Translate this page into:
Bioactive compounds, anti-inflammatory, anti-nociceptive and antioxidant potentials of ethanolic leaf fraction of Sida linifolia L. (Malvaceae)
⁎Corresponding authors at: Department of Biochemistry, Faculty of Biological Sciences, University of Nigeria, Nsukka, Enugu 410001, Nigeria (N.N. Emeka). nicodemus.nwankwo@unn.edu.ng (Nicodemus Emeka Nwankwo), emmanuel.ezeako.188647@unn.edu.ng (Emmanuel Chimeh Ezeako),
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
This study investigated the bioactive compounds, anti-inflammatory, anti-nociceptive, and antioxidant properties of the ethanolic leaf fraction of Sida linifolia (ELFSL). The in vitro anti-inflammatory study employed membrane stabilization, phospholipase A2, platelet aggregation, albumin denaturation, and protease inhibition assays. Intraperitoneal injection of freshly prepared carrageenan solution (0.1 mL of 0.01 g/mL), undiluted egg albumin (0.1 mL), acetic acid (0.6 % (v/v) (10 mL/kg bw), and formalin solution (0.02 mL of 1 % v/v) into mice hind paw, were used to evaluate the anti-inflammatory and anti-nociceptive mechanisms, respectively. In vitro antioxidant potentials were determined using 1,1-Diphenyl-2-picryl-hydrazyl (DPPH), nitric oxide (NO), ferric reducing power (FRAP), and total antioxidant capacity (TAC) assays. Varying quantities of flavonoids, phenols, tannins, saponins, terpenoids, steroids, and alkaloids, were detected in the fraction. GC-FID phytochemical profiling of ELFSL revealed a high level of epicatechin, moderate levels of catechin, kaempferol, flavone, naringenin, rutin flavanones, tannins, sapogenins, proanthocyanidin, and steroids, and small amounts of sparteine, resveratrol, and lunamarine. The ELFSL exerted excellent dose-dependent in vitro anti-inflammatory activities comparable with standard drugs (aspirin/prednisolone). The LD50 test showed safety up to 5000 mg/kg body weight (per oral) ELFSL. Interestingly, mice pre-administered various doses (200, 400, 600 mg/kg bw, po) of ELFSL showed significant (P < 0.05) reduction in edema, writhing, and time spent licking paw in all phases compared with control and were at par with 100 mg/kg bw (po) aspirin. The result also registered good concentration-dependent antioxidant potentials for ELFSL and was comparable to standards (gallic acid, butylated hydroxytoluene, and ascorbic acid). These imply that ELFSL possesses excellent antioxidant, anti-inflammatory, and anti-nociceptive potentials mediated by peripheral and central mechanisms.
Keywords
Anti-inflammation
Anti-nociceptive
Antioxidants
Malaria
Whitlow
Phenolics
1 Introduction
Consistent exposure of the human system to noxious or non-injurious immunogens of both exogenous and endogenous origins could trigger immune responses and result in deleterious inflammatory events (Ammendolia et al., 2021). The symptoms of inflammation, such as pain, heat, fever, redness, swelling of the infected tissue, fluid exudation from the tissue, and loss of tissue function, are the leading cause of high mortality associated with inflammatory diseases (Chen et al., 2017). Diverse vasoactive compounds, including histamine, serotonin, prostanoids, lymphokines, cytokines, chemokines, and transcription factors commonly synthesized in the tissues, have been implicated in the inflammatory process (Abdulkhaleq et al., 2018). Inflammatory response acts as a physical barrier to prevent the spread of infection or injury and prime tissue repair. Chemical substances, including prostaglandins, released during inflammation bind to receptors and nociceptors, sensitize pain, and prime a suitable environment for tissue repair and healing (Eming et al., 2014). However, a prolonged and excessive inflammatory response could cause adverse effects on healthy tissues and result in disease conditions (Chen et al., 2017). Several synthetic steroidal and non-steroidal anti-inflammatory agents, capable of assuaging acute and chronic inflammatory disorders fill the marketplace. However, their adverse side effects have prompted the recent shift in research efforts to screening medicinal plants for alternative anti-inflammatory drug candidates (Marcum and Hanlon, 2010). The use of natural products in the development of therapeutic agents has seen massive considerations. Plants have always been a good source of food, drugs, and templates for designing and developing pharmacologic agents and modern-day pharmaceutics dating from a time memorial (Dias et al., 2012; Sofowora et al., 2013). Fortunately, due to the tremendous stresses stemming from UV radiation, salinity, microbial infestation, drought, and chemical toxicants in the environment, plants have acquired genetic traits to drive biosynthetic pathways needed for producing bioactive compounds essential for their survival (Tungmunnithum et al., 2018). Consequently, this makes them an excellent source of compounds with therapeutic potential. Some phytochemicals are well-known for their anti-inflammatory and antioxidant potentials (Amarlal et al., 2009; Fürst and Zündorf, 2014).
Sida linifolia L., a member of the Sida genus and Malvaceae family, is an underexploited plant with medicinal applications in West African folklore medicines (Akubue et al., 1983; Kokwaro, 1993; Burkill, 1997; Neuwinger, 2000; Dinda et al., 2015; Saensouka et al., 2016). The African ethnomedicinal importance of S. linifolia leaves, such as its use in managing cutaneous and subcutaneous infections, whitlow, and malaria, gave the rationale for the study. The present study investigated the bioactive composition, antioxidant properties, and possible anti-inflammatory and anti-nociceptive mechanisms of the ethanolic leaf fraction of S. linifolia.
2 Materials and methods
2.1 Plant collection and identification
Fresh leaves of S. linifolia were collected from Nsukka metropolis, Nsukka LGA of Enugu State, Nigeria (6.8429° N, 7.3733° E) in May 2021. The plant identification was performed at the Bio-resources Development and Conservation Program Research Centre, Nsukka, Enugu-Nigeria. A sample of the plant leaves was deposited in the herbarium (voucher no: BDCP20210724).
2.2 Extraction and fractionation procedures
The extraction and fractionation were carried out according to Hwang et al. (2009) and Parvin et al. (2015) methods. Briefly, fresh leaves of S. linifolia plant were carefully washed free from debris with clean water and allowed to dry in an open space away from sun reach until a constant mass was attained. The resultant dried leaves were pulverized using an electric blender (High-Speed Grinder, China). A known weight (2500 g) of powdered leaves of S. linifolia was macerated in 8.0 L of absolute ethanol (98 %) in a flat-bottomed sterile vessel. The vessel, sealed with cotton was kept for 24 h in a rotating incubator (Kottermann, Germany) at 200 × g. Filtration and concentration of the resultant solution were performed with Whatman No. 1 filter paper and a rotary evaporator set at 45 °C and reduced pressure, respectively. The ethanolic extract thus obtained was partitioned (3 times each) in a fractionating column using different solvents (n-hexane, ethyl acetate, and ethanol) in order of their polarity. The combined leaf fraction in each instance was concentrated using a rotary evaporator (at a similar condition). The concentrated leaf fractions were stored in sterile and well-labeled screw-capped vessels at 3–5 °C until needed for the study. The ethanolic leaf fraction was used in the study.
2.3 Animals
The study deployed Swiss albino mice of either sex weighing 28–32 g. The study animals were gotten from the Department of Zoology and Environmental Biology animal holding unit, University of Nigeria, Nsukka. They were all kept in a highly hygienic environmental condition (relative humidity of 55 ± 5 %, temperature of 24 ± 1 °C, and a 12 /12 h light/dark period) in standard cages and fed with good quality rat chow (Vital feed) and left to acclimatize with the environment for 14 days, before the commencement of the study.
2.4 Ethical approval and informed consent
An ethical clearance the meticulous and safe conduction of experiment that adhered to conventional ethical rules was obtained from the ethics committee of the Department of the Biochemistry University of Nigeria, Nsukka in May 2021 (approval number: UNN/BCH/9014). The experiment complied with international ethics and protocols in research as enshrined in the revised National Institute of Health (NIH, 85–23; 1985) guide on the use of experimental animals. The informed consent and permission from all human blood donor volunteers were solicited and obtained appropriately before their participation.
2.5 Acute toxicity (LD50) study
The lethality dose study (LD50) of the leaf fraction was performed using Lorke (1983) method. Mice were randomly assigned into six groups (three mice per group) and were deprived of food and drink for 12 h before the commencement of the study. Animals in each group were administered oral doses of the leaf fraction in varying concentrations (10, 100, 1000, 1600, 2900, and 5000 mg/kg body weight) by gavage. Thereafter, they were observed for any distortion in behavior (nervousness, in-coordination, and dullness) or mortality for 24 h duration. The animals were further monitored for 7 days for any late symptom of toxicity.
2.6 Preliminary phytochemical screening
The ethanolic leaf fraction of Sida linifolia (ELFSL) was screened quantitatively for its phytochemical constituents, following established methods (Trease and Evans, 1985; Harborne, 1998). The quantity of the various phytoconstituents recorded in the leaf fraction was estimated using the following formulae:
2.7 Gas Chromatography-Flame ionization detection (GC-FID) analysis
2.7.1 Sample preparation
This study employed the methods outlined by Buss and Butle (2010) and Kelly and Nelson (2014). Graded amounts of ethanol (15 mL) and 50 % (m/v) potassium hydroxide (10 mL) were added into a test tube containing 1 g of the test sample. The mixture was allowed to stand in a water bath for 60 min at 60 °C. Thereafter, the resultant mixture was dispensed into a separatory funnel. The tube was washed successively with ethanol (20 mL), cold water (10 mL), hot water (10 mL), and hexane (3 mL) and collectively transferred into the funnel. After washing thrice with 10 mL of 10 % (v/v) aqueous solution of ethanol, drying with anhydrous sodium sulfate and solvent evaporation was performed. The resultant sample was dissolved in 1000 µL of pyridine, and 200 µL of it, was transferred to the vial for analysis.
2.7.2 GC-FID quantification
Phytochemical analysis of the test sample was performed on a BUCK M910 Gas chromatography Machine coupled with a flame ionization detector. The RESTEK 15-meter MXT-1 1 (15 m × 250 µm × 0.15 µm) column was used. Helium 5.0 pas was the carrier gas with a flow rate of 40 mL/min. The injector temperature was 280 °C with a splitless injection of 2 µL of sample and a linear velocity of 30 cm/s. The oven operated initially at 200 °C and was heated to 330 °C at a rate of 3 °C min−1 which was maintained for 5 min. The detector was operated at a temperature of 320 °C. Quantification of the phytochemicals was done by evaluating the ratio of the area and mass of internal standard and the area of the identified phytochemicals. The concentration of the different phytochemicals was expressed in µg/g (which is equivalent to µg/mL and ppm).
2.8 Determination of in vitro anti-inflammatory properties
2.8.1 Membrane stabilization tests
The preparation of red blood cells (RBC) suspension followed the Gandhisan et al. (1991) method with minor modifications. Blood samples were drawn from healthy human volunteers who had not taken any NSAIDs for at least three weeks before the collection date. The blood collection was done with tubes containing sodium oxalate. The whole blood was transferred into an equal volume of Alsever solution (0.8 % sodium citrate, 0.42 % NaCl, 2 % dextrose, and 0.5 % citric acid) and allowed to stand for 24 h at 4 °C. After then, the mixtures were centrifuged at 3000 × g for 10 min, and the packed cells volume was reconstituted to a 40 % (v/v) RBC suspension using phosphate-buffered saline (10 mM, pH 7.4), at room temperature (30 °C).
2.8.1.1 Hypotonicity-induced hemolysis
The procedure of the study followed Umapathy et al. (2010) method, with slight modifications. Measured volume (1 mL) of the reaction mixture which contained 37.5 µg/mL of the leaf fraction or aspirin (ASP) at varying concentrations (0.2, 0.4, 0.6, 0.8, and 1.0 mg/mL) and 950 µL of phosphate-buffered saline was transferred into 20 µL of 40 % (v/v) RBC suspension. One hour incubation at 30 °C followed by centrifugation at 5000 × g for 5 min was performed, after which 200 µL of the resultant supernatant was transferred into microtitre plates. A microplate reader (Molecular Devices, Inc., US) was used to estimate the free hemoglobin in the mixture at 540 nm spectrophotometrically. Aspirin (ASP) was employed as the standard drug. The positive and negative controls (0 % and 100 % hemolysis) were obtained by subjecting the RBC suspension in 0.1 % (w/v) phosphate-buffered saline and distilled water, respectively. The test was carried out in triplicates at each concentration, and the percentage of hemolysis inhibition was estimated using the formula as follows;
Ab1 = absorbance of isotonic solution (control I).
