Translate this page into:
Boswellic acid captivated hydroxyapatite carboxymethyl cellulose composites for the enhancement of chondrocytes in cartilage repair
⁎Corresponding author. liyuxu111@sina.com (Yuxu Li)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Recently tissue repairing bone grafted materials have been greater properties than the recapitulating intramembranous regeneration of natural bone especially cartilage damage regeneration. In this present work was designed and developed for the enhancement of chondrocyte generation for cartilage repair. Boswellic acid (BA) is a traditional compound used for the treatment of osteoarthritis. Boswellic acid subjected to involve the preparation of hydroxyapatite (HAP) and HAP-BA compounds were functionalized with carboxymethyl cellulose (CMC) to promote the extra-cellular matrix. HAP, HAP-BA and HAP-BA-CMC composites were characterized via their physicochemical properties through FTIR, XRD, SEM and TEM techniques. The antibacterial activity and chondrocyte cell variability were tested. At 14 days, HAP-BA-CMC composite was observed 67% of cell viability. The chondrocyte cell adhesion on the HAP-BA-CMC composite was investigated and it observed polygonal filopodia. From the results suggest that HAP-BA-CMC composite can improve the chondrocyte production for repair of damaged cartilage.
Keywords
Boswellic acid
Cartilage
Chondrocyte
Hydroxyapatite
Osteoarthritis
1 Introduction
Many numbers of peoples are affected by cartilage damage, and it is frequently observed is nearly 60% of patients who experienced knee arthroscopy replacement (Curl et al., 1997). Chondrocytes and avascular is the combination of cartilage and it has poor ability to mitosis and cytokinesis process since the cartilage has low regeneration capability (Ebihara et al., 2012). The complete cure of cartilage damage is not attained; the regeneration of cartilages is one of the possibilities. Because the cartilage in a joint is injured, it leads to inflammation, severe pain and some degree of disability (Setyawati et al., 2017). There is no effective and sturdy therapy for cartilage damage is accessible. In any case, regeneration of cartilage tissue has more attention in the researchers and as well as the scientific companies since it might be a promising technique for the regeneration of cartilage (Kock et al., 2012). The regeneration of cartilage has included the part of extracellular matrix (ECM) materials, growth factors, and seed cells. (Toyokawa et al., 2010). Chondrocytes are normal competitors of seed cell sources in the regeneration of cartilage since they can straightforwardly emit cartilage-site of extracellular lattice without inducible components (Maji and Dasgupta, 2015).
The chondrocytes are classified into two types, one is autologous chondrocytes and another one is allogenic chondrocytes (Bernhard et al., 2017). These two, autologous chondrocytes having minimum numbers of cells sources, while the practice of allogeneic chondrocytes has plentiful sources and need small operation (RoblaCostales et al., 2016; Lefebvre and Dvir-Ginzberg, 2017). If the cartilage is not cured properly; cartilage may start with degeneration and its leads to osteoarthritis. The momentum treatment accessible for joint inflammation is symptomatically based and intended to control the movement of the malady however numerous people don't react to this treatment. Dynamic research is being led to recognize more compelling regenerative and part of this concentration is redirected to elective prescription.
Since in the present work is planned to the synthesis of natural materials contain a scaffold for repair cartilage damage such as the synthesis of carboxymethyl cellulose reinforced boswellic directed hydroxyapatite (HAP) composites. Boswelliaserrata, regularly called Shallaki is a plant is known for anti-cancer, anti-inflammatory, asthma and analgesic activity, suggestively decreasing the count of WBC in the cartilage joint fluid, regaining the integrity of blood vessels destroyed by spasm or interior injury curing properties of Boswellic Acids got from it (Moreau et al., 2014; Kimmatkar et al., 2003). Boswellic acid is a major component of Boswelliaserrata with minimum amount carbohydrate by wide use in incense and aroma produce. Boswellic acid is reduced in joint pain, enhance joint fluid flow and aptitude to hike staircases was stated then the indications repaid on termination of the treatment.
