Bioaffinity ultrafiltration combined with UPLC-ESI-QTrap-MS/MS for screening of xanthine oxidase inhibitors from Paederia foetida L. leaves
⁎Corresponding author at: School of Food Science and Engineering, Hainan University, Haikou 570228, China. wangruimin2022@163.com (Ruimin Wang)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Paederia foetida L. leaf (PFL) is recognized for its uric acid-lowering. However, the key compounds and their mechanisms of countering hyperuricemia remain unclear. In this study, bioaffinity ultrafiltration in combination with UPLC-ESI-QTrap-MS/MS was used to screen and identify potential xanthine oxidase (XO) inhibitors in the purified PFL extract, and the possible mechanisms of their interaction were investigated by molecular docking. The experimental results showed that the purified PFL extract exhibited excellent antioxidant and anti-XO effect. Further the results of enzyme inhibition experiments in vitro showed that luteolin-7-O-glucoside (IC50 = 61.41 μg/mL), astragalin (IC50 = 114.89 μg/mL), quercitrin (IC50 = 124.50 μg/mL), dihydromyricetin (IC50 = 296.32 μg/mL), cyanidin-3-O-rutinoside (IC50 = 373.82 μg/mL), kaempferol-3-O-rutinoside (IC50 = 450.10 μg/mL) and (-)-epigallocatechin (IC50 = 610.48 μg/mL) in purified PFL extract were the major contributors to the XO inhibitory activity, and confirmed this finding. Molecular docking revealed these inhibitors interact with critical XO residues through hydrogen bonding, hydrophobic, and electrostatic interactions, highlighting flavonoids in purified PFL extract as potent XO inhibitors. This work provides insight into flavonoids in the purified PFL extract as potent XO inhibitors.
Keywords
Antioxidant activity
Bioaffinity ultrafiltration
Molecular docking
Paederia foetida L. leaf
Xanthine oxidase
- PFL
-
Paederia foetida L. leaf
- XO
-
xanthine oxidase
- HUA
-
hyperuricemia
- UA
-
uric acid
- TPC
-
total phenolic content
- TFC
-
total flavonoid content
- GAE
-
gallic acid equivalent
- DW
-
dry weight
- RE
-
rutin equivalent
- ABTS
-
2,2′-azino-bis (3-ethylbenzothiazoline-6-sulfonic acid)
- DPPH
-
1,1′-diphenyl-2–2′-picrylhydrazyl
- FRAP
-
reducing power (ferric ion reducing antioxidant power
- CUPRAC
-
cupric reducing antioxidant capacity
- BD
-
bio-affinity degree
- ESI
-
electrospray ionization
- RT
-
retention time
Abbreviations
1 Introduction
As living conditions improve worldwide, the incidence of hyperuricemia (HUA) has continued to increase in recent years, and it is showing a younger trend (Zheng et al., 2023). The main causes of this phenomenon are excessive production of uric acid (UA) and/or metabolic abnormalities (Liu et al., 2021). HUA has the potential to lead to several metabolic illnesses, including gout, diabetes, hypertension, cardiovascular diseases, and chronic renal disease (Wang et al., 2022). Xanthine oxidase (XO) is a crucial enzyme in the purine metabolism process that may catalyze the conversion of hypoxanthines to xanthines and their subsequent oxidation to uric acid, as well as directly catalyzing the synthesis of xanthines into uric acid (Liu et al., 2022b). Therefore, inhibition of XO activity is essential for treating HUA, gout, and other diseases associated with XO activity. Allopurinol and febuxostat are synthetic XO inhibitors commonly used in clinical settings to treat hyperuricemia and gout. But, the toxic side effects of synthetic drugs on the body should not be ignored. Allopurinol may cause a series of adverse symptoms, such as allergic, gastrointestinal, and nervous system reactions (Oh et al., 2019). Adverse effects of febuxostat typically include hypersensitivity, risk of cardiovascular death, hepatotoxicity, and possible immune system response with complications (Guan et al., 2022). Natural products have a wide range of pharmacological activities and are gaining attention in regulating uric acid levels and preventing related diseases. Phenolic acids or flavonoids in celery (Apium graveolens L.) seeds, Perilla frutescens (L.) Britt leaves, and green tea have been reported to have uric acid-lowering activity in vitro and in vivo (Chen et al., 2015; Li et al., 2019; Wang et al., 2017). Previous studies have further confirmed that flavonoids reduce uric acid levels by inhibiting XO activity, thereby reducing kidney damage and adverse effects (Meng et al., 2023).
Natural XO inhibitors screened from plants have received widespread attention for their low number of adverse effects. However, due to the complexity of plant composition, it is not easy to screen out the XO inhibitors. Bioaffinity ultrafiltration combined with the UPLC-ESI-QTrap-MS/MS method enables rapid screening and identification of bioactive components in natural products (Feng et al., 2022).
Paederia foetida L. (Rubiaceae) is widely distributed in tropical Asia. The plant contains various chemical substances, including flavonoids, alkaloids, iridoid glycosides, sitosterol, carbohydrates, and volatile oils (Wang et al., 2014). These substances have been demonstrated to have a variety of biological effects in both in vitro and in vivo experiments, including antioxidant, urate-lowering, anti-inflammatory, antibacterial, anticancer, hypoglycemic, and lipid-lowering activities (Ferdous et al., 2023). The PFL is not only a traditional Chinese medicine but has been used as a folk snack for thousands of years. These leaves are used to make traditional pastries, snacks, and teas that are enjoyed by the local populace in numerous locations. The plant releases sulfur chemicals with a pungent stench when its leaves or stems are harmed, which can be off-putting. Interestingly, these odors disappear when it is cooked. The PFL extract has been reported to be potent XO inhibitors and to reduce elevated uric acid levels in the blood (Yan et al., 2007; Yan et al., 2008; Houet al., 2014). However, the active substances in the PFL extracts and working mechanism with XO inhibitory activity remain unknown.
In this study, the total phenolic content (TPC) and total flavonoid content (TFC) of the PFL extract extracted at different ethanol concentrations were determined, and the antioxidation and XO inhibitory activities of the purified PFL extract were evaluated. Bioaffinity ultrafiltration combined with UPLC-ESI-QTrap-MS/MS screens potential XO inhibitors from purified PFL extract. In vitro enzyme inhibition experiments further confirmed the inhibitory action of these compounds on XO. Furthermore, molecular docking studies elucidated the interaction mechanisms between the identified ligands and XO receptor proteins. This research aims to shed light on the vital substances in PFL extract with XO inhibitory properties and provide valuable information for screening and identification of key active substances in complex medicinal plant extracts.
2 Materials and methods
2.1 Plant materials and chemicals
Paederia foetida L. leaves were sourced from a farm in Hainan province, China. These fresh leaves were freeze-dried in a vacuum freeze dryer (BK-FD18T, Beijing, China) for 48 h. The freeze-dried leaves were powdered with a grinder (DFY-500D, Zhejiang, China), filtered through a 60-mesh sieve, and then stored in a dryer for subsequent use.
