Translate this page into:
Berberrubine, an Attractive derivative of berberine with multiple pharmacological activities
⁎Corresponding authors. pengf@scu.edu.cn (Fu Peng), ailsa.mcgregor@otago.ac.nz (Ailsa McGregor), xiexiaofang@cdutcm.edu.cn (Xiaofang Xie)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Abstract
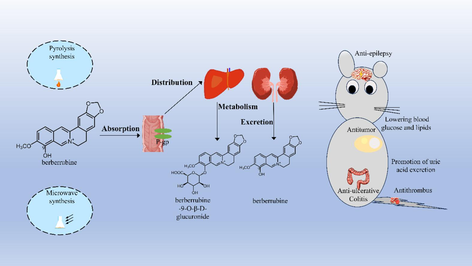
Berberrubine is the well-known metabolite of berberine that can be isolated from plants. It has increasingly gained attention with its biological properties that are at times more potent than berberine. Compared with berberine, the structure of berberrubine has better lipid solubility and P-gp receptor affinity, so it has better bioavailability. Equally, greater biological activity has emerged in the treatment of disease. Currently, berberrubine is mainly derived by chemical synthesis, which not only pollutes the environment but also cannot meet the market demand. In this paper, the source, pharmacokinetics, biological properties, and toxicity of berberrubine were systematically reviewed by collecting and summarizing the current relevant literature over the past few decades. Furthermore, the underlying molecular mechanism and dose–effect relationship of berberrubine and the prodrug berberine in the treatment of related diseases are also discussed to broaden the application and development prospects of berberrubine as a novel drug. Notably, the clinical studies of berberrubine are quite insufficient. Further high-quality studies are required to firmly establish the clinical efficacy of berberrubine.
Keywords
Berberrubine
Berberine
Synthesis methods
Biological properties
1 Introduction
Native flora is a treasure trove of natural medicinal compounds. One group of such compounds is called alkaloids, nitrogen-containing compounds that exist naturally in many plants. The presence of nitrogen lends most alkaloids their alkalinity and strong biological activity. One such alkaloid with potent properties is berberine. Berberine, an isoquinoline alkaloid isolated from several plant species including goldenthread (Coptidis chinensis), Oregon grape (Berberis aquifolium) and tree turmeric (Berberis aristate) has been used for many years in traditional Chinese and Ayurvedic medicine. Berberine is mainly used clinically to treat gastrointestinal infections, especially bacterial diarrhea, due to its excellent broad-spectrum antibacterial effect (Dehau et al., 2023), but also has proven anti-inflammatory, antioxidant and anticancer properties (Pacyga et al., 2024). Clinical trials have demonstrated that berberine also has lipid-lowering effects and improves insulin resistance highlighting its potential in the treatment of metabolic disorders (Xu et al., 2018). In terms of safety, the conventional dose of berberine seldom induces toxicity, although gastrointestinal discomfort has been reported in some cases(Imenshahidi and Hosseinzadeh 2019).
Although berberine is clinically effective and safe, its low bioavailability currently limits its applications. A popular strategy in drug discovery is the development of more effective and safer compounds based on the modification of the active structure of natural products. Such is the case of dihydroartemisinin, a derivative of artemisinin that exceeds the potency of the latter in the treatment of malaria (Yao et al., 2017). Structural modification of functional groups has a significant impact on the pharmacological activity of berberine and has been the focus of new drug development (Jin et al., 2016). Indeed, the main active metabolite of berberine, berberrubine, shows better bioavailability and retains its parent compound’s beneficial effects, promoting significant interest as a therapeutic product. Berberrubine regulates glucose and lipid metabolism (Yang et al., 2017); promotes uric acid excretion (Lin et al., 2021); alleviates ulcerative colitis (Yu et al., 2018); and has antitumor (Dian et al., 2022), antithrombotic (Wang et al., 2021), and antiepileptic (Zhang et al., 2020) properties.
2 Sources of berberrubine
2.1 Extraction from plants
Natural products can be isolated from plants. Berberine was first isolated from the rhizomes of a Coptidis plant (Kobayashi et al., 1995) and the expression of RNA related to the continuous synthesis of enzymes for berberine metabolism was found to be highest in rhizomes and lowest in leaves (Samanani et al., 2005). Similarly, berberrubine was first isolated from the rhizomes of a Coptidis plant (Kobayashi et al., 1995). However, the yield of natural products is low and high cost. Furthermore, the overuse of medicinal plant may disrupt the natural ecological balance (Zhang et al., 2020). The natural berberrubine content in certain plants is very low, and chemical synthesis methods are well developed; thus berberrubine is usually not obtained by extraction from plants (Zhong et al., 2014).
2.2 Synthesis from berberine
2.2.1 Pyrolysis assisted synthesis
Iwasa et al. (Iwasa et al., 1996) first reported that heating berberine at 190 °C for 15 min would break the 9-methyl group to obtain berberrubine. However, this method had a low yield (69 %), and the byproducts were difficult to separate from the desired compound. In 2011, Bodiwala et al.(Bodiwala et al., 2011) improved on Iwasa et al.’s method by heating the reaction under vacuum conditions to reduce byproducts and increase yield (80 %–90 %). Subsequently, Jin et al. (Jin et al., 2014) heated berberine with N,N-dimethylformamide (DMF) at 190 °C for 15 min to get an improved berberrubine yield of 90 %. This method is now commonly used. Berberrubine can also be prepared by reacting urea and berberine at 200 °C with stirring at 76 % yield (Hong et al., 2000). The current demand for berberine in China alone has reached thousands of tons. The partial synthesis of berberrubine from berberine isolated from natural plants is thus unrealistic and unsustainable. Therefore, establishing a method for the chemical total synthesis of berberine or berberrubine would be a significant advancement. Understanding the chemical total synthesis process of berberine would determine the foundation for the synthesis of its derivative berberrubine. In 1969, Kametani et al.(Kametani et al., 1969) announced a method for the synthesis of berberine iodide through eight steps of reaction with 3,4-methylenedioxyphenylamine and 5-benzyloxy-2-bromo-4-methoxy- phenylacetate as raw materials. The chemical total synthesis of berberrubine bromide was described for the first time in the fourth step reaction. Although the method is simple, it is not high-yielding. At present, the synthesis of berberine skeleton is the primary method for the Mannich and transition metal catalyzed reactions (Liu et al., 2022). The former reaction material is easy to obtain and the reaction is simple to perform. In contrast, the latter reaction has a higher atomic economy. Yan et al. (Yan et al., 2021) compared the reported synthesis methods for berberine. Of those evaluated, the use of 2,3-dimethoxybenzyl alcohol and 3,4-methylenedioxyphenethylamine as raw materials for berberine synthesis via alkylation, chloromethylation, cyanidation, alcoholysis, condensation, and cyclization was the most optimal with a yield of 67 %.
2.2.2 Microwave-assisted synthesis
Microwave-assisted synthesis is an environmentally friendly method conforming to the principle of green chemistry (Gabano and Ravera 2022). In the microwave irradiation environment, the high-speed movement and rotational friction of polar molecules generate heat to provide energy for chemical reactions, which is a new method for the structural modification of natural products (Hu et al., 2021). Berberine can thus be converted into berberrubine in a microwave irradiation environment, which is rapid and energy-saving. However, uneven heating is an issue. In 2002, Das and Srinivas (Das and Srinivas 2002) microwaved berberine in an alumina oxide bath for 5 min and achieved 98 % yield. In 2015, Delgado-Camon et al.(Delgado-Camon et al., 2015) heated berberine at 130 °C for 5 min in a vacuum at 300 W, cooled to room temperature, and again heated it at 180℃ for 10 min to obtain berberrubine at a yield of 85.3 %. In 2018, Han et al.(Han et al., 2018) reacted berberine, N,N-dimethylformamide, and anhydrous lithium chloride in the microwave at 550 W and 160 °C for 20 min and then cooled to room temperature. After storing overnight in the refrigerator, the filtered red-brown solid was recrystallized in 95 % ethanol, and the yield obtained for berberrubine was 85 %.
Overall, the current method for chemical total synthesis of berberine is relatively perfect. However, research is currently lacking on the chemical total synthesis of berberrubine, which is currently mainly obtained by the pyrolysis of berberine. Microwave-assisted synthesis of berberrubine currently has a higher yield than that of traditional pyrolysis. The detailed of these studies are presented in Table 1.
