Translate this page into:
Brassinin inhibits proliferation and induces cell cycle arrest and apoptosis in nasopharyngeal cancer C666-1 cells
⁎Corresponding author. gyf13529021251@sina.com (Yan-Fei Guan)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
Background
Nasopharyngeal cancer is a tumor that occurs in the mucous epithelium of the nasopharynx. Due to its rapid growth and early metastatic nature, the successful treatment of nasopharyngeal cancer is highly challenging.
Objective
Here, we intended to assess the in vitro anticancer property of brassinin against the nasopharyngeal cancer C666-1 cells.
Methodology
The in vitro free radical scavenging property of the brassinin was assessed by various free radical scavenging activities such as FRAP, DPPH, chemiluminescence (CL), and ORAC assays. The cytotoxic level of the brassinin (1–50 µM) against the nasopharyngeal cancer C666-1 cells and normal Vero cells were assessed by the MTT cytotoxicity assay. The levels of TBARS, GSH, and the SOD activity was assessed using kits. The level of ROS generation, MMP, and apoptosis were investigated by the respective fluorescent staining techniques. The flow cytometry analysis was done to scrutinize the cell cycle arrest. The Bax/Bcl-2 level and caspase activities were examined using respective kits.
Results
The brassinin treatment effectively scavenged the free radicals, which are assessed by the FRAP, DPPH, chemiluminescence (CL), and ORAC assays. The proliferation of brassinin treated C666-1 cells were decreased remarkably, while the same concentration of brassinin did not disturbed the Vero cell viability. The 30 µM of brassinin effectively increased the ROS production, depleted the MMP, and stimulated the apoptosis in the C666-1 cells. The brassinin increased the TBARS and depleted the GSH and SOD in the C666-1 cells. The flow cytometry analysis revealed that the brassinin administration improved the G0/G1 ratio and decreased the proportion of cells with ‘S’ and ‘G2/M’ phase. The Bax, caspase-3 and −9 were elevated and Bcl-2 level was decreased in the brassinin administered C666-1 cells.
Conclusion
Our findings discovered that the brassinin has the capacity to prevent the proliferation and stimulate the apoptotic cell death C666‐1 cells via blocking cell cycle and increasing oxidative stress and apoptotic markers. Hence, it can be a talented therapeutic agent to treat the nasopharyngeal cancer in the future.
Keywords
Cell cycle arrest
Bax/Bcl-2
Caspases
Brassinin
C666-1 cells
G2/M phase
Data Availability Statement
The data used to support the findings of this study are available from the corresponding author upon request.
1 Introduction
Nasopharyngeal cancer is a most aggressive tumor that arises in a mucous epithelial layer of nasopharynx. Due to the rapid growth and early metastatic nature of the nasopharyngeal cancer, its very challenging to the successful treatment (Sun and Wang, 2019). Nasopharyngeal cancer has a higher tendency to invade and metastasize to lymph nodes and distant organs at an early stage (Wang et al. 2014). At present, the post-operative radiotherapy has gained greater achievements in the management of nasopharyngeal cancer. Though, the survival rate of nasopharyngeal cancer patients within 5 years followed by the surgery is 50–70% only (Jin et al., 2014; Safavi et al., 2015). The previous etiological studies have highlighted that the development of nasopharyngeal cancer is a multifaceted process, in that environmental carcinogens, lifestyle history, and Epstein-Barr-Virus performs a critical functions in the initiation of nasopharyngeal cancer (Chua et al., 2016). In most incidences of nasopharyngeal cancer represents as progressed loco-regional disease (Brennan, 2006). The chances of survival of children with loco-regional disease varied from 80 to 90% and treatment failures are developed because of the distant relapses (Mertens et al., 2005). Additionally, chances of survival of children with metastatic nasopharyngeal cancer during diagnosis are <10%. However, the concurrent chemo-radiotherapies has been believed as a customary treatments for nasopharyngeal cancer (Buehrlen et al., 2012; Casanova et al., 2012). In despite of several improvements in the treatment of nasopharyngeal cancer with chemo and radiotherapies, there are several adverse effects were experienced in those therapies such as fatal toxicity and less effectiveness of recurrent disease (Chan et al., 2015; Luo et al. 2014). Hence, the need for the exploration of new antitumor drugs with less toxicity and improved effectiveness is in great demand.
The tumor cells often evade the normal cellular growth pathways regulation due to the activation of oncogenes, violation of cell cycle checkpoints and genetic instability. The inhibition of apoptotic pathways is also believed as a key factor of tumorigenesis. Apoptosis is a highly regulated cell death event with precise genetic and biochemical pathways that performs a crucial functions in the homeostasis of normal cells (Singh et al., 2019). It participates in the removal of unnecessary cells to preserve the balance between death/survival of normal cells (Ashkenazi et al., 2017). Certainly, the anti-apoptotic proteins are highly expressed in several cancer types (Liu et al., 2016). The elevated expression of anti-apoptotic genes reduces the proapoptotic response and leads to the resistance of tumor cells to the treatments (Tian et al., 2020). To inhibit the tumorigenesis, apoptosis helps to eliminate the potentially harmful DNA-damaged or altered cells under several stressful conditions like stimulation of DNA damage checkpoint pathway and precancerous lesions (Shakeri et al., 2017). Therefore, the apoptotic mechanism assist to protect the genomic integrity while deregulation of apoptotic signaling not only enhance the cancer progression but also increase the tumor cell resistance to the therapies. Accordingly, the escape of apoptosis is a major phenomenon of tumorigenesis (Ouyang et al., 2018).
