Translate this page into:
Biological role of the PAK4 signaling pathway: A prospective therapeutic target for multivarious cancers
⁎Corresponding author at: Genomic and Proteomic Research Division, Advanced Bioinformatics, Computational Biology and Data Science Laboratory, Bangladesh (ABCD Laboratory, Bangladesh), Chattogram 4226, Bangladesh. md.junaid@northsouth.edu (Md. Junaid) md.junaid@abcdlabbd.org (Md. Junaid)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
p21 activated kinase 4 (PAK4) along with PAK5 and PAK6 are members of the type II family of PAKs. From the type II family, PAK4 is being assessed as an expansively investigated area. PAK4 acts as a serine/threonine kinase in response to Rho GTPases, while interaction partners and substrates are rapidly extended, implying a diverse range of cellular functions regulated by PAK4, including cell cycle progression, proliferation, survival, cell adhesion, motility, neuronal development, and immune defense. Thus, altered expression and function of PAK4 imparts numerous pathological conditions. The overexpressed and, in some cases, amplified PAK4 gene is revealed to be associated with a diverse range of cancers. Ongoing findings explain how PAK4 is involved in cancer progression, promoting cancer cell growth, survival, and metastasis with the corresponding activation and signaling schemes. Meanwhile, upstream activating and downstream effector molecules are increasingly identified to elucidate the exact mode of action on the cancer progression role of PAK4. A plethora of approaches targeting PAK4 is continuing to assess the potentiality of inhibitors to eradicate the growth and metastasis of cancers overexpressing PAK4. Here, we summarize the findings on the role of PAK4 and its signaling pathways promoting cancers and examine PAK4 as a promising target for multitype cancers.
Keywords
PAK4
Cancer
Survival
Metastasis
Neuronal development
Immune defense
1 Introduction
Cancer denotes over and above 100 types of illness that violate the regular maintenance scheme of cells. Cells are unconscious to the general regulation of cell proliferation and comply with their mode of reproduction and achieve the ability to migrate and invade from the starting site to nearby and distant sites of the body (Weinberg 1996). Cancer is the main cause of premature death, which is also considered an eminent impediment to enhancing lifespan in the present world (Bray et al., 2021). Although the conventional therapeutic strategy has been improved, most therapies have low specificity acting on both on diseased and healthy tissues, generating severe adverse effects (Pucci et al., 2019), and cancer causes almost one million deaths worldwide in 2020, which refers to a major threat situation the management of human health (Sung et al., 2021). Hence, scientists are making great effort to reveal targeted therapy with higher specificity and minimal side effects (Pucci et al., 2019). Different kinase enzymes are a crucial target due to their prominent role in the development and progression of cancer (Bhullar et al., 2018).
PAK4, a serine/threonine kinase, is a constituent member of the type II subfamily of PAKs (Ha et al., 2012). It is expressed on a large scale at low and high levels in almost all adult tissues and embryogenesis, respectively (Rane and Minden 2014). PAK4 authorizes the cell to progress the cell cycle by reducing p21 levels, thus retracting the activity of CDK4/CDK6 kinases (Nekrasova and Minden 2011) and as a constituent of type II PAKs, is involved in cell motility through cytoskeletal reorganization and is also involved in cell proliferation, survival, and development (Ha et al., 2012, Ha and Boggon 2018).
The various studies elucidate the PAK4 mediated complex signaling mediated by PAK4 for deregulated biological events, that is, characteristics of cancer, including non-restraint cell proliferation, bypassing cell death, and obtaining immortality by resisting apoptosis and acquiring the capacity to invade and migrate the tissues (Minden 2012). A considerable inspection has proclaimed that PAK4 acts as a central signaling molecule that merges the major cancer-promoting signaling pathways like the Wnt/β-catenin, Ras–ERK, LIM kinase 1 (LIMK1) / Cofilin, PI3K / AKT / Bad and CREB. PAK4 is highly expressed in various cancers of diverse tissue origins, including breast, prostate, lung, gallbladder, ovarian, gastric cancers, etc (Won et al., 2019). and necessitated in cancer cells for oncogenic progression and anchorage-independent growth (Callow et al., 2002). PAK4 is also identified as a target for immune evasion of cancer, implying the potential to increase the effectiveness of immunotherapy for cancer (Gajewski and Fessler 2020).
In this review, we emphasize and accumulate the knowledge related to the role of PAK4 in cancers of ever-reported origins with the pathway involved and highlight the designed and reported inhibitors of PAK4 as a therapeutic strategy for cancers of different sources.
2 Methods
All of the information that has been reviewed in this article is retrieved from the well-known search engine. The most relevant article retrieval search engine was Google Scholar, PubMed, Science Direct, Web of Science, and Scopus. To gather information from these, several keywords were utilized such as PAK4 signaling pathways and cancer, the role of PAK4 in cancer, PAK4 and cancer, targeting PAK4 for cancer, and PAK4 inhibitors. The time restriction option was not imposed to retrieve relevant articles. Keywords along with Google Scholar provided hits from Google Scholar are listed in Table 1.
Keywords
Number of results
PAK4 signaling pathways and cancer
2,400
The role of PAK4 in cancer
6,300
PAK4 and cancer
6,850
Targeting PAK4 for cancer
5,420
Inhibitors of PAK4
7,490
3 Structural and functional properties of PAK4
In 1998, Abo et al. identified PAK4 as a component of the PAK family of kinase and effector signaling molecules for the Cdc42Hs (Abo et al., 1998). The PAK family is classified into type I (PAKs 1–3) and type II (PAKs 4–6). The PAK4 protein consists of 591 amino acids that are encoded in the chromosome 19q13.2 regions. From a structural perspective, it is made up of the N-terminal auto-inhibitory domain (AID) or autoinhibitory pseudosubstrate (PS) motif adjacent to a GTPase binding domain (GBD) and a C-terminal kinase domain (Baskaran et al., 2012, Ha et al., 2012, Rane and Minden 2014). Furthermore, PAK4 has been reported to contain four proline-rich SH3 binding sites and a GEF-H1 and Gab1-interacting domain (GID) in the central region in immediate proximity to the kinase domain. PAK4 also has a β5 integrin-binding domain within the C-terminal kinase domain (Dart and Wells 2013). The inactive state of PAK4 is constitutively maintained through the interaction of the AID and kinase domain in its monomer conformation, together with that A-loop is phosphorylated at the Ser 474 residue. That monomer form of inactive conformation is disrupted by binding of Cdc42 to the GBD, leading to the activation state of PAK4, implying conformational alteration-mediated activation of PAK4 activation in the AID without phosphorylation of A-loop (Baskaran et al., 2012). In addition to this regulatory scheme, a recently identified discrete regulatory mechanism describes that an autoinhibitory pseudosubstrate (PS) motif constitutively autoinhibits PAK4 independently of phosphorylation of the A loop through interaction with the kinase domain (Ha et al., 2012). The detailed structural features of PAK4 are mentioned in Fig. 1.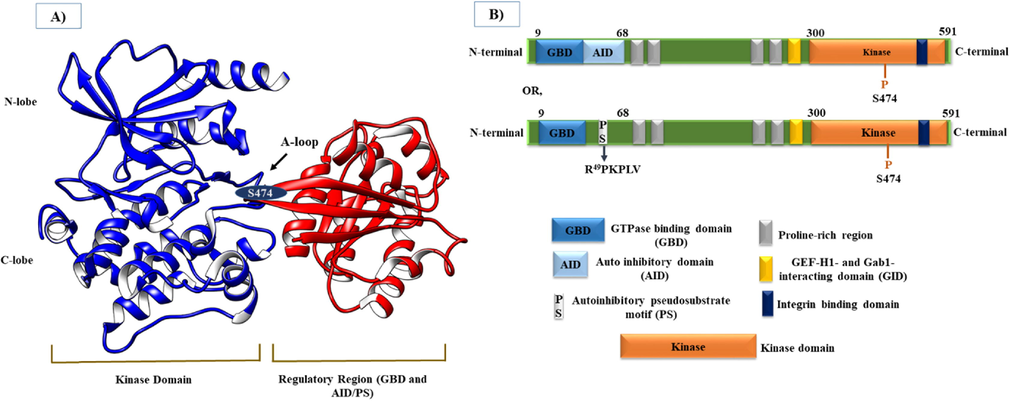
Architecture of the PAK4 protein. A) Crystal structure of PAK4 (PDB: 5UPL) highlighting the Kinase domain and Regulatory region with N-lobe, C-lobe, Activation loop (A-loop), GBD, AID/PS, and one phosphorylation site (S474) within the A loop. B) PAK4 domain structure showing important regions, including GBD, AID, PS motif, Proline-rich region, GID, β5 integrin-binding domain, and Kinase domain from the N-terminal to C-terminal.
PAK4 involves regulating many cellular processes such as cell cycle progression, proliferation, survival, cytoskeletal modeling-mediated alteration in cell adhesion, motility, and morphology. It is also involved in immune defense, embryonic development, and cancer development and progression (Nekrasova and Minden 2011, Dart and Wells 2013). The first documented function of PAK4 in response to activated Cdc42 is in the involvement of actin cytoskeletal remodeling and filopodia formation (Abo et al., 1998). PAK4 phosphorylates LIMK1, which consecutively phosphorylates cofilin to regulate the actin depolymerization process of actin (Dan et al., 2001, Ahmed et al., 2008). PAK4 inactivates SlingShot phosphatase (SSH-1L), a down-regulation of LIMK1, and activates LIMK1, resulting in the regulation of actin dynamics (Soosairajah et al., 2005). PAK4 also regulates the rate of focal adhesion turnover by phosphorylating paxillin at Ser 272 (Wells et al., 2010). In addition, PAK4 acts on the integrin to destabilize the adhesion structure, limiting the levels of cellular adhesion (Li et al., 2010).
The regulatory role of PAK4 in the cell cycle has been assessed by several studies and revealed exciting involvement phenomena (Nekrasova and Minden 2011). PAK4 expression levels during the cell cycle increase substantially and provisionally in the early G1 phase. The depletion of PAK4 in cells, which are in serum starved condition, causes reduction and elevation of cells in the G1 and G2/M phase, respectively. This indicates that PAK4 is involved in the initiation process of the cell cycle by regulating crucial checkpoints (Nekrasova and Minden 2011). PAK4 also regulates the progression of mitosis by phosphorylating the serine-135 residue of Ran GTPase (Bompard et al., 2010). Cell survival phenomena are substantiated to be promoted by the action of PAK4 through the inhibition of Bad and caspase 8, thus restricting the apoptosis of cells (Gnesutta et al., 2001, Gnesutta and Minden 2003). Evidence on the involvement of PAK4 in immune defense has come from the link between podosomes and PAK4. It is shown to regulate macrophage podosomes, while inhibition of PAK4 leads to reduction of podosomes and stimulates the generation of focal adhesions. This switch demonstrates an essential role for PAK4 in the migration scheme of macrophage (Foxall et al., 2019). Engulfment of IgG-coated particles by phagocytosis depends on the contractility of myosin. PAK4 phosphorylates MRLC thanks to regulating myosin-regulated contractility during phagocytosis (Bright and Frankel 2011). The requirement for PAK4 during development is proved by the knockout of the PAK4 gene in mice showing lethality on embryonic day 11.5, mainly due to the impediment in the fetal heart. These embryos (PAK4 deficient) also show striking defects in the development of neurons, neuronal tubes, and axon outgrowth of axons (Qu et al., 2003). More extensive investigation demonstrates that PAK4-deficient embryos have aberrations in extraembryonic tissues (such as the placenta and yolk sac) and vasculature (Tian et al., 2009). As an analogy to mammalian development, PAK4 in zebrafish is notably expressed and crucial for the development (Law and Sargent 2013).
4 Role of PAK4 in cancers
A plethora of studies provide extensive insight into the role of PAK4 in cancers. Overexpression, hyperactivation, and gene amplification of PAK4 have been considered to mediate the transformation and progression of numerous types of cancer (Won et al., 2019, Chandrashekar et al., 2020). Activation of PAK4 is implicated by various signaling molecules, hence activated PAK4 in returns acts on the downstream signaling pathway promoting cell proliferation, survival by restricting apoptosis, epithelial-mesenchymal transition (EMT), invasion and migration, and resistance to chemotherapeutic agents (Fig. 2) (Tyagi et al., 2014, Moon et al., 2015, Kesanakurti et al., 2017, Xie et al., 2017, Santiago-Gómez et al., 2019, Won et al., 2019, Dasgupta et al., 2020). In the following section, cancer-specific roles of PAK4 have been highlighted by accumulating studies, and inhibitors targeting PAK4 in vitro and/or in vivo as anticancer agents have also been mentioned in Table 2.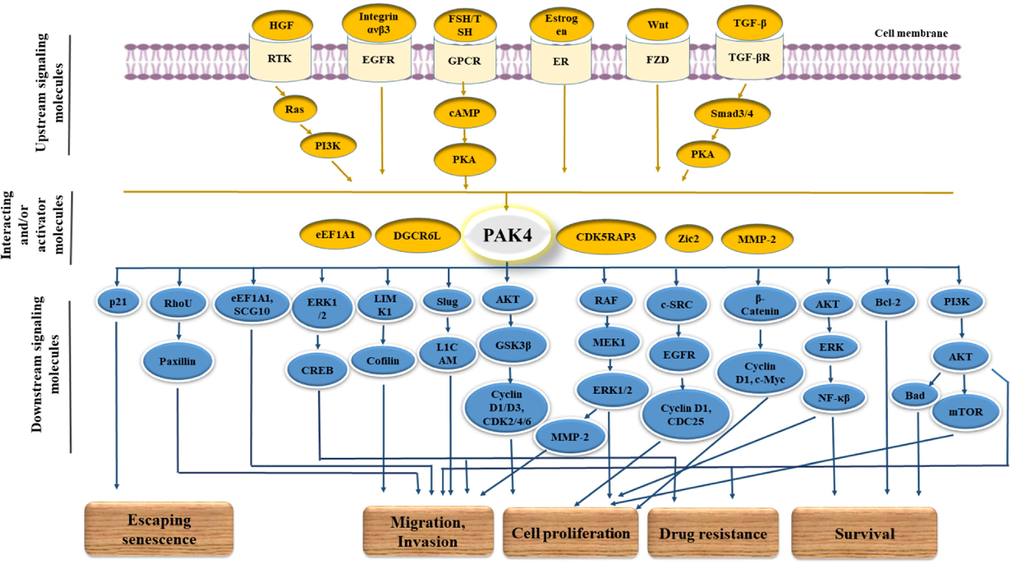
PAK4-mediated action on cancer transformation and progression of cancer along with the corresponding signaling molecules and pathways. PAK4 activation is mediated by various signaling molecules, including Ras, PI3K, HGF, FSH, Estrogen, Integrin ανβ3, etc using various types of corresponding receptors such as G protein-coupled receptors (GPCRs), receptor tyrosine kinases (RTKs), ERs, and FZD receptors, etc. That activated PAK4 contributes to cancer cell progression acting on various signaling pathways like RAF/MEK/ERK, AKT/ERK/NFκβ, PI3K/AKT, LIMK1/Cofilin, etc.
