Translate this page into:
Biological activities from andiroba (Carapa guianensis Aublet.) and its biotechnological applications: A systematic review
⁎Corresponding author. adrlui1@yahoo.com.br (Luís Adriano Santos do Nascimento), renatarcrn@gmail.com (Renata Coelho Rodrigues Noronha)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Carapa guianensis is a tree from Meliaceae family traditionally known as andiroba that has a wide range of biological properties, including therapeutic effects, antioxidant activities, insecticidal and repellent effects that can be used in biotechnological approaches to medicine, agriculture, and cosmetic products. Therefore, we aim to explore the biological activities exhibited by this species and their respective biotechnological applications of interest. For this, a systematic review was carried out following the PRISMA guidelines dated from 1993 to 2022 through the Scopus, Web of Science and Agricultural Research Database (Base de Dados da Pesquisa Agropecuária - BDPA), screened for biological activity/bioactive compounds. A total of 129 studies were included in the PRISMA flow analysis. Biological properties and major bioactive compounds, as well as biotechnological approaches could be identified. The biological activity from C. guianensis could be observed in different vegetative parts through diverse methods of extractions. These activities are mainly due to the unsaturated fatty acids and bioactive compounds, such as the limonoids and a small fraction of phenolic compounds. Gedunin-type limonoids, like gedunin and its derivatives, represent the class of compounds that show the highest bioactivities in different applications.
Keywords
Biological properties
Bioactive compounds
Limonoids
Fatty acids
Gedunin
Biomedical potential
- AOE
-
Andiroba essential oil
- AOF
-
Andiroba flower oil
- AOS
-
Andiroba seed oil
- AS
-
Andiroba seeds
- I2
-
Iodine
- AOS + I2
-
Andiroba oil associated with iodine
- BAT
-
Brown adipose tissue
- HFD
-
High-fat-diet
- LFD
-
Low-fat-diet
- MIC
-
Minimum inhibitory concentration
- MICA
-
Minimum Inhibitory Concentration of Adherence
- O2
-
Molecular oxygen
- OC
-
Copaiba oil
- OM
-
Oral mucositis
- WAT
-
White adipose tissue
- 5-FU
-
5-fluorouracil
- wt%
-
weight percentage
- ALT
-
Alanine aminotransferase
- C/EBPα
-
CCAAT/enhancer-binding proteins
- CCL11/eotaxin
-
Chemokine (C-C motif) ligand
- d-GalN
-
d-galactosamine
- DOX
-
Doxorubicin
- DPPH
-
2,2-Diphenyl-pycril-hydrazyl radical
- MTT
-
3-[4,5-dimethylthiazol-2-yl]-2,5 diphenyl tetrazolium bromide
- GABA
-
Gamma-aminobutyric acid
- GLUT4
-
Glucose transport
- IL
-
Interleukin
- iNOS
-
Inducible nitric oxide synthase
- IRS-1/Akt
-
insulin receptor
- l-NMMA
-
NG-monomethyl-l-arginine acetate
- LPS
-
Lipopolysaccharide
- NFκB
-
factor κB
- NO
-
Nitric oxide
- PAF
-
Platelet-activating factor
- PGE2
-
Prostaglandin
- PPARγ
-
receptor γ
- PTZ
-
Pentylenetetrazole
- RNS
-
Reactive nitrogen species
- ROS
-
Reactive oxygen species
- TNF-α
-
Tumor necrosis factor-α
- UCP
-
Uncoupling protein
- FA
-
Fatty acids
- FAA
-
Fatty acids amide
- FAEE
-
Fatty acid ethyl ester
- FFA
-
Free fatty acids
- LRFs
-
Limonoid-rich fractions
- TNTPs
-
Tetranortriterpenoids
- PCL
-
Poly (ε-caprolactone)
- XRD
-
X-ray diffraction
- p.o.
-
per oral
- DSC
-
Differential scanning calorimetry
- TG
-
triglyceride
- Nanoandi
-
Andiroba oil nanoemulsion
- Nanoandie
-
Andiroba essential oil nanoemulsion
- SPIONs
-
Superparamagnetism of iron oxide nanoparticles
Abbreviations
1 Introduction
Brazil covers an area of 8.5 million square kilometers, being the fifth biggest nation in the world, the largest country in South America and has the largest plant diversity on the planet. One of its more important biomes is Amazonia, the largest tropical forest in the world that occupies 49 % of Brazil and has one third of the planet’s tropical forests (4.2 million km2) containing a massive quantity of vegetation (1.5 million species) (Tappin et al. 2008; Alarcon et al. 2021). Then, it is not surprising that the gigantic biodiversity of Amazonia has been studied around the world. Among so many plant species, one receives a different look due to its versatility and multiple applications: Carapa guianensis Aublet.
C. guianensis is a tree belonging to the Meliaceae family and is popularly known as andiroba, a word derived from the tupi-guarani (an indigenous language of Brazil) which means “bitter taste” (Novello et al. 2015; Chia et al. 2018). This specie is a canopy tree, attaining 30 to 50 m height at maturity, with white flowers, slightly fragrant, round fruits, large and dark leaves and seeds of brown coloring and angular side (Enríquez, 2003). It grows in South and Central America, occurring preferably in wet-land forests, mainly in the Amazon River basin (Tappin et al. 2008; Londres et al. 2017).
Andiroba has a high economic value due its multiple uses, for instance, its wood has high value for solid products including furniture manufacturing, construction, veneers (wood sheets) and plywood (similar to medium-density fiberboard -MDF) (Firmino et al. 2019). On the other hand, C. guianensis usually produces 180–200 kg of seeds/tree/year, containing approximately 60 wt% (wt%) in oil (Embrapa, 2004; Lourenço et al. 2017). The oil extracted from the seeds has a transparent light-yellow color, solidifies at temperatures below 25 °C, has a bitter taste and reaches rancidity rapidly after extraction (Novello et al. 2015). This oil is mostly recognized for its uses in traditional medicine (Novello et al. 2015; Chia et al. 2018). Hammer and Johns (1993) interviewed a popular community of Marajó island, Pará, Brazil founding that C. guianensis cortex and the oil extracted from its seeds were used as insect repellent, wound healing, treatment of arthritis, throat inflammation, diarrhea, diabetes, ear infection and even uterine cancer (Enríquez, 2003; Tappin et al. 2008). Additionally, the by-product generated from the seed oil extraction can be used to manufacture insect repellants candles that can be used against the Anopheles mosquitoes that transmit malaria and Aedes (Stegomyia) aegypti L., the dengue vector (de Mendonca et al. 2005; Tappin et al. 2008).
The andiroba seed oil (AOS) is quite interesting to the cosmetics industry once it is composed mostly of triacylglycerols with high levels of unsaturated fatty acids (FA) such as oleic (51.81 %), palmitic (25.76 %), stearic (9.08 %), and linoleic acid (8.3 %). Its unsaponifiable content varies from 2 to 5 %, and is composed of triterpenes, steroids, alkaloids, coumarins, flavonoids and limonoids (Cabral et al. 2013). The oil is one of the best-selling medicinal products on Amazon, with an international demand, being exported to Europe and to the United State (Tappin et al. 2008). In the cosmetics industry, this oil is used in massage creams, soaps, conditioners and shampoos (de Santana et al. 2018). In another context, the density, viscosity and calorific value of the andiroba oil are quite similar to those of other vegetable oils extracted from traditional seeds, such as soybean and cotton, which has made it an alternative for biodiesel production (Cabral et al. 2013).
Thus, it is clear that andiroba has a worldwide range and is not restricted to the regional level and several research groups have studied this species and its fractions, extracts, oils and its metabolites around the world in order to test the most diverse activities, such as antioxidant, repellent, insecticide, anti-inflammatory, among others. Therefore, this systematic review aims to quickly present the current scenario related to the main biological activities presented by C. guianensis.
2 Methods
2.1 Search strategy
This systematic review was performed according to the PRISMA (Page et al. 2021) guidelines. The literature was retrieved from Scopus, Web of Science and Agricultural Research Database (Base de Dados da Pesquisa Agropecuária - BDPA) and for citation searching between 15 May 2022 and 30 June 2022. The keywords used were: “Andiroba”; “Andiroba OR Carapa guianensis”; “Andiroba OR “Carapa guianensis”AND “bioactive compounds”; “Reactive oxygen species” OR ROS and cytotoxic AND genotoxic AND hematotoxic”.
2.2 Selection criteria
The articles were firstly screened based on titles, abstracts and keywords. Next, refining tools were used: (1) limit to articles and reviews; (2) published between 1993 and 2022, (3) subject area; (4) not topic-related literature; (5) non-English or Portuguese literature; (6) duplication removal. A second following criteria included to literature was: (a) in vitro and in vivo studies; (b) provide biological activity; (c) biotechnology applications and the following literature were excluded: (i) did not produced significant bioactivity; (ii) unclear activity or doses concentrations; (iii) no access to full-text. Then, the literature data were extracted meeting the selection criteria and organized and stored in EndNote 20 libraries including the criteria steps in groups. The methodology flowchart can be seen Fig. 1.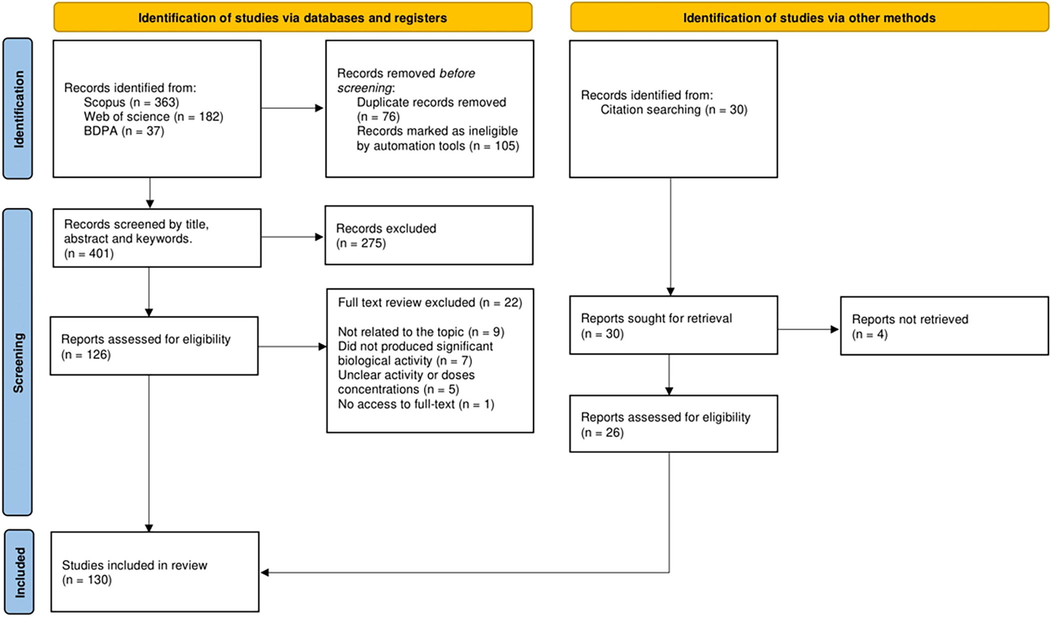
PRISMA flow diagram indicating the inclusion and exclusion criteria.
3 Results
A total of 582 articles were identified from the databases, of which 76 duplicates were removed by duplication and 105 by ineligibility. Then 401 articles were screened by titles, abstracts and keywords and 126 articles were selected for full-text reviewing, in which 22 did not match to selection criteria. A citation search identified 30 articles and then 4 articles did not retrieve. A total of 130 articles were eligible to the selection criteria and included in the analyses. The feasible bioactive effects for the application methods and bioactive compounds from C. guianensis of these reports are summarized in Table 1. The bioactive compounds were subsequently numbered in the text and their chemical structures were ilustred in figures. AS, andiroba seeds; AOS, andiroba seed oil; AOE, andiroba essential oil; AOF, andiroba flower oil; AOS + I2, andiroba oil associated with iodine; FFA, fatty acids amide; TNTPs, tetranortriterpenoids; LRFs, limonoid-rich fractions; *AOE, commercial oil (Beraca, RF3150) extracted from AS.
Sample
Bioactivity
Bioactive compounds or
oil application methods
References
AS
NO inhibition
Carapanolide J
Matsui et al. (2014)
Carapanolide T
AOS
Anticancer
Carapanolide A
Inoue et al. (2012)
Guianolide A
Inoue et al. (2013)
Carapanolide C
Inoue et al. (2014)
Carapanolide D
Carapanolide E
Carapanolide F
Carapanolide I
17β-hydroxyazaradione
Inoue et al. (2015)
Methylangolensate
Andirolide S
Sakamoto et al. (2013)
Andirolide T
Citotoxicity
Crude oil
Porfirio-Dias et al. (2020)
Genotoxicity
AOS diluted at 10 and 20 %
Wanzeler et al. (2018)
Therapeutic
Crude oil and TNTPs
Soares et al. (2021)
Antiallergic
Gedunin
Penido et al. (2005)
7-deacetoxy-7-oxogedunin
6α-acetoxygedunin
Methylangolensate
Andirobin
Antiallergic
Anti-inflammatoryTNTPs
Penido et al. (2006)
Antiallergic
Ferraris et al. (2011)
AS
Antiobesity
7-deacetoxy-7-Oxogedunin
Matsumoto et al. (2019a, 2019b)
AOS
Anticonvulsant
FAA
de Oliveira et al. (2020)
Collagen synthesis
Gedunin
Morikawa et al. (2018)
7-deacetoxy-7-oxogedunin
6α-acetoxygedunin
7-deacetoxy-7α-hydroxygedunin
Guianolide A
Methylangolensate
Healing
Crude oil
Santos et al. (2013)
Silva et al. (2015)
Ozonized AOS
Araújo et al. (2017)
Andiroba-based commercial emulsion (Tegum®)
Chia et al. (2018)
Antioxidant
Crude oil
Milhomem-Paixão et al. (2016)
Cytotoxicity
Nanoemulsion composed by AOS (10 g), KolliphorELP surfactant (20 g) and of Milli-Q water (10 g).
Milhomem-Paixão et al. (2017)
Therapeutic
Crude oil and nanoandi (Smix sufactant, Tween 80®: Span 80® (9:1, w/w) in 2000 mg.kg−1 prepared by a phase inversion temperature method
Melo et al. (2021)
Antibacterial
AOS diluted at 25 %
Santos et al. (2012)
Nanoemulsion prepared using oil and aqueous phase containing the lipophilic and hydrophilic surfactants Span 80® and Tween 20®, respectively, dispersed in AOS at 10 %
Vaucher et al. (2015)
Crude oil
Bataglion et al. (2014)
Araújo-Lima et al. (2018)
AOE*
Antifungal
AOE 0,2%, 0,4%, 0,6%, 0,8% e 1,0%
Sousa et al. (2012)
AOS
Crude oil
Sousa et al. (2018)
Nascimento et al. (2019)
AOS and AFO
Antimalarial
6α-acetoxyepoxyazadiradione
Tanaka et al. (2012)
Gedunin
6α-hydroxygedunin
6α-acetoxygedunin
Andirolides H
7-deacetoxy-7-oxogedunin
Pereira et al. (2014)
AOS
6α-acetoxygedunin
Andirobin
6α-hydroxydeacetylgedunin
7-deacetoxy-7-oxogedunin
6α-acetoxygedunin
Gedunin
7-deacetylgedunin
1,2-dihydro-3β-hydroxy-7-deacetoxy-7-oxogedunin
AndirobinMiranda Junior et al. (2012)
AOS and LRFs
Antileishmanial
7-deacetoxy-7-hydroxygedunin
Deacetyldihydrogedunin
Deoxygedunin
Gedunin
11β-hydroxygedunin
17-glycolyldeoxygedunin
6α-acetoxygedunin
6α,11β-diacetoxygeduninOliveira et al. (2018)
Nanoemulsion prepared by adding water and Tween 80® as aqueous phase; the organic phase was prepared using Span 80® and AOS (1 g)
Moraes et al. (2018)
AOE*
Trypanocidal
Nanoemulsion at 0.5–1.0 % of AOE composed by a organic phase containing a lipophilic surfactant (Span 80®) and acetone; the aqueous phase contained a hydrophilic surfactant (Tween 80®) and distilled water
Baldissera et al. (2013)
AOS
Insecticidal
AOS diluted at 10, 25, 30, 50, 100 %
Farias et al. (2009)
AOS diluted at 5, 10 and 20 %
Vendramini et al. (2012a, b)
Crude oil
Roma et al. (2013a, b, 2014, 2015)
AOE*
Larvicidal
AOE diluted at 10 %
Volpato et al. (2015)
AOS
AOS diluted at 25, 50, 75, 100 %
Barros et al. (2012)
Dry-scratched of AS
0.5 to 2 % and 0.5–4 % to treated the 3rd and 4th larval instar, respectevely
Silva et al. (2004)
AOS
The four larval stages of GCZ and Rockefeller strains of Aedes aegypti treated at the concentration of 80–489 ppm
Silva et al. (2006)
AOS
AOS at 500 µg.mL−1
de Mendonca et al. (2005)
AOS at 500 mg.mL−1
Prophiro et al. (2012)
Nanoemulsion composed by water (90 %), Sorbitan monooleate/polysorbate (5 %) and AOS (5 %)
Jesus et al. (2017)
Emulsion composed by a silk protein solution at 2 % (75 %), ethanol (24 %) and bioactive compounds (5 % AOS, FAEE or FFA)
Sarquis et al. (2020)
Crude oil
Santos et al. (2012)
Nanoemulsion prepared using oil and aqueous phase containing the lipophilic and hydrophilic surfactants Span 80® and tween 20®, respectively, dispersed in AOS at 10 %
Vaucher et al. (2015)
AOS diluted at 10 mL.L-1
Xavier et al. (2015)
Insecticidal
AOS diluted at 2L.100L-1
Nunes et al. (2015)
Crude oil
Wille et al. (2021)
6α-acetoxygedunin
Ambrozin et al. (2006)
AOS + I2
Sousa et al. (2019)
Arillus of C. guianensis
7-deacetoxy-7-oxogedunin
Sarria et al. (2011)
β-photogedunin
AOS
AOS + I2
Santos et al. (2015)
AOE*
AOE diluted at 1 % and 5 %
Klauck et al. (2014)
C. guianensis root and stem
Antihelmintic
Ethanolic extract at 1.06 mg.mL−1 and 0.34 mg.mL−1 of andiroba root and stem, respectively
Amorim et al. (2021)
AOS
Repellent
AOS diluted at 15 % and 100 %
Miot et al. (2004)
C. guianensis stem wood and seed hulls
Insecticidal
RepellentDilution at 250 µg.mL−1
Correa de Oliveira et al. (2022)
AOE*
Repellent
AOE diluted at 5 %
Klauck et al. (2015)
AOS
AOS 0.5–2 % associated with 5 % protein (Bioanastrepha™)
Machado da Rosa et al. (2013)
AOE*
AOE diluted at 5 %
Zortea et al. (2017)
AOS
Crude oil
Freire et al. (2006)
A set of biological activities were exhibited by C. guianensis in the main findings of the studies. This review identified the major biological activities and biotechnological approaches, those are: insecticidal (32.95 %), therapeutics (30.68 %), antimicrobial (15.91 %), toxicity (6,82 %), repellent effect (5.68 %) and biotechnological approaches (7.95 %) (Fig. 2). Furthermore, the major biological activities are related from the oil extraction method and its application, such as AOS (57,69 %), AOE (9,62 %), nanoandi (AOS nanoemulsion) (9,62 %), FA (7,69 %), TNTPs (3,85 %), AOS + I2 (3,85 %), LRFs (3,85 %), FAA (1,92 %) and nanoandie (AOE nanoemulsion) (1,92 %) (Fig. 3), as well as to the limonoids structure. The gedunin-type limonoids exhibit the highest and most diverse activities, followed by: mexicanolide-type > phragmalin-type > phragmalin 1,8,9-orthoacetate > chukrasone-type > andirobin-type > 9,10-seco-mexicanolide limonoids (Fig. 4).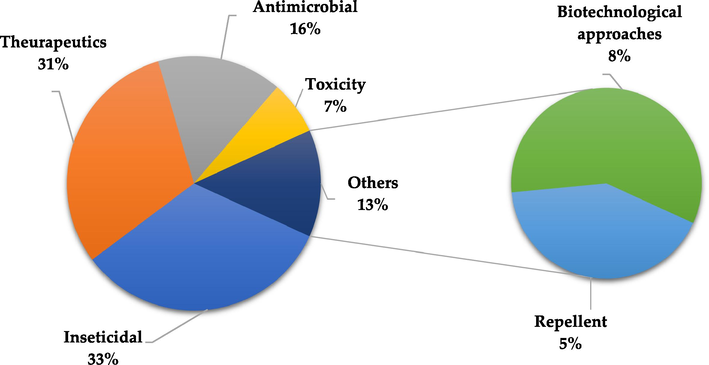
Major biological activities and biotechnological approaches displayed by C. guianensis in this review.