Brassinin (Fig. 1) is a well known phytoalexin, which extensively found in the cruciferous plants and vegetables e.g., Raphanus raphanistrum with several biological properties. A previous reports suggested that the brassinin triggered DNA fragmentation in human colon cancer and stimulated mitochondria-mediated apoptosis in prostate cancer (Chripkova et al., 2014; Kim et al., 2015). A latest literature highlighted that the brassinin inhibited the proliferation of human liver cancer cells (Hong et al., 2021). Izutani et al. (2012) reported that the brassinin induced the cell cycle arrest in the colon cancer cells. Brassinin repressed the invasion of lung cancer cells (Yang et al., 2019) and also demonstrated the potent anti-atherosclerotic activity (Han et al., 2017). However, the in vitro anticancer property of brassinin against the nasopharyngeal cancer was not studied yet. Hence, here we planned to assess the growth inhibition, cell cycle arrest, and apoptosis inducing potential of brassinin on the nasopharyngeal cancer C666-1 cells.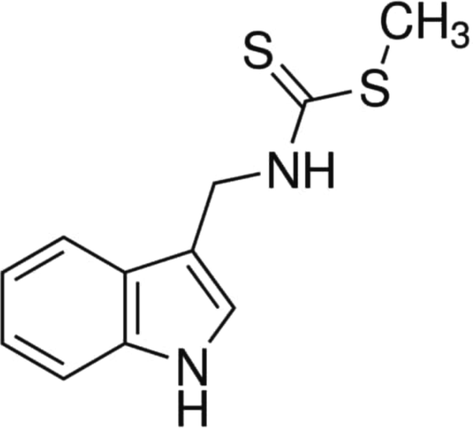
Chemical structure of brassinin.
2 Materials and methods
2.1 Chemicals
Brassinin, 3-(4,5-dimethylthiazoyl-2-yl)-2,5-diphenyltetrazolium bromide (MTT), dimethyl sulfoxide (DMSO), and other chemicals were purchased from the Sigma-Aldrich, USA. The assay kits for biochemical assessments were acquired from ThermoFisher Scientific, USA.
2.2 In vitro free radicals scavenging assays
2.2.1 Ferric reducing antioxidant power (FRAP) activity
The FRAP activity of the brassinin was examined by the method previously defined by the Benzie and Strain, (1996). The different concentrations of brassinin (1, 5, 10, 20, 30, 40, & 50 µM) were added with FRAP reagent (1 ml) along with the 300 mM of acetate buffer, 10 mM of TPTZ solution, and 20 mM of ferric chloride reagents. The 200 µl of the reaction solution was taken and loaded onto the 96-wellplate and stand for 10 min at 30 °C. Followed by the incubation, the developed final product were assessed using microplate reader at 593 nm wavelength. The final outcomes of FRAP activity of brassinin was represented as Trolox equivalent (mM).
2.2.2 DPPH radical scavenging activity
The DPPH scavenging effect of the brassinin was assessed by the approach of Brand-Williams et al. (1995). The DPPH (150 µl) was dissolved in ethanol (0.25 mM) and the mixed to the different concentrations of the brassinin (1–50 µM) and stand for 30 min at 37 °C. After that, the absorbance of final reaction suspension were measured at 515 nm. The final outcomes of the DPPH scavenging property of brassinin was represented as Trolox equivalent (mM).
2.2.3 Chemiluminescence (CL) assay
The superoxide scavenging property of the brassinin was examined by the technique of Shimada et al. (1992). The reaction solution was prepared by adding together of 10 µl of CL reagent, diverse concentrations of (1–50 µM) of brassinin, and 80 μl of xanthine oxidase. The control suspension was made using HEPES buffer. Afterward, the solution was placed onto the luminometer after the 200 µl of hypoxanthine substrate (0.72 mM). The CL activity was assessed for 10 min at the intervals of 10 s in luminometer. The assay was repeated in triplicates and final outcome was represented relative luminescence. The superoxide radical scavenging capacity of brassinin was represented as Trolox equivalent (mM).
2.2.4 Assessment of oxygen radical antioxidant capacity (ORAC)
The ORAC level of the brassinin was assessed by determining the peroxyl radical scavenging ability by using kit according to the guidelines described by the manufacturer (ThermoFisher Scientific, USA).
2.2.5 Collection of C666-1 cells
The nasopharyngeal cancer C666-1 cells and normal Vero cells were collected from the ATCC, USA. The purchased cells were then grown on the DMEM medium enriched with 10% of FBS at 37 °C in an humidified CO2 (5%) chamber. After the reaching of 80% of confluency, C666-1 cells were trypsinized and employed for the further examinations.
2.3 MTT assay
The viability of control and treated nasopharyngeal cancer C666-1 cells and normal Vero cells were examined by the MTT assay. For this, the both cells were grown onto the 96-wellplate loaded with DMEM medium at 5 × 103 cells/well density and incubated for 24 h. After that, C666-1 and Vero cells were administered with the various dosages (1–50 μM) of brassinin and maintained for 24 h at 37 °C. After that, MTT (20 μl) along with DMEM (100 µl) were added to all wells and further incubated for 4 h. The 100 μl of DMSO were added to dissolve the developed formazan stones. Finally, the absorbance of the control and treated cells were assessed at 570 nm by using microplate reader.
2.4 Measurement of oxidative stress and antioxidant markers
The level of TBARS, glutathione (GSH) level, and superoxide dismutase (SOD) activity in the control and brassinin (30 μM) administered C666-1 cells were assessed using respective assay kits using the protocols described by the manufacturer (Thermo Fisher, USA).
2.5 Measurement of ROS generation
The effect of brassinin treatment on the intracellular production of ROS in the C666-1 cells were examined by DCFH-DA staining technique. The cells were grown onto the 24-wellplate and sustained for 24 h. After that, cells were treated with 30 μM of brassinin and 2 μg of DOX for 24 h. The level of ROS generation in the brassinin treated C666-1 cells were assessed by staining with the 10 μl of DCFH-DA. The fluorescent intensity was examined under the fluorescent microscope to determine the ROS production level.
2.6 Mitochondrial membrane potential (MMP)
The MMP level was investigated by using Rh-123 staining assay. Briefly, the C666-1 cells were loaded onto the 24-wellplate with DMEM medium and treated with the 30 µM of brassinin and 2 μg of DOX for 24 h at 37 °C. Afterwards, the 10 μg/ml of Rh-123 were mixed to all wells and stand for 30 min. Lastly, the level of MMP was investigated using fluorescent microscope.
