Translate this page into:
Butter oil (ghee) enrichment with aromatic plants: Chemical characterization and effects on fibroblast migration in an in-vitro wound healing model
⁎Corresponding author. giovanni.caprioli@unicam.it (Giovanni Caprioli)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Ghee is a dairy product widely consumed in India, north-Africa, and Middle East countries, having beneficial pharmacological effects. This study aims to characterize the effects of aromatic plants addition (rosemary and clove) on the nutritional, volatile and oxidative profile of cow ghee and to evaluate the effect of flavored ghee on the fibroblasts migration during wound healing in vitro assay. Two flavored ghee products were obtained by adding clove (CG) and rosemary (RG) as aromatic plants through maceration in cattle traditional ghee (BT). It was revealed that enriched ghee samples had significantly lower peroxide values (6.76 and 6.80 meqO2 /kg) compared to control samples (8.20 meqO2 /kg). Moreover, the addition of rosemary and clove change the volatile profile, and increased the retinol levels of ghee (BT: 1.3 mg/kg; CG: 1.9 mg/kg; and RG: 3.05 mg/kg). Liquid-chromatography analyses revealed the presence of targeted phenolic compounds such as carnosic acid, rutin and gallic acid in CG and RG, showing thus, the transfer of polyphenols from aromatic plants into the ghee matrix. On the other hand, the fatty acid composition of ghee remained unchanged. The major components of the prepared ghee samples contributed to rising significantly the human fibroblast migration in wound healing in vitro assay. The results obtained underline that the flavored ghee samples could improve skin regeneration, making them potentials therapeutic ingredients in skincare formulations.
Keywords
Fibroblast migration
Ghee
Maceration
Natural antioxidant
Nutritional composition
Wound healing
1 Introduction
The wound is defined as loss or rupture of the cellular, anatomical, and functional continuity of living tissues. The healing of the wound consists of a process of coagulation, epithelialization, granulation, collagenation, and remodeling of tissues (Nandanwar et al., 2010). This tissue remodeling or tissue regeneration requires cells reprogramming, which involves development programs for healing, apoptosis and differentiation (Tottoli et al., 2020).
Another important element to take into consideration is the presence of oxidative reagents that can affect the healing process of the wound and provoke oxidative damage (Lin et al., 2019). Moreover, oxidative damage does not accumulate only with age, but also triggers the aging of individual and interferes with the process of reprogramming. Thanks to antioxidants and certain vitamins, it is possible to prevent and reduce this damage and considerably improve the efficiency of reprogramming (Jeon et al., 2018).
For the management of skin conditions, it is noted that the use of alternative and/or complementary therapeutic approaches, based on natural products, is remarkably increasing (Hajialyani et al., 2018). Indeed, Western society knows a kind of “green” consumerism and environmental awareness by using products that have no side effects on our natural environment. For these reasons, it would be interesting to develop local formulations based on natural products that improve the healing processes of wounds by acting on dermal fibroblasts. These cells synthesize the extracellular matrix and collagen and play an essential role in wound healing especially during the proliferation phase (Sabirin, & Yuslianti, 2016). After skin lesions, fibroblasts migrate into the clot of the wounds where they deposit their matrix rich in collagen, which contributes to the closure of the wound itself (Antognoni et al., 2016).
Indian traditional medicine, known as “Ayurveda”, selects ghee among the most important dairy products. One of the most widespread and well-known properties of ghee is its wound healing activity (Prasad & Dorle, 2006). The main world zones that consume ghee are India, north-Africa and Middle East countries, due to its pleasant flavor and valuable content of energy and fat-soluble vitamins (A, D, E and K). However, due to its high lipid content, ghee undergoes rapidly auto-oxidation with a reduction of its shelf-life (Lodh & Khamrui, 2017).
To solve this problem, a possible approach consists of adding antioxidants into ghee. Indeed, antioxidants can play an important role in the protection of dairy products from oxidative degradation (Gutierrez et al., 2018). Therefore, with the increased attention on naturally occurring antioxidants, some studies have assessed the effect of ghee enrichment with plants and plant extracts on its oxidative stability (Gandhi & Lal, 2018; Lodh et al., 2018). Researches pointed out that plant extracts with high polyphenols content can retard the oxidation processes in ghee (Hazra & Parmar, 2014).
Among the studied plants, just a few studies have been carried out on rosemary (Rahila et al., 2018; Pawar et al., 2014) and clove extracts (Patel et al., 2014; Shende et al., 2014). Rosemary and clove leaves are commonly used in the Mediterranean kitchen as flavoring agents. Moreover, due to their high content in phenolic compounds, these plants are characterized by an important antioxidant activity. Therefore, they can also be used as dried herbs, essential oils, or plant extracts to prevent lipid oxidation in oily products (Farhat et al., 2017). Most of the studies that assessed the effect of rosemary and clove on the oxidative stability of ghee, were carried out using extracts obtained with water or organic solvents. Interestingly, the direct use of plants (Kapadiya & Aparnathi, 2018) could enrich ghee with nutrients and could impact the flavor by transferring aromatic compounds into the food matrix. However, to our knowledge, no study has been conducted on these aspects of ghee research.
This study is therefore, one of the first to assess the effects of the addition of rosemary and clove leaves on the oxidative stability, the volatile profile, and the chemical composition of ghee. Finally, we assessed the capacity of our plant-enriched ghee samples to stimulate the migration of fibroblasts through an in vitro wound healing assay.
2 Materials and methods.
2.1 Chemicals and reagents.
High performance liquid chromatograhy (HPLC) grade methanol, isopropanol, and acetonitrile were purchased from Sigma–Aldrich (Milan, Italy). Milli-Q water (greater than18 MΩ cm resistivity) was obtained from a Milli-Q SP Reagent Water System (Millipore, Bedford, MA, USA). HPLC grade acetic acid was bought from J.T. Baker B.V. (Deventer, Netherlands). Sodium thiosulphate, NaCl, hexane, and cyclohexane were bought from Carlo Erba (Milan, Italy). The Folin-Ciocalteu reagent and the analytical standards of quercitrin, hyperoside, (+)-catechin, (-)-epicatechin, 3,5-di-O-caffeoylquinic acid, chlorogenic acid (3-CQA), neochlorogenic acid (5-CQA), rutin, rosmarinic acid, shikimic acid, carnosol, gallic acid, carnosic acid, retinol, and alpha-tocopherol were purchased from Sigma-Aldrich (Milan, Italy). For the identification of the volatile compounds by gas chromatography, the analytical standards of α-pinene, camphor, p-cymene, eugenol, (E)-caryophyllene and 1,8-cineole were purchased from Sigma Aldrich (Milan, Italy).
