Translate this page into:
Aryl-oxadiazole Schiff bases: Synthesis, α-glucosidase in vitro inhibitory activity and their in silico studies
⁎Corresponding author. fazalstar@gmail.com (Fazal Rahim)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
α-Glucosidase enzyme is a therapeutic target for diabetes mellitus and its inhibitors play a vital role in the treatment of this disease. A new series of aryl-oxadiazole Schiff bases (1–18) were synthesized and evaluated for α-glucosidase inhibitory potential. Fifteen compounds 1–8, 11–13, and 15–18 showed excellent inhibition with IC50 values ranging from 0.30 ± 0.2 to 35.1 ± 0.80 µM as compared to the standard inhibitor acarbose (IC50 = 38.45 ± 0.80 µM), nonetheless, the remaining compounds were found to have moderate activity. Among the series, compounds 7 (IC50 = 0.30 ± 0.2 μM) with hydroxy groups at phenyl rings on either side of the oxadiazole ring was identified as the most potent inhibitor of α-glucosidase. The molecular docking studies were conducted to understand the binding mode of active inhibitors with the active site of enzyme and results supported the experimental data.
Keywords
Synthesis
Aryl-oxadiazole Schiff bases
α-glucosidase
Molecular docking
Structure-activity relationship (SAR)
1 Introduction
Diabetes mellitus is a life threatening and chronic metabolic disorder caused by insufficient insulin secretion and characterized by hyperglycemia (Fatmawati et al., 2011). The enhanced level of post-prandial glucose is associated with type II diabetes mellitus and leads to the increased risk of developing atherosclerosis, stroke, and other coronary diseases (Rother, 2007). Thus reducing the post-prandial hyperglycemia by inhibiting the digestive enzymes such as α-glucosidase is an effective approach for the treatment of type II diabetes mellitus and other diabetic complications too (Casirola and Ferraris, 2006). α-Glucosidase is an enzyme located in the epithelium of small intestine and catalyzes the final step involved in the hydrolysis of disaccharides and polysaccharides into glucose. The activity of α-glucosidase is directly related to the concentrations of blood glucose, and inhibition of α-glucosidase is crucial due to the potential effects of decreased postprandial blood glucose levels (Chiasson et al., 2003). α-Glucosidase inhibitors such as voglibose and acarbose are clinically used for controlling the rapid increase of blood glucose. However, they often leads to some side effects including diarrhea, abdominal pain, and other gastrointestinal disorders in chronic therapy (Kawamori et al., 2009). Therefore, the search for efficient and safe α-glucosidase inhibitors are necessary for the therapy of post-prandial hyperglycemia (see Table 1). SEMa is the standard error of the mean, NAb Not active, Acarbosestd standard inhibitor for α-glucosidase inhibitory activity.
Compounds
Ring A
Ring B
IC50 ± SEMa (µM)
1
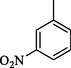
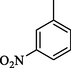
3.30 ± 0.1
2
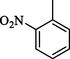

1.20 ± 0.1
3

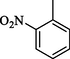
2.30 ± 0.1
4

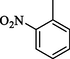
1.10 ± 0.10
5
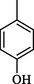

2.70 ± 0.1
6

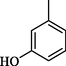
0.6 ± 0.05
7
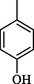

0.30 ± 0.2
8

3.60 ± 0.1
9
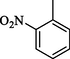
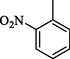
350.50 ± 0.6
10
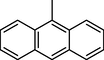

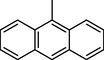
45.80 ± 1.1
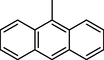
11

35.1 ± 0.80
12

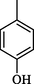
32.06 ± 0.70
13
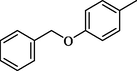
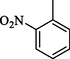
22.40 ± 0.6
14

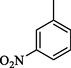
48.50 ± 0.6
15
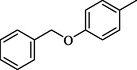
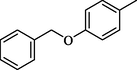
26.50 ± 0.4
16
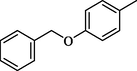
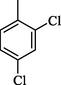
19.10 ± 0.4
17
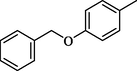
17.80 ± 0.50
18
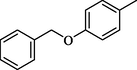
13.50 ± 0.40
Acarbose
38.45 ± 0.80
Among five-membered aromatic heterocycles, 1,3,4-oxadiazoles have attracted considerable attention in recent decades due to their broad spectrum of pharmaceutical and biological activities (Suwinski, 2008). Many compounds containing this scaffold display antimicrobial (Zheng et al., 2018; Salar et al., 2015), antiviral (Du and Luo, 2010), antiinflammatory (Palaska et al., 2002), antihypertensive (Zhu et al., 2016), analgesic (Husain et al., 2009), anticonvulsant (Dogan et al., 2002), antidiabetic (O’Neal et al., 1962); antileismanial (Taha et al., 2017), and antitubercular activities (Pattan et al., 2009). They have also attracted attention in medicinal chemistry as potential therapeutic agents for the treatment of cancer (Holla et al., 2005) and HIV infections (El-Emam et al., 2004). In addition, 1,3,4-oxadiazole derivatives have been utilized as bioisosteric replacements for carboxylic acid (Omar et al., 1996), ester (Orlek et al., 1991), and amide (Leung et al., 2005) functional groups in biologically active compounds.
Our research group had synthesized and reported a number of heterocyclic compounds for their different pharmacological activities (Kazmi et al., 2018; Rahim et al., 2016; Rashid et al., 2016; Rahim et al., 2015; Noreen et al., 2017; Taha et al., 2016; Taha et al., 2017; Taha et al., 2016; Rahim et al., 2015; Rahim et al., 2016; Taha et al., 2017). Oxadiazoles and Schiff bases are among the most diverse heterocycles that showed a range of biological activities including α-glucosidase and both these core structures are already reported by our group as potential α-glucosidase inhibitors (Taha et al., 2017; Rahim et al., 2015; Rahim et al., 2015; Rahim et al., 2015). Therefore, in continuation of our previous research, we have designed and synthesized the molecules (1–18) bearing both functionalities in search of potential α-glucosidase inhibitors (Fig. 1).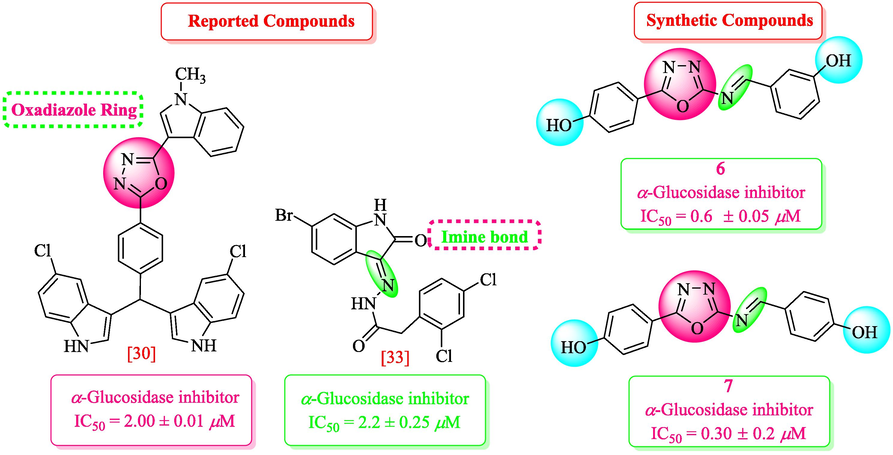
Rationale of current study.