Type
Condition
Yield
Reference
Pyrolysis assisted synthesis
190℃
69 %
(Iwasa et al., 1996)
Vacuum, 190℃
80 %-90 %
(Bodiwala et al., 2011)
DMF, 190℃
90 %
(Jin et al., 2014)
Urea, 200℃
76 %
(Hong et al., 2000)
Microwave-assisted synthesis
Alumina oxide bath
98 %
(Das and Srinivas 2002)
Vacuum, 300 W
85.3 %
(Delgado-Camon et al., 2015)
DMF, LiCl, 550 W
85 %
(Han et al., 2018)
2.3 Production by tissue culture of Coptidis plants
Unlike artificially cultivated plants, plant tissues are cultured in a well-controlled environment without dependence on the natural weather and climate and without the threat from pests and diseases. Most importantly, increasing the extraction yield with tissue culture can now be achieved with genetic engineering technology (Ochoa-Villarreal et al., 2016). Although the production of berberrubine by tissue culture has not been reported, berberine has been studied as early as the last century. In 1984, Fumihiko and Yasuyuki (Fumihiko and Yasuyuki 1984) cultured Coptis cells in White’s basal medium in darkness with high aeration and 3 % sucrose to obtain a berberine yield of about 1.39 mg/mL. In the same year, Nakagawa et al. cultured Thalictrum minus L. var. hypoleucum Miq. plant cells in LS medium. When the ratio of KNO3, the nitrogen source, and NH4Cl was 1:2, the berberrubine concentration obtained could be as high as 0.65 mg/mL (Nakagawa et al., 1984). The successful production of berberine by tissue culture suggests that berberrubine may also be obtained by tissue culture.
2.4 Microbial biosynthesis of berberrubine
Biosynthesis is a new research direction based on genetic and metabolic engineering, wherein organisms are engineered to obtain new functions(Patra et al., 2021). To enhance the output of natural products, biosynthesis can reduce the dependence on natural resources to meet the needs of business and scientific research (Cravens et al., 2019, Muhammad et al., 2020).
Understanding the biological synthesis of natural products is the basis of heterologous production. Currently, the biosynthesis of berberine is the conversion of L-tyrosine into 4-hydroxyphenylacetaldehyde and dopamine, which are processed by biological enzymes, such as synthetases and transferases, to synthesize the berberine skeleton (Huang et al., 2022). After modification of the skeleton, it is transformed into natural berberine. A small amount of berberrubine is also found in berberine-rich plants, indicating a biosynthetic pathway to synthesize berberrubine in plants the specific mechanism of which has not yet been established (Hayasaka et al., 2012). In 2015, Galanie and Smolke(Galanie and Smolke 2015) designed a S. cerevisiae strain that could express seven heterologous enzymes and optimized the culture conditions so that it could synthesize 1.8 mg/L berberine using norlaudanosoline. The founding that berberine is found to be metabolized into berberrubine by phytosynthesis in plants, gut microbiota, and hepatocytes can process berberine to, indicate biosynthesis will uncover feasible routes in berberrubine synthesis. Fig. 1 shows the biosynthetic pathway of berberrubine (Ding et al., 2012, Huang et al., 2022).The success of berberine biosynthesis will bring increasing yield of berberrubine. Researchers have begun to explore the biosynthesis of berberine.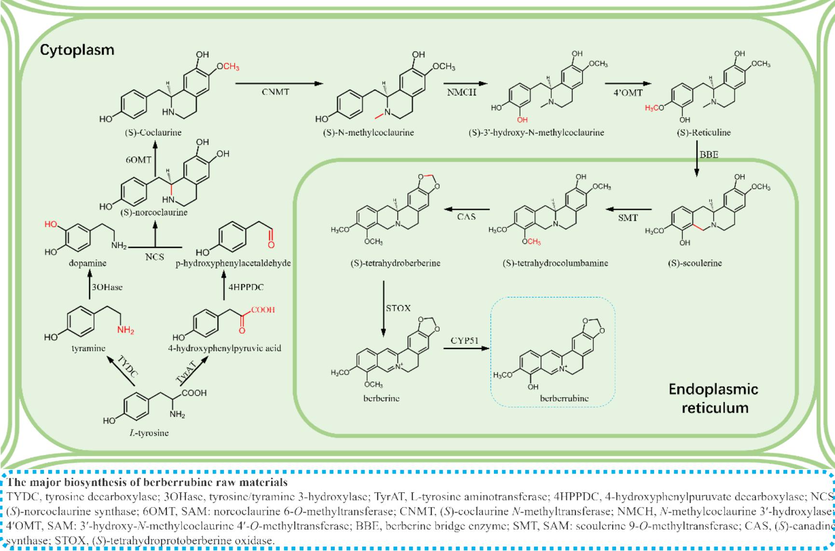
Biosynthesis pathway of berberrubine.
3 Pharmacokinetics of berberrubine
The absorption and metabolic transformation of berberine has been extensively studied to monitor its possible impact on various lifestyle-related diseases. Pharmacokinetic studies have shown a low plasma concentration after oral administration because of low absorption and utilization and the complex metabolic process it undergoes (Wang et al., 2017). The absolute bioavailability of berberine after oral administration in rats is below 1 % (Liu et al., 2016). Therefore, more and more researchers have focused on the active metabolites of berberine, such as berberrubine. Oral administration of 15 mg/kg berberrubine in rats yields an AUC0-∞ of berberrubine of 528.9 ± 78.5 ng·h/mL, whereas the AUC0-∞ for 5 mg/kg injection into the tail vein was 549.7 ± 51.2 ng·h/mL. This equates to an absolute bioavailability of berberrubine of 31.6 % (Wang et al., 2015). The structure of berberrubine determines its varying forms in different pH environments. It exists as an enol in an acidic environment (pH 4.5) (Spinozzi et al., 2014). In an alkaline environment (pH 8.5), such as in the intestine, berberrubine exists as a quinoid, a neutral molecule, which has better lipid solubility and can be better absorbed by the intestine. Fig. 2 shows the two structures of berberrubine. Fluorescence quenching of tryptophan revealed that berberine has two binding sites with bovine serum albumin, while berberrubine only has one. P-glycoprotein (P-gp) is a widely distributed transmembrane transport protein in the body that plays an important role in drug absorptionc is considered a key factor in mediating drug efflux. Berberine and berberrubine similarly act as substrates for P-gp; however, the affinity of berberrubine to P-gp is better than that of berberine (Zhang et al., 2019). In addition, the phenolic hydroxyl substitution of berberrubine can improve absorption rate (Li et al., 2010).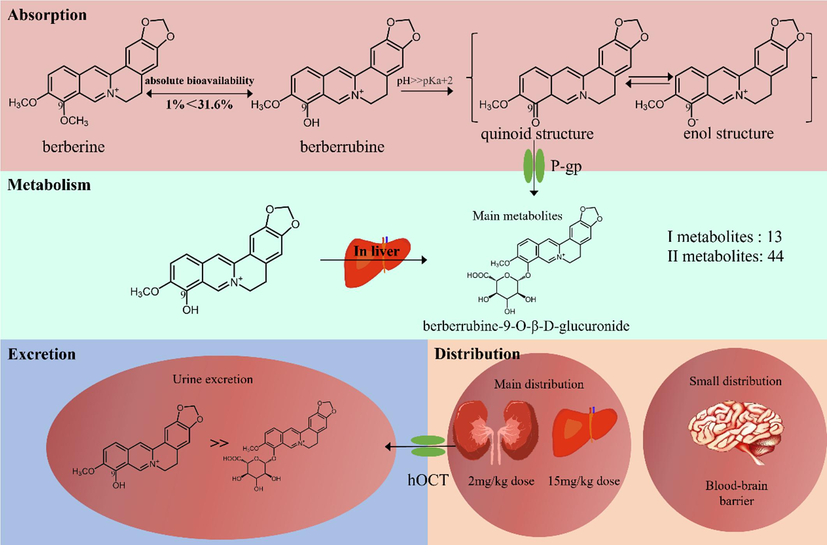
Pharmacokinetics of berberrubine.
In mice, berberrubine was mainly distributed in the liver, followed by the kidney and plasma, when it was orally administered at 15 mg/kg. Berberrubine concentration is lowest in the brain, indicating its poor penetration of the blood–brain barrier (Wang et al., 2015). Another study shows that after oral administration (2 mg/kg) in rats, beberrubine was mainly distributed in the kidney at the maximum concentration of 2070 ± 340 ng/g at 0.5 h, followed by the plasma and liver (Porru et al., 2018). The detailed pharmacokinetic parameters of these studies are presented in Table 2. In conclusion, berberrubine is more likely to accumulate in the kidney when taken orally at 2 mg/kg in rats, while it mainly accumulates in the liver when taken orally at 15 mg/kg in mice. These findings may be due to the inhibitory effect of berberrubine on transporters. Berberrubine can inhibit the activity of organic cationic transporters (hOCT) (Li et al., 2016, Zhang et al., 2022). hOCT are involved in renal excretion, and hOCT2 and hOCT3 may be expressed in the basolateral part of proximal tubule cells. The transport to the kidney is reduced when their activity is inhibited by berberrubine (Koepsell 2020, Samodelov et al., 2020). Tmax, time to reach this concentration; Cmax, maximum plasma concentration; AUC0-t, the area under the plasma concentration–time curve from zero to the time of the final measurable sample; AUC0-∞, the area under the plasma concentration–time curve from zero to infinity; CL, clearance; V, apparent volume of distribution.
