Translate this page into:
Bioactive Fluorenes. Part II. Unprecedented biologically active thiazole derivatives based-2,7-dichlorofluorene as competent DHFR inhibitors: Design, synthesis, and molecular docking approaches
⁎Corresponding authors at: Department of Chemistry, Faculty of Applied Science, Umm Al-Qura University, 21955 Makkah, Saudi Arabia. Tel./Fax: + 966 530435760 (E.M. Hussein) essam.hussein78@yahoo.com (Essam M. Hussein), amhfarghaly@uqu.edu.sa (Essam M. Hussein), saahmed@uqu.edu.sa (Saleh A. Ahmed)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
Abstract
In this study, a new series of (4-(2,7-dichloro-9H-fluoren-4-yl)thiazol-yl)acetamide derivatives was synthesized, and the new heterocycles were completely characterized, evaluated for their antimicrobial activity, and screened for cytotoxic activity against human lung carcinoma (A-549) and human breast carcinoma (MCF-7) cell lines. A molecular docking study was undertaken to identify the possible mode of action of the synthesized compounds, which suggested binding interactions with the dihydrofolate reductase (DHFR) active sites.
Most of the synthesized compounds displayed meaningful activity against A-549 and MCF-7 cell lines when compared to 5-fluorouracil (5-FU), which was used as a reference drug. Furthermore, some of the prepared compounds exhibited potent antibacterial and antifungal activities. The highly pronounced biological activities of the compounds under investigation offer such species as promising future drug prospects which may find applications in the fields of biological and medicinal sciences.
Keywords
Fluorene
Thiazole
Antimicrobial
Anti-cancer
Molecular docking
DHFR inhibitors
1 Introduction
In the last decades, fluorene and its derivatives have experienced extensive and effective uses as precursors in a wide range of of synthetic applications (Gualtieri et al., 1985). For example, in medical applications, 2,7-dichloro-4-(chloroacetyl)fluorene is considered as a key intermediate for the synthesis of the antimalarial agent known as benflumetol (Fun et al., 2010). On the other hand, in optical applications, 2,7-dichloro fluorenyldihydroindolizines (DHIs) represent multiaddressable photochromic properties in both solution, polymer materials and in solid state thin films (Ahmed et al., 2016, 2017). Indeed, fluorene derivatives display remarkable photophysical and spectroscopic properties (El Guesmi et al., 2017b). Fluorene-based fluorophores were significantly utilized for the improvement of dyes absorption properties (El Guesmi et al., 2017a) and fluorophore sensors (El Guesmi et al., 2018).
Nitrogen-based heterocycles containing sulfur atoms are an imperative class of compounds due to their broad applications in medicinal chemistry. The thiazole ring is a core structural feature found in a variety of biologically and medicinally active molecules. Furthermore, the thiazole ring is a structural constituent of natural compounds such as thiamine (vitamin B1) and penicillin. Additionally, thiazole derivatives demonstrate an extensive spectrum of medicinal and biological activities, such as antifungal, antibacterial, (Bharti et al., 2010) antiviral (Spector et al., 1998), anti-inflammatory (Yang et al., 2010), anti-HIV (Bell et al., 1995), antimalarial (Diego et al., 2011) and anticancer (Dos Santos et al., 2016; Silva et al., 2017; de Santana et al., 2018) activities.
One particular class of thiazole derivatives, the heterocyclic 2-aminothiazoles and their derivatives, are notable as fundamental intermediates for the synthesis of numerous biologically active compounds which include sulfur drugs, fungicides, biocides and intermediates in the synthesis of antibiotics, where many 2-aminothiazoles have been substituted with dissimilar groups for pharmaceutical applications purposes (Geronikaki et al., 2009; Papadopoulou et al., 2005; Kreutzberger and Tantawy, 1981).
Moreover, compounds incorporating the acetamide linkage exhibit a wide range applications and are well-recognized as chemotherapeutic agents (McCarthy et al., 2009; Liu et al., 2012). It's known that the acetamide functional group is responsible for inhibition of platelet aggregation (Xiang et al., 2018), and strong antimicrobial (Berest et al., 2011; Shams et al., 2011), anti-inflammatory (Dogruer et al., 2004; Raghavendra et al., 2012), antioxidant (Autore et al., 2010; Ley and Bertram, 2001) and urease inhibitory activities (Gull et al., 2016).
In cellular functions, dihydrofolate reductase (DHFR) is an enzyme that catalyzes the NADPH-dependent reduction of 7,8-dihydrofolate (DHF) to 5,6,7,8-tetrahydrofolate (THF): DHF + NADPH + H+ → THF + NADP+, which is the precursor of the co-factors required for the biosynthesis of purine nucleotides, thymidine (precursor for DNA replication) and numerous amino acids (Blakley, 1984). Thus, inhibition of dihydrofolate reductase can lead to the disruption of DNA synthesis and the rapid death of proliferating cells (Blakley, 1984; Brown and Kraut, 1992). Additionally, bacteria also need DHFR to grow and multiply and therefore inhibitors with high specificity and ability to discriminate between bacterial and host DHFR have found usage as antibacterial agents (Hawser et al., 2006). These two noteworthy features render the DHFR enzyme as a key target for both antimicrobial and antitumor drug design (Hussein et al., 2019a; Hussein et al., 2019b).
Based on these previous findings, we projected that combining the 2,7-dichlorofluorene moiety with the versatile thiazole ring bearing acetamide pharmacophores into a single chemical entity could produce competent antimicrobial and anticancer hybrids with highly enhanced biological activities. Thus, in continuation of our previous studies on the synthesis, antimicrobial and anticancer properties of bioactive organic molecules (Hussein et al., 2019a, 2019b, 2015a, 2015b, 2016), herein we report the synthesis of some novel (4-(2,7-dichloro-9H-fluoren-4-yl)thiazol-yl)acetamide derivatives and evaluate their cytotoxic activities against human lung carcinoma (A-549) and human breast carcinoma (MCF-7) cell lines. In addition, antimicrobial evaluations and molecular docking studies for the synthesized compounds have been carried out.
2 Experimental
2.1 Chemistry
2.1.1 General methods
All Chemicals and solvents used were purchased from Sigma-Aldrich (spectroscopic grade) and were used without further purifications. Melting points were determined on a Stuart SMP3 melting point apparatus and are uncorrected. FT-IR spectra were recorded on a Shimadzu IR-3600 FT-IR spectrometer using KBr pellets. NMR spectra were acquired on a Bruker Avance 500 instrument (500 MHz for 1H, 125 MHz for 13C) in DMSO‑d6 solutions, using residual solvent signals as internal standards. The starting materials, 2,7-dichloro-9H-fluorene (2) and 2-chloro-1-(2,7-dichloro-9H-fluoren-4-yl)ethanone (3) were prepared according to our previously reported method (Hussein et al., 2019b). 2-chloro-N-arylacetamides 7a-j were prepared according to our previously reported method (Hussein et al., 2019a).
