Biological evaluation, GC–MS profiling, and molecular docking studies of some essential oils against postharvest pathogens of maize
⁎Corresponding authors at: Department of Plant Pathology, Faculty of Agricultural Sciences, University of the Punjab, Lahore, Pakistan(W. Akram), Vegetable Research Institute, Guangdong Academy of Agricultural Sciences, Guangzhou, China(G. Li). liguihua@gdaas.cn (Guihua Li)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
Considering the use of essential oils (EOs) as biopesticides due to their ability to effectively control postharvest pathogens, EOs-based formulations, and coatings became a priority. The present work aimed to screen essential oils from eight different plant species with anti-fungal properties along with the phytochemical analysis and molecular docking insight. Furthermore, the selected essential oil was applied as an edible coating to examine its protective effect against the attack of isolated postharvest pathogens on maize seedlings and rescue the seedling’s growth. Eight EOs were screened for their antimicrobial potential against Fusarium oxysporum and Xanthomonas campestris. Later on, the identification of the chemical constituents of the best-performing essential oil (Bergamot oil) was performed by GC–MS analysis. Different coating formulations consisting of essential oils and chitosan at 0.25% and 0.5% concentrations were applied to maize seeds to protect against postharvest pathogens. Formulation containing the bergamot essential oil showed the highest protective potential against a fungal and bacterial postharvest pathogen of maize under in-vitro conditions. The molecular docking study showed that the linalool and linalyl acetate present in essential oil comprises strong interactions with antifungal target proteins.
Keywords
Essential oil
Bergamot
Antifungal
Fusarium oxysporum
Maize
Chitosan
1 Introduction
Maize (Zea mays L.) is known as the queen of cereals as it is an important source of food and fodder. After wheat and rice, it is the third most important cereal crop cultivated globally, with a total production of 1.16 billion metric tons (FAOSTAT 2021). Maize grain is a good substrate for fungal growth and mycotoxin synthesis that results in illness and economic losses (FAO 2010).
Fruits and grains are sources of minerals, fiber, and other nutrients that the human body needs. However, postharvest pathogens appear to be the primary cause of the decay, reduced shelf-life, and financial losses. The Postharvest pests of maize include some members of Aspergillus and Fusarium fungi. Fusarium is a ubiquitous soil-borne genus containing several toxigenic species, the most common maize pathogens. Fusarium spp. are detected, in soil and on crop residues. They are found on the surface and inside the grain of cereals [13]. Postharvest losses in maize have been estimated at 20–30 % of the total staple maize harvested (Yusuf and He 2011), valued at approximately US$4 billion annually (FAO 2010).
Although chemical preservatives can prevent pathogen growth and guarantee food safety, their use can impede adverse health effects and environmental issues (Novais et al., 2022). At present strict regulations are enforced by the fresh produce importing countries regarding the minimum pesticide residue levels in the edible portion of the fresh produce (Sivakumar & Bautista-Baños, 2014). Additionally, some fungal pathogens have developed resistance to synthetic fungicides. Therefore, the agricultural industry needs to find an alternative solution to postharvest fungicide applications. Hence, the research is aimed to implement the use of natural antimicrobial agents such as essential oils (EOs), studying their distinctive physicochemical characteristics and wide range of biological activities. EOs are a mixture of volatile compounds containing a variable number of polar and non-polar components (Raut & Karuppayil, 2014). Based on the chemical characterization of the natural compounds within EOs, these are mainly divided into the terpenes, aromatic, and aliphatic groups (Pavel, 2015). As a potential alternative for industrial chemicals, the U.S. Food and Drug Administration (USFDA) classifies them as Generally Recognized as Safe (GRAS). It is worth mentioning that the aroma of EOs is considered one of the main limitations of their use as food preservatives. Different strategies have been proposed to overcome this hurdle as lower concentrations of EOs or slow-released formulations of EOs, and encapsulation into micro- or nanoemulsions to overcome the organoleptic effects of EOs (Jemaa et al., 2017).
Additionally, due to consumer concerns about synthetic preservatives, numerous organic antimicrobials, such as essential oils are receiving more attention (Rout et al., 2022). The essential oils and EOs-based products have minor residual activity. Additionally, beneficial microbes present in the ecosystem suffer fewer negative impacts from these products (Azeem et al., 2022). Essential oils are organic substances that plants produce for defense against pathogens due to their biological properties (Tsai et al., 2013). They are made from a variety of plant organs.
Chitosan is a deacetylated form of chitin and is the second most abundant biopolymer found in nature after cellulose, with prominent film-forming properties, non-toxicity, biodegradable and biocompatible properties, high mechanical strength, and excellent antimicrobial activity (Hosseinnejad and Jafari 2016). The use of chitosan-based coatings to maintain the postharvest quality of fresh fruit is due to their ability to delay the metabolic processes and control microbial growth (Riaz Rajoka et al., 2019). The adequate adhesion of coatings on the food surfaces is essential to guarantee long-term performance. (de Oliveira Filho et al., 2021). Different types of essential oils can reduce pathogen infestation on cereal grains and increase protection during storage (Mongiano et al., 2021). Scientists have also developed seed-coating technology based on EOs to protect seeds from insects, pests, and diseases. Among these natural products, chitosan represents a potential candidate since it can protect both the seeds and the plant from pests by improving the quality of the seeds, their germination potential, and seedling growth (Mancini and Romanazzi 2014).
Molecular docking can be used to study the interactions between plant essential oil compounds and target proteins, which can provide insights into their potential therapeutic effects. Molecular docking is a computational technique that predicts the positioning of a ligand in a target interaction site. To explain and predict potential interactions, evaluate qualities, and examine relationships between chemical structure and biological activity docking is widely used.
Keeping this in view, this study was performed to analyze the antimicrobial properties of selected essential oils against postharvest pathogens of maize, chemical characterization of essential oil, preparation of seed coating emulsion, and molecular docking analysis.
2 Materials and methods
2.1 Essential oils (EOs)
The following eight EOs were selected for initial screening: Wintergreen (Gaultheria procumbens L.), Rose geranium (Pelargonium graveolens L. ‘Her’), Tansy blue (Tanacetum annum L.), Bergamot (Citrus bergamia Risso.), Geranium (Pelargonium distillates L. ‘Her’), Tuberose absolute (Polianthes tuberosa L.), Rose marry (Salvia rosmarinus Spenn.), and Cedarwood (Cedrus atlantica Endl.). These EOs were acquired from the local market of Lahore, Pakistan.
2.2 Isolation and identification of fungi and bacteria from corn seeds
The infected corn seeds were soaked in 5 % sodium hypochlorite solution (NaOCl) for about 5 min for surface sterilization (Sauer and Burroughs, 1986). After that, seeds were washed 3–4 times with distilled water, and then dried seeds were placed on 2 % Malt extract agar (MEA) plates for the isolation of fungi and on 2 % Luria Bertani agar (LBA) plates for the isolation of bacteria. MEA media plates were incubated at 28 °C for one week, whereas LBA media plates were incubated at 37 °C for 24 h. The obtained fungal and bacterial species were purified by successive sub-culturing on new media plates. The pure colonies were identified by the Fungal Culture Bank of Pakistan (FCBP), University of the Punjab, Pakistan.
2.3 Analysis of antimicrobial activity by agar well diffusion method
The agar well diffusion method was used for the evaluation of the antibacterial activity. Wells measuring approximately 6 mm in diameter and 5 mm in depth were made with the aid of a sterilized cork borer in the center of each medium plate (LB agar and potato dextrose agar for bacteria and fungus, respectively) to hold the EOs in the growth medium. Each EO was poured into wells separately, in an amount of about 50 μL. In another set of plates, 50 μL of the emulsion solution was poured into the wells according to the prepared emulsion (0.25 % CH + EO and 0.5 % CH + EO). The plates were then inoculated with a diluted spore suspension of the pathogens (Fusarium oxysporum, Xanthomonas campestris). No essential oil and coating agent was poured in the negative control and positive control was set with 50 μL of commercial fungicide (Difenoconazole). The plates were incubated for 5–7 days at 28 °C for fungi and 1 day at 37 °C for bacteria. The zones of inhibition were measured after incubation. The whole experiment was performed in triplicate and repeated twice.
2.4 Chemical characterization of bergamot essential oil
The best-performing oil was analyzed by GC–MS for phytochemical profiling as described by (Kumar et al., 2015). Then, the identification of the compounds was performed, based on a comparison with the NIST library and previously published literature.
