Translate this page into:
Biochemical and Anti-proliferative activities of seven abundant tropical red seaweeds confirm nutraceutical potential of Grateloupia indica
⁎Corresponding authors at: Division of Applied Phycology and Biotechnology, CSIR-Central Salt and Marine Chemicals Research Institute, G. B. Marg, Bhavnagar, (Gujarat), India (A. Mishra), and Plant Production Department, College of Food & Agriculture Sciences, King Saud University, Riyadh, Saudi Arabia (H.O. Elansary) avinash@csmcri.res.in (Avinash Mishra), helansary@ksu.edu.sa (Hosam O. Elansary),
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
Seaweeds are being used as food items in Asian countries from ancient times. Seaweeds mainly grow in intertidal zone and survive extreme environmental conditions and thus have developed metabolites, which help them survive in such conditions. Red seaweeds have proven to be rich in compounds, which are antioxidant and having many other health benefits. In this study, seven most abundantly grown red seaweeds, Gracilaria corticata (GC), Grateloupia indica (GI), Kappaphycus alvarezii (KA), Solieria robusta (SR), Amphiroa anceps (AA), Halymenia porphyriformis (HP), and Sarconema scinaioides (SS) were collected from Saurashtra coast of Arabian Sea. Harvested seaweeds were subjected to metabolite profiling, flavonoid and phenolic content analysis, different biological activities including radical scavenging, antioxidant, reducing and proliferation inhibition. Overall, GI was found to contain high contents (flavonoid and phenolic), biological activities and proliferation inhibition. Study confirms nutraceutical potential of red seaweed Grateloupia indica to be explored further for the bioactive compound or to be used as functional food.
Keywords
Anti-proliferation
Edible seaweed
Functional food
Marine algae
Metabolites
Metabolomics
Nutraceuticals
Red seaweeds
1 Introduction
Seaweeds, especially red seaweeds have proven to be rich source of dietary supplements (Tanna et al., 2019; Francavilla et al., 2013; Ragaza et al. 2015). Most of the seaweeds showed high antioxidant property, which can help decode their use as nutraceuticals application (Tanna and Mishra, 2018a; Tanna and Mishra, 2019; Choudhary et al. 2021). Total aquaculture production was 120 million tonnes in the year 2019, and algae-production contributed about 36 million tonnes (FAO, 2021). Out of total 36 million tonnes algal-production, about 50% algae were produced by cultivation. There is a large number of seaweeds available which includes 125 species of Rhodophyta (red algae), 64 Phaeophyceae (brown algae) and 32 Chlorophyta (green algae). Amongst available 221 species, 145 species are being used for foods while 101 species for the production of phycocolloid. There are many types of seaweeds, which are extensively cultivated that includes Kappaphycus alvarezii and Gracilaria spp. of red seaweeds. Maximum cultivation of seaweed has been observed in Chile and then in China (FAO, 2021). In East Asia countries specifically in China, Korea and Japan seaweeds are mainly used for food purpose (Nayar and Bott, 2014).
Red seaweeds from Rhodophyta phylum are widely used as seasonings, noodles, condiments, sushi wrappings and vegetables, also being use as food additive, functional-food, and in phycocolloid industry for agar and carrageenan due to their gelling, emulsifying, water retention and other physical properties (Arulkumar et al., 2018). Since the Viking age, in Norway red algae Palmaria palmata has been traditionally consumed (Roleda et al., 2019). The protein from red seaweed Palmaria palmata has shown hypotensive activity on rats by Renin Inhibitory mechanism (Fitzgerald et al., 2014).There is also edible film developed with antioxidant activity from Pyropia columbina (formerly Porphyra columbina) with their phycocolloids and phycobiliproteins enriched fractions (Cian et al., 2014). There have been many studies to check antioxidant activities of different seaweeds and have obtained good results like ethanolic extracts of two Gracilaria spp. from Brazil have shown high phenolic and antioxidant activities (Souza et al., 2011).
Metabolomics is newly emerged form of ‘omics’ study, which is biochemical phenotyping study to increase our knowledge about primary as well as secondary metabolites of given organism at a given time (Tanna and Mishra, 2018b). Tropical red seaweeds were reported to be rich in metabolites, flavonoid, and phenolic compounds (Tanna et al., 2019). Further seaweeds are considered natural antioxidants and possess high bioactivity (Choudhary et al., 2021; Tanna et al., 2018).
There are no reports, so far, on tropical red seaweeds from the Arabian Sea coast. Therefore, we studied the different biological activities of the selected red seaweeds. Seven abundantly growing red seaweeds (Gracilaria corticata, Grateloupia indica, Kappaphycus alvarezii, Solieria robusta, Amphiroa anceps, Halymenia porphyriformis, and Sarconema scinaioides) were analyzed for the different phytochemical composition (total flavonoid and phenolic contents) and biological activities including total antioxidant, reducing and scavenging activities, and proliferation inhibition on human cancer cell lines (HeLa and Huh7).
2 Materials and methods
2.1 Collection of seaweeds samples and preparation of extracts
Tropical seven red seaweeds; Gracilaria corticata (GC; family Gracilariaceae), Grateloupia indica (GI; family Halymeniaceae), Kappaphycus alvarezii (KA; family Solieriaceae), Solieria robusta (SR; family Solieriaceae), Amphiroa anceps (AA; family Lithophyllaceae), Halymenia porphyriformis (HP; family Halymeniaceae), and Sarconema scinaioides (SS; family Solieriaceae) were collected from Saurashtra coast (N. 22° 28′ 8.19″; E. 69° 04′8.24″) of Arabian Sea (Tanna et al., 2019). All collected samples were processed as described previously and stored for further analysis. Dried seaweed powder (10 g) was extracted overnight with aqueous methanol (70%, v/v) and supernatant was collected by centrifugation at 7000 g for 10 min. Supernatant was concentrated using rotary evaporator (100–150 mbar at 37 °C), lyophilized and stored at −20 °C until further use.
2.2 Estimation of total antioxidant activity
Total antioxidant of the red seaweed extracts were analyzed by measuring scavenging capacity of ABTS (2,2′-azino-bis(3-ethylbenzothiazoline-6-sulphonic acid)) free radicals and compared with standard trolox (Hazra et al., 2008; Re et al., 1999). Briefly, ABTS free radicals were generated by mixing ABTS diammonium salt (7 mM) with potassium persulphate (2.45 mM) in dark followed by incubation for 12–16 hrs at room temperature. ABTS free radicals (1 ml; OD734 = 0.70 ± 0.02) was mixed with various concentration (200, 400, 600, 800 and 1000 μg ml−1) of seaweed extracts or standard (1–5 μg ml−1 trolox), incubated for 90 min and absorbance was read at 734 nm. ABTS scavenging activity was compared with standard trolox and percentage inhibition was calculated.
