Cannabinoid receptors type 2: Function and development in agonist discovery from synthetic and natural sources with applications for the therapy of osteoporosis
⁎Corresponding authors at: School of Pharmaceutical Sciences, Zhejiang Chinese Medical University, #Binwen Road 584, Hangzhou 310053, China. hanting@smmu.edu.cn (Ting Han), zqy1965@163.com (Qiao-yan Zhang), lpqin@zcmu.edu.cn (Lu-ping Qin)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
Type-2 cannabinoid receptor (CB2R) is a member of the G protein-coupled receptor superfamily with various biological activities in pain regulation, anti-inflammation, anti-fibrosis, and bone metabolism regulation. CB2R modulators have been demonstrated to have significant regulatory effects on bone metabolism with no significant psychoactive adverse effects. In this article, we present a comprehensive review of the molecular mechanism of CB2R in regulating bone metabolism and summarize virtual screening and experimental screening methods for CB2R modulators from natural and synthetic products. Knowing that some botanical compounds are CB2R modulators involved in the regulation of bone metabolism, we also review the botanical and synthetic compounds currently known to be potential CB2R and summarize their regulatory effects on bone metabolism based on the results of animal, osteoblasts (OBs) and osteoclasts (OCs) experimental studies presently available, hoping that the findings and conclusions in this review could provide a scientific basis for the discovery of new CB2R regulators and the development of new anti-osteoporosis drugs.
Keywords
Cannabinoid receptors type 2
Osteoporosis
CB2R agonist discovery
Osteoblasts
Osteoclasts
- NF-κB
-
nuclear factor kappa-B
- PKA
-
protein kinase A
- ERK1/2
-
extracellular signal-regulated protein kinase 1/2
- JNK
-
c-Jun N-terminal kinase
- cAMP
-
cyclic adenosine monophosphate
- c-Fos
-
cellular oncogene fos
- c-JUN
-
Jun activation domain binding protein
- AP1
-
activator protein-1
- IκBα
-
inhibitor α of NF-κB
- MMP-9
-
matrix metalloproteinase-9
- PI3K
-
phosphatidylinositol 3-kinase
- p-AKT
-
phosphate protein kinases B
- PKC
-
protein kinase C
- DAG
-
diacylglycerol
- NFATc1
-
nuclear factor of activated T-cells, cytoplasmic 1
- CREB
-
cAMP-response element binding protein
- Cox
-
cyclooxygenase
- ATP
-
adenosine-triphosphate
- FAK
-
focal adhesion kinase
- Src
-
strict-regime camps
- RhoA
-
ras homolog gene family, member A
- TCF/LEF
-
ternary complex factor/lymphoid enhancing factor
- OCN
-
osteocalcin
- ALP
-
alkaline phosphatase
- OPG
-
recombinant osteoprotegerin
- Runx2
-
recombinant runt related transcription factor 2
- p38
-
cell signaling protein
- p52
-
proteins that play an important role in adhesion of eukaryotic cells
- p65
-
a marker of NF-κB pathway activation
Abbreviations
1 Introduction
Bone remodeling is the concerted interplay of two cellular activities: osteoblastic bone formation and osteoclastic bone resorption, which plays a key role in the maintenance of homeostasis of the skeleton and its physiologic functions (Sharma et al., 2024). Metabolic imbalance between osteoblasts (OBs) and osteoclasts (OCs) may lead to bone loss, eventually resulting in osteoporosis (OP) (Raggatt and Partridge, 2010, Chen et al., 2015), which is the most common degenerative skeleton disease characterized by decreased bone mineral density and bone structural change, thus increasing the risk of fracture and brittleness (Brown, 2017). Medical management of OP remains a clinical challenge. Accumulating evidence has demonstrated that cannabinoids and their receptors play important roles in bone metabolism by regulating the function of OBs and OCs (Apostu et al., 2019, Raphael-Mizrahi and Gabet, 2020). The endocannabinoid/endovanilloid (EC/EV) system receptors include type 1 cannabinoid receptor (CB1R), type 2 cannabinoid receptor (CB2R), and the transient receptor potential cation channel subfamily V member 1 (TRPV1). CB2R is involved in the regulation of bone metabolism, and some synthetic anti-osteoporotic drugs, such as raloxifene, tamoxifen, ospermine, bedoxifene and nafotin, have proved to be mediated by CB2R, which is accompanied by weak psychoactive effects like CB1R (Starowicz and Finn, 2017). Therefore, CB2R-specific ligands could offer an opportunity to prevent and/or rescue bone loss without producing significant adverse psychological effects of typical cannabinoids. Indeed, preclinical assessment has demonstrated that specific and non-psychoactive CB2R agonist HU308 can attenuate bone loss induced by estrogen depletion in ovariectomised (OVX) animals (Ossola et al., 2016). Some cannabinoid plants have proved to be a promising new entry point for the treatment of OP. In this review article, we will highlight the role of CB2R in bone metabolism and summarize the regulatory effects of synthetic and natural plant-derived CB2R modulators, hoping that the results could provide a scientific basis for discovery of novel anti-OP drugs.
2 Materials and methods
A systematic literature search was performed from the inception to June 2023 using the Scopus, Medline/PubMed and Web of Science databases. The following terms were used in various conjunctions or disjunctions to search for relevant literature: cannabinoid receptor 2, CB2, osteoporosis, osteoblast, osteoclast, synthetic compound, natural compound, botanical compound, plant medicine and phytomedicine. The inclusion studies in this paper shall satisfy the following criteria: (i) CB2R agonists were evaluated in terms of their regulatory effects on bone metabolism; and (ii) the studies on anti-osteoporotic activity were conducted on animals, or cultured OBs and OCs. The exclusion criteria consisted of (i) the regulatory effects on CB1R were more significant than those on CB2R; and (ii) the regulatory effects of CB2R modulators were assessed in inflammation and complicated disorders of bone metabolism. The compounds with Ki for CB2R less than 100 nM, or EC50 less than 200 nM for synthetic compounds, or Ki for CB2R less than 50 µM, or EC50 less than 10 µM for natural compounds were deemed as CB2R modulators.
3 Function and regulatory signaling pathway of CB2R in bone metabolism
In 1965, GAONI Y and MECHOULAM R isolated 9-tetrahydrocannabinol (9-THC) and firstly identified it as the psychoactive constituent in Cannabis sativa L. (Piomelli, 2014). In 1990, Matsuda et al cloned central CB1R of 9-THC from the rat brain tissue of the central nervous system (Matsuda et al., 1990). In 1993, Munro et al cloned the peripheral CB2R from the gene bank of human promyelocytic leukemia cell line HL60 and defined it as cannabinoid receptor 2 type (CB2R) (Munro et al., 1993). CB2R is widely distributed in cells and tissues of the immune system, including the thymus, tonsil, B lymphocyte, T lymphocyte, macrophage, monocyte, natural killer cell, and polymorphonuclear cell (Galiegue et al., 1995). The two most important cannabinoid receptor ligand Anandamide (AEA) and endogenous ligand 2-arachidonoylglycerol (2-AG), together with the enzymes involved in their biosynthesis and hydrolysis, form the endocannabinoid system (ECS) and represent the important therapeutic targets (Morales et al., 2016). The CB2R gene has a simple structure and contains a coding exon. As shown in Fig. 1, CB2R is structurally a member of G Protein-Coupled Receptors (GPCRs) on the cell membrane, which consists of a polypeptide chain with an extracellular N-terminal, 3 extracellular loops (EC1-3), 7 transmembrane domains (TM1-7), 3 intracellular loops (IC1-3), and an intracellular C-terminal domain (Munro et al., 1993). CB2R is located on chromosome 4 in mice and 1p36 in humans. There is a high level of CB2R sequence variation in humans, mice, and rats. There is 93 % amino acid homology between rat and mouse CB2Rs, and only 81 % amino acid homology between rat and human CB2Rs (Ndong et al., 2011). Compared with mouse and human CB2R sequence, rat CB2R sequence shows a completely different sequence homology at the carboxyl-terminal, and the intron DNA in rat CB2R is more different from that in mouse and human CB2Rs (Ndong et al., 2011).
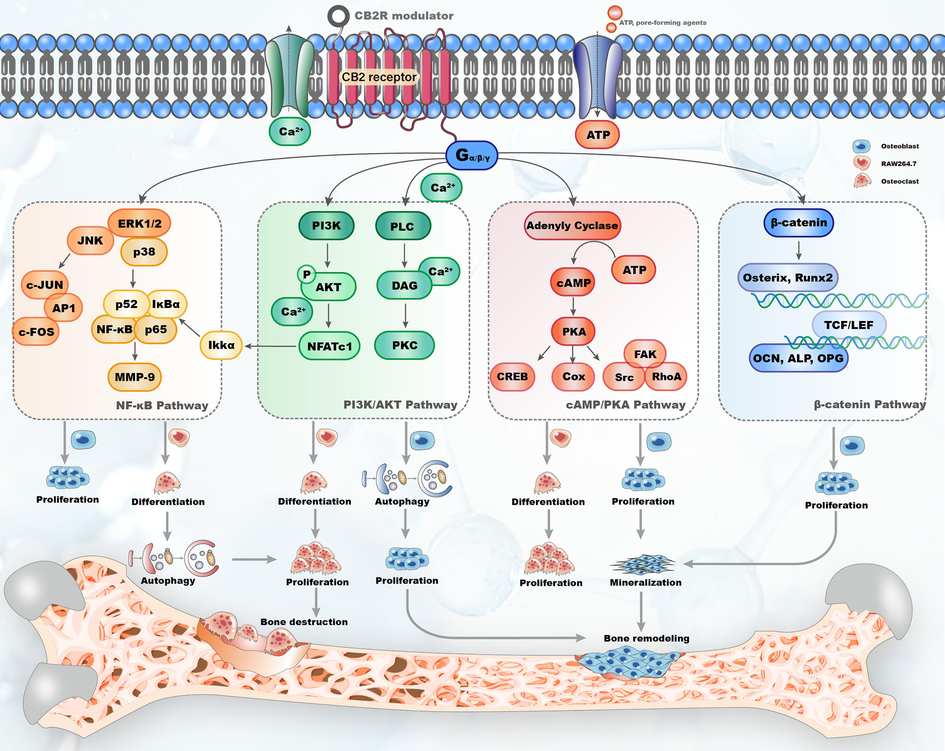
- The mechanism of CB2R regulation in bone metabolism.
CB2R negatively regulates the activity of adenylate cyclase and the expression and activity of cAMP protein kinase A (PKA), matrix metalloproteinase (MMP), focal adhesion kinase (FAK), recombinant human RhoA protein and other small molecules. Hence, CB2R activation decreases the level of intracellular cyclic adenosine monophosphate (cAMP), affects the motor ability of cells in the process of migration and invasion (Dhopeshwarkar and Mackie, 2016). In addition, CB2R ligand can activate p38 protein, extracellular-regulated kinase 1/2(ERK1/2) and c-JUN N-terminal kinase (JNK) in the mitogen-activated protein kinase (MAPK) pathway, and then selectively regulate cell proliferation, differentiation, transformation and death (Herrera et al., 2005, Li et al., 2022). CB2R ligand selectively regulate the expression of phosphatidylinositol 3-phosphatidylinositol 3-kinase (PI3K), phosphorylated protein kinase B (p-AKT) and synthesis of ceramide to control cell apoptosis, necrosis and autophagy (cell migration, cytokine production, proliferation and apoptosis) (Viscomi et al., 2009). CB2R activation is also known to inhibit nuclear factor kappa B (NF-κB) activation by abrogating phosphorylation of IκBα, thereby inhibiting its degradation and preventing the translocation of NF-κB to the nucleus (Juttler et al., 2004). In addition, CB2R activation is known to induce a transient rise in Ca2+ in calf pulmonary endothelial cells by activating protein lipase C and releasing intracellular Ca2+ stores in a CB2R-depenent manner (Huang et al., 2016).
In bone metabolism, CB2R on both OBs and OCs can also regulate the activity of Ca2+/K+/Na+ ion channels (De Petrocellis et al., 2017). CB2R activation in OCs can stimulate phospholipase C, regulate the level of intracellular calcium, inhibit the expression of NF-κB, and then reduce the activity of bone resorption (Geng et al., 2011). CB2R activation in OBs can regulate Wnt/β-catenin pathway and enhance the expression of main regulatory transcription factors of OB runt-related transcription factor 2 (Runx2), osteoprotegerin (OPG) and osteocalcin (OCN), which further regulates bone remodeling (Qiu et al., 2015)(Fig. 1).
4 The regulatory effect of CB2R on bone metabolism
Accumulating evidence has shown that the skeleton, OBs and OCs can synthesize and produce main endocannabinoids, anandamide and 2-AG in vitro, and also express diacylglycerol lipases (DAGLs), enzymes critically involved in 2-AG biosynthesis, and anandamide biosynthetic and degrading enzymes, N-Acylphosphatidylethanolamine-Hydrolyzing Phospholipase D (NAPE-PLD) and fatty acid amide hydrolase (FAAH) (Bab et al., 2008). Studies demonstrate that the CB2R-selective agonist AEA could stimulate OB proliferation in vitro, and that FAAH inhibitor URB597 could decrease the number of OCs and increase the AEA level endogenously in vitro (Bab et al., 2008). In addition, CB2R was found to be expressed in OBs, osteocytes, and OCs, and CB2R-deficient mice were characterized by age-related trabecular bone loss and cortical expansion, increased activity of trabecular OBs and OCs, and a marked decrease in the number of diaphyseal OBs precursors (Ofek et al., 2006), suggesting that CB2R signaling is essential for the maintenance of normal bone mass and can be considered as a molecular target for the diagnosis and treatment of OP (Ofek et al., 2006).
Postmenopausal OP is a high turnover type of bone metabolism caused by the decreased level of estrogen in postmenopausal women (North American Menopause, 2006). The polymorphism of gene encoding CB2R is closely related to bone mineral density in patients with postmenopausal OP (LaBuda et al., 2005). It was found that 17-β-estradiol increased the expression of CB2R by recruiting presumptive estrogen-responsive elements, suggesting that it may have impact on CB2R rather than hormone therapy to reduce postmenopausal bone resorption, exhibiting that CB2R is involved in the regulation of bone metabolism in postmenopausal OP (Rossi et al., 2013). With the increase of age, the level of estrogen in the body decreases, and the level of oxidative stress increases. As a result, bone formation is decreased and bone resorption is increased, leading to low-turnover bone metabolism (Coughlan and Dockery, 2014). It was found that CB2R-/- mice had a significant age-related trabecular loss and cortical dilatation (Ofek et al., 2006). The trabecular density of C57BL/6JCB2R-/- mice reached a normal peak in the first 2–3 months, followed by age-related bone loss (Sophocleous et al., 2017). At the age of 12 months, the trabecular volume density of CB2R-/- mice was about half of that of the wild-type control group, indicating that CB2R is involved in maintaining the balance of bone remodeling (Bab et al., 2009, Sophocleous et al., 2017). Several studies reported the use of CB2R as a new therapeutic target for inflammatory OP in different types of inflammatory models (Ashton, 2007). Fractures can cause inflammatory responses of nerve cells, and cannabinoid receptor modulators can further alleviate osteoporotic pain (Bruni et al., 2018). It was reported that the CB2R agonist 4-quinolone-3-carboxamide (4Q3C) can markedly inhibit the number of OCs in collagen-induced arthritis (CIA) in mice in a dose-dependent manner at doses of 1 mg/kg and 10 mg/kg, but CB2R antiagonist AM630 could counteract the protective effect of 4Q3C on the bone of CIA mice, suggesting that 4Q3C can reduce inflammatory cell infiltration and bone tissue destruction induced by rheumatoid arthritis by activating CB2R expression (Bai et al., 2019). Long-term use of retinoic acid, glucocorticoids, and proton pump inhibitors can cause bone loss, leading to drug-induced OP (Raterman et al., 2019). Methylprednisolone can disrupt the metabolism of the endogenous cannabinoid system, induce excessive activation of OCs, and reduce the activities of OBs, indicating that the CB2R is also involved into the regulation of glucocorticoid-induced bone loss (Bellini et al., 2017).
