Translate this page into:
Calixarene coated gold nanoparticles as a novel therapeutic agent
⁎Corresponding author. sadikhalid6@gmail.com (Sadia Khalid)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
In the current study, gold nanoparticles (AuC6NPs and AuC8NPs) were prepared through sodium borohydride reduction method by using Calix[6]rene and Calix[8]rene as a stabilizing agents. The synthesized AuNPs were screened for cytotoxic, phytotoxic, antifungal and antibacterial activities. The fabricated AuNPs were characterized by UV–visible spectroscopy, atomic force microscopy (AFM) and FTIR spectroscopy. Antibacterial activities of the AuNPs were tested against E. coli and S. aureus. The AuC6NPs were found to be effective against the growth of gram positive bacteria and inhibited the growth of S. aureus. AuC6NPs interact with bacterial cell and damaged cell membrane. Roughness of the bacterial surface and membrane rupture can be clearly observed by AFM images. The AuNPs possess insignificant antifungal activity against Aspergillus niger and Candida albicans. Moreover, AuC8NPs have significant phytotoxicity and moderate cytotoxicity.
Keywords
Gold nanoparticles
S. aureus
E. coli
Antibacterial mechanism
Phytotoxicity
Cytotoxicity and atomic force microscopy
1 Background
Applications of nanoparticles (NPs) in different research areas such as nonomedicine (Chen et al., 2016; Swierczewska et al., 2016), water treatment (Donovan et al., 2016), cosmetics (Jiménez-Pérez et al., 2017) and agriculture (Servin and White, 2016) has risen considerably in recent past. NPs have been used efficiently to develop various nano devices (Heerema and Dekker, 2016) and immunosensors for different biological, physical, pharmaceutical and biomedical applications (Ahmed et al., 2016; Anwar et al., 2018; Rao et al., 2017). Higher surface area to volume ratio and smaller size make them unique and active reagents for diverse applications. Functionalized gold nanoparticles are reported for their applications in a broad range due to their low toxicity, good stability and easy synthesis (Hornos Carneiro & Barbosa Jr, 2016). Functionalization of AuNPs with calixarenes opens new possibilities of applications. Calixarene is a macrocycle with unique three dimensional surfaces and is the subject of interest in biological domain since decades (Muneer et al., 2016; Nimse & Kim, 2013; Patil et al., 2016). Physical properties of NPs improve considerably by functionlaization with calixarene due to its unique architecture. Hence, calixarene coalesce with the nanomaterials serve as a potential and attractive component in the field of nanotechnology.
In this study, we report functionalization of gold nanoparticles with calixarene in order to develop a new therapeutic agent. Calixarene stabilized nanomaterials have gained tremendous attraction and have been used in various fields such as catalysis (Panchal et al., 2017), sensors (Maity et al., 2014) and molecular recognition (Göde et al., 2017). Recently, Hennie et al carried out controlled fictionalization of nanoparticles with calixarene ligand (Valkenier et al., 2017). Giuseppe et al used calixarene-based nanoassembly as a potential vehicle for ophthalmic drug delivery (Granata et al., 2017). Nilesh et al successfully released doxorubicin into cancer cell while using silica-calix hybrid composites. Resent research suggests the shift of calixarene functionalized nanomaterial towards biomedical application.
Metals have been used as an antimicrobial agent since ancient time. Therefore a recent increase in the use of metal nanoparticles for development new therapeutic agents is noticed (Balogh et al., 2001; Chen et al., 2010; Luo et al., 2014). Comprehension studies on calixarene functionalized gold nanoparticles for antimicrobial effect along with cytotoxic effect are rarely reported (Kellici et al., 2016). The prime focus of current project is evaluation of the antimicrobial activities of AuNPs. The efficiency of synthesized AuNPs was investigated against gram positive and gram negative bacteria and fungi along with its cytotoxic and phototoxic effect. Calixarene coated AuNPs were synthesized by a simple and economical chemical reduction process. Antibacterial mechanism was checked at different doses of AuNPs against human pathogen bacteria: E. coli, and S. aureus. Change in cellular morphology of the bacteria was evaluated through AFM. Aspergillus niger and Candida albicans strains of fungi were used for antifungal activity and the zones of inhibition were calculated by the Agar well diffusion method.
2 Results and discussion
2.1 Characterization of synthesized AuNPs
UV–visible spectroscopy is one of the basic technique to determine the structure and optical properties of metallic nanoparticles because the position and shape of the absorption band is associated with the diameter and aspect ratio of metallic nanoparticles (Noguez, 2007). Colloidal AuNPs show a characteristic red color as the surface electron cloud vibrates and absorbs the electromagnetic radiation of a particular energy. The samples of AuNPs were prepared by a chemical approach and the variation in the UV–visible spectrum of the resultant sol was observed to analyze the size effect of metallic nanoparticles on surface Plasmon band (SPB).
