Translate this page into:
Betanin ameliorates Lipopolysaccharide-induced acute lung injury in mice via inhibition of inflammatory response and oxidative stress
⁎Corresponding author at: Department of Emergency Medicine, Shaanxi Provincial People's Hospital, Xian, 710068, Shaanxi, China. chen1xin2jun@sina.com (Xinjun Chen)
-
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
Background
Acute lung injury (ALI) is the most common form of inflammatory disease, which has higher morbidity and mortality rates worldwide. ALI is characterized by alterations in the lungs such as epithelial dysfunction, excessive inflammation, and lung edema.
Objective
The current work was aimed to unveil the abrogative properties of betanin on the LPS-induced ALI in mice.
Methodology
In an in vitro assay, betanin-treated RAW 264.7 cells viability was evaluated by MTT assay. The TNF-α, IL-1β, and IL-6 levels in the cell lysates were estimated using assay kits. In in vivo studies, the ALI was initiated in the BALB/c mice by exposing them to a 3-day intra-tracheal challenge of 5 mg/kg of LPS and then treated with betanin (25 and 50 mg/kg) for three days. Later, the BALF samples were obtained from the mice and used to estimate the inflammatory biomarkers using assay kits. The levels of MDA and the antioxidants SOD and GSH were also examined using commerical kits. The iNOS, COX-2, and PGE-2 expressions were estimated using kits, and finally the histopathological study was conducted on the lung tissues.
Results
The outcomes of in vitro studies revealed that betanin appreciably improved the viability of LPS-exposed RAW 264.7 cells. The betanin effectively diminished the levels of IL-6, IL-1β, and TNF-α in LPS-induced RAW 264.7 cells. In in vivo studies, the findings demonstrated that betanin treatment diminished the lung/bodyweight, lung W/D weight, and protein level in the ALI mice. The inflammatory cell counts and inflammatory biomarkers such as COX-2, PGE2, and iNOS were substantially reduced by the betanin treatment in the ALI mice. Betanin treatment also increased the GSH and SOD and reduced the MDA in the ALI mice. The histopathological alterations in the lungs tissues of ALI mice were effectively ameliorated by the betanin.
Conclusion
In summary, the findings apparently suggest that betanin effectively ameliorated the LPS-triggered ALI in mice through its beneficial properties. Therefore, in the future, it can be used as a new salutary candidate to manage the ALI in medical settings.
Keywords
Alveolar macrophage
Interleukin-6
Sepsis
Inflammation
Betanin
Lung edema
1 Introduction
Acute lung injury (ALI) is a form of acute inflammatory lung disease that has a significant morbidity and fatality rate worldwide (Patel et al., 2019). Many factors can contribute to the progression of ALI, including pneumonia, trauma, and burns (Wang et al., 2020). ALI is defined by alterations in lung tissue such as epithelial dysfunction, excessive inflammation, and lung edema (Butt et al., 2016). The pathophysiology of ALI is unknown, but injury to the epithelium and endothelium of the lungs, the production of pro-inflammatory regulators, excessive oxidative stress, and a large influx of neutrophils into the lungs are all believed to be involved. Oxidative stress and severe inflammation are two major causes to ALI development. ALI can result in a variety of direct and indirect consequences, comprising pneumonia and other inflammation-related disorders, including acute cardiac failure, renal disease, liver function impairments, and life-threatening complications. At its core, ALI causes redox instability, oxidative injury, and DNA damage, which leads to the death of lung cells and a change in how the lungs work (Tan et al., 2017).
The discrepancy between the body's inflammatory and anti-inflammatory reactions is one of the probable pathogenesis of ALI, which contributes to the onset of numerous diseases (Wan et al., 2021). ALI is distinguished by the release of inflammatory mediators from the epithelium, which promotes neutrophil and macrophage influx into the injured sites, followed by a rise in inflammatory cytokines (Voiriot et al., 2017). The NF-kB pathway is linked to inflammatory mechanisms and promotes transcription of inflammatory proteins such as pro-inflammatory regulators released by activated macrophages. TNF-α and IL-1β trigger other inflammatory cells to produce more inflammatory cytokines, which are well-known chemoattractant factors for neutrophils. It has been shown that IL-6 is important for increasing the neutrophil numbers and lowering the bacterial load in the lungs (Zhao et al., 2016).
Lipopolysacharide (LPS) is a fundamental element of gram-negative bacteria’s cell membrane, which is made up of polysaccharides and lipids, which causes numerous organ failures, especially ALI and its more serious version, ARDS (Wang et al., 2021). LPS can cause inflammatory cell infiltrations that results in powerful immune responses. As a result, LPS has been routinely employed to trigger inflammation in animal models such as ALI (de Souza Xavier Costa N, Ribeiro Júnior G, Dos Santos Alemany AA, Belotti L, Zati DH, Frota Cavalcante M, , 2017). LPS has been proven in studies to cause lung damage via a variety of inflammatory pathways. When animals were administered with LPS, the immune mechanisms were stimulated and substantial pro-inflammatory mediators were generated, disrupting the inflammatory and anti-inflammatory homeostasis and resulting in lung inflammation (Mokhtari-Zaer et al., 2020). In innate and adaptive immune responses, lymphocytes, dendritic cells, granulocytes, and macrophages play important roles. Furthermore, research has shown that macrophages, RAW 264.7 cells are a useful in vitro inflammatory macrophage model (Lee et al., 2020). So, the LPS-stimulated inflammatory model can tell if a drug has properties that make it anti-inflammatory.