Ab2 = absorbance of test or reference drug.
Ab3 = absorbance of hypotonic solution (control II).
2.8.1.2 Heat-induced hemolysis
This study adopted Okoli et al. (2008) method, with slight modifications. A preliminary experiment was carried out to derive a suitable incubating time and temperature for the heat-induced hemolysis assay. Graded volume (20 μL) of RBC suspension was transferred into a 1.5 mL micro-tube containing a known volume (980 μL) of pre-incubated buffer and incubated at 55 ± 0.1 °C in a water bath (with thermostat regulator) regulated at constant temperature using a calibrated mercury thermometer. At 5 min intervals for 45 min, tubes were withdrawn from the water bath and spun at 5000 × g for 5 min in a centrifuge. The supernatant absorbance taken at 540 nm with a microplate reader (Molecular Devices, C.A. U.S.A.) was registered at each interval. Thus, an appropriate incubation temperature and time (54 °C for 20 min) for the heat-induced hemolysis assay was reached from the result of the preliminary study. Briefly, graded volume (30 μL) of various concentrations (0.2, 0.4, 0.6, 0.8, and 1.0 mg/mL) of the leaf fraction or ASP was mixed with 20 μL of RBC suspension and distributed (in duplicates) into a micro-tube (1.5 mL) containing pre-incubated buffer (950 μL). An equivalent volume (50 μL) of pre-incubated buffer (the vehicle) was transferred into another tube, which served as the negative control. One tube from each pair was heated at 54 °C for 20 min in a water bath. Whereas, the other tube from the pair was allowed to stand in an ice bath at 0–5 °C. Thereafter, the resultant mixtures were subjected to centrifugation at 5000 × g for 5 min, and the absorbance of the supernatant was recorded at 540 nm. The hemolysis inhibition percentage was evaluated with the formula as thus;
Abs1 = absorbance of test/reference drug/ control sample not heated.
Abs2 = absorbance of test/reference drug/vehicle sample that was heated.
Abs3 = absorbance of heated negative control sample.
2.8.2 Inhibition of platelet aggregation assay
This study adopted the Born and Cross (1963) method with slight modifications . The principle of the test lies in CaCl2 induction of platelet aggregation which is inversely proportional to the absorbance of the mixture at 520 nm. Essentially, the inhibitory effect of pharmacological agents on platelet aggregation correlates positively with the absorbance of the mixture after the addition of CaCl2. Blood samples drawn from healthy human volunteers into EDTA bottles were centrifuged for 10 min at 3000 × g and the resultant supernatant diluted (twice) in normal saline was adopted as the platelet-rich plasma (PRP). A known volume (0.2 mL) of PRP was transferred into sets of three test tubes containing 1 mL of various concentrations (0.2, 0.4, 0.6, 0.8, and 1.0 mg/mL) of the leaf fraction or reference drug (aspirin) in normal saline. The vehicle (normal saline) was used to raise the volume of the reaction mixtures to 2.2 mL. A test tube containing 2 mL normal saline and 0.2 mL PRP served as the control. Incubation at 30 °C for 5 min was performed after which CaCl2 (0.4 mL of 1.47 %) was added to the reaction mixtures. The blanks contained the leaf fraction or reference drug void of PRP. All The experiment was done in triplicates. The change in absorbance of the mixture (at 520 nm) at 2 min intervals for 8 min was recorded, and the mean % inhibition was evaluated.
2.8.3 Protease inhibition assay
This study employed the method outlined by Sakat et al. (2010) with minor modifications. Graded volume (2 mL) of the reaction mixture consisted of 1 mL of various concentrations (0.2, 0.4, 0.6, 0.8, and 1.0 mg/mL) of the leaf fraction or reference drug (ASP) and 0.06 mg trypsin dissolved in Tris HCl buffered solution (20 mM, pH 7.4). Incubation at 37 °C for 20 min and treatment with 70 % perchloric acid (2 mL) to halt the reaction was performed, after which the resultant cloudy suspension was centrifuged at 3000 × g for 10 min. Thereafter, the absorbance (Abs) of the supernatant was read at 210 nm (blanking with Tris buffer). The experiment was done in triplicates, and the inhibitory effect of the leaf fraction or standard drug at varying concentrations on protease activity compared with control was estimated with the formula as thus;
2.8.4 Inhibition of phospholipase A2 assay
This study adopted the protocol described by Vane (1971) with slight modifications. The activity of phospholipase A2 (PLA2) is implicated in several inflammatory cascades leading to inflammation. Activated PLA2 rips out membrane phospholipids (including arachidonic acid, the precursor molecule for the synthesis of several vasoactive molecules) and liberates hemoglobin (in erythrocyte) into the extracellular milieu. The ability of pharmacological agents to inhibit phospholipase A2 activity on the cell membrane (for erythrocyte is directly proportional to the amount of oxidized hemoglobin liberated into the medium which can be estimated spectrophotometrically at 418 nm) correlates with their anti-inflammatory potentials. Briefly; isolation of the enzyme, PLA2 was from a pure culture of the Aspergillus niger strain, while red cells suspension obtained from a volume (5 mL) of whole blood drawn from healthy human volunteers served as the substrate for PLA2. Two sets of test tubes (labeled Blank and Test) contained equal volumes of RBC suspension (0.2 mL), CaCl2 (0.2 mL), and phosphate-buffered saline (1 mL). A known volume (1 mL) of boiled and free enzymes was added to Blank and Test tubes, respectively. Thereafter, 1 mL of various concentrations (0.2, 0.4, 0.6, 0.8, and 1.0 mg/mL) of leaf fraction or prednisolone (in phosphate-buffered saline) were dispensed separately into test tubes. The control test tube contained red cell suspension, CaCl2, and free enzyme. Incubation at 30 °C for 1 h and centrifugation at 3000 × g for 10 min was performed on the mixtures after which, the supernatant absorbance of the mixtures was recorded at 418 nm. The peak % enzyme activity and inhibition were estimated using the formulae as follows;
2.8.5 Albumin denaturation assay
This study employed the method outlined by Mizushima and Kobayashi (1968) with minor modification. The reaction mixture contained varying concentrations (0.2, 0.4, 0.6, 0.8, and 1.0 mg/mL) of the leaf fraction or reference drug (ASP) and 1 % bovine albumin leaf fraction in distilled water. The mixture pH was adjusted occasionally by adding a few drops of 1 N HCl. Incubation at 30 °C for 20 min followed by heating in a water bath for 30 min at 57 °C, then cooling to room temperature was performed, after which the turbidity of the reaction mixture was estimated at 660 nm spectrophotometrically. The test was done in triplicates, and the percentage inhibitory effect of the leaf fraction or standard drug at varying concentrations on albumin denaturation compared with control were estimated with the formula as thus;
2.9 In vitro antioxidant analysis
2.9.1 Total antioxidant capacity (TAC)
This study adopted the Prieto et al. (1999) phosphomolybdenum method. The principle of the study lies in the propensity of antioxidant agents to reduce Mo6+ to Mo5+, which results in green phosphate/Mo5+complex formation in an acid medium. The experiment mixture contains a graded volume (0.1 mL) of the leaf fraction or gallic acid at various concentrations (0.2, 0.4, 0.6, 0.8, and 1.0 mg/mL) mixed with a known volume (1 mL) of the reagent solution [prepared by introducing 0.6 M sulfuric acid into tubes containing 28 mM sodium phosphate and 4 mM ammonium molybdate]. Incubation of the resultant mixtures for 90 min at 95 °C, was done, after which the test tubes were allowed to cool to room temperature, and the mixture absorbance was read at 695 nm in a spectrophotometer. Blanking was done with 0.1 mL methanol mixed with 1 mL of the reagent solution. The calibration curve was plotted by adding various concentrations of gallic acid into tubes containing methanol. The free radical scavenging activity was expressed as gallic acid-gram equivalent. The percentage free radical scavenging activity of the leaf fraction was evaluated using the formula as thus;
The IC50 value, the concentration of the sample that scavenged 50 % of the initial concentration of the free radical in the mixtures was also evaluated graphically.
2.9.2 1,1-Diphenyl-2-picryl-hydrazyl (DPPH) radical-scavenging assay
This study employed the method outlined by Liyana-Pathirana and Shahidi (2005). The test principle lies in the ability of antioxidant agents to donate an electron pair to DPPH radicals (resulting in the reduction of their distinctive purple colour to colorless). Briefly; 200 μL of 0.1 mM DPPH solution (in methanol) was dispensed into sets of test tubes containing 100 μL of various concentrations (0.2, 0.4, 0.6, 0.8, and 1.0 mg/mL) of the leaf fraction or butylated hydroxytoluene (BHT) (standard). The mixtures thus formed were allowed to incubate at 37 °C for 15 min. The mixture's absorbance was registered at 517 nm with a spectrophotometer. The experiment was done in triplicates. The calibration curve was plotted by adding various concentrations of BHT into tubes containing methanol. The results of the assay were expressed as BHT-gram equivalent; the percentage free radical scavenging activity of the leaf fraction was estimated using the formula above;
2.9.3 Ferric reducing antioxidant power (FRAP) assay
This test adopted the Sahreen et al (2014) method. The principle of the test lies in the propensity of antioxidant agents to reduce Fe3+ to Fe2+ in an acid medium. Briefly; graded volume (2.0 mL) of the leaf fraction or standard drug (ascorbic acid) at various concentrations were dispensed into different test tubes containing 2.0 mL of 10 mg/L (0.1 % (w/v) potassium ferricyanide and 2.0 mL of 0.2 M, phosphate-buffered solution of pH 6.6. Incubation for 20 min at 50 °C in a water bath, followed by the introduction of 2.0 mL of 100 mg/L (10 % (w/v) trichloroacetic acid solution into the reaction mixtures, was performed. Thereafter, graded volume (2.0 mL) of the resultant mixture was dispensed into 0.4 mL of 0.1 % (w/v) ferric chloride (FeCl3.6H20) solution, containing 2.0 mL of distilled water. After standing for 10 min, the absorbance of the mixture was recorded at 700 nm with a spectrophotometer. The calibration curve was plotted with various concentrations of ascorbic acid. The results of the assay were expressed as ascorbic acid-gram equivalent. The percentage scavenging activity of the leaf fraction and standard drug on the free radicals was evaluated using the formula above.
2.9.4 Nitric oxide free radical scavenging assay
This test adopted the Marcocci et al. (1994) method. Generation of nitric oxide radicals was done with sodium nitroprusside, and the Griess reaction was used to evaluate the degree of inhibition exerted by the test samples. Briefly; varying concentrations (0.2, 0.4, 0.6, 0.8, and 1.0 mg/mL) the leaf fraction or reference (ascorbic acid) was dispensed into graded volume (5.0 mL) of phosphate-buffered sodium nitroprusside (5 mM) solution. Incubation of the resultant mixture for 20 min at 25 °C was performed. An equal volume of the buffer treated in a like manner void of the test solution served as the control. Standing for 5 h, a graded volume (0.5 mL) of Griess solution (2 % Ophosphoric acid, 0.1 % naphthyl ethylenediamine dihydrochloride, and 1 % sulfanilamide) was added to the mixtures, thereafter, the mixture absorbance was recorded at 546 nm with a spectrophotometer. The calibration curve was plotted with various concentrations of ascorbic acid. The results were expressed as the quantity in gram-equivalent of ascorbic acid. The total antioxidant capacity (in %) of the leaf fraction compared to the reference drug was estimated with the formula above.
2.10 In vivo anti-inflammatory and anti-nociceptive tests
The anti-inflammatory and anti-nociceptive properties of ELFSL were determined using two models each. The dosages of fraction and standard drug employed in this study were adopted from that of Konaté et al. (2012). In each model, mice were randomly grouped into eight groups of four animals each (n = 4). Distilled water (10 mg/kg bw per oral), various dosages (200, 400, and 600 mg/kg bw, po) of ELFSL and 100 mg/kg bw, po ASP, respectively, were given to 24 h fasted mice in the various groups 1 h before the injection of phlogistic agents. Egg albumin and carrageenan and acetic acid and formalin models were employed in the anti-inflammatory and anti-nociceptive studies, respectively.