A suite of compounds, including kaempferol-3-O-rutinoside, dihydromyricetin, (-)-epigallocatechin, delphinidin-3-O-glucoside, protocatechualdehyde, epicatechin, (-)-gallocatechin gallate, rutin, isovitexin, astragalin, quercitrin, luteolin-7-O-glucoside, cyanidin-3-O-rutinoside, avicularin, myricetin, millipore ultrafiltration centrifugal tube (30 kDa) and AB-8 macroporous resins were procured from Solabao Technology Co., Ltd. (Beijing, China). Analytical grade reagents and antioxidants testing supplies were purchased from Aladdin Biochemical Technology Co., Ltd. (Shanghai, China). Allopurinol, xanthines, and XO were acquired from Sigma (Shanghai, China).
2.2 Extraction and purification of PFL
The 0.5 g PFL powder was mixed varying concentrations of ethanol solution (0, 20, 40, 60, 80, 100 %, v/v) maintaining a solid–liquid ratio of 1:40. The extraction process was performed in an ultrasonic water bath (KQ-400DE, Jiangsu, China) at 40℃, 320 W for 30 min. Based on the results, a 60 % ethanol solution was selected as the optimal extraction solvent for further experiments.
In these subsequent experiments, PFL powder was mixed with the 60 % ethanol solution and processed as described above to produce PFL extracts. These were then concentrated using a rotary evaporator at 45℃ until the ethanol was fully evaporated. The concentrated extract was transferred to a chromatography column filled with AB-8 macroporous resin. After standing overnight, the column was eluted with distilled water to remove macromolecular impurities such as pigments, sugars, proteins, etc. Flavonoids adsorbed to the AB-8 resin were eluted with 95 % ethanol. This eluate was collected, the ethanol was evaporated off, and the remaining substance was freeze-dried for further analysis.
2.3 Composition of the purified PFL extract
The Folin-Ciocalteu method was used to determine the TPC of the purified PFL extract with minor modifications (Lopusiewicz et al., 2019). Specifically, 100 μL of the purified PFL extract and 50 μL of Folin-calcium reagent were combined and incubated at 25 °C for 5 min in the dark. Subsequently, 300 μL of 20 % Na2CO3 solution was added, and the mixture was incubated for another 8 min. Absorbance at 765 nm was measured. The gallic acid was set as the standard, and the calibration curve was y = 0.0143x + 0.0188 (R2 = 0.9969). The findings were presented as gallic acid equivalent (GAE) per gram dry weight (DW) of the purified PFL extract (mg GAE/g DW).
The TFC was determined by the aluminum chloride colorimetric method (Wang et al., 2021). The rutin was applied as the standard, and the curve for calibration was y = 0.0007x + 0.0062 (R2 = 0.9977). The results are presented as rutin equivalent (RE) per gram DW (mg RE/g DW). The total sugar content was determined by the phenol–sulfuric acid method (Liu et al., 2022a), and the total protein content was measured using the Coomassie Brilliant Blue method (Mao et al., 2022).
2.4 Determination of antioxidant activities
The in vitro antioxidant activities of the purified PFL extract were determined using free radical scavenging capacity (2,2′-azino-bis (3-ethylbenzothiazoline-6-sulfonic acid) (ABTS) and 1,1′-diphenyl-2–2′-picrylhydrazyl (DPPH)) and reducing power (ferric ion reducing antioxidant power (FRAP) and cupric reducing antioxidant capacity (CUPRAC)) (Sieniawska et al., 2022). Trolox was regarded as standard, and the standard curves for DPPH, ABTS, FRAP, and CUPRAC were y = 0.9654x − 3.1705 (R2 = 0.9924), y = 0.9341x + 0.4765 (R2 = 0.994), y = 0.003x − 0.022 (R2 = 0.999) and y = 0.0008x − 0.0007 (R2 = 0.9965), respectively. The results are expressed as μmol TE/ g DW.
2.5 Enzyme inhibition activity assay
The XO inhibitory activity assay was slightly modified according to a recent method (Hu et al., 2021). Briefly, 50 μL of samples and 50 μL of XO (0.1 U/mL) were incubated for 5 min at 37 °C. Then, 150 μL of xanthine solution (0.2 mM) was introduced as the substrate. Absorbance was recorded at 295 nm at 2 min intervals for 10 min. Allopurinol served as the positive control. The XO inhibitory activity was calculated using the following formula (1):
2.6 Screening of potential XO Inhibitors using affinity ultrafiltration
The affinity ultrafiltration screening technique was established based on previous study with minor modifications (Dong et al., 2021). Briefly, 100 μL of sample (0.5 mg/mL) and 100 μL of XO (5 U/mL) were sequentially added to a Millipore ultrafiltration centrifugal tube. Next, the mixture was incubated (37℃, 10 min), followed by centrifugation at 12,000 rpm for 10 min. 100 μL of PBS (pH 7.5) was added, and the mixture was centrifuged 3–5 times to remove unbound compounds. 100 μL of methanol was added, the mixture was incubated for 10 min, and the ligands specifically bound to XO were then separated from the complex by centrifugation. The separation process was repeated two times. In addition, XO that was placed in boiling water for 10 min served as a negative control and was subjected to the same procedure. Finally, the filtrate was passed through the filtration membrane (0.22 μm) and analyzed using the UPLC-ESI-QTrap-MS/MS system. The bio-affinity degree (BD) of inhibitors with XO was calculated according to the following formula (2):
Among them, CC represents the concentration of compounds in the purified PFL extract bound with XO; CD indicates the concentration of compounds in the purified PFL extract bound with inactivated XO; C0 indicates the concentration of the compound in the purified PFL extract.
2.7 UPLC-ESI-QTrap-MS/MS and HPLC-DAD analysis
Flavonoids in the purified PFL extract were qualitatively analyzed using a broad-targeted metabolomic approach. Standards were used to build a plant database using the extensive targeting of the UPLC-MS/MS platform through Shanghai Biotree Biotech Co., Ltd (Shanghai, China). In this study, the Sciex Exion LC AD UPLC system, which is outfitted with an electrospray ionization (ESI) source and the Sciex QTrap 6500 mass detector (Framingham, MA, USA), was utilized to identify the flavonoids present in the extract. The Acquity UPLC HSS T3 column (2.1 mm x 100 mm, 1.8 μm, Waters, USA) was applied to separate the primary chemicals present in the extract. 0.1 % formic acid in water (A) and acetonitrile (B) were employed as the mobile phases. The gradient elution was programmed as follows: 0–0.5 min, 15 % B; 0.5–10 min, 2–50 % B; 10–13 min, 50–95 % B; 13–15 min, 95–2 % B. The injection volume was 2 μL, and the flow rate was 0.4 mL/min. Various fragmentation information was collected in positive/negative ion mode using an ESI source. The relevant parameters of the ion source were set as follows: Ion spray voltage was set at + 5500/-4500 V, curtain gas was 35 psi, the temperature was 400 °C, ion source gas of 1:60 and 2: 60 psi, and dp of ± 100 V. Quantitative analysis was performed following the procedures established in our laboratory (Wang et al., 2021). The results are presented as μg/g DW.