2.1.2 Synthesis of 4-(2,7-dichloro-9H-fluoren-4-yl)thiazol-2-amine (4)
A mixture of chloroacetyl derivative 3 (12.45 g, 40 mmol) and thiourea (3.80 g, 50 mmol) in ethanol (200 mL) was refluxed for 3 h. The reaction mixture was cooled and neutralized with saturated aqueous solution of sodium bicarbonate. The obtained solid product was filtered off, washed with cold water (3 × 50 mL), then with cold ethanol (3 × 10 mL), dried and recrystallized from ethanol to afford 12.90 g (97%) of pure 2-aminothiazole derivative 4 as pale yellow crystals, mp 199–200 °C. FT-IR (KBr): ν (cm−1) 3282, 3106 (NH2), 1639 (C⚌N); 1H NMR (DMSO‑d6): δ 7.66 (s, 1H, Flu-H), 7.63 (s, 1H, Flu-H), 7.55 (d, 1H, J = 5.5 Hz, Flu-H), 7.37 (s, 1H, Flu-H), 7.31 (d, 1H, J = 5.5 Hz, Flu-H), 7.18 (s, 2H, NH2), 6.77 (s, 1H, thiazolyl-H), 4.00 (s, 2H, CH2); 13C NMR (DMSO‑d6): δ 168.8 (C⚌N), 148.9 (C), 146.7 (C), 146.3 (C), 139.1 (C), 136.9 (C), 133.5 (C), 131.9 (C), 131.2 (C), 128.7 (CH), 126.9 (CH), 125.3 (CH), 125.1 (CH), 124.9 (CH), 105.5 (thiazole-CH), 36.7 (CH2).
2.1.3 Synthesis of 2-chloro-N-(4-(2,7-dichloro-9H-fluoren-4-yl)thiazol-2-yl)acetamide (5)
A mixture of 2-aminothiazole derivative 4 (10.0 g, 30 mmol) and chloroacetyl chloride (2.5 mL, 30 mmol) in DMF (10 mL) was stirred at room temperature for 3 h. The reaction mixture was poured onto ice–water. The solid obtained was filtered off, dried and recrystallized from ethanol to give 11.40 g (93%) of chloroacetyl derivative 5 as pale yellow crystals, mp 190–192 °C. FT-IR (KBr): ν (cm−1) 3190 (NH), 1670 (C⚌O), 1619 (C⚌N); 1H NMR (DMSO‑d6): δ 9.34 (s, 1H, NH), 7.96 (s, 1H, Flu-H), 7.78 (s, 1H, Flu-H), 7.52 (s, 1H, Flu-H), 7.39 (d, 1H, J = 6.5 Hz, Flu-H), 7.21 (d, 1H, J = 6.5 Hz, Flu-H), 7.05 (s, 1H, thiazolyl-H), 4.44 (s, 2H, CH2CO), 4.02 (s, 2H, CH2); 13C NMR (DMSO‑d6): δ 170.3 (C⚌O), 162.8 (C⚌N), 146.7 (C), 146.3 (C), 146.2 (C), 139.1 (C), 138.0 (C), 137.5 (C), 135.1 (C), 131.9 (C), 127.8 (CH), 126.5 (CH), 125.3 (CH), 125.1 (CH), 113.3 (CH), 107.2 (thiazole-CH), 43.5 (CH2), 36.9 (CH2).
2.1.4 Synthesis of N-(4-(2,7-dichloro-9H-fluoren-4-yl)thiazol-2-yl)-2-(arylamino)acetamides 6a-j
A mixture of chloroacetyl derivative 5 (0.41 g, 1 mmol) and different aryl amines (1 mmol) in absolute ethanol (15 mL) was refluxed for 5–6 h. The reaction mixture was concentrated under reduced pressure, the solid obtained was filtered off, washed with n-hexane (3 × 10 mL), dried and recrystallized from ethanol to give the title products 6a-j.
2.1.4.1 N-(4-(2,7-dichloro-9H-fluoren-4-yl)thiazol-2-yl)-2-(phenylamino)acetamide (6a)
Pale yellow crystals, yield 93%, mp 117–119 °C; FT-IR (KBr): ν (cm−1) 3349 (NH), 3197 (NH), 3054 (CH arom.), 2923 (CH aliph.), 1685 (C⚌O), 1602 (C⚌N); 1H NMR (DMSO‑d6): δ 7.70 (s, 1H, Flu-H), 7.65 (s, 1H, Flu-H), 7.59 (s, 1H, NH), 7.43 (s, 1H, Flu-H), 7.40 (d, J = 5.5 Hz, 1H, Flu-H), 7.25 (d, J = 5.5 Hz, 1H, Flu-H), 7.13–7.11 (m, 2H, Ph-H), 7.00 (s, 1H,NH), 6.63–6.61 (m, 3H, Ph-H), 6.06 (s, 1H, thiazolyl-H), 4.07 (s, 2H, CH2), 4.01 (s, 2H, CH2); 13C NMR (DMSO‑d6): δ 170.5 (C⚌O), 158.2 (C⚌N), 148.5 (C), 147.5 (C), 146.7 (C), 146.2 (C), 138.8 (C), 137.0 (C), 132.7 (C), 132.2 (C), 131.6 (C), 129.4 (CH), 129.2 (CH), 127.0 (CH), 125.5 (CH), 124.6 (CH), 119.5 (CH), 116.9 (CH), 112.7 (CH), 46.5 (CH2), 36.9 (CH2).
2.1.5 Synthesis of 2-(4-(2,7-dichloro-9H-fluoren-4-yl)thiazol-2-ylamino)-N-arylacetamides 8a-j
A mixture of aminothiazole 4 (0.33 g, 1 mmol) and 2-chloro-N-arylacetamides 7a-j (1 mmol) and triethylamine (0.1 mL) in absolute ethanol (15 mL) was refluxed for 6–9 h. The reaction mixture was cooled to room temperature, the solid obtained was filtered off, washed with cold ethanol, dried and recrystallized from ethanol to give the title products 8a-j.
2.1.5.1 2-(4-(2,7-Dichloro-9H-fluoren-4-yl)thiazol-2-ylamino)-N-phenylacetamide (8a)
Pale pink crystals, yield 81%, mp 160–162 °C; FT-IR (KBr): ν (cm−1) 3267 (NH), 3199 (NH), 3084 (CH arom.), 2930 (CH aliph.), 1671 (C⚌O), 1636 (C⚌N); 1H NMR (DMSO‑d6): δ 10.2 (s, 1H, NH), 7.64–7.62 (m, 3H, Flu-H), 7.51 (d, J = 10.0 Hz, 1H, Flu-H), 7.40–7.35 (m, 3H, Ph-H), 7.31 (d, J = 10.0 Hz, 1H, Flu-H), 7.19–7.17 (m, 2H, Ph-H), 6.77 (s, 1H, thiazolyl-H), 4.28 (s, 2H, CH2), 3.99 (s, 2H, CH2), 3.51 (s, 1H, NH); 13C NMR (DMSO‑d6): δ 168.9 (C⚌O), 165.2 (C⚌N), 148.6 (C), 146.8 (C), 146.1 (C), 139.1 (C), 137.9 (C), 136.9 (C), 133.5 (C), 131.6 (C), 131.4 (C), 129.2 (CH), 128.5 (CH), 127.3 (CH), 126.9 (CH), 125.8 (CH), 125.0 (CH), 122.8 (CH), 121.3 (CH), 105.6 (CH), 43.9 (CH2), 36.8 (CH2).
2.2 Antimicrobial screening
The antimicrobial activity was evaluated by the disc-agar diffusion method (Hay et al., 2000; Wilson, 2000; Saintigny et al., 2002), at 50-µg/disk concentration. Samples under testing were dissolved in dimethyl sulfoxide (DMSO) to 10 mg/mL. The disc (6 mm in diameter) containing the sample at certain concentration, are placed on the agar medium, which was seeded previously with 0.2 mL of broth culture of each organism for 18 h. Discs were incubated at 37 °C for 24 h for bacteria (and at 22 °C for 48 h; for fungi) and the zones of inhibition (ZI) determined in mm.
