Translate this page into:
Cerium (IV) ammonium nitrate-mediated reactions: Simple route to benzimidazole derivatives
*Corresponding author. Tel.: +20 862364806; fax: +20 862363011 kusadek@yahoo.com (Kamal Usef Sadek)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Available online 14 August 2010
Abstract
The reaction of o-phenylenediamine with aromatic aldehydes in MeOH at room temperature catalyzed by cerium (IV) ammonium nitrate (CAN) afforded either 2-aryl-1-arylmethyl-1H-benz-imidazoles and/or 2-aryl-substituted benzimidazoles.
Keywords
Aromatic aldehyde
o-Phenylenediamine
Benzimidazole derivatives
1 Introduction
The benzimidazole moiety is found in various biologically active compounds having antiviral (Gabril et al., 2001), antiulcer (Kim et al., 1996), antihypertensive (Roth et al., 1997), anticancer (Moukhoopadhyay et al., 2002), and antihistaminic properties (Junsens et al., 1985). In addition, benzimidazole derivatives have been used as topisomerase inhibitors, selective Neuropeptide yy1 receptor antagonists, smooth muscle cell proliferation inhibitors, a treatment for interstitial cystitis, as factor Xa inhibitors (Zarrinmayeh et al., 1999; Kohara et al., 1996; Lopez et al., 1990; Fonseca et al., 2001; Zhao et al., 2000). They also display a great affinity towards a variety of enzymes and protein receptors (Pason et al., 1999). The most common synthesis of benzimidazoles involves the reaction between o-phenylenediamine and a carboxylic acid (or their synthetic equivalents) or aromatic aldehydes (Wang et al., 2006; Lin et al., 2006; Preston and “ Chemistry of Heterocyclic Compounds, 1981; Chi and Sun, 2000; Dudd et al., 2003; Curini et al., 2004; Zheng et al., 2007) under harsh dehydrating conditions. Benzimidazoles have also been prepared on solid phase to provide a combinatorial approach (Mazurov, 2000). Recently, R. Kumar et al. Kumar and Joshi (2007) have reported a one-step synthesis of 2-aryl-1H-benzimidazoles from the reaction of o-phenylenediamine with various aldehydes in dichloromethane in the presence of CAN under reflux. Also, o-nitroaniline has evolved to include the synthesis of benzimidazoles via one step reduction and cyclisation (Kim et al., 2004; Wu et al., 2000). In all of these approaches, the sole isolable products were 2-aryl-1H-benzimidazoles. Recently, (Varala et al., 2007) a selective synthesis of 2-aryl-1-arylmethyl-1H-benzimidazoles from the reaction of o-phenylenediamine with aromatic aldehydes catalyzed by l-proline in which two molecules of aldehyde reacted with one molecule of o-phenylenediamine has been reported. Ceric ammonium nitrate (CAN) has emerged as a versatile reagent for a variety of synthetic transformations which have been well documented (Nair and Deepthi, 2007; Nair et al., 2004). In continuation to our interest in the synthesis of azoles and azines (Fawzia et al., 2008; Mekheimer et al., 2008a; Mekheimer et al., 2008b) via simple, efficient and environmentally friendly techniques we investigated the reaction of o-phenylenediamine with several aromatic aldehydes catalyzed by CAN in different solvents (CH3OH, CH3CN, C6H5CH3) at room temperature, both the expected products were obtained which revealed the dependence of such condensation on the nature of the solvent and enabled achieving a direct simple route to benzimidazole derivatives.
2 Results and discussion
Thus, when o-phenylenediamine 1 was treated with 4-methoxybenzaldehyde 2a in MeOH in the presence of CAN (10 mol%) at room temperature a compound of molecular formula C22H20N2O2 (MS = 344 m/e) was obtained in 90% yield. 13C NMR of the reaction product revealed a methylene carbon. at δ = 46.86 in addition to two methoxy carbons at δ = 54.97 and 55.27 as well as aromatic carbons. 1H NMR shows a band at δ = 5.49 ppm integrated for two protons in addition to two methoxy and 12 aromatic protons. This excludes structure 3a since it will reveal two azomethine protons at δ = 8.16 ppm. Consequently, the 2-aryl-1-arylmethyl-1H-benzimidazole derivative 5a was established for the reaction product. Similarly, 3,4-dimethoxybenzaldehyde 2b reacts with 1 under the same reaction conditions to afford the corresponding 2-aryl-1-arylmethyl-1H-benzimidazole derivative 5b (Scheme 1).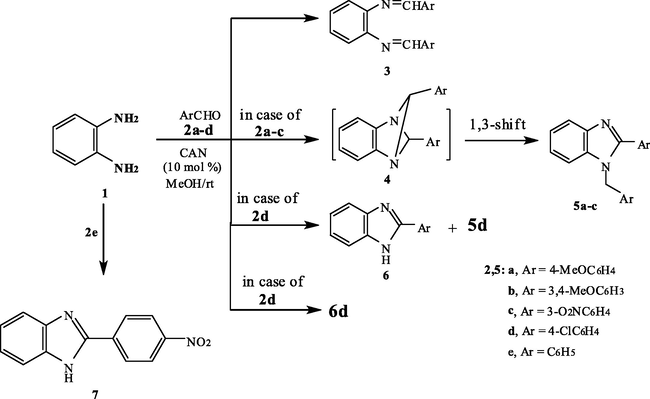