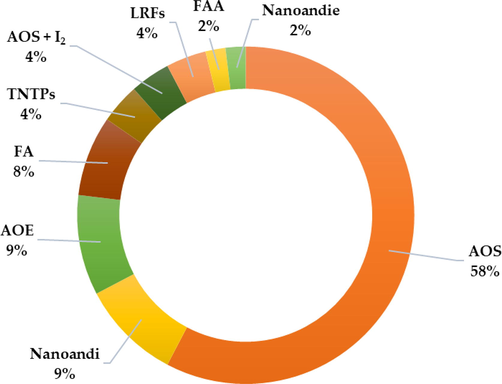
Bioactivities displayed through the oil extraction method and its applications. AOS = andiroba seed oil; Nanoandi: andiroba oil nanoemulsion; AOE = andiroba essential oil; FA: fatty acids; TNTPs = tetranortriterpenoids; AOS + I2 = andiroba oil associated with iodine; LRFs: limonoid-rich fractions; FFA: fatty acids amides; Nanoandie: andiroba essential oil nanoemulsion.
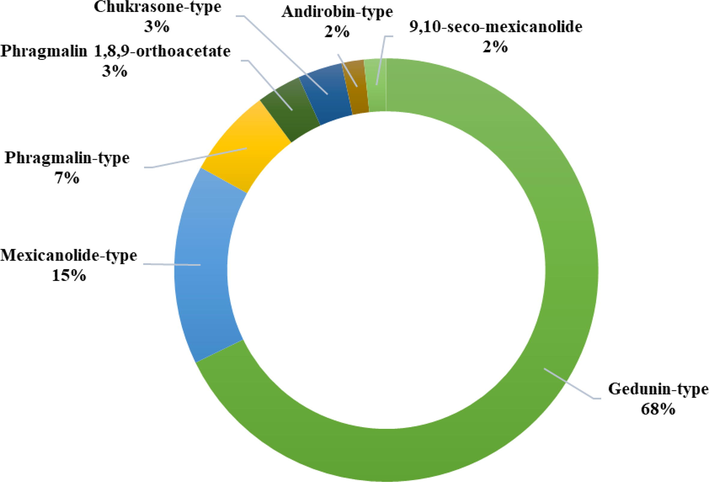
Structure of limonoids that exhibit biological activities.
4 Biological activity of Carapa guianensis
4.1 Limonoids
The bioactive properties of AOS are attributed to limonoids typically known for their chemical and biological properties. These compounds are commonly found in the plants of the Meliaceae and Rutaceae families (Inoue et al. 2018; Tsukamoto et al. 2019). Limonoids are tetranortriterpenoids (TNTPs) that are modified triterpenoids originated from a precursor with 4,4,8-trimethyl-17-furylsteroids that usually contains four highly oxidized (A, B, C, and D) rings. They are basically composed of 6/6/6/5-fused tetracyclic carbon skeletons processing five methyl groups at C-4, C-4, C-8, C-10 and C-13 positions and a furan ring at C-17. Cleavage and rearrangement of the carbon skeleton allows the occurrence of diverse structures that possess biological activities (Higuchi et al. 2017; Kikuchi et al. 2020).
Several structures of limonoids in AOS have been reported by researchers throughout the years, such as of carapanolides A and B (Inoue et al. 2012), carapanolides C–I (Inoue et al. 2014), carapanolides J–L (Matsui et al. 2014), carapanolides M−S (Inoue et al. 2015), carapanolides T–X (Miyake et al. 2015), carapanosins A–C (Higuchi et al. 2017); carapanosins D-F (Inoue et al. 2018), carapanins A-C (Kikuchi et al. 2020), guianolides A-B (Inoue et al. 2013), guianofruits A-B (Sasayama et al. 2018) and guianofruits C-I (Tsukamoto et al. 2019). Furthermore, the andiroba flower oil has also been reported, such as andirolides A-G (Tanaka et al. 2011), andirolides H—P (Tanaka et al. 2012), andirolides Q-V (Sakamoto et al. 2013) and andirolides W-Y (Sakamoto et al. 2015).
The limonoids presence in andiroba flower and seed oil have been report to exhibit highly efficient analgesic, anti-bacterial, anti-fungal, antimalarial agent (Matsui et al. 2014; Higuchi et al. 2017; Tsukamoto et al. 2019), anti-parasitic (Cabral et al. 2013), anti-inflammatory, anti-cancerous, anti-tumor, and antiallergic activities (Matsui et al. 2014; Higuchi et al. 2017; Tsukamoto et al. 2019). It's also a renowned leprostatic, antiulcer, antipyretic, anti-irritant, antifeedant and was also found to be effective against wounds, bruises, antiulcer, rheumatism, ear infections, insecticidal, repellent, has a growth-inhibiting properties, as well as acute and subacute toxicities (Sakamoto et al. 2015; Inoue et al. 2018; Tsukamoto et al. 2019; Kikuchi et al. 2020).
4.2 Inhibition of physiological nitric oxide
Reactive oxygen species (ROS) are chemical molecules with high oxidative reactivity that are produced by cellular metabolism and formed by the partial reduction of molecular oxygen (O2). In this group are include O2, superoxide, hydroxyl radical, singlet oxygen and hydrogen peroxide. In addition, the ROS contains a subclass of nitrogen formed by reaction of ROS with nitric oxide (NO), known as reactive nitrogen species (RNS). ROS at low levels plays essential roles in biological processes acting as key signaling molecules for cell metabolism, such as growth, development and deaths. However, once the level is exceed the tolerance threshold of cells, both ROS and RNS becomes lethal, causing cellular oxidative damage, for instance, the peroxidation of the biological structures of lipids, DNA and proteins macromolecules via oxidative stress (Liang et al. 2021; Salinas et al. 2021; Wang et al. 2021). The DNA repair system can reverse the damages, however, if it persists, induces genotoxicity and even mutagenesis (Milhomem-Paixão et al. 2017). The cells can scavenge intracellular ROS under normal conditions using antioxidants, including catalase, glutathione and ascorbic acid, to maintain the dynamic redox balance. The positive and negative effects of ROS on cells depend on their intracellular concentration and cellular context (Liang et al. 2021; Wang et al. 2021).
Macrophages plays important roles in biological defense, for this reason, they are potential targets in the therapeutic treatments of inflammatory diseases. When activated, they release pro-inflammatory mediators, such as NO, interleukin-1 beta, tumor necrosis factor-α (TNF-α), among others. NO is involved in blood pressure regulation and blood flow distribution, however, overexpression of these mediators has been reported to induce severe or chronic inflammatory diseases, such as tissue damage, rheumatoid arthritis, osteoarthritis, diabetes, multiple organs dysfunction and death, as well as systemic inflammatory responses in sepsis, such as hypotension, cardiodepression and vascular hyporeactivity (Higuchi et al. 2017; Tsukamoto et al. 2019).
There are several studies reporting bioactivity of compounds isolated from C. guianensis on the production of NO by lipopolysaccharide-activated (LPS-activated) mouse peritoneal macrophages (RAW264.7 cells). Using spectroscopy Matsui et al. (2014) isolated from andiroba seeds (AS) a novel gedunin and two novel phragmalin-type limonoids structures, named carapanolides J–L, as well as a known gedunin-type limonoid called epoxyazadiradione. From the results, carapanolide J (1) showed similar inhibitory activities compared to positive control, NG-monomethyl-l-arginine acetate (l-NMMA), an inducible nitric oxide synthase (iNOS) with no cytotoxicity. While the epoxyazadiradione (2) exhibited superior inhibitory NO production activities at non-toxic concentrations to those of l-NMMA (Table 2), suggesting that carapanolide J may be a valuable potential inhibitor of NO production. The chemical structures for these compounds can be seen in Fig. 5.
Compound
Produced NO (%) (μM)
IC50
(μM)
3
10
30
100
1
92.1 ± 1.5a
(103.4 ± 1.8) b
83.4 ± 3.1 a
(102.4 ± 0.8) b
61.8 ± 1.8 a
(101.0 ± 1.7) b
16.8 ± 0.0 a
(102.8 ± 0.6) b
37.4 a
>100b
2
74.0 ± 5.0a
(81.4 ± 0.8) b
30.0 ± 2.3 a
(65.6 ± 0.2)b
7.5 ± 1.0 a
(33.6 ± 6.3) b
3.9 ± 1.8 a
(0.4 ± 0.4) b
12.0 a
15.2b
l-NMM c
93.0 ± 3.3 a
(103.5 ± 0.5)b
79.3 ± 0.8a
(102.0 ± 1.5)b
58.2 ± 2.4a
(94.1 ± 1.4)b
39.9 ± 1.7a
(96.5 ± 2.5)b
53.7a
>100b
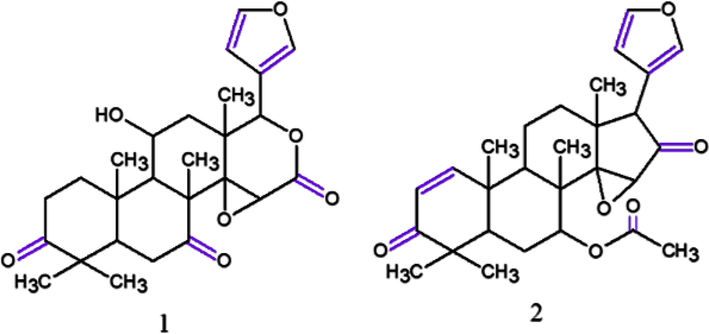
Chemical structures for compounds. (1): carapanolide J; (2): epoxyazadiradione. Source: adapted from Matsui et al. 2014.
AS are commonly studied, for instance, Miyake et al. (2015) isolated two novel mexicanolide-type limonoids from these seeds, the carapanolides T–U (mexicanolide-type limonoids that had OH in C-2 and C-8), as well as three novel phragmalin-type limonoids highly oxidized, the carapanolides V–X. In this study, four limonoids and l-NMMA were evaluated for their inhibitory effects on NO production in LPS-stimulated RAW264.7 cells by 3-[4,5-dimethylthiazol-2-yl]-2,5 diphenyl tetrazolium bromide (MTT) assay to determine safe concentrations of these limonoid cytotoxicities. Carapanolides T (3) and U (4) (Fig. 6) exhibited similar NO inhibitory activities (IC50: 22.0 μM; 23.3 μM, respectively) against l-NMMA (IC50 23.9 μM). Of these, 4 did not show cytotoxicities at 1–30 µM whereas 3 exhibited low cytotoxicity at 30 µM, however, not at the effective concentration, namely 10 µM, suggesting that both have potential as anti-inflammatory disease agents.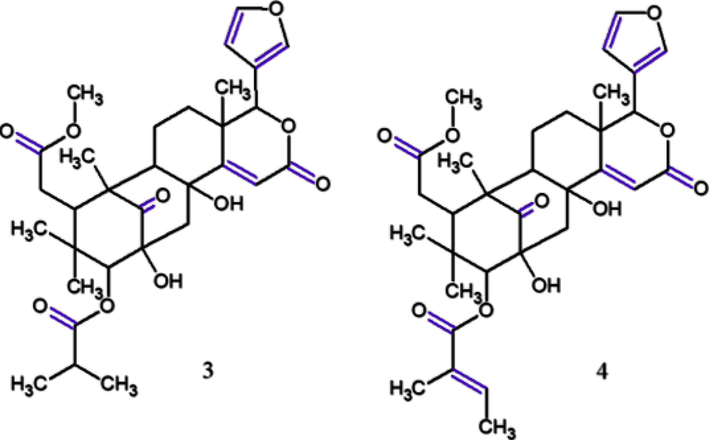
Chemical structures for compounds. (3): carapanolide T; (4): carapanolide U. Source: adapted from Miyake et al. 2015.
Higuchi et al. (2017) isolated eight limonoids from AOS and evaluated their inhibitory effects on NO production. All tested compounds did not exhibit cytotoxicity (cell viability 92.7–100.4 % at 30 μM). Of these, five compounds (Fig. 7) exhibited inhibitory activity on NO production, carapanosin C (5), swietephragmin D (7) and, 17-β-hydroxyazadiradione (9) (IC50: 13.7 μM; 4.9 μM; 10.8 μM, respectively) stronger than l-NMMA (IC50 23.9 μM) and carapanolide H (6) and 17-epi-17-hydroxyazadiradione (8) (IC50: 25.5 μM; 28.9 μM, respectively) showed moderate activity on NO production.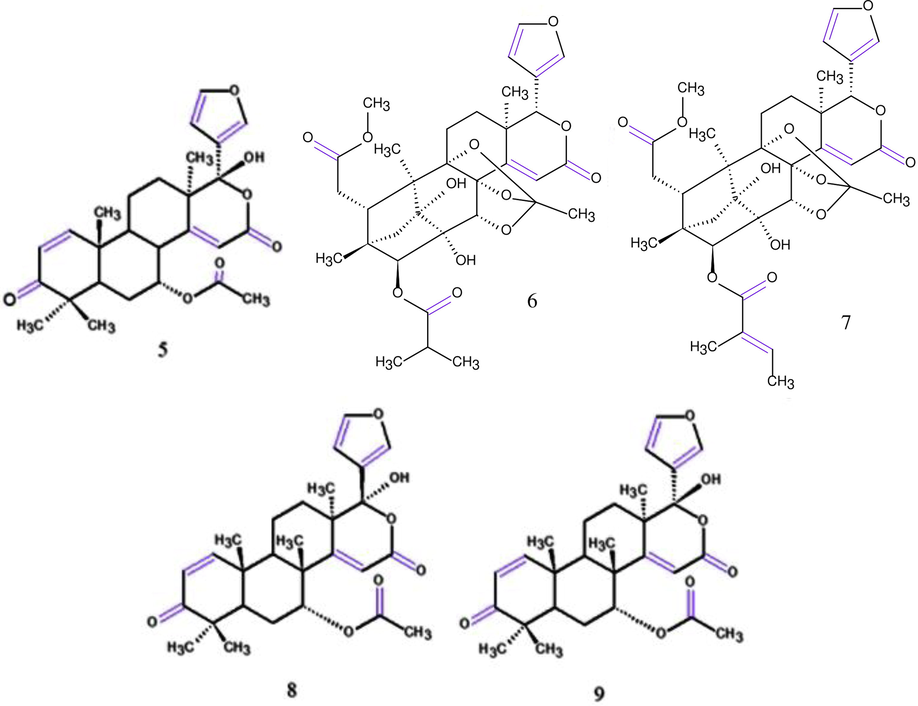
Chemical structures for compounds. (5): carapanosin C; (6): carapanolide H; (7): swietephragmin D; (8): 17-epi-17-hydroxyazadiradione and (9): 17-β-hydroxyazadiradion. Source: adapted from Higuchi et al. 2017.
Sasayama et al. (2018) isolated two chukrasone-type limonoids, guianofruits A (10) and B (11) (Fig. 8) from AOS and evaluated their effects on the production of NO in LPS-activated. The NO inhibitory assay showed that both compounds exhibited no toxicity at 1–30 µM. However, compound 10 showed similar inhibitory activities (produced NO 87.9 % at 10 µM; 47.5 % at 30 µM) to the positive control, l-NMMA (produced NO 68.9 % at 10 µM; 43.1 % at 30 µM), while 11 (NO produced 94.4 % at 10 µM; 65.6 % at 30 µM) exhibited moderate inhibitory activities on NO production.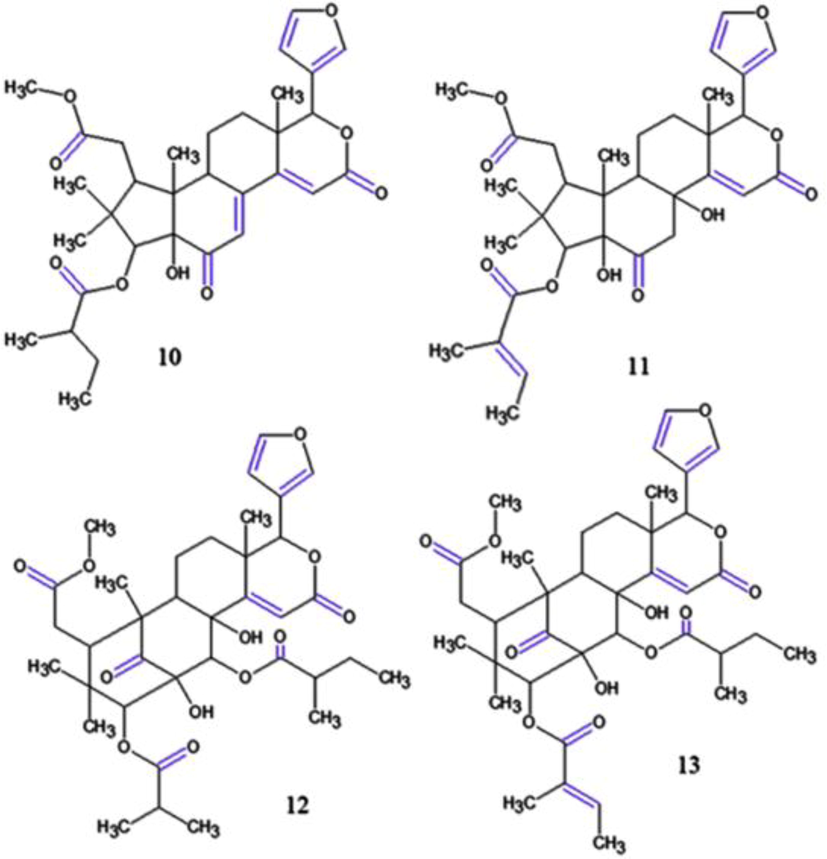
Chemical structures for compounds. (10): guianofruit A; (11): guianofruit B; (12): caraponosin E; (13): caraponosin F. Source: adapted from Sasayama et al. 2018 and Inoue et al. 2018.
