM KCl), and stored at 4 °C for later use.
2.4 Characterizations
The fluorescence quantum yields (QYs) of QDs were calibrated using a diluted solution of Rhodamine 6G (QY 95%). The absorption spectra of both Rhodamine 6G and QDs solutions were measured. The excitation wavelength was set at 350 nm, the ranges of integration were from 450 nm to 600 nm. The quantum yields were calculated from the following equation:
The fluorescence quantum yields of Rhodamine 6G and QDs are denoted as Φs and Фs, respectively. ODr and ODs represent the absorbance values of the Rhodamine 6G solution and QDs at an excitation wavelength of 350 nm, respectively. Is and Ir indicate the fluorescence intensities of Rhodamine 6G and QDs within the range of 450 nm to 600 nm, respectively. ηr and ηs correspond to the refractive indices of the dispersion media for Rhodamine 6G and QDs at a temperature of 25 °C.
High-resolution transmission electron microscopy (HRTEM) was conducted using a JEM-2100 instrument (JEOL Ltd, Japan) to characterize the morphology and size of CdTe@ZnS QDs. Infrared spectroscopy (IR) measurements of the QDs were obtained using a Tracer-100 infrared spectrometer (Shimadzu, Japan). Fluorescence spectra and UV–Vis absorption spectra were acquired using a SpectraMax M2e microplate reader (Molecular Devices, U.S.A.).
2.5 Detection and selectivity
The detection of thrombin activity based on static quenching was conducted as follows: the Apt15@QDs solution (300 nM) and the thrombin solution with varying concentrations (0, 20, 40, 80, 110, 140, 170, and 200 nM), or human serum samples were added to each well. After incubation at 25 °C for 15 min, the fluorescence intensity was measured at an excitation wavelength of 350 nm and emission wavelength of 520 nm. The Apt15@QDs solution (300 nM) and the interfering substance solution were added to further evaluate the selectivity in detecting thrombin. In the small-molecule selectivity experiments, the concentrations of Na+, K+, Cl-, Br-, F-, CH3COO–, SO42-, SCN- were all 10 mM; Ca2+, Mg2+, NH4+ were all 100 μM, and Zn2+, Fe3+, Mn2+, Ni2+ were all 5 μM. For macromolecular selectivity experiments, BSA, Trypsin, Pepsin, Proteinase K, and ALP concentrations were all 100 μg/ml. The concentration of thrombin was fixed at 100 nM.
3 Results and discussion
3.1 Characterization