It is reported that OBs and OBs progenitor cells can express CB2R. With the increase in the expression of CB2R in OBs, expression of OB marker genes such as tissue non-specific alkaline phosphatase, parathyroid hormone type 1 receptor and Runx2 is also enhanced (Bab et al., 2008). Mature OBs express CB2R, AEA, and 2-AG, which in turn enhances the receptor activator of OBs induced by the nuclear factor kappa-B ligand (RANKL), thus affecting the coupling between OBs and OCs (Kostrzewa et al., 2021). Cannabinoid ligands regulate bone formation by directly acting on CB2R in OBs, or by producing catecholamine, an inhibitor that inhibits OB differentiation. The CB2R signal in OBs is autonomic and does not depend on the existence of OBs or their precursors. In the process of differentiation and activation of OBs, CB2R activation triggers the proliferation and differentiation of pre-OBs and stimulates the function of differentiated OBs in bone remodeling(Ofek et al., 2011).
OCs can also express cannabinoid receptors and their related regulatory proteins, such as FAAH, and diacylglycerol lipase β (DAGLβ)(Bellini et al., 2017). OCs and their progenitor cells have relatively low levels of CB1R mRNA and express a large amount of CB2R mRNA, and CB2R inhibit the formation and differentiation of OCs after activation(Rossi et al., 2019). A cannabinoid acid is involved in the regulation of survival, polarization, and activity of OCs by acting on mature OCs via CB2R (George et al., 2008).
5 Screening for CB2R modulators
5.1 Virtual screening
The traditional way to discover new selective CB2R modulator faces many challenges, such as high costs and long cycle times(Nunes-Alves, 2015, Moore et al., 2018). Today, with the in-depth study of CB2R, abundant data have been accumulated, which lays a solid foundation for discovering new selective CB2R modulators by virtual screening. Typically, virtual screening methods can be roughly divided into two categories: structure-based virtual screening (SBVS), and ligand-based virtual screening (LBVS). SBVS is intended to search and score the poses resulting from possible interactions between ligands and proteins based on a three-dimensional structural model of the target complexes. The representative method is molecular docking (Method 1 in Fig. 3) which has been popular since the 1980 s(Kuntz et al., 1982). In addition, the multiple crystal structure of CB2R complexes has been revealed in recent years (Li et al., 2019) (Fig. 2), such as protein 5ZTY, 6KPF(Hua et al., 2020), and 6PT0(Xing et al., 2020), which greatly promotes the application of SBVS method for CB2R modulator discovery. LBVS is based on the assumption that compounds with similar structures are deemed to have similar biological activities(Johnson and Maggiora, 1990). Most current applications of LBVS are artificial intelligence-based quantitative structure–activity relationship (QSAR) modeling (Method 2 in Fig. 3). Applications of the virtual screening methods in CB2R agonist discovery are summarized in Table 1. In addition, our group also successfully established a CB2R ligand prediction model with high accuracy using a fingerprint combination method. The developed regression model that combines eXtreme Gradient Boosting (XGBoost) and AvalonFP + AtomPairFP + RDkitFP + MorganFP (AARM) showed better performance than that of the model reported by Mizra et al (R2 = 0.667 vs. R2 = 0.62). The combination of XGBoost and AtomPairFP + MorganFP + AvalonFP (AMA) in a classification model yielded an area under curve (AUC)-receiver operating characteristic (ROC) of 0.917 for the external verification set, and also exhibited significantly better performance than D-MPNN (AUC-ROC = 0.898) and Molmap (AUC-ROC = 0.912) (Zhou et al., 2023).
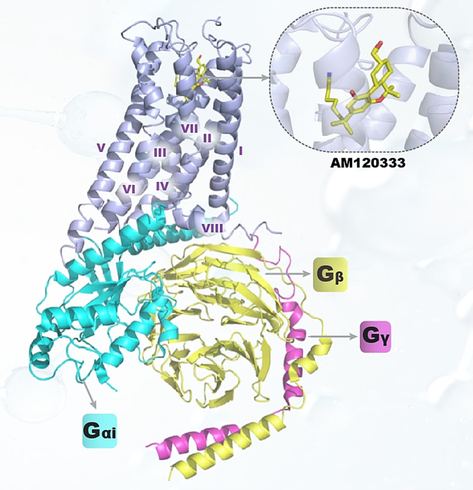
- Cryo-EM structures of the CB2R-Gi complexes Note: Protein PDB ID: 6kpf; the color code for the proteins is as follows: CB2R, light blue; Gαi, cyans; Gβ, yellow; Gγ, magenta; ligand AM12033, yellow.
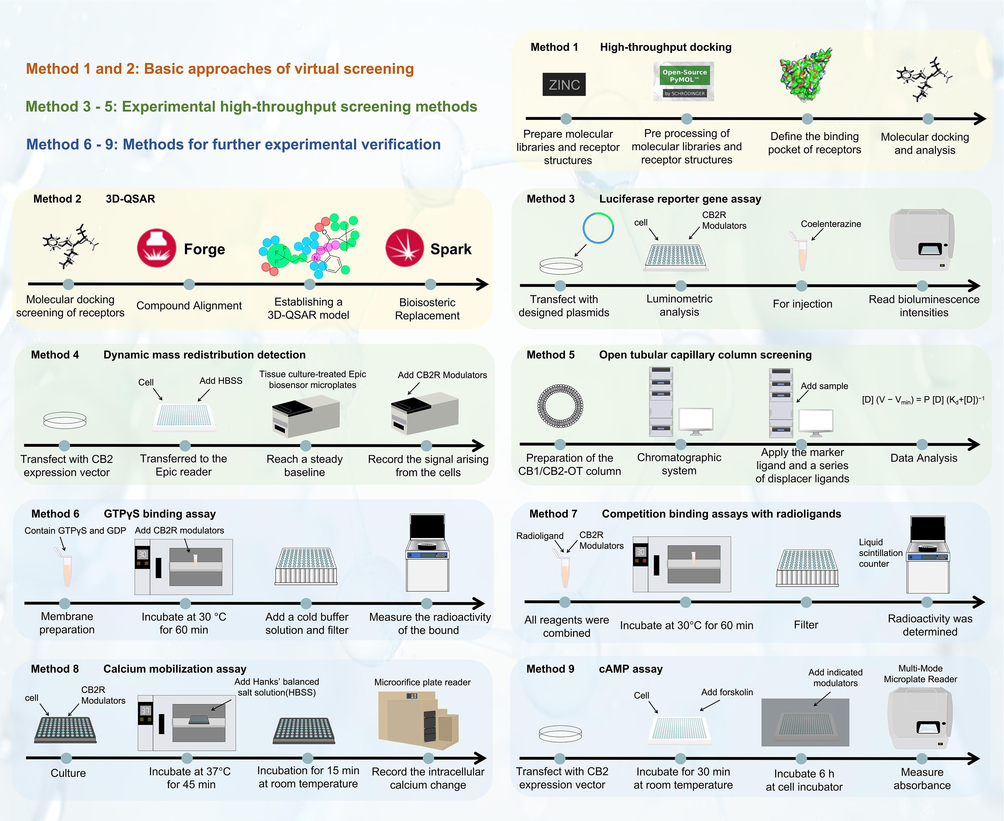
- Screening and validation methods for CB2R modulators.
| Year | Author | Method | Key findings | Representative ligand | Reference |
|---|---|---|---|---|---|
| 2006 | Tuccinardi et al. | Homologous modeling, docking | Whether the ligand interacts with the conserved residues, S3.31 and F5.46 in CB2R, is the key to CB2R/CB1R selectivity. |
(a)WIN-55212–2 |
(Tuccinardi et al., 2006) |
| 2007 | Chen et al. | Homologous modeling, 3D testing database query algorithm, MD, MM | The high precision homology model was established, which could distinguish the known antagonists from the randomly chosen molecules. |
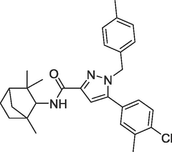
(a)SR144528 |
(Chen et al., 2007) |
| 2011 | Cichero et al. | 3D-QSAR model (CoMFA, CoMSIA) | CoMSIA exhibited the best performance where r2ncv = 0.96, r2cv = 0.713, and r2pred = 0.78. |
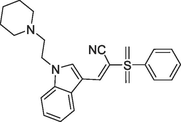
(b)48c, Ki = 0.0102 μM |
(Cichero et al., 2011) |
| 2013 | Ma et al. | Algorithm-based QSAR model | Classification model LiCABEDS was established and superior to SVM. |
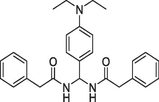
(b)XIE95PY1-61, Ki = 64 nM |
(Ma et al., 2013) |
| 2014 | Hickey et al. | Algorithm-based drug de novo designing and screening model | BI builder (a tool for de novo design) and PharmShape (a virtual screen package) methods were used to discover new chemotypes of CB2R agonists. |
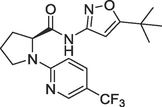
(b)17, Ki = 0.09 nM |
(Hickey et al., 2015) |
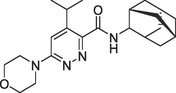
| 2016 | Romero-Parra et al. | Docking | A π-cation interaction with Lys109 has an important impact on the CB2R selectivity of ligands. |
(b)8f, Ki = 0.08 μM |
(Romero-Parra et al., 2016) |
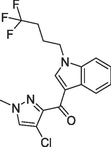
| 2017 | Qian et al. | Docking | The compound with high CB2R agonist activity was identified |
Compound 26(b), EC50 = 3.665 ± 0.533 nM |
(Qian et al., 2017) |
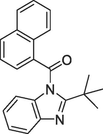
| 2018 | Tonelli et al. | Docking, ADMET analysis | Several benzimidazole derivatives were designed and synthesized, and 7 out of 18 compounds exhibited CB2R selective activity. |
(b)12a, Ki = 0.51 nM |
(Tonelli et al., 2018) |
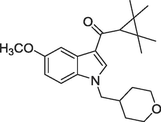
| 2018 | Floresta et al. | 3D-QSAR model | The model was uesd to evaluate the compounds derived from a thorough scaffold-hopping analysis and design of molecules with high affinity and novel scaffold. |
(a)Compound |
(Floresta et al., 2018) |
| 2019 | Vijayakumar et al. | Homology modeling, molecular docking, MD simulations, ADMET analysis | The binding mode of the TCN201 was analyzed, and a vision for designing new CB2R molecules was provided. |
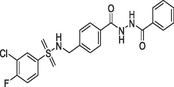
(a)TCN-201 |
(Vijayakumar et al., 2019) |
| 2020 | Yang et al. | Homology modeling, molecular dynamics simulation, and MM-PBSA | The binding affinity and binding mode of representative agonists and antagonists for CB1R and CB2R were systematically studied. |
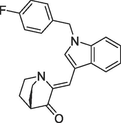
(a)PNR-4–20 |
(Yang et al., 2020) |
| 2021 | Wang et al. | Three-step virtual screening approach (deep learning-pharmacophore-molecular docking) | Novel CB2R antagonists were discovered, and 7 of the 15 screened compounds showed binding affinities against the CB2R in radioligand binding assay. |
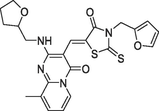
(b)4428–0510, Ki = 219 nM |
(Wang et al., 2021) |
| 2021 | Sadybekov et al. | Synthon-based virtual screening method | The high affinity compound was successfully obtained. |
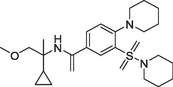
(b)747, Ki = 0.9 nM |
(Sadybekov et al., 2022) |
| 2022 | Morales et al. | High-Throughput Virtual Screening | Five compounds were identified to have dual agonistic effects on both CB2R and TRPV1. |
(a)151332252 |
(Morales et al., 2022) |
| 2022 | Yuan et al. | Algorithm toolset-MCCS | An algorithm toolset-MCCS was developed to predict the potential allosteric binding site, providing a new perspective to designing CB2R modulators. |
(a)CP-55940 |
(Yuan et al., 2022) |
Note: (a), This study was only used to dock the compound with CB2R, and the authors did not discover it; (b), The authors discovered this compound, and did some research on it. Abbreviations: LiCABEDS, ligand classifier of adaptively boosting ensemble decision stumps; SVM, support vector machine; MM-PBSA, molecular mechanics-poisson-boltzmann surface area; MCCS, molecular complex characterizing system.
5.2 Experiment-based screening methods
Several experimental screening methods have been developed to identify CB2R modulators from synthetic compounds or natural plant compounds (Fig. 3). Dynamic mass redistribution (DMR) is a label-free technology of using light to measure ligand-induced changes in the mass of cells proximate to the biosensor, which provides an integrated cellular response comprising multiple pathways and cellular events (Du et al., 2009). Zhou et. al applied DMR technology (Method 4 in Fig. 3) and stable CHO cell line transfected CB1R and CB2R genes to screen CB1R and CB2R modulators and obtained four natural CB2R modulators in Ganoderma species (Zhou et al., 2020).
Luciferase reporter gene assay is also used to screen CB2R modulators (Method 3 in Fig. 3). A drug screening model of CB2R agonist has been established, and used to discover active constituents from traditional Chinese medicine (Liu et al., 2012). Split luciferase complementation assay (SLCA) in HEK293 cells was developed to screen modulators of CB2R, and celastrol was identified as a novel modulator of CB2R (Jiang et al., 2020). With attempt to discover CB2R agonists with regulatory effects on bone metabolism, we established the screening system for CB2R modulators from botanical compounds, and identified nine CB2R agonists from 69 botanical compounds, and these CB2R agonists exhibited remarkable inhibitory effects on cAMP accumulation (Method 9 in Fig. 3) and good affinity to CB2R, as evidenced by the molecular docking and molecular dynamics (Hu et al., 2022).
An on-line screening method for CB1R and CB2R ligands was developed through immobilizing cellular membrane fragments of a chronic myelogenous leukemia cell line (KU-812) onto the surface of an open tubular capillary to create a CB1R/CB2R-OT column (Moaddel et al., 2011). The affinity chromatographic technique was applied to determine the binding activity of the immobilized CB1R/CB2R, and the binding affinity (the Ki value) for a single compound and then to screen individual or a mixture of multiple compounds. This method was used to screen a botanical matrix, Zanthoxylum clava-herculis, and the preliminary results suggest the presence of a high affinity phytocannabinoid in this plant (Moaddel et al., 2011).
