Translate this page into:
Biodiesel production evaluating the use and reuse of magnetic nanocatalysts Ni0.5Zn0.5Fe2O4 synthesized in pilot-scale
⁎Corresponding author. joelda.dantas@cear.ufpb.br (J. Dantas)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
Ni0.5Zn0.5Fe2O4 magnetic heterogeneous nanocatalyst synthesized by combustion reaction with the important differential of the production in pilot-scale of until 200 g/batch. Use of these materials in the reactions of transesterification and esterification via the methyl and ethyl route for an environmentally correct biodiesel production. Intrinsic magnetic characteristic of these nanocatalyst that allows its easy recovery for the reuse in other production cycles.
Abstract
Magnetic nanoparticles (MNP) of Ni0.5Zn0.5Fe2O4, successfully synthesized by combustion reaction, with special differential in the production, starting from the scale of 10 g/production to a pilot-scale reproducibility of up to 200 g/production, were used and reused as heterogeneous magnetic nanocatalysts in the reactions of biodiesel production. The obtained nanocatalysts were highly efficient in the biodiesel production with soybean oil, such by the methyl as ethyl routes. It was obtained the best activity in the esterification reaction, favoring conversions of up to 99.54 ± 0.16% on the methyl route and up to 99.38 ± 0.18% on the ethyl route. As regards transesterification, the maximum conversion achieved was 14%. Biodiesel was characterized in terms of viscosity, density, acidity and iodine ratios, whose obtained values place the produced biodiesel within the specifications applicable to the quality standards for its commercialization. MNP were characterized by XRD, BET, TEM, AGM and TPD. Besides, the nanocatalyst was recovered with the help of a simple external magnetic field (magnet) and reused in another 3 cycles, without significant loss in the catalytic activity, indicating considerable stability. Therefore, the nanoferrite Ni0.5Zn0.5Fe2O4 can be validated as a new environmentally correct catalyst for the heterogeneous catalysis in the field of biodiesel production.
Keywords
Biodiesel
Nanocatalyst Ni0.5Zn0.5Fe2O4
Pilot-scale
Magnetism
Reuse
1 Introduction
The interface between the society development, and consequently, of the technologies, make environmental issues worrying and prominent due to the increasing pollution raise that favors degradation. These are complex problems that involve effective difficulties and diverse interests, starting with the economic ones as in turn have social implications of national and international character. So much so that sustainable growth has become a fundamental contemporary theme.
Thus, energy discussions at a global level led to the rational use of biomass, of waste (industry, agriculture and urban waste), of biofuels (ethanol and biodiesel), as well as stimulating the development of new technologies for the use of alternative sources. Biofuels are part of these discussions as one of the most successful and promising strategies to minimize the use of fossil resources and environmental pollution. Studies (Azadi et al., 2017) report that because of their potential role to face the challenges about energy and environmental, biofuels have received significant attention from scientists across a range of disciplines and countries.
In the short term, biofuels are the only renewable resources that can meet the heavy dependence of the transport sector relating to petroleum without replacing the vehicles fleet, and they are rapidly advancing as a new research area to provide alternative sources of renewable energy. Because of this, the efforts made so far to contain greenhouse gases in the transport sector have focused on the biofuels production, and it is important to mention that the oils derived from biomass have advantages that overcome their failures as fuels. On the other hand, it is important to mention that in the rapid global growth of energy consumption, diesel engines play a key role, however, the use of diesel fuel contributes to the harmful pollution by the exhausted air from combustion. As a result, to overcome the serious problems caused, biodiesel extracted from many raw materials has been studied and implemented in the last decades (Yue et al., 2014; Isa and Ganda, 2018; Shameer and Ramesh, 2018).
It is clear that the substitution of the oil-fueled engines by others powered by renewable energy obtained from biomass has been widely studied as an alternative energy source such as biodiesel, that can be defined as a mixture of mono-alkyl esters of fatty acids derived from vegetable oils or animal fat, and that it is pointed out as an excellent alternative. This is because, biodiesel is a powerful alternative fuel and is less polluting and problematic to produce and implement (Yaakob et al., 2014; Hussain and Janajreh, 2018). In this way, being classified as a renewable fuel, biodiesel can solve a range of environmental and energy problems.
So a central question is being incorporated into researches, which is the concern on how to induce technological changes towards cleaner technologies to achieve environmental sustainability, where natural resources are preserved now and in the future, and that there is a pollution reduction even there been a production increase. An exponential proposal for this challenge has been through technological innovations for the production of fuels, such as biodiesel, which represents a less polluting alternative, since it is manufactured from renewable sources, being economically viable and presenting environmental advantages regarding to its fossil competitor, the diesel.
Biodiesel has great strategic importance. Economically because it reduces the demand for diesel of fossil origin and, therefore, the dependence of this derivative of petroleum. Environmentally, it is advantageous due to the reduction of greenhouse gas emissions when considering the whole production cycle and use of biodiesel and diesel. And socially, because it increases the possibilities of employment and income generation, favoring urban and industrial deconcentration and expanding opportunities for regional development and family farming.
The quality of the produced biodiesel is a fundamental factor, because it defines the operation mode and the engine life time, therefore, it is essential to guarantee a qualified product. The American quality standard was developed by the American Society of Testing and Materials (ASTM) through the norm ASTM D6751, and the European Union standard was established by the norm EN 14214 of the European Committee for Standardization (Comité Européen de Normalisation – CEN), which are listed as the best known and they are generally used as a reference or basis for other standards.
Commercially, biodiesel is produced by the transesterification or esterification reaction of vegetable oils or animal fat. These reactions are conducted in the presence of alkali or acid catalysts in the case of transesterification, and in presence of acid catalysts in the case of esterification. The use of each reaction will depend mainly of the vegetable oil or animal fat quality, that is, the fatty acid content and the water content. According to Moeinpour and Khojastehnezhad (2017) they reported that among other chemical reactions used in catalytic processes, the esterification of carboxylic acid with alcohol and the transesterification of fatty oils with alcohol are important for academic research, but also for industrial production.
The reactions of esterification and transesterification can be conducted in the absence of catalysts, but the time and energy cost involved in the completion of these reactions make them unviable (Otera and Nishikido, 2010; Veljković et al., 2014). In processes that use catalysts, the quality and type of them, directly influence the final obtained product, for example, an unwanted saponification reaction can be avoided using heterogeneous acid catalysts (Poonjarernsilp et al., 2015). These catalysts allow the transesterification of triacylglyceride containing high levels of fatty acids and offer the possibility to carry out simultaneous esterification and transesterification reactions. In fact, both esterification and transesterification can be catalyzed using acidic solid catalysts through a single reaction (Zhou et al., 2015).
It is known that to obtain biodiesel, homogeneous catalysts are used, however, the removal of this type of catalyst after the reaction is technically difficult and a large amount of waste water is produced. That is why the interest in replacing these catalysts by heterogeneous solid catalysts, since the latter are easier to operate, they allow less contamination of the product and co-products, easier separation of the catalyst from the reactive medium, possibility of regeneration and reuse (reduction of the corrosion problems) and they reduce significantly the environmental pollution.
Due to this, the heterogeneous catalysts have been the subject of several researches developed because of the improvement provided to the final product, because they do not produce emulsions, they reduce corrosion problem, they are easy to remove themselves, they generate less chemical effluents and allow the use of raw materials with different characteristics. In this last decade much attention has been paid to the conception of some heterogeneous long-cycle and multifunctional catalysts because these catalysts were essential to minimize energy consumption in all separation processes. So a multifunctional catalyst would be more competitive in industry, indicating that these types of catalysts would have a promising prospect in the near future, for example, in transesterification area (Wei et al., 2009; Ramos et al., Chap. 2014,; Xie and Fan, 2014; Zhang et al., 2016; Shi et al., 2017).
In both transesterification and esterification reactions, the use of solid catalysts has become increasingly promising in view of the possibility of recovery and reuse, besides these materials present the physical and chemical characteristics, such as acid and basic Lewis and Brönsted sites and surface area favorable to execute these reactions. In this way, one of the major challenges that industrial research companies, chemical industries and others research centers have faced today has been the catalysts development to produce biodiesel, which together exhibit high activity and selectivity. It is always sought to develop new materials that are selective, of highly dispersed phases, that result in high conversions and have active surfaces.
In the case of recyclability, heterogeneous catalysts based on ternary oxides with magnetic characteristics, such as spinel ferrites type AB2O4, are a promising alternative, because the magnetic separation avoids material loss and increases the reuse time (Shengyang et al., 2011). This is how that several studies have been carried out with the use of ferrites as biodiesel catalysts, because besides the easy separation of the reaction medium, these materials have considerable porosity, high contact area, high thermal stability and good chemical properties, culminating in a more effective participation in the chemical reactions to produce biodiesel (Dantas et al., 2017; Dantas et al., 2016; Zhang et al., 2015; Seo et al., 2014; Xue et al., 2014; Sankaranarayanan et al., 2013).
