Translate this page into:
Biphenyls and their derivatives as synthetically and pharmacologically important aromatic structural moieties
⁎Corresponding author. Tel.: +91 022 65797628, mobile: +91 9820291372; fax: +91 0253 2303203. zenishjain@gmail.com (Zenish J. Jain),
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.

Abstract
Biphenyls are an important intermediate in organic chemistry which constitutes the structural moiety of a wide range of compounds with profound pharmacological activity. Being a neutral molecule (due to absence of the active functional moiety on it), biphenyls are required to be functionalized i.e. introduction of active group on it. Thus, the substituted biphenyls can be used for synthesis or condensation of another active group with itself which are pharmacologically significant thereby forming the compounds with altogether new activity. Initially, biphenyls were widely used as intermediates in chemical synthesis and pesticides in the form of PCBs (polychlorinated biphenyls). But with the emerging trends in the synthetic chemistry, a number of derivatives are obtained which are of therapeutic significance. This review is summarized to know about synthesis of biphenyl and its derivatives as well as various biphenyl analogs that has potential to act against number of disease and disorders.
Keywords
Biphenyl synthesis
Functionalization
Halobenzene
Palladium
Pharmacological activity
1 Introduction
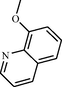
Biphenyls, as the name suggests, are two conjoined benzene rings attached through their 1,1′-positions. It is a white crystal with characteristic pleasant smell (Pagan, 2012). Biphenyls are important structural analog which are used considerably in synthesis of various compounds. Earlier, biphenyl derivatives were widely used as pesticides in the form of polychlorinated biphenyls (PCBs) (see Fig. 1).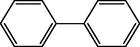
Biphenyl.
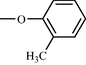
The biaryl axis is the central building block in a very large number of various molecules such as natural and pharmacologically active products, chiral reagents (Cram, 1988a,b), as the source of chiral phases for chromatography, (Mikes and Boshart, 1978) as inflexible “spacers” between two parts of a molecule(Brandmeier et al., 1989a,b; Tabushi et al., 1984), as the basis of liquid crystals (Yamamura et al., 1988, 1989) and fluorescent layers in OLEDs (Hohnholz et al., 2000). Different substituted biphenyls very often are used as suitable synthetic intermediates for synthesis heteroaromatic and polycyclic aromatic compounds (Yao et al., 2005). The key step in such synthesis almost always is the coupling of two matched aromatic parts of the target molecule. Another way is the directed modification of easily accessible biphenyls such as, for example, 4, 4′-biphenyldicarboxylic acid (Olkhovik et al., 2008) (see Fig. 2).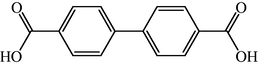
4,4′-Biphenyldicarboxylic acid.
Biphenyl is a neutral molecule and fairly non-reactive due to lack of functional group. However, biphenyl participates in many of the reactions that are typical for benzene, for example, substitution reactions upon treatment with halogens in the presence of a Lewis acid. Also, it is required to convert biphenyls into the structural analogs containing the active groups in order for it to be able to use for synthetic intermediate for the production of a host of other organic compounds such as emulsifiers, optical brighteners, crop protection products, plastics and pharmaceuticals (Johansson and Olsen, 2008). For this, it is important to consider the o,p-directing and/or m-directing effect, especially when substitution at a specific position is desired i.e. mono, di-, tri- or tetra- substitutions. It is possible to acetylate the carboxylic part, also various other biphenyl derivative synthesis are possible by carrying out the amination, halogenation, sulphonation, alkylation, hydroxylation, metal complexation etc.
Biphenyl derivative syntheses have been widely carried out and have yielded wide range of compounds with wide range of activities such as: Antimicrobial, antifungal, anti proliferative, anti diabetic, immunosuppressant, analgesic, anti inflammatory etc. Number of parent structures and their derivatives containing biphenyl moiety are synthesized and patented as well, by the research scientists and chemists. The review will start with general scheme for biphenyl synthesis, followed by which the various synthetic schemes and also the methods to synthesize various biphenyl derivatives have been summarized.
2 Synthesis of biphenyl
2.1 Biphenyl occurs naturally in coal tar, crude oil, and natural gas and can be isolated from these sources via distillation. It can also be synthesized synthetically by using a Grignard reagent such as phenylmagnesium bromide and reacting it with bromobenzene, reacting fluorobenzene with phenyl lithium or reacting benzyne with phenyl lithium as follows (Metasynthesis: The chemical theasaurus) (see Schemes 1–5).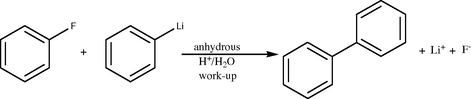
Synthesis of biphenyl from fluorobenzene and phenyl lithium.
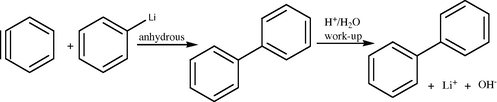
Synthesis of biphenyl from benzyne and phenyl lithium.
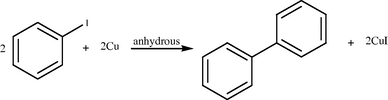
Synthesis of biphenyl from iodobenzene.
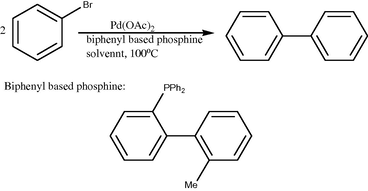
Synthesis of biphenyl from bromobenzene.
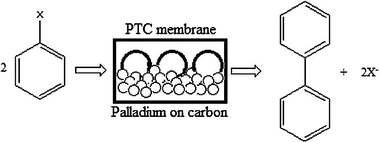
Synthesis of biphenyl from halobenzene using PTC membrane.
2.2 Ullmann et al. (1904), discovered that heating iodobenzene with a copper powder catalyst leads to the formation of biphenyl with high purity. Meanwhile, this reaction has become a textbook case and it is widely used in synthetic chemistry due to its versatility in the association of substituted phenyls to complex biphenyl derivatives.
The synthesis of biphenyl out of iodobenzene on copper essentially consists of three steps: dissociation of iodobenzene (C6H5I) to phenyl (C6H5) and iodine, diffusion of phenyl to find another phenyl as a reaction partner, and association to form biphenyl (C12H10). The dissociation of iodobenzene by thermal activation takes place at ∼180 K and biphenyl is formed at ∼210 K for multilayer (Weiss et al., 1998) and ∼300 K for submonolayer iodobenzene on Cu(III) [Yang et al., 1995; Xi and Bent, 1992]. Synthesis reported in reference (Hla et al., 2000).
