Translate this page into:
Biosynthesis of copperoxide nanoparticles using Abies spectabilis plant extract and analyzing its antinociceptive and anti-inflammatory potency in various mice models
⁎Corresponding author. nxl147868794@sina.com (Xiaoli Niu)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
Nanotechnology has shown rapid progress in various fields had made a remarkable change in the field of therapeutics too. The present study aims to synthesize and characterize the eco-friendly biocompatible copper oxide nanoparticles using Abies spectabilis plant extract (AS-CuONPs) and inspect its ameliorative potential against the different stimuli induced nociception and inflammatory reactions in mice. The formulated AS-CuONPs were characterized via the UV–vis spectroscopy, FT-IR, and TEM analysis. The nociception was induced by the method of acetic acid, glutamate, capsaicin, formalin, and thermal-induced techniques. The anti-inflammatory property of AS-CuONPs with carrageenan induced paw edema, peritoneal leukocyte infiltration, and carrageenan induced air pouch tests. Our result of UV–Visible spectroscopic analysis confirms the formation of AS-CuONPs and the characterization with FTIR and TEM proves the same. The result of various stimuli induced nociception models provide the evidence that formulated AS-CuONPs effectively inhibits the nociceptive responses in mice. Further the infiltration of leukocytes and inflammatory cytokines were drastically decreased by the AS-CuONPs treatment in the mice. The open field analysis confirms that the AS-CuONPs doesn’t impart any behavioral changes in mice. Over all our results confirms that AS-CuONPs is effective against different stimuli induced nociception and it act as a potent anti-inflammatory agent without rendering any side effects.
Keywords
Copper oxide nanoparticles
Green synthesis
Abies spectabilis
Antinociception
Anti-inflammation
1 Introduction
Inflammation is a common immunological protective mechanism educed in response to the various deleterious stimuli (Wang et al., 2014). This response is highly complex and engaged in numerous processes like cellular migration, plasma extravasations, vasodilatation, and release of different regulators that is primarily linked with pain (Sherwood and Toliver-Kinsky, 2004).
Pain is a typical sign of numerous inflammatory ailments distinguished as an obnoxious sensory incidence linked with definite tissue injury (IASP Taxonomy, 2017). The non-steroidal anti-inflammatory drugs (NSAIDs) are a primarily utilized therapeutic approach and it often experienced with the several complications in the long-term use (Bruno et al., 2014). Hence, the development of novel therapeutic agents for the treatment of pain and inflammatory ailments with minimal or null adverse effects are necessary (Knowles, 2014).
Nanoparticles possess unique physical properties such as small size, large surface area, electric conductivity, biomimetic property; targeted action that proves that the nanoparticles to be utilized in pharmaceutical field (Pugazhenthi et al., 2018; Venkatesan et al., 2020; Sathiyavimal et al., 2018). Nanodrugs shown increased bioavailability, sustained drug release, increased solubility and numerous nano based drugs were developed to treat ailments like cancer (Saratale et al., 2018), viral infection (Parboosing et al., 2012), neurological disease (Marta et al., 2016), diabetes, respiratory illness, pain, inflammatory diseases (Soares et al., 2018).
Copper oxides possess distinctive electrical, mechanical and optical properties which paved the utilization of copper oxide nanoparticles in industries as sensors, super capacitors, catalyst, semi conductors, viscosity enhancing agents, thermal conductors etc (Li et al., 2020; Dagher et al., 2014; Zhang et al., 2014). In therapeutic field copper oxide nanoparticles are used as antimicrobial, antioxidant, drug delivering agents (Vasanthraj et al., 2019; Khashan et al., 2016). Even though copper oxide nanoparticles possess several usages the major drawback of its utility is the severe side effects. Green nanotechnology is a potent alternative to overcome the side effects of copper oxide nanoparticles synthesized with physical and chemical reagents (Sahooli et al., 2012; Verma et al., 2019; Gomathi and Mayakrishnan, 2019). Eco friendly biocompatible nanoparticles were synthesized using plant extracts, fungi, bacteria algae without any toxic chemical reagents (Sheikholeslami et al., 2020). The phytochemicals aids the synthesis of non toxic metal as well as ideal nanoparticles to be utilized as nanomedicine (Prasad 2014; Prasad et al. 2016).
Non steroidal anti-inflammatory drugs (NSAID) were generally prescribed to treat inflammation which provide only temporary relief whereas the long term usage causes enormous side effects such as gastrointestinal disorders, hepatitis, nephritis etc. (Ong et al., 2007; Tielemans et al., 2014). Opioids are potent alternative for NSAID but it impairs psychological disturbances like depression, addiction, withdrawal symptoms (Kaplan et al., 2007). Hence it is need of today to discover a potent drug which effectively treats the inflammation and reduces pain without rendering any side effects. Abies spectabilis are evergreen coniferous trees belong to pinaceae family which is widely distributed in Eastern Asia (Wu et al., 2010). Abies sps consists of phytoconstituents such as terpenoids, flavonoids, lignans, steroids, phenols which proven to effective against ulcer, cancer, hypertension, microbial infection, neuronal disorders, respiratory disorders, inflammatory diseases, diabetes (Majeed et al., 2013). It was already proved that the A. spectabilis is a good source of metallic nanoparticles, and the gold nanoparticles synthesized from A. spectabilis showed a potent anticancer activity against the bladder cancer T24 cells (Wu et al., 2019). This present study aims to synthesize and characterize the eco-friendly biocompatible copper oxide nanoparticles using Abies spectabilis plant extract (AS-CuONPs) and inspect its ameliorative potential against the different chemicals induced nociception and inflammatory reactions in mice.
2 Materials & methods
2.1 Chemicals
Copper sulfate, diclofenac sodium, morphine, nalaoxone, acetic acid, Indomethacin, glutamate, capsaicin, formalin, and carrageenan were purchased from Sigma Aldrich Chemicals Co., USA. The each and every chemicals used for the present study is of analytical grade.
2.2 Experimental animals
Healthy young 8–10 weeks old mice weighing about 30–40 g were procured for the current nociceptive experiment protocols. The mice were maintained in a sterile laboratory environment with temperature of about 22–25 °C, 12 h/12 h light dark cycle and 55–60% of relative humidity. Only rice husk were used as bedding for mice and the animals were housed in polycarbonate cage with a standard size of 290 × 220 × 140 mm. The cages and the bedding of the mice cage were changed periodically to avoid any infection in mice. The mice were acclimatized in the laboratory condition for about two weeks before the initiation of experiments. The mice were fed ad libidum with laboratory pellet diet and water. The mice were restricted for food overnight only before the behavioral analyses. Each and every protocol performed with mice was approved by Institutional Ethical committee and utmost care and concern were taken to minimize the stress induced to mice due to the experimental procedures performed.
2.3 Synthesis and characterization of copper oxide nanoparticles from Abies spectabilis extract
The aerial parts of Abies spectabilis were washed thoroughly with double distilled water and air dried in room temperature until the sample is completely dried. The dried sample is then coarsely powdered using a clean blender. To 20 g of A. spectabilis aerial parts powder 200 mL of distilled water was added and boiled for 5 min. The solution was cooled and filtered using Whatmann filter paper, the filtrate was further subjected to centrifugation at 10,000g for 10 min and the supernatant was used for synthesis of copper oxide nanoparticles.