2.5 Preparation of chitosan emulsion for seed coating containing EOs
A coating of chitosan with selected EOs was prepared according to Mohamed et al., (2020). chitosan was used in emulsions at two different concentrations of 0.25 g and 0.5 g. Chitosan was dissolved in 80 mL of distilled water including 0.7 mL of acetic acid. To ensure that the chitosan was completely dissolved, the solution was stirred with a magnetic stirrer at room temperature for an entire night. The pH was adjusted to 5.5 using NaOH (10 %) and then distilled water was added to make the final volume 100 mL. To achieve a concentration of 1 percent (v/v) in the final volume, 1 mL of essential oil (EO) was added to each chitosan solution (0.25 and 0.5 %). To create the emulsion, the mixture was homogenized in a mixer for five minutes. Under the same conditions, a control solution was made with distilled water containing essential oil at a final concentration of 1 percent (v/v). The different concentrations of coating solution involved in this research were 0.25 % CH + EO, 0.5 % CH + EO, and 1 % EO. The control was without any coating and only had a pathogen.
2.6 Analysis of the antimicrobial potential of coating emulsion containing bergamot essential oil and chitosan
Three replicates of 5 corn seeds were surface disinfected by soaking in 2 % NaOCl solution for 2 min and then rinsed five times with sterile distilled water. Sterilized seeds were transferred in the pathogen inoculum for 1 min. The inoculated seeds were soaked for 16 h in a solution of 0.25 % CH + EO + Pathogen, 0.5 % CH + EO + Pathogen, 1 % EO + Pathogen, and control uncoated seeds only had pathogen. Afterward, these inoculated seeds were placed in a Petri dish on blotting paper that had been lightly moistened with sterilized distilled water. Petri plates were labeled according to the treatment and pathogen. The seeds were incubated at 25 ± 2 °C in the incubator. The observation was taken after 8–10 days. The contamination index after treatment was evaluated as follows:
2.7 Evaluation of the effect of coating emulsion on maize seed germination
The seeds were placed on wet filter paper in Petri dishes after soaking in the coating solution for 16 h. Five seeds were placed in each Petri dish, and each treatment was repeated three times. All Petri dishes were incubated at 25 ± 2 °C. After 6–8 days, the germination percentage (GP) was calculated according to the following formula:
where Sg is the number of germinated seeds and St is the number of total seeds investigated.
2.8 Evaluation of the effect of coating emulsion on seedling growth of maize
Various coating solutions were evaluated in-planta experiment. For 16 h, corn seeds were separately soaked in different coating solutions i.e., 0.25 % CH + EO + Pathogen, 0.5 % CH + EO + Pathogen, 1 % EO + Pathogen, and control uncoated seeds only had pathogen. Six of the corn seeds were planted in a pot in sterilized soil and all tests were carried out in triplicate. After 15 days the root length, shoot length, number of leaves, and fresh and dry weight of the plants were measured.
2.9 Molecular docking analysis
The Molecular docking analysis of the compounds present in essential oil and some common antifungal targets was performed to gain insight into molecular interactions. Four commonly used antifungal targets [G-protein beta subunit Fgb1 and FOXG_04696 protein of Fusarium oxysporum; lipid transfer protein “sec14p” of Saccharomyces cerevisiae and glycylpeptide N-tetradecanoyl transferase “NCC” of Candida albicans] were selected. The crystal structure of the proteins was retrieved from the Brookhaven protein data bank and Alpha Fold Protein Structure Database. Preparation of the antifungal protein structures involved the removal of co-crystallized ligands and waters in the protein, the addition of Kollmann charges and Polar Hydrogen atoms, and energy minimization using Chimera, MGLTools, and Swiss PDB Viewer software. Whereas ligand preparations involved the addition of hydrogen atoms and Gasteiger charge and energy minimization by PyRx Virtual Screening Tool and MGLTools. Docking was performed by AMDock tool; a user-friendly graphical tool to assist in docking using AutoDock Vina and AutoDock4. The analysis of the intermolecular interactions of best-scoring poses was performed by the Ligplot tool.
2.10 Statistical analysis
The means of the various treatments were compared using the DSAASTAT extension, which was collected from the experimental design. All the experiments were performed in triplicate and data was reported as means ± standard deviations. Means were separated by the Tukey multiple range test when ANOVA was significant (p < 0.05).
3 Results
3.1 Isolation and identification of fungi and bacteria from corn seeds
Fungus and bacteria isolated from corn seeds were Fusarium oxysporum, and Xanthomonas campestris respectively. The F. oxysporum possesses white cottony colonies whereas (Fig. 1A), Xanthomonas campestris formed slightly yellow colonies (Fig. 2A) on the Petri plate.
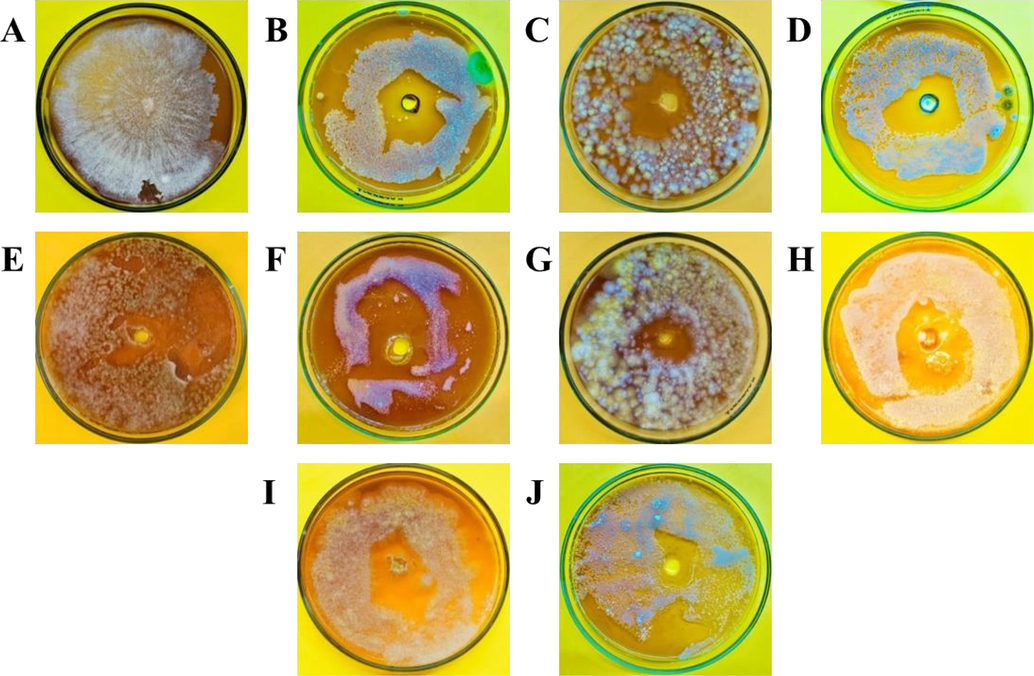
-
In-vitro antifungal potential of different essential oils on the growth of Fusarium oxysporum. A (Control), B (Fungicide), C (Tansy Blue Oil), D (Tuberose Absolute Oil), E (Rose Marry Oil), F (Bergamot Oil), G (Cedarwood Oil), H (Geranium Oil), I (Rose Geranium Oil), J (Winter Green Oil).