2.3 Estimation of radical scavenging activity
DPPH (2,2-diphenyl-1-picrylhydrazyl) is used for the study of scavenging activity (Saeed et al., 2012). About 1 ml of DPPH solution (0.024%, w/v in methanol; OD517 = 0.98 ± 0.02) was mixed with different concentration of seaweed extracts (200, 400, 600, 800 and 1000 μg ml−1) or trolox, incubated overnight and absorbance was read at 517 nm. The scavenging activity was determined by comparing the absorbance of extracts with standard trolox (Mishra et al., 2015; Patel et al., 2016).
2.4 Estimation of reducing power
The reducing power was determined using ascorbic acid as standard (Tanna et al., 2018; Tanna et al., 2021). Different concentration of seaweeds extracts (100, 200, 300, 400 and 500 μg ml−1) was mixed with 1 ml of phosphate buffer (0.2 M, pH 6.6) and 1 ml of K3Fe(CN)6 (10 mg ml−1). The reaction mixture was incubated at 50 °C for 20 min, 1 ml TCA (100 mg l−1) was added to terminate the reaction. Supernatant was collected by centrifugation at 7000 g for 10 min at room temperature. Freshly prepared FeCl3 (0.2 ml; 0.1%, w/v) was added to 1 ml of supernatant, incubated for 10 min at room temperature, absorbance was read at 700 nm, and reducing power was calculated.
2.5 Estimation of total phenolic and flavonoid contents
Total phenolic content (TPC) and total flavonoid content (TFC) were estimated with the help of gallic acid and quercetin as a standard, respectively (Tanna et al., 2019; Saeed et al., 2012; Zhishen et al., 1999). Different concentration of seaweed extracts (200, 400, 600, 800 and 1000 μg ml−1) was mixed with Folin-Coicalteu reagents (2.5 ml; 0.2 M), incubated for 90 min, 2 ml sodium carbonate (Na2CO3; 75 g l−1) was added, further incubated for 90 min in dark, and the absorbance was read at 760 nm. Total phenolic content was calculated using a standard curve of gallic acid and represented as mg ml−1 gallic acid per 100 mg of extract. Similarly, different concentration of seaweed extracts (200, 400, 600, 800 and 1000 μg ml−1) was mixed with 0.3 ml NaNO2 (5%, v/v), incubated at room temperature for 5 min, 0.3 ml AlCl3 (10%, v/v) and 2 ml NaOH (1 M) were added, and absorbance of the reaction mixture was read at 510 nm. TFC was determined as mg ml−1 quercetin per 100 mg of extract from a standard curve.
2.6 Cell culture and anti-proliferative activity
The human hepatoma cancer cell line (Huh-7) and human cervical cancer cell line (HeLa) were seeded in 96-well plate with a concentration of 104 cells in each well and incubated at 37 °C under 5% CO2 in the air for 24 h. Seaweed extracts were added and incubated further for 4–6 h. MTT based assay was performed for the study of anti-proliferative activity following the previously optimized protocol (Patel et al., 2018; Patel et al., 2019; Tanna et al., 2020). Absorbance was taken at 570 nm and following equations were used to calculate the percent anti-proliferative activity:
2.7 Extraction and analysis of metabolites
Previously optimized method was used for the extraction and quantification of metabolites (Pandey et al., 2015; Patel et al., 2020; Yadav et al., 2021). Briefly, dried seaweed samples were extracted with methanol (ice-cold) for 10 min at 70 °C. Supernatant was collected by centrifugation 11000 g for 10 min at 4° C, dried and proceed for derivatization (Mishra and Jha, 2009). Metabolites adonitol/ribitol was used as an internal standard for quantification, which was performed by GC–MS. Mass spectra peaks were compared with the library (NIST– National Institute of Standards and Technology) for the identification, whereas quantification was done using internal standard. Metabolite data were analyzed using partial least squares-discriminant analysis (PLS-DA) (Yadav et al., 2021].
2.8 Statistical analysis