Route of administration
Species
Dose
(mg/kg)
Tmax (h)
Cmax
(ng/mL)
AUC(0-t)
(ng·h/mL)
AUC(0-∞)
(ng·h/mL)
CL
(L·h/kg)
V
(L/kg)Reference
Oral
Rat
15
−
301.8 ± 19.1
519.7 ± 81.6
528.9 ± 78.5
28.8 ± 4.7
44.1 ± 20.0
(Wang et al., 2015)
Tail vein injection
Rat
5
−
1312.8 ± 482.3
547.8 ± 50.9
549.7 ± 51.2
9.2 ± 0.9
6.8 ± 1.9
Oral
mouse
2
0.5
204 ± 18
−
−
−
−
(Porru et al., 2018)
Wang et al. (Wang et al., 2018) found that after oral administration of berberrubine (30 mg/kg) in rats, 13 phase I metabolites were identified in the urine, plasma, bile, and feces, most of which were produced by demethylation, reduction, and hydroxylation. Most of the remaining 44 phase II metabolites were produced by glucuronidation and sulfation. Among them, berberrubine-9-O-β-D-glucuronide is the major metabolite in rat plasma, bile, and urine. Interestingly, berberrubine is rapidly converted to berberrubine-9-O-β-D-glucuronide in the liver but is excreted from the urine in its original form rather than as a metabolite. This may be because berberrubine-9-O-β-D-glucuronide is hydrolyzed to berberrubine by intestinal flora and reabsorbed during enterohepatic circulation (Zuo et al., 2006). Berberrubine exists as an active metabolite of berberine in vivo. Berberrubine was found in rats orally administered with berberine 200 mg/kg for 48 h at a conversion rate of 65.1 %, and berberine was continuously metabolized into grade II metabolite (Ma et al., 2013, Tan et al., 2013). Sterol 14α-demethylase (CYP51) belongs to the family of CYP450 enzymes and is present in many bacteria, lower eukaryotes, and mammals (Zhang et al., 2023). Studies have found that mouse hepatocytes and intestinal flora use the CYP51 enzyme to metabolize berberine to berberrubine and subsequently induce E. coli expressing the CYP51 gene to synthesize berberine. This study confirmed that CYP51 is involved in the demethylation metabolism of berberine (Zhang et al., 2021).
In conclusion, compared with berberine, berberrubine has a faster absorption and slower metabolism. In addition, understanding the metabolic pathway and products of berberrubine in animals is beneficial to further study these mechanisms to determine its physiological effects and toxicity.
4 Pharmacological activities of berberrubine
4.1 Antitumor
Cancer is the second leading cause of death, and the mortality rate has increased by 0.5 % in recent years (Ferlay et al., 2015). The China Cancer Report released by the National Cancer Center of China in 2022 registered 4.064 million new cases and 2.413 million cancer deaths, among which lung, colorectal, gastric, liver, and breast cancers were the most common (Zheng et al., 2022). Chemotherapy is widely used to treat cancer. Compared with the byproducts of synthetic drug production, the natural drugs isolated from plants are more easily available and have higher activity (Abu Samaan et al., 2019). From 1980 to 2019, 40 % of the antitumor drugs approved globally were developed from natural products and their derivatives (Newman and Cragg 2020). Natural products and their derivatives can effectively target many pathways involved in cancer pathogenesis (Cragg et al., 2009). For example, paclitaxel, a drug approved by the U.S. FDA for the clinical treatment of advanced ovarian cancer, inhibits the assembly of tubulin into microtubules, blocks the cell cycle, prevents mitosis, and hinders cancer cell growth (Zhu and Chen 2019).
Berberine is recognized as a drug with antitumor activity, with therapeutic effects on lung, gastric, breast, and colon cancers, among others (Samadi et al., 2020, Achi et al., 2022, Zhu et al., 2022). Berberrubine similarly has antitumor effects. In 2021, patients with breast cancer accounted for 30 % of female patients with malignant tumors, and 15 % of cancer deaths (Wang et al., 2021). The incidence of breast cancer has annually grown at a rate of 0.5 %, and has become the second leading cause of cancer death after lung cancer (Giaquinto et al., 2022). The discovery of new and effective drugs for breast cancer is thus urgent. Dian et al. (Dian et al., 2022) compared the anti-breast cancer activity of berberine and its derivatives, including berberrubine. They found that berberrubine (10–50 µM) can inhibit the proliferation of MCF7 and MDA-MB 231 breast cancer cells at the S phase and induce cell apoptosis by downregulating the expression of procaspase-3, procaspase-9, procaspase-8, and polyADP-ribose polymerase proteins. Furthermore, it can inhibit cell migration and invasion by upregulating the expression of GSK-3β and E-cadherin proteins and downregulating the expression of β-catenin and N-cadherin proteins. Compared with berberine, berberrubine can more significantly inhibit cell migration and invasion, suggesting that berberrubine has higher potency against breast cancer. The berberrubine and a carrier assembled into nanoparticles could disintegrate at the tumor site due to the acidic microenvironment, which allows the drug to be concentrated in the cancerous tissue, indicating a potential as targeted treatment for breast cancer (Jia et al., 2022). Berberrubine also has an inhibitory effect on other cancer types. Berberrubine inhibited the cell migration and proliferation of urothelial carcinoma cells and upregulated the expression of glutathione S-transferase (GST) active protein and mRNA (Shen et al., 2022). Berberrubine (20 µM) can reduce the wound healing rate of MGC-803 and HGC-27 gastric cancer cells and stop cell proliferation at the G2/M stage, inducing apoptosis in gastric cancer cells (Yu et al., 2023).
Topoisomerases (TOP) have attracted significant attention in anticancer drug development because they can regulate DNA topology and actively participate in chromosome replication, transcription, and segregation (Greco et al., 2022). For example, camptothecin derivatives topotecan and irinotecan, which have been approved by the U.S. FDA for clinical use, can treat cancer by blocking the activity of TOP to aggravate DNA damage (Vann et al., 2021, Talukdar et al., 2022). Tyrosyl-DNA phosphodiesterase (TDP) is a DNA repair enzyme that catalyzes the hydrolysis of phosphodiester bonds in the TOP1 covalent complex and repairs some other 3′-end DNA adducts (Delgado et al., 2018). The overexpression of TDP in tumor cells inhibited camptothecin activity on TOP, whereas, conversely, the inhibition of TDP activity enhanced the inhibitory effect of camptothecin on TOP (Zakharenko et al., 2023). Yang et al. proposed (Yang et al., 1996) to inhibit TDP1 enzyme activity to enhance the inhibition of TOP. In 2018, Zhang X.R. et al. (Kim et al., 1998) found that the natural product oxynitidine was a dual inhibitor of TDP and TOP. Similarly, berberrubine is an inhibitor of TOP (Kim et al., 1998) and TDP (Gladkova et al., 2021) during cell proliferation. Berberrubine and aliphatic sulfonyl chloride reaction substitutes more effectively inhibited TDP activity than berberine and exhibited a higher synthesis yield (Gladkova et al., 2021). Berberine and its derivatives isolated from Coptis rhizomes can inhibit TOP activity. However, berberrubine alone can inhibit the activity of TOP2 (Kobayashi et al., 1995). Kang and Chung (Kang and Chung 2002) induced berberrubine resistance in human colorectal cancer cells (AMC5) and found that the upregulation by berberrubine of TOP promoter activity was reduced in drug-resistant cells, which decreased TOP expression. Thus, TOP is a target of berberrubine antitumor activity. Overall, the antitumor mechanism of berberrubine may be related to the inhibition of TDP and TOP activities, which lead to DNA replication arrest and double-strand break formation (Zakharenko et al., 2019). In addition, TDP and TOP are important targets in cancer drug design, suggesting a potential research direction on berberrubine against tumor structure modification. Fig. 3 shows the antitumor mechanism of berberrubine.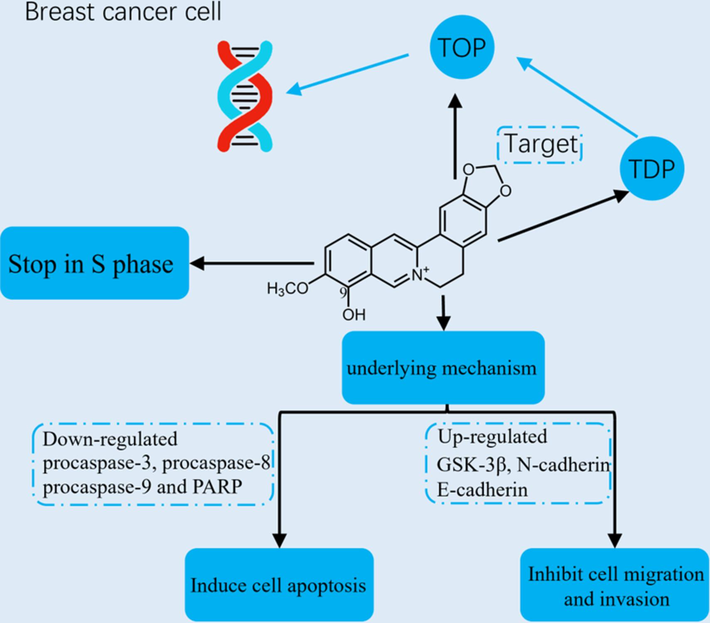
The anti-tumor mechanisms of berberrubine.