Eco friendly copper oxide nanoparticles were synthesized by reducing copper sulfate solution with A. spectabilis plant extract. To 50 mL of 1 mM copper sulfate solution 10 mL of A. spectabilis plant extract was added and placed on a stirrer at room temperature for 2 h. The color change from pale yellow to dark brown was considered to be the indication of synthesis of copper oxide nanoparticles. The reaction mixture was then centrifuged at 10,000g for 20 min, supernatant was discarded and the pellet was collected washed thrice with double distilled water (Sarkar et al., 2020). The nanoparticles were air dried at room temperature in a sterile environment and then resuspended in MilliQ water for further experiments.
To confirm the bioreduction of copper sulfate to copper oxide nanoparticles by the A. spectabilis plant extract the synthesized AS-CuONPs were subjected to UV–Vis Spectroscopic analysis using UV–vis spectrophotometer (Hitachi 330, Japan) at a spectra range of 300–800 nm.
The synthesized AS-CuONPs was vacuum dried and powdered using pestle and motor. It was then mixed with potassium bromide in a ratio of 1:100 (w/w) and made into pellets by applying pressure of maximum 25,000 psi. The pellets were then placed on the sample holder and subjected to FTIR analysis at a range of 4000–500 cm−1 spectra using spectrophotometer (Shimadzu 8400S, Kyoto, Japan). Totally 50 scans were performed at a resolution of 4 cm/scan and the data were analyzed using the software WINFIRST, Mattson, USA.
The topography, size and the morphology of biosynthesized AS-CuONPs were examined with high resolution transmission electron microscope. On a 30X30 µm carbon coated copper grid a drop of AS-CuONPs was placed and dried under mercury lamp for 10 min, then the sample was scanned under TEM instrument (Tecnai G2 spirit Biotwin (FP 5018/40), Hillsboro, USA), operating at accelerating voltage of 80 kV.
3 Assessment of AS-CuONPs antinociceptive property
3.1 Writhing test
Abdominal writhing was induced with acetic acid in mice and the antinociceptive property of AS-CuONPs was analyzed using the protocol of Parker et al. (2007). The mice were divided into five groups and pre treated with three different doses 5, 10, 15 µg of AS-CuONPs, negative control mice with 0.9% saline and the positive control mice with diclofenac sodium at dose of 10 mg/kg b.wt for 30 min prior to initiation of experiment. The mice were then intraperitoneally injected with 0.6% acetic acid at dose of 10 mL/kg b.wt and then placed in the observation chamber for a period of 30 min. The number of abdominal writhing performed by each group of mice for a period of 25 min were counted and recorded.
3.2 Glutamate induced nociception test
Nociception was induced in mice by injecting 10 μmol glutamate on the ventral surface of mice paw pretreated with drugs before 30 min of experiment. The nociception responses were observed and the number of licks performed by each group mice were observed and recorded for a period of 15 min (Beirith et al., 2002).
3.3 Capsaicin-induced paw licking test
The test was performed according to the protocol of Luiz et al. (2007). Capsaicin was prepared by dissolving capsaicin with 5% ethanol and 95% phosphate buffered saline. The mice were pretreated with AS-CuONPs before 30 min of experiment and capsaicin (1.6 µg/paw) was injected into the left of mice, placed in the observation chamber for a period of 5 min. The time spend by each mice on licking was observed and recorded.
3.4 Formalin induced paw licking test
The mice were treated with AS-CuONPs before 30 min of the experiment initiation. 20 μL of 0.5% formalin was injected subcutaneously into the right hind paw of pretreated mice and placed in the observation chamber for a period of 30 min (Miri et al., 2015). The licking time of each mouse was observed in two different phase i.e. initial phase (0–5 min) and secondary phase (16–30 min).
3.5 Hot plate test
The withdrawal latency of mice against the thermal induction was assessed using hot plate test (Vittalrao et al., 2011). After the pretreatment with AS-CuONPs, the mice were placed on the hot plate maintained at temperature of 55 °C ± 1 °C and observed for nociceptive responses such as licking the paws, jumping, the time was recorded. The experiment was repeated at different duration of time 0, 30, 60, 90 and 120 min. The maximum duration of time the experiment was performed was 60sec to avoid injury for animals.
The percentage of the maximal possible effect of each mice were calculated using the equation
4 Assessment of AS-CuONPs anti-inflammatory property
4.1 Carrageenan-induced paw edema test
Carrageenan induced paw edema test was performed according to the protocol of Chung et al. (2019). Followed by the pretreatment with AS-CuONPs, 100 µL of 1% carrageenan were injected to the right hind paw of mice and placed in the observation chamber for period of 5 h. The paw edema volumes were measured for every 60 min from 0th min to 4 h using the instrument plethysmometer.
4.2 Peritoneal cavity leukocyte infiltration test
The anti-inflammatory property of AS-CuONPs was analyzed with peritoneal cavity leukocyte infiltration test Vinegar et al. (1969). After the pretreatment with AS-CuONPs, 500 µg of 1% carrageenan inflammatory agent was intraperitoneally injected to the mice and placed in the cages for a period of 6 h. After 6 h the mice were euthanized and the infiltrates of peritoneal cavity was collected using 3 mL of phosphate buffered saline consisting 1 mM EDTA. The peritoneal fluid was centrifuged and the pellet was examined for the presence of leukocytes, mononuclear and polynuclear cells. The number of leukocytes, mononuclear and polynuclear cells were counted and recorded.
4.3 Air pouch test
The mice were subjected to air pouch test to assess the effect of AS-CuONPs on proinflammatory cytokines. Mice which formed pouches were divided into six groups and treated with following drugs control (0.9% saline), Carageenan control (0.5 mL carageenan), experimental group three different concentration of AS-CuONPs (5, 10, 15 µg/kg) along with carageenan and positive control dexamethasone along with carageenan. The mice were then euthanized and the pouch exudates were collected by injecting 5 mL of PBS along with 1 mM EDTA. The exudates were centrifuged and the pellet was utilized further to analysis the levels of TNF, IL-1β, IL-6 using commercially available ELISA kits.
4.4 Open field test
The mice were placed at the middle of the open field apparatus (50 cm × 50 cm × 50 cm) followed the AS-CuONPs pretreatment and allowed to explore the open field apparatus for a period 5 min. The number of squares crossed by the mice with all the paws was recorded. The apparatus were cleaned each time with mild ethanol before performing the experiment with new mice.
4.5 Statistical analysis
All the experiments were performed in triplicates and the data were analyzed statistically using One Way ANOVA followed by LSD test. The data were expressed as mean ± S.E.M.
p value < 0.05 is considered to be statistically significant.
5 Results
Fig. 1 depicts the UV spectroscopic pattern of biosynthesized AS-CuONPs. AS-CuONPs were subjected to spectroscopic analysis at varying spectra ranging from 300 to 800 nm. A Sharp absorption peak was observed at 403 nm which indicates the synthesis of copper oxide nanoparticles by the reducing agent Abies spectabilis plant extract.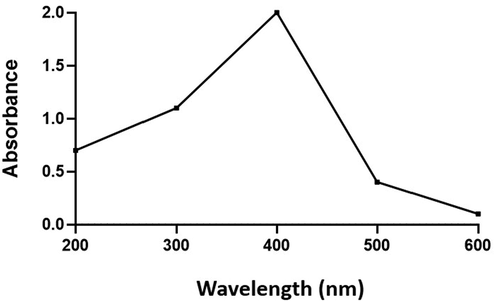
UV–Vis Spectroscopic pattern of copper oxide nanoparticles synthesized using Abies spectabilis plant extract as reducing agent.
