Translate this page into:
Bis-indole based triazine derivatives: Synthesis, characterization, in vitro β-glucuronidase anti-cancer and anti-bacterial evaluation along with in silico molecular docking and ADME analysis
⁎Corresponding author. sono_waj@yahoo.com (Wajid Rehman)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
The present work described the synthetic procedure adopted for the synthesis of bis-indole-based triazine derivatives via a series of reactions. All the compounds were synthesized through a stepwise reaction. After being confirmation through thin-layer chromatography (TLC) these compounds were characterized through various spectroscopic techniques including 1HNMR, 13CNMR and HREI-MS and evaluated against beta-glucuronidase in the presence of standard drug D-saccharic acid 1,4 lactone (IC50 = 31.2 ± 1.0 µM). Most of the synthesized derivatives were found with better to good inhibitory potentials (IC50 = 5.30 ± 2.0 µM to 33.10 ± 1.0 µM). SAR explains better results of analogs due to the presence of varied substituents on the aromatic rings. In this regard, the excellent potential was shown by analogs 1, 3, 6, 8 and 9 with IC50 = 5.30 ± 2.0, 7.10 ± 4.0, 6.10 ± 3.0, 8.40 ± 1.0 and 7.20 ± 3.0 µM respectively). In addition, all the synthesized analogs were evaluated for anti-cancer and anti-bacterial (E. coli) activities in which the targeted compounds were found with significant potentials in the presence of standard drugs Tetrandrineb (IC50 = 1.37 ± 0.10 µM) and streptomycin respectively. These derivatives were further subjected to molecular docking studies in order to investigate better binding sites with distance. Additionally, ADME properties for the synthesized compounds were also explored the drug like properties of the synthesized compounds.
Keywords
Indole
Triazine synthesis
SAR
β-Glucuronidase
Molecular docking & ADME
1 Introduction
The enzyme beta-glucuronidase catalyzes the glycosaminoglycans in the cell membrane as well as the extracellular matrix of both cancerous and healthy cells. The proliferation, invasion, and metastasis of cancer cells are all impacted by this catalysis (Khan et al., 2011; Chojnowska et al., 2011). The rise in β-glucuronidase activity is what causes liver cancer, colon carcinoma, and neoplastic bladder (Szajda et al., 2008; Kim & Jin 2001; Cheng et al., 2012; Zalewska-Szajda et al., 2018). In real-time monitoring, therapeutic therapy, early-stage diagnostics, and as a tumor marker, lucuronidase has been employed as a key tool (Juan et al., 2009; Murdter et al., 2002). Numerous diseases, including epilepsy (Falkenbach et al., 1993) and other physiological disorders, are linked to high levels of β-glucuronidase. Increased levels of β-glucuronidase result in liver injury, which is a significant problem worldwide (Byass 2014). The accumulation of glycosaminoglycans with glucuronic acid residues, which results in lysosomal storage in the brain, is the cause of mucopolysaccharidosis type VII (MPSVII, also known as Sly syndrome), which is brought on by a GUS deficiency in the human body (Hassan et al., 2013; Naz et al., 2013). Inhibiting this enzyme is crucial for preventing such a physiological problem. One of the key areas in medicinal chemistry is the study of heterocyclic molecules. They hold a significant place in the structures of many different natural products. Indole, one of the most useful structures, has drawn a lot of interest due to its pharmacological action and has been regarded as an essential scaffold (Cacchi & Fabrizi 2005; Imran et al., 2015; Elsonbaty et al., 2021). Several compounds based on indoles have been studied for their potential anticancer properties (Dadashpour & Emami 2018). In recent years, indole derivatives have become extremely important drugs. They are widely known for their important biological actions, including their cytotoxic, anti-bacterial, antidiabetic, and anti-inflammatory effects (Sravanthi & Manju 2016; Prakash et al., 2018; Singh & Singh 2018; Goyal et al., 2018). Our research group also works on the varied synthetic procedures of varied heterocyclic compounds (Khan et al., 2022; Khan et al., 2022; Khan et al., 2022; Khan et al., 2022; Khan et al., 2022; Hussain et al., 2022; Mumtaz et al., 2022; Khan et al., 2022; Hussain et al., 2022; Khan et al., 2023; Khan et al., 2022; Adalat et al., 2023; Khan et al., 2022; Khan et al., 2022).
In perspective of above details, in the current study we have planned to synthesize the novel bis-indole based triazine derivatives their characterization, beta-glucuronidase, anti-cancer and anti-bacterial activity, molecular docking and ADME-studies.
2 Results and discussion
2.1 Chemistry
Bis-indole bearing di-ketone moiety (I) was mixed along with thiosemicarbazide in ethanol, and the reaction mixture was refluxed condition for about 8hrs in the presence of potassium carbonate, afforded six-membered hydrazine bearing thiol group as an intermediate moiety (II) (Abdel‐Rahman et al., 2015). This intermediate moiety (II) were further mixed with varied substituted phenacyl bromide in ethanol in the presence of triethylamine (ET3N) and the reaction mixture were refluxed for about 6hrs to obtain bis-indole-based triazine derivatives (1–12). The synthesized derivatives (Scheme 1) (1–12) were collected and washed with n-hexane, given fine powder and were characterized through varied spectroscopic techniques such as 1HNMR, 13CNMR and HREI-MS.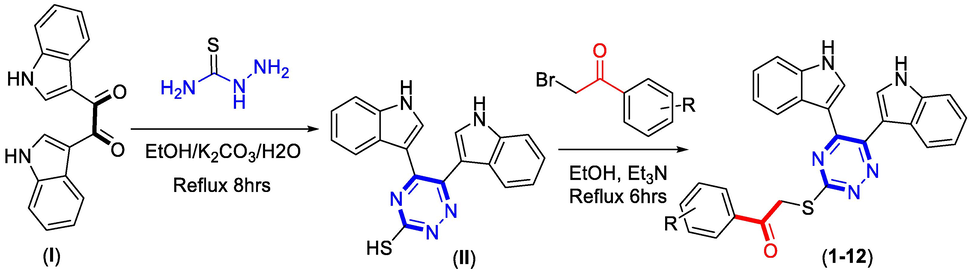
Represent adopted route for synthesis of bis-indole based triazine derivatives.
2.2 Material and methods
All the chemicals were purchased from Sigma Aldrich, USA. Avance Bruker machine was used to performed NMR experiment. Finnigan MAT-311A (Germany) mass spectrometer was used to recorded high resolution electron impact mass spectra (HR-EIMS). Thin layer chromatography (TLC) was done on pre-coated silica gel aluminium plates (Kieselgel 60, 254, E. Merck, Germany). UV at 254 and 365 nm was used to visualize the chromatograms.
2.3 Characterizations of bis-Indole based triazole derivatives (1–12)
2.3.1 2-((5,6-di(1H-Indol-3-yl)-1,2,4-triazin-3-yl)thio)-1-(4-(trifluoromethyl)phenyl)ethan-1-one (1)
Light yellow, 1H NMR (600 MHz, DMSO‑d6): δ 12.19 (s, 2H, N–H), 8.83 (s, 2H, Indole-H (2A/ 2B)), 8.52 (dd, J = 7.8, 2.4 Hz, 2H, Indole-H 4A/ 4B), 7.94 (dd, J = 8.2, 3.2 Hz, 2H, Indole-H 7A/ 7B), 7.90 (d, J = 7.8 Hz, 2H, Ar-H), 7.62–7.71 (m, 4H, Indole-H (5,6A/ 5,6B)), 7.18 (d, J = 6.9 Hz, 2H, Ar-H), 4.69 (s, 2H, CH2-S); 13C NMR (150 MHz, DMSO‑d6): δ 191.9, 182.6, 180.4, 177.6, 177.5, 175.0, 173.2, 171.1, 166.8, 165.6, 164.4, 162.9, 160.4, 160.1, 159.5, 159.3, 159.0, 158.9, 154.5, 150.5, 148.9, 146.6, 146.4, 138.8, 136.8, 135.3, 121.7, 41.6; HREI-MS: m/z calcld for C28H18F3N5OS, [M]+ 529.0153 Found 529.0121.
2.3.2 2-((5,6-di(1H-Indol-3-yl)-1,2,4-triazin-3-yl)thio)-1-(3-methyl-4-(trifluoromethyl)phenyl)ethan-1-one (2)
Light green, 1H NMR (600 MHz, DMSO‑d6): δ 12.04 (s, 2H, N–H), 8.52 (s, 2H, Indole-H (2A/ 2B)), 8.78 (dd, J = 7.3, 3.0 Hz, 2H, Indole-H 4A/ 4B), 7.83 (dd, J = 7.8, 3.0 Hz, 2H, Indole-H 7A/ 7B), 7.80 (s, 1H, Ar-H), 7.71–7.79 (m, 4H, Indole-H (5,6A/ 5,6B)), 7.62 (d, J = 7.1 Hz, 1H, Ar-H), 7.07 (d, J = 7.3 Hz, 1H, Ar-H), 4.72 (s, 2H, CH2-S) 3.03 (s, 3H, CH3); 13C NMR (150 MHz, DMSO‑d6): δ 196.7, 183.1, 180.9, 179.1, 179.0, 178.6, 176.7, 174.6, 169.3, 168.1, 167.9, 165.4, 163.9, 163.6, 161.5, 160.5, 160.3, 158.2, 157.0, 153.0, 151.4, 149.1, 146.5, 142.8, 140.3, 139.8, 126.2, 38.5, 22.4; HREI-MS: m/z calcld for C29H20F3N5OS, [M]+ 543.7236 Found 543.7196.