In the same year, Inoue et al. (2018) isolated a novel nor-phragmalin-type limonoid, named carapanosin D and two novel mexicanolide-type limonoids, carapanosins E (12) and F (13) (Fig. 8) from the AOS and were evaluated for their effects on NO production in LPS-activated mouse peritoneal macrophages. All of them showed non-toxicities at 0–30 µM. 12 and 13 showed superior inhibitory activities (IC50: 23.9 µM and 11.8 µM, respectively) when compared to the positive control, l-NMMA (IC50: 47.6 µM), which suggest that 12 and 13 are valuable and have high potential as inhibitors of macrophage activation.
Tsukamoto et al. (2019) isolated and elucidated the structure of 6 compounds from AOS, among them, guianofruits C (14) and D (15) (Fig. 9), two new chukrasone-type limonoids that exhibited moderate inhibitory activities (IC50: 80.4 µM; 61.0 µM, respectively) without cytotoxicities. Recently, (Kikuchi et al. 2020) isolated three new limonoids also from AOS, named carapanins A-C, being carapanins B (16) and C (17) (Fig. 9) were related as potent nitric oxide inhibitors (IC50: 12.6 μM; 29.5 μM, respectively) relative to l-NMMA (IC50 47.6 μM) without cytotoxicity exhibited at 1–30 μM.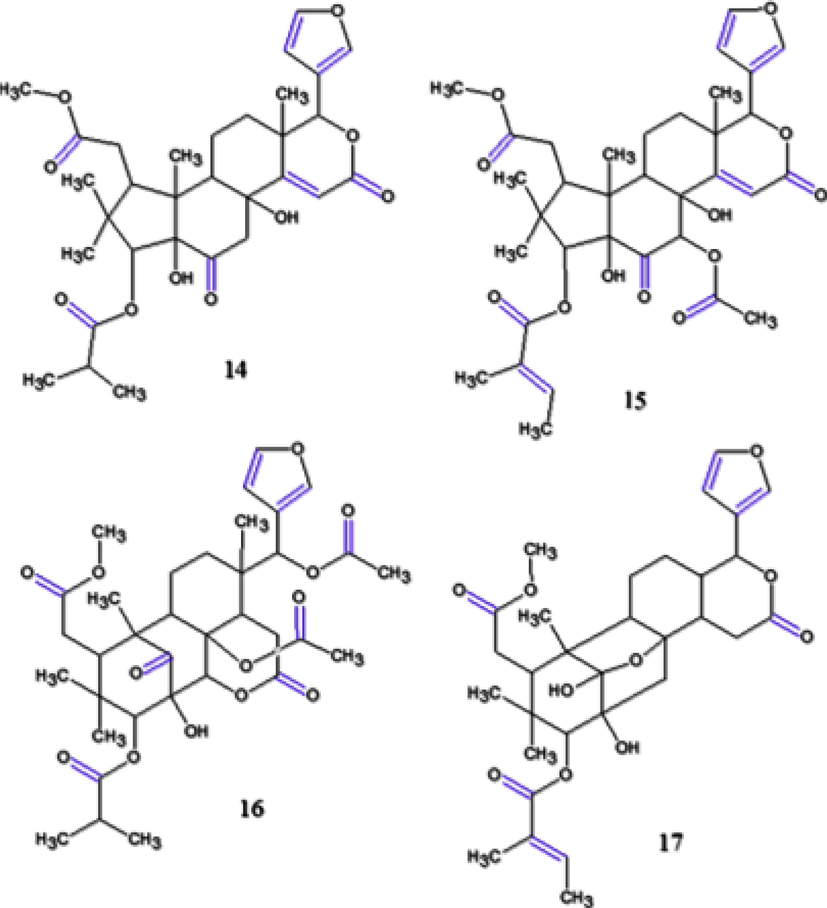
Chemical structures for compounds. (14): guianofruit C; (15): guianofruit D; (16): carapanin B and (17): carapanin C. Source: adapted from Tsukamoto et al. 2019.
In another study, 10 limonoids, seven known compounds and three new (andirolides W-Y) were obtained from the flower oil, a less usual source. Among them, 7-deacetoxy-7-oxogedunin (18), 6α-acetoxygedunin (19), 6α-hydroxygedunin (20), 6α-acetoxy-7α-deacetoxy-7α-hydroxygedunin (21), gedunin (22) and 7-deacetoxy-7-hydroxygedunin (23) (Fig. 10) were tested and exhibited inhibitory activities on NO inhibition (Table 3) without notable cytotoxic effects at effective concentrations in the MTT assays. 22 was found to be the most potent, being higher even than caffeic acid phenethyl ester (Sakamoto et al. 2015). CAPE, caffeic acid phenethyl ester; aNO inbihition (%) = values without parentheses; bindicate cell viability (%) in MTT assay = values between parentheses.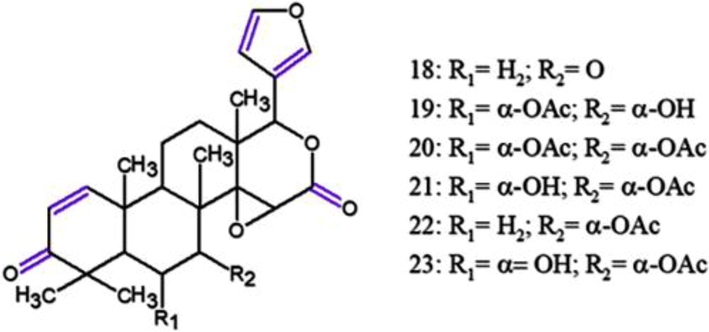
Chemical structures for compounds. (18): 7-deacetoxy-7-oxogedunin; (19): 6α-acetoxygedunin; (20): 6α-hydroxygedunin; (21): 6α-acetoxy-7α-deacetoxy-7α-hydroxygedunin; (22): gedunin; (23): 7-deacetoxy-7α-hydroxygedunin. Source: adapted from Sakamoto et al. 2015.
Authors
Compound
Inhibition (%) (μM)
IC50
(μM)
3
10
30
100
Sakamoto et al. (2015)
18
7.4 ± 5.2a
(100.3 ± 3.9) b
40.9 ± 4.7
(98.9 ± 3.2)94.0 ± 0.8
(98.8 ± 7.4)88.1 ± 2.1
(83.7 ± 1.2)12.8
19
16.9 ± 1.7
(96.8 ± 1.2)67.6 ± 4.6
(102.3 ± 2.2)88.4 ± 3.5
(92.5 ± 1.7)99.6 ± 0.2
(53.6 ± 5.1)7.9
20
7.7 ± 7.1
(88.4 ± 3.0)20.7 ± 4.3
(87.6 ± 4.0)64.0 ± 3.1
(90.4 ± 2.6)97.3 ± 0.3
(82.2 ± 4.2)19.1
21
5.8 ± 6.1
(99.8 ± 4.5)63.9 ± 3.0
(103.9 ± 6.9)97.2 ± 0.9
(108.9 ± 2.4)99.7 ± 0.5
(4.9 ± 0.5)9.4
22
25.1 ± 2.5
(102.2 ± 5.3)84.5 ± 2.3
(119.5 ± 5.3)101.8 ± 0.6
(94.8 ± 1.4)100.9 ± 0.4
(3.0 ± 0.4)4.6
23
15.7 ± 4.6
(110.3 ± 5.9)55.7 ± 4.0
(106.6 ± 3.1)98.8 ± 0.4
(96.3 ± 4.6)100.2 ± 0.2
(2.6 ± 0.5)8.7
Matsuda et al. (2009)Morikawa et al. (2014)
l-NMMA
1.4 ± 2.8
(101.1 ± 5.7)19.9 ± 2.8
(100.7 ± 6.2)43.0 ± 2.1
(102.6 ± 4.2)70.9 ± 1.6
(106.4 ± 4.6)36.0
CAPE
5.9 ± 5.2
(95.4 ± 0.7)44.4 ± 3.2
(70.0 ± 4.0)86.2 ± 1.1
(71.4 ± 6.0)99.6 ± 0.1
(53.0 ± 1.4)11.0
Ninomiya et al. (2016) examined 17 limonoids isolated from the seeds and/or flowers oil of C. guianensis related to hepatoprotective effects in order to clarify and characterize the action mechanisms and structural requirements against liver injury induced by d-galactosamine (d-GalN)/LPS in mice. The results showed that three gedunin-like limonoids, 7-deacetoxy-7-oxogedunin (18), 6α-acetoxygedunin (19) and gedunin (22) exhibited hepatoprotective effects at doses of 25 mg/kg, per oral (p.o), induced by d-GalN/LPS. Furthermore, they discovered that the action mechanisms are likely dependent on inhibition of LPS-induced macrophage activation and reduced sensitivity of hepatocytes to tumor necrosis TNF-α-induced cytotoxicity, however, these compounds did not decrease the cytotoxicity caused by d-GalN. The data from this work supports the inhibitory effects of 22 on NO production in LPS-activated macrophages previously reported by Borges et al. (2015) which gedunin suppressed the activation of macrophages through binding to myeloid differentiation protein 2 (MD-2) and not by affecting TLR4-mediated signaling. Moreover, the structural requirements of limonoids (1–17) with regard to inhibition of LPS-induced NO production in mouse peritoneal macrophages and TNF-α-induced cytotoxicity in L929 cells were found to show different tendencies.
From these data, it is possible observe that twenty-three limonoids from four structures, 10 gedunin-type, 6 mexicanolide-type, 4 chukrasone-type and 3 phragmalin-type exhibit strong and moderate inhibitory activity against NO production in LPS-activated RAW264.7 cells, as shown in Table 4. Between these structures, gedunin-type limonoids provide the most potent inhibition, especially 22 that exhibit the highest and most potent inhibitory activity on NO production.
Work
Limonoid Structures
Compound
IC50
µM
l-NMMA
µM
Inhibitory
Activity
Matsui et al. (2014)Higuchi et al. (2017)Sakamoto et al. (2015)
Gedunin-type
2
12.0
53.7
Strong
5
13.7
23.9
8
28.9
23.9
Moderate
9
10.8
Strong
18
12.8
36.0
Strong
19
7.9
20
19.1
Strong
21
9.4
22
4.6
Strong
23
8.7
Miyake et al. (2015)
Mexicanolide-type
3
22.0
23.9
Strong
4
23.3
Sasayama et al. (2018)Inoue et al. (2018)Tsukamoto et al. (2019)Kikuchi et al. (2020)
Chukrasone-type
12
23.9
47.6
13
11.8
16
12.6
17
29.5
10
–
–
Moderate
11
–
–
14
80.4
–
Moderate
15
61.0
–
Matsui et al. (2014)Higuchi et al. (2017)
Phragmalin-type
1
37.4
53.7
Strong
6
25.5
23.9
Moderate
7
4.9
Strong
4.3 Anticancer activity
Antineoplastic agents are a class of pharmaceuticals used for the treatment of various types of cancer through chemotherapy. Several anticancer drugs are capable of producing oxidative stress in biological systems, such as ROS generation in the patients who receive these drugs. Additionally, antineoplastics may interfere in the efficacy of the treatment (Conklin 2004), for this reason, phytotherapeutics have been extensively studied as alternative treatments for these drugs (Soares et al. 2021).
Due to the impact of cancer on humankind, the anticancer effects of AOS are of great pharmacological interest with a huge potential to become phytopharmaceutical products (Henriques and Penido 2014; Porfirio-Dias et al. 2020). Based on this, the anticancer properties of C. guianensis are one of the most important roles studied for compounds isolated from this species among researchers. Herein are described relevant discoveries about the action of these compounds.
Inoue et al. (2012) have started isolating and elucidating novel structures of limonoids, as well as testing their inhibition on cancer cell growth. In this report, carapanolide A (24) and B were isolated and tested in order to evaluate cytotoxic activities against three tumor cell lines, P388, L1210, and HL-60. As a primary screen for cancer cell growth inhibition, (24) showed moderate activity against L1210 cells, but none activity against HL-60 and P388 cells, whereas carapanolide B was inactive against all the cell lines. Next, Inoue et al. (2013) isolated two new compounds, guianolides A (25) and B and tested their cytotoxic activities against the same cell lines and only 25 showed a weak activity against the P388 cell lines.
In their continuous research, Inoue et al. (2014) isolated five new mexicanolide-type limonoids, carapanolides C–G together with two new phragmalin-type limonoids, carapanolides H–I from AOS. Carapanolides C (26), E (27), and I (28) exhibited moderate activity in the P388 and L1210 cell lines. Conversely, carapanolide D (29) exhibited a strong inhibitory effect in the HL-60 cell line and carapanolides F (30) showed inhibitory activity in the L1210 cell line. The cytotoxic activity of 28 was moderate in all cell lines.
Sakamoto et al. (2013) reported that andirolide S (31) has an γ-ethoxy-αβ-unsaturated γ-lactone as side chain and andirolide T (32) a mexicanolide-type limonoid having a hydroxy group at C-2, an acetyl group at C-3, and a 2-methylbutyryloxy group at C-30 showed significant cytotoxic activity against murine P388 and human HL-60 leukemia cell lines. Ning et al. (2010) reported that a 2-methylbutyryloxy moiety might be influential in the cytotoxicity of gedunin-type limonoids which that was also found that this influenced in the cytotoxicity of mexicanolide-type limonoids. Table 5 shows cytotoxic activities isolated from C. guianensis reported by Inoue et al. (2012, 2013, 2014) and Sakamoto et al. (2013).
Authors
Compound
IC50
µM
Linage
Cells
Inhibitory
Activity
Inoue et al.
(2012, 2013, 2014)24
8.7
L1210
Moderate
25
33.7
P388
Weak
26
13.3
P338
Moderate
17.9
L1210
52.3
HL-60
27
15.8
P388
Moderate
45.0
L1210
18.1
HL-60
28
89.8
P388
Moderate
24.3
L1210
90.8
HL-60
29
>100
P388
Weak
11.0
HL-60
Strong
27.1
L1210
Moderate
30
>100
P388
Weak
63.7
HL-60
Moderate
Sakamoto et al.
(2013)33
15.9
L1210
Strong
1.4
P388
1.3
HL-60
34
1.8
P388
1.3
HL-60
In another study, Inoue et al. (2015) also isolated five novel phragmalin-type limonoids, carapanolides M−Q and two mexicanolide-type limonoids, carapanolides R-S from AOS and evaluated their activities in the triglyceride metabolism-promoting in the high glucose-pretreated together with 12 known limonoids isolated from C. guianensis seed oil: 24, 25, 26, two gedunin-type limonoids, 17β-hydroxyazaradione (33) and methylangolensate (34) from AOS and 6 gedunin-type limonoids and an andirobin-type limonoid from the flower oil in human hepatocellular carcinoma cell line, HepG2. As shown in Table 6, only gedunin-type limonoids were capable of reducing triglyceride (TG) levels in hepatocytes: 7-deacetoxy-7-hydroxygedunin (23), gedunin (22) and 7-deacetoxy-7-oxogedunin (18) reduced significantly the TG levels, representing the strongest activities, respectively, whereas epoxyazadiradione (2), 31, 6α-acetoxy-7α-deacetoxy-7α-hydroxygedunin (21), 6α-hydroxygedunin (20) and 32 demonstrated moderate activities. The gedunin-type limonoids showed the strongest activities and were responsible for the fatty liver preventive effects of C. guianensis, besides had no substituent at C-6 exhibited which than those of moderate activities that have a hydroxyl or acetyl group at C-6. Furthermore, the reductions provided for these compounds were equal or stronger than those obtained using the hypoglycemic medicine, metformin. All related compounds (Inoue et al. 2012, 2013, 2014, 2015) and Sakamoto et al. (2013) can be seen in Fig. 11. Each value represents the mean ± S.E.M. (n = 4). Asterisks denote significant differences from the control, Crystal data of 2 *p < 0.05, **p < 0.01.
Treatment
TG/protein content in the homogenate (% of control)
Concentrarion (µM)
0
3
10
30
2
100.0 ± 4.6
96.4 ± 9.4
86.9 ± 8.2
65.0 ± 7.7**
19
100.0 ± 2.7
82.5 ± 1.2**
75.4 ± 4.2**
79.6 ± 3.0**
21
100.0 ± 2.9
93.6 ± 2.7
107.3 ± 8.3
79.8 ± 4.1**
22
100.0 ± 3.2
88.8 ± 3.5
86.0 ± 1.9*
70.2 ± 5.0**
23
100.0 ± 2.0
74.3 ± 2.2**
55.0 ± 3.6**
49.9 ± 2.5**
24
100.0 ± 3.9
71.9 ± 1.0**
35.4 ± 3.9**
32.0 ± 3.0**
33
100.0 ± 3.0
96.7 ± 5.5
93.0 ± 3.8
69.8 ± 3.1**
34
100.0 ± 3.1
89.5 ± 2.6*
96.8 ± 3.1
85.5 ± 3.7**
Metformin
100.0 ± 1.6
81.9 ± 2.7**
85.4 ± 1.5**
78.1 ± 2.8**
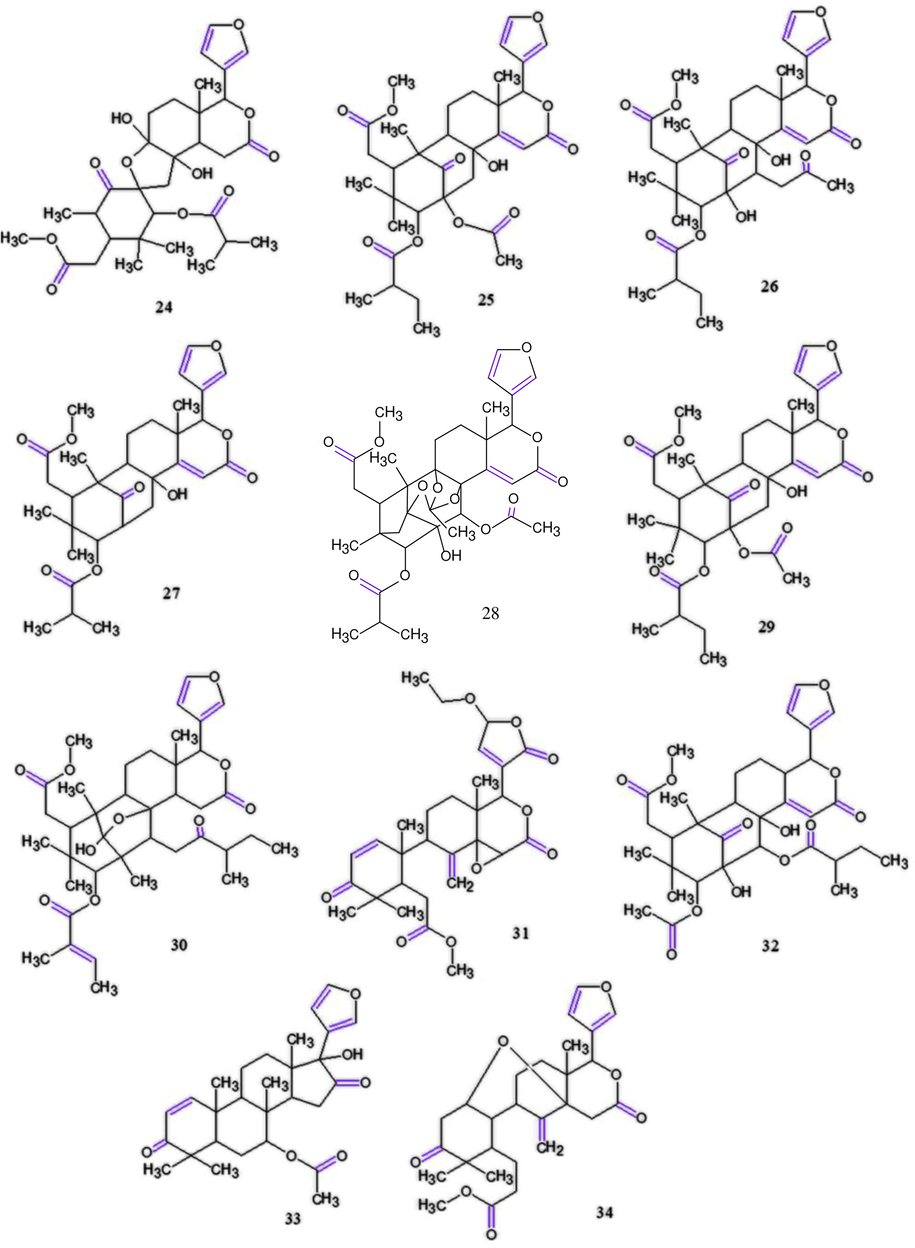
Chemical structures for compounds isolated and/or tested by the authors. (24): carapanolide A; (25): guianolide A; (26): carapanolide C; (27): carapanolide E; (28): carapanolide I; (29): carapanolide D; (30): carapanolide F; (31): andirolide S; (32): andirolide T; (33): 17β-hydroxyazaradione and (34): methylangolensate. Source: adapted from Inoue et al. 2012, 2013, 2014, 2015 and Sakamoto et al. 2013.