The biosynthesized AS-CuONPs was further subjected to Fourier transform infra red spectroscopic analysis to determine functional groups of the nanoparticles synthesized. The FTIR spectrum of AS-CuONPs was illustrated in the Fig. 2 which shows maximum peak between the ranges of 900–3500 cm−1. Characteristic peaks were seen at 916, 1059 (C—O—C stretch), 1537 (C—O stretch), 2988 cm−1 and broad blunt peaks were observed at 619, 689, 1288, 1653 (C⚌O stretch), 3285 (OH stretch), 3708 cm−1 indicating the presence of various functional groups.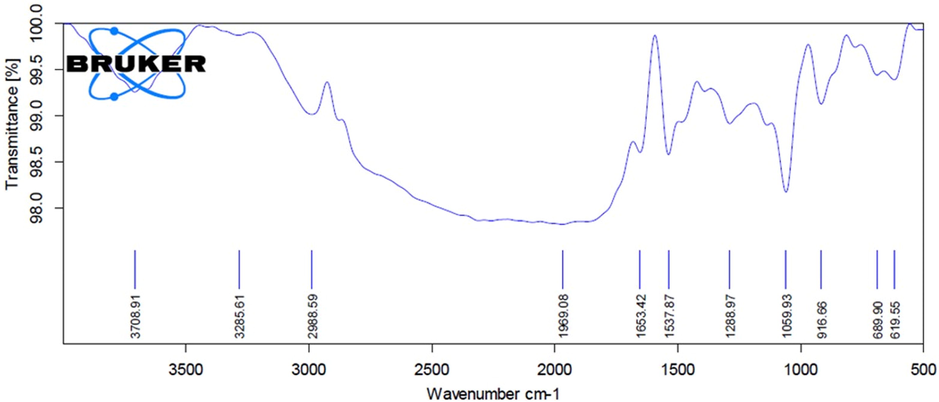
Fourier-transform infrared spectroscopy analysis of copper oxide nanoparticles synthesized using Abies spectabilis plant extract as reducing agent.
Fig. 3 represents the high resolution transmission electron microscopic images of biosynthesized AS-CuONPs. AS-CuONPs shown spherical morphology with a size of 5 nm and appears to be in clusters.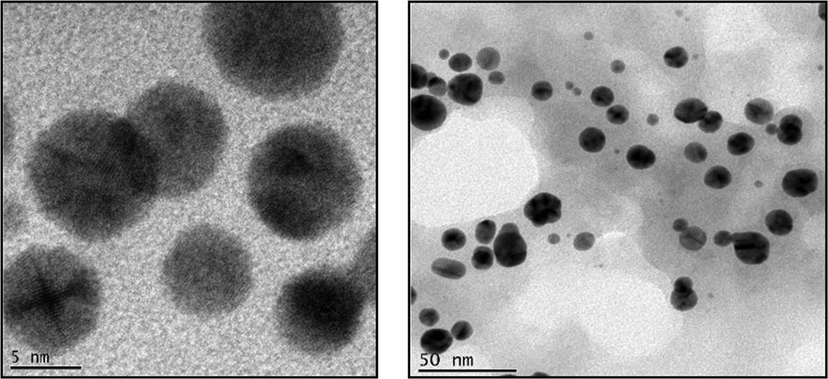
High Resolution Transmission electron microscopic images illustrating the copper oxide nanoparticles synthesized using Abies spectabilis plant extract as reducing agent. Scale bars: 5 nm and 50 nm.
The abdominal writhes performed by the AS-CuONPs treated and untreated mice in acetic acid induced writhing test were illustrated in the Fig. 4. The untreated control mice performed about 70 ± 2 writhes which significantly higher compared to the 5, 10 and 15 µg/kg AS-CuONPs treated mice which performed only about 49 ± 5, 36 ± 3, 31 ± 7 writhes. The standard drug diclofenac sodium treated mice shown comparatively decreased number of 22 ± 4 writhes compared to the control and AS-CuONPs treated mice.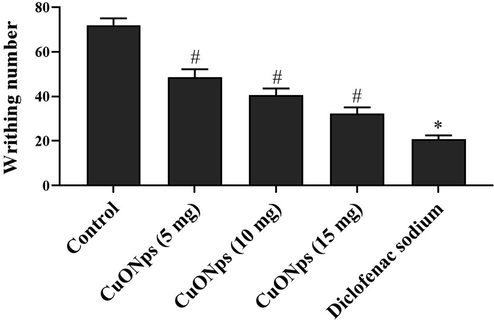
Effect of AS-CuONPs on acetic acid induced abdominal writhing. Mice were pre-treated with 5, 10, 15 µg/kg of AS-CuONPs and diclofenac sodium standard nociceptive drug used as positive control. Each group consists of six mice and the experiment was performed in triplicates. Data were statistically analyzed with One Way ANOVA followed by LSD test and the results were expressed as mean ± S.E.M. #p < 0.05, *p < 0.01 was considered to be statistically significant.
Fig. 5 depicts the number of licks performed by the control and AS-CuONPs treated mice in glutamate induced nociception analysis. AS-CuONPs treated mice shown significant reduction in the licking number when compared to the untreated mice. Untreated mice shown about 128 ± 9 number of licks whereas 5, 10 15 µg/kg AS-CuONPs treated mice shown 97 ± 12, 89 ± 7, 77 ± 6 number of licks respectively which is considerably decreased number. Standard drug diclofenac sodium treated mice shown 62 ± 9 licks which are comparatively equal to 15 µg/kg AS-CuONPs treated mice licking number.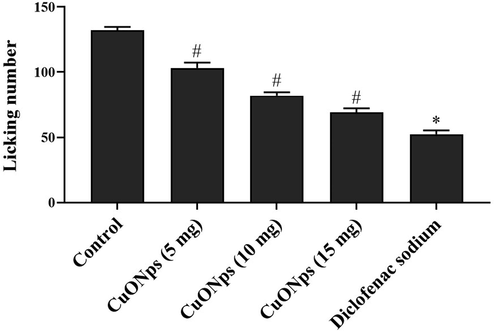
Effect of AS-CuONPs on Glutamate induced nociception. Mice were pre-treated with 5, 10, 15 µg/kg of AS-CuONPs and diclofenac sodium standard nociceptive drug used as positive control. Each group consists of six mice and the experiment was performed in triplicates. Data were statistically analyzed with One Way ANOVA followed by LSD test and the results were expressed as mean ± S.E.M. #p < 0.05, *p < 0.01 was considered to be statistically significant.
