Translate this page into:
Biosynthesis of Nickel oxide Nanoparticles from Euphorbia heterophylla (L.) and their biological application
⁎Corresponding author. rajanaika@tumkuruniversity.ac.in (H. Raja Naika)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract

Abstract
Nickel oxide Nanoparticles were prepared using ecofriendly bio synthesis route. Characterization of biosynthesized NiO NPs was confirmed by XRD, UV-DRS, FT-IR, SEM-EDAX and TEM techniques. Biosynthesized NiO NPs exhibiting Direct haemolytic and Anticoagulant properties. Biosynthesized NiO NPs shows significant antibacterial and cytotoxicity properties. Biosynthesis of Nickel oxide Nanoparticles from Euphorbia heterophylla (L.) and their biological application.
Abstract
Nickel oxide Nanoparticles (NiO NPs) were synthesized from E. heterophylla (L.) leaves extract act as reducing/capping agent by biosynthesis process. Further the synthesized NiO NPs was subjected for structural, optical and biological properties. The XRD pattern of NiO NPS exhibit face centred cubic (FCC) crystalline structure. The UV-DR spectrum of biosynthesized NiO NPs exhibited optical properties with well-defined at 321 nm and its exhibits optical band gap is 3.24 eV. The FT-IR spectrum of NiO NPs shows stretching vibration of Ni-O at 452 cm−1. The morphological features of NiO NPs are rhombohedra and slightly agglomerated and then size of the biosynthesized NiO NPs as found in the range of 12–15 nm. The NiO NPs shows vital non-toxic properties on human erythrocytes and its interference in activity coagulation cascade both on PRP and PPP on human blood. The Bactericidal activity of NiO NPs was shows significant inhibitory activity against pathogenic bacterial strains. Further, NiO NPs show significant cytotoxicity against human lung cancer cell line (A549) and human hepatocarcinoma (HepG2) cell lines. Therefore, the study reveals states that, the E. heterophylla (L.) leaves extract is an effective reducing/capping agent for the formation of NiO NPs and its exhibits biological properties.
Keywords
Biosynthesis
NiO NPs
Characterization
Biological applications
1 Introduction
In current years, among metal oxide nanoparticles have great attracted increasing attention due to the presence of different applications with highly remarkable with large surface area, band gap stability and reusability (Kaviyarasu et al., 2016; Sun et al., 2012; Hisatomi et al., 2014; Nandy et al., 2013; Pereira et al., 2014; Hoffmann et al., 1995; Ezhilarasi et al., 2018) Nickel oxide Nanoparticles (NiO NPs) has fascinated greater attention due to their different flexible properties (Singh et al., 2016; Jahromi et al., 2015). Different methods have been used to synthesize of Nickel oxide Nanoparticles (NiO NPs) such as the sol–gel, solvothermal, hydrothermal, thermal decomposition, precipitation (Sankar et al., 2016; Qing et al., 2015; Motevalli et al., 2016; Nassar et al., 2017; Gnanasekaran et al., 2017). Most of these methods are highly expensive, tedious protocol, highly sophisticated instruments, toxic, non-eco-friendly for synthesis of Nanoparticles (Mohammadijoo et al., 2014). To overcome the problem of toxic wastes and energy imbalance, biocompatibility, greener and ecofriendly process for the synthesis of nanoparticles been suggested (Madhumitha et al., 2016). Towards, the biocompatibility synthesis of NiO NPs from plants such as Moringa oleifera (Ezhilarasi et al., 2016), Tamarix serotina (Nasseri et al., 2016) Callistemon viminalis (Sone et al., 2016), Aegle marmelos (Ezhilarasi et al., 2018) and Agathosma betulina (Thema et al., 2016) contains different bioactive chemicals with various functional groups its exhibiting different applications (Roopan and Khan, 2011; Manivel et al., 2009). During the biocompatible metal oxide nanoparticles metabolites play a crucial role in converting metal into metal nano form by reduction mechanism (Zhi-Guo et al., 2011).
In the present study, a complete biocompatibility assisted synthesis of Nickel oxide Nanoparticles has been reported by using the aqueous leaves extract of medicinal plant Euphorbia heterophylla (Linn.). Euphorbia heterophylla (Linn.) belongs to the Euphorbiaceae family. The medicinal value of E. heterophylla is well documented and it is mostly used in the treatment of various disorders (Falodun and Agbakwuru, 2004; Uduak et al., 2010; Keerthana et al., 2014; Falodun et al., 2006; Meenakshi Sundaram et al., 2010). Biosynthesis of Nickel oxide Nanoparticles (NiO NPs) has been successfully demonstrated in recent years and therefore there is a growing interest in NiO nanoparticles synthesis via biosynthesis process. Further, the bio synthesized NiO NPs was confirmed by using various techniques such as XRD, UV-DRS, FT-IR, SEM-EDX and TEM techniques. Furthermore, the biosynthesized Nickel oxide Nanoparticles (NiO NPs) from E. heterophylla (L.) was investigated by various biological properties including such as direct haemolytic and Anticoagulant properties, antibacterial and cytotoxicity.