2.3.3 2-((5,6-di(1H-Indol-3-yl)-1,2,4-triazin-3-yl)thio)-1-(4-hydroxyphenyl)ethan-1-one (3)
White, 1H NMR (600 MHz, DMSO‑d6): δ 12.32 (s, 2H, N–H), 10.36 (s, 1H, Ar-OH), 8.78 (s, 2H, Indole-H (2A/ 2B)), 8.65 (dd, J = 7.7, 3.2 Hz, 2H, Indole-H 4A/ 4B), 8.02 (dd, J = 7.3, 2.9 Hz, 2H, Indole-H 7A/ 7B), 7.78–7.86 (m, 4H, Indole-H (5,6A/ 5,6B)), 7.75 (d, J = 6.8 Hz, 2H, Ar-H), 7.53 (d, J = 7.3 Hz, 2H, Ar-H), 4.72 (s, 2H, CH2-S);13C NMR (150 MHz, DMSO‑d6): δ 195.5, 181.6, 180.7, 179.4, 178.8, 175.8, 174.7, 171.6, 168.7, 166.9, 166.5, 162.7, 160.9, 160.6, 158.4, 157.5, 152.7, 150.4, 149.9, 148.0, 145.6, 144.9, 143.0, 140.3, 139.6, 137.8, 42.5; HREI-MS: m/z calcld for C27H19N5O2S, [M]+ 477.4328 Found 477.4292.
2.3.4 2-((5,6-di(1H-Indol-3-yl)-1,2,4-triazin-3-yl)thio)-1-(3-hydroxy-5-methylphenyl)ethan-1-one (4)
Light gray, 1H NMR (600 MHz, DMSO‑d6): δ 12.36 (s, 2H, N–H), 10.52 (s, 1H, Ar-OH), 8.43 (s, 2H, Indole-H (2A/ 2B)), 8.39 (dd, J = 7.1, 3.5 Hz, 2H, Indole-H 4A/ 4B), 8.21 (dd, J = 6.9, 3.1 Hz, 2H, Indole-H 7A/ 7B), 7.81–7.89 (m, 4H, Indole-H (5,6A/ 5,6B)), 7.58 (s, 1H, Ar-H), 7.42 (s, 1H, Ar-H), 7.38 (s, 1H, Ar-H), 4.72 (s, 2H, CH2-S) 2.95 (s, 3H, CH3); 13C NMR (150 MHz, DMSO‑d6): δ 188.2, 178.5, 173.1, 170.5, 169.3, 168.8, 167.2, 167.1, 164.7, 161.6, 158.2, 157.6, 157.3, 155.4, 154.9, 151.5, 149.4, 149.2, 149.1, 148.9, 145.4, 144.6, 141.6, 138.1, 137.6, 136.4, 49.4, 37.8; HREI-MS: m/z calcld for C28H21N5O2S, [M]+ 491.6456 Found 491.6432.
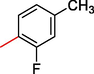
2.3.5 2-((5,6-di(1H-Indol-3-yl)-1,2,4-triazin-3-yl)thio)-1-(3-fluoro-4-methylphenyl)ethan-1-one (5)
Light gray, 1H NMR (600 MHz, DMSO‑d6): δ 12.53 (s, 2H, N–H), 8.42 (s, 2H, Indole-H (2A/ 2B)), 8.72 (dd, J = 7.2, 2.3 Hz, 2H, Indole-H 4A/ 4B), 7.66 (dd, J = 7.0, 3.2 Hz, 2H, Indole-H 7A/ 7B), 7.61 (s, 1H, Ar-H), 7.58–7.65 (m, 4H, Indole-H (5,6A/ 5,6B)), 7.33 (d, J = 7.5 Hz, 1H, Ar-H), 7.17 (d, J = 7.8 Hz, 1H, Ar-H), 4.47 (s, 2H, CH2-S) 3.09 (s, 3H, CH3); 13C NMR (150 MHz, DMSO‑d6): δ 194.5, 186.53 173.2, 172.8, 169.6, 169.4, 166.2, 166.0, 163.3, 162.3, 162.2, 159.7, 153.1, 152.5, 151.6, 148.6, 148.3, 147.4, 147.3, 146.9, 143.2, 141.4, 140.5, 139.8, 138.6, 136.4, 52.6, 38.2; HREI-MS: m/z calcld for C28H20FN5OS, [M]+ 493.5348 Found 493.5307.
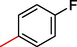
2.3.6 2-((5,6-di(1H-Indol-3-yl)-1,2,4-triazin-3-yl)thio)-1-(4-fluorophenyl)ethan-1-one (6)
Light yellow, 1H NMR (600 MHz, DMSO‑d6): δ 12.46 (s, 2H, N–H), 8.83 (s, 2H, Indole-H (2A/ 2B)), 8.59 (dd, J = 7.3, 2.8 Hz, 2H, Indole-H 4A/ 4B), 7.87 (dd, J = 8.3, 3.3 Hz, 2H, Indole-H 7A/ 7B), 7.72 (d, J = 7.2 Hz, 2H, Ar-H), 7.41–7.50 (m, 4H, Indole-H (5,6A/ 5,6B)), 7.23 (d, J = 7.4 Hz, 2H, Ar-H), 4.31 (s, 2H, CH2-S); 13C NMR (150 MHz, DMSO‑d6): δ 190.5, 182.6, 180.7, 176.4, 175.8, 173.5, 168.2, 164.0, 162.3, 160.6, 160.0, 158.6, 157.9, 157.9, 156.4, 153.5, 149.6, 149.5, 147.2, 147.0, 143.0, 142.9, 140.9, 139.7, 138.6, 136.4, 48.8; HREI-MS: m/z calcld for C27H18FN5OS, [M]+ 479.1264 Found 479.1230.
2.3.7 2-((5,6-di(1H-Indol-3-yl)-1,2,4-triazin-3-yl)thio)-1-(3-methyl-5-nitrophenyl)ethan-1-one (7)
Light yellow, 1H NMR (600 MHz, DMSO‑d6): δ 12.43 (s, 2H, N–H), 8.78 (s, 2H, Indole-H (2A/ 2B)), 8.61 (dd, J = 7.1, 3.5 Hz, 2H, Indole-H 4A/ 4B), 8.34 (dd, J = 8.4, 3.5 Hz, 2H, Indole-H 7A/ 7B), 7.86–7.92 (m, 4H, Indole-H (5,6A/ 5,6B)), 7.72 (s, 1H, Ar-H), 7.69 (s, 1H, Ar-H), 7.54 (s, 1H, Ar-H), 4.72 (s, 2H, CH2-S) 2.95 (s, 3H, CH3); 13C NMR (150 MHz, DMSO‑d6): δ 194.2, 177.5, 164.1, 163.4, 161.6, 160.8, 157.2, 157.0, 155.3, 154.6, 154.0, 151.6, 149.9, 148.9, 148.4, 142.5, 142.4, 140.5, 140.2, 140.0, 137.4, 137.2, 136.5, 133.8, 133.6, 130.4, 46.8, 32.6; HREI-MS: m/z calcld for C28H20N6O3S, [M]+ 520.6373 Found 520.6356.
2.3.8 2-((5,6-di(1H-Indol-3-yl)-1,2,4-triazin-3-yl)thio)-1-(4-nitrophenyl)ethan-1-one (8)
Light yellow, 1H NMR (600 MHz, DMSO‑d6): δ 12.58 (s, 2H, N–H), 8.61 (s, 2H, Indole-H (2A/ 2B)), 8.58 (dd, J = 7.7, 3.1 Hz, 2H, Indole-H 4A/ 4B), 8.02 (dd, J = 7.0, 3.7 Hz, 2H, Indole-H 7A/ 7B), 7.89 (d, J = 7.2 Hz, 2H, Ar-H), 7.66–7.74 (m, 4H, Indole-H (5,6A/ 5,6B)), 7.45 (d, J = 7.0 Hz, 2H, Ar-H), 4.73 (s, 2H, CH2-S); 13C NMR (150 MHz, DMSO‑d6): δ 178.4, 174.7, 161.3, 160.4, 158.7, 157.9, 154.7, 153.8, 152.4, 151.3, 151.0, 148.8, 146.9, 145.9, 145.5, 139.5, 139.2, 137.7, 137.3, 136.9, 134.7, 134.2, 133.4, 130.5, 129.2, 127.2, 42.9; HREI-MS: m/z calcld for C27H18N6O3S, [M]+ 506.2390 Found 506.2354.
2.3.9 1-(4-chlorophenyl)-2-((5,6-di(1H-Indol-3-yl)-1,2,4-triazin-3-yl)thio)ethan-1-one (9)
Light green, 1H NMR (600 MHz, DMSO‑d6): δ 12.47 (s, 2H, N–H), 8.74 (s, 2H, Indole-H (2A/ 2B)), 8.62 (dd, J = 7.2, 3.5 Hz, 2H, Indole-H 4A/ 4B), 8.32 (dd, J = 7.6, 3.0 Hz, 2H, Indole-H 7A/ 7B), 7.95 (d, J = 7.0 Hz, 2H, Ar-H), 7.78–7.83 (m, 4H, Indole-H (5,6A/ 5,6B)), 7.61 (d, J = 6.4 Hz, 2H, Ar-H), 4.82 (s, 2H, CH2-S); 13C NMR (150 MHz, DMSO‑d6): δ 184.7, 179.8, 165.6, 165.2, 163.8, 159.9, 159.7, 158.8, 158.2, 157.4, 154.7, 152.6, 150.7, 146.3, 145.6, 143.4, 142.8, 142.7, 142.3, 141.9, 139.7, 139.2, 138.3, 136.6, 133.4, 135.2, 41.8; HREI-MS: m/z calcld for C27H18ClN5OS, [M]+ 495.0260 Found 495.0224.
