Translate this page into:
Catalytic conversion of greenhouse gases (CO2 and CH4) to syngas over Ni-based catalyst: Effects of Ce-La promoters
⁎Corresponding author at: Chemical Engineering Department, Universiti Teknologi PETRONAS, 32610 Seri Iskandar, Malaysia. bawadi_abdullah@utp.edu.my (Bawadi Abdullah) bawadi73@gmail.com (Bawadi Abdullah)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
Dry reforming of methane (DRM) is an emerging technology as it can simultaneously serve as a prospective alternative energy source and mitigate greenhouse gases (e.g. CH4 and CO2). However, the industrial applications of DRM remain restricted due to the poor prospect of catalyst deactivation. In this study, the effects of adding CeO2 and La2O3 as promoters on the catalytic performance of Ni/Al2O3 catalyst were assessed. Catalysts such as Ni/Al2O3, Ni/Al2O3-La2O3, and Ni/Al2O3-CeO2 were synthesized at nano level using the sol-gel method. Citric acid was added to improve the reactivity of catalysts before the application of DRM. Various characterisation techniques were used to characterise synthesized catalysts, including Brunauer-Emmett-Teller (BET) analysis, temperature-programmed reduction (TPR), field emission scanning microscopy (FESEM), X-ray diffraction (XRD), and transmission electron microscopy (TEM). The results revealed that the BET surface area of the synthesized catalyst slightly decreased when CeO2 and La2O3 were added due to the deposition on the porous structure of the support. Meanwhile, the XRD results demonstrated the increase in reducibility and dispersion of Ni using CeO2 promoter and the inhibited development of the non-active phase of Ni/Al2O3 using La2O3 promoter (i.e. NiAl2O4), resulting in the carbon formation and reduced efficiency of the catalyst. The catalytic performance in DRM at 800 °C showed that Ni/Al2O3-CeO2 catalyst exhibited higher catalytic performance in terms of CH4 and CO2 conversion with 89.6% and 91.2% respectively. While Ni/Al2O3-La2O3 was found to play a substantial role in the stability of the chemical reaction during the 8 h reaction time-on-stream.
Keywords
Syngas
Reforming
Catalyst
Greenhouse gases
Promoters
1 Introduction
An increase of 30% in the global consumption of energy is expected by 2040 (Kargbo et al., 2010). Although renewable (e.g. solar and wind) and alternative (e.g. hydrogen cell) energy resources have recently become popular, fossil fuels like natural gas, oil, and coal still play a substantial role in meeting the global energy demands. The energy generation from fossil fuels release a significant amount of greenhouse gases (GHGs), which mainly comprise of carbon dioxide (CO2) and methane (CH4); thus, contributing to global warming and other adverse environmental changes (Mozammel, 2017; Oyama et al., 2012). There have been numerous attempts to minimise the emission of CO2 and CH4 in order to prevent global warming. The transformation of methane gas into synthesis gas (a mixture of H2 and CO) that can be conveniently processed to produce chemicals and fuels is one of the significant means to address these environmental issues (Djinović et al., 2012; Tao et al., 2014; Xu et al., 2009a; Zhao et al., 2017).
Accordingly, the composition of synthesis gas largely depends on the synthesis process and the composition of the raw materials. The syngas can be formed from a range of main fossil fuels, such as coal, petroleum coke, and natural gas (Abdullah et al., 2017; Ghoneim et al., 2016). However, the cheapest and most environmentally friendly methods to produce syngas are typically based on natural gas (Ghoneim et al., 2016; Fayaz et al., 2016). The reforming process is one of the most familiar methods used at the industrial scale to produce syngas, such as (1) partial oxidation of methane (POM), (2) dry reforming of methane (DRM), and (3) steam reforming of methane (SRM) (Jang et al., 2013). Among these three reforming processes, the catalytic SRM reaction is the most prevalent and conventional route to produce syngas (De Freitas Silva et al., 2013; Kim et al., 2015; Nieva et al., 2014; Wu et al., 2013). However, the main setbacks of this process include a higher ratio of H2 and CO (i.e. >3) and a large amount of undesirable production of CO2. Additionally, SRM requires intensive energy input due to its endothermic reaction and exorbitant in nature (Guo et al., 2004). Meanwhile, POM can generate syngas when the ratio between H2 and CO equals to 2 but the existence of hot areas and excessive reactivity make the handling process not easy (Xu et al., 2013). In addition, DRM has the capacity to handle two types of GHGs, specifically CO2 and CH4, for syngas production. The advantage of DRM for syngas production lies ideally in one molar ratio of H2 and CO, which is beneficial for the Fischer-Tropsch process (Guo et al., 2004; Wang et al., 1996, 2018b).
The utilisation of a catalyst is vital during the reforming process of CH4 for complete conversion of the reactants. Numerous past studies focused on improving the catalytic performance, such as the activity and stability of a catalyst, to support the DRM process. Nearly all such practices focus on overcoming the catalyst deactivation problem. The primary reason for the catalyst deactivation problem lies in the occurrence of coke deposition because, at higher temperature of reaction (Bartholomew, 2001; Zhang et al., 1996), the carbon formed covers the active sites of the catalyst (Abd Ghani et al., 2019; Siang et al., 2018b). Non-noble metals such as nickel- and cobalt-based catalysts have been widely used for the DRM reaction due to their high selectivity, catalytic activity, and low carbon formation (Al-Swai et al., 2019; Bian et al., 2017; Bradford and Vannice, 1999; Selvarajah et al., 2016; Siang et al., 2018a; Stroud et al., 2018; Xu et al., 2009b). Hence, numerous efforts have been made to acquire improved synthesis and design of stable Ni-based catalyst with high carbon resistance. One of the effective and practical strategy to overcome catalyst deactivation is utilizing nickel with the addition of support having strong Lewis basicity such as La2O3, CaO, and MgO (Al-Swai et al., 2019; Usman et al., 2015).