2 Results and discussion
2.1 Chemistry
Compounds 1–18 were synthesized in three steps, first step involves the formation of Schiff base via reaction of aldehyde with semicarbazide in the presence of NaOAc, water and methanol were used as the reaction medium and it was heated at 25 ˚C for 10 min. The Schiff base thus formed undergoes cyclization in the presence of iodine and potassium carbonate using 1,4-dioxane as solvent, the reaction mixture was then refluxed at 80 °C for 4 h to afford aryl-oxadiazoles. The mixture which contain the desired organic compound was cooled and treated with 5% Na2S2O3 followed by extraction using CH2Cl2/MeOH. The pure product which is obtained in the second step was further mixed and refluxed with different substituted benzaldehyde in methanol to give Schiff base of aryl-oxadiazole 1–18 which were crystallized from methanol (Scheme 1). The synthetic derivatives were characterized via different spectroscopic techniques such as 1H-, 13C NMR, and HREIMS.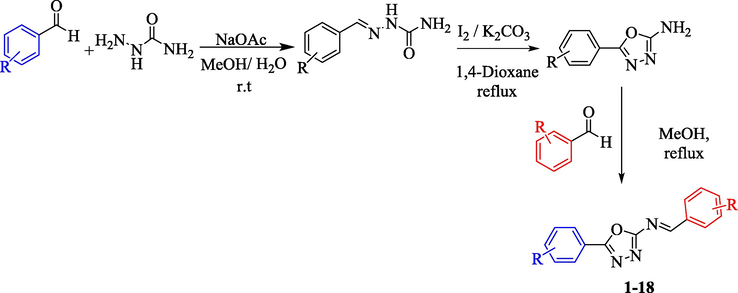
Synthesis of Aryl-oxadiazole bearing schiff bases derivatives (1–18).
2.2 α-Glucosidase inhibitory activity
We have synthesized aryl-oxadiazole bearing Schiff bases (1–18) and evaluated them for α-glucosidase inhibitory potential. All derivatives showed good to moderate inhibitory activities having IC50 values ranging between 0.30 ± 0.2–350.50 ± 0.6 µM as compared to the standard acarbose (IC50 = 38.45 ± 0.80 µM). Fifteen compounds 1–8, 11–13, and 15–18 showed superior inhibition with IC50 values of 3.30 ± 0.1, 1.20 ± 0.1, 2.30 ± 0.1, 1.10 ± 0.10, 2.70 ± 0.1, 0.6 ± 0.05, 0.30 ± 0.2, 3.60 ± 0.1, 35.1 ± 0.80, 32.06 ± 0.70, 22.40 ± 0.6, 26.50 ± 0.4, 19.10 ± 0.4, 17.80 ± 0.50 and 13.50 ± 0.40 µM, respectively, in comparison with the standard drug acarbose. However, three compounds 9, 10, and 14 displayed moderate inhibition with IC50 values of 350.50 ± 0.6, 45.80 ± 1.1, and 48.50 ± 0.6 µM, respectively. To develop the better structure-activity relationship, we have divided the molecule in four parts; ring A, oxadiazole part, imine part, and ring B as shown in Fig. 2. The variations were carried out mainly on ring A and ring B, and different substitutions at variable positions of both rings showed interesting pattern in the activity.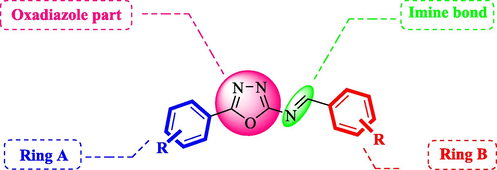
General structure of synthetic compounds (1–18).
2.2.1 Structure-activity relationship
Compound 7 (IC50 = 0.30 ± 0.2 μM) having hydroxyl groups at para position of ring A and ring B, respectively, was the most active compound of series. Its structurally similar compound 6 (IC50 = 0.6 ± 0.05 µM) with hydroxyl group at meta position of ring B displayed two folds less activity as compared to compound 7. However, both compounds exhibited potential inhibition in comparison with the standard acarbose. The activity of both these compounds may be due to the interaction of hydroxyl groups with the active site of enzyme. Compounds 4 and 5 with nitro groups on different positions of ring B showed a sharp decline in activity. Compound 4 (IC50 = 1.10 ± 0.10 μM) having nitro group at ortho position of ring B showed decreased activity, nevertheless, changing the position of nitro group from ortho to meta in compound 5 (IC50 = 2.70 ± 0.1 μM) resulted in further declined activity. It showed that the compounds with nitro group are less active as compared to hydroxyl substituted compounds (Fig. 3).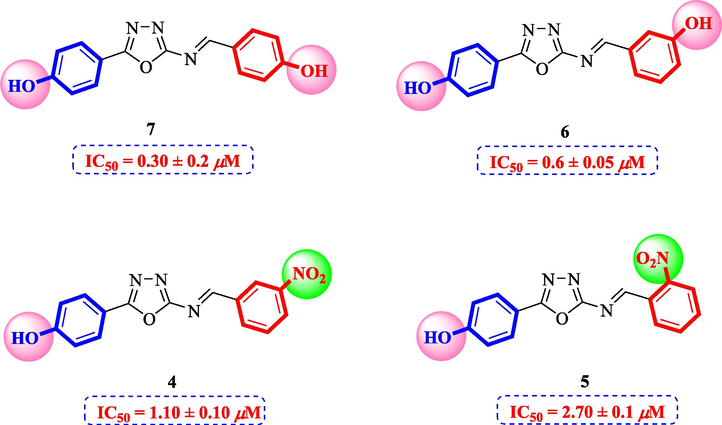
Structure-activity relationship of compounds 4, 5, 6, and 7.
Compounds 1 and 8 bearing nitro groups on different positions of rings A and B, respectively, displayed variable inhibitory potential which showed that the change in positions of these groups provide a different binding site of ligand-enzyme interaction. Compound 1 (IC50 = 3.30 ± 0.10 μM) having nitro group at meta positions of ring A and B, respectively, exhibited good inhibitory activity. Compound 8 (IC50 = 3.60 ± 0.1 μM) having nitro group at para position of ring A displayed comparable activity with compound 1. Nonetheless, compound 2 (IC50 = 1.20 ± 0.1 μM) having nitro at meta position of ring A and chloro group at para position of ring B showed better inhibitory potential as compared to compounds 1 and 8. Therefore, it can be concluded that the addition of chloro groups leads to the increased activity of compounds. Compound 3 (IC50 = 2.30 ± 0.1 µM) having dimethyl amino group at para position of ring A and nitro at ortho position of ring B also showed better inhibitory potential as compared to the standard acarbose. On comparison of compound 3 with 8, it can be said that the replacement of nitro with dimethyl amino group resulted in better activity (Fig. 4).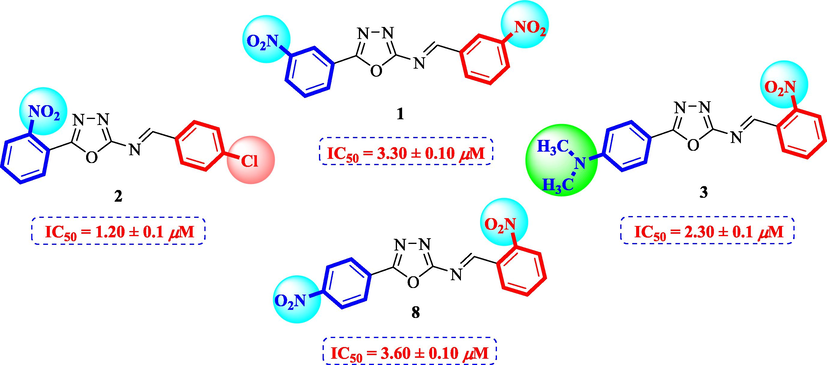
Structure-activity relationship of compounds 1, 2, 3, and 8.