UV–Visible spectra of the AuC6NCs showed SPB at 525 nm (Fig. 1A) and the SPB of AuC8NCs was observed at 540 nm (Fig. 1B), indicating reduction of Au3+ ions.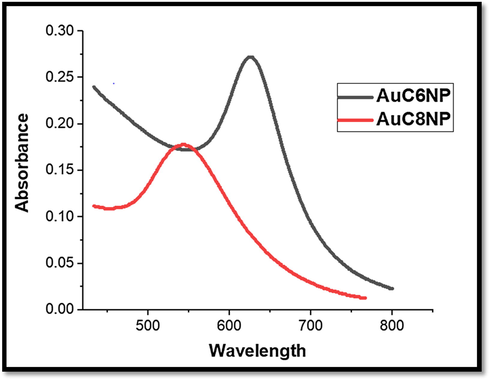
UV–visible spectrum for AuC6NPs and AuC8NPs respectively.
Atomic force microscopy (AFM) was executed to determine the particle size, and their size distribution of AuNPs (AuC6NPs and AuC8NPs). AFM analysis showed that fabricated AuNPs are spherical in shape however polydisperse. The analysis of histogram reveals that the AuC6NPs have size in range of 11–57 nm (Fig. 2A) whereas particle size of most of the NPs was found to be in the range of 31–34 nm. The sizes of AuC8NPs were in a range of 5–60 nm (Fig. 2B) with particles size of most of the NPs in the range of 41–44 nm. AFM results endorse the findings of UV–visible spectroscopy (Fig. 2A and B) (see Fig. 3).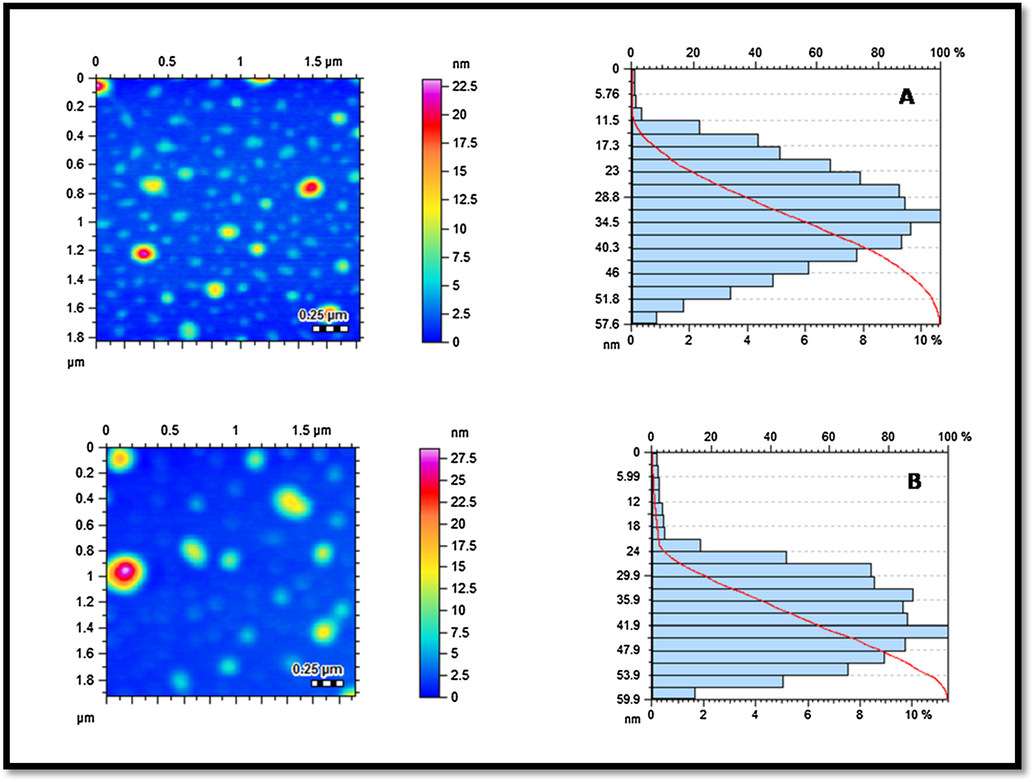
Atomic force microscopy (AFM) analysis of AuNPs: topographical images and histograms (A) AuC6NPs; and (B) AuC8NP.
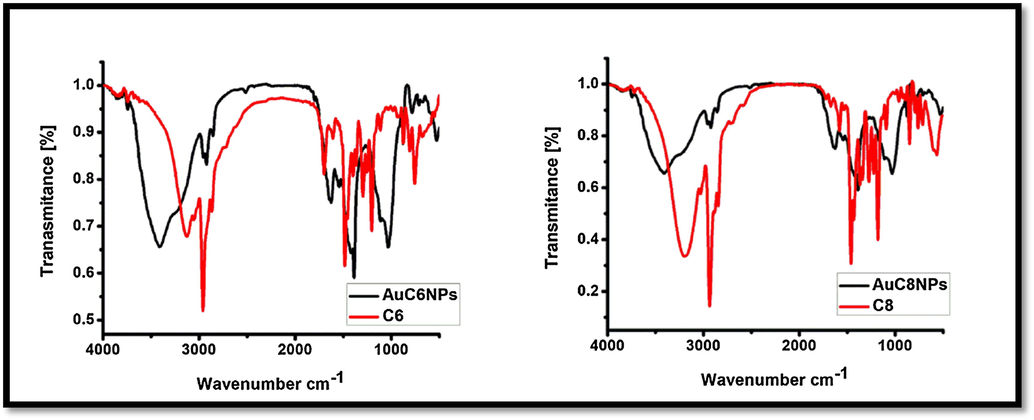
FT-IR spectrum of gold nanoparticles (AuC6NPs and AuC8NPs) and ligand (C6 and C8).