The use of nanomagnetic materials has been reported in pioneering research in the special field of catalysis for biodiesel, and as result were reported favorable and promising performances for the magnetic nanoferrites (NiCuZn)Fe2O4, (CuZn)Fe2O4, (NiZn)Fe2O4, CoFe2O4, MnFe2O4, NiFe2O4, CuFe2O4 and FeFe2O4 obtained by chemical synthesis by combustion reaction, with a scale of up to 10 g/product by batch, with expressive application in the biodiesel preparation by transesterification and esterification, coupled with easy recovery and aiming the reuse (Dantas et al., 2012a, 2012b, 2013, 2014).
The catalysts obtaining with a greater amount of active chemical sites aiming an expansion of the surface area has become the current challenge of the scientific and business community (Chaturvedi et al., 2012; Ong et al., 2014). Because of this, several studies focus on the particle size reducing of these materials, up to a nanometric scale. Then the nanoscience is the main responsible for such challenges, since it aims at the manipulation of nanoscale structures and their integration to form nanocatalysts with different applications. The nanostructured catalysts have attracted special attention because the activity of the heterogeneous catalyst is dependent on its surface area. Catalytically active materials reduced at the nanoscale (1–100 nm) may exhibit a much larger surface/volume ratio compared to their macroscale properties (Hernández-Hipólito et al., 2014).
The nanocatalysts provide an increase in surface area/volume ratio, inducing high catalytic activities when compared to traditional catalysts with large crystals, resulting in high chemical reactivity (Ong et al., 2014; Moshfegh, 2009). In particular, magnetic nanoparticles (MNP) have been presented as promising materials and stand out due to their diverse applications. The attractive differential in MNP is the high surface area of these materials, the ease of recovery by the use of magnetic field, the reuse and the presence of active sites (Guo et al., 2012).
Studies have shown that catalytic activity of a magnetic solid type in biodiesel synthesis is higher than traditional acid catalysts. This was attributed to the magnetic attraction between the particles, which provided strong ionic interactions, resulting in high activity and stability. Among the main characteristics of the solid catalyst of the magnetic acid type are the easily accessible acidic sites and the simple magnetic separation (Li and Liang, 2017).
Considering the total cycle of biodiesel production, it can say that the researches grow quickly in the search for new routes and new oilseeds sources, considering the content of fatty acids in the raw materials, the extraction efficiency and the possibilities of regionalization of the production. And equally it has an increase in the search for new catalysts, which have different characteristics and that can even be recovered and reused, like the nanomagnetic ones. In this context, biodiesel has been assuming special emphasis and increasing valuation, since its production is capable of harmonizing environmental, economic, social and strategic reasons.
In the light of what has been reported so far, among many already consolidated techniques, the combustion reaction method becomes attractive in the synthesis of a wide variety of advanced materials, including powders, intermetallic compounds, composites and functionally classified ceramic materials. According to the literature (Mukasyan et al., 2015; Xanthopoulou, 2010), the technique consists of an exothermic reaction that emits incandescent heat waves (combustion flame), with the capacity to self-propagate in heterogeneous environments causing the production of solid materials with the desired microstructures, as well as expected physical and chemical properties. The synthesis by combustion reaction has been outstanding in the production of magnetic ceramic systems, since it allows the obtaining of powders with nanometric characteristic, high surface area, high degree of purity, chemical homogeneity and with good crystallinity, besides being a simple and fast technique to obtain the desired end product. Among the main advantages, the technique requires low energy consumption in comparison to the conventional ceramic materials synthesis processes, as well as the significant reduction of the production time to a few minutes of processing, not requiring multiple steps, and still reducing the final cost of the product. In view of this, the search for large scale production is being desired and researched (Costa et al., 2009).
Based on this, the objective of this study was to provide an advanced material that increases the biodiesel production process, such as a catalyst synthesized in a simple and fast way, which exhibit high activity, selectivity, resistance, filterability and regenerability characteristics for reuse, and therefore minimize the overall costs to obtain this biofuel. Then, within the sustainable innovation concept (Barbieri et al., 2010), which is the introduction, production, assimilation or products exploitation, productive processes, new or just improved management or businesses methods that bring economic, social and environmental benefits compared to other alternatives, it was adopted in this work the use of MNP Ni0.5Zn0.5Fe2O4 synthesized by combustion reaction, with special differential of the reproducibility in pilot scale of 200 g/batch, that it was used as heterogeneous magnetic nanocatalyst in the biodiesel production reactions.
2 Experimental
2.1 Nanocatalysts synthesis
The synthesis of Ni0.5Zn0.5Fe2O4 by combustion reaction involved the mixing of metal ion salts as oxidizing reagents (nickel nitrates - Ni(NO3)2·6H2O and hexahydrate zinc - Zn(NO3)2·6H2O, nanohydrate iron nitrate - Fe(NO3)3·9H2O and urea - (NH2)2CO as a reducing agent to form a redox solution. The initial composition of the solution was calculated based on the total valence of the oxidizing and reducing reactants, using the propellants and explosives chemical concepts (Jain et al., 1981). The redox mixture of metallic nitrates and fuel was subjected to direct heating in conical reactors developed for the combustion synthesis that allows the production in different batches of 10, 100 and 200 g of the product (Costa and Kiminami, 2012). The nanocatalysts samples were designated by the nomenclatures PM, MM and GM (10, 100 and 200 g/product, respectively). To ensure the samples reproducibility on three different containers, we carried out 10 syntheses of each sample, PM, MM and GM.
2.2 Nanocatalysts characterization
Samples as synthesized PM, MM and GM were submitted to X-ray Diffraction (XRD), Textural Analysis (BET/BJH), Transmission Electron Microscopy (TEM), Magnetic Measurements (AGM) and Temperature-Programmed Desorption (TPD) characterizations. All characterizations were performed in triplicates to obtain a better accuracy of the results.
The determination of the present phases, crystallinity and the crystallite size of the MNP of Ni0.5Zn0.5Fe2O4 were determined from the diffraction data using a Bruker X-ray diffractometer (model D2 Phaser, Cu-Kα radiation), operating with tube of copper target at a voltage of 30.0 kV and 10.0 mA of current, with detector of 55D160. The crystallinity was determined from the ratio between the integrated area of the peak relative to the crystalline phase and the area related to the amorphous fraction using the Difract Eva software. The medium crystallite size was calculated from the X-ray enlargement line (d311) by deconvolution of the polycrystalline cesium secondary diffraction line (used as standard) using the Scherrer Equation (Sekar and Halliyal, 1998) with the Difract Eva software.
The surface area measurement was performed by the nitrogen/helium adsorption method developed by BET. A new equipment model Nova 3200 brand Micromeritics was used. This technique was also used to determine the average size of particle agglomerates (equivalent spherical diameter) by means of the Reed Equation (Reed, 1996). Theoretical density (ρ) used was 5.361 g/cm3 for Ni-Zn ferrite, obtained according to the crystal data sheet JCPDF 08-0278 from the Shimadzu program data package. The pore volume and the pore diameter were determined by the theory developed by BJH. The morphological aspect of the samples was obtained in a transmission electron microscope (TEM).
The magnetic hysteresis cycles (M x H) of the samples were obtained by an Alternate Gradient Magnetometer (AGM). By means of the M x H curves it was possible to determine some magnetic parameters values, such as: coercive field (Hc), remnant magnetization (Mr), saturation magnetization (Ms). The saturation magnetization was determined by fitting the field data applied to the function M = Sm (1–α/H), where M is the magnetization, Sm is the saturation magnetization, α is the fitting parameter and H is the applied field. Then the magnetic characteristics were obtained from the hysteresis graph, observing the curves behavior in the origin proximities of the Cartesian plane.
The active sites analysis was performed by the temperature-programmed desorption (TPD) method of NH3 molecules. In TPD studies, the solid, previously equilibrated with a gas, under well-defined temperature and partial pressure conditions, is subjected to a heating under temperature and flow schedule of an inert gas, that is, a flow gas that flows out on the sample, by monitoring the continuous gas desorption.
2.3 Catalytic performance
The chemical processes used to obtain biodiesel were the reactions of transesterification and esterification of commercial soybean oil, both by methyl and ethyl routes. For the esterification reactions the raw material of the soybean oil fat was used and artificially acidified with 15% by weight of oleic acid, in order to simulate high acidity, taking as reference some regional fats (castor oil, slaughterhouse fats and frying oil), which present contents between 10 and 20% of free fatty acids. Both reactions were conducted in a stainless steel reactor, which is equipped with a cup of borosilicate with useful volume of 80 mL, pressurized, composed of a duct for thermocouple input and coupled to a manometer. Heating and stirring of the system were promoted by a plate model IKA C-MAG HS 7, the stirring was by a magnetic bar of approximately 2.5 cm.
The catalytic reactions were carried out using 10 g of fatty acid, temperature of 180 °C, oil:alcohol molar ratio of 1:12, with 2% of catalyst for 1 h. These conditions were originated from a reaction optimization, in order to select the reaction and the experimental conditions that offered better conversions of fatty acids to esters. The tests were performed with the PM sample and reproduced in triplicate.
Through the results obtained with the PM nanocatalyst, the methyl and ethyl esterification reaction was selected for continuity of the catalytic tests, this time involving the three samples studied as nanocatalysts (PM, MM and GM), varying the amount of fatty acids from 10 to 30 g, the oil:alcohol molar ratio from 1:12 to 1:15 and the catalyst percentage from 2 to 3%, in order to further maximize the catalytic activity results. These tests were reproduced in septuplicate.