There are currently no efficient medications for the treatment of ALI (Sahu et al., 2020). The major medications utilized to treat ALI are NSAIDs and glucocorticoids, which have substantial side effects such as upper gastrointestinal responses, renal dysfunction, and arterial thrombotic complications, as well as poor outcomes (Tomazini et al., 2020; Cumhur Cure et al., 2020). As a result, new and potential medications that relieve the pathological signs of ALI should be discovered. Betanin, a nitrogenated heterocyclic compound that is the main bioactive substance of beetroot and confers its red-violet color (Silva et al., 2016). Additionally, betanin is characterized as a bioactive substance that can avert lipid peroxidation and boost the functions of antioxidant systems (Esatbeyoglu et al., 2015). Betanin has the potential to be employed as a complementary therapy to lessen the pathophysiological consequences of oxidative stress and inflammatory processes that result in CVD disorders (Fiordelisi et al., 2019; Satta et al., 2017). Several recent studies have also suggested that betanin has the potential to ameliorate oxidative stress in diabetic rats (Mousavi et al., 2022 Oct), allergic airway inflammation (Li et al., 2022 Mar 30), renal fibrosis in diabetic rats (Sutariya and Saraf, 2017 Feb), and showed cardioprotective effects (Da Silva et al., 2022). Nonetheless, the salutary role of betanin against LPS-induced ALI has not been systematically analyzed yet. Consequently, this work was undertaken to unveil the abrogative roles of betanin on the LPS-induced ALI in mice.
2 Materials and methods
2.1 Reagents
The following chemicals were acquired from Sigma-Aldrich, USA: betanin, LPS, 3-MTT, buffer solution, and other reagents. Thermofisher and Biocompare, USA, provided the ELISA test kits for evaluating biochemical parameters.
2.2 In vitro assays
2.2.1 Collection of cell line
The RAW 264.7 cells, which resemble macrophages, were purchased from the ATCC in the USA and kept on DMEM media supplemented with 10% FBS and 1% antimycotic cocktail at 37 °C in a dampened and CO2 (5%) supplied chamber. Later the cells reached 80% confluency, they were trypsinized, separated, and used in further experiments.
2.2.2 Cell viability assay
Betanin-treated RAW 264.7 cells' viability was determined by MTT test. Cells were cultivated in a 96-wellplate with DMEM media at 5 × 103 cells/well for 24 h at 37 °C. Later, different concentrations of betanin, ranging from 10, 15, 20, and 25 µM, were given to the cells at 37 °C for 24 hrs. Later, 20 µl of MTT and 100 µl of DMEM were mixed to each well. Then, 100 µl of DMSO was utilized to liquefy the formed formazan stones in each well, and using a microplate reader, finally the absorbance were taken at 570 nm.
2.3 Measurement of pro-inflammatory cytokines
The IL-6, IL-1β, and TNF-α levels in the cell lysates of control and betanin-administered RAW 264.7 cells were measured using assay kits from a commercial company while following the manufacturer's instructions (Thermofisher Scientific, USA).
2.4 Experimental animals
The mice used in this experiment were 3–4 weeks aged BALB/c mice weighing 27.5 g acquired from institutional animal care. All mice were kept in well-hygienic polypropylene containers with access to the typical rodent food and clean drinking water. Mice were kept in carefully planned laboratory settings with a 24 °C temperature, 60–70% air humidity, and a 12-hour cycle of darkness and light. Before to the start of the studies, the experimental mice were acclimated for a week in a lab setting.
2.5 Experimental design
The four groups of acclimatized animals, each with six animals, were randomly chosen. Animals in group I were designated as usual controls and were not given any pharmacological treatments. ALI was induced in Group-II mice by exposing them to a 3-day intra-tracheal challenge of 5 mg/kg of LPS. The animals in groups III and IV were ALI-initiated mice administered with betanin (25 and 50 mg/kg) for three days, respectively, before receiving an injection of LPS. After anesthesia and cervical dislocation death, the mice's lung tissues were gathered and precisely weighed to estimate the moist weight. Once the tissues had been dried out in an oven set to 80 °C, the dry weight was determined.
2.6 Collection of broncho alveolar lavage fluid (BALF) and total cell count
By injecting aliquots of 30 ml of saline solution into the right middle lobe of the mice—both control and experimental—the BALF was obtained. The BALF was then immediately centrifuged for 5 min at 6000 rpm. The centrifuged BALF sample was then relocated to a new tube and used for the biochemical calculations without any cell remnants. Differential cell labeling was used to figure out how many cells were in the BALF fluid pellets as a whole.
2.7 Quantification of oxidative stress markers
By using assay kits, the level of MDA, SOD, and GSH were estimated in the BALF fluids of both control and treated mice using the recommended protocols by the manufacturer (Biocompare, USA).
2.8 Determination of inflammatory biomarker levels
Using assay kits and in accordance to the suggested guidelines of the manufacturer, the status of inflammatory regulators like iNOS, COX-2, and prostaglandin-E2 (PGE-2) in the BALF of control and experimental mice were quantified (Biocompare, USA).
2.9 Assessment of lung histopathological score
The lung tissues from both experimental and control mice were taken out and treated with 10% neutral formalin in order to perform the histological analysis and calculate the lung histology scores. The lung tissues were then cut into 5 micro m-diameter and preserved in paraffin wax. The stain of hematoxylin and eosin were utilized to stain the deparaffinized tissues. The stained tissues were examined beneath the light microscope at a magnification of 100x, and the histopathological scores were determined based on the degrees of histological changes, such as neutrophil infiltration of the lungs, edema in the alveolar air sacs, and injuries to the alveolar epithelial cells.
2.10 Statistical analysis
One-way ANOVA and DMRT were performed to evaluate the data, which were statistically evaluated using SPSS software and given as the mean ± SD of three discrete assays. Significant was fixed as a “p” value < 0.05.
