Translate this page into:
Biosynthesis of silver nanoparticle using flowers of Calotropis gigantea (L.) W.T. Aiton and activity against pathogenic bacteria
⁎Corresponding author. silvymathew110@gmail.com (Silvy Mathew)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
Silver nanoparticles (AgNPs) from silver nitrate solution are carried out using the flower extract of Calotropis gigantea. Silver nanoparticles were characterized by UV–vis spectrophotometer, X-Ray diffractometer (XRD). Reduction of silver ions in the aqueous solution of silver during the reaction was observed by UV–vis spectroscopy. Crystalline nature of synthesized silver nanoparticles was studied by XRD pattern, refraction peak using the Scherrer’s equation. Antibacterial activity of the silver nanoparticles was performed by disc diffusion method against Bacillus subtilis, Pseudomonas putida and Escherichia coli. The antibacterial activity of synthesized silver nanoparticles by flower extract of C. gigantea was found against B. subtilis (10 mm). Synthesised AgNPs has the efficient antibacterial activity against Gram positive bacteria.
Keywords
Silver nanoparticles
Calotropis gigantea
Anti-bacterial UV–vis spectrophotometer
XRD
1 Introduction
The studies on silver nanoparticles (AgNPs) in the consumer products could be attributed to their potent antimicrobial activity against a wide range of pathogenic microorganisms and it is an exciting area of nanotechnology (Klaine et al., 2008; Ahmad et al., 2003; Kowshik et al., 2003; Sinha and Paul, 2015). Silver nanoparticles are known to possess antimicrobial activities against different pathogens including viruses and provide an excellent opportunity to develop new antiviral compounds.
The production and release of the AgNPS are to be continually documented (Impellitteri et al., 2009, Maregesi et al., 2008; Vorobyova et al., 1999; Zaidan et al., 2005). The recent studies also revealed that the AgNPs application have the capacity to control several plant diseases (Park et al., 2006). Nanoparticles play a pivotal role in the drug delivery due to high surface–to-volume ratio and thus can efficiently interact with various pathogens compare other biomolecules. Recent researches focus on the biologically synthesized nanoparticles with a size range from 1 to 100 nm (Zhang et al., 2016; Logeswari et al., 2015). Because medicinal and aromatic plants produce many secondary metabolites in the life cycle. (Gupta and Chadha, 1995; Singh et al., 2015).
Nanotechnology is an advanced technology, which deals with the synthesis of nano-particles, processing of the nanoparticles and their applications [Nazem and Mansoori, 2008; Lead et al., 2018]. The term nanotechnology was coined by Professor Norio Taniguchi of Tokyo Science University in the year 1974 to describe precision manufacturing of materials at the nanometer level. Physical Professor Richard. P. Feynman gave theconcept of nanotechnology in his lecture [Murty et al., 2013]. The medicinal application of nanotechnology is known as nanomedicine [Panneerselvam et al., 2011; Bhakya et al., 2015]. Today, nanotechnology and nanoscience approaches to particle design and formulating arestacking to develop the market for many drugs and are forming the basis for a highly profitable niche within the industry [Bandyopadhyay, 2009; Chaloupka et al., 2010]. Biosynthetic methods using plant extracts [Chandran et al., 2006; Gardea-Torresdey et al., 2002 or bacteria [Joerger et al., 2000], fungus [Shankar et al., 2003], honey [Philip, 2010] is a simple and viable process which is an alternative to yield nanoparticles.
Nano-sized particles ranging below 10 nm are of great interest, because of the chemical and physical behaviour of the particles arising from the quantum size effect. The various types of nanoparticles are inorganic nanoparticles, polymeric nanoparticles, solid lipid nanoparticles, liposomes, nanocrystal, nanotube [Ismail et al., 2016]. Silver ions are released by the nanoparticles which will enhance the bacterial activity. Bactericidal effect of silver nanoparticles is size dependent because nanoparticles smaller than 10 nm interact with bacteria and produce electronic effects, which enhances the reactivity of nanoparticles [Rishabh, 2019].