2.10.1 Carrageenan-induced rat paw edema test
This test was performed according to the protocol described by Winter et al. (1962). Thirty minutes post-administration, fresh carrageenan (0.1 mL, 0.01 g/mL) solution was injected into the subplantar region of the left hind paw of mice. The volume of the paw was measured using a digital Plethysmometer device. In effect, the animal paw is immersed in a particular water cell containing water; the resistance of which changes as a result of the animal paw's immersion. The change in resistance (which corresponds to the volume displacement) is measured in milliliters and displayed on the Plethysmometer's electronic monitor. The volume displacement change was measured at time zero of the carrageenan injection into the mice's hind paw and every 1 h thereafter for 5 h. The difference in volume displacement following phlogistic agent administration and zero time volume displacement of the induced paw (At – Ao) was used to calculate the average edema in each period (Anosike et al., 2012). Evaluation of the % inhibition of paw edema was done using the formula as thus;
Here; (At – Ao) = instanteneous change in mouse paw edema volume.
Ao = time zero mouse paw edema volume.
At = various time intervals mouse paw edema volume.
2.10.2 Egg albumin-induced rat paw edema test
This test was performed according to the method described by Okokon and Nwafor (2010). The egg albumin edema model is a useful inflammation model for screening anti-inflammatory agents. Increased vascular permeability and swelling of the paw are signs of induced edema. The amplitude and extent of the inflammation cascades are reduced when the attendant edema is inhibited, indicating anti-inflammatory activities. One hour after various treatments, 0.1 mL of undiluted fresh egg albumin was injected (i.p.) into the peritoneal cavity of the right hind paw of mice. Changes in the mice right hind paw volume was recorded starting at time zero of the experiment and every-one hour after the injection with egg albumin, for 5 h (Anosike et al., 2012). In each interval, the average edema and % paw edema inhibition were assessed.
2.10.3 Acetic acid-induced writhing test
This test was performed according to the method outlined by Mbagwu et al. (2007), with minor modifications. The acetic acid writhing model has been used to evaluate analgesic and anti-inflammatory drugs. Acetic acid is injected into the peritoneal cavity of animals to induce writhing, indicative of pain. A stretch, extension of the hind limbs, abdominal contraction so that the animal trunk touches the floor, or twisting of the trunk are all signs of pain (Koster et al., 1959). The analgesic activity of the test compounds is determined by their propensity to decrease writhing frequency. Sixty minutes post-treatment, the animals were injected (i.p.) 0.6 % (v/v) (10 mL/kg bw) acetic acid. Thirty minutes, post-acetic acid injection, the number of writhes (characterized by abdominal constrictions) was recorded for 30 min at 5 min intervals, and the average responses of mice pretreated with varying dosages of the leaf fraction were compared with the control and standard group. The % inhibition of writhing represents the anti-nociceptive activity of the vehicle/reference drug/fraction. The % inhibition of writhing was calculated using the expression as follows; where MWN = Mean number of writhing.
2.10.4 Formalin-induced arthritis test
This test was performed following the procedure described by Adeyemi and Akindele (2007), with minor modifications. Thirty minutes post-oral administration of the various treatment regimes, formalin (0.02 mL of 1 % v/v) solution was injected (i.p.) into the subplantar region of the right hind paw of mice. The amount of time (in sec.) spent by the mice licking and biting the injected paw, indicative of pain, was recorded for each animal. The formalin test is said to be biphasic. The first phase is characterized by a nociceptive response, which usually peaks 5 min after injection, while the second phase, indicated by a neurogenic and anti-inflammatory response peaks 15–30 min post-formalin injection. The mice responses of the first (0–5 min) and second phases (15–30 min) were recorded after formalin injection.
2.11 Statistical analysis
The data obtained in the study were analyzed using Statistical Package for Social Sciences (SPSS) version 23.0, and the results were expressed as mean ± standard error of the mean (SEM). Significant differences observed across various groups were determined using one-way and two-way ANOVA (Descriptive and Duncan post hoc). The established level of significance for the entire results was fixed at P < 0.05.
3 Results
3.1 Percentage yield
Extraction of a known amount (2500 g) of pulverized S. linifolia leaves yielded 70.65 g of crude ethanol extract, accounting for 2.83 % of the starting material. Further fractionation of the extract (70.65 g) yielded 15.82 g (22.39 %) n-Hexane fraction, 21.30 g (30.14 %) ethyl acetate leaf fraction 28.90 g (40.91 %) ethanolic fraction, and the residual mass was 4.63 g (6.55 %). The extraction yield may be influenced by several factors, including the time of harvest, drying temperature, storage condition, the texture of the test material, type and concentration of the solvent and extraction duration (Amah et al., 2022). Due to the affinity of most phenolics to polar fractions and the cumulative evidence of their potent pharmacological profile, the study further evaluated the bioactivities of the ethanolic leaf fraction (ELFSL). The choice of the fraction used in this study corresponds with Debalke et al. (2018).
3.2 Preliminary phytochemical screening of ELFSL
The result of the phytochemical screening of ELFSL is presented in Table 1. The result revealed high amounts of phenols, flavonoids, and tannins and moderate levels of steroids, alkaloids, glycosides, terpenoids, saponin, and cyanogenic compounds in the ELFSL. The rich composition of the plant leaf fraction in phenolic compounds, steroids, terpenoids, alkaloids, and saponins suggests a potent pharmacological profile. Studies have proposed a correlation between the phytochemical composition of plant materials and their pharmacological activities (Barbosa-Filho et al., 2006; Farooq et al., 2022). Steroids are well known to modulate inflammatory responses via their interactions with specific intracellular receptors which translates into either trans-repression (negative) or trans-activation (positive) of inflammation-related genes (Vandevyver et al., 2013; Timmermans et al., 2019). Flavonoids elicit anti-inflammatory and analgesic potentials by reducing intracellular Ca2+, thereby repressing and preventing the activation of phospholipase A2, whose activity produces the precursor molecules for the synthesis of a variety of inflammatory mediators (Mozaffarian and Wu, 2018; Yang et al., 2020; Yang et al., 2022; Waghole et al., 2022). Tannins are potent cyclooxygenase (COX) inhibitors with a notable propensity to suppress edemogenesis induced by phlogistic agents (Wagner, 1989; Attiq et al., 2018). The rich phenolic composition of the leaf fraction also implies excellent bioactivities, as phenolics are known to exhibit COX-1-sparing activities (Tungmunnithum et al., 2018). A recent study demonstrated the potential of some phenolics obtained from Canadian medicinal plants to selectively suppress COX-2 expression, sparing COX-1 (Ma et al., 2017). Prostaglandin E2 synthesized by COX-2 mediates nociception contrarily to COX-1, which plays the housekeeping functions and helps maintain the mucosal membrane integrity against aggressive activities of enterobacteria (Helicobacter pylori) and gastric contents (Attiq et al., 2018). These underpin the toxicity of NSAIDs, such as gastric ulcers and renal dysfunction caused by their indiscriminate inhibition of COX-1 and COX-2. Contrarily, agents that selectively suppress COX-2, sparing COX-1, are considered better anti-inflammatory drug options (Tungmunnithum et al., 2018). Results (analyzed using one-way ANOVA and Duncan post hoc) are expressed as Mean ± SEM (n = 3). Figures in a column having different alphabets as superscripts are significantly (P < 0.05) different.
Phytochemical Composition
Concentration (mg/100 g)
ELFSL
Flavonoids
118.0 ± 1.0d
Phenols
124.2 ± 0.6d
Tannin
74.2 ± 0.1c
Cyanogenic compounds
37.4 ± 4.3a
Glycoside
44.4 ± 1.0b
Terpenoid
43.9 ± 0.7b
Saponin
43.6 ± 0.8b
Steroids
54.2 ± 0.6b
Alkaloids
46.7 ± 0.3b
3.3 GC-FID profile of ELFSL
Further study to elucidate the different types of phytochemicals in the leaf fraction employed GC-FID analysis. The GC-FID chromatogram showing the phytochemical profile of ELFSL is shown in Fig. 1. The result revealed a higher level of epicatechin, moderate levels of sapogenins, tannins, proanthocyanidins, cardiac glycosides, cyanogenic glycosides, naringenin, rutin, kaempferol, flavan-3-ols, catechins, flavone, flavanones, steroids, lunamarine, sapogenin, oxalate and phytate, and small amounts of spartein and resveratrol in the leaf fraction (Table 2). * 1 ppm = 1 µg/mL = 1 µg/g.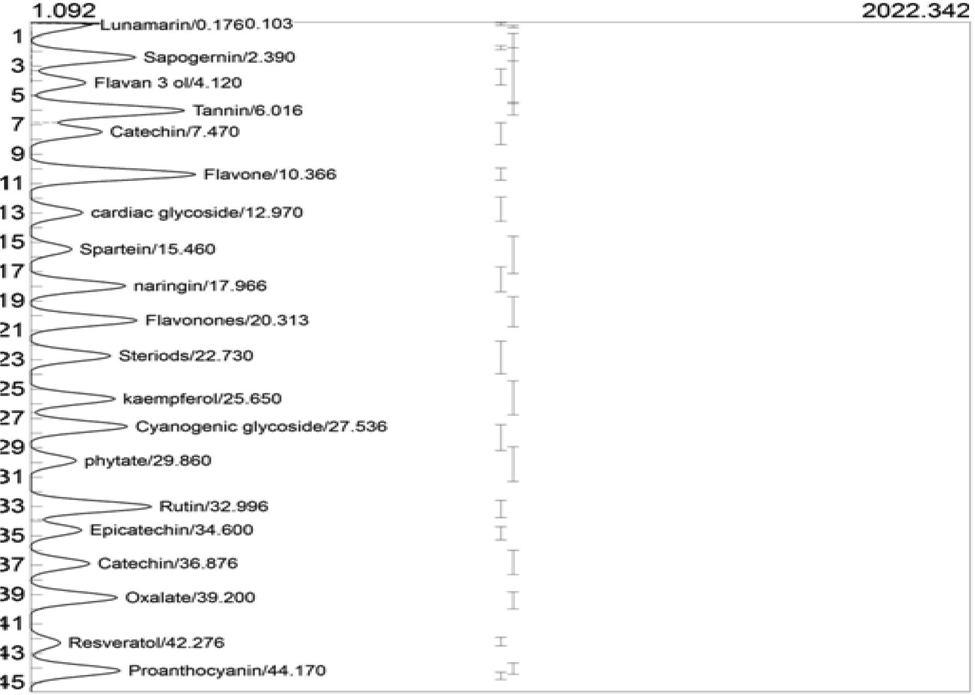
GC-FID chromatogram of ELFSL.
Compounds
Retention time (min)
Peak area
Peak height
Concentration (ppm)
Catechin
44.346
15059.251
279.336
10.8069
Proanthocyanin
44.170
10559.9278
191.980
5.0795
Resveratrol
42.276
3495.6477
63.894
1.9882
Oxalate
39.200
10092.8634
185.203
8.5897
Epicatechin
34.600
5872.8319
109.164
32.8801
Rutin
32.996
14308.4383
260.056
10.6547
Phytate
29.860
5353.2078
98.885
5.8161
Cyanogenic glycosides
27.536
11372.0654
208.605
10.6995
Kaempferol
25.650
10006.5650
182.904
9.0087
Steroids
22.730
9444.2704
173.278
12.8502
Flavonones
20.313
12481.4190
228.894
8.2466
Naringenin
17.966
11123.6380
204.891
8.2466
Spartein
15.460
4822.1464
89.919
2.0607
Cardiac glycosides
12.970
6155.5198
113.128
8.7167
Flavone
10.366
19532.1202
355.819
14.5446
Tannins
6.016
18223.8885
330.214
11.1121
Flavo-3-ol
4.120
6303.2894
116.993
4.6501
Sapogernin
2.390
12079.7082
223.098
10.5154
Lunamarin
0.279
4275.0512
368.972
8.2778
The rich composition of phenolic hydroxyl, electron-donating and electron-withdrawing groups in the ring structure of phenolic compounds endows them with excellent antioxidant potentials (Złotek et al., 2016). Stilbenoids, including resveratrol, are non-nitrogenous polyphenols with acidic and amphiphilic properties, well-known to possess anti-inflammatory potentials. Resveratrol (3,5,4′-trihydroxy trans-stilbene), a polyphenol stilbenoid containing two adjacently placed phenols that are linked by an ethylene bridge, has been shown to exhibit anti-inflammatory, neuroprotective and vasorelaxant potentials (Salehi et al., 2018). They act by inhibiting cyclooxygenases and 5-lipoxygenase (5-LOX) activity (Dvorakova and Landa, 2017; Kong et al., 2017). Studies have demonstrated the ability of resveratrol to inhibit the expression and release of inflammatory factors such as NF-κB, tumor necrosis factor-α and interleukin-6, macrophage inflammatory protein-2, and cyclooxygenase-2 activity, reactive oxygen species generation and caspase-3/9 in rabbit models (Zhou et al., 2018). Resveratrol was also reported to inhibit carrageenan-induced ear edema in mice (Wang et al., 2017), as well as attenuate neuropathic pain by balancing the release of pro-inflammatory and anti-inflammatory cytokines in mice models (Tao et al., 2016). Therefore, the presence of resveratrol in the leaf fraction suggests its anti-inflammatory and antioxidant potentials.