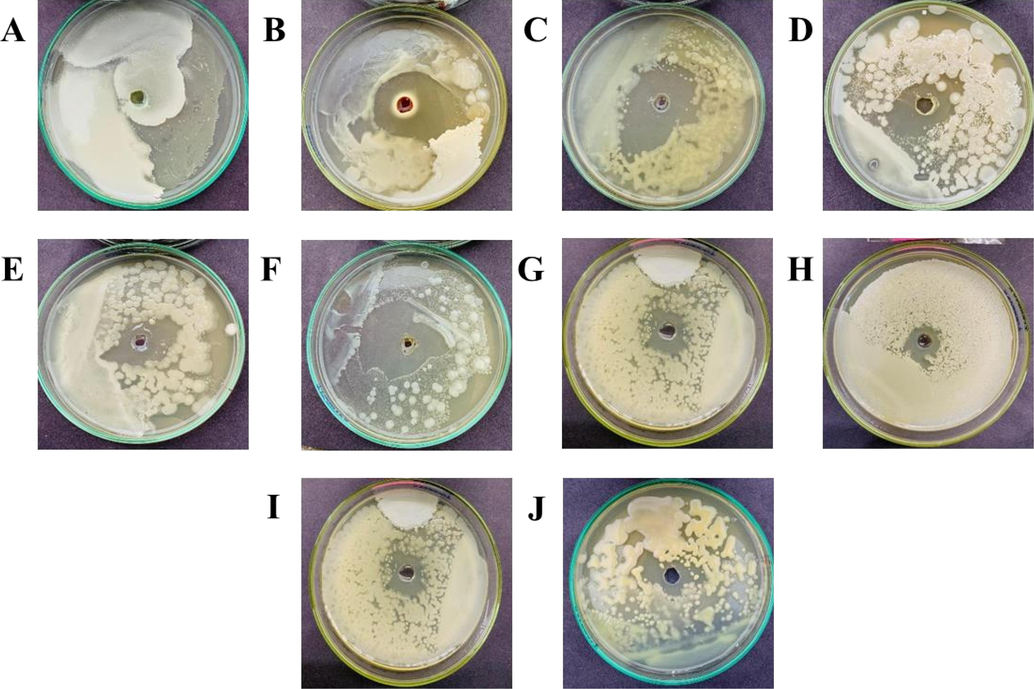
-
In-vitro antibacterial potential of different essential oils on the growth of Xanthomonas campestris. A (Control), B (Fungicide), C (Tansy Blue Oil), D (Tuberose Absolute Oil), E (Rose Marry Oil), F (Bergamot Oil), G (Cedarwood Oil), H (Geranium Oil), I (Rose Geranium Oil), J (Winter Green Oil).
3.2 Screening of the antimicrobial activity of essential oils
Selected eight essential oils significantly controlled the growth of tested bacteria and fungi (Fig. 3). However, slight variation was observed in the antimicrobial potential of selected essential oils. Bergamot essential oil (BMO) showed the highest potential to control the growth of both microbes. The zone of inhibition recorded in the case of X. campestris was 3.61 cm, whereas F. oxysporum showed a zone of inhibition of 3.58 cm. These results were found comparable to the one treated with selected fungicide (Difenoconazole). Cedarwood essential oil proved as the second most potent treatment (CO) against X. campestris (Fig. 1). It reduced bacterial growth by 4.75 % less than bergamot oil. However, in the case of F. oxysporum, rose geranium essential oil was found next to the bergamot oil, which showed 5.03 %less activity when compared to the bergamot oil (Fig. 2). Least antimicrobial activity was inferred by wintergreen (WGO) essential oil. The activity of this oil was 47.37 % and 28.49 % less than bergamot essential oil when checked against X. campestris and F. oxysporum respectively.
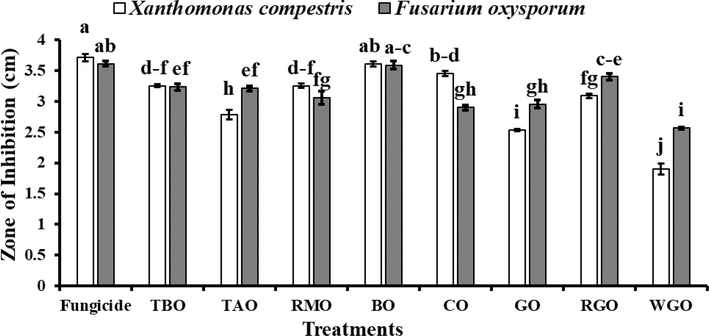
-
In-vitro antimicrobial potential of different essential oils on the growth of Xanthomonas campestris and Fusarium oxysporum. The error bars indicate the standard error. Different letters indicate significant differences (p < 0.05) among treatments according to Tukey’s multiple range test. Fungicide (Difenoconazole), TBO (Tansy Blue Oil), TAO (Tuberose Absolute Oil), RMO (Rose Marry Oil), BO (Bergamot Oil), CO (Cedarwood Oil), GO (Geranium Oil), RGO (Rose Geranium Oil), WGO (Winter Green Oil).
3.3 Antimicrobial potential of the combined effect of essential oil and chitosan
When bergamot essential oil was checked with chitosan, it significantly retained its antimicrobial potential. An increase in the concentration of chitosan decreased the zone of inhibition in both tested microbes. Bergamot essential oil along with 0.25 % chitosan produced a zone of inhibition of 3.6 and 3.5 cm in X. campestris and F. oxysporum respectively was 5.57 and 6.01 % more than when bergamot essential was tested with 0.5 % chitosan (Fig. 4,Fig. 5,Fig. 6).
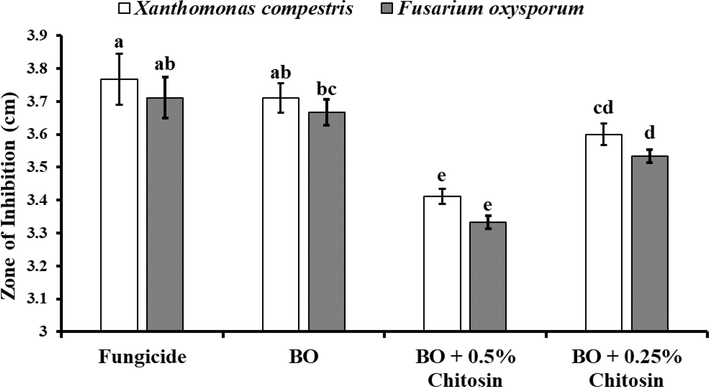
-
In-vitro antimicrobial potential of bergamot essential oil against Xanthomonas campestris and Fusarium oxysporum. The error bars indicate the estimated standard error. Different letters indicate significant differences (p < 0.05) among treatments according to Tukey’s multiple range test.
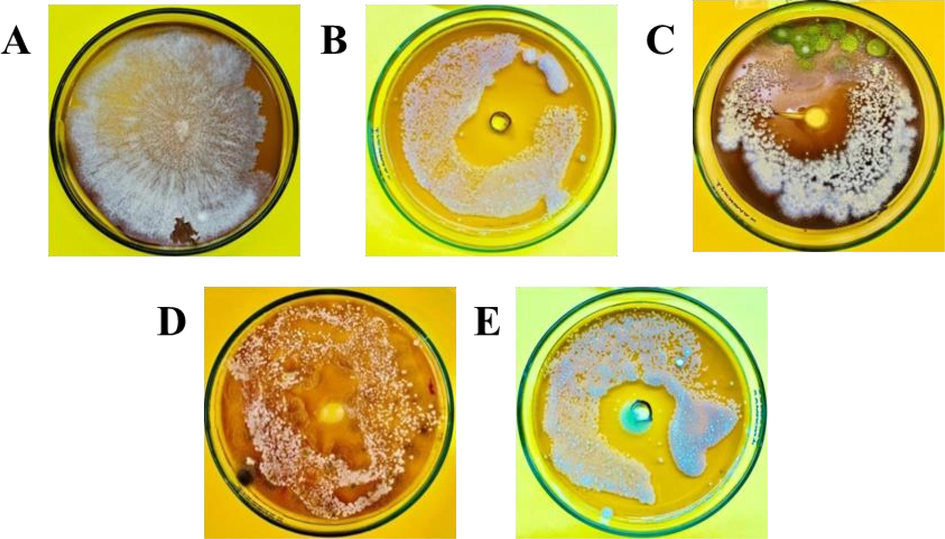
-
In-vitro antifungal potential of essential oils and coating solution against Fusarium oxysporum. A (Control), B (Fungicide), C (Bergamot Oil + 0.5 % Chitosan solution), D (Bergamot Oil + 0.25 % Chitosan solution), E (Bergamot Oil).