4.4 Healing properties
Some beneficial effects of AOS on health have been reported in vitro and in vivo, such as, antiallergic, anti-obesity, anticonvulsant, healing and collagen synthesis effects in rodents (Penido et al. 2005; Morikawa et al. 2018; Matsumoto et al. 2019a; de Oliveira et al. 2020). Furthermore, natural compounds are of great interest to the pharmaceutical industry, therefore, relevant studies on health effects tested in rats are described below. The antiallergic and anti-inflammatory properties of AOS, as well as its TNTPs fractions, including 7-deacetoxy-7-oxogedunin (18), 6α-acetoxygedunin (19), gedunin (22), methylangolensate (32) and andirobin (39) in rodents has been reported. Penido et al. (2005) showed that AOS and its TNTPs fractions displayed inhibition on allergen-induced in paw and ear edema (Swiss and C57/Bl10 mice) in formation and pleural exudation in previously sensitized mice via the impairment of signaling pathways triggered by histamine, serotonin, bradykinin and platelet-activating factor (PAF) and prostaglandin (PGE2). In addition, crude extract of C. guianensis seeds and TNTPs fractions also demonstrated an anti-nociceptive effect by oral treatment in Wistar rats through inhibition PGE2 generation, decreasing antigen-hyperalgesia.
Moreover, Penido et al. (2006) reported the inhibition of allergen-induced eosinophil recruitment and showed that the pooled TNTPs impaired the allergic response involved in the production of inflammatory mediators in the late phase, such as the eosinophilotactic mediators interleukin (IL)-5 and chemokine (C—C motif) ligand (CCL)11/eotaxin. They evidenced that impaired production of these mediators resulted in inhibition of nuclear factor κB (NFκB) by TNTPs observed in vitro and in vivo. Next, the anti-inflammatory activity isolated from C. guianensis on zymosan-induced arthritis in C57BL/10 mice showed that the pool of TNTPs inhibits neutrophil influx and edema formation in oral treatment via production of pro-inflammatory mediators, such as tumor necrosis factor (TNF)-α, KC/IL-8 in synovial washes in mice. The results of in vitro assay demonstrated that TNTPs is also able to impair murine macrophage activation by the inhibition of NFκB translocation induced by zymosan, which explain anti-inflammatory effect of these compounds in the experimental arthritis of zymosan-stimulated mice.
In turn, Ferraris et al. (2011) investigated the effect of TNTPs, as well as the individual effect of each of five TNTPs compounds mentioned above isolated from AOS on the modulating functions of T lymphocytes and eosinophils in vitro critically involved in allergic diseases. The eosinophils incubation in vitro of these compounds were able to impair the adhesion of eosinophils to TNF-α-primed tEND.1 endothelial cells. In addition, each one of the five limonoids and pooled TNTPs tested demonstrated impairment on the production of IL-2, CCL11 and CCL5/regulated upon activation of normal T cell expressed and secreted. On the other hand, pooled TNTPs did not inhibit adhesion and chemotaxis of T lymphocytes in vivo, but were able to impair anti-CD3 monoclonal antibody (mAb)-induced T cell proliferation and the expression of CD25, CD69 and NFκB. In the latter, 19 did not show any effects. The results demonstrated that in vivo assay, the anti-allergic activity is shared between the five TNTPs isolated from C. guianensis by the ability to inhibit the activation of T lymphocytes, enabling the production of eosinophilotactic mediators that causes the consequently impairment of eosinophil.
Another beneficial property of isolated compounds of C. guianensis is the effect against obesity, that is defined as an abnormal increase in adipose tissue mass, recognized as a worldwide health problem of an epidemic nature known related to lifestyle diseases, such as hyperlipidemia, diabetes mellitus, hypertension and cardiovascular disease by the interaction of three complex factors, genetic, behavioral and environmental effects. Obesity is the result of an excess of lipids in adipocyte cells whose function is to store lipids as a source of energy, regulate the lipid metabolism and balance the body's energy (Cornier et al. 2008; Attie and Scherer 2009; Finucane et al. 2011). Thus, there is great interest in the development of new anti-obesity drugs.
Matsumoto et al. (2019a, 2019b) investigated the antiadipogenic activity of the limonoid 7-Deacetoxy-7-Oxogedunin (18) isolated from C. guianensis seeds on mice 3 T3-L1 cells. The results showed that the ability of 18 to decrease intracellular accumulation of lipids was mediated by 3 factors, the reduction of glucose uptake by the insulin receptor (IRS-1)/Akt, expression of glucose transport (GLUT4) by CCAAT/enhancer-binding proteins (C/EBPα) and the expression of peroxisome proliferator-activated receptor γ (PPARγ) in adipocytes. Furthermore, no significant cytotoxicity was observed at concentrations up to 10 μM in WST-8 assay. Next, was tested the effect of 18 in low and high-fat-diet (HFD and LFD, respectively) on C57BL/6 administered by oral route (20 mg.mL−1) for 7 weeks. In this study, compound 18 decreased body weight gain, reduced serum TG levels and improved insulin sensitivity in HFD-fed mice, therefore, an improvement on the suppression development of obesity, as well as hypertriglyceridemia and insulin resistance were elucidated. Furthermore, 18 also showed an anti-inflammatory activity, for instance, the transcription levels of the M1 and the expression M2 macrophage-related genes decreased in the white adipose tissue (WAT) of HFD-fed mice. Regarding to brown adipose tissue (BAT), 18 enhanced the expression of uncoupling protein (UCP1), UCP2 and UCP3 and decreased the weight in both LFD and HFD-fed mice. The rectal temperature increased the energy expenditure under HFD-fed conditions. Thus, when BAT and UCP family proteins are activated, it is observed the enhancing of mRNA levels of the thermogenic PRDM16, Cidea, and PGC-1α genes, demonstrating that 18 has an ability to enhance thermogenesis. It is noteworthy that the UCP1 levels in WAT were also enhanced by 18 administrations. Thus, the results showed that andiroba contains anti-obesity and antidiabetic effects, as well as bioactive constituents.
The anticonvulsant activity from AOS has been widely used in traditional medicines and the substances present in this oil can be used as precursors for the synthesis of endocannabinoids, known as fatty acids amide (FAA). Due to their biological actions, such as the neuroprotective and antiepileptic effects, AOS have been attracting researchers attention (Nardi et al. 2016; de Oliveira et al. 2020). In this context, de Oliveira et al. (2020) evaluated the anticonvulsant activity of FAA synthesized via biocatalysis process of ethanolamine from AOS in pentylenetetrazole-induced (PTZ)-induced in Swiss mice on gamma-aminobutyric acid (GABA)A receptors. The results showed that FAA is effective in the control of seizures by decreasing PTZ-induced through potential mechanisms involved in neuroprotection, besides a significant change in myoclonic, tonic-clonic latency and duration of seizures. Additionally, the inhibitory action of flumazenil, which can reverse the FAA actions, provide the evidence that these compounds are involved in the regulation of GABAA receptors. In contrast, FAA was unable to affect behavioral seizures induced by PTZ, as well as the original composition of AOS which did not produce any effects mentioned above.
Morikawa et al. (2018) characterized the collagen synthesis-promoting effects of AOS and its 10 principal limonoid constituents. From these, 18, 19, 20, 22, 23, 25 and 32 significantly promoted collagen synthesis in normal human dermal fibroblasts without causing cytotoxic and the relativity potential activities were observed in 22 and 23. In regard to the structural requirements of gedunin-type limonoids in collagen synthesis-promoting activity, the groups: 6α-acetoxy and 6α-hydroxy moieties reduced the activity; the 7α-acetoxy group exhibited higher activity than that with 7α-hydroxy or 7-keto groups; compounds with an α,β-epoxy-g-lactone moiety in the d-ring exhibited higher activity than that with an α,β-unsaturated cyclopentanone moiety. In addition, Palheta et al. (2018) analyzed the effects of AOS associated with microneedling in Wistar rats skin and the results showed that AOS had inferior results as drug delivery when compared to vitamin C.
Santos et al. (2013) reported the healing process of gastropathies by gavage in male Wistar rats under the influence of AOS (100 mg.kg−1) treatment. The initial phase of the healing process started at 7th day, the intermediate and advanced phase at 14th and 21th days, respectively. They concluded that AOS treatment was able to accelerate the healing process of the stomach in rats. In addition, another study carried out by Silva et al. (2015) evaluated the effect of AOS in colonic healing also by gavage in male Wistar rats at the same period. According to results, animals treated with AOS showed better cecum healing when compared to the control group.
It is noteworthy that the healing process is subject of clinical, scientific, and economical interest, because it consists in a perfect and coordinated cascade of cellular, molecular and biochemical events that are interrelated in order to occur tissue reconstitution (Hussni et al. 2010; Silva et al. 2015). Therefore, compounds that accelerate the healing process, such as the combination of vegetable oils with medicinal ozone, are of great interest due its oxidant properties (Kim et al. 2009). In this manner, Araújo et al. (2017) analyzed the effects of topical application of pure and ozonized AOS on experimentally induced wounds in horses. There was no difference in healing process between the studied groups compared to control, however, it was possible to conclude that AOS and ozonized AOS may be good options to treat equine wounds.
Carmona et al. (2013) evaluated the effect of AOS on induced periodontitis in male Wistar rats treated with saline (0.63 mL.kg−1), AOS (0.63 mL.kg−1) and meloxicam (7.5 mL.kg−1) by gavage, for seven days, once a day. In regard to AOS, satisfactory effects on induced periodontitis were observed, like the decreased quantity of inflammatory cells in systemic administration. These effects are probably due to the anti-inflammatory and antibacterial agents of oil, however, the effectiveness on the measure of alveolar bone loss was only observed in meloxicam treatment.
Chia et al. (2018) investigated the healing effect of andiroba-based commercial emulsion (Tegum®) on full-thickness cutaneous wounds in Wistar rats via modulation of inflammation and transforming growth factor beta 3. This treatment resulted in a significant enhancement of wound closure rates in all times tested. In addition, the wounds were smaller and contracted and showed an increase in angiogenesis and decreased on the levels of CD68 + and M2 macrophages on the 7th and 15th day, but the higher effects were observed on the 20th day. The myofibroblasts appeared at the 7th and 15th day and TGFβ3 levels were higher in the treated wounds, besides a less dense collagen fibers, lower col I/III ratios and a higher tensile strength. Thus, andiroba-based emulsion was able to modulate all parameters tested in wounds, leading to an enhancement in the contraction and in the tensile strength and a better esthetic appearance on rats’ wounds.
As a last healing property reported is the effect of AOS on oral mucositis (OM), one of the most common, undesirable and painful side effects clinically significant in the cancer treatment by chemotherapy and radiation. Estimates have shown that more than 80 % of patients who undergo treatment for head and neck tumors develop OM. Furthermore, severe cases dramatically affect quality life of patients, which can result in the discontinuation of antineoplastic treatment (Wanzeler et al. 2018; Soares et al. 2021). In this context, a study developed by Wanzeler et al. (2018) was conducted to investigate the healing activity of AOS against OM induced by 5-fluorouracil (5-FU) in golden Syrian hamsters using three treatments: AOS in natura (100 %), a diluted oil (10 %) from AOS 100 % and a refined commercial AOS diluted (10 %), both mixed in an orabase base composition (pectin, gelatin, nipagin, ecgonine methyl ester and purified water) were tested. The results showed that the AOS 100 % group can reduce the healing OM when compared to the reported control group. However, they concluded that the percentage of micronuclei was concentration-dependent, as increasing the oil concentration, higher the percentage of micronuclei frequency, indicating that the extract AOS 100 % presents a genotoxic potential on the cells of male golden Syrian hamsters.
In a recent study, Soares et al. (2021) performed a clinical study on the effects of andiroba orabase 3 % (pectin, gelatin, nipagin, carboxymethylcellulose, and purified water) in the symptomatology and evolution of OM to establish its therapeutic effects in children with leukemia underwent chemotherapy compared with low power laser treatment. The andiroba group showed better and statistically significant results, without reported pain on the eighth day and any symptoms at the ninth day of follow-up OM. This successful management can be attributed to the analgesic and antimicrobial potential of AOS, which provides inhibited effects on the bacterial, as well as derived fraction of TNTPs obtained from the seeds of C. guianensis. In conclusion, the present study observed that andiroba is more effective in the treatment of OM (p < 0.05), when compared to low power laser, on account of the reduction in the degree of OM.
AOS and its innumerous extraction methods were able to provide a good profile of bioactivities and healing properties in animal models. The gedunin-type limonoids also displayed a potential inhibition in relation to antiallergic, anti-inflammatory and antiobesity activity. The direct application of andiroba crude oil and its emulsion or ozonized form demonstrated great results to promote a better healing process and collagen synthesis, demonstrating the high pharmacological potential of C. guianensis in the development of new bioproducts. In addition, further investigations are needed to assess the bioactivity and cytotoxicity of these compounds and applications in humans.
4.5 Genotoxic, hematotoxic, cytotoxic and mutagenic effects of C. guianensis
Bioactive compounds with potential antioxidant properties have been the focus of scientific interest to protect biological systems against ROS due to reduce the effects of toxic compounds on the environment and human health (Melo et al. 2018). Lipids play an important role in natural oil toxicities and the FA, such as, stearic, palmitic, oleic and linoleic acids can cause cellular apoptosis. However, FA from vegetable oils have an important antioxidant activity exerting a protective effect against ROS. Furthermore, the apoptosis caused by the FA may not originate from genetic damage, in a manner similar to that reported for limonoids that cause cell apoptosis via the mitochondrial route (Milhomem-Paixão et al. 2016; Melo et al. 2018).
In order to assess genotoxicity, cytotoxic, hematotoxicity and mutagenic parameters from AOS, some authors have reported the efficiency and safety of natural products used to treat diseases among Amazonian and other populations. In this context, it is particularly important to assess the potential genotoxicity since toxicity is considered to be fundamental in the development of diseases like cancer (Milhomem-Paixão et al. 2016).
Costa-Silva et al. (2008) carried out the acute and subacute toxicity of AOS in Wistar rats by oral administration oil route in order to investigate the hematological, biochemical and morphological parameters. They reported that at the doses up 5.0 g.kg−1 in acute test any sign of toxicity or death were produce in rats, suggesting a LD50 above 5.0 g.kg−1, in which according to Kennedy Jr. et al. (1986) substances that present LD50 higher than 5.0 g.kg-1 by oral route can be considered practically non-toxic. In the subacute treatment (1.5 g.kg−1) AOS did not change any biochemical parameters analyzed, except for an increase in alanine aminotransferase (ALT) serum levels in the group, indicating possible hepatotoxic effects. Furthermore, when the oral administration route of a drug is used, the biochemical toxicity of oil is almost null.
Henriques and Penido (2014) have also mentioned acute and subacute activities of AOS reported by their group. In the acute toxicity orally administrated in Swiss mice was reported that LD50 in AOS was 22.3 g.kg−1, which is superior to another drugs, such as the antihistamine fexofenadine (4.5 g.kg−1) and the corticosteroid dexamethasone (5.8 mg.kg−1) both used to treat acute and chronic allergic reactions. In the subacute standardized test, it was observed that as oil as pooled TNTPs orally administered for 21 days into infant mice did not induce significant changes in body weight gain, in spleen weight, in peripheral blood leukocytes in peripheral CD4 + T lymphocytes. Besides that, no changes were observed in red blood cell counts, inguinal lymph node or adrenal weights among control groups and dexamethasone-treated, oil-treated and tetranortriterpenoid-treated groups.
In another study, Milhomem-Paixão et al. (2016) used Swiss mice to evaluate genotoxic and mutagenic effects of AOS, besides its antioxidant properties and lipidome in order to address safety issues. Using comet and micronucleus assay and hematological analysis, the authors concluded that no clinical or behavioral alterations were observed in the treatment period and exposure to AOS at the dose concentrations (500, 1000 and 2000 mg.kg−1/day) by gavage for 14 consecutive days. The conditions used in this study did not result in hematotoxic, genotoxic or mutagenic effects, quite the opposite, the antioxidant activity of the oil would tend to protect cellular DNA from oxidative damage.
Milhomem-Paixão et al. (2017) also performed a comparative study to test the cytotoxicity, genotoxicity, and hematotoxicity of the AOS and its nanoemulsion (nanoandi) in vitro (fibroblasts, lineage NIH/3T3) and in vivo (Swiss mice) using the same method mentioned above. The nanoemulsion was prepared by a phase-inversion temperature method and its composition consists of 10 g of AOS, 20 g of Kolliphor ELP surfactant (Sigma) and 10 g of Milli-Q water. The nanoandi did not present genotoxic, cytotoxic, or mutagenic effects in vivo under the chosen experimental biological conditions, while the in vitro tests presented cytotoxicity at the highest concentrations. They also observed that there is an influence on cell morphology by the AOS, nanoandi and for the surfactant used in its composition, which means that probably AOS, nanoandi control and nanoandi have a profound influence on cell membranes.
Doxorubicin (DOX) is an anthracycline antibiotic from Streptomyces peucetius used as antineoplastics agent and quite effective in treating various types of cancer, including lymphomas, leukemias, ovarian, breast, lung, thyroid cancers, among others, however, its limited to clinical use because it causes severe side effects (Melo et al. 2021). The toxicity mechanism of DOX seems to be related to the production of ROS in the body (El-Moselhy and El-Sheikh 2014). In opposition, antioxidants might be used to decrease ROS generated by DOX. In this context, Melo et al. (2018) evaluated the genotoxic effects of AOS and its nanoemulsion on Swiss Mice, as well as its antigenotoxic effects using DOX as ROS inductor. In summary, the results indicated that both AOS and nanoandi did not cause genetic damage and showed protective effects against micronucleus formation in mice treated with DOX.