2.7 Dual staining
The level of apoptosis in control and brassinin administered C666-1 cells were assessed by using dual (AO/EB) staining technique. The C666-1 cells were loaded onto the 24-wellplate at 5 × 105 cell population/well in a DMEM and treated with the 30 μM of brassinin and 2 μg of DOX for 24 h at 37 °C. After that, the 100 μg/ml of AO/EB stain at 1:1 ratio was mixed to the each well for 5 min at 37 °C. Lastly, fluorescence intensity was assessed using fluorescent microscope to detect the apoptosis.
2.8 Propidium iodide (PI) staining
The level of apoptosis in the control and brassnin administered C666-1 cells were assessed by PI staining. The cells were loaded onto the 24-wellplate and maintained for 24 h. Afterward, cells were treated with 30 μM of brassinin and 2 μg of DOX at 37 °C for 24 h. Then the 5 µl of PI stain was used to stain the cells for 20 min and finally the fluorescence intensity was determined using fluorescent microscope.
2.9 Cell cycle analysis
The C666-1 cells at 5 × 106 cell density were collected and processed with 70% of ethanol and incubated for 12 h. Then, cells were cleansed and added to the 300 µl of staining solution, which contains 100 µl of PI, 0.5 mg/ml of RNase solution, and 0.08 mg/ml of proteinase inhibitors for 30 min. The flow cytometer was used to measure the PI fluorescence related with DNA. The nuclei percentages in every phase i.e., G1, S, G2/M of the cell cycle were determined by MultiCycle software (Phoenix Flow Systems, USA). The percentage of sub-diploid cells (apoptotic cells) were assessed by WinMDI 2.9 software installed on the flow cytometer.
2.10 Measurement of Bax/Bcl-2 level and caspase levels
The Bax and Bcl-2 contents and the activities of caspase-3 and −9 in the control and brassinin treated C666-1 cells were assessed with the help of commercial ELISA assay kits as per the protocols of the manufacturer (Thermofisher, USA).
2.11 Statistical analysis
The final results were assessed by using GraphPad Prism software. The outcomes were scrutinized by the one-way ANOVA and Tukey’s post hoc test. The outcomes were deliberated as a mean ± SD of three separate assays and ‘p’ value<0.05 were set as a significant.
3 Results
3.1 Effect of brassinin on the in vitro free radical scavenging property
The in vitro antioxidant property of the brassinin was scrutinized by several free radical scavenging assays and the findings were represented in the Fig. 2. The treatment with the different doses of brassinin remarkably scavenged the free radicals. The 1–50 µM of brassinin significantly (p < 0.05) decreased the several free radicals like DPPH, superoxide, and peroxyl radicals and also demonstrated the ferric reducing properties at the dose dependent manner. The increased dosage of brassinin significantly (p < 0.05) scavenged the levels of DPPH, superoxide, and peroxyl radicals and exhibited the ferric reducing power that proves in vitro free radical scavenging ability of the brassinin (Fig. 2). These outcome confirms the free radical scavenging property of brassinin.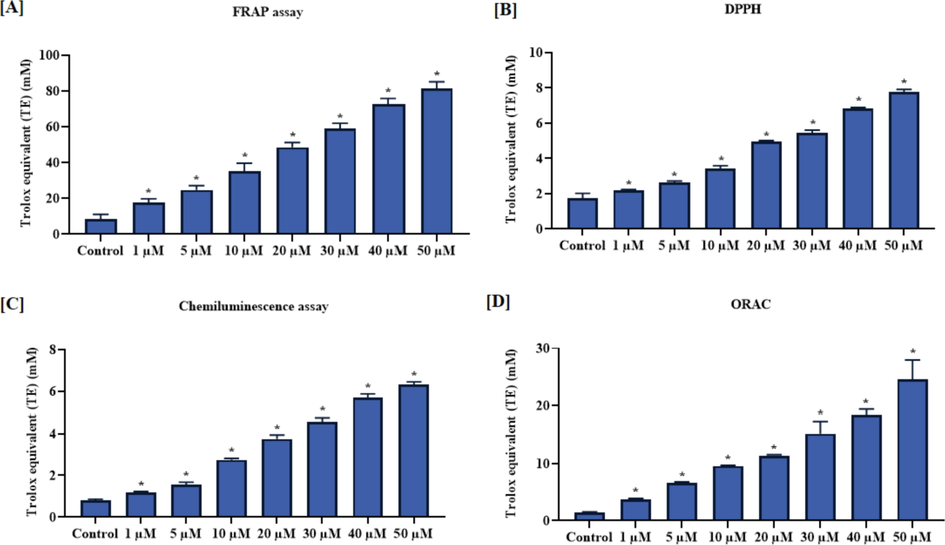
Effect of brassinin on the in vitro free radical scavenging property. The brassinin administration at different concentration (1–50 µM) exhibited the effective in vitro free radicals scavenging property. (A): FRAP activity, (B): DPPH scavenging activity, (C): Superoxide scavenging activity, (D): Peroxide scavenging activity. Outcomes were deliberated as a mean ± SD of three individual assays. The final data were assessed by the one-way ANOVA and Tukey’s post hoc study using GraphPad Prism software. Note: ‘*’ represents that data were significantly vary at p < 0.05 from control.