Al2O3 is the most suitable support for the most catalytic materials. But due to the acidic nature of Al2O3, it enhances the coke deposition which effects the catalyst deactivation (Wang et al., 2018a). Mo-promoted Ni/Al2O3 catalyst for DRM caused a considerable reduction in the catalytic activity compared to the non-promoted Ni/Al2O3 catalyst that showed high catalytic activity as reported by (Yao et al., 2017). This was primarily due to set up of a distinct Nio phase that disconnected from Mo and hence decreased the overall basicity of the catalyst. Meanwhile, the addition of La2O3 into Ni-Al catalyst can be beneficial as it inhibits the coke formation during the reaction of DRM and reduces the support acidity, which favour the chemisorption and dissociation of carbon dioxide and eliminate carbon via reverse Boudouard reaction (Al-Fatesh et al., 2014). Furthermore, the dispersion of La2O3 over Al2O3 and Ni crystallites prevents the growth of Ni grains at higher temperature, which minimises the sintering of catalyst due to the formation of La2O2CO3 (Charisiou et al., 2019). On the other hand, Ceria exhibits the characteristics of releasing and storing oxygen. As a result, the reduction of Ce4+ to Ce3+ leads to the formation of oxygen vacancies (Yao et al., 2018). The release of oxygen occurs in oxygen-poor medium whereas re-oxidation occurs in oxygen-rich medium (Xu et al., 1999). The effects of adding ceria (CeO2) doped with Pr, Zr and Nb to Pt/Al2O3 catalyst were evaluated by (da Fonseca et al., 2020) and found that there was a substantial increase in catalytic activity on doping the catalyst. The Pr exhibited maximum reducibility of ceria (23%) and Pt/CePr/Al2O3 displayed the paramount activity and stability. (Luisetto et al., 2015) studied the DRM reaction using different supports, specifically CeO2, MgO, and mixed oxide CeO2-MgO, on Ni/Al2O3 catalyst and proposed a lower temperature of reaction for improved Ni distribution.
In view of the above, finding a new catalyst that can resist the deposition of carbon using suitable support and promoter is vital in DRM. In the present study, the effects of Ce and La promoters on the physiochemical properties of Ni/Al2O3 catalysts, synthesized using the sol-gel method, were compared. The catalysts were characterized to describe the effects of Ce and La promoters on the catalyst performance.
2 Materials and methods
2.1 Materials
For the preparation of catalysts in the present study, Ni (NO3)2·6H2O served as the source of an active metal and Al (NO3)3·9H2O served as the source of catalyst support. In addition, La (NO3)3·6H2O and Ce (NO3)3·6H2O were utilised as catalyst promoters. Besides that, citric acid was utilised as the chelating agent. The gases for the reduction method before the reaction were H2 and N2, while CH4, CO2, and N2 were utilised during the test reaction.
2.2 Nano-catalyst synthesis
The sol-gel method was applied to produce Ni-based catalysts. There were various materials and reaction conditions involved. Typically, the stoichiometric amount of active metal, support, promoters, and citric acid were first mixed and dissolved in DI water. Ammonium hydroxide solution (28 vol.%) was added dropwise with continuous stirring to adjust the pH around 9–9.5. Following that, the solution was heated on the warm plate at 80 °C and continuously stirred for 6 h until the gel was formed. The gel was subsequently placed in an oven at 393 K for 12 h before it was calcined in static air for 5 h at 900 °C. The produced catalysts Ni/Al2O3, Ni/Al2O3-CeO2, Ni/Al2O3-La2O3 were donated as CAT-1, CAT-2 and CAT-3 respectively and their weight composition are presented in Table 1. As shown in the table, the nickel loading was 10 wt% whereas CeO2 and La2O3 promoters were 10 wt%.
Catalyst labelling
Catalyst
Support/Promoter
Composition (wt.%)
CAT-1
Ni/Al2O3
Al2O3/NA
Ni: 10%, Al2O3: 90%
CAT-2
Ni/Al2O3-CeO2
Al2O3/CeO2
Ni: 10%, Al2O3: 80%, CeO2: 10%
CAT-3
Ni/Al2O3-La2O3
Al2O3/La2O3
Ni: 10%, Al2O3: 80%, La2O3: 10%
2.3 Characterisation of catalyst
Surface area analysis, specifically Brunauer-Emmett-Teller (BET) analysis, was conducted using Micromeritics ASAP 2020 to measure the surface area of catalytic materials (by N2 adsorption). The specific surface areas and the pore size distribution were calculated using Brunauer–Emmett–Teller (BET) and Barrett–Joyner–Halenda (BJH) methods, respectively. Additionally, the reducibility of the catalysts was studied using Thermo Scientific TPD/R/O (1100). For this study, 50 mg catalyst was heated in a reactor with N2 flow of 20 mL min1 at 250 °C for 1 h. After the catalyst was degassed by injecting hydrogen flow (5% in N2), it was heated from 40 °C to 1000 °C. The basicity of the catalysts was measured using the same equipment of H2-TPR. Similarly, the materials were preheated for 1 h at 500℃, followed by cooling down at 80 °C. For the desorption of CO2, 10% CO2/He was injected for 1 h. Following that, pure N2 was injected to desorb CO2 for 15 min. The CO2 desorption was recorded while the samples were further heated to 800 °C at a constant rate of 10 °C min−1. Besides that, field emission scanning electron microscopy (FESEM) analysis was conducted using Zeiss Supra55 VP to obtain the topography of the surface of catalyst. Its topography provides information about the surface features in terms of surface irregularities and the size, shape, and distribution of particles and the elemental composition and internal structure of the catalyst. Apart from that, the XRD technique was applied in this study to characterise the calcined mixed oxide catalysts. Advance X-ray diffractometer (Bruker D8B) with a range of between 10° and 90° and step size of 0.04° per step was utilised. Phases existing in the samples were identified by comparison with reference patterns in the ICCD database.
