Translate this page into:
Benzimidazoles: A biologically active compounds
⁎Corresponding author. Tel.: +91 9891872142. sallu_05@yahoo.co.in (Salahuddin)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.

Abstract
Synthesis of commercially available benzimidazole involves condensation of o-phenylenediamine with formic acid. The most prominent benzimidazole compound in nature is N-riosyldimethylbenzimidazole, which serves as a axial ligand for cobalt in vitamin B12. The benzimidazole and its derivatives play a very important role as a therapeutic agent e.g. antiulcer and anthelmintic drugs. Apart from this the benzimidazole derivatives exhibit pharmacological activities such as antimicrobial, antiviral, anticancer, anti-inflammatory, analgesic, etc. The substituted benzimidazoles are summarized in this review to know about the chemistry as well as pharmacological activities.
Keywords
Benzimidazole
Pharmacological activity
Antimicrobial activity
1 Introduction
Imidazole is the accepted name for the parent compound in the series, the numbering of which follows the accepted pattern for heterocyclic compound. Imidazole or iminazoline is an azapyrrole, the nitrogen atom is separated by one carbon atom. This compound was earlier also called as glyoxalin as it was first prepared in 1958 from glyoxal and ammonia.
The benzo derivative of imidazole is referred to as benzimidazole (Bansal, 2002). Although benzimidazole is the commonest name of the parent compound of the series, other names such as benzimidazole and 1,3-benzodiazole (1) are often used.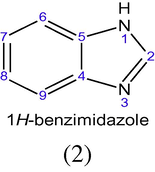
Mono acyl derivative of o-phenylenediamine is readily converted into the corresponding benzimidazole by the action of heat alone. These conversions are generally carried out at a temperature somewhat above the melting point of the starting compounds. This is a convenient method for preparing benzimidazoles when monoacyl derivatives are easily obtainable. The procedure may be improved by heating the monoacyl derivative of diamine in an atmosphere of nitrogen to prevent oxidation (Kelly, 1945).The diacyl derivatives of o-phenylenediamines are also converted into benzimidazoles but higher temperatures are required (Bistrzycki, 1980).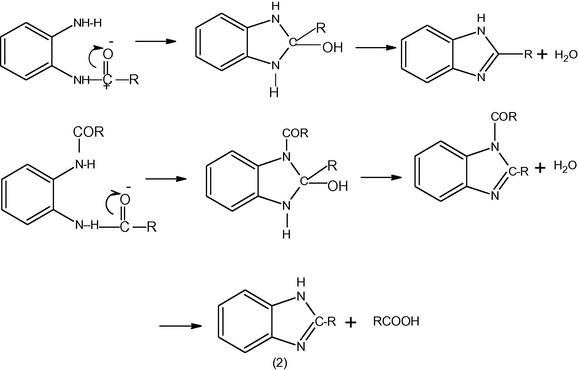
Benzimidazole is also synthesized from o-phenylenediamine and mono or di-basic acid. In this method, the diamine is simply heated with excess acid (Fischer, 1905).This procedure has been recommended as a means of identifying fatty acid α-hydroxy acid as well as phenylacetic acid and diphenylacetic acid are converted into the corresponding benzimidazoles when heated with o-phenylenediamine.
Phillips modification of the above procedure consists in refluxing with the (Philips, 1928) o-phenylenediamine and mono basic acid in 4 N hydrochloric acid. The benzimidazole is then precipitated by neutralizing the solution with ammonium hydroxide. Benzoic acid gives only traces of 2-phenylbenzimidazole. Apparently this method is not applicable to the aromatic monobasic acid.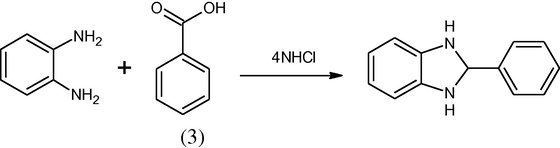
Benzimidazole derivative are associated with various types of pharmacokinetic and pharmacodynamic properties. Benzimidazole nucleus is one of the bioactive heterocyclic compounds that exhibit a range of biological activities. Specifically, this nucleus is a constituent of vitamin B12 (O'Neil et al., 2001).The pharmacological activities of the benzimidazole containing moiety have been well documented (Amari et al., 2002). Albendazole, Mebendazole and Thiabendazole are widely used as anthelmintic drugs (Kohler, 2001).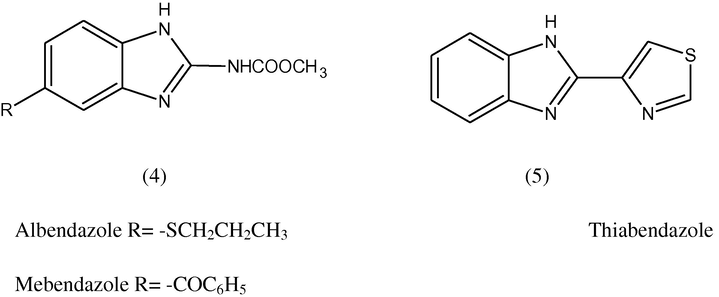
Literature survey reveals that the various derivatives of benzimidazole have been synthesized for their pharmacological activities. Some of the already synthesized compounds from the above mentioned field have found very strong application in medicine praxis (Mavrova et al., 2006). The activity against bacteria, fungi and helminthes resulted their mode of action, which resulted in the blockage of microtubule in various nematode, trematode and cystode. (Campbell and Denham, 1983).