The presence of catechin, epicatechin, kaempferol, naringenin, and rutin in ELFSL suggests its anti-inflammatory and antioxidant properties. Scientists have recognized flavonoids as a class of compounds with diverse therapeutic potentials. Classification of flavonoids on the premise of their molecular structure include flavones, flavanones (naringenin), flavonols (kaempferol), and flavononols (Kumar and Pandey, 2013; Panche et al., 2016; Ginwala et al., 2019). Flavan-3-ols, also known as flavanols, are a complex subclass of flavonoids, which include catechin and epicatechin (simple monomers). Flavan-3-ols is known to exhibit antioxidant and anti-inflammatory properties among other pharmacological profiles (Aron and Kennedy, 2008; Mena et al., 2014). Epicatechin, a natural phenolic compound widely found in plants, possesses several pharmacological potentials, including antioxidant, anticancer, and anti-inflammatory activities (Zhong et al., 2012; Zhou et al., 2012; Wang and Cao, 2014). Catechin, a polyphenolic compound found in food and medicinal plants, has been very effective in scavenging free radicals and may function indirectly as antioxidants through their interactions with transcription factors and enzymes in humans (Higdon and Frei, 2003; Fan et al., 2017). Plant-derived catechin also exerts an anti-inflammatory effect via its excellent antioxidant activities (Kim and Heo, 2022). Furthermore, catechin has been shown to exert its anti-inflammatory effects possibly by activating or deactivating cell signaling pathways associated with oxidative stress and inflammation, including nuclear factor-kappa B (NF-κB), mitogen-activated protein kinases (MAPKs), transcription factor nuclear factor (erythroid-derived 2)-like 2 (Nrf2), signal transducer and the activators of transcription 1/3 (STAT1/3) pathways (Fan et al., 2017). Kaempferol can reduce the risk of contracting chronic diseases, such as cancer, supplement the human antioxidant mechanisms, and modulate inflammation, apoptosis, angiogenesis, and metastasis (Chen and Chen, 2013). Naringenin, a type of flavanone, has several reported biological activities in humans, including the ability to inhibit lipid peroxidation and protein carbonylation, enhance carbohydrate metabolism, modulate immune response and inflammation, fortify the antioxidant mechanisms and scavenge free radicals (Salehi et al., 2019). Rutin is associated with several pharmacological activities, such as antioxidant, vasoprotective, cytoprotective, neuroprotective, cardioprotective, and anticarcinogenic activities (Ganeshpurkar and Saluja, 2017).
Lunamarine, a quinolone alkaloid with excellent immunomodulatory potentials, was also detected in the leaf fraction (Manu and Kuttan, 2009a; Manu and Kuttan, 2009b). Sparteine is a quinolizidine alkaloid with an excellent pharmacological profile, such as its ability to exert moderate analgesic and sedative effects in human and animal models (Pothier et al., 1998). The anticonvulsant and anticancer properties of sparteine have also been documented (Villalpando-Vargas et al., 2016; Liang and Liu, 2019). The chromatogram also detected the presence of sapogenins, which are known to modulate Ca+2 ions (Sun et al., 2016).
Tannins and Proanthocyanidin were also present in the ELFSL at moderate levels. Tannins are water-soluble polyphenols found in many plants. Generally, tannins possess anti-irritant and antiphlogistic potentials (Westendarp, 2006), while proanthocyanidins are condensed tannins in plants with various pharmacological properties, including free radicals scavenging, anti-inflammatory and anticancer effects (Ouedraogo et al., 2011; Dai et al., 2014; Rauf et al., 2019).
A moderate level of cardiac glycosides was also present in the leaf fraction. Cardiac glycosides are steroids capable of exerting specific effects on the cardiac muscle. A small amount of the compound can exert benefits in cardiac resuscitation. They manipulate the renin-angiotensin axis to improve cardiac output. Consequently, the myocardium becomes a more efficient pump and can meet the demands of the circulatory system (Haynes, 1906; Kelly, 1990; Farnsworth, 1966; Patel, 2016). Cyanogenic glycosides, oxalate, and phytate were also present in the ELFSL. A recent study has reported a dose-dependent anti-inflammatory and antiphlogistic effect of a cyanogenic glycoside (amygdalin) (Figurová et al., 2021). In addition, studies have suggested that a moderate amount of phytate may be beneficial as an antioxidant and anticancer agent (Goufo and Trindade, 2014; Gibson et al., 2018) and inhibit the formation of calcium oxalate kidney stones (Grases et al., 2000; Akter et al., 2020).
3.4 Anti-inflammatory activities of ELFSL
3.4.1 Membrane stabilizing effect of ELFSL
The membrane-stabilizing effect of ELFSL is shown in Table 3. From the result, ELFSL demonstrated excellent erythrocyte membrane stabilizing activity against hypotonicity- and heat-induced hemolysis in a concentration-dependent manner. The maximum membrane-stabilizing activities (63.19 ± 0.07 and 60.20 ± 0.71 %), exhibited by ELFSL against hypotonicity- and heat-induced hemolysis, respectively, were produced with the lowest dose (1.0 mg/mL) of the fraction; however, ASP showed more stabilizing potential (76.35 ± 0.48 and 76.69 ± 0.20 %), respectively, at a similar concentration (1.0 mg/mL). Our result agrees with Kumar et al. (2014), who reported effective membrane-stabilizing activity of extract from Sida cordata. The susceptibility of the lysosomal membrane to damage by injurious agents is critical in the modulation of inflammatory response. Injury on the lysosome may result in excessive release of proteolytic enzymes, which further exacerbates the inflammatory events (Chaitanya et al., 2011; Bukhari et al., 2013). Since, the erythrocyte membrane is an excellent model of the lysosomal membrane (Yoganandam et al., 2010), the ability of fraction to stabilize red cells membrane implies its lysosomal membrane-stabilizing potential (Anosike et al., 2012; Labu et al., 2015). Results (analyzed using one-way ANOVA and Duncan post hoc) are expressed as Mean ± SEM (n = 3). Figures in a column having different alphabets as superscripts are significantly (P < 0.05) different. π: indicates that prednisolone was the standard drug.
Treatments
Conc. (mg/mL)
Inhibition of hypotonicity-induced hemolysis (%)
Inhibition of heat-Induced hemolysis (%)
Inhibition of platelet aggregation (%)
Inhibition of Protease (%)
Inhibition of phospholipase A2 (%)
Inhibition of protein denaturation (%)
ELFSL
0.2
39.52 ± 0.17a
40.25 ± 0.63a
38.37 ± 0.13a
38.96 ± 0.12a
40.25 ± 0.63a
32.90 ± 0.50a
0.4
43.98 ± 0.14b
44.44 ± 0.41b
40.17 ± 0.30b
45.09 ± 0.31b
44.44 ± 0.41b
36.94 ± 0.38b
0.6
48.43 ± 0.16c
47.39 ± 0.69c
46.79 ± 0.10c
49.28 ± 0.40c
47.39 ± 0.69c
43.00 ± 0.52c
0.8
62.25 ± 0.16d
50.45 ± 1.76d
55.90 ± 0.17d
54.55 ± 0.37d
50.45 ± 1.76d
46.18 ± 0.52d
1.0
63.19 ± 0.07d
60.20 ± 0.71e
60.60 ± 0.19e
64.33 ± 0.17e
60.20 ± 0.71e
56.57 ± 1.01e
Aspirin
0.8
83.53 ± 0.62e
67.57 ± 0.20f
61.79 ± 0.58f
62.92 ± 0.17f
π 58.84 ± 0.59e
57.30 ± 0.09e
1.0
76.35 ± 0.48d
76.69 ± 0.20 g
69.00 ± 0.62 g
66.44 ± 0.13 g
π 67.11 ± 1.18f
69.73 ± 0.47f
3.4.2 Effect of ELFSL on platelet aggregation
The inhibitory effect of ELFSL on platelet aggregation is shown in Table 3. The ELFSL displayed excellent inhibitory potential on CaCl2-induced platelet aggregation in a concentration-dependent manner and was at par with the reference drug (aspirin). The peak inhibitory effect (60.60 ± 0.19 %) of the fraction was produced at the highest concentration (1.0 mg/mL). However, ASP demonstrated more potent (P < 0.05) anti-platelet aggregatory effect (76.35 ± 0.48 %) at similar concentration (1.0 mg/mL). The inhibitory effect of the leaf fraction on platelet aggregation may have followed the suppression of phospholipase A2 and cyclooxygenase activities, essential for the biosynthesis of thromboxane A2 (TXA2), a powerful platelet aggregator. When synthesized, TXA2 mediates platelet aggregation by increasing intracellular Ca2 + concentrations, facilitating the exocytosis of platelet granules and subsequent release of adenosine diphosphate (ADP) critical in platelet aggregation (Wang et al., 2021). The aggregation of platelets (thrombocytes) in the sight of injury initiates blood clotting and arrests bleeding; however, this facilitates the adhesion and diapedesis of leukocytes into the infected tissues and enhances endothelial responses to a variety of inflammatory stimuli (Hosseinzadegan and Tafti, 2017). Perhaps, the anti-platelet aggregatory action of ELFSL could also be related to a subsequent decrease in P-selectin and histamine activities, which, with platelet degranulation and aggregation, mediates an increase in vascular permeability and leucocyte extravasations (Zarbock et al., 2006). Platelet aggregation is associated with the pathophysiology of several diseases, including myocardial infarction, stroke, embolism, and other vascular thromboses (Fabre and Gurney, 2010). Therefore, the ability of the plant fraction to inhibit platelet aggregation suggests its antithrombotic and anti-inflammatory potentials. Our result correlates with Bahar et al. (2013) and Khan et al. (2014), which reported significant thrombolytic activity of Sida acuta and Sida rhombifolia, respectively.
3.4.3 Effect of ELFSL on protease activity
The inhibitory effect of ELFSL on protease activity is shown in Table 3. The ELFSL exhibited a significant (P < 0.05) degree of inhibition on protease activity that correlates positively with concentration. The maximum inhibition (64.33 ± 0.17 %) exerted by ELFSL occurred at the highest concentration (1.0 mg/mL). However, ASP showed significantly (P < 0.05) greater degree of protease inhibition (66.44 ± 0.13 %) at similar concentration (1.0 mg/mL). The observed inhibitory effect of the leaf fraction on protease activity may be attributable to its rich composition in phytochemicals known to inhibit protease activity, such as catechin, kaempferol, and other flavonoids. Studies have proposed the involvement of disproportionate release of proteases by immune cells or in lysosomal leakage in several inflammatory disorders (Bermúdez-Humarán et al., 2015; Enechi et al., 2019). Neutrophils being the first line of defense during an infection or tissue injury, upon activation and degranulation, secrete serine proteases such as proteinase 3, cathepsin G, and elastase into the extracellular environment, which degrade extracellular matrix proteins such as collagen, fibronectin, and elastin, facilitating leucocytes adhesion and diapedesis into the infected tissues (Pham, 2006; Korkmaz et al., 2008; Jakimiuk et al., 2021). Inhibition of proteases as a pharmacological target in treating various human diseases has proven to be a successful strategy in recent drug discoveries (Copeland et al., 2007). Elastase inhibitors are used to treat cancers, rheumatoid arthritis, and pulmonary disorders, among other ailments (Reboud-Ravaux, 2001; Tundis et al., 2015). Plants synthesize several protease inhibitors to protect them from insects, pest infestation, and herbivores (Srikanth and Chen, 2016). Flavonoids (flavan-3-ols, flavones, and flavanones) and other phenolics with two adjacent phenol groups are said to have inhibitory effects on trypsin-like serine proteases at the micromolar potency (Xue et al., 2017). As a result, the ability of ELFSL to efficiently block these hydrolytic enzymes could be due to its rich flavonoid composition.