2 Experimental
2.1 Chemicals
All the chemicals were purchased from Sigma Aldrich, SD-Fine and Himedia Laboratory Pvt. Ltd., India with analytical grade without further purification. All the aqueous solutions were prepared by using double distilled water throughout the experiments.
2.2 Preparation of extract and synthesis of NiO NPs
The preparation of extract from E. heterophylla (L.) leaves in aqueous medium by soxhlet extraction apparatus (Raja Naika and Krishna, 2006). In a typical experiment, 10 mL of aqueous leaves extract of E. heterophylla (1 mg/mL) and 90 mL of Nickel Nitrate hexahydrate (Ni (NO3)2·6H2O) (0.1 mM) were taken in a round bottomed flask. The mixture was then stirred vigorously with the help of magnetic stirrer at room temperature for 24 h. After stirring, the mixture was kept in dark condition to observe the colour and then bottom of the flask precipitate was formed. Then after, to collect the precipitate from bottom of the flask with the help of centrifuge at 10000 rpm for 30 min. After centrifugation, the clear supernatant was discarding and collects the pellet. The obtained pellet is again suspended in de-ionized distilled water and washed with several times to remove impurities for 2–3 times. Then after washing, the pellet was dried and calcined at 300 °C for 3 h. After calcination process, the product was crushed in to fine powder using mortar and pestle and then finally greyish black coloured product was obtained and kept it air tight container for further analysis.
2.3 Characterization
The phase purity and crystallinity of the biosynthesized NiO NPs was subjected to X-ray diffraction employing Shimadzu Powder X-ray diffractometer (PXRD) (CuKα, λ = 1.54 A°) radiation with nickel filter. The Ultra Violet Diffuse reflectance spectrum was recorded using Perkin Elmer Lambda-35 Spectrophotometer. The functional groups analysis of biosynthesized NiO NPs was using Perkin Elmer Spectrum BXFT-IR system. The morphological features of biosynthesized NiO NPs was studied by scanning electron microscopy (SEM, Hitachi – 3000), EDAX analysis (Nova Nano SEM 600, FEI Company) and transmission electron microscopy (TEM) (Jeol tem3010) fitted with a Gatan CCD camera operating at an accelerating voltage of 300 kV.
2.4 Direct haemolytic and Anticoagulant properties
2.4.1 Direct haemolytic activity
The direct haemolytic activity of NiO NPs (40, 60, 80 and 100 μg) was determined by using human erythrocytes (Chethana et al., 2017). The haemolytic activity was expressed as percentage of haemolysis against percentage of lysis of cells due to presence of water (positive control) and phosphate buffered saline (negative control).
2.4.2 Anticoagulant activity
2.4.2.1 Preparation of platelet-rich plasma and platelet-poor plasma
The preparation of human platelet-rich plasma (PRP) and Platelet-poor plasma (PPP) was followed method (Ardlie and Han, 1974). The platelet concentration of PRP was adjusted to 3.1 × 108 platelets/ml with PPP. Above content was maintained at 37 °C for 2 h due to the process of blood coagulation. All the above preparations were carried out using plastic wares or silicon zed glass wares.
2.4.2.2 Plasma recalcification time
The plasma recalcification time was determined was followed by Quick et al., method (Quick et al., 1935). In a briefly, the various concentration of biosynthesized NiO NPs (0–100 μg) was pre-incubated with 0.2 mL of citrated human plasma in the presence of 10 mM Tris HCl (20 μl) buffer pH 7.4 for 1 min at 37 °C. Then after, the addition of 20 μl of 0.25 M CaCl2 was pre incubated mixture and clotting time was recorded.
2.5 Antibacterial activity studies
The antibacterial activity of biosynthesized NiO NPs against pathogenic bacterial strains both Gram+ve Staphylococcus aureus (NCIM-5022) and Gram–ve bacteria Escherichia coli [NCIM-5051], Pseudomonas desmolyticum [NCIM-2028] and Klebsiella aerogenes [NCIM-2098] strains (Purchased from NCL Pune, India) by Agar well diffusion method (Manjunath et al., 2014). Nutrient agar plates were prepared by using 37.0 g of Nutrient agar media was dissolved in 1000 mL of distilled water, and then subject to the sterilized by autoclaved at (121 °C) 15 lbs pressure for 15–20 min. After sterilization, NA medium was poured into sterile petri-dishes and allowed to solidify then after, 100 µl of 24 h mature broth culture of individual pathogenic bacterial strains in nutrient broth while spreading all over the surface of agar plates using sterilized L-shaped glass rod. Thereafter, using the sterile steel cork borer 06 mm wells were made into the each petriplate under aseptic condition. Then after, different concentration of NiO NPs (200,400 and 600 µg/well) was dispersed in 10% DMSO solution and while standard antibiotic Ciprofloxacin used as a positive control and control in to the wells. The plates were incubated at 37 °C for 24 h. After the incubation period, to observe the Zone of inhibition around the wells was measured by using geometrical Vernier callipers in mm. The antibacterial bacterial activity was carried out in triplicate and then determining the bactericidal activity of NiO NPs.