2.3 One of the well-recognized mechanisms for Ullmann reaction (Hassan et al., 2001; Cepanec et al., 2007) involves consecutive oxidative addition steps of aryl halide on Pd(0) and Pd(II) species, respectively. The resulting Pd(IV) species is very unstable and readily undergoes reductive elimination to form Pd(II) species. A terminal reducing agent is required to regenerate Pd(0) from Pd(II) in the terminal reduction step and to complete the catalytic cycle. Several types of reductants have been reported such as zinc powder (Kende et al., 1975), tertiary amines (Hassan et al., 2001), hydrazine (Nakajima et al., 1980), molecular hydrogen (Mukhopadhyay et al., 1999) hydroquinone (Hennings et al., 1999). The main characteristic of this mechanism is the change of palladium oxidation state between zero, two and four.
2.4 In another typical reaction, the halobenzene and the reducing agent were stirred in the presence of the catalyst and a base to give biphenyl as the major product, together with minor amounts of benzene (the byproduct due to the parallel reduction reaction). No other products were observed (Mukhopadhyay et al., 2001).
Conversions and product selectivities for various halobenzenes are shown in Table 1.
Substrate
Time (H)
Conv. (%)
Ar–Ar
Ar–H
Chlorobenzene
8.5
100
83
17
Bromobenzene
8
100
92
8
Iodobenzene
6
100
93
7
Carbon was the obvious support of choice due to its high surface area and porosity, plus of course its high selectivity as a support in previous Pd-catalyzed reductive coupling studies. Using carbon-supported PTC, an increased product selectivity was observed (cf. Table 2, entries 1–3). When Na2CO3, TBAB (Tetra-n-butyl ammonium bromide), and Pd were all together supported on carbon, the rate and selectivity obtained were very high under the reaction conditions (Table 2, entries 4 and 5), while depositing the formate on the carbon support together with the other components resulted in a low selectivity to biphenyl due to an increase in reduction rate (Table 2, entry 6) (Mukhopadhyay et al., 2001). (Detailed procedures for the support preparation are given in reference Mukhopadhayay et al., 2001).
Entry
Support
Time (h)
Conv. (%)
Select. (%) Ar–Ar
1b
Support 1
6
100
34
2
Support 2
6.5
100
79
3
Support 2A
6
100
75
4
Support 3
8
100
92
5
Support 3A
7.5
100
89
6
Support 4
4
100
56
2.5 Biphenyl can also be synthesized with a high selectivity (88%) by the Pd-catalyzed dimerization of benzene in the presence of MoO2(acac)2 as a cocatalyst and O2 in acetic acid (Okamoto and Yamaji, 2001). In continuing study, recently investigated the effect of HPAs (heteropolyacids) of Keggin type containing molybdenum such as H4SiW12-nMon, H15-nPV12-nMonO40, and H3PW12-nMonO40 as a cocatalyst in the Pd(OAc)2-catalyzed dimerization of benzene in the presence of O2 and acetic acid (Scheme 6) have been investigated and found that HPAs of Keggin type containing molybdenum act as an excellent cocatalyst to obtain biphenyl from benzene in the Pd(OAc)2/HPA/O2/AcOH catalyst system (Okamoto et al., 2002).
Synthesis of biphenyl from Benzene.
3 Synthesis of biphenyl derivatives as intermediates: functionalization
As mentioned earlier it is necessary to functionalize the biphenyl in order for it to be used as an intermediate for synthesis of various compounds.
3.1 Miyaura and Suzuki (1995) pioneered the use of arylboronates, more readily accessible than boronic acids, for the cross-coupling reaction to afford biaryls (Ishiyama et al., 1995). The use of this methodology was recently expanded by developing a convenient one-pot protocol via aryl boronates generated in situ (Zhu et al., 2003). This one pot procedure allows coupling reactions between two aryl halides without the need for boronic acids. In search for a scaleable and economical synthesis of biphenyl aniline 1, amino and protected amino phenyl halides as boronate precursors in the one-pot Suzuki coupling reaction was investigated. Although amide aryl halides are known to cross couple with boronic acids (Wolfe et al., 1999) there is very limited literature precedent of amide aryl boronate in Suzuki coupling reactions.
The formamide derivatives 3(i-viii) were prepared in quantitative yields by treating appropriate anilines in formic acid/acetic anhydride (Sheehan and Yang, 1958). One-pot Suzuki coupling reaction between 3 and bromides 2 occurred smoothly (Scheme 7), and the results are shown in Table 3. In most cases, biphenyl4 was cleanly formed and isolated without chromatography. Specifically, biphenyls4(i-viii) were obtained cleanly in satisfactory yields (64–70%) after a simple trituration (entries i–v). Substituted biphenyl formanilides 4(vi–viii) were also prepared in moderate yields (entries vi–viii). Except for 4(vi) where a slower reaction was observed, hydrolysis of the biphenyl formanilides 4 readily occurred under mild acidic conditions to afford anilines 1 in near quantitative yields (Scheme 7) (Hett et al., 1998) (see Tables 4 and 5).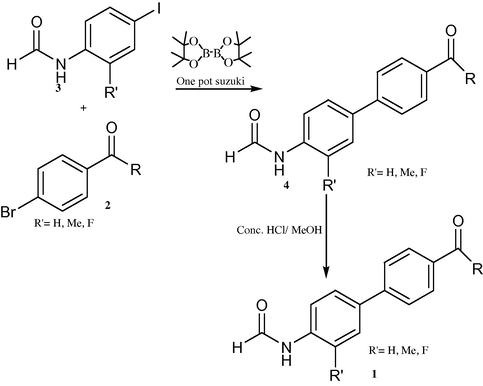
Biphenyl formamide and biphenyl aniline synthesis via one-pot Suzuki Coupling reaction.
Entry
Formamide 3R′=
Aryl Bromide 2R=
4 (% Yield)
1 (% Yield)
i
H
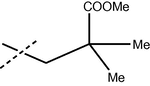
68
95
ii
H
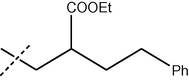
65
96
iii
H
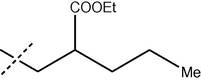
64
92
iv
H
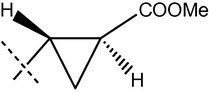
67
98
v
H
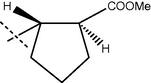
70
90
vi
Me
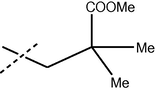
30
60
vii
F
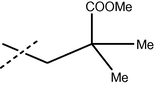
65
95
viii
F
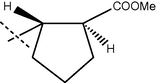
65
90
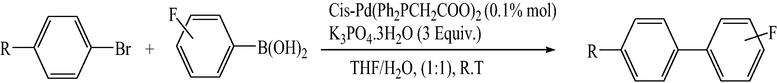
Haloarene
R
R′
X
t (h)
% Conversion
% Selectivity (Ar–Ar)
1
CH3
H
Cl
10
73
96
2
H
CH3
Cl
6
33
100
3
CF3
H
Cl
10
65
98
4
CHO
H
Cl
6
36
97
5
H
H
Cl
12
81
91
6
H
H
Br
12
84
98
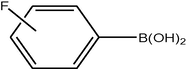
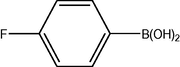
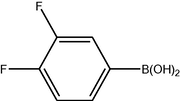
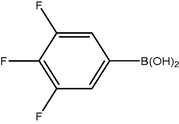
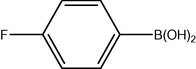
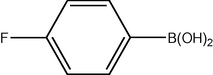
3.2 Fluorinated biphenyl derivatives are fundamental building blocks in fluorinated liquid crystals. The fluoro-substitution of benzene rings in mesomorphic molecules may lead to some changes in the melting point, viscosity, birefringence, dielectric anisotropy and other physical properties. The liquid crystalline materials containing monofluoro-, difluoro- or trifluoro-substituted phenyls (Goto and Kitanno, 1991; Nabor et al., 1991; Hird et al., 1992) are the most prominent for application in thin-film transistor liquid crystal displays (TFT-LCDS).