2 Antimicrobial
The two series of benzimidazole derivatives were synthesized, the first one was based on 2-thioalkyland thioaryl substituted benzimidazole (6), the second one was based on 5,6-dinitrobenzimidazole (7) and the antibacterial activity of the compound against nosocomial strains of Stenotrophomonas malthophilia was examined (Kazimierczuk et al., 2002).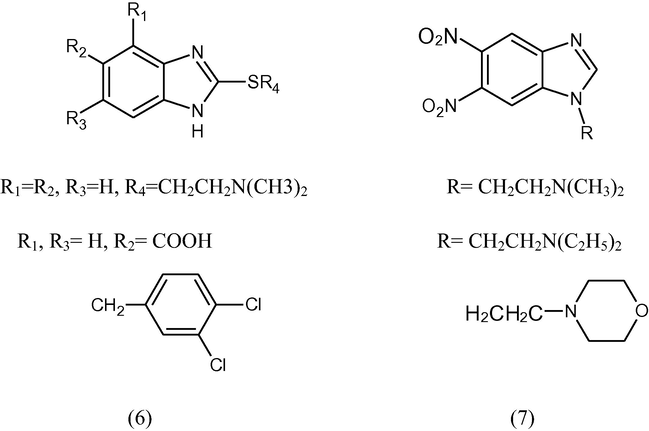
Novel azetidine-2-one (8) has been synthesized and evaluated for their antibacterial activity against Bacillus subtilis, Escherichia coli, Candida albicans, Aspergillus niger and Aspergillus flavus. The tested compounds are more effective against gram positive bacteria. The strong lipophilic character of molecule plays an essential role in producing antimicrobial effects (Ansari and Lal, 2009a,b).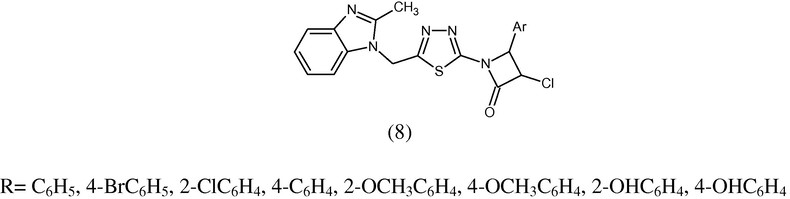
Some derivatives of benzimidazole were synthesized by nucleophilic substitution of substituted benzimidazole 2-substituted-1-[{(5-substituted-alkyl/aryl)-1,3,4-oxadziazolyl-2-yl}] (9) and were evaluated for antimicrobial activities toward Gram +ve and Gram –ve bacteria. Some of the synthesized compounds showed moderate activity against tested fungi (Ansari and Lal, 2009a,b).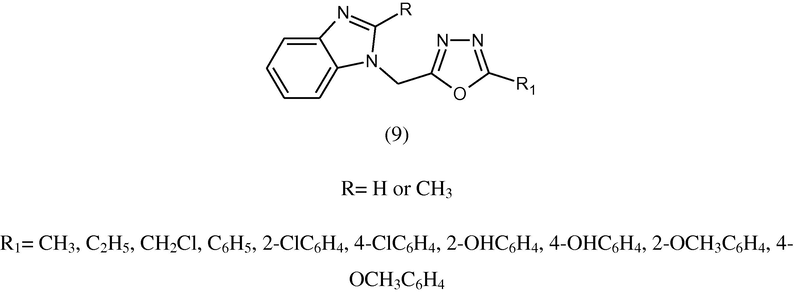
Gupta and Rani (1977) synthesized 2-thiohalogenonitrophenyl benzimidazole by the condensation of halogenonitrobenzenes and sodium salt of 2-mercaptobenzimidazole (10) and tested for their antifungal activity against Helmithosporium sativum, A. niger and Fusarium oxysporum by spore germination method. The percentage inhibition of the spores at 10 ppm has been recorded.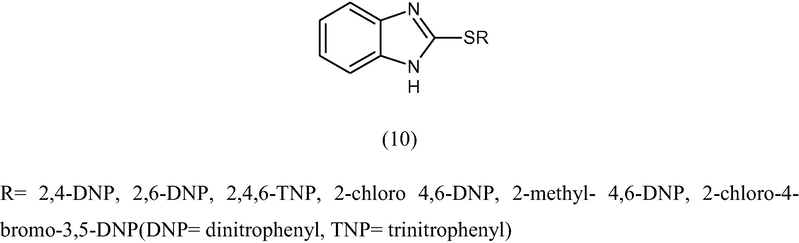
Ghoneim et al. (1998) synthesized 2-[(4-aminophenyl)sulphonyl] derivative (11) of benzimidazole and tested the antimicrobial activity of compounds against E. coli using agar diffusion method.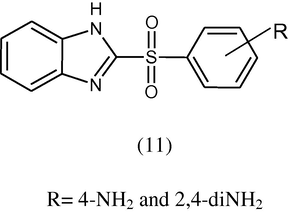
All 4-amino and 2,4-diaminophenylsulphonyl derivatives showed antimicrobial activity.
Various benzimidazoles (12) were prepared and evaluated by Mane et al. against Alternaria brassicicola, Fusarium, Staphylococcus (Gram +ve) and E. coli (Gram –ve) using filter paper disc method at 500 ppm concentration using 5 mm size filter paper. It was found that the compound having NO2 and chloro substituent showed good activity against fungi as well as bacteria (Mane et al., 1995).
A series of fused and spiropyrazolones (13), isoxazolines (14), pyrimidines (15), β-lactam (16) and thiazolidinones (17) incorporating 2-cyanomethyl benzimidazole were synthesized by Khalafallh et al. (1995).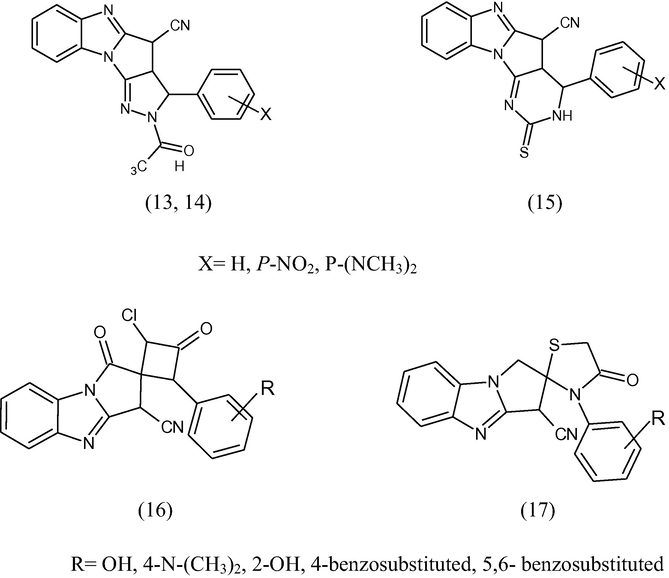
The synthesized compounds have been tested against some bacterial and fungal strains using the filter paper disc method and 3-cyano-2,3dihydropyrolo[1,2-a]benzimidazole-1(H)-one is more potent against bacteria and fungi than pyrazolines and pyrimidines.
Kumar et al. (2006) synthesized some novel 2-(6-flurochroman-2-yl)-1-alkyl/acy/aroyl-1-H-benzimidazoles (18) with different types of electrophiles. Some of the compounds show good antibacterial activity against Salmonella typhimurium and poor activity against Staphylococcus aureus.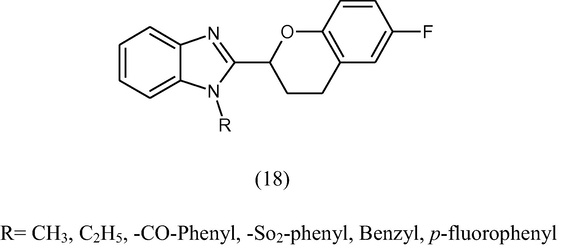
Kaghtara et al. (1999) reported when 2-(benzimidazol-2-yl) benzoyl hydrazide condensed with any aromatic acid and POCl3 gives 2-aryl-5-[(2′-benzimidazol-2″-yl)phenyl]-1,3,4-oxadiazoles (19). The acid hydrazide on cyclization with CNBr yields 2-amino-5-[2′-(benzimidazol-2″-yl-phenyl)-1,3,4-oxadiazole (20) which on reaction with any sulphonyl chloride and substituted benzoyl chloride gives the corresponding sulphonamides (21) and amides (22) respectively. All the synthesized products have been evaluated in vitro for their antimicrobial activity against several microbes.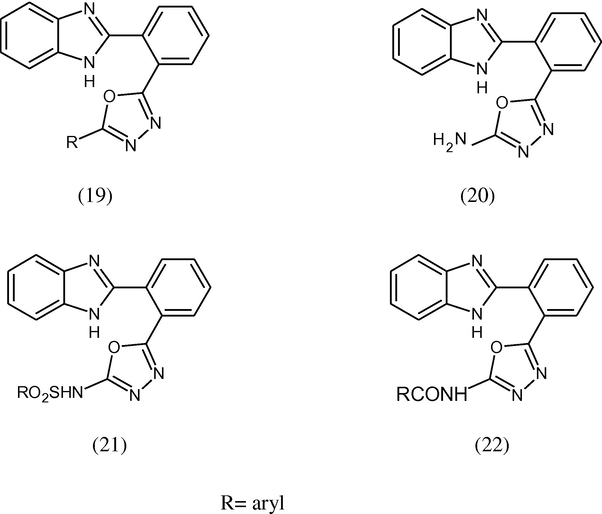
Bishnoi et al. (1978) reported various 10-(α-p-benzimidazolyl-aminobenzyl)phenothiazines (23). All the compounds were evaluated for their antifungal activity against Fusarium solani.