The synthesized AuNPs (AuC6NPs and AuC8NPs) were subjected to FTIR analysis for understanding of the nature of interactions. The FTIR spectra of ligand C6 and C8 shows absorption band of medium intensity at 3127 cm−1 for OH functionality (Gutsche, 2008). Absorption band at 2960 cm−1 corresponds to CH asymmetric stretch. Aromatic ring bending vibration appears at 1700 cm−1, C—O stretching vibration appears at 1482 cm−1 and tertiary alcohol vibration band is visible at 1205 cm−1 in calixarene. After the formation of metallic nanoparticle peak at 3127 cm−1 became more intense and broad that indicates the involvement of alcohol functionality in nanoparticles stabilization. On the other hand, shifting of band from 1700 cm−1 to 1798 cm−1 and 1482 cm−1 to1408 cm−1 shows interaction of aromatic ring with nanoparticles.
2.2 Stability of gold nanoparticles
The stability of AuNPs is of prime concern when its different applications are sought. The stability of NPs is generally required over a wide pH range, in presence of electrolyte, and on temperature treatment. In this context, the effect presence of electrolyte on the stability of AuNPs was studied by addition of NaCl solution of varying the concentration (0.5 to 1000 mM) and AuNPs were found to be stable over a wide range of electrolyte concentration. On the same line, the pH effect on the stability of AuNPs is evaluated. AuC6NPs were found to be stable over whole pH range while AuC8NPs were stable in basic condition only (Fig. 4B).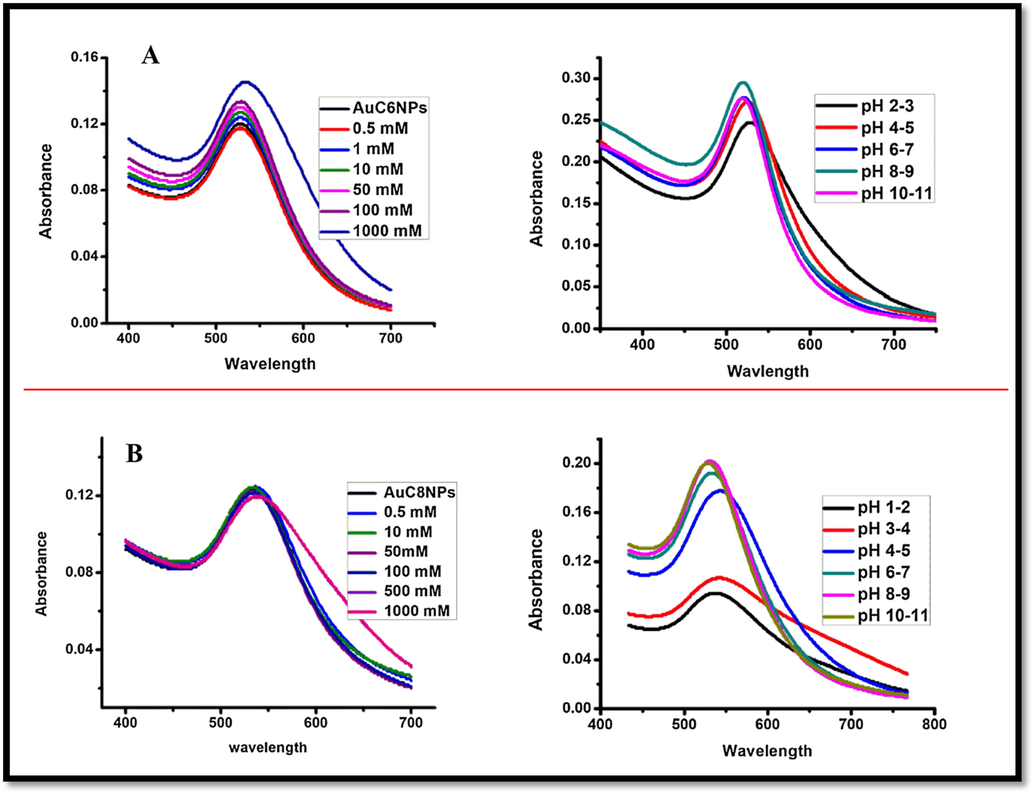
Representing stability of gold nanoparticles (A) Effect of pH and salt on AuC6NP (B) effect of pH and salt on AuC8NP.
2.3 Biological activities of synthesized AuNPs
The bactericidal effect of AuNPs (AuC8NPs and AuC8NPs) was evaluated against E. coli and S. aureus. The MIC value was calculated by monitoring the growth of S. aurues and E. coli. It is concluded that AuC6NPs have stronger effect on the growth of bacteria compared to AuC8NPs. Moreover, AuC6NPs inhibited the growth of both gram negative and gram positive bacteria.