2.6 Cytotoxicity of NiO NPs
The cytotoxicity of biosynthesized NiO NPs against human Lung cancer (A549) and Human Hepatocarcinoma (HepG2) cell line (National Centre for Cell Science, Pune, India) (Dhaneswar et al., 2013; Lingaraju et al., 2019; Ibraheem et al., 2019). The growing of cell lines in the presence of following Dulbecco's Modified Eagle's Medium (DMEM) with supplemented fetal bovine serum with antibiotics maintained at 37 °C in a humidified atmosphere of 5% CO2 for 24 h. The cells were seeded in 96 well plates at a density 25 × 103 cells/well. In the presence of MTT assay, the cytotoxicity of different concentration of biosynthesized NiO NPs (25, 50, 100, 200 and 400 µg/mL) against human cancer cell lines. MTT is a dye (Yellow in colour) which is reduced in to purple colour formazon crystals in the presence of activity shows in mitochondrial succinate dehydrogenase enzyme in viable cells. The statistical analysis of cytotoxicity of biosynthesized NiO NPs against cancer cell lines in the presence of MTT to viability of cell was evaluated the IC50 value was determined.
3 Results and discussion
3.1 Characterization
The PXRD pattern of biosynthesized NiO NPs from E. heterophylla as shown in Fig. 1. All the peaks at 37.24, 43.29, 62.85, 75.38, 79.37 assign to the planes were indexed with (1 1 1), (2 0 0), (2 2 0), (3 1 1) and (2 2 2) respectively well matched with JCPDS Card no.04–0835 with FCC (Face centered cubic) crystalline Bunsenite structure of NiO NPs. No impurities were observed which suggests the high purity of monophasic NiO nanoparticles. The average crystallite size of NiO NPs is found to be 15 nm in size was calculated using the Debye–Scherrer formula (Suresh et al., 2015).
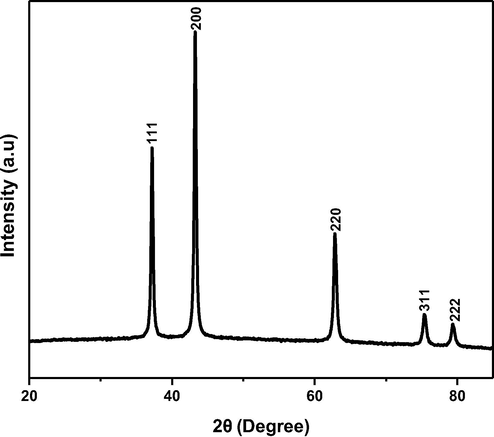
Show the PXRD pattern of biosynthesis of NiO NPs.
The UV-DR Spectrum of spectrum of biosynthesized NiO NPs was recorded between 300 and 800 nm as shown in Fig. 2. The UV-DR spectrum of NiO NPs at 321 nm (Fig. 2a) is due to the charge transfer from conduction band to valance band cations. The spectrum exhibits the toward shorter wavelength signals decrease in size of the particle.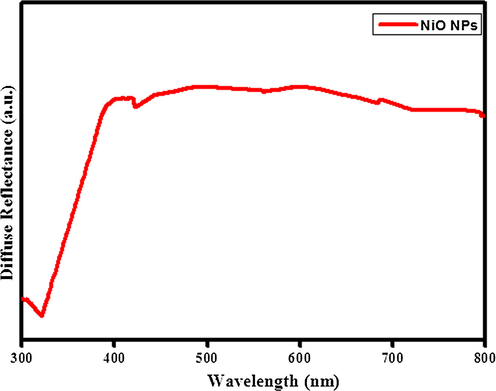
UV-Diffuse reflectance spectrum of biosynthesis of NiO NPs.