2.4 Experimental setup of DRM
The DRM reaction was recorded in a tube furnace-based reactor according to specific procedures. In particular, a 20 mg catalyst sample, which was sandwiched between quartz wool layers, was placed in the tube reactor. The bed was then positioned at the desired location within the reactor. Following that, N2 gas was injected at flow rate of 20 mL min−1 for 30 min to evacuate any air in the reactor. As the temperature was increased up to 800 °C, a mixture of H2/N2 gas was injected at a rate of 30/30 mL min−1. The reduction was carried out for 1 h to activate the catalyst. Subsequently, in order to remove H2 from the reactor, it was purged again for 15 min with a flow rate of 20 mL min−1. At the same time, the temperature was reduced to 650 °C and increased to 800 °C for 15 min. Once the temperature recorded 800 °C, N2, CO2, and CH4 gases were purged into the reactor at a ratio of 1:1:1 for about 8 h with a flow rate 60 mL min−1. Meanwhile, the volume fraction of the product stream was processed using an online gas chromatograph (GC). The percentage of reactant conversions, Xi (i denotes CH4 and CO2), and the syngas ratio (H2:CO) were estimated based on the following equations:
3 Results and discussion
3.1 Textural properties
Table 2 presents the BET surface area of the prepared catalysts. Overall, CAT-1 (the catalyst without any promoter) recorded the highest BET surface area measured due to its alumina trait. An area of 134.1 m2 g−1 was recorded. Besides that, the BET surface area decreased when CeO2 and La2O3 promoters were introduced, with the values of 126.9 m2 g−1 and 123.6 m2 g−1, respectively. The decrease in the BET surface area can be explained by the addition of CeO2 and La2O3 that cover alumina pores (De Freitas Silva et al., 2013). Moreover, there was a small difference in the BET values with the addition of promoters. Likewise, CAT-1 also recorded the highest average pore size (8.5 nm) and pore volume (0.27 cm3/g). However, these values decreased with the addition of CeO2 and La2O3 promoters due to the blockages of the pores.
Catalyst
BET surface area (m2/g)
Average pore size (nm)
Pore volume (cm3/g)
Average crystal sizea (nm)
CAT-1
134.1
8.5
0.27
4.35
CAT-2
126.9
6.2
0.25
2.41
CAT-3
123.6
6.1
0.22
7.86
Fig. 1(a) shows the isotherms of the N2 adsorption-desorption associated with the catalysts 1 (a), they are characterized as of group IV isotherm that represents materials of mesoporous (2–50 nm) characteristics, which are in accordance to IUPAC (Donohue and Aranovich, 1998). Moreover, the adsorption/desorption hysteresis loops took place within the range of P/Po, 0.60–0.90 and belong to H1 hysteresis loop. In other words, the catalysts contain highly uniform mesoporous channels and have agglomerates of approximately uniform spheres (Donohue and Aranovich, 1998). Referring to Fig. 1(b), the pore size distribution of the synthesized catalysts shows a relatively narrow distribution with the peak values of around 10 nm for CAT-1 and 8 nm for CAT-2 and CAT-3. With the addition of these promoters, the pore size of the catalysts reduced due to the partial blockage of the pores.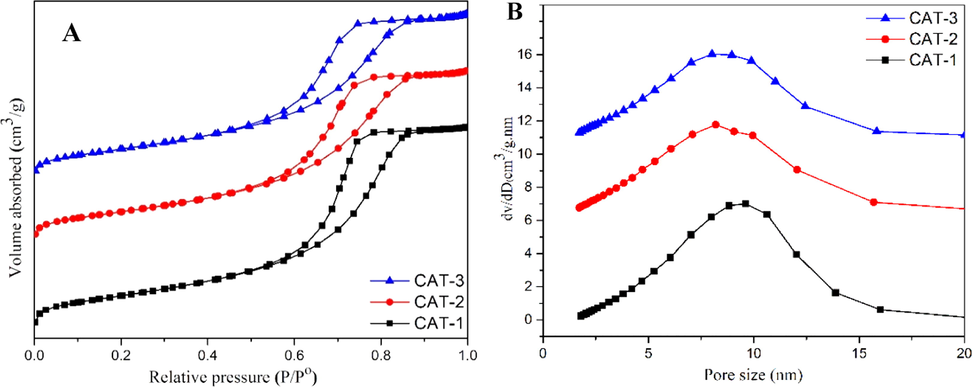
(a) N2 adsorption-desorption isotherm curves and (b) pore size distribution of the prepared catalysts.