Inhibitors
Cancer type
Cell lines
In vivo (mice)
Effects
Ref
All-trans retinoic acid (ATRA)
Pancreatic cancer
Wild-type (WT) and gemcitabine-resistant (GR) MiaPaCa-2, TB33117 cells
Inhibits cell proliferation, formation of colony, migration, and invasion in both cells of WT and GR.
Shows higher inhibitory impacts on GR cells.
ATRA sensitizes the GR cells to gemcitabine, and together they show synergistic effects on inhibiting the proliferation of both cell lines.
ATRA and gemcitabine-mediated actions on these cells are through the PAKs (PAK1, PAK2, and PAK4) and α-SMA downregulation.(Wang et al., 2019)
(2,4-diaminoquinazoline derivatives)
Lung cancer
A549 cells
Inhibits the PAK4 activity, proliferation, distribution of cell cycle, migration, and invasion.
(Wu et al., 2018)
CRT PAKi (PAK1/4-specific inhibitor)
ER + breast cancers
Samples derived from metastatic patients and Endocrine-resistant ER + MCF7 cells
Reduces the cancer stem cells (CSCs) activity of ER + metastatic breast cancer specimen.
Abrogates the self-renewal activity of TAMR and FULVR breast CSCs.
Reduces the cell proliferation and formation of colony tamoxifen-resistant (TAMR) and fulvestrant-resistant (FULVR) MCF7 cells.
CRT PAKi with tamoxifen/fulvestrant synergistically reduce the proliferation and formation of colony TAMR and FULVR MCF7 cells and CSC activity.
(Santiago-Gómez et al., 2019)
Fisetin
Oral squamous cell carcinoma (OSCC)
SCC9, SCC4 cells
Suppresses the PAK4 expression, Inhibits colony formation, cell cycle, and migration, and induces apoptosis of PAK4-highly expressing cells.
(Design et al., 2020)
SCC9 cells (PAK4 overexpressing)-derived xenograft
Dramatically decreases tumor growth.
Glaucarubinone
Pancreatic cancer
PANC-1, MiaPaCa-2, PAN02 cells
Inhibits proliferation, migration, and invasion.
(Yeo et al., 2014)
MiaPaCa-2 and PANC-1 derived xenograft
Significantly decreased weight and volume of MiaPaCa-2-derived tumor, did not have a notable effect on PANC-1-derived tumor.
Gemcitabine
Pancreatic cancer
PANC-1, MiaPaCa-2, PAN02 cells
Inhibits the proliferation
MiaPaCa-2 and PANC-1 derived xenograft
Significantly decreasing the weight and volume of MiaPaCa-2-derived tumor, did not have a notable effect on PANC-1-derived tumor.
Combination of Glaucarubinone and Gemcitabine
Pancreatic cancer
PANC-1, MiaPaCa-2, PAN02 cells
Synergistically inhibits cell growth.
MiaPaCa-2 and PANC-1 derived xenograft
Shows substantial synergistic effects on the reduction of the weight and volume of both MiaPaCa-2 and PANC-1-derived tumors.
GL-1196
Gastric cancer
MKN-45, SGC7901, BGC823
Suppresses the proliferation and arrests cell cycle at the G1 phase through the downregulation of PAK4/c-Src/EGFR/CyclinD1 signaling pathway and expression of CDK4/6.
Inhibits filopodia formation and invasion via affecting PAK4/LIMK1/cofilin signaling pathway.
(Zhang et al., 2016)
GNE-2861 (group II PAK inhibitor)
Breast cancer
MCF-7/LCC2 cells (tamoxifen-resistant)
PAK4 inhibition sensitizes the tamoxifen-resistant cell lines to tamoxifen and attenuates the signaling and levels of ERα protein, and 17β-estradiol (E2) stimulated cell proliferation.
(Zhuang et al., 2015)
KPT-9274 (dual inhibitor of PAK4/NAMPT)
Renal cell carcinoma
Caki-1, 786–0 cells
Attenuates the cell viability, G2–M transition, induces apoptosis and decreases the invasive and migratory potentiality.
Attenuates the PAK4/β-catenin signaling, resulting in the attenuation of cyclin D1 and c-Myc expression.(Aboud et al., 2016)
786-O cells-derived xenograft mice
Suppresses the growth of the tumor.
(Aboud et al., 2016)
KPT-9274
Glioblastoma (GBM)
Inhibits the GBM tumor-derived endothelial cells proliferation
(Ma et al., 2021)
GBM mouse
In the tumor vasculature, It alters the deranged morphology and causes a well-organized structure.It also reduces intratumoral
hypoxia.It
sensitizes tumors to immunotherapy (CAR-T), which results in the reduction (80 %) of tumor volume, and their combinatorial treatment increases the survival of mouse.
KPT-9274
Rhabdomyosarcoma
RH30, RD cells
Significantly reduces cell proliferation and induces apoptosis.
Blocks G1-S progression in RH30 cells. Arrest cell cycle at the G1-S phase in RD cells.
Substantially inhibits the motility and invasive properties of both cell lines.
Reduces PAK4 activity through the decreasing phosphorylation status of PAK4, hence reducing the target β-catenin activity.(Dasgupta et al., 2020)
Cell lines derived orthotropic xenograft, metastatic, and patients tumor pieces-derived xenograft mouse
Substantially reduces the tumor growth with the inhibition of PAK4 and metastasis potentiality.
Significantly reduces the tumor growth in xenograft of patients-derived tumor pieces.
Thyroid cancer
Lenvatinib-resistant 8505C cells
Suppresses the cell proliferation.
KPT-9274 with lenvatinib synergistically inhibit the cell proliferation, indicating KPT-9274-mediated PAK4 inhibition can sensitize the thyroid cancer lenvatinib-resistant cells to lenvatinib therapy.(Khan et al., 2019)
KPT-9274 and
Its analogsPancreatic ductal adenocarcinoma (PDAC)
MiaPaCa-2, L3.6pl cells
Inhibits the proliferation and migration, induces apoptosis, and arrests the cell cycle.
Reduces the total PAK4, p-PAK4, and cyclin D1 and other molecules, including ERK1/2, GEF-H1, and p-ERK1/2.
Overcomes the stemness and drug-resistant properties of PDAC.
KPT-9274 with gemcitabine and oxaliplatin shows synergistic effects on the inhibition of proliferation and colony formation and enhancement of apoptosis.(Aboukameel et al., 2017)
Xenografts mice model
KPT-9274 alone and combined with gemcitabine shows significant antitumor activity. KPT-9274 also almost completely suppresses the gemcitabine/ nab-paclitaxel resistant CSCs-derived tumors.
KPT-9274 (dual inhibitor of PAK4/NAMPT) and it's analog KPT-7523
Pancreatic Neuroendocrine Tumors
Everolimus resistant BON-1, QGP-1 cells
Inhibits proliferation and colony formation.
KPT-9274 with everolimus shows synergistic effects on the inhibition of proliferation and enhancement of apoptosis.
KPT-9274-everolimus in QGP-1 cells shows substantial down-regulation of mTOR, Akt, RAPTOR, FAK, and β-catenin.(Mpilla et al., 2019)
BON-1-derived xenograft
KPT-9274 substantially reduces the tumor and suppresses the Mcl-1 and Bcl-2 expression, and activates pro-apoptotic Bax.
KPT-9274, KPT-9307
PDAC
MiaPaCa-2, CSC cells
Induce Bad and Bax re-expression, miRNA (Tumor suppressive), and reduce the level of p-PAK4 and p-Bad
KPT-9274 causes activation of caspases-3, Bax upregulation, and Bcl-2 reduction.
KPT-9274 induces apoptosis and inhibits proliferation.
(Mohammad et al., 2019)
KPT-8752
KPT-9274TNBC
SUM159, MDA-MB-231, MDA-MB-468 cells
Substantially reduce PAK4 protein and phosphorylation status of downstream target including β-catenin and cofilin.
Almost entirely inhibits the growth of cells, Induces apoptosis.(Rane et al., 2017)
Mouse xenografts
Orally administered KPT-9274 substantially reduced the tumor volumes (for three cell lines) and weights (estimated for SUM159 and MDA-MB-231) without significantly affecting the mice's body weights.
Significantly decreased PAK4 protein level with non-significant off-targeting.
LC-0882
Gastric cancer
MKN-
45, BGC823, SGC7901
Suppresses the cell proliferation and arrests the cell cycle at the G1 phase via down-regulating the p-PAK4/cyclin D1 and CDK4/6.
Inhibits the filopodia formation and invasion via down-regulating the PAK4/LIMK1/cofilin signaling pathway.(Zhang et al., 2017)
LCH-7749944
Gastric cancer
MKN-1,
BGC823, MGC803, and SGC7901 cells
Inhibits the proliferation and induces apoptosis.
Arrest cell cycles at the G1-S phase.
Downregulates the level of p-PAK4, p-c-Src, p-EGFR, and expression of cyclin D1.
Decreases the migration and invasion of invasive cell lines and inhibits filopodia formation.
Decreases the levels of p-PAK4/p-LIMK1/p-cofilin and p-MEK-1/ERK1/2/ and MMP2 expression.
LCH-7749944
Gastric cancer
SGC7901 cells
Inhibits PAK4, hence inhibits the PAK4-mediated phosphorylation of SCG10, consequently suppressing the migration and invasion of SGC7901 cells.
(Guo et al., 2014)
PF-3758309
Pancreatic cancer
miaPaCa-2, PANC-1, and PAN02
Inhibits the PAK1 and PAK4 activities.Suppresses the proliferation and migration/
invasion.PF
-3758309 with gemcitabine synergistically suppresses the proliferation of all cell lines and anchorage-independent growth (except PAN02).
(Wang et al., 2018)
Orthotopic mouse model
Gemcitabine or PF-3758309 alone substantially reduces tumor growth (by decreasing proliferation, volume, and weight).Further, PF-3758309 with gemcitabine synergistically reduces the proliferation, volume, and weight of the
tumor.PF
-3758309 upregulates the immune responses to tumors.
PF 3,758,309
Endometrial cancer
RL95-2 cell
Attenuates the 17β-estradiol-stimulated cell proliferation.
(Su et al., 2017)
PF 3,758,309
Lung cancer
A549 cells
Induces apoptosis, inhibits migration and invasion.
Inhibits PAK4, which causes inhibition ERK1/2, CREB NF-κB, β-catenin, consequently down-regulating the expression of MMP-2/ MMP-9.(Ryu et al., 2014)
PF 3,758,309
Glioblastoma (GBM)
Inhibits the GBM tumor-derived endothelial cells proliferation
(Ma et al., 2021)
GBM Mouse
Reduces the abnormalities of mouse GBM tumor endothelial cells turning to the well-organized vascular structure.
PF-3758309
Breast cancer
MDA-MB-231 cells
Blocks PAK4-stimulated PI3K/AKT signaling activation.
(He et al., 2017)
PF-3758309
Breast cancer
HR-/
HER2 + and TNBC cell lines
It shows strong inhibitory activity on the tested cell lines and phosphorylation of PAK4 and LIMK1 and arrests the cell cycle at the G1 phase.
(Zhao et al., 2020)
BT474-derived tumor xenografts in mice
Do not show significant inhibitory effects on the xenografts.
PF-3758309
Rhabdomyosarcoma
RH30, RD cells
Significantly reduces cell proliferation and induces apoptosis.
Blocks G1-S progression in RH30 cells.
Arrest cell cycle at the G2/M phase in RD cells.
Substantially inhibits the motility of RD cells and invasive properties of both cell lines.
Reduces PAK4 activity through the decreasing phosphorylation status of PAK4, hence reducing the target β-catenin activity.(Dasgupta et al., 2020)
Orthotropic xenograft mouse model
Substantially reduces the tumor growth with the inhibition of PAK4.
PB-10
Colorectal cancer
HCT-116
Substantially inhibits the cell proliferation, formation of the colony and represses the cancer progression.
(Li et al., 2020)
SPU-106
Gastric cancer
SGC7901 cell lines
Decreases the invasion with the significant inhibition of p-PAK4, p- SCG10 and partial inhibition of p-LIMK1 and p-cofilin
(Song et al., 2019)
Vandetanib
Bladder cancer
VM-CUB1, RT-112 cells
Reduces cell proliferation and colony formation.
(Chandrashekar et al., 2020)
(-)-β-hydrastine
Lung cancer
A549, LTEP-A-2 cells
Inhibits the proliferation, Augments apoptosis, and Substantially decreases the invasive and migratory potentiality.Inhibits the expression of cyclin D1/D3, CDK2/4/6 via suppressing the PAK4/AKT/GSK3β signaling
pathway.It
also decreases the phosphorylated levels of LIMK1, Cofilin, and SCG10.
Guo et al., 2016)
1-phenanthryl-tetrahydroisoquinoline derivatives
Lung cancer
A549 cells
Inhibits PAK4 kinase activity and cell proliferation.
Compound 12a exhibits notable antiproliferative activity and arrests cell cycle at the G1/S transition with the reduced status of p-PAK4 and expression of CDK2, CDK4, CDK6, cyclin D1, and cyclin D3 protein.
Compound 12a induces apoptosis with reduced p-PAK4 and Bcl-2, augmented expression of BAX, caspase 3, and 8 protein.
Compound 12a decreases the invasion with the inhibition of activated PAK4 and phosphorylated levels of LIMK1, Cofilin, and SCG10.(Song et al., 2015b, Hao et al., 2016)
A549 cells-derived mouse xenograft
Oral administration of compound 12a substantially suppresses tumor growth.
1-phenanthryl-tetrahydroisoquinoline derivatives
Breast cancer
MCF-7 cells
Inhibits PAK4 kinase activity and cell proliferation.
Compound 21a exhibits notable antiproliferative activity and arrests cell cycle at the G1 phase with the reduced status of p-PAK4 and expression of CDK2, CDK4, CDK6, cyclin D1, and cyclin D3 protein.
Compound 21a induces apoptosis with reduced p-PAK4 and Bcl-2, augmented expression of BAX, caspase 3, and 8 protein.
Compound 21a decreases the migration and invasion with the inhibition of activated PAK4 and phosphorylated levels of LIMK1, Cofilin, and SCG10.
Compound 12a decreases the invasion in MCF-7 and MDA-MB-231 cells.(Song et al., 2015, Hao et al., 2016)
2, 4-diaminoquinazoline derivatives
Lung cancer
A549 cells
Compound 9d inhibits PAK4 kinase activity, cell proliferation, and arrests cell cycle at the G1 phase with increased expression of p21, p18 (CDK inhibitors), and decreased expression of cyclin D3 and CDK6.