2.8 Molecular docking analysis
The docking program Auto Dock Vina (https://vina.scripps.edu/) was used to simulate molecular modeling study and further explore the possible binding mechanisms of compounds to XO (Chen et al., 2023). XO (PDB: 2E1Q) was downloaded from RCSB Protein Data Bank. The ligand compound structure was drawn by ChemDraw and saved as a PDB file using Chem3D. The XO receptor protein was downloaded from the RCBS protein data bank (https://www.rcsb.org/). The water molecules and other small molecules in XO were removed by PyMol software (DeLano Scientific, San Carlos, CA, USA https://www.pymol.org/). Ligand compounds dock with XO, and the affinity values (kcal/mol) indicate their ability to bind.
2.9 Statistical analysis
Experiments were conducted in triplicate, with results reported as mean ± standard deviation (SD) was used to record the results. The IBM SPSS statistical program (New York, NY, USA) was used to conduct the statistical analysis. Images were drawn using Origin 2019b software (Origin Lab Corporation, Northampton, MA, USA). A p-value of less than 0.05 was considered to indicate statistical significance.
3 Results and discussion
3.1 Extraction and purification of flavonoids in the PFL extract
Previous research has demonstrated a positive correlation between the active functions of natural plants and the concentration of active substances in those plants (Tomasello et al., 2022). A positive correlation has been shown between polyphenol content and either uric acid production reduction or uric acid excretion capability promotion (Wang et al., 2019, Yusnaini et al., 2023). Compared with other organic extraction solvents, ethanol is a popular extraction solvent with low toxicity and safety. In this study, ethanol solutions at varying concentrations (0, 20, 40, 60, 80, 100 %, v/v) were used as extraction solvents to extract polyphenols in the PFL. The results showed significant differences were observed in the extraction efficiency of TPC and TFC in the PFL with different ethanol concentrations (p < 0.05, Fig. 1). Among them, the TPC and TFC in the PFL extracted with 60 % ethanol were 16.72 mg GAE/g DW and 29.83 mg RE/g DW, respectively, which were higher than those of other ethanol concentrations. The optimal extraction efficiency is attributed to the principle of similar miscibility, the polarity of 60 % ethanol content is comparable to that of polyphenols since water is a strongly polar fluid and ethanol is a weakly polar substance (Khanal et al., 2022). This finding is consistent with previous studies that 60 % ethanol is an extremely effective solvent for the extraction of polyphenols and flavonoids in Butea monosperma and Hyophorbe lagenicaulis leaf. (Farooq et al., 2020; William et al., 2019).
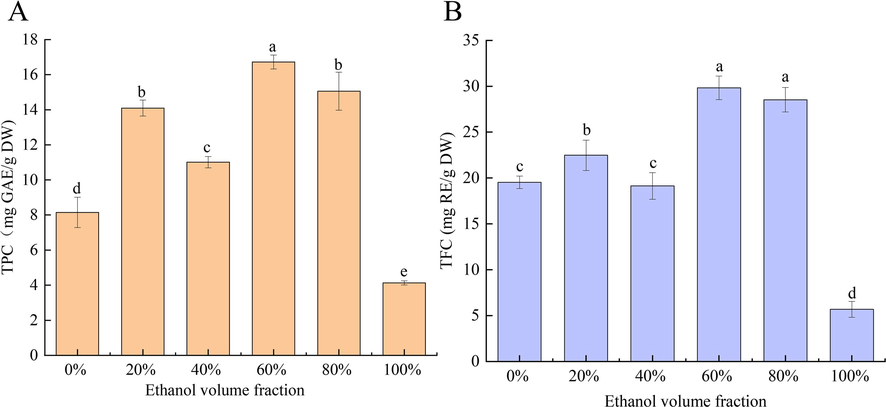
- Total phenolic content (TPC) (A) and total flavonoid content (TFC) (B) in the PFL extract.
The PFL extract underwent further purification using AB-8 macroporous resin. Analysis of the purified extract revealed a TFC of 760.33 mg RE/g DW, constituting 76.03 % of the extract composition, followed by total sugars (12.58 %), proteins (5.27 %), moisture (4.85 %), and ash (1.27 %) as shown in Fig. 2B. Compared to crude PFL extract (29.83 mg RE/g DW), TFC in the purified PFL extract increased by 25.49-fold (p < 0.05). The extract of fenugreek seeds was purified with AB-8 resin and the flavonoid purity reached to 71.84 % (Yang et al., 2022). The effectiveness of AB-8 resin in flavonoid purification is attributed to its weakly polar polystyrene structure, which does not bind to sugars, inorganic acids, alkalis, or small hydrophilic organic molecules. Therefore, AB-8 macroporous resin has excellent adsorption and desorption properties for flavonoids, and the concentration of flavonoids in extract is increased dramatically.
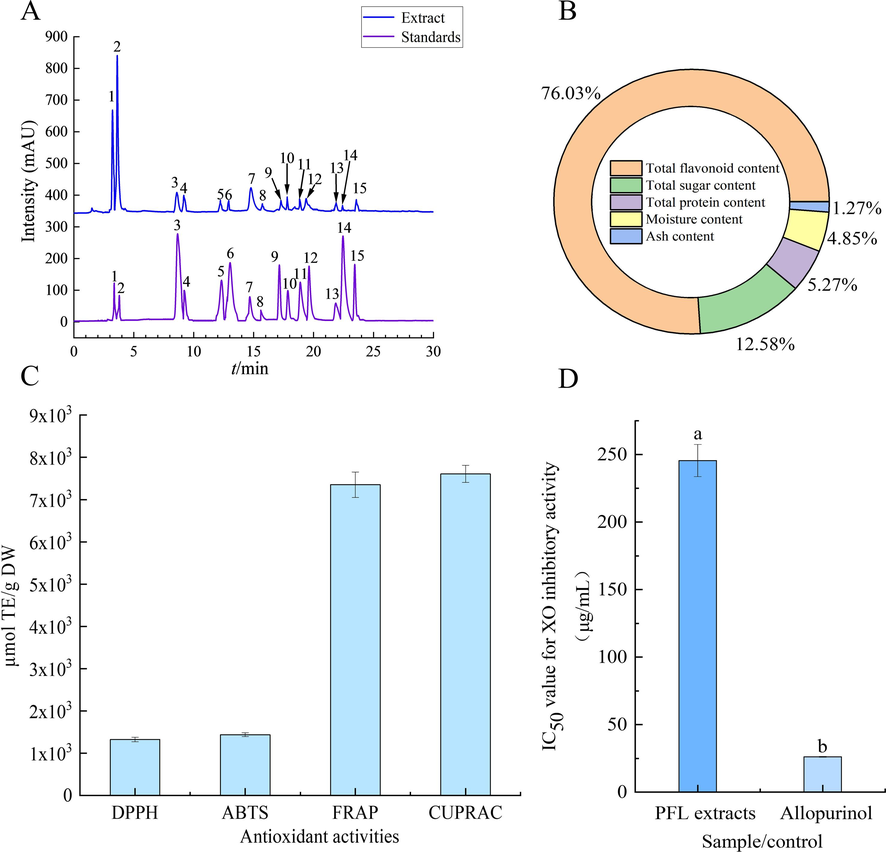
- UPLC-ESI-QTrap-MS/MS chromatograms (A), compounds (B), antioxidation (C), and IC50 value for XO inhibitory activity (D) of the purified PFL extract. 1, (-)-epigallocatechin; 2, delphinidin-3-O-glucoside; 3, protocatechualdehyde; 4, (-)-gallocatechin gallate; 5, epicatechin; 6, dihydromyricetin; 7, rutin; 8, isovitexin; 9, kaempferol-3-O-rutinoside; 10, luteolin-7-O-glucoside; 11, cyanidin-3-O-rutinoside; 12, astragalin; 13, quercitrin; 14, avicularin; 15, myricetin.