4.2 Lowering blood glucose and lipids
Long-term high-fat intake can cause obesity and lead to type II diabetes, hyperlipidemia, nonalcoholic fatty liver disease, and cardiovascular diseases, among others. At least 20,000 people die every year due to obesity (Duan et al., 2021). Berberine, a plant alkaloid with lipid and glucose-lowering properties, is widely used in the treatment of type II diabetes and hyperlipidemic diseases. Berberine and berberrubine can upregulate InsR and low-density lipoprotein receptor (LDLR) mRNA on HepG2 cell membrane and activate AMPK, thereby reducing blood glucose and lipid levels (Li et al., 2011). An in vitro study showed that berberine had better pharmacological activity than berberrubine in regulating glycolipid metabolism in vitro. However, berberrubine often showed a stronger lowering of blood glucose and lipid levels in vivo (Yang et al., 2017). On the one hand, this result may be related to the lower bioavailability of berberine; on the other, berberrubine may have stronger mediating effects on glucose and lipid metabolism (Pirillo and Catapano 2015).
The starches and disaccharides in food are hydrolyzed into glucose by α-glucosidase, a carbohydrate hydrolase. Inhibition of α-glucosidase activity can reduce the absorption of glucose in the small intestine and control blood glucose in patients with hyperglycemia, making α-glucosidase a key target enzyme for the treatment of type II diabetes (Zhang et al., 2020). Yang et al. (Yang et al., 2017) found that oral administration of berberrubine (50 mg/kg) could more potently inhibit intestinal α-glucosidase activity than berberine (120 mg/kg) in high-fat diet mice, thereby reducing polysaccharide absorption and blood glucose.
Berberrubine can regulate metabolic disorders by binding to farnesoid X receptor (FXR). FXR is mainly expressed in the intestinal tract and liver, and regulates bile acid metabolism to maintain glucose and lipid stability (Sepe et al., 2019). In the intestine, FXR can regulate transporters to inhibit the reabsorption of bile acid salts, while in the liver, it inhibits the synthesis of bile acids through a small heterodimer partner (SHP) (Mencarelli et al., 2013). Bile acids are derivatives of cholesterol, which can reduce blood glucose by regulating intestinal incretin secretion, hepatic gluconeogenesis and glycogen synthesis (Shapiro et al., 2018). Berberrubine can directly activate FXR in the gut, and increase the level of free bile acid in the liver and conjugated bile acid in the feces, thereby reducing the blood glucose level, and increasing the expression of organic solute transporter α(Osta) protein in the distal ileum of mice induced by a high-fat diet (Sun et al., 2021).
The metabolic-regulating effect of berberrubine may not only affect FXR activity but also the upregulation of LDLR and inhibition of lipid accumulation in HepG2 cells (Zhou et al., 2014). LDL is taken up by LDLR on the liver cell membrane and is degraded into cholesterol by lysosomes. The reduction of LDL levels is an important indicator for the treatment of hyperlipidemia, based on which LDL-lowering statins have been developed (Costet 2010). LDLR binds to proprotein convertase subtilisin kexin type 9(PCSK9) and its degradation increases LDL levels. Therefore, PCSK9 is a key regulator of LDLR. It has become the main target of therapeutic strategies for reduced circulating LDL levels (Maxfield and van Meer 2010). PCSK9 expression is regulated by the ERK signaling pathway downstream of JAK (Cao et al., 2011)Cao et al. (Cao et al., 2018) found that berberrubine can upregulate LDLR by inhibiting PCSK9 expression in HepG2 cells. This effect can, in turn, be blocked by ERK pathway inhibitor PD98059. These results indicate that the target of berberrubine action may be in the ERK signaling pathway.
In addition, Yang et al. (Yang et al., 2022) found that berberrubine administration (40 mg/kg, 4 weeks) improved glucose homeostasis in high-fat diet mice by reducing the expression of gluconeogenic proteins (G6Pase and PEPCK) and enhancing the expression of glucose uptake (GLUT2) and glycogen synthesis (GSK3β). Berberrubine also reduced the protein level of peroxisome proliferator-activated receptor γ (PPARγ) in liver tissue. PPARγ is the most important PPAR isoform in glucose and lipid metabolism and is mainly expressed in adipose tissue and vascular smooth muscle (Zhou et al., 2008). PPARγ activation can enhance the insulin sensitivity of cells, which is considered as a potential therapeutic target. Furthermore, molecular docking shows that berberrubine has a high intrinsic activity on PPARγ (Chen et al., 2012). Interestingly, berberrubine (Yang et al., 2022) and berberine (Wang et al., 2021) inhibit PPARγ expression during blood glucose regulation. Yang et al. (Yang et al., 2022) also found that berberrubine (4 μM) improved the lipid metabolism of oleic acid and in HepG2 cell by upregulating the expression of the proteins related to lipolysis (ATGL) and fatty acid β-oxidation (CPT-1 and PPARα). In the meantime, Yang et al. (Yang et al., 2022) also found that berberrubine improved the abundance of intestinal microbiota in mice to aid in glucose control, such as reducing the obesity-related Romboutsia, and increasing beneficial flora Roseburia and Mucispirillum, which were reported to produce short-chain fatty acids.
In conclusion, both berberine and berberrubine can regulate glucose and lipid metabolism, and berberrubine may exhibit stronger biological activity due to its higher bioavailability. On the hand, berberrubine regulates glucose and lipid metabolism in vitro mainly by inhibiting α-glucosidase and intestinal glucose absorption. In vivo, berberrubine selectively activates FXR in the gut to regulate glucose homeostasis and increases LDL consumption to reduce blood lipid levels through the ERK signaling pathway. In addition, berberrubine improves intestinal flora and helps regulate glucolipid metabolism. Berberrubine can reduce metabolic disorders induced by high-fat diet intake in many ways. Berberrubine combined with statins significantly reduced blood lipids. However, clinical studies are lacking on the regulation of glycolipid metabolism by berberine (Kong et al., 2008). Furthermore, a study on the effect of berberine on the L-O2 cell line and its major metabolite berberrubine-9-O-β-D-glucuronide found that G6Pase mRNA level in cells was decreased, which inhibited the metabolism of glucose 6-phosphate to glucose and enhanced the sensitivity of insulin-resistant cells (Yang et al., 2017). Glucoaldehyde is the main drug metabolite in liver, and many drugs can still maintain potency after metabolism. For example, morphine-6-glucuronic acid has similar pharmacological activity to morphine and has been successfully developed as a first-line analgesic drug (Strassburg et al., 2002). Therefore, the rapid transformation of berberrubine into metabolites in the liver does not affect its hypoglycemic activity Fig. 4 shows mechanisms of berberrubine in lowering blood glucose and lipids.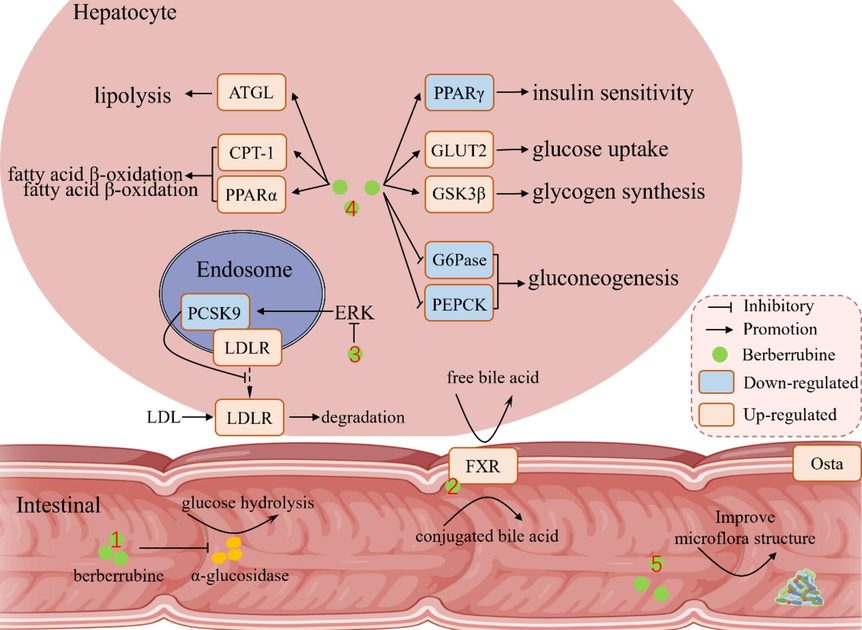
Underlying mechanisms of berberrubine in lowering blood glucose and lipids.