3.2 Effect of brassinin on the viabilities of C666-1 and Vero cells
The cytotoxicity of brassinin against the nasopharyngeal cancer C666-1 and normal Vero cells were assessed and outcomes were represented in the Fig. 3. Our findings demonstrated that the brassinin treatment at various concentrations (1–50 µM) significantly (p < 0.05) diminished the proliferation of C666-1 cells. Conversely, the same concentrations of brassinin did not disturbed the Vero cell viability. The mild reduction in the viability of Vero cells were noted at the high concentration of brassinin. This findings witnesses the cytotoxicity of brassinin against the C666-1 cells (Fig. 3). The IC50 level of brassinin for the C666-1 cells were noted at 30 µM, which is selected for the additional in vitro studies.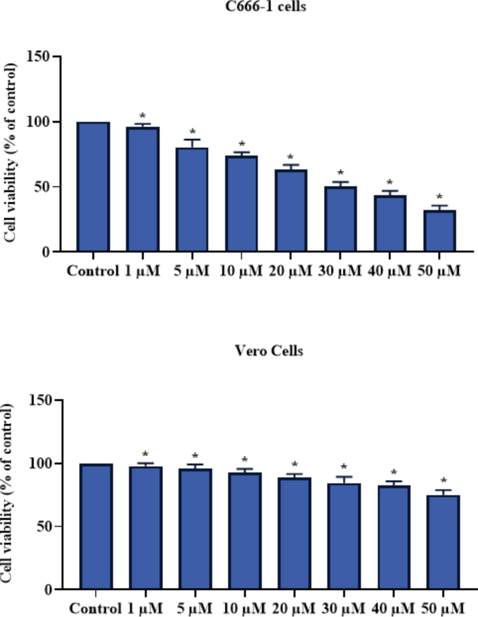
Effect of brassinin on the viabilities of C666-1 and Vero cells. Outcomes were deliberated as a mean ± SD of three individual assays. The final data were assessed by the one-way ANOVA and Tukey’s post hoc study using GraphPad Prism software. Note: ‘*’ represents that data were significantly vary at p < 0.05 from control.
3.3 Effect of brassinin on the antioxidants and TBARS level in the C666-1 cells
The effect of brassinin treatment on the level of TBARS and antioxidants in the nasopharyngeal cancer C666-1 cells were assessed and outcomes were demonstrated in the Fig. 4. The level of TBARS were significantly (p < 0.05) improved in the 30 µM of brassinin treated cells. The activity of SOD and GSH level was effectively decreased by the 30 µM of brassinin. These data revealed that the administration of brassinin significantly (p < 0.05) decrease the antioxidants and improve the oxidative stress in the C666-1 cells (Fig. 4). The standard drug DOX administration also remarkably elevated the TBARS level and decreased the GSH and SOD.
Effect of brassinin on the antioxidants and TBARS level in the C666-1 cells. Outcomes were deliberated as a mean ± SD of three individual assays. The final data were assessed by the one-way ANOVA and Tukey’s post hoc study using GraphPad Prism software. Note: ‘*’ represents significant at p < 0.05 between brassinin treatment and ‘#’ represents significant at p < 0.01 between control and DOX treatment.
3.4 Effect of brassinin on the ROS production, MMP level, and apoptosis in the C666-1 cells
Fig. 5(a) demonstrates the influence of brassinin treatment on the level of intracellular ROS generation in the C666-1 cells. The 30 µM of brassinin administered C666-1 cells demonstrated the increased green fluorescence than the control. The increased green fluorescence witnessed the improved intracellular production of ROS in the brassinin treated C666-1 cells. The standard drug DOX also demonstrated the augmented green fluorescence, which confirms the higher ROS production (Fig. 5a).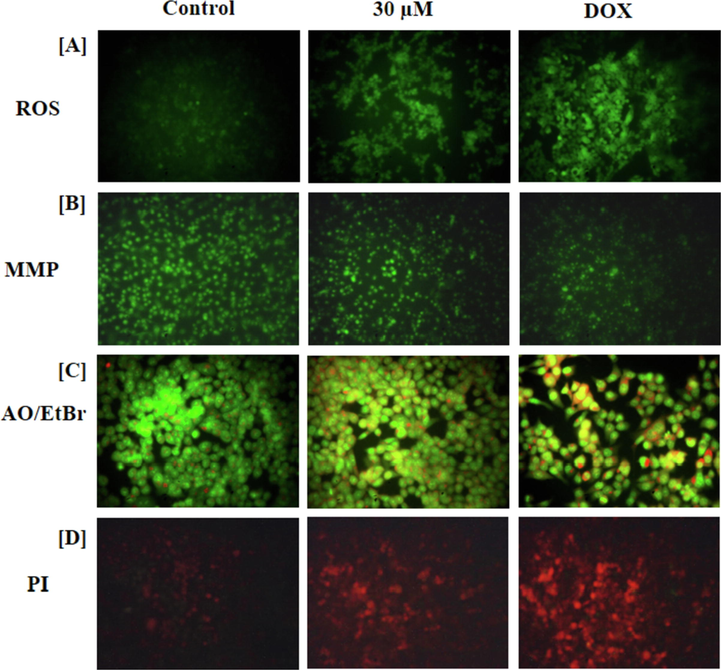
Effect of brassinin on the ROS production, MMP level, and apoptotic cell death in the C666-1 cells. The 30 µM of brassinin treated C666-1 cells represented the augmented green fluorescence than the control that confirms the increased ROS generation in the brassinin administered C666-1 cells (A). The 30 µM of brassinin treatment effectively depleted the MMP status in the C666-1 cells (B). The images of dual staining demonstrated the augmented orange and yellow in the 30 µM of brassinin treated C666-1 cells than control due to the increased apoptotic cells (C). The images of PI staining also demonstrated the augmented red fluorescence in the 30 µM of brassinin administered C666-1 cells that evidences the increased apoptosis (D).
Fig. 5(b) exhibits the MMP level of control and treated C666-1 cells. The increased MMP level was noted in the control cells that is identified by the intense green fluorescence. However, the 30 µM of brassinin administered C666-1 cells displayed the depleted MMP level that is evidenced by the decreased green fluorescence (Fig. 5b). The DOX also decreased the number of Rh-123 stained cells, which confirms the depleted MMP status.
Fig. 5(c) represents the influence of brassinin treatment on the apoptotic cell death events in the C666-1 cells. The 30 µM of brassinin administered cells exhibited the augmented apoptosis, which is witnessed by the intense yellow and orange fluorescence than the control, which evidences the increased number of early and late apoptotic cell deaths. The standard drug DOX treated C666-1 cells also exhibited the higher intense yellow/orange fluorescence, which evidences the increased apoptotic cell death (Fig. 5c).