All experiments were done in triplicates and three biological replicas were there in each experiment. Analysis of variance (ANOVA) was applied to all data and threshold for statistical significance was considered p < 0.05, all values are represented as mean ± SD (standard deviation of the mean), and significant difference is shown by different letters. Correlation studies and multivariate analysis were performed using principal component analysis and Pearson’s correlation matrix.
3 Results
3.1 Biological activities of red seaweed–extracts
All red seaweed–extracts showed dose dependent biological activities including total antioxidant, scavenging, and reducing activities (Fig. 1). Seaweeds, GI (70 ± 0.5 %) and AA (62 ± 0.3 %) have high total antioxidant activity at 400 μg concentration. Whereas, GC and HP showed 70 + 0.5 % and 65 ± 0.5 % total antioxidant activity at 1000 μg concentration. Seaweeds, SS and KA showed high antioxidant activity of 64 ± 0.7 % and 56 ± 0.3 % with 400 μg and 1000 μg concentration of extract, respectively (Fig. 1A). High scavenging activity, about 68 ± 0.3 % was observed with GI extract at 1000 μg concentration. Though >50% scavenging activity was noticed with AA (55 ± 1 %) and SS (52 ± 0.6 %) at 600 and 800 μg concentration, respectively. But, both (AA and SS) have shown similar about 58 ± 0.6 % scavenging activity at 1000 μg concentration. Other seaweeds, HP, SR and GC have shown comparatively less activity, about 51 ± 1, 49 ± 0.6 and 46 ± 0.4 %, respectively at 1000 μg concentration (Fig. 1B). Seaweed GI showed the highest reducing activity 40 ± 1 % at 100 μg concentration. Overall, SS has been observed to have the highest reducing activity of 68 ± 0.1 % with 500 μg concentration. The reducing activity was followed by AA, GI, GC and HP with 63 ± 0.2, 57 ± 0.5, 45 ± 0.4 and 43 ± 1%, respectively at 500 μg concentration (Fig. 1C).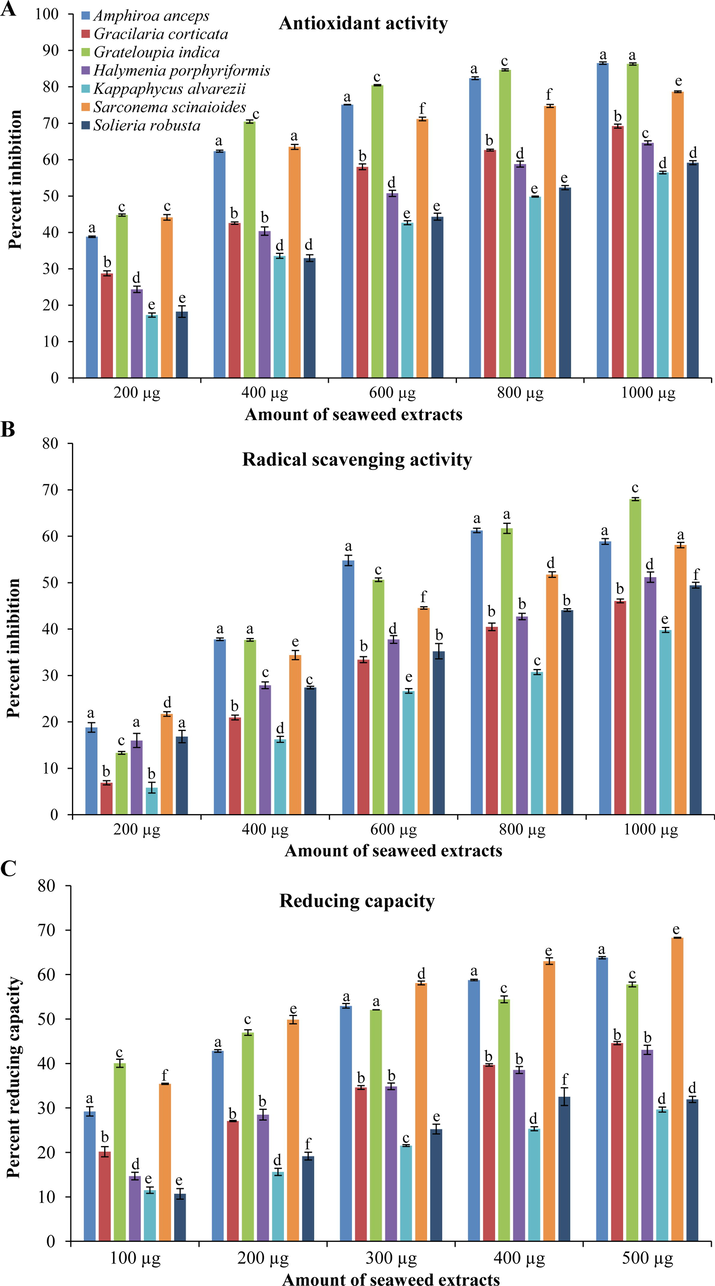
Different biological activities of selected tropical red seaweeds. (A) Total antioxidant, (B) scavenging, and (C) reducing activity. All activities are shown as mean ± SD (n = 3) and different small letters show a statistically significant difference at p < 0.05 (Tukey test).
For all above biological activities, half maximal concentration (EC50) was calculated for each red seaweed–extract (Fig. 2). Seaweed GI showed the highest antioxidant activity with the lowest EC50 values of 120 ± 4 μg ml−1 followed by SS (190 ± 10 μg ml−1), whereas the lowest activity was observed with SR as it showed the highest EC50 (770 ± 6 μg ml−1). Seaweed, GI also showed the highest scavenging activity with EC50 656 ± 1 μg ml−1, followed by AA (672 ± 4 μg ml−1). The lowest scavenging activity was found with KA extract (EC50 1235 ± 20 μg ml−1). In case of reducing power capacity, the lowest EC50 was observed with SS (237 ± 3 μg ml−1), followed by GI (294 ± 4 μg ml−1) and AA (306 ± 2 μg ml−1). Seaweed KA also showed the lowest reducing activity with the highest EC50 (938 ± 18 μg ml−1).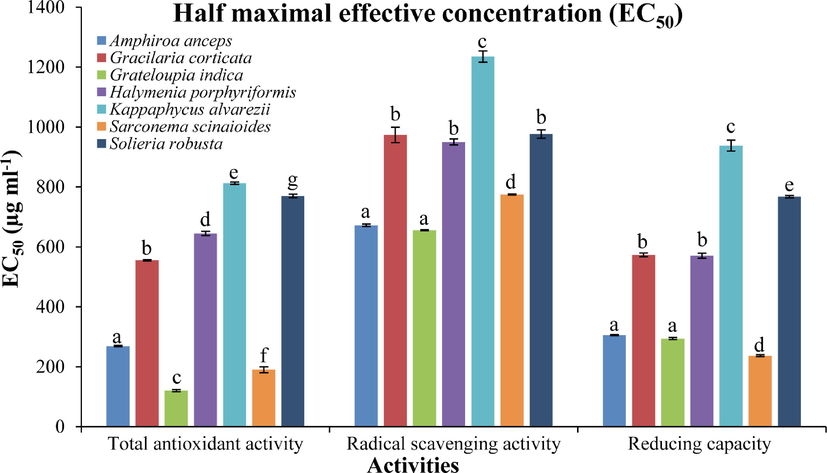
Half maximal effective concentration (EC50; μg ml-1) of red seaweeds for different biological activities. All activities are shown as mean ± SD (n = 3) and different small letters show a statistically significant difference at p < 0.05 (Tukey test).
3.2 Total phenolic and flavonoids contents
The maximum amount of total phenolic content was found with seaweed GI (80 ± 10 mg ml−1 per g extract), followed by AA and SS which contained 20 ± 2 and 15 ± 2 mg TPC per ml per g of extract, respectively (Fig. 3A). Similarly, the highest flavonoid content is also detected with GI (617 ± 72 mg ml−1 per g extract) followed by AA and SS, in which 472 ± 113 and 408 ± 103 mg TFC per ml per g of extract were determined (Fig. 3B). The lowest TPC (8–10 mg ml−1 per g extract) was found in SR, HP and KA; whereas KA also have the lowest TFC (352 ± 110 mg ml−1 per g extract).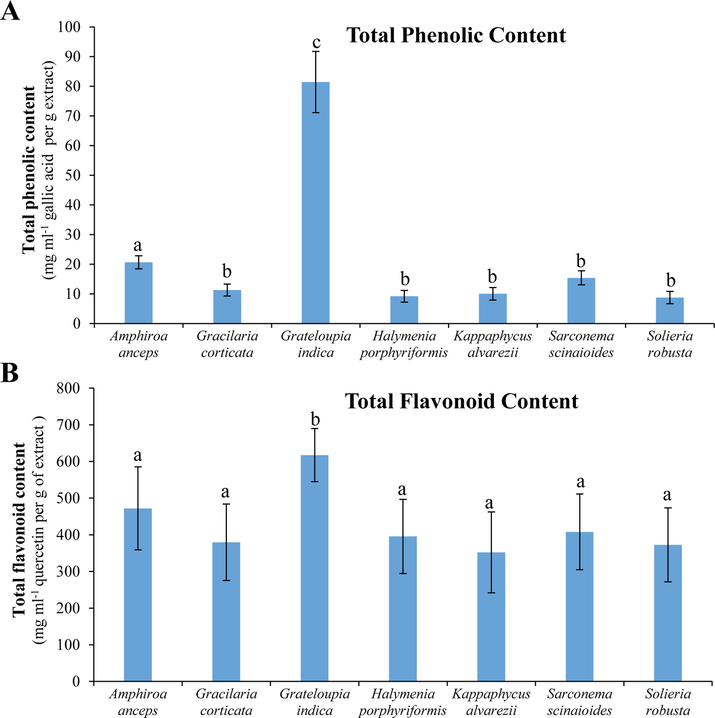
TPC and TFC of tropical red seaweeds. (A) TPC is shown as GAE (mg ml-1) per gram of extract. (B) TFC is shown as quercetin equivalent (mg ml-1) per gram of extract. All data are shown as mean ± SD (n = 3) and different small letters show a statistically significant difference at p < 0.05 (Tukey test).