In this present study, interactions of biologically synthesized silver nanoparticles from flower extracts of Calotropis gigantea (L).W.T.Aiton (Apocynaceae Juss.) and evaluated the antibacterial activity against Bacillus subtilis, Pseudomonas putida and Escherichia coli. The silver nanoparticles used in this study were synthesized by treating AgNO3 with flower extracts of C. gigantea reveals a source of antimicrobial effects. C. gigantea is a genus of plants that produce milky sap hence also commonly called milkweed [Kumar et al., 2010] and also known as Madar. It is considered as a common weed in some parts of the world mainly in Asian countries. The flowers are fragrant often used in making floral tassels in some mainland Southeast Asian cultures and their flowers are used for coughs, asthma, and loss of appetite and many other diseases. The use of silver nanoparticles from flowers of Tagetes erecta has been used against bacteria and showed an alternative to improve antimicrobial activity (Padalia et al. 2015). Here, we applied an eco-friendly route to produce silver nanoparticles from flowers of C. gigantea, combining natural products and metal nanoparticles, to obtain a therapy more efficient against bacteria.
2 Materials and methods
2.1 Collection and extraction of plant material
Fresh flowers of Calotropis gigantea L.W.T Aiton were collected from the campus of Vimala College (Autonomous) Thrissur, Kerala (Fig. 1) C.gigantea mediated synthesis of AgNPs was carried out based on green synthesis procedure with slight modifications (Babu and Prabu, 2011). C.gigantea fresh flowers (20 g) were washed thoroughly with distilled water for 15 min and dried in shade condition. Flowers were finely crushed and stirred with 200 mL distilled water at room temperature for 15 min and filtered. This filter was used as green chemical reducing as well as stabilizing agent. Flower extract was stored at4°C for further use.
(A). Arbustive habit of a specimen of Calotropis gigantea collected in the Vimala college (Autonomous) Thrissur, Kerala. (B). Flowery branches showing different developmental stages of white flowers.
2.2 Biosynthesis of silver nanoparticles
For the synthesis of AgNPs, 2 mL of flower extract and 15 mL of AgNO3 were used. The reaction mixture was prepared by adding 15 mL of AgNO3 (0.4 g AgNO3 in 250 mL water) and 2 mL of flower extract. This stoichiometric proportion was fixed based on initial testing with UV – Visible spectroscopy. The reaction mixture was kept at room temperature for 24 h in dark condition. Colour change pattern from yellow to dark brown indicates the formation of AgNPs (Fig. 2).
Solution of Silver Nitrate (1 mM) before (left) and after (right) exposure to the reaction (extract of Calotropis gigantea plus AgNO3) after 24 hrs incubation.
2.3 Characterization of silver nanoparticles
The colour change observed in the solution that showed the formation of silver nanoparticles. Synthesized silver nanoparticles were confirmed by sampling the reaction mixture at regular intervals and absorption maxima was scanned by UV– vis spectra, at the wave length 200–600 nm. Centrifugation of AgNP is widely used to get rid of unreacted materials and by products. Proper separation and purification are highly required for the characterization and applications of nanoparticles (Archna, 2016; Srikar et al., 2016). So, the reaction mixture was subjected to centrifugation at 1500 rpm for 10 min. Following which the pellet was re-dispersed in sterile distilled water was repeated thrice to ensure the better separation of free entities from the metal nanoparticles. The C.gigantea flower extract embedded with silver nanoparticles was freeze dried, powered and used for X-ray diffractometer (XRD) analysis. The diffracted intensities were recorded from 10 to 100 2 Ø angles.
2.4 Invitro inhibitory activity of silver nanoparticles against bacteria
The antibacterial activity of C. gigantea and green synthesized silver nanoparticles were tested by the disc diffusion method for the determination of antibacterial activity against Pseudomonas putida, Bacillus subtilis and Escherichia coli. A modified agar disc diffusion method was adopted to determine antibacterial activity (Bhakya et al., 2016; Rajkuberan et al., 2015). The bacterial suspension, 1x106CFU mL-3. These three bacteria were inoculated on Muller-Hinton agar plates.100 µl samples of synthesized AgNPs solution were added to individual discs in separate plates. After incubation at 37 °C for 24 h, the zone of inhibition was measured.
3 Results and discussion
Plants from Angiospermae bloom periodically, what produce natural mass in a renewable way, sustainable. Reports have been showed flowers from different species in synthesis of variety types of metallic nanoparticles and applied as antimicrobial and antioxidant (Eswaraiah et al., 2020; Dahibhate et al., 2020; Tan et al., 2002). This is the first assay using flowers of C. gigantea to synthesis of AgNPs.