All reagents used for cell culture and wound healing-migration assay were purchased from EuroClone (Milan, Italy).
2.2 Samples collection and preparation.
Wild rosemary dried leaves (Rosmarinus officinalis) were collected from the Zaghouan region in the north-east of Tunisia while dried leaves of cultivated clove (Syzygium aromaticum) were purchased from a local market (Tunis, Tunisia). Ghee was prepared by clarifying butter (Fig. 1.) that was previously separated from cow milk collected from farmers of the Soliman region in the north-east of Tunisia. Briefly, butter was heated (110–125 °C) to evaporate the water. The foam formed on the surface, consisting mainly of casein, was delicately eliminated. The obtained liquid was filtered to produce the cattle traditional ghee (BT) which was then enriched with plants by hot maceration (Saoudi et al., 2016). The dried plants were introduced in liquid ghee at a rate of 6% (w/w) and the mixture was kept in the dark at 60 °C for 21 days with daily agitation. The oils were filtered and stored at 4 °C until analysis. All the results obtained from the analysis of flavored ghee samples were compared with those of the non-macerated ghee (control ghee, BT: Bovin Traditional).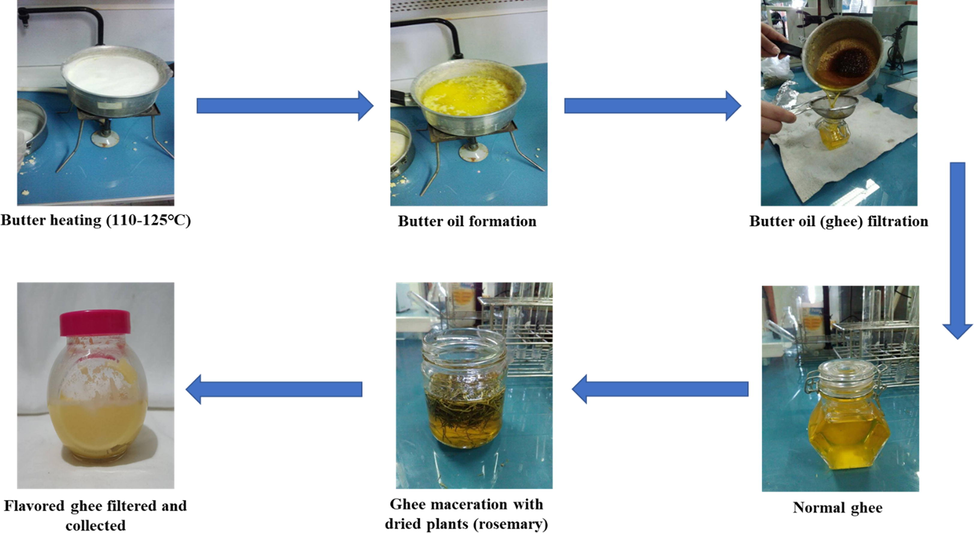
Illustration of ghee samples preparation.
2.3 Chemical characterization of the enriched samples.
2.3.1 Peroxide value.
Stability was evaluated in different ghee samples by monitoring the peroxide value (PV) during 21 days of accelerated incubation at 60 °C. The PV was determined following the standard method of the Association of Official Analytical Chemists Inc (AOAC) (Mehta et al., 2015). Five g of each ghee sample were placed in a flask and dissolved with 30 mL of acetic acid-chloroform mixture (60:40, v/v). Then, 0.5 mL of a saturated solution of Potassium iodide (KI) was added into the flask. The mixture was shaken for 1 min and then 15 mL of distilled water was added and the mixture was titrated against 0.01 N sodium thiosulphate (Na2S2O3) solution using a 1% starch solution as indicator. A blank sample was also titrated under the same conditions. PV was determined using the following equation:
PV = 1000* V*c/m;
Where PV is expressed as meqO2 /kg, V represents the volume of Na2S2O3 used for sample titration (mL), m was the mass of ghee (g) and c was the concentration (M) of sodium thiosulphate.
2.3.2 Fatty acids analyses.
2.3.2.1 Transmethylation.
The fatty acids composition of ghee samples was determined as the corresponding fatty acids methyl esters (FAMEs). For sample transmethylation and GC-FID analyses, the method of Caprioli et al (2016) was applied with some modifications. Briefly, 15 mg of ghee were dissolved in 1 mL of hexane. Then, 0.1 mL of 2 M KOH in methanol was added. After 2 min of vortex, 1.5 mL of 0.15 M of aqueous acetic acid was added and the solution was vortexed for 1 min and centrifuged at 5000 rpm for 10 min. The upper phase was collected and the operation was repeated three times. The final FAMEs solution was stored at −20 °C and before analysis, was evaporated to dryness with a nitrogen stream, dissolved in 1 mL of hexane and analyzed in triplicate.
2.3.2.2 Analysis by gas chromatography-flame ionization detector.
An Agilent Technologies 6850 gas chromatograph (GC) equipped with a flame ionization detector (FID) was used. The separation of FAMEs was carried out using a 50% cyanopropyl-phenyl-dimethylpolysiloxane column (30 m, 0.32 mm; film thickness, 0.25 mm i.d) (DB-225 Agilent Technologies, CA, USA). The injected volume was 1 µL in split mode with a ratio of 1:30 and an injector temperature of 260 °C. The oven temperature was kept at 60 °C for 3 min then it increased to 220 °C at 20 °C/min, and held at 220 °C for 8 min. The carrier gas was H2 at a flow of 3.7 mL/min and the FID detector temperature was 250 °C (airflow 400.0 mL/min, H2 flow 40.0 mL/min). Identification was performed by comparison with the standard « Supelco 37 Component FAME Mix » purchased from Supelco (Bellefonte, PA).
2.3.3 Extraction of retinol and alpha-tocopherol and analysis by HPLC-DAD.
The retinol and alpha-tocopherol extraction was performed according to the method of Caprioli et al. (2020) with slight modifications. Briefly, 3 g of ghee, 5 mL of a solution of sodium chloride (NaCl) 1% (w/v), 0.05 g of ascorbic acid, 9 mL of ethanol and 2 mL of a KOH solution (10 g KOH in 10 mL water) were placed in a centrifuged tube. The alkaline digestion was performed in water-bath at 65 °C for 1 h with continuous stirring. After digestion, tubes were cooled in an ice-bath and 15 mL of NaCl 1% were added. Vitamins A and E were extracted with three portions each of 6 mL of hexane. Then, the organic phase was evaporated under nitrogen stream and dissolved in 1 mL of acetonitrile. The prepared sample was filtered with and injected for analysis. The HPLC-DAD analysis of retinol and alpha-tocopherol was performed with a Hewlett Packard (Palo Alto, CA, USA) HP-1090 Series II, consisting of a binary pump, an auto-sampler, with a UV–Visible DAD-SL Diode Array Detector. A Chromolith® RP-18 HPLC Column (100 × 3 mm, macropore size 2 µm, mesopore size 13 nm; Merck) with pre-column (C18, Phenomenex), was used for the chromatographic separation and was thermostated at 30 °C. The mobile phase consisted of 90% acetonitrile (A) and 10% isopropanol (B) in an isocratic flow rate of 0.8 mL/min. The injection volume was 50 µL. Chromatograms were recorded at 292 nm for alpha-tocopherol and 326 nm for retinol.