As a result, assigned that the formation of oxides between the bond formations with functional groups to induced metal nucleation. The optical energy band gap (Eg) of the nanoparticles is estimated by Kubelka–Munk relation (K. Linga raju et al., 2019).The estimation of optical energy band gap (Eg) of biosynthesized NiO NPs is 3.24 eV as shown in the Fig. 2b.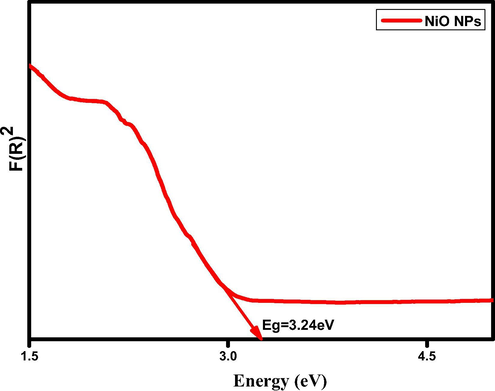
Show the direct energy band gap (Eg) of biosynthesis of NiO NPs.
The FTIR spectrum of biosynthesized NiO NPs from E. heterophylla as shown in the Fig. 3. The broad absorption peak at 3440 cm−1 belongs to O-H stretching vibrations indicates hydroxyl group which is generally phenolics and carboxylic acids. Another peak located at 1631 cm−1 is attributed to the bending mode (H-O-H) of water molecules. The characteristic absorption peaks of oxide groups belongs to 1037cm−1, 1112 cm−1, 1388 cm−1, 1462 cm−1 respectively. The other characteristics peak 2924 and 2849 cm−1 indicates the presence of OH group. The obtained peak at 452 cm−1 is caused by the Ni-O vibration. Thus, it is clearly indicating that, the phytochemicals take part in the biotransformation of nitrates to oxides. The phytochemicals from plant materials act as reducing agent during the formation of nanoparticles (Rajesh Kumar et al., 2013).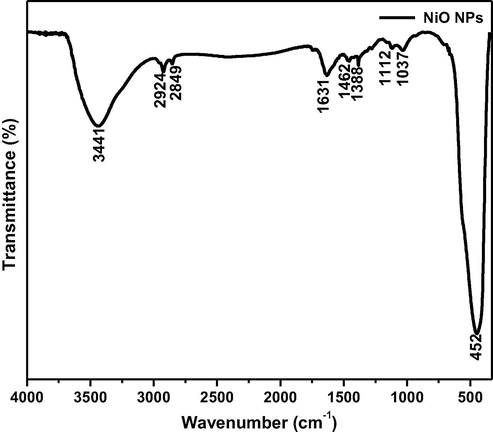
Show the FT-IR spectrum of biosynthesized NiO NPs.
The morphological studies of biosynthesis of NiO NPs from leaves extract of E. heterophylla as shown in Fig. 4.The SEM micrograph of NiO NPs are rhombohedral in shapes and its agglomeration with particles on the surface area as show in the Fig. 4(a). However, TEM images of NiO NPs with an average particles size around 12–15 nm as in Fig. 4(b). The particle size determined from the particle size distribution diagram was very similar to the value of 15 nm obtained based on XRD data using Scherrer's formula. The element analysis of NiO NPs such as Ni and O varies periodically along with percentage of atom and weight as shown in the Fig. 4(c), there is no evidence of impurity in the composition. The morphological analysis of NiO NPs showed the effect of plant extract of E. heterophylla.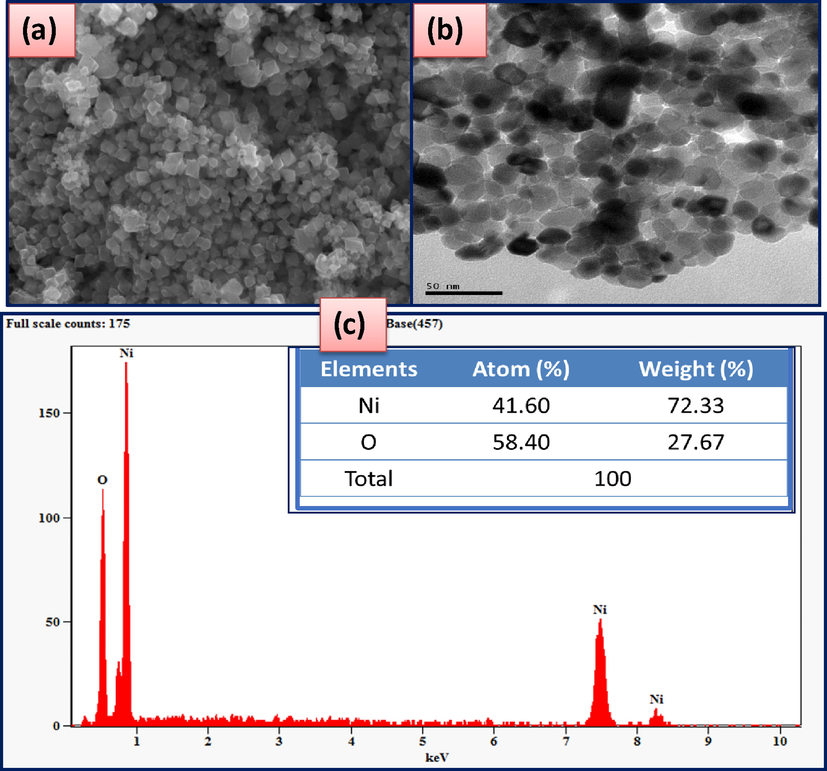
Show the morphological features of biosynthesized NiO NPs (a) SEM micrograph (b) TEM images (c) EDAX.
