Translate this page into:
Bioactive extracts of plant byproducts as sustainable solution of water contaminants reduction
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
Contamination by agro-waste materials after harvesting is a significant concern due to its negative environmental impacts. Similarly, it is rich in bioactive components that aid contaminant removal. The study aimed to formulate a membrane-loaded bioactive extract to reduce pesticide (PS) and aflatoxin (AFs) from simulated media. Bioactivity of Fenugreek (FE) and black cumin (CE) byproducts extracts were evaluated for antioxidants (DPPH and ABTS radicals), volatile content using the GC–MS, and phenolic compounds using the HPLC apparatus. A carboxy-methylcellulose/chitosan membrane, loaded with FE/CE (1:1 v/v), was utilized for PS and AFs removal from simulated media. The membrane was characterized by particle size, zeta-potential, viscosity, and stability—composite membrane ability for PS and AFs removal was evaluated. The results revealed a significant content of thymoquinone (30.05 %) and p-cymene (24.56 %) in black-cumin extract, while fenugreek significantly had β-pinene (18.14 %), neryl acetate (16.89 %), β-caryophyllene (15.81 %), and camphor (13.69 %). p-hydroxybenzoic and caffeic were valuable content in FE and CE, while gentisic (846.12 ± 1.41 µg/g) and methyl gallate (676.82 ± 1.88 µg/g) were dominant just in fenugreek. Active groups of the composite using FTIR showed that carbonyl, sulfhydryl, hydroxyl, and ester groups possess reaction functionality. The extracts were applied to form a composite with remarkable stability, as evidenced by its zeta potential (−28.8 ± 3.48) and particle size (51.78 ± 3.157 nm). The efficacy of composite emulsion in removing pesticides from simulated media was measured at 55.31 ± 1.62 % for chlorpyrifos and 61.54 ± 1.51 % for profenofos. The membrane reduced aflatoxin by more than 80 % compared to the crude extract, which reduced the content by more than 50 % of the total concentration. These results recommend the use of bioactive composites for contaminant removal. Further studies will be required for the mechanism illustrations.
Keywords
Bioactive extract
Pesticide and aflatoxin contaminants
Food wastewater
Membrane formation
Contaminant reduction
Extracts mix
Volatile components
Phenolic content
Fenugreek
DPPH
ABTS
HPLC
GC–MS
1 Introduction
Agro-food wastes pose environmental and health risks (Tonini et al., 2018; Socas-Rodríguez et al., 2021). Nonetheless, it may include valuable bioactive components with several advantages (Pérez-Marroquín et al., 2023). Several studies have shown that Saudi Arabia has significant agro-food-waste quantities (Baig et al., 2019; 2022). Fenugreek and black cumin are significant wastes because they contain phytochemicals and antioxidants (Gupta et al., 2017; Jain et al., 2020). These active compounds may be functionalized in extracts for food use and pollutant elimination. The body's fluid system benefits from fenugreek extract (FE)'s high total phenolic and flavonoid content (El-Wakf, Hassan et al., 2015). Black cumin extract (CE) has antibacterial and antifungal properties due to its bioactivity (Mnif and Aifa, 2015). Large amounts may have been produced postharvest without future use (Hodges et al., 2011).
Several mechanisms were applied to valorize the economical utilization of food waste materials. However, the accumulation of agricultural waste is more than 2 billion tons worldwide (Singh et al., 2021). Thermochemical or biochemical pathways and bioactive extraction can convert agro-industrial waste into valuable products. This paves the way for a circular economy model in agriculture, where waste becomes a transient phase and reintegrates into the economy. There are numerous ways to utilize agro-industrial wastes to produce value-added products, such as biofuels, microbial enzymes, single-cell protein, soil amendments, and value-added bioactive extracts. Using these novel products reduces investment and production costs.
However, agro-food waste extraction may be an intelligent answer for non-traditional contamination remedies like mycotoxin degradation. Toxigenic fungus inhibition and mycotoxin degradation depend on phenolics (Sabry et al., 2022). Composite nano-membranes loaded with active extract can preserve their activity and improve removal efficiency (Badr et al., 2020; Alharthi et al., 2021; Badr et al., 2022).
Composite membranes integrate two or more materials' characteristics (Padaki et al.,2015; Boudechicha et al., 2023). Industry uses these membranes for water treatment, gas separation, and food processing (Hussain et al., 2022). Composite membranes are sequentially made, where choosing the correct elements for a hybrid membrane is significant. One component forms the base or support layer, which gives the membrane its structure, while the others make the active layer selective or beneficial (Huang et al., 2000). Membrane component selection relies on the split method and required properties (Ulbricht, 2006).
The membrane's active component may need cross-linking to improve stability and function. Some polymers are suggested for their food and water treatment effectiveness and distinctive properties. Bioactive polysaccharides like carboxymethyl cellulose (CMC), chitosan, and gums degrade contaminants. Due to their biodegradability, lack of taste and odor, and widespread use, cellulose derivatives generate edible biofilms. Carboxymethyl cellulose membranes develop better due to their hydrophilicity (Kanikireddy et al., 2020). When taken in large concentrations, contaminants may harm health. Food, water, and agricultural goods may include pesticides and mycotoxins (Katole et al., 2013). Pesticides protect crops from insects, weeds, and fungi. They provide food security and avoid agricultural losses, but their residues may pose health risks (Hussain et al., 2022). Pesticides such as organophosphates, organochlorines, herbicides, and fungicides are often used to safeguard food and agricultural goods (Mahmood et al., 2016; Özkara et al., 2016). High pesticide exposure may cause neurological and developmental problems. Another persistent pesticide that builds in fatty foods is organochlorines.
Toxigenic fungi on crops may cause mycotoxins; thus, fungicides are employed to suppress them. Toxigenic fungi create mycotoxins, which are difficult to regulate. Mycotoxins such as aflatoxins, ochratoxins, fumonisins, and zearalenone have health risks for people and animals (Mirza Alizadeh et al., 2022). Lately, there has been a growing interest in adsorptive membranes, which have been used to eliminate diverse contaminants from wastewater (Qalyoubi et al., 2021). Adsorbent membranes effectively eliminate pollutants like pharmaceuticals, dyes, and heavy metals, but they could have disadvantages, like the limited adsorption capacity. Forming polymer membranes loaded with biologically active extracts could be a promising solution for eliminating residual pollutants, particularly in liquid systems (Aguilar-Pérez et al., 2020). The supporting step of the composite using bioactive extracts in the present investigation can ameliorate the membrane absorbency or increase pollutant degradation. The high content of bioactive applied extracts in the membrane will support the reduction of pollutant concentrations in liquid media (Tan et al., 2020).
In this regard, the present investigation aimed to utilize bioactive composite membrane loading by the FE/CE mix to remove spiked pesticides and aflatoxins from simulated media. This experiment will prove the efficient membrane utilization for getting rid of contaminants in solutions. Future studies will be required to illustrate the mechanism of action and ensure the removal mechanism.
2 Material and methods
2.1 Materials, reagents, and chemicals
Fresh fenugreek and black cumin byproducts were purchased from Alhajri Trading, Al-Anoud, Dammam, Saudi Arabia. Byproducts of fenugreek and black cumin are the waste collected after fruits or seeds are harvested and prepared as a primary product. These byproducts include the stem, leaves, and other plant materials, considered plant straws. The chemicals used in the present investigation, including 2, 2-Diphenyl-1-picrylhydrazyl (DPPH) radical, butylated hydroxytoluene (BHT), Gallic acid, Folin-Ciocalteu reagent, chitosan (low molecular weight, 50,000–190,000 Da), carboxymethyl cellulose (molecular weight 90,000), aflatoxins, chlorpyrifos, and profenofos were acquired from Sigma Chemical Co. (St. Louis, Minnesota, USA. Reagents and chemicals utilized in the investigation were ordered from Sigma Aldrich and were of analytical grade.
2.2 Extraction of byproduct bioactive content
A quantity of 100 g/each (CE or FE byproducts) was extracted by aqueous methanol (80 %) using an ultrasonic assessed method as described before with modifications (Afroz Bakht et al., 2019). In brief, plant byproduct samples were treated using ultrasonic bath extraction (40 kHz; 45 °C; 45 min; 230 V), and each sample was divided into two beakers (1L beaker held 50 g/500 mL methanol 80 %). The four beakers of extraction products after treatment (Ultrasonic bath DK-1500H, ul. Ustronna 41, 93–350 Łódź, Poland) were filtered using Whatman filter paper (No.1). The collected solutions were kept in amber vials (4 °C) till the lyophilization step in a tray diameter 180 mm, at (−80 °C/40 h/ 0,08 m2 freeze-drying area) using Chemica Freeze Dryer SH/0GJ/011, Viadella Quercia, 84,080 Pellezzano SA, Italy).
2.3 Preparation of the coarse emulsion for loading
A coarse emulsion consisting of fenugreek extract, black cumin extract, emulsifier, and soybean oil was prepared according to the methodology described before (Golden, Quinn, Shaaya, Kostyukovsky, and Poverenov, 2018). This oil was recommended as it has better efficiency in aiding pesticide removal (Cha et al., 2016). Briefly, 2 mL oil was mixed with 2 mL tween 80 using a mechanical overhead stirrer (SE-100, JP SELECTA, Highway A-2, Barcelona, Spain), 300 ppm of each extract dissolved in 100 µL ethanol (300 FE + 300 CE in 200 µL ethanol) and added dropwise to the oil mix. The coarse was stirred for 120 min until the solution turned stable. The final coarse oil loaded by the extract was added later to the membrane emulsion during the formation step.