The development of cartilage regeneration suffers from some drawbacks for the application of clinical level. Since there are many techniques and materials developed as a tissue-engineering approach to regeneration/repair injured cartilage in in-vivo and animal model (Yin et al., 2018). From the reports of the synthesized polymeric materials, chitosan, poly (glycolic acid), alginate, poly(ε-caprolactone), poly(lactic acid), carboxymethyl cellulose and their combined other polymers have been involved in extensive consideration for their biocompatibility and biodegradability properties for the cartilage repair (Sumathra et al., 2018). In addition, the hydroxyapatite (HAP) is the most important materials for bone regeneration and repairing because the HAP is osteoblast cell inducer. HAP is an inorganic material with the molecular formula of Ca5(PO4)3OH (Aruna et al., 2018). HAP used as a teeth and bones substitute, non-poisonous, should, therefore, be biocompatible and it can offer stability with natural bone and teeth. Be that as it may, the mechanical quality, strength, versatile modulus and surface properties of these polymers were lower than those of normal bone and ligament tissue was. In a previous couple of years, polymeric composites have been occupied with the tissue-designing field. Composites are connected to recreate tissues, for example, bone, ligament, ligament, and tendons since they are relied upon to enhance mechanical properties and biocompatibilities (Aruna et al., 2018). For enhancing mechanical properties contrasted with the perfect polymer framework, inorganic fillers were acquainted with biodegradable polymers with creating filler/polymer composites. In this manner, the carboxymethyl cellulose reinforced boswellic acid/hydroxyapatite composite was applied for cartilage restoration.
2 Materials and methods
2.1 Materials
Calcium chloride dihydrate (CaCl2·2H2O), Boswellic acid (BA), diammonium hydrogen phosphate (NH4)2HPO4, ethanol, and liquid ammonia were procured from Sigma-Aldrich (USA). Analytical grade chemicals were used all over experimentation. The deionized distilled water (DD) was applied for dilution and purifications of the experiments.
2.2 Synthesis of HAP/Boswellic acid composite
The template method of HAP formation was followed by a preceding report with minor modifications (Govindaraj and Rajan, 2016). For Ca2+, PO43−, and –OH precursors of Calcium chloride dihydrate (CaCl2·2H2O), diammonium hydrogen phosphate ((NH4)2HPO4), and ammonium hydroxide (NH4OH) were used as the preparation of hydroxyapatite respectively. The CaCl2·2H2O (0.05 M) solution was mixed with 10% (w/v) boswellic acid ethanol solution, and the solution was probe ultra-sonication (Vibra sonic cell, Germany) for 30 min (10 sec on 3 sec off) at ambient temperature (27 °C). Consequently, 0.03 M ((NH4)2HPO4) was supplemented slowly to the reaction mixture solution (Ca/P = 1.6) ratio. The solution pH was maintained at pH 10.0 by using aqueous ammonia with continuous mixing was extended to about 24 h. The obtained precipitate was subjected on probe ultra-sonication for 1 h at room temperature (27 °C) and pursued via heating in a micro oven at 150 W for about 10 min. The resulting composite was cleaned thrice with DD water continued by ethanol solution and then desiccated at 150 °C.
2.3 Synthesis of HAP/BA/CMC composites
Initially, HAP/BA was dispersed in 50 mL of DD water. Subsequently, 0.05 g CMC was dissolved in 10 mL deionized water by probe ultra-sonication and drops wise added into the solution of HAP/BA with probe ultra-sonication (Vibra sonic cell, Germany) for 30 min (10 sec on 3 sec off) at ambient temperature (27 °C). Finally, the white-colored mixture of HAP/BA/CMC was lyophilized using a freeze drier at −40 °C (SSIPL-LYF, Floor model, India) to form the composites. The HAP/BA and HAP/BA/CMC concentrations of precursors are given in Table 1 and the schematic representation of chemical reactions was given in Fig. 1.