2.3.4 Analysis of polyphenols in enriched ghee samples.
2.3.4.1 Polyphenols extraction.
Polyphenols were extracted from plant enriched ghee samples according to the method of Ricciutelli et al. (2017) with some modifications. Five g of ghee were dissolved in 10 mL of cyclohexane at 40 °C and extracted in a separating funnel at ambient temperature with 3 × 5 mL of methanol/water (80:20, v/v). The methanolic extracted solution was collected and evaporated until 4 mL with a nitrogen stream and was stored at − 6 °C for 2 h. Afterward, the extract was centrifuged and the supernatant was used for total polyphenols quantification (Folin Ciocalteu method) and HPLC-DAD analyses.
2.3.4.2 HPLC-DAD analyses.
The enriched samples were analyzed following the method developed by Bendif et al., (2017) to quantify 13 phenolic compounds by HPLC-DAD.
2.3.5 Characterization of the volatile profile of the enriched ghee samples.
2.3.5.1 Analysis of volatile organic compounds (VOCs).
The volatile organic compounds (VOCs) of different ghee samples were characterized by headspace solid-phase microextraction (HS-SPME) coupled with gas chromatography-mass spectrometry (GC–MS) (Nzekoue et al., 2019). Briefly, 1 g of ghee was placed in a 10 mL vial which was tightly capped with a PTFE-silicon septum. After 20 min of equilibration at 60 °C for 20 min, a 50/30 µm divinylbenzene/carboxene/polydimethylsiloxane (DVB/CAR/PDMS) fiber (Supelco, Bellofonte, PA, USA) was exposed to the headspace of the sample. After 20 min of exposition, the fiber was inserted into the GC injection port for 10 min of desorption, with the injector temperature of 260 °C in splitless mode (splitless time: 1 min). An Agilent 6890 N GC equipped with an Agilent 5973 N mass selective detector (GC–MSD) (Agilent, Santa Clara, CA) was used. Data were acquired in the electron impact (EI) mode with an ionization voltage of 70 eV, using the scan ion monitoring mode. A polyethylene glycol capillary column (DB–Wax, 60 m, 0.25 mm i.d., 0.25 µm film thickness, J & W Scientific, Folsom, CA, USA) was used. The carrier gas was He at a flow rate of 1 mL/min. The oven temperature was held at 50 °C for 3 min, then raised to 150 °C at a rate of 5 °C/min, to 250 °C at a rate of 10 °C/min and held at 250 °C for 7 min. The identification of VOCs was based on a comparison of their retention times and mass spectra with authentic standards and with reference spectra from the US National Institute of Standards and Technology (NIST, 2008). The results were expressed as peak area percentages, dividing the area of each component by the total area of all isolated components under the conditions described above. The values were the mean of three replicates of each sample. Data were analyzed by using MSD ChemStation software (Agilent, Version G1701DA D.01.00).
2.3.5.2 Multivariate analyses.
To reveal the relationship among ghee samples in terms of volatile components, and to identify the main constituents influencing variability, the composition data matrix of four samples (47 variables * 4 samples = 18 data) was analyzed using principal component analysis (PCA) with STATISTICA 7.1 (Stat Soft Italia srl, 2005, www.statsoft.it) (Sagratini et al., 2012). Eigenvalues were calculated using a covariance matrix among 154 chemical compounds and the two-dimensional PCA biplot, including both samples of different origins and compounds, was generated.
2.4 In vitro migration assay
2.4.1 Preparation of nanoemulsions.
The nanoemulsions of ghee in water were prepared using the high-pressure homogenization (HPH) technique. 2 g of Ghee and 0,7g of Tween 80 were melted together at 40–50 °C in a water bath. 7,3 g of water were heated at the same time. The previous mixture was added to the water and pre-emulsions were obtained by high-speed stirring using an UltraTurrax T25 (IKA Labortechnik, Jahnke und Kunkel, Germany) at 9500 rpm for 5 min. Then, pre-emulsions were passed 4 times through French press at 900–1000 bar (Donsì et al., 2012).
2.4.2 Cell culture.
Normal human fibroblasts (NHF-A12), kindly provided by Prof. Massimo Nabissi (School of Pharmacy, University of Camerino, Italy), were cultured in Minimum Essential Medium supplemented with penicillin (100 U/mL), streptomycin (100 µg/mL), 2 mM L-glutamine and fetal bovine serum (FBS, 15% v/v). They were grown in a humidified atmosphere at 37 °C with CO2 (5%) (Antognoni et al, 2016).
2.4.3 In vitro fibroblast-migration assay.
To evaluate cell migration ability a wound healing test was performed. Briefly, human fibroblasts were seeded on a 6-well plate at the density of five hundred thousand cells. The following day the medium was aspired and cells were scratched with a P10 pipette tip to create an artificial wound. Subsequently, 1 mL of fresh 2% FBS-medium with or without 50 μg/mL of each ghee sample was added to each well. Both controls, negative and positive (10 nM Epidermal Growth Factor, EGF), were also performed. Fibroblasts were examined at 0, 12 and 24 h after incubation. The wound healing was evaluated by measuring the wound area at different times. The area where cells migrated was photographed at the same location and measured using Image J analysis software (Antognoni et al, 2016).
2.5 Statistical analyses.
Analyses were performed in triplicate and values were expressed as mean ± standard deviation (S.D). To assess the statistical significance of the differences obtained between sample types, one-way ANOVA was used and probability values<0.05 (p ≤ 0.05) were considered statistically significant.