A new series of 2-substituted-1-[(5-substituted-phenyl-1,3,4-oxadiazole-2-yl)methyl-1H-benzimidazole (24) have been synthesized. The newly synthesized compounds were evaluated for their antibacterial activity (MIC in μg/mL) by the serial dilution method in vitro against Gram positive bacteria namely E. coli, S. aureus and Gram negative bacteria namely Pseudomonas aeruginosa (Gowda et al., 2010).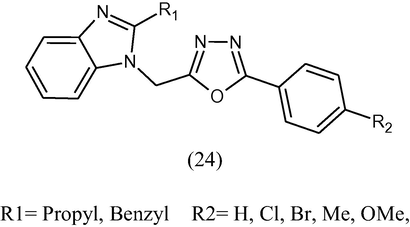
The 1H-benzimidazole-2-yl thioacetylpiperazine derivatives (25, 26, 27) were synthesized and evaluated for their in vitro activity against T. spiralis as well as their in vivo antinematode activity against S. obvelata (Mavrova et al., 2006). The in vitro activity showed that most of the tested compounds exhibit higher activity than albendazole against T. spiralis and comparable to that of ivermectin. Some of the compounds demonstrated 96.0%, 98.2% and 100% activities at a dose of 200 μg/mL after 48 h. Some of the compounds were found most active with 76%, 73% and 77% against S. obvelata.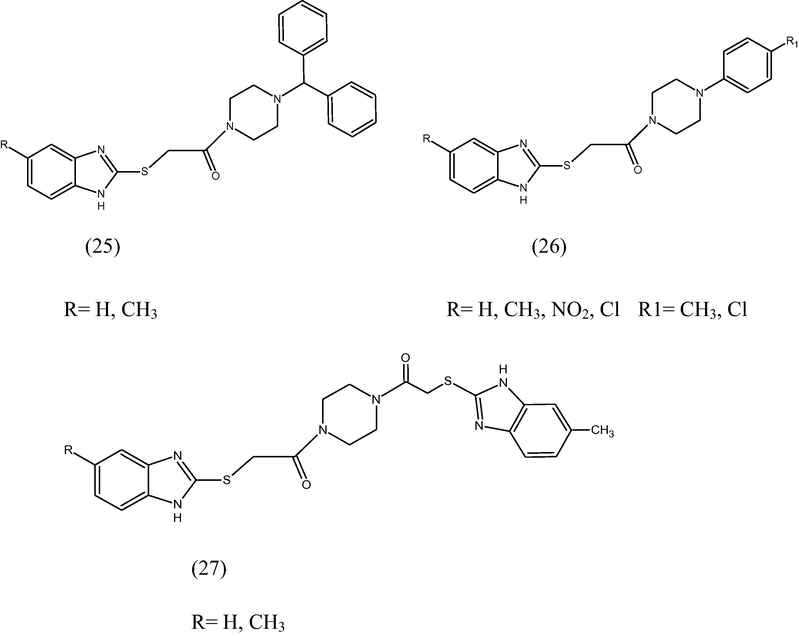
A series of 1-[(4-(4′-substituted)phenyl-3-alkyl/aralkyl-thio-4H-1,2,4-triazoles (28) have been prepared and screened for their antimicrobial activity against pathogenic organisms S. citrus, B. subtilis and E. coli and antifungal against A. fumigatus, C. albicans and F. heterosporum by single disc method at different concentration method (50 and 100 μg/mL) using ampicillin and miconazole as standard drugs for antibacterial and antifungal activities respectively (Shetgiri and Kokitkar 2001)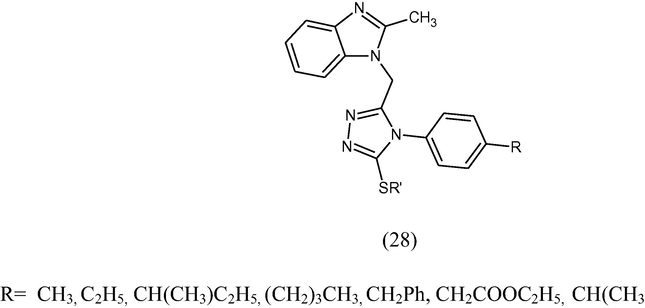
5-[2-(2-Methylbenzimidazol-1-yl)ethyl][1,3,4]oxadiazole-2(3H)-thione (29), 5-[2-methylbenzimidazol-1-yl)ethyl]-3-diethylaminoethyl (30), 5-[2-(2-methylbenzimidazol-1-yl)ethyl]-4-amino[1,2,4]triazole-3-thiol (31), 3-(2-methylbenzimidazol-1-yl)propionic acid hydrazide Schiff's base (32) have been synthesized and were tested for their antimicrobial activity against Gram +ve bacteria (Bacillus cereus), and Gram –ve bacteria (E. coli) (AfafH El-masry et al., 2000).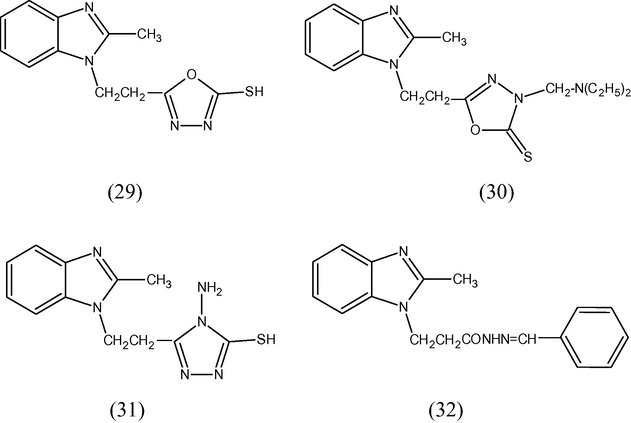
2-Arylamino-5-(p-nitrosothymoxymethyl)-1,3,4-oxadiazoles (33) were prepared along with their derivatives by chemoselective heterocyclization with NaOH/I2 of thiosemicarbazide. The compounds were screened for their antimicrobial activity against Gram +ve bacteria S. citrus and B. mega and against Gram –ve bacteria E. coli and S. typhosa and for antifungal activity against A. niger by the cup plate method at a concentration of 50 μg/mL using DMF as a solvent. The activity was compared with the known standard drug viz. ampicillin, chloramphenicol, norfloxacin and griseofulvin at same concentration (Vashi et al., 1996).