Fig. 5(d) represents the apoptotic cell morphology in the control and treated cells. The increased red fluorescence was noted in the 30 µM of brassinin administered C666-1 cells. The number of apoptotic cells were increased in the brassinin administered C666-1 cells, which is evidenced by the higher red fluorescence (Fig. 5d). The DOX also increased the apoptotic cell death in C666-1 cells, which is detected by the intense red fluorescence.
3.5 Effect of brassinin on the cell cycle arrest in the C666-1 cells
The distribution of cell cycle phases in both control and administered cells were examined by the flow cytometry and outcome was displayed in the Fig. 6. The 30 µM of brassinin treated C666-1 cells were exhibited the higher proportion of the cells with sub ‘G0/G1′ and ‘G1′ growth phase than the control. Furthermore, the proportion of the cells with ‘S’ phase and ‘G2/M’ phase were reduced in the 30 µM of brassinin administered C666-1 cells, which confirms that the brassinin administration blocked the cell cycle in the C666-1 cells (Fig. 6). The standard drug DOX also increased the cells with sub G0/G1 and G1 phase and diminished the cells with ‘S’ phase and ‘G2/M’ phase.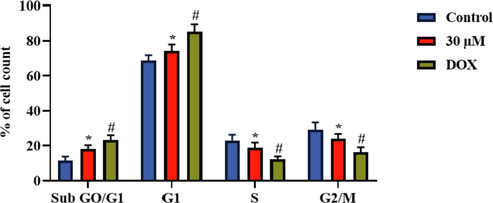
Effect of brassinin on the cell cycle arrest in the C666-1 cells. Outcomes were deliberated as a mean ± SD of three individual assays. The final data were assessed by the one-way ANOVA and Tukey’s post hoc study using GraphPad Prism software. Note: ‘*’ represents significant at p < 0.05 between brassinin treatment and ‘#’ represents significant at p < 0.01 between control and DOX treatment.
3.6 Effect of brassinin on the apoptotic biomarkers in the C666-1 cells
The effect of brassinin administration on the activities of caspase-3, −9 and Bax/Bcl-2 levels were examined and data were portrayed in the Fig. 7. The 30 µM of brassinin administered C666-1 cells exhibited the significantly (p < 0.05) increased activities of caspase-3 and −9, and Bax level. The brassinin administration also decreased the Bcl-2 level in the C666-1 cells. The DOX also increased the activities of caspase-3, −9, and Bax level and diminished the Bcl-2 level (Fig. 7).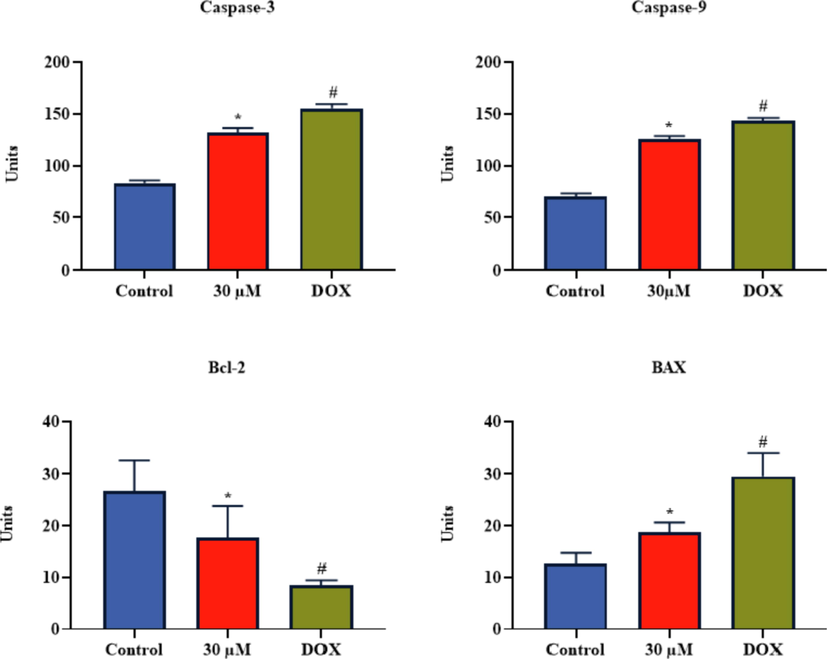
Effect of brassinin on the apoptotic biomarker levels in the C666-1 cells. Outcomes were deliberated as a mean ± SD of three individual assays. The final data were assessed by the one-way ANOVA and Tukey’s post hoc study using GraphPad Prism software. Note: ‘*’ represents significant at p < 0.05 between brassinin treatment and ‘#’ represents significant at p < 0.01 between control and DOX treatment.
4 Discussion
Nasopharyngeal cancer is a tumor arising from the epithelium of the nasopharynx (Chen et al., 2019). During early stages, nasopharyngeal cancer is likely to be undiagnosed due to its asymptomatic nature or only represents the insignificant clinical symptoms (Voon et al., 2015). The local recurrence of the disease were reported in nearly 5–10% of all nasopharyngeal cancer patients and 15–45% of those with stage IV followed by the treatment. In most cases of nasopharyngeal cancer experiences the distant metastases. Furthermore, the successful management of patients with developed stage of nasopharyngeal cancer, including metastasis, recurrent, and therapy-resistant still remains as a major challenge (Karam et al., 2016). The outcomes of patients with primary nasopharyngeal cancer has remarkably increased due to the developments in the chemo and radiotherapy (Blanchard et al., 2015; Yang et al., 2015). Though, the survival of the nasopharyngeal cancer patients with metastasis or recurrence is relatively poor with the mean survival rate is nearly 20 months (Miao et al., 2021). Hence, the need for the exploration of new anti-nasopharyngeal cancer drugs with more effectiveness and less toxicity was highly demanded. The investigation of cytotoxic levels of the sample drugs are crucial step in order to develop the novel anticancer candidates (Semlali et al., 2021). Here, our outcomes proved that the brassinin treatment demonstrated effective cytotoxicity to the nasopharyngeal cancer C666-1 cells. But, it did not disturbed the proliferation of normal Vero cells. This finding confirmed the cytotoxic nature of brassinin to the C666-1 cells.