3 Results and discussion.
3.1 Chemical characterization.
3.1.1 Oxidative stability of the enriched ghee samples.
The peroxide value was used to monitor the oxidative deterioration in ghee. Data obtained from control and plants enriched ghee during accelerated incubation (21 days at 60 °C) are shown in Table 1. It was revealed that the enriched ghee samples had significantly lower peroxide values compared to the control (p ≤ 0.05). This effect might be due to the antioxidant compounds present in plants which can delay the oxidative rancidity in fat (Gambacorta et al., 2007). Indeed, the PV (meqO2/kg) increased during the incubation (0.64 ± 0.02 at day 0) and the highest levels were observed in the control sample at day 5 (2.24 ± 0.07) and day 21 (8.20 ± 0.04). After 5 days of incubation, ghee macerated with clove showed the lowest PV (0.65 ± 0.02) followed in decreasing order by ghee macerated with rosemary (1.40 ± 0.03). The PV differences between the flavored ghee were statistically significant proving that the incorporation of clove allowed the best oxidative stability of ghee during the first five days. Moreover, clove and rosemary allowed better oxidative stability of ghee than the non-macerated ghee (p ≤ 0.05) after 21 days of incubation at 60 °C. These results agree with those of Saoudi et al. (2016) who reported that the rosemary incorporation in the soybean oil was active against thermal oxidation. In the same perspective, similar studies proved that the addition of clove essential oils slowed down the progress of peroxides under accelerated conditions (Shendea et al., 2014; Pawar et al., 2012; Wollinger et al., 2016). Results are expressed as mean ± standard deviation (n = 3). Values with different lower-case letters within the same column are statistically different (p < 0.05).
Day 0
Day 5
Day 21
Control (BT)
0.64 ± 0.02a
2.24 ± 0.07a
8.20 ± 0.04a
Clove ghee
0.64 ± 0.02a
0.65 ± 0.02b
6.80 ± 0.03b
Rosemary ghee
0.64 ± 0.02a
1.40 ± 0.03c
6.76 ± 0.08b
3.1.2 Fatty acids profiles.
The fatty acid composition of the three types of ghee samples is reported in Table 2. In all the analyzed samples, the most abundant fatty acid was palmitic acid (C16:0) followed by oleic (C18:1), myristic (C14:0) and stearic (C18:0) acids. These 4 fatty acids represent more than 81% of the total FAs identified. Moreover, in all ghee samples, the total percentage of saturated fatty acids (SFAs) was almost 70%, while those of monounsaturated and polyunsaturated fatty acids (MUFAs and PUFAs) were 25% and 4.5%, respectively. These results follow previous studies on ghee (Mariod et al., 2010; Kumar et al., 2015; Samet-Bali et al., 2017). According to the obtained results, the incorporation of plants did not have a significant effect on the fatty acid profile of ghee. However, some statistically significant differences (p ≤ 0.05) can be observed in single fatty acids such as conjugated linoleic acid (CLA). Indeed, the percentage of CLA (%) was significantly higher in ghee samples macerated with rosemary (1.50 ± 0.07) or with clove (1.24 ± 0.06) respect to control ghee (0.90 ± 0.04). Kravić et al., (2012) reported that α-linolenic acid and linoleic acid represent more than 50% of the total fatty acids of rosemary leaves. However, their incorporation did not significantly increase the percentage of α-linolenic acid and linoleic acid in ghee. Results are expressed as mean ± standard deviation (n = 3). CLA: conjugated linoleic acid, SFAs: saturated fatty acids, MUFAs: monounsaturated fatty acids, PUFAs: polyunsaturated fatty acids. Values with different lowercase letters within the same row are statistically different (p ≤ 0.05).
Fatty acid
Control(BT)
Clove ghee
Rosemary ghee
C10:0
3.51 ± 0.14 a
3.57 ± 0.23 a
3.35 ± 0.18 a
C12:0
4.42 ± 0.27 a
4.43 ± 0.19 a
4.50 ± 0.31 a
C14:0
13.99 ± 0.85 a
13.98 ± 0.95 a
14.11 ± 1.02 a
C14:1
1.27 ± 0.04 a
1.15 ± 0.02 a
1.53 ± 0.05 a
C15:0
1.25 ± 0.08 a
1.37 ± 0.04 a
1.51 ± 0.07 a
C16:0
36.10 ± 2.03 a
36.65 ± 1.96 a
37.00 ± 3.91 a
C16:1
1.60 ± 0.07 a
1.49 ± 0.05 a
1.59 ± 0.03 a
C17:0
0.98 ± 0.01 a
0.65 ± 0.03b
0.73 ± 0.07b
C18:0
9.85 ± 0.42 a
9.84 ± 0.73 a
10.01 ± 0.91 a
C18:1
22.89 ± 1.96 a
22.50 ± 1.07 a
21.15 ± 2.06 a
C18:2
2.61 ± 0.16a
2.55 ± 0.18a
2.40 ± 0.16a
C18:3
0.62 ± 0.02a
0.58 ± 0.02a
0.61 ± 0.03a
CLA
0.90 ± 0.04a
1.24 ± 0.06b
1.50 ± 0.07c
SFA
70.10 ± 3.80 a
70.48 ± 4.13 a
71.22 ± 6.47 a
MUFA
25.76 ± 2.13 a
25.14 ± 1.14 a
24.26 ± 2.14 a
PUFA
4.14 ± 0.17 a
4.37 ± 0.26 a
4.51 ± 0.26 a
In conclusion, although small differences were observed in some fatty acids, these variations were not important enough and therefore, the overall fatty acid profile of ghee did not significantly change with the incorporation at 6% of the studied plants.
3.1.3 Vitaminic profiles.
This study also evaluated for the first time the effect of plant enrichment on the vitamin composition of ghee. Notably, the retinol and α-tocopherol content of the different ghee samples were analyzed after 21 days of maceration (Table 3). Table 3 shows that the retinol levels significantly varied in the studied samples. Indeed, the highest level of retinol was observed in rosemary enriched ghee sample followed by clove enriched sample. These results can be explained by the retinol transfer into the ghee matrix by the 2 incorporated plants. The retinol levels (mg/100 g) were reported to be 0.94 and 0.048 in rosemary and clove respectively (United States Department of Agriculture [USDA], 2018). These levels could explain the highest values of vitamin A obtained in rosemary enriched ghee (3.05 ± 0.29 mg/kg). Moreover, according to the high retinol level observed in control ghee samples (1.30 ± 0.20 mg/kg) and the recommended daily allowances (RDA) values for vitamin A (800 µg/day), the cow milk ghee can be considered as a source of vitamin A since 100 g provide more than 15% of the RDA (EC regulation No 1924/2006, commission directive 2008/100/EC). The mean levels of α-tocopherol in the different ghee samples were in the range of 4.98–5.38 mg/kg. However, no significant differences were observed between the studied ghee samples although high levels of vitamin E are reported in clove (8.8 mg/100 g) (USDA, 2018). It is worth noting that the levels obtained in this study are lower than those reported in the literature for standard ghee made from cow cream (Kumar et al., 2010). This can be due to the vitamin deterioration in ghee samples which were maintained at 60 °C for 21 days (Hemery et al., 2018). Results are expressed as mean ± standard deviation (n = 3). Values with different lowercase letters within the same column are statistically different (p ≤ 0.05).