Compounds 13–18 having benzyloxy substituent at para position of ring A exhibited good inhibitory activity as compared to the standard. Among them, compound 18 (IC50 = 13.50 ± 0.40 μM) having hydroxyl group at para position of ring B was most active, however, its structurally similar analog 17 (IC50 = 17.80 ± 0.50 μM) bearing hydroxyl at meta position showed decreased inhibition. The activity was further decreased when hydroxyl groups were replaced by two chloro groups in compound 16 (IC50 = 19.10 ± 0.4 μM). Compound 13 (IC50 = 22.40 ± 0.6 μM) having nitro at ortho position of ring B also showed better activity as compared to the standard acarbose (IC50 = 38.45 ± 0.80 μM), however, changing the position of nitro group from ortho to meta resulted in two folds decreased activity in compound 14 (IC50 = 48.50 ± 0.6 µM). Compound 15 (IC50 = 26.50 ± 0.4 μM) having benzyloxy group on both rings also showed good inhibitory activity (Fig. 5).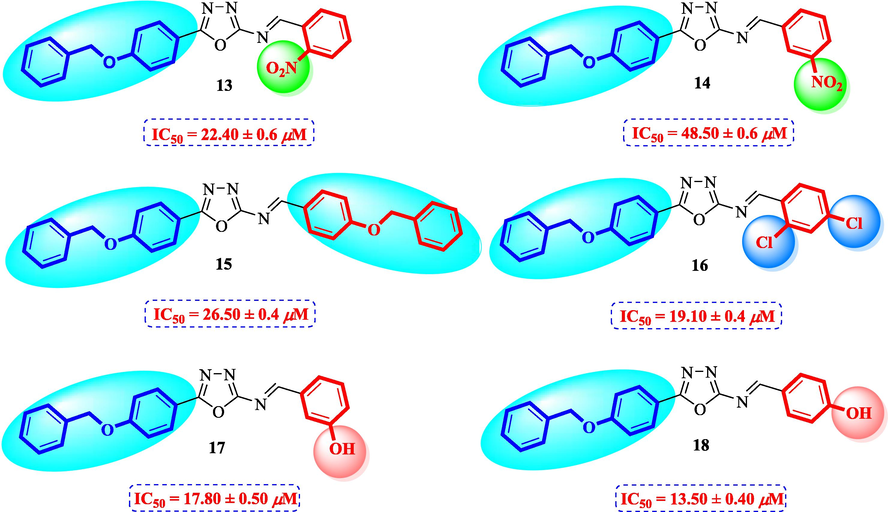
Structure-activity relationship of compounds 13, 14, 15, 16, 17, and 18.
The compounds with anthranyl group attached to oxadiazoles were less active as compared to other synthetic compounds. Among them, compound 12 (IC50 = 32.06 ± 0.70 μM) bearing hydroxyl group at para position of ring B was most active. The activity was slightly decreased when the position of hydroxyl group was shifted from para to meta in compound 11 (IC50 = 35.1 ± 0.80 μM). The activity was further decreased in case of nitro substituted derivatives 9 and 10. Compound 10 (IC50 = 45.80 ± 1.1 μM) having nitro group at meta position was less active as compared to standard. A sharp decline in the activity was observed when the nitro group was shifted to ortho position. This showed that the presence of bulky anthranyl group resulted in decreased activity of compounds and also the addition of nitro groups resulted in further decreased activity (Fig. 6).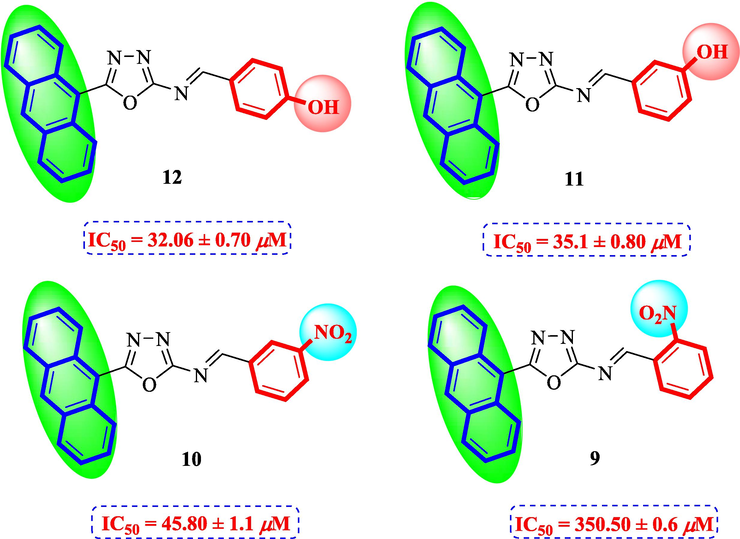
Structure-activity relationship of compounds 9, 10, 11, and 12.
On the basis of afore-mentioned observations it can be summarized that the compounds with hydroxyl groups on both rings exhibited good inhibitory potential as compared to the compounds with nitro and bulky groups like anthranyl and benzyloxy. It was also observed that the positions of certain groups at particular positions also altered the inhibitory activity, the substitutions at ortho and para positions were mainly contributing in the activity. To understand the binding interaction of the most active analogs molecular docking study was performed.
2.2.2 Molecular docking studies
It was observed from the molecular docking study that the top ranked confirmation of all derivatives fit well inside the active site of the homology model of α-glucosidase (Arg212, Asp214, Glu276, Asp349 and Arg439) (Rahim et al., 2015). From the docking confirmation of the derivatives, it was revealed that the most active derivative 7 (IC50 = 0.30 ± 0.2, docking score = −8.7632) formed five hydrogen bonds and one π-H linkage with the Lys155, Val303, Phe311, and Arg439 residues of the binding pocket as shown in Fig. 7a. Lys155 and Arg439 formed π-H and polar interaction with the 1,3,4-oxadiazole moiety of the ligand. Val303 and Phe311 formed polar interactions with the hydroxyl (—OH) moieties of the derivative. The good inhibitory activity of the derivative might be due to the availability of the electron donating groups (—OH) and electronic cloud system of benzene moieties of the compound. The docking conformation of the second most active compound 6 (IC50 = 0.6 ± 0.05) was observed having good interactions as well as good docking score (−8.6902). It was noticed that this compound has shown four hydrogen bonds with active site residues Phe311, Asp349 and Asn412 as shown in Fig. 7b. Phe311 and Asp349 were observed making hydrogen bonds with the —OH moieties of the compound while Asn412 formed two H-bonds with the nitrogen atom (—N) of the oxadiazole moiety of the inhibitor. The presence of the electron donating groups (—OH) and π-π electron system of this compound might be the reason of its high potency. The activity of the derivative 7 is to some extent higher to derivative 6 that may be due to different position of –OH group on benzene ring.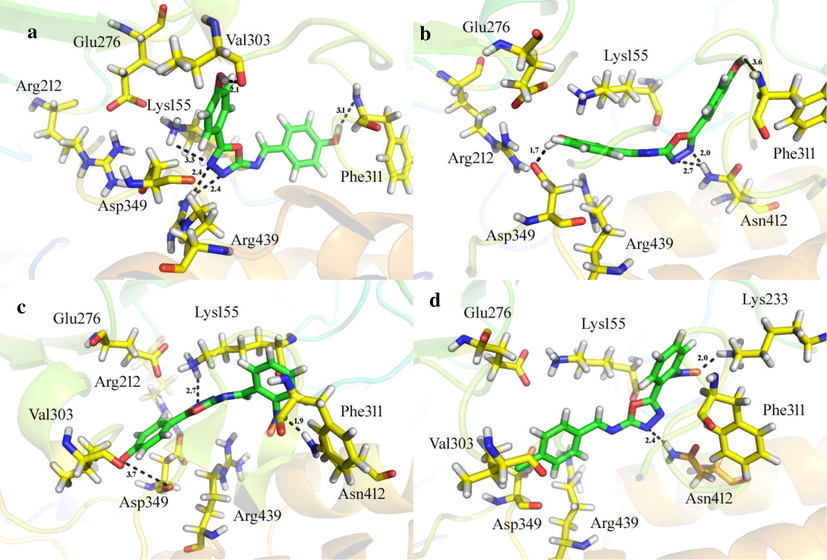
On active site of the α-glucosidase enzyme, the docking confirmations of the active analogs (a) three dimension binding mode of analog 7 (b) three dimension binding mode of analog 6 (c) three dimension binding mode of analog 4 (d) three dimension binding mode of analog 2.