In order to efficiently synthesize some fluorinated liquid crystals in industry goal, a new, air-stable and highly active P–O coordinated palladium-catalyst have been developed. These complexes are ideal catalysts for the coupling of deactivated aryl bromides as they are comparatively inexpensive, very easily synthesized and can be used to give conversions at low concentrations.
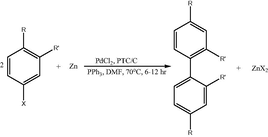
3.3 In another study, it was shown that PdCl2-catalyzed reductive coupling method can be employed to selectively synthesize biphenyls from haloarenes, namely, 4-chlorotoluene in the presence of an activated carbon supported phase-transfer catalyst (PTC), a ligand (PPh3), and a reducing agent such as zinc in anhydrous N,N-dimethylformamide (DMF). Similar attempts have been made before to utilize PdCl2 as the catalyst. However, only low reactivity was observed with chloroarenes, whereas bromo- and iodoarenes have reacted to give coupling products (Lee et al., 2001). In fact, NiCl2 has been employed (Kageyama et al., 1991, 1995) successfully as a catalyst in similar coupling reactions; however, the need for extremely toxic solvents (Kageyama et al., 1991, 1995) such as pyridine or quinoline invalidates the utility of such processes. Primarily, Zn was used as the reducing agent (Mukhopadhyay et al., 2000a,b).
3.4 Tyrosinase. is a multifunctional copper-containing enzyme, that is, key in melanin biosynthesis, melanisation in animals, and browning in plants. Tyrosinase inhibitors can therefore be clinically useful for the treatment of some dermatological disorders associated with melanin hyperpigmentation. In addition, these inhibitors are also known to be useful in cosmetics for whitening and depigmentation after sunburn (Chang, 2009; Kima and Uyamab, 2005). In the past few decades, a number of polyphenol tyrosinase inhibitors from both natural and synthetic sources, including flavonoids, stilbenes, and terpenoids, have been intensively investigated (Okombi et al., 2006; Karioti et al., 2007; Khan et al., 2006a,b; Shimizu et al., 2003). Fortuneanoside E is a new biphenyl glycoside isolated from the fruit of Pyracantha fortuneana that has shown tyrosinase inhibitory activity (see Fig. 3).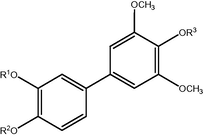
Fortuneanoside E: R1 = H, R2 = Glcosyl, R3 = H Compound I: R1 = Benzyl(Bn), R2 = H, R3 = CH3.
Generally, there are several methods for the construction of biphenyls, including Stille coupling, the Gomberg–Bachmann reaction, the Ullmann reaction, and Suzuki–Miyaura cross-coupling. Considered the particularities of these methods and the characteristics of the target compounds, Suzuki–Miyaura cross-coupling was applied in the first step. To find the optimal conditions, a model reaction was performed using 2-(benzyloxy)-4-bromobenzaldehyde and 3,4,5-trimethoxy-phenylboronic acid under different conditions. This reaction was found to be most efficient in the presence of K2CO3 and Pd(OAc)2 in DMF at 110 °C and using these conditions, compound 1 could be prepared in 72% yield (Bao et al., 2009). With these optimized condition, biphenyls 1–19 were prepared from various bromobenzenes (commercially available or prepared by conventional methods) by Suzuki–Miyaura cross-coupling with 3,4,5-trimethoxy-phenylboronic acid, resulting in yields ranging from 68% to 92%. Methyl and benzyl ethers have been widely used as stable protecting groups for hydroxyl groups in organic synthesis.
A number of methods have been reported to perform cleavage of the methyl and benzyl groups in these ethers (Nishioka et al., 2000; Punna et al., 2004). In a report, (Bao et al., 2009) a highly selective demethylation/debenzylation method that was effective in different kinds of pyrogallol trimethyl ethers using MgI2 under solvent-free conditions have been described. In these reactions, it was found that a variety of functional groups were tolerated under the stated reaction conditions. Consequently, this method was applied in the synthesis of biphenyls 20–36 (Table 6). Treatment of compounds 1–19 (Table 6) with MgI2 at 80 °C under solvent-free conditions gave compounds 20–36 in good to excellent yields (Scheme 8). If necessary, the amount of MgI2 and the reaction temperature could be adjusted to improve the reaction yield (Bao et al., 2010) (see Tables 7–9).
Compounds
R1
R2
R3
R4
R5
Compound I
H
OBn
OH
H
H
1
H
OBn
CHO
H
H
2
H
OH
CHO
H
H
3
H
OBn
COOH
H
H
4
H
OH
COOH
H
H
5
H
OH
COOEt
H
H
6
H
OBn
CONHBn
H
H
7
H
OH
Me
H
H
8
H
F
H
F
H
9
F
H
F
H
H
10
H
F
F
H
H
11
F
OMe
H
H
H
12
OMe
H
H
Ac
H
13
OMe
H
H
CHOHCH3
H
14
H
OH
H
H
H
15
H
OBn
H
H
H
16
Me
H
H
H
H
17
H
H
COOH
H
H
18
H
H
CONHBu
H
H
19
H
H
CONHBn
H
H
Fortuneonoside E
H
OH
OGly
H
H
20
H
OH
CHO
H
H
21
H
OH
COOH
H
H
22
H
OH
COOEt
H
H
23
H
OH
CONHBn
H
H
24
H
OH
Me
H
H
25
H
F
H
F
H
26
F
H
F
H
H
27
H
F
F
H
H
28
F
OMe
H
H
H
29
F
OH
H
H
H
30
OMe
H
H
Ac
H
31
OMe
H
H
CHOHCH3
H
32
H
OH
H
H
H
33
Me
H
H
H
H
34
H
H
COOH
H
H
35
H
H
CONHBu
H
H
36
H
H
CONHBn
H
H
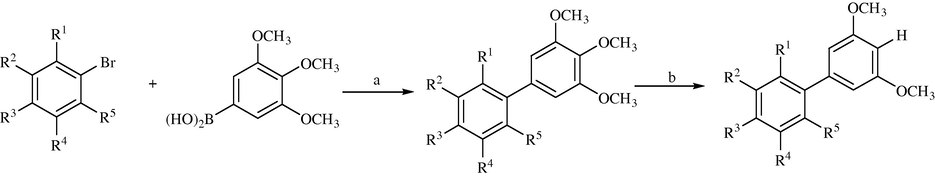
Synthesis of biphenyls 1–19 and 20–36. Reagents and conditions: (a) K2CO3 (2.0 equiv), Pd(OAc)2 (0.01 equiv), DMF, 110 °C; (b) MgI2 (3.0 or 6.0 equiv), 80 °C.