Efficacy of the novel compounds was evaluated against different strains of bacteria either gram-positive such as St. aureus (RCMB010010), and B. subtilis RCMB 015 (1) NRRL B-543 or gram-negative such as E. coli (RCMB 010052) ATCC 25955, and P. vulgaris RCMB 004 (1) ATCC 13315 (the reference drug is Gentamycin) as well as fungal strains include Aspergillus fumigatus (RCMB 002008), and Candida albicans RCMB 005003 (1) ATCC 10231 (the reference drug is Ketoconazol).
2.3 In vitro anticancer screening
The newly synthesized compounds were screened against human lung carcinoma (A-549) and breast carcinoma (MCF-7) by the Regional Center for Mycology and Biotechnology, Al-Azhar University as reported previously (Skehan et al., 1990). Cells were seeded for 24 h in ninety-six well plate (1 × 104 cells/well) in growth medium with 100-µL concentration. Then, fresh medium, which involve test sample with various concentrations, was added. Moreover, serial two-fold dilutions of the tested compound were added, flat-bottomed microtiter plates (Falcon, NJ, USA) using a multichannel pipette that incubated for 48 h at 37 °C and in presence of 5% carbon dioxide. Test samples were not added to the control cells. After incubation, different concentrations of sample (500, 250, 125, 62.5, 31.25, 15.6, 7.8, 3.9, 2 & 1 µg/L) were added, and continued the incubation for 48 h and viable cells were determined by a colorimetric method in which crystal violet solution (1%) was added to every well for 30 min. The excess stain was removed using tap water. After that, addition of well-mixed 30% glacial acetic acid, followed by gentle shaking on Microplate reader (TECAN, Inc.) and by using test wavelength of 490 nm, the absorbance of the plates was measured. Experiments were carried out in triplicates. The concentration of the compound that inhibits the tumor cell growth by 50% was determined (IC50 in µM/L) and the results summarized in Table 3. The reference control was 5-Fluorouracil (5-FU).
2.4 Docking assay
The study was performed in the Department of Pharmaceutical Organic Chemistry, Faculty of Pharmacy (Girls), Al‐Azhar University, Egypt, with computational software using Protein Data Bank, 5-Fluorouracil (PDB ID: 1BID) while dihydrofolate reductase (DHFR) (PDB ID: 4DFR) to predict the anticancer activity of the newly tested compounds.
3 Results and discussion
3.1 Chemistry
The current study deals with the synthesis of some acetamide derivatives incorporating different aryl substituents (tails) conjugated with biologically active 2,7-dichlorofluorene and thiazole moieties in order to assess their combined influence on the antimicrobial, anticancer activities, and study their structure–activity relationship (SAR).
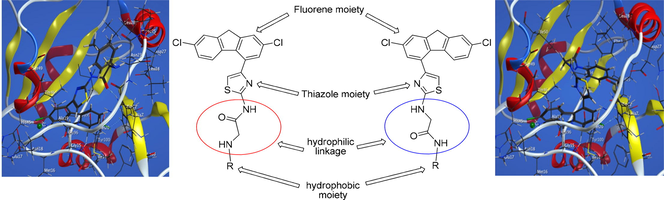
As the DHFR inhibition is considered one of the most prominent mechanism responsible for antimicrobial and anticancer activities (Bush et al., 1982; Rao and Venkatachalam, 1999; Patel et al., 2017), the synthesized compounds were designed in order to fulfill the following criteria: (i) inclusion of pharmacophores that may act as DHFR inhibitors; (ii) bearing of hydrophilic and hydrophobic moieties that can interact with the hydrophilic and hydrophobic regions of the DHFR active site, respectively, as presented in Fig. 1.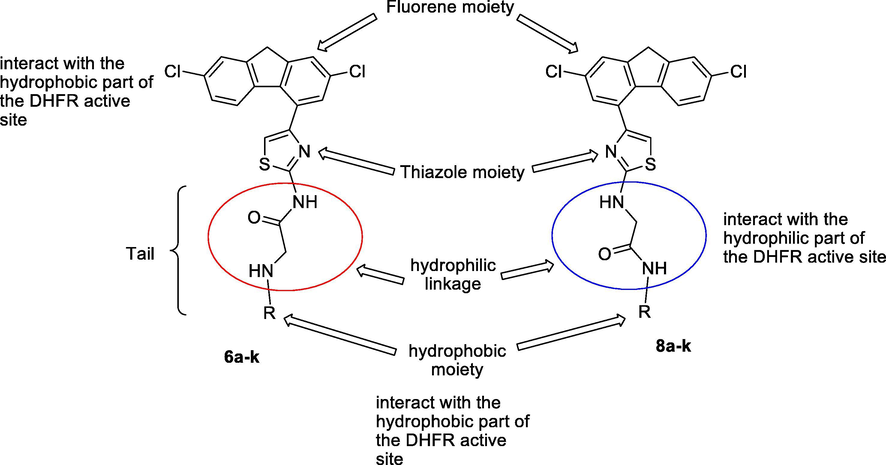
Structural elements of DHFR inhibitors in the DHFR enzymatic active site.
The main strategy for the synthesis of the target compounds 6a-j and 8a-j is outlined in Scheme 1. The synthetic approach begins with a simple and convenient approach to prepare 2,7-dichlorofluorene (2) which involves direct chlorination of fluorene (1) with NCS in a mixture of acetic acid AcOH and hydrochloric acid (HCl). Chloroacetylation of 2 is then accomplished in moderate to excellent yields by adding a solution of 2 in dichloromethane (DCM) at 0–5 °C to a suspension of chloroacetyl chloride and aluminum chloride in dichloromethane according to our previously reported method (Hussein et al., 2019b) after minor modification. As for the preparation of 2-aminothiazoles, and on account of their importance, numerous synthetic strategies have been formerly described. Hantzsch reaction of α-halocarbonyl compounds with thiourea affords an advantageous method for the synthesis of thiazoles (Hantzsch and Weber, 1887). Thus, 4-(2,7-Dichloro-9H-fluoren-4-yl)thiazol-2-amine (4) has been assembled through Hantzsch reaction of 2-chloro-1-(2,7-dichloro-9H-fluoren-4-yl)ethanone (3) with thiourea in refluxing ethanol. The aminothiazole derivatives 4 were converted to the corresponding chloroacetamide derivatives (5) in excellent yield by reaction with chloroacetyl chloride in dimethylformamide (DMF) at room temperature. The target compounds N-(4-(2,7-dichloro-9H-fluoren-4-yl)thiazol-2-yl)-2-(arylamino)acetamides 6a-j formed in good to excellent yields (67–93%) by refluxing chloroacetamide derivative 5 with arylamines (namely; aniline, 4-methylaniline, 4-chloroaniline, 4-bromoaniline, 4-methoxyaniline, 4-nitroaniline, 4-aminobenzoic acid, ethyl 4-aminobenzoate, 1-naphthyl amine and 2-naphthyl amine) in absolute ethanol for 4–7 h.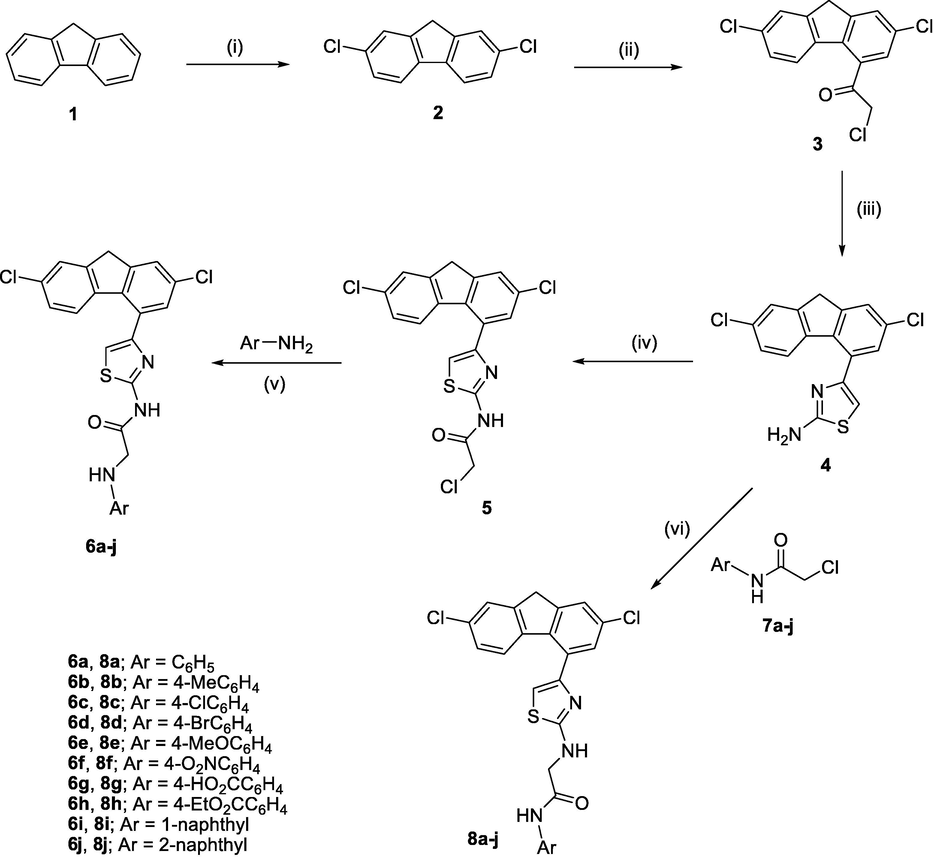