3.4.4 Effect of ELFSL on phospholipase A2 activity
The result of the inhibitory effect of ELFSL on phospholipase A2 activity is shown in Table 3. The ELFSL also inhibited phospholipase A2 in a concentration-dependent manner. The maximum inhibition (60.20 ± 0.71 %) occurred at the highest concentration (1.0 mg/mL). However, the reference drug (prednisolone) exhibited significantly (P < 0.05) greater phospholipase A2 inhibition (67.11 ± 1.18 %) at similar concentration (1.0 mg/mL). These depict a close resemblance in the mechanism of action between the leaf fraction and the reference drug (prednisolone), a typical steroidal anti-inflammatory drug. The leaf fraction may have acted by inhibiting the enzyme activity and preventing the release of arachidonic acid from the cell membrane lipid bilayers, which is essential for the biosynthesis of eicosanoids (Coutinho and Chapman, 2011). Steroidal anti-inflammatory drugs, such as corticosteroids and other glucocorticoids, have a similar mechanism of action; the ability to inhibit phospholipase A2 (Patel and Savjani, 2015). Moreover, an appreciable amount of steroids was detected in the leaf fraction, which may be attributable to the observed bioactivities. Our result correlates with that of Eze and Nwodo (2016).
3.4.5 Effect of ELFSL on protein denaturation
The result of the inhibitory effect of ELFSL on protein denaturation is shown in Table 3. The protein denaturation inhibition assay is a useful tool for determining the propensity of pharmacologic agents to reduce inflammation (Meredith, 2005; Enechi et al., 2019). The result showed that ELFSL exhibited an effective inhibitory effect on albumin denaturation in a concentration-dependent manner. The maximum inhibitory effect (56.57 ± 1.01 %) occurred at the highest concentration (1.0 mg/mL) of ELFSL. However, at similar concentration (1.0 mg/mL), ASP showed significantly (P < 0.05) higher inhibition (69.73 ± 0.47 %). The result suggests the ability of ELFSL to inhibit inflammation akin to the reference drug. Proteins lose their native structure and biological functions when they are denatured. This event usually results in increased recruitment of leukocytes into the infected tissues intending to eliminate the damaged proteins. However, this defensive response sometimes results in the indiscriminate destruction of healthy tissues by the immune cells (Ogbu et al., 2019). The ability of the fraction to inhibit protein denaturation suggests its anti-inflammatory properties. Our result correlates with that of (Shankar et al. 2021), who reported similar activities with Sida acuta ethanolic leaf extract.
3.5 Acute toxicity
Table 4 shows the result of the acute toxicity study. From the result, the administration of ELFSL revealed no toxicity up to 5000 mg/kg bw (po) over the period of observation. Studies have proposed a safe oral administration of pharmacologic substances with LD50 up to 5000 mg/kg bw (Anosike et al., 2012). Three submaximal doses (200, 400 and 600) which were found to be safe in mice (Konaté et al., 2012) were employed for further biochemical investigations. n = 3; po = per oral treatment; bw = body weight.
Treatments (mg/kg bw, po, ELFSL)
No. of animals used
No. of death recorded
10
3
0
100
3
0
1000
3
0
1600
3
0
2900
3
0
5000
3
0
3.6 In vivo anti-inflammatory activities of ELFSL
3.6.1 Effect of ELFSL on carrageenan-induced edema in mice
The effect of ELFSL on carrageenan-induced edema in mice is shown in Table 5. Injection of carrageenan into the peritoneal cavity of the left hind paw of mice in the control group resulted in edema progression, which peaked (6.30 ± 0.20 mL paw volume increase) at 4 h post-phlogistic agent injection. Mice pre-treated with various doses of ELFSL showed a significant (P < 0.05) decrease in paw volume in a timely and dose-dependent manner compared with the control and was similar to that produced by the standard drug (100 mg/kg bw, po ASP). The maximum inhibitory effect (77.53 %) of ELFSL on carrageenan-induced edema occurred at the highest dose (600 mg/kg bw po, ELFSL) at the 5th hour. This effect was not significantly (P > 0.05) lower than that (77.80 %) recorded for 100 mg/kg bw (po) ASP at the same duration. The plant fraction at various doses exerted significant inhibition on all three phases of carrageenan-induced inflammation and peaked at the third phase (the 5th hour). The edema inhibition was statistically significant (P < 0.05) compared with the control and did not differ significantly (P > 0.05) from the reference drug (ASP). The ability of the fraction to inhibit the early phase of inflammation (1–2 h) in the carrageenan model suggests that it may have suppressed the release of histamine, serotonin, and bradykinin (Georgewill and Georgewill 2010; Georgewill et al., 2010). The events of the late phase are mediated by excessive activity of bradykinin protease, cyclooxygenase (COX) synthesis of prostaglandins, and the release of proteolytic enzymes from the lysosome (Wallace, 2002; Kang et al., 2008). From the result, it could be that the fraction inhibited the late phase of inflammation in this model by suppressing the release and activities of prostanoids (Ricciotti and FitzGerald, 2011). Aspirin (ASP), a typical NSAID and an excellent cyclooxygenase inhibitor capable of suppressing prostaglandin synthesis, also showed a similar inhibitory effect on the carrageenan model, suggesting a similarity in activities between the NSAID and the fraction. Our result aligns with Sutradhar et al. (2006), which reported similar activity with principles from Sida cordifolia. Results (Two-way ANOVA performed with Duncan post hoc test) are expressed as Mean ± SEM (n = 8). Values in parentheses show the % inhibition of edema. Subsets of a column with different small lettered alphabets and rows with different capital alphabets as superscript vary significantly (P < 0.05). Control = 10 mg/kg bw distilled water; bw = body weight; po = per oral administration.
Treatments (mg/kg bw, po)
Mean oedema Volume (mL)
(% edema inhibition)
1 h
2 h
3 h
4 h
5 h
Toxic control
6.06 ± 0.10aC
–6.16 ± 0.07aBC
–6.26 ± 0.03aB
–6.30 ± 0.20aA
–6.05 ± 0.06aC
–
100 Aspirin
5.59 ± 0.07bA
(45.73 %)5.01 ± 0.10bB
(51.09 %)4.52 ± 0.39bC
(56.64 %)3.99 ± 0.12bD
(64.78 %)3.29 ± 0.07bE
(77.80 %)
200 ELFSL
5.19 ± 0.11cA
(40.12 %)4.53 ± 0.14cB
(45.64 %)3.98 ± 0.20cC
(52.15 %)3.18 ± 0.23cD
(64.65 %)2.76 ± 0.30cE
(74.99 %)
400 ELFSL
5.36 ± 0.14bcA
(43.85 %)4.92 ± 0.18bB
(47.77 %)4.43 ± 0.10bC
(52.94 %)3.66 ± 0.06bD
(63.92 %)3.10 ± 0.12bE
(75.50 %)
600 ELFSL
5.27 ± 0.06cA
(45.34 %)4.42 ± 0.07cB
(54.00 %)3.70 ± 0.11cC
(64.49 %)3.23 ± 0.12cD
(73.83 %)3.07 ± 0.11bDE
(77.53 %)
3.6.2 Effect of ELFSL on egg albumin-induced mice paw edema
The effect of ELFSL on egg albumin-induced edema in mice is shown in Table 6. Intraperitoneal injection of egg albumin into the right hind paw of mice in the control group resulted in edema progression, which peaked 1 h post-induction (6.90 ± 0.10 mL increase in paw volume). However, mice pre-treated with various dosages of the leaf fraction showed a significant (P < 0.05) decrease in paw volume in a time-related and dose-dependent manner compared with the control and was akin to that of the reference drug (100 mg/kg bw, po ASP). The maximum inhibition (78.27 %) of egg albumin-induced edema occurred at the lowest dose (200 mg/kg bw, po) of ELFSL in the 5th hour. Moreover, this effect was not significantly (P > 0.05) lower than that (81.40 %) recorded for 100 mg/kg bw (po) ASP at similar time interval. Intraperitoneal injection of egg albumin into animal paws is known to provoke the release of vasoactive compounds, like histamine, and serotonin, which results in increased vascular permeability and edemogenesis. Perhaps, the fraction may have exerted its inhibitory effect on egg albumin-induced edemogenesis by inhibiting the release and vasodilatory activities of histamine and serotonin (Akindele et al., 2015). Aspirin, a powerful cyclooxygenase inhibitor, also showed a significant (P < 0.05) inhibitory effect on egg albumin-induced edema formation akin to the fractions, implying a similarity in anti-inflammatory potential between ASP and the fraction. John-Africa et al. (2013) reported similar results with Sida corymbosa leaf extract. Results (Two-way ANOVA performed with Duncan post hoc test) are expressed as Mean ± SEM (n = 8). Values in parentheses show the % inhibition of edema. Subsets of a column with different small lettered alphabets and rows with different capital alphabets as superscript vary significantly (P < 0.05). Control = 10 mg/kg bw distilled water; bw = body weight; po = per oral administration.
Treatments (mg/kg bw, po)
Mean oedema Volume (mL)
(% edema inhibition)
1 h
2 h
3 h
4 h
5 h
Toxic control
6.90 ± 0.10abC
–6.37 ± 0.07aA –
6.51 ± 0.05aA
–6.63 ± 0.04aA
–6.48 ± 0.06aA
–
100 Aspirin
6.43 ± 0.17aA
(32.90 %)6.12 ± 0.24aB
(40.25 %)5.55 ± 0.09bC
(44.25 %)3.69 ± 0.09cD
(66.68 %)3.02 ± 0.10bE
(81.40 %)
200 ELFSL
6.51 ± 0.09aA
(39.50 %)5.84 ± 0.17bB
(44.06 %)5.05 ± 0.13bcC
(51.06 %)4.50 ± 0.06bD
(57.16 %)3.29 ± 0.14bE
(78.27 %)
400 ELFSL
6.42 ± 0.07aA
(37.41 %)5.63 ± 0.07bB
(37.41 %)4.92 ± 0.11cC
(48.65 %)4.10 ± 0.12bD
(58.45 %)3.09 ± 0.24bE
(77.80 %)
600 ELFSL
6.38 ± 0.07aA
(40.44 %)5.63 ± 0.13bB
(45.93 %)5.14 ± 0.10bcC
(50.26 %)4.40 ± 0.91bD
(58.66 %)3.36 ± 0.15bE
(74.84 %)
3.6.3 Effect of ELFSL on acetic acid-induced mice writhing
The effect of ELFSL on writhing induced in mice with acetic acid is shown in Fig. 2. Acetic acid injection into the peritoneal cavity of the control mice resulted in writhing syndrome, with 68.80 ± 1.10 writhes recorded in 30 min. The ELFSL significantly (P < 0.05) inhibited writhing syndrome in mice pre-treated with various doses of the leaf fraction in a dose-dependent fashion, compared with the control, and were comparable with the standard drug. The maximum inhibitory effect (66.54 %) exhibited by the leaf fraction occurred at the highest dose (600 mg/kg bw, po ELFSL). This did not differ significantly (P > 0.05) with the inhibition (62.20 %) demonstrated by 100 mg/kg bw ASP. Intraperitoneal injection of acetic acid in experimental animals increases vascular permeability and the levels of PGE2 and PGF2α in the peritoneal fluid, which results in writhing, indicative of pain (Bentley et al., 1983). Muscular contraction caused by acetic acid is seen as a nonselective nociceptive response because acetic acid interacts with nociceptors, sensitive to a variety of agonists, including narcotics, NSAIDs, and other CNSactive drugs such as morphine, by indirectly inducing the release of endogenous inflammatory mediators such as prostaglandin (Bighetti et al., 1999). The excellent inhibition of writhing, observed in mice treated with various dosages of ELFSL, which were at par with ASP, suggest the participation of a peripheral mechanism of anti-nociception by the fraction. Perhaps it could be that the model is associated with peripheral receptors stimulation, particularly the local peritoneal receptors expressed on the surface of the cells lining the peritoneal cavity (Zakaria et al., 2008). The NSAID, aspirin, also showed a significant (P < 0.05) anti-nociceptive effect in this model, although ELFSL was more potent at a higher dose (600 mg/kg bw, po). Our results correlate with those of Azad et al. (2017), which reported potent anti-nociceptive activities with S. rhombifolia to extract on the acetic acid model.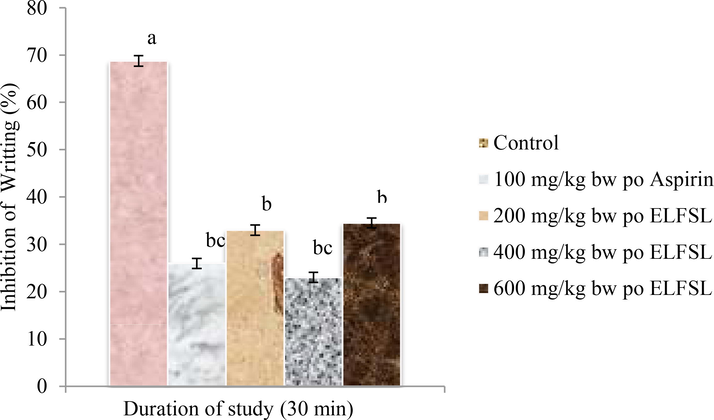
Effect of ELFSL on acetic acid induced writhing in mice, * Control = 10 mg/kg bw distilled water, n = 8; Values (analyzed with One-way ANOVA and Duncan post hoc) are presented as Mean ± SEM at P < 0.05.