3.2 XRD
Fig. 2 presents the XRD pattern of Ni/Al2O3 calcined catalysts with and without promoters. The pattern of the three prepared catalysts was recorded within the scan range of between 10° and 90°. Furthermore, XRD results revealed the presence of Al2O3, NiAl2O4, and CeO2 crystalline phases. However, crystalline peaks associated with Ni or NiO were found not visible due to the presence of small Ni particles and high dispersion on the support surface (Le Saché et al., 2018).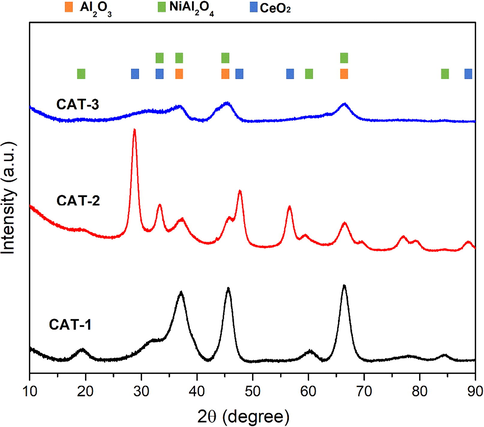
XRD analysis of calcined samples.
Meanwhile, diffraction peaks of alumina, Al2O3 (JCPDS 00-010-0339), appeared at (2θ) 37.2°, 45.4°, and 66.4° and corresponded to the respective index of 311, 400, and 440. The peaks due to the NiAl2O4 can be seen at 18.9°, 33.3°, 37.2ο, 45.5ο, 60.3°, 66.4ο and 84.4°; however, there was an overlap of that of Alumina peaks and NiAl2O4 as reported in a previous study (Le Saché et al., 2018). The coexistence of Al2O3 and NiAl2O4 phases indicate the inadequacy of 10 wt% Ni to transfer the gamma phase of alumina into NiAl2O4 (Penkova et al., 2011). Rahbar Shamskar et al. (2017) showed that NiO peaks were absent because of the strong interaction between NiO and Al2O3 which also indicates the high dispersion of NiO. CAT-2 with CeO2 revealed peaks at (2θ) 28.7°, 33.3°, 47.6°, 56.5° and 88.6ο (JCPDS 00-004-0593), which indicate the fluorite structure of Ceria. On the other hand, the diffractogram of CAT-3 with La2O3 demonstrated the least structure crystallinity, which indicates that La2O3 is amorphous and has a small reflection at peaks that are the same to that of Al2O3.
Table 2 presents the average crystal sizes of the catalysts that were calculated using the Debye–Scherrer equation. The average crystal size of NiO was estimated using the main peaks of NiAl2O4 as it is difficult to determine the crystal size of NiO due to the formation of NiAl2O4 spinal structure. The values thus signify CeO2 in lowering the crystal size of the catalyst, which indicates the higher dispersion of Ni on the surface of support.
3.3 Morphological analysis
As shown in Table 2, CAT-1 demonstrated high irregularity and more porous structure that supports the high surface area of Ni/Al2O3 catalyst. Meanwhile, CAT-2 demonstrated regular and uniform morphology, which indicate the distribution of Ceria on the surface of catalyst. The presence of CeO2 appears to obstruct the formation of NiAl2O4 and facilitates the dispersion of Ni on the support surface. On the other hand, CAT-3 with La2O3 demonstrated some irregular shapes that may be due to the growth of irregular crystalline grains during the synthesis (Aghamohammadi et al., 2017).
Fig. 3 presents the FESEM images and EDX results of the prepared catalysts. In particular, the EDX results reflect the elemental composition of catalysts. The amount of Ni found on the surface of catalyst increased with the addition of CeO2, which shows that Ce facilitates the dispersion of Ni and increases the weight percentage. The value increased from 12.73 wt% (CAT-1) to 15.05 wt% (CAT-2) as CeO2 was added as a promoter. Similarly, there was an increase of 14.43 wt% (CAT-3) when La2O3 was added as a promoter.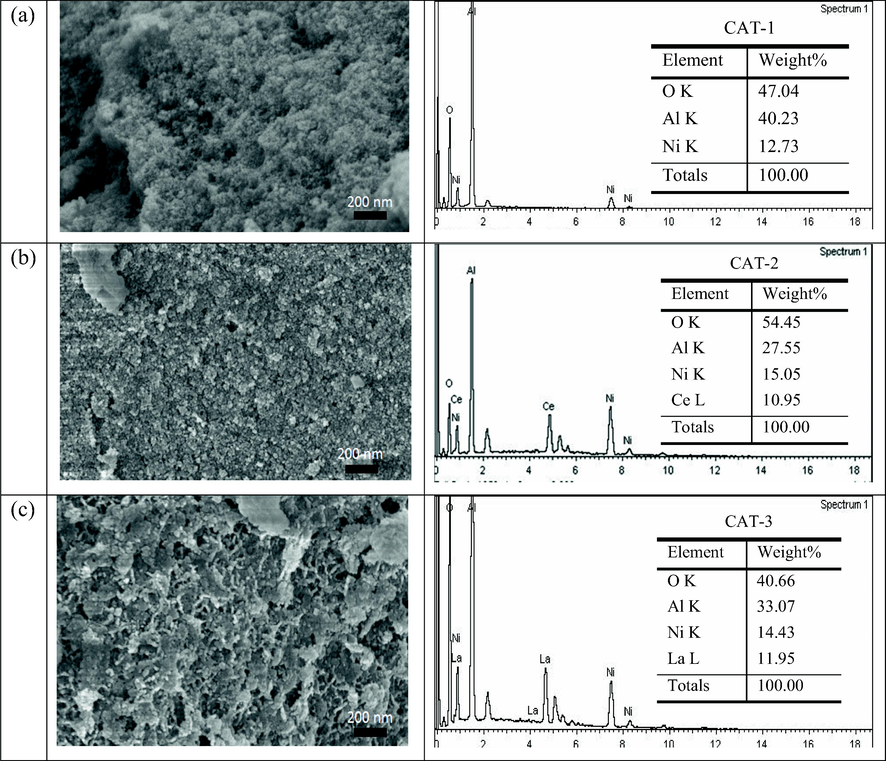
FESEM images and EDX graphs of pre reaction catalyst at 50.0 K× magnification.