3.2 Identification and quantification of flavonoid compositions of the PFL
UPLC-ESI-QTrap-MS/MS and HPLC-DAD were used to identify and quantify the purified PFL extract. A total of 15 compounds were characterized and identified based on retention time (RT), parent ion (m/z), and characteristic MS fragment ions (ms/ms) in comparison with standard compounds (Fig. 2A and Table 1). Three catechin compounds (peak 1, peak 4, peak 5) with the molecular ions at m/z of 306.9 [C15H14O7 + H]+, 457.0 [C22H18O11-H]-, and 291.1 [C15H14O6 + H]+, were temporarily assigned as (-)-epigallocatechin, (-)-gallocatechin gallate, and epicatechin, respectively (Choudhary et al., 2020). Peak 2 (RT = 3.47 min) and peak 3 (RT = 8.65 min) were tentatively assigned as delphinidin-3-O-glucoside and protocatechualdehyde according to their precursor ions of [C21H20O12 + H]+ and 138.9 [C7H6O3 + H]+ at m/z 465.1, product ions at m/z of 303.1 and 120.8, respectively. Peak 6 (RT = 13.00 min) with the precursor ions of [M + H]+ at m/z 320.9 and fragment ion at m/z of 302.8. Compared with the information in the database, it was designated as dihydromyricetin. Peak 7 (RT = 14.77 min) was recognized as rutin based on the parent ion [M + H]+ at m/z 611.0 and fragment ions at m/z of 465.0 (Abd Elrasoul et al., 2020). Peak 8 showed a precursor ion of [C21H20O10 + H]+, which was generated at m/z 433.0. It was preliminarily identified as isovitexin. Two isomers of compounds (peak 9 and peak 11), indicating the precursor ions at m/z 595.20 [C27H30O15 + H]+, with different product ions at m/z 449.0 and 287.1, were determined as kaempferol-3-O-rutinoside and cyanidin-3-O-rutinoside, respectively. The other two isomers (peak 10 and peak12), with the different characteristic fragment ions at m/z of 269.0 and 286.9, were temporarily assigned as luteolin-7-O-glucoside and astragalin, respectively. Peak 13 (RT = 21.81 min) and peak 14 (RT = 22.44 min) with deprotonated molecule ion at m/z 446.9 [M−H]- and 432.9 [M−H]- were recognized as quercitrin and avicularin owing to the characteristic fragment ions at m/z 303.0 and 300.4. The presence of the precursor ions of [M + H]+ at m/z 318.9 contributed to the tentative recognition of peak 15 (RT = 23.53 min) as myricetin.
| Retenion time (RT) | Parent ion (m/z) | Molecular weight | MS/MS (m/z) | Chemical formula | Compound | Contents (μg/g DW) | |
|---|---|---|---|---|---|---|---|
| 1 | 3.24 | 306.90[M + H]+ | 306 | 306.9, 180.9 | C15H14O7 | (-)-epigallocatechin | 367846.08a |
| 2 | 3.47 | 465.10[M + H]+ | 464 | 465.1, 303.1 | C21H20O12 | delphinidin-3-O-glucoside | 148505.58b |
| 3 | 8.65 | 138.90[M-H]- | 138 | 138.9, 120.8 | C7H6O3 | protocatechualdehyde | 4087.19d |
| 4 | 9.23 | 457.00[M-H]- | 458 | 457.0, 304.7 | C22H18O11 | (-)-gallocatechin gallate | 3698.38d |
| 5 | 12.26 | 291.10[M + H]+ | 290 | 291.1, 139.0 | C15H14O6 | epicatechin | 773.21d |
| 6 | 13.00 | 320.90[M + H]+ | 320 | 320.9, 302.8 | C15H12O8 | dihydromyricetin | 833.83d |
| 7 | 14.77 | 611.00[M + H]+ | 610 | 611.0, 465.0 | C27H30O16 | rutin | 38727.85c |
| 8 | 15.73 | 433.10[M + H]+ | 432 | 433.1, 415.1 | C21H20O10 | isovitexin | 1200.11d |
| 9 | 17.28 | 595.20[M + H]+ | 594 | 595.2, 449.0 | C27H30O15 | kaempferol-3-O-rutinoside | 2945.70d |
| 10 | 17.83 | 449.10[M + H]+ | 448 | 449.1, 269.0 | C21H20O11 | luteolin-7-O-glucoside | 1467.24d |
| 11 | 18.90 | 595.20[M + H]+ | 594 | 595.2, 287.1 | C27H30O15 | cyanidin-3-O-rutinoside | 6661.25d |
| 12 | 19.54 | 449.10[M + H]+ | 448 | 449.1, 286.9 | C21H20O11 | astragalin | 3824.20d |
| 13 | 21.81 | 446.90[M-H]- | 448 | 446.9, 303.0 | C21H20O11 | quercitrin | 525.09d |
| 14 | 22.44 | 432.90[M-H]- | 434 | 432.9, 300.4 | C20H18O11 | avicularin | 473.25d |
| 15 | 23.53 | 318.90[M + H]+ | 318 | 318.9, 272.9 | C15H10O8 | myricetin | 573.82d |
Note: Different lowercase letters indicate significant differences (p < 0.05).
The quantification results highlighted (-)-epigallocatechin (367846.08 μg/g DW), delphinidin-3-O-glucoside (148505.58 μg/g DW), and rutin (38727.85 μg/g DW) as the predominant compounds in the extract (Table 1). Notably, (-)-epigallocatechin and rutin are flavonoids that have strong antioxidant properties and can be applied as neutralizers to remove free radicals (Hassim et al., 2021). A detailed analysis revealed that many of these compounds, including (-)-epigallocatechin, protocatechualdehyde, epicatechin, rutin, isovitexin, kaempferol-3-O-rutinoside, astragalin, quercitrin, and myricetin, have been previously in PFL extract obtained using deep eutectic solvents (Liu et al., 2022c).
3.3 Biological activities
Flavonoids are widely found in plants as secondary metabolites with high antioxidant activity, including scavenging of free radicals and reduction of metal ions (Hassim et al., 2021). The antioxidant capabilities of the purified PFL extract were assessed through DPPH, ABTS+, FRAP, and CUPRAC assays. As displayed in Fig. 2C, the free radical scavenging capacity (DPPH· and ABTS+·) and reducing capacity (FRAP and CUPRAC) of the purified PFL extract were 1325.68, 1438.81, 7352.63, and 7608.77 μmol TE/g DW, respectively. Notably, the ABTS+ scavenging capacity of the PFL extract by a deep eutectic solvent was only 48.91 μmol TE/g DW (Liu et al., 2022c). Interestingly, the PFL extract purified with AB-8 macroporous resin significantly enhanced the ABTS+ scavenging capability of the extract by 29-fold, suggesting flavonoids might be the main contributor to the antioxidant activity. There are supported by the presence of potent antioxidants like cyanidin-3-O-rutinoside, (-)-epigallocatechin, delphinidin-3-O-glucoside, rutin, and other flavonoids in the PFL extract (Grzesik et al., 2018, Coklar and Akbulut, 2021).