Compound No.
Route
R
1
A
(CH2)2CH3
2
A
(CH2)3CH3
3
A
CH(CH3)2
4
A
CH2CH(CH3)2
5
A
Cyclohexyl
6
A
CH(Cyclohexyl)2
7
B
N(iPr)2
8
B
N(iBu)2
9
B
N(Phenyl)2
10
B
N(Cyclohexyl)2
11
B
NH(Adamantyl)
12
B
NH(3,5-bis(trifluoromethyl)phenyl)
13
B
Br
14
B
Cl
Compound No.
Ar
Ar′
1
C6H5−
C6H5−
2
C6H5−
3-NO2-C6H4−
3
C6H5−
4-Cl-C6H4−
4
C6H5−
3-Br-C6H4−
5
C6H5−
4-OCH3C6H4−
6
3-Br-C6H5−
C6H5−
7
3-Br-C6H5−
3-NO2C6H5−
8
3-Br-C6H5−
4-Cl-C6H4−
9
3-Br-C6H5−
3-Br-C6H4−
10
3-Br-C6H5−
4-OCH3C6H4−
11
4F-C6H5−
C6H5−
12
4F-C6H5−
3-NO2-C6H4−
Compound
R1
R2
R3
1
H
H
H
2
CH3
H
H
3
H
CH3
H
4
H
H
CH3
5
OCH3
H
H
6
H
H
OCH3
7
H
Cl
H
8
H
H
Cl
9
H
H
F
10
H
Cl
F
11
H
H
Br
3.5 The biphenyl structure is another steroidal A–C ring mimetic, which has been successfully used for the design of CYP 17 (P450 17) inhibitors (Zhuang et al., 2000; Wachall et al., 1999). Consequently, the syntheses of a series of substituted biphenyl-4-carboxylic acids designated as 5aR inhibitors have been described. Compounds 5,9 and 11 also showed good inhibition values (IC50 values of 0.64, 2.33, and 1.9 mM, respectively). Contrary to the results observed for type 2 isozyme, most of the biphenyl compounds exhibited a poor inhibitory activity versus human type 1 isozyme. The most active compound toward this enzyme was the dicyclohexyl amide 10 exhibiting 44% inhibition. Surprisingly the halogenated compounds 13 and 14 did not show significant inhibition toward human type 1 enzyme (DU 145 cells). It is striking that the biphenyl compounds are weak inhibitors of the human type 1 enzyme (Picard et al., 2002).
Two routes A and B were used for the syntheses of the substituted biphenyl-4-carboxylic acids 1–14 (Scheme 9). Route A is based on two successive Friedel–Crafts acylations. In the first one, biphenyl was reacted with the corresponding acid chlorides to yield compounds i. Subsequent reaction with oxalylchloride and methanol gave the corresponding methoxycarbonylbiphenylketones ii from which the corresponding carboxylic acids 1–14 were obtained by saponification. For route B, a Suzuki cross coupling reaction between 4-formyl-boronic acid and the corresponding N-substituted 4-bromobenzamides using Pd(Ph3P)4 as catalyst14 was applied. Compounds 13 and 14 were prepared as described (Tanaka et al., 1998). The substituted biphenyl-4-carboxaldehydes 7–14 were subjected to a Lindgren oxidation using NaClO2 as oxidative agent and 2-methyl-2-butene (Bal et al., 1981) or H2O2 (35%) (Dalcanale and Montanari, 1986) as a hypochloric acid scavenger to yield the corresponding carboxylic acids 7–14.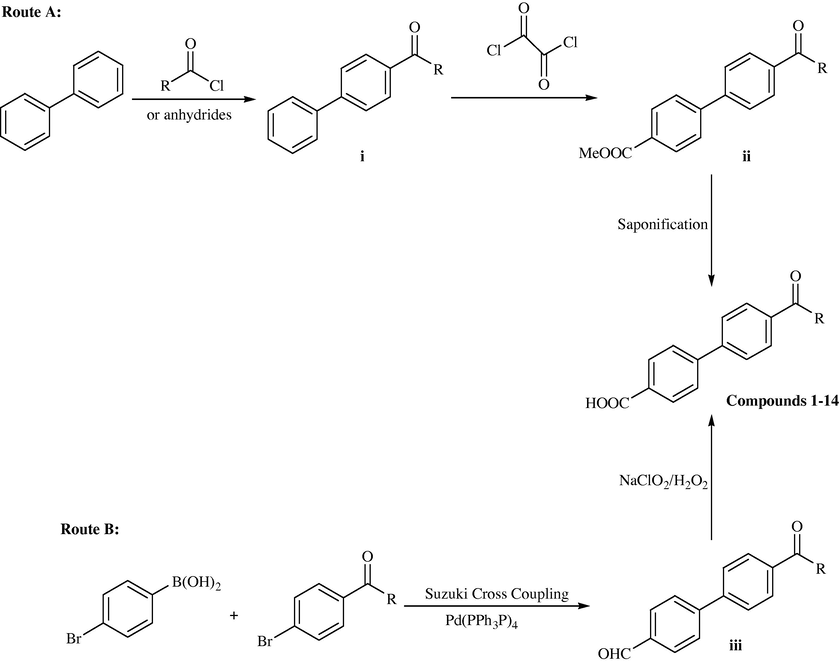
Synthesis of 4′-substituted biphenyl-4-carboxylic acids 1–14.