Retinol
α-tocopherol
Control (BT)
1.30 ± 0.20a
4.98 ± 0.32 a
Clove ghee
1.90 ± 0.30b
5.08 ± 0.48 a
Rosemary ghee
3.05 ± 0.29c
5.38 ± 0.36 a
3.1.4 Polyphenolic profiles.
The HPLC-DAD analysis allowed to identify 9 phenolic compounds (Table 4) in two enriched ghee samples. Rosemary-flavored samples showed higher levels of the targeted phenolic compounds (101.9 mg/kg). The major compound present in all samples analyzed was carnosic acid with levels ranging from 30 to 77 mg/kg. Rosemary-flavored ghee was also rich in terms of carnosol (8.6 mg/kg), epicatechin (5.9 mg/kg) and, rutin (5.6 mg/kg), while clove-flavored ghee was richer with gallic acid (5.0 mg/kg). These results indicate that maceration with aromatic plants transfers polyphenols into the ghee matrix.
Compounds
Clove ghee
Rosemary ghee
Shikimic acid
n.d.
0.6 ± 0.1
Gallic acid
5.0 ± 0.1
1.7 ± 0.1
3-CQA
n.d.
n.d.
5-CQA
n.d.
n.d.
Catechin
n.d.
n.d.
Epicatechin
1.7 ± 0.0
5.9 ± 0.3
Rutin
2.5 ± 0.0
4.6 ± 0.3
Hyperoside
0.2 ± 0.0
0.2 ± 0.0
Quercitrin
0.9 ± 0.1
1.4 ± 0.0
3,5-di-CQA
0.4 ± 0.0
1.8 ± 0.0
Rosmarinic acid
n.d.
n.d.
Carnosic acid
35.8 ± 4.9
77.1 ± 1.9
Carnosol
n.d.
8.6 ± 0.1
Total monitored polyphenols (mg/kg)
46.5 ± 5.1
101.9 ± 2.8
Many studies focused on the polyphenols composition of clove and rosemary (Hosseini et al., 2018). The polyphenols contained in these different plants are released into the ghee matrix increasing its oxidative stability. However, some problems can be linked to the transfer of phenolic compounds from plants to highly lipophilic matrix such as butter oil.
To solve this problem, some studies have used aqueous and ethanolic extracts of plants instead of proper plant leave for ghee enrichment (Pawar et al., 2014; Gandhi & Lal, 2018; Rahila et al., 2018). However, by using these extracts, it is difficult to flavor the ghee since the majority of plants flavoring compounds are in the essential oils which are not incorporated during extraction due to their lipophilic character.
3.1.5 Volatile profile of plant-enriched ghee.
From the volatile profiles obtained by the HS-SPME-GC–MS analysis of the 3 ghee sample types, 47 volatiles compounds have been identified. These compounds were unequally present in each sample type allowing to study the effect of the enrichment with aromatic plants on the VOCs of ghee. For better visualization, PCA was performed to identify patterns of correlation with individual composition variables involved in the discrimination among the normal (control) and the different flavored ghee samples. The 2D graphical representation of PCA is reported in Fig. 2. Samples were divided in three different clusters indicating the possibility of discrimination of ghee sample base on VOCs. The first cluster (type I) was made of clove-flavored ghee (ghee 2), the second group (type II) was made of rosemary-flavored ghee (ghee 3) and the third one was formed by the control ghee (ghee 1). Indeed, the type I was characterized by eugenol and α-humulene. These compounds are reported to be the main volatile compounds originating from clove (Kasai et al., 2016) and the main components of clove essential oil (de Oliveira et al., 2016). These two compounds have a strong antioxidant activity thus they can contribute to the observed oxidative stability of the macerated ghee. The second cluster (type II) was characterized by 1,8-cineole, camphor and α-pinene which are the main components of rosemary volatile fraction and essential oil (Sirocchi et al., 2013) and are also responsible for the antioxidant activity of the rosemary essential oil. To our knowledge, this is the first time that the influence of plants on the VOCs composition of ghee is reported in the literature. These results confirmed that the enrichment of ghee with aromatic plants can have a marked effect on its volatile profile and therefore on its flavor. This effect could be really important from a commercial point of view considering the high consumption of ghee in many countries and the good scents associated with these different flavoring compounds. Indeed, it can be possible with maceration, to produce naturally flavored ghee of different aromas according to the consumer preferences, opening thus, the way to a new commercial field not yet sufficiently exploited.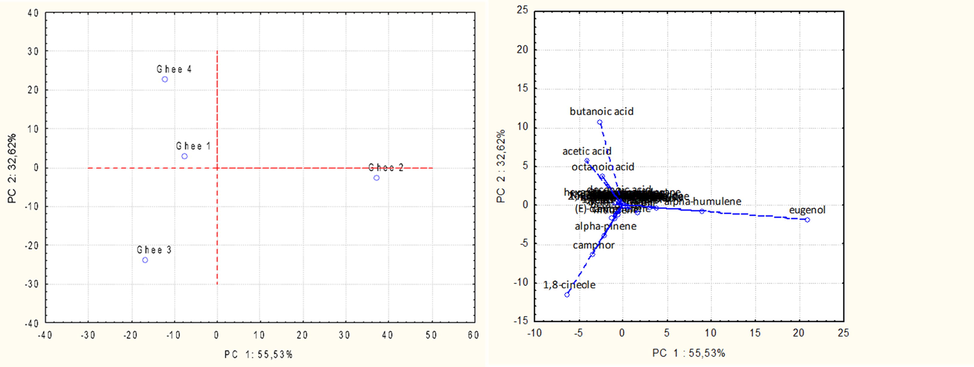
Principal component analysis (PCA) of volatile organic compounds (VOCs) in 3 types of ghee samples. Ghee1: non-macerated ghee; Ghee 2: clove-flavoured ghee; Ghee 3: rosemary-flavoured ghee.