3.6.4 Effect of ELFSL on formalin-induced hind mice paw licking
The effect of ELFSL on formalin-induced edema in mice is shown in Fig. 3. In the first phase, formalin injection into the peritoneal cavity of the left hind paw of mice in the control group produced nociceptive reactions of licking and biting of inflicted paws for an extended duration of 312.01 ± 14.01 sec. However, mice pre-administered various doses of ELFSL spent significantly (P < 0.05) lesser time range (196.2 ± 5.0–205.2 ± 6.0 sec) in licking and biting the inflicted paw compared with the control and was comparable with that (181.8 ± 9.0 sec) observed in the group administered 100 mg/kg bw, po ASP. The inhibitory effect of the leaf fraction followed a dose-dependent trend with peak inhibition (50.08 %) elicited by the 600 mg/kg bw po ELFSL dosage, which was not significantly (P > 0.05) different from that (54.20 % inhibition) of the reference drug. In the second phase, the time spent by the untreated animals (control) in biting and licking the affected paw, indicative of nociception, was 306.1 ± 14.06 sec, while mice pretreated with various doses of ELFSL spent significantly (P < 0.05) lesser time range (196.2 ± 5.0–205.2 ± 6.0 sec) in paw licking. This effect was comparable to that (181.8 ± 9.0 sec) observed in the group administered aspirin. The maximum inhibitory effect (78.28 %) demonstrated by the leaf fraction occurred at the 600 mg/kg bw (po) ELFSL dose, and this did not differ significantly (P > 0.05) from that (89.90 % inhibition) obtained with 100 mg/kg bw (po) ASP. The formalin model is proposed to complement the acetic acid writhing model to distinguish between central and peripheral mechanisms of anti-nociception (Chan et al., 1983; Okokon et al., 2012). Intraperitoneal injection of formalin induces an increase in spontaneous activity of C fiber afferents and instigates distinctive and measurable responses, such as animal paw licking, indicative of pains (Akindele et al., 2015). Paw licking induced by formalin injection into mice's paw peritoneal cavity produces two distinctive phases of nociceptive reactions (Hunskaar et al., 1985). The first phase of paw licking (0–5 min) is caused by the direct interaction of formalin with certain nociceptors (Zakaria et al., 2008), while the second phase (15–30 min) is characterized by inflammatory events (Tjolsen et al., 1992). Drugs that act via the central nervous system (CNS), such as morphine, suppress both phases of nociception, while NSAIDs, such as ASP, that act via the peripheral nervous system, inhibit only the later stage (Chan et al., 1983). The formalin test suggests that ELFSL significantly (P < 0.05) inhibited nociceptive responses in both phases in mice, depicting the participation of both peripheral and central mechanisms of anti-nociception. The result of our study correlates with that of Bonjardim et al. (2011), which demonstrated similar results using Sida cordifolia leaf extract.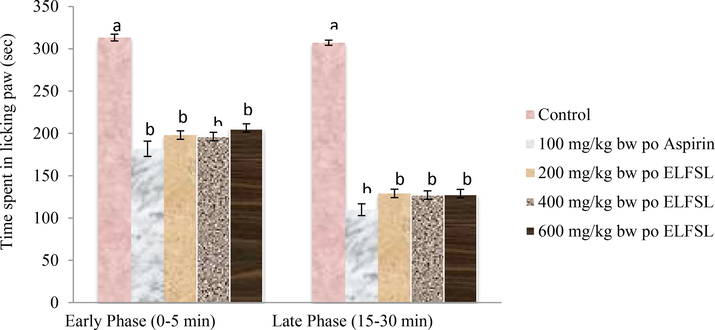
Effect of ELFSL on formalin-induced hind paw licking in mice, *Control = 10 mg/kg bw distilled water, n = 8; Values (analyzed with One-way ANOVA and Duncan post hoc) are presented as Mean ± SEM at P < 0.05.
3.7 In vitro antioxidant activities of ELFSL
Figs. 4–7 show the results of the in vitro antioxidant activities of ELFSL. The various in vitro antioxidant assays revealed that ELFSL possesses excellent antioxidant potential, which increased with concentration and was at par with the standards. The leaf fraction exhibited considerably high dose-dependent antioxidant capacities and ferric-reducing power akin to gallic acid and ascorbic acid, respectively. The fraction also exhibited remarkably high dose-dependent DPPH and nitric oxide scavenging activities akin to BHT and ascorbic acid, respectively. Our results correlate with Mah et al. (2017), which reported excellent antioxidant actions with leaf fractions of S. rhombifolia. Previously, Konaté et al. (2010) also reported effective radical scavenging properties with the whole plant extract of Sida alba and S. acuta. The screening of antioxidant agents of natural origin stems from the cumulative evidence of the involvement of oxidative stress in the etiology of several chronic diseases, including hemolytic and inflammatory diseases (Prieto et al., 1999). The observed excellent antioxidant potential of ELFSL could be due to its rich composition of phenolic compounds, known for their antioxidant potential (Del Rio et al., 2013; Cory et al., 2018). Perhaps some antioxidant phytochemicals in ELFSL may have exhibited synergistic or additive effects, resulting in the excellent antioxidant activities observed. This result agrees with the submission of Yao et al. (2012), which proposed the synergism of antioxidant phytochemicals in plant extracts. Oxidative stress ensues when free radical production exceeds the levels of available antioxidants responsible for checkmating their activities (Boots et al., 2008; Zhang et al., 2019). A disproportionate activity of immune cells results in the excessive release of free radicals (a process called respiratory burst) that is critical in pathogens' destruction and elimination (Reuter et al. 2010). These free radicals can disrupt the normal physiology of the body tissues, distort the primary structure of proteins, attack unsaturated fatty acids, break DNA strands, and aggravate immune response (Zhang et al., 2019). Hence, an agent that can scavenge free radicals and attenuate their destructive activities could inhibit inflammation. Some anti-inflammatory agents act via the inhibition of inducible nitric oxide synthase (iNOS) and suppress its free radical production (Ogbu et al., 2019). These result in the repression of the signaling pathways critical for expressing transcription factors such as TNF-α and NF-κB that mediate inflammation (Lawrence, 2009).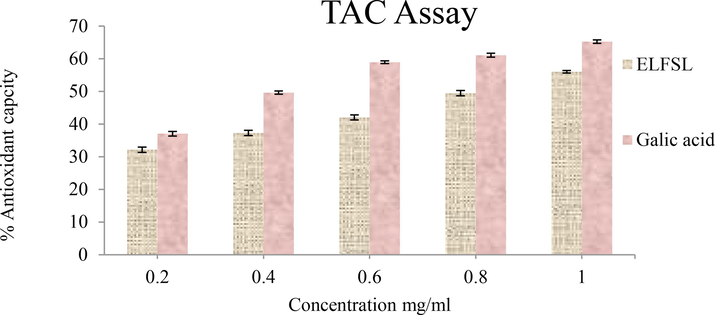
The total antioxidant capacity (TAC) of ELFSL compared with gallic acid (expressed in % gallic acid equivalent/gram).
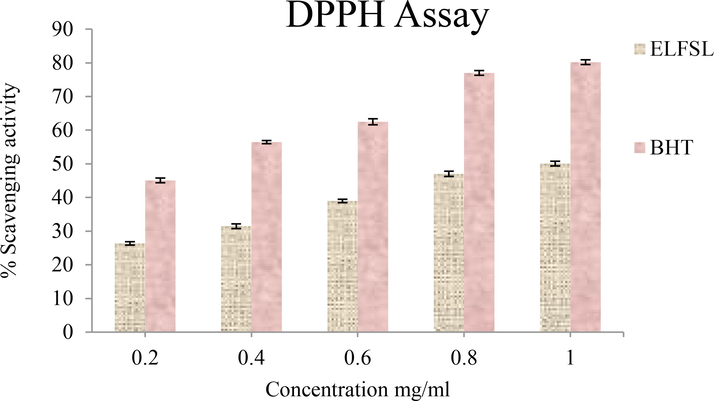
Effect of ELFSL on DPPH radicals compared with butylated hydroxytoluene (BHT) (expressed in % BHT equivalent/gram).
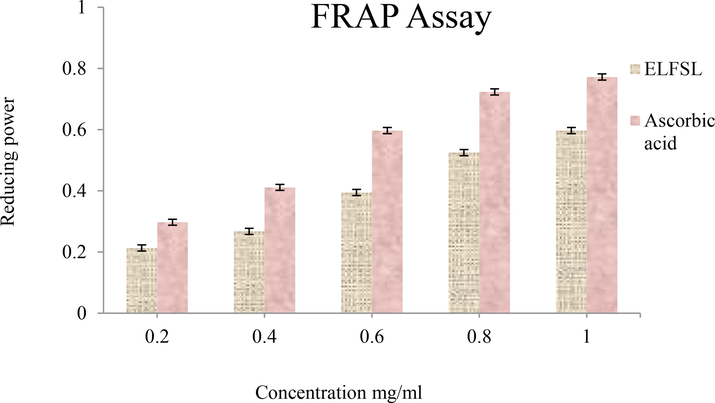
Ferric reducing antioxidant power (FRAP) of ELFSL compared with ascorbic acid (expressed in ascorbic acid equivalent/gram).
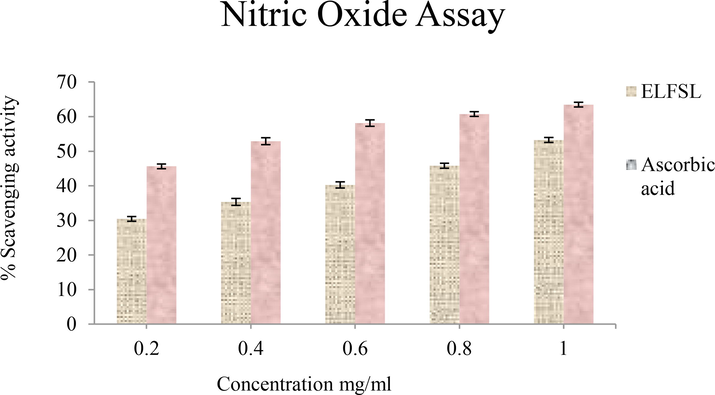
Effect of ELFSL on nitric oxide radicals compared with ascorbic acid (expressed in % ascorbic acid equivalent/gram).
Presented in Table 7 are the IC50 values of the antioxidant activities of ELFSL compared to the standard. The IC50 value ranges (in mg/mL) for ELFSL (0.80–0.96), imply that ELFSL possesses less potent antioxidant potential compared with gallic acid (0.47), ascorbic acid (0.32–0.50), and BHT (0.30). Although potent synthetic antioxidants agents such as 2,2′- methylenebis (6-tert-butyl-4-methylphenol) (AO2246), tert-butyl hydroquinone (TBHQ), butylated hydroxyanisole (BHA), and butylated hydroxytoluene (BHT), are available, they are not void of adverse side effects (Yang et al., 2018). These have necessitated the search for alternative antioxidant agents with mild activities and reduced toxicity. The higher IC50 values of the fraction, thus, imply that the fraction may represent a more suitable antioxidant agent compared to the standards. IC50 values expressed in mg/mL. BHT = butylated hydroxytoluene (BHT).
Test material
TAC
DPPH
FRAP
NO
ELFSL
0.82
0.96
0.80
0.92
Galic acid
0.47
–
–
–
BHT
–
0.30
–
–
Ascorbic acid
–
–
0.50
0.32
4 Conclusion
The results of this study suggest that the ethanolic leaf fraction of Sida linifolia possesses antioxidant, anti-inflammatory, and anti-nociceptive properties, due to its rich phytochemical composition. The anti-inflammatory activities of the leaf fraction were comparable with the standard drug (aspirin). The leaf fraction exerted an analgesic effect, perhaps via the participation of the central and peripheral mechanisms. The results also shed light on the folkloric use of the plant in managing inflammatory diseases. Further advanced studies on the characterization and structural elucidation of the individual bioactive principles responsible for the documented anti-nociception and anti-inflammatory activities of the plant leaf fraction are warranted.