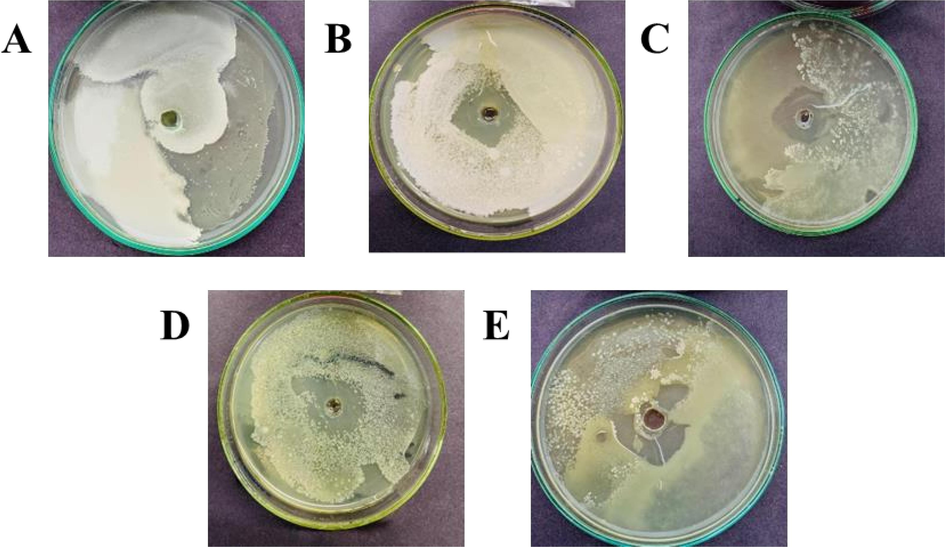
-
In-vitro antibacterial potential of essential oils and coating solution against Xanthomonas campestris. A (Control), B (Fungicide), C (Bergamot Oil + 0.5 % Chitosan solution), D (Bergamot Oil + 0.25 % Chitosan solution), E (Bergamot Oil).
3.4 GC–MS analysis of bergamot essential oil
The identification of the phytochemical constituents of bergamot essential oil was performed by GC–MS analysis. All the constituents of this essential oil are listed in Table 1. The most abundant peak was observed for Linalyl acetate (Fig. 7). The other characteristic peaks were α-Pinene, β-Pinene, and d-Limonene. Some compounds like β-Myrcene and Ocimene were found at relatively lower concentrations in the essential oil.
| Sr. No. | Compound Name | Retention Time (minutes) | Molecular Weight | Chemical Formula | % |
|---|---|---|---|---|---|
| 1 | α-Pinene | 5.877 | 136 | C10H16 | 06.13 |
| 2 | β-Pinene | 7.032 | 136 | C10H16 | 09.38 |
| 3 | β-Myrcene | 7.385 | 136 | C10H16 | 2.19 |
| 4 | d-Limonene | 8.457 | 136 | C10H16 | 21.37 |
| 5 | Ocimene | 8.945 | 136 | C10H16 | 01.18 |
| 6 | Linalool | 10.618 | 154 | C10H18O | 31.69 |
| 7 | Linalyl Acetate | 14.967 | 196 | C12H20O2 | 29.18 |
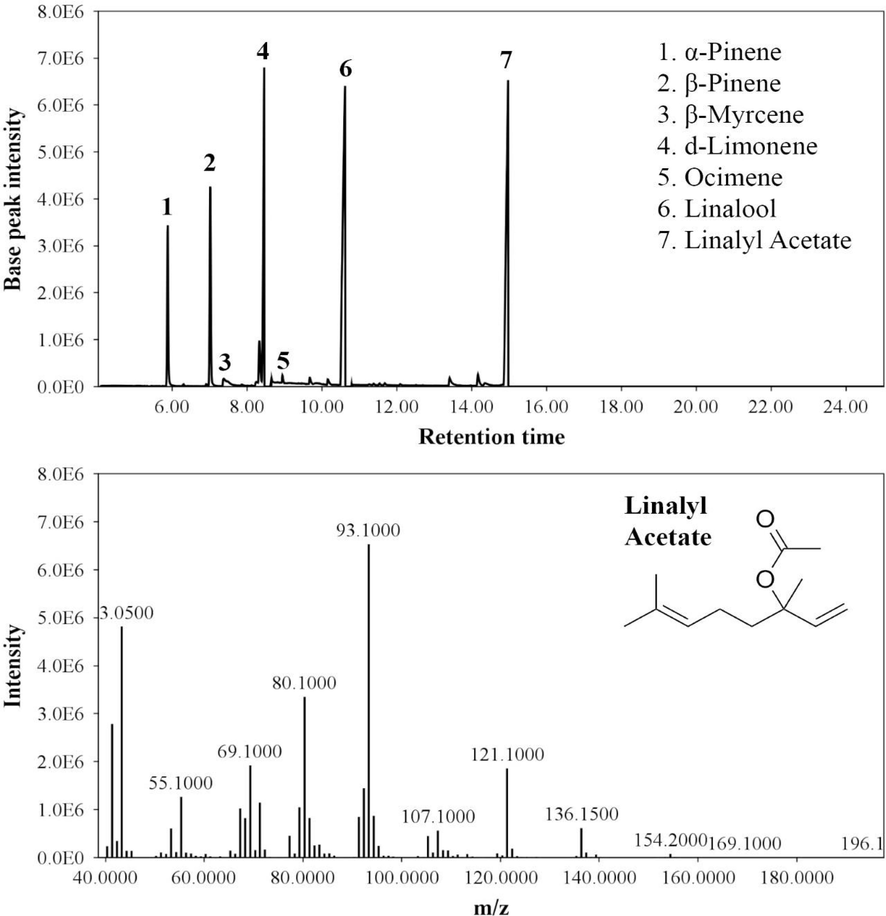
- Chromatogram of bergamot essential oil (up) and ionization pattern of Linalyl acetate (below).
3.5 Effects of chitosan and bergamot essential oil on seed germination
Both Bergamot essential oil alone and in combination with chitosan significantly increased seed germination. The highest tested concentration of chitosan i.e., 0.5 % along with bergamot essential oil showed an increase of 33.3 % in seed germination when compared to the control (Fig. 8). This increment in seed germination was 20 % greater than bergamot essential oil alone.
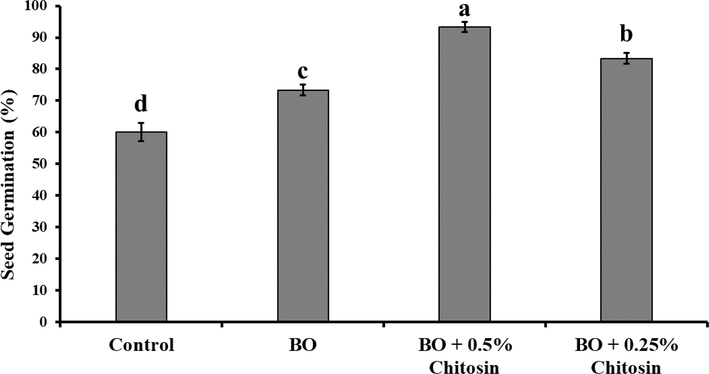
-
In-vitro effect of bergamot essential oil on seed germination. The error bars indicate the estimated standard error. Different letters indicate significant differences (p < 0.05) among treatments according to Tukey’s multiple range test.
3.6 Evaluation of the antimicrobial potential of chitosan and bergamot essential oil
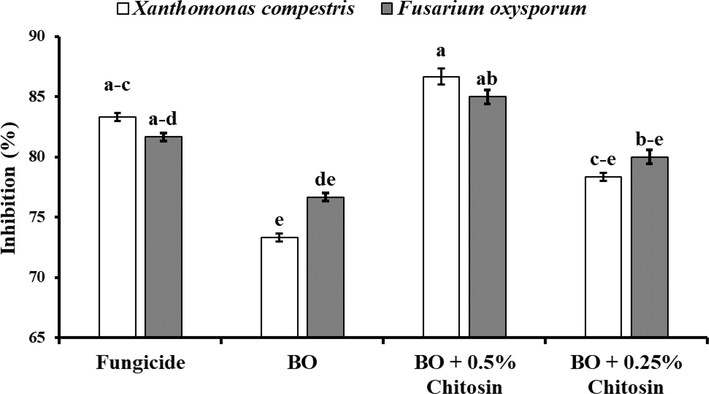
Bergamot essential oil alone and in combination with chitosan not only increased seed germination but also significantly restricted the colonization of selected microbes. In contrast to the effect of bergamot essential oil and chitosan on selected microbes, the inhibitory effect of a higher concentration of chitosan was significantly more than the lower one. Similarly, bergamot essential oil combined with 0.5 % chitosan showed a higher percentage of inhibition in both tested microbes when compared to the fungicides (Fig. 9,Fig. 10). Application of bergamot essential oil without any chitosan on corn seeds lost its effectivity and produced the least percentage of inhibition in both tested microbes.