Traditionally, Friedel–Crafts acylation of aromatic compounds (Scheme 9, route A) is carried out with classic Lewis acid catalysts such as AlCl3, BF3, etc. However, these acids do not behave as true catalysts since they areconsumed in stoichiometric amounts due to the formation of an adduct, and the ensuing separation of the product by hydrolysis generates a great deal of environmentally unfriendly waste. One of the advantages of using zeolites as catalyst is the possibility of providing shape selectivity. Chiche et al. (1986) obtained higher p-isomer yields when using CeNaY zeolite than with an AlCl3 catalyst. Also, Andy et al. (2000) demonstrated shape selectivity in the acylation of 2-methoxynaphthalene over Hβ by increasing the selectivity towards the 2,6-isomer by surface passivated Hβ zeolites. Unlike alkylation, acylation with zeolites may not need such a rigid control of both the SiO2/Al2O3 ratio of the zeolite and the operation conditions (temperature and propylene pressure) in order to avoid deactivation and decrease of selectivity towards 4,4′-disubstituted product (Sugi et al., 1999). Acylation has been shown to occur over zeolites with a wide range of reaction conditions and SiO2/Al2O3 ratios (Harvey and Maeder, 1992). Escola1 and Davis (2001) reported the results obtained in the acylation of biphenyl with acetic anhydride over zeolite Hβ, HY and Nafion/silica as well as a study of the main variables affecting the performance of the catalyst. In addition, the acylation of biphenyl with both hexanoic and trimethylacetic acid have been investigated (see Schemes 10 and 11).![Synthetic scheme for various chloromethylated biphenyls [65].](/content/184/2017/10/2_suppl/img/10.1016_j.arabjc.2013.07.035-fig13.png)
Synthetic scheme for various chloromethylated biphenyls [65].
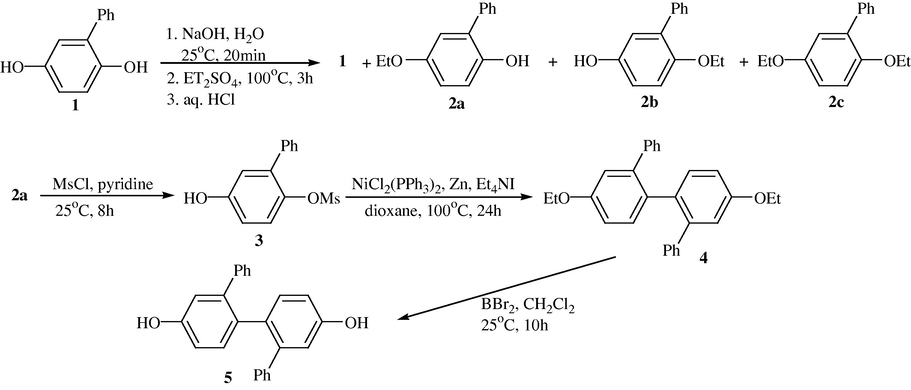
Synthetic route of 2,2′-Diphenyl-4,4′-dihydroxybiphenyl from Phenylhydroquinone. Yields: 2a = 49%, 3 = 90%, 4 = 66%, 5 = 91%.
3.6 The chloromethylation of biphenyl was examined with hydrochloric acid and trioxane as formaldehyde precursor by using some group 3 and 4 metal triflates as catalyst. The mixture of 4-chloromethylbiphenyl (Ia), 2-chloromethylbiphenyl (Ib), 4,4′-bis(chloromethyl)biphenyl (IIa) and 2,4′-bis(chloromethyl)biphenyl (IIb) obtained as the products. Each product was separated by silica gel column chromatography using hexane–chloroform (3/1 then 1/1) as eluent. Some group 3 and 4 metal triflates are highly active for the chloromethylation of biphenyl (BP) and 9,10-dihydrophenanthrene (DHP). The catalytic activity decreased in the order: Sc(OTf)3 > Yb(OTf)3 > Sm(OTf) >> In(OTf)3 > Hf(OTf)4. Sc(OTf)3 had the highest activity among them. The catalysts were stable under the reaction conditions, and catalytic amount of triflates was effective for the chloromethylation of BP and DHP. BP yielded a mixture of 4-chloromethylbiphenyl (Ia), 2-chloromethylbiphenyl (Ib), 4,4′-bis(chloromethyl)biphenyl (IIa) and 2,4′-bis(chloromethyl)biphenyl (IIb). The selectivity for IIa was around 70% among its isomers for all triflates (Kishida et al., 2006).
The catalysis by triflates occurs in heterogeneous mixture of hydrocarbon, trioxane and the triflate in aqueous hydrochloric acid. Since the triflate stayed in aqueous phase during the reaction, the catalyst solution was easily separated from the products and recycled for a new reaction. The addition of small amount of formic acid and acetic acid enhanced the chloromethylation of Ia to increase the yield of IIa (Kishida et al., 2006).
3.7 A new route for the synthesis of 2,2′-diphenyl-4,4’-dihydroxybiphenyl is described. This procedure starts from commercially available 2-phenol (33% overall yield) in five steps, in which the nickel(0) catalyzed homocoupling of the aryl mesylate of 4-protected-2-phenylhydroquinone was used as the key step reaction (Bae, 1998).
4 Pharmacologicaly active biphenyl containing compound derivatives
4.1 Antihypertensive activity
The renin–angiotensin system (RAS) (Quan et al., 1995) is known to play an important role in cardiovascular regulation (Claud et al., 1993) and the maintenance of blood pressure and electrolyte balance. AngiotensinII (AngII) (Quan et al., 1994) is active hormone of RAS and it mediates a variety of physiological functions through stimulation of specific receptors. There are at least two distinct receptor sub-types designed as AT1 and AT2. The AT1 receptor mediates most of the known AngII physiologic functions such as vasocontriction and aldosterone release. The potential role for non peptide AngII receptor antagonists in the treatment of hypertension has well been demonstrated by AT1-selective AngII such as losartan.
The compounds reported in this paper, 1, 2, 3 and 4 (Fig. 4) were the final compounds and prepared for antihypertensive activity. Taking Losartan as lead compound, the benzene ring with imidazole was fused and the coupling reaction with 4-chloromethyl biphenyl 2′- carboxylic acid was carried out to get the resulting compounds which show hypertensive activity. At the position 2, 2-phenyl and 2- ethyl were taken. From the study it was found that compounds that contain ethyl at position –2 gave better result than 2-phenyl. In the biphenyl ring carboxylic group at ortho position is necessary for pharmacological activity. At 2′ position tetrazolyl moiety had the best pharmacological activity was found in the literature. At position 5 of benzimidazole NH2 group gives good activity (Kumar et al., 2006).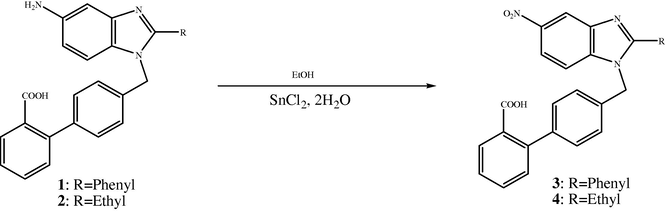
Chemical structure of biphenyl carboxylic benzimidazole derivatives 1,2,3 and 4.