The efficient and rapid synthesis of novel azetidin-2-ones (34) has been established. Thus, both microwave and conventional condensation of 2-{(1H-benzimidazol)-ylthio}-N0-2-(substituted phenyl) hydrazide with chloroacetylchloride were carried out in DMF-benzene solvent in the presence of Et3N catalyst. The resulting compounds have been evaluated for their antibacterial activity against B. subtilis, S. aureus and E. coli.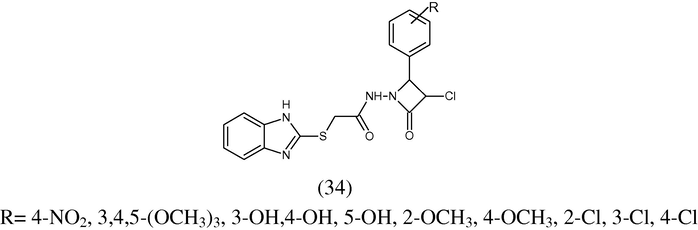
3 Antiviral
Some 7-(arylamidoalkyl)-3,4-diphenyl-isoquinolinyl-[1,5-c]-benzimidazoles (35) have been synthesized and were evaluated for their in vivo against influenza virus (IV) by inoculating it in 10 day old embryonated hen’s egg at the concentration of 0.5 mg per embryo in allantoic cavity. After 48 h it was found that the isoquinonyl benzimidazole derivative with nicotinamido group showed the maximum activity (Pandey and Shukla 1999).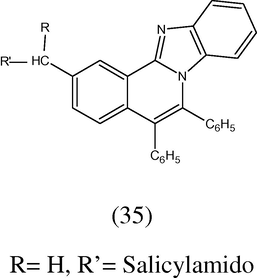
Various benzimidazol-2-ylalkyl N-aryldithiocarbamates (36), 2-arlimino-4-methyl/H-2H, 4H[1,3,4]dithiazino[4,5-a]benzimidazole (37), 1-aryl-4-methyl/H-1, 2-dihydro-4H-[1,3,4]thiadiazino[4,5-a]benzimidazole-2-thiones (38) 1-aryl-4-methyl/H-1, 2-dihydro-4H-[1,3,4]thiadiazino[4,5-a]benzimidazole-2-ones (39) were synthesized and tested for their antiviral activity (Yadav and Pal, 1996).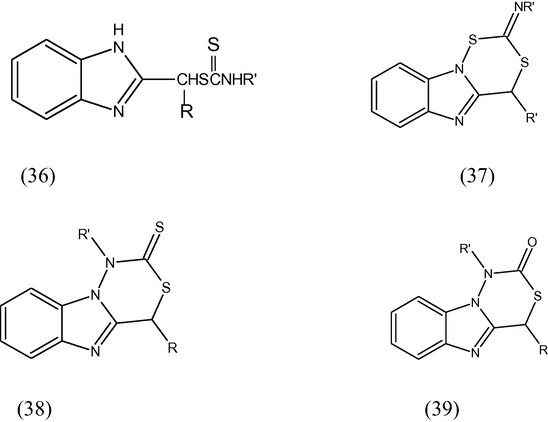
A set of 2-substituted-5-amidino-benzimidazole (40) derivatives bearing amidino substistuent at C-5 of benzimidazole ring were synthesized by introducing various heterocyclic nuclei at C-2 and were evaluated for their antiviral activity towards coxsackie viruses and echo viruses. The most selective activity towards coxsackie viruses and echo viruses was observed with the compound having pyridine ring at C-2 (Kristina et al., 2007).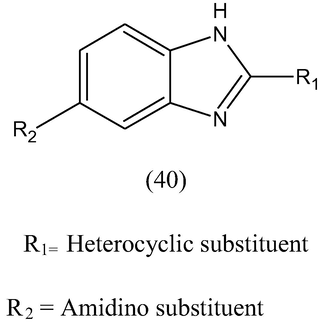
Some new 10-(α-p-benzimidazolyl-1-aminobenzyl) phenothiazines (41) have been synthesized and their antiviral activity was performed against JEV and HSV-1 (Bishnoi et al., 1978).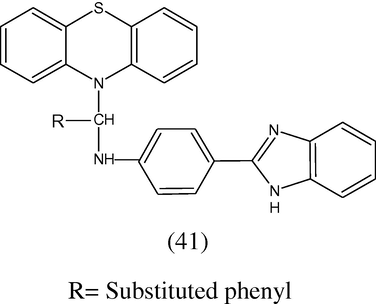
4 Anticancer
A novel series of benzimidazole substituted Schiff bases (42) were synthesized by the reaction of aromatic aldehydes with corresponding 2-aminobenzimidazoles. The synthesized Schiff bases were tested on their antiproliferative activity in vitro and exerted non-specific antiproliferative activity on the tested cell lines at the highest tested concentration (Hranjec et al., 2011).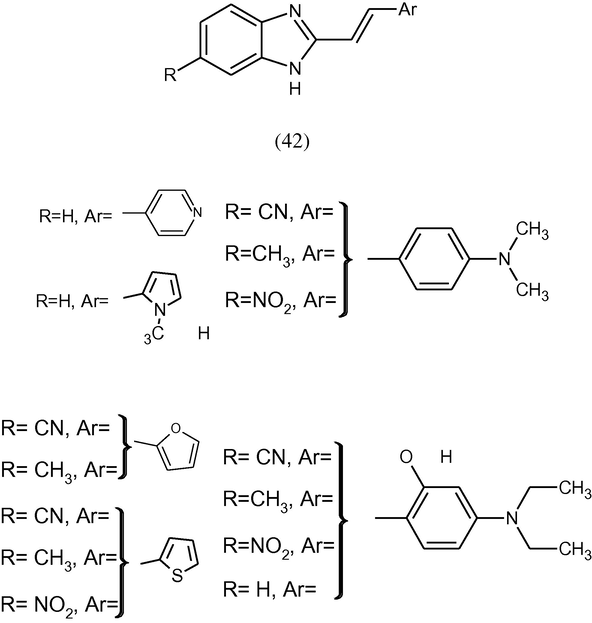
Benzimidazole-4,7-diones substituted at position-2 were synthesized via a microwave-assisted reaction using 2-chloromethyl-1,5,6-trimethyl-1H-benzimidazole-4,7-dione. Their anticancer activity has been evaluated on colon, breast and lung cancer cell lines. Among this 2,2′-bis(chloromethyl)-1,1′-dimethyl-5,5′-bi(1H-benzimidazole)-4,4′,7,7′-tetraone (43) was shown to possess excellent cytotoxicity comparable to that of mitomycin C (Gellis et al., 2008).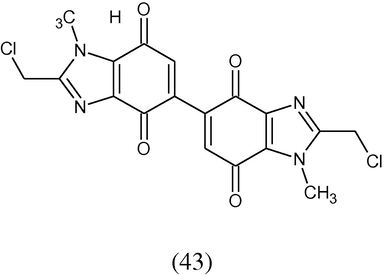
Various heterocyclic benzimidazole derivatives (44–47) have been synthesized by the condensation of succinic acid, homophthalic acid and 2,3-pyrazinedicarboxlic acid with various substituted diamines under microwave irradiation. All these compounds evaluated for anticancer activity at 50 mg/kg po exhibit good anticancer activity against ovary (IGR-OV-1), breast (MCF-7) and CNS (SF-295) human cancer cell lines (Sondhi et al., 2010).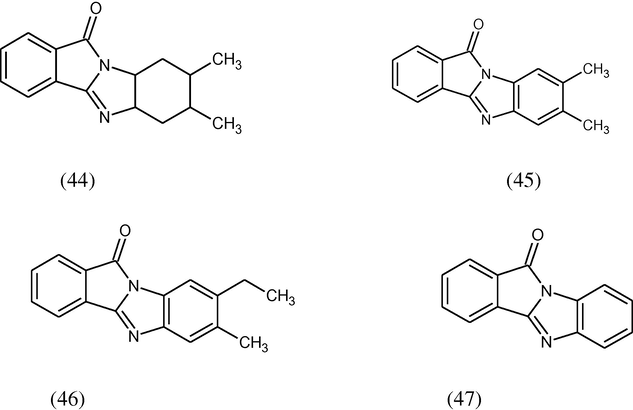