Reduction of silver ions in the aqueous solution of silver during the reaction with the ingredients present in the C. gigantea flower extracts was observed by UV–visible spectroscopy (Fig. 3). The change in colour was noted by visual observation in the flower extracts when it was incubated with silver nitrate solution. The colour of the flower extract changed to light brown within an hour and then later changed to dark brown during the 2 h incubation period (Fig. 2). The brown colour could be due to the excitation of surface plasmon vibrations, typical of the silver nanoparticles. Reduction of silver ions present in the aqueous solution of silver complex during the reaction with the ingredients present in the flower of C. gigantea extracts observed by the UV–vis spectroscopy revealed the presence of silver nanoparticles may be correlated with UV–vis spectra. These changes were attributed to the excitation of Surface Plasmon Resonance (SPR) in the metal nanoparticles [Prabhuram et al., 2003]. Besides, the plasmon bands are broadened with an absorption tail in the longer wavelengths which may be due to the size distribution of the particles.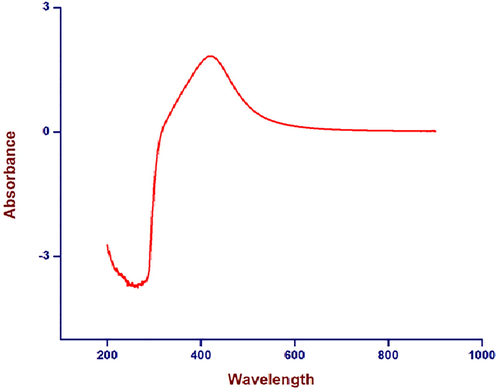
UV visible spectrum of C. gigantea flowers as a function of time. The absorbance peak corresponds to the plasmon resonance of silver nanoparticles at 422 nm.
To confirm the crystalline nature of the particles studies were carried out using X-ray diffraction. The XRD pattern obtained was shown in Fig. 4. It is important to know the exact nature of the silver particles formed and this can be deduced from the XRD spectrum of the sample. XRD spectra of pure crystalline silver structures have been published by the Joint Committee on Powder Diffraction Standards. The XRD pattern shows that the intense peaks in the whole spectrum of 2Ø values ranging from 10 to 100. The phytosynthesized silver nanostructures were characterized with powder XRD technique. The peaks at 37.8°, 44.0°, 64.2° and 77.0° in the XRD pattern corresponds to diffraction from (1 1 1), (2 0 0), (2 2 0) and (3 1 1) planes of Ag, respectively. In addition to this, some extra peaks (14.3°, 16.1°, 22.3°, 34. 0°) were also observed in the XRD pattern. This could be due to certain photochemical compounds of flower extract present in the sample (see Fig. 6).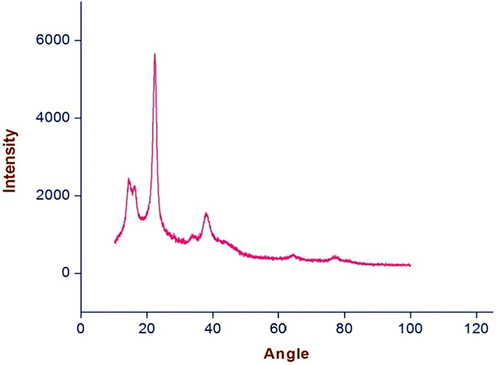
XRD spectrum of silvernanoparticles formed after 24 h of incubation of the C. gigantea flowers filtrate treated with silver nitrate solution.
Inhibition zone by Bacteria.
Schematic representation of the production of silver nanoparticles from Calotropis gigantea.
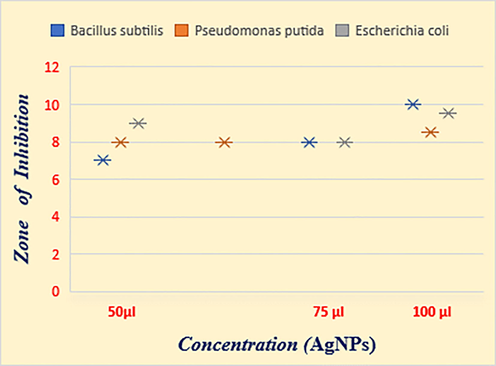
Antibacterial activity was evaluated for the synthesized silver nanoparticles against E. coli, B.subtilis, P. putida by using the disc diffusion method. The results showed that the biosynthesized silver particles from C. gigantea flower extracts have resistance against Gram positive bacteria like B. subtilis (10 mm) and at the same time gram negative bacteria like E.coli (9.5 mm) showed high resistance against the nanoparticles (Table 1& Fig. 4). The results were observed in terms of IZ around the disc caused by diffusion of antibacterial properties from the plant-based silver nanoparticles impregnated disc into the surrounding medium (Fig. 5).