3.4 TEM analysis
Fig. 4 shows the transmission electron microscope (TEM) images of the three synthesized catalysts. Two spots were detected—the presence of alumina causes the observed difference and the presence of Ni metal species causes the dark contrast (Kim et al., 2015). In particular, CAT-1 exhibited larger particle size with seemingly round shape particles whereas CAT-2 demonstrated higher dispersion of Ni particles. The clear particle agglomeration in Fig. 4(c) supported the FESEM results in Fig. 3(c).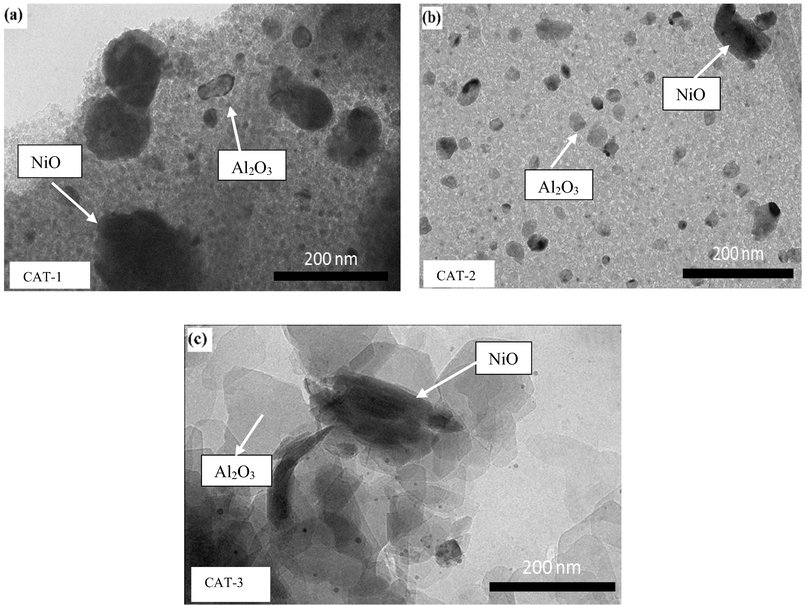
TEM micrographs of calcined samples.
3.5 H2-TPR
Fig. 5 presents the TPR plots for the three prepared catalysts, which revealed that NiO was reduced to metallic Ni with obvious peak differences when the temperature achieved below 850 °C. Unlike the case of Ni/Al2O3, both promoted catalysts showed higher peaks of hydrogen consumption. CeO2 and La2O3 are structural promoters that limit the aggregation of Ni particles during the reduction of H2 (Jiang et al., 2003).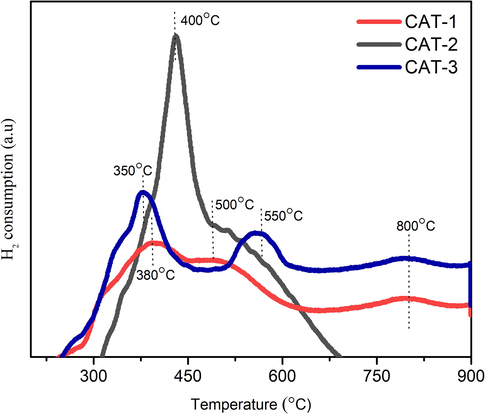
TPR analysis of calcined samples.
Meanwhile, the peaks at lower temperature (300–450 °C) can be attributed to the reduction of Ni that weakly interacts with the support. Referring to the TPR plots, CAT-1 revealed a small peak whereas striking peaks appeared for CAT-2 and CAT-3 (Farooqi et al., 2020). In other words, CeO2 and La2O3 improved the reduction of Ni.
Besides that, CAT-2 demonstrated the largest reduction peak for NiO at around 400 °C, which suggests that the presence of CeO2 did facilitate the dispersion of a large fraction of NiO species that are weakly attached to the support. The second peak for both CAT-1 and CAT-2 at 500 °C and CAT-3 at 550 °C can be ascribed to the reduction of Ni that is moderately attached to the support. Furthermore, the peak reduction appeared to be evident at 800 °C for CAT-1, which corresponded to the reduction of Ni with a strong bond formed between Ni and the support during the formation of Ni/Al2O3,(De Freitas Silva et al., 2013).
3.6 CO2-TPD
The basic sites present locations where the adsorbed CO2 has been desorbed. Generally, the Lewis alkaline sites are based on the desorption temperature of CO2: (1) less than 200 °C: weak; (2) between 200 °C and 400 °C: medium; (3) between 400 °C and 600 °C: strong; (4) more than 600 °C: very strong. Fig. 6 presents the CO2-TPD spectra of catalysts CAT-1, CAT-2, and CAT-3. Overall, the CO2-TPD profile for all catalysts appeared to be very similar in shape, as the main peak was stretched within the range of between 600 °C and 900 °C.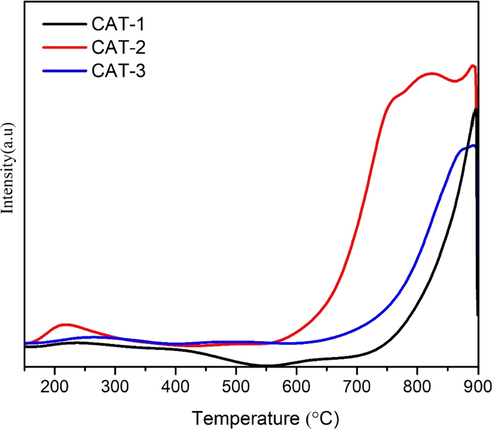
CO2-TPD analysis of calcined samples.