Some 1-methylene-2,3-diaryl-1,2-dihydropyrazino[1,2-a]benzimidazoles (48) and some 1-(2-arylvinyl)-3-arylpyrazino[1,2-a]benzimidazole derivatives (49) have been synthesized and their anticancer activity was reported. It can be seen that for all the compounds, log10GI50 values are smaller than –4. Melphalan cis-diaminodichloroplatinum, one of the chemotherapeutic agents, was used as standard compound (Demirayak et al., 2002).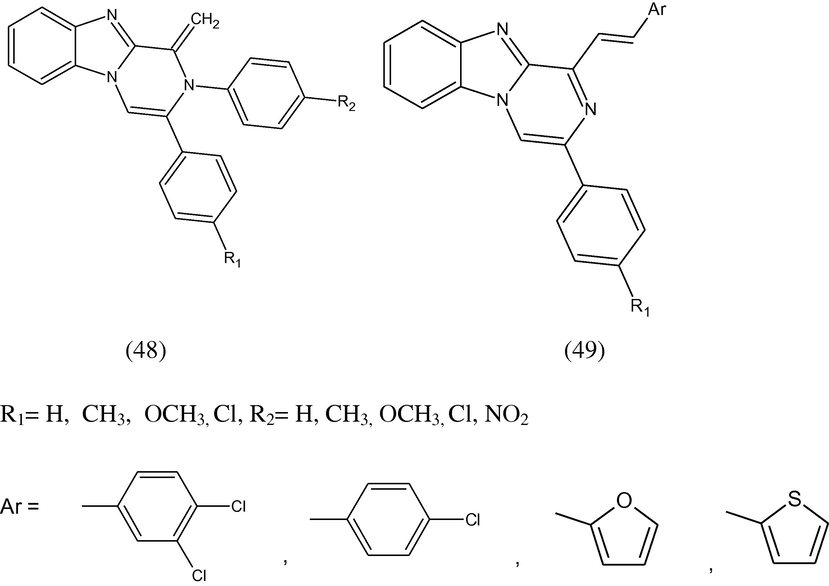
The synthesis of some series of benzimidazole like: 2-[(4-oxothiazolidin-2-ylidene)-methyl (50) and (4-amino-2-thioxothiazol-5-yl) benzimidazoles (51), 2-[(4-fluorobenzylidene (52) and cycloalkylidene)-cyanomethyl] benzimidazoles was carried out (53). All the synthesized compound were evaluated against three cell lines representing three common forms of human cancer i.e. human hepatocellular carcinoma cell line (HEPG2), human breast adenocarcinoma cell line (MCF7) and colon carcinoma cell line (HCT 116) (Refaat, 2010).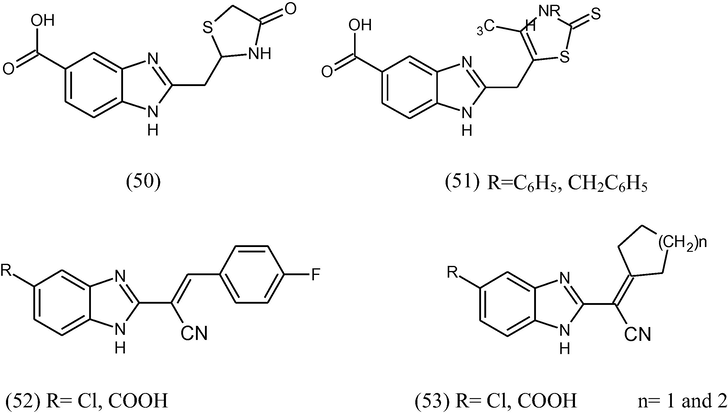
Shaharyar et al. (2010) synthesized 2-{5-[(substituted)phenyl]-4,5-dihydro-1H-3-pyrazolyl}-1H-benzoimidazole (54) and 2-{5-[(substituted)phenyl]-1-phenyl-4,5-dihydro-1H-3-pyrazolyl}-1H-benzimidazole (55) and screened at the National Cancer Institute (NCI), USA for anticancer activity at a single high dose (10 μM) in full NCI 60 cell panel. Among the selected compounds, 2-[5-(3,4-dimethoxyphenyl)-1-phenyl-4,5-dihydro-1H-3-pyrazolyl]-1H-benzimidazole was found to be the most active candidate of the series and selected for further evaluation at five dose level screening.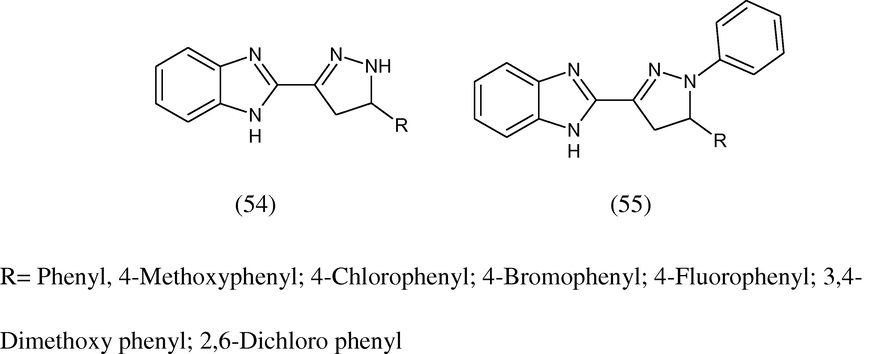
A novel series of trisubstituted benzimidazole and its precursors were synthesized. The title compounds were evaluated for inhibition against MDA-MB-231 breast cancer cell proliferation. The results revealed that the compound N-(4-cyano-3-(trifluoromethyl) phenyl)-4-fluoro-3-nitrobenzamide (56) was the potent inhibitor (Thimmegowda et al., 2008).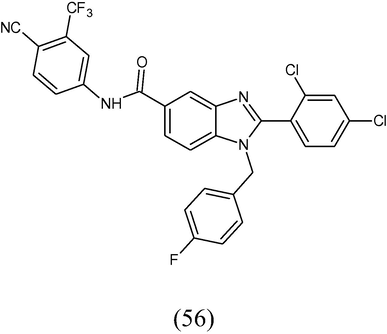
5 Antiprotozoal
Some thieno[2,3-d]pyrimidin-4(3H)-ones containing benzimidazol-2-yl-thioethyl- and benzimidazol-2-yl-methanethioethyl moiety in the second position of the pyrimidine ring were synthesized in order to determine their antitrichinellosis and antiprotozoal effects. The benzimidazole derivatives of thieno[2,3-d]pyrimidin-4-(3H)-ones (57) exhibited higher activity against Trichinella spiralis in vitro in comparison to albendazole. The most active compound, 2-[2-(5-nitro-1H-benzimidazol-1-yl)ethyl]-5,6,7,8-tetrahydro[1]benzothieno[2,3-d]pyrimidin-4(3H)-one (58) revealed 95% activity at a dosage of 5 mg/kg. The compound 2-{2-[(5(6)-nitro-1H-benzimidazol-2-yl)thio]ethyl}-5,6,7,8-tetrahydro[1]-benzothieno[2,3-d]pyrimidin-4(3H)-one exhibited 90% efficacy (Mavrova et al., 2010).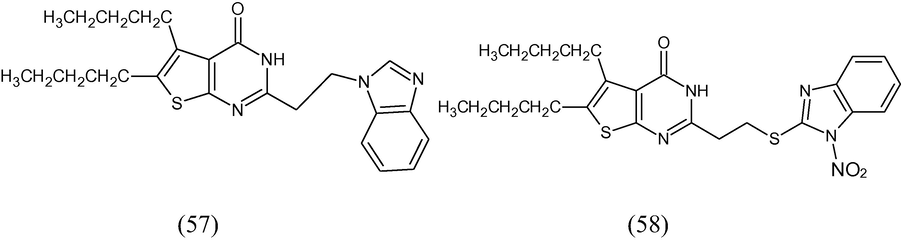
Novel series of some hybrids from benzimidazole and pentamidine (59) were prepared and each compound was tested in vitro against the protozoa Trichomonas vaginalis, Giardia lamblia, Entamoeba histolytica, Leishmania mexicana, and Plasmodium berghei. The tested compounds were compared with pentamidine and metronidazole (Gomez et al., 2008).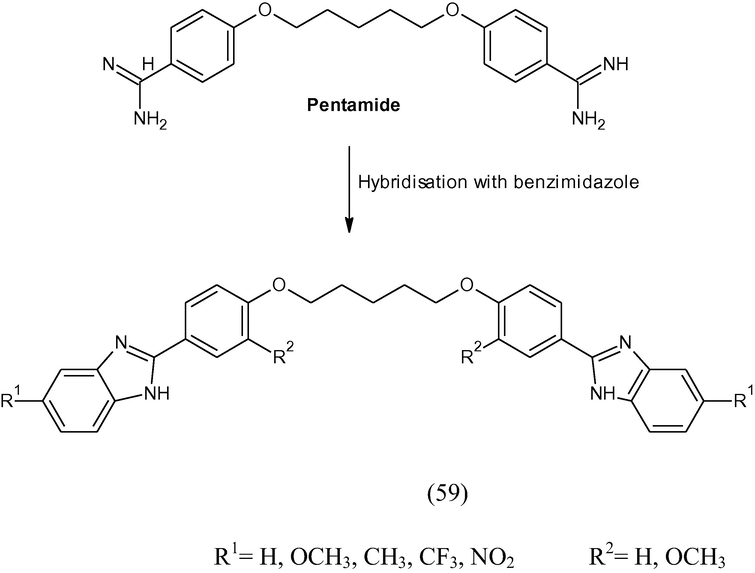