Melo et al. (2021) also evaluated the feasibility of AOS and nanoandi (Smix sufactant, Tween 80®:Span 80® (9:1, w/w) prepared by a phase inversion temperature method to prevent DOX damage to kidney, liver and spleen, as well as some biochemical and hematological parameters in Swiss mice by gavage for 14 days and simultaneously intraperitoneal route injection of DOX on 13 day. The results showed both AOS and nanoandi (2000 mg.kg−1) were capable to reduced several aspects in severity lesions caused by DOX (40 mg.kg−1), decreasing hematotoxicity and the histological changes, besides reducing the frequency of apoptotic cell death in the studied organs, mostly was established by nanoandi, which showed to be efficient to revert the deleterious DOX effects, proving to be a protective agent for the liver. These potential effects are believed due to the increase in the absorption in the liver where was observed the greatest tissue recovery. Thus, these data brought better applications to therapeutic properties of andiroba-based formulations.
In general, AOS and its nanoemulsions did not exhibit significant differences in respect of hematological, biochemical and morphological parameters, as well as hematotoxic, genotoxic, cytotoxic or mutagenic effects. On the contrary, it exhibits antioxidant activities capable of modulating oxidative damage in DNA and reducing side effects of antineoplastic agents.
4.6 Antimicrobial activity
The natural prospection of new products with deleterious effects against diseases caused by microorganisms in plants and animals through secondary plant metabolites has increased, showing to be potentially useful for the pharmaceutical industry, medicine and agriculture (Nascimento et al. 2019). In this sense, efforts towards drug discovery and prudent use of antimicrobial agents are the basis for overcoming the worldwide problem of microbial resistance (Santos et al. 2010). The antimicrobial activity of some essential oils against pathogenic microorganisms has been recognized and explored as their mechanical procedures and/or specific conditions, successfully releasing bactericidal or bacteriostatic substances, such as quaternary ammonium compounds, bisbiguanides, enzymes, metallic salts, however, the high volatility of the compounds present in the composition of the oils represent the cause of a sharp decline in antimicrobial activity (Conde et al. 2015; Vaucher et al. 2015).
The minimum inhibitory concentration (MIC) is used to determine the smallest concentration of the substance capable of inhibit microbial growth. There are several methods to assess antibacterial and antifungal of vegetal extracts, such as agar diffusion method, macrodilution and microdilution method (Ostrosky et al. 2008; Conde et al. 2015). Antimicrobial activities of AOS have been reported while its antibacterial, antifungal and antiprotozoal activity, therefore, relevant findings are discussed below.
4.6.1 Antibacterial
The antimicrobial activity of commercial andiroba (RF3150) extracted from AS and copaiba (Copaifera officinalis) (RF3350) oils (Beraca Sabará, Químicos e Ingredientes S/A (São Paulo, Brazil) against bacteria of the genus Paenibacillus was determined for the first time by Santos et al. (2012). In this research, both oils demonstrated a MIC value range 1.56–25 %, nonetheless, copaiba oil (OC) showed the best efficiency, excepting for the higher MIC value observed for P. azotofixans, as well as, in the AOS that showed a MIC of 25 % against P. azotofixans and P. larvae. The time-response effect of AOS and OC on P. larvae were determined for up to 48 h of exposure. No viable cells of P. larvae ATCC 9545 were observed after 24 h treatment with AOS (25 %) and after 48 h treatment with OC (1.56 %). Besides that, a steep decline in CFU.mL−1 was observed after incubation with AOS and OC oils for 12 and 24 h, respectively.
Subsequently, Vaucher et al. (2015) evaluated the physicochemical properties of nanoemulsions of OC and AOS oils using an oil and an aqueous phase containing the lipophilic and hydrophilic surfactants Span 80® and Tween 20®, respectively, dispersed in AOS or OC at 10 % and tested its antibacterial activity also against Paenibacillus species and showed that all species tested were susceptible to both nanoemulsions. The MIC values were lower than 0.39 %, except for P. gluconolyticus and P. validus, which showed MIC of 0.78 % for AOS 10 % and of 6.25 % for OC 10 %, respectively, differently of control (medium-chain triglyceride 10 %), in which none of the strains was inhibited. These results demonstrate that both AOS and OC and their respectively nanoemulsions presented a high activity against Paenibacillus species, which may be candidates for the treatment or prevention of American foulbrood in honeybee diseases. In addition, the potential protective effects of the oils nanoemulsions and the protection against volatility were also confirmed.
In another research, Bataglion et al. (2014) analyzed the antibacterial activity in three Amazonian oils, coconut, andiroba and castor seed oils. The experiment's results showed significant but moderate activity only for AOS and solely against Enterococcus aeruginosa (MIC 0.25 mg.mL−1). According to the authors, this activity can be a synergetic effect of the presence of all those compounds present in the oil, wherein FA are the most abundant. In contrast, Conde et al. (2015) tested the antimicrobial activity in vitro of the Amazon plants on oral biofilm microorganisms and concluded that AOS did not exhibited antibacterial effects, however, was able to inhibited the microbial adherence in Minimum Inhibitory Concentration of Adherence (MICA) in concentrations varying between 100 and 500 mg.mL−1.
Araújo-Lima et al. (2018) extracted the oil of C. guianensis by three different methods (oil 1 was obtained by pressing the dried seeds at room temperature, oil 2 by autoclaving, drying, and pressing; oil 3 by Soxhlet extraction at 30–60 °C using petroleum ether) and evaluated their chemical composition, free-radical scavenging activity, and mutagenic and genotoxic properties. It was observed that oils presented differential yields, physicochemical properties, and phenolic contents. In 2,2-Diphenyl-pycril-hydrazyl radical (DPPH), oil 1 showed the higher scavenging activity compared to oil 2 and 3, which suggested a significant antioxidant activity. Furthermore, all oils showed cytotoxicity against the bacteria Salmonella enterica serovar Typhimurium strains TA97, TA98, TA100, TA102, and TA1535 and to CHO-K1 and RAW264.7 cells. Mutagenicity was observed in S. typhimurium at noncytotoxic concentrations in oil 2 and induced micronuclei in both cell types, as well as oil 3 that also induced micronucleus formation. Thus, it was concluded that oil 1 was the safest for use by not showing mutagenicity or micronucleus induction compared to the other two oils.
4.6.2 Antifungal
Studies on essential oils effects as alternative control to anthracnose caused by the fungus Colletotrichum gloeosporioides in peppers were reported by Sousa et al. (2012). In this study, a commercial andiroba essential oil (AOE) was tested in vitro at different concentrations against C. gloeosporioides and in post-harvest pepper fruits, for which two variables were chosen: the mycelial growth rate and mycelial growth velocity index. The ability of AOE to inhibit fungal growth was concentration-dependent, for instance, as the concentration increased, greater inhibitions were observed, which differed from control concentration at 1.0 %. In contrast, Sousa et al. (2018) evaluated the fungicidal effects of pure AOS and its association with sublimated iodine (I2) against the growth and development of fungi that cause brown and white rot using the toxicity assay in culture medium. The results showed that the best growth inhibitions were 83.62 % and 79.10 % against Trametes versicolor (treatment with 1 % of I2) and Postia placenta (treatment with 5 % of I2), respectively, during 20 days.
Fungal pathogens cause significant global income losses in relation to agriculture and the food industry. In this sense, Nascimento et al. (2019) characterized the lipidomic profiles of oils obtained from the seeds of two Carapa species, C. guianensis and C. vasquezii and their synergistic implications for the inhibition on phytopathogenic fungi (Aspergillus flavus, A. niger and Fusariumoxysporum). The analyzes were carried out using the inhibition test of fungal growth in liquid medium and inhibition of conidial germination and the IC50 was also determined (Table 7). In summary, C. vasquezii oil reveal the highest inhibitory effect against all strains tested when compared with C. guianensis, that means an 8-fold difference on inhibition of F. oxysporum and A. flavus and an 2-fold for A. niger, while the mycelial growth and conidial germination were inhibited using 125 μL.mL−1 from both oils. Curiously, C. vasquezii was the species that had the highest average results for fungal inhibition and presented the highest concentrations of FA in its oil composition. These facts, collaborates to the literature, wherein some FA has been shown an inhibitory effect on fungal germination and sporulation (Urbanek et al. 2012; Golebiowski et al. 2014). MIC expressed in μL.mL − 1. IC50, concentration in μL.mL − 1 that inhibits 50 % fungal growth.
Specie
Aspergillus
flavus
Aspergillus
niger
Fusarium
oxysporum
Carapa
vasquezzi
MIC
31.2
125
15.6
IC50
6.5
50.8
4.08
Carapa
guianensis
MIC
125
250
250
IC50
71.2
47.8
19.7
Another relevant factor is that the fungal composition of the cell wall influences in greater or lesser extent the sensitivity to substances with antifungal potential, for this reason, the fungal cell wall is important and serves as a defense mechanism. Fungi with lower levels of steroids has been reported as more sensitive, therefore, increases the fungal fluid on the cell wall membrane when induced by the inhibitory effects of AOS (Avis and BéLanger, 2001). In addition, the antifungal efficiency of FA is associated with increased carbon chain length, however, the excess length in FA carbon chains reduces the solubility, making difficult to mix the oil into the fungal growth medium (Sado-Kamdem et al. 2009; Pohl and Thibane, 2011).
4.6.3 Antiprotozoal
Regarding the antiprotozoal activity of AOS, some reports have shown this activity against the pathogenic protozoans to humans, like the etiological agents of malaria and leishmania, Plasmodium falciparum, P. vivax, P. ovale, and P. malaria and a several species of Leishmania genus, respectively (Miranda Junior et al. 2012; Moraes et al. 2018; Oliveira et al. 2018).
The antimalarial activity was reported by Tanaka et al. (2012), where nine new limonoids, three new gedunin, an andirobin, three mexicanolides, and two phragmalin-type, named andirolides H—P were isolated from C. guianensis flower oil. The antimalarial activity was assessed against the P. falciparum FCR-3 strain (ATCC 30932, chloroquine-sensitive) in gedunin-type limonoids, andirolides H (35), 6α-acetoxyepoxyazadiradione (36) (Fig. 12), 7-deacetoxy-7-oxogedunin (18), 6α-acetoxygedunin (19), 6α-hydroxygedunin (20) and gedunin (22). The best result was obtained with 36, that is because it bore a furan ring, α,β-unsaturated ketone on the A ring, as well as in acetoxy group at the C-6 position.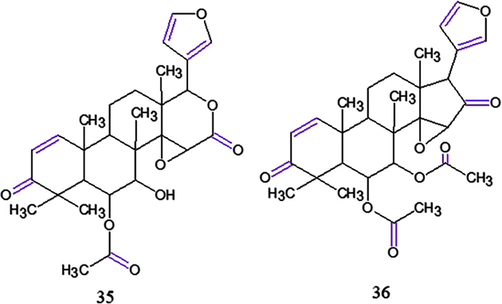
Chemical structures for compounds: (35): andiroloide H; (36): 6α-acetoxyepoxyazadiradione. Source: adapted from Tanaka et al. 2012.
In a previously report by MacKinnon et al. (1997) the conjugated enone, the furan ring and the acetoxy group at the C-7 position was presumed to be crucial functional groups for the antimalarial activity in 22, likewise, 20 also satisfied these requisites exhibiting a lower antimalarial activity, while 19 and 35 showed a more potent activity compare to 22. Thus, the 7α-acetoxy group was not required for significant in vitro antiplasmodial activity against P. falciparum FCR-3 strain, nonetheless, the low in vitro activity of gedunin derivative 20 (IC50 = 90 μM) was attributed to the presence of a 6α-hydroxy group. In addition, 18 did not carry an acetoxy group at the C-6 or C-7 positions, showing better selectivity than 22.
Miranda Junior et al. (2012) also analyzed the antiplasmodial activity in vitro and in vivo from AOS and its limonoid-rich fractions (LRFs) against P. falciparum Dd2 (resistant to chloroquine, mefloquine and pirimetamin) and W2 (resistant to chloroquine and sensitive to mefloquine). As shown in Table 8, AOS and its LRFs were able to inhibit the growth of both clones W2 (100 %) and Dd2 (88 %), between 24 and 72 h. In the acute toxicity test using Swiss albino mice, the AOS fixed dose was determined (LD50 > 2.0 g/kg) was nontoxic, which differs from the values obtained by Costa-Silva et al. (2008), as mentioned above. Therefore, the authors highlighted that in toxicity bioassays using mice and rats could be accepted doses>2.0 g/kg and 5.0 g/kg, respectively. Furthermore, the antimalarial activities were attributed to limonoids isolated from AOS, 18, 19, 22 and 7-deacetylgedunin (37), 1,2-dihydro-3β-hydroxy-7-deacetoxy-7-oxogedunin (38) and andirobin (39) (Fig. 13). Being in conflict with the results in the Tanaka et al. (2012) in terms of 39 activity, that could be explained by the manners in which the samples were tested, LRFs and individually, respectively, however, 39 activity it is support by Pereira et al. (2014) as mentioned below.
Sample
Concentration (µg.mL−1)
W2 inhibition (%)
24 h
48 h
72 h
AOS
8.2
100
100
100
LRFs
3.1
100
100
100
Quinine
0.016
71
73
75
Dd2
Inhibition (%)
IC50 (µg.mL−1)
Inhibition (%)
IC50 (µg.mL−1)
Inhibition (%)
IC50 (µg/mL−1)
AOS
8.2
31
>82
71
9.4
88
8.4
LRFs
3.1
56
2.8
64
2.4
82
0.4
Chloroquine
0.031
10
>1
35
0.1
60
0.01
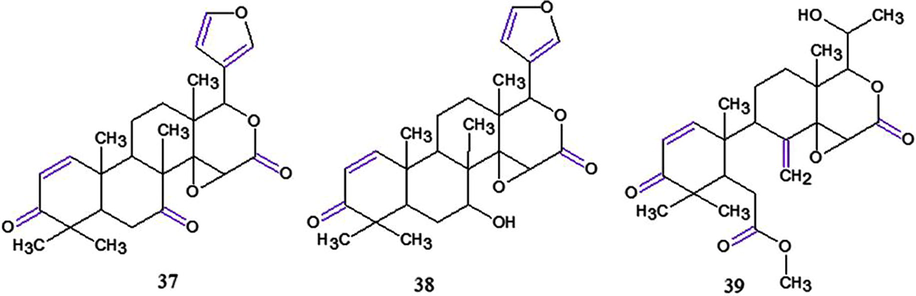
Chemical estructures for compounds: (37): 7-deacetylgedunin; (38): 1,2-dihydro-3β-hydroxy-7-deacetoxy-7-oxogedunin and (39): andirobin. Source: adapted from Miranda Junior et al. 2012.
In the research of Pereira et al. (2014) were evaluated the in vitro and in vivo antimalarial activity against P. falciparum K1 and P. berghei NK65, respectively, and the cytotoxicity of limonoids isolated from the residual biomass from C. guianensis RPSM oil production. The in vitro antimalarial assay of compounds 18, 19, 36, 39 exhibited a moderate activity, whereas the semi-synthetic derivative 6α-hydroxydeacetylgedunin (40) (Fig. 14) obtained from deacetylation of 19 exhibited the most inhibitory activity, besides 18 exhibited the lower activity of all limonoids tested. The IC50 values for these compounds ranged from 5.0 to 20.7 μM and none of the were toxic to the human fibroblasts MRC-5 cell lines (18: IC50 = 47.3 μg.mL−1, all other were: > 100 μg.mL−1) over a period of 48 h. In this work, the in vitro anti-plasmodial activity of 40 was reported for the first time and shown to be greater than the four natural isolates against the strain P. falciparum K1 (IC50 = 5.0 μM), consisting to the previously generated data by Tanaka group in which the notion that within this group of gedunin derivatives, an O-acetyl group at the 6 and/or 7 position is not a required for significant in vitro antiplasmodial activity against P. falciparum. Another data can be compared between K1 and FCR-3 strain inhibition for compound 19 that exhibited an IC50 = 7.0 μM and 2.8 μM, respectively. Finally, larger quantities of 18 and 19 allowed the in vivo assay adult female BALB/c mice and the greatest activity was detected to 19, representing 65.7 % of parasitemia suppression against P. berghei when compared to controls under administered orally doses at 100 mg/kg/day.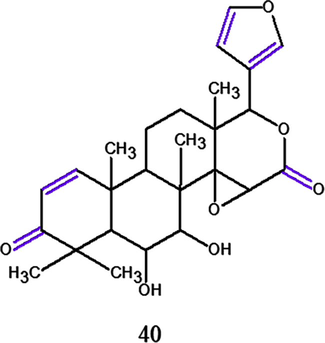
Chemical structure for compound (40): 6α-hydroxydeacetylgedunin. Source: adapted from Pereira et al. 2014.
In order to classify antiplasmodial activity in vitro, Batista et al. (2009) have used combined criteria: IC50: <1 µM, excellent/potent activity; IC50: 1–20 µM, good activity; IC50: 20–100 µM, moderate activity; IC50: 100–200 µM, low activity; and IC50: > 200 µM, inactive. Based on this, Table 9. shows the efficiency of antiplasmodial activity herein cited by EC50 and IC50.
P. falciparum strains
Sample
EC50 (mol/L)
IC50 (µg.mL−1)
IC50 µM
Authors
FCR-3
18
2.5x10-6
–
–
Tanaka et al. (2012)
19
2.8x10-6
–
–
20
9.0x10-5
–
–
22
2.5x10-6
–
–
35
4.0x10-6
–
36
4.5x10-6
–
–
W2
AOS
–
8.2
–
Miranda Junior et al. (2012)
LRFs
–
3.31
–
Dd2
AOS
–
9.4
–
LRFs
–
2.4
–
K1
18
–
–
20.7
Pereira et al. (2014)
19
–
–
7.0
36
–
–
15.4
39
–
–
15.3
40
–
–
5.0
Regarding the antileishmanial activity, Moraes et al. (2018) reported the nanoandi prepared by adding water and Tween 80® as aqueous phase; the organic phase was prepared using Span 80® and AOS (1 g) against Leishmania infantum and L. amazonensis. The nanoemulsions were analyzed by direct observation under optical microscopy and reveal to be toxic for parasites, mainly by reducing promastigotes of both Leishmania species. The data showed that the nanoandi was capable to kill L. amazonensis (IC50: 260 µg.mL−1) and L. infantum (IC50: 320 µg.mL−1) promastigotes at 48 h. Furthermore, treated cells infected by these parasites showed significant reduction in level infections around 36–89 % to L. infantum and 54–96 % to L. amazonensis between 200 and 300 µg.mL−1 on the macrophage cultures. Ultrastructural changes in oval cell shape and flagella were observed after 1 h of treatment. The leishmanicidal activity was also evaluated in vivo on BALB/c mice treated for 8 weeks with nanoandi and showed significant beneficial effects on lesion size, parasite burden and histopathology induced by L. amazonensis, whereas the group infected with L. infantum was effective in reducing the parasite burden around 50 % within livers and spleens and liver and also to improve histopathological features.