The signaling of CB1R and CB2R is mediated via Gi proteins. CB1R and CB2R receptor activation can activate Gα16 protein, and then activate phospholipase C (PLC) to produce inositol triphosphate IP3 and diacylglycerol DAG. Binding of IP3 to its IP3 receptor in the endoplasmic reticulum was found to induce the release of intracellular calcium, and therefore change in intracellular calcium flow was used as an indicator of CB1R and CB2R activation (Method 8 in Fig. 3) (Jiang et al., 2020). Some studies used cell-based calcium mobilization assay to evaluate the functional activity of coumarin derivatives and found that some synthesized coumarin derivatives exhibited high selectivity towards CB2R against and CB1R (Xu et al., 2020).
6 Synthetic CB2R modulators and their potential effects on bone metabolism
Along with endocannabinoids, some synthetic compounds have been shown to bind and signal through CB2R via G proteins, leading to both inhibitory or stimulating signals depending on the biological process(Alexandre et al., 2020), including the non-selective CB2R agonists WIN552122 and CP55940, selective CB2R agonists HU308 and JWH133, and CB2R selective inverse agonists AM630 and SR144528 (Table 2). These synthetic CB2R modulators are reported to possess the potential effects on bone metabolism, and their chemical structures and regulatory effects on bone metabolism are summarized in Fig. 4.
| Name | Mode of action | Method of assay | Activity | Selectivity vs CB1R | Reference |
|---|---|---|---|---|---|
| HU308 | CB2R selective agonist | Competition binding assay with radioligands | Ki = 22.7 ± 3.9 nM | Ki > 10 μM | (Hanus et al., 1999) |
| GTPγS binding assay | EC50 = 6.4 nM | / | (Smoum et al., 2015) | ||
| HU433 | CB2R selective agonist | GTPγS binding assay | EC50 = 130 nM | / | |
| JWH133 | CB2R selective agonist | Competition binding assay with radioligands | Ki = 3.4 nM | Ki = 677 nM | (Huffman et al., 1999) |
| JWH015 | CB2R selective agonist | Antagonist pre-treatment | Verified by using inhibitor | / | (Mao et al., 2019) |
| AM630 | CB2R selective inverse agonist | Competition binding assay with radioligands | Ki = 31.2 nM | Ki = 5 μM | (Ross et al., 1999) (Bolognini et al., 2012) |
| GTPγS binding assay | EC50 = 76.6 nM | / | |||
| Ridaifen-B | CB2R partial agonist | Competition binding assay with radioligands | Ki = 43.7 ± 14.6 nM | Ki = 732 ± 168 nM | (Franks et al., 2018) |
| γ-Sanshool | CB1R antagonist CB2R agonist |
Competition binding assay with radioligands | EC50 = 41.7 nM | IC50 = 99.6 nM | (Dossou et al., 2013) |
| 3, 3-diindolylmethane | CB2R partial agonist | β-Arrestin assay | EC50 = 1.7 ± 1.3 μM | IC50 > 30 μM | (Yin et al., 2009) |
| Competition binding assay with radioligands | Ki = 1.1 ± 0.3 μM | Ki = 4.3 ± 0.3 μM | |||
| GTPγS binding assay | EC50 = 0.98 ± 0.22 μM | IC50 = 11.1 μM | |||
| cAMP assay | EC50 = 0.42 ± 0.23 μM | EC50 > 30 μM | |||
| β-Caryophyllene | CB2R selective agonist | Competition binding assay with radioligands | (E)-BCP: Ki = 155 ± 4 nM(Z) -BCP: Ki = 485 ± 36 nM |
/ | (Gertsch et al., 2008) |
| Euphol | CB2R partial agonist | Antagonist pre-treatment or knockdown gene | Verified by using inhibitor | Verified by using inhibitor | (Dutra et al., 2012) |
| Betulinic acid | CB2R partial agonist | Competition binding assay with radioligands | Ki = 41.21 ± 12.08 μM | Ki = 36.71 ± 4.12 μM | (Liu et al., 2012) |
| Celastrol | CB2R selective agonist | Split luciferase complementation assay | EC50 = 1.77 ± 0.23 nM | / | (Jiang et al., 2020) |
| Anthocyanins | CB2R partial agonist | Competition binding assay with radioligands | Cyanidin: Ki = 33.5 μM Delphinidin: Ki = 34.3 μM Peonidin: Ki = 46.4 μM |
Cyanidin: Ki = 16.2 μM Delphinidin: Ki = 21.3 μM |
(Korte et al., 2009) |
| 4-O-Methylhonokiol | CB2R selective agonist | Competition binding assay with radioligands | Ki = 43.9 ± 5.2 nM | Ki = 2.4 ± 0.2 μM | (Schuehly et al., 2011) |
| Magnolol | CB2R partial agonist | Competition binding assay with radioligands | Ki = 3.15 ± 1.65 μM | Ki = 1.44 ± 0.10 μM | (Rempel et al., 2013) |
| cAMP assay | EC50 = 3.28 ± 2.01 μM | EC50 = 18.3 ± 8.6 μM | |||
| Curcumin | CB2R partial agonist | Antagonist pre-treatment | Verified by using inhibitor | Verified by using inhibitor | (Aguiar et al., 2022) |
| Biochanin A | CB2R partial agonist | Competition binding assay with radioligands | Inhibition 33 ± 5 % of control | Inhibition 27 ± 7 % of control | (Thors et al., 2010) |
| Paeonol | CB2R selective agonist | Antagonist pre-treatment | Verified by using inhibitor | / | (Peng et al., 2020) |
| N-acylethanolamines (eicosapentaenoyl, docosapentaenoyl and docosahexaenoyl derivatives) | CB2R selective agonist | Rapid response assay | / | / | (Alharthi et al., 2018) |
| Rutamarin | CB2R selective agonist | Competition binding assay with radioligands | Ki = 7.4 μM | / | (Rollinger et al., 2009) |
Abbreviations: ↑: Up-regulation of expression; ↓: Down-regulation of expression. OVX, Ovariectomized; DMSO, dimethyl sulfoxide; BM-MSCs, bone marrow mesenchymal stem cells; BMMs, bone marrow-derived macrophages; BMD, bone mineral density; BV/TV, the percentage of trabecular bone volume (BV) to total volume (TV) of bone tissue; BS/TV, bone surface area density; Tb.Sp, the average width of the trabecular bone marrow; Tb.Th, the average thickness of trabecular bone; ES/BS, eroded surfaced; ROS, reactive oxygen species; RANKL, the receptor activator of nuclear factor-κB ligand; TNF-α, serum tumor necrosis factor α; TRACP, tartrate-resistant acid phosphatase; ALP, alkaline phosphatase; GLU, glutamic acid; OCN, osteocalcin; IL-6, interleukin-6; EZH2, enhancer of zeste homolog 2; Conn.D, dynamic histomorphometric variables; SMI, structure model index; ITB3, Integrin beta-3; PI3K, phosphatidylinositol 3-kinase; p-AKT, phosphorylated protein kinase B; p-GSK-3, phosphorylated glycogen synthase kinase-3; CCL3, chemokine (C–C motif) ligand 3; CTX, cyclophosphamide; MMP-9, matrix metalloproteinase-9; CtsK, cathepsin K.
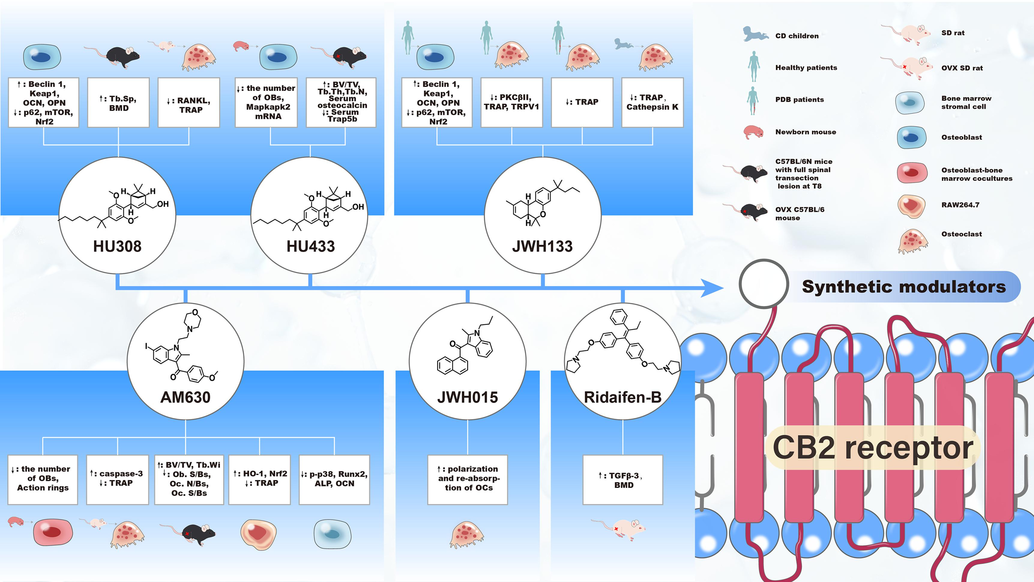
- Synthetic CB2R modulators and their regulatory effects on bone metabolism Abbreviations: OBs, osteoblast cells; OCs, osteoclast cells; OCN, osteocalcin; OPN, osteopontin; BMD, bone mineral density; Keap1, Kelch-like ECH-associated protein 1; mTOR, mammalian target of rapamycin; Nrf2, nuclear factor erythroid 2-related factor 2; PKC, protein kinase C; TRAP, tartrate resistant acid phosphatase; Mapkapk2, recombinant mitogen activated protein kinase activated protein kinase 2; BV/TV: the percentage of trabecular bone volume (BV) to total volume (TV) of bone tissue; TRPV1, transient receptor potential vanilloid-1; Runx2, recombinant runt related transcription factor 2; HO-1, heme oxygenase 1; ALP, alkaline phosphatase; TGF, transforming growth factor; RANKL, Receptor Activator for Nuclear Factor-κB Ligand; Tb.Sp, the average width of the trabecular bone marrow; Tb.Th, the average thickness of trabecular bone; Tb.N, trabecular number, Tb.Wi, trabecular width; Ob.S/BS, osteoblast surface; Oc.S/BS, osteoclast surface; Oc.N/BS, osteoclast number.
6.1 (6AR,10AR)-3-(1,1-dimethylbutyl)-6A,7,10,10A-tetrahydro-6,6,9-trimethyl-6H-dibenzo[B,D]pyran (JWH133) and (2-methyl-1-propylindol-3-yl)-naphthalen-1-ylmethanone (JWH015)
JWH133, selectively binds to the peripheral CB2R with Ki as 3.4 nM, but weakly binds to central CB1R with Ki as 677 nM (Huffman et al., 1999). JWH133 was demonstrated to be a CB2R agonist participating in systemic metabolic regulation in the EV/EC system as evidenced by improving tremor and spasm in diseased mice (Blazquez et al., 2003). JWH133 prevented bone loss induced by methylprednisolone through decreasing the expression of PKCβII, which significantly antagonized the overexpression of TRAP and TRPV1, and reversed the over-activation of OCs (Bellini et al., 2017). JWH133 induced OB differentiation of human bone marrow mesenchymal stem cells (BM-MSCs) by inducing autophagy. JWH133 enhanced the ALP activity and mineralization, and upregulated the expression of osteogenic markers, osteopontin and osteocalcin in hFOB 1.19 cells through CB2R activation (Xu et al., 2020). JWH133 significantly reduced multi-nucleated TRAP-positive OCs and the resorption areas in in vitro samples of patients with paget’s disease of the bone (Paoletta et al., 2021). While JWH133 reduced the OC activity of patients with celiac disease (Tortora et al., 2020). Stimulating CB2R combined iron metabolism in counteracting OC overactivity in childhood cancer survivors could attenuate OP and improve their quality of life. (Rossi et al., 2022). JWH015, a selective agonist of CB2R, stimulated polarization and re-absorption of human OCs, and increased the formation and re-absorption of actin rings in OCs (Mao et al., 2019).
6.2 [(1R,4R,5R)-4-[2,6-dimethoxy-4-(2-methyloctan-2-yl)phenyl]-6,6-dimethyl-2-bicyclo[3.1.1]hept-2-enyl]methanol (HU308) and its enantiomer HU433
HU308, a new type of non-psychotic and selective CB2R agonist, could effectively bind to CB2R with Ki as 22.7 ± 3.9 nM, but could not bind to CB1R with Ki being more than 10 μM (Hanus et al., 1999). HU308 promoted mitosis of the OBs line but had an anti-mitosis effect in the monocyte OC line and reduced the expression of RANKL mRNA in bone marrow stromal cells during OBs differentiation. HU433, an enantiomer of HU308, has 3S, 4R, 6R configuration. HU308 is conducive to the accumulation of GTPγS (Method 6 in Fig. 3), while HU433 transmits signals through ERK1/2. HU433 retains the HU308 specificity for CB2R, as shown by its failure to bind to the CB1R and has no activity in CB2R-deficient cells and animals. The CB2R binding affinity of HU433 in terms of [3H]CP55,940 displacement (Method 7 in Fig. 3) and its effect on [35S] GTPγS accumulation was substantially lower compared with HU308 (Smoum et al., 2015). A molecular-modeling analysis suggests that HU433 and HU308 have two different binding conformations within CB2R, with one of them possibly responsible for the affinity difference, involving [35S] GTPγS and cAMP synthesis. HU433 is more effective than HU308 in reducing bone loss induced by ovariectomy, but its binding to CB2R is much lower than that of HU308, and HU-433 is 3–4 orders of magnitude more potent in OB proliferation and OC differentiation, as well as in mouse models, for the rescue of ovariectomy-induced bone loss and ear inflammation(Smoum et al., 2015).
6.3 (6-iodo-2-methyl-1-(2-morpholinoethyl)-1H-indol-3-yl)(4-methoxyphenyl)methanone (AM630)
AM630 is a selective and competitive CB2R reverse agonist acting on CB1R and CB2R with a Ki value of 5 μM and 31.2 nM, respectively (Bolognini et al., 2012). AM630 could increase the production of cAMP stimulated by trichostatin in cells transfected with CB2R with EC50 as 230.4 nM and inhibit the binding of GTPγS to the cell membrane transfected with CB2R cells. A bone histomorphometry analysis showed that AM630 could completely prevent bone loss in ovariectomized wild-type mice by regulating CB2R (Idris et al., 2008). AM630 inhibited OC formation, reduced the number of TRAP-positive cells, inhibited the expression of genes related to OC differentiation and activation by inhibiting RANKL-induced ERK activation, and also increased the expressions of heme oxygenase 1 (HO-1) and nuclear factor erythroid 2-related factor 2 (Nrf2) (Li and Sun, 2020).
6.4 Ridaifen-B (RID-B)
RID-B is a novel derivative of tamoxifen (a selective estrogen receptor modulator) and a high affinity reverse agonist of CB2R with Ki as 43.7 nM (Franks et al., 2018). It could regulate the activity of G protein and adenylate cyclase with an Imax of 398 ± 30 % and exerted an anti-inflammatory activity by reducing the concentration of nitrogen monoxide (NO), interleukin 6 (IL-6) and IL-1α (Franks et al., 2018). It also inhibited OC differentiation and activity through inducing the production of transforming growth factor β-3 in ovariectomized mice, and reduced differentiation of bone marrow macrophages into OCs like estrogen (Bryant et al., 1999).