Compound 9d decreases the migration and invasion with the inhibition of activated PAK4 and phosphorylated levels of LIMK1.(Hao et al., 2017)
4.1 Cervical cancer
Shu et al. 2015 showed that the higher expression of PAK4 in cervical cancer patients was significantly associated with the FIGO stage, metastasis (lymph node and distance), inferior histological grade, and the overall survival time (OS) time of these patients was dramatically mitigated. Furthermore, overexpressed PAK4 was conferred resistance to the chemotherapeutic agent cisplatin through the PI3K/Akt-dependent signaling pathway. Hence, they concluded PAK4 as a hopeful marker for the treatment of patients with cervical cancer patients (Shu et al., 2015). High expression of PAK1 and PAK4 were found in HeLa cells, but in hypoxic conditions, the only knockdown of PAK4 decreased the expression of hypoxia-inducible factor-1a (HIF-1α) expression. PAK4 discovered that the Akt-mTOR-4E-BP1 signaling pathway is stimulated by PAK4 for the regulation of HIF-1α translation in hypoxia. A similar result was also observed in colon cancer cells, HCT116 (Kim et al., 2017). Small nucleolar RNA host gene 7 (SNHG7), a competitive endogenous RNA (ceRNA) for miR-485 in the cervical cancer cell, highly expressed in cervical cancer, and this up-regulation is markedly associated with the FIGO stage, metastasis (lymph node), increasing the depth of cervical invasion and reducing OS time in patients. To further confirm the function of SNHG7, SNHG7 was removed where the diminished capacity for cell proliferation, migration, invasion, and increased apoptosis capacity was observed in cervical cancer cell lines and decreased PAK4 mRNA expression level of PAK4 mRNA. It was also confirmed that miR-485 targets PAK4 mRNA, showing a significant negative correlation between miR-485 and PAK4 mRNA expression levels. In vivo experiment, by knocking down, SNHG7 also inhibited cervical cancer growth by down-regulating PAK4 by miR-485. Thus, the SNHG7-miR-485-PAK4 pathway may play a significant role against cervical cancer in the design of promising diagnostic and treatment strategies (Wu et al., 2020).
4.2 Prostate cancer
PAK4 has been reported to be involved in prostate cancer cell polarity and migration of prostate cancer as a downstream signaling molecule of hepatocyte growth factor (HGF) through the use of LIMK1 and cofilin (Ahmed et al., 2008). Furthermore, the regulatory role of PAK4 (induced by HGF) was demonstrated by GEF-H1 and paxillin in the adhesion of prostate cancer cell adhesion and then ultimately in migration by causing disassembly of focal adhesions and stress fibers (Wells et al., 2010). In vitro studies in prostate cancer cell lines indicated that protein kinase A (PKA) regulated PAK4. Tumor formation using PAK4 knockdown cell lines is almost wholly blocked in athymic mice. Furthermore, PAK4 was revealed to have a role in the regulation of cAMP Response Element-Binding Protein (CREB) regulation, neuroendocrine differentiation to promote tumor progression, and chemo-resistance in prostate cancer. Therefore, it was shown that PAK4 involved in the PKA-PAK4-CREB signaling pathway contributed to tumor progression in prostate cancer through the hormone-refractory and chemo-resistance (Park et al., 2013). In vitro and in vivo regarding prostate cancer revealed that epithelial-mesenchymal transition (EMT) was regulated by PAK4 through the slug, thus indicating its role in prostate cancer progression through stimulation of invasion and metastasis. It was also reported that TGF-β stimulated EMT, also mediated by the PAK4 slug pathway. They also suggested that the PAK4 slug pathway could be a promising therapeutic target for prostate cancer (Park et al., 2018). Both in vitro and in vivo assays revealed that higher expression of miR608 in prostate cancer was associated with significant antiproliferative activity and inhibition of the G2 / M transition, EMT-independent migration, and induction of apoptosis was also demonstrated through the in vitro study. This function was revealed to be carried out targeting RAC2, BCL2L1, and PAK4, modulating the signaling pathway RAC2 / PAK4 / LIMK1 / cofilin, and BCL2L1/caspase‐3 signaling pathway (Zhang et al., 2019).
4.3 Ovarian cancer
Siu et al. reported a substantial up-regulated pattern of PAK4 and phosphorylated PAK4 (p-PAK4) in cancer tissue, ascitic fluid, and cell lines. Higher PAK4 expression was also significantly associated with advanced stages of cancer, attenuated overall and disease-free survival, and chemotherapeutic resistance. The in vitro study revealed that PAK4 knockdown caused a reduction of cell proliferation, migration, and invasion, and these events were inversely related to PAK4 overexpression. The proliferation, migration, and invasion action of PAK4 in ovarian cancer depended on the c-Src, ERK1/2, EGFR, and MMP2 signaling molecules. PAK4 in ovarian cancer was also reported to be regulated by HGF and follicle-stimulating hormone (FSH). Furthermore, the removal of PAK4 led to the attenuation of tumor growth and dissemination of tumor in vivo (Siu et al., 2010). An in vitro study reported that miR126 negatively regulated PAK4 expression in ovarian cancer (Luo et al., 2015). Long intergenic nonprotein-coding RNA 1224 (LINC01224) was shown to be up-regulated. Its depletion has also been reported to inhibit the proliferation, migration, invasion, and stimulation of apoptosis in epithelial ovarian cancer (EOC). LINC01224 was confirmed to be a ceRNA for miR-485-5p that directly targets the PAK4 in EOC cells. The in vivo assay demonstrated that silencing of LINC01224 caused inhibition of tumor growth in EOC (Xing et al., 2020). MiR-425 was found to suppress ovarian cancer cell proliferation, migration, and inhibition, and promote apoptosis through inhibition of PAK4 (Wang et al., 2021).
4.4 Endometrial cancer (EC)
PAK4 was found to be overexpressed in human EC cell lines, and total and activated PAK4 expression (PAK4 and p-PAK4, respectively) was also substantially increased in EC tissues. Overexpression of both of them was significantly correlated with invasion of the myometrial and vascular space. Their expression levels are also notably associated with lymph node metastasis, advanced stages (stages III and IV) of the disease, and grade-3 histological differentiation. These findings ultimately suggest that overexpressed and/or activated PAK4 play a crucial role in the malignant progression of EC. Furthermore, studies on cell lines confirmed the involvement of PAK4 in metastasis through ERK1 / 2 guided MMP-2 secretion (Lu et al., 2013). PAK4 (in the cytoplasm and nucleus) and p-PAK4 (in the nucleus) were highly expressed and inversely related to histological grade and were involved in the postmenopausal pathogenesis of endometrial cancer in women. At the same time, myometrial invasion and PAK4 cytoplasmic expression of PAK4 also showed an inverse correlation. Furthermore, lower expression of PAK4 in the cytoplasm was reported to be associated with shorter survival (Siu et al., 2015). Estrogen-induced activation of PAK4 was reported through the PI3K / AKT pathway in EC cells, which in turn augmented the transcription of the estrogen receptor alpha (ERα) transcription subsequently, ERα mediated expression of target genes. Both inhibition and suppression of PAK4 in the EC cell caused suppression of estrogen-induced proliferation. The in vivo study also showed a substantial reduction in estrogen-stimulated tumor growth by knocking down PAK4. Therefore, targeting PAK4-ERα would be a promising therapeutic option against EC (Su et al., 2017).
4.5 Colorectal cancer (CRC)
MiR-145 was shown to act on the PAK4 and retard the PAK4 and MAPK pathway, ultimately inhibiting cell proliferation of colorectal cancer cells (Wang et al., 2012). Knockdown of PAK4 or PAK1 in KRAS or BRAF mutant colon cancer cell lines showed significant antiproliferative action in both anchorage-dependent and independent growth. This inhibitory action was independent of the expression levels of PAK4 or PAK1 protein and the PI3K / AKT or RAF/MEK/ERK signaling pathway. Alteration in the actin cytoskeleton also appeared to be independent of the phosphorylation of LIMK/cofilin/paxillin. Augmented cell death and apoptosis were also associated with the addition of ABT-737 (antibody of prosurvival proteins Bcl-XL, Bcl-2, and Bcl-w) after PAK4 or PAK1 Knockdown (Tabusa et al., 2013). Overexpression of PAK4, which follows PAK1, was demonstrated in tissues of colorectal cancer with advanced stages (stage III and IV). Higher expression of PAK4 and PAK1 was found in patients with infiltrated serous layer and lymph node positive, respectively. PAK1 was also mentioned to be a prognostic marker due to its association with overexpression and progression-free survival. Metastasis and invasion of colorectal cancer were suggested to be associated with PAK4 and PAK1 (Song et al., 2015a). MicroRNA-145 has been reported to subdue the migration and invasion of the colorectal cancer cell line by targeting PAK4 by downregulating LIMK1 and cofilin phosphorylation levels (Sheng et al., 2017). PAK4 was to be associated with increased cell line proliferation of colon cancer by promoting glucose uptake, NADPH, and lipid biosynthesis through regulation of glucose-6-phosphate dehydrogenase (G6PD) via p53 degradation. This regulation of G6PD was also confirmed by an investigation in tissues of colon cancer tissues. Hence, targeting PAK4 and / or G6PD was suggested to be a potential therapeutic option in patients with colon cancer patients (Zhang et al., 2017). Another study showed overexpression of PAK4 in colorectal cancer tissues, and its elimination in the cancer cell lines caused significant suppression of cell proliferation and promoted apoptosis (Wang et al., 2019).
4.6 Kidney cancer/ renal cell carcinoma
PAK4 overexpression in patients of nonmetastatic clear cell renal cell carcinoma (ccRCC) with grade 1 + 2 Fuhrman grade and early T category undergoing surgery was reported to be significantly associated with lower overall and recurrence-free survival. Therefore, PAK4 could be a potential target for the treatment of nonmetastatic ccRCC postsurgical patients (Liu et al., 2015). KPT-9274, a dual inhibitor of PAK4/NAMPT, was shown to significantly attenuate viability, metastasis (invasion and migration), G2–M transition, and promote apoptosis through modulation of PAK4/β-catenin and NAD biosynthetic pathway in renal cell carcinoma cell lines. Furthermore, an in vivo study of renal cell carcinoma was shown to inhibit tumor growth by KPT-9274 in a dose-dependent manner (Aboud et al., 2016). Later, in vivo assay described that KPT-9274 and anti-programmed cell death 1 (anti-PD1) antibody significantly reduced kidney tumor growth (Trott et al., 2020). PAK4 expression was reported to be up-regulated and inversely related to miR-663 expression level of miR-663 in the ccRCC tissues. MiR-663 was also determined to exert an inhibitory role in the proliferation and invasion of ccRCC cell lines by targeting PAK4 (Liu et al., 2019). Out of 131 specimens from surgically treated patients with RCC, 29 and 75 samples exhibited high expression of PAK4 and pPAK4S474 expression, respectively. High expression of pPAK4S474 but not PAK4 was correlated with advanced grade and tumor stage. A subgroup of localized CCR and the total cohort of CCR showed lower recurrence-free and cancer-specific survival, respectively. Thus, pPAK4S474 may be considered a perfect prognostic factor (Kang et al., 2021).
4.7 Liver cancer
Studying the cell line and mouse model revealed that miR-199a/b-3p suppressed the growth of hepatocellular carcinoma (HCC) by targeting PAK4 through the PAK4 / Raf / MEK / ERK signaling pathway. The role of PAK4 in HCC was also ascertained by knockdown in vitro and in vivo, causing suppression of tumor growth by decreasing cell growth, stimulating apoptosis, and inhibiting cell cycle progression of the cell cycle (Hou et al., 2011). Experiments with transgenic TG221 HCC mice showed that miR-199a-3p has substantial antitumor activity by down-regulating PAK4 and MTOR (Callegari et al., 2018). Overexpression of CDK5RAP3 was reported and associated with an aggressive phenotype and poor cell differentiation in human HCC. In vitro and in vivo studies confirmed the tumor growth-promoting properties of CDK5RAP3. CDK5RAP3 knockdown suppressed tumorigenic, migration, and invasiveness properties in HCC cell lines. CDK5RAP3 was also shown to exert its role in HCC by interacting and activating PAK4 (Mak et al., 2011). PAK4 could stimulate proliferation in HCC cell lines of HCC. miRNA-433 suppresses PAK4-dependent proliferation of HCC cells by targeting PAK4 and subsequently inhibiting the PI3K/AKT signaling pathway (Xue et al., 2015). Frequent overexpression of PAK4 was reported in HCC tissue samples of HCC and associated with a higher aggressive behavior of tumors and metastases. Furthermore, an in vitro study reported that the metastatic role of PAK4 in HCC was mediated by attenuating p53 activity through phosphorylation (Xu et al., 2016). In vitro and in vivo studies revealed that Zic2 expression increased and was associated with increased proliferation of cells and migration in HCC through up-regulation of PAK4 as a transcriptional activator, subsequently activating the MAPK (Raf/MEK/ERK) pathway. The high expression of Zic2 and PAK4 was also reported to be related to advanced stage and poor tumor as well as lower disease-free and overall survival (Lu et al., 2017). Significant antitumor activity of miR-199a-3p was confirmed in HCC transgenic mice targeting MTOR and PAK4, which consequently repressing the FOXM1 (Callegari et al., 2018).
4.8 Lung cancer
Migration and invasion of lung cancer have been reported to be inhibited by a PAK4 inhibitor PF-3758309. That PAK4 inhibition led to suppression of the cAMP/PKA signaling pathway by inactivating ERK1/2 and CREB. Along with that, it also inhibited NF-κB, β-catenin signaling pathways; ultimately, inhibition of these pathways would down-regulate two gelatinases such as MMP-2 and MMP-9 to cause inhibition of metastasis (Ryu et al., 2014). Nonsmall cell lung cancer (NSCLC) showed a higher expression of PAK4 in both cell lines and human tissues of NSCLC, significantly related to NSCLC progression and a poor overall survival rate. Knockdown of PAK4 in cell lines revealed the action of PAK4-mediated metastasis action in NSCLC through the phosphorylation of LIMK1, which was further confirmed by the in vivo study (Cai et al., 2015). (-)-β-hydrastine is involved in inhibiting lung adenocarcinoma cell line proliferation, migration, and invasion, as well as inducing apoptosis by inhibiting PAK4. These actions have been reported to be mediated by inhibition of cyclin D1/3 and CDK2/4/6 expression for cell cycle capture, decreasing the level of Bcl-2 and the potentiality of mitochondrial membrane for induction of apoptosis, as well as inhibition of cofilin, LIMK1, SCG10, and MMP2 for suppression of migration and invasion (Guo et al., 2016). The computational and experimental studies indicated that CDK2 and PAK4 were associated with improved prognostic performance to classify high-risk patients with poor outcome under standard therapy and could be used as a potential drug target in the NSCLC (Wang et al., 2016). Inhibition of PAK4 by miR-193a-3p was revealed to cause suppression of aggressive NSCLC aggressive behavior by modulating the p53/Slug/L1CAM signaling pathway (Liu et al., 2019). PAK4 showed a higher expression pattern in both clinical samples and lung cancer cells of the lung. PAK4-mediated phosphorylation of fumarase (FH) on the S46 residue in lung NSCLC cell lines was found to inhibit the formation of FH / CSL / p53 and subsequently inhibit the TGF-β stimulated growth arrest (Chen et al., 2019).