The obtained products were analyzed on a gas chromatograph Varian 450c with Flame Ionization Detection (FID) on a short capillary column of stationary phase Varian Ultimetal “Select Biodiesel Glycerides + RG” (15 m × 0.32 mm × 0.45 μm). The detector temperature was 250 °C and the injector temperature was 240 °C. The oven temperature was programmed from 150 up to 260 °C at a heating rate (ramp) of 10 °C/min. The carrier gas used was H2 of high purity. The samples preparation consisted in the dilution of 50 mg of these in 5 mL of standard n-hexane UV/HPLC (Vetec P.A./A.C.S.) and subsequent injection of 1 μL of the solution into the equipment. The standard used was the internal standard provided by Varian Inc.
2.3.1 Recovery and reuse
The reuse tests were carried out under the best reaction conditions established by the experiments and using the selected sample because of the greater catalytic activity and also whose synthesis was in a larger production scale (GM). The formed products were separated from the mixture and the remaining nanocatalyst was separated by external field and then subjected to some procedures for their recovery and subsequent reuse.
Then we tried to develop a simple and fast methodology that would allow the nanocatalyst recovery, which consisted in the application of an external magnetic field (magnet) to separate the reaction medium, a first wash with hot distilled water (approximately 60 °C), a chemical washing treatment with the N-hexane solvent, centrifugation for 10 min, drying in an oven at 120 °C for 12 h and finally being tested for reuse.
3 Results and discussion
3.1 X-ray diffraction (XRD)
The X-ray diffractograms of the nanocatalysts Ni0.5Zn0.5Fe2O4 obtained by combustion reaction in different containers (10, 100 and 200 g of production) are shown in Fig. 1(a, b and c). X-ray diffraction curves has shown the presence of the main characteristic peaks of the inverse spinel structure, evidenced by the appearance of the main peak 2θ = 35.5°, according to crystal card JCPDF 52-0278, regardless of the type of vessel used (10, 100 and 200 g of production). All the diffractograms has shown peaks with high intensity and high basal width for all the reflections, revealing that the synthesized materials are crystalline and with nanostructural characteristics.
X-ray diffractograms of Ni0.5Zn0.5Fe2O4 nanocatalyst: (a) PM, (b) MM and (c) GM.
Moreover, it can observe that the diffractograms presented in addition to the ferrite spinel Ni-Zn major phase, the presence of characteristic peaks of hematite (Fe2O3) and zinc oxide (ZnO) segregated phases. The phase Fe2O3 was identified by the standard card JCPDS 40-1139, and the phase ZnO was identified by standard card JCPDS 36-1451.
As for the presence of these segregated phases, it can say that they were generated possibly by the rapidity of the syntheses, which did not allow the complete formation and crystallization of the spinel phase under study, which does not compromise the syntheses efficiency, but rather indicates that it can change the synthesis conditions and obtain a single-phase product, if it is essentially necessary. However, another possibility for the second phase traces formation can be attributed to the maximum temperatures reached in the combustion syntheses, because the maximum temperatures reached in all the reactions were very close, not exceeding 800 °C.
Then it would probably be necessary, higher temperature to effectively meet the reagents demand to be dissolved for single-phase formation (spinel). Sinthiya et al. (2015), when they synthesized by the hydrothermal method the ferrite ZnFe2O4 using different fuels, observed the presence of ZnO as a segregated phase, and also attributed to the temperature.
The batch synthesis reproducibility in pilot scale and the efficiency of the combustion method were demonstrated by the diffraction curves reproduced, since the presence of the majority inverse spinel phase of the Ni-Zn ferrite was evidenced with intensity and basal width of the similar diffraction peaks, indicating good crystallinity and crystallite size very close, despite the discrete traces of hematite (Fe2O3) and zinc oxide (ZnO). Moreover, the presence of these oxides may possibly act beneficially for many applications, for example in catalytic reactions, as active metals and promoters.
Structural data such as crystallinity and crystallite size, which were obtained from the XRD results of the nanocatalyst Ni0.5Zn0.5Fe2O4 are shown in Table 1.
Samples
Crystallinity (%)
Crystallite size (nm)
Average crystallinity (%)
Average crystal size (nm)
PM1
PM2
PM362.2
63.9
64.142.1
42.6
41.763.40 ± 1.04
42.13 ± 0.45
MM1
MM2
MM366.1
69.1
66.631.7
32.8
31.167.27 ± 1.61
32.07 ± 0.63
GM1
GM2
GM358.7
56.1
55.638.2
37.1
35.556.80 ± 1.66
36.93 ± 1.36
In general, it was observed a low variation in crystallite sizes that varied from 31.1 to 42.6 nm, indicating that the identified basal reflections diffracted with good uniformity, suggesting that the nanoparticles of Ni0.5Zn0.5Fe2O4 presented characteristic of low anisotropy in diffraction. Thus, it was possible to obtain crystallites at a nanometric scale, without further sintering, confirming the efficiency of the combustion reaction method for the synthesis of these materials. Leal et al. (2018) when they synthesized spinel type MFe2O4 ferrites by combustion reaction, also proved the efficiency of the method in obtaining powders with nanometric characteristics, presenting crystallite sizes varying from 12 to 45 nm. Thakur et al. (2016), they produced the ferrite Ni0.5Zn0.5Fe2O4 by coprecipitation, and only reached crystallite size values of 38.36 to 42.16 nm, when they sintered the material.
Although the crystallite sizes of the three samples were close, the value determined for PM was 23% higher than the value of the MM sample, and approximately 11% in relation to the value of the GM sample, which in turn were closer to each other. This is possibly related to the combustion temperatures reached during the syntheses and can also be attributed to the variation in the amount of the segregated phases reported for the samples.
These assignments are consistent with those reported in the literature, for example, the study of the ferrite Ni-Zn developed by the authors Srinivas et al. (2016), when they concluded that temperature (heat treatment mode), secondary phases formation, preparation method, additives nature, among others, influence crystallite sizes. Saba et al. (2011), they observed an increase in crystallite size and then a decrease with concentration increase of ions Ni2+ in thin films of Ni-Zn. They reported that this variation is due to the formation of the secondary phase of α-Fe2O3 at higher concentrations of Ni2+.
In relation to the crystallinity of the samples, it was also observed that they presented close values ranging from 55.6 to 69.1%, due to the structural organization and the crystals periodicity within the network. In all cases, the combustion reaction process proved to be an excellent technique to obtain crystalline magnetic catalysts, at nanometer scale and large batch.
3.2 Textural analysis by nitrogen adsorption (BET)
As a result of the textural characterization, the adsorption/desorption isotherms of N2 for samples PM, MM and GM are shown in Fig. 2. The study of the adsorption phenomenon was done aiming to obtain information concerning the specific area and the porous structure of the samples, since the construction of the adsorption isotherm curve is of fundamental importance, because its format reveals details about the morphological characteristic, that is, the curve shape is a function of the type of material porosity.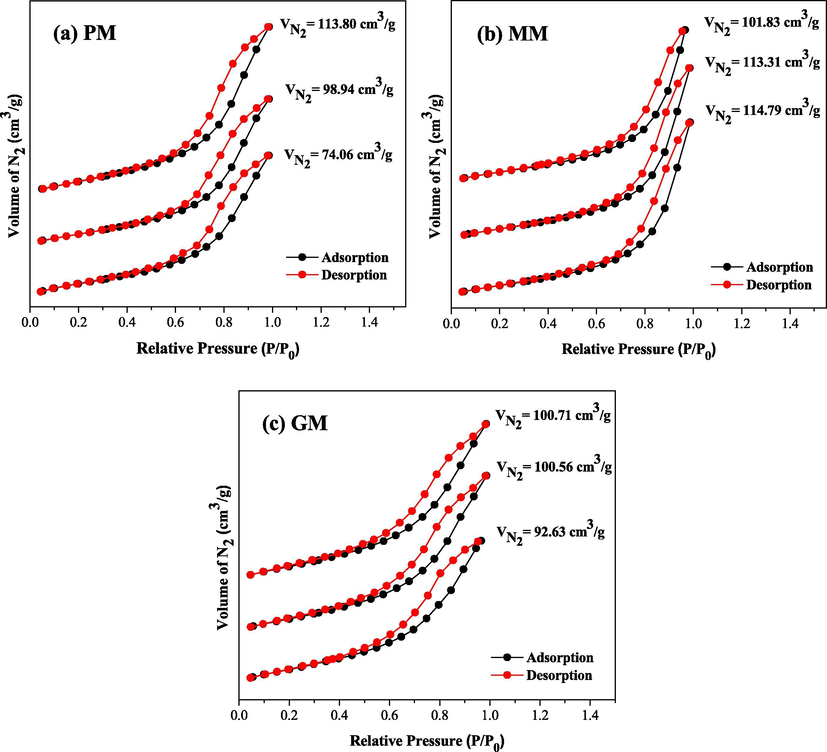
Adsorption/desorption isotherms of N2 in triplicates of the samples (a) PM, (b) MM and (c) GM.