Furthermore, the excessive activity of certain enzymes is linked to many human diseases, making enzyme inhibition a critical strategy for disease prevention and treatment. XO, as a crucial enzyme in purine metabolism, is of particular interest for managing uric acid levels in the body (Dong et al., 2021). In this study, the in vitro XO inhibitory activity of the purified PFL extract was studied. As shown in Fig. 2D, the purified PFL extract showed a promising inhibition effect on XO with an IC50 value of 253.40 μg/mL and a positive control of 25.29 μg/mL. The inhibitory effect of the purified PFL extract was more pronounced than the results of previous studies involving Clinacanthus nutans (Burm.f.) Lindau (304.00 μg/mL), Hibiscus sagittifolius (Kurz) Merr. (311.00 μg/mL), and Baeckea frutescens L. (662.00 μg/mL) (Duong et al., 2017), the PFL has a stronger inhibition effect on XO (0.1 U/mL), which indicates that PFL has a promising future in lowering uric acid levels.
3.4 Identification of XO inhibitors using ultrafiltration-UPLC-ESI-QTrap-MS/MS
Following in vitro enzyme inhibition experiments, substances exhibiting XO inhibitory activity were detected within the purified PFL extract. These inhibitors, forming complexes with XO, were quantitatively analyzed using UPLC-MS/MS upon release. The results of the identification were presented in Fig. 3A, (-)-epigallocatechin, kaempferol-3-O-rutinoside, astragalin, dihydromyricetin, luteolin-7-O-glucoside, cyanidin-3-O-rutinoside and quercitrin were identified as the potential XO inhibitors. The bio-affinity degree (BD%) of these compounds with XO varied from 10.40 % to 64.67 %, with luteolin-7-O-glucoside showing the highest BD% at 64.67 % (Fig. 3C). These results demonstrate the differences in bio-affinity degree (BD%) values of different compounds with XO. The alterations in BD% among the compounds are attributed to the structural diversity of flavonoids, which influences their binding affinity to XO (Ferro et al., 2022).
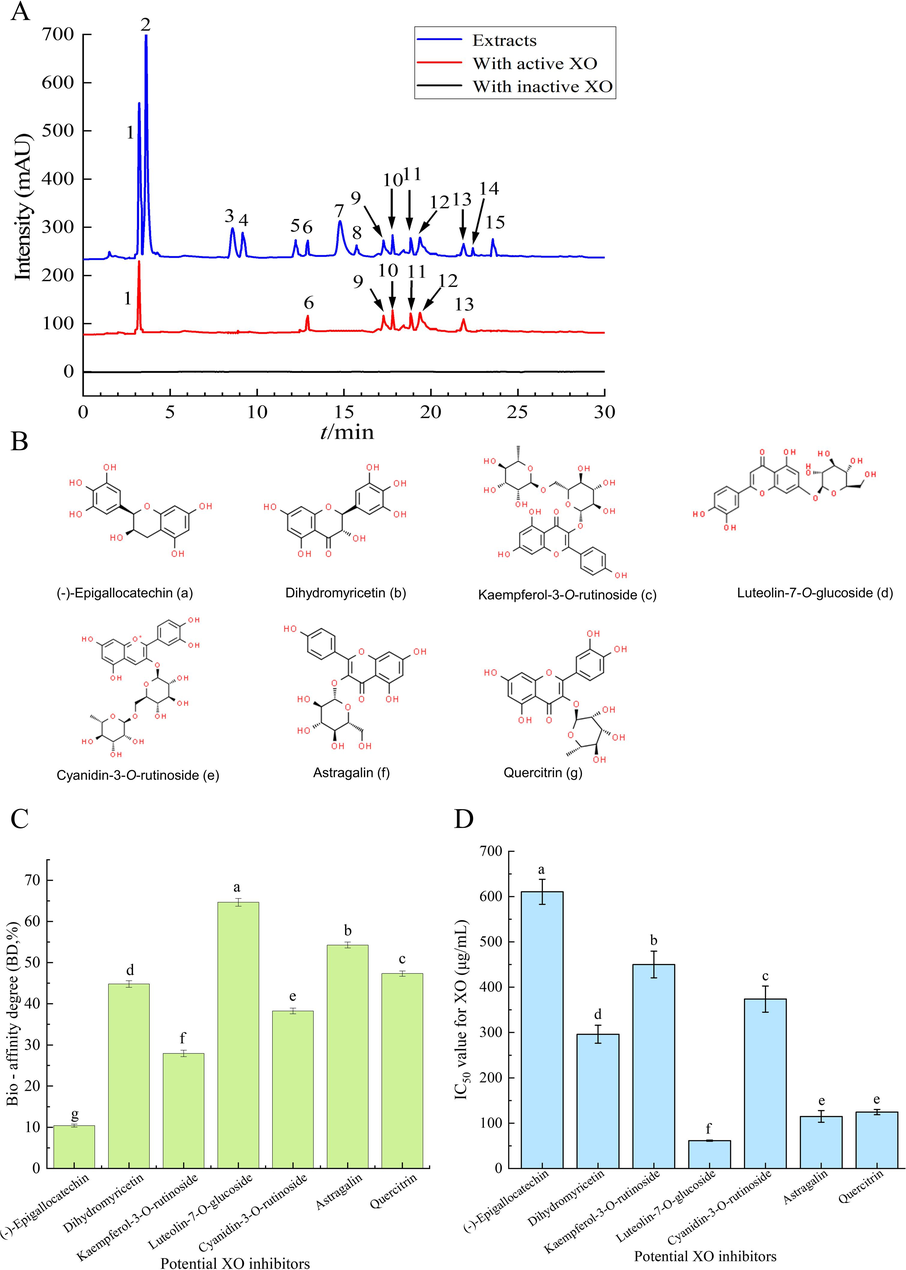
- Chromatograms (A) and chemical structures (B) of XO inhibitors screened in the purified PFL extract by ultrafiltration. The different solid lines denoted HPLC-DAD chromatograms of the purified PFL extract without ultrafiltration (blue), with active (red) and inactive (black) XO after ultrafiltration. Binding capacity (C) and IC50 value (D) of the compounds screened from the purified PLF extract to XO.