The CdTe@ZnS QDs exhibited a nearly spherical shape with a well-defined lattice structure (Fig. 2A), and the average diameter was approximately 3.08 ± 0.56 nm (Fig. 2B). Additionally, EDS analysis revealed that the CdTe@ZnS QDs had a higher concentration of Zn and S elements, which originated from the ZnS shell surrounding them (Fig. 2C). In the FT-IR spectra (Fig. 2D) of CdTe QDs, two distinct absorption peaks at 3305 cm−1 and 1597 cm−1 were respectively attributed to the N—H stretching vibration and full vibration in the amide bond, while the shoulder peak at 1643 cm−1 represented the C⚌O stretching vibration in the amide bond. Furthermore, the peak observed at 1402 cm−1 corresponded to asymmetric stretching vibration of C⚌O after ionization of carboxyl groups. The FT-IR spectra of CdTe QDs with ZnS shell did not exhibit significant changes since quantum dot epitaxial growth resulted in a less dense ZnS shell formation. Thus, characteristic peaks related to abundant amide bonds from glutathione ligands on CdTe QD surfaces could still be observed. According to the results of UV–Vis absorption spectra, it is evident that CdTe QDs exhibit a distinct excitonic absorption peak at 460 nm. Upon coating with a ZnS shell, the excitonic absorption peak of CdTe@ZnS QDs undergoes a red-shift to 485 nm, which can be attributed to the increase in particle size (Fig. 3A). Furthermore, the fluorescence emission spectra also demonstrate that the maximum emission wavelength of CdTe@ZnS QDs experiences a red-shift from 495 nm (the maximum emission wavelength of CdTe QDs) to 515 nm (Fig. 3B). Importantly, the fluorescence quantum yield of CdTe@ZnS QDs (44.9%) exhibits significant improvement compared to that of CdTe QDs (12.4%), owing to the formation of a ZnS shell which reduces surface defects and enhances exciton recombination efficiency (Zare et al., 2015). These findings confirm successful coverage of CdTe QD surfaces with an appropriate amount of ZnS and highlight how the ZnS shell greatly enhances the fluorescence quantum yield of CdTe@ZnS QDs.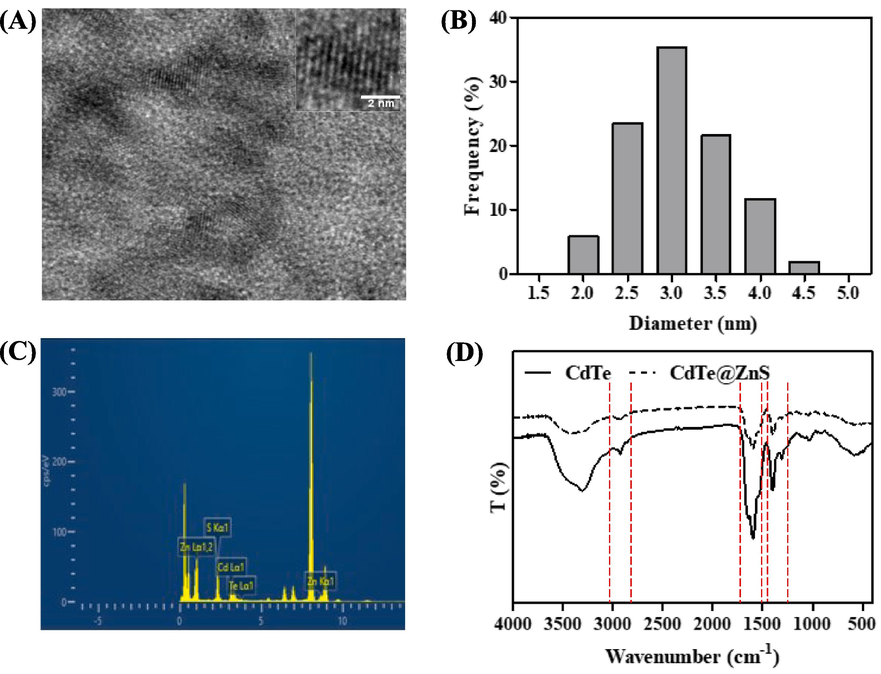
The HRTEM images (A), size distribution histogram (B), EDS spectra (C) and FT-IR spectra (D) of CdTe@ZnS QDs.
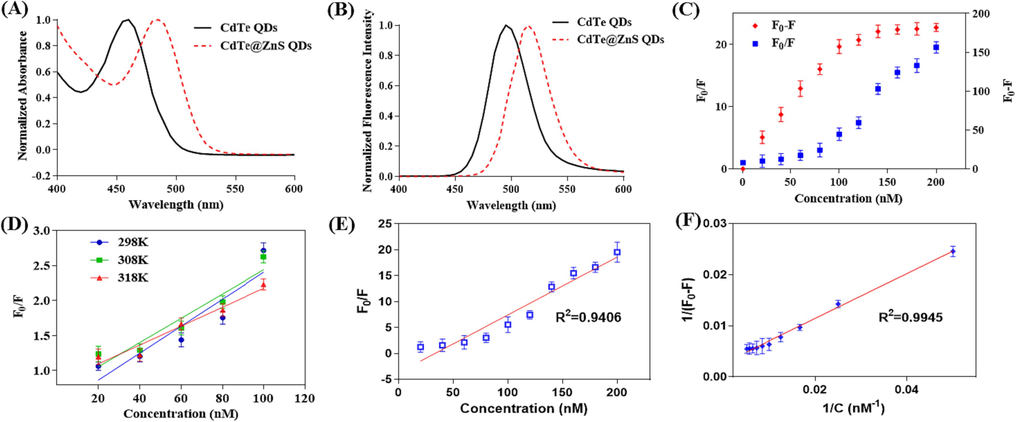
The UV–Vis absorption spectra (A) and fluorescence spectra (B) of CdTe QDs and CdTe@ZnS QDs. (C) The relationship between fluorescence intensity changes and thrombin concentration. (D) The relationship between fluorescence intensity ratio and thrombin concentration at different incubation temperatures. (E) When incubated at 310 K, the relationship between the fluorescence intensity ratio and thrombin concentration. (F) When incubated at 310 K, the relationship between the reciprocal of the fluorescence intensity difference and the thrombin concentration. (F0 and F represent the fluorescence intensity of the mixture at 520 nm before and after the addition of thrombin, respectively, and the excitation wavelength is 350 nm.).