4.9 Pancreatic cancer
One hundred and five genes with increased copy numbers, including PAK4, were identified and found to be associated with higher expression levels in the 13 pancreatic cancer cell lines of pancreatic cancer (Mahlamäki et al., 2004). Analysis of pancreatic cancer samples identified that PAK4 was recurrently amplified and highly activated and correlated with the oncogenic and activated form of the KRAS2 gene and its amplification (Chen et al., 2008). Experiments on cell lines and primary tumors revealed amplified PAK4; Furthermore, 22 % (14 out of 63) of pancreatic ductal adenocarcinoma (PDAC) samples showed PAK4 amplification. Western blot and RT-qPCR analysis of cell lines showed higher expression of PAK4 expression regardless of PAK4 amplification; subsequently, the migratory and invasive roles of PAK4 in PDAC were also revealed (Kimmelman et al., 2008). The higher expression of PAK4 was revealed in the cell lines and pancreatic cancer. This higher expression was demonstrated in the cell lines associated with increased growth (promoting cell cycle progression, conferring apoptosis resistance), platting efficiency, and clonogenic potential. These cancer-promoting functions of PAK4 was revealed to be mediated by AKT and ERK-guided NF-κB activation (Tyagi et al., 2014). The in vitro study determined that glaucarubinone restricted the proliferation, migration, and invasion of pancreatic cancer cells and decreased tumor growth in the in vivo model. The synergistic effect of glaucarubinone and gemcitabine on reducing cell proliferation and tumor growth was demonstrated by in vitro and in vivo assays, respectively. The aforementioned antitumor activities of glaucarubinone and gemcitabine were mediated by inhibition of PAK1 and PAK4 (Yeo et al., 2014). Pancreatic cancer cell lines of pancreatic cancer showed a higher expression of PAK4, and an inverse correlation was found between the expression level of PAK4 and the human equilibrative nucleoside transporter 1 (hENT1), which allowed the penetration of gemcitabine into the cell. Substantially increased sensitivity to gemcitabine was observed by knocking down PAK4 in gemcitabine resistant cell lines (Moon et al., 2015). Higher expression of PAK4 was observed in the stem cells of pancreatic cancer stem cells, as well as a correlation was revealed between the expression of PAK4 and stemness-related markers. Diminished sphere formation property and increased sensitivity to gemcitabine were also associated with the silencing of PAK4. PAK4-mediated activation of the signal transducer and activator of transcription 3 activator (STAT3) was ascertained to maintain the stemness potential mentioned above potentiality of pancreatic cancer cells (Tyagi et al., 2016). PAK4 allosteric modulators (PAM) were construed to show anticancer activity against PDAC cells through induction of apoptosis, cell cycle arrest, and suppression of migration through reduction of total and activated PAK4 as well as cyclin D1, ultimately affecting PAK4-related signaling. The PAMs also showed stem strength and chemoresistant overcoming activities. PAM (KPT-9274) with two drugs (gemcitabine and oxaliplatin) also synergistically affected PDAC cell lines. The previous findings led them to evaluate the antitumor activity of the in vivo model. KPT-9274 showed substantial antitumor activity as a single and combined with gemcitabine in the PDAC animal tumor model. KPT-9274 also mostly eliminated chemoresistant cancer stem cell (CSC) tumors of PDAC CSCs-derived xenograft model (Aboukameel et al., 2017). The higher level of PAK4 expression was discerned and associated with the migration and invasion induced by hepatocyte growth factor (HGF) of pancreatic cancer cell lines by interacting with the p85α subunit of PI3K, which subsequently acting on the Akt (King et al., 2017). In resected patients with pancreatic adenocarcinoma, it was determined that PAK4 negativity or absentees were associated with poorer overall and disease-free survival, as well as histological differentiation (Park et al., 2017). The proliferation and migration of pancreatic cancer cell lines (human miaPaCa-2 and PANC-1 and murine PAN02) were suppressed by PF-3758309 targeting PAK1 in all cell lines and PAK4 in miaPaCa-2 and PAN02 cell lines. PF-3758309 with gemcitabine was revealed to have a synergistic effect on reducing cell proliferation and anchorage-independent growth in vitro, as well as tumor growth in vivo. Inhibition of PAKs by administering PF-3758309 or gemcitabine caused a significant reduction in tumor growth in vivo. PF-3758309-mediated inhibition of PAKs enhanced the immune response to tumors in vivo (Wang et al., 2018). Both pancreatic neuroendocrine tumor cell lines and patient-derived tissues expressed higher levels of PAK4 and nicotinamide phosphoribosyltransferase (NAMPT). Down-regulated PAK4 and NAMPT were associated with the reduction of growth, survival, and anti-apoptotic factors in PNET cell lines of PNET, and that growth inhibition was also consistent in the BON-1-derived in vivo tumor model. Dual inhibition of PAK4 and NAMPT by KPT-9274, as well as its analog decreased the growth and survival of PNET cell lines. KPT-9274, as well as Everolimus, were shown to have a synergistic effect on everolimus-resistant PNET cell lines by downregulating everolimus-resistant factors. In vivo experiment on the antitumor activity of KPT-9274 in BON-1 xenograft revealed significant growth suppression property of KPT-9274 by downregulating NAMPT, β-catenin (a PAK4 target), antiapoptotic molecules (Mcl-1 and Bcl-2), and activating Bax (a pro-apoptotic molecule) (Mpilla et al., 2019). Gemcitabine resistant cell lines including PDAC CSCs of PDAC harbored higher level of PAK4 expression and inhibition of PAK4 by only PAK4 inhibitors (such as KPT-9274 and KPT-9307) or in combination with gemcitabine led to induction of apoptosis, subsequently inhibition of proliferation of PDAC cells proliferation via induction of Bad and Bax reexpression, down-regulation of p-PAK4, p-Bad, and Bcl-2, up-regulation of miRNAs (involved in tumor suppression) and Bax, and activation of caspase-3 (Mohammad et al., 2019). An in vitro study of gemcitabine resistant (GR) and wild-type (WT) pancreatic cancer cell lines revealed that all-trans retinoic acid (ATRA) alone and combination of ATRA with gemcitabine exhibited antitumor properties through the inhibition of cell proliferation, colony formation, migration / invasion and synergistically inhibition of proliferation, respectively, by downregulating PAK1, PAK2, PAK4, and α-SMA (Wang et al., 2019). Higher expression of LINC00657, which is a long non-coding RNA, was found both cell lines and PDAC. A significant association was also observed between the higher expression of LINC00657 and clinical properties such as stage T, lymph node metastasis, and poorer overall survival in patients with PDAC. The in vitro assay indicated the involvement of LINC00657 in the growth (proliferation, restriction of apoptosis) and metastasis (migration, invasiveness) promoting activities by acting as a ceRNA for miR-433, which directly targeted PAK4, thus up-regulating the expression of PAK4. Furthermore, restriction of PDAC tumor growth was also confirmed in an in vivo experiment by LINC00657 knockdown. These data suggested that LINC00657 was involved in the progression of PDAC progression via up-regulation of PAK4 (Bi et al., 2020). In vitro and in vivo assays reported that suppression of PAK4 utilizing exosome-guided RNAi caused decreased tumor growth and prolongation of mice survival (Xu et al., 2021).
4.10 Glioma/Neuroblastoma
PAK4 was revealed to be highly expressed in glioma cell lines and glioblastoma multiforme (GBM) tissues. At the same time, a very small / no and no significant amount of PAK4 was observed in the astrocytes and normal brain (NB) / tumor adjacent NB (ANB) tissues of humans, respectively. A positive correlation was found between increased PAK4 expression of PAK4 and pathological grades of glioma. PAK4 was determined to play a role in the anoikis resistance, migration, and invasion of glioma cells to anoikis by interacting with MMP-2, subsequently regulating the survival signaling of ανβ3 integrin/EGFR. Transcriptionally suppressed proteins involved in cell proliferation and adhesion were also reported in PAK4 knockdown cells. In vivo experiments in orthotopic tumors demonstrated that PAK4 suppression led to inhibition of tumor growth and corroborated the previous findings of interaction between PAK4 and MMP-2, subsequent regulation of integrin ανβ3/EGFR signaling. Therefore, targeting PAK4 and MMP-2 was suggested to be a promising therapeutic option in the glioma (Kesanakurti et al., 2012). Integrin ανβ3 activates PAK4 to inhibit p21, subsequently allowing glioblastoma cells of glioblastoma to escape senescence. Thus, glioblastoma cells depend on Integrin ανβ3/PAK4 signaling to evade senescence (Franovic et al., 2015). MiR485was found to be downregulated in cell lines and GBM tissues. That miR 485 can inhibit cell proliferation, colony formation, and metastasis and induce apoptosis by directly targeting PAK4, subsequently inactivating ERK and AKT signaling in GBM. The in vivo assay also showed that miR 485 has significant antitumor activity, targeting PAK4 and modulating ERK and AKT signaling pathways (Mao et al., 2017). Overexpression and suppression of PAK4 showed that PAK4 was involved in the improvement of Epithelial-to-Mesenchymal Transition (EMT) in glioma. Furthermore, ionizing radiation therapy (IR) was also reported that ionizing radiation (IR) therapy significantly increase PAK4 expression of PAK4 and nuclear localization, as well as PAK4 interaction of PAK4 with peroxisome proliferator-activated receptor-gamma (PPARγ). Furthermore, the PAK4/PPARγ complex acted on the Nox1 promoter to increase its expression and reactive oxygen species (ROS) levels, and then EMT in glioma. In vivo experiments on orthotopic tumors showed that PAK4 knockdown caused significant suppression of tumor growth, more suppression was also found in a combination of IR therapy in PAK4 knockdown tumors by down-regulating PAK4, PPARγ, and Nox1. IR therapy in in vivo control tumor model improved the expression level of PAK4, PPARγ, Nox1, and N-cadherin, implying EMT induction. Targeting PAK4 would be a potential therapeutic option to overcome resistance to IR resistance in glioma (Kesanakurti et al., 2017). Tissue samples and cell lines appeared to have significantly higher expression was of PAK4 and that higher expression strongly related with the diagnosis grade, stage of TNM (implying a degree of primary tumor invasiveness, distant and lymph node metastasis), and undesirable phenotype (indicating a poor prognosis). PF-3758309, a potent PAK4 inhibitor, showed substantial antiproliferative, cell cycle arrest, and apoptosis activities in highly expressed cell lines by inhibiting the MEK/ERK pathway (Li et al., 2017). The down-regulated expression of miR-342 was narrated in tissues, and glioma cell lines of glioma and its expression levels were associated with advanced grades of the WHO advanced grades and fewer KPS scores. Overexpression of miR-342 can cause inhibition of proliferation and invasion, as well as activation of glioma cell lines. Furthermore, PAK4 was identified as a direct target of miR-342, which caused inhibition of PAK4 expression and inactivation of the AKT/ERK pathway. An inverse correlation was also reported between the expression of miR-342 and PAK4. The underexpression and reintroduction of PAK4 can mimic and rescue the role of overexpressed miR-342, respectively, in glioma cells (Lu et al., 2017). Vascular abnormalities in solid malignant tumors cause the formation of immune-hostile microenvironment formation and resistance to immunotherapy. Genetic reprogramming and activation of the proangiogenic pathway in endothelial cells (ECs) of tumors may be responsible for that aberrant vascularity. Analysis of mesenchymal-like transcription activation in ECs derived from human glioblastoma (GBM) identified the selective regulatory role of PAK4 in vascular abnormalities and genetic reprogramming. PAK4 deletion led to the reexpression of adhesion protein in EC, reduction of aberrant vascularity, improvement of T cell, and inhibition of GBM growth of GBM in mice, as well as increased mouse survival. Furthermore, inhibition of PAK4 also normalized the tumor vascular microenvironment of the tumor and sensitized the chimeric antigen receptor T cell-mediated immunotherapy. Enhancement of vessel permeability by PAK4 has been reported to suppress the expression of claudin-14 through the expression mediated by myocyte-specific enhancer factor 2D (MEF2D) of ZEB1. It was suggested that the PAK4/SLUG pathway was involved in reducing T cell adhesion through down-regulated expression of intercellular adhesion molecule 1 (ICAM-1) and VCAM-1 in GBM ECs (Ma et al., 2021).