The first peak at the range of between 150 °C and 300 °C can be attributed to the weakly adsorbed CO2 on the basic sites of the surface of catalysts. The main peak of the CO2-TPD profile was centered at 800 °C for CAT-2 and 850 °C for CAT-1, which may be due to desorption of CO2 that strongly attached to the basic sites (Xu et al., 2017). Meanwhile, CO2 desorption peak appeared to be higher for CAT-2 with CeO2 which is expected to exhibit strong resistance against deactivation considering that catalysts with higher basic sites can reduce coke formation and prolong the activity of the catalysts (Radlik et al., 2015).
3.7 Catalytic methane dry reforming evaluation
It is well known that the extent of a chemical reaction only depends on the thermodynamics of the reaction, and catalyst could only change the rate of the reaction. Fig. 7 presents the occurrence of DRM reaction at 800 °C for 8 h. The conversion of CH4 and the perceptible influence of CeO2 and La2O3 on the catalytic performance can be observed in Fig. 7(a). In particular, CAT-1 and CAT-3 recorded the same initial conversion of 75%. The conversion of CH4 subsequently dropped to 64% after 8 h of reaction. CAT-2 with La2O3 seemed to maintain a relatively stable conversion with a conversion of 67% after 8 h. Noticeably, La2O3, as a promoter, only influences the stability of catalyst (not its activity). Furthermore, Al-Fatesh et al. (2014) previously demonstrated that La2O3, as a promoter, can accomplish a dual role in dry reforming reaction—it can restrict the disposition of carbon and reduce the acidity of the support. With that, the formation of pyrolytic carbon can be stopped since it favours the dissociation of CO2. These characteristics accelerate the formation of carbon through the reverse Boudouard reaction (BR).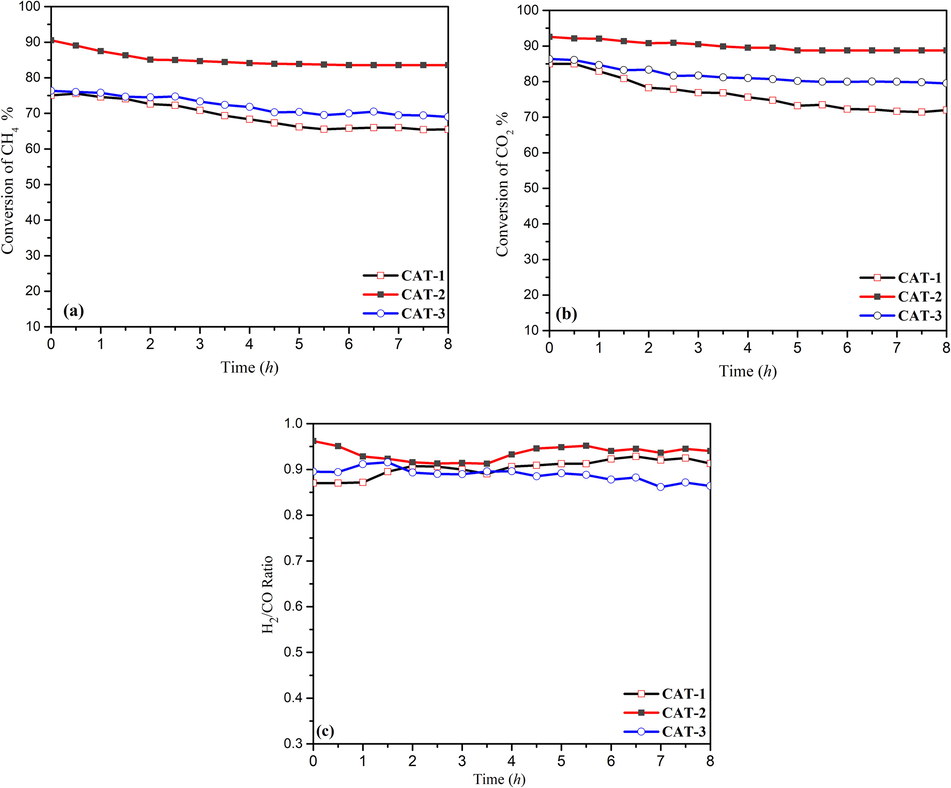
(a) Conversion of CH4, (b) Conversion of CO2 and (c) syngas ratio (H2/CO) at 800 °C.
In contrast, CAT-2 recorded the highest conversion with an initial conversion of 88%. Besides that, the catalyst demonstrated prominent stability for 8 h. This can be associated with the properties of catalyst, as demonstrated by H2-TPR (cf. Fig. 5) and CO2-TPD (Fig. 6). Furthermore, CeO2 is known as a promoter that facilitates or improves the dispersion of the active species on the surface of catalyst, as compared to the mono-oxides or bare support without the addition of promoter (Al-Swai et al., 2019). The coexistence of CeO2 with other oxides increases the reducibility and oxygen storage capacity of the catalyst (Li and Gong, 2014). Damyanova et al. (2009) previously demonstrated that a slight amount of CeO2 (e.g. 6 wt%) can stabilise the textural properties of the modified ZrO2 having CeO2.