7 The regulatory effect of plant-derived CB2R modulators on bone metabolism
Plant CB2R modulators include those derived from marijuana or plants other than marijuana. More than 120 cannabinoid compounds have been isolated from cannabis plants, and dozens of CB2R modulators have been isolated and identified from plants other than marijuana. Some natural CB2R modulators from plants other than marijuana, including alkaloids, terpenes, polyphenols, fatty acid derivatives and phenylpropanoids, have been demonstrated to regulate the expression of CB2R. The chemical structures of these natural monomer compounds are shown in Fig. 5 and Table 2. Some plant extracts, such as Echinacea purpurea (Linn) Moench. and Morinda citrifolia L. also showed regulatory effects on CB2R (Fig. 6). In this article, we will focus on CB2R modulators from plants other than marijuana and discuss their potential effects on bone metabolism (Table 3).
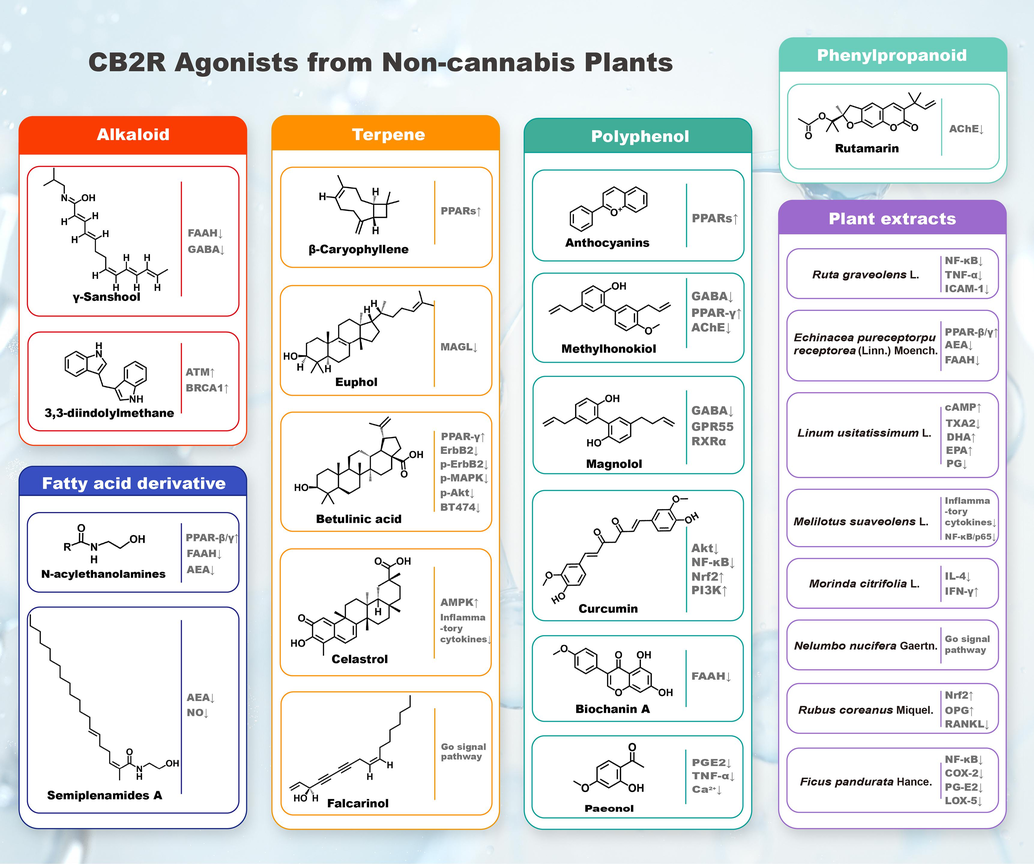
- Representative CB2R agonists from non-cannabis plants.
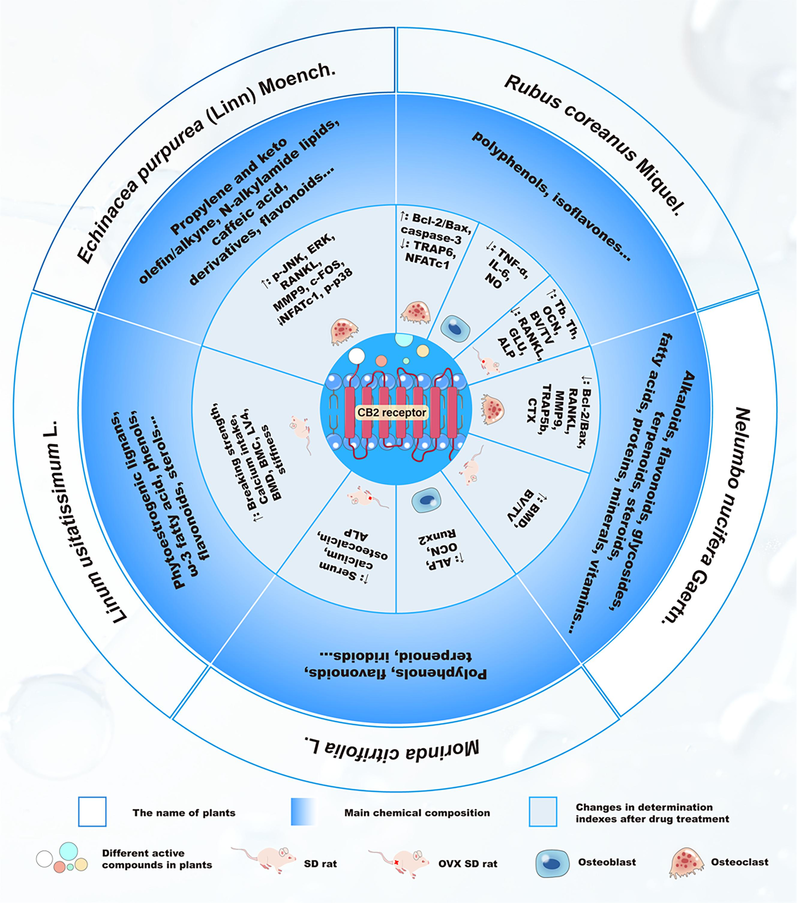
- The medicinal plants with agonistic activities on CB2R and their regulatory effects on bone metabolism Abbreviations: ↑, Up-regulation of expression; ↓, Down-regulation of expression; COX-2, cyclooxygenase-2; GPR55, G Protein-Coupled Receptor 55; RXRα, retinoic acid X receptor-α; GABA, gamma-aminobutyric acid; PTP-1B, ProteinTyrosine Phosphatase-1B; ATM, ataxia telangiectasia mutated; BRCA1, breast cancer 1; BT474, Human Breast Cancer Cells E; AMPK, Adenosine 5′-monophosphate (AMP)-activated protein kinase; Nrf2, NF-E2-related factor 2; NF-κB, nuclear factor kappa-B; PI3K, phosphatidylinositol 3-kinase; NO, nitric oxide; DHA, docosahexaenoic acid; EPA, Eicosapntemacnioc Acid; PG, Prostaglandine; GO, Gene ontology; cAMP, Cyclic Adenosine monophosphate; TXA2, thromboxaneA2; IL-4, Interleukin-4; TNF-α, serum tumor necrosis factor α; ICAM-1, intercellular cell adhesion molecule-1; OPG, Osteoprotegerin; RANKL, Receptor Activator of Nuclear Factor-κB Ligand; LOX-5, Human 5 lipoxygenase; PPAR, peroxisome proliferators-activated receptors; CREB, cAMP-response element binding protein; 2-AG, anterior gradient 2; MAGL, monoacylglycerol lipase; FAAH, fatty acid amide hydrolase; ErbB2, epidermal growth factor receptor; MAPK, mitogen-activated protein kinase; p-Akt, phosphate protein kinases B; EAATs, excitatory amino acid transporters; PKC, protein kinase C; ECM, extracellular matrix; AEA, Anandamide, N-arachidonoylethanolamine; C/EBP-α, recombinant human CCAAT/enhancer binding protein-α; AChE, acetylcholinesterase; GO: Gene ontology; PGE2, Prostaglandin E2.
| Name | Model | Dosage and duration of administration | Changes of related indexes | Reference |
|---|---|---|---|---|
| Betulinic acid | OVX C57/BL6 mice BMMs of mice |
5 or 10 mg/kg, i.p. once every 2 days for 6 weeks Co-culture with OCs induced by RANKL |
↑: BV/TV ↓: BS/TV, Tb.Sp, ROS, RANKL, TNF-α, TRAP, F-actin |
(Choi et al., 2016) |
| 3,3-diindolylmethane | OVX C57/BL6 mice | 0.1 mg/kg, i.p. twice a week for 4 weeks | ↑: BMD, BV/TV, Tb. N, Conn.D ↓: Tb.Sp, SMI, number and area of OCs |
(Yu et al., 2015) |
| Biochanin A | OVX Sprague-Dawle rat | Oral 25 mg/kg, once daily for 14 weeks | ↑: BMD, BMC, BV/TV, ALP, Osteocalcin ↓: TNF-α, IL-1 β, DPD/Creatine, TRAP, RANKL/OPG |
(Su et al., 2013) |
| Anthocyanins | OVX Sprague-Dawle rat OVX C57/BL6 mice |
Oral, 10 % dried blueberry powder aqueous solution, daily for 12 weeks Take 1 % blackcurrant extract with a diet for 4, 8, 12 weeks |
↑: OPG, ALP, BV/TV, Tb.N, Tb.Th ↓: Tb.Sp, ES/BS, O.Pm, L.Pm ↑: BMD, BV/TV ↓: TRAP, ES / BS |
(Melough et al., 2017) |
| Celastrol | Male C57BL/6 mcie | 1 mg/kg, i.p. daily for 12 weeks | ↑: PI3K, p-AKT, p-GSK-3 ↓: TRAP, Urinary calcium / creatinine, TRACP-5b, CTX, CtsK |
(Xi et al., 2018) |
| OVX C57BL/6 mice | 2.5, 5 mg/kg, i.p. daily for 2 weeks | ↑: BV/TV, Tb.N, Tb.Th ↓: Tb.Sp, TRAP, Ocs/BS |
(Xu et al., 2021) | |
| BM-MSCs of female C57BL/6 mice and RAW264.7 cells | 25, 50,100 nM for 2–4 days | ↓: NF-ATc1, TRAP, c-Fos, CtsK, CTR, c-Fos, DC-STAMP, NF-ATc1, V-ATPase d2, V-ATPase a3, p-ERK, p-p38, p-JNK, TAK1, p-IκB, p-p65 | ||
| Male C57BLKS/J mcie | 1 mg/kg, i.p. daily for 4 weeks | ↓: TRAP-5b, PTH, CTX, DPD, NF-κB(P65), MMP-1, MMP-9 | (Liu et al., 2016) | |
| OVX C57BL/6J mice | 200 μg/kg, i.p. every 2 days for 4 weeks | ↑: Tb.BV/TV, Tb.N, Ct.Th, Vt.BV, PGC-1α ↓: Tb.Sp, Es.Pm, |
(Li et al., 2020) | |
| BM-MSCs of mice | 0.25, 0,5, 1.0 μM for 7 days | ↑: PGC-1α, BGLAP, ALP, Osterix, Runx2, SIRT1, AMPK ↓: Pparg, Fabp4, ROS, |
||
| Male DBA/1J mice | 1 mg/kg, i.p. daily for 15 days | ↓: TRAP5b, TRAP, CtsK, CTR, MMP-9, c-Fos, c-JUN, NF-ATc1 | (Gan et al., 2015) | |
| RAW264.7 cells | 0.1, 0.2, 0.3 μM for 3 days | ↓: TTRAP, CtsK, CTR, MMP-9, c-Fos, c-JUN, NF-ATc1, p-p65, p-p38, p-ERK, p-JNK | ||
| β-Caryophyllene | Bone marrow cells of C57BL/6 mice | 0.1–100 μM for 3 days | ↑: OBs mineralization ↓: The number of adipocytes, only OCs in TNF-α |
(Yamaguchi and Levy, 2016) |
| OVX C57BL/6 mice | Oral 25 mg and 50 mg BCP in IV saline for 2 weeks | ↑: BV, BV/TV, BMD, klotho, Tb.Sp ↓: Tb.N |
(Dong et al., 2022) | |
| MC3T3-E1 cells | 0.5, 1.0, 5 μM; 24 h | ↑: Nrf2, HO-1, GSH ↓: ROS |
(Shan et al., 2017) | |
| Methylhonokiol | BMMs of mice | 0.1, 1 μM; 3–5 days | ↑: JNK, p38 ↓: TRAP, c-Fos, NF-ATc1, TRAP |
(Park et al., 2017) |
| Magnolol | MC3T3-E1 cells | 0.01, 0.1, 1 μM; Cultivate for 2 days | ↑: ALP, Bone matrix protein, Glutathione content ↓: Bone resorption factor, RANKL, TNF – α, IL-6 |
(Kwak et al., 2012) |
| BMMs of old male ICR mice | 1.25–20 μM; 7 days | ↓: IL-1, TRAP, RANKL, PGE2 | (Hwang et al., 2018) | |
| OVX C57BL/6 mice BMMCs RAW264.7 cells |
Oral 10 mL/kg daily for 6 weeks 5, 10, 20 μg/mL; 7 days |
↑: BMD, BS/BV, BS/TV, BV/TV, Tb.N ↓: CTX-1, IL-6, TNF-α, Tracp 5B ↓: NF-ATc1, TRAP, F-actin, resorption area, Cathepsin K, CTR, MMP9, TRAF6, p-IκB, p-p65, p-p50, p-ERK, p-p38, p-JNK |
(Fei et al., 2019) | |
| Echinacea purpurea (Linn.) Moench. | BMMs of C57BL/6 mice | 30 μM; 0, 24, 48, 72, 96 h | ↓: p-JNK, ERK, RANKL, MMP-9, c-Fos, NF-ATc1, p-p38 | (Chang et al., 2020) |
| Nelumbo nucifera Gaertn. | Tibial BMMs of ICR mice female Balb/c nude mice implanted MDA-MB-231 cells into the medullary cavity |
10, 20, 40, 60 μM; 5 days Oral 10, 20 mg/kg liensinine, 5 times a week for 5 weeks |
↑: BMD, BV/TV ↓: Bcl-2/Bax, RANKL, MMP-9, TRAP5b, CTX |
(Kang et al., 2017) |
| Morinda citrifolia L. | OVX Wistar Rat | Oral administration, 2, 10, 50 mg/kg, daily for 7 days | ↑: Serum calcium, osteocalcin, ALP ↓: Number of OCs |
(Wattanathorn et al., 2018) |
| Rubus coreanus Miquel. | OVX Wistar rat | 400 mg/kg, 5 days a week for 6 weeks. | ↑: Tb.Th, OCN, BV/TV ↓: GLU, ALP, RANKL |
(Choi et al., 2012) |
| Linum usitatissimum L. | male Wistar rat | 250 g/kg with a diet daily once a day for 160 days | ↑: BMD, BMC, Calcium intake, LV, stiffness, breaking strength | (Maira et al., 2018) |
Abbreviations: p-JNK, phosphorylated c-Jun N-terminal kinase; ERK, extracellular regulated protein kinases; RANKL, Receptor Activator for Nuclear Factor-κB Ligand; MMP9, matrix metalloprotein9; c-Fos, cellular oncogene fos; NFATc1, nuclear factor of activated T-cells, cytoplasmic 1; BMC, bone mineral content; OCN, osteocalcin; Runx2, recombinant runt related transcription factor 2; BV/TV: the percentage of trabecular bone volume (BV) to total volume (TV) of bone tissue; OPN, osteopontin; BMD, bone mineral density; ALP, alkaline phosphatase; Bcl-2, B-cell lymphoma-2; Bax, Bcl2-associated X; GLU, glucose; IL-6, interleukin-6; NO, nitric oxide; TNF, tumor-necrosis factor; CTX, cyclophosphamide; TRAP, tartrate resistant acid phosphatase; Tb.Th, the average thickness of trabecular bone; p-p38, phosphorylated p38; LV4, Lumbar vertebra.