3.5 Validation of the XO-inhibiting activities of the identified compounds
The affinity ultrafiltration-UPLC-ESI-QTrap-MS/MS technique proved to be an effective method for isolating XO ligands from natural sources, though non-specific binding can lead to false positives. To verify the XO inhibitory potential of the identified compounds, standards were tested for their XO inhibition efficacy. The results showed that these screened monomer compounds significantly differed in their inhibitory effects on XO (Fig. 3D, p < 0.05). Compared with the purified PFL extract (IC50 = 253.40 μg/mL), luteolin-7-O-glucoside (IC50 = 61.41 μg/mL), astragalin (IC50 = 114.89 μg/mL), quercitrin (IC50 = 124.50 μg/mL) showed stronger XO inhibition ability. Followed by dihydromyricetin (IC50 = 296.32 μg/mL), cyanidin-3-O-rutinoside (IC50 = 373.82 μg/mL), kaempferol-3-O-rutinoside (IC50 = 450.10 μg/mL) and (-)-epigallocatechin (IC50 = 610.48 μg/mL). The positive effects of luteolin-7-O-glucoside, quercitrin, and kaempferol-3-O-rutinoside on XO inhibitory activity have been demonstrated in many studies (Ozyel et al., 2021, Ferro et al., 2022, Idoudi et al., 2023). These results indicate that the methods used to screen XO inhibitors from natural plants are effective. In summary, seven flavonoid compounds were identified as potent XO inhibitors, the findings underscore the potential of flavonoids in PFL extract can be used as natural sources for developing new XO inhibitors.
3.6 Molecular docking analysis
Seven potential XO inhibitors were screened, but the mechanism of interaction between these compounds and XO remains unclear. Molecular docking technology further elucidates the interaction mechanism between compound ligands and XO receptors. Before docking, it was necessary to remove excess ions and water molecules, optimize energy, and adjust force field parameters to meet the low-energy conformation of the compound ligand structure. As shown in Table 2, the binding ability of these seven potential inhibitors to XO can be represented by different binding affinity values. The binding strength of the ligand to XO increases with a decrease in the binding affinity value. Affinity values of less than −7.0 kcal/mol are often interpreted as the ligand has strong binding activity with the enzyme (Liao et al., 2023). From an energy perspective, the binding affinity values of the seven ligand compounds range from −8.6 to −10.4 kcal/mol. The compounds that docked with XO were ranked according to their binding affinity values in the following order: kaempferol-3-O-rutinoside (−10.4 kcal/mol), luteolin-7-O-glucoside (−9.4 kcal/mol), dihydromyricetin (−9.4 kcal/mol), quercitrin (-9.0 kcal/mol), cyanidin-3-O-rutinoside (−8.8 kcal/mol), astragalin (−8.8 kcal/mol), and (-)-epigallocatechin (−8.6 kcal/mol). The experimental results showed that these flavonoids were defined as having excellent binding capacity with XO, especially their binding affinity values were all below −7.0 kcal/mol.
| Compound | Binding affinity (kcal/mol) | H-bond atom |
|---|---|---|
| kaempferol-3-O-rutinoside | −10.4 | Glu45, Ser356, Asp360, Asn351, His1221 |
| dihydromyricetin | −9.4 | Ser347 |
| luteolin-7-O-glucoside | −9.4 | Lys256, Arg394, Lys249 |
| quercitrin | −9.0 | Thr354, Asn261, Thr262 |
| cyanidin-3-O-rutinoside | −8.8 | Arg394, Ala432, Asp360, Glu45, Asn351 |
| astragalin | −8.8 | Clu402, Clu367, Leu257, Lys249, Thr354 |
| (-)-epigallocatechin | −8.6 | Arg1053,Gln568, Leu1245, Gln562 |
At the same time, flavonoids containing two phenolic hydroxybenzene ring structures can bind to the vital catalytic site residue of the enzyme through hydrogen bonds and hydrophobic interactions to inhibit XO activity (Zhu et al., 2021). The molecular docking results are shown in Fig. 4, (-)-epigallocatechin interacts with residues Gln562, Gln568, Arg1053, Leu1245 to form hydrogen bonds and Pro565 to form hydrophobic interactions. Similarly, astragalin creates hydrogen bonds with Clu402, Clu367, Leu257, Lys249, and Thr354 and hydrophobic interactions with Ile353. The binding affinity predictions of dihydromyricetin and luteolin-7-O-glucoside for XO were equal. Among them, one hydroxyl group of dihydromyricetin interacts with Ser347 by hydrogen bonding, and the aromatic ring binds to Leu257, Val259, and Ile353 to form pi-alkyl, pi-donor hydrogen bond and pi-sigma interactions, respectively. The reactive residues Lys256, Lys249, and Arg394 interact with the hydroxyl groups of luteolin-7-O-glucoside to form hydrogen bonds, and Leu257 and Ile353 to produce pi-alkyl hydrophobic and carbon-hydrogen bond interaction. In addition to hydrogen bonding and pi-sigma hydrophobic interactions with surrounding Glu45, Asn351, Ser356, Asp360, His1221, Ser359, Ser1226, Phe337, and Thr1227. Generation of pi-cation electrostatic interactions with Ysl271 and Arg394 also contributes to the enhancement of the binding of kaempferol-3-O-rutinoside and XO. These interactions are important in the reduction of XO activity by kaempferol-3-O-rutinoside. The predicted binding affinity of cyanidin-3-O-rutinoside and astragalin with XO were −8.8 kcal/mol, and the number of hydrogen bonds produced by the residue was equal. Cyanidin-3-O-rutinoside produces hydrogen bonds with Glu45, Asn351, Asp360, Arg394, Ala432 and Ile431, and pi-alkyl hydrophobic interactions with Ala346. The interactions between the residues and Thr354, Asn261, and Thr262, including hydrogen bonds, pi-sigma, and pi-alkyl hydrophobic effects with Leu257, Ile264, and Ile353, are what cause quercitrin to bind to XO. Allopurinol is a purine analog XO inhibitor that generates hydrogen bonds with Gly798, Phe799, Met1039 and Gln1195 residues, hydrophobic interactions with Cys150 and Phe799 residues, and electrostatic interactions with Arg913. Previous studies have reported that quercetin and kaempferol-3-O-rutanoside are inhibitors of XO. Lys256, Leu257, Val259, Asn261, Thr262, Ile353, and Thr354 were the vital amino acid residues that the inhibitors bind to XO (Li et al., 2022). This is highly consistent with our results. In this study, in addition to the reported interactions between hydrogen bonding and hydrophobicity, electrostatic interactions are also important reasons for the tight binding of inhibitors to XO. The mechanism by which flavonoids inhibit the catalytic activity of XO is summarized as the insertion of flavonoids into the active cavity of XO, occupying the active site of its catalytic center and impeding the entry of xanthines to reduce the catalytic activity of XO.