S.No
CaCl2·2H2O (M)
(NH4)2HPO4 (M)
Boswellic acid Wt (%)
CMC Wt(%)
Sample name
1
0.5
0.3
10
–
HAP/BA
2
0.5
0.3
10
5
HAP/BA/CMC
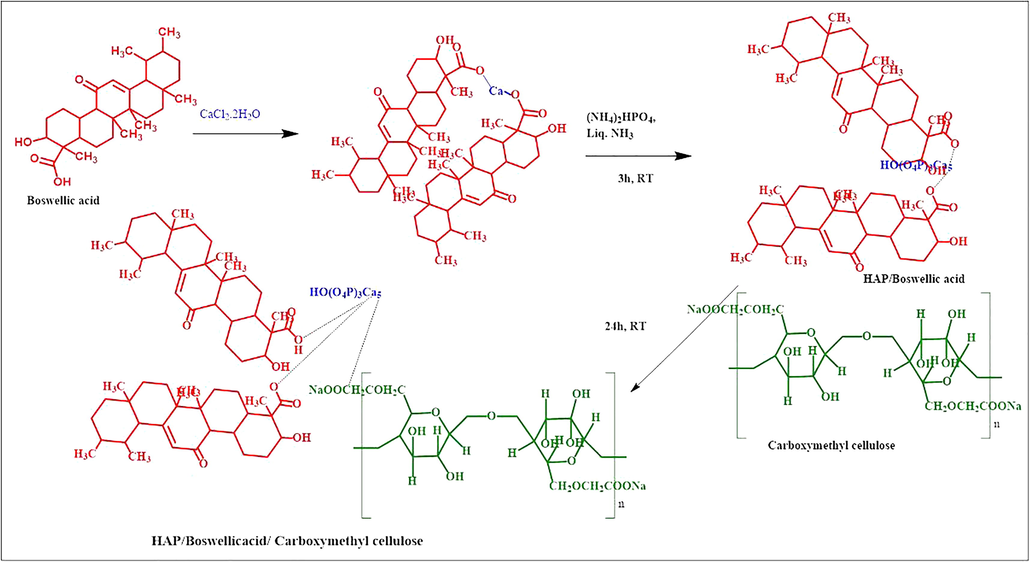
The chemical reaction of hydroxyapatite/boswellic acid/carboxymethyl cellulose composite formation.
2.4 Characterizations
2.4.1 Functionality analysis by Fourier transform infrared spectroscopy (FTIR)
The functionality of HAP, HAP/BA, and HAP/BA/CMC materials were recorded using a Bruker tensor 27 series FTIR spectrometer in the range of 400–4000 cm−1 with 2 cm−1 resolution and 16 scans. The 1 g of KBr and 0.2 g of sample were adhesive by mixing to make a pellet.
2.4.2 X-ray diffraction
For the phase composition and crystallinity analysis of prepared HAP nanoparticles and its composites was carried out via X-ray diffraction (XRD) determination. Bruker D8 Advance Diffractometer with the monochromatic CuKα source was used for the observation of XRD pattern operated at 40 kV and 30 mA with an acceleration voltage of 30 kV and a current of 15 mA. The investigation was executed over the 2θ range of 10–80° in step scan mode with a size of 0.02°and at a scan rate of 0.02°/min.
2.4.3 Scanning Electron Microscopy (SEM) and Transmission Electron Microscopy (TEM) characterizations
The morphology and microstructure of the HAP and Boswellic acid (BA), Carboxy Methyl Cellulose (CMC) composites were inspected using FESEM (VEGA3 TESCAN) handled at an accelerating voltage of 10 kV outfitted with energy dispersive X-ray analysis (EDAX) and high-resolution transmission electron microscopy (HRTEM, TECNAI F30).
2.5 Antibacterial analysis
2.5.1 Strains and culture conditions
Anti-bacterial activity was tested against Bacillus cereus (B. cereus) (ATCC 11778) and Pseudomonas aeruginosa (P. aeruginosa) (ATCC 25619) pathogens. Tryptic soy broth (TSB) consisting 0.5% glucose (pH 7 ± 0.2) at 37 °C was the condition of pathogens culture. The Cultures were vaccinated from overnight inoculum at a dilution of 1/100 and incubated at 37 °C with shaking at 170 rpm.
2.5.2 Minimum inhibitory concentration (MIC) determination
The vulner capacities of planktonic cells of P. aeruginosa and B. cereus to the HAP and HAP/BA/CMC compounds were analyzed controlled by microtiter broth dilution, as depicted by the Clinical and Laboratory Standards Institute. Restraint examines were performed in sterile 96-well plates (Corning Co., NY, USA) in the last volume of 150 μL, containing 50 μL of microbial societies (3 × 106 CFU/mL) and 100 μL of the successively weakened HAP and HAP/BA/CMC compounds (100 μg/mL). The plates were stored at 37 °C for 24 h, the development of planktonic cells was captured by using Normal canon camera.