The surface area of a catalyst determines the reactants accessibility to the active sites, and the magnitude of this area determines that a catalyst satisfactorily promotes a chemical reaction. In majority particles their surfaces are quite irregular and such irregularities can range from atomic scale to relatively large pores. Thus in heterogeneous catalysis the gaseous adsorption method of N2 is widely used to study the textural properties of a catalyst and is based on the determination of the amount of an inert gas required to form a mono molecular layer on the catalyst surface, at a constant temperature. Thus, the catalyst surface area is equal to the area to be occupied by each gas molecule under pre-established conditions.
Then, regarding to the particle size obtained by the surface area, there is a gas passing between the particles, then, so thinner they are and more interparticle porosity exists, the reading made in the gas passage will indicate a larger surface area, which is the contact area. This context refers to the importance exerted by the porous characteristic of the studied samples.
Then the curves of Fig. 2 show that the samples PM, MM and GM presented characteristics of mesoporous materials, however, PM and GM characteristics were more similar in some parameters when compared to the sample MM. Then PM and GM presented surface structures with profiles of the isothermal adsorption curve, which according to the IUPAC classification (Sing et al., 1985), they are classified as type V, suggesting a mesoporous material characteristic (pores with rays varying from 10 to 250 Å). Also analyzing the hysteresis form that corresponds to different pore geometries, it can say that the nanoparticles of cited samples are represented by hysteresis loop type 3 (H3) (constituting the formation of wedge-shaped pores, cones or parallel plates), and they are also associated with hysteresis type 2 (H2) (open and closed cylindrical pores with strangulations, bottle-like morphology).
The sample MM has shown a surface structure with a profile of the isothermal adsorption curve framed in type V (solids with mesopores) and together type III (nonporous solids associated with mesopores). The hysteresis form was represented by hysteresis loop type 3 (H3).
Table 2 shows the specific surface area (SBET), particle size (DBET), pore volume (Pv), pore radius (Pr), relation between particle size and crystallite size (DBET/Cs) and the adsorbed volume of N2, corresponding to samples PM, MM and GM.
Samples
SBET (m2g−1)
DBET (nm)
Pv (cm3/g)
Pr (Å)
DBET/Csa
PM
65.289 ± 0.324
17.14 ± 0.08
0.167 ± 0.015
42.754 ± 4.739
0.41
MM
50.942 ± 0.076
21.97 ± 0.04
0.171 ± 0.011
48.042 ± 10.228
0.69
GM
64.171 ± 1.074
17.44 ± 0.29
0.152 ± 0.007
32.630 ± 5.1215
0.47
The samples presented high surface areas. The highest medium specific surface area of 65.29 m2/g was obtained by PM, and the lowest surface area was of 50.94 m2/g, obtained by MM. Consequently, these samples presented, respectively, the lowest (17.14 nm) and the largest (21.97 nm) particle size value. Comparing the particle size results presented by the samples obtained by combustion reaction in different production batches with the particle size reported by Ghasemi and Mousavinia (2014), when synthesizing the same system by the sol–gel method, whose values were between 1 and 4 μm (1000–4000 nm), the technique efficiency is verified by combustion reaction in the pilot-scale nanomaterials production.
It was also sought to correlate the pores volume (which are the voids between the nanoparticles agglomerates, which form their structure) with their respective adsorbed volumes of N2. Fig. 2 shows that sample MM presented the highest volume of adsorbed N2, with a value of 109.98 cm3/g, followed by GM and PM, with values of 97.97 and 95.60 cm3/g, respectively. This is probably related to the porosity of each of them, that is, as larger the porosity, as greater the volume of adsorbed N2, thus confirming the values of obtained pore volumes, which averaged 0.171, 0.152 and 0.162 cm3/g.
Consequently, in the same order, the pore radio values that were 48.042; 32.630 and 42.754 Å, that is, as larger they were, as larger the respective associated volume. Therefore, this corroborates the pore shapes obtained by PM and GM, which according to their hysteresis presented more varied geometries, and even closed pores, which hinders the adsorption/desorption process, possibly leading to a lower porosity, when compared to the sample MM.
Regarding the DBET/CS ratio (particle size/crystallite size), it can be said that the closer to 1, the more it suggests that the particle size is close to the size of the crystal, thereby indicating that the particle tends to be monocrystalline. And that the greater than 1, more polycrystalline is the material. However, in the present study this DBET/CS ratio was below 1, and this is probably related to the differences between the techniques and methodologies used in the collection of these results. In the X-ray diffraction technique, the Scherrer equation (Sekar and Halliyal, 1998) was used, and the textural analysis by adsorption/desorption of N2 was performed based on BET/BJH methodologies (Reed, 1996). For the calculation of particle size (DBET), it was taken into account the surface area of the sample as a whole, ie the crystalline and non-crystalline regions present, in which the N2 gas flows through and fills all the voids present in the sample, leading to a particle size value closer to the real one. However, for the calculation of crystallite size (CS), only the main peak 311 of the crystalline phase Ni0.5Zn0.5Fe2O4 was considered, disregarding the presence of the segregated and/or deleterious phases present in the sample, besides the possible presence of non-crystalline (amorphous) material, possibly leading to less precise (not near real) results of crystallite sizes and with higher values.
Therefore, the obtained results are very attractive for catalysis, because the materials with a greater amount of active chemical sites obtained by the expansion of its surface area, that is, by the particle size reducing to a nanoscale has been the target of several researches, because the increase in the surface area/volume ratio favors high chemical reactivity and high catalytic activity.
3.3 Transmission electron microscopy (TEM)
Fig. 3 shows the light field micrographs of the nanocatalysts PM, MM and GM under study. In these micrographs are observed agglomerates of weakly interconnected nanoparticles with average sizes of 325, 540 and 330 nm, respectively, and consisting of nanometric particles having both irregular and approximately spherical shapes, with average sizes of 18, 23 and 25 nm, respectively. This agglomeration state is probably related to the method of chemical synthesis adopted.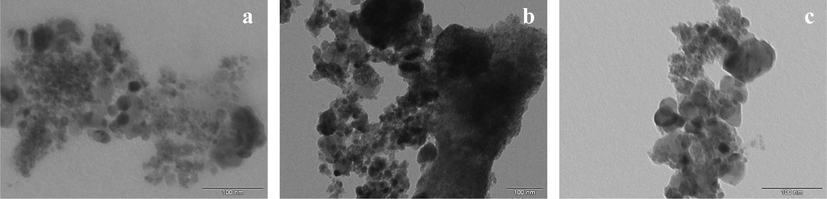
Transmission electron microscopy of the samples: (a) PM, (b) MM and (c) GM.
Particle size values were considerably lower than reported by Wang et al. (2016), which synthesized the ferrite Ni0.8Zn0.2Fe2O4 by the sol gel method, and observed much larger particles with size around 50 nm. Abbas et al. (2014), when studying the nanoferrites Ni0.5Zn0.5Fe2O4 synthesized by two different routes, the sonochemical method and the polyol, reported that the average size obtained by polyol and sonochemical method were 25 and 40 nm, respectively. In this way, stands out once again the efficiency of the combustion reaction technique to obtain particles at nanometric scale, in addition to its pilot-scale production.
These results also confirm the particle sizes obtained in the N2 adsorption (BET) textural analysis, whose values ranged from approximately 17 to 22 nm, proving the nanometric characteristic of the samples.
3.4 Magnetic measurements
In the catalysis, the most evident points in the nanomagnetic materials are the high surface area, the ease of recovery through magnetic field, the reuse and the presence of basic or acidic active sites (Guo et al., 2012; Jing et al., 2015). In addition, heterogeneous catalysts are usually separated from the reaction medium by intensive filtration and/or centrifugation steps, thus much research has been devoted to the development of heterogeneous magnetic catalysts which are readily separable. Therefore, the fact that the nanocatalysts PM, MM and GM have high surface areas (which also favors the presence of active sites), as well as having magnetic properties (which effectively helps to prevent catalyst loss and to increase the recovery rate during the separation process), this places the magnetic catalysts as more advantageous than the conventional heterogeneous catalysts as potential products in the catalytic area.
Fig. 4 shows the dependence of the magnetization M as a function of the applied magnetic field H for the samples PM, MM and GM resulting from the synthesis by combustion reaction in different batches. The curves were plotted with their respective magnifications to visualize the determination of remnant magnetization (Mr) and coercive field (Hc). The magnetic properties of ferrites are appreciably dependent on structure, composition, defects, crystallite size, internal stress and cation distribution.
Hysteresis curves M x H of samples (a) PM, (b) MM, (c) GM and their respective magnifications for determination of Mr and Hc.
It was observed that the three samples have hysteresis curves of ferrimagnetic behavior, which are characteristics of soft magnetic materials, which magnetizes and demagnetizes with low field values, due to their small values of remnant magnetization and coercivity, but different of zero, thereby revealing the complete formation of the narrow magnetic hysteresis cycle.
In Table 3 it has the magnetic parameters (saturation magnetization (Ms), remnant magnetization (Mr) and coercive field (Hc)) calculated from the hysteresis curves of the samples PM, MM and GM.