Apoptosis is a multifaceted event, which involves several pathways and leads to the chromatin and nuclear membrane fragmentation. Although, when the apoptotic mechanisms tend to be deregulated, several pathological manifestations were arises to promote tumorigenesis (Koff et al., 2015). Apoptosis is one of the active cell death mechanisms, which preforms a pivotal roles in the maintenance of normal tissue homeostasis (Shrivastava et al., 2020). The deregulation apoptotic pathways is one of the major phenomenon of tumorigenesis (Neophytou et al., 2021). The tumor cells use several molecular mechanisms to inhibit the apoptosis. The tumor cells can attain resistance to the apoptosis via triggering of increased Bcl-2 expression or by the prevention or inhibition of Bax expression (Dabrowska et al., 2016). The induction of apoptosis is believed to be the critical strategy to prevent and treat the cancers. Additionally, the stimulation of apoptotic cell death in tumor cells are seems to be a most imperative activity for antitumor candidates. The several studies were directed to examine whether the cytotoxicity of chemotherapeutic drugs are connected with the apoptotic events (Qi et al., 2015; Zhang et al., 2014). The most conformist chemotherapeutic candidates like cisplatin, shows a anticancer effects via promoting the apoptosis (Matsuura et al., 2017). Here, our findings demonstrates that the brassinin treatment effectively stimulated the apoptosis in the C666-1 cells, which is confirmed by the AO/EB and PI staining techniques.
In tumor cells, mitochondria usually overproduce the ROS. The increased level of ROS trigger DNA-damage and cell death (Kocyigit and Guler, 2017). The improved production of ROS and depletion of MMP was reported to be an imperative factor of stimulating apoptosis in tumor cells (He et al., 2019). It was reported that the natural products have received greater attention as good source of chemo-therapeutic drugs for cancer treatment.
Several previous literatures reported that the natural compounds has stimulated the apoptosis in tumor cell via increasing the ROS production (Tripathi et al., 2020; Zhang et al., 2016). Our results evidenced that the brassinin appreciably increased the ROS accumulation and depleted the MMP level in the C666-1 cells.
The cell death mechanisms is generally connected with the proliferating cells. This infers the presence of molecules in late S and G1 phase, whose functions enables the execution of apoptotic mechanisms (Li et al., 2020). The G1, G2, and M checkpoints are the most crucial phases that make sure cells can develop to each step during the cell cycle without the faults (Rattanapornsompong et al., 2021). The flaws in those checkpoints can stimulate the apoptosis and remove the malignant cells (Luo et al., 2021). The arresting of cell cycle is an imperative task that affects the cell growth. In majority of cancers, cell cycle is often dysregulated. The controlling of cell cycle progression is believed as an effective strategy to prevent the cancer growth (Hoffmann et al., 2021). The cell cycle is a multifaceted process, and changes in its regulation may leads to the abnormal cell metabolism and proliferation. Several previous literatures found that the numerous natural compounds effectively inhibits the tumor cell viability via improving the cell cycle arrest (Wang et al., 2017; Zhang et al., 2018). In this study, we performed the flow cytometry study to explore whether brassinin could disturb the cell cycle mechanisms of C666-1 cells. Here, our findings revealed that the brassinin could regulate the cell cycle pattern in the C666-1 cells. Brassinin remarkably increased the G0/G1 ratio, whereas reduced the proportion of cells with ‘S’ and ‘G2/M’ phase. This outcomes witnessed that the cell cycle in the C666-1 cells by brassinin treatment may one of the mechanisms of attenuation of proliferative ability of nasopharyngeal cells. Our present findings were consistent with the previous literatures (Liu et al., 2019).
The Bcl-2 family proteins are the well‑established group of proteins, which is actively participates in the mitochondria-mediated apoptotic pathway (Nocquet et al., 2020). The balance between Bax and Bcl-2 genes regulates the normal survival/death events in cells. The Bcl-2 is tightly connected with the chemo-resistance of tumor cells, as it is one of the imperative regulators of apoptosis. Furthermore, tumor cells can escape apoptosis by several mechanisms to gain immortalization. The escape of apoptosis is a imperative cause of the chemo-resistance in tumor cells (Hassan et al., 2014; Su et al., 2014). Certainly, a depletion in the Bax/Bcl-2 ratio is believed as a critical biomarker of apoptosis (Kapoor et al., 2020; Pucci et al., 2019). Here, we observed that the Bax level was improved, while the Bcl-2 level was reduced in the brassinin administered C666-1 cells.
Caspase-3 is a critical player, which is essential to the regulation of cell apoptosis (Julien and Wells, 2017). Caspase-3 and −9 stimulates the apoptosis in tumor cells, which is triggered by the pro-apoptotic genes (Crowley and Waterhouse, 2016; Li et al., 2019). Caspases-3 is an “effector” caspase that can cleave the cellular products during the later phases of apoptosis (Carneiro and El-Deiry, 2020). Caspase-9 can cleave the caspase-3, which ultimately causes the DNA cleavage and degradation of cytoskeleton and nuclear proteins (Brown-Suedell A, Bouchier-Hayes, 2020). In this study, our findings evidenced that the brassinin treatment remarkably improved the caspase-3 and −9 activities in the C666-1 cells. Overall, the current findings revealed that the brassinin induced oxidantive stress and apoptosis in the C666-1 cells (Fig. 8).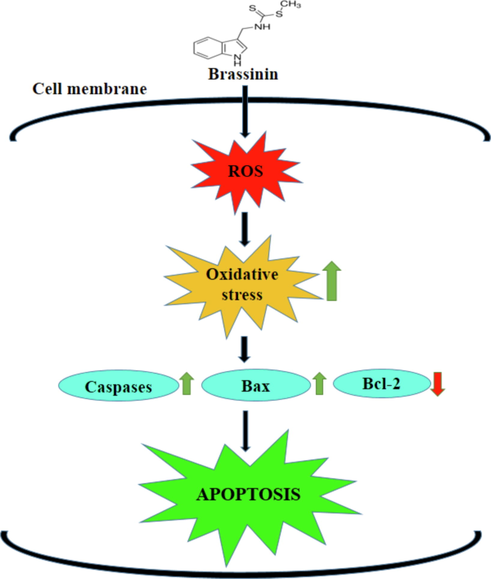
Schematic representation of probable molecular mechanisms of brassinin on the C666-1 cells.