The effect of AS-CuONPs against neuropathic pain was assessed using irritant capsaicin and the results were depicted in the Fig. 6. AS-CuONPs treated mice shown significantly decreased number of licks in dose dependent manner compared to the untreated mice. 15 µg/kg AS-CuONPs treated mice shown significantly decreased number of 36 ± 5 licks compared to the untreated mice which shown 78 ± 9 licks whereas it is slightly increased than the diclofenac sodium treated mice which performed only 23 ± 5 licks.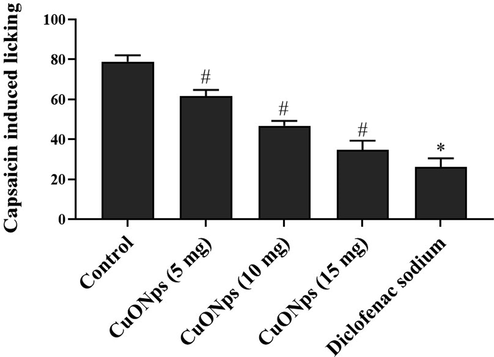
Effect of AS-CuONPs on Capsaicin-induced nociception. Mice were pre-treated with 5, 10, 15 µg/kg of AS-CuONPs and diclofenac sodium standard nociceptive drug used as positive control. Each group consists of six mice and the experiment was performed in triplicates. Data were statistically analyzed with One Way ANOVA followed by LSD test and the results were expressed as mean ± S.E.M. #p < 0.05, *p < 0.01 was considered to be statistically significant.
The antinociception property of AS-CuONPs was assessed in two phases using the chemical formalin and the results were depicted in Fig. 7. Irrespective of control and experimental mice all the mice performed increased number of licking during the second phase 16–30 min compared to the initial phase of 0–5 min compared. AS-CuONPs treated mice shown decreased number of licks in a dose dependent manner compared to the control. 15 µg/kg AS-CuONPs treated mice shown 46 ± 9 licks during initial phase which was comparatively equal to standard drug diclofenac sodium treated mice which shown 32 ± 5 licks. Whereas in second phase 15 µg/kg AS-CuONPs treated mice performed 67 ± 7 licks and diclofenac sodium treated mice performed 44 ± 8 licks.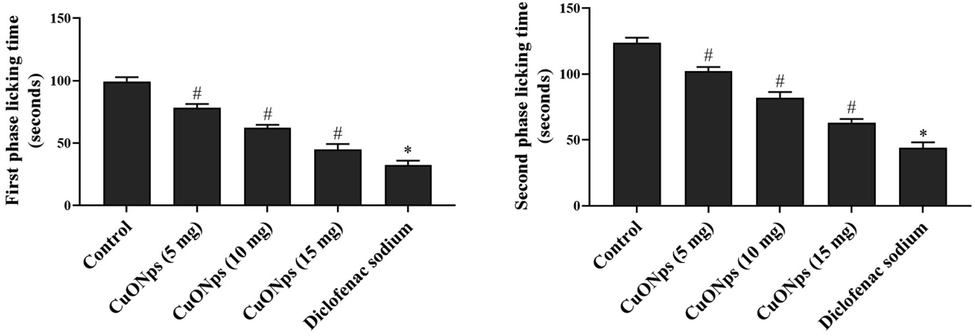
Effect of AS-CuONPs on Formalin induced nociception. Mice were pre-treated with 5, 10, 15 µg/kg of AS-CuONPs and diclofenac sodium standard nociceptive drug used as positive control. Each group consists of six mice and the experiment was performed in triplicates. Experiment was performed in two phases (A) initial phase (0–5 min) and (B) second phase (16–30 min). Data were statistically analyzed with One Way ANOVA followed by LSD test and the results were expressed as mean ± S.E.M. #p < 0.05, *p < 0.01 was considered to be statistically significant.
Table 1 illustrates the antinociceptive property of AS-CuONPs against thermal induced nociception in mice. AS-CuONPs treated shown significantly increased delay in response time compared to the untreated mice. The delay in response time increases with the dosage of AS-CuONPs treatment and also with the duration of incubation period. 15 µg/kg AS-CuONPs treated mice show maximum delay in response at 120 min compared to the 5 and 10 µg/kg AS-CuONPs treated mice. Even when treated with opioid antagonist nalaoxone the 15 µg/kg AS-CuONPs treated mice shown delay in response time of 11.66 ± 0.14 which was comparatively equal to standard morphine treated mice response time of 13.83 ± 0.77. Each group consists of six mice and the experiment was performed in triplicates. Data were statistically analyzed with One Way ANOVA followed by LSD test and the results were expressed as mean ± S.E.M. #p < 0.05, *p < 0.01 was considered to be statistically significant.
Treatment (mg/kg)
pre treatment
Response time(s)(%MPE)
30 min
60 min
90 min
120 min
Control
7.12 ± 0.26
7.25 ± 0.72
7.51 ± 0.54
7.93 ± 0.12
8.08 ± 0.37
CuNp (5 µg)
7.61 ± 0.14
9.33 ± 0.19 (13.25)
11.26 ± 0.20 (25.41) #
11.83 ± 0.73 (32.88) *
12.01 ± 0.61 (40.29) *
CuNp (10 µg)
7.39 ± 0.36
9.52 ± 0.42 (17.96)
11.43 ± 0.73 (33.41) #
12.27 ± 0.11 (44.30) *
12.63 ± 0.16 (48.57) *
CuNp (15 µg)
7.86 ± 0.41
10.51 ± 0.14 (22.40)
12.97 ± 0.54 (46.38) #
13.83 ± 0.96 (54.47) *
14.42 ± 1.10 (58.97) *
Morphine (5 mg)
7.22 ± 0.74
12.36 ± 0.35 (43.22)
14.87 ± 0.11 (55.36) #
15.97 ± 0.80 (61.33) *
17.23 ± 1.31 (69.41) *
NLX 2 mg) + Control
7.55 ± 0.01
7.63 ± 0.77
8.11 ± 0.43
8.41 ± 0.62
8.96 ± 0.71
NLX (2 mg) + CuNp (5 µg)
7.78 ± 0.11
8.02 ± 0.41 (9.82)
8.72 ± 0.69 (11.30) #
9.13 ± 0.33 (16.67) *
10.33 ± 0.71 (22.14) *
NLX(2 mg) + CuNp (10 µg)
7.33 ± 0.35
7.79 ± 0.74 (10.76)
8.69 ± 0.23 (14.42) #
9.41 ± 0.64 (19.36) *
10.27 ± 0.83 (26.41) *
NLX(2 mg) + CuNp (15 µg)
7.21 ± 0.58
7.96 ± 0.30 (11.66)
8.87 ± 0.65 (19.77) #
10.21 ± 0.86 (26.71) *
11.66 ± 0.14 (33.32) *
NLX(2 mg) + Morphine (5 mg)
7.53 ± 0.12
7.78 ± 0.36 (5.62)
9.22 ± 0.47 (13.51) #
10.36 ± 0.10 (19.96) *
13.83 ± 0.77 (36.44) *
Carrageenan induced paw edema test was performed in control and AS-CuONPs treated mice to assess the anti-inflammatory property of AS-CuONPs against the inflammatory reagent carrageenan. The maximum possible effect of AS-CuONPs was significantly increased during the initial one hour after the treatment whereas it decreased on second, third and fourth hour of post treatment. AS-CuONPs treated mice shown maximum possible effect which was comparatively equal to the mice treated with standard drug morphine (Table 2). Mice were pretreated with 5, 10, 15 µg/kg of AS-CuONPs and indomethacin standard nociceptive drug used as positive control. Each group consists of six mice and the experiment was performed in triplicates. Data were statistically analyzed with One Way ANOVA followed by LSD test and the results were expressed as mean ± S.E.M. #p < 0.05, *p < 0.01 was considered to be statistically significant.