3.2 Feasibility and principle
After incubating thrombin samples with Apt15@QDs for 15 min, it was discovered that the fluorescence intensity at 520 nm gradually decreased as the concentration of thrombin increased. Moreover, both the difference in fluorescence intensity (F0-F) and the ratio of fluorescence intensity (F0/F) before and after adding thrombin exhibited a gradual increase with increasing thrombin concentration (Fig. 3C). The concentration-dependent changes in thrombin were evidenced by the difference and ratio of fluorescence intensity, indicating the feasibility of detecting thrombin concentration using Apt15@QDs.
Generally, the fluorescence quenching mechanism of quenchers towards fluorophores encompasses dynamic and static quenching modes. During the dynamic quenching process, when excited state fluorophores collide with quenchers, a portion of energy is dissipated as heat, resulting in fewer radiative transitions back to the ground state and consequently reducing the number of fluorophores returning to the excited state, thereby diminishing fluorescence intensity (Soemo and Pemberton, 2014; Wang et al., 2015). The relationship between fluorescence intensity ratio and quencher concentration can be described by formula (2), which is derived from the Stem-Volmer equation where Ksv represents the Stem-Volmer constant. In cases involving dynamic quenching, an increase in temperature enhances molecular thermal motion, thereby increasing collision probability between excited state fluorophores and quenchers. Consequently, Ksv increases with rising temperature. Apt15@QDs were incubated with thrombin at different temperatures (298 K, 308 K, 318 K), and linear regression analysis was performed using the fluorescence intensity ratio before and after thrombin addition (F0/F) to determine thrombin concentration. As depicted in Fig. 3D, it was observed that the slope of linear regression equation (Ksv) gradually decreased with increasing incubation temperature (Table 1). This suggests that thrombin may not be responsible for Apt15@QDs' quenching through dynamic mechanisms.
Temperature (K)
Ksv (L·mol−1)
R2
298
1.925*107
0.8573
308
1.731*107
0.9148
318
1.350*107
0.9526
Furthermore, Apt15@QDs was co-incubated with thrombin at 37℃ (310 K) for 15 min. Subsequently, regression analysis (Fig. 3E) reveals a significant deviation from the linear regression equation and demonstrates hyperbolic characteristics in the relationship between F/F0 and the concentration of thrombin. This suggests that Apt15@QDs undergoes dynamic quenching by thrombin. Static quenching occurs due to the formation of weakly fluorescent complexes between fluorophore and quencher through hydrogen bonding, hydrophobic interactions, and electrostatic interactions (Chaves et al., 2018; Derayea et al., 2019). In the process of static quenching, the formation of a complex between the fluorescence group and the quencher leads to fluorescence quenching. This phenomenon can be described by the Lineweaver-Burk double reciprocal curve (Equation (3). As depicted in Fig. 3F, at 37℃ (310 K), there is a strong linear correlation (R2 = 0.9945) between the reciprocal of the difference in fluorescence intensity before and after thrombin addition (1/(F0-F)) and the reciprocal of thrombin concentration. This suggests that Apt15@QDs may form a weakly fluorescent complex with thrombin, resulting in fluorescence quenching within the system. Previous studies have indicated that electrostatic forces and hydrogen bonding play significant roles in this combination process, as Apt15 can bind to basic amino acid residues at Exosite I on thrombin through numerous negatively charged phosphate groups. In summary, the proposed detection mechanism can be summarized as follows: initially, the oligonucleotide segment of Apt15@QDs folds into a G-tetra-chain conformation. Subsequently, under specific pH conditions, the phosphoric acid group undergoes ionization and forms a binary complex with weak fluorescence by electrostatically binding to the thrombin anion binding site. This ultimately leads to fluorescence quenching in the system. On one hand, as temperature rises, the stability of the binary complex decreases or diminishes. On the other hand, increased molecular thermal motion accelerates collision chances between excited state Apt15@QDs and thrombin, resulting in dynamic quenching characteristics during the quenching process. As shown in Table 1, these results also explain why Ksv decreases with increasing temperature and why the regression coefficient R2 approaches 1.