4.2 Combination of antihypertensive activity and calcium channel blocking activity (Mojarrad et al., 2011)
Angiotensin receptor blockers (ARBs) such as Losartan and Telmisartan (Fig. 5) are potent chemicals, which antagonize angiotensin II (Ang II) by preventing Ang II from binding to Ang II receptor (AT1) on vascular smooth muscle. As a result, blood vessels dilate and blood pressure is reduced. The vasodilatation actions of ARBs are due to reduced concentration of intracellular Ca2+ ions (Williams and Lemke, 2002).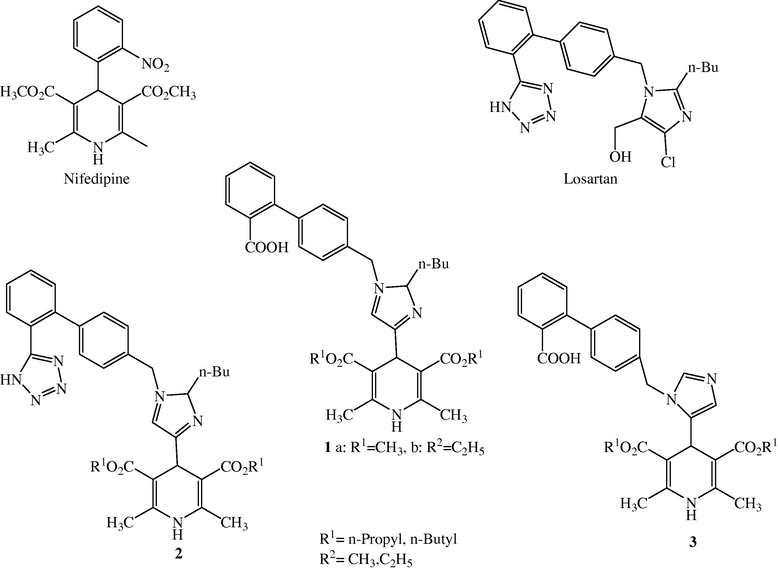
Chemical structures of Nifedipine, Losartan and designed dual CCB-ARB (Compounds 1,2,3).
On the other hand, 1,4-dihydropyridines (DHP) containing substituted heterocycles on the C4 position, such as Nifedipine (Shafiee et al., 1997, 2002a,b; Foroumadi et al., 2002; Hosseini et al., 2007; Mojarrad et al., 2004, 2005), have shown to reduce the influx of extracellular Ca2+ ions through the L-type potential-dependent calcium channel therefore reducing the hypertension (Williams and Lemke, 2002).
It has been hypothesized that merging the key structural elements present in an AT1 receptor antagonists such as [2′-(acidic moiety)biphenyl-4-yl] imidazole pharmacophores with key structural elements in 1,4-dihydropyridine calcium channel blockers (CCBs) would yield compounds with potential dual activity for both receptors. Advantages of combination therapy of ARBs and CCBs which include low dose, low side effect, cardioprotection, renoprotection and anti-atherosclerosis are reported in literatures (Hasebea and Kikuchi; 2005; Okuda et al., 2005; Swales and Williams, 2002).
4.3 Anti inflammatory activity
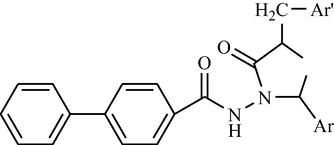
Some new biphenyl-4-carboxylic acid 5-(arylidene)-2-(aryl)-4-oxothiazolidin-3-yl amides have been synthesized and evaluated for anti-inflammatory activity. Biphenyl-4-carboxylic acid hydrazide was converted to the corresponding aryl hydrazones using aryl aldehydes in the presence of catalytic amount of glacial acetic acid. The aryl hydrazones on reaction with thioglycolic acid in the presence of anhydrous zinc chloride yielded the biphenyl-4-carboxylic acid 2-(aryl)-4-oxothiazolidin-3-yl amides, which again on reaction with aromatic aldehydes in the presence of anhydrous sodium acetate and glacial acetic acid furnished the title compounds (Deep et al., 2010a).
4.4 Diuretic activity
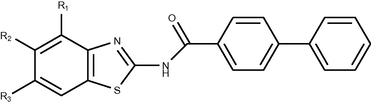
Diuretics are important class of drugs used in the treatment of edema, heart failure or in hepatic, renal or pulmonary disease. They are used, alone or in combination of antihypertensive agents, in the treatment of high blood pressure. So there exist urgent clinical requirements for novel, selective diuretics (high ceiling (loop)/potassium sparing/ osmotic) devoid of many of the unpleasant side effects viz. hypokalemia, hyperuricemia etc. associated with current diuretic regimens. A series of N-{(substituted)1,3-benzothiazol-2-yl}-1,1i-biphenyl-4-carboxamides was synthesized by reaction between biphenyl acid chloride and 2-aminobenzothiazole (Yar and Ansari, 2009).
4.5 Anti microbial activity: biphenyl hydrazide–hydrazone
Development of novel chemotherapeutic agents is an important and challenging task for the medicinal chemists and many research programs are directed towards the design and synthesis of new drugs for their chemotherapeutic usage. Hydrazone compounds constitute an important class for new drug development in order to discover an effective compound against multidrug resistant microbial infection. Here, the synthesis of the novel derivatives of biphenyl-4-carboxylic acid hydrazide hydrazone [Deep et al., 2010b] (see Fig. 6).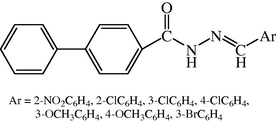
Chemical structure of biphenyl-4-carboxylic acid hydrazide-hydrazone derivatives.
4.6 Anti microbial activity: thiazolidinone
The design of novel chemotherapeutic agents is particularly beneficial due to their dissimilar mode of action which can avoid cross resistance to known drugs. 4-thiazolidinones have received considerable attention due to their wide range of biological activities. Some derivatives of biphenyl-4-carboxylic acid 2-(aryl)-4-oxo-thiazolidin-3-yl–amide were synthesized and studied for their antimicrobial activity. These compounds were prepared from biphenyl-4-carboxylic-acid hydrazides. Biphenyl-4-carboxylic-acid hydrazides on refluxing with aryl aldehydes in the presence of catalytic amount of glacial acetic acid furnish the biphenyl-4-carboxylic acid hydrazone. The aryl hydrazones on reaction with thioglycolic acid in the presence of anhydrous zinc chloride yielded the biphenyl-4-carboxylic acid-2-(aryl)-4-oxo-thiazolidin-3-yl-amides (Fig. 7) (Madhukar et al., 2009) (see Fig. 8).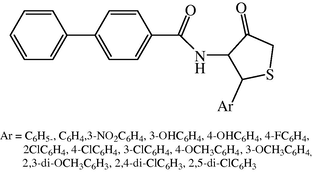
Chemical structure of 2-substituted-4-thiazolidinones.