Funding
The project was funded by the authors.
Acknowledgment
The authors gratefully acknowledge the support of staff of the University of Nigeria Nsukka and the Taif University Researchers Supporting Project number (TURSP-2020/38), Taif University, Taif, Saudi Arabia. They wholeheartedly laud the assistance, solidarity and contributions from the researchers in the different institutions consulted during the experimental period.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- The crucial roles of inflammatory mediators in inflammation: a review. Vet. World. 2018;11(5):627-635.
- [Google Scholar]
- Evaluation of the anti-inflammatory activity of the aqueous leaf extract of Byrsocarpus coccineus. Fitoterapia. 2007;78:25-28.
- [Google Scholar]
- Antinociceptive and anti-inflammatory activities of Telfairia occidentalis hydroethanolic leaf extract (Cucurbitaceae) J. Med. Food.. 2015;18:1157-1163.
- [Google Scholar]
- Interactions between phytochemicals and minerals in Terminalia ferdinandiana and implications for mineral bioavailability. Front. Nut.. 2020;7
- [CrossRef] [Google Scholar]
- Preliminary pharmacological study of some Nigerian medicinal plants. 1. J. Ethnopharm.. 1983;8:53-63.
- [Google Scholar]
- Ethyl acetate leaf fraction of Fagara zanthoxyloides root-bark possess antidiabetic property against alloxan-induced diabetes and its complications in Wistar rat model. J. Ethnopharm.. 2022;293:115259
- [Google Scholar]
- Plant extracts with anti-inflammatory properties; a new approach for characterization of their bioactive compounds and establishment of structure-antioxidant activity relationships. Bioorg. Med. Chem.. 2009;17:1876-1883.
- [Google Scholar]
- Plasma membrane integrity: implications for health and disease. BMC Biol.. 2021;19:71.
- [Google Scholar]
- The anti-inflammatory activity of garden egg (Solanum aethiopicum) on egg albumin-induced oedema and granuloma tissue formation in rats Asian Pac. J. Trop. Biomed.. 2012;1:62-66.
- [Google Scholar]
- Flavan-3-ols: nature, occurrence and biological activity. Mol Nutr Food Res.. 2008;52(1):79-104.
- [CrossRef] [Google Scholar]
- Hypoglycemic, analgesic and anti-inflammatory activities of methanol extract of Sida rhombifolia L. leaves on experimental mice. Int. J. Pharm. Sci. Scient. Res.. 2017;3(7):82-87.
- [Google Scholar]
- Anti-inflammatory activity of alkaloids: A twenty-century review. Braz. J. Pharmacogn.. 2006;16:109-139.
- [Google Scholar]
- Studies on the antinociceptive action of agonist drugs and their interaction with opoid mechanisms. Br J Pharm.. 1983;79:125-134.
- [Google Scholar]
- Serine protease inhibitors protect better than IL-10 and TGF-β anti-inflammatory cytokines against mouse colitis when delivered by recombinant lactococci. Microb. Cell. Fact.. 2015;14:26.
- [Google Scholar]
- Anti-inflammatory and anti-nociceptive effects in rodents of the essential oil of Croton cajucara Benth. J. Pharmacol. Methods.. 1999;51:1447-1453.
- [Google Scholar]
- Sida cordifolia leaf extract reduces the orofacial nociceptive response in mice. Phytother. Res.. 2011;25(8):1236-1241.
- [CrossRef] [Google Scholar]
- Health effects of quercetin: From antioxidant to nutraceutical. Eur. J. Pharmacol.. 2008;585:325-337.
- [Google Scholar]
- The analgesic and anticonvulsant effects of piperine in mice. J Physiol Pharmacol.. 2013;64:789.
- [Google Scholar]
- Burkill, H.M., 1997. The useful plants of West Tropical Africa. 2nd Edition. Volume 4, Families M–R. Royal Botanic Gardens, Kew, Richmond, United Kingdom. pp. 969.
- Natural product chemistry for drug discovery,. The Royal Society of Chemistry, Cambridge; 2010. p. :153.
- HRBC membrane stabilizing property of root, stem and leaf of glochidion velutinum. Int. J. Res. Pharmaceut. Biomed. Sci.. 2011;2:256-259.
- [Google Scholar]
- Chan, T.F., Tsai, H.Y., Tian-Shang, W., 1983. Anti-inflammatory and Antinociceptive activities from the roots of Angelica pubescens.
- A review of the diatary flavonoids, kaempferolon human health and cancer chemoprevention. Food Chem.. 2013;138(4):2099-12017.
- [Google Scholar]
- Inflammatory responses and inflammation-associated diseases in organs. Oncotarget.. 2017;9:7204-7218.
- [Google Scholar]
- Targeting enzyme inhibitors in drug discovery. Expert. Opin. Ther. Targ.. 2007;11:967-978.
- [Google Scholar]
- The role of polyphenols in human health and food systems: a mini-review. Front. Nutr.. 2018;21:87.
- [Google Scholar]
- The anti-inflammatory and immunosuppressive effects of glucocorticoids, recent developments and mechanistic insights. Mol. Cell. Endocrinol.. 2011;335:2-13.
- [Google Scholar]
- Antioxidant properties of proanthocyanidins attenuate carbon tetrachloride (CCl4)-induced steatosis and liver injury in rats via CYP2E1 regulation. J. Med. Food. 2014;17(6):663-669.
- [CrossRef] [Google Scholar]
- Assessments of antibacterial effects of aqueous-ethanolic extracts of Sida rhombifolia’s aerial part. Sci. World J.. 2018;2018:1-8.
- [CrossRef] [Google Scholar]
- Dietary (poly)phenolics in human health: structures, bioavailability, and evidence of protective effects against chronic diseases. Antioxid. Redox Signal.. 2013;18:1818-1892.
- [Google Scholar]
- A historical overview of natural products in drug discovery. Metabolites.. 2012;2:303-336.
- [Google Scholar]
- The genus Sida L. – A traditional medicine: Its ethnopharmacological, phytochemical and pharmacological data for commercial exploitation in herbal drugs industry. J. Ethnopharm.. 2015;176:135-176.
- [Google Scholar]
- Anti-inflammatory activity of natural stilbenoids: a review. Pharmacol. Res.. 2017;124:126-145.
- [CrossRef] [Google Scholar]
- Wound repair and regeneration: mechanisms, signaling, and translation. Sci Transl Med.. 2014;6(265)
- [CrossRef] [Google Scholar]
- Evaluation of in vitro anti-inflammatory and antioxidant potentials of methanol leaf extract of Fagara zanthoxyloides Indo Am. J. Pharm. Sci.. 2019;6:14365-14371.
- [Google Scholar]
- Limitations of current therapies to prevent thrombosis : a need for novel strategies. Mol. Biosyst.. 2010;6:305-315.
- [Google Scholar]
- Catechins and their therapeutic benefits to inflammatory bowel disease. Molecules (Basel, Switzerland). 2017;22(3):484.
- [CrossRef] [Google Scholar]
- Preliminary phytochemical analysis: in-vitro comparative evaluation of anti-arthritic and anti-inflammatory potential of some traditionally used medicinal plants. Dose-Response.. 2022;20(1) 15593258211069720
- [CrossRef] [Google Scholar]
- Inflammation, it's regulation and antiphlogistic effect of the cyanogenic glycoside amygdalin. Molecules (Basel, Switzerland). 2021;26(19):5972.
- [CrossRef] [Google Scholar]
- Plant-derived anti-inflammatory compounds: hopes and disappointments regarding the translation of preclinical knowledge into clinical progress. Med. Inflam.. 2014;2014:1-9.
- [Google Scholar]
- Anti-inflammatory action of Lannea coromandelica on HRBC membrane stabilization. Fitotherapia.. 1991;62:82-83.
- [Google Scholar]
- The Pharmacological Potential of Rutin. Saudi Pharm. Soc.. 2017;25(2):149-164.
- [CrossRef] [Google Scholar]
- Anti-inflammatory effects of Morninga oleifera lam extract in rats. Asian Pac J. Trop. Med.. 2010;3:133-135.
- [Google Scholar]
- Evaluation of the anti-inflammatory activity of extract of Vernonia amygdalina. Asian Pac. J. Trop. Med.. 2010;3:150-151.
- [Google Scholar]
- Implications of phytate in plant-based foods for iron and zinc bioavailability, setting dietary requirements, and formulating programs and policies. Nut. Rev.. 2018;76(11):793-804.
- [CrossRef] [Google Scholar]
- Potential role of flavonoids in treating chronic inflammatory diseases with a special focus on the anti-inflammatory activity of apigenin. Antioxidants (Basel, Switzerland). 2019;8(2):35.
- [CrossRef] [Google Scholar]
- Rice antioxidants: phenolic acids, flavonoids, anthocyanins, proanthocyanidins, tocopherols, tocotrienols, γ-oryzanol, and phytic acid. Food Sci. Nutr.. 2014;2:75-104.
- [CrossRef] [Google Scholar]
- Urinary phytate in calcium oxalate stone formers and healthy people: dietary effects on phytate excretion. Scand. J. Urol. Nephrol.. 2000;34:162-164.
- [CrossRef] [Google Scholar]
- Phytochemical methods: a guide to modern techniques of plant analysis (3rd ed.). Germany.: Springer; 1998. p. :302.
- The pharmacological action of Digitalis, Strophanthus, and Squill on the heart. Biochem. J. 1906;1:62-87.
- [Google Scholar]
- Tea catechins and polyphenols: health effects, metabolism, and antioxidant functions. Crit Rev Food Sci Nutr.. 2003;43(1):89-143.
- [CrossRef] [Google Scholar]
- Mechanisms of platelet activation, adhesion, and aggregation. Res. Pract. Thromb. Haemost.. 2017;1:1008-1015.
- [Google Scholar]
- Formalin test in mice: A useful technique for evaluating mild anti-nociceptives. J. Neurosci. Methods.. 1985;14:69-76.
- [Google Scholar]
- Effects of grape seed extract and its ethylacetate/ethanol leaf fraction on blood glucose levels in a model of type 2 diabetes. Phytother. Res.. 2009;23:1182-1185.
- [Google Scholar]
- Flavonoids as inhibitors of human neutrophil elastase. J. Enz. Inhib. Med. Chem.. 2021;36(1):1016-1028.
- [CrossRef] [Google Scholar]
- Anti-ulcer and wound healing activities of Sida corymbosa in rats. Afr. J. Tradi.t Complement. Altern. Med.. 2013;11(1):87-92.
- [Google Scholar]
- Anti-inflammatory activity of arctigenin from Forsythiae fructus. J. Ethnopharmacol.. 2008;116:305-310.
- [Google Scholar]
- Characterization and quantification by gas chromatography of phytochemicals. J. -Braz. Chem. Soc.. 2014;25(2):109-115.
- [Google Scholar]
- The roles of catechins in regulation of systemic inflammation. Food Sci. Biotechnol. 2022
- [CrossRef] [Google Scholar]
- Kokwaro, J.O., 1993. Medicinal plants of East Africa. 2nd ed.tion. Kenya Literature Bureau, Nairobi, Kenya. pp. 401.
- Toxicity assessment and analgesic activity investigation of aqueous acetone extracts of Sida acuta Burn f and Sida cordifolia L. (Malvaceae), medicinal plants of Burkina Faso. BMC Complement Altern. Med.. 2012 Aug;11(12):120.
- [CrossRef] [Google Scholar]
- In vitro antioxidant, lipoxygenase and xanthine oxidase inhibitory activities of fractions from Cienfuegosia digitata Cav., Sida alba L. and Sida acuta Burn f. (Malvaceae) Pak. J. Biol. Sci.. 2010;13:1092-1098.
- [Google Scholar]
- Resveratrol raises in vitro anticancer effects of paclitaxel in NSCLC cell line A549 through COX-2 expression. Korea. J. Physiol. Pharmacol.. 2017;21:465-474.
- [CrossRef] [Google Scholar]
- Neutrophil elastase, proteinas 3 and cathepsin G: physicochemical properties, activity and physiopathological functions. Biochimie. 2008;90:227-242.