Oliveira et al. (2018) characterized the chemical composition and tested the antileishmanial activity and the cytotoxicity AOS and its LRFs extracts on L. amazonensis. The limonoids were obtained by silica gel column chromatography and yield six LRFs (LF1-LF6) identified as 7-deacetoxy-7-hydroxygedunin (23), deacetyldihydrogedunin, deoxygedunin, andirobin (39), gedunin (22), 11β-hydroxygedunin, 17-glycolyldeoxygedunin, 6α-acetoxygedunin (19), and 6α,11β-diacetoxygedunin. Only three fractions, LF3, LF4, and LF5 exhibited leishmanicidal activity against promastigotes and amastigotes forms, however, they also showed cytotoxicity to peritoneal macrophage. LF3 revealed the most consistent activity against amastigote forms, inducing a reduction in the percentage of infected cells and the mean amastigotes per 200 cells at 20 µg.mL−1. The LRFs were more effective against promastigote forms than intracellular amastigote forms and these activities were assigned by the gedunin-type limonoids, especially 11β-hydroxygedunin and 6α,11β-diacetoxygedunin (Fig. 15), which were more concentrated in these fractions. In contrast, AOS, LF1, LF2, and LF6 exhibited antileishmanial activity higher than 500 µg.mL−1 for the promastigote forms after 72 h of treatment and did not demonstrate in vitro cytotoxicity, except to LF2.
Chemical structure for compounds (41): 11β-hydroxygedunin and (42): 6α,11β-diacetoxygedunin. Source: adapted from Oliveira et al. 2018.
The trypanocidal activity of a comercical AOE (RF3150) extracted from AS and its respective nanoemulsion (nanoandie) was analyzed in vitro by Baldissera et al. (2013) against Trypanosoma evansi Steel. After 1, 3 and 6 h of treatment, a dose-dependent trypanocidal effect could be observed through the reduction of 66 %, 71 % and 86 % in the number of live trypomastigotes at AOS concentrations of 0.5 %, 1.0 % and 2.0 %, respectively. The nanoandie was prepared by spotaneous emulsificantion methoad and is composed by the AOE, lipophilic surfactant (Span 80®) and acetone; the aqueous phase contained thehydrophilic surfactant (Tween 80®) and distilled water. The nanoandie treatment significantly reduced the living trypanosomes after 1 h in 94 % and 100 % at the concentration of 0.5 % and 1.0 %, respectively. Therefore, as conventional oil as its nanoemulsion form showed to have a high activity against T. evansi in vitro, which suggests a possible alternative treatment for this disease. However, when the AOE were tested in mice infected with T. evansi, trypanocidal activity or curative effect were not found (Baldissera et al. 2014).
A potential activity against microbial agents has been elucidated from C. guianensis oil and its derivatives. The FA composition from AOS and AOE and its respective nanoemulsions exhibit a high and moderate efficiency to inhibit phytopathogenic bacterial and fungal strains due their antioxidant activities, which demonstrate a great alternative to control these microorganisms, reducing the impact on food and agriculture industries. On the other hand, the antiplasmodial activity is attributed to AOS and AOE and their nanoemulsions, but mainly by gedunin-type limonoids against the promastigotes and amastigotes protozoal forms.
4.7 Insecticidal activity
4.7.1 Ectoparasites
Due to the environmental impacts caused by synthetic chemical insecticides, several plant products with insecticidal properties have been used to control parasites, because of the slower resistance development and its biodegradable characteristics, which leads to lower environmental impact (Farias et al. 2009; Roma et al. 2014). Ticks are one of ectoparasites with the most important among arthropods, being obligatory hematophagous and vectors of biopathogens. Therefore, some researchers have reported the effect of AOS against many arthropods because of its compounds that are repellent or lethal. Rhipicephalus sanguineus Latreille is an urban plague of great medical-veterinary importance that has the dog as its main host and can also parasite other mammals, including humans (Vendramini et al. 2012a; Roma et al. 2013b).
As a first report, Farias et al. (2009) evaluated the acaricide potential on engorged adult females of R. sanguineus and Anocentor nitens Neumann by immersion test using five AOS dilutions (10 %, 25 %, 30 %, 50 %, 100 %). It was possible to observe a mortality and oviposition reduction with infertile eggs of 100 % in all tested dilutions, showing the potential effects of AOS extract against these two species. In this same line, Vendramini et al. (2012a, 2012b) analyzed the action of different concentrations of AOS (5, 10, and 20 %) on the female reproductive system of R. sanguineus. The results showed that there were important structural and physiological differences in oocytes induced by AOS, as well as the drastic reductions in proteins, polysaccharides and lipids of these cells (essential components for embryo viability) compromised reproductive success. Furthermore, in the highest concentration (20 %), oviposition was stimulated by the organism's defense mechanism in order to ensure the reproductive success of the species, however, the results suggested that there was no viability in these eggs due to the changes caused in the oocytes.
Roma et al. (2013a, 2013b) performed studies in order to analyze the potential toxic effect of the AOS on the central nervous system of R. sanguineus and the results demonstrated that this natural oil interferes in the synganglion by inducing structural and enzymatic changes, leading to the consequently impairment on the transmission of nervous impulses to the different organs of these parasites. Among the changes, damages represented by empty spaces between the perineurium and the cortical region were observed even at low concentrations, showing the feasibility of the oil to cause structural damage to the nervous tissue. According to these data, it was possible to observe that neurotoxicity depends on the oil concentration, as the higher concentration, greater damages are evidenced by the presence of several cytoplasmic vacuoles in the cortex. Furthermore, no changes have been observed in the genetic material of ovary cells exposed to the AOS, as well as permethrin, however, both causes damage to physiology of the synganglion through neurotoxic action, which leads to a loss integrity of the genetic material, resulting in the impairment of the metabolism in another systems of R. sanguineus.
Besides that, Roma et al. (2014, 2015) induced the ultrastructural changes in the synganglion of R. sanguineus female ticks by the management of AOS in order to provide scientific grounds in regard to the creation of more specific and efficient methods of control. The neurotoxic action of the AOS to the exposed synganglion of females promoted structural changes in the irregular and apparently thinner neural lamella, perineurium glial cells presenting large cytoplasmic vacuoles, decrease in the extensions of glial cells, separation of cortex cells, which were formerly attached through their membranes, neural cells presenting irregular plasma membranes, cytoplasm with autophagic vacuoles, as well as mitochondria with disorganized cristae and in process of degeneration. These effects confirmed that AOS would probably be able to impair the neural functions, which suggests that this product has a potential as an alternative method to be used as control ticks. It is noteworthy that the use of AOS is not harmful to the environment, due to its rapid biological degradation compared to the synthetic acaricides, which have a high residual power, remaining in the environment for a longer period of time.
Volpato et al. (2015) accomplished a study to verify in vitro influence of the commercial rosemary (STD Comércio e Exportação Ltda, Brazil) andiroba code (RF3150) and copaiba essential (RF3350) oils on different stages of the cattle tick Rhipicephalus microplus at the concentrations of 5 % and 10 %. In regard to AOE an inhibition of 77.5 % at 5 % and 92 % at 10 % in female reproduction was observed, moreover, AOE (10 %) had an acaricidal activity (100 %) and ovicidal effects. Based on this, the authors concluded that AOE may directly affect the oviposition and hatchability of cattle ticks’ due to a decrease in the number of females that have performed oviposition. In addition, many engorged ticks present an absence in oviposition, which may be due to the death or injury of the reproductive system.
Barros et al. (2012) studied the in vitro efficacy in four concentrations of AOS (25, 50, 75 e 100 %) dispersed in Tween 80® against the cat lice Felicola subrostratus. The analysis persisted in an immersion bath in 50 mL disposable cups containing 3 mL of the respective solutions, mixed constantly for three minutes, next the liquid excess was removed and the specimens were transferred to paper-filter envelopes and mortality rates were observed for 72 h. The best results reported 100 % mortality at concentrations of 50 and 100 % after the first hour and for the third hour of the test at concentrations of 10 and 25 %. These results demonstrate the possible use of AOS in the F. subrostratus control.
4.7.2 Insecticidal and larvicidal effect against urban and agriculture pests
Silva et al. (2004) evaluated the larvicidal effect on dry-scratched of AS (0.5–4 g.100 mL−1) against a sylvatic F1 progeny and a laboratory-colonized population of Aedes albopictus Skuse larvae (3rd and 4th instars) for 24 and 48 h. Both populations showed a high mortality rate after 24 h. The laboratory colony treated with 0.5–4 % of C. guianensis, however, the higher mortality was observed in the field population treated with 0.5–2 % of C. guianensis (Table 10). Next, de Mendonca et al. (2005) evaluated the larvicidal activity of AOS at 500 µg.mL−1 against Ae. aegypti and the results showed a mortality of 100 % (LC50 = 57 µg.mL−1) to 4th instar larvae of this mosquito.
Species
Population
Larval
Instar
LC50 (µg.mL−1)
24 h
48 h
Ae. albopictus
Sylvatic F1 progeny
3rd
0.74
0.68
4th
0.66
0.55
laboratory-colonized
3rd
1.81
–
4th
1.82
–
A. aegypti
–
Larval
Instar
CGZ strain
Rockefeller strain
24 h
48 h
24 h
48 h
1st
36
24
48
30
2nd
40
38
126
102
3rd
48
34
106
78
4th
128
114
234
138
In the followed year, Silva et al. (2006) evaluated the larvicidal activity from AOS at concentrations of 80–489 ppm against the strains GCZ and Rockefeller of Ae. aegypti It was observed a dose-dependent mortality caused by AOS to both strains after 24 and 48 h (Table 10). Furthermore, the toxic effect of AOS was significantly more sensitive to GCZ strain larvae in all instars than Rock strain, which requires approximately double of concentration to kill 90 % of 1st instars, demonstrating a significant variation in the susceptibility of larvae to AOS.
Prophiro et al. (2012) evaluated the initial time of larvicidal activity and the residual effect of AOS on Ae. aegypti. The lethal effect to larvae expo-sure to the oil started between the first 2 and 3 h at 500 mg.L-1; the toxic effect of residual activity remained with a total efficiency of 100 % in larvae mortality until 12th day. When sublethal dosage (LC50 = 140 mg.L-1) was used, the mortality could be observed after 72 h in the larval molt. In light of this, a recent study reported by Oliveira et al. (2022) carried out an ethnobotanical study in the Amazonian São Sebastião de Marinaú riverside community in order to provide new larvicides against Ae. aegypti Rockefeller strain, thus, extracts from stem wood and seed hulls of C. guianensis were obtained by hexane:ethyl acetate:dichloromethane (45:45:10) and tested at 250 µg.mL−1 on larvae and pupae for 24, 48 and 72 h. C. guianensis seed hulls extract caused a mortality of 100 % (LC50 = 70 μg.mL−1) after 72 h of exposure, however, the mixture was inactive against pupae. Furthermore, the residual activity of the initial C. guianensis seed hulls extract was also investigated and achieved 100 % mortality by day 6 (250 μg.mL−1), decreasing to 62 % on day 9 and inactive on day 10, differing briefly from results obtained by Prophiro et al. (2012). The authors suggests that these differences may be attributed to the higher concentration employed and/or the different mosquito strains tested. In addition, the increased susceptibility of larvae to larvicidal compounds is likely due to filter feeding during the developmental stages, whereas pupae are less susceptible to harmful agents, because they do not feed at this stage of development (da Silva Costa et al. 2016; Silva et al. 2020).
Jesus et al. (2017) prepared a nanoandi by a Low-Energy/Solvent-Free method contaning water (90 %), Sorbitan monooleate/polysorbate (5 %) and AOS (5 %) and tested its preliminary residual larvicidal activity against the late 3rd instar/early 4th instar larvae of Rockefeller strain of Ae. aegypti. Therefore, 250 µg.mL−1 of AOS were diluted in the optimal nanoemulsion and then mortality was recorded in three cycles of 48 h. Each cycle represents the removal of all larvae after 24 h, followed by filtration of the aqueous medium with the nanoandi left in the beaker for an additional period of 24 h. Only in the third cycle it was possible to observe a significant mortality (53.33 ± 15.30 %), while in the first (13.33 ± 11.55 %) and in the second cycle (16.6.7 ± 15.30 %) there were no significant differences in mortality compared to the control group (3.33 ± 5.77 %). Thus, these results demonstrated an increase in mortality as a function of time. Furthermore, a potential bioactive nanoemulsion of AOS in water was achieved with an ecofriendly approach, which means that these characteristics may be promising to controlled release system and for valorization of this Amazon raw material.
In another research, Sarquis et al. (2020) reported the use of silk fibroin solution (2 %) as a natural surfactant on preparation of an oil-in-water emulsion mixed with ethanol (24 %) and bioactive compounds of AOS(5 %) and their derivatives, free fatty acids (FFA) (5 %) and fatty acid ethyl ester (FAEE) (5 %) to evalute the activity against the larvae of Ae. aegypti. The AOS was extracted from Carapa trees in the dry and rainy seasons, therefore, two groups with two samples were generated, AOS1 and their derivatives FFA1 and FAEE1 and AOS2 and their derivatives FFA2 and FAEE2. The most active emulsion was FFA2 (LC50 = 94.45 and 16.79 μg.mL−1), followed by FFA1 (LC50 = 212.33 and 129.45 μg.mL−1) at 24 and 48 h, respectively. The effects of FFA solubilized in DMSO were also evaluated and the LC50 showed to be higher than those of the respective solution in fibroin. Thus, the carrying capacity of the fibroin emulsion and its positive impact on the biological activity were emphasized, suggesting an increase on the biodistribution and bioavailability of activity in aqueous medium promoted by biopolymeric matrix of silk fibroin.
The studies of Santos et al. (2012) and Vaucher et al. (2015), mentioned above also evaluated the toxicity of AOS against adults and larvae of honey bees Apis mellifera were also investigated. The results obtained in the first study showed that after AOS exposition, an index of about 20 % per day could be observed in bee mortality until day 4, and the survival rate was approximately 20 % at the 10th day of the experiment. In the second study, mortality of 8.33 % of adult worker bees and 26.2 % of larvae was observed after 24 h of treatment with nanoandi 10 % and these percentage did not increase after 48 h. Thus, nanoandi provoked a low toxicity at these conditions. In this same line, Xavier et al. (2015) evaluated the acute toxicity and sublethal effects of AOS (10 mL.L-1) against A. mellifera and the results showed that only AOS demonstrated no lethality to A. mellifera adult workers, however, an acute toxicity to larvae was observed.
Anastrepha fraterculu Widemann is a fruit fly species that causes damage to several fruit plants and losses in fruit production that can reach up to 100 % (Freire et al. 2006). In this sense, Nunes et al. (2015) analyzed the toxic effect of AOS (2L.100L-1) on the oviposition and mortality in adults of A. fraterculu by no-choice and free-choice tests in pear fruits. In the no-choice test an intermediary efficacy on adult mortality (26 %), whereas in free-choice test, the AOS showed mortality of 15.9 %. In addition, there was no larval emergence, pupae and adult development in fruits in both tests treated with AOS.
Recently, Wille et al. (2021) evaluated the mortality and offspring effects treated with AOS in control of A. fraterculu using strawberry guava, peach and apple as fruits hosts. A higher mortality rate was observed on strawberry guava and apple fruits 35.0 % and 18.4 %, respectively, as well as prevented its complete proliferation in the same fruits, while in peach fruits treatment, the only effect was observed in the offspring, reducing the number of pupae by 68.3 %. Thus, these results suggest the AOS as an alternative product to control A. fraterculus, however, further studies are needed to consider the effectiveness of AOS under field conditions.
Ambrozin et al. (2006) assayed the insecticidal activities of five limonoids: 7-deacetoxy-7-oxogedunin (18), 6α-acetoxygedunin (19), 17β-hydroxyazaradione (31), methylangolensate (32), 1,2-dihydro-3β-hydroxy-7-deacetoxy-7-oxogedunin (38) towards Atta sexdens rubropilosa. The results showed that all limonoids tested exhibit moderate insecticidal actvities, compound 19 showed a significant difference to the control group in the log-rank test. Regarding termites, Sousa et al. (2019) evaluated the efficiency of AOS enriched with 1 %, 3 %, and 5 % of I2 against dry-wood termites (Cryptotermes brevis Walkerre). The greater efficiency against termites was observed in the concentrations of AOS at 3 and 5 % I2, which provided the total mortality of these pests.
In another study of da Silva Costa et al. (2016) evaluated the insecticidal activity of AOS against the melonworm Diaphania hyalinata L. and their toxicity and phytotoxicity to the predatory ant Paratrechina sp. and to pumpkin plants, respectively. Through the results, no significant acute toxicity was observed on D. hyalinata larvae, however, there was an inhibition in feeding and oviposition. The predatory ant Paratrechina sp. in contact with AOS showed a mortality of<20 % and non-phytotoxicity to pumpkin plants was observed.
Sarria et al. (2011) tested the effect of limonoids of 18 and β-photogedunin (43) (Fig. 16) from arillus of C. guianensis against the larvae and pupae of armyworm Spodoptera frugiperda J. E. Smith. These compounds affected the larval and pupal development and prolongated the larval phase in 1.4 and 0.6 days at 50.0 mg.kg−1 by 18 and 43, respectevely, when compared with the 14.9 days using 22 as control and by reducing the pupal weight in 17.8 and 31.5 mg by 18 and 43, respectevely, compared with the control (272.2 mg). In summary, a moderate insecticidal activity at 50.0 mg.kg−1 could be observed, causing a mortality of 33.3 % and 53.3 % in the larval phase by 18 and 43, respectively. In addition, the highest insecticidal activity against S. frugiperda was presented by 43 and then was suggested for the control of this worm. As mentioned before by Silva et al. (2020), susceptible harmful agents against pupae are less available commercially due non feeding at this stage, however, the results obtained in this study suggested a new source of botanical insecticides from C. guianensis, constituting a promising alternative large scale eco-friendly larvicidal and pupicidal.
Chemical structure for compound (43): β-photogedunin. Source: adapted from Sarria et al. 2011.
Later, Santos et al. (2016) evaluated the effect of AOS in the eggs and 3rd instar caterpillars of S. frugiperda after exposition of 24 h. The data obtained showed a significant efficiency on eggs (64.7 % ± 6.4 %) and 3rd instar caterpillars (97.5 % ± 2.2 %) under the influence of undiluted oils at 200 µL, furthermore, the lethal concentration was established at 60.84 % on 3rd instar caterpillars of S. frugiperda. In addition, another study accomplished by Santos et al. (2015) the insecticidal potential of C. guianensis against the maize weevil (Sitophilus zeamais Motschulsky) was evaluated. From the results, the lethal dose was established at 60 % and the mortality reached 100 % after 24 h.
Klauck et al. (2014) evaluated the insecticidal and repellent effects in vitro and in vivo of the commercial AOE (RF3150) at concentrations of 1.0 % and 5.0 % on two species flies, Haematobia irritans L. and Musca domestica L. The insecticidal efficiency of 67 % was observed to AOE at 5.0 % against M. domestica after 12 h. In contrast, 100 % efficacy was observed against H. irritans at both concentrations for up to 4 h. Holstein cows naturally infested by H. irritans were used to assess the repellency effects of the oil at 5 % and a reduction of 57.7 % at 24 h could be observed in the cows treated when compared to control.