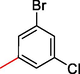
2.3.10 1-(3-bromo-5-chlorophenyl)-2-((5,6-di(1H-Indol-3-yl)-1,2,4-triazin-3-yl)thio)ethan-1-one (10)
Light green, 1H NMR (600 MHz, DMSO‑d6): δ 12.50 (s, 2H, N–H), 8.75 (s, 2H, Indole-H (2A/ 2B)), 8.71 (dd, J = 8.0, 3.4 Hz, 2H, Indole-H 4A/ 4B), 8.65 (dd, J = 7.2, 3.0 Hz, 2H, Indole-H 7A/ 7B), 7.94–8.02 (m, 4H, Indole-H (5,6A/ 5,6B)), 7.81 (s, 1H, Ar-H), 7.72 (s, 1H, Ar-H), 7.69 (s, 1H, Ar-H), 4.79 (s, 2H, CH2-S); 13C NMR (150 MHz, DMSO‑d6): δ 179.9, 175.8, 161.6, 160.9, 158.8, 155.2, 155.8, 154.8, 154.4, 153.4, 150.5, 147.1, 145.6, 141.3, 140.7, 139.2, 138.8, 138.7, 138.3, 138.0, 135.7, 135.4, 133.2, 131.6, 128.6, 118.2, 47.8; HREI-MS: m/z calcld for C27H17 BrClN5OS, [M]+ 574.5279 Found 574.5250.
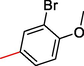
2.3.11 1-(3-bromo-4-methoxyphenyl)-2-((5,6-di(1H-Indol-3-yl)-1,2,4-triazin-3-yl)thio)ethan-1-one (11)
Light brown, 1H NMR (600 MHz, DMSO‑d6): δ 12.44 (s, 2H, N–H), 8.45 (s, 2H, Indole-H (2A/ 2B)), 8.76 (dd, J = 7.9, 2.1 Hz, 2H, Indole-H 4A/ 4B), 7.69 (dd, J = 7.2, 3.4 Hz, 2H, Indole-H 7A/ 7B), 7.64 (s, 1H, Ar-H), 7.61–7.68 (m, 4H, Indole-H (5,6A/ 5,6B)), 7.37 (d, J = 7.1 Hz, 1H, Ar-H), 7.21 (d, J = 7.8 Hz, 1H, Ar-H), 4.47 (s, 2H, CH2-S) 3.97 (s, 3H, OCH3); 13C NMR (150 MHz, DMSO‑d6): δ 182.7, 177.5, 163.3, 162.7, 158.6, 157.9, 157.6, 156.6, 156.4, 154.2, 152.5, 149.1, 147.4, 143.3, 142.8, 141.2, 140.7, 140.6, 140.3, 140.0, 137.5, 137.5, 135.6, 133.6, 133.6, 121.2, 59.5, 44.8; HREI-MS: m/z calcld for C28H20BrN5O2S, [M]+ 570.4236 Found 570.4203.
2.3.12 1-(3-bromo-4-chlorophenyl)-2-((5,6-di(1H-Indol-3-yl)-1,2,4-triazin-3-yl)thio)ethan-1-one (12)
Light brown, 1H NMR (600 MHz, DMSO‑d6): δ 12.52 (s, 2H, N–H), 8.58 (s, 2H, Indole-H (2A/ 2B)), 8.55 (dd, J = 7.6, 3.2 Hz, 2H, Indole-H 4A/ 4B), 7.73 (dd, J = 7.0, 3.9 Hz, 2H, Indole-H 7A/ 7B), 7.58 (s, 1H, Ar-H), 7.67–7.74 (m, 4H, Indole-H (5,6A/ 5,6B)), 7.43 (d, J = 8.2 Hz, 1H, Ar-H), 7.38 (d, J = 8.1 Hz, 1H, Ar-H), 4.59 (s, 2H, CH2-S); 13C NMR (150 MHz, DMSO‑d6): δ 186.9, 179.7, 162.3, 160.1, 153.6, 153.4, 150.6, 150.4, 150.0, 148.7, 144.5, 141.6, 140.0, 139.3, 132.8, 132.7, 130.9, 130.8, 130.5, 130.4, 128.8, 128.6, 126.5, 119.6, 119.4, 117.1, 45.5; HREI-MS: m/z calcld for C27H17BrClN5OS, [M]+ 574.3147 Found 574.3125.
3 Biological profile
3.1 3.1Beta-Glucuronidase inhibitory activity
The aforementioned synthesized derivatives (1–12) were evaluated against beta-glucuronidase to investigate the inhibitory potentials and leads to the potent candidates among the series. The inhibitory profiles was compared with standard drug D-saccharic acid 1,4 lactone (IC50 = 31.2 ± 1.0 µM). In this various substituted derivatives exhibited varied ranges of inhibitory profile (IC50 = 5.30 ± 2.0 µM to 33.10 ± 1.0 µM). Number, nature and position of substituents increase and decrease the inhibitory profile. By this analysis various same substituted derivatives were compared in biological inhibitory profiles as well as with standard drug (Table 1).
S/No
R
IC50 (µM ± SEM
IC50 Anticancer (mM ± SEM)
S/No
R
IC50 (µM ± SEM
IC50 Anticancer (mM ± SEM)
1
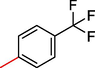
5.30 ± 2.0
0.80 ± 0.10
7
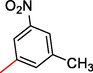
21.30 ± 2.0
10.0 ± 0.30
2
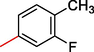
15.30 ± 3.0
4.02 ± 0.20
8
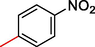
8.40 ± 1.0
3.60 ± 0.10
3
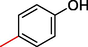
7.10 ± 4.0
1.40 ± 0.10
9
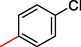
7.20 ± 3.0
3.80 ± 0.10
4
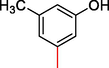
19.30 ± 1.0
7.10 ± 0.20
10
29.10 ± 2.0
16.20 ± 0.20
5
13.10 ± 1.0
9.10 ± 0.10
11
33.10 ± 1.0
16.20 ± 0.30
6
6.10 ± 3.0
1.80 ± 0.20
12
29.20 ± 1.0
17.20 ± 0.10
Standard drug
D-saccharic acid 1,4 lactone (IC50 = 31.2 ± 1.0 µM) Tetrandrineb (IC50 = 1.37 ± 0.10 µM)
Based on the presence of different functionalities molecule was represented via different parts in order to compare the biological profiles of the synthesized derivatives. For this purpose structure activity relationships (SAR) were made on the basis of same substituted compounds in order to make the comparison among the analogs (Fig. 1). In this regard analog-1 (IC 50 = 5.30 ± 2.0 µM) was found with excellent potential when compared with standard drug D-saccharic acid 1,4 lactone (IC50 = 31.2 ± 1.0 µM). The better biological activity of analog-1 might be the presence of triflouro moiety at para-position of aromatic ring. Therefore it was considered as to be much potent analog of the series. Similarly fluoro substituted analogs (2, 5 and 6) exhibited significant biological potential such as IC50 = 15.30 ± 2.0 µM, IC50 = 13.10 ± 1.0 µM and IC50 = 6.10 ± 3.0 µM respectively. Among the flouro-substituted analog (6) the most effective potential was shown by analog-6 due to the presence of flouro group at para-position on aromatic ring which dominantly reduce the enzymatic activity due to much negative charge in the ring by flouro group, while ortho-Substituted analog (2) found with somewhat lower in potential as compared to analog-6. This might be the presence of methyl moiety at para-position on aromatic ring. Likewise analog-5 also contain methyl moiety at para-position on aromatic ring and flouro group at meta-position which also found with lower in potential due to the presence of methyl group which reduce the biological potential by steric hindrance.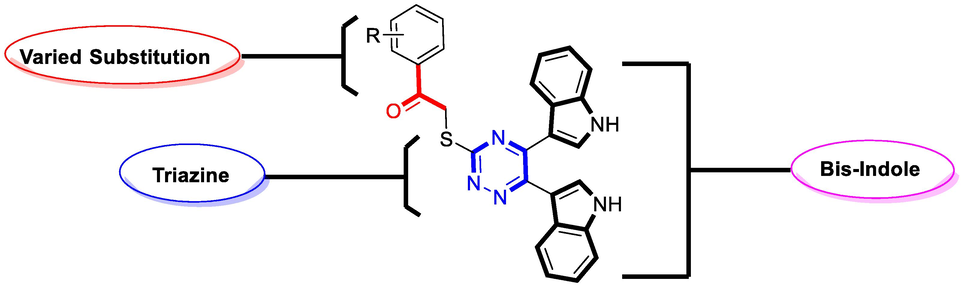
General representation of molecule.