4.11 Gastric cancer (GC)
PAK4 and DGCR6L were reported to be highly expressed in an in vitro study, and a novel interaction between them, that interaction was reported to mediate the formation of a complex with β-actin and gastric cancer cells by acting on the LIMK1 and cofilin (Li et al., 2010). Eighteen GC cell lines were selected to determine the PAK4 expression, where strong and weak overexpressed PAK4 was reported in five (AGS, MKN-28, MKN-74, SNU-216, and SNU-601) and four (KATOIII, MKN-1, SNU-620, and SNU-719) GC cell lines, respectively. SiRNA-mediated knockdown of PAK4 in AGS cells (PAK4 overexpressed cells) induced apoptosis, which was confirmed by caspase 3 & 9 as well as PARP cleavages. The immunohistochemical staining method exposed that 4 out of 49 metastatic GC samples from patients exhibited a higher expressed PAK4; interestingly, these four patients did not respond to capecitabine / cisplatin chemotherapy based on capecitabine/cisplatin and had a scheme of poorer survival (Ahn et al., 2011). LCH-7749944 was confirmed as a potent and novel inhibitor of PAK4 and significantly suppressed GC cell proliferation of cells of GC and arrested the cell cycle by inhibiting PAK4, subsequently the c-Src / EGFR / cyclin D1 pathway, as well as induced apoptosis. LCH-7749944 substantially inhibited migration and invasion by blocking PAK4 and its related pathway of LIMK1/cofilin and MEK-1/ERK1/2/MMP2. LCH-7749944 also exhibited an inhibitory action on EGFR and filopodia formation, as well as cell elongation induction activity (Zhang et al., 2012). TGF-β1-mediated activation mediated by TGF-1 of Smad2/3 through the phosphorylation, subsequent interaction with Smad4 to form Smad2/3/4, and the nuclear localization of activated Smad2/3 can be blocked by PAK4 in GC cells by interacting with the Smad2/3/4, which is independent of PAK4 kinase activity as well as TGF-β1 signaling. Furthermore, in vitro and in vivo assays found that PAK4 phosphorylates the Ser465 residue in Smad2 and Ser423 in Smad3 that phosphorylated Smad2 undergoes degradation through the ubiquitin proteasome pathway in response to HGF stimulation. These interactions and modulation of the phosphorylated state by PAK4 suppressed TGF-β1 mediated growth of GC cells; therefore, PAK4 exerts oncogenic function and develops GC. Clinical samples of GC analysis deduced an inverse and positive correlation between PAK4 expressions of PAK4 with p-Smad2 Ser465/467 (TGF-β1-mediated site) and p-Smad2 Ser465 (PAK4-mediated site), respectively. Immunochemical staining of GC samples also showed substantially increased expression of HGF, p-PAK4 Ser474, and p-Smad2 Ser465 and decreased expression of Smad2 p-PAK4 Ser474 showed a positive correlation with p-Smad2 Ser465 and a negative correlation between Smad2 and p-Smad2 Ser465 (Wang et al., 2014). In a study, the interaction of PAK4 with the superior cervical ganglia 10 (SCG10) in GC cell lines and tissues of GC as well as PAK4-mediated phosphorylation on the SCG10 Ser50 (p-SCG10) residue as a downstream effector of Cdc42 and HGF in GC cell lines was observed. That phosphorylation of SCG10 on Ser50 by PAK4 was found to facilitate and promote microtubule instability, migration, and invasion of GC cells in vitro, and metastasis in vivo. Inhibition of PAK4 markedly decreased p-SCG10-mediated migration and invasion. To get more information on the role of p-SCG10 in GC, GC tissue samples of GC were analyzed and found higher expressed p-SCG10 substantially associated with poor pT and pTNM stage as well as distant metastasis, along with the fact that a notable positive correlation was revealed between PAK4 and p-SCG10. Thus, high levels of PAK4 and p-SCG10 are assumed to be related to the progression and metastasis (Guo et al., 2014). A significant correlation was observed between the level of p-PAK4 level and grosstype (advanced vs early stages of GC) by analyzing GC tissues. Kaplan–Meier survival analysis revealed that a higher level of p-PAK4 was associated with a poorer prognosis. Cox regression for multivariate analysis found independent factors for the prognosis of GC patients such as high phosphorylated PAK4 level, metastasis to lymph nodes, and advanced stage. Studies in GC cell lines and tissues of GC demonstrated that PAK4 regulated cell migration, which was dependent on kinase activity, through the LIMK1 and cofilin pathway (Li et al., 2015). 95 of 217 tumors (43.8 %) expressed a high level of PAK4. Overexpressed PAK4 was remarkably associated with deep invasion (T2 / T3 / T4), lymph node metastasis, pathological stage (II/III/IV), recurrence or remote metastasis, lower survival related to disease specific (DS) and relapse-free (RF). Multivariate analysis also confirmed PAK4 as a distinct prognostic factor for disease stage (II & III), DS, and RF survival (Kobayashi et al., 2016). The MKN-45, BGC-823, and especially the SCG-7901 cell lines of human GC expressed a higher level of miR-224 and that miR-224 mediated significant cell proliferative and migratory promoting activities of SCG-7901 in vitro by targeting PAK4 (Xia et al., 2016). GL-1196 showed the down-regulating effect on the c-Src / EGFR / cyclin D1 pathway of c-Src/EGFR/cyclin D1 and the expression of CDK4/6 and LC-0882 in cyclin D1 and CDK4/6 to inhibit proliferation and induce cell cycle arrest in the G1 phase of GC cells by targeting PAK4. GL-1196 and LC-0882 also significantly suppressed GC cell invasion of GC cells by blocking PAK4 and its mediated LIMK1 and cofilin pathway. Furthermore, inhibition of filopodia formation and induction of cell elongation of GC cells were also observed using GL-1196 and LC-0882 (Zhang et al., 2016, Zhang et al., 2017). PAK4 interacted with eukaryotic elongation factor 1 α1 (eEF1A1) and colocalized in the cytoplasm with its interacted state in vitro and in vivo. In BGC823 cells, overexpressed PAK4 substantially enhanced the expression of eEF1A1 in a PAK4 kinase-independent manner. Separate transfection of PAK4 and eEF1A1 and co-transfection of both in BGC823 cells were done to analyze their role in GC migration and invasion of GC. Higher expressed eEF1A1 or PAK4 significantly improved migration and invasion, as well as cotransfection, showed higher migration and invasion properties than their alone. Analysis of human GC tissues revealed the higher expression level of PAK4 and eEF1A1 and revealed a positive correlation between them (Li et al., 2017). Expression of miR-199a/b-3p profoundly decreased in the tissues and cell lines of GC. The overexpression of miR-199a/b-3p and the silencing of PAK4 led to inhibition of cell proliferation, and miR-199a / b-3p suppressed the expression level of PAK4, which later became the PAK4 / MEK / ERK pathway of PAK4/MEK/ERK in vitro. Furthermore, miR-199a/b-3p inhibited GC cell proliferation by down-regulating PAK4 expression in vivo (Zeng et al., 2018).
4.12 Breast cancer (BC)
Western blot inspection of the level of the PAK4 protein level in BC cell lines, including triple negative types, showed a high PAK4 level. KPT-8752 and KPT-9274 substantially reduced the PAK4 level, consequently the phosphorylated state of a downstream target of PAK4 (β-catenin and Cofilin), substantially blocking cell growth and induced apoptosis in triple negative breast cancer (TNBC) cell lines (MDA-MB-231, MDA-MB-468 and SUM159). These TNBC cell lines were also subjected to assess the efficacy of KPT-9274 by oral administration in vivo, which caused a substantial reduction in tumor volumes and weights as the well as PAK4 level (Rane et al., 2017). An immunohistochemical study on 93 BC tissue samples of BC inferred a relationship between high levels of PAK4 expression with more metastasis to lymph nodes, the larger tumor size, and advanced stages of AJCC. The Kaplan-Meier plotter deduced a significant association between highly expressed PAK4 with short disease-free and overall survival; along with that, in multivariate and univariate analysis, PAK4 remained as a distinct untoward prognostic factor. In vitro assay on higher expressed PAK4 from BC cell lines (MDA-MB-231 cells) reported increased proliferation, migration and invasion, while PAK4 knockdown of PAK4 reversed these actions. The aforementioned cancer progression actions of PAK4 were mediated by activating the PI3K/AKT pathway through the independent and dependent ways. Furthermore, it was also confirmed that PAK4 overexpression enhanced tumor growth in the MDA-MB-231 cells derived xenograft mouse model. Hence, dual targeting of PAK4 and PI3K/AKT would be a promising therapeutic strategy for BC therapy (He et al., 2017). Analysis of the Cancer Genome Atlas (TCGA) dataset revealed a positive correlation between PAK4 and Claudin4 mRNA expression (CLDN4), which was further confirmed in 135 BCE samples by immunohistochemical staining (IHC). Additionally, in vitro analysis reported that PAK4 was involved in BC migration and invasion of BC through the up-regulation of CLDN4 by phosphorylating on the Thr-235 residue of CCAAT/enhancer-binding protein β (CEBPB), consequently activating the CEBPB (Wang et al., 2019). Cell lines of breast cancer (MDA-MB-231, CAL-120, SUM149PT, and HCC1395), having high metastatic potential, expressed lower miR-199a/b-3p level. The transfection of miR-199a / b-3p in MDA-MB-231 indicated that it had a role in inhibiting cell growth, reducing migration and invasion. Furthermore, miR-199a / b-3p was elicited that miR-199a/b-3p directly act on the mRNA of PAK4 in MDA-MB-231 cells, subsequently inhibiting the MEK/ERK pathway. To confirm the antitumor activity of miR-199a/b-3p by targeting PAK4, silencing PAK4 was performed, which led to significant suppression of cell growth via affecting the MEK / ERK pathway, and invasion of MDA-MB-231, as well as PAK4 overexpression, reversed the inhibitory properties of miR-199a/b-3p (Li et al., 2015). Studies on BC MCF-7 cell lines reported that PAK4 can down-regulate the cyclin-dependent kinase inhibitor 1C (p57Kip2) both in protein expression and mRNA levels. In addition, further experiments also confirmed that PAK4 caused p57Kip2 in the ubiquitin-guided proteasome-dependent pathway. An inverse correlation was suggested between PAK4 and p57Kip2 in BC tissue samples of BC by the IHC analysis (Li et al., 2013). High-throughput drug screening identified several PAK4 inhibitors from Cancer Research UK's Commercial Partnerships Team (formerly known as Cancer Research Technology, CRT). Hit compounds were selected based on the structure–activity relationship against ATP competitive inhibitors of PAK1 and PAK4. These selected hit compounds were subjected to a review of their properties, including pharmacodynamics, inhibitory potentiality of PAK4 kinase activity, and toxicity in terms of drug metabolism and pharmacokinetic properties in vitro. These evaluations recognized a potent inhibitor of CRT PAKi with 49 % bioavailability in vivo. A detailed survival analysis of 669 and 343 patients with tamoxifen treated and untreated estrogen receptor positive (ER + ) BC, respectively, from published gene expression data sets based on Affymetrix microarray and subsequently Kaplan-Meier plotter analysis revealed that elevated PAK4 was remarkably associated with metastatic relapse. Furthermore, impoverished clinical outcome displaying reduced OS in untreated ER + BC patients was correlated with higher PAKs (PAK1 and PAK4). Thus, PAK4 could be a prognostic marker in determining the elevated risk of endocrine-resistant ER + BC patients. Metastatic analysis of 18 BC, analysis of patient samples analysis, determined a correlation and suggested that PAK4 expression of PAK4 was a maintenance factor for CSC activity in patients with ER + BC. Two samples from ER + BC metastatic patients indicated that cancer progression was positively associated with PAK1 / 4 and CSC activity expression, implying their role in endocrine resistant. Tamoxifen (TAMR) and fulvestrant (FULVR) resistant MCF-7 cells (BC cell lines) showed higher expressed PAK1 and PAK4. To gain more insight on the endocrine resistance and stem-related role of PAK1/4 in TAMR cells and FULVR cells, the inhibition of PAK1/4 was carried out by CRT PAKi. That inhibition led to the abrogation of the self-renewal property of CSCs more than 95 % and 80 % in TAMR and FULVR, respectively, along with that PAK4 silencing led to the restoration of tamoxifen and fulvestrant sensitivity. These results confirmed that PAK4 involved in the activity of endocrine-resistant cells’ CSCs could be used as a target to conquer endocrine resistance. They hypothesized that combinatorial treatment, using the PAK4 inhibitor and endocrine, will benefit patients of ER + BC. To test this hypothesis, the effect of CRT PAKi was screened, revealing a significant inhibition of proliferation and colony formation in cells resistant to endocrine conditions, especially TAMR and FULVR. Furthermore, a synergistic effect of CRT PAKi and tamoxifen / fullvestrant was observed in decreasing colony formation and proliferation of endocrine resistant cells. To further confirm this finding, single and combinatorial treatment of CRT PAKi and fulvestrant in metastatic patient samples derived from ER + BC with high PAK4 showed a synergistic effect in the decrease in CSC activity (>half). Therefore, PAK4 could be used as a potential prognostic tool and target to reduce CSC activity in metastatic ER + BC (Santiago-Gómez et al., 2019). LIMKi 3, a LIMK1 inhibitor, showed antiproliferative activity against all luminal, hormone receptor (HR) negative and human epidermal growth factor receptor 2 (HER2) positive (HR-/HER2 + ), and TNBC, especially BC (MDA-MB-157 and MDA-MB-468) cell lines. On the contrary, PF-3758309, an inhibitor of PAK4, possessed strong inhibitory activity against all subjected cell lines; those were used for LIMKi 3 while showing a weak inhibitory effect against luminal cell lines. PF-3758309 and LIMKi 3 exhibited a synergistic effect in 4 out of 5 used luminal (very low doses) used, 1 out of 4 TNBC, and 3 out of 4 HR-/HER2 + cell lines. LIMKi 3 arrested the G2/M phase in both BT474 (Hr+/HER2 + ) and T47D (HR+/HER2-), while PF-3758309 in BT474 induced G1 arrest and reduced cells in the S and G2/M phase. Combinatorial application of PF-3758309 and LIMKi 3 in BT474 cells increased the number of significant cell number in the phase of the cell cycle phase as follows sub-G1, G1, and G2 / M, while the sub-G1 phase in T47D cells. Combining PF-3758309 and LIMKi 3 treatment in these two cell lines also led to reduced activity of ERα and myc activity through the inhibition of phosphorylation and reduction of C-MYC expression, respectively. Tumor xenografts derived from BT474 (HR+/HER2 + ) cell lines were used to assess the combined effect of PF-3758309 and LIMKi 3 in vivo. LIMKi 3 alone and combined treatment dramatically decreased tumor growth, while the effect of PF-3758309 on tumors was not significant. The combined treatment showed a synergistic effect in reducing tumor growth and phosphorylated ERα (p- ERα) expressions were also found to decrease in tumor xenografts undergoing combined treatment. The functional activity of combined therapy was manifested by reduction of tumor volume, cell density, and inhibition of Ki-67, ERα signaling, as well as alteration in the checkpoint of the cell cycle. Tumor lysate analysis after treatment with LIMKi 3, PF-3758309 alone and a combination of them revealed that PF-3758309 efficiently abated the phosphorylated levels of PAK4 / 7 / 7 (p-PAK4 / 7) and LIMKi 3 also lessened the p-PAK4/6/7 level, possibly through inhibition of AKT phosphorylation, as well as their combined therapy almost completely eliminate the PAK and AKT phosphorylation. LIMKi 3 and PF-3758309 alone or combinedly also hindered the ERα phosphorylation. Extensive computational studies on BC cell line protein and gene expression datasets imparted a positive correlation between the inhibitory actions of PF-3758309 on ERα + tumor and LIMK1, giving a theoretical basis for combinatorial treatment of PAK4 and LIMK1 inhibitors (Zhao et al., 2020). IHC stain analysis of bone and non-bone metastatic breast cancer samples (BMBC, 95 cases & NBMBC, 187 cases) exhibited a substantial positive correlation between nuclear PAK4 (nPAK4) and ERα in BMBC. Further analysis of tumors from patients with BMBC with nPAK4 expression showed shorter bone metastasis-free survival (BMFS) times; a similar outcome was also found in the ERα-positive BMBC patients with ER-positive BMBC with nPAK4. BC tissues with ERα + were subjected to immunofluorescence staining to study the association between PAK4 and ERα, which revealed a notable nuclear colocalization of PAK4 and ERα. In vitro assays unveiled a novel interaction and nuclear cotranslocation of PAK4/ERα/17β-estradiol (E2) after E2 stimulation. Subsequently, PAK4 inhibited the ERα-guided transactivation and acted as a negative regulator of E2-mediated target gene expression of ERα in ERα + BC cell lines, which may be responsible for progression and subsequently increased aggressiveness in ERα + BC cells. Overexpression and knockdown studies in ERα + BC cells suggested that PAK4 stimulated migration in noninvasive cell lines through promoting E-dependent cellular epithelial-mesenchymal transition (EMT). In vivo assay on MCF-7-luc-F5 cells (ERα + BC cells) derived mice model revealed ERα + BC cell metastasis to the bone from the breast was mediated by PAK4 by inhibiting the LIFR expression. Invasion of ERα + MCF-7 BC cells after E2 treatment was mediated by up-regulation and downregulation of PAK4 and LIFR, respectively (Li et al., 2019). Analysis of the Metabric and KMplot database found an association between high expression of PAK4 and an unfavorable outcome in endocrine specifically tamoxifen-treated ERα + BC patients, indicating the involvement of PAK4 in resistance to tamoxifen. To investigate the role of PAK4 in affecting the sensitivity of tamoxifen, MCF-7 (ERα + BC cells) were studied and revealed that overexpressed PAK4 markedly decreased the sensitivity of tamoxifen to human MCF-7 BC cells. The application of group II inhibitors GNE-2861, which is highly specific for PAK4, in MCF-7 and tamoxifen-resistant BC cells significantly improved the sensitivity of tamoxifen in these cells. Treatment with E2 of MCF-7 cells increased PAK4 expression by binding ERα to PAK4 intron 1. Inhibition of PAK4 decreased protein levels and signaling of ERα as well as E2-guided proliferation of T47D and MCF-7 BC cell lines. Furthermore, overexpressed PAK4 suppressed proteasomal degradation of ERα, regulating protein stability due to increasing protein levels of ERα. ER phosphorylation ERα also mediated increased stability and regulation of ERα signaling at Serine-305 by PAK4 (Zhuang et al., 2015).