2.6 Cell viability
The chondrocytes cells were collected from the joints of cartilage by crumbling method into minor fragments and relocated into the 2 mg/ml of collagenase type II of DMEM/F12 solution (Worthington, Lakewood, NJ, USA). Cell harvest and viability were analyzed with a hemocytometer and trypan blue segregation. Chondrocytes cell were loaded at 5 × 103 cells/cm2 in T-175 dishes and preserved in Lower level Glucose– Dulbecco's Modified Eagle Medium(LG-DMEM) complemented with 10% fetal ox-like serum (FBS), penicillin (100 U/ml) – streptomycin (100 mg/ml) and amphotericin B (0.25 mg/ml) (all Sigma-Aldrich). The composition was extended to 1, 7 and 14 days in a moistened atmosphere at 37◦C and 5% CO2. The cell adhesion analysis carried out by the same procedure. The cell-seeded sample was cleaned with PBS and immobile with 3% glutaraldehyde at 4 °C after the treatment of 1, 7 and 14 days and the sample observed their morphology for same protocol followed by the SEM characterization (Table 2).
Gene
Forward
Reverse
Collagen type I
5′-GGGCTCTAATGATGTTGAACTTGT-3′
5′-ATGATTGTCTTTCCCCATTCATTT-3′
Aggrecan
5′-AGTCACACCTGAGCAGCATC-3′
5′-AGTTCTCAAATTGCATGGGGTGTC-3′
Collagen type II
5′-GGATGGCTGCACGAAACATACCGG-3′
5′-CAAGAAGCAGACCGGCCCTATG-3′
Collagen type X
5′-GTGTTTTACGCTGAACGATACCAA-3′
5-ACCTGGTTTCCCTACAGCTGATG-3′
Alkaline phosphatase
5′-ATCTTTGGTCTGGCCCCCATG-3′
5-AGTCCACCATGGAGACATTCTCTC-3′
3 Results and discussion
3.1 Functional elucidations
Fig. 2 represents the HAP, BA, CMC, HAP/BA, HAP/BA/CMC composites FTIR spectrum. The Fig. 2a illustrates the entire representative absorption features of HAP compound, such as the stretching and vibrational frequencies of the PO43— group at 569, 879, and 1033 cm−1 and O—H groups at 3345 cm−1 and 610 cm−1 (Sumathra et al., 2018). In the boswellic acid, the peaks were found at 3453 cm−1 corresponding to a hydroxyl group, 2954 cm−1 of C—H stretching and 1735 cm−1 of the carboxyl group and were presented in Fig. 2(b) (Saji et al., 2012). Broadband was observed between 3800 and 3000 cm−1 in Fig. 1c, this is due to the intra-molecular hydrogen bonding of free hydroxyl groups of the CMC. The peak 2967 cm−1 denotes the presence of C—H stretching in CMC. The peak in wavenumber 1738 and 1422 cm−1 elucidate the existence of C⚌O stretching and CH2 bending confirms the functional groups of CMC constituent (Fig. 2c) (Enas et al., 2016). Fig. 1d confirms the COO-Ca2+ interaction between HAP and 5 wt% of BA at 1649 cm−1 which denotes that acid binding with HAP (Sumathra et al., 2017). In addition, the COO-Ca2+ peak was shifted to the lower region at 1593 cm−1 which were due to CMC interaction with HAP/BA composites (Fig. 2e) (Masayuki et al., 2013) for the confirmation of HAP/BA/CMC composite formation. This was also coincides with the previous report suggested by the Surmenevaa et al. (2019).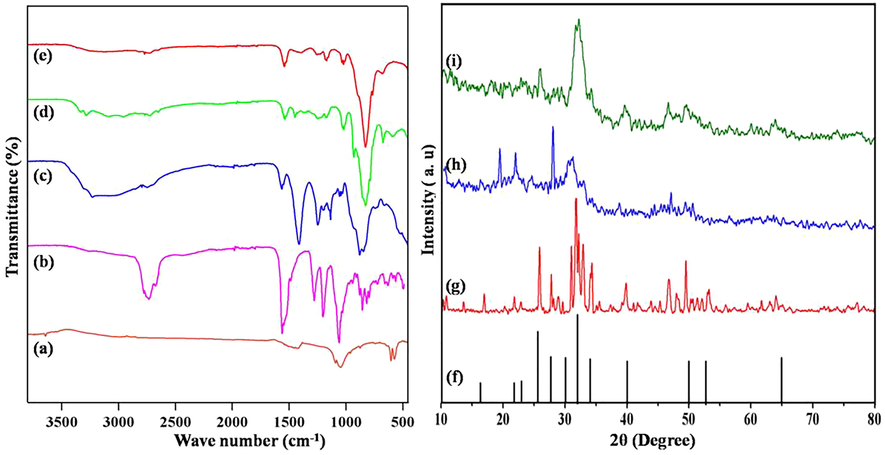
FTIR spectra of (a) HAP, (b) BA, (c) CMC, (d) HAP/BA and (e) HAP/BA/CMC composites and XRD patterns of (f) JCPDS data of HAP, (g) Synthesized HAP, (h) HAP/BA and (i) HAP/BA/CMC.