3 Results
3.1 Effect of betanin on the viability of LPS-induced RAW 264.7 cells
The effects of betanin on the LPS-exposed RAW 264.7 cell viability are shown in Fig. 1. The outcomes proved that the survival of RAW 264.7 cells was not impacted by betanin alone. The 15 and 20 µM of betanin concentrations were chosen for further testing because the 20 µM of betanin concentration only slightly decreased cell viability. Nevertheless, the cells that had received LPS demonstrated a significant decline in survival. Surprisingly, when cells were administered with 15 and 20 µM of betanin and then exposed to LPS, the viability of the cells increased noticeably (Fig. 1). In light of this, it was evident that the betanin therapy increased the survival of LPS-exposed RAW 264.7 cells.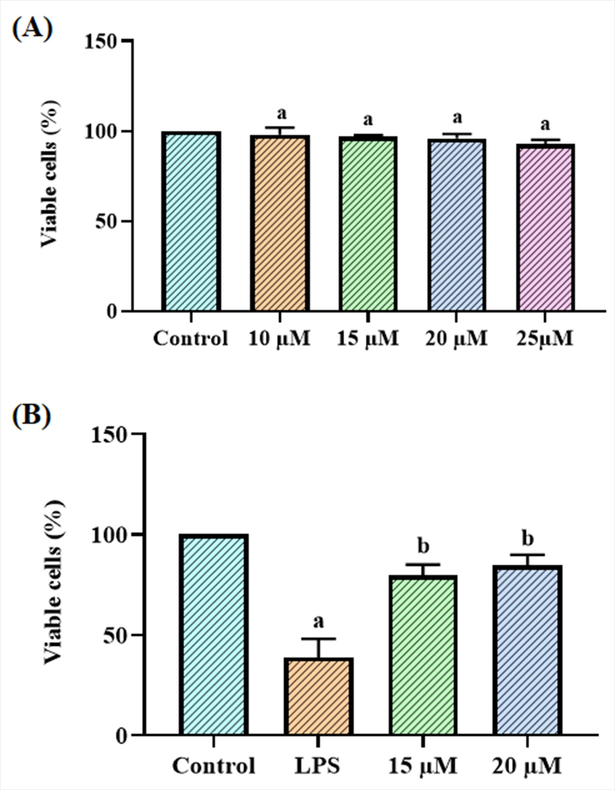
Effect of betanin on the viability of LPS-induced RAW 264.7 cells The viability of RAW 264.7 cells was unaffected by the betanin treatment alone at various dosages (10–25 µM) (A). Additionally, the treatments with 15 and 20 µM of betanin significantly increased the viability of LPS-exposed RAW 264.7 cells (B). Results presented as the mean ± SD of three replicated tests. The SPSS program was used to analyze the results using one-way ANOVA and DMRT. Note: “a” denotes p < 0.01 when compared to control, while “b” denotes p < 0.05 when compared to cells that had been treated to LPS.
3.2 Effect of betanin on the pro-inflammatory cytokine levels in the LPS-exposed RAW 264.7 cells
Fig. 2 reveals the results of measuring the status of IL-6, IL-1β, and TNF-α in control and LPS-exposed RAW 264.7 cells using assay kits. As contrasted with the control cells, the LPS-exposed cells displayed elevated IL-6, IL-1β, and TNF-α. Nevertheless, when cells were given 15 and 20 µM of betanin and then exposed with LPS, the IL-6, IL-1β, and TNF-α were considerably depleted (Fig. 2). These results show the anti-inflammatory property of betanin.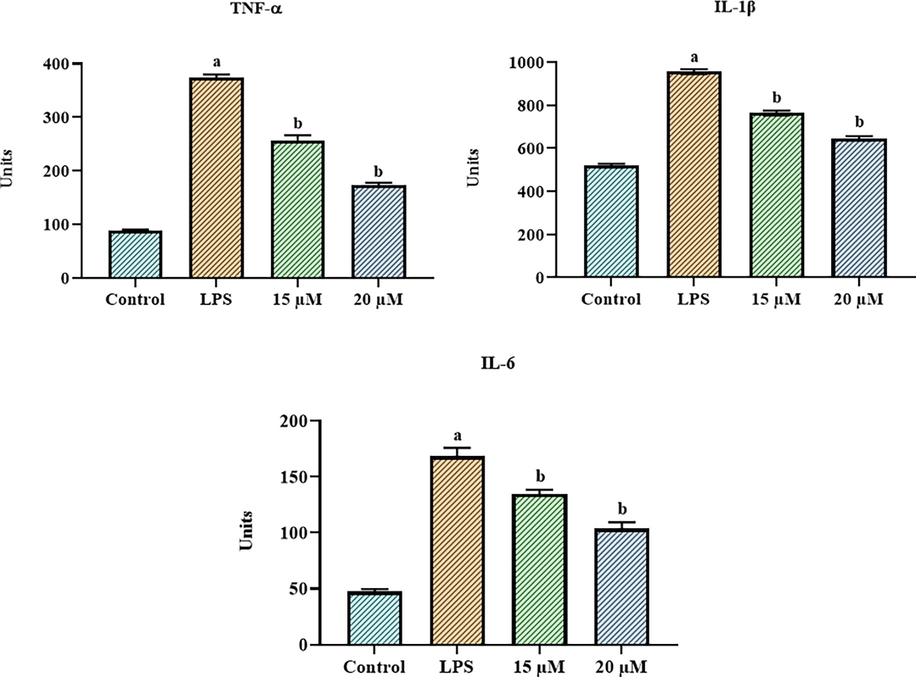
Effect of betanin on the pro-inflammatory cytokine levels in the LPS-exposed RAW 264.7 cells Betanin treatments at concentrations of 15 and 20 µM reduced the levels of inflammatory cytokines such IL-6, IL-1β, and TNF-α in LPS-exposed RAW 264.7 cells. Results presented as the mean ± SD of three replicated tests. The SPSS program was used to analyze the results using one-way ANOVA and DMRT. Note: “a” denotes p < 0.01 when compared to control, while “b” denotes p < 0.05 when compared to cells that had been treated to LPS.