The docking conformation of the third one most active compound 4 (IC50 = 1.10 ± 0.10, docking score = −8.3487) was observed that this compound formed three polar interactions with the Lys155, Phe311 and Asp349 residues of the target enzyme Fig. 7c. Lys155 formed H-bond with the nitrogen atom of the oxadiazole moiety while Phe311 and Asp349 made H-bonds with nitro (—NO2) and –OH moieties of the derivative, respectively. The potency of the derivative might be due to electron donating group (—OH) and electron withdrawing group (—NO2).
The docking conformation of the compound 2 (IC50 = 1.20 ± 0.1, docking score = −8.1076) also showed good interactions with the active site residues of the target enzyme, however, slightly inferior inhibitory potential as compared to compound 4 may be due to halogen groups, hydroxyl (—OH) group is electron donating while —Cl group is electron withdrawing. Compound 2 formed two H-acceptor and one π-H interactions with the Lys233, Asn412 and Asp349 residues of the enzyme as shown in Fig. 7d, Table 2.
Compounds
Docking score
Interactions Report
1
−6.6230
Ligand
Receptor
Interaction Distance E (kcal/mol)
O22
31
ND2
ASN
153
H-acceptor
3.01
−0.8
O24
33
NZ
LYS
155
H-acceptor
3.34
−1.2
2
−8.1076
N2
2
ND2
ASN
412
H-acceptor
2.41
−2.8
O23
32
NZ
LYS
233
H-acceptor
2.00
−4.1
6-ring
CB
ASP
349
π-H
4.72
−0.7
3
−7.0524
O22
31
NZ
LYS
155
H-acceptor
2.77
−0.9
6-ring
CA
PHE
311
π -H
4.35
−0.4
6-ring
6-ring
PHE
300
π-π
3.63
−0.0
4
−8.3487
O20
29
OD1
ASP
349
H-donor
3.74
−3.8
N2
2
ND2
LYS
155
H-acceptor
2.70
−4.9
6-ring
CA
PHE
311
π-H
1.92
−0.4
5
−6.6586
5-ring
CA
LYS
155
pi-H
4.05
−1.5
6
−8.6902
O20
29
NH
PHE
311
H-donor
2.70
−2.1
O21
31
OD2
ASP
349
H-donor
2.63
−1.3
N3
3
ND2
ASN
412
H-acceptor
2.71
−4.7
N2
2
ND2
ASN
412
H-acceptor
2.00
−4.9
7
−8.7632
O20
29
O
VAL
303
H-donor
2.12
−1.0
O21
31
O
PHE
311
H-donor
3.12
−2.6
N2
2
NH1
ARG
439
H-acceptor
2.42
−3.4
N3
3
NH1
ARG
439
H-acceptor
2.42
−0.8
5-ring
CE
LYS
155
π-H
3.31
−2.0
8
−6.0100
O21
30
N
GLN
238
H-acceptor
3.08
−2.0
6-ring
CA
LYS
155
π-H
4.27
−1.0
5-ring
CE
LYS
155
π-H
4.07
−1.4
9
−2.7615
5-ring
CE
LYS
155
π-H
3.47
−1.3
10
−4.1123
O30
44
NZ
LYS
155
H-acceptor
3.33
−1.2
11
−4.2398
6-ring
CE
LYS
155
π-H
3.38
−0.5
6-ring
N
ARG
312
π-H
3.69
−0.6
12
−4.8103
O28
42
O
ASN
153
H-donor
2.86
−2.9
N2
2
CA
PHE
311
H-acceptor
3.51
−0.7
N3
3
N
ARG
312
H-acceptor
3.10
−3.5
13
−5.3956
5-ring
CA
PHE
311
π-H
4.20
−1.1
14
−4.0081
N3
3
CA
PRO
309
H-acceptor
2.41
23.6
O29
45
CB
ASN
153
H-acceptor
2.30
36.7
O29
45
ND2
ASN
153
H-acceptor
2.64
−0.7
6-ring
CD
ARG
439
π-H
4.33
−1.1
15
−5.0782
6-ring
N
ARG
312
π-H
3.81
−0.7
16
−5.4913
C16
22
O
ASP
349
H-donor
2.46
2.7
6-ring
CA
LYS
155
π-H
4.04
−0.3
17
−5.9653
6-ring
CD
ARG
439
π-H
4.33
−1.0
18
−5.9891
C14
18
O
ASP
349
H-donor
3.30
−0.6
6-ring
CA
LYS
155
π-H
3.88
−0.3
6-ring
CD2
PHE
300
π-H
3.41
−0.4
6-ring
CD
ARG
439
π-H
4.47
−0.8
Standard Acarbose
−5.8934
O
29
ND2
ASN
412
H-acceptor
2.94
−2.6
O
67
NZ
LYS
155
H-acceptor
2.94
−1.7
N
58
OE1
GLU
276
ionic
2.71
−6.7
O
67
O
ASP
349
H-donor
2.76
−2.9
O
84
NH1
ARG
439
H-acceptor
2.75
0.4
3 Conclusion
Eighteen derivatives of aryl-oxadiazole bearing Schiff bases (1–18) were synthesized and evaluated for α-glucosidase inhibitory potential. All synthetic derivatives showed good inhibitory activity. Compounds 6 and 7 were found to have hundred folds superior inhibitory activity as compared to the standard acarbose. SAR study revealed that the compounds with hydroxyl groups particularly at ortho and para positions were more active as compared to the compounds with nitro and Cl group. Molecular docking studies confirmed the binding sites and interactions of the synthetic ligands with the enzyme. So, it can be concluded that further structural modification of these active analogs may help to find a prospective anti-diabetic lead molecule.
4 Materials and methods
1H- and 13C NMR spectra were recorded on Bruker 500 MHz spectrometers. Mass experiments were carried out on a Finnigan MAT-311A (Germany) mass spectrometer. Thin-layer chromatography (TLC) was monitored on pre-coated silica gel aluminum plates (Kieselgel 60, 254, E. Merck, Germany). Visualization of TLC chromatograms was performed at wavelengths of 254 and 365 nm. All reagents of analytical grades were purchased from Merck, Germany.
4.1 General method for the synthesis of oxadiazole derivatives (1–18)
To a stirred solution of aldehyde (1 mmol) in methanol (10 mL), semicarbazide (1 mmol) in distilled H2O (10 mL) was added and refluxed in the presence of NaOAc (2 mmol) at 25 °C for 10 min. Progress of the reaction was monitored by TLC, upon completion, the solvent was evaporated under reduced pressure on a rotary evaporator. The residue obtained was further refluxed with iodine and potassium carbonate in 1,4-dioxane at 80 °C for 4 h. The reaction was continued until the starting material was completely consumed. On disappearance of starting material, the reaction mixture was cooled to room temperature and then it was reacted with 5% Na2S2O3 followed by extraction with CH2Cl2/MeOH (9:1). The organic layer was then dried and washed with ether and ethyl acetate as eluent to remove impurities. The pure products thus obtained in the second step was further mixed and refluxed with different substituted benzaldehydes (1 mmol) in methanol (10 mL) to give desired products (1–18). The synthetic derivatives were crystallized from methanol and characterized through different spectroscopic techniques such as 1H-, 13C NMR, and HREI-MS.
4.2 (E)-1-(3-Nitrophenyl)-N-(5-(3-nitrophenyl)-1,3,4-oxadiazol-2-yl)methanimine (1)
Yield: 82%, 1H NMR (500 MHz, DMSO‑d6) δ 8.97 (s, 1H, —CH⚌N), 8.58 (d, J = 1.4 Hz, 1H, H-2), 8.48 (d, J = 1.6 Hz, 1H, H-2′), 8.42(dd, J = 8.1, 1.2 Hz, 1H, H-6), 8.30 (d, J = 8.5 Hz, 1H, H-4), 8.23(dd, J = 8.3, 1.1 Hz, 1H, H-6′), 8.07 (d, J = 8.4 Hz, 1H, H-4′), 7.82 (dd, J = 8.4, 8.1 Hz, 1H, H-5), 7.61 (dd, J = 8.2, 8.4 Hz, 1H, H-5′), 13C NMR (125 MHz, DMSO‑d6): δ 164.0, 164.0, 160.2, 148.3, 148.1, 137.0, 135.1, 133.4, 130.0, 129.6, 127.1, 126.0, 125.7, 123.7, 122.6, HREI-MS m/z : Calcd for C15H9N5O5, 339.0604, Found: 339.0600.