Treatment (mg/kg)
Response time(s)(%MPE)
Basal
1st h
2nd h
3rd h
4th h
Control
25.65 ± 3.12
147.37 ± 13.96
134.86 ± 9.63
128.72 ± 7.27
119.41 ± 5.46
CuNp (5 µg)
28.53 ± 9.41
96.72 ± 7.24 (47.26%)
94.06 ± 5.77 (36.04%)#
91.86 ± 4.43 (29.31%)*
83.52 ± 6.41 (19.03%)*
CuNp (10 µg)
26.43 ± 4.86
93.48 ± 7.32 (43.64%)
90.72 ± 9.53 (41.72%)#
82.14 ± 5.33 (36.33%)*
79.65 ± 6.37 (33.79%)*
CuNp (15 µg)
29.32 ± 3.21
90.21 ± 5.34 (36.77%)
77.26 ± 4.72 (34.96%) #
65.43 ± 7.67 (31.87%)*
61.68 ± 4.15 (32.93%)*
Indomethacin (10 mg)
25.53 ± 4.72
77.26 ± 7.33 (42.11%)
75.92 ± 6.23 (40.76%)#
68.33 ± 8.82 (38.62%) *
62.73 ± 5.33 (33.48%)*
Fig. 8 depicts the number of leukocytes infiltrated in the peritoneal cavity of mice treated with AS-CuONPs along with inflammatory agent carageenan. All the three doses 5, 10 and 15 µg/kg of AS-CuONPs treated mice shown significantly decreased infiltration of leukocytes compared to the untreated control mice. 15 µg/kg of AS-CuONPs treated mice shown comparatively equal number of leukocytes infiltration as that of standard drug diclofenac sodium treated mice.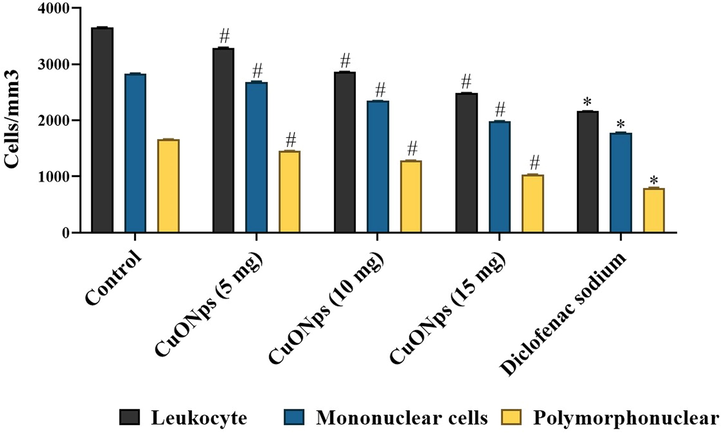
Effect of AS-CuONPs on leukocyte infiltration. Mice were pre-treated with 5, 10, 15 µg/kg of AS-CuONPs and diclofenac sodium standard nociceptive drug used as positive control. Each group consists of six mice and the experiment was performed in triplicates. Data were statistically analyzed with One Way ANOVA followed by LSD test and the results were expressed as mean ± S.E.M. #p < 0.05, *p < 0.01 was considered to be statistically significant.
The levels of inflammatory cytokines TNF-α, IL-1β and IL-6 were measured from the air pouch exudates of experimental mice and the results were represented in Fig. 9. AS-CuONPs treated mice shown significantly decreased levels of TNF-α, IL-1β and IL-6 in dose dependent manner compared to the carageenan alone treated mice. Even though standard drug dexamethasone treated mice drastically decreased levels of inflammatory cytokines compared to the carageenan alone treated mice the 15 µg/kg of AS-CuONPs treated mice also shown 50% reduction in levels of inflammatory cytokines.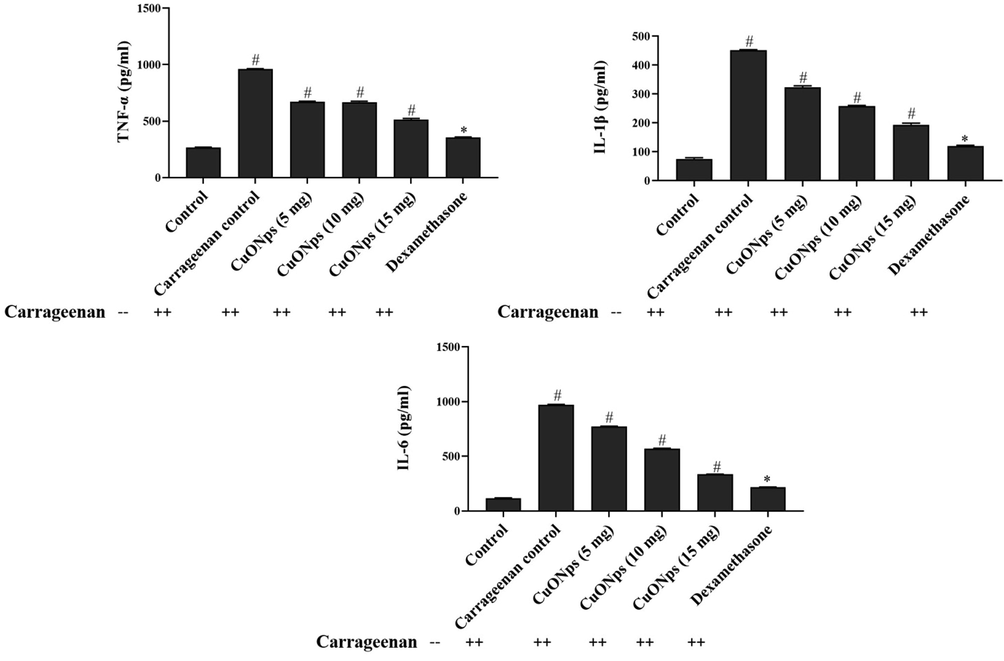
Effect of AS-CuONPs on inflammatory cytokines. Mice were pre-treated with 5, 10, 15 µg/kg of AS-CuONPs and diclofenac sodium standard nociceptive drug used as positive control. (A) TNF-α, (B) IL-1β and (C) IL-6. Each group consists of six mice and the experiment was performed in triplicates. Data were statistically analyzed with One Way ANOVA followed by LSD test and the results were expressed as mean ± S.E.M. #p < 0.05, *p < 0.01 was considered to be statistically significant.
The spontaneous locomotor action and exploratory behaviour of untreated and AS-CuONPs treated mice were assessed using open field apparatus. AS-CuONPs treated mice shown significantly decreased exploratory behaviour compared to the control mice. Whereas even the higher dosage 15 µg/kg of AS-CuONPs treated mice also shown increased exploratory behaviour compared to standard drug morphine treated mice (Fig. 10).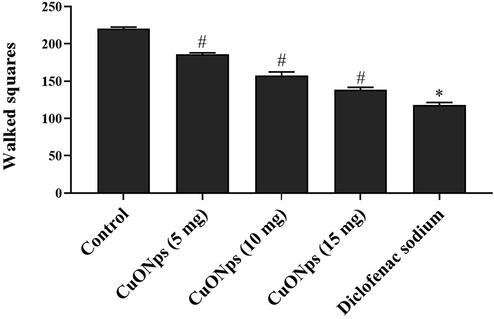
Effect of AS-CuONPs on Open Field analysis. Mice were pre-treated with 5, 10, 15 µg/kg of AS-CuONPs and morphine standard nociceptive drug used as positive control. Each group consists of six mice and the experiment was performed in triplicates. Data were statistically analyzed with One Way ANOVA followed by LSD test and the results were expressed as mean ± S.E.M. #p < 0.05, *p < 0.01 was considered to be statistically significant.