7.1 Chemical compounds from plants other than marijuana
7.1.1 β-caryophyllene (BCP)
BCP or (1R,4Z,9S)-4,11,11-trimethyl-8-methylidenebicyclo[7.2.0]undec-4-ene is a common constituent of the essential oil from numerous plants and a major component in Cannabis with antidepressant, antianxiety, analgesic and anticonvulsant activities (Hashiesh et al., 2021). (E)-BCP selectively binds to CB2R with Ki as 155 ± 4 nM and acts as a functional CB2R ligand to induce Gi/0 signals, and then activates CB2R-mediated intracellular signal transduction, such as inhibition of adenylate cyclase, intracellular calcium release and activation of mitogen-activated kinases Erk1/2 and p38 (Gertsch et al., 2008). BCP can relieve osteoporotic pain and reduce inflammation and pain response. In addition, it increased the mineralization of OBs derived from BM-MSCs of 8-week-old female C57BL/6 mice, reduced the number of adipocytes, and inhibited the formation and differentiation of mouse bone marrow monocytes into OCs induced by tumor necrosis factor α (TNF-α) through mediating the NF-κB signal pathway (Yamaguchi and Levy, 2016). CD300a gene and CB2R had a strong positive correlation (r = 0.774) in female C57BL/6 mice, and klotho enzyme regulated the expression level of CB2R. BCP can intervene CD300a gene through klotho enzyme to significantly prevent trabecular bone defects caused by vitamin D deficiency (Dong et al., 2022). CB2R has been shown expressed in MC3T3-E1 OBs. The effect of trans-caryophyllene on osteocalcin secretion and matrix mineralization was found to be directly dependent on CB2R, which interfered with the antioxidant system in OBs (Shan et al., 2017).
7.1.2 Magnolol
Magnolol or 4-allyl-2-(5-allyl-2-hydroxyphenyl) phenol is an active component from the bark of Magnolia officinalis, with an antibacterial activity and an inhibitory effect on platelet aggregation and muscle relaxation (Zhang et al., 2019). It is a selective partial agonist of CB2R with EC50 as 3.28 μM in cAMP accumulation studies, and its metabolite (tetrahydro)magnolol is 19-fold more potent than magnolol with EC50 as 0.170 µM. Additionally, β-arrestin translocation assay showed that (tetrahydro)magnolol (a CB2R-related orphan receptor) behaved as an antagonist at GPR55 with kB value as 13.3 µM (Rempel et al., 2013). 2-(2-hydroxy-5-propylphenyl)-4-pentylphenol, a CB2R partial agonist (Ki = 0.0371 µM), also showed potency to select versus GPR18 and GPR55 (Fuchs et al., 2013). Magnolol and its metabolites can protect MC3T3-E1 cells against cytotoxicity of antimycin A, increase OB proliferation, alkaline phosphatase activity, bone collagen synthesis, and bone matrix mineralization by activating mitochondrial function (Kwak et al., 2012). Magnolol did not directly affect OB precursors, but indirectly inhibited OC differentiation by inhibiting PGE2 synthesis and RANKL expression in OBs (Hwang et al., 2018). Magnolol prevented bone loss caused by ovariectomy through inhibiting OCs formation via regulating NF-κB and mitogen-activated protein kinase pathway (Lu et al., 2015). Methylmagnolol inhibited the activity of TRAP, formation of F-actin ring and expression of c-Fos, NF-ATc1, TRAP and ITB3 in OCs, and magnolol inhibited the formation and function of OCs derived from RAW264.7 with RANKL (Fei et al., 2019).
7.1.3 4-O-methylhonokiol
4-O-methylhonokiol or 2-(4-methoxy-3-prop-2-enylphenyl)-4-prop-2-enylphenol is a novel neolignan compound from Magnolia officinalis with anti-osteoporosis, anti-Alzheimer’s disease, anti-inflammatory, anti-oxidative, anti-cancer, and anti-aging activities (Lee et al., 2011). The chemical structure of 4-O-methylhonokiol is similar with that of HU308, which has the characteristics of the CB2R selective agonist with Ki as 43.9 ± 5 nM and could inhibited the release of cAMP (Schuehly et al., 2011). 4-O-methylhonokiol inhibited the release of cAMP and also increased the [35 s]GTPγS binding induced by methylmagnolol with its EC50 value as 285.7 nM (Chicca et al., 2015). 4-O-methylhonokiol protected OB MC3T3-E1 cells against injuries induced by antimycin A through activating mitochondrial function. 4-O-methylhonokiol inhibited TRAP-positive multinuclear OCs and F-actin ring formation via the receptor activator during NF-κB ligand (RANKL)-mediated osteoclasteogenesis, and also suppressed RANKL-induced critical factors, such as c-Fos, NF-ATc1, TRAP and ITB3 for OC differentiation and function through inhibiting activation of the ERK1/2, AKT, and NF-κB pathways (Park et al., 2017).
7.1.4 Celastrol
Celastrol or 3-hydroxy-24-nor-2-oxo-1(10),3,5,7-friedelatetraen-29-oic acid is a methylated quinone methyl triterpene from the roots of Tripterygium wilfordii Hook. f., with anti-oxidation, anti-rheumatoid, anti-inflammation, anti-cancer neo-vascularization and immunosuppression activities (Hou et al., 2020, Xu et al., 2021). Celastrol has an excitatory effect on CB2R with an EC50 value of 1.81 ± 0.16 μM, and a weak activating effect on CB1R with an EC50 value of more than 10 μM as determined by using the SLCA's new CB2R screening method (Jiang et al., 2020). Celastrol inhibited bone resorption and enhanced bone formation in glucocorticoid-induced osteoporotic rats through inhibiting prostaglandin E2 and caspase-3 protein expression, and inducing PI3K, phosphorylated AKT and glycogen synthase kinase-3 phosphorylation and Wnt and β-catenin protein expression (Xi et al., 2018). Celastrol promoted OB differentiation and prevented adipocyte differentiation in BM-MSCs in vitro by stimulating PGC-1α signaling (Li et al., 2020). Celastrol was used as an NF-κB inhibitor to improve articular cartilage and cancellous bone structure in dexamethasone-induced OP mice (Liu et al., 2016). It could also alleviate bone loss and bone marrow adipose tissue accumulation in OVX and aged mice and inhibit the expression of pro-inflammatory cytokines and metalloproteinase through NF-κB inhibition via stimulating CB2R on osteocytes (Gan et al., 2015). In CIA mice, daily injection of celastrol markedly suppressed arthritis, and reduced bone damage in the joints as demonstrated by serum biochemical parameters, histology and bone micro-computed tomography (Li et al., 2020). In OCs derived from RAW264.7 induced with RANKL, celastrol inhibited the formation of TRAP-positive multinucleated cells and the bone-resorbing activity in a dose-dependent manner, and reduced the RANKL-induced expression of OCs genes and transcriptional factors, as well as phosphorylation of NF-κB and MAPK (Gan et al., 2015).
7.1.5 Betulinic acid
Betulinic acid or 3-beta-Hydroxy-lup-20(29)-en-28-oic acid is a naturally occurring triterpenoid found in the bark of plants in the family of Betulaceae and Sambucus williamsii Hance, with a broad spectrum of pharmacologic properties, including anti-viral, anti-bacterial, anti-inflammatory, anti-malarial, and anti-cancer activities (Rios and Manez, 2018). It is an agonist of the CB2R, with binding affinity to CB2R with Ki as 36.71 ± 4.12 μM, and to CB1R with Ki as 41 ± 12.1 μM(Liu et al., 2012). Betulinic acid at the dose of 10 mg/kg slowed down bone loss and degeneration of the bone trabecular microstructure in ovariectomized mice (Wei et al., 2020). Combined use of 25 or 50 μg Betulinic acid with BMP2 (3 μg) markedly increased ectopic bone formation in male C57BL/6 mice. Betulinic acid increased OB formation through activating BMP/Smad/Runx2 and β-catenin signal pathways and increased increased the alkaline phosphatase (ALP) activity and expression level of osteogenic marker genes without decreasing cell viability of MC3T3-E1 pre-OBs (Choi et al., 2016). Betulinic acid inhibited bone resorption, formation of F-actin and expression of cathepsin K and MMP-9 in OCs via suppressing the expression of ERK, JNK, and p38 in MAPK signaling pathways (Wei et al., 2020).
7.1.6 3, 3-diindolylmethane (DIM)
DIM is a major acid-condensation product or metabolite of indole-3-carbinol found in cruciferous vegetables, with anti-cancer, anti-inflammatory, and immune stimulating activities (Biersack, 2020, Vermillion Maier et al., 2021). Four independent radioligand binding assays including β-arrestin assay, GTP-γS binding assay, radioligand binding assay and cAMP analysis of human CB1R and CB2R demonstrated that DIM had an agonistic effect on CB2R with the EC50 value ranging from 0.42 μM to 1.7 μM, and the Ki as 1.1 ± 0.3 μM (Yin et al., 2009). DIM treatment increased bone mass in female mice by suppressing OC bone resorption under both physiological and pathological conditions (Yu et al., 2015). Prostate cancer bone metastasis has long been believed to be related to OBs because of bone remodeling leading to the formation of new bone. Combined use of isoflavone and DIM inhibited OC and OB differentiation through regulating multiple signaling pathways such as high androgen receptor/prostate-specific antigen (AR/PSA), homo sapiens NK3 homeobox 1 (NKX3-1)/Akt/p27 and microphthalmia-associated transcription factor (MITF) in prostate cancer bone metastasis (Li et al., 2012).
7.1.7 Anthocyanins
Anthocyanin or 2-phenylchromenylium is derived from blueberry, cranberry, red cabbage, and other common plants, with anti-mutation, anti-oxidation, free radical scavenging, neuro-protective, anti-inflammatory, analgesic activities (Li et al., 2014, Panchal et al., 2022). Competitive radioligand binding assay for CB2R identified that the affinity of anthocyanin was achieved by cyanidin (Ki = 33.5 μM), delphinidin (Ki = 34.3 μM), and peonidin (Ki = 46.4 μM) (Korte et al., 2009). Anthocyanin decreased the activity of NF-κB, the mRNA expression level of TNF-α and IL-1β, and formation and differentiation of OCs derived from RAW264.7 macrophages(Melough et al., 2017).
7.1.8 Biochanin A
Biochanin A or 5,7-dihydroxy-4′-methoxy isoflavone derived from red clover, soybean, alfalfa sprout, peanut, chickpea, and other like plants, has potential for the treatment of climacteric syndrome, OP, cancer, aging, and cardiovascular disease (Sarfraz et al., 2020). 30 µM Biochanin A was demonstrated to reduce 33 ± 5 % (compared with control) specific binding of [3H] CP55,940 to CB2R as compared with control (Thors et al., 2010). Biochanin A increased the expression of OB gene osterix, type I collagen, ALP and osteocalcin, and the mRNA level of OPG secreted by OBs in OVX SD rats. In addition, it has an inhibitory effect on OCs differentiation and formation by inhibiting mature OC proliferation and decreasing the expression and activity of TRAP of OCs in a dose-dependent manner (Su et al., 2013).
7.2 Medicinal plants
7.2.1 Echinacea purpurea (Linn) Moench
Echinacea purpurea (Linn) Moench., which belongs to the family of Compositae, is widely cultivated in the United States, Canada, and Germany. Echinacea extracts have been shown to have anti-oxidant, anti-bacterial, anti-inflammatory, and immunostimulatory properties (Xu et al., 2021). Phytochemical investigations have shown that Echinacea purpurea contains propylene and keto olefin/alkyne, N-alkylamide lipids, caffeic acid derivatives and flavonoids (Aarland et al., 2017). Some N-isobutyl amides and N-methyl-butyl amides from this plant bind and partially activate CB2Rs as evidenced by alteration of the release of intracellular calcium. The Ki value of the n-hexane extract from the Echinacea purpurea root to CB2R is 269 ng/mL, with the content of total N-alkylamides reaching 7.01 % (Chicca et al., 2009). The Echinacea purpurea extract can inhibit the expression of NFATc1 and c-Fos of OCs induced by RANKL, and subsequently decrease the activity of TRAP, cathepsin K (CtsK) and MMP-9 in OCs by inactivating the MAPK signaling pathway (Chang et al., 2020). The echinalkamide isolated from cell cultures of this plant was shown to have an inhibitory effect on RANKL-induced OC proliferation and differentiation and attenuate OC bone resorption by disturbing phosphorylation of MAPK and inhibiting activation of the OC transcription factors c-Fos and NFATc1(Chang et al., 2020).
7.2.2 Linum usitatissimum L
Linum usitatissimum L., which is commonly known as flax or flaxseed, comes from the family of Linaceae and is grown in many countries and regions (Akter et al., 2021). The seeds of this plant, flaxseed, have been broadly utilized in numerous milieus worldwide as a primeval medicinal plant because of its health benefits in diverse types of diseases (Shayan et al., 2020). Phytochemical analysis shows that the major chemical constituents of Linum usitatissimum L. include ω-3 fatty acid, phytoestrogenic lignans, phenols, flavonoids, and sterols. Among them, secoisolariciresinol diglycosides (SDG) is the major bioactive compound of Linum usitatissimum L. with prospective pharmacological accomplishments(Ansari et al., 2019, Akter et al., 2021). The extracts or compounds from Linum usitatissimum L. exhibit significant anti-cancer, anti-oxidant, anti-microbial, anti-inflammatory, anti-obesity, anti-diabetic, anti-diarrheal, anti-malarial, hepato-protective, reno-protective, immunosuppressive, antiarrhythmic, and cognitive effects. The cannabinoid-like compound in its fiber extract is known as a new terpenoid compound that can activate the gene expression of specific peripheral CB2R (Styrczewska et al., 2012). Flaxseed can help improve osteoporotic bone, and this bone protective effect may be attributed to α-linolenic acid (ALA) rather than lignans in flaxseed. Dietary flax seed supplements or ALA were shown to retain bone mass in Wistar male rats by increasing the expression of osteocalcin, thus enhancing the ability of pre-OB differentiation to mature OBs (Maira et al., 2018). When combined with estrogen therapy, ω-3 polyunsaturated fatty acids, ALA in particular, from flaxseeds or flaxseed oil are beneficial to the bone health (Kim and Ilich, 2011). Flaxseed flour could improve bone mass and biomechanical properties, decrease total polyunsaturated fatty acids and arachidonic acids, and enhance ALA and eicosapentaenoic acid (EPA) in dam rats during the post-weaning growing phase (Ribeiro et al., 2017). Flaxseed oil, which is rich in ALA, could attenuate the high-fat-diet-induced decrease in bone biomechanical properties, bone mineral density, and bone micro-archiatexue by promoting osteogenesis as evidenced by serum bone formation markers ALP and the bone resorption marker human C-telopeptide of typeⅠcollagen (CTX-1) in HFD-induced bone loss of rats (Chen et al., 2019). Cyclolinopeptides, cyclic peptides extracted from flaxseed, could act directly on bone marrow-derived macrophages and reduce OC formation, maturation, and bone resorption by down-regulating the RANKL receptor activity (Kaneda et al., 2019).