2.4 Preparation of bioactive composites
With modifications, the composite was fabricated using a process outlined by Zhao et al (Zhao et al., 2020). The primary solution of the carboxy methyl cellulose (CMC; 3 g) was prepared by dissolving the powder with ethyl alcohol (10 mL) in a clean beaker. Afterward, 100 mL of distilled water was gradually added to the beaker while stirring. The CMC powder was wholly dissolved using a mechanical overhead stirrer at 40℃; then, the bioactive solution was inserted for loading. The coarse solution prepared in the previous step intended for loading was added (2 mL oil containing 600 ppm FE/CE) to the prepared CMC solution.
Concurrently, a chitosan solution was prepared to be a part of the formulated composite using the methodology described by Wang et al. (Wang et al., 2021). Chitosan (ChS; 3 g) was weighed and dissolved in 100 mL of distilled water containing (1 % lactic acid). The acid was added in this step to serve as a cross-linking agent (Mannai et al., 2023). The resultant was then agitated (2 h/25℃) and added sodium tri-poly-phosphate solution (0.4 %; w/v). The suspension was overnight stirred (600 rpm /min; 20 ± 3℃). After the solutions of CMC and ChS were ready for mixing (2:1; v/v), followed by stirring using the overhead stirrer (4 h; 20 + 3; 600 rpm) until the complete preparation step (Zhu et al., 2021; Li et al., 2023). A saturated solution (3 mL NaHCO3) was added to the solution for the pH raising. According to the preliminary experiment, a mix of glycerol-sorbitol (2:1; v/v) is a suitable co-surfactant and co-emulsifier for ameliorating the composite formation, and the appropriate ratio for the addition was 3 % (v/v of the total mix). After the final additive, the prepared composite solution was stirred for three hours (800 rpm) until the complete homogenization (IKA mechanical stirrer, RW 20 digital with R 1382 Propeller stirrer, IKA −Werke GmbH & Co., Staufen, Germany).
The solution portions of loaded CMC-CS were homogenized using the IKA Ultra-Turrax T25 (21000 rpm/10 min/4C). Subsequently, ultrasonic probe homogenizers were used until perfect homogeneity was achieved (700 W/20KHz, 50 % pulse, Fisherbrand™ 705 Sonicator with Probe, Milan, Italy). The film solution was introduced into the film-forming medium and subjected to dryness in a hot-air oven (40℃/16 h). The resulting film was then maintained in a desiccated state for up to 48 hrs until further analyses.
2.5 Evaluation of the extract contents of phenolic compounds
Estimating the extracted materials' phenolic constituents (phenolic acid and Flavonoids) was conducted using methods established according to the previously described methodologies (Stuper-Szablewska et al., 2017; Mizzi et al., 2020). The phenolic content was determined using Use HPLC model 1100 (Agilent Technologies, CA, USA) with a quaternary pump, auto-sampler injector, and diode array detector. Samples were inserted into a 35 °C Agilent Eclipse XDB C18 column (150 × 4.6 μm; 5 μm). Gradient elution used acetonitrile (solvent A) and 2 % acetic acid in water (solvent B) at 0.8 mL/min. Elution began at 0 % solvent A, grew to 15 % in 30 min, 50 % in 20 min, and 100 % for 5 min. Reconditioning took 10 min after the run. Target compounds were identified and quantified by correlating retention durations and peak areas to standards. The limit of quantification was 10 ng/g. The data were obtained via three independent measurements and are shown as the mean value ± standard deviation.
2.6 Determination of volatile contents
The volatile contents of subjected byproducts were analyzed using a gas chromatography system (the GC–MS System, Agilent 8890-GC attached to Agilent 5977B GC/MSD). The system had an HP-5MS fused silica capillary column (30 m × 0.25 mm × 0.25 µm). The oven-programmed temperature was from 50 to 220 °C (5 °C /min), then subsequently from 220 °C to 280 °C (10 °C/ min). Helium was carrier gas (1 mL/min). The isolated peaks were identified by matching with data from the mass spectra database maintained by the National Institute of Standards and Technology. The SPME extraction and desorption were carried out automatically with a Multipurpose Sampler (MPS-2, Gerstel). In headspace-solid phase micro extraction (HS-SPME), lyophilized extract (1 g) was placed into a 20 mL headspace-sealed vial; volatiles were sampled using the HS-SPME (60 min/30 °C). Volatiles were desorbed (250C/2 min) in the GC–MS inlet.
2.7 Determination of antioxidant activity
The investigation of antioxidant activity for the two kinds of extracts was conducted using two assays, DPPH and ABTS, following the methodology outlined by Chaves et al. (Chaves, Santiago, and Alías, 2020). The scavenging values were determined using a UV-spectrophotometer (UV-3600i Plus, Shimadzu, Kyoto, Japan) for the DPPH● and ABTS+ at wavelengths 517 nm and 734 nm, respectively. For the DPPH assay briefly, each extract (0.2 mL) extract received 1 mL of a 0.5 mmol/L methanol solution (of DPPH) and 2 mL of methanol. After mixing the reaction mixture, it was left in the dark for 30 min. The absorbance of the solution was calculated using a blank made of methanol. The ABTS measuring activity was determined by dissolving the chemical in water at a concentration of 7 mmol/L. The ABTS radical cation was prepared by combining ABTS stock solution with 2.45 mmol/L potassium persulfate and keeping the mixture at 22 °C for 16 h before use. The ABTS radical cation solution was diluted with ethanol to achieve an absorbance of 0.70 at 734 nm and equilibrated. Antioxidant activity was assessed to evaluate the extract's potency to interact with tested contaminants. Antioxidant activity was quantified IC50 values and expressed as a percentage (%).
2.8 Determination of the FTIR characteristics
The active group of the extracts and their mix loaded to the membrane were identified using Fourier transform infrared spectroscopy (FTIR) and absorption spectroscopy in the infrared range, as Vicentini, Smania, and Laranjeira (2010) described. The Fourier Transform Infrared (FTIR) spectra were acquired via a Bruker IFS48 spectrometer. The absorbance spectra were estimated within a resolution range of 500 cm−1 to 4000 cm−1 using the triangle apodization technique implemented in the Bruker software. The FTIR spectrometer underwent a purging process to mitigate spectral interferences arising from background carbon dioxide and water vapor. Subsequently, the average of four spectra obtained from distinct pellets of the same sample was calculated.
2.9 Determination of membrane and its emulsion characteristics
The emulsion viscosity was measured during the storage. Viscosity values were obtained by calculating the mean reading (4 times, 3 reads each) at 1, 7, 14, and 21 days of storage time. The viscosity was assessed using a viscometer manufactured by Brookfield Engineering Laboratories (DV-E Model, Middleboro, MA, USA), fitted with a spindle-type configuration (CPA-40Z) measurement system. The samples were transferred to the apparatus and allowed to reach thermal equilibrium at (20 ± 1 °C) for 5 min before measurement. The measurements were recorded as mean ± SD and expressed in centipoise (cP).
To evaluate the emulsion size, zeta-potential, and polydispersity, the emulsion (0.5 mL) was placed into a cuvette and diluted in water (0.5 mL) to measure the particle size (using Zetasizer Nano-ZS equipment). Also, the zeta potential values were calculated using (50 µL) placed in a capillary cell and diluted in water (1 mL). The particle size was measured as Z-average, applying the Stokes-Einstein relation with its corresponding polydispersity index (PDI). Each sample's particle size and zeta potential were carried out three times. Centrifugation and separation tests were done according to the methodology described before (Hosseini et al., 2015). In brief, the centrifugation test was done by transferring 5 mL of the emulsion into a falcon tube and processing centrifugation at 2278 Xg/ 15 min at 22 ± 2 ℃. The following equation was utilized to calculate the value:
Cfs is the percentage of separation after centrifugation, Vf is the final volume, and Vi is the initial volume.
The separation test was determined at 1, 7, 14, and 21 days for the emulsion, and the results were calculated from the equation (2):
The SPT is the emulsion separation ratio, Lu is the higher separated layer (mL), and Lt is the total emulsion volume (mL).
The titrable acidity of the membrane emulsions was calculated by titrating 10 mL with sodium hydroxide solution (NaOH, 0.1 N) until it reached an 8.1 pH value. The titrable acidity was expressed as a citric acid percentage. The pH value was measured using a pH meter (GenWay, pH 2001-Genway equipment, UK). The formed membrane underwent sputter coating with gold ions using the Edwards' model S 140A to create a conducting medium for inspection under a scanning electron microscope (SEM). The membrane was subjected to scanning electron microscopy (JEOL Model JSM-T20) to analyze the surface properties.
2.10 Application of membrane-loaded extracts to reduce contaminants
2.10.1 Simulation of pesticide reduction
As an application, a beaker contained spiked water injected with one type of pesticide residue (chlorpyrifos or profenofos) at 100 ppm each. A simulated experiment was conducted to evaluate the potency of bioactive membrane sheets in reducing pesticide content. In this experimental study, cleaned beakers were filled with deionized water and injected with a singular pesticide. The beakers were categorized into seven sets:
S1: replicates (in beakers) of pure deionized water as control;
S2: replicates of 100 ppm chlorpyrifos;
S3: replicates of 100 ppm profenofos;
S4: replicates of 100 ppm chlorpyrifos contained FE-CE membrane sheet;
S5: replicates of 100 ppm profenofos contained FE-CE membrane sheet;
S6: replicates of 100 ppm chlorpyrifos with membrane-free of FE-CE;
S7: replicates of 100 ppm profenofos with membrane-free of FE-CE.