Synthetic routes to the target compounds 6a-j and 8a-j. Reagents and conditions: (i) NCS, AcOH/ HCl, rt; (ii) ClCH2COCl, AlCl3, DCM, 0–5 °C; (iii) thiourea, EtOH, reflux, then aq. NaHCO3; (iv) ClCH2COCl, DMF, rt; (v) EtOH, reflux; (vi) EtOH/ Et3N, reflux.
On the other hand, 2-chloro-N-arylacetamides 7a-j were promptly prepared by the reaction of arylamines (namely; aniline, 4-methylaniline, 4-chloroaniline, 4-bromoaniline, 4-methoxyaniline, 4-nitroaniline, 4-aminobenzoic acid, ethyl 4-aminobenzoate, 1-naphthyl amine and 2-naphthyl amine) with chloroacetyl chloride in dimethylformamide at room temperature.
Reaction of 2-chloro-N-arylacetamides 7a-j with 4-(2,7-dichloro-9H-fluoren-4-yl)thiazol-2-amine (4) in ethanol under refluxing conditions yielded the target compounds 2-(4-(2,7-dichloro-9H-fluoren-4-yl)thiazol-2-ylamino)-N-arylacetamides 8a-j in excellent yields (72–92%). The reactions were activated by addition of catalytic amount of triethylamine (TEA) as a basic catalyst with a reaction period of 5–7 h. The chemical structures of all synthesized compounds 4, 5 and 6a-j and 8a-j were well-established on the basis of spectroscopic data such as FT-IR, 1H NMR, 13C NMR, and DEPT-135 data (c.f. experimental section and supporting information).
The FT-IR spectra of compounds 6a-j showed the characteristic absorption bands at 3376–3343 and 3203–3191 cm−1 for the two NH groups, 1689–1672 cm−1 for the C⚌O groups, and 1632–1600 cm−1 for the C⚌N groups. Compounds 6g and 6h showed additional absorption bands at 1690 and 1744 cm−1 corresponding to the carbonyl of the carboxylic and the ester groups, respectively. Furthermore, to fully elucidate the chemical structures of all products, intensive 1D (1H, 13C, and DEPT-135) NMR studies were conducted in DMSO‑d6. As a representative example, the 13C and 13C-DEPT-135 NMR spectra of 6a showed 22 signals (10 aromatic quaternary carbons, 9 aromatic CH’s, two methylene carbons, and one carbonyl carbon). Its 1H NMR spectrum showed three singlets at δ 7.70, 7.65, and 7.43 ppm, two doublets at 7.40 and 7.25 ppm (J = 5.5 Hz) for five protons of the fluorene moiety. Two multiplets at δ 7.13–7.11 and 6.63–6.61 ppm are corresponding to five protons of the phenyl ring. The thiazole ring proton appeared as a singlet at δ 6.06 ppm. In addition, two singlets appeared at 7.59 and 7.00 ppm for the two NH protons, and two other singlets corresponding to the two methylene protons resonated at δ 4.07 and 4.01 ppm.
Furthermore, the FT-IR spectra of compounds 8a-j displayed characteristic absorption bands at 3317–3257 and 3204–3119 cm−1 for two NH groups, 1694–1665 cm−1 for (C⚌O) groups, and 1638–1624 cm−1 for C⚌N groups. As a typical example, NMR analysis of the 13C and 13C-DEPT-135 NMR spectra of 8h indicated the presence of 25 signals (11 aromatic quaternary carbons, 8 aromatic CH’s, 3 methylene carbons, 2 carbonyl carbons, and one methyl carbon). Its 1H NMR spectrum showed two singlets at δ 4.07 and 4.01 ppm (NCH2 protons) and δ 8.18, 7.28 ppm for two fluorene protons, two multiplets at δ 7.61–7.59 and δ 7.34–7.32 ppm for three fluorene and four phenyl protons. A singlet appearing at δ 6.75 ppm corresponds to a proton of the thiazole ring. In addition, two NH protons appeared as two singlets at δ 6.12 and 4.31 ppm. Additionally, two singlet at δ 3.98 and 3.84 ppm correspond to two methylene protons. The ester group protons appeared as a quartet and a triplet signals at δ 4.70 and 2.82 ppm (J = 7.5 Hz), respectively.
3.2 Biological activity
3.2.1 Antimicrobial activity
The target compounds presented in this investigation were evaluated for antimicrobial activity against different strains of bacteria, either Gram-positive (St. aureus (RCMB010010), and B. subtilis RCMB 015 (1) NRRL B-543) or Gram-negative (E. coli (RCMB 010052) ATCC 25955, and P. vulgaris RCMB 004 (1) ATCC 13315 (the reference drug is Gentamycin)), as well as fungal strains which include Aspergillus fumigatus (RCMB 002008), and Candida albicans RCMB 005003 (1) ATCC 10231 (the reference drug is Ketoconazol).
The results of the antimicrobial assay of the synthesized compounds is given in Table 1 and Fig. 2. It is noted that some compounds have proven highly effective inhibitors of different types of bacteria and fungi. Acetamide derivatives 8c and 8i exhibited promising activity against P. vulgaris with zone of inhibition (ZI) value of 18 mm, while, compound 6i bearing naphthyl-1-amino acetamide moiety is approximately equipotent to the reference drug against C. Albicans. However, compounds 6i, 6j, 8c, 8a, 8h, 8e, 8i and 8b showed excellent activity against A. fumigatus. From the previous results it is noteworthy that both compounds 8c and 8i are active as antimicrobial agents (have higher antibacterial and antifungal activities), while compounds 6i, 6j, 8a, 8b, 8e and 8h have only antifungal activity. This underscores the selectivity of the synthesized compounds towards bacteria and fungi which bestows significant importance to these compounds for medicinal applications.