7.2.3 Morinda citrifolia L
Morinda citrifolia L., which belongs to the family of Rubiaceae, is native to Southeast Asia and has long been used as a food supplement and medicinal plant(Wang et al., 2002). The fruit and leaf of Morinda citrifolia are rich in polyphenols, flavonoids, terpenoids, and iridoids, and have been demonstrated to have diverse biological and therapeutic properties, such as immunomodulatory, anti-oxidant, anti-osteoporotic and anti-cancer activities (Chanthira Kumar et al., 2022, Choi et al., 2022). It was reported that the fruit juice of this plant could potently activate CB2R and inhibit CB1R in a concentration-dependent manner (Palu et al., 2008). The leaf extract of Morinda citrifolia could protected the bone micro-architecture as assessed by serum levels of calcium, osteocalcin, alkaline phosphatase, and the density of OBs and OCs in the tibia (Wattanathorn et al., 2018). Morinda officinalis capsules were also shown to possess an anti-osteoporotic effect in OVX rats (Li et al., 2014). The fruit juice of Morinda citrifolia could enhance bone marrow derived stroma cells could enhance the proliferation of bone marrow-derived stroma cells and up-regulate the osteogenic differentiation marker genes ALP and OCN and Runx2 (Hussain et al., 2016).
7.2.4 Nelumbo nucifera Gaertn
Nelumbo nucifera Gaertn., also known as the sacred lotus, is mainly used as food throughout the Asian regions, and its seeds, rhizomes, leaves, flowers, and roots have been described in Ayurveda and traditional Chinese medicine for various disorders including stress, fever, depression, insomnia and cognitive problems. Phytochemical and pharmacological investigations have demonstrated that this plant contains diverse chemical constituents, and its bioactive phytocompounds including alkaloids, polyphenols, offering distinct pharmacological activities, mainly against cancer, microbial infection, diabetes, inflammation, atherosclerosis and obesity(Kumarihamy et al., 2015, Shen et al., 2019, Wang et al., 2023). A competitive radioligand assay demonstrated that the Nelumbo nucifera petal extract was a novel agonist with an antagonist activity towards CB2R as evidenced by competitive radioligand assay, which found that 10 μg/mL methanol extract of Nelumbo nucifera petals could antagonize the binding of 26.2 % excitatory ligand CP55940 to CB2R(Velusami et al., 2013). Neferine, a natural compound isolated from Nelumbo nucifera, was found to have a protective effect against OVX-induced bone loss in rats, inhibit RANKL-induced OC formation and the bone resorptive activity of mature OCs by suppressing RANKL-induced activation of the NF-κB signaling pathway, and inhibiting induction and activation of NFATc1. It could also enhance the differentiation and bone mineralization activity of MC3T3-E1 pre-OBs (Chen et al., 2019). Liensinine and nuciferine, major active components in Nelumbo nucifera, could prevent breast cancer cell-mediated bone destruction, and inhibit RANKL-induced OCs differentiation from mouse bone marrow macrophage cells and mature OC-mediated bone resorption. Liensinine could reduce osteolysis in nude mice with the intratibial injection of MDA-MB-231 cells (Kang et al., 2017).
7.2.5 Rubus coreanus Miquel
Rubus coreanus Miquel., black raspberry, belongs to the family of Rosaceae. Its fruit has long been used as traditional medicine, knowing that it contains various chemical compounds such as polyphenols and isoflavones (Lee et al., 2011, Kim et al., 2013). Radioligand displacement assay showed that Rutamarin from Rubus coreanus had a selective activity on CB2R with Ki as 7.4 μM (Rollinger et al., 2009). Treatment with Rubus coreanus could benefit osteoporotic rats caused by prostatic hyperplasia (Lim et al., 2015). Rubus coreanus extract could prevent bone loss in ovariectomized rats and aged rats by up-regulating the ESC (Choi et al., 2012). The Rubus coreanus extract could repair the H2O2 damaged function of MC3T3-E1 OBs by attenuating their apoptosis and reducing the production of TNF-α, IL-6, and NO in OBs, and also reduce the formation and differentiation of OCs through down-regulating the expression of TNF receptor associated factor 6 (TRAF6) and NFATc1 and increasing the ratio of B-cell lymphoma/leukemia 2 (Bax/Bcl-2) and expression of caspase-3 of OCs (Do et al., 2008).
8 Conclusions and future perspectives
The endocannabinoid system plays a vital role in the regulation of numerous physiological and pathological processes, and CB2R has been extensively investigated and recognized as a potential target for OP treatment. Bone metabolism is a process of bone remodeling with the participation of OBs and OCs, and the imbalance between OBs and OCs often leads to bone loss, finally resulting in OP. CB2R expression is shown to be involved in the regulation of OB bone formation and OC bone resorption. Some synthetic and botanical CB2R agonists could increase OB bone formation and decrease OC bone resorption without causing psychotropic adverse effects. Therefore, the investigation of CB2R agonists and their regulatory effects on bone metabolism could provide lead compounds for the research and development of new anti-OP drugs.
Although CB2R participates in many processes at the behavioral level through neuronal regulation, it often exists in the peripheral tissue, such as the bone and immune system compared to CB1R (Zou and Kumar, 2018, Grabon et al., 2023). The agonist of CB2R is considered safer and well tolerated in clinical applications, without psychoactive side effects observed after CB1R activation. However, the agonist binding pockets of CB1R and CB2R are structurally similar, including the binding modes and key amino acids, which may explain the poor selectivity of cannabinoid receptor agonists structurally. Most CB2R agonists so far identified are based on the structural modification of the chemical components of the plant cannabis, which may lead to some adverse effects such as affecting the mental activity. In addition, functional selectivity may be another concern for CB2R agonists relevant to antiosteoporosis. Different CB2R agonists may activate different populations of receptors or produce different conformational changes in the receptor allowing to produce different signaling outputs depending on the cell type and the fate of the cell. Therefore, the compounds, which can activate CB2R and have no effects on CB1R, need further continue to verify the regulatory effects on OBs and OCs, and upon validation, these compounds may be worthy to be developed as a new anti-osteoporotic drug.
CB2R is a very promising therapeutic target for its regulatory effects on both OB bone formation and OC bone resorption, but none of CB2R agonist have yet been approved as an antiosteoporotic drug owing to poor drug-like properties (Saponaro et al., 2021). Therefore, the design and synthesis of selective CB2R agonists should consider the balance between selectivity, activity, and pharmacokinetic properties, such as CB2R-β-arrestin2 dependent endocytosis (Steinmueller et al., 2023). Most CB2R ligands possess poor pharmacokinetic properties including high lipophilicity, low solubility, tight plasma protein binding, high in vivo clearance, and low oral bioavailability. Hence, it is a key challenge to optimize the ligand physicochemical properties to improve the pharmacokinetic properties of CB2R ligands through rational drug design and structural optimization strategies. The discovery of LEI-102, a new CB2R agonist, proved to be able to improve the lipophilicity of oral administration in a chemotherapy-induced nephropathy model (Li et al., 2023). Modification of the chemical structure of natural CB2R ligands also can achieve the good “drug-like” properties. A deep learning approach allows for precise design of selective CB2R agonists by reducing the unnecessary structural optimizations and lowering the cost of drug development. We have proposed a deep learning-based approach to de novo drug design, knowing that the structure of the active site of the target protein is essential for new molecular design (Zhou et al., 2023). Soon, artificial intelligence is expected to be applied to the design of selective CB2R agonists and provide more ligands for clinical and commercial use.
CB2R activation launches its downstream signaling cascades, including P38/MAPK/ NF-κB pathway, which are involved in regulating the function of both OCs and OBs (Qian et al., 2010, Li et al., 2022). Therefore, it is necessary to evaluate the regulatory effects of CB2R agonists on downstream pathways in OBs and OCs. In addition, CB2R activation may cause a series of responses of OBs and OCs that may alter the function of OBs and OCs. Hopefully, various currently available technologies and tools, including the omics and bioinformatic methods, as well as system biology approaches, will help disclose the complexity of ECS and CB2R and discover novel potential therapeutic opportunities for bone metabolism disorders. For instance, proteomics and transcriptomics can be used to analyze alterations in biological processes and molecular functions of OBs and OCs under CB2R deletion or overexpression conditions, which would contribute to a better understanding of CB2R contribution in OP pathogenesis, and the underlying mechanism of CB2R agonists on bone metabolism.
CB2R agonists exhibit promising antiosteoporosis potential, but some limitations need to be overcome before these agonists can be applied to clinical practice. Firstly, OP therapy requires the long-term use of medications, and therefore it is necessary to elucidate the pharmacological and toxicological properties of CB2R agonists based on in vivo and in vitro experiments to identify an effective and safe drug dosage and treatment time, especially, the long-term effects of CB2R-targeted drug therapy, for there is a lack of information regarding the efficacy or toxicity of such compounds in humans at present. Secondly, as CB2R also exists in the peripheral immune system, whether the immune regulatory effect CB2R agonists is beneficial or unfavorable to OP treatment needs to be verified. So, the immune regulatory effect and related effects of CB2R agonists on bone metabolism should be further investigated. Thirdly, CB2R agonists could non-specifically bind to other receptors in the skeleton system and reduce the drug concentration targeting CB2R, which may affect their therapeutic efficacy. Finally, osteoporotic patients may use multiple medications simultaneously and therefore the interactions of CB2R agonists with other drugs need to be further investigated.
Accumulating evidence has demonstrated that natural medicines are the important resources of antiosteoporotic drugs, and plenty of natural medicines have showed anti-osteoporotic activities, such as cimicifuga racemosa L.(Walji et al., 2007) and Humulus lupulus L.(Gonzalez-Salitre et al., 2023). Screening of CB2R agonists from anti-osteoporotic natural medicines can not only lay the foundation for the discovery of new lead compounds but further explain the anti-ostoporotic activities and mechanisms of these natural medicines(Hu et al., 2022). Therefore, researchers should focus on medicinal plants other than marijuana to discover novel CB2R agonists with anti-osteoporotic activities and without CB1R-like psychoactive side effects to develop new anti-osteoporotic drugs with novel structures, remarkable activities, and fewer adverse effects.
In conclusion, increasing evidence has demonstrated that CB2R plays a vital role in bone homeostais throughout human life by participating in both OB bone formation and OC bone resorption. CB2R agonists may be applied for prevention and treatment of OP induced by aging, estrogen deficiency, medication abuse and other reasons. A rational drug design strategy and modification of natural compounds are needed to improve the drug-like properties of CB2R agonists, including selectivity, specificity, activity, and pharmacokinetic properties. Further experimental and clinical studies are needed to warrant the efficacy and safety of CB2R agonists for the treatment of diseases associated with bone metabolic disorders.
CRediT authorship contribution statement
Si-jing Hu: Conceptualization, Writing – original draft, Formal analysis, Visualization, Writing – review & editing. Gang Cheng: Conceptualization, Writing – original draft. Gao-ce Chen: Validation, Investigation. Hao Zhou: Methodology, Data curation. Qi Zhang: Methodology, Data curation. Qi-ming Zhao: Methodology, Data curation. Chen-xia Lian: Validation, Formal analysis, Investigation, Visualization. Zi-hui Zhao: . Quan-long Zhang: . Ting Han: Validation, Supervision. Qiao-yan Zhang: Conceptualization, Validation, Writing – review & editing, Project administration, Funding acquisition. Lu-ping Qin: Conceptualization, Validation, Writing – review & editing, Project administration, Funding acquisition.
Acknowledgements
This work was supported by the National Natural Science Foundation of China (No. 82374098; 82374099; 81974534); the Shanghai Municipal Science and Technology Commission (No. 22S21901600); the Opening Project of Zhejiang Provincial Preponderant and Characteristic Subject of Key University (Traditional Chinese Pharmacology) and Zhejiang Chinese Medical University (No. ZYAOX2018018).
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Studies on phytochemical, antioxidant, anti-inflammatory, hypoglycaemic and antiproliferative activities of Echinacea purpurea and Echinacea angustifolia extracts. Pharm. Biol.. 2017;55:649-656.
- [CrossRef] [Google Scholar]
- Curcumin induces peripheral antinociception by opioidergic and cannabinoidergic mechanism: Pharmacological evidence. Life Sci.. 2022;293:120279
- [CrossRef] [Google Scholar]
- A comprehensive review on Linum usitatissimum medicinal plant: Its phytochemistry, pharmacology, and ethnomedicinal uses. Mini Rev. Med. Chem.. 2021;21:2801-2834.
- [CrossRef] [Google Scholar]
- Synthetic cannabinoids and their impact on neurodevelopmental processes. Addict. Biol. 2020
- [Google Scholar]
- n-3 polyunsaturated N-acylethanolamines are CB(2) cannabinoid receptor-preferring endocannabinoids. Biochim. Biophys. Acta Mol. Cell Biol. Lipids. 2018;1863:1433-1440.
- [CrossRef] [Google Scholar]
- A review on pharmacological and clinical aspects of Linum usitatissimum L. Curr. Drug Discov. Technol.. 2019;16:148-158.
- [CrossRef] [Google Scholar]
- Cannabinoids for the treatment of inflammation. Curr. Opin. Investig. Drugs. 2007;8:373-384.
- [Google Scholar]
- Endocannabinoids and the regulation of bone metabolism. J. Neuroendocrinol.. 2008;20(Suppl 1):69-74.
- [CrossRef] [Google Scholar]
- Cannabinoids and the skeleton: from marijuana to reversal of bone loss. Ann. Med.. 2009;41:560-567.
- [CrossRef] [Google Scholar]
- A selective CB(2) agonist protects against the inflammatory response and joint destruction in collagen-induced arthritis mice. Biomed. Pharmacother.. 2019;116:109025
- [CrossRef] [Google Scholar]
- PKCbetaII-mediated cross-talk of TRPV1/CB2 modulates the glucocorticoid-induced osteoclast overactivity. Pharmacol. Res.. 2017;115:267-274.
- [CrossRef] [Google Scholar]
- 3,3'-Diindolylmethane and its derivatives: nature-inspired strategies tackling drug resistant tumors by regulation of signal transduction, transcription factors and microRNAs. Cancer Drug Resist.. 2020;3:867-878.
- [CrossRef] [Google Scholar]
- Inhibition of tumor angiogenesis by cannabinoids. FASEB J.. 2003;17:529-531.
- [CrossRef] [Google Scholar]
- AM630 behaves as a protean ligand at the human cannabinoid CB2 receptor. Br. J. Pharmacol.. 2012;165:2561-2574.
- [CrossRef] [Google Scholar]
- Cannabinoid delivery systems for pain and inflammation treatment. Molecules. 2018;23
- [CrossRef] [Google Scholar]
- An estrogen receptor basis for raloxifene action in bone. J. Steroid Biochem. Mol. Biol.. 1999;69:37-44.
- [CrossRef] [Google Scholar]
- Effect of echinalkamide identified from Echinacea purpurea (L.) Moench on the inhibition of osteoclastogenesis and bone resorption. Sci. Rep.. 2020;10:10914.