3.3 Effect of betanin on the lung weight, lung/bodyweight ratio, and total protein levels in the LPS-induced ALI mice
The changes in the W/D weight of the lungs, lung/bodyweight ratio, and protein status in the control and LPS-exposed ALI mice are revealed in Fig. 3. It was discovered that the LPS-exposed ALI mice had higher levels of total protein, lung W/D weight, and lung/bodyweight. Nonetheless, the use of betanin substantially reduced these alterations (Fig. 3). When betanin at the dose of 25 and 50 mg/kg were given to ALI mice that had been exposed to LPS, the lung W/D weight, the lung/bodyweight ratio, and protein content in the BALF all went down in a noticeable way.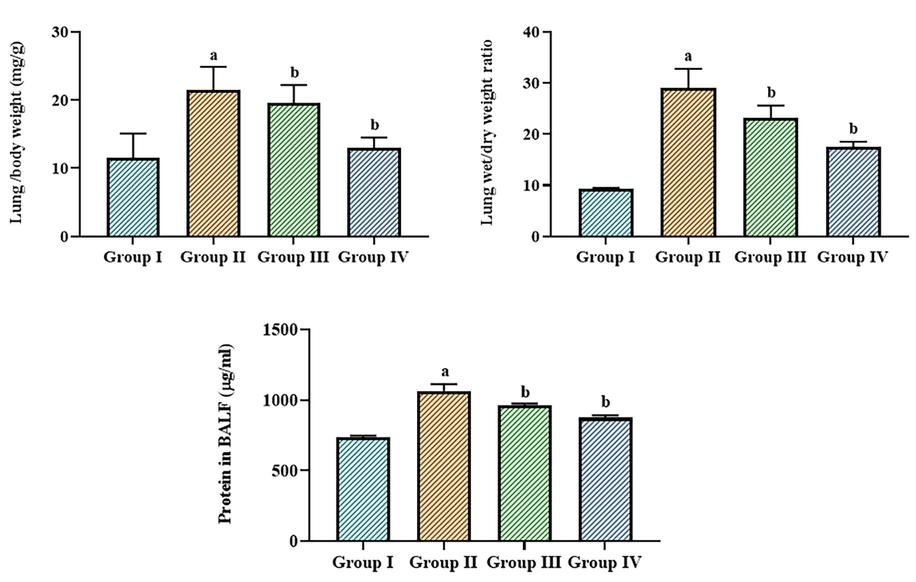
Effect of betanin on the lung weight, lung/bodyweight ratio, and total protein levels in the LPS-induced ALI mice The lung weight, lung/bodyweight ratio, and protein content in the LPS-exposed ALI mice were successfully decreased by the treatment of betanin (25 and 50 mg/kg). Results presented as the mean ± SD of three replicated tests. The SPSS program was used to analyze the results using one-way ANOVA and DMRT. Note: “a” denotes p < 0.01 when compared to control, while “b” denotes p < 0.05 when compared to cells that had been treated to LPS.
3.4 Effect of betanin on the inflammatory cell counts in the BALF of LPS-induced ALI mice
Fig. 4 shows data from the assessment of the levels of inflammatory cells, i.e., macrophages, neutrophils, lymphocytes, and total cell counts. The total cells in the BALF of LPS-exposed ALI mice were augmented. Intriguingly, the LPS-exposed ALI mice were given betanin at 25 and 50 mg/kg, and the outcomes revealed a striking reduction in these increments in the BALF (Fig. 4).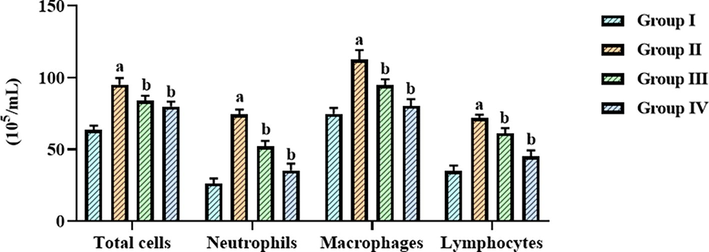
Effect of betanin on the inflammatory cell counts in the BALF of LPS-induced ALI mice Betanin treatment at doses of 25 and 50 mg/kg significantly reduced the number of lymphocytes, neutrophils, macrophages, and total cells in the BALF of LPS-exposed ALI mice. Results presented as the mean ± SD of three replicated tests. The SPSS program was used to analyze the results using one-way ANOVA and DMRT. Note: “a” denotes p < 0.01 when compared to control, while “b” denotes p < 0.05 when compared to cells that had been treated to LPS.
3.5 Effect of betanin on the oxidative and antioxidative biomarker levels in the LPS-induced ALI mice
The levels of oxidative and antioxidative indicators including MDA, SOD, and GSH in control and LPS-exposed ALI mice are presented in Fig. 5. The LPS-exposed ALI mice displayed an upsurge in MDA and a diminution in GSH and SOD levels. It's interesting to note that the betanin treatment successfully reversed these alterations. Fig. 5 shows that when betanin (25 and 50 mg/kg) was given to animals with ALI caused by LPS, the amount of MDA was greatly reduced and SOD and GSH were raised.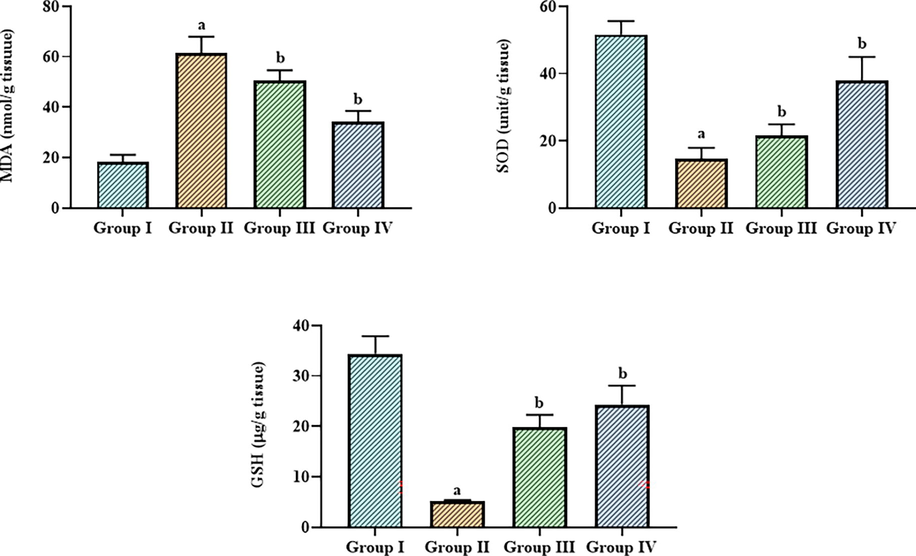
Effect of betanin on the levels of oxidative and antioxidative biomarkers in the LPS-induced ALI mice The GSH level was decreased and SOD and GSH was elevated by the 25 and 50 mg/kg of betanin treatement in the LPS-induced ALI mice. Results presented as the mean ± SD of three replicated tests. The SPSS program was used to analyze the results using one-way ANOVA and DMRT. Note: “a” denotes p < 0.01 when compared to control, while “b” denotes p < 0.05 when compared to cells that had been treated to LPS.