4.3 Promotion of uric acid excretion
Hyperuricemia is a metabolic disease caused by abnormal purine metabolism, which has become a global health problem. Hyperuricemia is considered as the fourth common metabolic disease after hypertension, hyperlipidemia, and hyperglycemia (Xu et al., 2021). The key to treating hyperuricemia is to control serum uric acid levels. Therefore, reducing uric acid production and promoting its excretion are the key mechanisms in the treatment of hyperuricemia, whereas the auxiliary treatment involves dietary and lifestyle changes. Allopurinol and phenylbromarone are currently used in the clinical treatment of hyperuricemia. Although they help reduce uric acid levels in the blood, their side effects, including allergic reactions, cardiovascular diseases, and hepatorenal toxicity, limit their long-term application (Kang et al., 2021). Among numerous natural active products, researchers found that berberine can inhibit the activities of urate transporter 1 (URAT1) and glucose transporter 9 (GLUT9) to alleviate hyperuricemia (Li et al., 2021, Shan et al., 2022). Lin et al. (Lin et al., 2021) found that berberrubine, as one of the active forms of berberine, can inhibit the activities of URAT1, GLUT9, and xanthine oxidase (XOD) to reduce uric acid production, as well as upregulate the expression of protein and mRNA of organic anion transporter 1/3 (OAT1/3) and ATP-binding cassette transporter (ABCG2) to promote uric acid excretion. Recent studies have found that xanthine, a metabolite of adenine and guanine in food and dying cells, is oxidized into uric acid by XOD in the liver. Uric acid is distributed to the kidney by blood circulation. OAT1/3 in the kidney transports uric acid from the renal interstium to the proximal tubular epithelial cells. Furthermore, ABCG2 functions as a high-capacity urate secretion transporter, and some uric acid can be reabsorbed by URAT1 and GLUT9. Berberine can regulate many mechanisms, such as uric acid production and excretion, and has development prospects (Yanai et al., 2021). Zhong et al. (Zhong et al., 2023) administered 8-oxyberberrubine, a novel metabolite in liver microsomes, to mice with hyperuricemia and found that it was more effective than berberrubine. 8-oxyberberrubine was more potent than berberrubine, and a series of inflammatory reactions caused by hyperuricemia were alleviated through the inflammasome NLRP3. Overall, berberrubine demonstrates good anti-hyperuricemia activity.
4.4 Antithrombosis
A high-fat diet can not only induce hyperlipidemia and diabetes, but also cause cardiovascular diseases, including thromboembolic diseases (Miszta et al., 2020). In thromboembolic disease, platelet activation promotes the conversion of fibrinogen to fibrin to form thrombi; thus, inhibiting platelet activity, such as by aspirin intake, has become treatment for thromboembolic diseases. Because PI3K/AKT has no significant effect on primary hemostasis, it has become the therapeutic target of anti-platelet aggregation drugs (Su et al., 2016). Some studies have found that berberine can improve the blood hypercoagulation state of rats induced by a high-fat diet, but the effect is not ideal (Wang et al., 2018). Their in vitro studies on berberrubine revealed that it can inhibit ADP-activated platelets and the binding of activated platelets to fibrinogen for antithrombosis. The potential mechanism may be related to the specific inhibition of PI3Kβ and Rasa3 proteins and the subsequent inhibition of Rap1 activation to inhibit platelet activation (Wang et al., 2021). Vitamin K deficiency interferes with the synthesis of coagulation proteins and, consequently, with the coagulation process (Mishima et al., 2023). Warfarin, a commonly used oral anticoagulant, prevents the synthesis of vitamin K-dependent coagulation proteins. This means that the coagulation factors already synthesized by the body are not affected, and the effect of warfarin is slow but lasting. Furthermore, the resulting bleeding becomes a common and serious adverse reaction (Mega and Simon 2015). An in vivo study showed that orally administered berberrubine (100 mg/kg) prolonged prothrombin time and inhibited carrageenan-induced tail thrombosis in mice. A metabolomics study found that berberrubine could regulate the vitamin K catalytic cycle, while subsequent molecular docking revealed that berberrubine could bind to VKOR and GGCX, two important enzymes in the vitamin K cycle, but would not prolong the tail bleeding time (Wang et al., 2023). In conclusion, berberrubine exerts an antithrombotic effect by inhibiting platelet activation and participating in the vitamin K catalytic cycle pathway, with good biological activity. However, berberrubine does not interfere with primary hemostasis or prolong bleeding time. Therefore, berberrubine is safer than aspirin and warfarin, which broadens its clinical application prospects Fig. 5 shows underlying mechanisms of berberrubine in antithrombosis.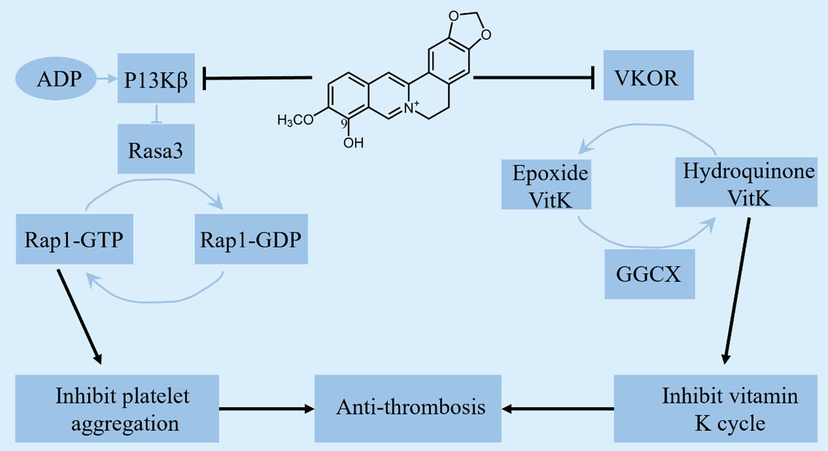
Underlying mechanisms of berberrubine in antithrombosis.
4.5 Anti-epilepsy
Epilepsy, a common and recurrent nervous system disease, is characterized by convulsive behavior caused by the abnormal discharge of neurons due to microneuron damage, proliferation of neuroglia, and increase of microglia. Most studies on epilepsy focus on the hippocampus. When seizure occurs, inflammatory factors in the hippocampus are upregulated, and inflammatory factors further promote the development of epilepsy (Rana and Musto 2018). Although the intestinal absorption rate of berberine is very low, it can penetrate the blood–brain barrier and accumulate in the hippocampus to act as neurons. Berberine mainly exerts anti-apoptosis, anti-inflammatory, and antioxidant effects to treat neurological diseases through the Pl3K/Akt/Bcl-2 and MAPK signal pathways (Lin and Zhang 2018). Some studies have found that berberine can alleviate kainic acid-induced status epilepticus in rats and protect neurons by exerting antioxidative and anti-inflammatory effects, including reducing the levels of reactive oxygen species (ROS), NF-κB, TLR4, TNFα, and IL-1β (Sedaghat et al., 2017). In addition, berberine and berberrubine can prolong the latency of pentylenetetrazolium (PTZ)-induced epilepsy in zebrafish; inhibit epileptiform behavior; restore c-fos and neuronal discharge during seizures; inhibit the recruitment of neutrophils and macrophages in zebrafish brain; and reduce TNFα, IL-1β, and IL-6 levels. Thus, berberine and berberrubine can alleviate epilepsy symptoms by inhibiting inflammatory pathways. However, at the same dose (100 μM) berberrubine has a stronger effect on alleviating PTZ-induced epilepsy than berberine (Zhang et al., 2020).
4.6 Anti-ulcerative colitis
Ulcerative colitis is a chronic, frequently recurring inflammation that is characterized by the development of ulcers in the colon and rectal mucosa, causing abdominal pain, diarrhea, and even hematochezia. In clinical practice, aminosalicylic acid and glucocorticoid are often administered to alleviate the inflammation in ulcerative coliti. While immunosuppressive drugs can be used for severe cases, they do not achieve satisfactory effects and even have high costs and adverse reactions (Cao et al., 2019). As an antibacterial drug, berberine is mainly used in the treatment of dysentery and intestinal infection and can reduce damage to the intestinal mucosa induced by proinflammatory cytokines. Berberine can alleviate the symptoms of ulcerative colitis induced by dextran sodium sulfate in mice, including diarrhea, bloody stool, and weight loss. In addition, berberine can regulate the expression of Bcl-2, Bax, and Caspase-3 to inhibit the apoptosis of colon cells and reduce the levels of inflammatory factors TNF-α, IFN-γ, and IL-1β to alleviate inflammation (Wang et al., 2021). Compared with berberine (50 mg/kg), berberrubine (20 mg/kg) can alleviate the symptoms of ulcerative colitis induced by dextran sodium sulfate in mice and alleviate the damage to the intestinal mucosal barrier caused by colonic inflammation by inhibiting the expression of TJ proteins and mucins (Yu et al., 2018). The occurrence and spread of intestinal inflammation can destroy barrier function. Some studies have shown that berberrubine can preserve barrier function from inflammation damage (Cui et al., 2006). The 9-hydroxy group in the berberrubine structure can increase the clearance of free radicals, such as ROS, without significant negative effects (Jang et al., 2009).