6 Discussion
Nanomedicine is a blooming interdisciplinary field which utilizes nanomaterials to diagnose and treat diseases (Sheikholeslami et al., 2020; Tinkle et al., 2014). Nanomedicine increases the efficacy of drug, enhances the bioavailability, reduces the toxicity and also increases the distribution of drug (Pugazhenthi et al., 2018; Sathiyavimal et al., 2020). Nanotechnology pioneers the invention of potent alternative medicine to treat various deadly diseases (Li et al., 2020; Pita et al., 2016). Nanoparticles were synthesized using physical, chemical and biological methods compared to physiochemical methods the biologically synthesized nanoparticles were widely used in medical field (Lee and Jun 2019; Jerome et al., 2020). Metal nanomaterials were synthesized from metallic salts using the plant extract, microorganisms, fungi, marine algae, and fruits as reducing agent (Hamouda et al., 2019; Samuel et al., 2020). Nanomaterials synthesized using plant extract had drawn greater attention since the phytochemicals influences the physiochemical and biological properties of nanomaterials. Earlier reports have confirmed the green synthesis of nanomaterials with plant extracts is cost effective, simple and non toxic (Yu et al., 2019; Sheikholeslami et al., 2019). Therefore in the present study, the copper oxide nanoparticles from the Abies spectabilis were synthesized and characterized using different methods.
Nanoformulations should possess certain physiochemical properties such as ideal size, surface area, particle size distribution to be utilized as nanodrug (Commission Recommendation, 2011; Bleeker et al., 2013; Boverhof et al., 2015). Conventional nanodrug size should be at range of 1–100 nm since minimal sized nanoparticles between 20 and 50 nm are cleared rapidly through renewal excretion whereas the larger sized nanomaterials above 200 nm are engulfed by phagocytic cells (Burlacu et al., 2019; Adabi et al., 2017; Louro, 2018). In our study the copper oxide nanoparticles synthesized using Abies spectabilis plant extract were of optimal size of 50 nm which is ideal for usage as a nanodrug. The UV visible spectroscopic analysis confirms revealed that the maximum absorption peak was noted at 403 nm that proves the bioreduction of copper oxide nanoparticles by the reducing agent Abies spectabilis plant extract. TEM images of AS-CuONPs are spherical in shape with the size of 50 nm. FTIR analysis was performed to analyze the functional groups present in AS-CuONPs, which identifies the biomolecules with the energy absorption. Characteristic peaks of copper oxide nanoparticles were observed in FTIR spectra of AS-CuONPs that indicates the presence various functional groups in the formulated AS-CuONPs. The findings of the synthesis and characterization of copper oxide nanoparticles from the Abies spectabilis plant extract was supported by the many other previous reports (Varghese et al., 2020; Sackey et al., 2020; Zou et al., 2020).
Green synthesized AS-CuONPs was further assessed for its antinociceptive and anti-inflammatory properties using various mice models. The antinociceptive property of AS-CuONPs was analyzed with acetic acid induced writhing method, glutamate induced nociception, and capsaicin induced nociception, formalin test and hot plate method. Acetic acid induced writhing test was performed to assess the potency of AS-CuONPs inhibiting peripheral pain (Zhang et al., 2015). Acetic acid stimulates the production of inflammatory cytokines, interleukins prostaglandins thereby induces abdominal writhes in mice (Murugan & Parimelazhagan, 2013). In the present study AS-CuONPs effectively decreased the writhes compared to the control mice which indicate AS-CuONPs suppresses peripheral pain in mice model.
Various studies have reported elevated levels excitatory neurotransmitter glutamate in damage tissues. Synovial fluid of arthritis patients shown increased glutamate levels compared to normal individuals (Alfredson and Lorentzon 2002; Carlton et al. 2003). Inflammed tissues induce the release of vesicular glutamate in neuronal terminals which provokes pain (Wen et al., 2015; deGroot et al. 2000). Our AS-CuONPs treated mice shown decreased number of licks after glutamate injection compared to control which shows AS-CuONPs significantly decreases the levels of glutamate thereby suppresses pain in mice.
Non-Reflexive Pain Tests were performed with capsaicin and formalin induced nociception test. The spontaneous pain behavior of mice was assessed with capsaicin and mustard oil model test (Deuis et al., 2017). Capsaicin, irritant activates the TRPV1 containing nociceptors thereby inducing local neurogenic inflammation leading to pain (Illie et al., 2019; Chu et al., 2020). Formalin induced nociception model elucidates the analgesic effect of drug in two phases initial phase which last of first minutes is neurogenic phase where pain occurs due to stimulation of nerve terminals. Whereas second phase pain occurs due to the release of bradykinin, inflammatory cytokines and prostaglandins (Hong et al., 2020; Le Bars et al., 2001). AS-CuONPs treatment significantly decreased the pain induced licking in both capsaicin induced nociception and formalin induced nociception model. In formalin induced model AS-CuONPs effectively decreased the licking time in both the phases which confirms AS-CuONPs efficiently suppresses pain during both neurogenic and inflammatory phases. These findings were coincides with the previous findings done by Mahmoudvand et al. (2020).
Further the hot plate test was performed to analyze the potency of AS-CuONPs against the supraspinal response induced due to the thermal stimuli (Le Bars et al., 2001). AS-CuONPs shown increased response time even in the presence of opioid antagonist nalaoxone compared to the control mice. AS-CuONPs shown remarkable analgesic effect in various nociceptive mice model therefore the anti-inflammatory potency of AS-CuONPs was analyzed. This result was supported by the previous research work done on antianalgesic activity of gold nanoparticles in animal model by Araujo Junior et al. (2017).
Anti-inflammatory property of AS-CuONPs was assessed with carrageenan induced paw edema test, peritoneal leukocyte infiltration test and carrageenan induced air pouch test. Carrageenan induced paw edema test was widely used test to assess the anti-inflammatory response of drug in three phases which involves the suppression of histamine, serotonin in first phase and bradykinin, prostaglandins respectively in second and third phases of inflammation (Zazquez et al., 2015). AS-CuONPs effectively decreased the size of paw edema which proves AS-CuONPs inhibited the synthesis of histamine, serotonin, prostaglandins thereby decreased the vascular permeability and vascular response against inflammation.
Peritoneal leukocyte infiltration test was performed to assess the infiltration of leukocytes which is the key event occurs during the acute phase of inflammation. Prostaglandins, interleukins, nitric oxide, tumor necrosis factor (TNF-α) are the inducers of inflammation, carrageenan increases the production of these inflammatory inducers (Mansouri et al., 2015). AS-CuONPs significantly decreased the infiltration of leukocytes and also inhibited the synthesis of IL-1β, IL-6 and TNF-α. Our results of various nociceptive analysis and inflammatory test confirmed the analgesic and anti-inflammatory property of AS-CuONPs, which may be due to the bioactive compounds limonene, α-pinene, bornyl acetate, β-phellandrene, camphene, β-pinene and terpenoids present in Abies spectabilis extract which reduced copper sulfate salt into copper oxide nanoparticles.