3.6 Effect of betanin on the inflammatory biomarker levels in the LPS-induced ALI mice
Fig. 6 shows the status of inflammatory biomarkers i.e., iNOS, COX-2, and PGE2 in the control and LPS-exposed ALI who were given betanin. When compared to control, the ALI mice demonstrated a substantial elevation in iNOS, COX-2, and PGE2. It's interesting to note that the betanin treatment successfully reversed these alterations. In the LPS-exposed ALI mice, administering with betanin (25 and 50 mg/kg) considerably depleted the iNOS, COX-2, and PGE2 levels (Fig. 6).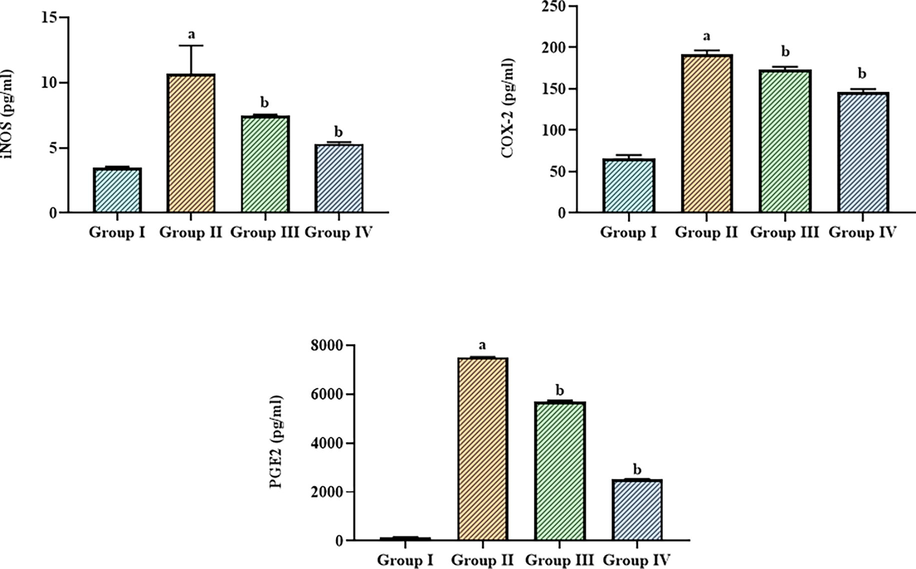
Effect of betanin on the levels of inflammatory biomarkers in the LPS-induced ALI mice The treatment with 25 and 50 mg/kg of betanin effectively decreased the levels of COX-2, iNOS, and PGE-2 in the LPS-induced ALI mice. Results presented as the mean ± SD of three replicated tests. The SPSS program was used to analyze the results using one-way ANOVA and DMRT. Note: “a” denotes p < 0.01 when compared to control, while “b” denotes p < 0.05 when compared to cells that had been treated to LPS.
3.7 Effect of betanin on the lung histopathology in the LPS-induced ALI mice
The influence of betanin on the lung histopathology in the LPS-exposed ALI mice is depicted in Fig. 7. The lung injury score was raised in the LPS-exposed ALI mice when compared to control due to the enhanced neutrophil penetration, lung edema, and alveolar epithelial cell destruction, as shown in Fig. 7. It's interesting to note that ALI animals treated with betanin (25 and 50 mg/kg) indicated a noticeable diminution in inflammatory signs, lung edema, alveolar damage, and lung injury scores (Fig. 7). These results indicate that betanin therapy reduced the lung tissues' histological changes brought on by LPS.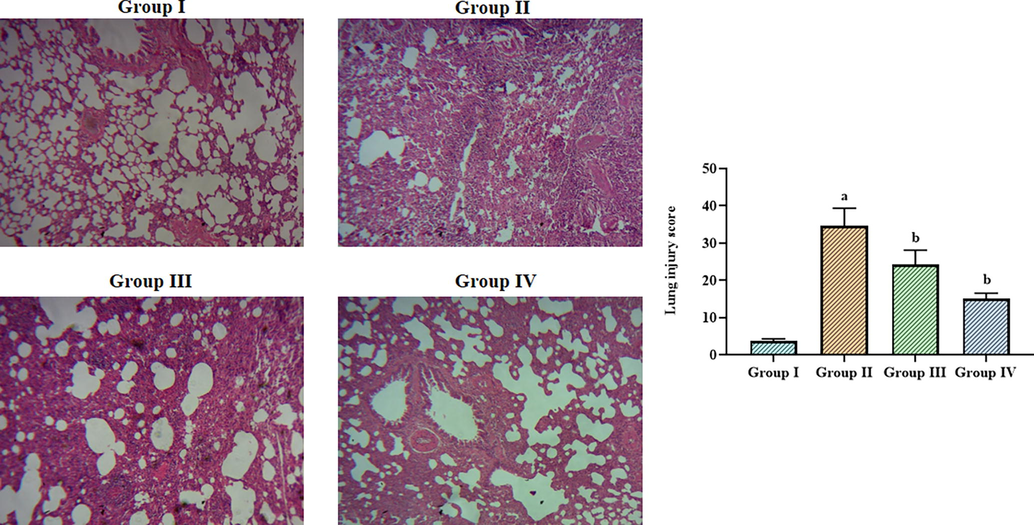
Effect of betanin on the lung histopathology in the LPS-induced ALI mice The lung tissues of control mice showed normal cellular arrangements (Group I). The LPS-induced ALI mice demonstrated increased inflammatory cell infiltration, lung edema, and alveolar epithelial disruption in the lung tissues (Group II). The treatment with 25 and 50 mg/kg of betanin substantially reduced the histological changes in the lung tissues of ALI mice (Group III & IV). Results presented as the mean ± SD of three replicated tests. The SPSS program was used to analyze the results using one-way ANOVA and DMRT. Note: “a” indicates p < 0.01 when compared to control, while “b” indicates p < 0.05 when compared to LPS-induced ALI mice.