The reduction of pesticide residues (% PR)can be calculated from equation (3):
IPc and IFc are the primary and final pesticide concentrations, respectively.
The samples conducted agitation in a shaking incubator (ZWYR-211D, Shanghai Analytical Manufacturing Co., China) at a speed of 200 rpm round per minute for 20 min at a temperature of 27 °C. The suspensions underwent centrifugation with a force of 2000 times the acceleration due to gravity (M1416R, High-Speed Centrifuge, RWD, Shenzhen, Guangdong, China) to separate the membrane. The samples underwent filtration, and the resulting filtrate solutions were analyzed for pesticide residues using gas chromatography-tandem mass spectrometry (GC–MS-MS).
The system of Gas Chromatography 7890B, coupled with the tandem mass spectrometer 7010A Quadrupole (Agilent Technologies, USA), was utilized, and chromatographic separations were successfully conducted using the HP5MS ultra inert capillary column (30 mm × 0.25 × 0.25 µm). The data acquisition was performed using Mass Hunter software version 7.01(Agilent Technologies USA), with spectrum library (NIST 14). The temperature of the GC oven is maintained at 70 °C /1 min and increased to 150 °C (50 °C/min), then raised to 260 °C (6 °C/min), and finally was ramped from 260 to 310 °C (20 °C/min/for 1.6 min).
The carrier gas was ultra-high quality helium (>99.999 %) at a 1.6 mL/min flow rate. The flow rates of helium and nitrogen gases were recorded at 2.25 mL/min and 1.5 mL/min, respectively. The injector, transfer line, ion source, and quadrupole temperatures were recorded as 260, 280, 300, and 180 °C, respectively. During a solvent delay of 2 min, the filament current of 100 μA was deactivated. The acquisition approach used MRM mode, where one MRM transition was employed for quantification purposes, while the other transitions were used for confirmation purposes. The experimental procedure included chlorpyrifos, Profenofos, and Parathion standards.
2.10.2 Simulation of mycotoxin reduction
The impact of the membrane sheet on removing aflatoxin residue from the horticulture and vegetable wastewater was assessed utilizing a simulated model designed by spiked samples. Mixed spiked toxins were injected into deionized water at 1200 ng/mL (300 ng/mL of each). The toxin solution was prepared by mixing relative amounts of aflatoxins (Aflatoxin B1, aflatoxin B2, aflatoxin G1, and aflatoxin G2) at 1:1:1:1 before it was injected into the spiked sample. Four groups were prepared as follows: G1 was the control positive and contained 1200 ng/mL of toxin mix; G2 was the spiked sample (1200 ng/mL) with a membrane sheet without bioactive extracts; and G3 was treated with a membrane sheet with bioactive CE and FE; G4 was treated with FE and CE mix without membrane, at the same concentration.
Following specified HPLC parameters, the AFs determination was performed using HPLC Series 1260 (Agilent, Santa Clara, CA, USA). The analytical column was Zorbax Eclipse XDB-C18 (4.6 mm × 150 mm × 3.5 µm). Aflatoxins were detected using a mobile phase of water, methanol, and acetonitrile (50:40:10; v/v/v) at 1 mL/min flow rate and temperature (20 ± 1 °C) using a UV detector (at 256 nm). The injection volume for each sample was 10 µL, with a concentration of 0.05 mg/mL.
2.11 Statistical analysis
The investigations were carried out in triplicates, where the results were expressed as the mean ± standard deviation. The data was analyzed using the software of the Statistical Program of Social Sciences (SPSS V.16). The analysis of variance (ANOVA- one way) test was utilized to assess the significant existence disparity between the mean values at p ≤ 0.05, and a post hoc analysis was done.
3 Results and discussion
3.1 Phenolic and flavonoid contents
Research and innovation are necessary to optimize the use of agricultural waste in food production. The bioactive components of these substances may be discovered by efficient extraction, processing, and application techniques. Advocating for circular economy solutions incorporating trash into diverse value chains may also result in sustainable risk reduction and environmental stewardship.The plant waste extract included various phenolic acids and flavonoids (Table 1). About 14 major phenolic acids are found in fenugreek byproduct extract. Gentisic (846.12 ± 1.41 µg/g) is the most potent phenolic acid in the FE extract, followed by methyl gallate (603.91 ± 2.08) and p-hydroxybenzoic acid (377.81 ± 2.02 µg/g). Coumaric has the lowest phenolic acid level in FE (51.84 ± 0.64 µg/g). The CE byproduct extract lacks chlorogenic, gentisic, methyl gallate, and ellagic phenolic acids. Both FE and CE byproduct extracts include significant levels of p-hydroxybenzoic (377.81 ± 2.02 µg/g) and caffeic acid (116.27 ± 2.56 µg/g). Although the CE extract contains 10 phenolic acids, cinnamic has a low concentration (0.21 ± 0.01 µg/g). Ferulic acid concentration (248.12 ± 1.67 and 38.87 ± 0.64 µg/g for FE and CE extracts, respectively) is high, indicating bioactivity against pollutants and microbiological infections (Anjali et al., 2022). * Data are represented in mean ± SD (n=5, P ≤ 0.05), SD: Standard deviation, and ND: represent the not-detected values.
Compounds
Phenolic acids (µg/g)
Fenugreek
Black cumin
Gallic acid
507.68 ± 2.57
43.21 ± 0.67
p-hydroxybenzoic acid
603.91 ± 2.08
377.81 ± 2.02
Chlorogenic acid
428.55 ± 1.67
ND
Gentisic acid
846.12 ± 1.41
ND
Methyl gallate
676.82 ± 1.88
ND
Caffeic acid
202.66 ± 1.54
116.27 ± 2.56
Syringic acid
96.37 ± 1.02
0.84 ± 0.11
Ellagic acid
349.27 ± 2.08
ND
Coumaric acid
51.84 ± 0.64
0.48 ± 0.05
Vanillic acid
86.23 ± 0.49
3.17 ± 0.21
Ferulic acid
248.12 ± 1.67
38.87 ± 0.64
Sinapic acid
191.96 ± 1.55
27.92 ± 1.05
Cinnamic acid
79.16 ± 1.02
0.21 ± 0.01
Salicylic acid
84.29 ± 1.66
7.97 ± 0.34
Compounds
Flavonoids (µg/g)
Fenugreek
Black cumin
Procatechin
18.14 ± 0.37
ND
Catechin
41.38 ± 1.05
112.97 ± 1.02
Epicatechin
ND
235.67 ± 1.01
Catechol
128.74 ± 2.21
ND
Rutin
12.96 ± 0.22
21.18 ± 1.66
Naringenin
175.46 ± 1.28
0.19 ± 0.02
Daidzein
89.74 ± 0.84
ND
Quercetin
96.12 ± 1.08
5.21 ± 0.27
Apigenin
43.21 ± 0.67
1.41 ± 0.56
Kaempferol
39.66 ± 0.28
10.99 ± 0.86
Hesperetin
ND
4.72 ± 0.52
Flavonoids are lower in FE and CE than phenolic acids. The CE contains eight flavonoid compounds, whereas the FE has nine. Naringenin (175.46 ± 1.28 µg/g) and catechol (128.74 ± 2.21 µg/g) are the highest flavonoids in the FE solution (Table 1). The highest flavonoid components in CE extract are epicatechin (235.67 ± 1.01 µg/g) and catechin (112.97 ± 1.02 µg/g). The two extracts have considerable apigenin, rutin, and quercetin levels. The extracts include bioactive fungi-fighting chemicals kaempferol, quercetin, and naringenin (El-Shahir et al., 2022). Blending plant extracts to boost phenolic and volatile content may work because bioactive components from diverse plants work together. Flavor, scent, and health benefits are linked to phenolic and volatile chemicals. In addition to volatiles, phenolic compounds may boost the extract's bioactivity and degradation of unwanted substances (Pinto et al., 2021).
Previous studies examined fenugreek or black cumin extract phenolic and flavonoid content. Fenugreek seeds have 81.96 mg GAE per g of dry extract using aqueous methanol for total phenolic content (Lohvina et al., 2022). Many fenugreek seed preparations included roughly fifteen phenolic components. Caffeic, luteolin, coumaric, and apigenin had the most phenolics (Kenny et al., 2013).
At the same time, black cumin extract analysis manifested a content of eight phenolic compounds, while dihydroxy benzoic, ferulic, and gallic acids were reported to have a higher content in the extract (Ansary et al., 2022). One more point: the mixture of several extracts together could be the direct reason for changes regarding phenolic compound content (García-Pérez et al., 2020). These changes or enhancements of phenolic compound content are also linked with the antioxidant and antimicrobial properties of the new extract consisting of a mixture (Estevinho et al., 2008; Abdul Qadir et al., 2017).
3.2 Volatile content of byproduct extracts
Valued volatile chemicals are detected in the FE and CE extracts. Table 2 shows that the CE byproduct extract includes 21 volatile compounds, whereas the fenugreek extract has 18. Four chemicals dominate the FE: β-pinene (18.14 %), neryl acetate, β-caryophyllene, and camphor. The CE extract is dominated by thymoquinone (30.05 %), followed by p-cymene (24.56 %) and α-thujene (14.65 %) (Table 2). Although carvacrol is absent, the FE contains several terpene chemicals. Two extract forms include bioactive volatile compounds: α-Pinene, sabinene, and β-pinene. Four chemicals (thymoquinone, carvacrol, α-longipinene, and longifolene) are found in the CE extract but not in the FE (Table 2). These chemicals are associated with the CE extract bioactivity and may be essential for future use. Alpha- and beta-pinene (monoterpene) are bioactive against pollutants and infection (Salehi et al., 2019; Das et al., 2021). It also reduces toxigenic fungi and mycotoxins (Das et al., 2021; Makhuvele, 2020). RI1: Alkane's standards calculated retention indices in the DB-5 column; MS: mass spectra; RI: retention index; ST: standard applied for identification. LRI2: Retention indices according to literature. ID3: confirmed by comparison with the retention indices, the mass spectrum of the authentic compounds, and the NIST mass spectra library data; (−): represents the non-reported compounds in the tested sample.