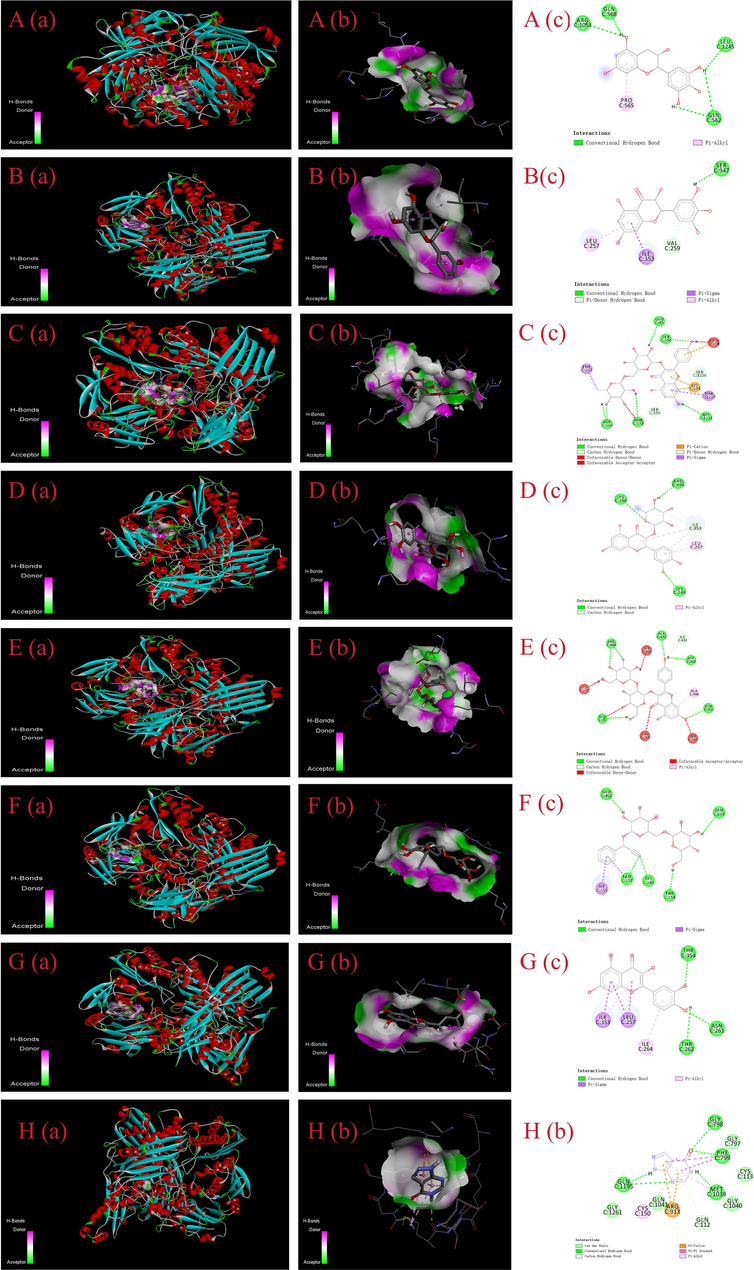
- The conformations of the screened XO inhibitors docked with XO by molecular docking analysis. A, (-)-epigallocatechin; B, dihydromyricetin; C, kaempferol-3-O-rutinoside; D, luteolin-7-O-glucoside; E, cyanidin 3-O-rutinoside; F, astragalin; G, quercitrin; H, allopurinol.
4 Conclusions
This study successfully identified 15 bioactive compounds within the purified extract of PFL utilizing UPLC-MS/MS technology. The purified PFL extract had good antioxidant and XO inhibitory activities. Among these compounds, seven flavonoids were pinpointed as potential XO inhibitors, including kaempferol-3-O-rutinoside, dihydromyricetin, luteolin-7-O-glucoside, quercitrin, cyanidin-3-O-rutinoside, astragalin and (-)-epigallocatechin for their potent activity. In addition, molecular docking analysis showed that seven ligand compounds were inserted into the active cavity of XO, thereby occupying the active site of catalytic center, hindering the entry of xanthines and reducing the catalytic activities of XO. These findings underscore the potential of PFL as a valuable source of natural compounds for developing new antioxidant and anti-hyperuricemia treatments. Future research should continue to explore the therapeutic applications of these bioactive compounds.
CRediT authorship contribution statement
Yuyi Liu: Conceptualization, Methodology, Software, Writing – original draft. Xuan Hu: Investigation, Resources, Validation. Enhui Li: Investigation, Resources, Validation. Yajing Fang: Investigation, Resources, Validation. Hui Xue: Supervision, Methodology, Software. Jiachao Zhang: Supervision, Methodology, Software. Rajesh Jha: Supervision, Methodology, Software. Ruimin Wang: Project administration, Writing – review & editing, Funding acquisition, Supervision.
Acknowledgment
This study was supported by “Nan Hai Xin Xing” Science and Technology Innovation Talent Platform Project Funding of Hainan Province (NHXXRCXM202312) and the Scientific Research Foundation of Hainan University(No. KYQD(ZR)23016).
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Antioxidant, anti-inflammatory, and anti-apoptotic effects of Azolla pinnata ethanolic extract against lead-induced hepatotoxicity in rats. Antioxidants.. 2020;9(10):1014.
- [CrossRef] [Google Scholar]
- Xanthine oxidase inhibitory peptides from Larimichthys polyactis: characterization and in vitro/in silico evidence. Foods.. 2023;12(5):982.
- [CrossRef] [Google Scholar]
- Green tea polyphenols decreases uric acid level through xanthine oxidase and renal urate transporters in hyperuricemic mice. J. Ethnopharmacol.. 2015;175:14-20.
- [CrossRef] [Google Scholar]
- Characterization, inhibitory activity and mechanism of polyphenols from faba bean (gallic-acid and catechin) on α-glucosidase: insights from molecular docking and simulation study. Prep. Biochem. Biotech.. 2020;50(2):123-132.
- [CrossRef] [Google Scholar]
- Changes in phenolic acids, flavonoids, anthocyanins, and antioxidant activities of Mahonia aquifolium berries during fruit development and elucidation of the phenolic biosynthetic pathway. Hortic. Environ. Biotechnol.. 2021;62(5):785-794.
- [CrossRef] [Google Scholar]
- Ligand fishing based on bioaffinity ultrafiltration for screening xanthine oxidase inhibitors from citrus plants. J. Sep. Sci.. 2021;44(7):1353-1360.
- [CrossRef] [Google Scholar]
- Duong, N.T., Vinh, P.D., Thuong, P.T., Hoai, N.T., Thanh, L.N., Bach, T.T., Nam, N.H., Anh, N.H., 2017. Xanthine oxidase inhibitors from Archidendron clypearia (Jack.) IC Nielsen: Results from systematic screening of Vietnamese medicinal plants. Asian Pacific Journal of Tropical Medicine. 10(6), 619-626. https://doi.org/10.1016/j.apjtm.2017.06.002.
- UHPLC-QTOF-MS/MS based phytochemical characterization and anti-hyperglycemic prospective of hydro-ethanolic leaf extract of Butea monosperma. Sci. Rep.. 2020;10(1):3530.
- [CrossRef] [Google Scholar]
- Potential multifunctional bioactive compounds from dysosma versipellis explored by bioaffinity ultrafiltration-HPLC/MS with topo I, topo II COX-2 and ACE2. J. Inflamm. Res.. 2022;15:4677-4692.
- [CrossRef] [Google Scholar]
- Assessment of the hypoglycemic and anti-hemostasis effects of Paederia foetida (L.) in controlling diabetes and thrombophilia combining in vivo and computational analysis. Comput. Biol. Chem.. 2023;107:107954.
- [CrossRef] [Google Scholar]
- Citrus bergamia and cynara cardunculus reduce serum uric acid in individuals with non-alcoholic fatty liver disease. Medicina-Lithuania.. 2022;58(12):1728.
- [CrossRef] [Google Scholar]
- Antioxidant properties of catechins: comparison with other antioxidants. Food Chem.. 2018;241:480-492.