A comparison criterion was set for –OH containing analog-3 having IC50 = 7.10 ± 4.0 µM and analog-4 with IC50 = 19.30 ± 1.0 µM. The significant activity of analog-3 may be the strong involvement of –OH moiety present at para-position while the addition of methyl moiety at meta-position of the ring which indicates a clear decline in the activity profile of analog-4 due to its steric hindrance in the ring. A similar case was observed in –NO2 substituted analogs 7 (IC50 = 21.30 ± 2.0 µM) and 8 (IC50 = 8.40 ± 1.0 µM) the activity profile was found lower in case of methyl substituted analog-7 while simple nitro-substituted analog-8 was found with much better result as compared to standard drug. Moreover, Chloro-substituted analogs-9 (IC50 = 7.20 ± 3.0 µM), 10 (IC50 = 29.10 ± 2.0 µM) and 12 (IC50 = 29.20 ± 1.0 µM) the significant results was found in case of analog-9 due to the presence of chloro moiety whereas the presence of bromo moiety in case of analog 10 and 12 the inhibitory profile were found somewhat lower than the profile of analog-9. This is due to the bulky nature of bromine which reduces the interactive property of molecule thus shown lower potentials. A similar decline was also found in case of –OMe substituted analog-11 (IC50 = 33.10 ± 1.0 µM), due to the presence of bromo group the inhibitory profile was found lower as compare to standard drug.
3.2 In vitro anti-cancer inhibitory activity
All the synthesized analogs (1–12) were also evaluated for anti-cancer activity in order to investigate the biological inhibitory profile of the potent analog among the synthesized moieties. A similar inhibitory profile was observed when all the analogs were evaluated in the presence of standard drug, Tetrandrineb (IC50 = 1.37 ± 0.10 µM). Among the tested series analog-1 bearing triflouro moiety displayed significant biological activity having IC50 = 0.80 ± 0.10 µM. This analog was also found with potent; mention in this activity might be the involvement of flouro groups which might block the enzymatic actions. Moreover, flouro–substituted analogs 2 (IC50 = 4.02 ± 0.20 µM), 5 (IC50 = 9.10 ± 0.10 µM) and 6 (IC50 = 1.80 ± 0.20 µM) were comparable activity in this regard, analog bearing only flouro moiety was found to be most effective among the flouro-substituted analogs while the remaining were few fold less potent due to the presence of methyl moiety which decreases the inhibitory profile by its steric hindrance. Likewise –OH containing analogs-3 (IC50 = 1.40 ± 0.10 µM) and 4 (IC50 = 7.10 ± 0.20 µM) were also found with remarkable activity profile as compare to standard drug. Both –OH bearing analogs, the potential inhibition was shown by analog-3. While analog-4 was somewhat lower due to the presence of –CH3 group which cause reduction in the inhibitory profile. Nitro-substituted analog-7 (IC50 = 10.0 ± 0.30 µM) and analog-8 (IC50 = 3.60 ± 0.10 µM) also showed moderate to good activity, in this the presence of methyl moiety (analog-7) also decrease the activity. A similar decline was also observed in case of chloro-substituted analogs due to the attachment of bromo moiety 10 (IC50 = 16.20 ± 0.20 µM) and 12 (IC50 = 17.20 ± 0.10 µM) whereas simple chloro-substituted analog-9 (IC50 = 3.80 ± 0.10 µM) was found with better potential. It was concluded that bromo moiety decrease the inhibitory profile due to its bulky nature. Bromo-substituted analog-11 was also found with lower activity profile which confirms the bulky nature of bromo group that inhibits the inhibitory action of molecules (Table 1).
3.3 Anti-bacterial activity
All these compounds were also screened for their inhibitory profile against E. coli in order to investigate their anti-bacterial profile. Almost all analogs were found with better to good inhibitory profile but some analogs like 1, 6, 8 and 9 were found with maximum inhibition such as 38.12%, 36.3%, 35.21% and 36% respectively. All the analogs were compared with inhibitory profile of standard drug streptomycin (STM inhibitory profile 40.33%). In this activity it was also concluded that all these analogs having varied substituents which display different inhibitory profile but in this regard the strong inhibition was shown by those analogs having triflouro, hydroxyl, nitro and chloro moieties respectively.
The mechanism related with the insertion of active triflouro group derivatives into the nuclei of E. Coli which eventually can bind with DNA and can also suppress the mRNA expression and stop the synthesis of protein results the death of E. Coli cell. Enhanced activity of trifluro group derivatives could be well explained by generation of bonds formed between the active trifluro groups and the peptidoglycan of the cell wall of E. Coli and lipoprotein in the outer membrane in addition to blocking the feeding channels which finally results in the death of E. Coli.
Due to their effective nature these substituted analogs were maintain their significances as compared to standard drug streptomycin. The excellent potency shown by analog having triflouro group a para-position (1) of aromatic ring. Inhibitory profiles of these analogs have been incorporated in the graph-1. Form these inhibition it was concluded that position, nature and number of substituents affect the biological activity of analogs. Bulky group reduce the inhibitory profile also observed in this biological activity (Graph 1).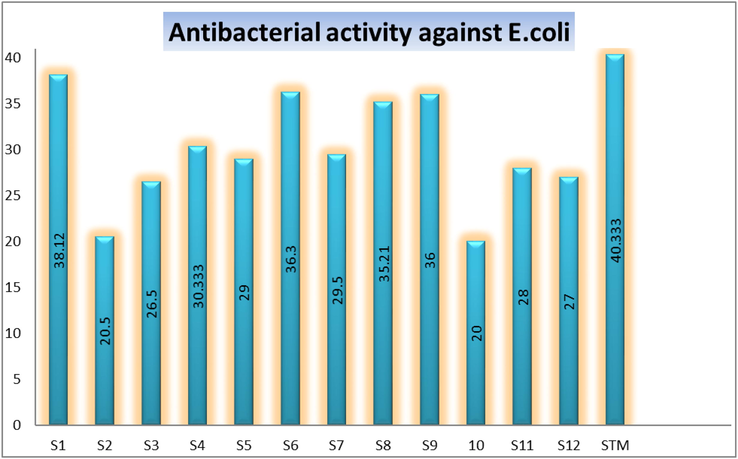
Represent the anti-bacterial activity of analogs along with streptomycin (STM) a standard drug against E. coli specie.
4 Docking studies
4.1 Docking modes of beta gulcuronidase studies
Molecular docking studies were performed by using Autodock, Discovery Studio Visualizer (DSV) and Pymol. These software were used for different purposes such autodock for molecular docking, DSV and Pymol used for visualization of results (Khan et al., 2023; Khan et al., 2022). Number of compounds were docked and visualized among the potent molecules are listed below (Figs. 2 to 6). In addition, type of receptor, distance and interactions is necessary to visualize the ligand in the protein complex that how the ligand linked to the amino acid with distance due to these reason its interactive tendency can be measured (Elsonbaty et al., 2022; Elsonbaty & Attala 2021; Abdel Raoof et al., 2023; Elsonbaty et al., 2023).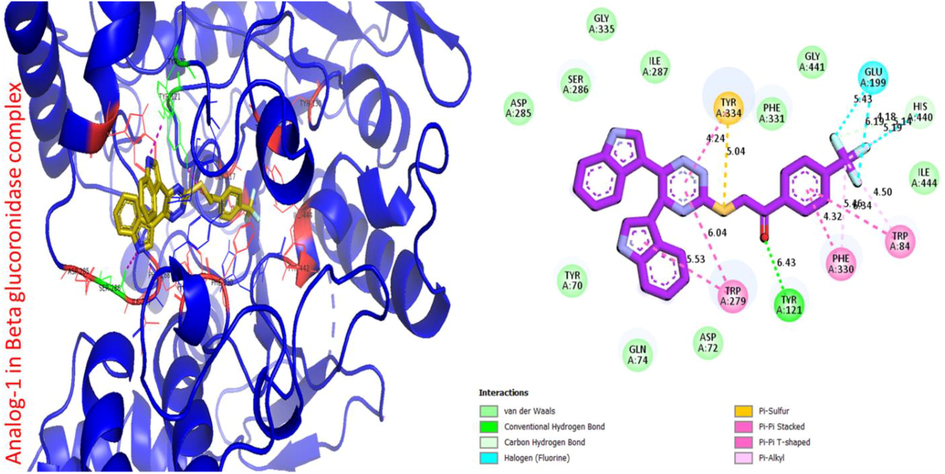
Protein ligand interaction (PLI) of analog-1 in beta-glucuronidase complex.
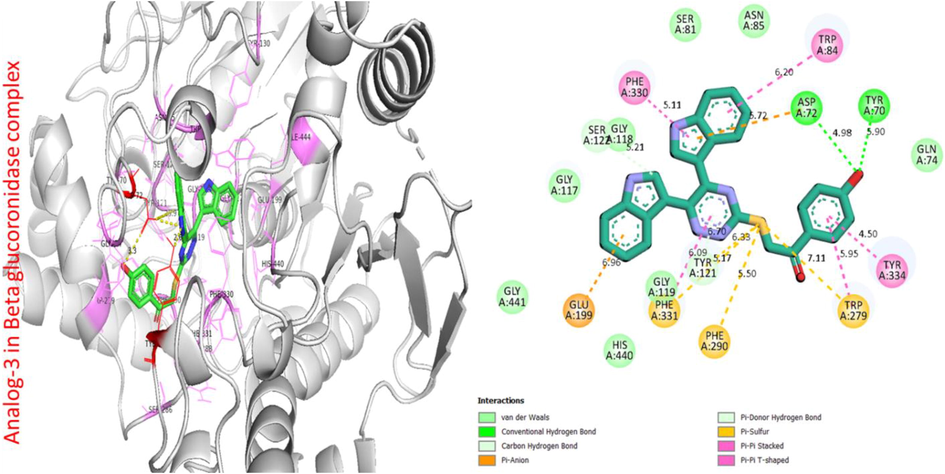
Protein ligand interaction (PLI) of analog-3 in beta-glucuronidase complex.