Similarly, the conversion of CO2, as shown in Fig. 7(b), appeared to exhibit the same trend as that of the conversion of CH4. CAT-1 and CAT-3 recorded the same initial conversion of 83%. However, CAT-3 maintained a more stable conversion than CAT-1. Meanwhile, CAT-2 demonstrated the best conversion and stability but its conversion of CO2 was higher than that of CH4 due to the simultaneous generation of reverse water gas shift (RWGS) reaction (Dȩbek et al., 2016; Farooqi et al., 2020). Essentially, RWGS involves the reaction of CO2 and H2 to form CO. As shown in Fig. 6(c), the obtained results can be observed in terms of the molar ratio of H2 and CO, where the ratio was found to be lower than 1 for CAT-1 (0.86), CAT-2 (0.95), and CAT-3 (0.89).
4 Conclusion
The performance of CAT-1, CAT-2, and CAT-3 were compared in the present study. The addition of CeO2 as a promoter in Ni/Al2O3 catalyst appeared to improve the dispersion. Furthermore, the obtained results on the performance of Ni/Al2O3-CeO2 catalyst clearly reflected enhanced reducibility and basicity of catalyst through the active metal content on the surface of catalyst, where a higher and stable conversion was achieved. On the other hand, the addition of La2O3 in this study did not increase the conversion of the reactant but successfully maintained a relatively stable conversion over 8 h on stream. This may be due to the contribution of La2O3 in eliminating deposited carbon during the reaction. Besides that, the characterisation of fresh catalysts for FESEM characterisation revealed a homogeneous composition of catalysts for all samples. Nevertheless, CAT-3 yielded the best particle size and sharp FESEM image.
Acknowledgement
The authors would like to thank Ministry of Education (MOE), Malaysia for providing financial assistance under FRGS/1/2018/TK02/UTP/02/10 and Universiti Teknologi PETRONAS for providing the required facilities to conduct this research work.
Declaration of Competing Interest
The authors declared that there is no conflict of interest.
References
- Dry reforming of methane for hydrogen production over Ni-Co catalysts: Effect of Nb-Zr promoters. Int. J. Hydrogen Energy. 2019;44(37):20881-20888.
- [Google Scholar]
- Recent advances in dry reforming of methane over Ni-based catalysts. J. Clean. Prod.. 2017;162:170-185.
- [Google Scholar]
- Sequential impregnation vs. sol-gel synthesized Ni/Al2O3-CeO2 nanocatalyst for dry reforming of methane: Effect of synthesis method and support promotion. Mol. Catal.. 2017;431:39-48.
- [Google Scholar]
- Role of La2O3 as promoter and support in Ni/γ-Al2O3 catalysts for dry reforming of methane. Chin. J. Chem. Eng.. 2014;22:28-37.
- [Google Scholar]
- Syngas production via methane dry reforming over ceria-magnesia mixed oxide-supported nickel catalysts. Ind. Eng. Chem. Res.. 2019;58:539-552.
- [Google Scholar]
- A review on bimetallic nickel-based catalysts for CO2 reforming of methane. ChemPhysChem. 2017;18:3117-3134.
- [Google Scholar]
- Investigating the correlation between deactivation and the carbon deposited on the surface of Ni/Al2O3 and Ni/La2O3-Al2O3 catalysts during the biogas reforming reaction. Appl. Surf. Sci.. 2019;474:42-56.
- [Google Scholar]
- Pt supported on doped CeO2/Al2O3 as catalyst for dry reforming of methane. Int. J. Hydrogen Energy. 2020;45:5182-5191.
- [Google Scholar]
- The effect of CeO2 on the surface and catalytic properties of Pt/CeO2-ZrO2 catalysts for methane dry reforming. Appl. Catal. B Environ.. 2009;89:149-159.
- [Google Scholar]
- Ni/Al2O3 catalysts: Effects of the promoters Ce, La and Zr on the methane steam and oxidative reforming reactions. Catal. Sci. Technol.. 2013;3:635-643.
- [Google Scholar]
- Methane dry reforming over hydrotalcite-derived Ni-Mg-Al mixed oxides: The influence of Ni content on catalytic activity, selectivity and stability. Catal. Sci. Technol.. 2016;6:6705-6715.
- [Google Scholar]
- Efficient catalytic abatement of greenhouse gases: Methane reforming with CO2 using a novel and thermally stable Rh-CeO2 catalyst. Int. J. Hydrogen Energy. 2012;37:2699-2707.
- [Google Scholar]
- Classification of Gibbs adsorption isotherms. Adv. Colloid Interface Sci.. 1998;76–77:137-152.
- [Google Scholar]
- Syngas production via dry reforming of methane over Ni based catalysts. IOP Conf. Ser.: Mater. Sci. Eng.. 2020;736:42007.
- [Google Scholar]
- Promotional Effect of Ce-dopant on Al2O3-supported Co Catalysts for Syngas Production via CO2 Reforming of Ethanol. Procedia Eng.. 2016;148:646-653.
- [Google Scholar]
- Review on innovative catalytic reforming of natural gas to syngas. World J. Eng. Technol.. 2016;04:116-139.
- [Google Scholar]
- Dry reforming of methane over nickel catalysts supported on magnesium aluminate spinels. Appl. Catal. A Gen.. 2004;273:75-82.
- [Google Scholar]
- H2 and CO production over a stable Ni-MgO-Ce0.8Zr0.2O2 catalyst from CO2 reforming of CH4. Int. J. Hydrogen Energy. 2013;38:4508-4512.
- [Google Scholar]
- Methane decomposition over Ni/α-Al2O3 Promoted by La2O3 and CeO2. J. Nat. Gas Chem.. 2003;12:183-188.
- [Google Scholar]
- Natural gas plays in the marcellus shale: Challenges and potential opportunities. Environ. Sci. Technol.. 2010;44:5679-5684.