The antibacterial effect of the AuNPs on different doses indicates that antibacterial action of AuNPs and ligands have dose dependency i.e. high antibacterial activity at high concentrations and vice versa. Maximum percent inhibition was observed at higher doses for both S. aureus and E. coli, Fig. 5. Having said that, AuC6NPs showed stronger effect against S. aureus, hence, are more potent compared to AuC8NPs. Maximum percent inhibition of AuC6NPs was calculated 90% for S. aureus and 60% for E. coli at 500 µg/mL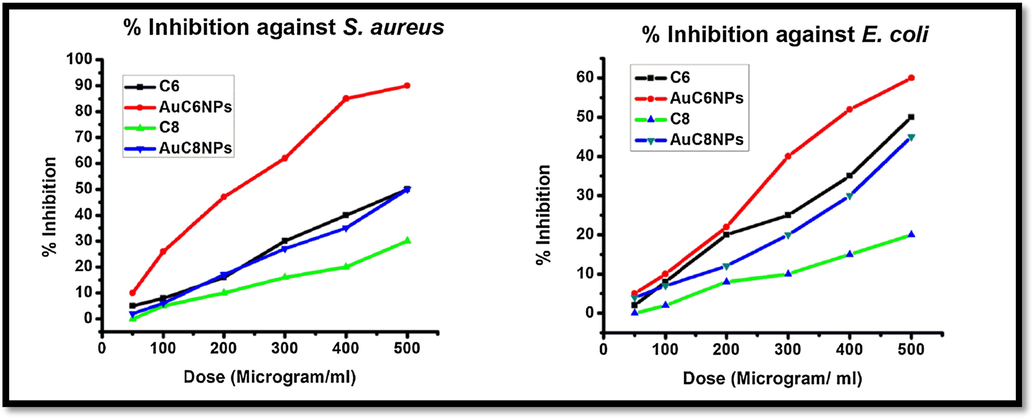
% inhibition of gold nanoparticles (AuC6NPs and AuC8NPs) and ligand (C6 and C8) against E. coli and S. aureus.
Subsequent changes in cell morphology of E. coli and S. aurues were examined by AFM, Figs. 6 and 7. The untreated bacterial strains of E. coli and S. aurues are compared with treated bacterial strains with C6 and AuC6NPs. Untreated E. coli and S. aureus were investigated and cultures exhibited cells