4 Discussion
The primary clinical manifestations of ALI include breathing problems, hypoxemia, significant capillary extravasation, and noncardiogenic pulmonary embolism (Kotas and Matthay, 2018; Li et al., 2020). Unrestrained and severe lung inflammation is an underlying fundamental immunological process and contributes to its high fatality rate (Fan and Fan, 2018). The buildup of neutrophils in the interstitial, bronchoalveolar, and lung microvasculature is believed as a pivotal causative factor to ALI/ARDS (Blazquez-Prieto and I. Ĺopez-Alonso, C. Huidobro, and G. M. Albaiceta, , 2018). Macrophages are diverse cell types that perform a pivotal function in the ALI pathophysiology. This macrophage aggregation is often seen at the locations of tissue damage resulting from exposure to pulmonary toxicants (Laskin et al., 2019). Following LPS activation, macrophages release a variety of proinflammatory mediators to further worsen the inflammatory response, thereby attracting neutrophils (Hollingsworth et al., 2005). Neutrophils release powerful antibacterial chemicals, such as protease, cationic compounds, and reactive oxidants (Lakshmi et al., 2012). Both neutrophil accumulation in lung capillaries and changes in neutrophil deformability are caused by LPS. Vascular permeability and pulmonary embolism can result from activated neutrophils damaging endothelial cells and destroying basement membranes (Kral-Pointner et al., 2019). In this work, our findings demonstrated a drastic elevation in the inflammatory cell accumulations such as macrophages and lymphocytes in the BALF of the LPS-activated ALI mice. However, these increments were effectively ameliorated by the betanin treatment, which is evidenced by the reduced inflammatory cell accumulations.
Lung edema is a primary feature of ALI (Hu et al., 2017). The excessive inflammatory response, oxidative stress, and endothelial injury brought on by an inflammatory process that causes microvascular permeability are the main factors contributing to pulmonary edema (Elicker et al., 2016). An essential marker of lung edema is the lung W/D ratio. An assessment of the level of water in the lungs is provided by the W/D weight level. Another crucial sign of lung edema is a rise in BALF protein content brought on by protein vasodilatation (Muller-Redetzky et al., 2014). Our findings revealed that the LPS-exposed ALI mice demonstrated increased lung W/D weight and protein levels in the BALF than the control. Interestingly, the treatment with betanin appreciably reduced the lung W/D weight and protein level in the BALF of the LPS-exposed ALI mice. These outcomes demonstrated that the betanin effectively reduced the lung edema in ALI mice.
Inflammation may lead to oxidative stress, and oxidative stress may worsen inflammation. These two processes can interact in a variety of ways (Mittal et al., 2014; Biswas, 2016). According to a previous report, l oxidative stress and excessive inflammation causes lung injury (Zhou et al., 2019). Moreover, MDA is a byproduct of lipid peroxidation catalyzed by ROS that harms cells. Overproduction of ROS also leads to oxidative injury that modifies the body's natural antioxidant defenses by reducing SOD activity (Arunachalam et al., 2019). As a significant antioxidant in the body, SOD protects cells from free radical damage and keeps the body's metabolism in balance (Barrera et al., 2018).
SOD is found in lung tissue in large amounts and defends it against ALI by scavenging ROS (Zhang et al., 2017). GSH is crucial to the antioxidant mechanism because of its potential free radicals scavenging properties.. GSH level fluctuations reflect fluctuations in the body's oxidative state, and when oxidation stimulates the body, GSH biosynthesis rises (Khazri et al., 2016). An earlier finding highlighted that LPS exposure increases the MDA level while decreasing the antioxidant capacity in the LPS-induced ALI model (Dikmen et al., 2021). In line with this statement, the current findings also demonstrated a significant increase in MDA and a diminution in SOD and GSH in the LPS-exposed ALI mice. However, the treatment with betanin appreciably depleted the MDA content and heightened both SOD and GSH in the ALI mice. These observations reveal the antioxidant properties of betanin.
Several inflammatory-related cytokines and chemokines are released in response to LPS exposure (Tirunavalli et al., 2021). The inflammation in ALI is not only triggered but also amplified and prolonged by activated key cytokines. The augmented cytokines are thought to be directly linked to lung damage (Yang et al., 2020). TNF-α is the first and most significant regulator of an inflammatory reaction. It is mostly generated by monocytes or macrophages that have been stimulated, and it triggers an inflammatory reaction that harms the vascular system's endothelial cells even while causing the pulmonary epithelial network to secrete other cytokines, like IL-6 (Chen et al., 2021; Guo et al., 2022). TNF-α, in along with IL-1β, commences the inflammation by increasing the synthesis of other cytokines (Thompson et al., 2017). TNF-α and IL-1β exacerbate inflammation throughout the body. TNF-α and Il-1β activity was linked to the severity of lung damage. The rate of survival of the ALI mice is also improved by inhibiting TNF-α or IL-6. In order to effectively treat ALI in clinical settings, it is necessary to decrease the abnormal secretion of inflammatory regulators (Hu et al., 2019). When RAW 264.7 cells are administered with LPS, the TLR-4 pathway was activated to identify and bind to LPS, which further led to the stimulation of intracellular signaling cascades comprising NF-κB. This, in turn, encourages the discharge of significant inflammatory signaling molecules, resulting in a cytokine flood (Byun et al., 2013). The current study found significantly higher levels of IL-6, IL-1, and TNF- in LPS-exposed RAW 264.7 cells than in control. However, the exposure of LPS along with the betanin considerably reduced these cytokines. These findings witnessed the in vitro anti-inflammatory effects of betanin.
In LPS-evoked inflammation, a pro-inflammatory mediator secretated by stimulated macrophages performs a pivotal function in commencing and fueling inflammation (Lee et al., 2016). Numerous biological mechanisms, such as neurotransmission, wound repair, apoptosis, and inflammation, are influenced by iNOS. Massive amounts of NO are produced by the iNOS enzymes in living things (Lee et al., 2018). The process mediated by COX-2 results in the production of PGE2, a key lipid that may induce the induction of inflammation (Jiang et al., 2020). Therefore, to research the anti-inflammatory property of betanin, we assessed the status of iNOS, PGE2, and COX-2. The current study found that LPS-exposed ALI mice had significantly higher status of iNOS, PGE2, and COX-2. Interestingly, the betanin treatment exhibited a substantial reduction of these key inflammatory markers in the ALI mice. These results also evidenced the anti-inflammatory roles of betanin.