3.2 XRD analysis
According to JCPDS value (Card no. 9-432), the crystalline nature of HAP is characterized by XRD as shown in Fig. 2(f) The consequent distinguished peaks at 26, 32, 33, 40 recognized to (0 0 2), (2 1 1), (3 0 0), and (3 1 0) planes, correspondingly. The hydroxyapatite XRD spectrum was shown in Fig. 2(g) (Sumathra et al., 2017). The synthesized hydroxyapatite, boswellic acid XRD spectrum was revealed in Fig. 2(h). The development of new peaks along with HAP XRD spectrum nearly 10–20° indicates the presence of BA in HAP (Enas et al., 2016). There is a decrease in the intensity of the peaks confirms the formation of HAP/BA composite. Fig. 2(i) corresponds to the XRD spectrum of the addition of CMC on HAP/BA composite. The existence of an amorphous component, CMC influences only the intensity of the XRD peaks. The broad peaks appeared between 10O and 20O stand for the presence of CMC in the composite (Zakharov et al., 2005). The CMC macromolecule created no drastic modification in the structure HAP/BA sample.
3.3 SEM & TEM characterizations
The synthesis of boswellic acid assisted HAP morphological observation was investigated through SEM and TEM technologies. Fig. 3 demonstrated the SEM and TEM images of HAP, BA, HAP/BA and HAP/BA/CMC composites. The pure HAP shows aggregated with spherical particles Fig. 3(a). This HAP was prepared via precipitation method since the particles are aggregated with no stabilizing factor involved. The similar report was mentioned earlier by Sumathra et al, by the preparation of HAP through precipitation method (Sumathra et al., 2017). This finding was supported by the previous research work done by the Ivanovaa et al. (2018). The boswellic acid morphology is presented in Fig. 3(b) and it like resins. The boswellic acid aided synthesis of HAP was presented in Fig. 3(c) and the surfaces of the particle