3.3 Correlation analysis
A correlation analysis was performed individually for all seven red seaweeds (Table S1). A very strong (0.9–1.0; R2 > 0.9) significant (p < 0.05) correlation was observed among all biological activities (total antioxidant, scavenging and reducing) and contents (TPC and TFC) for red seaweeds AA, GC, GI, HP and SR. Seaweed SS also showed strong significant correlations (0.9–1.0; R2 > 0.9; p < 0.05) among biological activities and content, except TFC, which showed moderate (0.5–0.7; R2 = 0.3–0.6) insignificant (p > 0.05) correlation with other biological activities including TPC. Seaweed KA showed strong significant correlation (0.9–1.0; R2 > 0.9; p < 0.05) amongst TPC, total antioxidant and scavenging activities. However, reducing activity showed week (0.35–0.45) while TFC showed strong (0.6–0.7) correlations with other biological activities.
3.4 Anti-proliferative activity
Seaweeds, HP and GI were found to have the highest proliferation-inhibition activities (about 40 ± 0.5%) on HeLa cells. Similarly, GI and KA showed the maximum 44 ± 2% proliferation-inhibition on Huh-7 cells (Fig. 4). Other red seaweeds showed low anti-proliferation activities; GC and AA showed 32–35%, and SR and KA showed 26–28% proliferation-inhibition on HeLa cells. Similarly, seaweeds, AA, GC, HP, SR and SS showed<40% proliferation-inhibition on Huh-7 cells.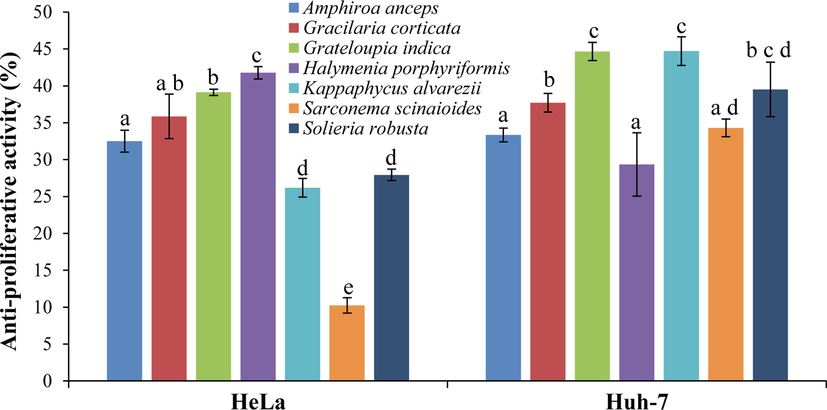
Proliferation–inhibition of tropical red seaweed extracts on carcinoma cell (HeLa and Huh-7) lines using MTT assay. All data are shown as mean ± SD (n = 3) percent inhibition and different small letters show a statistically significant difference at p < 0.05 (Tukey test).
3.5 Multivariate correlation analysis
A cumulative multivariate correlation analysis was performed between biological activities and phytochemical composition (Table S2). Overall total antioxidant of tropical red seaweeds showed a significant (p < 0.5) and very strong (0.9–1.0) correlation with scavenging activity (0.940; R2 = 0.833), TFC (0.870; R2 = 0.757) and proliferation–inhibition of Huh-7 cells (0.708; R2 = 0.501). Overall total antioxidant activity showed a significant strong (0.7–0.9) correlation with reducing activities (0.792; R2 = 0.627), and a moderate (0.4–0.7) correlation with TPC (0.628; R2 = 0.395) and proliferation–inhibition of HeLa cells (0.538; R2 = 0.290). Similarly, overall scavenging activity was strongly correlated with reducing activity (0.763; R2 = 0.582), TFC (0.897; R2 = 0.804) and proliferation–inhibition of Huh-7 cells (0.801; R2 = 0.642). Within phytochemical composition, TPC showed a strong correlation with TFC; whereas TFC not only showed strong correlation with TPC but also with antioxidant and scavenging activities.
A cumulative principal component analysis of different biological activities and contents were performed for red seaweeds (Fig. 5). The biplot inferred from PCA showed 82.42% variation (PC1), and categorized red seaweeds based on their biological activities. The analysis revealed that GI has nutraceutical potential as it showed high biological activities including total antioxidant, total scavenging, anti-proliferative activity of HeLa cells and also have maximum TPC.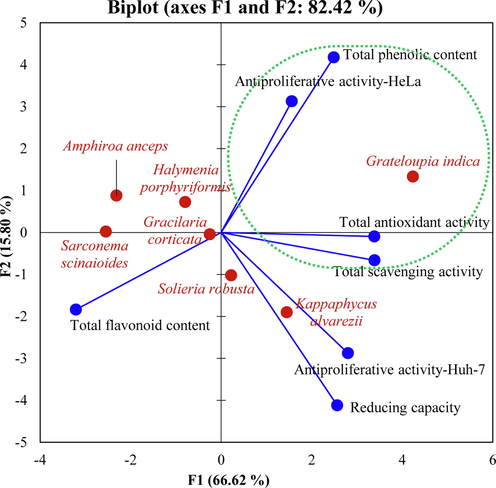
A cumulative principal component analysis. A Bi-plot of different biological activitiesof red seaweeds deduced from the Pearson correlation matrix.
3.6 Metabolite profiling
A total of 57 different metabolites were detected in red seaweeds (Table 1). These metabolites were grouped in the different categories; about 25 types of sugars, 15 fatty acids, 9 sugar derivatives, 7 different amino acids and one polyol were quantified. Maximum 39 different metabolites were detected in SS followed by 23 in GI, 22 in HP, 21 in GC and AA, 19 in KA, and lowest 13 was found in SR. It was also observed that seaweed SS have maximum 12 exclusive metabolites, followed by 4 unique metabolites owned by GI, HP contained 2 while GC have 1 specific metabolites. No exclusive metabolites were detected in other seaweeds AA and SR. Sugar mannobiose was abundantly detected in seaweeds GI (5600 ± 500 µg g−1 DW) followed by AA (4000 ± 250 µg g−1 DW) and SS (2500 ± 150 µg g−1 DW). Seaweed GC contained maximum glucose (11160 ± 185 µg g−1 DW), and KA contained maximum galactose (1275 ± 260 µg g−1 DW), while no abundance of sugars were found in HP and SR seaweeds. Amino acid valine was detected in all seaweeds in the range of 0.5–7 µg g−1 DW. Among fatty acids, palmitic acids were detected in all seaweeds with maximum amount 83 ± 7 µg g−1 DW in GI, followed by SS (40 ± 5 µg g−1 DW). About 20 µg g−1 DW palmitic acids were estimated in seaweeds HP and KA, 17 ± 6 µg g−1 DW palmitic acids was found in AA. Comparatively low amount of palmitic acid was detected in seaweeds SR (10 ± 2 µg g−1 DW) and GC (5 ± 2 µg g−1 DW). Interestingly, GC contained high amount of heptanoic acid (252 ± 43 µg g−1 DW) and octanoic acid (123 ± 7 µg g−1 DW). Metabolites allose, galactonic acid, glucuronic acid and pinitol were exclusively detected in GI. Similarly, glucose and tagatofuranose were found exclusively in GC and KA, respectively. Two sugar-metabolites lyxose and ribofuranose were detected exclusively in HP. About 12 different metabolites, decanoic acid, dodecanoic acid, lignoceric acid, octadecenoic acid, allopyranose, mannitol, sucrose, tagatose, talose, trehalose, xylose and arabinitol were observed exclusively in seaweed SS. ^Seaweed codes used in the study; AA: Amphiroa anceps, GC: Gracilaria corticata, GI: Grateloupia indica, HP: Halymenia porphyriformis, KA: Kappaphycus alvarezii, SS: Sarconema scinaioides, and SR: Solieria robusta. ‘––’ not detected or negligible amount detected.