Furthermore, the role of epithelial damage in the course of ALI has now become increasingly clear. This epithelial damage impairs cell fluid transport and decreases surfactant synthesis (Manicone, 2009). Usually, lung damage reduces oxygenation and exacerbates the systemic inflammatory reaction. Endothelial damage appears minutes to hours following ALI stimulation and leads to endothelial intercellular gaps. Improved microvascular permeability can be attributed to the formation of intercellular gaps (Mao et al., 2009). In this work, the findings of the histopathological study demonstrated neutrophil infiltration, lung edema, and alveolar epithelial membrane destruction. It's interesting to note that ALI mice treated with betanin effectively ameliorated the LPS-triggered histological injuries in ALI mice.
5 Conclusion
The current results first show that betanin effectively ameliorated LPS-induced ALI in mice through its potential anti-inflammatory and antioxidant properties. The betanin substantially inhibited the inflammatory response and augmented the antioxidants in the ALI mice. It also improved the proliferation and abridged the inflammatory cytokines in the RAW 264.7 cells in vitro. This study suggested that betanin could effectively ameliorate the LPS-exposed ALI in mice and could be utilized as an anti-inflammatory and anti-oxidant candidate to deal with ALI in medical settings.
Funding
Clinical study of lung ultrasound to predict the prognosis of acute lung injury due to paraquat, Item level: Shanxi Social Development Guide Project, Item Number: 2018SF-225. This project was supported by Researchers Supporting Project number (RSPD2023R712), King Saud University, Riyadh, Saudi Arabia.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Cochlospermum regium (Mart. ex Schrank) Pilg.: evaluation of chemical profile, gastroprotective activity and mechanism of action of hydroethanolic extract of its xylopodium in acute and chronic experimental models. J. Ethnopharmacol.. 2019;233:101-114.
- [Google Scholar]
- Barrera G, Pizzimenti S, Daga M, Dianzani C, Arcaro A, Cetrangolo GP, et al. Lipid peroxidation-derived aldehydes, 4-hydroxynonenal and malondialdehyde in aging-related disorders. Antioxid (Basel) (2018) 7(8)
- Does the interdependence between oxidative stress and inflammation explain the antioxidant paradox? Oxid. Med. Cell Longev.. 2016;2016:5698931.
- [Google Scholar]
- J. Blazquez-Prieto, I. Ĺopez-Alonso, C. Huidobro, and G. M. Albaiceta, “The Emerging Role of Neutrophils in Repair after Acute Lung Injury,” American Journal of Respiratory Cell and Molecular Biology, vol. 59, no. 3, pp. 289–294, 2018.
- Acute lung injury: a clinical and molecular review. Arch. Pathol. Lab. Med.. 2016;140(4):345-350.
- [Google Scholar]
- The procyanidin trimer C1 inhibits LPSinduced MAPK and NF-κB signaling through TLR4 in macrophages. Int. Immunopharm.. 2013;15(2):450-456.
- [Google Scholar]
- D. Chen, C. Chen, X. Xiao, Z. Huang, X. Huang, and W. Yao, “TNF-α induces neutrophil apoptosis delay and promotes intestinal ischemia- reperfusion-induced lung injury through activating JNK/FoxO3a pathway,” Oxidative Medicine and Cellular Longevity, vol. 2021, Article ID 8302831, 2021.
- NSAIDs may increase the risk of thrombosis and acute renal failure in patients with COVID-19 infection. Thérapie. 2020;75(4):387-388.
- [Google Scholar]
- Betanin as a multipath oxidative stress and inflammation modulator: a beetroot pigment with protective effects on cardiovascular disease pathogenesis. Crit. Rev. Food Sci. Nut.. 2022;62(2):539-554.
- [Google Scholar]
- de Souza Xavier Costa N, Ribeiro Júnior G, Dos Santos Alemany AA, Belotti L, Zati DH, Frota Cavalcante M, et al. Early and late pulmonary effects of nebulized LPS in mice: An acute lung injury model. PloS One (2017) 12(9): e0185474.
- Dikmen N, Cellat M, Etyemez M, Isler CT, Uyar A, Aydın T, et al. Ameliorative effects of oleuropein on lipopolysaccharide-induced acute lung injury model in rats. Inflammation (2021) 44(6): 2246–59.
- Elicker BM, Jones KT, Naeger DM, JA. Imaging of acute lung injury. Radiol Clin North Am (2016) 54(6): 1119–32
- Betanin-a food colorant with biological activity. Mol. Nutr. Food Res.. 2015;59(1):36-47.
- [Google Scholar]
- Regulation of alveolar macrophage death in acute lung inflammation. Respir. Res.. 2018;19(1):50.
- [Google Scholar]
- NF kappa B is a key player in the crosstalk between inflammation and cardiovascular diseases. Int. J. Mol. Sci.. 2019;20(7):1599.
- [Google Scholar]
- Lactobacillus paracasei CCFM1223 protects against lipopolysaccharide-induced acute liver injury in mice by regulating the “gut–liver” axis. Microorganisms. 2022;10:1321.
- [Google Scholar]
- The critical role of hematopoietic cells in lipopolysaccharide-induced airway inflammation. Am. J. Respir. Crit. Care Med.. 2005;171:806-813.
- [Google Scholar]
- Protective effect of TM6 on LPS-induced acute lung injury in mice. Sci. Rep.. 2017;7(1):572.
- [Google Scholar]
- The protective effect of hyperin on LPS-induced acute lung injury in mice. Microb. Pathog.. 2019;127:116-120.
- [Google Scholar]
- CircC3P1 attenuated pro-inflammatory cytokine production and cell apoptosis in acute lung injury induced by sepsis through modulating miR-21. J. Cell. Mol. Med.. 2020;24(19):11221-11229.
- [Google Scholar]
- Grape seed and skin extract protects against bleomycin-induced oxidative stress in rat lung. BioMed. Pharmacother.. 2016;81:242-249.
- [Google Scholar]
- Mesenchymal stromal cells and macrophages in sepsis: new insights. Eur. Respir. J.. 2018;51(4)
- [Google Scholar]
- Platelet PI3K modulates innate leukocyte extravasation during acid-induced acute lung inflammation. Thromb. Haemost.. 2019;119(10):1642-1654.