Comp. No.
Gram (+ve) Bacteria
Gram (-ve) Bacteria
Fungi
S. aureus
B. subtilis
E. coli
P. vulgaris
A. fumigatus
C. albicans
6a
---
---
---
---
---
---
6b
---
---
---
---
---
---
6c
---
---
---
---
12
---
6d
---
---
---
---
13
---
6e
---
---
---
---
11
---
6f
---
---
---
---
15
---
6g
---
---
---
---
---
---
6h
---
---
---
---
---
---
6i
14
15
12
17
30
17
6j
---
8
---
12
27
---
8a
---
15
14
17
22
10
8b
---
16
13
16
18
12
8c
12
20
16
18
25
11
8d
---
12
---
---
---
---
8e
---
16
13
14
20
12
8f
---
14
---
---
---
---
8g
---
---
---
---
---
---
8h
---
19
12
16
22
10
8i
10
17
11
18
20
12
8j
15
17
12
---
---
---
Sta
24
26
30
25
17
20
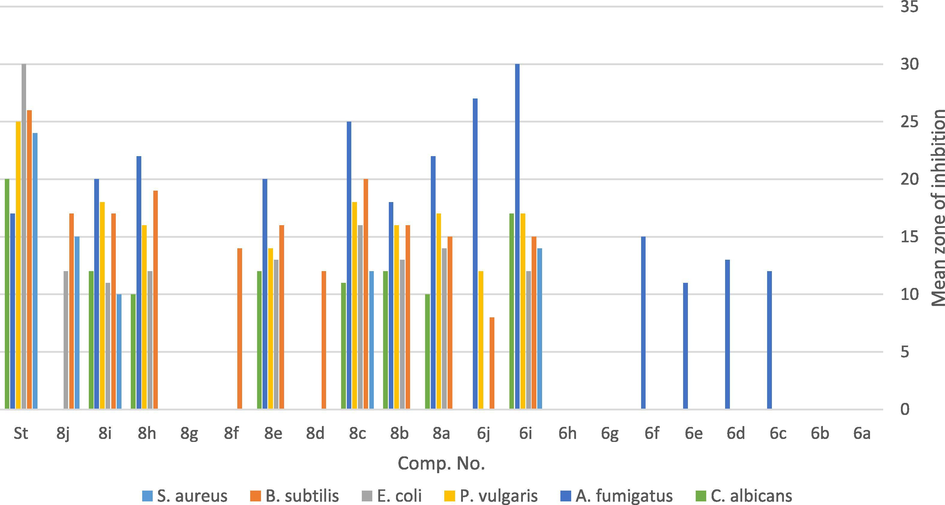
Comparison of the antimicrobial activity of the newly synthesized compounds 6a-j and 8a-j.
3.2.2 In vitro anticancer activity
According to the tabulated results listed in Table 2, the high efficiency of the synthesized target compounds displayed against cancer cell lines such as human lung (A-549 and MCF-7) it’s a noteworthy observation. For example, compound 6j having naphthalen-2-amino acetamide moiety exhibited IC50 7.67 µM and 9.53 µM, respectively, and compound 8a bearing phenylacetamido group produced IC50 values of 11.43 µM and 13.34 µM, respectively. While, compound 6h incorporating 4-(ethoxycarbonyl)phenylamino acetamide moiety displayed IC50 values of 13.00 µM and 27.49 µM, respectively. Furthermore, compound 8b having p-tolylacetamide moiety showed IC50 values of 13.30 µM and 15.02 µM, respectively. On the other hand, compound 6f having 4-nitrophenyl amino acetamide moiety showed IC50 values of 19.75 µM and 39.31 µM, respectively. Compound 8c having 4-chlorophenyl acetamide moiety showed IC50 values of 23.47 µM and 18.71 µM, respectively. Furthermore, compound 8i having 1-naphthyl acetamide moiety revealed IC50 values of 27.30 µM and 57.70 µM, respectively. While, compound 8h having 4-(ethoxycarbonyl)phenyl acetamide moiety showed IC50 values of 28.85 µM and 48.80 µM, respectively. IC50 value: concentration causing 50% inhibition of cell viability.
Comp.
A-549
MCF-7
IC50a (μg/mL)
IC50a (μM)
IC50a (μg/mL)
IC50a (μM)
6a
247
529.61
229
491.02
6b
234
487.08
228
474.59
6c
114
227.63
188
375.38
6d
62
113.70
107
196.23
6e
23.5
47.34
33.6
67.69
6f
10.1
19.75
20.1
39.31
6g
99.6
195.14
82.4
161.45
6h
7
13.00
14.8
27.49
6i
24
46.47
29.4
56.93
6j
3.96
7.67
4.92
9.53
8a
5.49
11.43
6.41
13.34
8b
6.66
13.30
7.52
15.02
8c
12.8
23.47
10.2
18.71
8d
90.3
181.91
61.1
123.08
8e
18.3
35.79
27.1
52.99
8f
>500
1036.53
>500
1036.53
8g
202
375.16
234
434.59
8h
14.9
28.85
25.2
48.80
8i
14.1
27.30
29.8
57.70
8j
371
772.26
>500
1040.78
5FU
43.9
337.48
27.8
213.71
However, compound 8e bearing 4-methoxyphenyl acetamide moiety showed relatively higher IC50 values of 35.79 µM and 52.99 µM, respectively. Compound 6i having naphthalen-1-amino acetamide moiety showed IC50 values of 46.47 µM and 56.93 µM, respectively. Compound 6e having 4-methoxyphenyl amino acetamide moiety showed IC50 values of 47.34 µM and 67.69 µM, respectively. Moreover, compound 6d having 4-bromophenyl amino acetamide moiety showed IC50 values of 113.70 µM and 196.23 µM, respectively. Compound 8d having 4-bromophenyl acetamide moiety showed IC50 values of 181.91 µM and 123.08 µM, respectively. Finally, compound 6g having 4-carboxyphenyl amino acetamide moiety showed IC50 values of 195.14 µM and 161.45 µM, respectively.