- [CrossRef] [Google Scholar]
- Chanthira Kumar, H., X. Y. Lim, F. H. Mohkiar, et al., 2022. Efficacy and Safety of Morinda citrifolia L. (Noni) as a Potential Anticancer Agent. Integr Cancer Ther. 21, 15347354221132848. Doi: 10.1177/15347354221132848.
- Neferine suppresses osteoclast differentiation through suppressing NF-kappaB signal pathway but not MAPKs and promote osteogenesis. J. Cell Physiol.. 2019;234:22960-22971.
- [CrossRef] [Google Scholar]
- The osteocyte plays multiple roles in bone remodeling and mineral homeostasis. Med. Mol. Morphol.. 2015;48:61-68.
- [CrossRef] [Google Scholar]
- GPCR structure-based virtual screening approach for CB2 antagonist search. J. Chem. Inf. Model.. 2007;47:1626-1637.
- [CrossRef] [Google Scholar]
- Flaxseed oil ameliorated high-fat-diet-induced bone loss in rats by promoting osteoblastic function in rat primary osteoblasts. Nutr. Metab. (Lond).. 2019;16:71.
- [CrossRef] [Google Scholar]
- Synergistic immunomopharmacological effects of N-alkylamides in Echinacea purpurea herbal extracts. Int. Immunopharmacol.. 2009;9:850-858.
- [CrossRef] [Google Scholar]
- 4'-O-methylhonokiol increases levels of 2-arachidonoyl glycerol in mouse brain via selective inhibition of its COX-2-mediated oxygenation. J. Neuroinflamm.. 2015;12:89.
- [CrossRef] [Google Scholar]
- Choi, S. I., I. J. La, X. Han, et al., 2022. Immunomodulatory Effect of Polysaccharide from Fermented Morinda citrifolia L. (Noni) on RAW 264.7 Macrophage and Balb/c Mice. Foods. 11, Doi: 10.3390/foods11131925.
- Betulinic acid synergically enhances BMP2-induced bone formation via stimulating Smad 1/5/8 and p38 pathways. J. Biomed. Sci.. 2016;23:45.
- [CrossRef] [Google Scholar]
- Effect of Rubus coreanus extracts on diabetic osteoporosis by simultaneous regulation of osteoblasts and osteoclasts. Menopause. 2012;19:1043-1051.
- [CrossRef] [Google Scholar]
- Homology modeling in tandem with 3D-QSAR analyses: a computational approach to depict the agonist binding site of the human CB2 receptor. Eur. J. Med. Chem.. 2011;46:4489-4505.
- [CrossRef] [Google Scholar]
- Osteoporosis and fracture risk in older people. Clin. Med. (Lond).. 2014;14:187-191.
- [CrossRef] [Google Scholar]
- Actions and regulation of ionotropic cannabinoid receptors. Adv. Pharmacol.. 2017;80:249-289.
- [CrossRef] [Google Scholar]
- Functional selectivity of CB2 cannabinoid receptor ligands at a canonical and noncanonical pathway. J. Pharmacol. Exp. Ther.. 2016;358:342-351.
- [CrossRef] [Google Scholar]
- Bone-protecting effect of Rubus coreanus by dual regulation of osteoblasts and osteoclasts. Menopause. 2008;15:676-683.
- [CrossRef] [Google Scholar]
- Beta-caryophyllene prevents the defects in trabecular bone caused by Vitamin D deficiency through pathways instated by increased expression of klotho. Bone Joint Res.. 2022;11:528-540.
- [CrossRef] [Google Scholar]
- Identification of CB1/CB2 ligands from Zanthoxylum bungeanum. J. Nat. Prod.. 2013;76:2060-2064.
- [CrossRef] [Google Scholar]
- Distinct growth factor-induced dynamic mass redistribution (DMR) profiles for monitoring oncogenic signaling pathways in various cancer cells. J. Recept. Signal. Transduct. Res.. 2009;29:182-194.
- [CrossRef] [Google Scholar]
- Euphol, a tetracyclic triterpene produces antinociceptive effects in inflammatory and neuropathic pain: the involvement of cannabinoid system. Neuropharmacology. 2012;63:593-605.
- [CrossRef] [Google Scholar]
- Magnolol prevents ovariectomy-induced bone loss by suppressing osteoclastogenesis via inhibition of the nuclear factor-kappaB and mitogen-activated protein kinase pathways. Int. J. Mol. Med.. 2019;43:1669-1678.
- [CrossRef] [Google Scholar]
- Discovery of high-affinity cannabinoid receptors ligands through a 3D-QSAR ushered by scaffold-hopping analysis. Molecules. 2018;23
- [CrossRef] [Google Scholar]
- The tamoxifen derivative ridaifen-B is a high affinity selective CB(2) receptor inverse agonist exhibiting anti-inflammatory and anti-osteoclastogenic effects. Toxicol. Appl. Pharmacol.. 2018;353:31-42.
- [CrossRef] [Google Scholar]
- The natural product magnolol as a lead structure for the development of potent cannabinoid receptor agonists. PLoS One. 2013;8:e77739.
- [Google Scholar]
- Expression of central and peripheral cannabinoid receptors in human immune tissues and leukocyte subpopulations. Eur. J. Biochem.. 1995;232:54-61.
- [CrossRef] [Google Scholar]
- Celastrol attenuates bone erosion in collagen-Induced arthritis mice and inhibits osteoclast differentiation and function in RANKL-induced RAW264.7. Int. Immunopharmacol.. 2015;24:239-246.
- [CrossRef] [Google Scholar]
- Cannabinoid receptor-2 selective antagonist negatively regulates receptor activator of nuclear factor kappa B ligand mediated osteoclastogenesis. Chin. Med. J. (Engl).. 2011;124:586-590.
- [Google Scholar]
- Ajulemic acid, a nonpsychoactive cannabinoid acid, suppresses osteoclastogenesis in mononuclear precursor cells and induces apoptosis in mature osteoclast-like cells. J. Cell Physiol.. 2008;214:714-720.
- [CrossRef] [Google Scholar]
- Beta-caryophyllene is a dietary cannabinoid. Proc. Natl. Acad. Sci. U. S. A.. 2008;105:9099-9104.
- [CrossRef] [Google Scholar]
- Humulus lupulus L. a potential precursor to human health: High hops craft beer. Food Chem.. 2023;405:134959
- [CrossRef] [Google Scholar]
- CB2 receptor in the CNS: From immune and neuronal modulation to behavior. Neurosci. Biobehav. Rev.. 2023;150:105226
- [CrossRef] [Google Scholar]
- HU-308: a specific agonist for CB(2), a peripheral cannabinoid receptor. Proc. Natl. Acad. Sci. U. S. A.. 1999;96:14228-14233.
- [CrossRef] [Google Scholar]
- A focused review on CB2 receptor-selective pharmacological properties and therapeutic potential of beta-caryophyllene, a dietary cannabinoid. Biomed. Pharmacother.. 2021;140:111639
- [CrossRef] [Google Scholar]
- p38 MAPK is involved in CB2 receptor-induced apoptosis of human leukaemia cells. FEBS Lett.. 2005;579:5084-5088.
- [CrossRef] [Google Scholar]
- Selective CB2 receptor agonists. Part 1: the identification of novel ligands through computer-aided drug design (CADD) approaches. Bioorg. Med. Chem. Lett.. 2015;25:575-580.
- [CrossRef] [Google Scholar]
- Celastrol: Progresses in structure-modifications, structure-activity relationships, pharmacology and toxicology. Eur. J. Med. Chem.. 2020;189:112081
- [CrossRef] [Google Scholar]
- Identification of novel cannabinoid CB2 receptor agonists from botanical compounds and preliminary evaluation of their anti-osteoporotic effects. Molecules. 2022;27
- [CrossRef] [Google Scholar]
- Activation and signaling mechanism revealed by cannabinoid receptor-G(i) complex structures. Cell. 2020;180:655-665.e618.
- [CrossRef] [Google Scholar]
- Cannabinoid receptor subtype 2 (CB2R) agonist, GW405833 reduces agonist-induced Ca(2+) oscillations in mouse pancreatic acinar cells. Sci. Rep.. 2016;6:29757.
- [CrossRef] [Google Scholar]
- 3-(1',1'-dimethylbutyl)-1-deoxy-delta8-THC and related compounds: synthesis of selective ligands for the CB2 receptor. Bioorg. Med. Chem.. 1999;7:2905-2914.
- [CrossRef] [Google Scholar]
- Assessment of the role of noni (Morinda citrifolia) juice for inducing osteoblast differentiation in isolated rat bone marrow derived mesenchymal stem cells. Int J Stem Cells.. 2016;9:221-229.
- [CrossRef] [Google Scholar]
- Magnolol inhibits osteoclast differentiation via suppression of RANKL expression. Molecules. 2018;23
- [CrossRef] [Google Scholar]
- Regulation of bone mass, osteoclast function, and ovariectomy-induced bone loss by the type 2 cannabinoid receptor. Endocrinology. 2008;149:5619-5626.
- [CrossRef] [Google Scholar]
- Celastrol is a novel selective agonist of cannabinoid receptor 2 with anti-inflammatory and anti-fibrotic activity in a mouse model of systemic sclerosis. Phytomedicine. 2020;67:153160
- [CrossRef] [Google Scholar]
- Johnson, M. and G. M. Maggiora, 1990. Concepts and applications of molecular similarity.
- The cannabinoid dexanabinol is an inhibitor of the nuclear factor-kappa B (NF-kappa B) Neuropharmacology. 2004;47:580-592.
- [CrossRef] [Google Scholar]
- Cyclolinopeptide F, a cyclic peptide from flaxseed inhibited RANKL-induced osteoclastogenesis via downergulation of RANK expression. J. Nat. Med.. 2019;73:504-512.
- [CrossRef] [Google Scholar]
- Liensinine and nuciferine, bioactive components of nelumbo nucifera, inhibit the growth of breast cancer cells and breast cancer-associated bone loss. Evid. Based Complement. Alternat. Med.. 2017;2017:1583185.
- [CrossRef] [Google Scholar]
- Implications of dietary alpha-linolenic acid in bone health. Nutrition. 2011;27:1101-1107.
- [CrossRef] [Google Scholar]
- Anti-inflammatory and anti-superbacterial activity of polyphenols isolated from black raspberry. Korean J. Physiol. Pharmacol.. 2013;17:73-79.
- [CrossRef] [Google Scholar]
- An examination of anthocyanins' and anthocyanidins' affinity for cannabinoid receptors. J. Med. Food. 2009;12:1407-1410.
- [CrossRef] [Google Scholar]
- Modulation of endocannabinoid tone in osteoblastic differentiation of MC3T3-E1 cells and in mouse bone tissue over time. Cells. 2021;10
- [CrossRef] [Google Scholar]
- In vitro opioid receptor affinity and in vivo behavioral studies of Nelumbo nucifera flower. J. Ethnopharmacol.. 2015;174:57-65.
- [CrossRef] [Google Scholar]
- A geometric approach to macromolecule-ligand interactions. J. Mol. Biol.. 1982;161:269-288.
- [CrossRef] [Google Scholar]
- Effect of magnolol on the function of osteoblastic MC3T3-E1 cells. Mediators Inflamm.. 2012;2012:829650
- [CrossRef] [Google Scholar]
- Cannabinoid CB2 receptor agonist activity in the hindpaw incision model of postoperative pain. Eur. J. Pharmacol.. 2005;527:172-174.
- [CrossRef] [Google Scholar]
- Therapeutic applications of compounds in the Magnolia family. Pharmacol. Ther.. 2011;130:157-176.
- [CrossRef] [Google Scholar]
- Effects of a Rubus coreanus Miquel supplement on plasma antioxidant capacity in healthy Korean men. Nutr. Res. Pract.. 2011;5:429-434.
- [CrossRef] [Google Scholar]
- Structural basis of selective cannabinoid CB(2) receptor activation. Nat. Commun.. 2023;14:1447.
- [CrossRef] [Google Scholar]
- Celastrol regulates bone marrow mesenchymal stem cell fate and bone-fat balance in osteoporosis and skeletal aging by inducing PGC-1alpha signaling. Aging (Albany NY). 2020;12:16887-16898.
- [CrossRef] [Google Scholar]
- Cannabidiol promotes osteogenic differentiation of bone marrow mesenchymal stem cells in the inflammatory microenvironment via the CB2-dependent p38 MAPK signaling pathway. Int. J. Stem Cells. 2022;15:405-414.
- [CrossRef] [Google Scholar]
- Crystal structure of the human cannabinoid receptor CB2. Cell. 2019;176:459-467.e413.
- [CrossRef] [Google Scholar]
- Targeting bone remodeling by isoflavone and 3,3'-diindolylmethane in the context of prostate cancer bone metastasis. PLoS One. 2012;7:e33011.
- [CrossRef] [Google Scholar]
- Effect of Morinda officinalis capsule on osteoporosis in ovariectomized rats. Chin. J. Nat. Med.. 2014;12:204-212.
- [CrossRef] [Google Scholar]
- Nrf2 is required for suppressing osteoclast RANKL-induced differentiation in RAW 264.7 cells via inactivating cannabinoid receptor type 2 with AM630. Regen. Ther.. 2020;14:191-195.
- [CrossRef] [Google Scholar]
- Rabbiteye blueberry prevents osteoporosis in ovariectomized rats. J. Orthop. Surg. Res.. 2014;9:56.
- [CrossRef] [Google Scholar]
- Stimulation of cannabinoid receptors by using Rubus coreanus extracts to control osteoporosis in aged male rats. Aging Male. 2015;18:124-132.
- [CrossRef] [Google Scholar]
- Betulinic acid targets YY1 and ErbB2 through cannabinoid receptor-dependent disruption of microRNA-27a:ZBTB10 in breast cancer. Mol. Cancer Ther.. 2012;11:1421-1431.
- [CrossRef] [Google Scholar]
- Celastrol, an NF-kappaB inhibitor, ameliorates hypercalciuria and articular cartilage lesions in a mouse model of secondary osteoporosis. J. Pharmacol. Sci.. 2016;130:204-211.
- [CrossRef] [Google Scholar]
- Establishment of a high throughput screening model of cannabinoid receptor 2 agonist. Xi Bao Yu Fen Zi Mian Yi Xue Za Zhi. 2012;28:194-196.
- [Google Scholar]
- Magnolol Inhibits RANKL-induced osteoclast differentiation of raw 264.7 macrophages through heme oxygenase-1-dependent inhibition of NFATc1 expression. J. Nat. Prod.. 2015;78:61-68.
- [CrossRef] [Google Scholar]
- LiCABEDS II. Modeling of ligand selectivity for G-protein-coupled cannabinoid receptors. J. Chem. Inf. Model.. 2013;53:11-26.
- [CrossRef] [Google Scholar]
- Flaxseed (linum usitatissimum) flour contributes to bone health in adult male rats. Nutrition. 2018;49:48-50.
- [CrossRef] [Google Scholar]
- Cannabinoid receptor 2-selective agonist JWH015 attenuates bone cancer pain through the amelioration of impaired autophagy flux induced by inflammatory mediators in the spinal cord. Mol. Med. Rep.. 2019;20:5100-5110.
- [CrossRef] [Google Scholar]
- Structure of a cannabinoid receptor and functional expression of the cloned cDNA. Nature. 1990;346:561-564.