Biphenyl benzimidazolesdiamidines (60) were synthesized from their respective diamidoximes, through the bis-o-acetoxyamidoxime followed by hydrogenation. The target compounds contain hydroxy and/or methoxy substituted 1,3-phenyl groups as the central space between the two amidino bearing aryl groups. All the compounds have performed DNA binding studies [ΔTm values for poly(dA.dT)2] and in vitro evaluation against Trypanosoma b. rhodesiense (T.b.r.) and P. falciparum for the diamidino biphenyl benzimidazoles (Ismail et al., 2004).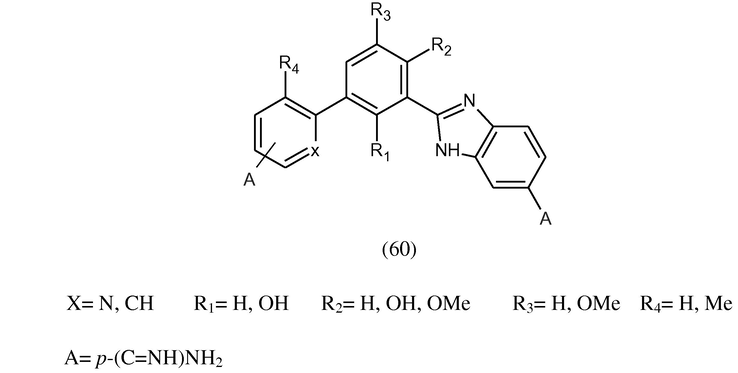
Various chloro-, bromo and methyl-analogues of 1H-benzimidazole (61–64), 1H-benzotriazole and their N-alkyl derivatives (65–68) have been synthesized and evaluated in vitro against the protozoa Acanthamoe bacastellanii. It was found that 5,6-dimethyl-1Hbenzotriazole (11) and 5,6-dibromo-1H-benzotriazole (14) have higher efficacy than the antiprotozoal agent chlorohexidine (Kopanska et al., 2004).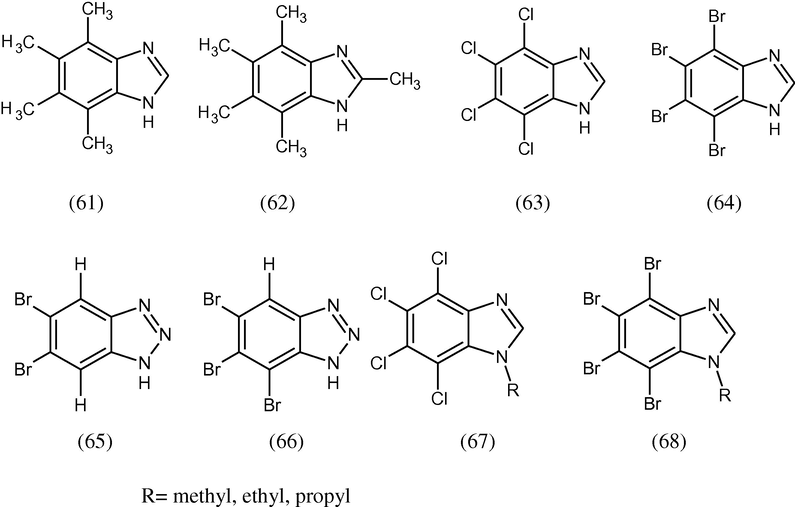
Padilla et al. (2009) reported the synthesis and antiprotozoal activity in vitro of 1-methylbenzimidazolederivatives (69) substituted with chlorine atoms at the benzenoid ring and aminocarbonyl, N-methylaminocarbonyl, N,N-dimethylaminocarbonyl,ethoxycarbonyl, 1-hydroxyethyl and acetyl at position-2. Some of the compounds are more active than metronidazole, the drug of choice against Giardia intestinalis and most of them against T. vaginalis. The most active group of compounds for both parasites in this series is substituted with 2-ethoxycarbonyl.
The derivatives of 2-(tri-fluoromethyl)benzimidazole (70) substituted at the 1-, 5-, and 6-positions have been synthesized and tested in vitro against the protozoa G. lamblia, E. histolytica, and the helminth T. spiralis. The tested compounds are more active as antiprotozoal agents than albendazole and metronidazole. One compound (20) was as active as albendazole against T. spiralis. These compounds were also tested for their effect on tubulin polymerization and none inhibited tubulin polymerization (Vazquez et al., 2001).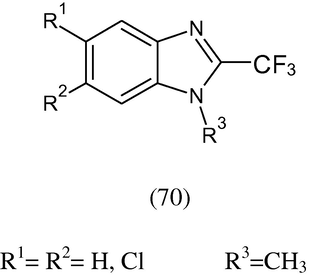
Various 1-H-benzimidazoles (71) have been synthesized and tested in vitro against the protozoa G. lamblia, E. histolytica and the helminth T. spiralis. The compounds were also tested for the inhibition of rat brain tubulin polymerization and compared with standard drug. Most of the compounds tested were more active as antiprotozoal agents than metronidazole and albendazole. None of the compounds was as active as albendazole against T. spiralis (Valdez et al., 2002).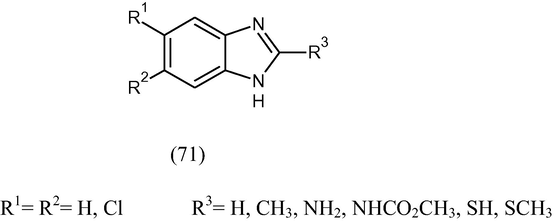
2-(Trifluoromethyl)-1H-benzimidazole derivatives (72) with various bioisosteric substitutents (-Cl, -F, -CF3, -CN) were prepared and tested in vitro against G. intestinalis and T. vaginalis in comparison with albendazole and metronidazole. Some analogues had IC50 values <1 μM against both species, which make them more potent than either standard (Vazquez et al., 2006).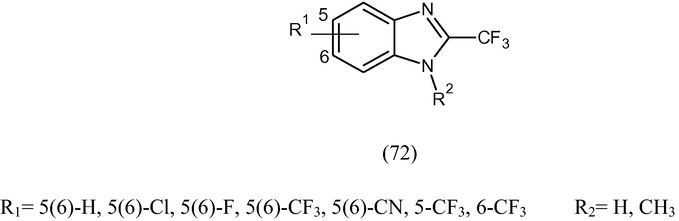
Some thio-alkylated and thio-arylated derivatives of c-substituted benzimidazole (73) have been synthesized and evaluated as antiprotozoal activity against nosocomial strains of S. malthophilia using metronidazole as standard. One of the tested compounds, 4,6,-dichloro-2-(4-nitrobenzylthio)-benzimidazole showed the most distinct antiprotozoal activity (Kazimierczuk et al., 2002).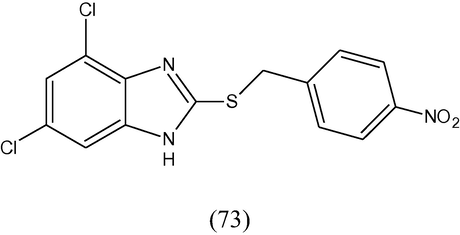