3.2 In vitro assay on fibroblasts.
3.2.1 Nanoemulsions
Pre emulsions, obtained using the ultra turrax T25 on 3 ghee samples, were used to prepare the 3 different sterile nanoemulsions systems. Nanoemulsions preparation was carried out by high-pressure homogenization (4 passes at 900–1000 bar), droplet sizes ranged from of 20 to 200 nm and the emulsion denoted a uniform distribution of narrow size particle (Donsì et al., 2012). Thanks to their small droplet size, nano-emulsions have resistance against sedimentation or creaming and ensure a fine particle size and physical stability over 1 month under refrigerated conditions (Fig. 3).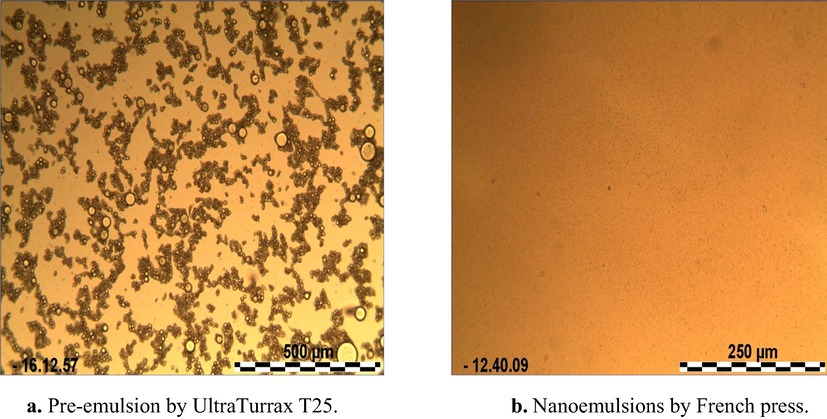
Effect of the high-pressure homogenization on the droplets size distribution (nanoemulsions of ghee in water). a. Pre-emulsion by UltraTurrax T25. b. Nanoemulsions by French press.
3.2.2 In vitro fibroblast-migration assay.
The effect of ghee samples on the migration of human fibroblasts was examined by the scratch wound assay. Fig. 4 indicates the migration activity of these cells after 0, 12, and 24 h of incubation with 50 µg/mL of each ghee sample, only medium (negative control), and EGF (positive control). After 12 h, the wound closure percentage of human fibroblasts in 50 µg/ml rosemary and clove enriched ghee groups was 45.27% and 44.54%, respectively, (Fig. 5 B and C) which marks that these groups had a higher migratory activity compared with EGF used as the positive control (33.23%) (p ≤ 0.05). The non-enriched ghee group (Fig. 5 A) showed a wound closure percentage of 29.6% after 12 h, which was anyway quite high if compared with the negative control group (18.8%), an expected result considering the presence in ghee of PUFAs and MUFAs, key molecules in wound healing. In fact, olive oil and cocoa butter, which are rich in oleic acid, have also shown good results in wound healing (Saporito et al., 2018; Bayir et al., 2019). After 24 h, the scratch in ghee treated groups were completely closed, while the wound closure percentages in negative and positive control were 81.84% and 91.57%, respectively (Fig. 5), underlining once more the positive effect of ghee in wound closure.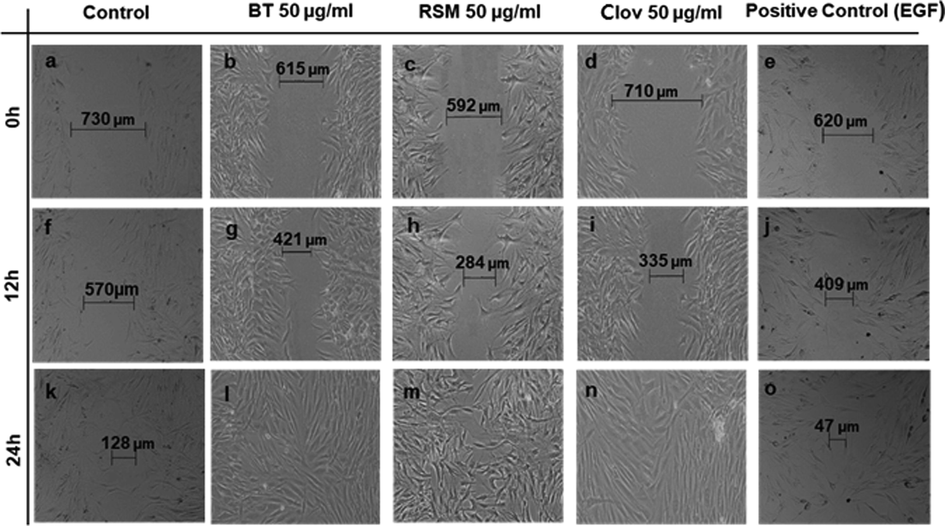
Effects of ghee samples on the migration of human fibroblasts. The extent of wound closure in scratch assays at doses of 50 μg/mL after 0, 12 and 24 h in comparison with positive and negative controls. Fibroblasts migrated until complete closure to fill the wound. BT: non-macerated ghee; RSM: Rosemary; Clov: Cloves.
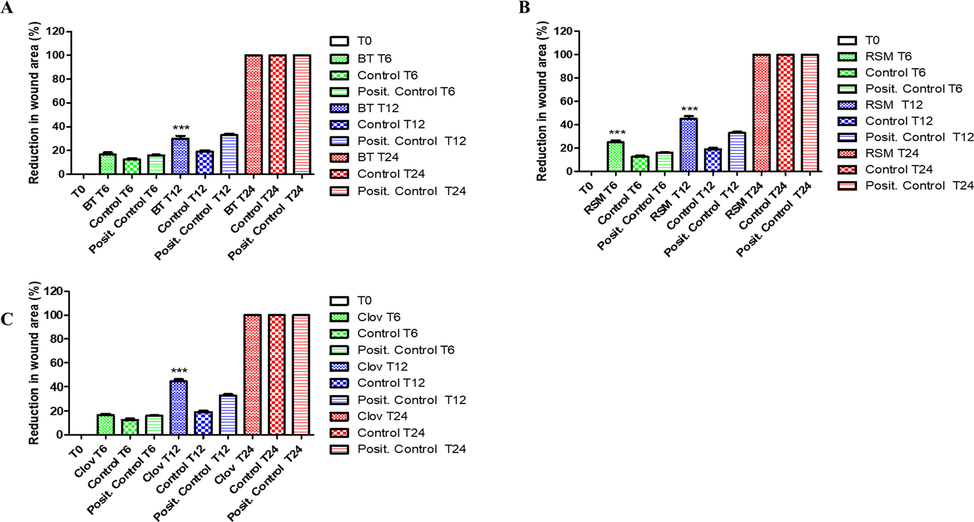
Reduction in wound areas measured at different time points for human Fibroblasts, following treatments with BT (A), RSM (B) and Glov (C)samples. Negative and positive controls are constituted by medium and epidermal growth factor (EGF), respectively. Results are expressed as the mean ± SE of three independent replicates.***p < 0.05 with respect to Control. BT: non-macerated ghee; RSM: Rosemary; Clov: Cloves.