In vitro assays confirmed that PAK4 interacted and colocalized with Runt-related transcription factor 1 (RUNX1), as well as PAK4-mediated phosphorylation of RUNX1 at Thr-207 was also confirmed in the nucleus of ERα + BC cell lines under physiological circumstances, subsequently that phosphorylation led to cytoplasmic translocation (Tang et al., 2020). Association of RUNX1 with the SIN3A / HDAC1 corepressor complex SIN3A/HDAC1 causes repression of target genes and interaction of PRMT1 and PRMT1-mediated methylation of RUNX1 residues (R206 and R210) abrogate binding with that corepressor complex, implying activation of transcriptional activity (Imai et al., 2004). Co-immunoprecipitation assays in MCF-7 cells found that RUNX1 interacted with the PRMT1 and SIN3A/HDAC1 complex. Further studies revealed that PAK4-mediated phosphorylation of RUNX1 phosphorylation inhibited and improved the association of RUNX1 with the SIN3A/HDAC1 corepressor complex and PRMT1, respectively, as well as the level of methylation of RUNX1 methylation was increased. Consequently, the activated transcriptional activity of RUNX1 led to the expression of osteoclast differentiation and maturation-related genes. An in vitro study indicated that phosphorylation of RUNX1 at the Thr-207 residue increased the metastatic potentiality of ER + BC cells in bone and enhanced the osteolytic destruction of bone in vivo. These results reveal PAK4-mediated and enhanced bone destruction in ERα + BC through osteolysis (Tang et al., 2020). Eighty patient samples from different pathological categories were analyzed to determine the interaction and expression patterns of PAK4 and P54 in BC, where the IHC S—P technique demonstrated that the expression of PAK4 and P54 was markedly higher than breast fibroma.
Interestingly, their expression patterns gradually increased as BC progressed (non-invasive < early invasive < advanced invasive). The immunofluorescence assay and the confocal experiment showed the cytoplasmic localization of P54 and the colocalization of PAK4 (Bi et al., 2016). To determine the role of PAK4 in mammary cell tumorigenesis, PAK4 expression was reduced by elimination, which reported that knockdown of PAK4 decreased proliferation, delayed motility, and recuperated acinar development of MDA-MB-231 BCE cells. Furthermore, PAK4 knockdown MDA-MB-231 cells were implanted in athymic mice and observed for 40 days, which entirely failed to develop tumors, while MDA-MB-231 cells as control developed large tumors (Wong et al., 2013). A wide variety of tumors and cell lines showed PAK4. Immortalized mouse mammary epithelial cells (iMMECs) from wild-type mice poorly expressed PAK4, while oncogene-expressing iMMECs exhibited high levels of PAK4. High levels of PAK4 in the 2D culture of iMMEC did not cause an increase in growth rate. 3D cultured iMMECs with high expression of PAK4 were found to be abrogated and attenuated in acinar lumen formation and luminal apoptosis, respectively. 3D cultured iMMECs having higher levels of PAK4 promoted cell proliferation and led to prolonged activation of the ERK pathway activation. Inhibition of MEK with U0126 to investigate the role of the MEK-ERK pathway in PAK4 overexpressing iMMECs caused restoration of luminal apoptosis and attenuation of cell proliferation in the acinar structure.
Furthermore, it was also revealed that PAK4 overexpressed iMMEC altered the cell polarity in vitro and generated mammary tumors in vivo (Liu et al., 2010).. Costa et al. 2019 reported that all subtypes of human BC have highly expressed PAK4 and are correlated with poor outcomes in patients. In mice, higher expression of Mouse mammary tumor virus (MMTV) -PAK4 stimulated mammary tumors, while PAK4 depletion of PAK4 impeded MMTV- Polyoma Middle T (PyMT) guided mammary tumors. PAK4 knockdown in BC prompted senescence hallmarks in vitro, in vivo, and ex vivo, but not in untransformed human mammary epithelial cells (HMECs), while higher expression of PAK4 in non-immortalized HMECs revoked H-RAS-V12-mediated senescence. The regulation of senescence property by PAK4 was mediated by phosphorylation of RELB (a subunit of NF-κB) at the Ser151 residue, negatively affecting the binding of RELB-DNA, subsequently RELB-guided transcription of the beta CCAAT / enhancer binding protein beta (C/EBPβ), a critical factor for senescence (Hoare et al., 2016, Costa et al., 2019). In BC, PAK1, PAK2, PAK4, and PAK6 mRNA levels were up-regulated and down-regulated for PAK3 and PAK5 by analyzing the different databases compared to normal tissues. The Human Protein Atlas (HPA) database showed higher levels of PAK1, PAK2, and PAK4 protein expression in BC compared to normal tissues. IHC analysis in 90 BC patients’ samples revealed an increased expression pattern of PAK4, which was consistent with HPA. Survival analysis of the Kaplan-Meier plotter predicted worse relapse-free survival (RFS) for higher PAK4 levels (Dang et al., 2020).
4.13 Squamous cell carcinoma (SCC)
PAK4 was identified as an amplicon and highly expressed gene in SKN-3, an OSCC cell line with a proliferation-boosting effect. Further analysis of 105 primary tumors of head and neck squamous cell carcinoma (HNSCC), including oral, hypopharyngeal, oropharyngeal SCC, revealed that higher PAK4 levels have potential in proliferation and survival of tumor cells. Kaplan–Meier plotter analysis showed that PAK4 positive tumor in patients were remarkably associated with a worse prognosis (Begum et al., 2009). Western blot and RT-PCR analysis revealed that mRNA and protein levels were higher in Hep-2 laryngeal carcinoma Hep-2 cells. In vitro downregulation of PAK4 in Hep-2 cells reduced proliferation, increased apoptosis, induced S / G2. Down-regulation of PAK4 in established Hep-2 cells derived xenografted tumors caused by reducing the size and weight of the tumors. Additionally, mice with tumors expressing PAK4 showed a significantly lower survival rate than mice with tumors not expressing PAK4.
Further experiments confirmed the involvement of the ATM/Chk1/2/p53 activated pathway in PAK4-mediated cell cycle arrest (Sun et al., 2013). Thirty-four oral SCC tissue samples and cell lines (SAS, Tca8113, and SKN3) showed notably downregulated expression of miR140 5p compared with the normal tissues and human normal oral keratinocytes (NOKs). Substantially weakened proliferative potentiality, elevated apoptosis rate and cells in the G0 / G1 phage, and reduced cells in the S and G2 phages were observed in oral SCC cell lines overexpressed with miR 140 5p, where miR‐140‐5p executed this function by directly targeting PAK4. Furthermore, the elimination and overexpression of PAK4 in oral SCC confirmed its role in affecting proliferation, apoptosis, and arresting the cell cycle. Overexpressed miR1405p caused a notable reduction in tumor growth (in both weight and volume) in SKN3 cell lines derived from oral SCC xenograft mice. Thus, miR1405p suppressed the tumorigenesis of oral SCC by directly targeting PAK4 (Peng and Pang 2019). PAK4 expression was down-regulated by applying fisetin in oral SCC cell lines of oral SCC. Furthermore, it was also observed that fisetin suppressed colony formation, cell cycle, migration, and induced apoptosis in oral SCC cell lines of oral SCC having highly PAK4. These in vitro outcomes were subjected to in vivo, where fisetin dramatically inhibited oral SCC growth (Design et al., 2020). Linc01234 was markedly overexpressed in oral SCC samples and cell lines compared to normal adjacent tissues and NOK cells, and was closely correlated with the prognosis of oral SCC patients. Knockdown of Linc01234 in oral SCC led to repression of cell growth, migration, and invasion. Linc01234 was further revealed to be a sponge for miR-433-3p, which directly targeted PAK4 to show antiproliferative and anti-metastasis activities. Thus, Linc01234 is involved in the progression of oral SCC by acting on the miR-433-3p/PAK4 pathway (Liu et al., 2020). The suppression of SET domain-containing protein 6 (SETD6) substantially suppressed proliferation, metastasis, and induced apoptosis in oral SCC cells. The silence of SETD6 led to up-regulated expression and hindered the methylation of PAK4 and P65 (RelA) (Huang et al., 2021).
4.14 Miscellaneous cancers
Osteosarcoma tissues and cell lines, especially MG63 cells, highly expressed PAK4, LIMK1, and Cofilin-1 compared to normal tissues and osteoblasts, as well as their positive expression, were reported to be notably correlated with tumor grade, clinical stage, and distant metastasis. Knockout of the PAK4 gene can inhibit the proliferation and metastasis of MG63 cells, while overexpression of LIMK1 overexpression can encourage its growth and migration. The co-immunoprecipitation experiment confirmed that PAK4 specifically interacted with LIMK1. Mechanically, PAK4 inhibition reduced p-LIMK/LIMK and p-Cofilin-1/Cofilin-1 levels, implying the PAK4/LIMK1/involvement Cofilin-1 pathway. The growth and weight of MG63 cell-derived subcutaneous tumors was significantly reduced in PAK4 knockout groups, compared to control groups but was substantially augmented by LIMK1 (Yao et al., 2020). Highly expressed PAK4 was demonstrated at both levels of mRNA and protein in clinical samples from gestational trophoblastic disease (GTD) and malignant choriocarcinoma (CCA) cell lines compared to normal tissues (placental tissues) and cell lines (trophoblastic). The aggressive phenotype of GTD was also demonstrated to be correlated with overexpressed PAK4. Investigation on human CCA cell lines (JAR and JEG3 cells) identified human chorionic gonadotropin (hCG) and the PI3K / PBB pathway as upregulation of PAK4 expression and activator of PAK4, respectively. Furthermore, PAK4 transfection and knockdown of PAK4 revealed the PAK4 action in enhancing proliferation, migration, and invasion of CCA cell lines through down-regulation of p16 and up-regulation of CDK6 for proliferation and stimulation of membrane-type 1 matrix metalloproteinase (MT1-MMP) transcription for migration and invasion (Zhang et al., 2011). Overexpressed PAK4 was observed in the various primary tumors such as the esophageal, mammary, and colon. The overexpressed and activated mutant PAK4 of wild type due to its ability of cell survival and uncontrolled proliferation in athymic mice led to the formation of the tumor with features of sarcoma (Liu et al., 2008). Cancer cells during invasion were believed to form invasive protrusions Invadopodia rich in actin (Stylli et al., 2008, Buccione et al., 2009). Later, Invadopodia was reported to be a metastasis-promoting factor both in vitro and in vivo (Yamaguchi et al., 2005, Blouw et al., 2008, Gligorijevic et al., 2012). In vitro and in vivo assays demonstrated that depletion of PAK1 and PAK4 can suppress the invasive potentiality of melanoma cells. Later, the dynamics of melanoma cells invadopodia were explored to largely depend on PAK1 and PAK4, where PAK1 is involved in the formation, and PAK4 inhibits PDZ-RhoGEF for promoting the maturation of invadopodia (Nicholas et al.,). Elevated PAK4 and p-PAK4 levels were observed in tissues of papillary thyroid carcinoma (PTC), and their elevated levels were found to be substantially correlated with tumor size and stage of TNM. The removal of PAK4 from PTC cell lines markedly suppressed the growth, migration, and invasion. TSH stimulated an increase in PAK4 activity of PAK4 through the increase in p-PAK4 levels, which was reported to promote the growth and colony formation potential of PTC cells through the TSHR-cAMP-PKA-PAK4 signaling pathway. The involvement of this pathway was further confirmed in PTC tissue samples (Xie et al., 2017). Various tissue-derived Human 60 cell lines of tumor were analyzed, where substantially high levels of PAK4 expression were detected in 47 cell lines compared to the normal counterpart. The PAK4 serine residue at 474 (Ser-474) was identified as an autophosphorylated site, and the mutation of this residue to glutamic acid (S474E) constitutively caused kinase activation of the kinase. Furthermore, the active variant of PAK4 (S474E) was documented to have transformation potential, resulting in anchorage-independent growth of cancer cells (Callow et al., 2002). Nuclear exporter protein exportin 1 (XPO1) inhibitor and PAK4-NAMPT dual inhibitor could inhibit the proliferation of lenvatinib-resistant anaplastic 8505C cells of thyroid cancer. Next, combined application of these inhibitors with lenvatinib showed a substantial synergistic effect on inhibiting the growth of 8505C cells in vitro and 8505C cells derived xenograft in vivo, implying that targeting XPO1 and PAK4 would be a viable option to overcome resistance to lenvatinib treatment resistance (Zhuang et al., 2015). The clinical specimen analysis showed higher expression of PAK4 and elevated levels of total and p-PAK4 in Rhabdomyosarcoma (RMS) cell lines. Stimulation of basal fibroblast growth factor 2 (bFGF2) in RD and RH30 (RMSD cell lines) and insulin-like growth factor 2 (IGF2) in RD only resulted in increased activated PAK4 levels with increasing phospho-β-catenin and phospho-ERK1 / 2. Furthermore, both molecular inhibition (knockdown by short hairpin RNA) and pharmacological inhibition (KPT-9274 and PF-3758309) of p21-activated kinase 4 (PAK4) provided strong evidence of a significant reduction in cell proliferation and metastasis in vitro and in vivo assays. Therefore, for the first time, PAK4 was reported that PAK4 could be a promising and exclusive therapeutic option for the RMS (Dasgupta et al., 2020). Amplification, increased kinase activity, and PAK4 overexpression of PAK4 have been documented in bladder cancer (up to 12 %). Both inhibition and suppression of PAK4 in cell lines indicated that it was involved in the proliferation, progression, and colony formation of bladder cancer cells. Therefore, they also reported that targeting PAK4 and protein tyrosine kinase 6 (PTK6), which is up-regulated due to inhibition of PAK4, could be a potential therapeutic strategy for overexpressing PAK4 bladder cancer patients (Chandrashekar et al., 2020).
5 Conclusions
According to global cancer statistics, 19.3 and 10 million cancer patients have been diagnosed and died, respectively, in 2020. Despite the advancement of treatment strategies, cancer remains the leading cause of death worldwide. Due to the hundreds of types and enormous means of a complex mechanism, cancer is a great burden in medical science in developing precise and effective therapeutics. Recently, kinases have gained great interest as a target-specific therapy, because they play an important role in carcinogenesis. PAK4 is one of them, which involves regulating the hallmarks of cancer. Tremendous efforts are underway to elucidate the regulatory role of PAK4 in all aspects of cancer progression and to develop PAK4-specific inhibitors to be used as an anti-cancer therapeutic strategy. Recent studies have demonstrated the involvement of PAK4 from the escape of senescence to the transformation and progression of numerous forms of cancers. Innumerable studies have also demonstrated the potentiality of PAK4-targeted inhibitors in vitro and/or in vivo to substantially stop the growth and metastasis of many forms of cancerous cells. Although PAK4-targeted drugs are yet to be approved, the ongoing investigation of PAK4 will facilitate the establishment of effective and novel therapeutic options.