Bacteria
Silver nanoparticle from Calotropis gigantea
Diameter of Zone of Inhibition(mm) of aqueous concentration of NPs
50 µl
75 µl
100 µl
Bacillus subtilis
7 ± 0.2
8 ± 0.3
10 ± 0.3
Pseudomonas putida
8 ± 0.1
8 ± 0.1
8.5 ± 0.1
Escherichia coli
9 ± 0.3
8 ± 0.3
9.5 ± 0.1
The antimicrobial properties of silver nanoparticles depend on size and environmental conditions (size, pH, ionic strength and capping agent). The exact mechanisms of antimicrobial or toxicity activities by silver nanoparticles are still in investigation and a well debated topic. The positive charge on the Ag ions is suggested vital for antimicrobial activities. In order for silver to have any antimicrobial properties, it must be in its ionized form. In its ionized form, silver is inert but on coming in contact with moisture it releases silver ions [Klueh et al., 2000, Mushir et al., 2016, Reddy et al., 2014]. Several phyto constituents have been isolated and identified from different parts of the C. gigantea belonging to the category of alkaloids, glycosides, flavanols, tannins, saponins, sterols and triterpenoids and these phytocompounds are the responsive group for the synthesis of silver nanoparticles [Joshi et al., 2011].
Ag+ ions are able to form complexes with nucleic acids and preferentially interact with the nucleosides rather than with the phosphate groups of nucleic acids. Thus, all forms of silver or silver containing compounds with observed antimicrobial properties are in one way or another sources of silver ions (Ag+); these silver ions may be incorporated into the substance and released slowly with time as with silver sulfadiazine, or the silver ions can come from ionizing the surface of a solid piece of silver as with silver nanoparticles [Sondi and Salopek-Sondi, 2004, Roucoux et al., 2003].
The results from this study reveal that Gram negative bacteria are resistant to the action of tested extracts and the same have been reported by several researchers for other plant based extracts. (Ncube et al., 2008). Cos et al., 2006 evaluated that Gram negative bacteria are generally more resistant when compared to the gram positive ones. This is because of Gram negative bacteria strains have an outer phospholipids membrane with the structural lipopolysaccharide components, which make their cell wall impermeable to antimicrobial agents (Nikaido and Vaara, 1985). The Gram-positive bacteria should be more susceptible since they have only an outer peptidoglycan layer which is not an effective permeability barrier (Scherrer and Gerhardt, 1971). Antibacterial study reveals that silver nanoparticles possess mild potential antibacterial activity against B. sutbilis. Maximum zone of inhibition was observed in Bacillus.
4 Conclusion
From the results obtained, the biosynthesized nanoparticles from C.gigantea flower extracts showed that the environmentally benign and renewable source of C.gigantea can be used as an effective reducing as well as stabilizing agent for the synthesis of AgNPs. The formation of AgNPs was observed by visible colour change from yellow to dark brown and confirmed by UV vis analysis. These nanoparticles showed characteristic absorption peak 422 nm in UV spectra. Crystalline nature of silver nanoparticles was studied using X-ray diffraction. Antibacterial activity evaluated for synthesized AgNPs showed that biosynthesized AgNPs from the flower extract have resistance against Gram positive bacteria like B. subtilis. This type of nanoparticle biosynthesis is an environmentally acceptable, nontoxic approach.
Acknowledgement
The authors wish to acknowledge DST- FIST, Department of Science and Technology, Govt.of India [Grant No. SR/FST/College-046/2011] and DBT-STAR College Scheme, Department of Biotechnology, Govt.of India [No. 102/IFD/SAN/3988/201-20 dated 29.02.2020] for the financial assistance to the Science Departments of Vimala College (Autonomous), Thrissur,Kerala, India.
References
- Extracellular biosynthesis of silver nanoparticles using the fungus Fusarium oxysporum. Colloids Surf. B Biointerf.. 2003;28(4):313-318.
- [CrossRef] [Google Scholar]
- Bandyopadhyay A K, Text book of Nano materials, New Age Science, ISBN- 10: 1906574278,1-320.
- Biogenic synthesis of silver nanoparticles and their antioxidant and antibacterial activity. Appl. Nanosci.. 2016;6(5):755-766.
- [Google Scholar]
- Catalytic degradation of organic dyes using synthesized silver nanoparticles: a green approach. J Bioremediat Biodegrad.. 2015;1:6(5)
- [CrossRef] [Google Scholar]
- Nanosilver as a new generation of nanoproduct in biomedical applications. Trends biotechnol.. 2010;28(11):580-588.