In a recent study, of Amorim et al. (2021) analyzed the effect of the ethanolic extract (1.06 mg.mL−1 and 0.34 mg.mL−1) from root and stem of C. guianensis, respectively, in vitro and in vivo on gastrointestinal nematodes of sheep naturally infected in the Western Amazon. Both extracts demonstrated an anthelmintic activity against Artemia saline Leach with LC50 = 530 μg.mL−1 and 170 μg.mL−1, respectively. Extracts of root and stem were orally administered in a single treatment at the days 1, 2, 3, 4, 15, 16, 17 and 18 in 2 groups of crossbred sheep at 1.06 mg.mL−1 and 0.34 mg.mL−1, respectively, for 30 days. The blood and feces samples collected at intervals of 7 and 15 days to assess the parasitic and hematological profile. From the results it was possible to observe a reduction in parasitic load of 86 and 59 % in egg count per gram of feces to the root and stem of andiroba, respectively. It was also observed a reduction of 70 and 55 % in the 3rd instar larvae recovered from the sheeps after the treatment with the root and stem of andiroba, respectively. Thus, the both extracts of C. guianensis showed an anthelmintic potential against these nematodes, representing a good alternative for the control of sheep endoparasitosis.
From these reports discussed here, it is possible to infer that the greatest and most expressive biological activity of C. guianensis is the biopesticide activity, reaching inhibitory activities and mortality of up to 100 % in insects, larvae and eggs. The AOS and AOE and its respective dilutions or nanuemulsions represent the major applications, leading to a great and potential mortality rate to several pests that cause notable losses in agriculture. Only gedunin-type limonoids were used to control these parasites and exhibited moderate activities, wherein compound 19 displays the most significant activity, whereas 43 is capable of affect pupal development.
4.8 Repellent effect
Miot et al. (2004) performed a comparative study between the effectiveness of AOS 15 % and 100 % and DEET 50 % as repellent for female Aedes sp on forearms of volunteers. From the results it was possible observe that the median of the first and third bite was 17.5 s and 40.0 s without any product, respectively, whereas AOS 15 % and 100 % exhibited 63.0 s and 97.5 % and 56.0 s and 142,5s to the first and third bite, respectively. Bites on the volunteers that used DEET 50 % after 3600 s were not observed. Therefore, the pure andiroba oil presented discreet repellent effect against bite of Aedes sp., however was significantly inferior to DEET 50 %.
The in vitro repellent effect in the commercial AOE (RF3150) at 5 % against Haematobia irritans L. and Chrysomya megacephala Fabricius were evaluated by Klauck et al. (2015). The results demonstrated a 100 % effective repellency to both species until 240 min, nevertheless, a lower efficiency of 75 % against C. megacephala was observed when compared to citronella oil, however, death of flies remained in the compartments with AOE.
Machado da Rosa et al. (2013) evaluated the repellent effect of AOS in vitro at 0.5–2 % associated with 5 % protein (Bioanastrepha™) on fruit fly A. fraterculus and conclude that this association significantly reduced the capture potential of A. fraterculus in the treatments containing 1 and 2 % of AOS. Furthermore, the authors give attention to the needing studies for this application in field on leaves and/or fruits.
A least reported of repellent effects on flies was accomplished by Zortea et al. (2017), wherein the effect of commercial AOE (RF3150) at 5 % was assessed on Musca domestica L., as well as the ecotoxicological effects on the environment. A significant reduction was observed after 2 and 24 h in the number of flies and at 48 h no difference in this number could be observed. In the ecotoxicological tests, the presence of the oil in soil did not inhibit the microbial activity or affect the survival and reproduction of springtails Folsomia candida Willem.
In another study, Freire et al. (2006) evaluated the effect of AOS on phorids, a hives’s prague, by monitoring the female phorids posture in a box containing a pot of 60 mL of AOS plus pollen. The repellent effect was observed against this prague by reducing the female oviposition reaching no posture held in the most repetitions. Furthermore, the repellent effect was also analyzed on Melipona Compressipes Manaosensis Fabricius bees and after 3 days the adults and larvae phorids presence were not observed, highlighting the repellent effect of AOS.
The repellent effects displayed by C. guianensis were attributed to pure AOS and AOE and its respective dilutions. In most of the reports mentioned above, AOS or AOE represented a significant repellency effect on flies, phorids and Aedes mosquitoes. However, just a few reports explored this activity, therefore, further investigations are needed to evaluate these parameters using other methods applications of the AOS and AOE, besides their isolated bioactive compounds, as well as their application in the field.
5 Biotechnological applications
Several biological activities from different vegetative parts of C. guianensis and its different manipulations methods were discussed here and proven to be a potential source to biotechnological applications, mainly to pharmaceutical, agriculture and cosmetic industries. Among the reports in this review, the nanoemulsions were the main applications tested in C. guianensis to the development of new drugs derived from plants. Interestingly, through the development of new tools by nanobiotechnology to natural products have been reported in the literature, such as the nanoemulsions used for pharmaceutical and biomedical aids and vehicles exhibiting a promise to drug therapy and biotechnology. It is noteworthy that nanoemulsions have been used to disperse oil extracts and compounds in aqueous media (Vaucher et al. 2015; Milhomem-Paixão et al. 2017). Furthermore, some reports in the literature have been demonstrating the use of C. guianensis in the formulation of products and new technologies, such as biomaterials, biodiesel and recovery of fermentable sugars through waste treatment in the andiroba production chain (Iha et al. 2014; Stachiw et al. 2016; Gaspar et al. 2017; Souza et al. 2019; Santos et al. 2020; Silva et al. 2021; Ferreira et al. 2022).
Nanoprobes synthetized from high quality magnetic nanoparticles are of great interest to biomedical applications, such as imaging techniques and cancer therapy, as well as the ability to control and drive the nanoparticles through bloodstream by using an external magnetic field to a specific target (Lu et al. 2007; Gaspar et al. 2017). In this context, Gaspar et al. (2017) developed a biocompatible magnetic nanofluid by incorporating superparamagnetism of iron oxide nanoparticles (SPIONs) associated with C. guianensis oil benefits. The MTT and Propidium iodide assays were used to assess the biocompatibility in human cells SW480 colon cancer line and L929 fibroblasts in the best SPIONs (A) and none effect was observed on cells viability up to 200 μg in iron, for 24 h incubation. Moreover, SPIONs A were able to internalize into colon cancer cells within 3 h and to preserve the superparamagnetic behavior, nonetheless, exhibited a 4-fold lower saturation magnetization when compared to native SPIONs.
Emulgels composed by Carbopol 934P and AOS for application in the topical propolis delivery were prepared by Santos et al. (2020) investigated the bioadhesion, drug release profile, and ex vivo skin pig permeation. The emulsion was composed by 1.0 % and 8.0 % of C934P and AOS, respectively and the bioadhesive was 0.0692 ± 0.0051 N. It was possible observe that this formulation displayed a great bioadhesive properties, modified (prolonged) propolis release profile, as well as skin permeation, and retention.
Silva et al. (2021) created a multifunctional wound dressing through the Poly (ε-caprolactone) (PCL)/AOS hybridization and assessed the thermal, surface area and the biological properties of this material in order to obtain a more natural and straightforward treatment. The hybridization showed that AOS affects some parameters, such as the decreasing hydrophilicity and porosity in interaction with water, the evaporation rate of solvent, besides an increase in the crystals domains. Triglycerides analysis suggested a higher thermal stability of this hybrid material due the barrier effect caused by polymer chains to AOS molecules. No cytotoxicity was observed under L929 cells on PCL/AOS viability tests, moreover, exhibited the ability of PCL hybrid film as a matrix for cell growth. Therefore, the authors highlighted that the absorption capacity of this material can be suitable for biomedical applications, such as wound dressings, which can be able to assist covering, preventing infection in the wound healing process.
Next, Ferreira et al. (2022) prepared and analyzed a polymeric membrane composed of chitosan, green banana peel extract and AOS by macroscopic and morphological analyses and the surface of membranes by swelling and moisture tests, contact angles, X-ray diffraction (XRD) and Differential scanning calorimetry (DSC). The membranes composed of green banana peel extract, chitosan and AOS exhibited a superior percentage of moisture, as well as a higher percentage of swelling in synthesized membranes, demonstrating the inverse proportionality between the swelling and the moisture. The addition of the plant constituents, such green banana peel extract and AOS to chitosan membranes caused a decreasing in the crystallinity, which have characteristics to make up a biomaterial to treat epithelial lesions, gathering essential properties, like the absorption capacity and fluid retention, cellular adhering facilitated by decreased crystallinity and thermal degradation. However, biological assays were not carried out, therefore, the ideal concentrations and the effectiveness of this material in tissue regeneration must be adequately studied.
Iha et al. (2014) produced a renewable biodiesel by analyzing the physicochemical properties of AOS and despite that this bio-oil was not completely deoxygenated, a potential production for a diesel engines was demonstrated, adding value to this raw material and promising. Moreover, Stachiw et al. (2016) also evaluated the potential biodiesel production from AOS by methyl route and concluded that there is a high potential for biofuel production, nonetheless, it was possible to observe a high acidity value (85 %) from this oil.
A recently study reported by Souza et al. (2019) elucidated the recovery of fermentable sugars through optimization of alkaline pretreatment from C. guianensis residues that are a great source of carbohydrates that can be used for production of organic acids and ethanol. The alkaline pretreatment optimized the production of fermentable sugar and the highest concentration was obtained at 100 min reaction time, 4 % (m/v) and 120 °C, which demonstrated the promising production of fermentable sugars from this biomass.
The high viability of C. guianensis was testified due its great benefits in a wide range of bioactivities displayed here, therefore, this specie demonstrates the great interest in the advance and development of novel bioproducts and biotechnologies to enhance these properties and its applications in order to generate economically viable and eco-friendly biotechnologies, which aggregate values at all stages of the cycle production chain of andiroba tree.
6 Conclusion
It is possible to observe that different vegetative parts of C. guianensis exhibit a wide range of biological activities of interest to pharmaceutical, medicinal, cosmetological and insecticidal applications. These activities are mainly due to fatty acids and the presence of bioactive compounds, such as limonoids and a small fraction of phenolic compounds. Gedunin-type limonoids, such as gedunin and its derivatives or synthesized from it, represent the class of compounds that show the highest bioactivities in different applications, in which gedunin exhibits the best activities with the lowest inhibitory concentration. Most of the studies were carried out in vitro and in vivo tests using the animal model and reported a broad therapeutic benefit from C. guianensis, which arouses the interest of researchers in the evaluation of these properties and enhance them through biotechnological approaches, such as emulsions and nanoemulsions, emulgels, polymeric membranes and hybrid films in wound healing, repellents and bioinsecticides, representing an alternative and eco-friendly approaches that adds value to Amazon raw material. It is noteworthy that there is a lack of studies in the analysis of the therapeutic effects in a human model, therefore, further studies are necessary to evaluate the viability of these properties, as well as investigations on the bioproducts and biotechnologies generated from the bioactive fractions of C. guianensis to assess their potential properties.
CRediT authorship contribution statement
Kaio Kelvin Barros Dias: Methodology. Adauto Lima Cardoso: Methodology, Visualization. Ana Alice Farias da Costa: Methodology. Marcele Fonseca Passos: Methodology, Visualization. Carlos Emmerson Ferreira da Costa: Resources. Geraldo Narciso da Rocha Filho: Conceptualization, Resources, Supervision. Eloísa Helena de Aguiar Andrade: Resources, Supervision. Rafael Luque: Conceptualization, Supervision. Luís Adriano Santos do Nascimento: Conceptualization, Visualization, Supervision. Renata Coelho Rodrigues Noronha: Conceptualization, Resources, Visualization, Supervision.
Acknowledgments
The authors thank PROPESP/UFPA, CNPQ (315279/2021-4 and 313798/2022-2), and FAPESPA (2022/1437745) for their support.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Opportunities for the use of Brazilian biomass to produce renewable chemicals and materials. ChemSusChem. 2021;14:169-188.
- [CrossRef] [Google Scholar]
- Limonoids from andiroba oil and Cedrela fissilis and their insecticidal activity. J. Braz. Chem. Soc.. 2006;17
- [CrossRef] [Google Scholar]
- Anthelmintic activity of the ethanolic extract of Carapa guianensis (Meliaceae) on gastrointestinal nematodes of sheep in the Western Amazon. Semin Ciências Agrárias. 2021;42:2371-2388.
- [CrossRef] [Google Scholar]
- Effects of topical application of pure and ozonized andiroba oil on experimentally induced wounds in horses. Brazilian J Vet Res. Anim. Sci.. 2017;54
- [CrossRef] [Google Scholar]
- Antioxidant activity and genotoxic assessment of crabwood (andiroba, Carapa guianensis Aublet) seed oils. Oxid. Med. Cell. Longev.. 2018;2018
- [CrossRef] [Google Scholar]
- Specificity and mode of action of the antifungal fatty acid cis -9-heptadecenoic acid produced by Pseudozyma flocculosa. Appl. Environ. Microbiol.. 2001;67:956-960.
- [CrossRef] [Google Scholar]
- Trypanocidal activity of the essential oils in their conventional and nanoemulsion forms: in vitro tests. Exp. Parasitol.. 2013;134:356-361.
- [CrossRef] [Google Scholar]
- Using of essential oils in the treatment of mice infected with Trypanosoma evansi. Rev MVZ CORDOBA. 2014;19:4109-4115.
- [Google Scholar]
- In vitro efficacy of oil from the seed of Carapa guianensis (andiroba) in the control of Felicola subrostratus. Rev Bras Farmacogn. 2012;22:1130-1133.
- [CrossRef] [Google Scholar]
- Comprehensive characterization of lipids from Amazonian vegetable oils by mass spectrometry techniques. Food Res. Int.. 2014;64:472-481.
- [CrossRef] [Google Scholar]
- Plant-derived antimalarial agents: new leads and efficient phytomedicines. Part II. non-alkaloidal natural products. Molecules. 2009;14:3037-3072.
- [CrossRef] [Google Scholar]
- Gedunin binds to myeloid differentiation protein 2 and impairs lipopolysaccharide-induced toll-like receptor 4 signaling in macrophages s. Mol. Pharmacol.. 2015;88:949-961.
- [CrossRef] [Google Scholar]
- Typification and quality control of the Andiroba (Carapa guianensis) oil via mass spectrometry fingerprinting. Anal. Methods. 2013;5
- [CrossRef] [Google Scholar]
- Effect of andiroba oil on periodontitis in Wistar rats. Acta Cir. Bras.. 2013;28:430-434.
- [CrossRef] [Google Scholar]
- Healing effect of andiroba-based emulsion in cutaneous wound healing via modulation of inflammation and transforming growth factor beta 31. Acta Cir. Bras.. 2018;33:1000-1015.
- [CrossRef] [Google Scholar]
- Atividade antimicrobiana in vitro de plantas da Amazônia sobre alguns micro-organismos formadores do biofilme dental. Rev Odonto Ciência. 2015;30
- [CrossRef] [Google Scholar]
- Chemotherapy-associated oxidative stress: impact on chemotherapeutic effectiveness. Integr. Cancer Ther.. 2004;3:294-300.
- [CrossRef] [Google Scholar]
- Bioprospection for new larvicides against Aedes aegypti based on ethnoknowledge from the Amazonian Sao Sebastiao de Marinau riverside community. J. Ethnopharmacol.. 2022;293:115284
- [CrossRef] [Google Scholar]
- Acute and subacute toxicity of the Carapa guianensis Aublet (Meliaceae) seed oil. J. Ethnopharmacol.. 2008;116:495-500.
- [CrossRef] [Google Scholar]
- Multiple modes of action of the squamocin in the midgut cells of Aedes aegypti Larvae. PLoS One. 2016;11:e0160928.
- [Google Scholar]
- Activities of some Brazilian plants against larvae of the mosquito Aedes aegypti. Fitoterapia. 2005;76:629-636.
- [CrossRef] [Google Scholar]
- Rapid discrimination between authentic and adulterated andiroba oil using FTIR-HATR spectroscopy and random forest. Food Anal. Methods. 2018;11:1927-1935.
- [CrossRef] [Google Scholar]
- Protective mechanisms of atorvastatin against doxorubicin-induced hepato-renal toxicity. Biomed. Pharmacother.. 2014;68:101-110.
- [CrossRef] [Google Scholar]
- Embrapa, (Projeto Dendrogene. Espécies Arbóreas da Amazônia 2), 2004. Andiroba, Carapa guianensis. In: (CPATU) EAO (ed). Embrapa Amazônia Oriental, p 6.
- Biodiversidade da Amazônia: usos e potencialidades dos mais importantes produtos naturais do Pará. NUMA/UFPA; 2003.
- Potencial acaricida do óleo de andiroba Carapa guianensis Aubl. sobre fêmeas adultas ingurgitadas de Anocentor nitens Neumann, 1897 e Rhipicephalus sanguineus Latreille, 1806. Arq Bras Med Veterinária e Zootec. 2009;61:877-882.
- [CrossRef] [Google Scholar]
- Modulation of T lymphocyte and eosinophil functions in vitro by natural tetranortriterpenoids isolated from Carapa guianensis Aublet. Int. Immunopharmacol.. 2011;11:1-11.
- [CrossRef] [Google Scholar]
- Synthesis and characterization of natural polymeric membranes composed of chitosan, green banana peel extract and andiroba oil. Polym. 2022;14
- [CrossRef] [Google Scholar]
- Re: National, regional, and global trends in body-mass index since 1980: Systematic analysis of health examination surveys and epidemiological studies with 960 country-years and 9.1 million participants. J. Urol.. 2011;186:1982-1983.
- [CrossRef] [Google Scholar]
- Wood properties of Carapa guianensis from floodplain and upland forests in Eastern Amazonia. Brazil. Sci Rep. 2019;9:10641.
- [CrossRef] [Google Scholar]
- Efeito dos óleos vegetais de andiroba (Carapa sp.) e Copaíba (Copaifera sp.) sobre forídeo, pragas de colméias, (Diptera: Phoridae) na Amazônia Central. Acta Amaz. 2006;36:365-368.
- [CrossRef] [Google Scholar]
- Development of a biocompatible magnetic nanofluid by incorporating SPIONs in Amazonian oils. Spectrochim. Acta A Mol. Biomol. Spectrosc.. 2017;172:135-146.
- [CrossRef] [Google Scholar]
- The antifungal activity of fatty acids of all stages of Sarcophaga carnaria L. (Diptera: Sarcophagidae) Microbiol. Res.. 2014;169:279-286.
- [CrossRef] [Google Scholar]
- Tapping an Amazônian plethora: four medicinal plants of Marajó island, Pará (Brazil) J. Ethnopharmacol.. 1993;40:53-75.
- [CrossRef] [Google Scholar]
- The therapeutic properties of Carapa guianensis. Curr. Pharm. Des.. 2014;20:850-856.
- [CrossRef] [Google Scholar]
- Carapanosins A-C from Seeds of Andiroba (Carapa guianensis, Meliaceae) and Their Effects on LPS-Activated NO Production. Molecules. 2017;22
- [CrossRef] [Google Scholar]
- Phenylbutazone effects on experimental wound healing in horses. Brazilian J Vet Res Anim Sci. 2010;47:262-267.
- [CrossRef] [Google Scholar]
- Potential application of Terminalia catappa L. and Carapa guianensis oils for biofuel production: Physical-chemical properties of neat vegetable oils, their methyl-esters and bio-oils (hydrocarbons) Ind. Crop. Prod.. 2014;52:95-98.
- [CrossRef] [Google Scholar]
- Carapanolides A and B: unusual 9,10-seco-mexicanolides having a 2R,9S-oxygen bridge from the seeds of Carapa guianensis. Tetrahedron Lett.. 2012;53:6685-6688.
- [CrossRef] [Google Scholar]
- Guianolides A and B, new carbon skeletal limonoids from the seeds of Carapa guianensis. Org. Lett.. 2013;15:3018-3021.