-
In-vitro effect of bergamot essential oil on seed colonization of Xanthomonas campestris and Fusarium oxysporum. The vertical bars indicate the standard error. Different letters indicate significant differences (p < 0.05) among treatments according to Tukey’s multiple range test.
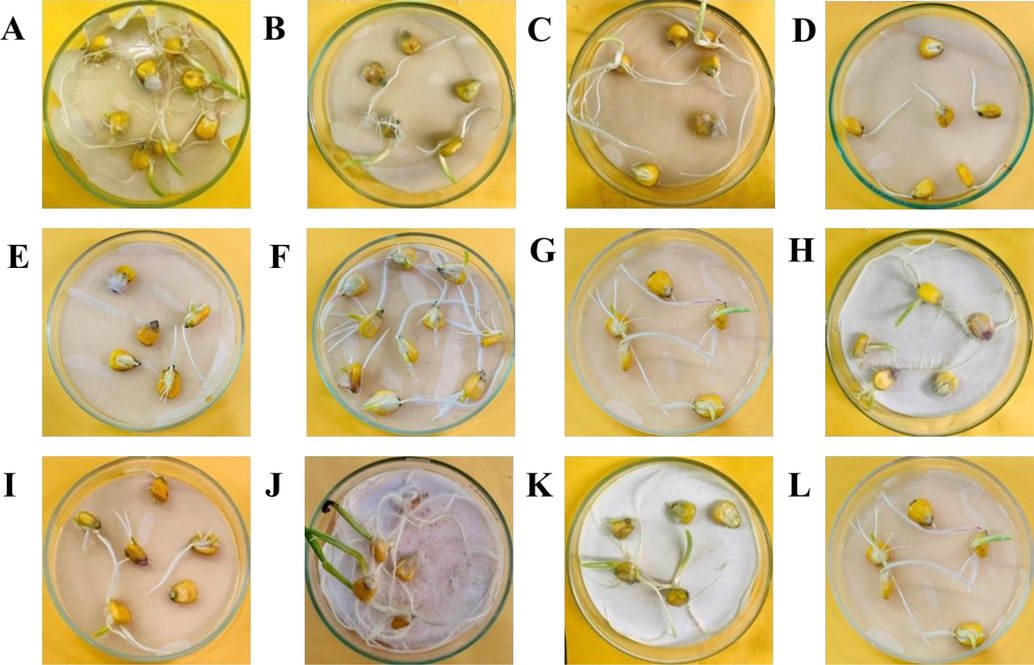
- The effect of essential oil and coating emulsion on the seedling growth (A-D) and infestation with F. oxysporum (E-H) and X. campestris (I-L). A (Control), B (Bergamot Oil + 0.5 % Chitosan solution), C (Bergamot Oil + 0.25 % Chitosan solution), D (Bergamot Oil). E (Control), F (Bergamot Oil + 0.5 % Chitosan solution), G (Bergamot Oil + 0.25 % Chitosan solution), H (Bergamot Oil). I (Control), J (Bergamot Oil + 0.5 % Chitosan solution), K (Bergamot Oil + 0.25 % Chitosan solution), L (Bergamot Oil).
3.7 Effect of bergamot essential oil and chitosan emulsion on the growth of maize seedlings
The impacts of seed coating on the growth of maize seedlings is presented in Table 2. The seedling growth was positively affected when the seeds were coated with chitosan and EOs emulsion. The plant raised from uncoated seeds (negative control) showed reduced plant growth. Moreover, an increase in plant growth was noted when the concentration of chitosan solution was increased from 0.25 % to 0.5 %. Results from this study have revealed a significantly positive effect on the seedling growth obtained from the coated seeds with chitosan with EOs on maize seeds.
| Treatments | Shoot Length (cm) | Root Length (cm) | No. of Leaves | Fresh Weight (g) | Dry Weight (g) |
|---|---|---|---|---|---|
| Fusarium oxysporum | |||||
| Control (FO) | 4.667 ± 0.33c | 10.67 ± 0.333c | 3 ± 0b | 0.37 ± 0.0057c | 0.163 ± 0.008c |
| B oil + 0.5 % CH + FO | 11.3 ± 0.333a | 33.3 ± 0.667a | 6 ± 0a | 1.187 ± 0.043a | 0.767 ± 0.033a |
| B oil + 0.25 % CH + FO | 10.33 ± 0.333a | 32.67 ± 0.33a | 5.67 ± 0.333a | 1.1 ± 0.057ab | 0.6 ± 0.057b |
| B oil + 0 % CH + FO | 7.67 ± 0.333b | 29.67 ± 0.33b | 4.67 ± 0.333b | 0.967 ± 0.033b | 0.467 ± 0.03b |
| Xanthomonas campestris | |||||
| Control (XC) | 3.33 ± 0.33a | 7.6 ± 0.333d | 2.66 ± 0.333b | 0.22 ± 0.0145c | 0.111 ± 0.0072c |
| B oil + 0.5 % CH + XC | 11 ± 0.577a | 31.3 ± 0.88a | 6 ± 0a | 1.2 ± 0.05a | 0.53 ± 0.033a |
| B oil + 0.25 % CH + XC | 9 ± 0.577b | 29 ± 0.577b | 5.66 ± 0.333a | 1.06 ± 0.066ab | 0.53 ± 0.033a |
| B oil + 0 % CH + XC | 7.3 ± 0.33b | 26.6 ± 0.33c | 5.33 ± 0.333a | 0.93 ± 0.066b | 0.46 ± 0.033a |
The maximum average root length observed was 33.3 cm in the case of bergamot + 0.5 % Chitosan and control (F. oxysporum) was minimum with 10.67 cm length of roots. In the case of the control (X. campestris) mean was 7.6 cm and the highest at bergamot + 0.5 % chitosan was 31.3 cm (Table 2). While the shoot length was significantly increased from a control of 4.67 cm to bergamot oil + 0.5 % chitosan of 11.3 cm (F. oxysporum). In the case of X. campestris, the bergamot EO + 0.5 % chitosan showed a maximum shoot length of 11 cm, as compared to the control (3.3 cm) (Table 2). The maximum number of leaves was significantly observed when bergamot oil with 0.5 % chitosan was used.
The fresh and dry weights of plants were calculated immediately after the harvested plant. The EO with 0.5 % chitosan solution showed the best result of the fresh and dry weight of plants. The control (F. oxysporum) plant showed minimum fresh and dry weight (0.37 & 0.163 g) and maximum fresh and dry weight was noticed (1.187 & 0.77 g) in the case of bergamot oil with 0.5 % chitosan, respectively (Table 2). Furthermore, the pathogen (X. campestris) interferes with plant growth hormones, the highest fresh and dry weight was 1.2 and 0.53 g in the case of bergamot + 0.5 % chitosan and the minimum fresh and dry weight was 0.22 and 0.11 g for the control (X. campestris), respectively (Table 2).
3.8 Molecular docking analysis
The analysis of the intermolecular interactions between compounds of essential oils and some antifungal targets was performed by Ligplot. For that purpose, representative structures obtained after molecular docking were used. The prevalent interactions included hydrophobic and hydrogen bonding.
The interactions included hydrophobic and hydrogen bonding interactions on the most representative structure obtained from the two MD trajectories using cluster analysis performed by Ligplot. As shown in Fig. 11 in all antifungal target proteins, both linalool and linalyl acetate formed hydrogen bonds with the receptor residues. The oxygen atom of the linalool was engaged in hydrogen bonding with the (Tyr205, Arg208, Met 209) and (Ans93, Arg18, Gly15, Gly21) from FOXG_04696 and sec14p proteins, respectively (Fig. 11). Similarly, linalool showed hydrogen bonding with the (Met17, Tyr75) and (Thr211) residues of Fgb1 and NCC, respectively. The oxygen 2 atoms in the linalyl acetate contributed H bonds with the different residues of FOXG_04696, NCC, and sec14P target proteins (Table 3). Along with the hydrogen bonding, all the compounds present in essential oil were involved in the hydrophobic interactions with different residues of antifungal target proteins.