- [CrossRef] [Google Scholar]
- Synthesis of Gold Nanotriangles and Silver Nanoparticles Using Aloe vera Plant Extract. Biotechnol. Prog.. 2006;22(2):577-583.
- [CrossRef] [Google Scholar]
- Anti-infective potential of natural products: How to develop a stronger in vitro ‘proof-of-concept’. J. Ethnopharmacol.. 2006;106(3):290-302.
- [CrossRef] [Google Scholar]
- Phytochemical Screening, Antimicrobial and Antioxidant Activities of Selected Mangrove Species. Current Bioactive Compounds. 2020;16(2):152-163.
- [CrossRef] [Google Scholar]
- Studies on phytochemical, antioxidant, antimicrobial analysis and separation of bioactive leads of leaf extract from the selected mangroves. J. King SaudUniversity-Sci.. 2020;32(1):842-847.
- [CrossRef] [Google Scholar]
- Gardea-Torresdey JL, Parsons JG, Gomez E, Peralta-Videa J, Troiani HE, Santiago P, Yacaman MJ. Formation and growth of Au nanoparticles inside live alfalfa plants. Nano letters. 2002,10;2(4):397-401. https://doi.org/10.1021/nl015673+
- Medicinal and aromatic plants research in India. Adv. Hort. Med. Aromatic Plants. 1995;11:1-43.
- [Google Scholar]
- The Speciation of Silver Nanoparticles in Antimicrobial Fabric Before and After Exposure to a Hypochlorite/Detergent Solution. J. Environ. Qual.. 2009;38(4):1528-1530.
- [CrossRef] [Google Scholar]
- Plant mediated green synthesis of anti-microbial silver nanoparticles—a review on recent trends. Rev. Nanosci. Nanotechnol.. 2016;5:119-135.
- [CrossRef] [Google Scholar]
- Biologically produced silver–carbon composite materials for optically functional thin-film coatings. Adv. Mater.. 2000;12(6):407-409.
- [Google Scholar]
- Nanomaterials in the environment: behavior, fate, bioavailability, and effects. Environ. Toxicol. Chem.. 2008 Sep;27(9):1825-1851.
- [CrossRef] [Google Scholar]
- Efficacy of silver-coated fabric to prevent bacterial colonization and subsequent device-based biofilm formation. J. Biomed. Mater. Res. Off. J. Soc. Biomater. Japanese Soc. Biomater. Australian Soc. Biomater. Korean Soc. Biomater.. 2000;53(6)
- [Google Scholar]
- Extracellular synthesis of silver nanoparticles by a silver-tolerant yeast strain MKY3. Nanotechnology. 2003;14(1):95-100.
- [CrossRef] [Google Scholar]
- Antibacterial activity of aqueous extract of Calotropis gigantea leaves–an in vitro study. Int J Pharm Sci Rev Res.. 2010;4(2)
- [Google Scholar]
- Nanomaterials in the environment: Behavior, fate, bioavailability, and effects-An updated review: Nanomaterials in the environment. Environ. Toxicol. Chem.. 2018;37(8):2029-2063.
- [CrossRef] [Google Scholar]
- Synthesis of silver nanoparticles using plants extract and analysis of their antimicrobial property. J. Saudi Chem. Soc.. 2015;19(3)
- [CrossRef] [Google Scholar]
- Screening of some Tanzanian medicinal plants from Bunda district for antibacterial, antifungal and antiviral activities. J. Ethnopharmacol.. 2008;119(1):58-66.
- [Google Scholar]
- Murty BS, Shankar P, Raj B, Rath BB, Murday J. Textbook of nanoscience and nanotechnology. Springer Science & Business Media; 2013 Dec 6.
- Mushir A, Jahan N, Ahmed A. A review on phytochemical and biological properties of Calotropis gigantea (Linn.) R. Br. Discovery Phytomedicine. 2016;3(3):15.
- Nanotechnology solutions for Alzheimer's disease: advances in research tools, diagnostic methods and therapeutic agents. J. Alzheimers Dis.. 2008;13(2):199-223.
- [Google Scholar]
- Assessment techniques of antimicrobial properties of natural compounds of plant origin: current methods and future trendsEnglish. Afr. J. Biotechnol.. 2008;7(12)
- [CrossRef] [Google Scholar]
- Molecular basis of bacterial outer membrane permeability. Microbiol. Rev.. 1985;49(1):1.