3.3 Optimization of conditions
In order to achieve a sensitive signal, the optimization of incubation temperature, incubation time, and buffer pH was conducted as a preliminary step. As depicted in Fig. 4A, when incubated in a buffer with pH 7.4 at 25 °C, the detection signal (1-F/F0) exhibited significantly lower values compared to that of buffers with pH 7.7 and 8.0. Notably, the signals from the latter two were found to be quite similar within a 15 min incubation period. With prolonged incubation time, there was a slight attenuation observed in the detection signal for the buffer solution with pH 8.0. However, this attenuation was more pronounced for buffers with pH 7.7 and 7.4. A similar trend was observed when incubated at 37 °C. However, it should be noted that overall detection signals were generally lower (Fig. 4B). It is worth mentioning that lower pH levels can adversely affect QDs stability and this effect becomes more prominent over extended periods of incubation time (Mandai and Tamai, 2008). During our investigation into the detection principle employed here, we have classified thrombin's quenching effect on Apt15@QDs as static quenching which necessitates initial formation of a binary complex between thrombin and Apt15@QDs molecules. Furthermore, previous reports have indicated an inverse correlation between static quenching degree and temperature primarily due to increased molecular thermal motion at higher temperatures which hampers binary complex formation (Gadallah et al., 2021). Therefore, to obtain robust and consistent results, a standardized protocol involving an incubation temperature of 25 °C, a buffer pH value set at 8.0, and an optimal duration of 15 min has been established.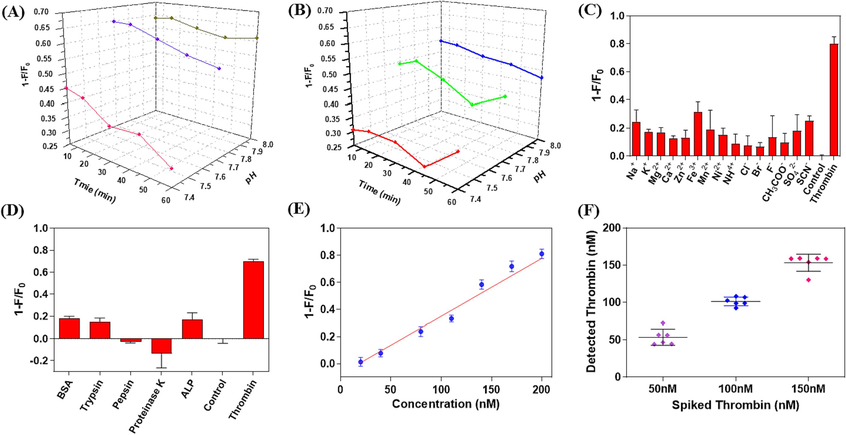
The effects of incubation time and buffer pH on the detection signal (1-F/F0) at 25 °C (A) and 37 °C (B). The effect of interfering ions (C) and biomacromolecule interfering substances (D) on detection signal (1-F/F0). (E) Regression curve of detection signal (1-F/F0) versus thrombin concentration in diluted human serum. (F) The distribution of thrombin detected in spiked samples at high (150 nM), medium (100 nM) and low (50 nM) concentration levels (n = 6). (F0 and F represent the fluorescence intensity of the mixture at 520 nm before and after the addition of thrombin, respectively, and the excitation wavelength is 350 nm.).
The signal-to-noise ratio (SNR) was determined by calculating the signal ratio between the low-concentration sample and the blank sample. Results indicated that an SNR greater than 3 was achieved at a thrombin concentration of 8 nM, which was therefore established as the detection limit for this method. Apt15@QDs performance in detecting thrombin was evaluated under optimal conditions within a range of 20 to 200 nM. The detection signals (1-F/F0) exhibited a strong linear relationship with thrombin concentration (1-F/F0 = 0.005207[thrombin]-0.08674, R2 = 0.9870). Furthermore, accuracy and precision of the detection method were assessed using lower limit of quantitation (LLOQ, 20 nM), low quality control (LQC, 60 nM), medium quality control (MQC, 100 nM), and high quality control (HQC, 160 nM). Table 2 presents the results showing relative standard deviations below 15% for all concentration levels and accuracy ranging from 82.6% to123.7%. conc.: concentration; SD: standard deviation; RSD: relative standard deviation; n: number of replicates.