Funding
This work did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Acknowledgments
Declared None.
References
- PAK4, a novel effector for Cdc42Hs, is implicated in the reorganization of the actin cytoskeleton and in the formation of filopodia. EMBO J.. 1998;17:6527-6540.
- [CrossRef] [Google Scholar]
- Dual and specific inhibition of NAMPT and PAK4 by KPT-9274 decreases kidney cancer growth. Mol. Cancer Ther.. 2016;15:2119-2129.
- [CrossRef] [Google Scholar]
- Novel p21-activated kinase 4 (PAK4) allosteric modulators overcome drug resistance and stemness in pancreatic ductal adenocarcinoma. Mol. Cancer Ther.. 2017;16:76-87.
- [CrossRef] [Google Scholar]
- A PAK4-LIMK1 pathway drives prostate cancer cell migration downstream of HGF. Cell. Signal.. 2008;20:1320-1328.
- [CrossRef] [Google Scholar]
- P21-Activated Kinase 4 Overexpression in Metastatic Gastric Cancer Patients. Transl. Oncol.. 2011;4:345-349.
- [CrossRef] [Google Scholar]
- Group I and II mammalian PAKs have different modes of activation by Cdc42. EMBO Rep.. 2012;13:653-659.
- [Google Scholar]
- Begum, A., I. Imoto, K.-i. Kozaki, et al., 2009. Identification of PAK4 as a putative target gene for amplification within 19q13 . 12-q13 . 2 in oral. 100, https://doi.org/10.1111/j.1349-7006.2009.01252.x.
- Kinase-targeted cancer therapies: progress, challenges and future directions. Mol. Cancer. 2018;17:1-20.
- [Google Scholar]
- Study on the expression of PAK4 and P54 protein in breast cancer. World J. Surg. Oncol.. 2016;14:10-14.
- [CrossRef] [Google Scholar]
- Long noncoding RNA LINC00657 enhances the malignancy of pancreatic ductal adenocarcinoma by acting as a competing endogenous RNA on microRNA-433 to increase PAK4 expression. Cell Cycle. 2020;19:801-816.
- [CrossRef] [Google Scholar]
- A role for the podosome/invadopodia scaffold protein Tks5 in tumor growth in vivo. Eur. J. Cell Biol.. 2008;87:555-567.
- [CrossRef] [Google Scholar]
- Subgroup II PAK-mediated phosphorylation regulates Ran activity during mitosis. J. Cell Biol.. 2010;190:807-822.
- [CrossRef] [Google Scholar]
- The ever-increasing importance of cancer as a leading cause of premature death worldwide. Cancer 2021
- [Google Scholar]
- PAK4 phosphorylates myosin regulatory light chain and contributes to Fcγ receptor-mediated phagocytosis. Int. J. Biochem. Cell Biol.. 2011;43:1776-1781.
- [Google Scholar]
- Buccione, R., G. Caldieri and I. Ayala, 2009. Invadopodia : specialized tumor cell structures for the focal degradation of the extracellular matrix. 137-149. https://doi.org/10.1007/s10555-008-9176-1.
- Overexpression of P21-activated kinase 4 is associated with poor prognosis in non-small cell lung cancer and promotes migration and invasion. J. Exp. Clin. Cancer Res.. 2015;34:1-10.
- [CrossRef] [Google Scholar]
- miR-199a-3p Modulates MTOR and PAK4 Pathways and Inhibits Tumor Growth in a Hepatocellular Carcinoma Transgenic Mouse Model. Molecular therapy. Nucleic acids.. 2018;11:485-493.
- [CrossRef] [Google Scholar]
- Requirement for PAK4 in the anchorage-independent growth of human cancer cell lines. J. Biol. Chem.. 2002;277:550-558.
- [Google Scholar]
- Callow, M. G., F. Clairvoyant, S. Zhu, et al., 2002. Requirement for PAK4 in the Anchorage-independent Growth of Human Cancer Cell Lines *. 277, 550-558. https://doi.org/10.1074/jbc.M105732200
- Therapeutically actionable PAK4 is amplified, overexpressed, and involved in bladder cancer progression. Oncogene. 2020;39:4077-4091.
- [CrossRef] [Google Scholar]
- Copy number alterations in pancreatic cancer identify recurrent PAK4 amplification. Cancer Biol. Ther.. 2008;7:1793-1802.
- [CrossRef] [Google Scholar]
- PAK4 phosphorylates fumarase and blocks TGFβ-induced cell growth arrest in lung cancer cells. Cancer Res.. 2019;79:1383-1397.
- [CrossRef] [Google Scholar]
- PAK4 suppresses RELB to prevent senescence-like growth arrest in breast cancer. Nat. Commun.. 2019;10
- [CrossRef] [Google Scholar]
- Cytoskeletal changes regulated by the PAK4 serine/threonine kinase are mediated by LIM kinase 1 and cofilin. J. Biol. Chem.. 2001;276:32115-32121.
- [Google Scholar]
- Genomics Systemic analysis of the expression and prognostic signi fi cance of PAKs in breast cancer. Genomics. 2020;112:2433-2444.
- [CrossRef] [Google Scholar]
- P21-activated kinase 4–not just one of the PAK. Eur. J. Cell Biol.. 2013;92:129-138.
- [Google Scholar]
- Targeting PAK4 inhibits Ras-mediated signaling and multiple oncogenic pathways in high-risk Rhabdomyosarcoma. Cancer Res 2020
- [CrossRef] [Google Scholar]
- Design, D., Y. Li, S. Jia, et al., 2020. Fisetin Modulates Human Oral Squamous Cell Carcinoma Proliferation by Blocking PAK4 Signaling Pathways. 773-782.
- PAK4 kinase activity plays a crucial role in the podosome ring of myeloid cells. Cell Rep.. 2019;29:3385-3393.
- [Google Scholar]
- Glioblastomas require integrin αvβ3/PAK4 signaling to escape senescence. Cancer Res.. 2015;75:4466-4473.
- [CrossRef] [Google Scholar]
- Gligorijevic, B., J. Wyckoff, H. Yamaguchi, et al., 2012. N-WASP-mediated invadopodium formation is involved in intravasation and lung metastasis of mammary tumors. 724-734. https://doi.org/10.1242/jcs.092726.
- Death receptor-induced activation of initiator caspase 8 is antagonized by serine/threonine kinase PAK4. Mol. Cell. Biol.. 2003;23:7838-7848.
- [Google Scholar]
- The serine/threonine kinase PAK4 prevents caspase activation and protects cells from apoptosis. J. Biol. Chem.. 2001;276:14414-14419.
- [Google Scholar]
- Guo, B., X. Li, S. Song, et al., 2016. Hydrastine suppresses the proliferation and invasion of human lung adenocarcinoma cells by inhibiting PAK4 kinase activity. 35: 2246-2256.
- PAK4 kinase-mediated SCG10 phosphorylation involved in gastric cancer metastasis. Oncogene. 2014;33:3277-3287.
- [CrossRef] [Google Scholar]
- CDC42 binds PAK4 via an extended GTPase-effector interface. Proc. Natl. Acad. Sci.. 2018;115:531-536.
- [Google Scholar]
- Type II p21-activated kinases (PAKs) are regulated by an autoinhibitory pseudosubstrate. Proc. Natl. Acad. Sci.. 2012;109:16107-16112.
- [Google Scholar]
- Advances in the 1-phenanthryl-tetrahydroisoquinoline series of PAK4 inhibitors: potent agents restrain tumor cell growth and invasion. Org. Biomol. Chem.. 2016;14:7676-7690.
- [CrossRef] [Google Scholar]
- Development of 2, 4-diaminoquinazoline derivatives as potent PAK4 inhibitors by the core refinement strategy. Eur. J. Med. Chem.. 2017;131:1-13.
- [CrossRef] [Google Scholar]
- Activated-PAK4 predicts worse prognosis in breast cancer and promotes tumorigenesis through activation of PI3K/AKT signaling. Oncotarget. 2017;8:17573-17585.
- [CrossRef] [Google Scholar]
- NOTCH1 mediates a switch between two distinct secretomes during senescence. Nat. Cell Biol.. 2016;18:979-992.
- [CrossRef] [Google Scholar]
- Identification of miRNomes in Human Liver and Hepatocellular Carcinoma Reveals miR-199a/b-3p as Therapeutic Target for Hepatocellular Carcinoma. Cancer Cell. 2011;19:232-243.
- [CrossRef] [Google Scholar]
- Huang, W., H. Liu and T. Lv, 2021. Silencing of SETD6 inhibits the tumorigenesis of oral squamous cell carcinoma by inhibiting methylation of PAK4 and RelA.
- The Corepressor mSin3A Regulates Phosphorylation-Induced Activation, Intranuclear Location, and Stability of AML1. Mol. Cell. Biol.. 2004;24:1033-1043.
- [CrossRef] [Google Scholar]
- Expression of phosphorylated p21-activated kinase 4 is associated with aggressive histologic characteristics and poor prognosis in patients with surgically treated renal cell carcinoma. Investigative and clinical urology.. 2021;62:399-407.
- [CrossRef] [Google Scholar]
- Functional cooperativity by direct interaction between PAK4 and MMP-2 in the regulation of anoikis resistance, migration and invasion in glioma. Cell Death Dis.. 2012;3:1-13.
- [CrossRef] [Google Scholar]
- Kesanakurti, D., D. Maddirela, Y. K. Banasavadi-Siddegowda, et al., 2017. A novel interaction of PAK4 with PPARγ to regulate NOX1 and radiation-induced epithelial-to-mesenchymal transition in glioma. 36: 5309-5320.
- Targeting XPO1 and PAK4 in 8505C Anaplastic Thyroid Cancer Cells: Putative Implications for Overcoming Lenvatinib Therapy Resistance. Int. J. Mol. Sci.. 2019;21
- [CrossRef] [Google Scholar]
- Biochemical and Biophysical Research Communications p21-activated kinase 4 regulates HIF-1 a translation in cancer cells. Biochem. Biophys. Res. Commun.. 2017;486:270-276.
- [CrossRef] [Google Scholar]
- Kimmelman, A. C., A. F. Hezel, A. J. Aguirre, et al., 2008. Genomic alterations link Rho family of GTPases to the highly invasive phenotype of pancreas cancer. Proceedings of the National Academy of Sciences of the United States of America. 105, 19372-19377. https://doi.org/10.1073/pnas.0809966105.
- PAK4 interacts with p85 alpha: Implications for pancreatic cancer cell migration. Sci. Rep.. 2017;7:1-12.
- [CrossRef] [Google Scholar]
- Prognostic significance of PAK4 expression in gastric cancer. J. Clin. Pathol.. 2016;69:580-585.
- [CrossRef] [Google Scholar]
- Maternal pak4 expression is required for primitive myelopoiesis in zebrafish. Mech. Dev.. 2013;130:181-194.
- [Google Scholar]
- DGCR6L, a novel PAK4 interaction protein, regulates PAK4-mediated migration of human gastric cancer cell via LIMK1. Int. J. Biochem. Cell Biol.. 2010;42:70-79.
- [CrossRef] [Google Scholar]
- P21 activated kinase 4 binds translation elongation factor eEF1A1 to promote gastric cancer cell migration and invasion. Oncol. Rep.. 2017;37:2857-2864.
- [CrossRef] [Google Scholar]
- Integrin-mediated cell attachment induces a PAK4-dependent feedback loop regulating cell adhesion through modified integrin αvβ5 clustering and turnover. Mol. Biol. Cell. 2010;21:3317-3329.
- [Google Scholar]
- Inhibition of neuroblastoma proliferation by PF-3758309, a small-molecule inhibitor that targets p21-activated kinase 4. Oncol. Rep.. 2017;38:2705-2716.
- [CrossRef] [Google Scholar]
- P21-Activated Kinase 4 Regulates the Cyclin-Dependent Kinase Inhibitor P57Kip2 in Human Breast Cancer. Anat. Rec.. 2013;296:1561-1567.
- [CrossRef] [Google Scholar]
- MiR-199a/b-3p suppresses migration and invasion of breast cancer cells by downregulating PAK4/MEK/ERK signaling pathway. IUBMB Life. 2015;67:768-777.
- [CrossRef] [Google Scholar]
- PB-10, a thiazolo[4,5-d] pyrimidine derivative, targets p21-activated kinase 4 in human colorectal cancer cells. Bioorg. Med. Chem. Lett.. 2020;30:126807.
- [CrossRef] [Google Scholar]
- Activated Pak4 expression correlates with poor prognosis in human gastric cancer patients. Tumor Biol.. 2015;36:9431-9436.
- [CrossRef] [Google Scholar]
- A mandatory role of nuclear PAK4-LIFR axis in breast-to-bone metastasis of ERα-positive breast cancer cells. Oncogene. 2019;38:808-821.
- [CrossRef] [Google Scholar]
- The protein kinase Pak4 disrupts mammary acinar architecture and promotes mammary tumorigenesis. Oncogene. 2010;29:5883-5894.
- [CrossRef] [Google Scholar]
- Liu, D., X. Jian, P. Xu, et al., 2020. Linc01234 promotes cell proliferation and metastasis in oral squamous cell carcinoma via miR-433 / PAK4 axis. 1-10.
- Liu, Y., D. Jiao and Z. Tian, 2019. MicroRNA-663 inhibits the proliferation and invasion of clear cell renal cell carcinoma cells by directly targeting PAK4. 19: 711-718.
- miR-193a-3p inhibition of the Slug activator PAK4 suppresses non-small cell lung cancer aggressiveness via the p53/Slug/L1CAM pathway. Cancer Lett.. 2019;447:56-65.
- [CrossRef] [Google Scholar]
- Liu, Y., H. Xiao, Y. Tian, et al., 2008. The Pak4 Protein Kinase Plays a Key Role in Cell Survival and Tumorigenesis in Athymic Mice. 6, 1215-1225. https://doi.org/10.1158/1541-7786.MCR-08-0087
- P21-Activated kinase 4 predicts early recurrence and poor survival in patients with nonmetastatic clear cell renal cell carcinoma. Urologic Oncology: Seminars and Original Investigations.. 2015;33:205.e213-205.e221.
- [CrossRef] [Google Scholar]
- Lu, X., H. Wang, Z. Su, et al., 2017. MicroRNA-342 inhibits the progression of glioma by directly targeting PAK4. 1240-1250. https://doi.org/10.3892/or.2017.5783.
- p21-activated kinase 4 regulation of endometrial cancer cell migration and invasion involves the ERK1/2 pathway mediated MMP-2 secretion. Neoplasma. 2013;60:493-503.