Category
Metabolites↓
Amount (µg g−1 DW)
Seaweeds^ →
AA
GC
GI
HP
KA
SS
SR
Amino acids
Alanine
50 ± 10
44 ± 16
––
––
––
35 ± 7
22 ± 6
Glutamic acid
––
1 ± 0.5
––
––
––
20 ± 7
––
Glycine
3 ± 1
––
––
5 ± 1
1 ± 0.5
––
1.5 ± 0.5
Leucine
––
5.5 ± 2.0
––
2 ± 1
––
––
––
Threonine
1 ± 0.5
3.5 ± 1
––
––
––
2 ± 0.5
––
Tyramine
––
2.5 ± 2
––
––
1.5 ± 0.2
1.5 ± 0.5
Valine
2 ± 0.5
7 ± 2
––
4 ± 2
0.5 ± 0.1
3 ± 1
1.5 ± 0.5
Fatty acids
Arachidic acid
––
––
1 ± 0.5
––
––
2.5 ± 1
––
Decanoic acid
––
––
––
––
––
0.2 ± 0.05
––
Dodecanoic acid
––
––
––
––
––
1 ± 0.1
––
Eicosanoic acid
––
––
1.5 ± 0.5
––
––
2 ± 0.2
––
Heptanoic acid
0.2 ± 0.1
2 ± 0.2
––
––
0.5 ± 0.1
1.5 ± 0.5
1.5 ± 0.1
Hexanoic acid
0.6 ± 0.02
––
2.5 ± 2
0.2 ± 0.03
1 ± 0.5
––
2 ± 0.05
Lignoceric acid
––
––
––
––
––
0.5 ± 0.1
––
Myristic acid
1 ± 0.05
––
4 ± 0.5
1.5 ± 0.5
1 ± 0.1
2 ± 0.5
1 ± 0.05
Octadecenoic acid
––
––
––
––
––
8.5 ± 5
––
Octanoic acid
1 ± 0.2
5 ± 1
1.5 ± 0.5
2 ± 1
2 ± 1
3 ± 0.1
––
Oleic Acid
1 ± 0.5
4.5 ± 2
––
––
––
––
––
Palmitic Acid
17 ± 6
125 ± 7
83 ± 7
20 ± 0.5
20 ± 1
40 ± 5
10 ± 2
Pentanoic acid
1 ± 0.5
––
1.3 ± 0.7
1 ± 0.5
0.5 ± 0.1
7 ± 1
––
Stearic acid
2.5 ± 0.5
20 ± 0.5
15 ± 2
3 ± 0.1
3 ± 0.1
7 ± 1
2 ± 0.5
Valeric acid
––
5 ± 1
––
––
––
8 ± 1
––
Sugars
Allopyranose
––
––
––
––
355 ± 15
––
Allose
––
––
1 ± 0.1
––
––
––
––
Arabinofuranose
––
––
––
4 ± 0.2
1 ± 0.5
––
––
Arabinose
1 ± 0.3
––
––
1 ± 0.05
––
––
––
Cellobiose
––
––
––
––
30 ± 15
0.5 ± 0.1
3 ± 0.1
Deoxyribose
––
6 ± 0.5
––
––
––
7 ± 0.5
––
Fructose
––
2 ± 0.5
1 ± 0.1
1 ± 0.5
––
0.5 ± 0.05
––
Galactofuranose
1.5 ± 1.0
4 ± 1
––
––
1.5 ± 0.5
2.5 ± 1
1 ± 0.1
Galactose
1 ± 0.7
––
2 ± 1
––
1275 ± 260
1 ± 0.2
––
Glucopyranose
––
––
15 ± 5
––
––
0.5 ± 0.1
2 ± 0.3
Glucose
––
255 ± 45
––
––
––
––
––
Glyceryl-glycoside
90 ± 10
58 ± 22
510 ± 80
10 ± 0.5
20 ± 2
70 ± 12
––
Lyxose
––
––
––
0.5 ± 0.2
––
––
––
Maltose
––
11160 ± 185
––
––
––
1 ± 0.1
––
Mannitol
––
––
––
––
––
1 ± 0.5
––
Mannobiose
4000 ± 250
––
5600 ± 500
––
––
2500 ± 150
––
Myo-Inositol
1 ± 0.5
2 ± 0.5
3 ± 1.5
1 ± 0.1
1 ± 0.5
1 ± 0.1
––
Ribofuranose
––
––
––
1 ± 0.1
––
––
––
Scyllo-Inositol
––
––
––
4 ± 0.5
2 ± 0.2
10 ± 6
––
Sucrose
––
––
––
––
––
6 ± 2
––
Tagatofuranose
––
––
––
––
0.2 ± 0.1
––
––
Tagatose
––
––
––
––
––
0.5 ± 0.1
––
Talose
––
––
––
––
––
1 ± 0.1
––
Trehalose
––
––
––
––
––
5 ± 1
––
Xylose
––
––
––
––
––
2 ± 1
––
Sugar derivatives
Arabinitol
––
––
––
––
––
2 ± 1
––
Erythritol
––
––
2 ± 0.5
1.5 ± 0.5
––
2 ± 0.5
1 ± 0.5
Galactonic acid
––
––
1 ± 0.1
––
––
––
––
Glucitol
0.5 ± 0.2
2 ± 0.1
––
2 ± 0.05
––
––
––
Glucuronic acid
––
––
2 ± 1
––
––
––
––
Threitol
––
0.5 ± 0.1
2 ± 0.1
––
––
––
––
Threonic acid
115 ± 55
35 ± 5
10 ± 3
5 ± 1
1 ± 0.2
3 ± 0.2
––
Xylitol
0.3 ± 0.1
––
0.5 ± 0.2
3 ± 0.2
0.5 ± 0.1
––
––
Xylonic acid
––
––
––
1 ± 0.1
––
––
––
Polyol
Pinitol
––
––
1 ± 0.05
––
––
––
––
A score plot was inferred using PLS-DA (Partial Least Squares Discriminant Analysis) for metabolites which were commonly detected in all seaweeds (Fig. 6). PLS-DA plot showed two important key measures; one is variable importance in projection (VIP) and the second is the weighted sum of absolute regression coefficients. The colored boxes on the right indicate the relative concentrations of the corresponding metabolite in each red seaweed group. A hierarchical cluster analysis was done and a heat map was inferred using Spearman’s rank correlation coefficients (Supplementary Figure S1). The deduced heat map showed differential accumulation of metabolites in different red seaweeds.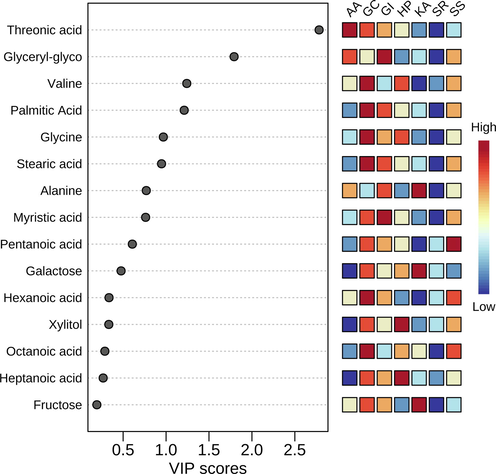
A score plot inferred by partial least squares discriminant analysis for metabolites commonly detected in all red seaweeds. AA: Amphiroa anceps, GC: Gracilaria corticata, GI: Grateloupia indica, HP: Halymenia porphyriformis, KA: Kappaphycus alvarezii, SS: Sarconema scinaioides, and SR: Solieria robusta.