Sample
Ms (emu/g)
Mr (emu/g)
HC (KOe)
PM
36.5702
2.7717
0.08965
MM
20.9721
1.7996
0.09650
GM
19.8312
1.3244
0.09280
The maximum saturation magnetization value for the sample PM was of 36.5702, for MM was of 20.9721 and for GM was of 19.8312 emu/g. The maximum magnetization presented by PM was 42.65 and 45.77% higher than those presented by MM and GM respectively. These magnetization values for PM, MM and GM were 54.29; 73.78 and 75.21% lower than the value of 80.0 emu/g reported by Jacobo and Bercoff (2016), when they synthesized by the sol gel method the ferrite Ni—Zn to investigate the structural and electromagnetic properties of this pure material and when in replacing to the yttrium ions.
This discrepancy is mainly due to the type of method employed for the synthesis, where the combustion reaction was considerably more efficient for nanoscale production, whose particle sizes (17.14; 21.97; 17.44 nm) were lower to the value reported for the sol gel method (350 nm). This statement can be supported by the report by Ramesh et al. (2016), that ferrites Ni—Zn are excellent soft magnetic materials and that it is well known that ferrite properties are highly sensitive to the preparation method, sintering conditions and chemical modifications.
According to Das and Singh (2016), in general, magnetic properties depend on several factors, such as the size, shape and cations distribution. These authors obtained particle sizes of 40 to 110 nm for the ferrite Ni0.5Zn0.5Fe2O4 synthesized by the self-combustion method, whose magnetization was 73.13 emu/g, which value is higher than the magnetization of 59.0 emu/g reported by Masrour et al. (2014) for particles Ni0.5Zn0.5Fe2O4 produced in bulk by the solid state method. The authors mentioned that the magnetization increase can be explained by changes in the interchange interactions between the tetrahedral and octahedral subunits due to crystallinity, particle shape and magnetization direction.
Therefore, the magnetization values of this work in function of the obtained particle sizes are consistent with the magnetization and particle sizes reported by other authors, because the magnetization is influenced by the particles size. This happens because for different synthesis methods, the magnetization occurs according to the cations distribution in the available interstices, which affects the exchange interactions and, therefore, the total magnetic moment of the ferrite.
This can be supported by Bohr's magnetic moment, which is related to greater or lesser occupation of ions at the octahedral sites (spin down or spin up, respectively), causing increase or reduction of the total magnetic moment of the network. However, as reported by Castro et al. (2014), on the other hand, extrinsic characteristics such as grain and/or particle size influence the magnetic domain area and may contribute to the magnetization increase because as larger the particle and/or grains size as lower the number of barriers, favoring a greater magnetization.
Thus, it is evidenced that the samples synthesized by combustion reaction in different batches are extremely nanometers, have good saturation magnetization and are very reactive, which is perfectly adequate for many applications, such as catalysis.
3.5 Thermal analysis by temperature-programmed desorption (TPD)
In the catalysis area, the MNP can act as promising catalysts, because the manipulation of nanoscale structures leads to obtain nanocatalysts with a greater number of active chemical sites, thus promoting an expansion of the surface area. Netto et al. (2013) they reported that nanoparticles have large surface area as well as high mass transfer area, ideal criteria, for example, for a catalysis support.
The reactivity of the catalysts surface is a direct consequence of its intrinsic characteristics as well as of its synthesis method; however, the reactivity can be affected by conditions that are imposed on these materials to prepare them for a given application. Therefore, a better accuracy of surface reactivity can validate the materials as catalytic active, and this is done with some specific investigations, such as to measure the surface area, to study the shape and distribution of the pores, and to study the acid and basic sites present in the surface. Therefore, the structure, morphology, chemical and thermal state and surface reactivity exert a strong interaction with the nature, quantity and strength of the active sites.
Table 4 shows the results obtained from the TPD analysis. It should be noted that the final temperature results correspond to the average of three results obtained from GM, as well as the volume of adsorbed NH3 corresponds to the average of three results obtained from this same sample after respective correction of the results, considering the deconvolution of the desorption peaks.
GM
Final temperature (°C)
Volume (mLg−1)
Type of acidity
Event 1
197
2.01
Weak
Event 2
309
1.18
Moderate
Event 3
478
0.44
Strong
Thus, it appears that MNP are composed of weak, moderate and strong acid sites, but almost 88% of the volumes of their sites are of a weak and moderate nature, since the results obtained from the TPD, where the desorption events of NH3, considered as the acidic sites present in the MNP, occurred at the final temperatures of 197, 309 and 478 °C, with volumes of 2.01; 1.18 and 0.44 mLg−1, respectively. Therefore, the total volume of acid sites was of 3.63 mLg−1.
Then we can say that the nanoparticles of the samples PM, MM and GM, synthesized by combustion reaction, which have the same chemical composition, structure and morphology, are formed by acid sites. Kurian et al. (2015) they investigated the effect of the preparation method on the structure, magnetism and acid properties of cobalt ferrite nanoparticles synthesized by two different methods (co-precipitation and sol–gel). The authors confirmed the presence of weak, moderate and strong acid sites in the nanoparticles produced by both methods and related the amounts of the acid sites and their variations as a function of the particles agglomeration. The authors justified this according to the results obtained from XRD and SEM.
3.6 Selection of the catalytic process
Fig. 5 shows the results of the catalytic tests obtained in the transesterification and esterification reactions by means of the methyl and ethyl routes of the soybean oil to obtain biodiesel. The reactions were obtained at 180 °C with a molar ratio of 1:12, in the presence of 2% of the sample PM, and reaction time of 1 h, in order to select the process in which MNP of Ni0.5Zn0.5Fe2O4 presented more active.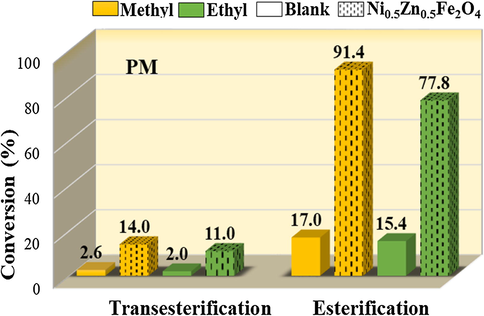
Results of the catalytic tests with the sample PM, in the methyl and ethyl transesterification and esterification reactions.
In general, the analysis of these results confirms that the sample was catalytically active for the reactions of methyl and ethyl transesterification and esterification of soybean oil, since they presented average efficiency (between the methyl and ethyl route), in both investigated reactions, of 12.5 and 84.6%, respectively, that is, the reactions conducted without the presence of the sample (blank test) were higher, where the conversion was on average of 2.3 and 16.2%, respectively.
However, it is observed that the catalytic activity was significantly more satisfactory in the esterification reaction, to the detriment of the transesterification reaction. It can also observe that as in transesterification as in esterification, the nanocatalyst was more active when the alcoholizing agent was methanol. This may have occurred because the methanol is most reactive, whereas the ethyl esters have a higher affinity for glycerin, making it difficult to separate them. That is, these results can be attributed to the fact that, the transesterification and esterification reactions by the ethyl route is significantly more complex than by the methyl one, because with the increase of the carbonic chain of the used alcohol, greater will be the spatial impediment of alcohol with the triacylglyceride.
Taking into account the conversion values in the esterification, it can be inferred that there was a difference of approximately 15% in favor of the methyl route. In transesterification, there was a difference of approximately 21% in the conversion values in favor also of methanol. This may have occurred because, in relation to alcohol, methanol is the most used in these reactions for reasons of physical and chemical nature (short chain and polarity), leading to higher conversions.
Then, the combination of these characteristics of short chain and polarity, coupled with the fact that methanol is also free of water, makes it easier to separate the esters and glycerin, leading to greater conversions. Thus, possibly the water present in the ethanol interfered, for example, in the transesterification reaction, reducing the amount of transesterified product, and provided the emulsion formation in the esters mixture due to the vegetable oil saponification.
Therefore, the alcohol type may possibly be attributed as one of the factors for the disparity in conversions from the transesterification and esterification reactions. Murugesan et al. (2009), they reported that ethyl esters present greater difficulty in formation when compared to methyl esters; biodiesel produced from ethanol has the kinematic viscosity higher than the biodiesel produced from methanol. The authors also reported that during the transesterification reaction there may be the emulsions formation, due to the non-miscibility of the ethanol with triacylglycerides, which makes it difficult to separate and purify the esters.
Although ethanol is also polar in nature because it has a hydroxyl group, it is an oil solubilizing agent, weaker than for example methanol and hexane. Dagostin et al. (2015) they reported that ethanol has limited solubility with triacylglycerols and is able to solubilize the oil not only present in the matrix, but also water and other polar components. If the hydrated ethanol is applied as a solvent, its ability to solubilize triacylglycerides is further reduced, so, gradually, the efficiency process is limited.
This fact assumes that probably the esterification results have been highlighted, because in the transesterification may have occurred pores clogging and active surface poisoning caused by organic species, such as triacylglycerols and glycerol, which led to the decrease of the active sites for the reagents, reducing the conversions of the oil into esters.
On the other hand, a factor that must be attributed as a preponderant to the excellent catalytic activity obtained in the esterification reaction is related to the acidity of the MNP given by TPD (that presented on its surface weak, moderate and strong acidic sites, considered as propitious to promote an efficient catalytic activity), added to the soybean oil acidification used as raw material, since for the transesterification reactions neutral soybean oil was used. A previous acidity indication of the reactional product (biodiesel) of the esterification was verified by the pH measurement of the washing water, which confirmed the simulation efficiency of an acidic raw material promoted by the oleic acid addition.