S/N
Compound
RI1
LRI2
Area%
ID3
Fenugreek
Cumin
1
α-Thujene
926
930
1.37
14.65
RI, MS, STD
2
α-Pinene
935
939
3.58
4.12
RI, MS, STD
3
Sabinene
969
975
4.25
1.21
RI, MS, STD
4
β-Pinene
973
979
18.14
2.96
RI, MS, STD
5
α-phyellandrene
1005
1007
0.96
3.05
RI, MS
6
α-Terpinene
1013
1017
2.18
0.41
RI, MS
7
p-cymene
1019
1024
0.61
24.56
RI, MS, STD
8
D-limonene
1033
1029
−
1.37
RI, MS, STD
9
γ-Terpinene
1055
1059
3.05
0.36
RI, MS
10
Fenchone
1094
1095
−
1.65
RI, MS
11
trans-4-Methoxy thujane
1105
1110
0.41
5.76
RI, MS
12
Terpinen-4-ol
1169
1177
1.46
0.74
RI, MS
13
α-Terpineol
1189
1188
0.97
1.18
RI, MS
14
6-methyl-5-heptan-2-one
1206
1208
5.44
−
−
15
Carvone
1240
1243
−
1.21
RI, MS
16
Cuminal
1248
1249
0.41
2.32
RI, MS
17
Thymoquinone
1255
1258
−
30.05
RI, MS, STD
18
Carvacrol
1292
1299
−
1.56
RI, MS, STD
19
α-Longipinene
1350
1352
−
0.22
RI, MS
20
Longifolene
1409
1410
−
0.54
RI, MS
21
β-caryophyllene
1416
1418
15.81
0.67
RI, MS
22
α-Campholenal
−
−
3.67
−
RI, MS, STD
23
Camphor
1514
1517
13.69
−
RI, MS
24
Geranial
1579
1977
6.95
−
RI, MS
25
Neryl acetate
1722
1726
16.89
−
RI, MS
26
Ethyl phenol
2042
2045
−
1.42
RI, MS
Total
−
−
99.84
100.1
−
The synergistic impact of mixing plant extracts may increase their volatile concentration. Since each plant extract offers distinct strengths, this synergistic interaction may create a more complex and strong mix with bioactivity or sensory effects (Jha and Sit, 2022). Plant extracts may have different phenolic and volatile compounds (Pinto et al., 2021). These compounds may interact synergistically, increasing antioxidant or taste effects beyond each extract (Soleimani et al., 2022). Complementary effects may occur when extracts are combined. Phenolic chemicals may stabilize volatile substances, preserving product properties (Cheynier, 2012). Multiple extracts may also stabilize bioactive chemicals (Joana Gil-Chávez et al., 2013). When combined with other extracts, certain chemicals may be more stable and less susceptible to degradation.
Fenugreek and black cumin agro-waste were chosen to reduce pollutants. This selection was made by utilizing simulated media. Analysis of bioactive molecules in FE or CE extracts revealed substantial percentages of phenolic compounds and volatile or essential constituents. The chemicals mentioned in toxigenic fungi, mycotoxin reduction, and pesticide removal have all been associated with these effects (Aguilar-Pérez et al., 2020; Loi et al., 2020).
A study conducted by Gavahian et al. (Gavahian et al., 2020), Dey (Dey, 2021), and Hsu et al. (Hsu et al.,2022) has determined the worth of agro-waste extracts in eliminating pesticides from media or soil. The antioxidant effectiveness of FE or CE agro-waste extracts remained significant even after loading onto the bioactive membrane composite, as shown in Table 4. The presence of antioxidants in agricultural and food waste helps to decrease the levels of pollutants, particularly fungi that produce toxins (Loi et al., 2020). The activity may be attributed to the response mechanisms of the simulated medium in eliminating pesticides or aflatoxins. Phenolic and flavonoid levels and membrane components might enhance antioxidant action.
3.3 Evaluation of active group using FTIR
Regarding the fenugreek extract, it has several peaks that are linked to its active group content, which can be shown in (Fig. 1). The area 1000 to 1300 cm−1 is reflected by a sharp peak, particularly for the CE, which is linked with the presence of ester group. At 1450 cm−1, the methyl group could be noticed in the FE, also connected with C–H bending. The area between 1780 and 1820 cm−1 shows a clear peak linked to phenyl ester and other aromatic compounds (C–H stretching). The region between 1700 and 2700 cm−1 is displayed without any peak, which reflects no active group for this region. The carbonyl group, which ranged between 1660 and 1820 cm−1, is present in both extracts and their mix, but the curve has the most significant area in the case of CE extract. The peak was characterized by the most muscular shape and medium width, which recommended the rich presence of the carbonyl group (C = O stretching) in the CE extract. The peak that existed at 2550 to 2650 cm−1 is referred to as the sulfhydryl group, also known as thiol, and the curve of the CE can show it; it is also found in the mixture solution. There is a peak at 3200 cm−1, shown in the three solutions (FE, CE, and their mix), which is mainly linked to the alcohol group in the extract. Also, double peaks were recorded near 3400 cm−1, which recommended the existence of an amide group in the CE extract.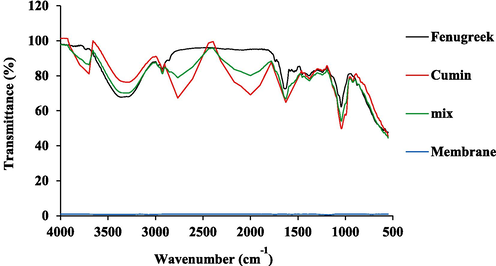
The FTIR curves for the free membrane formed using (CMC/ChS) and loaded membrane of the FE, BC, and their mix.
Generally, it can be concluded that mixed solutions have a group of characteristics as the sum of the active groups found in the two types of extracts. The presence of an active group can support the functionality of the extract by allowing it to link with contaminants. The active group enrichment also facilitates the capacity of their loaded materials to absorb or adsorb the undesirable content from applied solutions. The mixing process of the two extracts, as their loading on a membrane, showed more activity as a result of the active groups, which may play a prominent role in enhancing the membrane's ability to biologically interact and contribute to the removal of various contaminants as a result of interference, whether by degradation or by binding or chelation.
A membrane bioactive composite was prepared using chitosan and CMC to load the extracts (FE/CE; 600 ppm). The resulting membrane was evaluated for its distinguishing characteristics. Particle size (51.78 ± 3.157 nm), zeta-potential (−28.8 ± 3.48), and a polydispersing index (0.288) were shown by a significant value, and it ensured the nano size of prepared material inside the membrane. Other properties, including viscosity, pH, acidity, and stability of the membrane emulsion, reflect better characteristics during the storage time (Table 3). The membrane characteristics play a crucial function in supporting the delivery properties of active ingredients (Nedovic et al., 2011, Yasamineh et al., 2022). The nano size of the extracted molecule may enhance the bioactivity delivered and facilitate the accuracy of the reaction (Liao et al., 2018). * The data is expressed as mean ± SD (n = 5; P ≤ 0.05; SD: standard deviation). * STP: emulsion separation ratio; cp: centipose. * The data is expressed as mean ± SD (n = 5; P ≤ 0.05; SD: standard deviation). * (−): represents the non-reported compounds in the tested sample.
pH
Centrifugation (%)
SPT (%)
Acidity (g lactic/L)
Viscosity (cp)
Day 1
7.41 ± 0.021
89.51 ± 0.67
0.122 ± 0.001
−
432.27 ± 5.41
Day 7
7.16 ± 0.011
85.16 ± 1.02
0.146 ± 0.002
−
476.51 ± 3.27
Day 14
7.01 ± 0.034
84.05 ± 1.31
0.237 ± 0.007
−
567.41 ± 5.81
Day 21
6.98 ± 0.026
81.36 ± 1.27
0.368 ± 0.005
0.101 ± 0.002
637.74 ± 6.45
Type
Antioxidant activity
Phenolic (µg/g)
Flavonoids (µg/g)
DPPH (%)
ABTS (%)
Fenugreek
67.51 ± 0.48
64.18 ± 0.73
88.65 ± 1.54
7258.15 ± 7.91
Black cumin
74.55 ± 0.31
71.16 ± 0.55
258.26 ± 3.16
5315.22 ± 6.24
Membrane mix-loaded (1:1)
76.72 ± 0.77
75.21 ± 0.41
32.45 ± 4.66
138.25 ± 14.47
Trolox (ST)
99.86 ± 0.21
99.54 ± 0.26
−
−
Moreover, the content of the bioactive group was determined using the FTIR apparatus (Fig. 3). The result reflects a variable content of active groups on the bioactive composite membrane. These groups include carbonyl, sulfhydryl, amide, ester, alcohols, and hydroxyl groups. The presence of these active groups may support the bioactive composite's activity in removing contaminants, either by binding covalent bonds or through an antioxidant reaction. Several phytochemical extracts were reported to have activity in contaminant removal, such as mycotoxin content (Abu-Sree et al., 2021, Badr et al., 2022a; 2022b). Moreover, applying bioactive extracts in a nanoform could ameliorate their activity for the reduction process of mycotoxin (Abdel-Razek et al., 2021; Alharthi et al., 2021).