- [Google Scholar]
- Zic2 promotes tumor growth and metastasis via PAK4 in hepatocellular carcinoma. Cancer Lett.. 2017;402:71-80.
- [CrossRef] [Google Scholar]
- Luo, P., J. Fei, J. Zhou, et al., 2015. microRNA-126 suppresses PAK4 expression in ovarian cancer SKOV3 cells. 9: 2225-2229.
- Targeting PAK4 to reprogram the vascular microenvironment and improve CAR-T immunotherapy for glioblastoma. Nature Cancer.. 2021;2:83-97.
- [CrossRef] [Google Scholar]
- High-resolution genomic and expression profiling reveals 105 putative amplification target genes in pancreatic cancer. Neoplasia. 2004;6:432-439.
- [CrossRef] [Google Scholar]
- Overexpression of a novel activator of PAK4, the CDK5 kinase-associated protein CDK5RAP3, promotes hepatocellular carcinoma metastasis. Cancer Res.. 2011;71:2949-2958.
- [CrossRef] [Google Scholar]
- MicroRNA-485 inhibits malignant biological behaviour of glioblastoma cells by directly targeting PAK4. Int. J. Oncol.. 2017;51:1521-1532.
- [CrossRef] [Google Scholar]
- Targeting Rho GTPase effector p21 activated kinase 4 (PAK4) suppresses p-Bad-microRNA drug resistance axis leading to inhibition of pancreatic ductal adenocarcinoma proliferation. Small GTPases.. 2019;10:367-377.
- [CrossRef] [Google Scholar]
- p21-activated kinase 4 (PAK4) as a predictive marker of gemcitabine sensitivity in pancreatic cancer cell lines. Cancer Res. Treat.. 2015;47:501-508.
- [CrossRef] [Google Scholar]
- PAK4-NAMPT dual inhibition as a novel strategy for therapy resistant pancreatic neuroendocrine tumors. Cancers. 2019;11
- [CrossRef] [Google Scholar]
- PAK4 is required for regulation of the cell-cycle regulatory protein p21, and for control of cell-cycle progression. J. Cell. Biochem.. 2011;112:1795-1806.
- [Google Scholar]
- Nicholas, N. S., A. Pipili, M. S. Lesjak, et al., PAK4 suppresses PDZ-RhoGEF activity to drive invadopodia maturation in melanoma cells. 7,
- Prognostic value of p21-activated kinase 4 in resected pancreatic cancer. APMIS. 2017;125:699-707.
- [CrossRef] [Google Scholar]
- The p21-activated kinase 4-Slug transcription factor axis promotes epithelial−mesenchymal transition and worsens prognosis in prostate cancer. Oncogene. 2018;37:5147-5159.
- [CrossRef] [Google Scholar]
- Park, M. h., H. s. Lee, C. s. Lee, et al., 2013. p21-Activated kinase 4 promotes prostate cancer progression through CREB. 2475-2482. https://doi.org/10.1038/onc.2012.255.
- MicroRNA - 140–5p inhibits the tumorigenesis of oral squamous cell carcinoma by targeting p21 - activated kinase 4. Cell Biol. Int.. 2019;1–10
- [CrossRef] [Google Scholar]
- Innovative approaches for cancer treatment: Current perspectives and new challenges. Ecancermedicalscience.. 2019;13
- [Google Scholar]
- PAK4 kinase is essential for embryonic viability and for proper neuronal development. Mol. Cell. Biol.. 2003;23:7122-7133.
- [Google Scholar]
- P21 activated kinases: structure, regulation, and functions. Small GTPases.. 2014;5
- [CrossRef] [Google Scholar]
- A novel orally bioavailable compound KPT-9274 inhibits PAK4, and blocks triple negative breast cancer tumor growth. Sci. Rep.. 2017;7:1-12.
- [CrossRef] [Google Scholar]
- PF-3758309, p21-activated kinase 4 inhibitor, suppresses migration and invasion of A549 human lung cancer cells via regulation of CREB. NF-κB, and β-catenin signalings.. 2014;389:69-77.
- [Google Scholar]
- PAK4 regulates stemness and progression in endocrine resistant ER-positive metastatic breast cancer. Cancer Lett.. 2019;458:66-75.
- [CrossRef] [Google Scholar]
- MiR-145 inhibits human colorectal cancer cell migration and invasion via PAK4-dependent pathway. Cancer Med.. 2017;6:1331-1340.
- [CrossRef] [Google Scholar]
- PAK4 confers the malignance of cervical cancers and contributes to the cisplatin-resistance in cervical cancer cells via PI3K/AKT pathway. Diagn. Pathol.. 2015;10:1-9.
- [CrossRef] [Google Scholar]
- Siu, M. K. Y., H. Y. Chan, D. S. H. Kong, et al., 2010. P21-Activated Kinase 4 Regulates Ovarian Cancer Cell Proliferation, Migration, and Invasion and Contributes To Poor Prognosis in Patients. Proceedings of the National Academy of Sciences of the United States of America. 107, 18622-18627. https://doi.org/10.1073/pnas.0907481107.
- P21-Activated Kinases 1, 2 and 4 in endometrial cancers: Effects on clinical outcomes and cell proliferation. PLoS One. 2015;10:1-13.
- [CrossRef] [Google Scholar]
- Design, synthesis and biological evaluation of 1-phenanthryl-tetrahydroisoquinoline derivatives as novel p21-activated kinase 4 (PAK4) inhibitors. Org. Biomol. Chem.. 2015;13:3803-3818.
- [CrossRef] [Google Scholar]
- P21-activated kinase 1 and 4 were associated with colorectal cancer metastasis and infiltration. J. Surg. Res.. 2015;196:130-135.
- [CrossRef] [Google Scholar]
- Strategy and validation of a structure-based method for the discovery of selective inhibitors of PAK isoforms and the evaluation of their anti-cancer activity. Bioorg. Chem.. 2019;91:103168.
- [CrossRef] [Google Scholar]
- Interplay between components of a novel LIM kinase–slingshot phosphatase complex regulates cofilin. EMBO J.. 2005;24:473-486.
- [Google Scholar]
- Stylli, S. S., A. H. Kaye and P. Lock, 2008. Invadopodia : At the cutting edge of tumour invasion. 15, 725-737. https://doi.org/10.1016/j.jocn.2008.03.003
- Su, T., J.-J. Qu, K. Wang, et al., 2017. Cross-talk between p21-activated kinase 4 and ERα signaling triggers endometrial cancer cell proliferation. 8: 68083-68094.
- Sun, X. I. N., B. I. N. Liu, J. I. N. Wang, et al., 2013. Inhibition of p21-activated kinase 4 expression suppresses the proliferation of Hep-2 laryngeal carcinoma cells via activation of the ATM / Chk1 / 2 / p53 pathway. 683-689. https://doi.org/10.3892/ijo.2012.1718.
- Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin.. 2021;71:209-249.
- [Google Scholar]
- Knockdown of PAK4 or PAK1 inhibits the mroliferation of mutant KRAS colon cancer cells independently of RAF/MEK/ERK and PI3K/AKT signaling. Mol. Cancer Res.. 2013;11:109-121.
- [CrossRef] [Google Scholar]
- PAK4 phosphorylating runx1 promotes erα-positive breast cancer-induced osteolytic bone destruction. Int. J. Biol. Sci.. 2020;16:2235-2247.
- [CrossRef] [Google Scholar]
- Essential role for the Pak4 protein kinase in extraembryonic tissue development and vessel formation. Mech. Dev.. 2009;126:710-720.
- [Google Scholar]
- Anti-Cancer Activity of PAK4/NAMPT Inhibitor and Programmed Cell Death Protein-1 Antibody in Kidney Cancer. Kidney360.. 2020;1:376-388.
- [CrossRef] [Google Scholar]
- p-21 activated kinase 4 promotes proliferation and survival of pancreatic cancer cells through AKT- and ERK-dependent activation of NF-κB pathway. Oncotarget. 2014;5:8778-8789.
- [CrossRef] [Google Scholar]
- P-21 activated kinase 4 (PAK4) maintains stem cell-like phenotypes in pancreatic cancer cells through activation of STAT3 signaling. Cancer Lett.. 2016;370:260-267.
- [CrossRef] [Google Scholar]
- Antitumor effects of all-trans retinoic acid and its synergism with gemcitabine are associated with downregulation of p21-activated kinases in pancreatic cancer. Am. J. Physiol. Gastrointest. Liver Physiol.. 2019;316:G632-G640.
- [CrossRef] [Google Scholar]
- A novel PAK4-CEBPB-CLDN4 axis involving in breast cancer cell migration and invasion. Biochem. Biophys. Res. Commun.. 2019;511:404-408.
- [CrossRef] [Google Scholar]
- PAK4, a target of miR-9-5p, promotes cell proliferation and inhibits apoptosis in colorectal cancer. Cell. Mol. Biol. Lett.. 2019;24:1-12.
- [CrossRef] [Google Scholar]
- Inhibition of p21 activated kinase enhances tumour immune response and sensitizes pancreatic cancer to gemcitabine. Int. J. Oncol.. 2018;52:261-269.
- [CrossRef] [Google Scholar]
- Oncogenic PAK4 regulates Smad2/3 axis involving gastric tumorigenesis. Br. Dent. J.. 2014;217:3473-3484.
- [CrossRef] [Google Scholar]
- A two kinase-gene signature model using CDK2 and PAK4 expression predicts poor outcome in non-small cell lung cancers. Neoplasma. 2016;63:322-329.
- [CrossRef] [Google Scholar]
- miR-425 regulates ovarian cancer proliferation, metastasis, and apoptosis by repressing PAK4 expression. Asia Pac. J. Clin. Oncol. 2021
- [CrossRef] [Google Scholar]
- MiR-145 regulates PAK4 via the MAPK pathway and exhibits an antitumor effect in human colon cells. Biochem. Biophys. Res. Commun.. 2012;427:444-449.
- [CrossRef] [Google Scholar]
- PAK4: A pluripotent kinase that regulates prostate cancer cell adhesion. J. Cell Sci.. 2010;123:1663-1673.
- [CrossRef] [Google Scholar]
- PAK4 signaling in health and disease: Defining the PAK4–CREB axis. Exp. Mol. Med.. 2019;51:1-9.
- [Google Scholar]
- The Pak4 protein kinase is required for oncogenic transformation of MDA-MB-231 breast cancer cells. Oncogenesis.. 2013;2:2-7.
- [CrossRef] [Google Scholar]
- Discovery of 2- (4-Substituted-piperidin/piperazine-1-yl)-N- (5-cyclopropyl-1H-pyrazol-3-yl)-quinazoline-2,4-diamines as PAK4 Inhibitors with Potent A549 Cell Proliferation, Migration, and Invasion Inhibition Activity. Molecules (Basel, Switzerland).. 2018;23
- [CrossRef] [Google Scholar]
- Long noncoding RNA SNHG7, a molecular sponge for microRNA-485, promotes the aggressive behavior of cervical cancer by regulating PAK4. OncoTargets and Therapy.. 2020;13:685-699.
- [CrossRef] [Google Scholar]
- MIR-224 promotes proliferation and migration of gastric cancer cells through targeting PAK4. Pharmazie. 2016;71:460-464.
- [CrossRef] [Google Scholar]
- Xie, X., X. Shi, H. Guan, et al., 2017. P21-activated kinase 4 involves TSH induced papillary thyroid cancer cell proliferation. 8, 24882-24891.
- Linc01224 exhibits cancer-promoting activity in epithelial ovarian cancer through microrna-485-5p-mediated pak4 upregulation. OncoTargets and Therapy.. 2020;13:5643-5655.
- [CrossRef] [Google Scholar]
- Exosome-mediated RNAi of PAK4 prolongs survival of pancreatic cancer mouse model after loco-regional treatment. Biomaterials. 2021;264:120369.
- [CrossRef] [Google Scholar]
- PAK4 phosphorylates p53 at serine 215 to promote liver cancer metastasis. Cancer Res.. 2016;76:5732-5742.
- [CrossRef] [Google Scholar]
- MicroRNA-433 inhibits cell proliferation in hepatocellular carcinoma by targeting p21 activated kinase (PAK4) Mol. Cell. Biochem.. 2015;399:77-86.
- [CrossRef] [Google Scholar]
- Yamaguchi, H., J. Wyckoff and J. Condeelis, 2005. Cell migration in tumors. 559-564. https://doi.org/10.1016/j.ceb.2005.08.002.
- Yao, Z.-f. L. Y.-d., Y.-y. Z. Yan, L. Z.-h. Liu, et al., 2020. Effects of PAK4 / LIMK1 / Cofilin-1 signaling pathway on proliferation , invasion , and migration of human osteosarcoma cells. 1-9. https://doi.org/10.1002/jcla.23362.
- Glaucarubinone and gemcitabine synergistically reduce pancreatic cancer growth via down-regulation of P21-activated kinases. Cancer Lett.. 2014;346:264-272.
- [CrossRef] [Google Scholar]
- MiR-199a/b-3p inhibits gastric cancer cell proliferation via down-regulating PAK4/ MEK/ERK signaling pathway. BMC Cancer. 2018;18:1-7.
- [CrossRef] [Google Scholar]
- Nonconserved miR-608 suppresses prostate cancer progression through RAC2/PAK4/LIMK1 and BCL2L1/caspase-3 pathways by targeting the 3′-UTRs of RAC2/BCL2L1 and the coding region of PAK4. Cancer Med.. 2019;8:5716-5734.
- [CrossRef] [Google Scholar]
- Zhang, H.-j., M. K. Y. Siu, M. C. W. Yeung, et al., 2011. Overexpressed PAK4 promotes proliferation , migration and invasion of choriocarcinoma. 32, 765-771. https://doi.org/10.1093/carcin/bgr033
- LCH-7749944, a novel and potent p21-activated kinase 4 inhibitor, suppresses proliferation and invasion in human gastric cancer cells. Cancer Lett.. 2012;317:24-32.
- [CrossRef] [Google Scholar]
- GL-1196 suppresses the proliferation and invasion of gastric cancer cells via targeting PAk4 and inhibiting PAK4-mediated signaling pathways. Int. J. Mol. Sci.. 2016;17:1-15.
- [CrossRef] [Google Scholar]
- LC-0882 targets PAK4 and inhibits PAK4-related signaling pathways to suppress the proliferation and invasion of gastric cancer cells. Am. J. Transl. Res.. 2017;9:2736-2747.
- [Google Scholar]
- PAK4 regulates G6PD activity by p53 degradation involving colon cancer cell growth. Cell Death Dis.. 2017;8:e2820-e.
- [CrossRef] [Google Scholar]
- Combined LIM kinase 1 and p21-Activated kinase 4 inhibitor treatment exhibits potent preclinical antitumor efficacy in breast cancer. Cancer Lett.. 2020;493:120-127.
- [CrossRef] [Google Scholar]
- p21-activated kinase group II small compound inhibitor GNE-2861 perturbs estrogen receptor alpha signaling and restores tamoxifen-sensitivity in breast cancer cells. Oncotarget. 2015;6:43853-43868.
- [CrossRef] [Google Scholar]