Based on the tabulated results shown in Table 2, it is noted that, the cytotoxic activity of the investigated compounds against human lung carcinoma (A-549) follow the order: 6j > 8a > 6h > 8b > 6f > 8c > 8i > 8h > 8e > 6i > 6e > 6d > 8d > 6g > 6c > 5-FU > 8g > 6b > 6a > 8j > 8f (Fig. 3). However, the cytotoxic activity of the tested compounds against Human breast carcinoma cell line (MCF-7) followed the order: 6j > 8a > 8b > 8c > 6h > 6f > 8h > 8e > 6i > 8i > 6e > 8d > 6g > 6d > 5-FU > 6c > 8g > 6b > 6a > 8f > 8j (Fig. 3). The preceding data concerning the biological screening for the synthesized compounds helped identify the relevant structural motifs as necessary features required in the investigated compounds to produce optimum enhancement in anticancer properties and render such species effective anticancer agents. Further, it is worth highlighting the biological screening results obtained with compounds 8c and 8i which clearly exhibit dual biological activities as they are active as antimicrobial and anticancer agents.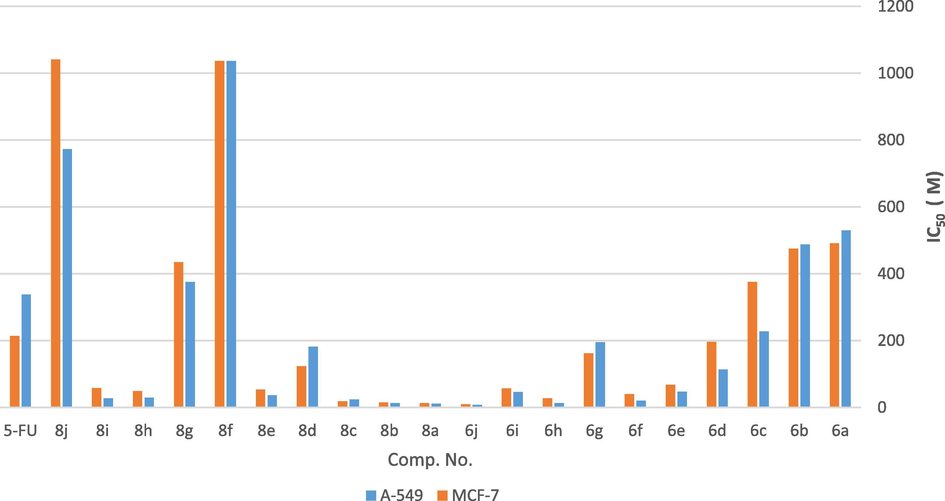
Comparison of the cytotoxic activity of the newly synthesized compounds 6a-j and 8a-j against human lung carcinoma (A-549) and human breast carcinoma (MCF-7).
3.2.3 Docking and molecular modeling study
The most prominent enzymes used for the development of anticancer and antimicrobial agents are thymidylate synthase and dihydrofolate reductase (DHFR) (Rao and Venkatachalam, 1999; Du et al., 2013). Molecular modeling is considered as an outstanding way to study molecular interactions and binding site. In the present work, Molecular Operating Environment (MOE) module was adapted to verify the cytotoxic potency of the tested compounds (Vilar et al., 2008). Moreover, study of molecular docking aids in explaning how compounds behave through their reaction with the enzyme’s active sites. Twenty active anticancer compounds 6a-j and 8a-j were subjected to docking using Molecular Operating Environment (MOE) program on the 3D structure of dihydrofolate reductase (DHFR). Furthermore, it was used to demonstrate the enzyme interactions with its substrate doxorubicin (DOX). Binding free energy data obtained after the docking procedure revealed that the above compounds exhibit favorable docked complexes with the target. Docking was performed for the synthesized compounds 6a-j and 8a-j on DHFR to predict their activity as anticancer agents (c.f. SI file). The tested compounds garnered interactions with the DHFR active sites to different extents. Compounds 8h, 6g, 8g, 8b, 6h and 6j produced cytotoxic activity by inhibiting the active sites of DHFR (Table 3). Docking score energy of the tested compounds found to follow this order: 8h > 6g > 8g > 8b > 6h > 6j > 6c > 6d > 6e > 8f > 6f > 8j > 8e > 8d > 6b > 8i > 6i > 8c > 8a > 6a, as shown in Fig. 4. Score: lower scores are more favorable. The unit for scoring functions is kcal/mol. E-conf: energy of the conformer. E-place: Score from the placement stage. E-score 1: Score from the first rescoring stage. E-score 2: Score from the second rescoring stage. E-refine: Score from the refinement stage.
Comp. No.
Score
E_conf
E_place
E_score1
E_score2
E_refine
6a
−7.2787
11.8659
−86.1684
−9.8410
−7.2787
−44.9553
6b
−7.5857
19.1284
−54.1995
−10.3997
−7.5857
−34.3523
6c
−7.7689
17.0702
−56.2519
−10.7120
−7.7689
−38.0747
6d
−7.7625
24.7109
−94.0857
−9.4051
−7.7625
−48.2892
6e
−7.7217
17.8309
−76.4011
−10.4083
−7.7217
−47.0829
6f
−7.6817
56.2074
−76.6499
−10.8973
−7.6817
−39.0149
6g
−8.2413
−51.8593
−84.6688
−12.7100
−8.2413
−56.2588
6h
−7.8777
25.0914
−38.1129
−10.3773
−7.8777
−36.7423
6i
−7.5599
38.3409
−70.3623
−11.1607
−7.5599
−45.3069
6j
−7.8543
20.4799
−47.9571
−10.9165
−7.8543
−40.1227
8a
−7.3022
−50.3011
−74.7956
−10.3966
−7.3022
−38.7188
8b
−7.9480
−56.3332
−40.1580
−10.7623
−7.9480
−39.5560
8c
−7.4879
−58.3923
−63.1983
−9.7065
−7.4879
−46.3605
8d
−7.5962
−46.4119
−66.0624
−10.5870
−7.5962
−37.5610
8e
−7.6346
−58.2125
−42.5277
−10.8762
−7.6346
−45.6290
8f
−7.7121
−17.3436
−51.2697
−11.6343
−7.7121
−36.2258
8g
−8.2263
−114.3670
−52.3371
−13.8340
−8.2263
−46.2320
8h
−8.2943
−36.7831
−44.7879
−10.8018
−8.2943
−42.2573
8i
−7.5817
−42.5465
−66.4027
−10.4089
−7.5817
−46.4740
8j
−7.6730
−56.4827
−60.1864
−10.8810
−7.6730
−45.5533
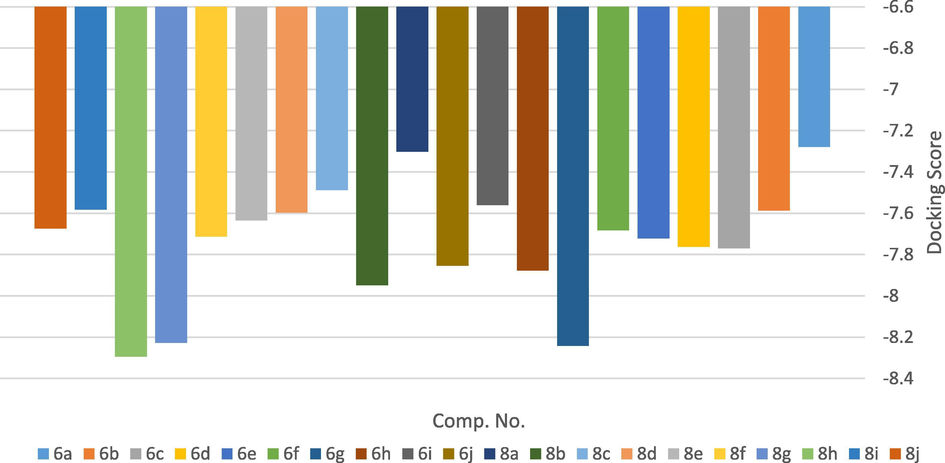
Docking Score energy of the tested compounds 6a-j and 8a-j.
3.2.3.1 Docking study of 6j and 8a in the DHFR active sites
The active site docking studies of 6j having naphthalen-2-amino acetamido group showed that (N) atom acted as a H-bond donor with Ser 49 (3.26 Å) with energy −1.7 kcal/mol. However, there are several hydrophobic interactions with: Phe 31, Leu 28, Leu54, Lys 32, Ile 50, Ala 19, Met 16, His 45, Glu 17, Thr 46, Asn 18, Gly 15, Gly 96, Ala 6, Ile 14, tyr 100, Ala 7, Leu 8, Met 20, Arg 52.