Later, the acidity of the soybean oil with oleic acid mixture was confirmed by titration, presenting acidity of 0.360 ± 0.006 mg KOH/g, thus meeting the maximum limit of 0.5 mg KOH/g, established by law instrument as biodiesel quality standard. Martins et al. (2016) they reported that the quality of the oil used to produce biodiesel, especially in terms of acidity, is an important index to be appreciated, and should be within specifications, because high acidity, for example, can lead to corrosion or deterioration of the engine. Thus, it is important to analyze the oil acidity and then compare the results with the pre-set parameters.
Because of this, and still in relation to the samples acidity, it is possible to consider the presence of Brönsted and Lewis’s acid sites. It is worth mentioning that although the literature reports that the Brönsted’s acid sites presented in the catalysts are more suitable for the esterification reaction (Di Serio et al., 2008), the catalytic activity may be related to the presence of Lewis’s acidic sites, even in a small amount. For the esterification reaction, Lewis’s acids may be more suitable than Brönsted’s acids, especially because in the presence of these sites, dehydration and racemization of the involved alcohol can be avoided (Narasimharao et al., 2007).
In order to evaluate the performance of the three studied samples (PM, MM and GM), the results of this study showed that the PM sample exhibited a greater efficiency in the esterification, under different reaction conditions, whose results are plotted in Fig. 6. The reactions were performed maintaining the same temperature of 180 °C and the same reaction time of 1 h, varying only the molar ratio from 1:12 to 1:15, as well as the amount of nanocatalyst sample, from 2 to 3%.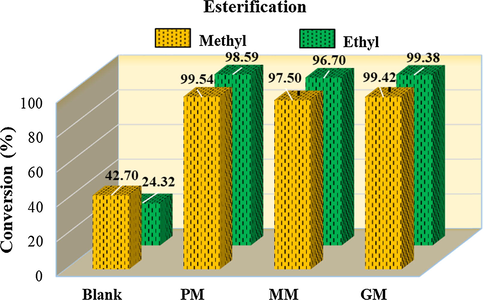
Results of the catalytic tests for the samples PM, MM and GM on methyl and ethyl esterification.
In general, the obtained results express the direct effect promoted by the oil:alcohol molar ratio in the esterification reactions. Firstly, the molar ratio increase was an extremely important variable because it resulted in a very considerable increase in the conversion rate, possibly due to the greater glycerol solubilization remnant by the alcohol. Then the amount of alcohol was predominant for the excellent obtained results, and these results can be understood like have done a more adequate alcohol concentration to execute the esterification reactions.
So much so that even the tests carried out without the presence of the nanocatalyst PM (blank test), realized at a molar ratio between oil and alcohol of 1:12, whose conversions were of 17.0 and 15.4%, increased of about 26% in the methyl route and of 9% in the ethyl route, when compared to the blank tests in ratio of 1:15, whose conversions were 42.70 and 24.32%, respectively. The esterification can be self-catalyzed due to the presence of Brunsted’s functional acids, although weak, in fatty acids and for this reason the catalytic activity should always be compared to a reaction conducted without the presence of the catalyst.
On the other hand, the nanocatalyst increase was also an important variable that contributed to the conversions increase, because probably it propitiated that more nanoparticles per volume were dispersed in the reactional medium where they were involved, since being magnetic a good part of them were retained in the magnetic bar used for stirring.
It was observed that methyl and ethylic routes presented excellent results for the three samples PM, MM and GM as nanocatalysts, whose conversions were respectively 99.54 ± 0.16; 97.50 ± 0.34 and 99.42 ± 0.40% in the methyl route, and 98.59 ± 0.13; 96.70 ± 0.54 and 98.38 ± 0.18% on the ethylic route. Thus, taking into account the blank tests, it is evidenced the strong influence that the presence of nanocatalysts imposed on the conversion of soybean oil to esters, thus revealing the high performance of the studied samples as catalysts for biodiesel production.
Thus, the conversion results, which were all above the minimum limit required for the biodiesel use, which is 96.5%, validate both routes as promising. However, it should be emphasized that the choice, as well as the care with the limits or amount of alcohol involved in the reactions, should be carefully analyzed, because the recovery system of unreacted alcohol is an important variable in the biodiesel production when we have to decide which route to use. For example, in the ethyl route the ethanol recovery makes this process more expensive regarding to energy consumption, to the detriment of the methyl route, due to the dehydration stage of the ethanol in order to be returned to the reaction. Moreover, according to Lora et al. (2012), ethanol must be completely anhydrous, because any presence of water greater than 2% can cause the chemical reaction reverse, and also the triacylglycerides molecular breakdown.
In addition, the type and quantity that make up the mixture species to obtain biodiesel, such as oil, alcohol and catalysts, are closely related to the independent variables such as time, temperature and agitation, thus the properties of the final product are strongly influenced by the involved reactional conditions.
The excellent obtained results can also be attributed to the texture and morphological characteristics presented by these materials, which have high surface areas, composed of mesopores and with presence of active acid sites, which culminated in the high conversion values, because such characteristics are structures for catalysis. The reactions using the samples PM and GM showed conversions a little larger than the reactions with the sample MM, this possibly is related to their respective surface areas, since it is a crucial parameter in catalysis, because it is on it that all reaction is processed, as well as the type and amount of active acid sites present in the nanoparticles.
In addition, it may be that part of the reaction has possibly occurred on the pores surface, and less in its interior, due to its surface shape. This is supported by the argument that in practice, a catalyst does not have its energetically homogeneous surface, that is, with all its equivalent adsorption sites and with the same amount of energy to interact with the reagent molecules. Therefore, that is why in catalysis it is necessary to determine the actually active surface, usually constituted by a set of atoms called sites, which have catalytic activity and are accessible to the reagents (Silva et al., 2008).
Also, the catalytic efficacy of the nanocatalyst can be attributed due to its unique structural properties beyond its thermal and chemical stability. A spinel is formed by the distribution of divalent and trivalent metal ions between the tetrahedral and octahedral coordination sites resulting from the cubic and narrow oxide ion packing. The catalytic activity of the spinel ferrites depends exclusively on the redox properties of the metal ions present in the octahedral sites, since the surface of the spinel structure contains mainly octahedral sites and, consequently, its catalytic activity is directly related to the octahedral cations. Thus, the catalytic activity of these materials is due to the ability of the metal ions to migrate between the subnets without altering the crystalline structure. These properties make this material an efficient catalyst for many chemical reactions (Dantas et al., 2017; Nair and Kurian, 2017).
Then, from the obtained results, it is evident that through combustion reaction, it is possible to start the production from a small scale of 10 g for a production in large scale of 200 g/product, without any loss in the structure of these materials for application in catalysis, in the field of biodiesel production. In addition, the conversions results place the obtained products above the minimum quality limit required by law to use biodiesel in diesel engines. Both esterification reactions by methyl and ethyl route showed excellent conversion values, thus demonstrating the high efficiency that the samples PM, MM and GM can offer to catalyze the biodiesel production reactions, even at the industrial level.
The promising results of achieved catalytic activity corroborate Buller and Strunk (2016) report, that nanostructured materials are widely adequate to face the pressing challenges associated with energy conversion, because they work effectively in the major domains of energy conversion investigation (chemical, thermal, electrochemical and solar). These authors reported that in many cases the catalyst activity and their nanostructure are inseparably correlated.
3.7 Biodiesel characterization
Other quality specifications, in addition to the conversion of triacylglycerides to esters (which is within the standards established by current legislation), should be met as criteria to apply this biodiesel at industrial level, such as viscosity (40 °C), density (20 °C), acidity and iodine index, flammability, peroxides, among others. The first four specifications were investigated for soybean biodiesel catalyzed by the sample GM, and the results are presented in Table 5.
Biodiesel characterization
Catalyst GMa
Acidified oilb
Commercial biodieselc
Acidity index (mg KOH/g)
0.41 ± 0.02
0.48 ± 0.23
0.5
Iodine index (g I2/100 g)
119.17 ± 1.15
125.1 ± 2.23
120
Kinematic viscosity 40 °C (mm2/s)
5.34 ± 0.01
29.50 ± 0.12
3–6
Density 20 °C (g/cm3)
888.30 ± 0.87
917.67 ± 6.02
850–900
These obtained values place the soybean biodiesel produced within the specifications applicable to the commercial biodiesel quality standards, governed by ANP, ASTM and EN standards.
3.8 Recovery and reuse
Regarding the recyclability of heterogeneous catalysts, it is said that it is one of the most important constraints to consider, and in this context, those with inherent magnetic characteristics are more advantageous because they promote different results when it comes to their recovery and reuse.
Fig. 7 shows the excellent magnetic response of the sample GM, synthesized in large scale, where its nanoparticles involved in the oily reaction medium respond promptly to the stimuli of an external magnetic field (magnet), facilitating its recovery after each reaction, by means of a simple, fast and efficient method.
Magnetic response of the nanoparticles of the sample GM against a magnet stimulus.