Spiked conc.
(nM)Measured conc.
(mean ± SD, nM)Accuracy
(%)RSD
(%)
20
24.75 ± 3.36
123.7
13.6
60
49.57 ± 6.38
82.6
12.9
100
92.78 ± 13.29
92.8
14.3
160
170.24 ± 4.44
106.4
2.6
3.4 Selectivity
In order to further assess the specificity of Apt15@QDs for thrombin detection, we investigated the potential interference of common ions and proteins on Apt15@QDs. Fig. 4C demonstrates that the detection signals from all tested interfering ion samples are significantly lower than those from thrombin (100 nM). Additionally, Fig. 4D clearly illustrates that common proteins do not result in a significant increase in detection signals. Hence, it can be concluded that Apt15@QDs exhibits satisfactory selectivity towards common interfering ions and proteins. This further validates the applicability of Apt15@QDs for rapid and specific thrombin detection.
3.5 Biological sample analysis
In order to further assess the performance of Apt15@QDs in the rapid and specific detection of thrombin in complex matrices, we investigated the recovery and precision of the detection method for human serum samples using the standard addition method. All experiments related to human serum were approved by ethics committee of Sir Run Run Hospital of Nanjing Medical University (approval no. 2022-SR-S033). As depicted in Fig. 4E, within the range of 20~200 nM, there was a strong linear correlation between the concentration of thrombin in human serum (1-F/F0 = 0.00426 [Thrombin]-0.07761, R2 = 0.9755) and the detection signal (1-F/F0). The same method was employed to detect spiked and human serum samples containing thrombin, as shown in Fig. 4F. It can be observed that at high (150 nM), medium (100 nM), and low (50 nM) concentration levels, the detection values were concentrated with minimal standard deviation. With an exception for a low concentration level of 20.1%, all other concentrations exhibited standard deviations below 10%. Moreover, recoveries ranged from 101.1% to 106.9% (Table 3). The above results indicate that Apt15@QDs can detect thrombin rapidly, accurately and specifically in human serum compared with other detection methods (Table 4), and exhibits broad prospects for application to thrombin in complex matrices (Lao et al., 2016, Li et al., 2018, Sadik et al., 2019, Yu and Wu 2019, Zeng et al., 2021). conc.: concentration; SD: standard deviation; RSD: relative standard deviation; n: number of replicates.
Spiked conc.
(nM)Measured conc.
(mean ± SD, nM)Recovery
(%)RSD
(%)
50
53.44 ± 10.77
106.9
20.1
100
101.09 ± 5.82
101.1
5.8
150
153.13 ± 11.47
102.1
7.5
Materials/Probes
LLOQ (nM)
LOD (nM)
Analysis Period
Reference
AuNPs
10
7.5
30 min
Li et al.
SITS-TA
300
205
10 s
Zeng et al.
OEPSi
10
8.21
–
Yu et al.
QDs
10
10
120 min
Lao et al.
mSAMS
30
6
100 min
Ataman et al.
CdTe@ZnS-Apt15
20
20
15 min
This Work
4 Conclusion
In this study, we have developed a straightforward static quenching method for the rapid and sensitive detection of thrombin. CdTe@ZnS QDs were employed as fluorophores, while the aptamer Apt15 served as a recognition tool that specifically binds to thrombin by adopting a G-quadruplex conformation. Our results demonstrate that Apt15@QDs selectively interact with thrombin to form a binary complex with diminished fluorescence intensity, thereby facilitating the static quenching of CdTe@ZnS QDs and enabling the sensitive detection of thrombin in both buffer solution and human serum (with LLOQ was as low as 20 nM). Notably, this approach obviates the need for additional reagents and offers an expedited detection time of only 15 min per sample, thus providing a simple, rapid, and highly sensitive methodology for detecting thrombin.
Acknowledgment
This study was supported by the China Pharmaceutical University's “Double First Class” initiative (No. CPU2022QZ16).