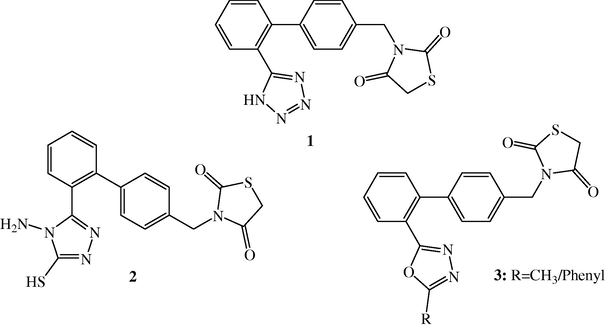
Chemical Structures of biphenyl tetrazole derivative 1; 1,3,4-triazole derivative 2 and 1,3,4-oxadiazole derivative 3.
4.7 Anti Diabetic Activity
Non-insulin-dependent diabetes mellitus (NIDDM) is a metabolic disorder characterized by hyperglycemia as well as insulin resistance and/or impaired insulin secretion (De Fronzo, 1988; DeFronzo et al., 1983). Therapy for NIDDM has been aimed at improving glycemic control by a combination of diet, exercise, and oral agents (Gerich, 1989). These oral agents stimulate the secretion of insulin in response to glucose and may have extra pancreatic effects as well (Joost, 1985; Beck-Nielsen et al., 1988).
One such strategy is to design and synthesize inhibitors for protein tyrosine phosphatase 1B (PTP 1B), which is a legitimate target for the treatment of Type 2 diabetes by attenuating insulin resistance. PTP 1B inhibitors could potentially ameliorate insulin resistance and normalizes plasma glucose and insulin without inducing hypoglycemia and could be potential pharmacological agents for the treatment of obesity and Type 2 diabetes mellitus (Non insulin dependent diabetes mellitus). Following, is the structure of biphenyl ethanone derivatives (Table 10) whose synthesis and structural characteristics has been reported in reference (Sachan et al., 2009).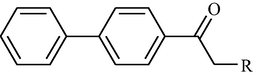
Compound No
R
Compound No
R
a
i
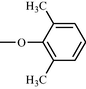
b
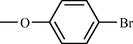
j
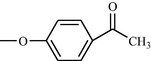
c
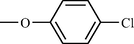
k
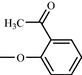
d
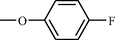
l
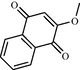
e
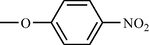
m
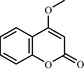
f
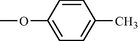
n
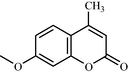
g
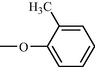
o
h
During the last few decades, considerable attention has been devoted also to the synthesis of 1,2,4-triazole derivatives possessing antibacterial, antifungal, anticancer and antiviral activities (Demirba et al., 2002; El-Essawy et al., 2008). The oxadiazole chemistry has been developed extensively. Presently, there are large number of drugs used clinically which comprise 1,3,4-oxadiazole moiety in association with various heterocyclic rings. Also, it has been established that coupling of two or more biodynamic moieties results into enhanced pharmaceutical property. In the light of these observations, the present paper illustrates the synthesis of 1,3-thiazolidin-2,4-dione derivatives containing biphenyl ring system derivatised with the tetrazole (1), 1,2,4-triazoles (2), and 1,3,4- oxadiazole (3). Also, the tetrazole moiety functions as a carboxylic acid isostere (Bradbury et al., 1993), which imparts greater metabolic stability and increased absorption relative to the carboxylic acid (Kamble et al., 2011).
4.8 Antipsychotic and anxiolytic activity
In the present study, we describe an alternate synthesis and further pharmacological characterization of a recently reported positive allosteric modulator of Metabotropic Glutamate Receptor Subtype 2 (mGluR2) termed biphenyl-indanone A (BINA). In recombinant systems, BINA produced a robust and selective potentiation of the response of mGluR2 to glutamate with no effect on the glutamate response of other mGluR subtypes. In hippocampal brain slices, BINA (1 μM) significantly potentiated the mGluR2/3 agonist-induced inhibition of excitatory synaptic transmission at the medial perforant path-dentate gyrus synapse. BINA was also efficacious in several models predictive of antipsychotic- and anxiolytic-like activity in mice. These results indicate that BINA is a selective mGluR2 positive allosteric modulator and provide further support for the growing evidence that selective allosteric potentiators of mGluR2 mimic many of the in vivo actions of mGluR2/3 agonists that may predict therapeutic utility of these compounds. In the present study, BINA also mimicked the effects of group II mGluR agonists and mGluR2 allosteric potentiators in animal models used to predict potential antipsychotic-like and anxiolytic-like activity (Ruggero et al., 2006) (see Fig. 9).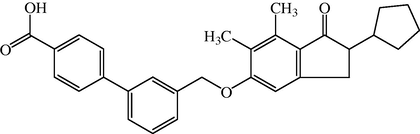
Chemical structure of Biphenylindanone A (BINA).
4.9 Polymer formation
The high molecular weight biphenyl-based polyarylene ether nitrile (PEN) copolymers were synthesized by nucleophilic substitution reaction of 2,6-dichlorobenzonitrile (DCBN) with varying molar ratios of 4,4′-dihydroxybiphenyl (BP) and hydroquinone (HQ) (Fig. 10). The BP content of the copolymers has influence on glass transition temperature (Tg), initial decomposition temperature (Tid), mechanical properties and the crystallinity. All the copolymers could be dissolved in NMP (N-methyl pyrrolidone), DMF (N,N-dimethyl formamide) and DMAc (Dimethyl acetate) on heating, and were stable up to 450 °C with a high char yield above 50% at 800 °C in nitrogen atmosphere. The glass transition temperature, the melting temperature and tensile strength of copolymers were found to increase with increase in concentration of the BP units in the polymer (Liu et al., 2007) (see Figs. 8 and 9).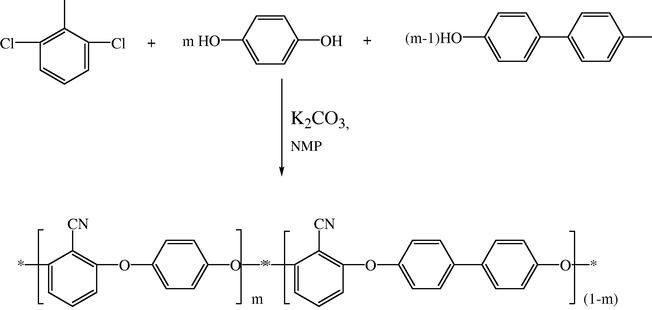
Polymerization of PEN (BP/HQ) copolymer.
4.10 Anti cancer activity
Biphenyl derivatives have also been found to show anti cancer profile (against breast cancer). Within in the literature so far, a range of derivatives include nitro, chalcone, bis indolyl, triclosan, eugenol based biphenyls and the series can further be explored.