4.3 (E)-1-(4-Chlorophenyl)-N-(5-(2-nitrophenyl)-1,3,4-oxadiazol-2-yl)methanimine (2)
Yield: 78%, 1H NMR (500 MHz, DMSO‑d6) δ 9.02 (s, 1H, —CH⚌N), 8.01 (d, J = 8.3 Hz, 1H, H-6), 7.98 (dd, J = 8.4, 1.4 Hz, 1H, H-3), 7.94 (dd, J = 8.0, 1.4 Hz, 2H, H-2′/6′), 7.87 (m, 1H, H-5), 7.70 (m, 1H, H-4), 7.57 (dd, J = 8.2, 1.5 Hz, 2H, H-3′/5′), 13C NMR (125 MHz, DMSO‑d6): δ 164.0, 164.0, 160.2, 146.7, 136.5, 135.1, 134.4, 131.4, 130.3, 130.3, 129.5, 128.7, 128.7, 128.2, 124.2, HREI-MS m/z : Calcd for C15H9ClN4O3, 328.0363, Found: 328.0360.
4.4 (E)-N,N-Dimethyl-4-(5-((2-nitrobenzylidene)amino)-1,3,4-oxadiazol-2-yl)aniline (3)
Yield: 73%, 1H NMR (500 MHz, DMSO‑d6) δ 9.04 (s, 1H, —CH⚌N), 8.04 (d, J = 8.5 Hz, 1H, H-6′), 7.96 (dd, J = 8.1, 1.3 Hz, 1H, H-3′), 7.70 (m, 1H, H-5′), 7.62 (m, 1H, H-4′), 7.57 (dd, J = 8.0, 1.4 Hz, 2H, H-2/6), 6.90 (dd, J = 8.2, 1.6 Hz, 2H, H-3/5), 3.00 (s, 6H, —CH3), 13C NMR (125 MHz, DMSO‑d6): δ 164.0, 164.0, 160.2, 155.1, 147.6, 134.7, 131.8, 130.0, 128.3, 128.3, 128.3, 124.1, 115.4, 112.5, 112.5, 41.1, 41.1, HREI-MS m/z : Calcd for C17H15N5O3, 337.1175, Found: 337.1170.
4.5 (E)-4-(5-((2-Nitrobenzylidene)amino)-1,3,4-oxadiazol-2-yl)phenol (4)
Yield: 80%, 1H NMR (500 MHz, DMSO‑d6) δ 9.69 (s, 1H, OH), 9.06 (s, 1H, —CH⚌N), 8.08 (d, J = 8.6 Hz, 1H, H-6′), 8.00 (dd, J = 8.3, 1.7 Hz, 1H, H-3′), 7.74 (m, 1H, H-5′), 7.64 (m, 1H, H-4′), 7.87 (dd, J = 8.2, 1.1 Hz, 2H, H-2/6), 6.89 (dd, J = 8.4, 1.3 Hz, 2H, H-3/5), 13C NMR (125 MHz, DMSO‑d6): δ 164.0, 164.0, 160.2, 158.3, 147.5, 134.7, 131.7, 130.0, 128.3, 124.1, 118.6, 116.2, 116.2, 116.1, 116.1, HREI-MS m/z : Calcd for C15H10N4O4, 310.0702, Found: 310.0700.
4.6 (E)-4-(5-((3-Nitrobenzylidene)amino)-1,3,4-oxadiazol-2-yl)phenol (5)
Yield: 84%, 1H NMR (500 MHz, DMSO‑d6) δ 9.72 (s, 1H, OH), 9.10 (s, 1H, —CH⚌N), 8.53 (d, J = 1.6 Hz, 1H, H-2′), 8.28 (dd, J = 8.5, 1.4 Hz, 1H, H-6′), 8.12 (d, J = 8.6 Hz, 1H, H-4′), 7.88 (dd, J = 8.4, 1.4 Hz, 2H, H-2/6), 7.66 (dd, J = 8.3, 8.1 Hz, 1H, H-5′), 6.94 (dd, J = 8.2, 1.4 Hz, 2H, H-3/5), 13C NMR (125 MHz, DMSO‑d6): δ 164.0, 164.0, 160.2, 158.3, 148.1, 137.0, 135.1, 129.5, 126.0, 125.7, 118.6, 116.2, 116.2, 116.1, 116.1, HREI-MS m/z : Calcd for C15H10N4O4, 310.0702, Found: 310.0700.
4.7 (E)-3-(((5-(4-Hydroxyphenyl)-1,3,4-oxadiazol-2-yl)imino)methyl)phenol (6)
Yield: 86%, 1H NMR (500 MHz, DMSO‑d6) δ 9.74 (s, 1H, OH), 9.47 (s, 1H, OH), 9.12 (s, 1H, —CH⚌N), 7.87 (dd, J = 8.1, 1.2 Hz, 2H, H-2/6), 7.35 (dd, J = 8.3, 1.6 Hz, 1H, H-6′), 7.27 (d, J = 1.5 Hz, 1H, H-2′), 7.16 (dd, J = 8.0, 8.3 Hz, 1H, H-5′), 6.97 (d, J = 8.2 Hz, 1H, H-4′), 6.95 (dd, J = 8.5, 1.7 Hz, 2H, H-3/5), 13C NMR (125 MHz, DMSO‑d6): δ 164.0, 164.0, 160.2, 158.4, 158.3, 138.5, 130.0, 121.7, 118.5, 118.0, 116.2, 116.2, 116.1, 116.1, 114.7, HREI-MS m/z : Calcd for C15H11N3O3, 281.0800, Found: 281.0798.
4.8 (E)-4-(5-((4-Hydroxybenzylidene)amino)-1,3,4-oxadiazol-2-yl)phenol (7)
Yield: 77%, 1H NMR (500 MHz, DMSO‑d6) δ 9.77 (s, 2H, OH), 9.15 (s, 1H, —CH⚌N), 7.89 (dd, J = 8.4, 1.5 Hz, 2H, H-2/6), 7.70 (dd, J = 8.2, 1.3 Hz, 2H, H-2′/6′), 6.96 (dd, J = 8.1, 1.6 Hz, 2H, H-3/5), 6.88 (dd, J = 8.2, 1.3 Hz, 2H, H-3′/5′), 13C NMR (125 MHz, DMSO‑d6): δ 164.0, 164.0, 160.7, 160.2, 158.3, 130.5, 130.5, 129.1, 118.5, 116.2, 116.2, 116.1, 116.1, 116.1, 116.1, HREI-MS m/z : Calcd for C15H11N3O3, 281.0800, Found: 281.0798.
4.9 (E)-1-(2-Nitrophenyl)-N-(5-(4-nitrophenyl)-1,3,4-oxadiazol-2-yl)methanimine (8)
Yield: 79%, 1H NMR (500 MHz, DMSO‑d6) δ 9.04 (s, 1H, —CH⚌N), 8.45 (dd, J = 8.7, 1.6 Hz, 2H, H-3/5), 8.27 (dd, J = 8.4, 1.7 Hz, 2H, H-2/6), 8.10 (d, J = 8.5 Hz, 1H, H-6′), 7.95 (d, J = 8.4 Hz, 1H, H-3′), 7.67 (m, 1H, H-5′), 7.55 (m, 1H, H-4′), 13C NMR (125 MHz, DMSO‑d6): δ 164.0, 164.0, 160.2, 147.7, 147.6, 134.7, 132.0, 131.7, 130.7, 130.7, 130.0, 128.6, 128.6, 128.2, 124.2, HREI-MS m/z : Calcd for C15H9N5O5, 339.0604, Found: 339.0600.