- [Google Scholar]
- Effects of JAM-A deficiency or blocking antibodies on neutrophil migration and lung injury in a murine model of ALI. Am. J. Physiol. Lung Cell. Mol. Physiol.. 2012;303:L758-L766.
- [Google Scholar]
- Role of macrophages in acute lung injury and chronic fibrosis induced by pulmonary toxicants. Toxicol Sci.. 2019;168(2):287-301.
- [Google Scholar]
- Socheongryongtang suppresses COPD-related changes in the pulmonary system through both cytokines and chemokines in a LPS COPD model. Pharm. Biol.. 2020;58(1):538-544.
- [Google Scholar]
- Protective effect of Tremella fuciformis Berk extract on LPS-induced acute inflammation via inhibition of the NF-κB and MAPK pathways. Food Funct.. 2016;7(7):3263-3272.
- [Google Scholar]
- Anti-inflammatory effect of quercetin and galangin in LPS-stimulated RAW264.7 macrophages and DNCB induced atopic dermatitis animal models. Int. J. Mol. Med.. 2018;41:888-898.
- [Google Scholar]
- Hydrogen treatment prevents lipopolysaccharide-induced pulmonary endothelial cell dysfunction through RhoA inhibition. Biochem. Biophys. Res. Commun.. 2020;522(2):499-505.
- [Google Scholar]
- Betanin dose-dependently ameliorates allergic airway inflammation by attenuating Th2 response and upregulating cAMP-PKA-CREB pathway in asthmatic mice. J. Agric. Food Chem.. 2022 Mar 30;70(12):3708-3718.
- [Google Scholar]
- Role of the pulmonary epithelium and inflammatory signals in acute lung injury. Expert Rev. Clin. Immunol.. 2009;5:63-75.
- [Google Scholar]
- Hydrogen-rich saline reduces lung injury induced by intestinal ischemia/reperfusion in rats. Biochem. Biophys. Res. Commun.. 2009;381(4):602-605.
- [Google Scholar]
- Reactive oxygen species in inflammation and tissue injury. Antioxid. Redox. Signal. 2014;20(7):1126-1167.
- [Google Scholar]
- The protective effect of nigella sativa extract on lung inflammation and oxidative stress induced by lipopolysaccharide in rats. J. Ethnopharmacol.. 2020;253:112653
- [Google Scholar]
- Betanin alleviates oxidative stress through the Nrf2 signaling pathway in the liver of STZ-induced diabetic rats. Mol. Biol. Rep.. 2022 Oct;49(10):9345-9354.
- [Google Scholar]
- Dynamics of pulmonary endothelial barrier function in acute inflammation: mechanisms and therapeutic perspectives. Cell Tissue Res.. 2014;355(3):657-673.
- [Google Scholar]
- Extracorporeal membrane oxygenation as rescue therapy for severe hypoxemic respiratory failure. J. Thorac. Dis.. 2019;11(Suppl 14):S1688-S1697.
- [Google Scholar]
- Pharmacological inhibition of poly (ADPribose) polymerase by olaparib, prevents acute lung injury associated cognitive deficits potentially through suppression of inflammatory response. Eur. J. Pharmacol.. 2020;877:173091
- [Google Scholar]
- The role of Nrf2 in cardiovascular function and disease. Oxid. Med. Cell. Longev.. 2017;2017:9237263.
- [Google Scholar]
- Physicochemical, nutritional, and sensory analyses of a nitrate enriched beetroot gel and its effects on plasmatic nitric oxide and blood pressure. Food & Nutr. Res.. 2016;60:29909.
- [Google Scholar]
- Betanin, isolated from fruits of Opuntia elatior Mill attenuates renal fibrosis in diabetic rats through regulating oxidative stress and TGF-β pathway. J. Ethnopharmacol.. 2017 Feb;23(198):432-443.
- [Google Scholar]
- Pharmacologic protection of mitochondrial DNA integrity may afford a new strategy for suppressing lung ischemia-reperfusion injury. Ann. Am. Thorac. Soc.. 2017;14(Suppl 3):S210-S215.
- [Google Scholar]
- Dehydrozingerone ameliorates lipopolysaccharide induced acute respiratory distress syndrome by inhibiting cytokine storm, oxidative stress via modulating the MAPK/NF-κB pathway. Phytomedicine. 2021;92:153729
- [Google Scholar]
- Effect of dexamethasone on days alive and ventilator-free in patients with moderate or severe acute respiratory distress syndrome and COVID-19: the CoDEX randomized clinical trial. J. Am. Med. Assoc.. 2020;324(13):1307-1316.
- [Google Scholar]
- Interleukin-6 displays lung anti-inflammatory properties and exerts protective hemodynamic effects in a double-hit murine acute lung injury. Respir. Res.. 2017;18:64.
- [Google Scholar]
- Chimonanthus nitens oliv. essential oil mitigates lipopolysaccharide-induced acute lung injury in rats. Food Chem. Toxicol.. 2021;156:112445
- [Google Scholar]
- NLRC5 negatively regulates inflammatory responses in LPS-induced acute lung injury through NF-kB and p38 MAPK signal pathways. Toxicol. Appl. Pharmacol.. 2020;403:115150
- [Google Scholar]
- EGCG promotes PRKCA expression to alleviate LPS-induced acute lung injury and inflammatory response. Sci. Rep.. 2021;11(1):11014.
- [Google Scholar]
- Pterostilbene prevents LPS-induced early pulmonary fibrosis by suppressing oxidative stress, inflammation and apoptosis in vivo. Food Funct.. 2020;11:4471-4484.
- [Google Scholar]
- Dexmedetomidine alleviates hyperoxia-induced acute lung injury via inhibiting NLRP3 inflammasome activation. Cell. Physiol. Biochem.. 2017;42(5):1907-1919.
- [Google Scholar]
- Protective effect of suppressing STAT3 activity in LPS-induced acute lung injury. Am. J. Physiol. Lung Cell. Mol. Physiol.. 2016;311:L868-L880.
- [Google Scholar]
- Isorhynchophylline exerts anti-inflammatory and anti-oxidative activities in LPS-stimulated murine alveolar macrophages. Life Sci.. 2019;223:137-145.
- [Google Scholar]