- [Google Scholar]
- Green synthesis of silver nanoparticles from marigold flower and its synergistic antimicrobial potential. Arab. J. Chem.. 2015;1;8(5):732–741
- [CrossRef] [Google Scholar]
- Potential anti-plasmodial activity of synthesized silver nanoparticle using Andrographis paniculata Nees (Acanthaceae) Arch. Appl. Sci. Res.. 2011;3(6)
- [Google Scholar]
- A new composition of nanosized silica-silver for control of various plant diseases. Plant Pathol. J.. 2006;22(3):295-302.
- [CrossRef] [Google Scholar]
- Honey mediated green synthesis of silver nanoparticles. Spectrochimica Acta Part A.. 2010;75(3)
- [Google Scholar]
- Synthesis and characterization of surfactant-stabilized Pt/C nanocatalysts for fuel cell applications. J. Phys. Chem. B. 2003;107(40):11057-11064.
- [CrossRef] [Google Scholar]
- Evaluation of antioxidant, antibacterial and cytotoxic effects of green synthesized silver nanoparticles by Piper longum fruit. Mater. Sci. Eng. C.. 2014;34:115-122.
- [CrossRef] [Google Scholar]
- Rishabh Anand, Essentials of Nanotechnology, Medtech Publisher,1-365.
- Arene hydrogenation with a stabilised aqueous rhodium (0) suspension: A major effect of the surfactant counter-anion. Adv. Synth.. 2003;345(1–2):222-229.
- [CrossRef] [Google Scholar]
- Molecular sieving by the Bacillus megaterium cell wall and protoplast. J. Bacteriol.. 1971;107(3):718-735.
- [Google Scholar]
- Geranium Leaf Assisted Biosynthesis of Silver Nanoparticles. Biotechnol. Prog.. 2003;19(6):1627-1631.
- [CrossRef] [Google Scholar]
- Antibacterial and antioxidant activities of Mentha piperita L. Arab. J. Chem.. 2015;8(3):322-328.
- [CrossRef] [Google Scholar]
- Phytosynthesis of Silver Nanoparticles Using Andrographis paniculata Leaf Extract and Evaluation of Their Antibacterial Activities. Spectrosc. Lett.. 2015;48(8):600-604.
- [CrossRef] [Google Scholar]
- Sondi I, Salopek-Sondi B. Silver nanoparticles as antimicrobial agent: a case study on E. coli as a model for Gram-negative bacteria. J. Colloid Interface Sci. 2004, 1; 275(1):177-82. https://doi.org/10.1016/j.jcis.2004.02.012
- Thiosalicylic Acid-Functionalized Silver Nanoparticles Synthesized in One-Phase System. J. Colloid Interf. Sci.. 2002;249(2):336-345.
- [CrossRef] [Google Scholar]
- Preparation of silver nanoparticles by interphase reduction. Colloids Surf. A Physicochem. Eng. Asp.. 1999;152(3):375-379.
- [CrossRef] [Google Scholar]
- Zaidan MR, Noor Rain A, Badrul AR, Adlin A, Norazah A, Zakiah I. In vitro screening of five local medicinal plants for antibacterial activity using disc diffusion method. Trop biomed. 2005, 1;22(2):165-70.
- Silver nanoparticle-mediated cellular responses in various cell lines: an in vitro model. Int. J. Mol. Sci.. 2016;17(10):1603.
- [CrossRef] [Google Scholar]
- Archna HR. A review on green synthesis of silver nanoparticle, characterization and optimization parameters. Int. J. Res. Eng. Technol. 2016.
- Srikar SK, Giri DD, Pal DB, Mishra PK, Upadhyay SN. Green synthesis of silver nanoparticles: a review. Green Sustain Che. 2016, 16;6(1):34-56. http://dx.doi.org/10.4236/gsc.2016.61004
- Joshi H, Gururaja MP, Suares D. Calotropis gigantea R. Br. (Asclepiadaceae): A Review. Int J Pharm Res 2011; 3: 10-14.
- Babu SA, Prabu HG. Synthesis of AgNPs using the extract of Calotropis procera flower at room temperature. Materials Letters. 2011; 15;65(11):1675-1677. https://doi.org/10.1016/j.matlet.2011.02.071.
- Antibacterial and cytotoxic potential of silver nanoparticles synthesized using latex of Calotropis gigantea L. Spectrochim. Acta Part A Mol. Biomol. Spectros.. 2015;5:924-930.
- [CrossRef] [Google Scholar]