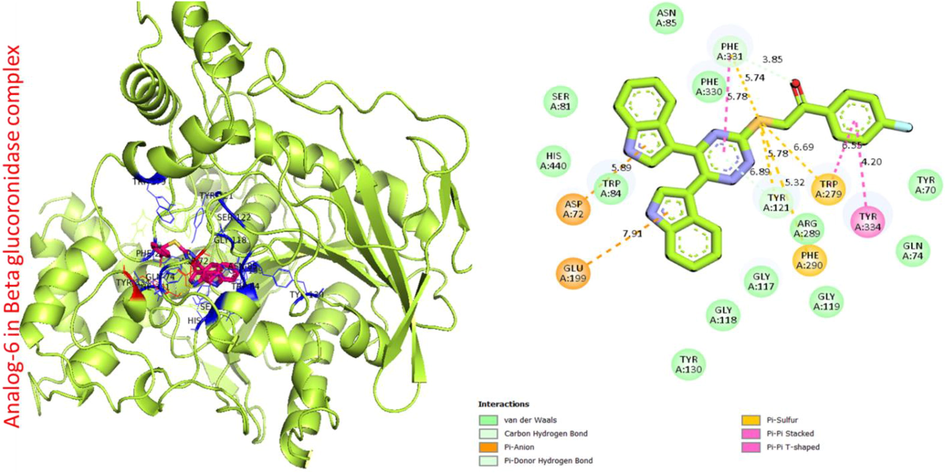
Protein ligand interaction (PLI) of analog-6 in beta-glucuronidase complex.
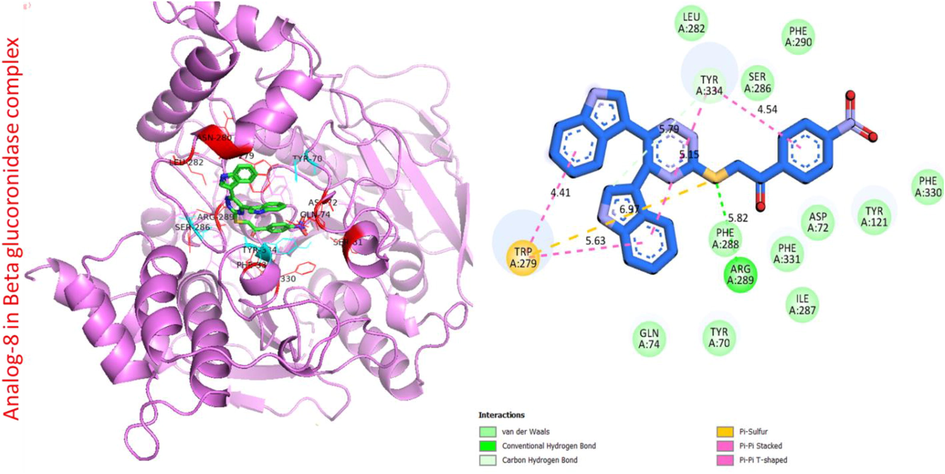
Protein ligand interaction (PLI) of analog-8 in beta-glucuronidase complex.
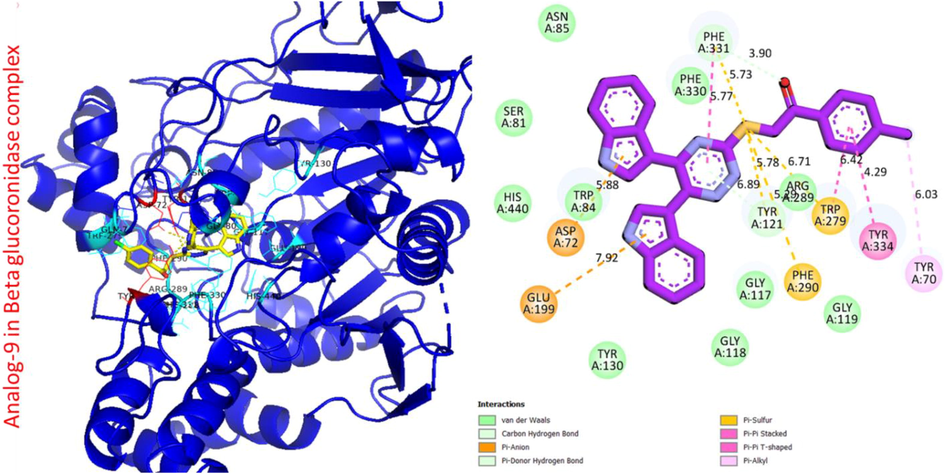
Protein ligand interaction (PLI) of analog-9 in beta-glucuronidase complex.
The following potent analogues (1, 3, 6, 8 and 9) were found with varied interactions in active sites of enzymes it might be the presence of different substituents such as triflouro, hydroxyl, flouro, nitro and chloro respectively. The better binding interaction of molecules in beta-glucuronidase complex, showed significant results (Table 2).
Compound
Receptor
Interaction
Distance
Docking Score
Analog 1 in beta-glucuronidase
TRP-A-334
Pi-S
4.24A°
−12.6
TRP-A-334
Pi-S
5.04A°
GLU-A-199
H-F
5.43A°
GLU-A-199
H-F
6.19A°
HIS-A-440
C-H
4.18A°
TRP-A-84
Pi-Pi Stacked
4.50A°
PHE-A-330
Pi-Pi Stacked
5.46A°
PHE-A-330
Pi-Pi T-Shaped
4.32A°
TYR-A-121
H-B
6.43A°
TRP-A-279
Pi-Pi Stacked
6.04 A°
TRP-A-279
Pi-Pi T-Shaped
5.53 A°
Analog 3 in beta-glucuronidase
PHE-A-330
Pi-Pi Stacked
5.11A°
−11.6
TRP-A-84
Pi-Pi Stacked
6.20A°
ASP-A-72
H-B
5.72A°
ASP-A-72
H-B
4.98A°
TRP-A-70
H-B
5.90A°
TYR-A-334
Pi-Pi T-Shaped
4.50A°
TRP-A-279
Pi-S
5.95A°
TRP-A-279
Pi-S
7.11A°
PHE-A-290
Pi-S
5.95A°
PHE-A-290
Pi-S
6.33A°
PHE-A-290
Pi-Pi Stacked
6.09A°
GLU-A-199
Pi-Cation
6.96 A°
SER-A-122
C-H
5.21A°
Analog 6 in beta-glucuronidase
PHE-A-331
Pi-Pi Stacked
5.78A°
−11.4
PHE-A-331
Pi-S
5.74A°
PHE-A-331
C-H
3.85A°
TYR-A-334
Pi-Pi Stacked
4.20A°
TRP-A-279
Pi-S
6.55A°
TRP-A-279
Pi-S
6.69A°
TYR-A-121
H-C
5.32A°
PHE-A-290
Pi-S
5.78A°
GLU-A-199
Pi-anion
7.91A°
ASP-A-72
Pi-anion
5.89A°
Analog 8 in beta-glucuronidase
TRP-A-279
Pi-S
6.97A°
−10.2
TRP-A-279
Pi-S
5.63A°
TRP-A-279
Pi-S
4.41A°
TYR-A-334
C-H
5.79A°
TYR-A-334
C-H
5.15A°
TYR-A-334
C-H
4.54A°
PHE-A-288
H-B
5.82A°
Analog 9 in beta-glucuronidase
GLU-A-199
Pi-Anion
7.92A°
−9.8
ASP-A-72
Pi-Anion
5.88A°
PHE-A-331
C-H
5.73A°
PHE-A-331
C-H
5.77A°
TYR-A-70
Pi-R
6.03A°
TYR-A-334
Pi-Pi-Stacked
4.29A°
TRP-A-279
Pi-Pi-T Shaped
6.42A°
TRP-A-279
Pi-S
6.71A°
TYR-A-121
Pi donor H-B
6.89A°
PHE-A-290
Pi-S
5.29A°
D_Saccharic acid 1,4 lactone in beta-glucuronidase
TYR-A-175
H-B
4.30A
−5.4
GLN-A-202
H-B
4.08A
PHE-A-200
Polar H-B
4.08A
HIS-A-94
H-B
3.67A
HIS-A-94
H-B
3.67A
4.2 Docking modes of anticancer studies
Docking studies were also performed for anti-cancer studies against Human ROS1 Kinase Domain. The selected analogs were also subjected in this study to explore the binding interactions of ligand with active site of enzyme (Figs. 7 to 11). The following candidate (1,3,6,8 and 9) and their interaction are incorporated in Table 3.(See Fig. 12).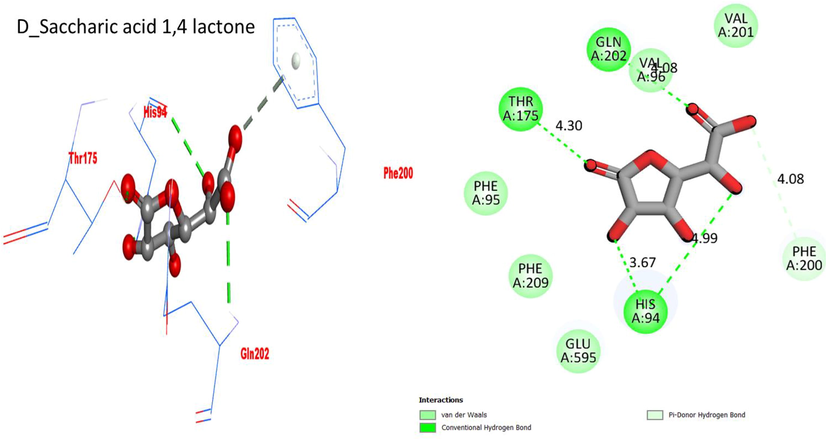
Protein ligand interaction (PLI) D_saccharic acid 1,4 lactone in beta-glucuronidase complex.