3.2 Biological properties
3.2.1 Direct haemolytic and Anticoagulant properties
3.2.1.1 Direct haemolytic activity
The direct haemolytic activity of biosynthesized NiO NPs on human RBC blood cells as shown in the Fig. 5. As a result, shows that the different concentration of biosynthesized NiO NPs did not hydrolyse on RBC while comparing the positive control (water) and negative control (PBS) on Human erythrocytes.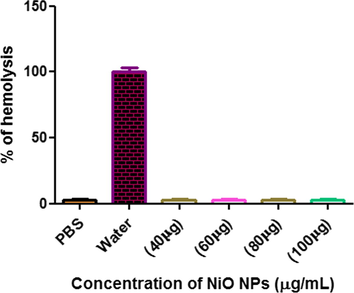
Show the direct hemolytic activity of biosynthesized NiO NPs.
3.2.1.2 Anticoagulant activity
The identified the probable role of biosynthesized NiO NPs in coagulation cascade, plasma re-calcification time was performed using both human platelet rich plasma (PRP) and platelet poor plasma (PPP) as shown in the Fig. 6. The significance of biosynthesized NiO NPs exhibited anticoagulant effects by enhancing the clotting time of both PRP and PPP from control 200 s to 350 s and 210 s to 379 s respectively. The different concentration of biosynthesized NiO NPs shows the maximum concentration consumed in both the cases was found to be 100 µg/mL and remain unchanged up on increased dose to 90–100 µg/mL.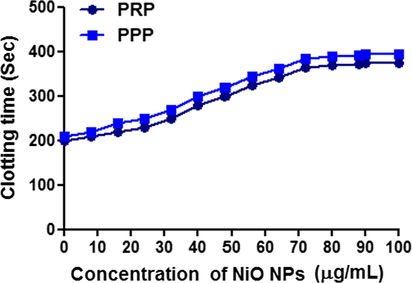
Show the plasma re-calcification time was performed using both human platelet rich plasma (PRP) and platelet poor plasma (PPP) of biosynthesized NiO NPs.
3.2.2 Antibacterial activity
The antibacterial activity of biosynthesized NiO NPs was screened against pathogenic bacterial strains Gram+ve Staphylococcus aureus and Gram−ve Escherichia coli Pseudomonas desmolyticum and Klebsiella aerogenes by agar well diffusion method. The inhibitory effect of biosynthesized NiO NP with different concentrations (200, 400 and 600 μg/μL) with respective positive control (Ciprofloxacin) and control as shown in Fig. 7. The result of antibacterial activity of NiO NPs shows higher significant in E. coli and moderate in S. aureus, P. desmolyticum and K. aerogenes as shown in the Table 1. The exact mechanism of bactericidal activity of NPs is not reported yet, but assumption for the studies, the nanoparticles were interact with bacterial cell effects change in morphology of cell membrane and their increasing the permeability, the interrupts the regular transport through plasma membrane and then finally cell was death. As a results indicates that, the biosynthesized NiO NPs as size as size is very less, stability is high convoyed with large surface area to volume ratio, it simplifies that, the cell was interaction and increased the bactericidal activity with releases of Ni2+ ions released, easily penetrate to the cell wall and then later disturb the electron transport system and affect the components of cells, protein, DNA and oxidative stress was induced, which finally cell was damaged. The subsequently, the bactericidal activity and stability of NiO nanoparticles are high effective, they could be employed in as antimicrobial coatings that will be helpful for various environmental and biomedical applications (Nagajyothi et al., 2014). Values are the mean ± SE of inhibition zone in mm.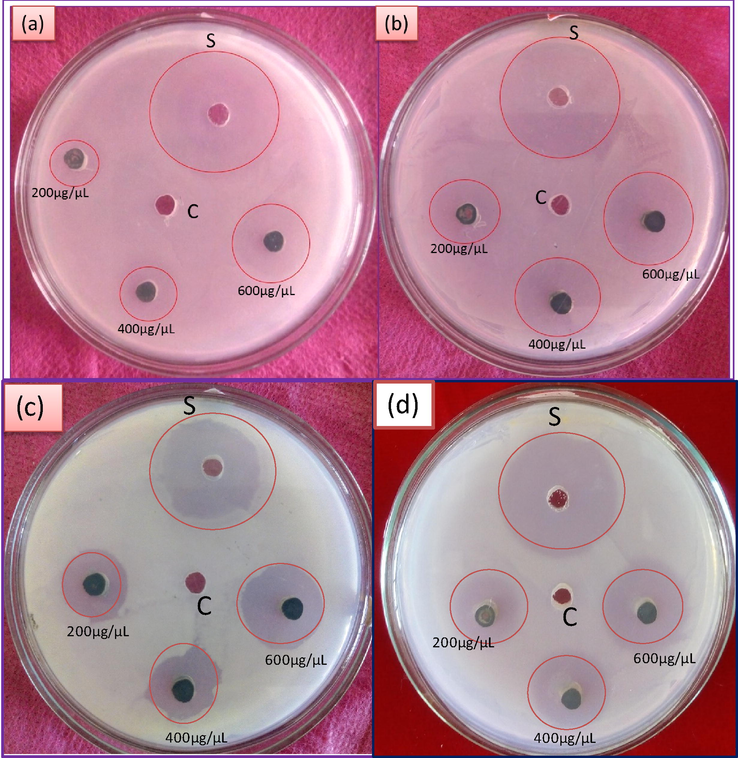
The antibacterial activity of biosynthesized NiO NPs against pathogenic bacterial strains (a) S.aureus (b) E.coli (c) Pseudomonas desmolyticum and (d) Klebsiella aerogenes.