4 Discussion
Recently the priority for consumers turned to the healthiness, lifestyle and comfort without neglecting environmental and sustainability concerns (Santos et al., 2020). The phrase “Good food, good health” means a lot itself. In the last few decades, seaweeds get attention of researchers because it offers the opportunity of discovering a broad range of both primary and secondary metabolites which have interesting properties and applications (Tanna and Mishra, 2018a; Tanna and Mishra, 2019; Choudhary et al., 2021). Seaweeds possess valuable metabolites like vitamins, polyphenols, minerals, as well as strong balance of n-3 to n-6 ratio of PUFA. Seaweeds can live and adapt in harsh and extreme conditions and in addition to that they are easily cultivated and thus economical (Ragaza et al., 2015). Researchers mainly focus on the seaweeds extracts and their chemical characterization which publicized their variety of components with very attractive biological activities such as antimicrobial, antiviral, anti-inflammatory, antitumor, fat-lowering, neuroprotective and antihypertensive activities (Hakim and Patel, 2020). In the present study, seven red seaweeds which are abundantly growing in their natural habitat were studied for their phytochemical composition and biological activities, keeping their further exploration in the future.
Primary metabolites contribute their role directly in physiological function of organism, under normal growth which includes photosynthesis, whereas secondary metabolites are produces under diverse environmental stress conditions which are temperature and salinity fluctuations, ultraviolet exposure and environmental pollutants (El-Beltagi et al., 2019). Primary metabolites include normal ones, polysaccharides, lipids and proteins while the secondary metabolites produced in seaweeds tissues which are halogenated compound, phenolic compounds, terpenes, phenolic compounds and small peptides along with their bioactivity (Rico et al., 2017). GC has shown the presence of essential amino acids such – leucine, threonine and valine. Amongst which valine and leucine are dietary branched chain amino acid that has shown to be useful for immune response (Calder, 2006) as well as injury induced cognitive repair system (Ibuki et al., 2011). Specifically, valine was found to be present in all selected seaweeds except for GI. Seaweeds AA, GI, SS showed very high levels of Mannobiose sugar, which has proven to activate innate immune system against Salmonella enteritidis (SE) infection which is food-born illness in humans (Ibuki et al., 2011). Other sugars like glyceride-glycoside was present in all collected seaweeds except for Solieria robusta. Glucose was found to be present in very high amount in Gracilaria corticata. Other sugars such as arabinofuranose, fructose, galactofuranose were found to be present in multiple selected red seaweeds in significant quantity. Various sugar derivatives such as threonic acid was also found to be present in all selected seaweeds except for Solieria robusta. There has been study-showing magnesium salt of threonic acid to have beneficial effect on Alzheimer’s patient condition (Wroolie et al., 2017). Grateloupia indica showed presence of important omega-6 polyunsaturated fatty acid- arachidic acid. In previous study also it has shown that tropical seaweeds are good source of PUFAs (Kumar et al., 2011).
Polyphenolics are secondary metabolites which are major antioxidants containing one or more hydroxyl group mainly found in plants, fruits, beverages such as wine, tea and coffee and also in vegetables (Machu et al., 2015). There have been many studies showing phenolic compounds are directly correlated to antioxidant activity in various red seaweeds. Marine seaweeds are exposed to light and oxygen variations, which generate stress and radicals in them, but they are stable to these oxidation conditions, which indicate that they have well established defense mechanism (Matanjun et al., 2008). There have been studies involving direct correlation of brown (Tanna et al., 2021) and green seaweeds with their biological activities such as antioxidant activities with polyphenolic and flavonoid contents. In this study, we have shown the correlation of red seaweeds selected along the Gujarat coast.
Polyphenols from Neopyropia tenera (formerly Porphyra tenera), Palmaria palmata (Machu et al., 2015), Kappaphycus alvarezii (formerly Eucheuma cottonii), Eucheuma denticulatum (formerly E. spinosum) and Halymenia durvillei (Matanjun et al., 2008), Gracilaria birdiae and Crassiphycus corneus (Gracilaria cornea) from Brazil (Souza et al., 2011), Gracilaria corticata of Indian origin (Sachindra et al., 2010) have shown direct correlation between phenolic content and antioxidant activity of their extracts. Antioxidant activity and phenolic content of Gracilaria gracilis (collected in summer from Italy) has shown to be higher in comparison to commercial antioxidant products. In this study, red seaweeds, including Amphiroa anceps, Gracilaria corticata, Grateloupia indica, Halymenia porphyriformis and Solieria robusta have shown significant correlation between phenolic content and antioxidant activity. Grateloupia indica has shown highest total phenolic (80 ± 10 mg ml−1 per g extract) and flavonoid (617 ± 72 mg ml−1 per g extract) content (Fig. 3) amongst all studied red seaweeds. The lowest EC50 of antioxidant (120 ± 4 μg ml−1) and scavenging (656 ± 1 μg ml−1) activity was detected for Grateloupia indica, while the lowest reducing power was observed in Sarconema scinaioides extract (237 ± 3 μg ml−1), followed by Grateloupia indica (294 ± 4 μg ml−1) (Fig. 2). Grateloupia indica has also shown the highest anti-proliferative activity on Huh-7 cell lines. Various species of Grateloupia have been shown to possess high antioxidant activities such as Grateloupia livida (Tang et al., 2017) due to presence of sulfated polysaccharides; Grateloupia turuturu with its UV-shielding capability due to antioxidant compounds (Félix et al., 2020).