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Molecular imprinted polymer combined with aptamer (MIP-aptamer) as a hybrid dual recognition element for bio(chemical) sensing applications. Review. Talanta.. 2022;236:122878
- [CrossRef] [Google Scholar]
- Nanozyme and Stimulated Fluorescent Cu-Based Metal-Organic Frameworks (Cu-MOFs) Functionalized with Engineered Aptamers as a Molecular Recognition Element for Thrombin Detection in the Plasma of COVID-19 Patients. ACS Omega. 2022;7:36804-36810.
- [CrossRef] [Google Scholar]
- Ultrasensitive aptamer-functionalized Cu-MOF fluorescent nanozyme as an optical biosensor for detection of C-reactive protein. Anal. Biochem.. 2022;658:114928
- [CrossRef] [Google Scholar]
- Facile synthesis of carbon nitride quantum dots as a highly selective and sensitive fluorescent sensor for the tetracycline detection. RSC Adv.. 2021;11:24892-24899.
- [CrossRef] [Google Scholar]
- In vitro analysis of the interaction between human serum albumin and semi–synthetic clerodanes. J. Braz. Chem. Soc.. 2018;29:1786-1795.
- [CrossRef] [Google Scholar]
- A Review of Three Stand-Alone Topical Thrombins for Surgical Hemostasis. Clin. Ther. 2009
- [CrossRef] [Google Scholar]
- Pathologies at the nexus of blood coagulation and inflammation: Thrombin in hemostasis, cancer, and beyond. J. Mol. Med. 2013
- [CrossRef] [Google Scholar]
- Investigating erythrosine B as a fluorimetric probe for the determination of benzimidazole drugs via facile complexation reaction. Microchem. J.. 2019;149
- [CrossRef] [Google Scholar]
- A Sandwich-Type Electrochemical Immunosensor Using Antibody-Conjugated Pt-Doped CdTe QDs as Enzyme-Free Labels for Sensitive HER2 Detection Based on a Magnetic Framework. Front. Chem.. 2022;10
- [CrossRef] [Google Scholar]
- Towards understanding of the interaction of certain carbapenems with protein via combined experimental and theoretical approach. Spectrochim. Acta A Mol. Biomol. Spectrosc.. 2021;246
- [CrossRef] [Google Scholar]
- Ultrasmall near-infrared Ag 2Se quantum dots with tunable fluorescence for in vivo imaging. J. Am. Chem. Soc.. 2012;134:79-82.
- [CrossRef] [Google Scholar]
- A design of red emission CDs-based aptasensor for sensitive detection of insulin via fluorescence resonance energy transfer. Spectrochim. Acta A Mol. Biomol. Spectrosc.. 2022;280
- [CrossRef] [Google Scholar]
- Catalytic Molecular Imaging of MicroRNA in Living Cells by DNA-Programmed Nanoparticle Disassembly. Angew. Chem.. 2016;128:3125-3128.
- [CrossRef] [Google Scholar]
- Folic acid functionalized molybdenum oxide quantum dots for the detection of Cu2+ ion and alkaline phosphatase via fluorescence turn off–on mechanism. Spectrochim. Acta A Mol. Biomol. Spectrosc.. 2022;268
- [CrossRef] [Google Scholar]
- Signal-on Protein Detection via Dye Translocation between Aptamer and Quantum Dot. ACS Appl. Mater. Interfaces. 2016;8:12048-12055.
- [CrossRef] [Google Scholar]
- Target binding and DNA hybridization-induced gold nanoparticle aggregation for colorimetric detection of thrombin. Sens. Actuators B-Chem.. 2018;262:733-738.
- [CrossRef] [Google Scholar]
- A novel fluorescent strategy for Golgi protein 73 determination based on aptamer/nitrogen-doped graphene quantum dots/molybdenum disulfide @ reduced graphene oxide nanosheets. Spectrochim. Acta A Mol. Biomol. Spectrosc.. 2023;294
- [CrossRef] [Google Scholar]
- Strong two-photon-induced fluorescence from photostable, biocompatible nitrogen-doped graphene quantum dots for cellular and deep-tissue imaging. Nano Lett.. 2013;13:2436-2441.
- [CrossRef] [Google Scholar]
- Detection of Thrombin Based on Fluorescence Energy Transfer between Semiconducting Polymer Dots and BHQ-Labelled Aptamers. Sensors (Basel, Switzerland).. 2018;18
- [CrossRef] [Google Scholar]
- Electrogenerated chemiluminescence resonance energy transfer between ZnGa2O4/g-C3N4and gold nanoparticles/graphene and its application in the detection of thrombin. Analyst. 2020;145:7412-7420.