5 Conclusion
In summery, this work discovered that brassinin has the capacity to remarkably prevent the proliferation and stimulate apoptosis in nasopharyngeal cancer C666‐1 cells via blocking cell cycle and increasing oxidative stress and apoptotic markers. The brassinin treatment exhibited a cytotoxicity to the C666-1 cells. The brassinin effectively arrested the cell cycle, improved the oxidative stress and apoptosis in the C666-1 cells. Hence, our findings suggest the brassinin as a hopeful therapeutic agent to treat the nasopharyngeal cancer in the future. However, the further confirmative studies still required in the future to understand the clear molecular mechanisms of anticancer property of the brassinin against the nasopharyngeal cancer.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- From basic apoptosis discoveries to advanced selective BCL-2 family inhibitors. Nat. Rev. Drug Discov.. 2017;16:273-284.
- [Google Scholar]
- The ferric reducing ability of plasma (FRAP) as a measure of “antioxidant power”: the FRAP assay. Anal. Biochem.. 1996;239:70-76.
- [Google Scholar]
- Chemotherapy and radiotherapy in nasopharyngeal carcinoma: an update of the MAC-NPC meta-analysis. Lancet Oncol.. 2015;16:645-655.
- [Google Scholar]
- Use of free radical method to evaluate antioxidant activity. LWT-Food Sci. Technol.. 1995;28:25-30.
- [Google Scholar]
- Caspase-2 Substrates: To Apoptosis, Cell Cycle Control, and Beyond. Front. Cell Dev. Biol.. 2020;8:610022
- [Google Scholar]
- Multimodal treatment, including interferon beta, of nasopharyngeal carcinoma in children and young adults: preliminary results from the prospective, multicenter study NPC-2003-GPOH/DCOG. Cancer. 2012;118:4892-4900.
- [Google Scholar]
- and Rare Tumors in Pediatric Age Group. A prospective protocol for nasopharyngeal carcinoma in children and adolescents: the Italian Rare Tumors in Pediatric Age (TREP) project. Cancer. 2012;118:2718-2725.
- [Google Scholar]
- Therapeutic targeting of CBP/β-catenin signaling reduces cancer stem-like population and synergistically suppresses growth of EBV-positive nasopharyngeal carcinoma cells with cisplatin. Sci. Rep.. 2015;5:9979.
- [Google Scholar]
- Brassinin and its derivatives as potential anticancer agents. Toxicol. Vitro. 2014;28:909-915.
- [Google Scholar]
- Crowley, L.C., Waterhouse, N.J., 2016. Detecting cleaved caspase-3 in apoptotic cells by flow cytometry. Cold Spring Harb Protoc.
- Apoptotic Caspases in Promoting Cancer: Implications from Their Roles in Development and Tissue Homeostasis. Adv. Exp. Med. Biol.. 2016;930:89-112.
- [Google Scholar]
- Inhibitory effect of brassinin on TNF-α-induced vascular inflammation in human umbilical vein endothelial cells. Mol. Med. Rep.. 2017;16(5):6890-6895.
- [Google Scholar]
- Apoptosis and molecular targeting therapy in cancer. Biomed Res. Int.. 2014;2014:150845
- [Google Scholar]
- Phillygenin exerts in vitro and in vivo antitumor effects in drug-resistant human esophageal cancer cells by inducing mitochondrial-mediated apoptosis, ROS generation and inhibition of the nuclear factor kappa B NF-κB signalling pathway. Med. Sci. Monit.. 2019;25:739-745.
- [Google Scholar]
- Downregulation of Cell Cycle and Checkpoint Genes by Class I HDAC Inhibitors Limits Synergism with G2/M Checkpoint Inhibitor MK-1775 in Bladder Cancer Cells. Genes (Basel).. 2021;12(2):260.
- [Google Scholar]
- Brassinin Inhibits Proliferation in Human Liver Cancer Cells via Mitochondrial Dysfunction. Cells.. 2021;10(2):332.
- [Google Scholar]
- Brassinin induces G1 phase arrest through increase of p21 and p27 by inhibition of the phosphatidylinositol 3-kinase signaling pathway in human colon cancer cells. Int. J. Oncol.. 2012;40(3):816-824.
- [Google Scholar]
- Meta-analysis of the association between GSTT1 null genotype and risk of nasopharyngeal carcinoma in Chinese. Tumour Biol.. 2014;35:345-349.
- [Google Scholar]
- Targeting BCL-2 in B-cell malignancies and overcoming therapeutic resistance. Cell Death Dis.. 2020;11(11):941.
- [Google Scholar]
- Outcomes after reirradiation for recurrent nasopharyngeal carcinoma: North American experience. Head Neck. 2016;38(Suppl 1):E1102-E1109.
- [Google Scholar]
- Brassinin combined with capsaicin enhances apoptotic and anti-metastatic effects in PC-3 human prostate cancer cells. Phytother. Res.. 2015;29:1828-1836.
- [Google Scholar]
- Curcumin induce DNA damage and apoptosis through generation of reactive oxygen species and reducing mitochondrial membrane potential in melanoma cancer cells. Cell. Mol. Biol.. 2017;63:97-105.
- [Google Scholar]
- A time to kill: Targeting apoptosis in cancer. Int. J. Mol. Sci.. 2015;16:2942-2955.
- [Google Scholar]
- Metformin induces cell cycle arrest, apoptosis and autophagy through ROS/JNK signaling pathway in human osteosarcoma. Int. J. Biol. Sci.. 2020;16(1):74-84.
- [Google Scholar]
- Oridonin synergistically enhances the anti-tumor efficacy of doxorubicin against aggressive breast cancer via pro-apoptotic and anti-angiogenic effects. Pharmacol. Res.. 2019;146:104313
- [Google Scholar]
- Brevilin A Induces Cell Cycle Arrest and Apoptosis in Nasopharyngeal Carcinoma. Front. Pharmacol.. 2019;24(10):594.