5 Toxicity of berberrubine
Few studies have explored the toxicity of berberine and berberrubine. The median lethal dose (LD50) of berberine administered by intraperitoneal injection in rats and mice is 205 and 30 mg/kg, respectively (Singh et al., 2021). However, berberine is non-toxic at clinical doses, although gastrointestinal discomfort and blood pressure drop are occasionally observed at higher doses of oral berberine (Zhang et al., 2020, Zhao et al., 2021). Berberine (10 µM) exhibited inhibitory toxicity to primary myocardial cells of neonatal rats, but berberrubine did not at the same dose (Zhang et al., 2018). Similarly in zebrafish larvae, the LD50 of berberine is 623.3 μM. Compared with berberine, berberrubine has an LD50 of 3012 μM and is thus less toxic and safer (Zhang et al., 2020). Studies have found that berberrubine (100 μM) can significantly inhibit the growth of gastric cancer cells but has no significant inhibitory effect on normal human epithelial cells (Yu et al., 2023). The liver is the main organ for drug metabolism and has attracted much attention in the study of toxicity and side effects. Berberrubine (100 mg/kg) can cause liver injury in mice 2 h after intraperitoneal injection, which was manifested as alanine aminotransferase (ALT) and aspartate aminotransferase (AST) activities enhanced in serum. Significant inflammatory cell infiltration and edema were observed in hepatocytes. However, berberrubine administered orally 100 mg/kg/day to rats showed only mild hepatotoxicity after 6 weeks. The mechanism of berberrubine-induced liver injury may be related to the modification of cysteine residues of proteins in the liver by its metabolites (Wang et al., 2020). The kidney is the main organ for drug excretion and therefore is often of interest in the study of side effects. One study found that after oral administration of berberrubine (50 mg/kg/day) for 6 weeks, mice on a high-fat diet had higher serum levels of blood urea nitrogen (BUN) and renal tubular and interstitial lesions. Berberrubine also reduces the mRNA levels of NPHS1 and SYNPO, two genes that encode proteins essential to renal filtration function (Yang et al., 2016). Endogenous substances, such as glutaric acid and linoleic acid, in mice fed with a high-fat diet inhibited the activity of UDP-glucuronosyltransferases (UGTs) in the liver, which are essential to the metabolism of glucuronic acid in the liver. This means that the main metabolic pathway of berberrubine in the liver is inhibited, and other metabolites are produced, resulting in renal toxicity (Yang et al., 2018). These findings indicate that berberrubine induces liver and kidney toxicity when used long-term at high doses. Furthermore, conformational studies on the safety of berberrubine at a reasonable dose are needed.
6 Concluding remarks and outlook
Natural products have been increasingly recognized as excellent sources of medications. Berberrubine, a bioactive metabolite and possible alternative to berberine, shows good bioavailability and has multiple pharmacological effects. Currently, berberrubine is obtained by chemical synthesis using berberine as raw material. Although the chemical total synthesis of berberine has been well-defined, its production is still limited (Chen et al., 2017). Therefore, the methods of obtaining berberrubine sustainably need to be established. The synthesis of berberrubine in plants is unclear, but the pathway of berberrubine as a metabolite in the body has been elucidated. Whether berberrubine can be obtained by modifying berberine biosynthesis is a promising future research direction.
In summary, berberrubine has better bioavailability than berberine, has a wide range of pharmacological effects, and exerts therapeutical potential for many diseases. Studies with a focus on the potential molecular mechanism, dose–effect relationship, toxicity, and clinical efficacy are required to promote its clinical application.
CRediT authorship contribution statement
Yi Li: Writing – original draft, Investigation. Gangmin Li: Writing – original draft. Cheng Peng: Funding acquisition. Xiaodong Shi: Supervision. Fu Peng: Writing – review & editing. Ailsa McGregor: Writing – review & editing. Xiaofang Xie: Writing – review & editing, Conceptualization.
Acknowledgements
This work was supported by the Regional Joint Fund of the National Natural Science Foundation of China (no. U19A2010); National Natural Science Foundation of China (no. 81891012); National Natural Science Foundation of China (no. 82003879); National Inter disciplinary Innovation Team of Traditional Chinese Medicine (no. ZYYCXTD-D-202209); Sichuan provincial innovation team of Chinese medicine science and technology industry (no. 2022C001); Sichuan Province Natural Science Foundation Program (Grant NO. 24NSFSC0627); Xinglin Scholars“ Nursery Talent Special Program(grant number MPRC2023032);and also supported by the National Scholarship Fund of China and Fund of the State Key Laboratory of Southwestern Chinese Medicine Resources.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Paclitaxel's Mechanistic and Clinical Effects on Breast Cancer. Biomolecules.. 2019;9
- [CrossRef] [Google Scholar]
- Multi-Target Potential of Berberine as an Antineoplastic and Antimetastatic Agent: A Special Focus on Lung Cancer Treatment. Cells.. 2022;11
- [CrossRef] [Google Scholar]
- Synthesis of 9-substituted derivatives of berberine as anti-HIV agents. Eur J Med Chem.. 2011;46:1045-1049.
- [CrossRef] [Google Scholar]
- Janus kinase activation by cytokine oncostatin M decreases PCSK9 expression in liver cells. J Lipid Res.. 2011;52:518-530.
- [CrossRef] [Google Scholar]
- Berberrubine and its analog, hydroxypropyl-berberrubine, regulate LDLR and PCSK9 expression via the ERK signal pathway to exert cholesterol-lowering effects in human hepatoma HepG2 cells. J Cell Biochem 2018
- [CrossRef] [Google Scholar]
- Progress in active compounds effective on ulcerative colitis from Chinese medicines. Chin J Nat Med.. 2019;17
- [CrossRef] [Google Scholar]
- Three-in-one agonists for PPAR-α, PPAR-γ, and PPAR-δ from traditional Chinese medicine. J Biomol Struct Dyn.. 2012;30:662-683.
- [Google Scholar]
- A Concisely Convergent Synthesis of Berberine Chloride. Chinese Journal of Organic Chemistry.. 2017;37:503-507.
- [CrossRef] [Google Scholar]
- Molecular pathways and agents for lowering LDL-cholesterol in addition to statins. Pharmacol Ther.. 2010;126:263-278.
- [CrossRef] [Google Scholar]
- Impact of natural products on developing new anti-cancer agents. Chem Rev.. 2009;109:3012-3043.
- [CrossRef] [Google Scholar]
- Synthetic biology strategies for microbial biosynthesis of plant natural products. Nat Commun.. 2019;10:2142.
- [CrossRef] [Google Scholar]
- Effect of berberrubine on interleukin-8 and monocyte chemotactic protein-1 expression in human retinal pigment epithelial cell line. Life Sci.. 2006;79:949-956.
- [Google Scholar]
- Conversion of berberine into berberrubine by selective demethylation under microwave irradiation. Synthetic Communications.. 2002;32:3027-3029.
- [CrossRef] [Google Scholar]
- A High Dose of Dietary Berberine Improves Gut Wall Morphology, despite an Expansion of Enterobacteriaceae and a Reduction in Beneficial Microbiota in Broiler Chickens.. 2023;mSystems. 8:e0123922.
- Resonance driven regioselective demethylation of berberine. Microwave assisted synthesis of berberrubine and its assessment as fluorescent chemosensor for alkanes. Tetrahedron.. 2015;71:6148-6154.
- [CrossRef] [Google Scholar]
- Berberine alkaloids inhibit the proliferation and metastasis of breast carcinoma cells involving Wnt/β-catenin signaling and EMT. Phytochemistry.. 2022;200:113217
- [CrossRef] [Google Scholar]
- Progress in Synthesis and Physiological Activity of Berberine Derivatives. Chinese Journal of Organic Chemistry.. 2012;32:677-685.
- [CrossRef] [Google Scholar]
- Flavonoids from Whole-Grain Oat Alleviated High-Fat Diet-Induced Hyperlipidemia Regulating Bile Acid Metabolism and Gut Microbiota in Mice. J Agric Food Chem.. 2021;69:7629-7640.
- [CrossRef] [Google Scholar]
- Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer.. 2015;136:E359-E386.
- [CrossRef] [Google Scholar]
- High berberine-producing cultures of coptis japonica cells. Phytochemistry.. 1984;23
- [Google Scholar]
- Microwave-Assisted Synthesis: Can Transition Metal Complexes Take Advantage of This “Green” Method? Molecules.. 2022;27
- [CrossRef] [Google Scholar]
- Optimization of yeast-based production of medicinal protoberberine alkaloids. Microb Cell Fact.. 2015;14:144.