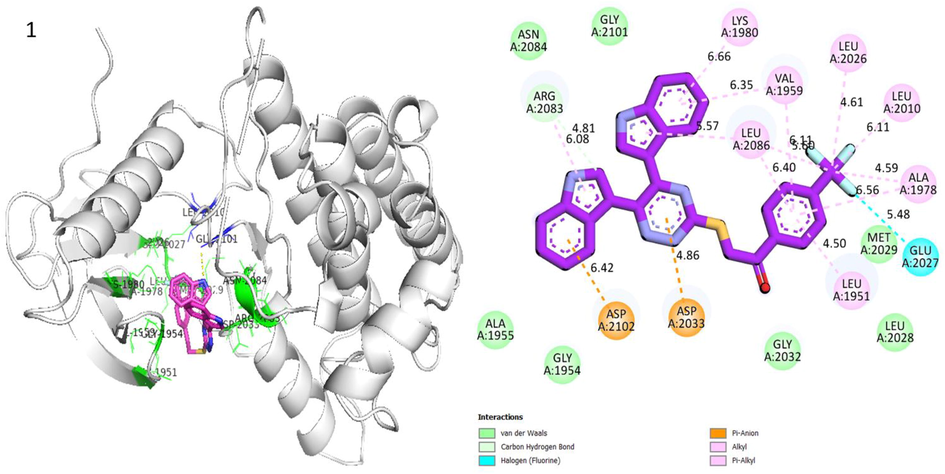
Protein ligand interaction (PLI) of analog-1 in Human ROS1 Kinase Domain.
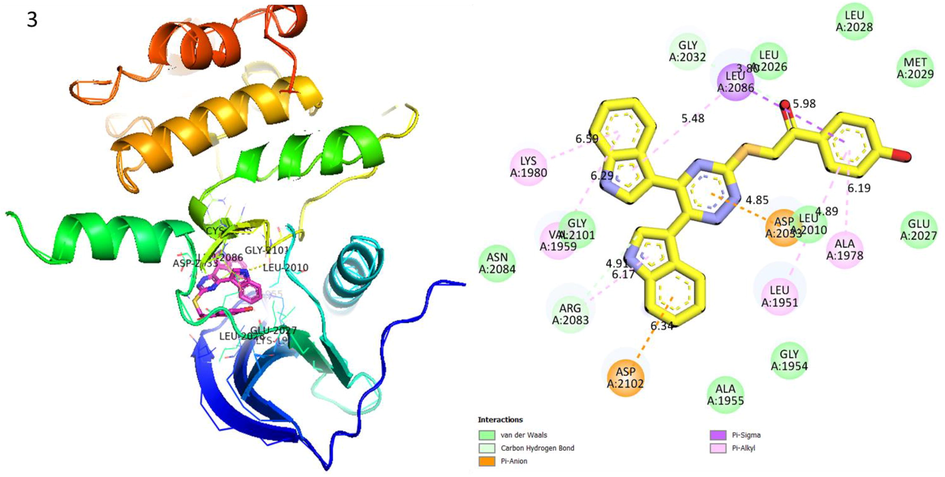
Protein ligand interaction (PLI) of analog-3 in Human ROS1 Kinase Domain.
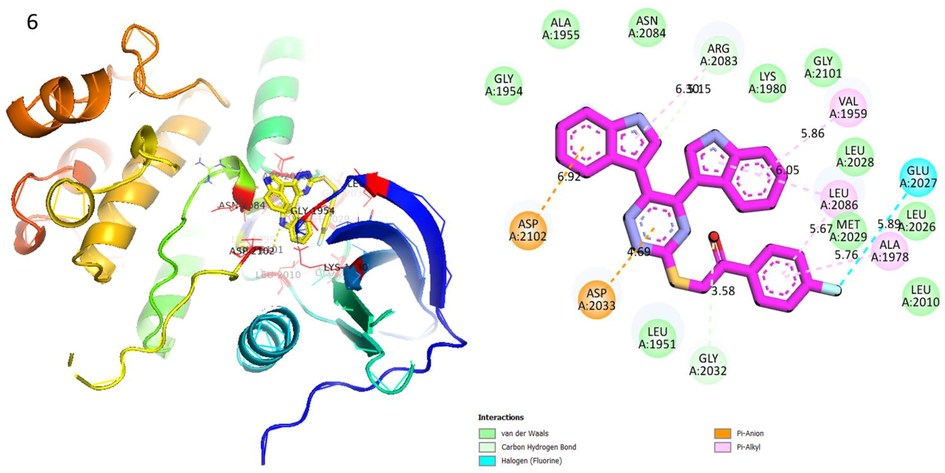
Protein ligand interaction (PLI) of analog-6 in Human ROS1 Kinase Domain.
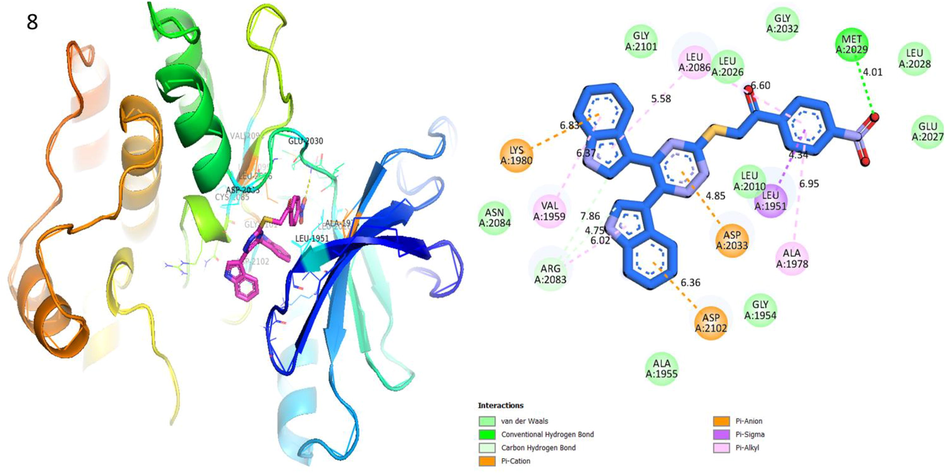
Protein ligand interaction (PLI) of analog-8 in Human ROS1 Kinase Domain.
Compound
Receptor
Interaction
Distance
Docking Score
Analog 1 in Human ROS1 Kinase Domain
ARG-A-2083
C-H
4.81A°
−8.6
LYS-A-1980
Pi-R
6.66A°
VAL-A-1959
Pi-R
6.35A°
VAL-A-1959
Pi-R
6.11A°
LEU-A-2086
R
5.57A°
LEU-A-2086
R
6.40A°
LEU-A-2026
Pi-R
4.61A°
LEU-A-2010
Pi-R
6.11A°
ALA-A-1978
Pi-R
4.59A°
ALA-A-1979
Pi-R
6.56 A°
GLU-A-2277
H –F
5.48 A°
LEU-A-1951
Pi-R
4.50A°
ASP-A-2033
Pi-Anion
4.86A°
ASP-A-2102
Pi-Anion
6.42A°
Analog 3 in Human ROS1 Kinase Domain
LYS-A-1980
Pi-R
6.99A°
−8.3
VAL-A-1959
Pi-R
6.29A°
ARG-A-2083
H-C
4.91A°
ASP-A-2102
Pi-Anion
6.34A°
ASP-A-2033
Pi-Anion
4.85A°
LEU-A-1951
Pi-R
4.89A°
ALA-A-1978
Pi-R
6.19A°
LEU-A-2086
Pi-Sigma
5.98A°
LEU-A-2086
Pi-Sigma
5.48A°
Analog 6 in Human ROS1 Kinase Domain
ASP-A-2102
Pi-Anion
6.92A°
−7.9
ASP-A-2033
Pi-Anion
4.69A°
GLY-A-1951
C-H
3.58A°
GLU-A-2077
H-F
5.89A°
TLA-A-1978
Pi-R
5.76A°
LEU-A-2086
Pi-R
5.67A°
LEU-A-2086
Pi-R
5.05A°
VAL-A-1959
Pi-R
5.86A°
ARG-A-2083
Pi-anion
6.15A°
Analog 8 in Human ROS1 Kinase Domain
LYS-A-1980
Pi- anion
6.83A°
−8.2
VAL-A-1959
Pi-R
6.37A°
ARG-A-2083
C-H
4.75A°
ASP-A-2102
Pi- anion
6.36A°
ASP-A-2033
Pi- anion
4.85A°
LEU-A-1951
Pi- Sigma
4.34A°
ALA-A-1978
Pi- R
6.95A°
MET-A-2029
H-B
4.01A°
LEU-A-2026
C-H
6.60A°
LEU-A-2086
Pi- R
5.58A°
Analog 9 in Human ROS1 Kinase Domain
ASP-A-2102
Pi-Anion
6.33A°
−7.3
ASP-A-2033
Pi-Anion
4.89A°
GLY-A-2032
C-H
3.83A°
LEU-A-1951
Pi-R
4.86A°
ALA-A-1978
Pi-R
6.22A°
LEU-A-2086
Pi-Sigma
4.29A°
LEU-A-2086
Pi-Sigma
5.48A°
VAL-A-1959
Pi-R
6.23A°
LYS-A-1980
Pi-R
6.54A°
ARG-A-2083
C-H
5.01A°
ARG-A-2083
C-H
6.26A°
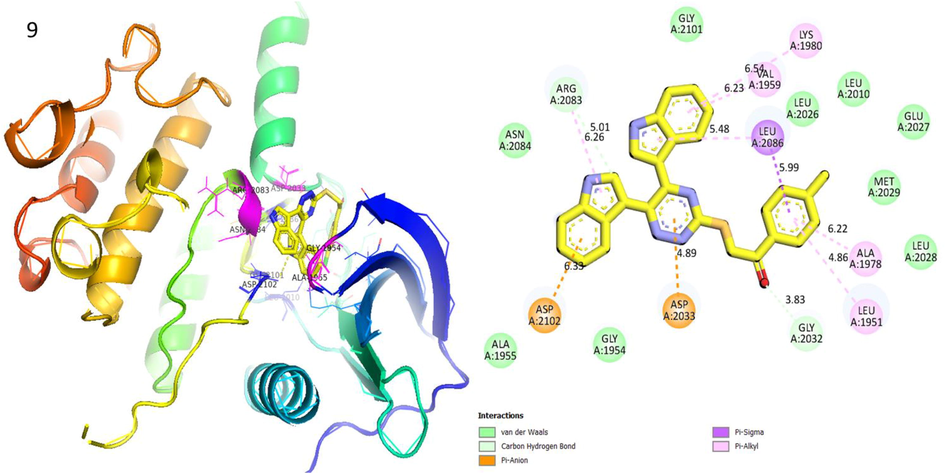
Protein ligand interaction (PLI) of analog-9 in Human ROS1 Kinase Domain.