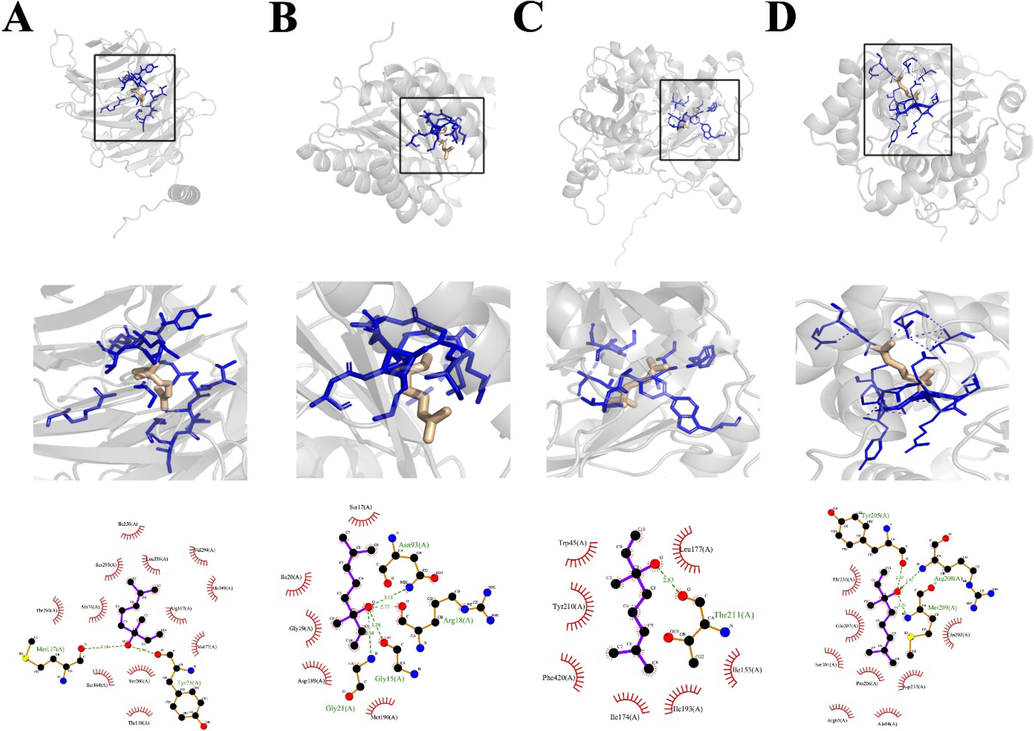
- Molecular docking and interaction analysis of linalool with target antifungal proteins. A = FGB1, B = FOXG_04696, C = NCC, D = SECP14.
| Receptor | Compound | VS | HBI | OI | Contacting receptor residues |
|---|---|---|---|---|---|
| FOXG_04696 | ɑ-Pinene | −5.2 | 7 | 122PHE, 126NLG, 129TYR, 173ASP, 174PHE | |
| β-Pinene | −5.0 | 7 | 122PHE, 126NLG, 129TYR, 173ASP, 174PHE | ||
| d-Limonene | −4.7 | 10 | 122PHE, 125ALA, 126GLN, 129TYR, 170LEU, 174PHE | ||
| Linalool | −4.7 | 3 | 2 | 18ARG, 20ILE, 21GLY, 93ASN | |
| Linalyl acetate | −4.7 | 3 | 3 | 18ARG, 20ILE, 21GLY, 93ASN, 189ASP | |
| Myrcene | −4.0 | 6 | 122PHE, 126GLN, 129TYR, 170LEU, 173ASP | ||
| Ocimene | −4.4 | 99PHE, 151PRO, 197LYS, 200LYS | |||
| FGB1 | ɑ-Pinene | −4.9 | 1 | 76ALA | |
| β-Pinene | −4.9 | – | – | – | |
| d-Limonene | −4.9 | 1 | 76ALA | ||
| Linalool | −4.8 | 1 | 2 | 76ALA, 117MET, 249ALA | |
| Linalyl acetate | −5.2 | 2 | 167ARG, 251GLN | ||
| Myrcene | −4.4 | – | – | ||
| Ocimene | −4.6 | 6 | 32ILE, 33LYS, 36LYS, 277ARG | ||
| SEC14 | ɑ-Pinene | −5.8 | 6 | 194VAL, 212PHE, 214ILE, 225PHE, 228PHE, 232LEU | |
| β-Pinene | −5.9 | 7 | 214ILE, 225PHE, 228PHE, 232LEU | ||
| d-Limonene | −6.5 | 13 | 126LEU, 179LEU, 182ILE, 187ALA, 190VAL, 221PHE, 228PHE, | ||
| Linalool | −5.7 | 2 | 2 | 64ALA, 208ARG, 209MET, 263THR | |
| Linalyl acetate | −6.2 | 14 | 179LEU, 190VAL, 194VAL, 212PHE, 214ILE, 221PHE, 228PHE, 232LEU, 240ILE | ||
| Myrcene | −5.9 | 17 | 126LEU, 179LEU, 182ILE, 187ALA, 190VAL, 194VAL, 212PHE, 221PHE, 225PHE, 228PHE, 232LEU, 240ILE | ||
| Ocimene | −5.9 | 13 | 194VAL, 212PHE, 214ILE, 221PHE, 225PHE, 228PHE, 232LEU, 240ILE | ||
| NCC | ɑ-Pinene | −5.3 | 6 | 108VAL, 110ASP, 115PHE, 339PHE | |
| β-Pinene | −5.2 | 4 | 117PHE, 339PHE | ||
| d-Limonene | −7.6 | 12 | 153ILE, 174ILE, 193ILE, 196ILE, 210TYR, 420PHE | ||
| Linalool | −6.0 | 1 | 11 | 45TRP, 153ILE, 174ILE, 177LEU, 193ILE, 210TYR, 216LEU, 420PHE | |
| Linalyl acetate | −5.9 | 8 | 110ASP, 117PHE, 225TYR, 227HIS, 240PHE, 339PHE | ||
| Myrcene | −7.2 | 15 | 172VAL, 174ILE, 193ILE, 197THR, 200VAL, 210TYR, 420PHE | ||
| Ocimene | −7.0 | 12 | 45TRP, 153ILE, 174ILE, 193ILE, 196ILE, 208ALA, 210TYR, 420PHE |
VS = Vina Score, HBI = Hydrogen bond interactions, OI = other interactions.
4 Discussion
The use of essential oils instead of fungicides to control postharvest fungal pathogens is a good alternative. Numerous secondary metabolites found in EOs could stop or impede the growth of bacteria, yeast, and mold (De Martino et al., 2009). When added to fresh fruits, vegetables, and grains, EOs do not only slow microbial growth but also maintain sensory acceptability. The effects of various functional groups of essential oils including alcohols, aldehydes, phenolics, terpenes, ketones, and other antimicrobial compounds, effectively destroy a number of pathogens (Cai et al., 2022). In the present study, we have studied the antimicrobial properties of eight essential oils, and their major components. Initially, the antimicrobial activity of the test essential oils was screened using the agar well diffusion method, and their inhibition zones were measured. Bergamot performed as the best antimicrobial agent, which was then mixed with various concentrations of chitosan (as a coating agent) and then their combined antimicrobial potential was evaluated. The best-selected concentrations were then tested against the seed germination potential in-vitro as well as in the pot experiment.
The prominent isolated pathogens from corn seeds were F. oxysporum and X. campestris. The fungi have been reported to be responsible for losses of seed viability that occurred during seed development, and storage, and may have hazardous impacts as seedling infection after germination. Similar isolation was observed by Adejumo et al., (2007), who isolated various fusarium species from different maize samples consumed in Nigeria and concluded that these pathogens significantly damaged the stored seeds. These isolated pathogens were evaluated for their antimicrobial potential by using various essential oils and coating solutions.
Eight essential oils were tested in the current experiment for their antimicrobial efficacy against corn-stored pathogens (F. oxysporum and X. axonopodis) using the agar well diffusion method. Results revealed that essential oils performed as antimicrobial agents in the following order, Bergamot > Tansy blue > Rose geranium > Cedarwood > Rosemarry > Tuberose Absolute > Geranium > Winter green. Studies conducted in recent years show that the application of EOs to control the pathogenic Fusarium spp. that attacks cereals and cereal products is very effective. For instance, mycelial growth was significantly or completely inhibited in the tested isolates of Fusarium (F. oxysporum, F. solani, F. verticillioides, and F. subglutinans) by EOs of eucalyptus, clove, lemongrass, and mint (Sharma et al., 2017). According to Reyes-Jurado et al., (2020), the vapor phase of EOs has a particular impact against molds due to their superficial growth, which makes them more susceptible to EOs volatile compounds. EOs vapors affect molds at different stages of their life cycle, such as germination, hyphae growth, and sporulation. The results in another study are in consistent with the present study indicating that all four essential oils extracted by hydro distillation applied in the same concentration showed significant antimicrobial efficacy against S. aureus, S. pyogenes, S. typhi, S. epidermidis, E. coli, Shigella spp., P. aeruginosa, two Trichophyton spp., and two Aspergillus spp. in vitro (Mekonnen et al., 2016). In another study, the antimicrobial potential of lemongrass, Palm rosa, eucalyptus, Tagetus minuta, geranium, citronella, and mentha essential oils were evaluated. All the above-mentioned essential oils showed promising antimicrobial properties against various fungal and bacterial pathogens than streptomycin and chloramphenicol, and comparable activity with ampicillin and gentamicin (Mangalagiri et al., 2021).