Treatment
Bacterial strains
Sample
Concentration (µg/mL)
S. aureus (Mean ± SE)
E. coli (Mean ± SE)
P. desmolyticum (Mean ± SE)
K. aerogenes (Mean ± SE)
Ciprofloxacin
10
14.08 ± 0.22
11.43 ± 0.23
11.50 ± 0.29
12.02 ± 0.13
NiO NPs
200
2.43 ± 0.15
3.17 ± 0.17
3.23 ± 0.15
3.43 ± 0.12
400
4.23 ± 0.15
5.27 ± 0.15
5.30 ± 0.06
5.33 ± 0.09
600
8.23 ± 0.15
9.23 ± 0.15
7.33 ± 0.09
7.10 ± 0.21
3.2.3 Cytotoxicity activity
The evaluation of cytotoxicity of biosynthesized NiO NPs against A549 and HepG2 cell line cancer cells were measured on cellular reduction of MTT based on invitro analysis. The treated with NiO NPs was screened against cell lines with respective positive control as Cisplatin and control as shown in the Fig. 8. As a result shown that, the viability assay of cytotoxicity of NiO NPs against cancer cell line as shown in the Table 2. The decrease of cell viability with increasing the concentration of NiO NPs as shown significant cytotoxicity to accumulate the internal cells and higher stress, ultimately leading to apoptosis (Song et al., 2012).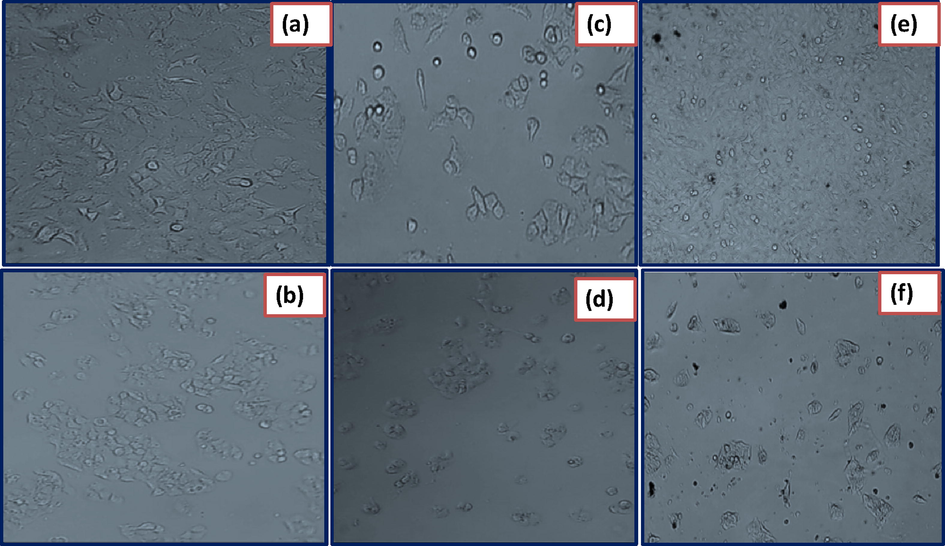
Photographs on cytotoxicity studies against cancer cell lines such as A549 (a) HepG2 (b) control-cell untreated. A549 (c) HepG2 (d) cell lines treated with standard drug Cisplatin and A549 (e) HepG2 (f) Cells treated with biosynthesized NiO NPs.