However, the active site docking studies of 8a bearing phenylacetamido group showed that (O) atom of (C⚌O) group acted as a H-bond acceptor with Arg 52 (3.14 Å) having energy −1.1 kcal/mol. Moreover, it showed an arene-H interaction between the phenyl ring and Thr 46 (3.88 Å) having energy −0.6 kcal/mol. Additionally, there are various hydrophobic interactions with; Pro 55, Lys 32, Ile 5, Phe 31, Leu 28, Ala 6, Ala 7, Tyr 100, Ile 94, Gly 15, Ser 49, Met 16, Ile 50, His 45, Asp 27, Leu 54 (Fig. 5).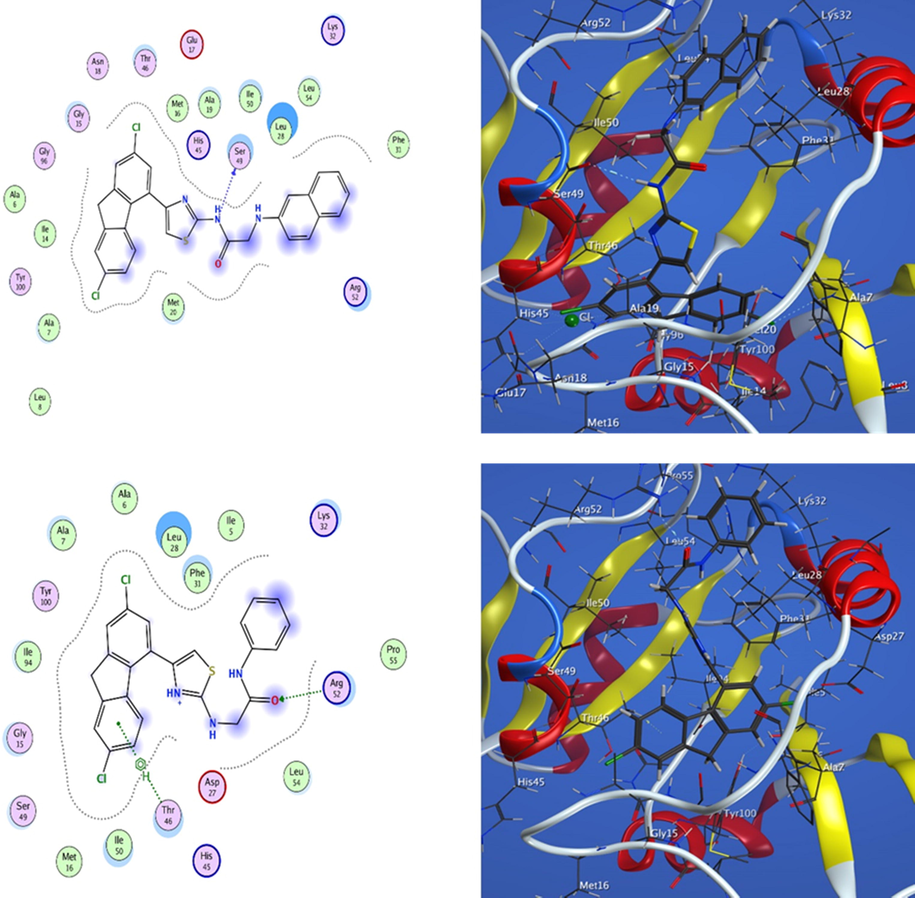
Docking of compounds 6j and 8a into DHFR active sites.
4 Conclusion
In this report, the synthesis and characterization of a novel series of (4-(2,7-dichloro-9H-fluoren-4-yl)thiazol-yl)acetamide derivatives have been achieved. Most of the newly synthesized compounds exhibited potent anticancer activity against human lung carcinoma (A-549) and human breast carcinoma (MCF-7) cell lines compared with 5-Fluorouracil which used as a reference drug in this study. Furthermore, the synthesized compounds were evaluated as antimicrobial agents where several showed remarkable activity as antibacterial and antifungal agents. A dual biological activity of some investigated compounds against microbial strains and cancer cell lines has been observed. The results indicated that 2-(4-(2,7-dichloro-9H-fluoren-4-yl)thiazol-2-ylamino)-N-arylacetamides such as 2-(4-(2,7-Dichloro-9H-fluoren-4-yl)thiazol-2-ylamino)-N-phenylacetamide (8a), 2-(4-(2,7-Dichloro-9H-fluoren-4-yl)thiazol-2-ylamino)-N-p-tolylacetamide (8b), and N-(4-chlorophenyl)-2-(4-(2,7-dichloro-9H-fluoren-4-yl)thiazol-2-ylamino)acetamide (8c) are more efficacious antimicrobial and anticancer agents compared to N-(4-(2,7-dichloro-9H-fluoren-4-yl)thiazol-2-yl)-2-(arylamino)acetamide derivatives. The findings described in this work will open a new alternative area in the field of medicinal chemistry of fluorene derivatives that can be considered as multi-addressable pharmacophores.
Acknowledgments
The authors are highly indebted to the Deanship of the Scientific Research (DSR), Umm Al-Qura University, Saudi Arabia for the financial support of this work through project number 18-SCI-1-01-0009. S. A. Ahmed is highly indebted to Alexander von Humboldt Foundation (AvH) and Prof. Dr. Karola Rück-Braun, Technical University Berlin (TU-Berlin), Germany for their endless help and support.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Photochromism of dihydroindolizines. Part XX: Synthesis and photophysical behavior of fluorenyldihydro-indolizines photochromes based “Click” chemistry strategy. J. Photochem. Photobiol. A.. 2016;328:163-170.
- [Google Scholar]
- Photochromism of dihydroindolizines Part XIX. Efficient one-pot solid-state synthesis, kinetic, and computational studies based on dihydroindolizine photochromes. J. Phys. Org. Chem.. 2017;30:e3614
- [Google Scholar]
- Ammonium chloride catalyzed synthesis of novel Schiff bases from spiro[indoline-3,4′-pyran]-3′-carbonitriles and evaluation of their antimicrobial and anti-breast cancer activities. SpringerPlus. 2016;5:887.
- [Google Scholar]
- Acetamide derivatives with antioxidant activity and potential anti-inflammatory activity. Molecules. 2010;15:2028-2038.
- [Google Scholar]
- Phenethylthiazolethiourea (PETT) compounds, a new class of HIV-1 reverse transcriptase inhibitors. 1. Synthesis and basic structure-activity relationship studies of PETT analogs. J. Med. Chem.. 1995;38:4929-4936.
- [Google Scholar]
- Synthesis and biological activity of novel N-cycloalkyl-(cycloalkylaryl)-2-[(3-R-2-oxo-2H-[1,2,4]triazino[2,3-c]quinazoline-6-yl)thio]acetamides. Eur. J. Med. Chem.. 2011;46:6066-6074.
- [Google Scholar]
- Synthesis, anti-bacterial and anti-fungal activities of some novel Schiff bases containing 2,4-disubstituted thiazole ring. Eur. J. Med. Chem.. 2010;45:651-660.
- [Google Scholar]
- Dihydrofolate reductase. In: Blakley R.L., Benkovic S.J., eds. Folates and pteridines. New York: Wiley; 1984. p. :191-253.
- [Google Scholar]
- Exploring the molecular mechanism of dihydrofolate reductase. Faraday Discuss.. 1992;93:217-224.
- [Google Scholar]
- Activity of sulfa drugs and dihydrofolate reductase inhibitors against Candida albicans. Experientia. 1982;38:436-437.
- [Google Scholar]
- Synthesis, anticancer activity and mechanism of action of new thiazole derivatives. Eur. J. Med. Chem.. 2018;144:874-886.