Anyway, rosemary and clove enriched ghee have shown excellent results, stimulating the fibroblasts migration and favoring the wound repair. The importance of the obtained data is confirmed by other studies reported in literature carried out on fibroblasts and keratinocytes. In fact, the difference in effect found between untreated and treated cells with non-enriched ghee after 24 h is similar to that obtained in this study after only 12 h of treatment with rosemary and clove enriched ghee (Kotian et al., 2017; Kotian et al., 2019). This result can be explained by the enrichment of flavored ghee by therapeutic (vitamin A), and antioxidant (vitamin E) vitamins that perform an important role in skincare (Stanley Shapiro & Saliou, 2001), especially when they are associated with the other antioxidants (polyphenols) from rosemary and cloves.
The exact mechanism that takes places in wound healing is very complex and includes several molecules and pathways. Anyway, some molecules present in ghee preparations exert beneficial effects on wound healing and skin recovery. In fact, some studies revealed that CLA has positive repercussions on scar healing processes through a modulation of inflammatory response and oxidative stress (Park, Valacchi & Lim, 2010). The effect is due to a reduced activation of NFκB, which may prevent prolonged inflammatory responses by decreased oxidative stress. These results were corroborated by HO-1, CuZnSOD, and MnSOD expression. In addition, further studies showed that the encapsulation of CLA was useful for the wound healing process, suggesting promising clinical applications for the treatment of chronic wounds (Zhang & Liu, 2020).
Moreover, in this article it has been demonstrated the presence of vitamin A and E, which stimulate epithelial growth, collagen synthesis, epithelialization, and increase dermal collagen deposition restoring epithelial structure (Zinder et al., 2019; Polcz & Barbul, 2019). Other evidences showed instead the importance of vitamin E in healing processes mainly in eliminating and reducing the negative impacts of ROS damage on healing tissues (Hobson, 2016).
Taken together, these results indicate that enriched ghee could be useful to accelerate the wound healing process and to reduce the inflammation, which in turn promotes more even skin tone and helps reduce acne and wrinkles.
4 Conclusions
The maceration of ghee with rosemary and clove showed a beneficial effect on the oxidative stability of ghee through the release of polyphenols and other antioxidant compounds. It was also demonstrated that the obtained flavored ghee got different retinol contents and VOCs profiles, while the overall fatty acid composition remained unchanged. Ghee macerated with natural antioxidants is better than the non-enriched ghee despite its high vitamin A content, suggesting that the improved migration ability of fibroblasts observed during in-vitro assays is probably due to the presence of other components such as phenolic acids. The results of this study suggest that ghee could be useful for the skincare, especially when it is enriched with natural antioxidants from rosemary or clove leaves. Future studies will be performed to improve the plant-enrichment of ghee using lower temperatures and innovative processes. Furthermore, in-vivo studies will be performed to deeply assess the wound healing property of plant-enriched ghee.
Acknowledgments
The authors are grateful to the School of Pharmacy and the School of Science and Technology of the University of Camerino (Italy) for supporting the research work.
References
- Polar extracts from the berry-like fruits of Hypericum androsaemum L. as a promising ingredient in skin care formulations. Journal of Ethnopharmacology. 2016;195:255-265.
- [CrossRef] [Google Scholar]
- Physicochemical characteristics and storage stability of clarified butter fat «smen» produced from pasteurized and non-pa1steurized milk. Journal of Pharmaceutical & Health Sciences. 2017;5:195-205.
- [Google Scholar]
- The effects of Beeswax, Olive oil and Butter impregnated bandage on burn wound healing. Burns. 2019;45(6):1410-1417.
- [CrossRef] [Google Scholar]
- The effects of feeding supplementation on the nutritional quality of milk and cheese from sheep grazing on dry pasture. International Journal of Food Sciences and Nutrition. 2020;71(1):50-62.
- [CrossRef] [Google Scholar]
- Lipid Nutritional Value of Legumes: Evaluation of different extraction methods and determination of fatty acid composition. Food Chem.. 2016;192:965-971.
- [Google Scholar]
- Design of nanoemulsion-based delivery systems of natural antimicrobials: Effect of the emulsifier. Journal of Biotechnology. 2012;159:342-350.
- [CrossRef] [Google Scholar]
- Efficiency of the optimized microwave assisted extractions on the yield, chemical composition and biological activities of Tunisian Rosmarinus officinalis L. essential oil. Food and Bioproducts Processing. 2017;105:224-233.
- [CrossRef] [Google Scholar]
- Changes in the chemical and sensorial profile of extra virgin olive oils flavored with herbs and spices during storage. Journal of Food Lipids. 2007;14:202-215.
- [CrossRef] [Google Scholar]
- Potential of Herbal Nutraceuticals in Ghee: A Review. Research & Reviews: Journal of Dairy Science and Technology. 2018;4(2):1-5.
- [Google Scholar]
- Effects of Pro-Oxidants and Antioxidants on the Total Antioxidant Capacity and Lipid Oxidation Products of Milk During Refrigerated Storage. Journal of Food Science. 2018;83(2):275-283.
- [CrossRef] [Google Scholar]
- Natural product-based nanomedicines for wound healing purposes: therapeutic targets and drug delivery systems. International journal of nanomedicine. 2018;13:5023.
- [CrossRef] [Google Scholar]
- Natural antioxidant used in ghee-A mini review. Journal of Food Research and Technology. 2014;2(3):101-105.
- [Google Scholar]
- Storage conditions and packaging greatly affects the stability of fortified wheat flour: Influence on vitamin A, iron, zinc, and oxidation. Food Chemistry. 2018;240:43-50.
- [CrossRef] [Google Scholar]
- Vitamin E and wound healing: an evidence-based review. International Wound Journal. 2016;13(3):331-335.
- [CrossRef] [Google Scholar]
- Optimization of heat-and ultrasound-assisted extraction of polyphenols from dried rosemary leaves using response surface methodology. Journal of Food Processing and Preservation. 2018;e13778
- [CrossRef] [Google Scholar]
- Effects of the Extracts from Fruit and Stem of Camellia japonica on Induced Pluripotency and Wound Healing. Journal of Clinical Medicine. 2018;7(11):449.
- [CrossRef] [Google Scholar]
- Evaluation of commonly used herbs to enhance shelf life of ghee against oxidative deterioration. Journal of Food Processing and Preservation. 2018;e13658
- [CrossRef] [Google Scholar]
- Influence of traditional medicines on the activity of keratinocytes in wound healing: an in-vitro study. Anatomy & Cell Biology. 2019;52(3):324-332.