with regular shapes and smooth membranes (Figs. 6A and 7A). This serves as a control for monitoring the changes after treatment. Cell degradation and membrane rupture were observed upon treatment with AuC6NPs and C6. AuC6NPs show extensive degradation unlike pure C6 ligand while, complete degradation of S. aureus cells is noticed after 24 h incubation leading to rough cell surface. Like behavior is observed for E. coli cells. Extensive membrane damage leads to the leakage of interacellular components and ultimately causing cell lysis. Morphological changes and cell rupture endorsed the binding and internalization of AuC6NPs. Hence, AFM images show clear evidence for antibacterial effect on AuC6NPs.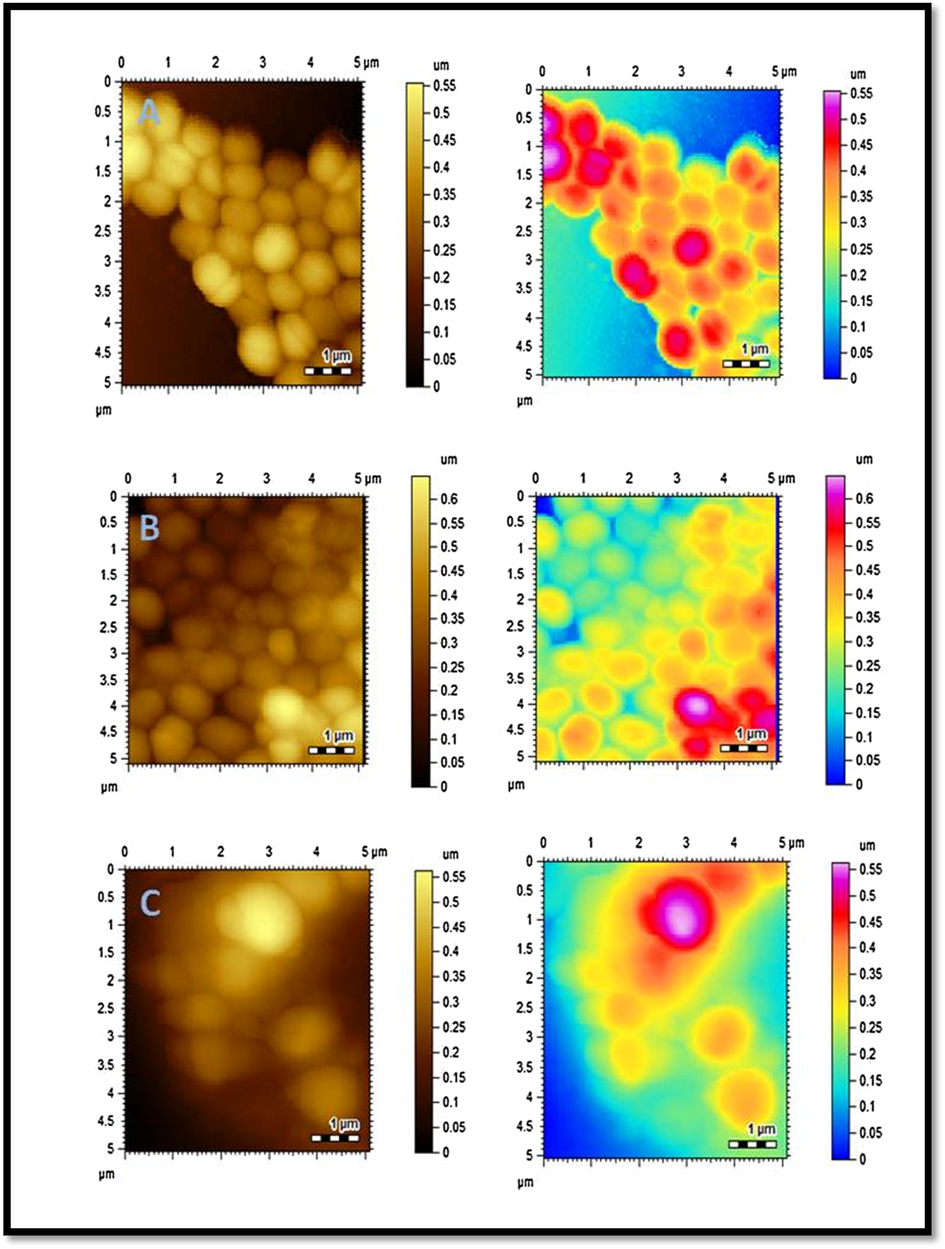
AFM images of S. aureus. (A) Control (B) C6 treated S. aurues (C) AuC6NPs treated S. aurues.
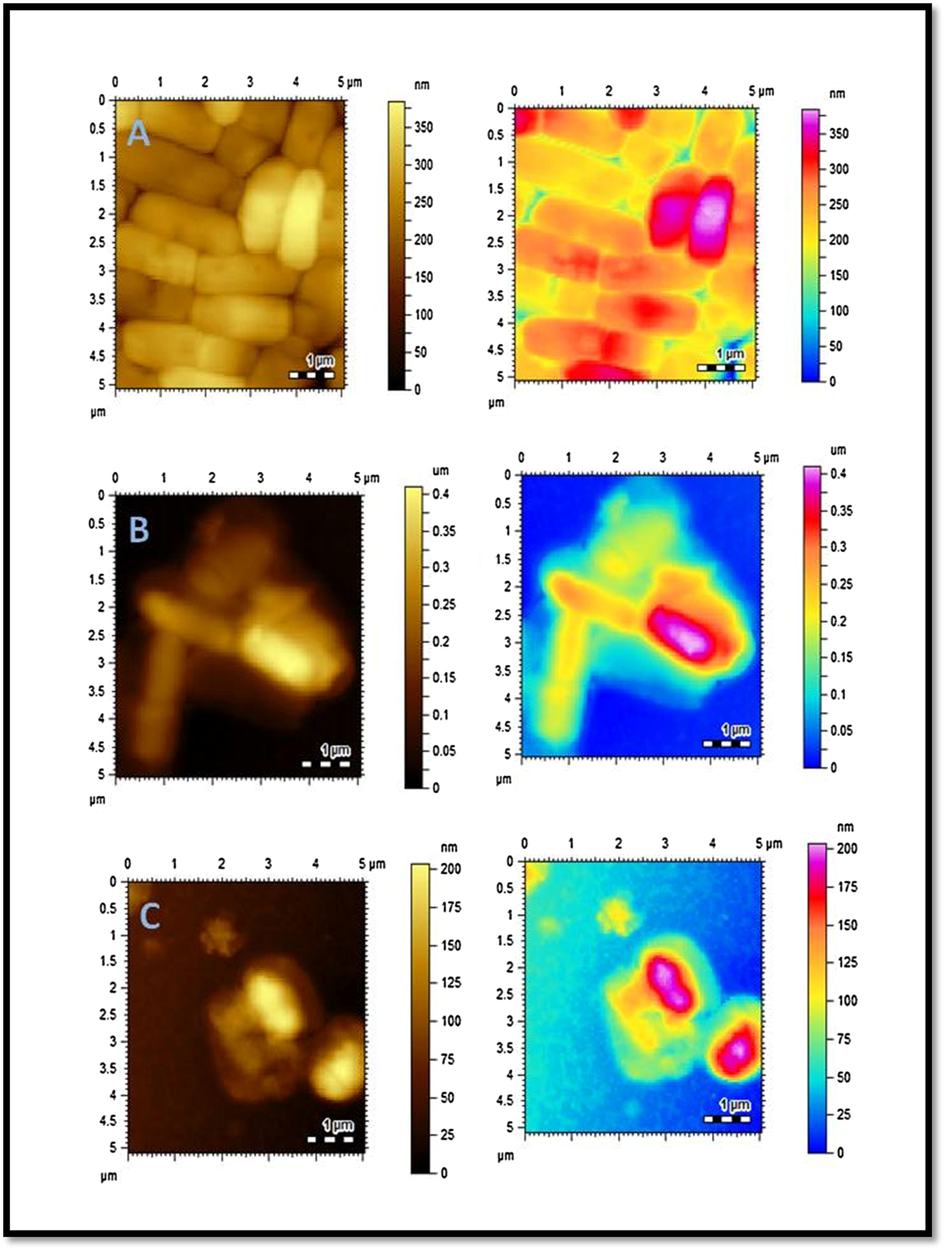
AFM images of E. coli. (A) Control (B) C6 treated E. coli (C) AuC6NPs treated E. coli.