- [CrossRef] [Google Scholar]
- Exploration of Fluorescence Quenching Mechanism in Tryptophan Induced by Norfloxacin: Analytical Applications. Macromol. Symp.. 2019;387
- [CrossRef] [Google Scholar]
- Influence of acid on luminescence properties of thioglycolic acid-capped CdTe quantum dots. J. Phys. Chem. C. 2008;112:8244-8250.
- [CrossRef] [Google Scholar]
- Diagnosis of prostate cancer and prostatitis using near infra-red fluorescent aginse/zns quantum dots. Int. J. Mol. Sci.. 2021;22
- [CrossRef] [Google Scholar]
- Recent Progress in Aptamer Discoveries and Modifications for Therapeutic Applications. ACS Appl. Mater. Interfaces 2021
- [CrossRef] [Google Scholar]
- Coagulation and non-coagulation effects of thrombin. J. Thromb. Haemost. 2016
- [CrossRef] [Google Scholar]
- CdS quantum dots generated in-situ for fluorometric determination of thrombin activity. Mikrochim. Acta. 2019;186:657.
- [CrossRef] [Google Scholar]
- Mixed monolayer decorated SPR sensing surface for thrombin detection. J. Pharm. Biomed. Anal.. 2019;176
- [CrossRef] [Google Scholar]
- Combined quenching mechanism of anthracene fluorescence by cetylpyridinium chloride in sodium dodecyl sulfate micelles. J. Fluoresc.. 2014;24:295-299.
- [CrossRef] [Google Scholar]
- An antifouling electrochemical aptasensor based on hyaluronic acid functionalized polydopamine for thrombin detection in human serum. Bioelectrochemistry. 2022;145:108073
- [CrossRef] [Google Scholar]
- An electrochemical dual-aptamer biosensor based on metal-organic frameworks MIL-53 decorated with Au@Pt nanoparticles and enzymes for detection of COVID-19 nucleocapsid protein. Electrochim. Acta. 2021;387
- [CrossRef] [Google Scholar]
- Quantifying the dynamic fluorescence quenching of phenanthrene and ofloxacin by dissolved humic acids. Environ. Pollut.. 2015;196:379-385.
- [CrossRef] [Google Scholar]
- Quantum dots derived from two-dimensional materials and their applications for catalysis and energy. Chem. Soc. Rev. 2016
- [CrossRef] [Google Scholar]
- Evaluation of COVID-19 coagulopathy; laboratory characterization using thrombin generation and nonconventional haemostasis assays. Int. J. Lab. Hematol.. 2021;43:123-130.
- [CrossRef] [Google Scholar]
- An instrument-free visual quantitative detection method based on clock reaction: the detection of thrombin as an example. Anal. Methods. 2022;15:48-55.
- [CrossRef] [Google Scholar]
- Aptamer-Based Detection of Circulating Targets for Precision Medicine. Chem. Rev. 2021
- [CrossRef] [Google Scholar]
- Rapid and reagentless detection of thrombin in clinic samples via microfluidic aptasensors with multiple target-binding sites. Biosens. Bioelectron.. 2019;146
- [CrossRef] [Google Scholar]
- High-efficiency CdTe/CdS core/shell nanocrystals in water enabled by photo-induced colloidal hetero-epitaxy of CdS shelling at room temperature. Nano Res.. 2015;8:2317-2328.
- [CrossRef] [Google Scholar]
- A Fluorescence Kinetic-Based Aptasensor Employing Stilbene Isomerization for Detection of Thrombin. Materials.. 2021;14
- [CrossRef] [Google Scholar]
- Label-free sensing of thrombin based on quantum dots and thrombin binding aptamer. Talanta. 2013;107:140-145.
- [CrossRef] [Google Scholar]
- GSH capped Cu-Zn-In-S quantum dots: one-pot aqueous synthesis and cell labeling applications. Chalcogenides Lett. 2021
- [Google Scholar]
- Ascorbic acid detector based on fluorescent molybdenum disulfide quantum dots. Microchim. Acta. 2022;189
- [CrossRef] [Google Scholar]