are smooth and the spherical nature of particles gets retained in the boswellic acid and the hydroxyapatite composite. As final composites of HAP/BA/CMC was inspected and portrayed in Fig. 3(d) and the addition of CMC form a porous structure. The HAP/BA was entrapped on the CMC porous and the entrapment of the HAP/BA was stabilized by the interaction of Ca2+ of HAP and COO— of CMC of the composite. The HAP/BA, HAP/BA/CMC composites were also confirmed by TEM images Figs. 3 and 4(e and f). TEM micrographs of HAP/BA, HAP/BA/CMC composites are well correlated with SEM images and it revealed that the spherical and HAP/BA entrapped porous structure of HAP/BA, HAP/BA/CMC composites respectively. The insert images are corresponding to the EDAX spectrum of HAP/BA and SEAD HAP/BA/CMC were shown in Fig. 3(g and h).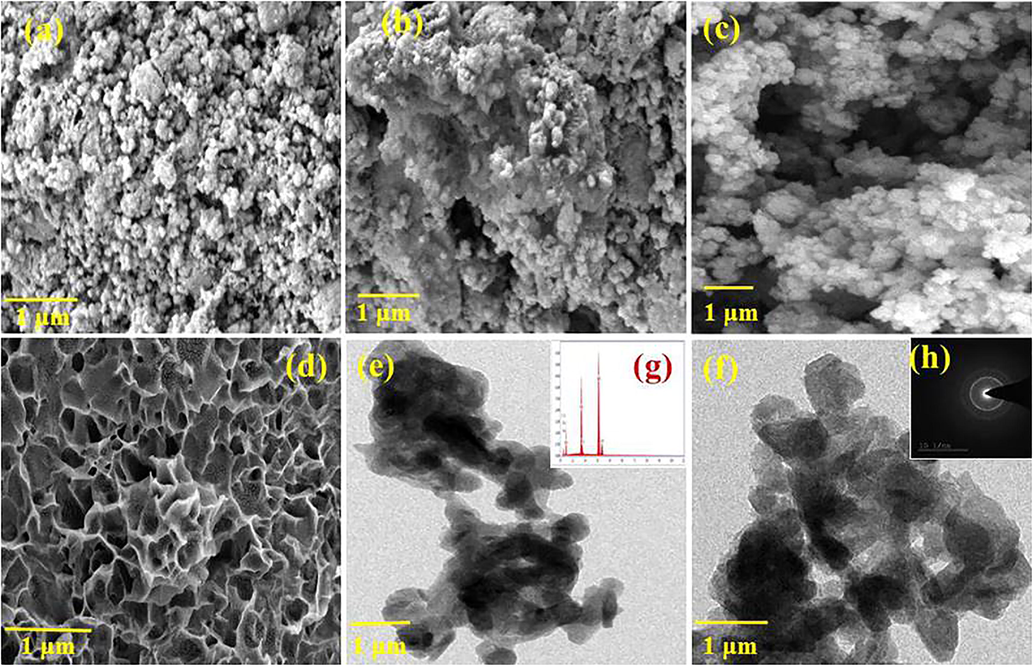
SEM images of (a) HAP, (b) BA (c) HAP/BA and (d) HAP/BA/CMC, TEM images of (e) HAP/BA and (g) HAP/BA/CMC, SAED pattern of (f) HAP/BA and (h) HAP/BA/CMC composite.
(i) Antimicrobial activities of HAP.
3.4 MIC determination
The antibacterial activity of HAP and HAP/BA/CMC compound were performed through the broth dilution assay method. The obtained results show that the HAP and HAP/BA/CMC compound possess actual antibacterial activity against B. cereus and P. aeruginosa. However, the HAP/BA/CMC compound has higher anti-bacterial activity (Fig. 5) compound than pure HAP. This is due to the presence of boswellic acid in the HAP/BA/CMC composite. Number of previous researchers was reported that boswellic acid is one of the potential anti-bacterial agents (Raja et al., 2011; Raja et al., 2011).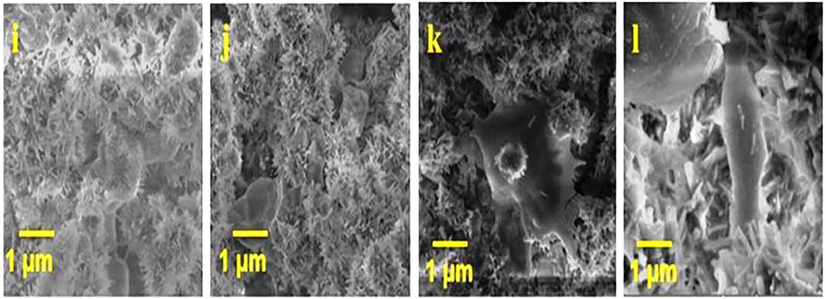
(A) and HAP/BA/CMC composites; (ii) cell viability of HAP (A) and HAP/BA/CMC composites and (iii) Optical image of cell viability for 1, 7 and 14 days of HAP (b, c and d).