Antifungal activity was monitored by using two antifungal strains namely, Aspergillus niger and Candida albicans. None of the synthesized AuNPs showed significant activity against any strain of fungi. Thirty % inhibition of AuC8NPs against Aspergillus niger and twenty % against Candida albicans was observed. In case of AuC6NPs 25% inhibition was noticed against Aspergillus niger and 30% against Candida albicans. Comparison of both samples of gold nanoparticles against Aspergillus niger and against Candida albicans is demonstrated Fig. 8A. The results of phytotoxic analysis of AuNPs is depicted in Fig. 8B where AuC8NPs show 70% growth regulation while 45% growth regulation was observed for AuC6NPs. AuC8NPs have shown to be the more phytotoxic compared to AuC6NPs. Paraquat was used as an standard drug.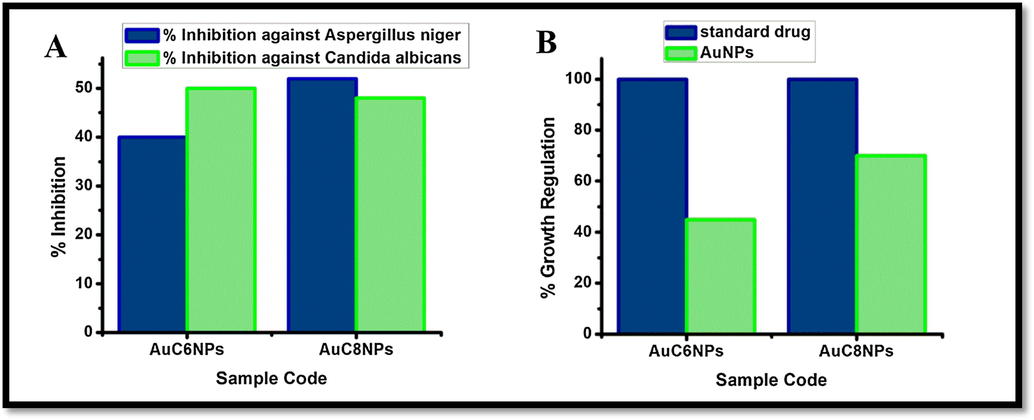
Antifungal and phytotoxic analysis of AuNPs.
Brine shrimp viability bioassay was used to examine the potential toxicity of prepared AuNPs. Results of the toxicity evaluation against brine shrimp on both samples of gold nanoparticles (AuC6NPs and AuC8NPs) showed the mortality at high concentration as shown in Table 1. Lethality was found to be directly proportional to the concentration. However, cytotoxic effects of gold nanoparticles toward shrimp’s larvae can be linked to anticancer activity and can be used as a potential source of anticancer drugs. Paraquat is used as a standard drug. Incubation conditions = 28 ± 1 °C.
Sample code
Dose (μg/ml)
No. of shrimps
No. of survivors
LD50 of nanoparticle (μg/ml)
LD50 of Std. Drug (μg/ml)
AuC8NPs
0.0129
30
25
66.7184
7.2539
0.129
24
1.29
20
AuC6NPs
0.013
30
23
21.37
20.5459
0.13
22
1.3
18
Various AuNPs-based systems have been employed for antimicrobial applications. Recently Elisabetta de Alteriis and co-workers demonstrated in vitro antifungal activity of indolicidin-capped AuNPs (AuNPs-indolicidin) against Candida albicans. Similarly, Webster and co-workers reported mono and bimetallic NPs which are active against MDR E. coli (Webster & l cholula-Díaz, 2019). Above therapeutic agent is only active against gram negative strain whereas, our fabricated system is equally effective against both gram negative and positive becteria. Major advantage of this work is, our synthesized NPs are economical, environmentally friendly and have shown competent results against both bacterial and fungal strains at the same time. Overall, our research offers a tremendous platform to design antimicrobial therapeutics in future studies. The present work suggests the nanoparticles may have potent anticancer activity. Further research is required to investigate the anticancer potential of the particles
2.4 Conclusion
In this study, we report the successful preparation of gold nanoparticles by using Calix[6]rene and Calix[8]rene as a stabilizing agent. Synthesized gold nanoparticles were characterized by UV–vis spectroscopy, FT-IR and AFM. It is believed that hydroxyl group present in the ligand (C6 and C8) has stabilized the gold ions into metallic nanoparticles. The synthesized AuC6NPS exhibited strong antibacterial activity against S. aureus. Furthermore, changes in cell structure and roughness on surface of the bacteria verify cell lysis. AuC6NPS killed bacteria with greater efficiency in comparison to ligand C6. Both samples of gold nanoparticles possess promising phytotoxicity and moderate cytoxicity. Our synthesized gold nanoparticles may have commercial viability to develop nanomedicine against different microbes and human pathogens. Taken together, cytotoxic and phytotoxic effects of gold nanoparticles can be linked with anticancer activity and hence, the synthesized nanoparticles could be alternative source of anticancer drugs.