6 Anti-inflammatory and analgesic activities
Some 2-substituted benzimidazoles (74, 75) have been synthesized by the condensation of o-phenylenediamine with 2-coumaranonyl acetic acid derivatives and indole 3-acid and evaluated their anti-inflammatory and analgesic activities. The compounds were found to have significant anti-inflammatory activity at 50 mg/kg dose (Khan and Nandan, 1997).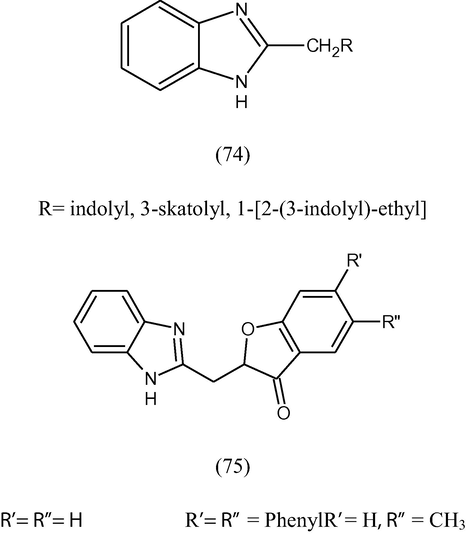
A new synthesis and their anti-inflammatory activity of a group of 1H-benzimidazole (76) were reported. The compounds were assessed on rat adjuvant arthritis screen and indomethacin as standard compound. The result gave 30% or greater reduction in non injected paw volume compared to control together with the result for indomethacin (Evans et al., 1996).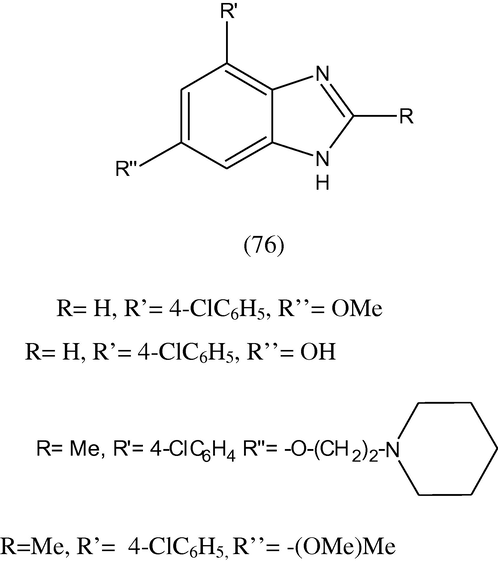
Some imino sugars of methylbenzimidazole (77) have been prepared and anti-inflammatory activity of the compounds was studied by employing the cotton pellet granuloma bioassay in rats using indomethacin as reference standard. The granuloma% inhibition values were determined for each compound (Taha, 2005).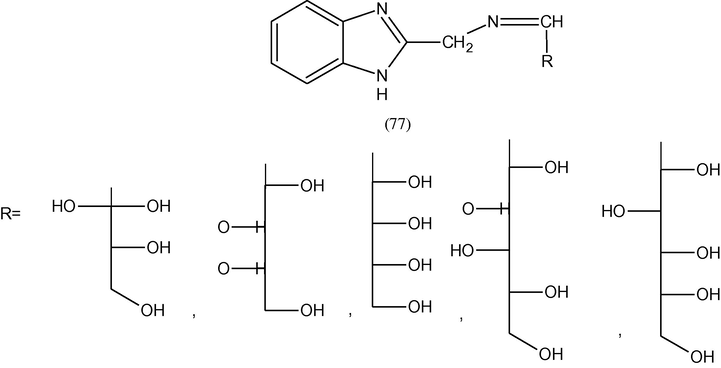
1-[2,3-(2-Phenylbenzimidazole)]2-methyl/phenyl-4-(3,4-disubstituted benzylidine)-5-oxoimidazoles (78) have been synthesized by condensing 2-(2/3 aminophenyl)benzimidazoles with appropriate 2-methyl/phenyl-4-(3,4-disubstituted) oxazoline-5-ones in dry pyridine and screened anti-inflammatory activity against carrageenan induced oedema (Mohan et al., 1984).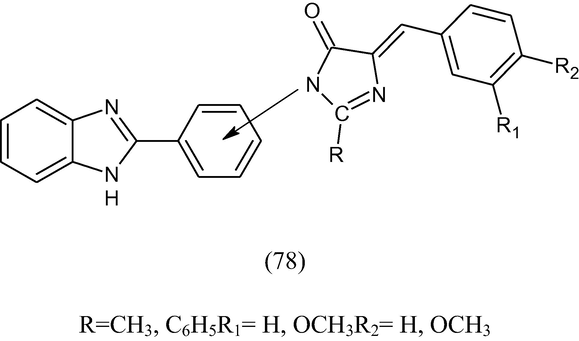
A series of novel 5-substituted-1-(phenylsulphonyl)-2-methylbenzimidazole derivatives (79) have been synthesized. Compounds were evaluated for their anti-inflammatory and analgesic activities as well as gastric ulcerogenic effects by carrageenan-induced rat paw edema and acetic acid-induced writhing in mice using indomethacin as standard (Gaba et al., 2010).
7 Conclusion
The new classes of reviewed substituted benzimidazole have a wide variety of biological activities. The 2-substituted-1-[{(5-substituted-alkyl/aryl)-1,3,4-oxadziazolyl-2-yl}] 3-cyano-2,3dihydropyrolo[1,2-a]benzimidazole-1(H)-one has good antibacterial and antifungal activities respectively. The significant antiviral and anticancer activities are shown by 7-(arylamidoalkyl)-3,4-diphenyl-isoquinolinyl-[1,5-c]-benzimidazole and benzimidazole substituted Schiff bases, but they are associated with some drawbacks like side effect, toxicity. So for minimizing these drawbacks there is a need to synthesize some new chemical compounds with better results.
References
- Molecules. 2000;5:1429.
- Reactivity studies on 4-aminopyrones: Access to benzimidazole and benzimidazolone derivatives. J. Heterocycl. Chem.. 2002;39:811.
- [Google Scholar]
- Synthesis and evaluation of some new benzimidazole derivatives as potential antimicrobial agents. Eur. J. Med. Chem.. 2009;44:2294.
- [Google Scholar]
- Synthesis, physicochemical properties and antimicrobial activity of some new benzimidazole derivatives. Eur. J. Med. Chem.. 2009;44:4028.
- [Google Scholar]
- Herocyclic Chemistry (third ed.). New Age International: Publisher, New Delhi; 2002. 401 pp
- Synthesis and characterization of benzimida-zolylphenothiazine derivatives and a study of their antiviral and antifungal activities. Ind. J. Chem.. 1978;41B:1978.
- [Google Scholar]
- Ueber Diacyl-o-Diamine. Ber.. 1980;23:1876.