- [CrossRef] [Google Scholar]
- Carapanolides C-I from the seeds of andiroba (Carapa guianensis, Meliaceae) Fitoterapia. 2014;96:56-64.
- [CrossRef] [Google Scholar]
- Carapanolides M-S from seeds of andiroba (Carapa guianensis, Meliaceae) and triglyceride metabolism-promoting activity in high glucose-pretreated HepG2 cells. Tetrahedron. 2015;71:2753-2760.
- [CrossRef] [Google Scholar]
- Carapanosins D—F from the seeds of andiroba (Carapa guianensis, Meliaceae) and their effects on LPS-activated NO production. Molecules. 2018;23
- [CrossRef] [Google Scholar]
- Preparation of a nanoemulsion with Carapa guianensis Aublet (Meliaceae) oil by a low-energy/solvent-free method and evaluation of its preliminary residual larvicidal activity. Evid Based Complement Altern. Med.. 2017;2017:6756793.
- [CrossRef] [Google Scholar]
- Estimation of acute oral toxicity in rats by determination of the approximate lethal dose rather than the LD50. J. Appl. Toxicol.. 1986;6:145-148.
- [CrossRef] [Google Scholar]
- Carapanins A-C: new limonoids from andiroba (Carapa guianensis) fruit oil. Org. Biomol. Chem.. 2020;18:9268-9274.
- [CrossRef] [Google Scholar]
- Therapeutic effects of topical application of ozone on acute cutaneous wound healing. J. Korean Med. Sci.. 2009;24:368-374.
- [CrossRef] [Google Scholar]
- Insecticidal and repellent effects of tea tree and andiroba oils on flies associated with livestock. Med. Vet. Entomol.. 2014;28(Suppl 1):33-39.
- [CrossRef] [Google Scholar]
- In vitro repellent effect of tea tree (Melaleuca alternifolia) and andiroba (Carapa guianensis) oils on Haemotobia irritans and Chrysomya megacephala flies. Trop. Biomed.. 2015;32:160-166.
- [Google Scholar]
- Role of reactive oxygen species in tumors based on the “seed and soil” theory: A complex interaction (Review) Oncol. Rep.. 2021;46
- [CrossRef] [Google Scholar]
- Population Structure and Fruit Production of Carapa guianensis (Andiroba) in Amazonian Floodplain Forests. Trop Conserv Sci. 2017;10
- [CrossRef] [Google Scholar]
- Lourenço, J., Ferreira, L., Martins, G., Nascimento, D., 2017. Produção, biometria de frutos e sementes e extração do óleo de andiroba (Carapa guianensis Aublet.) sob manejo comunitário em Parintins, AM. Embrapa Amazônia Ocidental (CPAA), p 36.
- Magnetic nanoparticles: synthesis, protection, functionalization, and application. Angew. Chem. Int. Ed. Engl.. 2007;46:1222-1244.
- [CrossRef] [Google Scholar]
- Andiroba oil (Carapa guianensis Aubl) in the capture of the fruit fly (Anastrepha fraterculus Wiedemann) in Feijoa (Acca sellowiana (Berg) Burret) Idesia (Arica). 2013;31:97-101.
- [CrossRef] [Google Scholar]
- Antimalarial activity of tropical Meliaceae extracts and gedunin derivatives. J. Nat. Prod.. 1997;60:336-341.
- [CrossRef] [Google Scholar]
- Hepatoprotective amide constituents from the fruit of Piper chaba: Structural requirements, mode of action, and new amides. Bioorganic Med Chem. 2009;17:7313-7323.
- [CrossRef] [Google Scholar]
- Carapanolides J-L from the seeds of Carapa guianensis (Andiroba) and their effects on LPS-activated NO production. Molecules. 2014;19:17130-17140.
- [CrossRef] [Google Scholar]
- A Limonoid, 7-Deacetoxy-7-Oxogedunin (CG-1) from Andiroba (Carapa guianensis, Meliaceae) Lowers the Accumulation of Intracellular Lipids in Adipocytes via Suppression of IRS-1/Akt-Mediated Glucose Uptake and a Decrease in GLUT4 Expression. Molecules. 2019;24
- [CrossRef] [Google Scholar]
- Limonoid 7-Deacetoxy-7-oxogedunin from Andiroba, Carapa guianensis, Meliaceae, Decreased Body Weight Gain, Improved Insulin Sensitivity, and Activated Brown Adipose Tissue in High-Fat-Diet-Fed Mice. J. Agric. Food Chem.. 2019;67:10107-10115.
- [CrossRef] [Google Scholar]
- Evaluation of the Genotoxic and Antigenotoxic Effects of Andiroba (Carapa guianensis Aublet) Oil and Nanoemulsion on Swiss Mice. J. Nanomater.. 2018;2018:1-8.
- [CrossRef] [Google Scholar]
- Andiroba oil and nanoemulsion (Carapa guianensis Aublet) reduce lesion severity caused by the antineoplastic agent doxorubicin in mice. Biomed. Pharmacother.. 2021;138:111505
- [CrossRef] [Google Scholar]
- The lipidome, genotoxicity, hematotoxicity and antioxidant properties of andiroba oil from the Brazilian Amazon. Genet. Mol. Biol.. 2016;39:248-256.
- [CrossRef] [Google Scholar]
- Andiroba Oil (Carapa guianensis Aublet) Nanoemulsions: Development and Assessment of Cytotoxicity, Genotoxicity, and Hematotoxicity. J. Nanomater.. 2017;2017:1-11.
- [CrossRef] [Google Scholar]
- Comparative study of the topical effectiveness of the Andiroba oil (Carapa guianensis) and DEET 50% as repellent for Aedes sp. Rev. Inst. Med. Trop. Sao Paulo. 2004;46:253-256.
- [CrossRef] [Google Scholar]
- Antiplasmodial activity of the andiroba (Carapa guianensis Aubl., Meliaceae) oil and its limonoid-rich fraction. J. Ethnopharmacol.. 2012;142:679-683.
- [CrossRef] [Google Scholar]
- Carapanolides T-X from Carapa guianensis (Andiroba) Seeds. Molecules. 2015;20:20955-20966.
- [CrossRef] [Google Scholar]
- Hepatoprotective triterpenes from traditional Tibetan medicine Potentilla anserina. Phytochemistry. 2014;102:169-181.
- [CrossRef] [Google Scholar]
- Effects of nanoemulsions prepared with essential oils of copaiba- and andiroba against Leishmania infantum and Leishmania amazonensis infections. Exp. Parasitol.. 2018;187:12-21.
- [CrossRef] [Google Scholar]
- Collagen Synthesis-Promoting Effects of Andiroba Oil and its Limonoid Constituents in Normal Human Dermal Fibroblasts. J. Oleo Sci.. 2018;67:1271-1277.
- [CrossRef] [Google Scholar]
- Artisanal extraction and traditional knowledge associated with medicinal use of crabwood oil (Carapa guianensisAublet.) in a Peri-Urban Várzea environment in the Amazon Estuary. Evidence-Based Complement Altern. Med.. 2016;2016:1-12.
- [CrossRef] [Google Scholar]
- Lipidomic profiles from seed oil of Carapa guianensis Aubl. and Carapa vasquezii Kenfack and implications for the control of phytopathogenic fungi. Ind. Crop. Prod.. 2019;129:67-73.
- [CrossRef] [Google Scholar]
- Limonoids from the leaves of Cipadessa baccifera. J. Nat. Prod.. 2010;73:1327-1331.
- [CrossRef] [Google Scholar]
- Hepatoprotective Limonoids from Andiroba (Carapa guianensis) Int. J. Mol. Sci.. 2016;17
- [CrossRef] [Google Scholar]
- Extraction, chemical characterization and antioxidant activity of andiroba seeds oil obtained from pressurized n-butane. Ind. Crop. Prod.. 2015;76:697-701.
- [CrossRef] [Google Scholar]
- Control of the South American fruit fly in pear with natural-based products. Comun Sci. 2015;6
- [CrossRef] [Google Scholar]
- Carapa guianensis Aublet (Andiroba) Seed Oil: Chemical Composition and Antileishmanial Activity of Limonoid-Rich Fractions. Biomed Res. Int.. 2018;2018:5032816.
- [CrossRef] [Google Scholar]
- Métodos para avaliação da atividade antimicrobiana e determinação da Concentração Mínima Inibitória (CMI) de plantas medicinais. Rev Bras Farmacogn. 2008;18:301-307.
- [CrossRef] [Google Scholar]
- PRISMA 2020 explanation and elaboration: updated guidance and exemplars for reporting systematic reviews. BMJ. 2021;372:n160
- [CrossRef] [Google Scholar]
- Efeitos da andiroba associada ao microagulhamento na pele de ratos. Surg Cosmet Dermatology. 2018;10
- [CrossRef] [Google Scholar]
- Anti-allergic effects of natural tetranortriterpenoids isolated from Carapa guianensis Aublet on allergen-induced vascular permeability and hyperalgesia. Inflamm. Res.. 2005;54:295-303.
- [CrossRef] [Google Scholar]
- Inhibition of allergen-induced eosinophil recruitment by natural tetranortriterpenoids is mediated by the suppression of IL-5, CCL11/eotaxin and NFκB activation. Int. Immunopharmacol.. 2006;6:109-121.
- [CrossRef] [Google Scholar]
- In vitro and in vivo anti-malarial activity of limonoids isolated from the residual seed biomass from Carapa guianensis (andiroba) oil production. Malar. J.. 2014;13:317.
- [CrossRef] [Google Scholar]
- Antifungal free fatty acids: a review. Sci. Against Microb. Pathog. Commun. Curr. Res. Technol. Adv. 2011:61-71.
- [Google Scholar]
- Andiroba oil (Carapa guianensis Aubl) shows cytotoxicity but no mutagenicity in the ACPP02 gastric cancer cell line. J. Appl. Toxicol.. 2020;40:1060-1066.
- [CrossRef] [Google Scholar]
- Evaluation of time toxicity, residual effect, and growth-inhibiting property of Carapa guianensis and Copaifera sp. in Aedes aegypti. Parasitol. Res.. 2012;110:713-719.
- [CrossRef] [Google Scholar]
- Fatty acid amides synthesized from andiroba oil (Carapa guianensis Aublet.) exhibit anticonvulsant action with modulation on GABA-A receptor in mice: a putative therapeutic option. Pharm.. 2020;13
- [CrossRef] [Google Scholar]
- Morphological and cytochemical changes in synganglion of Rhipicephalus sanguineus (Latreille, 1806) (Acari: Ixodidae) female ticks from exposure of andiroba oil (Carapa guianensis) Microsc. Res. Tech.. 2013;76:687-696.
- [CrossRef] [Google Scholar]
- Action of andiroba oil and permethrin on the central nervous and reproductive systems of Rhipicephalus sanguineus (Latreille, 1806) (Acari: Ixodidae) ticks females. A confocal study. Res Vet Sci. 2013;95:529-536.
- [CrossRef] [Google Scholar]
- Changes in the synganglion of Rhipicephalus sanguineus (Latreille, 1806) (Acari: Ixodidae) female ticks exposed to permethrin: An ultrastructural overview. Acta Trop.. 2014;136:19-26.
- [CrossRef] [Google Scholar]
- Effects of andiroba (Carapa guianensis) oil in ticks: ultrastructural analysis of the synganglion of Rhipicephalus sanguineus (Latreille, 1806) (Acari: Ixodidae) Acta Trop.. 2015;141:7-15.
- [CrossRef] [Google Scholar]
- Effect of alpha-linolenic, capric and lauric acid on the fatty acid biosynthesis in Staphylococcus aureus. Int. J. Food Microbiol.. 2009;129:288-294.
- [CrossRef] [Google Scholar]
- Andirolides Q-V from the flower of andiroba (Carapa guianensis, Meliaceae) Fitoterapia. 2013;90:20-29.
- [CrossRef] [Google Scholar]
- Andirolides W-Y from the flower oil of andiroba (Carapa guianensis, Meliaceae) Fitoterapia. 2015;100:81-87.
- [CrossRef] [Google Scholar]
- Antimicrobial activity of Amazonian oils against Paenibacillus species. J. Invertebr. Pathol.. 2012;109:265-268.
- [CrossRef] [Google Scholar]
- Use of plant oils from the southwestern Amazon for the control of maize weevil. J. Stored Prod. Res.. 2015;63:67-70.
- [CrossRef] [Google Scholar]
- Inseticidal Oils from Amazon Plants in Control of Fall Armyworm. Rev Caatinga. 2016;29:642-647.
- [CrossRef] [Google Scholar]
- Anti-inflammatory and Immunomodulatory Effects of Ulomoides dermestoides on Induced Pleurisy in Rats and Lymphoproliferation In vitro. Inflammation. 2010;33:173-179.
- [CrossRef] [Google Scholar]
- Influence of Schinus terebinthifolius Raddi (aroeira) and Carapa guianensis Aublet (andiroba) in the healing process of gastrorraphies] Arq. Bras. Cir. Dig.. 2013;26:84-91.
- [CrossRef] [Google Scholar]
- Emulgels containing Carbopol 934P and different vegetable oils for topical propolis delivery: bioadhesion, drug release profile, and ex vivo skin permeation studies. AAPS PharmSciTech. 2020;21:209.
- [CrossRef] [Google Scholar]
- Reactive oxygen species: Role in carcinogenesis, cancer cell signaling and tumor progression. Life Sci.. 2021;284:119942
- [CrossRef] [Google Scholar]
- Carapa guianensis Aubl. (Meliaceae) oil associated with silk fibroin, as alternative to traditional surfactants, and active against larvae of the vector Aedes aegypti. Ind Crops. Prod. 2020;157
- [CrossRef] [Google Scholar]
- Effect of triterpenoids and limonoids isolated from Cabralea canjerana and Carapa guianensis (Meliaceae) against Spodoptera frugiperda (J. E. Smith) Z. Naturforsch., C: J. Biosci.. 2011;66:245-250.
- [CrossRef] [Google Scholar]
- Guianofruits A and B from the Fruit Oil of Andiroba (Carapa guianensis, Meliaceae) and Their Effects on LPS-Activated NO Production. ChemistrySelect. 2018;3:6056-6060.
- [CrossRef] [Google Scholar]
- Residual Larvicidal Activity of Quinones against Aedes aegypti. Molecules. 2020;25:3978.
- [CrossRef] [Google Scholar]
- PCL/Andiroba Oil (Carapa guianensis Aubl.) Hybrid Film for Wound Healing Applications. Polym. 2021;13
- [CrossRef] [Google Scholar]
- The use of andiroba Carapa guianensis as larvicide against Aedes albopictus. J. Am. Mosq. Control Assoc.. 2004;20:456-457.
- [Google Scholar]
- Larvicidal Effect of Andiroba Oil, Carapa guianensis(Meliaceae), against Aedes Aegypti. J. Am. Mosq. Control Assoc.. 2006;22:699-701.
- [CrossRef] [Google Scholar]
- Effect of Carapa guianensis Aublet (Andiroba) and Orbignya phalerata (Babassu) in colonic healing in rats. Rev. Col. Bras. Cir.. 2015;42:399-406.
- [CrossRef] [Google Scholar]
- Therapeutic effects of andiroba (Carapa guianensis Aubl) oil, compared to low power laser, on oral mucositis in children underwent chemotherapy: A clinical study. J. Ethnopharmacol.. 2021;264:113365
- [CrossRef] [Google Scholar]
- Valorization of andiroba (Carapa guianensis Aubl.) residues through optimization of alkaline pretreatment to obtain fermentable sugars. BioResources. 2019;15:894-909.
- [CrossRef] [Google Scholar]
- Análise física e avaliação do efeito antifúngico dos óleos de andiroba, copaíba e pinhão-manso. Floresta. 2018;48
- [CrossRef] [Google Scholar]
- Efficiency of Vegetable Oils in Wood Resistance to Cryptotermes brevis Termites. Floresta e Ambient. 2019;26
- [CrossRef] [Google Scholar]
- Efeito de óleos essenciais como alternativa no controle de Colletotrichum gloeosporioides, em pimenta. Summa Phytopathol.. 2012;38:42-47.
- [CrossRef] [Google Scholar]
- Potencial de produção de biodiesel com espécies oleaginosas nativas de Rondônia, Brasil. Acta Amaz. 2016;46:81-90.
- [CrossRef] [Google Scholar]
- Absolute stereostructure of Andirolides A-G from the flower of Carapa guianensis (Meliaceae) Tetrahedron. 2011;67:782-792.
- [CrossRef] [Google Scholar]
- Andirolides H-P from the flower of andiroba (Carapa guianensis, Meliaceae) Tetrahedron. 2012;68:3669-3677.
- [CrossRef] [Google Scholar]
- Development of an HPLC method for the determination of tetranortriterpenoids in Carapa guianensis seed oil by experimental design. J. Pharm. Biomed. Anal.. 2008;48:1090-1095.
- [CrossRef] [Google Scholar]
- Guianofruits C-I from fruit oil of andiroba (Carapa guianensis, Meliaceae) Tetrahedron. 2019;75:1149-1156.
- [CrossRef] [Google Scholar]
- Composition and antimicrobial activity of fatty acids detected in the hygroscopic secretion collected from the secretory setae of larvae of the biting midge Forcipomyia nigra (Diptera: Ceratopogonidae) J. Insect Physiol.. 2012;58:1265-1276.
- [CrossRef] [Google Scholar]
- Antimicrobial activity of nanostructured Amazonian oils against Paenibacillus species and their toxicity on larvae and adult worker bees. J. Asia Pac. Entomol.. 2015;18:205-210.
- [CrossRef] [Google Scholar]
- Cytotoxic effects of andiroba oil (Carapa guianensis) in reproductive system of Rhipicephalus sanguineus (Latreille, 1806) (Acari: Ixodidae) semi-engorged females. Parasitol. Res.. 2012;111:1885-1894.
- [CrossRef] [Google Scholar]
- Action of andiroba oil (Carapa guianensis) on Rhipicephalus sanguineus (Latreille, 1806) (Acari: Ixodidae) semi-engorged females: morphophysiological evaluation of reproductive system. Microsc. Res. Tech.. 2012;75:1745-1754.
- [CrossRef] [Google Scholar]
- Influence of rosemary, andiroba and copaiba essential oils on different stages of the biological cycle of the tickRhipicephalus microplus in vitro. J. Essent. Oil Res.. 2015;27:244-250.
- [CrossRef] [Google Scholar]
- Reactive oxygen species in immune cells: A new antitumor target. Biomed. Pharmacother.. 2021;133:110978
- [CrossRef] [Google Scholar]
- Therapeutic effect of andiroba oil (Carapa guianensis Aubl.) against oral mucositis: an experimental study in golden Syrian hamsters. Clin. Oral Invest.. 2018;22:2069-2079.
- [CrossRef] [Google Scholar]
- Use of andiroba oil to control Anastrepha fraterculus (Diptera: Tephritidae) in different fruit hosts. Rev. Colomb. Entomol.. 2021;47:2-4.
- [CrossRef] [Google Scholar]
- Acute Toxicity and Sublethal Effects of Botanical Insecticides to Honey Bees. J. Insect Sci.. 2015;15
- [CrossRef] [Google Scholar]
- Repellent Effects of Andiroba and Copaiba Oils against Musca domestica (Common House Fly) and Ecotoxicological Effects on the Environment. Acta Sci. Vet.. 2017;45
- [CrossRef] [Google Scholar]