5 Conclusion
It is concluded that biphenyls can be easily synthesized as well as functionalized by the use of adequate catalysts such as palladium derivatives, suitable reagents and reactants such as haloarenes. Also, the substituted biphenyls can be synthesized by Suzuki cross coupling reactions. The substituted biphenyls in turn can be used as an intermediate in the synthesis of pharmacologically active molecules. Compounds containing biphenyl moiety possess a wide range of activity such as antihypertensive, antimicrobial, diuretic, anti diabetic, antipsychotic and anxiolytic etc. Thus, there is a wide scope for the synthetic scientists and chemists to synthesize more and more compounds with different substitutions using substituted biphenyl along with an adequate heterocycle as a basic moiety. The investigation on this ring can still be continued and a number of such therapeutic importances of the biphenyl moiety can be explored.
Acknowledgements
The authors thank Mr. Bhavin. N. Chaudhari and Ms. Rohini Kakad, M.Pharm, Department of pharmaceutical chemistry, M.E.T’s Institute of Pharmacy, Nashik for excellent technical assistance and critical proof reading of the manuscript; Dr Paraag. S. Gide, Principal, M.E.T’s Institute of Pharmacy, Nashik; The Trustee, Bhujbal Knowledge City, M.E.T’s Institute of Pharmacy, Nashik for providing the necessary facilities for conduct of review work.
References
- J. Catal.. 2000;192:215.
- J. Ind. Eng. Chem.. 1998;4:319.
- Tetrahedron. 1981;37:2091.
- Bioorg. Med. Chem.. 2010;18:6708.
- Org. Biomol. Chem.. 2009;7:5084.
- J. Med. Chem.. 1993;36:1245.
- Angew. Chem.. 1989;101:466.
- Angew. Chem. Int. Ed.. 1989;28:486.
- Tetrahedron. 2007;63:5614.
- Int. J. Mol. Sci.. 2009;10:2440.
- J. Org. Chem.. 1986;51:2128.
- J. Med. Chem.. 1993;36:3371.
- Angew. Chem.. 1988;100:1041.
- Angew. Chem. Int. Ed.. 1988;27:1009.
- J. Org. Chem.. 1986;51:567.
- Diabetes. 1988;37:667.
- Acta Pol. Pharm. Drug Res.. 2010;67:63.
- Acta Pol. Pharm. Drug Res.. 2010;67:255.
- Am. J. Med.. 1983;75:52.
- Bioorg. Med. Chem.. 2002;10:3717.
- Naturforsch A S. 2008;63:667.
- Appl. Catal. A. 2001;214:111.
- IL Farmaco. 2002;57:195.
- Engl. J. Med.. 1989;321:1231.
- Goto, Y., Kitano, K., 1991. European Patent 387, 032.
- J. Fluorine Chem.. 2006;127:177.
- Coll. Czech. Chem. Commun.. 1992;57:862.
- J. Hypertens.. 2005;23:445.
- Tetrahedron. 2001;57:7845.
- Org. Lett.. 1999;1:1205.
- Org. Process Res. Dev.. 1998;2:96.
- Liquid Cryst.. 1992;4:531.
- Phys. Rev. Lett.. 2000;85:2777.
- Synth. Met.. 2000;110:141.
- Arch. Pharm.. 2007;340:549.
- J. Org. Chem.. 1995;60:7508.
- J. Chem. Theory Comput.. 2008;4:1460.
- Trends Pharmocol Sci.. 1985;6:239.
- Diabetic Med.. 1988;5:613.
- Chem. Express. 1991;6:229.
- Chem. Express. 1995;5:645.
- J. Chem. Sci.. 2011;123:393-401.
- Bioorg. Med. Chem.. 2007;15:2708.
- Tetrahedron Lett.. 1975;16:3375.
- Bioorg. Med. Chem.. 2006;14:6027.
- Bioorg. Med. Chem.. 2006;14:938.
- Cell. Mol. Life Sci.. 2005;62:1707.
- J. Mol. Catal. A: Chem.. 2006;246:268.
- Eur. J. Chem.. 2006;3:278.
- Bull. Korean Chem. Soc.. 2001;22:375.
- Express Polym. Lett.. 2007;1:499-505.
- Int. J. ChemTech Res.. 2009;1:1376.
- Metasynthesis, 2012. The chemical thesaurus. <http://www.chemthes.com/rxn-type_dp.php> (accessed 14.05.12).
- Chromatography. 1978;149:455.
- Chem. Rev. 1995:2457.
- Adv. Pharm. Bull.. 2011;1:9.
- Bioorg. Med. Chem.. 2004;12:3215.
- Bioorg. Med. Chem.. 2005;13:4085.
- J. Chem. Soc. Perkin Trans.. 2000;2:1809.
- Org. Lett.. 2000;2:211.
- Tetrahedron Lett.. 2001;42:6117.
- Tetrahedron. 1999;55:14763.
- Liquid Cryst.. 1991;10:785.
- Bull. Chem. Soc. Jpn.. 1980;53:1767.
- Synthesis. 2000;2:243.
- J. Organomet. Chem.. 2002;664:59.
- Chem. Lett.. 2001;212:9.
- Bioorg. Med. Chem. Lett.. 2006;16:2252.
- Hypertens. Res.. 2005;28:431.
- Arkivoc. 2008;9:69-93.
- Pagan, A., 2012. <http://mustang.millersville.edu/∼iannone/research/Pagan07.pdf> (accessed 30.05.12).
- Bioorg. Med. Chem.. 2002;10:437.
- Org. Lett.. 2004;6:2777.
- Adv. Synth. Catal.. 2002;344:1079.
- J. Med. Chem.. 1995;38:2938.
- Bioorg. Med. Chem.. 1994;4:2011.
- J. Pharmacol. Exp. Ther.. 2006;318:173.
- Int. J. ChemTech Res.. 2009;1:1625-1631.
- Ind. J. Chem.. 1997;36:813.
- Arzneimittelforschung. 2002;52:537.
- Arch. Pharm.. 2002;335:69.
- J. Am. Chem. Soc.. 1958;80:1154.
- Chem. Pharm. Bull.. 2003;51:318.
- Appl. Catal. A. 1999;189:251.
- J. Renin-Angiotensin-Aldosterone Syst.. 2002;3:79.
- J. Am. Chem. Soc.. 1984;106:5267.
- Bioorg. Med. Chem.. 1998;6:15.
- Justus Liebig’s Ann. der Chemie. 1904;331:38.
- Bioorg. Med. Chem.. 1999;7:1913.
- Langmuir. 1998;14:1284.
- Foyés Principles of Medical Chemistry (fifteenth ed.). Philadelphia: Lippincott Williams & Wilkins; 2002. pp. 533–561
- J. Am. Chem. Soc.. 1999;121:9550.
- Surf. Sci.. 1992;278:19.
- Tetrahedron Lett.. 1988;29:1797.
- Synlett 1989:18.
- Surf. Sci.. 1995;341:9.
- J. Org. Chem.. 2005;70:3511.
- Acta Pol. Pharm. Drug Res.. 2009;66:387.
- J. Org. Chem.. 2003;68:3729.
- Tetrahedron Lett.. 2008;49:2734.