4.10 (E)-N-(5-(Anthracen-9-yl)-1,3,4-oxadiazol-2-yl)-1-(2-nitrophenyl)methanimine (9)
Yield: 83%, 1H NMR (500 MHz, DMSO‑d6) δ 9.11 (s, 1H, —CH⚌N), 8.51 (s, 1H, H-6), 8.17 (d, J = 8.6 Hz, 2H, H-2/10), 8.03 (d, J = 8.7 Hz, 1H, H-6′), 7.99 (d, J = 8.2 Hz, 2H, H-5/7), 7.92 (d, J = 8.6 Hz, 1H, H-3′), 7.64 (m, 1H, H-5′), 7.52 (m, 1H, H-4′), 7.46 (m, 2H, H-4/8), 7.41 (m, 2H, H-3/9), 13C NMR (125 MHz, DMSO‑d6): δ 164.0, 164.0, 160.2, 147.6, 134.7, 134.0, 132.0, 132.0, 131.7, 130.2, 130.2, 130.0, 129.7, 128.3, 128.0, 128.0, 125.5, 125.5, 125.5, 125.5, 124.2, 124.2, 124.2, HREI-MS m/z : Calcd for C23H14N4O3, 394.1066, Found: 394.1061.
4.11 (E)-N-(5-(Anthracen-9-yl)-1,3,4-oxadiazol-2-yl)-1-(3-nitrophenyl)methanimine (10)
Yield: 81%, 1H NMR (500 MHz, DMSO‑d6) δ 8.94 (s, 1H, —CH⚌N), 8.61 (s, 1H, H-6), 8.53 (d, J = 1.6 Hz, 1H, H-2′), 8.31 (d, J = 8.5 Hz, 1H, H-6′), 8.24 (dd, J = 8.5, 1.4 Hz, 2H, H-2/10), 8.13 (d, J = 8.0 Hz, 1H, H-4′), 8.05(d, J = 8.4 Hz, 2H, H-5/7), 7.60 (dd, J = 8.7, 1.6 Hz, 1H, H-5′),7.52 (m, 2H, H-4/8), 7.40 (m, 2H, H-3/9), 13C NMR (125 MHz, DMSO‑d6): δ 164.0, 164.0, 160.2, 148.2, 137.0, 135.1, 134.0, 132.0, 132.0, 130.2, 130.2, 129.7, 129.5, 128.0, 128.0, 126.0, 125.7, 125.4, 125.4, 125.4, 125.4, 124.2, 124.2, HREI-MS m/z : Calcd for C23H14N4O3, 394.1066, Found: 394.1061.
4.12 (E)-3-(((5-(Anthracen-9-yl)-1,3,4-oxadiazol-2-yl)imino)methyl)phenol (11)
Yield: 74%, 1H NMR (500 MHz, DMSO‑d6) δ 9.50 (s, 1H, OH), 8.91 (s, 1H, —CH⚌N), 8.63 (s, 1H, H-6), 8.26 (dd, J = 8.3, 1.6 Hz, 2H, H-2/10), 8.07 (d, J = 8.6 Hz, 2H, H-5/7), 7.57 (m, 2H, H-4/8), 7.38 (m, 2H, H-3/9), 7.28 (dd, J = 8.3, 1.3 Hz, 1H, H-6′), 7.21 (dd, J = 1.2 Hz, 1H, H-2′), 7.10 (dd, J = 8.5, 8.3 Hz, 1H, H-5′), 6.90 (d, J = 8.0 Hz, 1H, H-4′), 13C NMR (125 MHz, DMSO‑d6): δ 164.0, 164.0, 160.2, 158.5, 138.6, 134.0, 132.0, 132.0, 130.3, 130.3, 130.0, 129.7, 128.0, 128.0, 125.4, 125.4, 125.4, 125.4, 124.2, 124.2, 121.7, 118.0, 114.7, HREI-MS m/z : Calcd for C23H15N3O2, 365.1164, Found: 365.1160.
4.13 (E)-4-(((5-(Anthracen-9-yl)-1,3,4-oxadiazol-2-yl)imino)methyl)phenol (12)
Yield: 76%, 1H NMR (500 MHz, DMSO‑d6) δ 9.77 (s, 1H, OH), 8.90 (s, 1H, —CH⚌N), 8.69 (s, 1H, H-6), 8.30 (dd, J = 8.6, 1.2 Hz, 2H, H-2/10), 8.11 (d, J = 8.3 Hz, 2H, H-5/7), 7.61 (dd, J = 8.5, 1.7 Hz, 2H, H-2′/6′), 7.62 (m, 2H, H-4/8), 7.35 (m, 2H, H-3/9), 6.82 (dd, J = 8.0, 1.2 Hz, 2H, H-3′/5′), 13C NMR (125 MHz, DMSO‑d6): δ 164.0, 164.0, 160.6, 160.2, 134.0, 132.0, 132.0, 130.4, 130.4, 130.2, 130.2, 129.6, 129.2, 128.0, 128.0, 125.4, 125.4, 125.4, 125.4, 124.2, 124.2, 116.1, 116.1, HREI-MS m/z : Calcd for C23H15N3O2, 365.1164, Found: 365.1160.
4.14 (E)-N-(5-(4-(Benzyloxy)phenyl)-1,3,4-oxadiazol-2-yl)-1-(2-nitrophenyl)methanimine (13)
Yield: 85%, 1H NMR (500 MHz, DMSO‑d6) δ 9.09 (s, 1H, —CH⚌N), 8.09 (d, J = 8.4 Hz, 1H, H-6′), 8.04 (dd, J = 8.7, 1.6 Hz, 2H, H-2/6), 7.87 (d, J = 8.3 Hz, 1H, H-3′), 7.60 (m, 1H, H-5′), 7.51 (m, 1H, H-4′), 7.45 (dd, J = 8.2, 1.4 Hz, 2H, H-2″/6″), 7.37 (dd, J = 8.5, 1.7 Hz, 2H, H-3″/5″), 7.29 (m, 1H, H-4″), 7.00 (dd, J = 8.7, 1.2 Hz, 2H, H-3/5), 5.13 (s, 2H, —CH2), 13C NMR (125 MHz, DMSO‑d6): δ 164.0, 164.0, 160.2, 159.2, 147.7, 136.5, 134.7, 131.7, 130.0, 128.8, 128.8, 128.3, 127.4, 127.0, 127.0, 124.2, 118.3, 115.7, 115.7, 114.6, 114.6, 70.6, HREI-MS m/z : Calcd for C22H16N4O4, 400.1172, Found: 400.1168.
4.15 (E)-N-(5-(4-(Benzyloxy)phenyl)-1,3,4-oxadiazol-2-yl)-1-(3-nitrophenyl)methanimine (14)
Yield: 87%, 1H NMR (500 MHz, DMSO‑d6) δ 8.84 (s, 1H, —CH⚌N), 8.65 (d, J = 1.8 Hz, 1H, H-2′), 8.35 (dd, J = 8.5, 1.6 Hz, 1H, H-6′), 8.15 (d, J = 8.1 Hz, 1H, H-4′), 8.07 (dd, J = 8.0, 1.5 Hz, 2H, H-2/6), 7.71 (dd, J = 8.3, 8.4 Hz, 1H, H-5′), 7.50 (dd, J = 8.2, 1.4 Hz, 2H, H-2″/6″), 7.33 (dd, J = 8.5, 1.1 Hz, 2H, H-3″/5″), 7.22 (m, 1H, H-4″), 6.98 (dd, J = 8.4, 1.4 Hz, 2H, H-3/5), 5.11 (s, 2H, —CH2), 13C NMR (125 MHz, DMSO‑d6): δ 164.0, 164.0, 160.2, 159.2, 148.2, 137.0, 136.5, 135.1, 129.5, 128.7, 128.7, 127.5, 127.0, 127.0, 126.0, 125.7, 118.3, 115.7, 115.7, 114.6, 114.6, 70.6, HREI-MS m/z : Calcd for C22H16N4O4, 400.1172, Found: 400.1168.