- [Google Scholar]
- Synthesis of new 2-[1(2H)-phthalazinon-2-yl]acetamide and 3-[1(2H)-phthalazinon-2-yl]propanamide derivatives as antinociceptive and anti-inflammatory agents. Archiv. der Pharmazie. 2004;337:303-310.
- [Google Scholar]
- Antitumor and immunomodulatory activities of thiosemicarbazones and 1,3-thiazoles in Jurkat and HT-29 cells. Biomed. Pharmacother.. 2016;82:555-560.
- [Google Scholar]
- Novel 1,3,4-oxadiazole thioether derivatives targeting thymidylate synthase as dual anticancer/antimicrobial agents. Bioorg. Med. Chem.. 2013;21:2286-2297.
- [Google Scholar]
- Photochromism of dihydroindolizines. Part XXI: multiaddressable photochromic performances based on pyrrolo[1,2-b]pyridazine photochromes: kinetics, substituent effect and solvatochromism. J. Photochem. Photobiol. A. 2017;346:287-295.
- [Google Scholar]
- Photochromism of dihydroindolizines. Part XXII: Significant effect of region B substituents on tuning the photophysical properties of photochromic dihydroindolizines: absorption, kinetic and computational studies. J. Photochem. Photobiol. A.. 2017;348:125-133.
- [Google Scholar]
- Photochromism of dihydroindolizines. Part XXIV: Exploiting “Click” chemistry strategy in the synthesis of fluorenyldihydroindolizines with multiaddressable photochromic properties. J. Photochem. Photobiol. A. 2018;360:210-223.
- [Google Scholar]
- Evaluation of the local anaesthetic activity of 3-aminobenzo[d]isothiazole derivatives using the rat sciatic nerve model. Eur. J. Med. Chem.. 2009;44:473-481.
- [Google Scholar]
- Structure-activity relationship studies in the field of calcium(II) antagonists. Effect of modifications at the tetrasubstituted carbon of verapamil-like compounds. J. Med. Chem.. 1985;28:1621-1628.
- [Google Scholar]
- Synthesis of N-(6-arylbenzo[d]thiazole-2-acetamide derivatives and their biological activities: an experimental and computational approach. Molecules. 2016;21:266-282.
- [Google Scholar]
- Ber. Dtsch. Chem. Ges.. 1887;20:3118-3132.
- Dihydrofolate reductase inhibitors as antibacterial agents. Biochem. Pharmacol.. 2006;71:941-948.
- [Google Scholar]
- Hay, R.J., Cleland, M.M., Durkin, S., Reid, Y.A., 2000. Cell line preservation and authentication in animal cell culture. In: Masters, J.R.W. (Ed.). Oxford University Press, New York.
- Design, synthesis, and biological evaluation of novel N4-substituted sulfonamides: acetamides derivatives as dihydrofolate reductase (DHFR) inhibitors. BMC Chem.. 2019;13:91.
- [Google Scholar]
- Bioactive fluorenes. Part I. Synthesis, pharmacological study and molecular docking of novel dihydrofolate reductase inhibitors based-2,7-dichlorofluorene. Heliyon. 2019;5:e01982
- [Google Scholar]
- Synthesis of some novel 6′-(4-chlorophenyl)-3,4′-bipyridine-3′-carbonitriles: assessment of their antimicrobial and cytotoxic activity. Z. Naturforsch.. 2015;70b:783-795.
- [Google Scholar]
- Efficient synthesis and antimicrobial evaluation of some Mannich bases from 2-arylidine-1-thia-4-azaspiro[4.5]decan-3-ones. Chem. Cent. J.. 2015;9:25.
- [Google Scholar]
- Antibacterial agents. 7. Aminomethinylation of guanidino heterocycles. Arch. Pharm.. 1981;314:968-969.
- [Google Scholar]
- Synthesis of polyhydroxylated aromatic mandelic acid amides and their antioxidative potential. Tetrahedron. 2001;57:1277-1282.
- [Google Scholar]
- Discovery of novel 2-N-aryl-substituted benzenesulfonamidoacetamides: orally bioavailable tubulin polymerization inhibitors with marked antitumor activities. Chem. Med. Chem.. 2012;7:680-693.
- [Google Scholar]
- Design, synthesis and evaluation of novel uracil acetamide derivatives as potential inhibitors of Plasmodium falciparum dUTP nucleotidohydrolase. Eur. J. Med. Chem.. 2009;44:678-688.
- [Google Scholar]
- Synthesis and biological evaluation of new thiazolyl/benzothiazolyl-amides, derivatives of 4-phenyl-piperazine. Farmaco. 2005;60:969-973.
- [Google Scholar]
- Novel 2,3-disubstituted quinazoline-4(3H)-one molecules derived from amino acid linked sulphonamide as a potent malarial antifolates for DHFR inhibition. Eur. J. Med. Chem.. 2017;129:251-265.
- [Google Scholar]
- Synthesis, pharmacological evaluation and docking studies of N-(benzo[d]thiazol-2-yl)-2-(piperazin-1-yl)acetamide analogs as COX-2 inhibitors. Bioorg. Med. Chem. Lett.. 2012;22:820-823.
- [Google Scholar]
- Dihydrofolate reductase and cell growth activity inhibition by the β-carboline-benzoquinolizidine plant alkaloid deoxytubulosine from Alangium lamarckii: its potential as an antimicrobial and anticancer agent. Bioorg. Med. Chem.. 1999;7:1105-1110.
- [Google Scholar]
- Homologous recombination resolution defect in werner syndrome. Mol. Cell. Biol.. 2002;22:6971-6978.
- [Google Scholar]
- Design and synthesis of novel antimicrobial acyclic and heterocyclic dyes and their precursors for dyeing and/or textile finishing based on 2-N-acylamino-4,5,6,7-tetrahydro-benzo[b]thiophene systems. Molecules. 2011;26:6271-6305.
- [Google Scholar]
- Anti-liver cancer activity in vitro and in vivo induced by 2-pyridyl 2,3-thiazole derivatives. Toxicol. Appl. Pharmacol.. 2017;329:212-223.
- [Google Scholar]
- New colorimetric cytotoxicity assay for anticancer-drug screening. J. Nat. Cancer Inst.. 1990;82:1107-1112.
- [Google Scholar]
- Inhibition of herpes simplex virus replication by a 2-aminothiazole via interactions with the helicase component of the UL5-UL8-UL52 complex. J. Virol.. 1998;72:6979-6987.
- [Google Scholar]
- Medicinal chemistry and the Molecular Operating Environment (MOE): application of QSAR and molecular docking to drug discovery. Curr. Top. Med. Chem.. 2008;8:1555-1572.
- [Google Scholar]
- Wilson, A.P., 2000. Cytotoxicity and viability assays in animal cell culture: a practical approach. third Ed. In: Masters, J.R.W. (Ed.). Oxford University Press, New York.
- Rational design, synthesis, and biological activity of N-(1,4-benzoxazinone)acetamide derivatives as potent platelet aggregation inhibitors. Bull. Korean Chem. Soc.. 2018;39:146-155.
- [Google Scholar]
- Dimethyl-diphenyl-propanamide derivatives as nonsteroidal dissociated glucocorticoid receptor agonists. J. Med. Chem.. 2010;53:8241-8251.
- [Google Scholar]
Appendix A
Supplementary material
Supplementary data to this article can be found online at https://doi.org/10.1016/j.arabjc.2020.03.024.
Appendix A
Supplementary material
The following are the Supplementary data to this article:Supplementary Data 1
Supplementary Data 1