Therefore, this magnetic property contributes effectively to avoid the nanoparticles loss and to increase their recovery rate during the separation process, because these materials are normally separated from the reaction medium by means of intense filtration and/or centrifugation steps, then, many investigation has been devoted to develop magnetic nanoparticles that are easily separable, and this work not only effectives this research, but it also does the differential to obtain these nanoparticles with highly nanometric characteristics and in large scale.
Bet-Moushoul et al. (2016), they used heterogeneous nanocatalysts CaO—Au to produce sunflower biodiesel, and obtained conversions up to 97% in transesterification. After nanocatalyst recovery by filtration and washing steps, a conversion of 89% was obtained after 10 reuses. The authors attributed the reduction of the catalytic activity to the catalyst solubilization in methanol during the recovery, because it caused loss of catalyst and of the active sites. They also attributed the formation of triacylglycerides and glycerol, which filled the pores, causing the active sites decrease, which consequently reduced the yield.
MNP were reused in the methyl esterification reactions under the same optimized conditions. The conversions results obtained on reuse are illustrated in Fig. 8.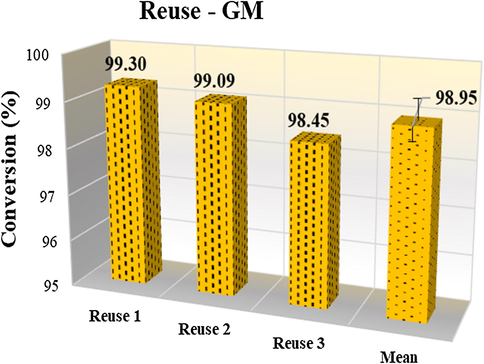
Results of the reuse tests for the sample GM in the methyl esterification.
After three cycles, there was no significant loss of efficiency in the catalytic activity, which presented a mean conversion of 98.95 ± 0.44%. Therefore, the magnetic nanocatalyst sample is economically viable for practical application from the industrial point of view. The magnetic nanoparticles recovery for reuse in catalytic processes, in the most varied fields, has been highly reverberated in the specialized literature. In the biodiesel production field, some authors have reported excellent performances, for example, Liu et al. (2016), which obtained 98.3% of conversion in the transesterification, using the magnetic nanoparticles of the catalyst MgFe2O4@CaO. The catalyst was recovered by external magnetic field and reused 5 times, and still showed activity above 89%. Feyzi and Norouzi (2016), using magnetic nanocatalysts of Ca/Fe3O4@SiO2, obtained high catalytic activity for biodiesel production, which reached 97%. The authors reported that the catalyst was recovered simply by using an external magnetic field and reused several times without appreciable loss of its catalytic activity.
It can be inferred from the achieved results that the proposed structure could be applied successfully in biodiesel production (except for some adjustments that contribute to a reduction in the operating temperature), because the benefits have surpassed the traditional methods, since that in the last few years the proper choice of raw material and catalyst has been seen as a critical point to determinate the cost of biodiesel production in refineries and try to minimize it.
This contributes to the technical-economic approach of the researchers Abubakar et al. (2015) regarding to the production processes in biodiesel refineries. The authors reported that biodiesel production, like other engineering projects, involves important decisions that have to be made under uncertainties arising from a variety of issues, such as the inherent variation in operating conditions and market forces that characterize the inflation, depreciation factors and variations in equipment and production costs.
That is, the authors listed as a critical point in the biodiesel production chain, operational, financial and management issues in the face of present challenges. In this way, the results obtained with the samples PM, MM and GM express great prospects for, in addition to other studies, to reach some constraint aims in the biofuel production, and eventually to offer additional measures of performance that are necessary for potential investors, governments, engineers and other interested parts in order to ensure the safety of the refineries installations and their cost-effectiveness.
Therefore, it was possible to obtain a final product with differential to be produced on a large scale, with wide possibilities of application, thus providing more arguments in the researches sum about magnetic ceramics, which in turn revolves around to present a new material or for new applications, with relatively low cost, and especially with the possibility of having a technological product directed to the society benefit, regarding to the life and the environment preservation, through the contribution for biodiesel production, complying the true role of science, which must be to promote the common good of society in general.
4 Conclusions
The synthesis by combustion reaction was efficient in the production of nanocatalysts Ni0.5Zn0.5Fe2O4 in small and larger production scale (pilot scale), with excellent reproducibility.
The magnetic nanocatalysts of Ni0.5Zn0.5Fe2O4 with spinel-like structure are active and promising materials for esterification and transesterification reactions of soybean oil to obtain biodiesel, regardless of the amount of material produced in the synthesis, which highlights the great viability to produce nanocatalysts by combustion reaction in large scale for industrial use.
The nanocatalyst Ni0.5Zn0.5Fe2O4 was more active in the methyl and ethyl esterification reaction than in the transesterification reactions, showing conversion of the fatty acids in biodiesel of 99.54%, above the minimum limit ruled by law. Specifications such as acidity and iodine index, viscosity and density, are within the specifications applicable to the biodiesel quality standards, ruled by ANP, ASTM and EN.
The magnetic characteristics of the nanocatalyst effectively helped to prevent the nanoparticles loss and to increase their recovery rate during the separation process, which was performed using a magnet. This underscores the advantage that goes beyond other heterogeneous catalysts that do not exhibit magnetic properties.
After 3 reuses of the sample GM, it was observed no significant loss of efficiency in the catalytic activity, which presented an average conversion of 98.95 ± 0.44%. Therefore, the magnetic nanocatalyst sample is economically viable for practical application from the industrial point of view.
From the obtained results, it can conclude that the studied nanocatalyst could be successfully applied in the biodiesel production, because the advantages exceed the traditional methods, because in the last years the adequate choice of raw material and of the catalyst has been a critical point to determinate the cost of biodiesel production in refineries and try to minimize it.
Acknowledgements
The authors thank PNPD/CAPES: Process Number: 23038.007104/2011-84, AUX PE – PNPD – 2490/2011 and CNPq Process Number: 404395/2013-9 and Process Number: 402029/2013-5, MCTM/CNPq Process Number: 190822/2013-9 for financial support.
References
- Shape and size-controlled synthesis of NiZn ferrite nanoparticles by two different routes. Mater. Chem. Phys.. 2014;147(3):443-451.
- [Google Scholar]
- Stochastic techno-economic considerations in biodiesel production. Sustain. Energy Technol. Assess.. 2015;9:1-11.
- [Google Scholar]
- The evolution of the biofuel, Science. Renew. Sustain. Energy Rev.. 2017;76:1479-1484.
- [Google Scholar]
- Innovation and sustainability: New models and propositions. Revista de Administração de Empresas. 2010;50(2):146-154.
- [Google Scholar]
- Application of CaO-based/Au nanoparticles as heterogeneous nanocatalysts in biodiesel production. Fuel. 2016;164:119-127.
- [Google Scholar]
- Síntese por reação por combustão de nanopós de hexaferrita de estrôncio dopada com cromo. Cerâmica. 2014;60:254-258.
- [Google Scholar]
- Dispositivo para produção de nanomateriais cerâmicos em larga escala por reação de combustão e processo contínuo de produção dos nanomateriais, Brasil. Revista de Propriedade Industrial - RPI BR. 2012;10:002181-2183.
- [Google Scholar]
- Combustion synthesis processing of nanoceramics. In: Handbook of Nanoceramics and Their Based Nanodevices (Synthesis and Processing) (first ed.). American Scientific Publishers; 2009. p. :375-392.
- [Google Scholar]
- Liquid–liquid phase equilibrium measurements and modeling for systems involving {soybean oil + ethyl esters + (ethanol + water)} Fuel. 2015;141:164-172.
- [Google Scholar]
- Síntese, caracterização dos espinélios NiFe2O4 e CoFe2O4 e avaliação do desempenho na transesterificação e esterificação do óleo de algodão. Revista Eletrônica de Materiais e Processos (ISSN 1809‐8797). 2012;7(3):174-179.
- [Google Scholar]
- Evaluation of catalyst Ni0.4Cu0.1Zn0.5Fe2O4 on methyl esterification of free fatty acid present in cottonseed oil. Mater. Sci. Forum. 2012;727–728:1302-1307.
- [Google Scholar]
- C.F.M. Use of Ni-Zn ferrites doped with Cu as catalyst in the transesterification of soybean oil to methyl esters. Mater. Res.. 2013;16(3):625-627.
- [Google Scholar]
- Study of nanoferrites Ni0.5Zn0.5Fe2O4 and Ni0.1Cu0.4Zn0.5Fe2O4. Mater. Sci. Forum. 2014;775–776:705-711.
- [Google Scholar]
- Síntese, caracterização e performance catalítica de nanoferritas mistas submetidas a reação de transesterificação e esterificação via rota metílica e etílica para biodiesel. Revista Matéria (ISSN 1517-7076). 2016;21(4):1080-1093.
- [Google Scholar]
- Magnetic nanocatalysts of Ni0.5Zn0.5Fe2O4 doped with Cu and performance evaluation in transesterification reaction for biodiesel production. Fuel. 2017;191:463-471.
- [Google Scholar]
- Structural, magnetic and dielectric study of Cu substituted NiZn ferrite nanorod. J. Magn. Magn. Mater.. 2016;1:918-924.