FTIR in plant extract bioactivity study provides valuable insights into chemical composition, notably functional group identification and characterization (Bajrami et al., 2023). FTIR may reveal the extract's chemical composition. Functional groups absorb infrared light at specific wavelengths, giving each molecule a unique spectral pattern (Dong et al., 2022). The FTIR spectroscopy can identify functional groups of plant extracts. FTIR can identify plant extract organic compounds. FTIR analysis may identify functional groups, including bioactive compounds with proven medicinal properties (Poojary et al., 2015). Antioxidant and anti-inflammatory activities are associated with phenolic compounds. The bioactive groups revealed by FT-IR in this study may also reveal molecules connected to them. FTIR data may be compared to bioassay results to determine plant extract chemical composition and biological activity correlations. This method helps identify biomolecules responsible for bioactivity. This work uses FTIR analysis to identify unique active groups in the freshly prepared solution comprising a mix of extracts.
3.4 Membrane characteristic evaluation
The characteristics of the formed solution for the membrane were evaluated as follows to reflect the membrane quality. The result is shown by significant values recorded for the particle size diameter inside the formed solution (51.78 ± 3.157 nm) and the value recorded for the polydispersity index (0.228). Moreover, the value of the zeta average is shown at (−28.8 mV ± 3.48), as represented in Fig. 2. These values reflect the excellent characteristics of the formed solution as the particle size recorded by nanosize; also, the values indicate a high quality that shows the solution stability of Z-potential and the PDI. These parameters can support the membrane activity for the removal characteristics of pollutants and contaminants from liquid materials application. Membrane characteristics of particle size and zeta potential activity were involved in its activity for pollutants and contaminants removal of the fluid system (Kárászová et al., 2020; Mamba et al., 2021).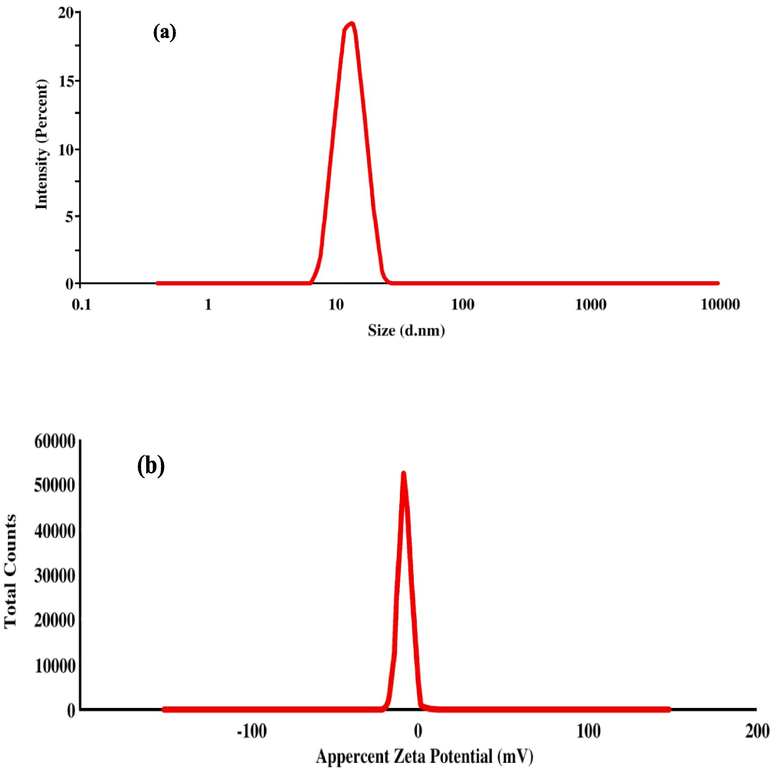
(a) Particle size, zeta potential, and the (b) Polydispersity index of the solution prepared to form the membrane.

The scanning electron microscope capture of the membrane formed using (CMC/ChS) and loaded with fenugreek-cumin extract.
Other characteristics of the CMC/ChS solution loaded by the byproduct extracts of the FE and the CE (1:1, v/v) are represented in Table 3. The pH values measured during the emulsion storage time of up to 21 days showed slight changes. The data represent an acceptable decrease of the pH values (7.41 ± 0.021 to 6.98 ± 0.026). The results expressed the stability of the formed emulsion by measuring two variables of the centrifugation and separation ratio. Regarding the centrifugation, the ratios ranged between 89.51 ± 0.67 % and 81.36 ± 1.27 %, reflecting high stability, even during the storage period (up to 21 days). Again, the separation ratio of the loaded materials from the emulsion has not exceeded 0.4 %. The acidity was not detected, except for the emulsion stored for up to 21 days. At that time, a slight acidity is recorded by a value of 0.101 ± 0.002 g lactic acid/L. Moreover, the viscosity of the formed emulsion (432.27 ± 5.41 cp.) is recorded to increase by the time incubation to reach (637.74 ± 6.45 cp.) after 21 days of incubation. This increase in viscosity could be linked to the evaporation that may happen to the emulsion during the storage period.
It could be concluded from Table 3 that the formed emulsion is slightly alkaline at the prepared stage, with significant stability and a low separation ratio. The viscosity value is not high but is raised slowly by storage in a liquid phase, which is recommended to turn the emulsion into a dry membrane.
The emulsion of this composite was dried to form a membrane loaded with the bioactive components sourced from applied extracts (FE and CE). This step was performed for two reasons: scanning electron microscope (SEM) evaluation of the emulsion and facilitating the composite application for pollution removal. The SEM evaluation of the composite membrane gives a vision of the structure and surface morphology of the performed membrane (Fig. 3). The capture showed a particle size of fewer than 50 nm in diameter. The capture reflects a homogeneous distribution of bioactive molecules inside the membrane, with low bioactive-free interfacial areas. The capture also showed a better bioactive loading density of molecules, increasing the membrane potency.
3.5 Bioactivity of the extracts and loaded membrane
Antioxidant activity is determined and represented in Table 4 as an indicator that reflects the membrane activity compared to the raw materials of inserted extracts. The antioxidant activity, determined by two assays (DPPH and ABTS), records the enhancement of the membrane antioxidant value. The better antioxidant values recorded for the formed membrane could be linked to its composition, including bioactive extracts, chitosan, the CMC, and coarse emulsion. The value is considered significant following the value recorded for the standard antioxidant material of Trolox. Moreover, the data represented the total phenolic and flavonoid values, which were spectrophotometrically determined (Table 4). The results showed a perfect relative content of total phenols and flavonoids compared to the crude extracts. The recorded content in the formed membrane performed using CMC/ChS loaded by FE-CE was also consistent with the amount loaded in membrane formation (600 ppm).
3.6 Pesticide removal using FE/CE extract
In the simulation experiment of the residues of the pesticides chlorpyrifos and profenofos removal, the utilization of a previously prepared extract showed significant efficiency in reducing their concentration. The reduction amount for profenofos was more than that recorded for chlorpyrifos (Fig. 4a). The removal percentage appeared in Fig. 4b, where the efficiency of a loaded mix of the extract on the membrane was higher for the pesticide profenofos than the one recorded for the chlorpyrifos.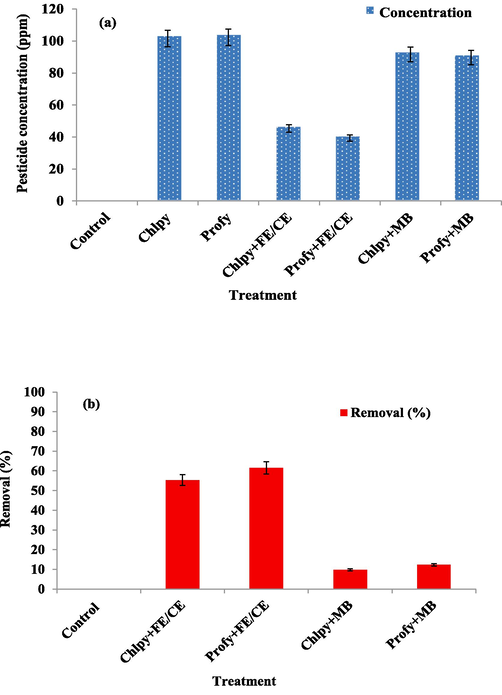
Byproducts extract (FE-CE) impact on reducing pesticide residues: (a) Pesticide reduction in concentration, (b) Pesticide removal ratio. FE: Fenugreek extract; CE: black cumin extract; Chlpy: chlorpyrifos; Profy:profenofos; MB: Membrane free of the extracts.
Evaluation of the extract-free membrane showed a weak ability to remove pesticides, which confirms the vital action of the extracts loaded on the membrane in reducing pesticide levels in the simulated media. The negative control used was deionized water to evaluate the efficiency of the experiment and ensure its validity.
The reduction recorded using the free membrane is recorded with a meager reduction, which may be linked to a chelating or binding process inside the membrane ingredients. Clearly, extract mix significantly impacts pesticide reduction (Fig. 4 a, b). Again, a significant reduction is shown between chlorpyrifos and the profenofos removal, with better results for profenofos.