- [CrossRef] [Google Scholar]
- Discovery of Novel Sultone Fused Berberine Derivatives as Promising Tdp1 Inhibitors. Molecules.. 2021;26
- [CrossRef] [Google Scholar]
- Marine-Derived Compounds Targeting Topoisomerase II in Cancer Cells: A Review. Mar Drugs.. 2022;20
- [CrossRef] [Google Scholar]
- Synthesis of 9-Substituted Berberine Derivatives with Microwave Irradiation. Chemical Research in Chinese Universities.. 2018;34:571-577.
- [CrossRef] [Google Scholar]
- Traditional Japanese herbal (kampo) medicines and treatment of ocular diseases: a review. Am J Chin Med.. 2012;40:887-904.
- [CrossRef] [Google Scholar]
- Antimicrobial activity of 9-O-acyl- and 9-O-benzoyl-substituted berberrubines. Planta Med.. 2000;66:361-363.
- [Google Scholar]
- Microwave technology: a novel approach to the transformation of natural metabolites. Chin Med.. 2021;16:87.
- [CrossRef] [Google Scholar]
- Biosynthesis Investigations of Terpenoid, Alkaloid, and Flavonoid Antimicrobial Agents Derived from Medicinal Plants. Antibiotics (basel).. 2022;11
- [CrossRef] [Google Scholar]
- Berberine and barberry (Berberis vulgaris): A clinical review. Phytother Res.. 2019;33:504-523.
- [CrossRef] [Google Scholar]
- Antibacterial activity and structure-activity relationships of berberine analogs. Eur J Med Chem.. 1996;31:469-478.
- [CrossRef] [Google Scholar]
- Hydroxyl radical scavenging activities of isoquinoline alkaloids isolated from Coptis chinensis. Arch Pharm Res.. 2009;32:341-345.
- [CrossRef] [Google Scholar]
- Dual-responsive nanoparticles with transformable shape and reversible charge for amplified chemo-photodynamic therapy of breast cancer. Acta Pharmaceutica Sinica. b.. 2022;12:3354-3366.
- [CrossRef] [Google Scholar]
- Pharmacological effects of berberine and its derivatives: a patent update. Expert Opin Ther Pat.. 2016;26:229-243.
- [CrossRef] [Google Scholar]
- Design, synthesis, and anticancer activity of novel berberine derivatives prepared via CuAAC “click” chemistry as potential anticancer agents. Drug Des Devel Ther.. 2014;8:1047-1059.
- [CrossRef] [Google Scholar]
- Studies on the syntheses of heterocyclic compounds. CCCII. Alternative total syntheses of (+ -)-nandinine, (+ -)-canadine, and berberine iodide. J Chem Soc Perkin. 1969;1. 15:2036-2038.
- [Google Scholar]
- Down-regulation of DNA topoisomerase IIalpha in human colorectal carcinoma cells resistant to a protoberberine alkaloid, berberrubine. Mol Pharmacol.. 2002;61:879-884.
- [Google Scholar]
- Cardiovascular risk associated with allopurinol vs. benzbromarone in patients with gout. Eur Heart J.. 2021;42:4578-4588.
- [CrossRef] [Google Scholar]
- Induction of topoisomerase II-mediated DNA cleavage by a protoberberine alkaloid, berberrubine. Biochemistry.. 1998;37:16316-16324.
- [Google Scholar]
- Inhibitors of DNA topoisomerase I and II isolated from the Coptis rhizomes. Planta Med.. 1995;61:414-418.
- [Google Scholar]
- Organic Cation Transporters in Health and Disease. Pharmacol Rev.. 2020;72:253-319.
- [CrossRef] [Google Scholar]
- Combination of simvastatin with berberine improves the lipid-lowering efficacy. Metabolism.. 2008;57:1029-1037.
- [CrossRef] [Google Scholar]
- Effect of Berberine on Hyperuricemia and Kidney Injury: A Network Pharmacology Analysis and Experimental Validation in a Mouse Model. Drug Des Devel Ther.. 2021;15:3241-3254.
- [CrossRef] [Google Scholar]
- Design, synthesis, and cholesterol-lowering efficacy for prodrugs of berberrubine. Bioorg Med Chem.. 2010;18:6422-6428.
- [CrossRef] [Google Scholar]
- Bioactivities of berberine metabolites after transformation through CYP450 isoenzymes. J Transl Med.. 2011;9:62.
- [CrossRef] [Google Scholar]
- Interaction of six protoberberine alkaloids with human organic cation transporters 1, 2 and 3. Xenobiotica.. 2016;46:175-183.
- [CrossRef] [Google Scholar]
- Berberrubine attenuates potassium oxonate- and hypoxanthine-induced hyperuricemia by regulating urate transporters and JAK2/STAT3 signaling pathway. Eur J Pharmacol.. 2021;912:174592
- [CrossRef] [Google Scholar]
- Berberine: Pathways to protect neurons. Phytother Res.. 2018;32:1501-1510.
- [CrossRef] [Google Scholar]
- Research Progress on the Synthesis of Protoberberine Skeleton and Its Anti-inflammatory Activity. Chinese Journal of Organic Chemistry 2022
- [CrossRef] [Google Scholar]
- Research progress on berberine with a special focus on its oral bioavailability. Fitoterapia.. 2016;109:274-282.
- [CrossRef] [Google Scholar]
- Excretion of berberine and its metabolites in oral administration in rats. J Pharm Sci.. 2013;102:4181-4192.
- [CrossRef] [Google Scholar]
- Cholesterol, the central lipid of mammalian cells. Curr Opin Cell Biol.. 2010;22:422-429.
- [CrossRef] [Google Scholar]
- Pharmacology of antithrombotic drugs: an assessment of oral antiplatelet and anticoagulant treatments. Lancet.. 2015;386:281-291.
- [CrossRef] [Google Scholar]
- Dissociation of intestinal and hepatic activities of FXR and LXRα supports metabolic effects of terminal ileum interposition in rodents. Diabetes.. 2013;62:3384-3393.
- [CrossRef] [Google Scholar]
- Diverse biological functions of vitamin K: from coagulation to ferroptosis. Nat Metab.. 2023;5:924-932.
- [CrossRef] [Google Scholar]
- A high-fat diet delays plasmin generation in a thrombomodulin-dependent manner in mice. Blood.. 2020;135:1704-1717.
- [CrossRef] [Google Scholar]
- Production of plant natural products through engineered Yarrowia lipolytica. Biotechnol Adv.. 2020;43:107555
- [CrossRef] [Google Scholar]
- Release and crystallization of berberine in the liquid medium of Thalictrum minus cell suspension cultures. Plant Cell Rep.. 1984;3:254-257.
- [CrossRef] [Google Scholar]
- Natural Products as Sources of New Drugs over the Nearly Four Decades from 01/1981 to 09/2019. J Nat Prod.. 2020;83:770-803.
- [CrossRef] [Google Scholar]
- Plant cell culture strategies for the production of natural products. BMB Rep.. 2016;49:149-158.
- [Google Scholar]
- Bioactive Compounds from Plant Origin as Natural Antimicrobial Agents for the Treatment of Wound Infections. Int J Mol Sci.. 2024;25
- [CrossRef] [Google Scholar]
- Recent advances in systems and synthetic biology approaches for developing novel cell-factories in non-conventional yeasts. Biotechnol Adv.. 2021;47:107695
- [CrossRef] [Google Scholar]
- Berberine, a plant alkaloid with lipid- and glucose-lowering properties: From in vitro evidence to clinical studies. Atherosclerosis.. 2015;243:449-461.
- [CrossRef] [Google Scholar]
- Combined analytical approaches to define biodistribution and biological activity of semi-synthetic berberrubine, the active metabolite of natural berberine. Anal Bioanal Chem.. 2018;410:3533-3545.
- [CrossRef] [Google Scholar]
- The role of inflammation in the development of epilepsy. J Neuroinflammation.. 2018;15:144.
- [CrossRef] [Google Scholar]
- Berberine: A novel therapeutic strategy for cancer. IUBMB Life.. 2020;72:2065-2079.
- [CrossRef] [Google Scholar]
- Cell type-specific localization of transcripts encoding nine consecutive enzymes involved in protoberberine alkaloid biosynthesis. Plant Cell.. 2005;17:915-926.
- [Google Scholar]
- Organic Cation Transporters in Human Physiology, Pharmacology, and Toxicology. Int J Mol Sci.. 2020;21
- [CrossRef] [Google Scholar]
- Berberine ameliorates intrahippocampal kainate-induced status epilepticus and consequent epileptogenic process in the rat: Underlying mechanisms. Biomed Pharmacother.. 2017;87:200-208.
- [CrossRef] [Google Scholar]
- Novel Isoxazole Derivatives with Potent FXR Agonistic Activity Prevent Acetaminophen-Induced Liver Injury. ACS Med Chem Lett.. 2019;10:407-412.