- [Google Scholar]
- Heterogeneous catalysts for biodiesel production. Energy Fuels. 2008;22(1):207-217.
- [Google Scholar]
- Preparation and kinetic study of magnetic Ca/Fe3O4@SiO2 nanocatalysts for biodiesel production. Renew. Energy. 2016;94 (C):579-586.
- [Google Scholar]
- Structural and magnetic evaluation of substituted NiZnFe2O4 particles synthesized by conventional sol-gel method. Ceram. Int.. 2014;40(2):2825-2834.
- [Google Scholar]
- Solid acid mediated hydrolysis of biomass for producing biofuels. Prog. Energy Combust. Sci.. 2012;38(5):672-690.
- [Google Scholar]
- Biodiesel production with nanotubular sodium titanate as a catalyst. Catal. Today. 2014;220–222:4-11.
- [Google Scholar]
- Acousto-chemical analysis in multi-transducer sonochemical reactors for biodiesel production. Ultrason. Sonochem.. 2018;40(A):184-193.
- [Google Scholar]
- Bio-oil as a potential source of petroleum range fuels. Renew. Sustain. Energy Rev.. 2018;81(1):69-75.
- [Google Scholar]
- Structural and electromagnetic properties of yttrium-substituted Ni–Zn ferrites. Ceram. Int.. 2016;42(6):7664-7668.
- [Google Scholar]
- A new approach to thermo chemical calculations of condensed fuel. Combust. Flame. 1981;40(1981):71-79.
- [Google Scholar]
- Preparation of magnetic nanocomposites of solid acid catalysts and their applicability in esterification. Chin. J. Catal.. 2015;36(2):244-251.
- [Google Scholar]
- Structural, magnetic, and acidic properties of cobalt ferrite nanoparticles synthesised by wet chemical methods. J. Adv. Ceram.. 2015;4(3):199-205.
- [Google Scholar]
- Effect of the surface treatment on the structural, morphological, magnetic and biological properties of MFe2O4 iron spinels (M = Cu, Ni Co, Mn and Fe) Appl. Surf. Sci.. 2018;455C:635-645.
- [Google Scholar]
- Magnetic solid acid catalyst for biodiesel synthesis from waste oil. Energy Convers. Manage.. 2017;141:126-132.
- [Google Scholar]
- Biodiesel production from soybean oil catalyzed by magnetic nanoparticle MgFe2O4@CaO. Fuel. 2016;164:314-321.
- [Google Scholar]
- Lora, E.E.S., Venturini, O.J., 2012. Biocombustíveis, Rio de Janeiro: Interciência, v. 1.
- Effectiveness of methods for reducing tilapia oil acidity for biodiesel production. J. Food Agric. Environ.. 2016;14(1):124-127.
- [Google Scholar]
- Polyphosphoric acid supported on Ni0.5Zn0.5Fe2O4 nanoparticles as a magnetically-recoverable green catalyst for the synthesis of pyranopyrazoles. Arabian J. Chem.. 2017;10(2):S3468-S3474.
- [Google Scholar]
- Combustion synthesis in nanostructured reactive systems. Adv. Powder Technol.. 2015;8366(3):954-976.
- [Google Scholar]
- Production and analysis of biodiesel from non-edible oils—A review. Renew. Sustain. Energy Rev.. 2009;13(4):825-834.
- [Google Scholar]
- Heterogeneous catalytic oxidation of persistent chlorinated organics over cobalt substituted zinc ferrite nanoparticles at mild conditions: Reaction kinetics and catalyst reusability studies. J. Environ. Chem. Eng.. 2017;5(1):964-974.
- [Google Scholar]
- Catalysts in production of biodiesel: A review. J. Biobased Mater. Bioenergy. 2007;1(1):19-30.
- [Google Scholar]
- Superparamagnetic nanoparticles as versatile carriers and supporting materials for enzymes. J. Mol. Catal. B Enzym.. 2013;85–86:71-92.
- [Google Scholar]
- Synthesis and characterization of CuO/C catalyst for the esterification of free fatty acid in rubber seed oil. Fuel. 2014;120:195-201.
- [Google Scholar]
- Esterification (second ed.). Weinhein, Germany: Wiley-VCH Verlog GmbH e Co; 2010. p. :376.
- Simultaneous esterification and transesterification for biodiesel synthesis by a catalyst consisting of sulfonated single-walled carbon nanohorn dispersed with Fe/Fe2O3 nanoparticles. Appl. Catal. A. 2015;497:145-152.
- [Google Scholar]
- Effect of Mn/Co substitutions on the resistivity and dielectric properties of nickel–zinc ferrites. Ceram. Int.. 2016;42(8):9591-9598.
- [Google Scholar]
- Applications of heterogeneous catalysts in the production of biodiesel by esterification and transesterification. Bioenergy Res.: Adv. Appl.. Chap. 2014,;17:255-276.
- [Google Scholar]
- Principles of Ceramics Processing. New York, EUA: John Wiley & Sons; 1996.
- Structure and magnetic properties of NixZn1−xFe2O4 thin films prepared through electro deposition method. J. Mater. Sci.. 2011;46:3574-3582.
- [Google Scholar]
- Catalytic properties of spinel-type mixed oxides in transesterification of vegetable oils. J. Mol. Catal. A: Chem.. 2013;379:234-242.
- [Google Scholar]
- Low-temperature synthesis, characterization, and properties of lead-based ferroelectric niobates. J. Am. Ceram. Soc.. 1998;88:380-388.
- [Google Scholar]
- Effect of barium ferrite particle size on detachment efficiency in magnetophoretic harvesting of oleaginous Chlorella sp. Bioresour. Technol.. 2014;152:562-566.
- [Google Scholar]
- Assessment on the consequences of injection timing and injection pressure on combustion characteristics of sustainable biodiesel fuelled engine. Renew. Sustain. Energy Rev.. 2018;81(1):45-61.
- [Google Scholar]
- Nano-magnetic catalyst KF/CaO–Fe3O4 for bio-diesel production. Appl. Energy. 2011;88(8):2685-2690.
- [Google Scholar]
- Influence of crystal of Fe2O3 in magnetism and activity of nanoparticle CaO@Fe2O3 for biodiesel production. Fuel. 2017;197:343-347.
- [Google Scholar]
- Silva, J.B., Rodrigues, J.A.J., Nono, M.C.A., 2008. Caracterização de materiais catalíticos, Instituto Nacional de Pesquisas Espaciais (INPE), 15252-PUD/198.
- Reporting physisorption data for gas/solid systems - with special reference to the determination of surface area and porosity. Pure Appl. Chem. (IUPAC). 1985;57:603-619.
- [Google Scholar]
- Synthesis of zinc ferrite (ZnFe2O4) nanoparticles with different capping agents. Int. J. ChemTech Res.. 2015;7(5):2144-2149.
- [Google Scholar]
- Structural and magnetic characterization of co-precipitated NixZn1−xFe2O4 ferrite nanoparticles. J. Magn. Magn. Mater.. 2016;407:135-141.
- [Google Scholar]
- Enhancement of magnetic properties of Ni0.5Zn0.5Fe2O4 nanoparticles prepared by the co-precipitation method. Ceram. Int.. 2016;42(9):10664-10670.
- [Google Scholar]
- The wastewater treatment in the biodiesel production with alkali-catalyzed transesterification. Renew. Sustain. Energy Rev.. 2014;32:40-60.
- [Google Scholar]
- Synthesis and electromagnetic properties of La-doped Ni–Zn ferrites. J. Magn. Magn. Mater.. 2016;398:90-95.
- [Google Scholar]
- Application of waste eggshell as low-cost solid catalyst for biodiesel production. Bioresour. Technol.. 2009;100(11):2883-2885.
- [Google Scholar]
- Catalytic properties of the SHS products – Review. Adv. Sci. Technol.. 2010;63:287-296.
- [Google Scholar]
- Biodiesel production by transesterification using tetraalkylammonium hydroxides immobilized onto SBA-15 as a solid catalyst. Chem. Eng. J.. 2014;239:60-67.
- [Google Scholar]
- Biodiesel production from soybean and Jatropha oils by mag-netic CaFe2O4–Ca2Fe2O5-based catalyst. Energy. 2014;68:584-591.
- [Google Scholar]
- A review on the oxidation stability of biodiesel. Renew. Sustain. Energy Rev.. 2014;35:136-153.
- [Google Scholar]
- Biomass-to-bioenergy and biofuel supply chain optimization: Overview, key issues and challenges. Comput. Chem. Eng.. 2014;66:36-56.
- [Google Scholar]
- Biodiesel production directly from oils with high acid value by magnetic Na2SiO3@Fe3O4/C catalyst and ultrasound. Fuel. 2015;150:370-377.
- [Google Scholar]
- Production of biodiesel and hydrogen from plant oil catalyzed by magnetic carbon-supported nickel and sodium silicate. Green Chem.. 2016;18:3302-3314.
- [Google Scholar]
- Nano La2O3 as a heterogeneous catalyst for biodiesel synthesis by transesterification of Jatropha curcas L. oil. J. Ind. Eng. Chem.. 2015;31:385-392.
- [Google Scholar]