5 ADME results
Most of the drugs failed during clinical development had inadequacies in ADME/Tox. Therefore, the purpose of virtual screening should not only be to increase selectivity and enhance binding affinity, but also to consider pharmacokinetic factors as important filters (Khan et al., 2023). Using Qikprop (QikProp, version 3.5, Schrödinger), 44 descriptors and pharmaceutically important characteristics of substituted bis-indole based triazine analogues were examined. Some of the key terms need to envision molecules' drug-like characteristics are reported. Most synthetic compounds have complied with Lipinski's requirements, which are as follows: molecular weight 500, octanol/water partition coefficient (QPlogPo/w), < 5 hydrogen bond acceptor < 10 and donor < 5. For the majority of the derivatives, the oral absorption rate was determined to be between 80 and 100% are shown in graph-2–5. The potent analogs (1,6,8 and 9) were identified for log Kp, Lipinski, Ghose, Veber, Egan, Muegge, Bioavailability, Pains, Brenk, Leadlikensee and synthetic accessibility via ADME study (Graph 2 Graph 3 Graph 4 Graph 5 Graph 6). ADME properties help us to predict the properties of specific molecules which shows drug like properties.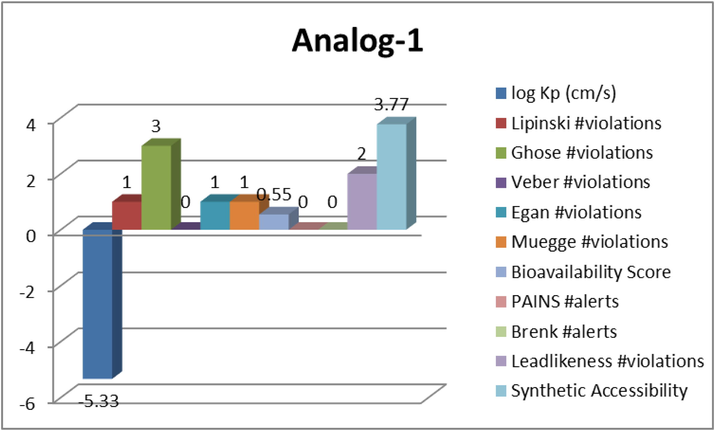
Represent the ADME properties of the synthesized analog-1.
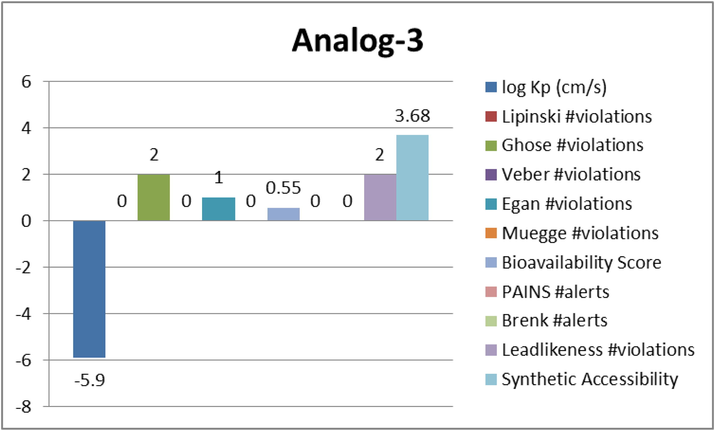
Represent the ADME properties of the synthesized analog-3.
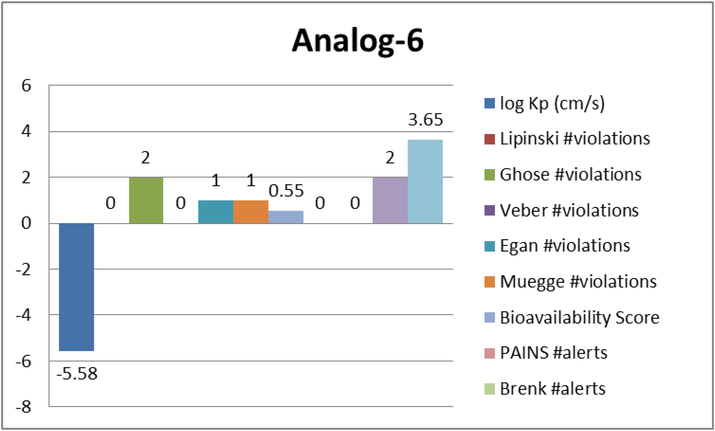
Represent the ADME properties of the synthesized analog-6.
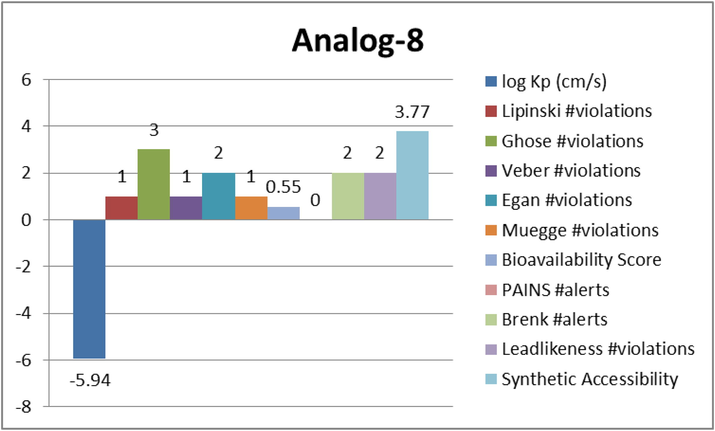
Represent the ADME properties of the synthesized analog-8.
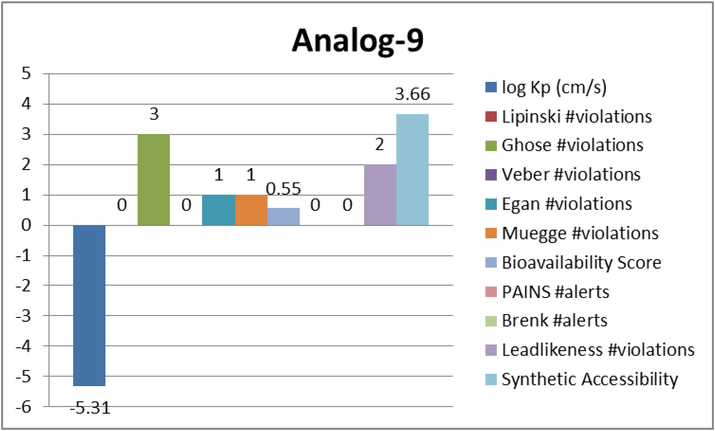
Represent the ADME properties of the synthesized analog-9.
6 Conclusion
It was observed form the targeted compounds that nature, number and position of substituents has a great impact on the properties of analogs. Hence in this study we have synthesized, characterized and evaluated for B-glucuronidase, anti-cancer and anti-bacterial activities. Almost all analogs were found with significant results but few analogs such as 1 (2-((5,6-di(1H-indol-3-yl)-1,2,4-triazin-3-yl)thio)-1-(4-(trifluoromethyl)phenyl)ethan-1-one), 3 (2-((5,6-di(1H-indol-3-yl)-1,2,4-triazin-3-yl)thio)-1-(4-hydroxyphenyl)ethan-1-one) 6 (2-((5,6-di(1H-indol-3-yl)-1,2,4-triazin-3-yl)thio)-1-(4-fluorophenyl)ethan-1-one), 8 (2-((5,6-di(1H-indol-3-yl)-1,2,4-triazin-3-yl)thio)-1-(4-nitrophenyl)ethan-1-one) and 9 (1-(4-chlorophenyl)-2-((5,6-di(1H-indol-3-yl)-1,2,4-triazin-3-yl)thio)ethan-1-one), showed much potent potentials as compared to standard drugs (D-saccharic acid 1,4 lactone, Tetrandrineb and Streptomycin). Moreover, these selected analogs were further subjected for ADME analysis and molecular docking studies in which the physiochemical properties and binding interactions were explored respectively. Finally, it was concluded that the above mention analogs, compound-1, was the most effective inhibitor might be the efficacy of triflouro group, given ranked-1. The other compounds (3, 6, 8 and 9) being strong inhibitory profile were also ranked based on their inhibitory activity. Moreover, these compounds were found few folds better profile when compared to their referenced drugs.