Myristic acid has shown to possess antimicrobial activity in previous studies (Liu and Huang 2012). Red seaweed Ceramium rubrum possessed high content of myristic acid and also shown for high antimicrobial activity (Cortés et al., 2014). Similarly, red seaweeds Polysiphonia virgata and Grateloupia turuturu showed high antibacterial activities (Saravanakumar et al., 2008; García-Bueno et al., 2015). In our study, high content of myristic acid was detected in GI which suggests that this seaweed could explore further for antimicrobial activities. High anti-inflammatory activity was reported for Kandelia candel due to high content of glyceride glycosides (Dat et al., 2015). In this study, high glyceride glycoside content was found in GI, this could be responsible for the high antioxidant activity. Seaweed GI could also be explored for the anti-inflammatory activity.
Previous studies confirmed that Grateloupia indica possessed high antioxidant and flavonoids including ascorbic acid, catechins, gallic acid and luteolin (Tanna et al., 2019). Previously, Amphiroa anceps also reported to have high gallic acid and catechin along with low quantity of proanthocyanine and apigenin (Tanna et al., 2019). Overall study demonstrates that red seaweed, Grateloupia indica contains high TPC and TFC, also showed high proliferation inhibition.
5 Conclusions
Among all seven studied red seaweeds, high antioxidant activities were found in GI followed by SS. Similarly, GI showed high scavenging activity followed by AA. However, SS showed high reducing activity followed by GI and AA. Seaweed, GI was found to be rich in phenolics and flavonoid contents, followed by AA and SS. HP and GI showed high proliferation inhibition on Hela while GI and KA showed on Huh-7 cell lines. Overall, GI (Grateloupia indica) considered the potential candidate for nutraceutical applications followed by AA (Amphiroa anceps) and SS (Sarconema scinaioides). HP (Halymenia porphyriformis) and KA (Kappaphycus alvarezii) also showed potential proliferation inhibition activities along with Grateloupia indica. The study confirms that flavonoid and phenolic compounds are major contributors for biological activities. Based on study, Grateloupia indica is recommended for nutraceutical application as it contain high nutritional values and antioxidant properties with anticancer activities.
Funding
King Saud University, RSP-2021/118.
Data Availability Statement
Not applicable.
Acknowledgement
The authors extend their deep appreciation to the Researchers Supporting Project (RSP-2021/118), King Saud University, Riyadh, Saudi Arabia for OA support. Defence Research and Development Organization (DRDO), New Delhi, India is gratefully acknowledged for the research support to A.M. Authors, B.T. and B.C. are thankful to CSIR and UGC, respectively, for research fellowships. AESD & CIF of the Institute (CSIR-CSMCRI, Bhavnagar) is duly acknowledged for running the samples. C.R.K. Reddy and Santlal Jaiswar are duly acknowledged for identifying the collected seaweeds. CSIR-CSMCRI Communication No. is PRIS-242/2021
References
- Phytochemical composition, in vitro antioxidant, antibacterial potential and GC-MS analysis of red seaweeds (Gracilaria corticata and Gracilaria edulis) from Palk Bay India. Biocatal. Agric. Biotechnol.. 2018;15:63-71.
- [CrossRef] [Google Scholar]
- Edible seaweeds: a potential novel source of bioactive metabolites and nutraceuticals with human health benefits. Front. Mar. Sci.. 2021;8:740054
- [CrossRef] [Google Scholar]
- Development of naturally activated edible films with antioxidant properties prepared from red seaweed Porphyra columbina biopolymers. Food Chem.. 2014;146:6-14.
- [CrossRef] [Google Scholar]
- Novel antimicrobial activity of a dichloromethane extract obtained from red seaweed Ceramium rubrum (Hudson) (Rhodophyta: Florideophyceae) against Yersinia ruckeri and Saprolegnia parasitica, agents that cause diseases in salmonids. Electron. J. Biotechnol.. 2014;17(3):126-131.
- [Google Scholar]
- Anti-inflammatory Triterpenes and Glyceryl Glycosides from Kandelia candel (L.) Druce. Nat. Product Sci.. 2015;21(3):150-154.
- [Google Scholar]
- El-Beltagi, H.S., Mohamed, H.I., Abou El-Enain, M.M,. 2019. Role of secondary metabolites from seaweeds in the context of plant development and crop production. In Seaweeds as Plant Fertilizer, Agricultural Biostimulants and Animal Fodder, Pereira, L., Bahcevandziev, K., Joshi, N.H., Eds.; CRC Press: Boca Raton, Florida, USA, pp. 64-79.
- FAO (Food and Agriculture Organisation of the United Nations). 2021. Fishery and Aquaculture Statistics. Global Production Statistics 1950–2019. Food and Agriculture Organisation of the United Nations, Rome. www.fao.org/fishery/statistics/software/fishstatj/en.
- Industry-friendly hydroethanolic extraction protocols for Grateloupia turuturu UV-shielding and antioxidant compounds. Appl. Sci.. 2020;10(15):5304.
- [CrossRef] [Google Scholar]
- Potential of a renin inhibitory peptide from the red seaweed Palmaria palmata as a functional food ingredient following confirmation and characterization of a hypotensive effect in spontaneously hypertensive rats. J. Agric. Food Chem.. 2014;62(33):8352-8356.
- [CrossRef] [Google Scholar]
- The red seaweed Gracilaria gracilis as a multi products source. Mar. Drugs.. 2013;11(10):3754-3776.
- [CrossRef] [Google Scholar]
- Seasonal variation in the antivibrio activity of two organic extracts from two red seaweed: Palmaria palmata and the introduced Grateloupia turuturu against the abalone pathogen Vibrio harveyi. Aquatic Living Resources. 2015;28(2–4):81-87.
- [Google Scholar]
- A review on phyto constituents of marine brown algae. Future J. Pharm. Sci.. 2020;6(1):1-11.
- [CrossRef] [Google Scholar]
- Antioxidant and free radical scavenging activity of Spondias pinnata. BMC Complement. Altern. Med.. 2008;8:63.
- [CrossRef] [Google Scholar]
- β 1–4 mannobiose enhances Salmonella-killing activity and activates innate immune responses in chicken macrophages. Vet. Immunol. Immunopathol.. 2011;139:289-295.
- [CrossRef] [Google Scholar]
- Minerals, PUFAs and antioxidant properties of some tropical seaweeds from Saurashtra coast of India. J Appl Phycol.. 2011;23:797-810.