3.7 Aflatoxin degradation using FE/CE extract
The simulated experiment for the aflatoxin reduction in media showed significant results, particularly for the extract loaded on the membrane (Fig. 5). It is noticed that the crude extract showed an ability to reduce aflatoxin from the media but with less efficiency compared to the loaded one. The results point out a lesser ability of the mix-extract loaded to the membrane in the AFB2 reduction from the media. This impact could be linked to each toxin's chemical structure and the suggested interactions between aflatoxin and bioactive molecules in the formed membrane.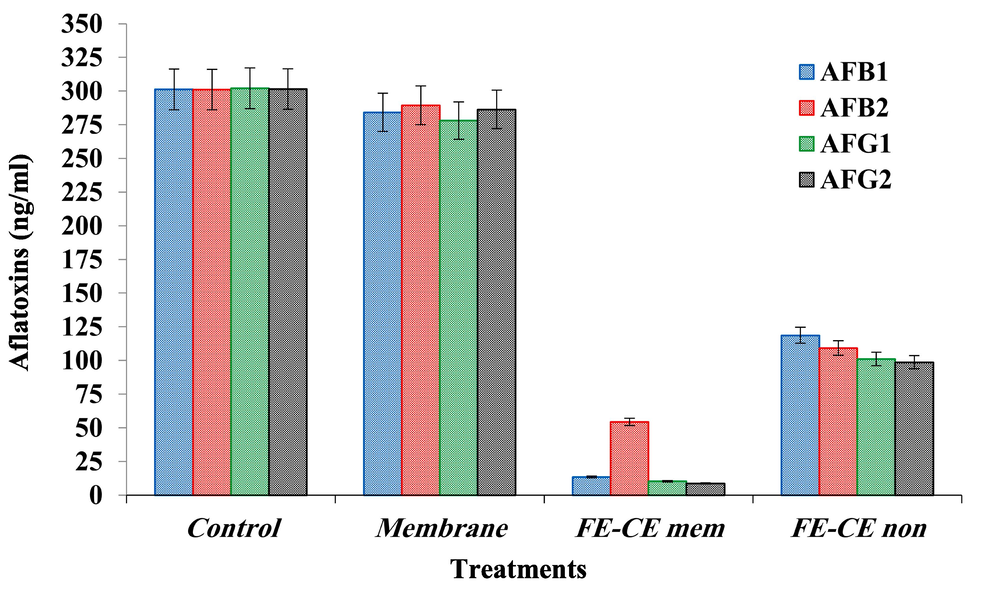
Byproduct extract (FE-CE) impact to degrade Aflatoxins (AFB1; AFB2; AFG1; AFG2) from the simulated solution contained spiked concentration. AFB1: aflatoxin B1; AFB2: aflatoxin B2; AFG1: aflatoxin G1; AFG2: aflatoxin G2. FE: fenugreek extract; CE: black cumin extract; FE-CE mem: extracts (1:1) loaded on membrane; FE-CE non: crude extracts (1:1) without membrane. The group of column with different asterisk number are significantly different.
The results showed a reduction in the percentage of aflatoxins concentration using the crude extract, whether for the AFB1, AFB2, AFG1, or AFG2 applied in the simulation media. The reduction was higher than 50 % for all aflatoxins under investigation. Moreover, the results of reducing aflatoxins by the crude extract application without a membrane loading indicated effectiveness in the toxin content reduction with a varying degree as the following order (AFG2 > AFG1 > AFB2 > AFB1).
Although the membrane functionality provides a contact medium between bioactive components and mycotoxins, which allows the occurrence of the changes that are manifesting in the recorded results, likely, the reduction that is occurring in the proportions and quantities of aflatoxins in the simulation media could be due to the oxidation or reduction occurrence that is linked to the activity of the biological components present in the extract-mix that is being loaded to the membrane utilized.
3.8 Suggested mechanism of the reactions
Nanomaterials possess significant characteristics, including substantial specific surface areas, size distribution, molecular structure, hydrophilicity/lipophilicity, and elevated reactivities. The various physicochemical properties of nanomaterials make them well-suited for a diverse array of environmental applications, including their use as adsorbents, catalysts, and biodegradable material (Neto et al., 2019). Several techniques, including polymer application, ozonation, and chelating using active biochar were effectively remove the pesticide residue (Dumitriu et al., 2022). It is noticed that two volatile compounds of p-cymene and thymoquinone are present in the FE. Also, β-Pinene, Neryl acetate, β-caryophyllene, and camphor are the dominant volatiles in the CE. All of these volatile compounds are distinguished by enrichment of the methyl group, which could play a significant function in formulating an umbrella of the organic group that chelates or binds to the pesticides' active sites of chlorpyrifos or profenofos. Otherwise, several essential constituents were applied instead of chemical pesticides (Assadpour et al., 2023). Another bioactive molecule that could have an impact on reducing the pesticide residues or aflatoxin contamination is linked to the ones rich in the hydroxyl group (Fig. 6). The presence of the hydroxyl group regarding the extract-mix (FE/CE) is related to the suggested reduction occurred to the contaminants of pesticides and aflatoxins. It may recommend the existence of an oxidation or reduction reaction between the bioactive molecules rich in the hydroxyl group and the targeted compounds (Venkatachalapathy et al., 2020; Hussain et al., 2022). This reaction may not be chemical (covalent bonds between the functional groups on both compounds). These bonds can illustrate the reduction recorded by the simulated media evaluation.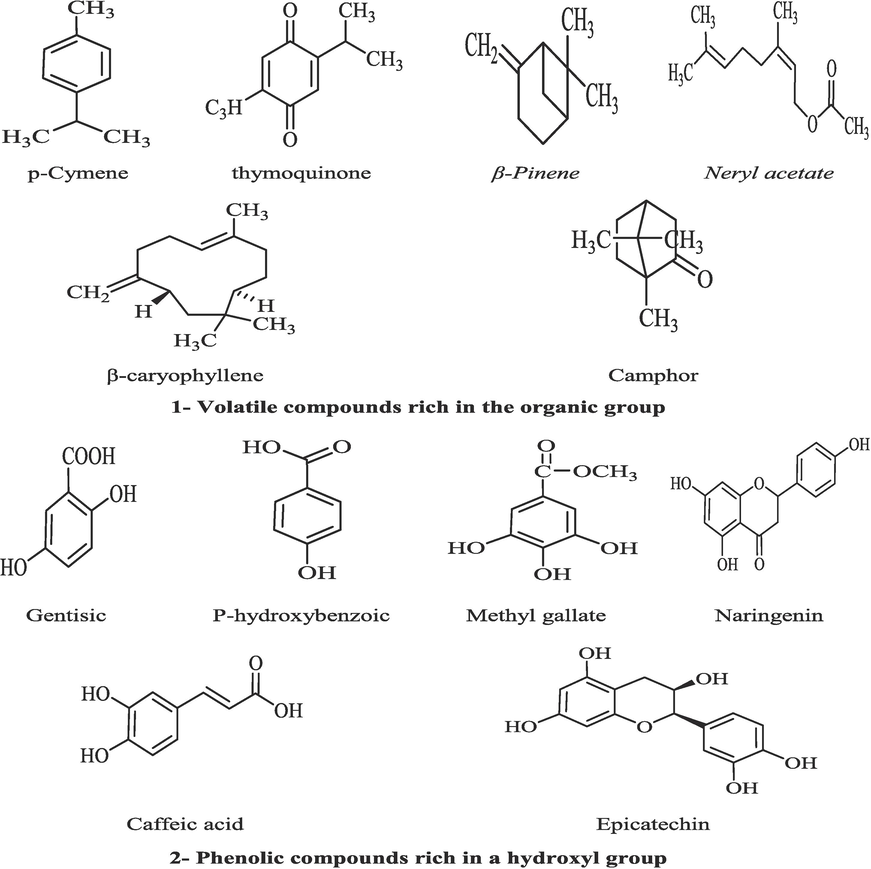
Chemical structures of bioactive molecules present in the applied extract (FE-CE).
However, the chemical composition of the pesticides or aflatoxins used is characterized by several active groups that are connected to the structure of the substance. The chlorpyrifos and profenofos differ due to the inclusion of organic (methyl) groups, which provide a deteriorating quality to the halogen atoms. The disparities between the two compounds in terms of the phosphorus group are clearly shown. The chlorpyrifos molecule has a double bond between the phosphorus group and sulfur in its structure. Conversely, the phosphorus atom is connected to three oxygen atoms at three equal locations. The spatial arrangement of the phosphorus atom in Profenophos is distinct, since it retains its five-point valence, but forms a double connection with an oxygen atom and a single bond with a sulfur atom (Hussain et al., 2022). The variation in the spatial arrangement and the disparity in the positioning of the double bond on the phosphorus atom might account for the discrepancy in the observed clearance rates of the two pesticides in the same extract.
The positioning of the double bond on the mycotoxin molecule distinguished the four aflatoxins (AFB1, AFB2, AFG1, and AFG2). Aflatoxin B1 and G1 possess a double bond on the first ring, absent in B2 and G2 molecules. The last ring of aflatoxins G1 and G2 had an extra oxygen atom, in contrast to aflatoxins of the (B) category (Abdel-Fattah et al., 2018). However, aflatoxin compounds include many oxygen atoms, which might facilitate their involvement in oxidation or reduction reactions under ideal conditions. Aflatoxins possess free radical activity, which significantly enhances their propensity to undergo reactions. These factors could account for the decline in levels of various contaminants in the simulated medium, resulting in reduced pollution rates (Farouk et al., 2023). Additional research might provide a more precise understanding and explanation of the mechanisms via which the levels of pollutants in the simulated medium dropped. The forthcoming investigation will elucidate the reaction mechanisms and their proposed sites on the specific compounds via covalent bonds that could connect to their binding reaction, clarifying their removal from the medium or through oxidation/reduction reactions anticipated in a surplus of hydroxyl groups.