3 Experimental section
3.1 Materials
Analytical grade reagents such as hydrogen tetrachloroaurate (III) trihydrate (HAuCl4·3H2O) 99.995%, sodium borohydride (NaBH4) 98%, calix[6]arene 97% and calix[8]arene 90% were purchased from Sigma Aldrich. The solutions were prepared in deionized water. For antibacterial activity E. coli and S. aureus bacterial strains were used whereas antifungal activities were carried out by using Aspergillus niger and Candida albicans fungal strains.
3.2 Synthesis of gold nanoparticles
The AuNPs were synthesized by using sodium borohydride as a reducing agent. Initially the solutions of calix[6]arene (C6) and calix[8]arene (C8) were prepared in DMF which were further diluted in deionized water to prepare 0.1 mM solution. Two samples of AuNPs (AuC6NPs and AuC8NPs) were synthesized by adding 5 mL C6 to sample 1 and 3 mL C8 to sample 2 in tetrachloroaurate (III) trihydrate solution (0.1 mM, 30 mL). The samples were reduced by drop wise addition of sodium borohydride (4 mM). Pink color was obtained immediately after adding NaBH4, the mixture was stirred till wine red is obtained.
3.3 Methodology of antifungal activity
The Agar tube dilution Method was used to determine antifungal activity (Atta-ur-Rahman & Abbas, 1991) and Amphotericin B was used as an standard. All the samples and standard were dissolved in sterilized distilled water/DMSO (24 mg/mL). Samples with various concentrations (50–400 μg /ml) were placed in test tube containing sterile Sabouraud’s Dextrose agar medium and were inoculated in slanting position at room temperature for overnight. After inoculation fungal cultures the samples were left to incubate for 7 days at 29 °C. While, Growth inhibition was calculated against reference to the negative control by applying following formula:
% inhibition of fungal growth = 100 − linear growth and test (mm)/linear growth in control (mm) × 100
3.4 Methodology of antibacterial activity
Bacterial cultures were grown in nutrient agar medium at the concentration of 105 cells/mL. Later, a hole of 60 mm was made in each plate by using a borer, Different concentrations ranging from 10 to 500 µg/ mL of Ceftriaxone, and AuNPs were inserted in each hole simultaneously to evaluate the efficiency of methodology. However, 500 µg mL−1 stock solutions of Ceftriaxone, and AuNPs were used to avoid nonspecific merged zones of inhibition. The plates were incubated at 37 °C ± 1 for 24 h. After 24 h incubation, zone of inhibition was calculated at millimeter scale. Afterwards Minimal Inhibitory Concentration (MIC) of gold nanoparticles was calculated against each bacterial strain, for E. coli = Ceftriaxone MIC was calculated 10 µg/mL and for S. aureus was calculated 20 µg/mL. Two bacterial strains S. aureus ATCC 9144 and E. coli ATCC 10536 were used to evaluate antibacterial activity of samples.
3.5 Brine shrimp lethality assay
Cytotoxic activity of synthesized AuNPs was determined by brine shrimp lethality assay. Sample was prepared by taking 2 mL of AuNPs solutions in a vial and 5, 50, 500 μL of prepared solution of AuNPs was transferred to other three vials. Brine shrimp (Artemia saline) eggs were hatched in artificial sea water (prepared by dissolving 38 g/L sea salt in water) and were incubated for 48 h at 37 °C. After 2 days nauplii were transferred with Pasteur pipette in the vials containing 2 mL of sample. Teen larvae were transferred to each vials and sea water was added to make volume up to 5 mL. A vial containing 50 μL DMSO instead of sample was used as control. After 24 h of incubation the number of survived shrimps were counted and recorded in each vial. Etoposide was used as a positive control. Final data was analyzed with Finnery computer program to determine the LD50 values with 95% confidence level.
3.6 The Lemna bioassay
Phytotoxic activity of AuNPs was determined by using the modified protocol of Lemna minor. Different constituents were mixed in 1000 mL distilled water to prepare medium and the pH (6.0 to 7.0) of the medium was adjusted by KOH solution. The E-medium was prepared by adding 100 mL of stock solution into 900 mL of distilled water. Three flasks were inoculated with 10, 100, and 1000 μL from the stock solution of AuNPs for 10, 100, and 1000 μg/mL respectively. The solution was left to evaporate under sterile conditions for overnight. 20 mL of E-medium was added to each flask containing a rosette of three fronds of Lumna minor was added. All flasks were plugged with cotton and placed in the growth cabinet for 7 days. The number of fronds per flask was counted and their growth regulation was calculated with the reference to the negative control.
3.7 Characterization technique
UV–visible spectroscopy (Thermo Scientific Evolution 300 spectrophotometer) and FT-IR (Bruker Victor 22 spectrophotometer) were employed for analysis.