- [Google Scholar]
- Effect of natural medicines on dermal fibroblasts in wound healing: an in-vitro study. Advanced Science Letters. 2017;23(3):1949-1956.
- [CrossRef] [Google Scholar]
- Fatty acid composition of rosemary (Rosmarinus officinalis L.) leaves. Novi Sad (Serbia): Institute of Food Technology; 2012.
- Detection of Vegetable Oil and Animal Depot Fat Adulteration in Anhydrous Milk Fat (Ghee) using Fatty Acid Composition. MOJ Food Processing Technology. 2015;1:13-19.
- [CrossRef] [Google Scholar]
- A comparison of the physico-chemical properties of low-cholesterol ghee with standard ghee from cow and buffalo creams. International journal of dairy technology. 2010;63(2):252-255.
- [CrossRef] [Google Scholar]
- Gastrodin alleviates oxidative stress-induced apoptosis and cellular dysfunction in human umbilical vein endothelial cells via the Nrf2/HO-1 pathway and accelerates wound healing in vivo. Frontiers in pharmacology. 2019;10:1273.
- [CrossRef] [Google Scholar]
- Evaluation of physico-chemical changes in Curcumin fortified buffalo Ghee during storage at 30±1 C. IJCS. 2017;5(2):141-144.
- [Google Scholar]
- Optimization of heat treatment and curcumin level for the preparation of anti-oxidant rich ghee from fermented buffalo cream by Central Composite Rotatable Design. Journal of Food Science and Technology. 2018;55:1832-1839.
- [CrossRef] [Google Scholar]
- Effect of the method of processing on quality and oxidative stability of anhydrous butter fat (samn) Afr J Biotechnol. 2010;9:1046-1051.
- [CrossRef] [Google Scholar]
- Comparison of five analytical methods for the determination of peroxide value in oxidized ghee. Food Chemistry. 2015;185:449-453.
- [CrossRef] [Google Scholar]
- N Nandanwar, R., Gurjar, H., Sahu, V. K., & Saraf, H. (2010). Studies on wound healing activity of gel formulation containing cow ghee and Aloe vera. International journal of pharmaceutical sciences and research, 1(3), 50-54.
- HS-SPME-GC-MS technique for FFA and hexanal analysis in different cheese packaging in the course of long term storage. Food Research International. 2019;121:730-737.
- [CrossRef] [Google Scholar]
- Effect of dietary conjugated linoleic acid supplementation on early inflammatory responses during cutaneous wound healing. Mediators of Inflammation. 2010;2010
- [CrossRef] [Google Scholar]
- Antioxidant potential of herbs and spices during deep frying of ghee. International Journal of Dairy Technology. 2014;67(3):365-372.
- [CrossRef] [Google Scholar]
- The effects of Asparagus racemosus (shatavari) extract on oxidative stability of ghee, in relation to added natural and synthetic antioxidants. International Journal of Dairy Technology. 2012;65(2):293-299.
- [CrossRef] [Google Scholar]
- Effect of added herb extracts on oxidative stability of ghee (butter oil) during accelerated oxidation condition. Journal of Food Science and Technology. 2014;51(10):2727-2733.
- [CrossRef] [Google Scholar]
- The role of vitamin A in wound healing. Nutrition in Clinical Practice. 2019;34(5):695-700.
- [CrossRef] [Google Scholar]
- Evaluation of ghee based formulation for wound healing activity. Journal of Ethnopharmacology. 2006;107:38-470.
- [CrossRef] [Google Scholar]
- Rosemary (Rosmarinus officinalis Linn.) extract: A source of natural antioxidants for imparting autoxidative and thermal stability to ghee. Journal of Food Processing and Preservation. 2018;42(2):e13443
- [CrossRef] [Google Scholar]
- Regulation (EC) No 1924/2006 - EUR-Lex - europa.eu. (2006, December 20). Retrieved from https://eur-lex.europa.eu/legal-content/EN/TXT/HTML/?uri=CELEX:02006R1924-20121129&from=EN
- Benefits of Ethanol Based Noni Leaf (Morinda citrifolia L.) Extract on Oral Mucosal Wound Healing by Examination of Fibroblast Cells. Journal of Dentistry. Indonesia. 2016;23(3):1.
- [Google Scholar]
- Comparative study of aroma profile and phenolic content of Montepulciano monovarietal red wines from the Marches and Abruzzo regions of Italy using HS-SPME-GC-MS and HPLC-MS. Food Chem.. 2012;132:1592-1599.
- [Google Scholar]
- Saoudi, S., Chammem, N., Sifaoui, I., Bouassida-Beji, M., A.Jiménez, I., L.Bazzocchi, I., Silva, S. D., Hamdi, M., & Bronze, M. R., (2016). Influence of Tunisian aromatic plants on the prevention of oxidation in soybean oil under heating and frying conditions. Food Chemistry, 212, 503-511. https://doi.org/10.1016/j.foodchem.2016.05.186.
- Essential oil-loaded lipid nanoparticles for wound healing. International journal of nanomedicine. 2018;13:175.
- [CrossRef] [Google Scholar]
- Oxidative stability of ghee incorporated with clove extracts and BHA at elevated temperatures. International Journal of Food Properties. 2014;17(7):1599-1611.
- [CrossRef] [Google Scholar]
- Biogenic amines as freshness index of meat wrapped in a new active packaging system formulated with essential oils of Rosmarinus officinalis. International Journal of Food Sciences and Nutrition. 2013;64(8):921-928.
- [CrossRef] [Google Scholar]
- Stanley S.Shapiro & Claude Saliou (2001). Role of vitamins in skin care. journal of Nutrition 17, 839- 844. https://doi.org/10.1016/S0899-9007(01)00660-8.
- Skin Wound Healing Process and New Emerging Technologies for Skin Wound Care and Regeneration. Pharmaceutics. 2020;12(8):735.
- [CrossRef] [Google Scholar]
- Antioxidant activity of hydro distillation water residues from Rosmarinus officinalis L. leaves determined by DPPH assays. ComptesRendusChimie. 2016;19(6):754-765.
- [CrossRef] [Google Scholar]
- Enhancement of Skin Wound Healing by rhEGF-Loaded Carboxymethyl Chitosan Nanoparticles. Polymers. 2020;12(7):1612.
- [CrossRef] [Google Scholar]
- Vitamin A and wound healing. Nutrition in Clinical Practice. 2019;34(6):839-849.
- [CrossRef] [Google Scholar]