EOs are known for harboring a broad spectrum of biological activities (Calo et al., 2014). Concerning the effectiveness of bergamot essential oil in this study, different mechanisms would have been responsible for its anti-bacterial and antifungal activities. The efficacy of EOs is either biostatic or biocide which means that EOs kill living cells (Calo et al., 2014). The exact mechanisms of action of EOs are not yet established due to the great variability of their chemical compounds (Hyldgaard et al., 2012). It is most likely that EO’s antimicrobial propriety is not attributable to a single or a specific mechanism but it is probably related to many targets in the bacterial cell (Calo et al., 2014). Generally, EOs permeabilize layers of fatty acids in the cytoplasmic membrane (Rodriguez-Garcia et al., 2016) and disintegrate bacterial cell wall and cell membrane structures by altering the conformation of fatty acids, polysaccharides, and phospholipids layers by raising their permeability (Prakash et al., 2015). Additionally, EOs are able to coagulate the cytoplasm and inhibit several enzyme systems responsible for energy regulation and the synthesis of structural components (Burt et al., 2004). All these mechanisms would have acted synergistically to hinder the growth of bacteria and fungi as observed in this study.
The bergamot essential oil which showed good antimicrobial activity, was selected for GC analysis. Our GC analysis indicated linalyl acetate and linalool content in the bergamot essential oil. Based on the literature available about the bergamot essential oil, used in the present studies contains limonene, linalyl acetate, and linalool as active antimicrobial compounds (Lin et al., 2015, Xing et al., 2019). According to previous studies, limonene has broad-spectrum bactericidal activity. D-limonene can significantly inhibit gram-negative and gram-positive bacteria as well as fungal activity (Semeniuc et al., 2017). Han et al., (2020) showed that limonene caused the destruction of the cell integrity and wall structure of bacteria Listeria monocytogenes. Similarly, linalool and linalyl acetate has been reported as a strong antimicrobial agent (Pitarokili et al., 2002; D'Auria et al., 2005; Sokovic et al., 2020). According to a previous study, linalyl acetate may cause perturbation of the lipid fraction of microorganism plasma membrane, resulting in alterations of membrane permeability and leakage of intracellular materials (Trombetta et al., 2005). In another study, scientists showed that lavender oil successfully inhibited spore germination, germ tube formation, and hyphal elongation of Candida albicans (D'Auria et al., 2005). They attribute these aspects to the presence of two major components (linalool and linalyl acetate) in the essential oil of Lavender (D'Auria et al., 2005). Hence, the activity of bergamot oil against test microbes may depend on an additive effect of its major components.
The beneficial effect of chitosan on seed conservation before planting has been revealed in recent years. The present study was conducted to assess not only the antifungal activity of chitosan associated with bergamot EO but also the impact of these coating solutions on maize seed germination and plant growth. Our research indicated that chitosan and bergamot EO acted as seed coating agents able to control F. oxysporum and X. axonopodis. According to the results, bergamot essential oil with a 0.5 % chitosan emulsion performed best against F. oxysporum and X. campestris. Indeed, chitosan has been used to coat many seeds and demonstrated an efficient activity to protect seeds against Aspergillus ssp. (Adejumo et al., 2007, Pichyangkura and Chadchawan 2015, Praveen Kumar et al., 2019). Additionally, it could increase soluble sugar content and enhance protease activity hence leading to an increasing level of free amino acids which have demonstrated obvious inhibiting effects for many plant's pathogenic fungi. Chitosan inhibits the growth of various harmful bacteria, including Xanthomonas (Li et al., 2008) and Erwinia carotovora (Badawy et al., 2014).
Studies for crop protection led to the development of seed coating technology as a pesticide against crop pests and diseases. Among these natural products, chitosan represents a potential candidate since it can protect both the seeds and the plant from pests by improving the quality of the seeds, their germination potential, and seedling growth (Mancini and Romanazzi 2014). After the initial in-vitro screening of bergamot essential oil with various concentrations of chitosan, further evaluation of their combined effect was performed on the seed germination and growth. On seed germination, a significant increase in the germination time and vigor was observed which was also evident from some of the previous research. In an experiment, it was found that a coating formulation containing chitosan and essential oils effectively protected maize seeds (Noshirvani et al., 2017). Soaking cereal seeds in essential oil solutions without coating solution may inhibit their germination (Anžlovar et al., 2014). Other results available from the literature also indicate that soaking wheat grains in a solution of clove oil can significantly reduce their germination (Karaca et al., 2017).
Chitosan has previously been reported to boost protease activity and increase soluble sugar content, which in turn raises the level of free amino acids, which has been shown to have a clear inhibiting effect on many plants' pathogenic fungi. Results of the present study showed a positive pathogen control as well as seed germination and growth. Similar results were observed by (Santamaria et al., 2016) who obtained positive results in managing phytopathogenic fungi (Alternaria alternata, Bipolaris oryzae, F. graminearum, F. equiseti and F. verticillioides) in stored rice grains by using clove essential oil, which contains high percentages of eugenol, while both (Daferera et al., 2003) and (Stević et al., 2014) demonstrated the efficacy of thymol to inhibit several fungal species.
The FOXG_04696, Fgb1, NCC, and sec14p proteins are widely known antifungal targets for drug development and in-silico studies due to their vital role in fungal physiology (Hosseinnejad and Jafari 2016, Karaca et al., 2017, Kalboush and Hassan 2019). Their agonists/modulators have a potential role in the development of antifungal formulations (Che et al., 2009, Gao et al., 2021). Thus, interaction analysis of compounds present in essential oil with antifungal target proteins can shed light on the ligand-dependent inactivation of these proteins. The molecular docking conformations with the best score were used for interaction analysis presented in Table 3. The 2D maps of interactions made by LigPlot are shown in Fig. 11. These descriptors included hydrophobic effects, van der Waals, and hydrogen bond effects. These results support the hypothesis that the presence of strong hydrogen bonds between the essential oil compounds and the active sites of antifungal target proteins markedly blocks the necessary enzymatic machinery and provides antifungal capabilities (Okpareke et al., 2020).
The essential oils and EOs-based products used as biopesticides only have a minor residual activity, and non-target living organisms in the ecosystem may be less negatively affected (Azeem et al., 2022). Yet even though the goal of EO formulation was to increase EO stability and effectiveness, most of the time, the coating materials themselves are not toxic to living things. As a way to improve both their biological activity and stability, new emerging techniques like EO formulations through emulsion like with chitosan coating might enable EOs to appear on a larger scale.
The main goal of this research was to find the most effective control for corn storage using essential oils to reduce post-harvest losses and replace synthetic chemicals with natural essential oils. Plant essential oils are one of several promising environmentally friendly alternatives to conventional synthetic pesticides used to control several fungal and bacterial pathogens and food contaminants. The active ingredients of the essential oils and their isomers can be used effectively as seed and soil treatments controlling most of the post-harvest decay pathogens and in plant and food protection.
5 Conclusion
The antimicrobial activities of essential oils, along with the knowledge about their safe uses, emphasize the possible applications of essential oils in many fields including agriculture and the food industry. This will help to the reduction of the use of toxic synthetic agrochemicals and additives. bergamot oil was the most effective against postharvest pathogens of maize. An important outcome was the significant protective effect of the edible coating of bergamot oil applied on maize seeds during in-vivo assay. The study proves that Essential oils have wide acceptance as alternatives to traditional toxic agrochemicals.
Funding
This study was financially supported by the Science and Technology Planning Project of Guangdong Province (Project NO: 2023B03J1270); Special Fund Project for Science and Technology Innovation Strategy of Guangdong Province (Project No: 2022KJ122 and 2023KJ122) and China Young Scientist Talent Program (Project No: QN2022030024L).