The behavior impact of AS-CuONPs treatment on mice was assessed with open field analysis. Even the high dose 15 µg/kg AS-CuONPs treated mice shown increased exploratory behavior than the standard drug morphine treated mice which confirm AS-CuONPs doesn’t imparts any behavioral changes in mice.
7 Conclusion
The findings of this proved that the Abies spectabilis plant is a good source of metallic copper oxide nanoparticles, which is evidenced by our results of various characterization techniques. Further, the copper oxide nanoparticles from the Abies spectabilis (AS-CuONPs) potentially ameliorates the various stimuli induced nociception and inflammation in mice. The findings of the various nociceptive mice model authentically proved that the AS-CuONPs inhibited both central and peripheral nociception. AS-CuONPs treatment also attenuates the carrageenan induced paw edema, peritoneal leukocyte infiltration that confirms the inhibitory action of AS-CuONPs against carrageenan induced inflammation. These findings were proved that the AS-CuONPs can be a promising therapeutic agent to treat the nociception and inflammatory ailments. However, further studies still needed in future to understand the exact therapeutic mechanisms of AS-CuONPs against inflammatory diseases.
Acknowledgments
The authors extend their appreciation to the Researchers supporting project number (RSP-2020/173) King Saud University, Riyadh, Saudi Arabia.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Biocompatibility and nanostructured materials: applications in nanomedicine. Artif. Cells Nanomed. Biotechnol.. 2017;45(4):833-842.
- [Google Scholar]
- Chronic tendon pain: no signs of chemical inflammation but high concentrations of the neurotransmitter glutamate. Implications for treatment? Curr. Drug Targets.. 2002;3:43-54.
- [Google Scholar]
- Anti-inflammatory, analgesic and anti-tumor properties of gold nanoparticles. Pharmacol. Rep.. 2017;69(1):119-129.
- [Google Scholar]
- Mechanisms underlying the nociception and paw oedema caused by injection of glutamate into the mouse paw. Brain Res.. 2002;924(2):219-228.
- [Google Scholar]
- Considerations on the EU definition of a nanomaterial: science to support policy making. Regul. Toxicol. Pharm.. 2013;65(1):119-125.
- [Google Scholar]
- Comparative assessment of nanomaterial definitions and safety evaluation considerations. Regul. Toxicol. Pharm.. 2015;73(1):137-150.
- [Google Scholar]
- Variability in the response to non-steroidal anti-inflammatory drugs: mechanisms and perspectives. Basic Clin. Pharmacol.. 2014;114:56-63.
- [Google Scholar]
- A review of bark-extract-mediated green synthesis of metallic nanoparticles and their applications. Molecules. 2019;24(23):4354.
- [Google Scholar]
- Peripheral glutamate receptors: novel targets for analgesics? In: Dostrovsky J.O., Carr D.B., Koltzenburg M., eds. Proceedings of the 10th world congress on pain, progress in pain research and management. Vol 24. Seattle: IASP Press; 2003. p. :125-139.
- [Google Scholar]
- A single TRPV1 amino acid controls species sensitivity to capsaicin. Sci. Rep.. 2020;10:8038.
- [Google Scholar]
- Antinociceptive and anti-inflammatory effect of the citrus flavanone naringenin. Ci Ji Yi Xue Za Zhi.. 2019;31(2):81-85.
- [Google Scholar]
- Commission Recommendation, 2011. Commission Recommendation of 18 October 2011 on the definition of nanomaterial 2011/696/EU. Off. J. Eur. Union L. 275, 38–40.
- Synthesis and optical properties of colloidal CuO nanoparticles. J. Lumin.. 2014;151:149-154.
- [Google Scholar]
- Peripheral glutamate release in the hindpaw following low and high intensity sciatic stimulation. Neuro Rep.. 2000;11:497-502.
- [Google Scholar]
- Methods used to evaluate pain behaviors in rodents. Front. Mol. Neurosci. 2017
- [CrossRef] [Google Scholar]
- Evaluation of antioxidant and anticorrosion activities of Ligularia fischeri plant extract. Chem. Sci. Eng. Res.. 2019;1(1):16-24.
- [Google Scholar]
- Synthesis and biological characterization of silver nanoparticles derived from the cyanobacterium Oscillatoria limnetica. Sci. Rep.. 2019;9:13071.
- [Google Scholar]
- Antinociceptive effect of chrysin in diabetic neuropathy and formalin-induced pain models. Animal Cell Syst.. 2020;24(3):143-150.
- [Google Scholar]
- IASP Taxonomy, 2017. (Accessed 02 February 2018), http://www.iasp-pain.org/.
- Capsaicin: physicochemical properties, cutaneous reactions and potential applications in painful and inflammatory conditions. Exp. Ther. Med.. 2019;18(2):916-925.
- [Google Scholar]
- Controlled green synthesis of polymer functionalized zinc oxide nanoparticles. Green Rep.. 2020;1(1):16-20.
- [Google Scholar]
- Use of herbal preparations in the treatment of oxidant-mediated inflammatory disorders. Complement Ther. Med.. 2007;15(3):207-216.
- [Google Scholar]
- Synthesis and antibacterial activity of CuO nanoparticles suspension induced by laser ablation in liquid. Arab. J. Sci. Eng.. 2016;41:301-310.
- [Google Scholar]
- Development of anti-Inflammatory drugs- the research and development process. Basic Clin. Pharmacol. Toxicol.. 2014;114:7-12.
- [Google Scholar]
- Silver nanoparticles: synthesis and application for nanomedicine. Int. J. Mol. Sci.. 2019;20(4):856.
- [Google Scholar]
- Transient pool boiling and particulate deposition of copper oxide nano-suspensions. Int. J. Heat Mass Transf.. 2020;155:119743
- [Google Scholar]
- Pool boiling heat transfer to CuO-H2O nanofluid on finned surfaces. Int. J. Heat Mass Transfer.. 2020;156:119780
- [Google Scholar]
- Relevance of physicochemical characterization of nanomaterials for understanding nano-cellular interactions. In: Saquib Q., Faisal M., Al-Khedhairy A., Alatar A., eds. Cellular and Molecular Toxicology of Nanoparticles. Advances in Experimental Medicine and Biology. Vol Vol. 1048. Cham: Springer; 2018. p. :123-142.
- [Google Scholar]
- Antinociceptive action of ethanolic extract obtained from roots of Humirianthera ampla Miers. J. Ethnopharmacol.. 2007;114(3):355-363.
- [Google Scholar]
- Antinocicpetive effects of green synthesized copper nanoparticles alone or in combination with morphine. Ann. Med. Surg. (Lond).. 2020;51:31-36.
- [Google Scholar]
- An overview of biological, phytochemical, and pharmacological values of Abies pindrow. JPP. 2013;2(3):182-187.
- [Google Scholar]
- A study of the mechanisms underlying the anti-inflammatory effect of ellagic acid in carrageenan-induced paw edema in rats. Indian J Pharmacol.. 2015;47(3):292-298.