Under controlled settings using spiking pesticides (chlorpyrifos or profenofos; 100 ppm), a bioactive composite membrane successfully eliminated more than 50 % of the pollutants. The membrane, when not treated with extract, exhibits a pesticide clearance of less than 10 % (Fig. 4). The bioactive membrane composite effectively decreases the presence of aflatoxins in a medium that contains elevated levels of AFB1, AFB2, AFG1, and AFG2 (Fig. 5). Prior studies have shown that nanoemulsion effectively decreases the presence of mycotoxin (Mirza Alizadeh et al., 2022; Hassanein, 2023). Applying a nanoform extract to a membrane might decrease mycotoxin's presence in the medium or substrate (Loi et al., 2021; Khojah, 2022). The main constituents of the nanoemulsion are bioactive phenolic, flavonoid, or antioxidant chemicals that reduce mycotoxin levels via several mechanisms (Jafarzadeh et al., 2022; Chen et al., 2023). The clearance ratio of pesticides that were added to a simulated medium was found to be significant, with just a little difference between the two pesticides that were used (Fig. 4). The administration of the extract mix (FE/CE) was effective in reducing aflatoxin levels in spiked simulated medium, regardless whether the extracts were crude or loaded. This is seen in Fig. 5. However, more research is necessary to establish the mechanism of action for each instance and validate the appropriate approach for eliminating pesticides or aflatoxins.
4 Conclusion
Fenugreek extract included phenolic compounds such as gentisic (846.12 ± 1.41 µg/g) and methyl gallate (676.82 ± 1.88 µg/g), whereas the black cumin extract had significant amounts of p-hydroxybenzoic and caffeic acids. In FE from wastes, thymoquinone (30.05 %) and p-cymene (24.56 %) were the most common volatile compounds, followed by β-pinene (18.14 %), neryl acetate (16.89 %), β-caryophyllene (15.81 %), and camphor (13.69 %). The bio-membrane of carboxymethyl cellulose and chitosan, loaded with both extracts at 600 ppm (1:1, FE/CE), showed notable qualitative features, such as a particle size of 51.78 ± 3.157 nm, a zeta potential of (−28.8 ± 3.48), and a PDI of The membrane's surface contained carbonyl, sulfhydryl, esters, and hydroxyl groups, either alcohols or aldehydes, according to FTIR examination. Simulated experiments showed the produced extract lowered two pesticide residues. The membrane-loaded extract combination (FE/CE) was compared to the membrane-free to evaluate the enhancement in pollution removal percentage. In a simulated media, the composite membrane removed 55.31 ± 1.62 % chlorpyrifos and 61.54 ± 1.51 % profenofos. The membrane reduced over 80 % of aflatoxin, compared to 50 % in crude extract. These results propose bioactive composites for pollutant removal. More studies are needed on the mechanisms and drawings of contaminants' active groups and natural extract components.
Acknowledgment
The author extends his appreciation to Taif University, Saudi Arabia, for supporting this work through project number (TU-DSPP-2024-111).
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Antifungal and anti-mycotoxigenic impact of eco-friendly extracts of wild stevia. J. Biol. Sci.. 2018;18(8):488-499.
- [CrossRef] [Google Scholar]
- Efficacy of bottle gourd seeds' extracts in chemical hazard reduction secreted as toxigenic fungi metabolites. Toxins. 2021;13(11):789.
- [CrossRef] [Google Scholar]
- Abdul Qadir, M., Shahzadi, S. K., Bashir, A., Munir, A., Shahzad, S., 2017. Evaluation of Phenolic Compounds and Antioxidant and Antimicrobial Activities of Some Common Herbs. International Journal of Analytical Chemistry 2017, 3475738. https://doi.org /10.1155/2017/3475738.
- Neoteric approach for peanuts biofilm using the merits of Moringa extracts to control aflatoxin contamination. Toxicol. Rep.. 2021;8:1685-1692.
- [CrossRef] [Google Scholar]
- Ultrasound-assisted extraction of some branded tea: Optimization based on the polyphenol content, antioxidant potential, and thermodynamic study. Saudi J. Biol. Sci.. 2019;26(5):1043-1052.
- [CrossRef] [Google Scholar]
- Nano-sorbent materials for pharmaceutical-based wastewater effluents - An overview. Case Stud. Chem. Environ. Eng.. 2020;2:100028
- [CrossRef] [Google Scholar]
- Bioactive molecules of mandarin seed oils diminish mycotoxin and the existence of fungi. Molecules. 2021;26(23):7130.
- [CrossRef] [Google Scholar]
- Ferulic acid incorporated antimicrobial self cross-linking hydrogel: A promising system for moderately exudating wounds. J. Drug Delivery Sci. Technol.. 2022;73:103446
- [Google Scholar]
- Evaluation of the in vitro bioaccessibility of phenolic compounds of black cumin (BARI-1cumin) methanolic extract. eFood. 2022;3(3):e15.
- [Google Scholar]
- Assadpour, E., Karaça, C. A., Fasamanesh, M., Mahdavi, S. A., Shariat-Alavi, M., Feng, J., Kharazmi, M. S., Rehman, A., Jafari, S. M., 2023. Application of essential oils as natural biopesticides; recent advances. Critical Reviews in Food Science and Nutrition 1-21. https://doi: 10.1080/10408398.2023.2170317.
- Bioactive components of pomegranate oil and their influence on mycotoxin secretion. Toxins. 2020;12
- [CrossRef] [Google Scholar]
- Spent coffee grounds valorization as bioactive phenolic source acquired antifungal, anti-mycotoxigenic, and anti-cytotoxic activities. Toxins. 2022;14
- [CrossRef] [Google Scholar]
- Synergistic impact of bioactive byproduct extract leads to anti-fusarium and anti-mycotoxin secretion. J. Fungi. 2022;8
- [CrossRef] [Google Scholar]
- Food waste posing a serious threat to sustainability in the Kingdom of Saudi Arabia - A systematic review. Saudi J. Biol. Sci.. 2019;26(7):1743-1752.
- [CrossRef] [Google Scholar]
- Food waste in Saudi Arabia: Causes, consequences, and combating measures. Sustainability. 2022;14(16):10362.
- [CrossRef] [Google Scholar]
- A phytochemical analysis of mint and Salvia officinalis L. tea using FTIR technique. Eur. J. Agric. Food Sci.. 2023;5(4):55-59.
- [CrossRef] [Google Scholar]
- Microfluidizing technique application for Algerian Cymbopogon citratus (DC.) Stapf effects enhanced volatile content, antimicrobial, and anti-mycotoxigenic properties. Molecules. 2023;28(14)
- [CrossRef] [Google Scholar]
- Canola oil is an excellent vehicle for eliminating pesticide residues in aqueous ginseng extract. J. Ginseng Res.. 2016;40(3):292-299.
- [CrossRef] [Google Scholar]
- Quantification of the antioxidant activity of plant extracts: Analysis of sensitivity and hierarchization based on the method used. Antioxidants. 2020;9
- [CrossRef] [Google Scholar]
- Effects and mechanisms of plant bioactive compounds in preventing fungal spoilage and mycotoxin contamination in postharvest fruits: A review. Food Chem.. 2023;415:135787
- [CrossRef] [Google Scholar]
- Phenolic compounds: From plants to foods. Phytochem. Rev.. 2012;11(2):153-177.
- [CrossRef] [Google Scholar]
- Exploration of some potential bioactive essential oil components as green food preservative. Lwt. 2021;137:110498
- [CrossRef] [Google Scholar]
- Valorization of agro-waste into value-added products for sustainable development. Bioresour. Technol. Rep.. 2021;16:100834
- [CrossRef] [Google Scholar]
- Mechanistic insights into the chemical structural changes of lignite on the potential formation of the polycyclic aromatic hydrocarbons. Chemosphere. 2022;307:135916
- [CrossRef] [Google Scholar]
- Dumitriu (Gabur), G.-D., Teodosiu, C., Cotea, V. V., 2022. Management of Pesticides from Vineyard to Wines: Focus on Wine Safety and Pesticides Removal by Emerging Technologies. IntechOpen. 1-26. .
- Bioactive compounds and antifungal activity of leaves and fruits methanolic extracts of Ziziphus spina-christi L. Plants. 2022;11(6):746.
- [Google Scholar]
- Fenugreek potent activity against nitrate-induced diabetes in young and adult male rats. Cytotechnology. 2015;67(3):437-447.
- [CrossRef] [Google Scholar]
- Antioxidant and antimicrobial effects of phenolic compounds extracts of Northeast Portugal honey. Food Chem. Toxicol.. 2008;46(12):3774-3779.
- [CrossRef] [Google Scholar]
- In-vitro and in-silico investigation for the spent-coffee bioactive phenolics as a promising aflatoxins production inhibitor. Toxins. 2023;15(3)
- [CrossRef] [Google Scholar]
- Combining medicinal plant in vitro culture with machine learning technologies for maximizing the production of phenolic compounds. Antioxidants. 2020;9
- [CrossRef] [Google Scholar]
- Recent advances in the application of innovative food processing technologies for mycotoxins and pesticide reduction in foods. Trends Food Sci. Technol.. 2020;106:209-218.
- [CrossRef] [Google Scholar]
- Golden, G., E. Quinn, E., Shaaya, M. Kostyukovsky and E. Poverenov (2018). “Coarse and nanoemulsions for effective delivery of the natural pest control agent pulegone for stored grain protection.” Pest Management Science 74(4): 820-827. DOI: Doi: 10.1002/ps.4787.
- Antioxidant potential of some agri-horticultural wastes. Int. Food Res. J.. 2017;24(3)
- [Google Scholar]
- Application of lime peel oil composite nanoemulsion to prevent toxigenic fungi in nuts. Heliyon. 2023;9(8)
- [CrossRef] [Google Scholar]
- Postharvest losses and waste in developed and less developed countries: opportunities to improve resource use. J. Agric. Sci.. 2011;149(S1):37-45.