- [Google Scholar]
- Chemistry and biological activities of flavonoids: an overview. Sci. World J.. 2013;2013:162750
- [CrossRef] [Google Scholar]
- Membrane stabilization as a mechanism of anti-inflammatory and thrombolytic activities of ethanolic extract of arial parts of Spondias pinnata (Anacardiaceae) Pharmacologyonline. 2015;2:44-51.
- [Google Scholar]
- The nuclear factor NF-kappaB pathway in inflammation. Cold Spring Harbor Perspect. Biol.. 2009;1:1651.
- [Google Scholar]
- Sparteine exerts anticancer effect on human cervical cancer cells via induction of apoptosis, G0/G1 cell cycle arrest and inhibition of VEGFR2 signalling pathway. Trop. J. Pharm. Res.. 2019;18(7):1455-1460.
- [Google Scholar]
- Antioxidant activity of commercial soft and hard wheat (Triticum aestivum L.) as affected by gastric pH conditions. J. Agric. Food Chem.. 2005;53:2433-2440.
- [Google Scholar]
- A new approach to practical acute toxicity testing. Arch. Toxicol.. 1983;35:275-287.
- [Google Scholar]
- Taheebo polyphenols attenuate free fatty acid-induced inflammation in murine and human macrophage cell lines as inhibitor of cyclooxygenase-2. Front. Nutr.. 2017;4:63.
- [Google Scholar]
- Antiinflammatory, anti-cholinergic and cytotoxic effects of Sida rhombifolia. Pharm. Biol.. 2017;55(1):920-928.
- [CrossRef] [Google Scholar]
- Punarnavine induces apoptosis in B16F–10 melanoma cells by inhibiting NF-kappaB signaling. Asian Pac. J. Cancer Prev.. 2009;10(6):1031-1037.
- [CrossRef] [PubMed] [Google Scholar]
- Immunomodulatory activities of Punarnavine, an alkaloid from Boerhaavia diffusa. Immunopharmacol. Immunotoxicol.. 2009;31(3):377-387.
- [CrossRef] [Google Scholar]
- The nitric oxide scavenging activity of Ginko biloba extract. Biochem. Biophys. Res. Commun.. 1994;201:748-755.
- [Google Scholar]
- Recognizing the Risks of Chronic Nonsteroidal Anti-Inflammatory Drug Use in Older Adults. JAMDA.. 2010;18:24-27.
- [Google Scholar]
- Anti-nociceptive, antipyretic and anti-inflammatory properties of Mezoneuron benthamianum Baill (Caesalpiniaceae) Niger Q J Hosp Med.. 2007;17:35-41.
- [Google Scholar]
- Protein denaturation and aggregation: cellular responses to denatured and aggregated proteins. Ann. N. Y. Acad. Sci.. 2005;1066:181-221.
- [Google Scholar]
- Interaction of anti-inflammatory drugs with serum proteins, especially with some biologically active proteins. J. Pharm. Pharmacol.. 1968;20:169-173.
- [Google Scholar]
- Flavonoids, dairy foods, and cardiovascular and metabolic health. Circ. Res.. 2018;122:369-384.
- [Google Scholar]
- Neuwinger, H.D., 2000. African traditional medicine: a dictionary of plant use and applications. Medpharm Scientific, Stuttgart, Germany. pp. 589.
- Anti-inflammatory activities of crude ethanol extract of Combretum zenkeri Engl. and Diels leaves. Comp. Clin. Path.. 2019;2019:1-13.
- [Google Scholar]
- Antiinflammatory, analgesic and antipyretic activities of ethanolic root extract of Croton zambesicus. Pak. J. Pharm. Sci.. 2010;23:383-390.
- [Google Scholar]
- Anti-inflammatory and analgesic activities of Melanthera scandens. Asian Pac. J. Trop. Biomed.. 2012;2:144-148.
- [Google Scholar]
- Acanthus montanus: An experimental evaluation of the antimicrobial, anti-inflammatory and immunological properties of a traditional remedy for furuncles. BMC Complement Altern. Med.. 2008;8:27.
- [Google Scholar]
- An overview of cancer chemopreventive potential and safety of proanthocyanidins. Nutr. Cancer. 2011;63:1163-1173.
- [Google Scholar]
- Evaluations of in vitro anti-inflammatory and antibacterial potential of Crescentia cujete leaves and stem bark. BMC Res. Notes.. 2015;4:412.
- [Google Scholar]
- Plant-derived cardiac glycosides: Role in heart ailments and cancer management. Biomed. Pharmacother.. 2016;84:1036-1041.
- [CrossRef] [Google Scholar]
- Systematic review of plant steroids as potential antiinflammatory agents: Current status and future perspectives. J. Phytopharmacol.. 2015;4:121-125.
- [Google Scholar]
- Neutrophil serine proteases: specific regulators of inflammation. Nat. Rev. Immunol.. 2006;6:541-550.
- [Google Scholar]
- A comparative study of the effects of sparteine, lupanine and lupin extract on the central nervous system of the mouse. J Pharm Pharmacol. J Pharm Pharmacol. Aug;(:. 1998;50(8):949-954..
- [CrossRef] [Google Scholar]
- Spectrophotometric quantitation of antioxidant capacity through the formation of a phosphomolybdenum complex: specific application to the determination of vitamin E. Anal. Biochem.. 1999;269:337-341.
- [Google Scholar]
- Proanthocyanidins: a comprehensive review. Biomed. Pharmacother.. 2019;116:108999
- [CrossRef] [Google Scholar]
- Oxidative stress, inflammation, and cancer: how are they linked? J. Free Radic Biol. Med.. 2010;49:23-28.
- [Google Scholar]
- Prostaglandins and inflammation. Arterioscler. Thromb. Vasc. Biol.. 2011;31:986-1000.
- [Google Scholar]
- Sida linifolia (Malvaceae), a new record for Thailand. J. Jpn. Bot.. 2016;91(5):295-297.
- [Google Scholar]
- Comprehensive assessment of phenolics and antiradical potential of Rumexhastatus D. Don. roots. BMC Complement. Altern. Med.. 2014;14:47.
- [Google Scholar]
- In vitro antioxidant and anti-inflammatory activity of methanol extract of Oxalis corniculataLinn. Int. J. Pharm. Sci. Res.. 2010;2:146-155.
- [Google Scholar]
- Resveratrol: a double-edged sword in health benefits. Biomed.. 2018;6(3):91.
- [CrossRef] [Google Scholar]
- The therapeutic potential of naringenin: a review of clinical trials. Pharmaceuticals (Basel, Switzerland). 2019;12(1):11.
- [CrossRef] [Google Scholar]
- Antidiabetic and anti-inflammatory potentials of Sida acuta leaf ethanolic extract. J. Phamaceut. Res. Int.. 2021;33(64):96-103.
- [Google Scholar]
- The role and place of medicinal plants in the strategies for disease prevention. Afr. J. Traditional, Complement. Alternative Med.: AJTCAM.. 2013;10:210-229.
- [Google Scholar]
- Plant protease inhibitors in therapeutics-focus on cancer therapy. Front. Pharm.. 2016;7:1-19.
- [Google Scholar]
- Analgesic and anti-inflammatory principle from Sida cordifolia Linn. J. Bio. Sci.. 2006;6(1):160-163.
- [CrossRef] [Google Scholar]
- Resveratrol attenuates neuropathic pain through balancing pro-inflammatory and anti-inflammatory cytokines release in mice. Int. Immunopharmacol.. 2016;34:165-172.
- [CrossRef] [Google Scholar]
- Trease, G.E., Evans, W.C., 1985. Pharmacognsy, Eleventh edition. In: Brailliar Tiridel Can. Macmillian Publishers, London. pp. 135–203.
- Potential role of natural compounds against skin aging. Curr. Med. Chem.. 2015;22:1515-1538.
- [Google Scholar]
- Flavonoids and other phenolic compounds from medicinal plants for pharmaceutical and medical aspects: an overview. Medicines (Basel, Switzerland).. 2018;5:93.
- [Google Scholar]
- An experimental evaluation of Albucasetosa aqueous extract on membrane stabilization, protein denaturation and white blood cell migration during acute inflammation. J. Med. Plant Res.. 2010;4:789-795.
- [Google Scholar]
- New insights into the anti-inflammatory mechanisms of glucocorticoids: an emerging role for glucocorticoid-receptor-mediated transactivation. Endocrin.. 2013;154:993-1007.
- [Google Scholar]
- Inhibition of prostaglandin synthesis as a mechanism of action for aspirin like drugs. Nat. New Biol.. 1971;231:232-235.
- [Google Scholar]
- Sparteine as an anticonvulsant drug: Evidence and possible mechanism of action. Seizure.. 2016;39:49-55.
- [CrossRef] [Google Scholar]
- In vitro and in vivo anti-inflammatory activity of Tetrastigmasulcatum leaf extract, pure compound and its derivatives. Inflammopharm.. 2022;30:291-311.
- [Google Scholar]
- Search for new plant constituents with potential antiphlogistic and antiallergic activity. Planta Med.. 1989;55:235-241.
- [Google Scholar]
- Nutritional and botanical modulation of the inflammatory cascade–eicosanoids, cyclooxygenases, and lipoxygenases–as an adjunct in cancer therapy. Integr. Cancer Ther.. 2002;1:7-37.
- [Google Scholar]
- Anti-inflammatory effects of (-)-epicatechin in lipopolysaccharide-stimulated raw 264.7 macrophages. Trop. J. Pharm. Res.. 2014;13(9):1415-1419.
- [Google Scholar]
- Analgesic and anti-inflammatory activities of resveratrol through classic models in mice and rats. Evid. Based Complement. Alternat. Med.. 2017;2017:9.
- [CrossRef] [Google Scholar]
- Metabolism pathways of arachidonic acids: mechanisms and potential therapeutic targets. Sig Transduct Target Ther. 2021;6:94.
- [CrossRef] [Google Scholar]
- Zur Wirkung von Gerbstoffen in der Tierernährung [Effects of tannins in animal nutrition] Dtsch Tierarztl Wochenschr.. 2006;113(7):264-268.
- [Google Scholar]
- A structural mechanism of flavonoids in inhibiting serine proteases. Food Funct.. 2017;8:2437-2443.
- [Google Scholar]
- Characterization and Evaluation of Antioxidant and Anti-Inflammatory Activities of Flavonoids from the Fruits of Lyciumbarbarum. Foods.. 2022;11:306.
- [Google Scholar]
- Developmental toxicity of synthetic phenolic antioxidants to the early life stage of zebrafish. Sci. Tot. Environ.. 2018;643:559-568.
- [CrossRef] [Google Scholar]
- The in vitro and in vivo anti-inflammatory activities of triterpene saponins from Clematis Florida. Nat. Prod. Res. 2020:1-4.
- [Google Scholar]
- Synergism of antioxidant phytochemicals: comparisons among purified polyphenols and dietary-plant extracts. Acta Hortic.. 2012;939:121-127.
- [Google Scholar]
- Evaluation of anti-inflammatory and membrane stabilizing properties of various extracts of Punicagranatum L. (Lythraceae) Int. J. Pharmtech. Res.. 2010;2:1260-1263.
- [Google Scholar]
- Anti-nociceptive, anti-inflammatory, and antipyretic properties of an aqueous extract of Dicrariopteris linearis leaves in experimental animal models. J. Nat. Med.. 2008;62:179-187.
- [Google Scholar]
- Complete reversal of acid-induced acute lung injury by blocking of platelet-neutrophil aggregation. J. Clin. Investig.. 2006;116:3211-3219.
- [Google Scholar]
- Synergistic anti-inflammatory effects and mechanisms of combined phytochemicals. J. Nut. Biochem.. 2019;3:42.
- [Google Scholar]
- Antiinflammatory activity of lipophilic epigallocatechin gallate (egcg) derivatives in lps-stimulated murine macrophages. Food Chem.. 2012;134(2):742-748.
- [Google Scholar]
- Advance in anticancer studies on catechins and their derivatives. Zhongguo Zhong Yao Za Zhi. 2012;37(17):2510-2518.
- [Google Scholar]
- Anti-inflammatory activity of resveratrol prevents inflammation by inhibiting NF-kB in animal models of acute pharyngitis. Mol. Med. Rep.. 2018;17:1269-1274.
- [Google Scholar]
- The effect of different solvents and number of extraction steps on the polyphenol content and antioxidant capacity of basil leaves (Ocimum basilicum L.) extracts. Saudi J. Bio. Sci.. 2016;23(5):628-633.
- [CrossRef] [Google Scholar]