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Occurrence of Fusarium species and trichothecenes in Nigerian maize. Int. J. Food Microbiol.. 2007;116:350-357.
- [Google Scholar]
- Essential oil of common thyme as a natural antimicrobial food additive. Food Technol. Biotechnol.. 2014;52:263-268.
- [Google Scholar]
- Enhanced antibacterial and antioxidant properties of Chitosan-quercetin complex containing polycaprolactone microspheres for the treatment of gastroenteritis: An in-vitro and in-vivo analysis. Mater. Today Commun.. 2022;31:103780
- [Google Scholar]
- Antimicrobial and inhibitory enzyme activity of N-(benzyl) and quaternary N-(benzyl) Chitosan derivatives on plant pathogens. Carbohydr. Polym.. 2014;111:670-682.
- [Google Scholar]
- Essential oils: Their antibacterial properties and potential applications in foods—a review. Int. J. Food Microbiol.. 2004;94:223-253.
- [Google Scholar]
- Antifungal and mycotoxin detoxification ability of essential oils: A review. Phytother. Res.. 2022;36:62-72.
- [Google Scholar]
- New azoles with potent antifungal activity: design, synthesis and molecular docking. Eur. J. Med. Chem.. 2009;44:4218-4226.
- [Google Scholar]
- The effectiveness of plant essential oils on the growth of Botrytis cinerea, Fusarium sp., and Clavibacter michiganensis subsp. michiganensis. Crop Prot.. 2003;22:39-44.
- [Google Scholar]
- Antifungal activity of Lavandula angustifolia essential oil against Candida albicans yeast and mycelial form. Med. Mycol.. 2005;43:391-396.
- [Google Scholar]
- De Martino, L., V. De FEOSs, F. Fratianni, et al., 2009. Chemistry, antioxidant, antibacterial, and antifungal activities of volatile oils and their components. Natural product communications. 4, 1934578X0900401226.
- Arrowroot starch-based films incorporated with a carnauba wax nanoemulsion, cellulose nanocrystals, and essential oils: a new functional material for food packaging applications. Cellul.. 2021;28:6499-6511.
- [CrossRef] [Google Scholar]
- FAO, 2010. Reducing Post-harvest Losses in Grain Supply Chains in Africa: Lessons Learned and Practical Guidelines. FAO/World Bank, Rome, Italy.
- FAOSTAT, 2021. Food and Agriculture Organization of the United Nations Statistical Database; Statistical Division. FAO: Rome, Italy.
- Streptochlorin analogues as potential antifungal agents: Design, synthesis, antifungal activity and molecular docking study. Bioorg. Med. Chem.. 2021;35:116073
- [Google Scholar]
- Evaluation of different factors affecting antimicrobial properties of Chitosan. Int. J. Biol. Macromol.. 2016;85:467-475.
- [Google Scholar]
- Quality preservation of deliberately contaminated milk using thyme free and nanoemulsified essential oils. Food Chem.. 2017;217:726-734.
- [Google Scholar]
- Antifungal potential and characterization of plant extracts against Fusarium fujikuroi on rice. J. Plant Protect. Pathol.. 2019;10:369-376.
- [Google Scholar]
- Effects of some plant essential oils against fungi on wheat seeds. Indian J. Pharma. Edu. Res.. 2017;51:S385-S388.
- [Google Scholar]
- Chemical composition and in vitro cytotoxicity of essential oils from leaves and flowers of Callistemon citrinus from western Himalayas. PLoS One. 2015;10:e0133823.
- [Google Scholar]
- Antibacterial activity of Chitosan solution against Xanthomonas pathogenic bacteria isolated from Euphorbia pulcherrima. Carbohydr. Polym.. 2008;72:287-292.
- [Google Scholar]
- Research on the extraction of bergamot essential oil by steam distillation and component analysis. J. Food Saf. Qual.. 2015;6:619-625.
- [Google Scholar]
- Seed treatments to control seed-borne fungal pathogens of vegetable crops. Pest Manag. Sci.. 2014;70:860-868.
- [Google Scholar]
- Antimicrobial activity of essential plant oils and their major components. Heliyon. 2021;7:e06835.
- [Google Scholar]
- Mekonnen, A., B. Yitayew, A. Tesema, et al., 2016. In vitro antimicrobial activity of essential oil of Thymus schimperi, Matricaria chamomilla, Eucalyptus globulus, and Rosmarinus officinalis. International journal of microbiology. 2016.
- Cinnamon and ginger essential oils to improve antifungal, physical, and mechanical properties of Chitosan-carboxymethyl cellulose films. Food Hydrocoll.. 2017;70:36-45.
- [Google Scholar]
- Natural food colorants and preservatives: a review, a demand, and a challenge. J. Agric. Food Chem.. 2022;70:2789-2805.
- [Google Scholar]
- Synthesis, structure, computational and molecular docking studies of asymmetrically di-substituted ureas containing carboxyl and phosphoryl hydrogen bond acceptor functional groups. J. Mol. Struct.. 2020;1203:127360
- [Google Scholar]
- Composition and antifungal activity on soil-borne pathogens of the essential oil of Salvia sclarea from Greece. J. Agric. Food Chem.. 2002;50:6688-6691.
- [Google Scholar]
- Plant essential oils as food preservatives to control moulds, mycotoxin contamination and oxidative deterioration of agri-food commodities–Potentials and challenges. Food Control. 2015;47:381-391.
- [Google Scholar]
- Praveen Kumar, G., S. Desai, B. M. Moerschbacher, et al., 2019. Seed treatment with Chitosan synergizes plant growth promoting ability of Pseudomonas aeruginosa-P17 in sorghum (Sorghum bicolor L.). BioRxiv. 601328.
- Raut J.S., Karuppayil, S.M. 2014. A status review on the medicinal properties of essential oils Industrial Crops and Products, 62, 250-264.
- Essential oils in vapor phase as alternative antimicrobials: A review. Crit. Rev. Food Sci. Nutr.. 2020;60:1641-1650.
- [Google Scholar]
- Chitosan and its derivatives: synthesis, biotechnological applications, and future challenges. Appl. Microbiol. Biotechnol.. 2019;103:1557-1571.
- [Google Scholar]
- Oregano essential oil as an antimicrobial and antioxidant additive in food products. Crit. Rev. Food Sci. Nutr.. 2016;56:1717-1727.
- [Google Scholar]
- Rout, S., S. Tambe, R. K. Deshmukh, et al., 2022. Recent trends in the application of essential oils: The next generation of food preservation and food packaging. Trends in Food Science Technology.
- Improvement in the performance of low-temperature H2–O2 fuel cell with Chitosan–phosphotungstic acid composite membranes. Int. J. Hydrogen Energy. 2016;41:5389-5395.
- [Google Scholar]
- Disinfection of seed surfaces with sodium hypochlorite. Phytopathology. 1986;76:745-749.
- [Google Scholar]
- Antibacterial activity and interactions of plant essential oil combinations against Gram-positive and Gram-negative bacteria. J. Food Drug Anal.. 2017;25:403-408.
- [Google Scholar]
- Antifungal activities of selected essential oils against Fusarium oxysporum f. sp. lycopersici 1322, with emphasis on Syzygium aromaticum essential oil. J. Biosci. Bioeng.. 2017;123:308-313.
- [Google Scholar]
- A review on the use of essential oils for postharvest decay control and maintenance of fruit quality during storage. Crop Prot.. 2014;64:27-37.
- [Google Scholar]
- Antifungal activity of selected essential oils against fungi isolated from medicinal plants. Indus. Crops Prod.. 2014;55:116-122.
- [Google Scholar]
- Mechanisms of antibacterial action of three monoterpenes. Antimicrob. Agents Chemother.. 2005;49:2474-2478.
- [Google Scholar]
- Chemical composition and biological properties of essential oils of two mint species. Trop. J. Pharm. Res.. 2013;12
- [CrossRef] [Google Scholar]
- Chemical composition and biological activities of essential oil isolated by HS-SPME and UAHD from fruits of bergamot. LWT. 2019;104:38-44.
- [Google Scholar]
- Design, development, and techniques for controlling grains post-harvest losses with metal silos for small and medium-scale farmers. Afr. J. Biotechnol.. 2011;10:14552-14561.
- [Google Scholar]