- [CrossRef] [Google Scholar]
- Berberine Attenuates Hyperuricemia by Regulating Urate Transporters and Gut Microbiota. Am J Chin Med.. 2022;50:2199-2221.
- [CrossRef] [Google Scholar]
- Bile acids in glucose metabolism in health and disease. J Exp Med.. 2018;215:383-396.
- [CrossRef] [Google Scholar]
- The suppressive role of phytochemical-induced glutathione S-transferase Mu 2 in human urothelial carcinoma cells. Biomed Pharmacother.. 2022;151:113102
- [CrossRef] [Google Scholar]
- Plant isoquinoline alkaloids: Advances in the chemistry and biology of berberine. Eur J Med Chem.. 2021;226:113839
- [CrossRef] [Google Scholar]
- Berberine and its metabolites: relationship between physicochemical properties and plasma levels after administration to human subjects. J Nat Prod.. 2014;77:766-772.
- [CrossRef] [Google Scholar]
- Developmental aspects of human hepatic drug glucuronidation in young children and adults. Gut.. 2002;50:259-265.
- [Google Scholar]
- The pyrrolidinoindoline alkaloid Psm2 inhibits platelet aggregation and thrombus formation by affecting PI3K/Akt signaling. Acta Pharmacol Sin.. 2016;37:1208-1217.
- [CrossRef] [Google Scholar]
- The Hypoglycemic Effect of Berberine and Berberrubine Involves Modulation of Intestinal Farnesoid X Receptor Signaling Pathway and Inhibition of Hepatic Gluconeogenesis. Drug Metab Dispos.. 2021;49:276-286.
- [CrossRef] [Google Scholar]
- Topoisomerase I inhibitors: Challenges, progress and the road ahead. Eur J Med Chem.. 2022;236:114304
- [CrossRef] [Google Scholar]
- Tissue distribution of berberine and its metabolites after oral administration in rats. PLoS One.. 2013;8:e77969.
- [Google Scholar]
- Topoisomerase II Poisons: Converting Essential Enzymes into Molecular Scissors. Biochemistry.. 2021;60:1630-1641.
- [CrossRef] [Google Scholar]
- The metabolism of berberine and its contribution to the pharmacological effects. Drug Metabolism Reviews.. 2017;49:139-157.
- [CrossRef] [Google Scholar]
- Metabolic Activation and Covalent Protein Binding of Berberrubine: Insight into the Underlying Mechanism Related to Its Hepatotoxicity. Drug Des Devel Ther.. 2020;14:4423-4438.
- [CrossRef] [Google Scholar]
- Pharmacokinetics in rats and tissue distribution in mouse of berberrubine by UPLC-MS/MS. J Pharm Biomed Anal.. 2015;115:368-374.
- [CrossRef] [Google Scholar]
- Integrated metabolomics and molecular docking reveal berberrubine inhibits thrombosis by regulating the vitamin K catalytic cycle in mice. Eur J Pharmacol.. 2023;938:175436
- [CrossRef] [Google Scholar]
- Chinese Medicine in the Battle Against Obesity and Metabolic Diseases. Front Physiol.. 2018;9:850.
- [CrossRef] [Google Scholar]
- Anti-Hyperuricemic and Nephroprotective Effects of Dihydroberberine in Potassium Oxonate- and Hypoxanthine-Induced Hyperuricemic Mice. Front Pharmacol.. 2021;12:645879
- [CrossRef] [Google Scholar]
- Studies on the Chemical Synthesis of Natural Drugs Berberine. Chinese Journal of Organic Chemistry.. 2021;41:2217-2227.
- [CrossRef] [Google Scholar]
- Molecular Biological and Clinical Understanding of the Pathophysiology and Treatments of Hyperuricemia and Its Association with Metabolic Syndrome, Cardiovascular Diseases and Chronic Kidney Disease. Int J Mol Sci.. 2021;22
- [CrossRef] [Google Scholar]
- A eukaryotic enzyme that can disjoin dead-end covalent complexes between DNA and type I topoisomerases. Proc Natl Acad Sci U S a.. 1996;93:11534-11539.
- [Google Scholar]
- Berberrubine, a Main Metabolite of Berberine, Alleviates Non-Alcoholic Fatty Liver Disease Modulating Glucose and Lipid Metabolism and Restoring Gut Microbiota. Front Pharmacol.. 2022;13:913378
- [CrossRef] [Google Scholar]
- High fat diet aggravates the nephrotoxicity of berberrubine by influencing on its pharmacokinetic profile. Environ Toxicol Pharmacol.. 2016;46:319-327.
- [CrossRef] [Google Scholar]
- In vitro assessment of the glucose-lowering effects of berberrubine-9-O-β-D-glucuronide, an active metabolite of berberrubine. Acta Pharmacol Sin.. 2017;38:351-361.
- [CrossRef] [Google Scholar]
- Inhibitory Effects of Endogenous Linoleic Acid and Glutaric Acid on the Renal Glucuronidation of Berberrubine in Mice and on Recombinant Human UGT1A7, 1A8, and 1A9. Mol Pharmacol.. 2018;93:216-227.
- [CrossRef] [Google Scholar]
- The structural modification of natural products for novel drug discovery. Expert Opin Drug Discov.. 2017;12:121-140.
- [CrossRef] [Google Scholar]
- Bioactive compounds and mechanism of Xianglian pill in the treatment of gastric cancer: Network pharmacology analysis and experimental validation. J Ethnopharmacol.. 2023;314:116573
- [CrossRef] [Google Scholar]
- Berberrubine attenuates mucosal lesions and inflammation in dextran sodium sulfate-induced colitis in mice. PLoS One.. 2018;13:e0194069.
- [Google Scholar]
- Dual DNA topoisomerase 1 and tyrosyl-DNA phosphodiesterase 1 inhibition for improved anticancer activity. Med Res Rev.. 2019;39:1427-1441.
- [CrossRef] [Google Scholar]
- Natural Products and Their Derivatives as Inhibitors of the DNA Repair Enzyme Tyrosyl-DNA Phosphodiesterase 1. Int J Mol Sci.. 2023;24
- [CrossRef] [Google Scholar]
- Transformation of berberine to its demethylated metabolites by the CYP51 enzyme in the gut microbiota. J Pharm Anal.. 2021;11:628-637.
- [CrossRef] [Google Scholar]
- The interaction of alkaloids in Coptis chinensis Franch -Tetradium ruticarpum (A. Juss.) T.G. Hartley with hOCT1 and hOCT2. J Ethnopharmacol.. 2022;295:115395
- [CrossRef] [Google Scholar]
- Strategies of targeting CYP51 for IFIs therapy: Emerging prospects, opportunities and challenges. Eur J Med Chem.. 2023;259:115658
- [CrossRef] [Google Scholar]
- Cardiotoxicity evaluation of nine alkaloids from Rhizoma Coptis. Hum Exp Toxicol.. 2018;37:185-195.
- [CrossRef] [Google Scholar]
- Different structures of berberine and five other protoberberine alkaloids that affect P-glycoprotein-mediated efflux capacity. Acta Pharmacol Sin.. 2019;40:133-142.
- [CrossRef] [Google Scholar]
- Berberine is an insulin secretagogue targeting the KCNH6 potassium channel. Nat Commun.. 2021;12:5616.
- [CrossRef] [Google Scholar]
- Cancer incidence and mortality in China, 2016. Journal of the National Cancer Center.. 2022;2:1-9.
- [CrossRef] [Google Scholar]
- Oxyberberrubine, a novel liver microsomes-mediated secondary metabolite of berberine, alleviates hyperuricemic nephropathy in mice. Phytomedicine.. 2023;108:154521
- [CrossRef] [Google Scholar]
- A new quaternary protoberberine alkaloid isolated from Dicranostigma leptopodum (Maxim) Fedde. Natural Product Research.. 2014;28:507-510.
- [CrossRef] [Google Scholar]
- Berberine metabolites could induce low density lipoprotein receptor up-regulation to exert lipid-lowering effects in human hepatoma cells. Fitoterapia.. 2014;92:230-237.
- [CrossRef] [Google Scholar]
- Chronic effects of berberine on blood, liver glucolipid metabolism and liver PPARs expression in diabetic hyperlipidemic rats. Biol Pharm Bull.. 2008;31:1169-1176.
- [Google Scholar]
- Progress in research on paclitaxel and tumor immunotherapy. Cell Mol Biol Lett.. 2019;24:40.
- [CrossRef] [Google Scholar]
- Berberine a traditional Chinese drug repurposing: Its actions in inflammation-associated ulcerative colitis and cancer therapy. Front Immunol.. 2022;13:1083788.
- [CrossRef] [Google Scholar]
- Pharmacokinetics of berberine and its main metabolites in conventional and pseudo germ-free rats determined by liquid chromatography/ion trap mass spectrometry. Drug Metab Dispos.. 2006;34:2064-2072.
- [Google Scholar]