- [CrossRef] [Google Scholar]
- Systematically exploring the chemical ingredients and absorbed constituents of Polygonum capitatum in hyperuricemia rat plasma using UHPLC-Q-orbitrap HRMS. Molecules. 2022;27(11):3521.
- [CrossRef] [Google Scholar]
- Scale-up approach for supercritical fluid extraction with ethanol-water modified carbon dioxide on Phyllanthus niruri for safe enriched herbal extracts. Sci. Rep.. 2021;11(1):15818.
- [CrossRef] [Google Scholar]
- Protective effect of iridoid glycosides from Paederia scandens (lour.) Merrill (rubiaceae) on uric acid nephropathy rats induced by yeast and potassium oxonate. Food Chem. Toxicol.. 2014;64:57-64.
- [CrossRef] [Google Scholar]
- Purification and identification of novel xanthine oxidase inhibitory peptides derived from round scad (Decapterus maruadsi) protein hydrolysates. Mar. Drugs.. 2021;19(10):538.
- [CrossRef] [Google Scholar]
- Influence of extraction techniques and solvents on the antioxidant and biological potential of different parts of Scorzonera undulata. Life-Basel.. 2023;13(4):904.
- [CrossRef] [Google Scholar]
- Phytochemical analysis and in vitro antioxidant and antibacterial activity of different solvent extracts of Beilschmiedia roxburghiana nees stem barks. The Scientific World J.. 2022;2022:6717012.
- [CrossRef] [Google Scholar]
- In vitro inhibitory effects of polyphenols from Tartary buckwheat on xanthine oxidase: identification, inhibitory activity, and action mechanism. Food Chem.. 2022;379:132100
- [CrossRef] [Google Scholar]
- Anti-gouty arthritis and anti-hyperuricemia properties of celery seed extracts in rodent models. Mol. Med. Rep.. 2019;20(5):4623-4633.
- [CrossRef] [Google Scholar]
- Network pharmacology- and molecular docking-based analyses of the antihypertensive mechanism of ilex kudingcha. Front. Endocrinol.. 2023;14:1216086.
- [CrossRef] [Google Scholar]
- Inflammatory response and oxidative stress as mechanism of reducing hyperuricemia of Gardenia jasminoides-Poria Cocos with network pharmacology. Oxid. Med. Cell. Longev.. 2021;2021:8031319.
- [CrossRef] [Google Scholar]
- Characterization of xanthine oxidase inhibitory activities of phenols from pickled radish with molecular simulation. Food Chemistry-x.. 2022;14:100343
- [CrossRef] [Google Scholar]
- Ultrasonic-assisted extraction of polyphenolic compounds from Paederia scandens (lour.) merr. using deep eutectic solvent: optimization, identification, and comparison with traditional methods. Ultrason. Sonochem.. 2022;86:106005
- [CrossRef] [Google Scholar]
- Biological characteristics, bioactive compounds, and antioxidant activities of off-season mulberry fruit. Front. Plant Sci.. 2022;13:1034013.
- [CrossRef] [Google Scholar]
- Development, characterization, and bioactivity of non-dairy kefir-like fermented beverage based on flaxseed oil cake. Foods.. 2019;8(11):0544.
- [CrossRef] [Google Scholar]
- Impact of high hydrostatic pressure on the micellar structures and physicochemical stability of casein nanoemulsion loading quercetin. Food Chemistry-x.. 2022;14:100356
- [CrossRef] [Google Scholar]
- Chimonanthus nitens oliv. leaves flavonoids alleviate hyperuricemia by regulating uric acid metabolism and intestinal homeostasis in mice. Food Sci. Human Wellness.. 2023;12(6):2440-2450.
- [CrossRef] [Google Scholar]
- Effects of chondroT on potassium oxonate-induced hyperuricemic mice: downregulation of xanthine oxidase and urate transporter 1. BMC Complement. Altern. Med.. 2019;19:10.
- [CrossRef] [Google Scholar]
- Anti-inflammatory effects of quercetin on high-glucose and pro-inflammatory cytokine challenged vascular endothelial cell metabolism. Mol. Nutr. Food Res.. 2021;65(6):2000777.
- [CrossRef] [Google Scholar]
- Phytochemical insights into Ficus Sur extracts and their biological activity. Molecules.. 2022;27(6):1863.
- [CrossRef] [Google Scholar]
- Phytocomplex of a standardized extract from red orange (Citrus sinensis L. osbeck) against photoaging. Cells.. 2022;11(9):1447.
- [CrossRef] [Google Scholar]
- A Phytochemical, Pharmacological and Clinical Profile of Paederia foetida and P-scandens. Natural Product Communications.. 2014;9(6):879-886.
- [CrossRef] [Google Scholar]
- The flavonoid-rich fraction from rhizomes of Smilax glabra roxb. ameliorates renal oxidative stress and inflammation in uric acid nephropathy rats through promoting uric acid excretion. Biomed. Pharmacother.. 2019;111:162-168.
- [CrossRef] [Google Scholar]
- Tailor-made deep eutectic solvents-based green extraction of natural antioxidants from partridge leaf-tea (Mallotus furetianus L.) Sep. Purif. Technol.. 2021;275:119159
- [CrossRef] [Google Scholar]
- Competitive binding experiments can reduce the false positive results of affinity-based ultrafiltration-HPLC: a case study for identification of potent xanthine oxidase inhibitors from Perilla frutescens extract. J. Chromatography B-Anal. Technol. Biomed. Life Sci.. 2017;1048:30-37.
- [CrossRef] [Google Scholar]
- Research progress of risk factors and early diagnostic biomarkers of gout-induced renal injury. Front. Immunol.. 2022;13:908517
- [CrossRef] [Google Scholar]
- Antioxidant activity, α-glucosidase inhibition and phytochemical profiling of Hyophorbe lagenicaulis leaf extracts. PeerJ.. 2019;7:7022.
- [CrossRef] [Google Scholar]
- Effect of extracts of Paederia scandens on mouse hyperuricemia induced by yeast and potassium oxonate. Acta Universitatis Medicinalis Anhui.. 2007;06:676-678.
- [Google Scholar]
- The dual actions of Paederia scandens extract as a hypouricemic agent: xanthine oxidase inhibitory activity and uricosuric effect. Planta Med.. 2008;74(11):1345-1350.
- [CrossRef] [Google Scholar]
- Multiple responses optimization of antioxidative components extracted from fenugreek seeds using response surface methodology to identify their chemical compositions. Food Sci. Nutr.. 2022;10(10):3475-3484.
- [CrossRef] [Google Scholar]
- Ethanolic extract from Limonia acidissima L. fruit attenuates serum uric acid level via URAT1 in potassium oxonate-induced hyperuricemic rats. Pharmaceuticals.. 2023;16(3):419.
- [CrossRef] [Google Scholar]
- Hyperuricemia as an effect modifier of the association between metabolic phenotypes and nonalcoholic fatty liver disease in Chinese population. J. Transl. Med.. 2023;21(1):39.
- [CrossRef] [Google Scholar]
- Epicatechin gallate as xanthine oxidase inhibitor: inhibitory kinetics, binding characteristics, synergistic inhibition, and action mechanism. Foods.. 2021;10(9):102191
- [CrossRef] [Google Scholar]