Funding
This work was supported by Princess Nourah bint Abdulrahman University researchers supporting project number (PNURSP2023R205), Princess Nourah bint Abdulrahman University, Riyadh, Saudi Arabia. This work was supported by the Researchers Supporting Project number (RSPD2023R620), King Saud University, Riyadh, Saudi Arabia.
Acknowledgment
The authors extend their appreciation to the Researchers Supporting Project number (RSPD2023R620), King Saud University, Riyadh, Saudi Arabia. The authors also extend their appreciation to Princess Nourah bint Abdulrahman University researcher supporting project number (PNURSP2023R205), Princess Nourah bint Abdulrahman University, Riyadh, Saudi Arabia.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- 1, 2, 4-triazine chemistry part IV: synthesis and chemical behavior of 3-functionalized 5, 6-diphenyl-1, 2, 4-triazines towards some nucleophilic and electrophilic reagents. J. Heterocycl. Chem.. 2015;52(6):1595-1607.
- [Google Scholar]
- Potentiometric determination of mebeverine hydrochloride antispasmodic drug based on molecular docking with different ionophores host–guest inclusion as a theoretical study. RSC Adv.. 2023;13(2):1085-1093.
- [Google Scholar]
- Biologically potent Benzimidazole-based-substituted benzaldehyde derivatives as potent inhibitors for Alzheimer’s disease along with molecular docking study. Pharmaceuticals. 2023;16(2):208.
- [Google Scholar]
- The global burden of liver disease: a challenge for methods and for public health. BMC Med.. 2014;12(1):1-3.
- [Google Scholar]
- Synthesis and functionalization of indoles through palladium-catalyzed reactions. Chem. Rev.. 2005;105(7):2873-2920.
- [Google Scholar]
- An activity-based near-infrared glucuronide trapping probe for imaging β-glucuronidase expression in deep tissues. J. Am. Chem. Soc.. 2012;134(6):3103-3110.
- [Google Scholar]
- Indole in the target-based design of anticancer agents: a versatile scaffold with diverse mechanisms. Eur. J. Med. Chem.. 2018;150:9-29.
- [Google Scholar]
- Electrochemical determination of amprolium hydrochloride in chicken meats and eggs: food safety control and theoretical study. J. Electrochem. Soc.. 2021;168(3):037518
- [Google Scholar]
- Application of experimental design approaches and in silico molecular docking on the host-guest complexes with Cyclodextrin for the analysis of Benazepril hydrochloride in pharmaceutical formulation. J. Electrochem. Soc.. 2021;168(5):057515
- [Google Scholar]
- Computational design for eco-friendly visible spectrophotometric platform used for the assay of the antiviral agent in pharmaceutical dosage form. Spectrochim. Acta Part A: Mol. Biomol. Spectrosc.. 2022;271:120897
- [Google Scholar]
- Current advances in computer-aided design of electrochemical sensors: an analytical review. Records Pharma. Biomed. Sci.. 2023;7(1):65-96.
- [Google Scholar]
- Cyclosporin treatment in rheumatoid arthritis is associated with an increased serum activity of β-glucuronidase. Scand. J. Rheumatol.. 1993;22(2):83-85.
- [Google Scholar]
- Benzofuran and indole: promising scaffolds for drug development in Alzheimer's disease. ChemMedChem.. 2018;13(13):1275-1299.
- [Google Scholar]
- High resolution crystal structure of human β-glucuronidase reveals structural basis of lysosome targeting. PLoS One. 2013;8(11):e79687.
- [Google Scholar]
- Hussain, R., Shah, M., Iqbal, S., Rehman, W., Khan, S., Rasheed, L., Naz, H., Al-Ghulikah, H.A., Elkaeed, E.B., Pashameah, R.A. and Alzahrani, E., 2022. Molecular iodine-promoted oxidative cyclization for the synthesis of 1, 3, 4-thiadiazole-fused-[1, 2, 4]-thiadiazole incorporating 1, 4-benzodioxine moiety as potent inhibitors of α-amylase and α-glucosidase: In vitro and in silico study. Frontiers in Chemistry, 10.
- Synthesis of novel Benzimidazole-based Thiazole derivatives as multipotent inhibitors of α-Amylase and α-Glucosidase. in vitro evaluation along with molecular docking study. Molecules. 2022;27(19):6457.
- [Google Scholar]
- A review of bisindolylmethane as an important scaffold for drug discovery. Curr. Med. Chem.. 2015;22(38):4412-4433.
- [Google Scholar]
- Antiangiogenesis targeting tumor microenvironment synergizes glucuronide prodrug antitumor activity. Clin. Cancer Res.. 2009;15(14):4600-4611.
- [Google Scholar]
- New quinoline-based triazole hybrid analogs as effective inhibitors of α-amylase and α-glucosidase: preparation, in vitro evaluation, and molecular docking along with in silico studies. Front. Chem.. 2022;10
- [Google Scholar]
- Design, synthesis, in silico testing, and in vitro evaluation of Thiazolidinone-based Benzothiazole derivatives as inhibitors of α-Amylase and α-Glucosidase. Pharmaceuticals. 2022;15(10):1164.
- [Google Scholar]
- New biologically hybrid pharmacophore Thiazolidinone-based indole derivatives: synthesis, in vitro alpha-amylase and alpha-glucosidase along with molecular docking investigations. Molecules. 2022;27(19):6564.
- [Google Scholar]
- Synthesis, in vitro anti-microbial analysis and molecular docking study of aliphatic hydrazide-based benzene sulphonamide derivatives as potent inhibitors of α-glucosidase and urease. Molecules. 2022;27(20):7129.
- [Google Scholar]
- Synthesis, In vitro biological evaluation and in silico molecular docking studies of indole based thiadiazole derivatives as dual inhibitor of acetylcholinesterase and butyrylchloinesterase. Molecules. 2022;27(21):7368.
- [Google Scholar]
- Synthesis, Molecular docking and ADMET studies of bis-benzimidazole-based thiadiazole derivatives as potent inhibitors, in vitro α-amylase and α-glucosidase. Arab. J. Chem. 2023:104847.
- [Google Scholar]
- Synthesis of novel inhibitors of β-glucuronidase based on benzothiazole skeleton and study of their binding affinity by molecular docking. Bioorg. Med. Chem.. 2011;19(14):4286-4294.
- [Google Scholar]
- New biologically potent benzimidazole-based-triazole derivatives as acetylcholinesterase and butyrylcholinesterase inhibitors along with molecular docking study. J. Heterocycl. Chem.. 2022;59:2225-2239.
- [Google Scholar]
- Synthesis, in vitro α-amylase, α-glucosidase activities and molecular docking study of new benzimidazole bearing thiazolidinone derivatives. J. Mol. Struct.. 2022;1269:133812
- [Google Scholar]
- New thiazole-based thiazolidinone derivatives: synthesis, in vitro α-amylase, α-glucosidase activities and silico molecular docking study. Chem. Data Collect.. 2022;42:100967
- [Google Scholar]
- New benzoxazole-based sulphonamide hybrids analogs as potent inhibitors of α-amylase and α-glucosidase: synthesis and in vitro evaluation along with in silico study. Arab. J. Chem.. 2022;15(12):104341
- [Google Scholar]
- Synthesis, DFT studies, molecular docking and biological activity evaluation of thiazole-sulfonamide derivatives as potent Alzheimer’s inhibitors. Molecules. 2023;28(2):559.
- [Google Scholar]
- Intestinal bacterial β-glucuronidase activity of patients with colon cancer. Archives Pharma. Res.. 2001;24:564-567.
- [Google Scholar]
- New Triazinoindole bearing Benzimidazole/Benzoxazole hybrids analogs as potent inhibitors of urease: synthesis, in vitro analysis and molecular docking studies. Molecules. 2022;27(19):6580.
- [Google Scholar]
- Dose optimization of a doxorubicin prodrug (HMR 1826) in isolated perfused human lungs: low tumor pH promotes prodrug activation by β-glucuronidase. J. Pharmacol. Exp. Ther.. 2002;301(1):223-228.
- [Google Scholar]
- Human β-glucuronidase: structure, function, and application in enzyme replacement therapy. Rejuvenation Res.. 2013;16(5):352-363.
- [Google Scholar]
- Novel indole derivatives as potential anticancer agents: design, synthesis and biological screening. Med. Chem. Res.. 2018;27:321-331.
- [Google Scholar]
- Recent progress in biological activities of indole and indole alkaloids. Mini Rev. Med. Chem.. 2018;18(1):9-25.
- [Google Scholar]
- Indoles—a promising scaffold for drug development. Eur. J. Pharm. Sci.. 2016;91:1-10.
- [Google Scholar]
- Urinary exoglycosidases, reference values in healthy children. Adv. Med. Sci.. 2018;63(2):224-229.
- [Google Scholar]