4.16 (E)-1-(4-(Benzyloxy)phenyl)-N-(5-(4-(benzyloxy)phenyl)-1,3,4-oxadiazol-2-yl)methanimine (15)
Yield: 81%, 1H NMR (500 MHz, DMSO‑d6) δ 8.86 (s, 1H, —CH⚌N), 8.00 (dd, J = 8.3, 1.6 Hz, 2H, H-2/6), 7.97 (dd, J = 8.5, 1.6 Hz, 2H, H-2″/6″), 7.43 (dd, J = 8.2, 1.4 Hz, 4H, H-2″/6″/2‴/6‴), 7.32 (dd, J = 8.6, 1.4 Hz, 4H, H-3″/5″/3‴/5‴), 7.27 (m, 2H, H-4″/4‴), 7.10 (dd, J = 8.7, 1.5 Hz, 2H, H-3″/5‴), 6.96 (dd, J = 8.2, 1.2 Hz, 2H, H-3/5), 5.18 (s, 4H, —CH2), 13C NMR (125 MHz, DMSO‑d6): δ 164.0, 164.0, 161.1, 160.2, 159.2, 136.5, 136.5, 130.0, 130.0, 128.7, 128.7, 128.7, 128.7, 128.5, 127.4, 127.4, 127.0, 127.0, 127.0, 127.0, 118.3, 115.7, 115.7, 114.6, 114.6, 114.2, 114.2, 70.6, 70.6, HREI-MS m/z : Calcd for C29H23N3O3, 461.1739, Found: 461.1736.
4.17 (E)-N-(5-(4-(Benzyloxy)phenyl)-1,3,4-oxadiazol-2-yl)-1-(2,4-dichlorophenyl)methanimine (16)
Yield: 72%, 1H NMR (500 MHz, DMSO‑d6) δ 8.96 (s, 1H, —CH⚌N), 8.06 (dd, J = 8.6, 1.3 Hz, 2H, H-2/6), 8.01 (d, J = 8.8 Hz, 1H, H-6′), 7.68 (s, 1H, H-3′), 7.45 (d, J = 8.5 Hz, 1H, H-5′), 7.41 (dd, J = 8.3, 1.7 Hz, 2H, H-2″/6″), 7.36 (dd, J = 8.2, 1.3 Hz, 2H, H-3″/5″), 7.25 (m, 1H, H-4″), 7.07 (dd, J = 8.4, 1.4 Hz, 2H, H-3/5), 5.20 (s, 2H, —CH2), 13C NMR (125 MHz, DMSO‑d6): δ 164.0, 164.0, 160.2, 159.2, 136.5, 131.4, 131.1, 129.2, 129.1, 128.7, 128.7, 128.1, 127.4, 127.0, 127.0, 127.0, 118.3, 115.7, 115.7, 114.6, 114.6, 70.6, HREI-MS m/z : Calcd for C22H15Cl2N3O2, 423.0541, Found: 423.0538.
4.18 (E)-3-(((5-(4-(Benzyloxy)phenyl)-1,3,4-oxadiazol-2-yl)imino)methyl)phenol (17)
Yield: 78%, 1H NMR (500 MHz, DMSO‑d6) δ 9.51 (s, 1H, OH), 8.92 (s, 1H, —CH⚌N), 8.10 (dd, J = 8.4, 1.5 Hz, 2H, H-2/6), 7.66 (dd, J = 8.5, 1.6 Hz, 2H, H-2″/6″), 7.31 (dd, J = 8.4, 1.4 Hz, 2H, H-3″/5″), 7.23 (m, 1H, H-4″), 7.19 (dd, J = 8.1, 1.2 Hz, 1H, H-6′), 7.15 (d, J = 1.1 Hz, 1H, H-2′), 7.10 (dd, J = 8.6, 8.4 Hz, 1H, H-5′), 7.09 (dd, J = 8.1, 1.5 Hz, 2H, H-3/5), 6.95 (d, J = 8.3 Hz, 1H, H-4′), 5.22 (s, 2H, —CH2), 13C NMR (125 MHz, DMSO‑d6): δ 164.0, 164.0, 160.2, 159.2, 158.4, 138.5, 136.5, 130.0, 128.7, 128.7, 127.4, 127.0, 127.0, 121.7, 118.3, 118.0, 115.7, 115.7, 114.7, 114.6, 114.6, 70.6, HREI-MS m/z : Calcd for C22H17N3O3, 371.1270, Found: 371.1265.
4.19 (E)-4-(((5-(4-(Benzyloxy)phenyl)-1,3,4-oxadiazol-2-yl)imino)methyl)phenol (18)
Yield: 81%, 1H NMR (500 MHz, DMSO‑d6) δ 9.80 (s, 1H, OH), 8.90 (s, 1H, —CH⚌N), 8.00 (dd, J = 8.6, 1.3 Hz, 2H, H-2/6), 7.54 (dd, J = 8.2, 1.5 Hz, 2H, H-2′/6′), 7.38 (dd, J = 8.2, 1.5 Hz, 2H, H-2″/6″), 7.33 (dd, J = 8.7, 1.6 Hz, 2H, H-3″/5″), 7.21 (m, 1H, H-4″), 7.11 (dd, J = 8.3, 1.2 Hz, 2H, H-3/5), 6.80 (dd, J = 8.0, 1.1 Hz, 2H, H-3′/5′), 5.19 (s, 2H, —CH2), 13C NMR (125 MHz, DMSO‑d6): δ 164.0, 164.0, 160.6, 160.2, 159.2, 136.5, 130.4, 130.4, 129.2, 128.7, 128.7, 127.4, 127.0, 127.0, 118.3, 116.2, 116.2, 115.7, 115.7, 114.6, 114.6, 70.6, HREI-MS m/z : Calcd for C22H17N3O3, 371.1270, Found: 371.1265.
5 Biological assays
5.1 α-Glucosidase inhibitory assay
In 96 well plate, 135 µL of 50 mM phosphate saline buffer pH (6.8) and 20 µL of the test sample with 70% DMSO were added. The plate was incubated for 15 min after the addition of 20 µL of enzyme to each well. SpectraMax Microplate reader was used to pre-read the plate after incubation. Subsequently 25 µL of the substrate (pNPG) was added and samples were read using SpectraMax Microplate reader at 400 nm for 30 min. The normal reading was taken and the percent inhibition was calculated (Taha et al., 2017).
5.2 Molecular docking assay
The interactions of inhibitor molecule with protein target are easily explored through molecular docking study (Rahim et al., 2015). MOE-Dock program was used to carry out molecular docking, to guess the binding interactions of these molecules in the active sites of α-glucosidase enzyme. We used homology model as defined by Rahim et al. (2015).
Acknowledgements
Authors would like to acknowledge Higher Education Commission of Pakistan for providing a research grant under National Research Program for Universities under Project No. 5721.
References
- α-Glucosidase inhibitors prevents diet-induced increases in intestinal sugar transport in diabetic mice. Metabolism. 2006;55:832-841.
- [Google Scholar]
- Acarbose treatment and the risk of cardiovascular disease and hypertension in patients with impaired glucose tolerance. J. Am. Med. Assoc.. 2003;290:486-494.
- [Google Scholar]
- Synthesis of new 2,5-Disubstituted-1,3,4-thiadiazoles and preliminary evaluation of anticonvulsant and antimicrobial activities. Bioorg. Med. Chem.. 2002;10:2893-2898.
- [Google Scholar]
- Du, Li-H., Luo, Xi-P., 2010. Efficient one-pot synthesis of benzimidazoles under solvent-free conditions. Syn. Comm. 1, 2880–2886.