- [Google Scholar]
- Application of image processing to assess emulsion stability and emulsification properties of Arabic gum. Carbohydr. Polym.. 2015;126:1-8.
- [CrossRef] [Google Scholar]
- Application of saponins extract from food byproducts for the removal of pesticide residues in fruits and vegetables. Food Control. 2022;136:108877
- [CrossRef] [Google Scholar]
- Pervaporation dehydration of aqueous ethanol and isopropanol mixtures through alginate/chitosan two ply composite membranes supported by poly(vinylidene fluoride) porous membrane. J. Membr. Sci.. 2000;167(2):275-289.
- [CrossRef] [Google Scholar]
- Total phenolics, flavonoids, and antioxidant activity of agricultural wastes, and their ability to remove some pesticide residues. Toxicol. Rep.. 2022;9:628-635.
- [CrossRef] [Google Scholar]
- Recent advances in plant-based compounds for mitigation of mycotoxin contamination in food products: current status, challenges and perspectives. Int. J. Food Sci. Technol.. 2022;57(4):2159-2170.
- [CrossRef] [Google Scholar]
- Vegetable residue of fenugreek (Trigonella Foenum-Graecum), waste biomass for removal of Basic Violet 14 from wastewater: Kinetic, equilibrium, and reusability studies. Sustain. Chem. Pharm.. 2020;16:100269
- [CrossRef] [Google Scholar]
- Extraction of bioactive compounds from plant materials using a combination of various novel methods. A review. Trends Food Sci. Technol.. 2022;119:579-591.
- [CrossRef] [Google Scholar]
- Technologies for extraction and production of bioactive compounds to be used as nutraceuticals and food ingredients: An overview. Compr. Rev. Food Sci. Food Saf.. 2013;12(1):5-23.
- [CrossRef] [Google Scholar]
- Carboxymethyl cellulose-based materials for infection control and wound healing: A review. Int. J. Biol. Macromol.. 2020;164:963-975.
- [CrossRef] [Google Scholar]
- Membrane removal of emerging contaminants from water: which kind of membranes should we use? Membranes. 2020;10(11):305.
- [Google Scholar]
- Environmental pollutants and livestock health: A review. Vet. Res. Int.. 2013;1(1):1-13.
- [Google Scholar]
- Antioxidant properties and quantitative UPLC-MS analysis of phenolic compounds from extracts of fenugreek (Trigonella foenum-graecum) seeds and bitter melon (Momordica charantia) fruit. Food Chem.. 2013;141(4):4295-4302.
- [CrossRef] [Google Scholar]
- Bioactives of pomegranate by-products and barley malt grass engage in cereal composite bar to achieve antimycotic and anti-aflatoxigenic attributes. Foods. 2022;11
- [CrossRef] [Google Scholar]
- Preparation of sodium carboxymethyl cellulose-chitosan complex membranes through sustainable aqueous phase separation. ACS Appl. Polym. Mater.. 2023;5(3):1810-1818.
- [CrossRef] [Google Scholar]
- Liao, W., Z. Liu, T. Zhang, S. Sun, J. ye, Z. Li, L. Mao and J. Ren (2018). “Enhancement of Anti-Inflammatory Properties of Nobiletin in Macrophages by a Nanoemulsion Preparation.” Journal of Agricultural and Food Chemistry 66(1): 91-98. DOI: 10.1021/acs.jafc.7b03953.
- Effect of ethanol solvents on total phenolic content and antioxidant properties of seed extracts of fenugreek (Trigonella foenum-graecum L.) varieties and determination of phenolic composition by HPLC-ESI-MS. Diversity. 2022;14
- [CrossRef] [Google Scholar]
- Plant bioactive compounds in pre- and postharvest management for aflatoxins reduction. Front. Microbiol.. 2020;11
- [CrossRef] [Google Scholar]
- Mahmood, I., S. R. Imadi, K. Shazadi, A. Gul and K. R. Hakeem (2016). “Effects of pesticides on environment.” Plant, soil, and microbes: volume 1: implications in crop science: 253-269.
- The use of plant extracts and their phytochemicals for control of toxigenic fungi and mycotoxins. Heliyon. 2020;6(10)
- [Google Scholar]
- Recent advances in biopolymeric membranes towards the removal of emerging organic pollutants from water. Membranes. 2021;11(11):798.
- [Google Scholar]
- Carboxymethyl cellulose from Opuntia ficus-indica (Cactaceae) for cross-linked films. Cellul.. 2023;30:9575-9591.
- [CrossRef] [Google Scholar]
- Recent advances on the efficacy of essential oils on mycotoxin secretion and their mode of action. Crit. Rev. Food Sci. Nutr.. 2022;62(17):4726-4751.
- [Google Scholar]
- Mizzi, L., Chatzitzika, C., Gatt, R., Valdramidis, V., 2020. HPLC Analysis of Phenolic Compounds and Flavonoids with Overlapping Peaks. Food Technology and Biotechnology 58 (1), 12-19. Doi: Doi: 10.17113/ftb.58.01.20.6395.
- Cumin (Cuminum cyminum L.) from traditional uses to potential biomedical applications. Chem. Biodivers.. 2015;12(5):733-742.
- [CrossRef] [Google Scholar]
- An overview of encapsulation technologies for food applications. Procedia Food Sci.. 2011;1:1806-1815.
- [CrossRef] [Google Scholar]
- Neto, V. D. O. S., Freire, P. T. C., do Nascimento, R. F., 2019. Removal of pesticides and volatile organic pollutants with nanoparticles. Nanomaterials Applications for Environmental Matrices 405-426. Doi: 10.1016/B978-0-12-814829-7.00013-6.
- Özkara, A., D. Akyıl and M. Konuk (2016). Pesticides, environmental pollution, and health. Environmental health risk-hazardous factors to living species, IntechOpen.
- Membrane technology enhancement oil–water separation. A review. Desalination. 2015;357:197-207.
- [CrossRef] [Google Scholar]
- Agro-food waste as an ingredient in functional beverage processing: Sources, functionality, market and regulation. Foods. 2023;12
- [CrossRef] [Google Scholar]
- Bioactive (Poly)phenols, volatile compounds from vegetables, medicinal and aromatic plants. Foods. 2021;10
- [CrossRef] [Google Scholar]
- Extraction, characterization and biological studies of phytochemicals from Mammea suriga. J. Pharm. Anal.. 2015;5(3):182-189.
- [CrossRef] [Google Scholar]
- Recent progress and challenges of adsorptive membranes for the removal of pollutants from wastewater. Part II: Environmental applications. Case Stud. Chem. Environ. Eng.. 2021;3:100102
- [Google Scholar]
- Utilizing lemon peel extract and its nanoemulsion to control aflatoxin toxicity in rats. Food Biosci.. 2022;50:101998
- [CrossRef] [Google Scholar]
- Salehi, B., Upadhyay, S., Erdogan Orhan, I., Kumar Jugran, A., LD Jayaweera, S., A. Dias, D., ... & Sharifi-Rad, J. (2019). Therapeutic potential of α-and β-pinene: A miracle gift of nature. Biomolecules, 9(11), 738.
- Utilization of agro-industrial waste for sustainable green production: a review. Environ. Sustain.. 2021;4(1):619-636.
- [CrossRef] [Google Scholar]
- Food byproducts and food wastes: are they safe enough for their valorization. Trends Food Sci. Technol.. 2021;114:133-147.
- [CrossRef] [Google Scholar]
- Phenolic compounds and antimicrobial properties of mint and thyme. J. f Herb. Med.. 2022;36:100604
- [CrossRef] [Google Scholar]
- Response of non-enzymatic antioxidative mechanisms to stress caused by infection with Fusarium fungi and chemical protection in different wheat genotypes. Chem. Ecol.. 2017;33(10):949-962.
- [CrossRef] [Google Scholar]
- A review on microalgae cultivation and harvesting, and their biomass extraction processing using ionic liquids. Bioengineered. 2020;11(1):116-129.
- [CrossRef] [Google Scholar]
- Environmental impacts of food waste: Learnings and challenges from a case study on the UK. Waste Manag.. 2018;76:744-766.
- [CrossRef] [Google Scholar]
- Venkatachalapathy, R., Chandra, I. R. A., Das, S., 2020. Effective removal of organophosphorus pesticide residues in tomatoes using natural extracts. Journal of Food Process Engineering 43 (2). 43:e13351. Doi: 10.1111/jfpe.13351.
- Chitosan/poly (vinyl alcohol) films containing ZnO nanoparticles and plasticizers. Mater. Sci. Eng. C. 2010;30(4):503-508.
- [CrossRef] [Google Scholar]
- Chitosan/montmorillonite coatings for the fabrication of food-safe greaseproof paper. Polymers. 2021;13
- [CrossRef] [Google Scholar]
- A state-of-the-art review on the recent advances of niosomes as a targeted drug delivery system. Int. J. Pharm.. 2022;624:121878
- [CrossRef] [Google Scholar]
- Preparation of chitosan and carboxymethylcellulose-based polyelectrolyte complex hydrogel via SD-A-SGT method and its adsorption of anionic and cationic dye. J. Appl. Polym. Sci.. 2020;137(34):48980.
- [CrossRef] [Google Scholar]
- Tuning complexation of carboxymethyl cellulose/cationic chitosan to stabilize Pickering emulsion for curcumin encapsulation. Food Hydrocoll.. 2021;110:106135
- [CrossRef] [Google Scholar]