- [CrossRef] [Google Scholar]
- Antimicrobial activity of curcumin-loaded myristic acid microemulsions against Staphylococcus epidermidis. Chem. Pharm. Bull.. 2012;60(9):1118-1124.
- [Google Scholar]
- Phenolic content and antioxidant capacity in algal food products. Molecules.. 2015;20:1118-1133.
- [CrossRef] [Google Scholar]
- Antioxidant activities and phenolics content of eight species of seaweeds from north Borneo. J. Appl. Phycol.. 2008;20:367.
- [CrossRef] [Google Scholar]
- Non-targeted metabolomics and scavenging activity of reactive oxygen species reveal the potential of Salicornia brachiata as a functional food. J. Funct. Foods.. 2015;13:21-31.
- [CrossRef] [Google Scholar]
- Isolation and characterization of extracellular polymeric substances from micro-algae Dunaliella salina under salt stress. Bioresour. Technol.. 2009;100(13):3382-3386.
- [CrossRef] [Google Scholar]
- Current status of global cultivated seaweed production and markets. World Aquac.. 2014;45(2):32-37.
- [Google Scholar]
- Physio-biochemical composition and untargeted metabolomics of cumin (Cuminum cyminum L.) make it promising functional food and help in mitigating salinity stress. PLoS One.. 2015;10(12):e0144469
- [CrossRef] [Google Scholar]
- Physicochemical characterization, antioxidant and anti-proliferative activities of a polysaccharide extracted from psyllium (P. ovata) leaves. Int. J. Biol. Macromol.. 2018;118:976-987.
- [CrossRef] [Google Scholar]
- Metabolic profiling and scavenging activities of developing circumscissile fruit of psyllium (Plantago ovata Forssk.) reveal variation in primary and secondary metabolites. BMC Plant Biol.. 2020;20:116.
- [CrossRef] [Google Scholar]
- Non-targeted metabolite profiling and scavenging activity unveil the nutraceutical potential of psyllium (Plantago ovata Forsk) Front. Plant Sci.. 2016;7:431.
- [CrossRef] [Google Scholar]
- Physicochemical, scavenging and anti-proliferative analyses of polysaccharides extracted from psyllium (Plantago ovata Forssk) husk and seeds. Int. J. Biol. Macromol.. 2019;133:190-201.
- [CrossRef] [Google Scholar]
- Dietary supplemental effects of red seaweed Eucheuma denticulatum on growth performance, carcass composition and blood chemistry of juvenile Japanese flounder, Paralichthys olivaceus. Aquac. Res.. 2015;46:647-657.
- [CrossRef] [Google Scholar]
- Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic. Biol. Med.. 1999;26:1231-1237.
- [CrossRef] [Google Scholar]
- Production of primary and secondary metabolites using algae. In: Prospects and Challenges in Algal Biotechnology. Singapore.: Springer; 2017. p. :311-326.
- [CrossRef] [Google Scholar]
- Variations in polyphenol and heavy metal contents of wild-harvested and cultivated seaweed bulk biomass: Health risk assessment and implication for food applications. Food Control.. 2019;95:121-134.
- [CrossRef] [Google Scholar]
- Radical scavenging and singlet oxygen quenching activity of extracts from Indian seaweeds. J. Food Sci. Technol.. 2010;47:94-99.
- [CrossRef] [Google Scholar]
- Antioxidant activity, total phenolic and total flavonoid contents of whole plant extracts Torilis leptophylla L. BMC Complement. Altern. Med.. 2012;12:221.
- [CrossRef] [Google Scholar]
- Unraveling the lipidome and antioxidant activity of native Bifurcaria bifurcata and invasive Sargassum muticum seaweeds: a lipid perspective on how systemic intrusion may present an opportunity. Antioxid.. 2020;9:642.
- [CrossRef] [Google Scholar]
- Antimycobacterial Activity of the Red Alga Polysiphonia virgata. Pharm. Biol.. 2008;46(4):254-260.
- [Google Scholar]
- Antioxidant potential of two red seaweeds from the Brazilian coasts. J. Agric. Food Chem.. 2011;59:5589-5594.
- [CrossRef] [Google Scholar]
- Purification, partial characterization and bioactivity of sulfated polysaccharides from Grateloupia livida. Int. J. Biol. Macromol.. 2017;94:642-652.
- [CrossRef] [Google Scholar]
- Phenolic, flavonoid, and amino acid compositions reveal that selected tropical seaweeds have the potential to be functional food ingredients. J. Food Process Pres.. 2019;43(12):e14266
- [CrossRef] [Google Scholar]
- Antioxidant, Scavenging, reducing, and anti-proliferative activities of selected tropical brown seaweeds confirm the nutraceutical potential of Spatoglossum asperum. Foods.. 2021;10(10):2482.
- [CrossRef] [Google Scholar]
- Metabolites unravel nutraceutical potential of edible seaweeds: An emerging source of functional food. Compr. Rev. Food Sci. Food Saf.. 2018;17(6):1613-1624.
- [CrossRef] [Google Scholar]
- Tanna, B., Mishra, A., 2018b. Metabolomics of seaweeds: Tools and techniques. In Plant Metabolites and Regulation under Environmental Stress; Academic Press: Cambridge, MA, USA, pp. 37–52. 10.1016/B978-0-12-812689-9.00002-9.
- Nutraceutical potential of seaweed polysaccharides: Structure, bioactivity, safety, and toxicity. Compr. Rev. Food Sci. Food Saf.. 2019;18(3):817-831.
- [CrossRef] [Google Scholar]
- Metabolite profiling, antioxidant, scavenging and anti-proliferative activities of selected tropical green seaweeds reveal the nutraceutical potential of Caulerpa spp. Algal Res.. 2018;36:96-105.
- [CrossRef] [Google Scholar]
- Anti-proliferative and ROS-inhibitory activities reveal the anticancer potential of Caulerpa species. Mol. Biol. Rep.. 2020;47(10):7403-7411.
- [CrossRef] [Google Scholar]
- An 8-week open label trial of L-threonic acid magnesium salt in patients with mild to moderate dementia. Pers. Med. Psychiatry.. 2017;4–6:7-12.
- [CrossRef] [Google Scholar]
- Differential Accumulation of Metabolites in Suaeda Species Provides New Insights into Abiotic Stress Tolerance in C4-Halophytic Species in Elevated CO2 Conditions. Agronomy.. 2021;11:131.
- [CrossRef] [Google Scholar]
- The determination of flavonoid contents in mulberry and their scavenging effects on superoxide radicals. Food Chem.. 1999;64:555-559.
- [CrossRef] [Google Scholar]
Appendix A
Supplementary material
Supplementary data to this article can be found online at https://doi.org/10.1016/j.arabjc.2022.103868.
Appendix A
Supplementary material
The following are the Supplementary data to this article:Supplementary data 1
Supplementary data 1