- [CrossRef] [Google Scholar]
- The role of AOPP in age-related bone loss and the potential benefits of berry anthocyanins. Nutrients. 2017;9
- [CrossRef] [Google Scholar]
- Development and characterization of immobilized cannabinoid receptor (CB1/CB2) open tubular column for on-line screening. Anal. Biochem.. 2011;412:85-91.
- [CrossRef] [Google Scholar]
- Estimated costs of pivotal trials for novel therapeutic agents approved by the US Food and Drug Administration, 2015–2016. JAMA Intern. Med.. 2018;178:1451-1457.
- [CrossRef] [Google Scholar]
- Cannabinoid receptor 2 (CB2) agonists and antagonists: a patent update. Expert. Opin. Ther. Pat.. 2016;26:843-856.
- [CrossRef] [Google Scholar]
- Targeting CB2 and TRPV1: Computational approaches for the identification of dual modulators. Front. Mol. Biosci.. 2022;9:841190
- [CrossRef] [Google Scholar]
- Molecular characterization of a peripheral receptor for cannabinoids. Nature. 1993;365:61-65.
- [CrossRef] [Google Scholar]
- Cloning and pharmacological characterization of the dog cannabinoid CB(2)receptor. Eur. J. Pharmacol.. 2011;669:24-31.
- [CrossRef] [Google Scholar]
- North American Menopause, S., 2006. Management of osteoporosis in postmenopausal women: 2006 position statement of The North American Menopause Society. Menopause. 13, 340-367; quiz 368-349. Doi: 10.1097/01.gme.0000222475.93345.b3.
- Nunes-Alves, C., 2015. New tricks for old drugs. Nature Reviews Microbiology. 13, 68-68. Doi: 10.1038/nrmicro3421.
- Peripheral cannabinoid receptor, CB2, regulates bone mass. Proc. Natl. Acad. Sci. U. S. A.. 2006;103:696-701.
- [CrossRef] [Google Scholar]
- CB2 cannabinoid receptor targets mitogenic Gi protein-cyclin D1 axis in osteoblasts. J. Bone Miner. Res.. 2011;26:308-316.
- [CrossRef] [Google Scholar]
- Anti-inflammatory and osteoprotective effects of cannabinoid-2 receptor agonist HU-308 in a rat model of lipopolysaccharide-induced periodontitis. J. Periodontol.. 2016;87:725-734.
- [CrossRef] [Google Scholar]
- The effects of Morinda citrifolia L. (noni) on the immune system: its molecular mechanisms of action. J. Ethnopharmacol.. 2008;115:502-506.
- [CrossRef] [Google Scholar]
- Anthocyanins in chronic diseases: The power of purple. Nutrients. 2022;14
- [CrossRef] [Google Scholar]
- Role of the endocannabinoid/endovanilloid system in the modulation of osteoclast activity in paget's disease of bone. Int. J. Mol. Sci.. 2021;22
- [CrossRef] [Google Scholar]
- RANKL-induced osteoclastogenesis is suppressed by 4-O-methylhonokiol in bone marrow-derived macrophages. Arch. Pharm. Res.. 2017;40:933-942.
- [CrossRef] [Google Scholar]
- Paeonol alleviates primary dysmenorrhea in mice via activating CB2R in the uterus. Phytomedicine. 2020;68:153151
- [CrossRef] [Google Scholar]
- Piomelli, D., 2014. More surprises lying ahead. The endocannabinoids keep us guessing. Neuropharmacology. 76 Pt B, 228-234. Doi: 10.1016/j.neuropharm.2013.07.026.
- Developing pyridazine-3-carboxamides to be CB2 agonists: The design, synthesis, structure-activity relationships and docking studies. Eur. J. Med. Chem.. 2017;137:598-611.
- [CrossRef] [Google Scholar]
- Activation of cannabinoid receptor CB2 regulates osteogenic and osteoclastogenic gene expression in human periodontal ligament cells. J. Periodontal. Res.. 2010;45:504-511.
- [CrossRef] [Google Scholar]
- Type-2 cannabinoid receptor regulates proliferation, apoptosis, differentiation, and OPG/RANKL ratio of MC3T3-E1 cells exposed to Titanium particles. Mol. Cell. Biochem.. 2015;399:131-141.
- [CrossRef] [Google Scholar]
- Cellular and molecular mechanisms of bone remodeling. J. Biol. Chem.. 2010;285:25103-25108.
- [CrossRef] [Google Scholar]
- The cannabinoids effect on bone formation and bone healing. Curr. Osteoporos. Rep.. 2020;18:433-438.
- [CrossRef] [Google Scholar]
- Current treatments and new developments in the management of glucocorticoid-induced osteoporosis. Drugs. 2019;79:1065-1087.
- [CrossRef] [Google Scholar]
- Magnolia extract, magnolol, and metabolites: Activation of cannabinoid CB2 receptors and blockade of the related GPR55. ACS Med. Chem. Lett.. 2013;4:41-45.
- [CrossRef] [Google Scholar]
- Incorporation of flaxseed flour as a dietary source for ALA increases bone density and strength in post-partum female rats. Lipids. 2017;52:327-333.
- [CrossRef] [Google Scholar]
- New pharmacological opportunities for betulinic acid. Planta Med.. 2018;84:8-19.
- [CrossRef] [Google Scholar]
- In silico target fishing for rationalized ligand discovery exemplified on constituents of Ruta graveolens. Planta Med.. 2009;75:195-204.
- [CrossRef] [Google Scholar]
- Synthesis, binding assays, cytotoxic activity and docking studies of benzimidazole and benzothiophene derivatives with selective affinity for the CB2 cannabinoid receptor. Eur. J. Med. Chem.. 2016;124:17-35.
- [CrossRef] [Google Scholar]
- Agonist-inverse agonist characterization at CB1 and CB2 cannabinoid receptors of L759633, L759656, and AM630. Br. J. Pharmacol.. 1999;126:665-672.
- [CrossRef] [Google Scholar]
- The 17-beta-oestradiol inhibits osteoclast activity by increasing the cannabinoid CB2 receptor expression. Pharmacol. Res.. 2013;68:7-15.
- [CrossRef] [Google Scholar]
- The endocannabinoid/endovanilloid system in bone: From osteoporosis to osteosarcoma. Int. J. Mol. Sci.. 2019;20
- [CrossRef] [Google Scholar]
- Alteration of osteoclast activity in childhood cancer survivors: Role of iron and of CB2/TRPV1 receptors. PLoS One. 2022;17:e0271730.
- [Google Scholar]
- Synthon-based ligand discovery in virtual libraries of over 11 billion compounds. Nature. 2022;601:452-459.
- [CrossRef] [Google Scholar]
- The role of cannabinoids in bone metabolism: A new perspective for bone disorders. Int. J. Mol. Sci.. 2021;22
- [CrossRef] [Google Scholar]
- Biochanin A: A novel bioactive multifunctional compound from nature. Sci. Total Environ.. 2020;722:137907
- [CrossRef] [Google Scholar]
- Mechanisms of osteoclastogenesis inhibition by a novel class of biphenyl-type cannabinoid CB(2) receptor inverse agonists. Chem. Biol.. 2011;18:1053-1064.
- [CrossRef] [Google Scholar]
- Protective effects of trans-caryophyllene on maintaining osteoblast function. IUBMB Life. 2017;69:22-29.
- [CrossRef] [Google Scholar]
- Epigenetic regulation of bone remodeling and bone metastasis. Semin. Cell Dev. Biol.. 2024;154:275-285.
- [CrossRef] [Google Scholar]
- Flaxseed for health and disease: Review of clinical trials. Comb. Chem. High Throughput. Screen.. 2020;23:699-722.
- [CrossRef] [Google Scholar]
- Polyphenols extract from lotus seedpod (Nelumbo nucifera Gaertn.): Phenolic compositions, antioxidant, and antiproliferative activities. Food Sci. Nutr.. 2019;7:3062-3070.
- [CrossRef] [Google Scholar]
- CB2 cannabinoid receptor agonist enantiomers HU-433 and HU-308: An inverse relationship between binding affinity and biological potency. Proc. Natl. Acad. Sci. U. S. A.. 2015;112:8774-8779.
- [CrossRef] [Google Scholar]
- Combined deficiency of the Cnr1 and Cnr2 receptors protects against age-related bone loss by osteoclast inhibition. Aging Cell. 2017;16:1051-1061.
- [CrossRef] [Google Scholar]
- Cannabinoids and pain: Sites and mechanisms of action. Adv. Pharmacol.. 2017;80:437-475.
- [CrossRef] [Google Scholar]
- Steinmueller, S. A. M., J. Fender, M. H. Deventer, et al., 2023. Visible-Light Photoswitchable Benzimidazole Azo-Arenes as beta-Arrestin2-Biased Selective Cannabinoid 2 Receptor Agonists. Angew Chem Int Ed Engl. e202306176. Doi: 10.1002/anie.202306176.
- Cannabinoid-like anti-inflammatory compounds from flax fiber. Cell Mol. Biol. Lett.. 2012;17:479-499.
- [CrossRef] [Google Scholar]
- The preventive effect of biochanin a on bone loss in ovariectomized rats: involvement in regulation of growth and activity of osteoblasts and osteoclasts. Evid. Based Complement. Alternat. Med.. 2013;2013:594857
- [CrossRef] [Google Scholar]
- Biochanin A, a naturally occurring inhibitor of fatty acid amide hydrolase. Br. J. Pharmacol.. 2010;160:549-560.
- [CrossRef] [Google Scholar]
- Exploring the effectiveness of novel benzimidazoles as CB2 ligands: synthesis, biological evaluation, molecular docking studies and ADMET prediction. Medchemcomm. 2018;9:2045-2054.
- [CrossRef] [Google Scholar]
- The role of cannabinoid receptor type 2 in the bone loss associated with pediatric celiac disease. J. Pediatr. Gastroenterol. Nutr.. 2020;71:633-640.
- [CrossRef] [Google Scholar]
- Cannabinoid CB2/CB1 selectivity. Receptor modeling and automated docking analysis. J. Med. Chem.. 2006;49:984-994.
- [CrossRef] [Google Scholar]
- Effect of Nelumbo nucifera petal extracts on lipase, adipogenesis, adipolysis, and central receptors of obesity. Evid. Based Complement. Alternat. Med.. 2013;2013:145925
- [CrossRef] [Google Scholar]
- 3,3'-diindolylmethane exhibits significant metabolism after oral dosing in humans. Drug Metab. Dispos.. 2021;49:694-705.
- [CrossRef] [Google Scholar]
- A pharmacoinformatic approach on Cannabinoid receptor 2 (CB2) and different small molecules: Homology modelling, molecular docking, MD simulations, drug designing and ADME analysis. Comput. Biol. Chem.. 2019;78:95-107.
- [CrossRef] [Google Scholar]
- Selective CB2 receptor agonism protects central neurons from remote axotomy-induced apoptosis through the PI3K/Akt pathway. J. Neurosci.. 2009;29:4564-4570.
- [CrossRef] [Google Scholar]
- Black cohosh (Cimicifuga racemosa [L.] Nutt.): safety and efficacy for cancer patients. Support Care Cancer. 2007;15:913-921.
- [CrossRef] [Google Scholar]
- Identification of novel antagonists targeting cannabinoid receptor 2 using a multi-step virtual screening strategy. Molecules. 2021;26
- [CrossRef] [Google Scholar]
- Alkaloids from lotus (Nelumbo nucifera): recent advances in biosynthesis, pharmacokinetics, bioactivity, safety, and industrial applications. Crit. Rev. Food Sci. Nutr.. 2023;63:4867-4900.
- [CrossRef] [Google Scholar]
- Morinda citrifolia (Noni): a literature review and recent advances in Noni research. Acta Pharmacol. Sin.. 2002;23:1127-1141.
- [Google Scholar]
- Wattanathorn, J., C. Thipkaew, W. Thukham-Mee, et al., 2018. Morinda citrifolia L. Leaf Extract Protects against Cerebral Ischemia and Osteoporosis in an In Vivo Experimental Model of Menopause. Oxid Med Cell Longev. 2018, 1039364. Doi: 10.1155/2018/1039364.
- Betulinic acid protects from bone loss in ovariectomized mice and suppresses RANKL-associated osteoclastogenesis by inhibiting the MAPK and NFATc1 pathways. Front. Pharmacol.. 2020;11:1025.
- [CrossRef] [Google Scholar]
- Celastrol inhibits glucocorticoid-induced osteoporosis in rat via the PI3K/AKT and Wnt signaling pathways. Mol. Med. Rep.. 2018;18:4753-4759.
- [CrossRef] [Google Scholar]
- Cryo-EM structure of the human cannabinoid receptor CB2-G(i) signaling complex. Cell. 2020;180:645-654.e613.
- [CrossRef] [Google Scholar]
- Celastrol Attenuates RANKL-induced osteoclastogenesis in vitro and reduces titanium particle-induced osteolysis and ovariectomy-induced bone loss in vivo. Front. Pharmacol.. 2021;12:682541
- [CrossRef] [Google Scholar]
- Celastrol in metabolic diseases: Progress and application prospects. Pharmacol. Res.. 2021;167:105572
- [CrossRef] [Google Scholar]
- Activation of cannabinoid receptor type 2-induced osteogenic differentiation involves autophagy induction and p62-mediated Nrf2 deactivation. Cell Commun. Signal.. 2020;18:9.
- [CrossRef] [Google Scholar]
- Echinacea in hepatopathy: A review of its phytochemistry, pharmacology, and safety. Phytomedicine. 2021;87:153572
- [CrossRef] [Google Scholar]
- beta-Caryophyllene promotes osteoblastic mineralization, and suppresses osteoclastogenesis and adipogenesis in mouse bone marrow cultures in vitro. Exp. Ther. Med.. 2016;12:3602-3606.
- [CrossRef] [Google Scholar]
- Binding modes and selectivity of cannabinoid 1 (CB1) and cannabinoid 2 (CB2) receptor ligands. ACS Chem. Neurosci.. 2020;11:3455-3463.
- [CrossRef] [Google Scholar]
- Lipid G protein-coupled receptor ligand identification using beta-arrestin PathHunter assay. J. Biol. Chem.. 2009;284:12328-12338.
- [CrossRef] [Google Scholar]
- 3,3'-Diindolylmethane increases bone mass by suppressing osteoclastic bone resorption in mice. J. Pharmacol. Sci.. 2015;127:75-82.
- [CrossRef] [Google Scholar]
- In silico prediction and validation of CB2 allosteric binding sites to aid the design of allosteric modulators. Molecules. 2022;27
- [CrossRef] [Google Scholar]
- Insights on the multifunctional activities of magnolol. Biomed. Res. Int.. 2019;2019:1847130.
- [CrossRef] [Google Scholar]
- Identification of novel phytocannabinoids from Ganoderma by label-free dynamic mass redistribution assay. J. Ethnopharmacol.. 2020;246:112218
- [CrossRef] [Google Scholar]
- Reliable prediction of cannabinoid receptor 2 ligand by machine learning based on combined fingerprints. Comput. Biol. Med.. 2023;152:106379
- [CrossRef] [Google Scholar]
- Cannabinoid receptors and the endocannabinoid system: Signaling and function in the central nervous system. Int. J. Mol. Sci.. 2018;19
- [CrossRef] [Google Scholar]