3.8 Analysis of cellular morphology
Change in morphology on bacterial surface was monitored by Agilent 5500 Atomic Force Microscopy (AFM), (Arizona USA). Initially mica slides were prepared by adding 10 mL polylysine. Treated and untreated S. aureus, Ecoli cultures were placed on already prepared mica slides and air-dried. These air-dried slides were then subjected for AFM analysis. The variations in the morphology of treated and untreated samples were evaluated. The images were acquired in tapping mode.
3.9 Abbreviations
NP: nanoparticles AuNP: gold nanoparticles AFM: atomic force microscopy SPB: surface Plasmon band C6: calix[6]arene C8: calix[8]arene
4 Declarations
Authors contributions
SK Synthesized and characterized the nanoparticles and wrote the manuscript. SP and SA performed biological work. MRS designed project and supervised studies. SR interpreted biological analyses. MIM corrected and finalized the manuscript. All authors read and approved the final manuscript.
Acknowledgement
All authors are thankful to the ICCBS, University of Karachi for instrumental support.
Competing interest
The authors declare that they have no competing interests.
References
- Biofilm inhibitory effect of chlorhexidine conjugated gold nanoparticles against Klebsiella pneumoniae. Microb. Pathog.. 2016;98:50-56.
- [Google Scholar]
- Synthesis of 4-(dimethylamino) pyridine propylthioacetate coated gold nanoparticles and their antibacterial and photophysical activity. J. Nanobiotechnol.. 2018;16(1):6.
- [Google Scholar]
- Dendrimer-silver complexes and nanocomposites as antimicrobial agents. Nano Lett.. 2001;1(1):18-21.
- [Google Scholar]
- Nanochemistry and nanomedicine for nanoparticle-based diagnostics and therapy. Chem. Rev.. 2016;116(5):2826-2885.
- [Google Scholar]
- Functional gold nanoclusters as antimicrobial agents for antibiotic-resistant bacteria. Nanomedicine. 2010;5(5):755-764.
- [Google Scholar]
- Single particle ICP-MS characterization of titanium dioxide, silver, and gold nanoparticles during drinking water treatment. Chemosphere. 2016;144:148-153.
- [Google Scholar]
- A novel electrochemical sensor based on calixarene functionalized reduced graphene oxide: application to simultaneous determination of Fe (III), Cd (II) and Pb (II) ions. J. Colloid Interface Sci.. 2017;508:525-531.
- [Google Scholar]
- Potential eye drop based on a calix [4] arene nanoassembly for curcumin delivery: enhanced drug solubility, stability, and anti-inflammatory effect. Mol. Pharm.. 2017;14(5):1610-1622.
- [Google Scholar]
- Gutsche, C.D., 2008. Calixarenes: an introduction. Royal Society of Chemistry.
- Gold nanoparticles: a critical review of therapeutic applications and toxicological aspects. J. Toxicol. Environ. Health B. 2016;19(3–4):129-148.
- [Google Scholar]
- Applications of Panax ginseng leaves-mediated gold nanoparticles in cosmetics relation to antioxidant, moisture retention, and whitening effect on B16BL6 cells. J. Ginseng Res. 2017
- [Google Scholar]
- Calixarene assisted rapid synthesis of silver-graphene nanocomposites with enhanced antibacterial activity. ACS Appl. Mater. Interfaces. 2016;8(29):19038-19046.
- [Google Scholar]
- Engineering ultrasmall water-soluble gold and silver nanoclusters for biomedical applications. Chem. Commun.. 2014;50(40):5143-5155.
- [Google Scholar]
- Calix [4] arene functionalized gold nanoparticles: application in colorimetric and electrochemical sensing of cobalt ion in organic and aqueous medium. Sens. Actuat. B. 2014;191:757-764.
- [Google Scholar]
- Synthesis and antimicrobial activity of p-tetranitrocalix [4] arene derivative. Polycyclic Aromat. Compd.. 2016;36(4):554-563.
- [Google Scholar]
- Biological applications of functionalized calixarenes. Chem. Soc. Rev.. 2013;42(1):366-386.
- [Google Scholar]
- Surface plasmons on metal nanoparticles: the influence of shape and physical environment. J. Phys. Chem. C. 2007;111(10):3806-3819.
- [Google Scholar]
- Heck-type olefination and Suzuki coupling reactions using highly efficient oxacalix [4] arene wrapped nanopalladium catalyst. J. Saudi Chem. Soc. 2017
- [Google Scholar]
- Encapsulation of rhodamine-6G within p-sulfonatocalix [n] arenes: NMR, photophysical behaviour and biological activities. RSC Adv.. 2016;6(111):110206-110220.
- [Google Scholar]
- Gum tragacanth stabilized green gold nanoparticles as cargos for Naringin loading: a morphological investigation through AFM. Carbohydr. Polym.. 2017;174:243-252.
- [Google Scholar]
- Nanotechnology in agriculture: next steps for understanding engineered nanoparticle exposure and risk. NanoImpact. 2016;1:9-12.
- [Google Scholar]
- Polysaccharide-based nanoparticles for theranostic nanomedicine. Adv. Drug Deliv. Rev.. 2016;99:70-84.
- [Google Scholar]
- Controlled functionalization of gold nanoparticles with mixtures of calix [4] arenes revealed by infrared spectroscopy. Langmuir. 2017;33(33):8253-8259.
- [Google Scholar]