- Chemotherapy. In: Campbell W.C., ed. Trichinilla and Trichinosis. USA and London, UK: Plenum Press; 1983. 340 pp
- [Google Scholar]
- Synthesis and anticancer and anti-HIV testing of some pyrazino[1,2-a]benzimidazole derivatives. Eur. J. Med. Chem.. 2002;37:255.
- [Google Scholar]
- Synthesis of a group of 1H–benzimidazoles and their screening for antiinflammatory activity. Eur. J. Med. Chem.. 1996;31:635.
- [Google Scholar]
- The chemistry of benzimidazoles. Ber.. 1905;38:320.
- Synthesis and pharmacological evaluation of novel 5-substituted-1-(phenylsulfonyl)-2-methylbenzimidazole derivatives as anti-inflammatory and analgesic agents. Eur. J. Med. Chem.. 2010;45:2245.
- [Google Scholar]
- Synthesis and cytotoxicity evaluation of some benzimidazole-4,7-diones as bioreductive anticancer agents. Eur. J. Med. Chem.. 2008;43:1858.
- [Google Scholar]
- Synthesis of 2-[(4-amino or 2,4-diaminophenyl)sulfonyl] derivatives of benzimidazole, benzothiazole and 6-methyluracil as potential antimicrobial agents. Ind. J. Chem.. 1998;37B:904.
- [Google Scholar]
- Design, synthesis and in vitro antiprotozoal activity of benzimidazole-pentamidine hybrids. Bioorg. Med. Chem. Lett.. 2008;18:3147.
- [Google Scholar]
- Microwave assisted synthesis of 1,3,4-oxadiazoles carrying benzimidazole moiety and their antimicrobial properties. Ind. J. Chem.. 2010;49B:1130.
- [Google Scholar]
- Synthesis and fungitoxicity of some 5-substituted- 3-polynitrophenyl rhodanines. LIV: J. Ind. Chem. Soc.; 1997. 478
- Synthesis, spectroscopic characterization and antiproliferative evaluation in vitro of novel Schiff bases related to benzimidazoles. Eur. J. Med. Chem.. 2011;46:2274.
- [Google Scholar]
- Dicationic biphenyl benzimidazole derivatives as antiprotozoal agents. Bioorg. Med. Chem.. 2004;12:5405.
- [Google Scholar]
- Synthesis of 2,5-disubdtituted 1,3,4-oxadiazoles as biologically active heterocycles. Ind. J. Chem.. 1999;38B:572.
- [Google Scholar]
- Synthesis, antiprotozoal and antibacterial activity of nitro- and halogeno-substituted benzimidazole derivatives. Acta Biochim. Pol.. 2002;49:185.
- [Google Scholar]
- Preparation of 2-phenylnaphth [1,2] imidazole and 2-methylnaphth [1,2] imidazole. J. Am. Chem. Soc.. 1945;67:1074.
- [Google Scholar]
- Novel synthesis of some new fused/spiro heterocyclic compounds and their biological activity. Ind. J. Chem.. 1995;34B:1066.
- [Google Scholar]
- 2-Substituted benzimidazoles as anti-inflammatory and analgesic agents. Indian J. Heterocycl. Chem.. 1997;7:55.
- [Google Scholar]
- The biochemical basis of anthelmintic action and resistance. Int. J. Parasitol.. 2001;31:336.
- [Google Scholar]
- Synthesis and activity of 1H-benzimidazole and 1H-benzotriazole derivatives as inhibitors of Acanthamoeba castellanii. Bioorg. Med. Chem.. 2004;12:2617.
- [Google Scholar]
- Synthesis, antiviral and antitumor activity of 2-substituted-5-amidino-benzimidazoles. Bioorg. Med. Chem.. 2007;15:4419.
- [Google Scholar]
- Synthesis and anti-bacterial activity of some novel 2-(6-fluorochroman-2-yl)-1-alkyl/acyl/aroyl-1H-benzimidazoles. Eur. J. Med. Chem.. 2006;41:599.
- [Google Scholar]
- Synthesis and biological activity of some new 2-alkyl-1-(l-dihydroxypyridyl-methyl)benzimidazoles. Ind. J. Chem.. 1995;34B:917.
- [Google Scholar]
- Antihelminthic activity of some newly synthesized 5(6)-(un)substituted-1H-benzimidazol-2-ylthioacetylpiperazine derivatives. Eur. J. Med. Chem.. 2006;41:1412.
- [Google Scholar]
- Synthesis, antitrichinnellosis and antiprotozoal activity of some novel thieno[2,3-d]pyrimidin-4(3H)-ones containing benzimidazole ring. Eur. J. Med. Chem.. 2010;45:5856.
- [Google Scholar]
- CNS, anthelmintic and antiinflammatory activities of some 1-[2/3-(2-phenyl benzimidazole)]2-methyl/phenyl-4-(3,4-disubstituted benzylidene)5-oxo-imidazoles. Pharma-col. Res. Commun.. 1984;16:321.
- [Google Scholar]
- O’Neil, M.J., Smith, M., Heckelman, 2001. The Merck Index, 13th ed. Merck & Co Inc., P-1785, 10074.
- Synthesis and antiprotozoal activity of novel 1-methylbenzimidazole derivatives. Bioorg. Med. Chem.. 2009;17:1724.
- [Google Scholar]
- Synthesis and biological activity of isoquinolinyl benzimidazoles. Ind. J. Chem.. 1999;38B:1381.
- [Google Scholar]
- Philips, 1928. The formation of 2-substituted benziminazoles. J. Chem. Soc. 2393.
- Synthesis and anticancer activity of some novel 2-substituted benzimidazole derivatives. Eur. J. Med. Chem.. 2010;45:2949.
- [Google Scholar]
- Pyrazoline bearing benzimidazoles: Search for anticancer agent. Eur. J. Med. Chem.. 2010;45:114.
- [Google Scholar]
- Synthesis and biological activity of new benzimidazoles. Ind. J. Chem.. 2001;40B:163.
- [Google Scholar]
- Solvent free synthesis, anti-inflammatory and anticancer activity evaluation of tricyclic and tetracyclic benzimidazole derivatives. Bioorg. Med. Chem. Lett.. 2010;20:2306.
- [Google Scholar]
- Synthesis and antiinflammatory evaluation of some imino-sugars of methylbenzimidazole. J. Ind. Chem. Soc.. 2005;82:180.
- [Google Scholar]
- Synthesis, characterization and evaluation of benzimidazole derivative and its precursors as inhibitors of MDA-MB-231 human breast cancer cell proliferation. Bioorg. Med. Chem. Lett.. 2008;18:432.
- [Google Scholar]
- Synthesis and antiparasitic activity of 1H-benzimidazole derivatives. Bioorg. Med. Chem. Lett.. 2002;12:2221.
- [Google Scholar]
- Synthesis of 2,5-disubstituted-1,3,4-oxadiazole, 1,5-disubstituted-2- mercapto-1,3,4-triazole and 2,5-disubstituted-1,3,4-thiadiazole derivatives as potential antimicrobial agents. Ind. J. Chem.. 1996;35B:111.
- [Google Scholar]
- Synthesis and antiparasitic activity of 2-(Trifluoromethyl)benzimidazole derivatives. Bioorg. Med. Chem. Lett.. 2001;11:187.
- [Google Scholar]
- Synthesis and antiprotozoal activity of some 2-(trifluoromethyl)-1H-benzimidazole bioisosteres. Eur. J. Med. Chem.. 2006;41:135.
- [Google Scholar]
- Synthesis and antiviral activity of new 1,3,4-dithi(thiadi)azino(4,5-a) benzimidazole derivatives involving oxidative N-S and N-N bond formations. Ind. J. Chem.. 1996;35B:748.
- [Google Scholar]