- [Google Scholar]
- What is the role of nanotechnology in diagnosis and treatment of metastatic breast cancer? Promising scenarios for the near future. J Nanomater. 20165436458
- [Google Scholar]
- Antinociceptive and anti-inflammatory activities of Teucrium persicum boiss. extract in mice. Scientifica (Cairo).. 2015;2015:972827
- [Google Scholar]
- Study of anti-nociceptive, anti-inflammatory properties and phytochemical profiles of Osbeckia parvifolia Arn. (Melastomataceae) Ind. Crops Prod.. 2013;51:360-369.
- [Google Scholar]
- An evidence-based update on nonsteroidal anti-inflammatory drugs. Clin. Med. Res.. 2007;5(1):19-34.
- [Google Scholar]
- Antinociceptive effects of the aqueous extract of Brugmansia suaveolens flowers in mice. Biol. Res. Nurs.. 2007;8(3):234-239.
- [Google Scholar]
- Synthesis of silver nanoparticles in photosynthetic plants. J. Nanopart. 2014963961
- [Google Scholar]
- Engineering tailored nanoparticles with microbes: quo vadis. WIREs Nanomed. Nanobiotechnol.. 2016;8:316-330.
- [Google Scholar]
- Inorganic nanoparticles: a potential cancer therapy for human welfare. Int. J. Pharm.. 2018;539(1–2):104-111.
- [Google Scholar]
- Pugazhenthi, A., Kumar, S.S., Manikandan, M., Saravanan, M., 2018. Photocatalytic properties and antimicrobial efficacy of Fe doped CuO nanoparticles against the pathogenic bacteria and fungi. 122, 84–89.
- Assessment of antioxidant, anticholinesterase and antimyloidogenic 7-Methyl gallic acid-an in vitro and in silico studies. J. Mol. Liquid.. 2018;257:69-81.
- [Google Scholar]
- Electrochemical properties of Euphorbia pulcherrima mediated copper oxide nanoparticles. Mater. Chem. Phys.. 2020;244(1):122714
- [Google Scholar]
- Synthesis and characterization of mono sized CuO nanoparticles. Mater. Lett.. 2012;8:169-172.
- [Google Scholar]
- Immobilization of Cu3(btc)2 on grapheme oxide-chitosan hybrid composite for the adsorption and photocatalytic degradation of methylene blue. J. Photochem. Photobiol. B: Biol.. 2020;204:111809
- [Google Scholar]
- Photocatalytic activity of CuO/Cu(OH)2 nanostructures in the degradation of reactive green 19A and textile effluent, phototoxicity studies and their biogenic properties (antibacterial and anticancer) J. Environ. Manage.. 2018;223:1086-1097.
- [Google Scholar]
- Green synthesized copper oxide nanoparticles ameliorate defence and antioxidant enzymes in lens culinaris. Nanomaterials (Basel).. 2020;10(2)
- [Google Scholar]
- Biogenesis of copper oxide nanoparticles (CuONPs) using Sida acuta and their incorporation over cotton fabrics to prevent the pathogenecity of gram negative and gram positive bacteria. J. Photochem. Photobiol. B: Biol.. 2018;188:126-134.
- [Google Scholar]
- Eco-biocompatibility of chitosan coated biosynthesized copper oxide nanocomposite for enhanced industrial (Azo) dye removal from aqueous solution and antibacterial properties. Carbo. Polym.. 2020;241:116243
- [Google Scholar]
- Sheikholeslami, M., Haq, R., Shafee, A., Li, Z., Elaraki, Y.G., Tlili, I., 2019. Heat transfer simulation of heat storage unit with nanoparticles and fins through a heat exchanger. 135, 470–478.
- Sheikholeslami, M., Jafaryar, M., Abohamzeh, E., Shafee, A., Babazadeh, H., 2020. Sustainable energy technologies and assessments. 39, 100727.
- Acceleration of discharge process of clean energy storage unit with insertion of porous foam considering nanoparticle enhanced paraffin. J. Clean. Prod.. 2020;261:121206
- [Google Scholar]
- Mechanisms of the inflammatory response. Best Pract. Res. Clin. Anaesthesiol.. 2004;18:385-405.
- [Google Scholar]
- Nanomedicine: principles, properties, and regulatory issues. Front. Chem.. 2018;6:360.
- [Google Scholar]
- Gastrointestinal symptoms in NSAID users in an 'average risk population': results of a large population-based study in randomly selected Dutch inhabitants. Int. J. Clin. Pract.. 2014;68(4):512-519.
- [Google Scholar]
- Nanomedicines: addressing the scientific and regulatory gap. Ann. N. Y. Acad. Sci.. 2014;1313:35-56.
- [Google Scholar]
- Biochemical synthesis of copper nanoparticles using Zingiber officinalis and Curcuma longa: characterization and antibacterial activity study. Mater. Today: Proc.. 2020;25(2):302-306.
- [Google Scholar]
- Synthesis of ecofriendly copper oxide nanoparticles for fabrication over textile fabrics: characterization of antibacterial activiry and dye degradation potential. J. Photochem. Photobiol. B: Biol.. 2019;191:143-149.
- [Google Scholar]
- Green synthesis of silver nanoparticles for antimicrobial activity. Green Rep.. 2020;1(1):8-15.
- [Google Scholar]
- Green nanotechnology: advancement in phytoformulation research. Medicines (Basel).. 2019;6(1):E39.
- [Google Scholar]
- Biphasic development of carrageenan oedema in rats. J. Pharmacol. Exp. Ther.. 1969;166:96-103.
- [Google Scholar]
- Evaluation of antiinflammatory and analgesic activities of alcoholic extract of Kaempferia galanga in rats. Indian J. Physiol. Pharmacol.. 2011;55(1):13-24.
- [Google Scholar]
- Antinociceptive and anti-inflammatory activities of extract and two isolated flavonoids of Carthamus tinctorius L. J. Ethnopharmacol.. 2014;151:944-950.
- [Google Scholar]
- Excitatory amino acid glutamate: role in peripheral nociceptive transduction and inflammation in experimental and clinical osteoarthritis. Osteoarthritis Cartilage.. 2015;23:2009-2016.
- [Google Scholar]
- Systematic phytochemical investigation of Abies spectabilis. Chem. Pharm. Bull. (Tokyo).. 2010;58(12):1646-1649.
- [Google Scholar]
- Synthesis and characterization of gold nanoparticles from Abies spectabilis extract and its anticancer activity on bladder cancer T24 cells. Artif. Cells Nanomed. Biotechnol.. 2019;47(1):512-523.
- [Google Scholar]
- Green biosynthesis of silver nanoparticles using Eriobotrya japonica (Thunb.) leaf extract for reductive catalysis. Materials (Basel). 2019;12(1):189.
- [Google Scholar]
- Systemic changes following carrageenan-induced paw inflammation in rats. Inflamm. Res.. 2015;64(5):333-342.
- [Google Scholar]
- CuO nanostructures: Synthesis, characterization, growth mechanisms, fundamental properties, and applications. Prog. Mater. Sci.. 2014;60:208-337.
- [Google Scholar]
- Anti-inflammatory and antinociceptive activities of non-alkaloids fractions from Aconitum flavum in vivo. Rev. Bras. Farmacogn.. 2015;25(1):47-52.
- [Google Scholar]
- Bio-mediated synthesis of copper oxide nanoparticles using Pogestemon benghalensis extract for treatment of the esophageal cancer in nursing care. J. Drug Delivery Sci. Tech.. 2020;58:101759
- [Google Scholar]