- [Google Scholar]
- Direct Activation of Bax Protein for Cancer Therapy. Med. Res. Rev.. 2016;36(2):313-341.
- [Google Scholar]
- Clinical outcomes for early-stage nasopharyngeal carcinoma with predominantly WHOII histology treated by intensity-modulated radiation therapy with or without chemotherapy in nonendemic region of China. Head Neck. 2014;36:841-847.
- [Google Scholar]
- 18β-Glycyrrhetinic Acid Has Anti-Cancer Effects via Inducing Apoptosis and G2/M Cell Cycle Arrest, and Inhibiting Migration of A549 Lung Cancer Cells. Onco Targets Ther.. 2021;14:5131-5144.
- [Google Scholar]
- Downregulation of the proapoptotic protein MOAP-1 by the UBR5 ubiquitin ligase and its role in ovarian cancer resistance to cisplatin. Oncogene. 2017;36:1698-1706.
- [Google Scholar]
- Treatment of nasopharyngeal carcinoma in children and adolescents: definitive results of a multicenter study (NPC-91-GPOH) Cancer. 2005;104:1083-1089.
- [Google Scholar]
- IAP-1 promoted cisplatin resistance in nasopharyngeal carcinoma via inhibition of caspase-3-mediated apoptosis. Am J Cancer Res.. 2021;11(3):640-667.
- [Google Scholar]
- Apoptosis Deregulation and the Development of Cancer Multi-Drug Resistance. Cancers (Basel).. 2021 Sep;13(17):4363.
- [Google Scholar]
- Mitochondria at Center of Exchanges between Cancer Cells and Cancer-Associated Fibroblasts during Tumor Progression. Cancers (Basel).. 2020;12(10):3017.
- [Google Scholar]
- SIRT6 overexpression induces apoptosis of nasopharyngeal carcinoma by inhibiting NF-κB signaling. Onco Targets Ther. 2018;11:7613-7624.
- [Google Scholar]
- Innovative approaches for cancer treatment: current perspectives and new challenges. Ecancermedicalscience.. 2019;13:961.
- [Google Scholar]
- MiR-142-3p Suppresses SOCS6 Expression and Promotes Cell Proliferation in Nasopharyngeal Carcinoma. Cell. Physiol. Biochem.. 2015;36:1743-1752.
- [Google Scholar]
- Impaired G2/M cell cycle arrest induces apoptosis in pyruvate carboxylase knockdown MDA-MB-231 cells. Biochem. Biophys. Rep.. 2021;25:100903
- [Google Scholar]
- Epidemiology of nasopharyngeal cancers in Iran: A 6-year report. Asian Pac J. Cancer Prev.. 2015;16:4447-4450.
- [Google Scholar]
- The curcumin analog (PAC) suppressed cell survival and induced apoptosis and autophagy in oral cancer cells. Sci. Rep.. 2021;11:11701.
- [Google Scholar]
- Antioxidative properties of xanthone on the auto oxidation of soybean in cylcodextrin emulsion. J. Agr. Food Chem.. 1992;40:945-948.
- [Google Scholar]
- Molecular targeted therapy: novel therapeutic approach for head and neck cancer. Ther Deliv.. 2020;11(10):637-651.
- [Google Scholar]
- Regulation of apoptosis in health and disease: the balancing act of BCL-2 family proteins. Nat. Rev. Mol. Cell Biol.. 2019;20(3):175-193.
- [Google Scholar]
- 13-acetoxysarcocrassolide induces apoptosis on human gastric carcinoma cells through mitochondria-related apoptotic pathways: P38/JNK activation and PI3K/AKT suppression. Mar. Drugs. 2014;12:5295-5315.
- [Google Scholar]
- Correlation between COX-2 gene polymorphism and susceptibility to nasopharyngeal carcinoma. Eur. Rev. Med. Pharmacol. Sci.. 2019;23:5770-5778.
- [Google Scholar]
- MiRNAs in Radiotherapy Resistance of Nasopharyngeal Carcinoma. J Cancer.. 2020;11(13):3976-3985.
- [Google Scholar]
- Plumbagin engenders apoptosis in lung cancer cells via caspase-9 activation and targeting mitochondrial-mediated ROS induction. Arch Pharm Res.. 2020;43:242-256.
- [Google Scholar]
- Nutlin-3 sensitizes nasopharyngeal carcinoma cells to cisplatin-induced cytotoxicity. Oncol. Rep.. 2015;34:1692-1700.
- [Google Scholar]
- Effect of IL-17A on the migration and invasion of NPC cells and related mechanisms. PLoS ONE. 2014;9:e108060
- [Google Scholar]
- Chen YC Anti-proliferative effect and cell cycle arrest induced by saponins extracted from tea (Camellia sinensis) flower in human ovarian cancer cells. J. Funct. Foods. 2017;37:310-321.
- [Google Scholar]
- Development and external validation of nomograms for predicting survival in nasopharyngeal carcinoma patients after definitive radiotherapy. Sci. Rep.. 2015;5:15638.
- [Google Scholar]
- Brassinin Represses Invasive Potential of Lung Carcinoma Cells through Deactivation of PI3K/Akt/mTOR Signaling Cascade. Molecules. 2019;24(8):1584.
- [Google Scholar]
- Celecoxib enhances radiosensitivity via induction of G(2)-M phase arrest and apoptosis in nasopharyngeal carcinoma. Cell. Physiol. Biochem.. 2014;33:1484-1497.
- [Google Scholar]
- Effects of ophiopogonin B on the proliferation and apoptosis of SGC-7901 human gastric cancer cells. Mol. Med. Rep.. 2016;13:4981-4986.
- [Google Scholar]
- Chen YC Dietary compound proanthocyanidins from Chinese bayberry (Myrica rubra Sieb. et Zucc.) leaves inhibit angiogenesis and regulate cell cycle of cisplatin-resistant ovarian cancer cells via targeting Akt pathway. J. Funct. Foods. 2018;40:573-581.
- [Google Scholar]