Treatment
Cell lines
Standard (Cisplatin) (25 µM)
Control
NiO NPs (µg/mL)
IC50 (µg/mL)
25
50
100
200
400
A549
49.09 ± 0.002
100 ± 0.00
93.15 ± 0.005
84.15 ± 0.002
78.95 ± 0.003
67.17 ± 0.003
45.16 ± 0.003
353.161
HepG2
54.48 ± 0.00
100 ± 0.00
95.205 ± 0.001
84.31 ± 0.002
70.97 ± 0.001
58.48 ± 0.002
47.23 ± 0.005
344.26
4 Conclusion
In conclusion, NiO Nanoparticles (NiO NPs) was successfully prepared via biosynthesis route using aqueous leaves extract of E. heterophylla [L.] leaf extract act as reducing/capping/stabilizing agent for the first time. Formation of NiO NPs was confirmed by analytical techniques such as XRD, UV–Vis, FT-IR, SEM-EDAX and TEM. Further, the application of Direct haemolytic and Anticoagulant properties, antibacterial and cytotoxicity of biosynthesized NiO NPs consists of nano holes with various pore size was confirmed. As results show that, biosynthesized NiO nanoparticles are biocompatible material for finding the various applications.
Acknowledgement
The authors express sincere thanks to Tumkur University, Tumkur, for providing the lab facilities to carry out a part of this work.
Declaration of Competing Interest
All the authors states that there is no conflict of interest regarding this manuscript to publish in your reputed journal.
References
- Enzymatic basis for platelet aggregation and release: the significance of the ‘platelet atmosphere’ and the relationship between platelet function and blood coagulation. J. Haematol. 1974;26:331-356.
- [Google Scholar]
- Evaluation of anticoagulant and antiplatelet activity of Pisum sativum pod extract. J. Blood Res Hematol Dis.. 2017;2(1):1-8.
- [Google Scholar]
- Synthesis of NiO nanoparticles and evaluation of antioxidant and cytotoxic activity. Colloid Surf. B: Biointer.. 2013;11(1):556-560.
- [Google Scholar]
- Green synthesis of NiO nanoparticles using Aegle marmelos leaf extract for the evaluation of in-vitro cytotoxicity, antibacterial and photocatalytic properties. J. Photochem. Photobiol. B Biol.. 2018;180:39-50.
- [Google Scholar]
- Green synthesis of NiO nanoparticles using Moringa Oleifera extract and their biomedical applications: cytotoxicity effect of nanoparticles against HT-29cancer cells. J. Photochem. Photobiol. B Biol.. 2016;164:352-360.
- [Google Scholar]
- Phyto-chemical analysis and laxative activity of the leaf extracts of Euphorbia heterophylla L. (Euphorbiaceae) Pak. J. Sci. Ind. Res.. 2004;47(5):345-348.
- [Google Scholar]
- Phytochemical screening and anti-inflammatory evaluation of methanolic and aqueous extracts of Euphorbia heterophylla Linn (Euphorbiaceae) African J. Biotechnol.. 2006;5(6):529-531.
- [Google Scholar]
- Synthesis and characterization of metal oxides (CeO2, CuO, NiO, Mn3O4, SnO2 and ZnO) nanoparticles as photo catalysts for degradation of textile dyes. J. Photochem. Photobiol. B. 2017;173:43-49.
- [Google Scholar]
- Recent advances in semiconductors for photocatalytic and photo electro chemical water splitting. Chem. Soc. Rev.. 2014;43:7520-7535.
- [Google Scholar]
- Environmental applications of semiconductor photocatalysis. Chem. Rev.. 1995;95:69-96.
- [Google Scholar]
- In vitro cytotoxicity, MMP and ROS activity of green synthesized nickel oxide nanoparticles using extract of Terminalia chebula against MCF-7 cells. Mater. Lett.. 2019;234(1):129-133.
- [Google Scholar]
- Influence of particle size on performance of a nickel oxide nanoparticle-based supercapacitor. RSC Adv.. 2015;5(18):14010-14019.
- [Google Scholar]
- Synthesis and characterization studies of NiO Nano rods for enhancing solar cell efficiency using photonupconversion materials. Ceram. Int.. 2016;42:8385-8394.
- [Google Scholar]
- Preliminary phytochemical screening and invitro antioxidant potential of Euphorbia heterophylla (L.) Inter. J. Pharm. Pharm. Sci.. 2014;6(8):(15).
- [Google Scholar]
- Euphorbia heterophylla (L.) mediated fabrication of ZnO NPs: Characterization and evaluation of antibacterial and anticancer properties. Biocatal. Agri. Biotech.. 2019;18 100894
- [Google Scholar]
- Biotechnological aspects of ZnO nanoparticles: overview on synthesis and its applications. Appl. Microbiol. Biotechnol.. 2016;100(2):571-581.