3.5 Cell viability and cell adhesion
The cell viability of the isolated chondrocytes was tested using HAP and HAP/BA/CMC at 1, 7 and 14 days. The good viable nature was observed in both samples. Compare with HAP, HAP/BA/CMC having higher viable nature and it enhances the cell growth. The percentage of cell viability was observed 74% ± 25% of HAP/BA/CMC. The presence of boswellic acid and carboxymethyl cellulose in HAP has enhanced the viability compared with pure HAP (Mahesh et al., 2014; Ko et al., 2005). The chondrocyte cell growth on the HAP/BA/CMC composite was tested with the seeding of the cells for 1, 7 and 14 days. The cell growth morphology was observed through scanning electron microscope instrument (Fig. 6). Enhanced chondrocyte cell adhesion on the HAP/BA/CMC composites surface is a primary requirement of cartilage repair. Form the results of cell adhesion, the HAP/BA/CMC composites having well biocompatible and it able chondrocyte attach on the surface of the materials. From the SEM image demonstrate the chondrocyte cell spread on surface material with the increasing time. From 1 to 14 days, the maximum cell was spared on the material surface and it mainly influences by the presence of HAP and boswellic acid (Caron et al., 2012). Presence of Ca, boswellic acid and CMC the chondrocyte cells grew significantly on the HAP/BA/CMC and quantity of cells increased over time by eyes (Figs. 7 and 8).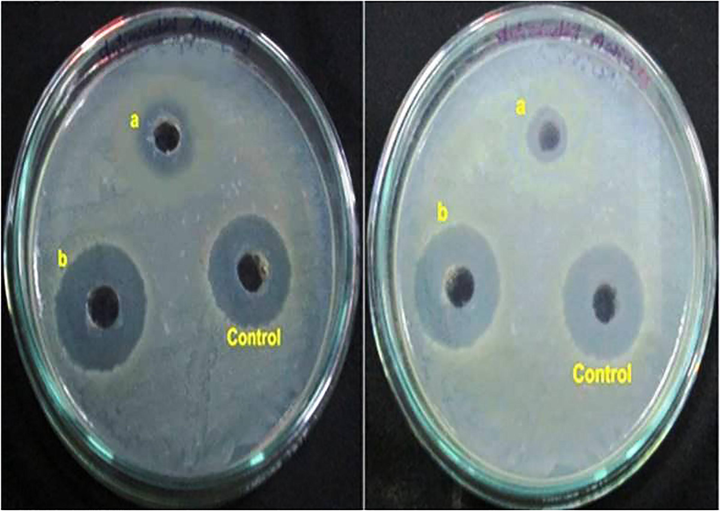
HAP/BA/CMC composites (f, g and h) respectively, image j, k and l is SEM image of cell adhesion on HAP/BA/CMC composites (a, e and J is control).
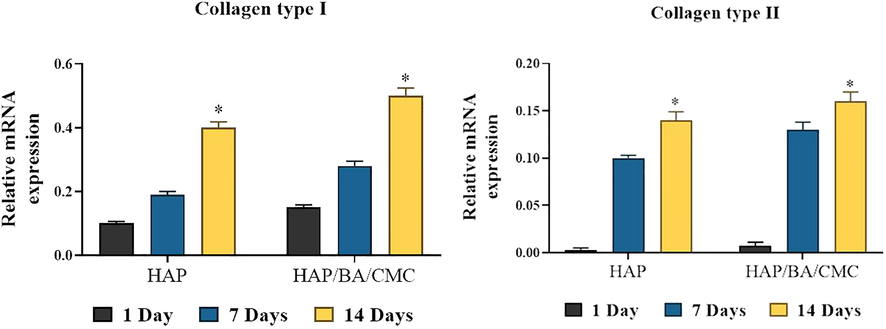
RT-PCR analysis of expression levels of chondrogenic genes in chondrocytes growing on HAP and HAP/BA/CMC for 1, 7 and 14 days, both of which have undergone similar rounds of proliferation or population doubling. Collagen type II stands and collagen type I stands. Results are normalized to those in cells prior to seeding, and shown as mean ± SD (*p < 0.05).