- Synthesis, antimicrobial, and anti-HIV-1 activity of certain 5-(1-adamantyl)-2-substituted thio-1,3,4-oxadiazoles and 5-(1-adamantyl)-3-substituted aminomethyl-1,3,4-oxadiazoline-2-thiones. Bioorg. Med. Chem.. 2004;12:5107-5113.
- [Google Scholar]
- A potent α-glucosidase inhibitor isolated from the fruiting body of Ganoderma lucidum. Phytomedicine. 2011;18:1053-1055.
- [Google Scholar]
- Synthesis and anticancer activity studies on some 2-choloro-1,4-bis-(5-substituted-1,3,4-oxadiazole-2-ylmethyleneoxy)phenylene derivatives. Ind. J. Chem.. 2005;44:1669-1673.
- [Google Scholar]
- Fenbufen based 3-[5-(substituted aryl)-1,3,4-oxadiazol-2-yl]-1-(biphenyl-4-yl)propan-1-ones as safer antiinflammatory and analgesic agents. Eur. J. Med. Chem.. 2009;44:3798-3804.
- [Google Scholar]
- Voglibose for prevention of type 2 diabetes mellitus: a randomised, double-blind trial in Japanese individuals with impaired glucose tolerance. Lancet. 2009;373:1607-1614.
- [Google Scholar]
- A new entry into the portfolio of a-glucosidase inhibitors as potent therapeutics for type 2 diabetes: Design, bioevaluation and one-pot multi-component synthesis of diamine-bridged coumarinyl oxadiazole conjugates. Bioorg. Chem.. 2018;77:190-202.
- [Google Scholar]
- Discovery of an exceptionally potent and selective class of fatty acid amide hydrolase inhibitors enlisting proteome-wide selectivity screening: concurrent optimization of enzyme inhibitor potency and selectivity. Bioorg. Med. Chem. Lett.. 2005;15:1423-1428.
- [Google Scholar]
- Synthesis of Alpha Amylase Inhibitors Based on Privileged Indole Scaffold. Bioorg. Chem.. 2017;72:248-255.
- [Google Scholar]
- Potential Hypoglycemic Agents: 1,3,4-Oxadiazoles and Related Compounds. J. Med. Chem.. 1962;5:617-626.
- [Google Scholar]
- Design, synthesis and antiinflammatory activity of some 1,3,4-oxadiazole derivatives. Eur. J. Med. Chem.. 1996;31:819-825.
- [Google Scholar]
- Comparison of azabicyclic esters and oxadiazoles as ligands for the muscarinic receptor. J. Med. Chem.. 1991;34:2726-2735.
- [Google Scholar]
- Synthesis and antiinflammatory activity of 1-acylthiosemicarbazides, 1,3,4-oxadiazoles, 1,3,4-thiadiazoles and 1,2,4-triazole-3-thiones. IL Farmaco. 2002;57:101-107.
- [Google Scholar]
- Synthesis and evaluation of some novel substituted 1,3,4-oxadiazole and pyrazole derivatives for antitubercular activity. Ind. J. Chem.. 2009;48:1453-1456.
- [Google Scholar]
- Synthesis, Molecular Docking, Acetylcholinesterase and Butyrylcholinesterase Inhibitory Potential of Thiazole Analogs as New Inhibitors for Alzheimer Disease. Bioorg. Chem.. 2015;62:106-116.
- [Google Scholar]
- Synthesis of 4-thiazolidinone Analogs as potent in vitro Anti-urease Agents. Bioorg. Chem.. 2015;63:123-131.
- [Google Scholar]
- Isatin based Schiff bases as inhibitors of α-glucosidase: Synthesis, characterization, in vitro evaluation and molecular docking studies. Bioorg. Chem.. 2015;60:42-48.
- [Google Scholar]
- Triazinoindole analogs as potent inhibitors of α-glucosidase: Synthesis, biological evaluation and molecular docking studies. Bioorg. Chem.. 2015;58:81-87.
- [Google Scholar]
- Synthesis, in vitro evaluation and molecular docking studies of thiazole derivatives as new inhibitors of α-glucosidase. Bioorg. Chem.. 2015;62:15-21.
- [Google Scholar]
- Synthesis and in vitro Acetylcholinesterase and Butyrylcholinesterase Inhibitory Potential of Hydrazide based Schiff Bases. Bioorg. Chem.. 2016;68:30-40.
- [Google Scholar]
- Development of bis-Thiobarbiturates as Successful Urease Inhibitors and their Molecular Modeling Studies. Chin. Chem. Lett.. 2016;27:693-697.
- [Google Scholar]
- Synthesis of 2-Acylated and Sulfonated 4-hydroxycoumarins: In vitro Urease Inhibition and Molecular Docking Studies. Bioorg. Chem.. 2016;66:111-116.
- [Google Scholar]
- Focus on research: diabetes treatment-bridging the divide. New Engl. J. Med.. 2007;356:1499-1501.
- [Google Scholar]
- Biology-Oriented Syntheses (BIOS) of Novel Santonic-1,3,4-oxadiazole Derivatives under Microwave-Irradiation and their Antimicrobial Activity. J. Chem. Soc. Pak.. 2015;37:1020-1029.
- [Google Scholar]
- J. Suwinski, W. Szczepankiewicz, Comprehensive Heterocyclic Chemistry III, Ed. Chap. 6. Elsevier Science Ltd., Oxford 5 (2008) 398
- Synthesis and biological evaluation of novel N-arylidenequinoline-3-carbohydrazides as potent β-glucuronidase inhibitors. Bioorg. Med. Chem.. 2016;24:3696-3704.
- [Google Scholar]
- Synthesis, β-Glucuronidase Inhibition and Molecular Docking Studies of Hybrid Bisindole-Thiosemicarbazides Analogs. Bioorg. Chem.. 2016;68:56-63.
- [Google Scholar]
- Biology-oriented drug synthesis (BIODS) of 2-(2-methyl-5-nitro-1Himidazol-1-yl)ethyl aryl ether derivatives, in vitro α-amylase inhibitory activity and in silico studies. Bioorg. Chem.. 2017;74:1-9.
- [Google Scholar]
- Synthesis, α-glucosidase inhibitory activity and in silico study of tris-indole hybrid scaffold with oxadiazole ring: As potential leads for the management of type-II diabetes mellitus. Bioorg. Chem.. 2017;74:30-40.
- [Google Scholar]
- Synthesis and study of the a-amylase inhibitory potential of thiadiazole quinoline derivatives. Bioorg. Chem.. 2017;74:179-186.
- [Google Scholar]
- Synthesis and molecular modelling studies of phenyl linked oxadiazole-phenylhydrazone hybrids as potent antileishmanial agents. Eur. J. Med. Chem.. 2017;126:1021-1033.
- [Google Scholar]
- Antimicrobial activity of 1,3,4-oxadiazole derivatives against planktonic cells and biofilm of Staphylococcus aureus. Fut. Med. Chem.. 2018;10:283-296.
- [Google Scholar]
- Design, synthesis, and pharmacological evaluation of 5-oxo-1,2,4-oxadiazole derivatives as AT1 antagonists with antihypertension activities. Clin. Exp. Hyper.. 2016;38:435-442.
- [Google Scholar]
Further reading
- Prediction of protein–ligand interactions. Docking and scoring: successes and gaps. J. Med. Chem.. 2006;49:5851-5855.
- [Google Scholar]
- Synthesis and α-Glucosidase Inhibitory Mechanisms of Bis (2,3-dibromo-4,5-dihydroxybenzyl) Ether, a Potential Marine Bromophenol α-Glucosidase Inhibitor. Mar. Drugs. 2011;9:1554-1565.
- [Google Scholar]
- Ferreira, Synthesis, Biological Activity, and Molecular Modeling Studies of 1H–1,2,3-Triazole Derivatives of Carbohydrates as α-Glucosidases Inhibitors. J. Med. Chem.. 2010;53:2364-2375.
- [Google Scholar]