- [Google Scholar]
- Synthesis of 3 substituted isoquinolin-1-yl-2-(cycloalk-2-enylidene) hydrazines and their antimicrobial properties. J. Chil. Chem. Soc.. 2009;54:183-185.
- [Google Scholar]
- Facile combustion synthesis of ZnO nanoparticles using Cajanuscajan (L.) and its multidisciplinary applications. Mater. Res. Bull.. 2014;7:325-334.
- [Google Scholar]
- Antimicrobial and anticancer studies on Euphorbia heterophylla. J. Pharm. Res.. 2010;3(9):2332-2333.
- [Google Scholar]
- Synthesis and characterization of nickel oxide nanoparticle with wide band gap energy prepared via thermochemical processing. Nano Sci. Nanotechnol.: Int. J.. 2014;4:6-9.
- [Google Scholar]
- Simple, novel and low-temperature synthesis of rod-Like NiO nanostructure via thermal decomposition route using anew starting reagent and its photocatalytic activity assessment. J. Mater. Sci. Mater. Electron.. 2016;27:4794-4799.
- [Google Scholar]
- Characterization antibacterial, antioxidant, and cytotoxic activities of ZnO nanoparticles using Coptidis Rhizoma. Bio. Org. Med. Chem. Lett.. 2014;24(17):4298-4303.
- [Google Scholar]
- Current transport mechanism at metal-semiconductor nanoscale interfaces based on ultrahigh density arrays of p-type NiO nano-pillars. Nanosca.. 2013;5:11699-11709.
- [Google Scholar]
- Synthesis, characterization and biological activity of some novel Schiff bases and their Co(II) and Ni(II)complexes: a new route for Co3O4 and NiO nanoparticles for photocatalytic degradation of methylene blue dye. J. Mol. Struct.. 2017;1143:462-471.
- [Google Scholar]
- Green Biosynthesis of NiO nanoparticles using aqueous extract of Tamarix serotina and their characterization and application. Appl. Organomet. Chem.. 2016;30:978-984.
- [Google Scholar]
- Electro chromic behavior of NiO thin films deposited by e-beam evaporation at room temperature. Sol. Energy Mater. Sol. Cell. 2014;120:109-115.
- [Google Scholar]
- Solvothermal synthesis and photocatalytic properties of NiO ultrathin nanosheets with porous structure. Appl. Surf. Sci.. 2015;328:525-530.
- [Google Scholar]
- A study of the coagulation defect in hemophilia and in jaundice. Am. J. Med. Sci.. 1935;190:501-511.
- [Google Scholar]
- Antimicrobial activity of extracts from the leaves of Clematis gouriana Roxb. Inter. J. Biomed. Pharma. Sci.. 2006;1:69-72.
- [Google Scholar]
- Preparation and characterization of NiO nanoparticles by co-precipitation method. Inter. J. Eng. Appl. Manag. Sci. Para.. 2013;06:64-67.
- [Google Scholar]
- SnO2 nanoparticles mediated non-traditional synthesis of biologically active 9-chloro-6,13-dihydro-7-phenyl-5H-indolo [3,2-c]- acridine derivatives. Med. Chem. Res.. 2011;20:732-737.
- [Google Scholar]
- Photocatalytic properties of Mn-doped NiO spherical nanoparticles synthesized from sol-gel method. Optik Int. J. Light Electron. Opt.. 2016;127:10727-10734.
- [Google Scholar]
- Biological synthesis of nanoparticles from plants and microorganisms. Trends Biotechnol.. 2016;34(7):588-599.
- [Google Scholar]
- Physical & electrochemical properties of green synthesized bunsenite NiO nanoparticles via Callistemon viminalis' extracts. Int. J. Electrochem. Sci.. 2016;11:8204-8220.
- [Google Scholar]
- Nanostructured ceria-based materials: synthesis, properties, and applications. Energy Environ. Sci.. 2012;5:8475-8505.
- [Google Scholar]
- Green synthesis of multifunctional zinc oxide (ZnO) nanoparticles using Cassia fistula plant extract and their photodegradative, antioxidant and antibacterial activities. Mater. Sci. Semicond. Process. 2015;31 446-454
- [Google Scholar]
- Single phase bunsenite NiO nanoparticles green synthesis by Agathosma betulina natural extract. J. Alloy. Comp.. 2016;657:655-661.
- [Google Scholar]
- Antimicrobial activities of some euphorbiaceae plants used in the traditional medicine of Akwa Ibom state of Nigeria. Ethnobotanical Leaflets. 2010;14:654-664.
- [Google Scholar]
- Preparation and band-gap modulation in MgxNi1-xO thin films as a function of Mg contents. Thin Solid Films. 2011;519:5174-5177.
- [Google Scholar]