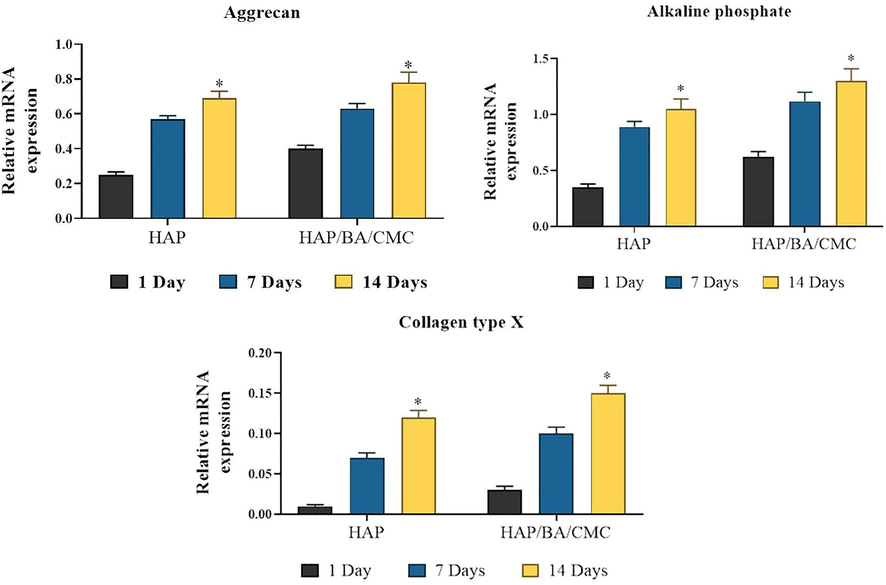
RT-PCR analysis of expression levels of hypertrophic genes in chondrocytes growing on HAP and HAP/BA/CMC for 1, 7 and 14 days, both of which have undergone similar rounds of proliferation or population doubling. Collagen type II stands and collagen type I stands. Results are normalized to those in cells prior to seeding, and shown as mean ± SD (*p < 0.05).
4 Conclusion
The HAP-BA-CMC composite was developed in the first time for the enhancement of chondrocyte cell to cartilage repair. From the physicochemical characterizations was confirmed for the formation of composites and ECM resamples like morphologies. Comparative anti-bacterial activity studies against B. cereus and P. aeruginosa which were higher antibacterial activity observed HAP-BA-CMC composites indicated that the presence of boswellic acid. The cell viability and cell adhesion on chondrocyte cells were confirmed as biocompatible with polygonal filopodia cell growth. Additional in vivo investigations in future may be valuable to examinethe sustained period effectiveness of the HAP-BA-CMC. This exploration evidenced the future emergence of HAP-BA-CMC as biocompatible, stable for chondrocyte enhancement in cartilage repair.
5 Availability of data and material
The data used in the study is confidential so we don’t want to share
Funding
The Project Supported by Natural Science Basic Research Plan in Shaanxi Province of China (Program No. 2019JM-493).
7 Authors' contributions
Bin Jia – manuscript preparation; DingjunHao – data analysis, FengQiao – manuscript preparation, Xiaoqing Zhou – methodology design, Yuming Zhang –result interpretation; Tao Wang – project management.
Acknowledgements
We thank Joint Surgery Department, Hip Ward, Hong-Hui Hospital, Xi’an Jiaotong University College of Medicine, Xi'an City, Shaanxi Province, PR China for laboratory facilities.
Declaration of Competing Interest
The authors declared that there is no conflict of interest.
References
- Trans. Indian Ceram. Soc.. 2018;77
- Biomaterials. 2017;139:202-212.
- Osteoarthrit. Cartilage. 2012;20:1170-1178.
- Arthroscopy. 1997;13:456-460.
- Biomaterials. 2012;33:3846-3851.
- Int. J. Chem. Tech. Res.. 2016;9:270-281.
- Mater. Today: Proc.. 2016;3:2394-2398.
- Mater. Charact.. 2018;142:261-269.
- Phytomedicine. 2003;10:3-7.
- J. Bio. Mater. Sci. Polym. Ed.. 2005;16:1277-1291.
- Cell Tissue Res.. 2012;347:613-627.
- Connect. Tissue Res.. 2017;58:1.
- Chin. J. Natural Med.. 2014;12(9):663-671.
- Trans. Indian Ceram. Soc.. 2015;74:4.
- Biochim. Biophys. Acta. 2013;1828:2319-2327.
- Resear. Veteri. Sci.. 2014;97:574-581.
- BMC Microbiol.. 2011;11:54.
- BMC Res. Notes. 2011;4:406.
- J. Cranio-Maxillo Fac. Sur. 2016
- [CrossRef]
- J. Natural Prod.. 2012;5:100-108.
- Orient. J. Chem.. 2017;33(6):3003-3008.
- Sustain. Chem. Pharm.. 2017;5:46-53.
- New J Chem. 2018 (in press)
- [CrossRef]
- New J. Chem.. 2018;42:725-734.
- Mater. Chem Phys.. 2019;221:89-98.
- Arthroscopy. 2010;26:375-383.
- Biomaterials. 2018;165:66-78.
- Inorg. Mater.. 2005;41:509-515.