- [Google Scholar]
- The kinetics of steam methane reforming over a Ni/γ-Al2O3 catalyst for the development of small stationary reformers. Int. J. Hydrogen Energy. 2015;40:4512-4518.
- [Google Scholar]
- Multicomponent Ni-CeO2 nanocatalysts for syngas production from CO2/CH4 mixtures. J. CO2 Util.. 2018;25:68-78.
- [Google Scholar]
- Strategies for improving the performance and stability of Ni-based catalysts for reforming reactions. Chem. Soc. Rev.. 2014;43:7245-7256.
- [Google Scholar]
- Ni/CeO2-Al2O3 catalysts for the dry reforming of methane: The effect of CeAlO3 content and nickel crystallite size on catalytic activity and coke resistance. Appl. Catal. A Gen.. 2015;500:12-22.
- [Google Scholar]
- Mozammel, T., 2017. Catalyst development and process intensification towards syngas production through methane reforming.
- Steam-methane reforming at low temperature on nickel-based catalysts. Chem. Eng. J.. 2014;235:158-166.
- [Google Scholar]
- Dry reforming of methane has no future for hydrogen production: Comparison with steam reforming at high pressure in standard and membrane reactors. Int. J. Hydrogen Energy. 2012;37:10444-10450.
- [Google Scholar]
- Hydrogen production by methanol steam reforming on NiSn/MgO-Al2O3 catalysts: The role of MgO addition. Appl. Catal. A Gen.. 2011;392:184-191.
- [Google Scholar]
- Dry reforming of methane over Ni/Ce0.62Zr0.38O2 catalysts: Effect of Ni loading on the catalytic activity and on H2/CO production. Comptes Rendus Chim.. 2015;18:1242-1249.
- [Google Scholar]
- The influence of Ni loading on the activity and coke formation of ultrasound-assisted co-precipitated Ni–Al2O3 nanocatalyst in dry reforming of methane. Int. J. Hydrogen Energy.. 2017;42:4155-4165.
- [Google Scholar]
- Syngas production from methane dry reforming over Ni/Al2O3 catalyst. Res. Chem. Intermed.. 2016;42:269-288.
- [Google Scholar]
- Combined steam and CO2 reforming of methane for syngas production over carbon-resistant boron-promoted Ni/SBA-15 catalysts. Microporous Mesoporous Mater.. 2018;262:122-132.
- [Google Scholar]
- Hydrogen production from CH4 dry reforming over bimetallic Ni–Co/Al2O3 catalyst. J. Energy Inst.. 2018;91:683-694.
- [Google Scholar]
- Chemical CO2 recycling via dry and bi reforming of methane using Ni-Sn/Al2O3 and Ni-Sn/CeO2-Al2O3 catalysts. Appl. Catal. B Environ.. 2018;224:125-135.
- [Google Scholar]
- Syngas production by CO2 reforming of coke oven gas over Ni/La2O3-ZrO2 catalysts. Int. J. Hydrogen Energy. 2014;39:18650-18658.
- [Google Scholar]
- Dry reforming of methane: Influence of process parameters – A review. Renew. Sustain. Energy Rev.. 2015;45:710-744.
- [Google Scholar]
- Carbon dioxide reforming of methane to produce synthesis gas over metal-supported catalysts: State of the art. Energy Fuels. 1996;10:896-904.
- [Google Scholar]
- Low-temperature catalytic CO2 dry reforming of methane on Ni-based catalysts: A review. Fuel Process. Technol.. 2018;168:199-206.
- [Google Scholar]
- Low-temperature catalytic CO2 dry reforming of methane on Ni-Si/ZrO2 catalyst. ACS Catal.. 2018;6:6495-6506.
- [Google Scholar]
- Ni-based catalysts for low temperature methane steam reforming: Recent results on Ni-Au and comparison with other bi-metallic systems. Catalysts. 2013;3:563-583.
- [Google Scholar]
- Studies of reforming natural gas with carbon dioxide to produce synthesis gas. X. The role of CeO2 and MgO promoters. J. Mol. Catal. A: Chem. 1999:47-54.
- [Google Scholar]
- Biogas reforming for hydrogen production over nickel and cobalt bimetallic catalysts. Int. J. Hydrogen Energy. 2009;34:6646-6654.
- [Google Scholar]
- Characterization and analysis of carbon deposited during the dry reforming of methane over Ni/La2O3/Al2O3 catalysts. Cuihua Xuebao/Chin. J. Catal.. 2009;30:1076-1084.
- [Google Scholar]
- Alkaline-promoted Ni based ordered mesoporous catalysts with enhanced low-temperature catalytic activity toward CO2 methanation. RSC Adv.. 2017;7:18199-18210.
- [Google Scholar]
- Small-scale reforming of diesel and jet fuels to make hydrogen and syngas for fuel cells: A review. Appl. Energy. 2013;108:202-217.
- [Google Scholar]
- Synthesis gas production via dry reforming of methane over manganese promoted nickel/cerium-zirconium oxide catalyst. Ind. Eng. Chem. Res. 2018
- [Google Scholar]
- Mo-promoted Ni/Al2O3 catalyst for dry reforming of methane. Int. J. Hydrogen Energy. 2017;42:23500-23507.
- [Google Scholar]
- Reforming of methane with carbon dioxide to synthesis gas over supported rhodium catalysts: I. Effects of support and metal crystallite size on reaction activity and deactivation characteristics. J. Catal.. 1996;158:51-63.
- [Google Scholar]
- Effect of mineralizers for preparing ZrO2 support on the supported Ni catalyst for steam-CO2 bi-reforming of methane. Int. J. Hydrogen Energy. 2017;42:6598-6609.
- [Google Scholar]