The formation of 5a and b was assumed to proceed via the formation of the dihydro-2-arylbenzimidazole intermediate 8 which condenses with another molecule of the aldehyde under the effect of the catalyst to afford the 1,4-diazbicyclo (Kim et al., 1996; Gabril et al., 2001; Gabril et al., 2001) hexane intermediate 4, which undergoes a thermally allowed 1,3-hydrogen shift to yield the final isolable product 5 (Scheme 2).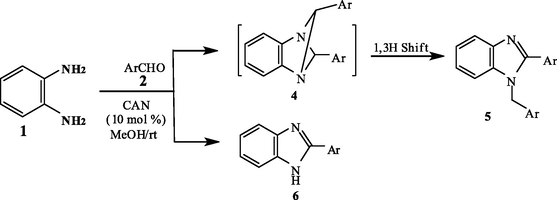
In contrast, the reaction of 3-nitrobenzaldehyde 2c with 1 afforded a mixture of 2-(3-nitrophenyl)-benzimidazole 6c and the 1-arylmethyl derivative 5c in 2:1 ratio. The structure proposed for the reaction products was established based on analytical and spectral data (see Section 4). The reaction of 1 with 4-chlorobenzaldehyde 2d afforded mainly the corresponding 2-(4-chlorophenyl)-benzimidazole derivative 6d. The structure of 6d was established based on 1H NMR which revealed besides the aromatic protons an (NH) band at δ = 8.2 ppm. The formation of 6d was proposed to be formed via condensation of one molecule of 2d followed by oxidation of the formed adduct by the effect of (CAN). The oxidizing effects of (CAN) are well known and have been established. (Varala et al., 2007) Interestingly, the reaction of benzaldehyde 2e with 1 afforded a product of molecular formula C13H9N3O2 (Ms = 239 m/e). The 1H NMR spectrum of this product revealed a singlet at δ = 11.62 integrated for one (NH) proton besides aromatic protons. Moreover, its 13C NMR reveals absorption bands for aromatic carbons and 2-benzimadazoyl carbons. Accordingly, structure 7 was established for the reaction product. It is assumed to be formed via the formation of the corresponding condensation product 6e followed by nitration of the phenyl group at position 2 of the benzimidazole by the action of (CAN). If nitration of benzaldehyde is prior to the condensation step it will afford 6c. The nitration process was illustrated in Scheme 3 in which (CAN) acts as a carrier of nitronium species and is similar to that observed with metal nitrates (Goonbes and Russel, 1971) (Table 1).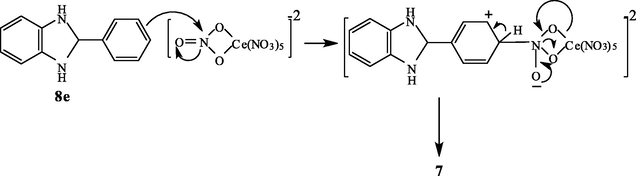
Product
Ar
Yield (%)
m.p. (°C)
Published data m.p. (°C)
5a
4-MeOC6H4
90
130–131
129–130
5b
3,4-MeOC6H3
89
170–172
–
5c
3-NO2C6H4
15
162–164
–
6c
3-NO2C6H4
55
206–207
207–208
6d
4-ClC6H4
70
293–294
292–293
7
4-O2NC6H4
82
323–325
327
In order to investigate the quantity effect of CAN on the reaction yield we studied the efficiency of the catalyst ratio (1, 2, 5, 10, 15 moL%) and we revealed that 10 moL% of the catalyst was the optimum ratio to carry out the forward reaction. For less than 10% the yield decreases to 65–70% while an increase in the quantity of CAN has no significant effect on the overall yield. In addition, methanol was the best solvent among others (CH3CN, C6H5CH3).
3 Conclusion
The results obtained in our investigation clearly revealed that the reaction of o-phenylenediamine with aromatic aldehydes proceeds mainly via two different routes. The first is a 1:1 condensation followed by oxidation to afford the 2-aryl-substituted benzimidazoles. Second is a 1:2 condensation to afford 2-aryl-1-arylmethyl-1H-benzimidazole. Both the expected products were obtained whose ratios depended on the nature of the solvent. It was previously reported that in methanol as a solvent both 5 and 6 were obtained with 5 as a predominant one (Salehi et al., 2006).
4 Experimental section
Melting points were determined on a Gallenkamp apparatus and are uncorrected. The reactions were monitored by TLC (thin layer chromatography) and visualized with UV light. IR spectra (KBr) were recorded on Perkin–Elmer FTIR model 2000 spectrometer and the NMR spectra were measured on Bruker-DPX-400 (400 MHz) spectrometer using TMS as an internal standard. Mass spectra were carried out on a VG-Autospac-Q high performance tri-sector GC/MS/MS Spectrometer. Microanalyses were performed by the Microanalytical Data Unit at Kuwait University.
4.1 General procedure of the reaction 1 and 2a–d
A mixture of o-phenylenediamine 1 and aromatic aldehydes 2a–d in MeOH (10 mL) was treated with Ceric ammonium nitrate (CAN) (10 mol%). The reaction mixture was stirred at room temperature overnight and then treated with brine solution. The solid formed was collected by filtration and crystallized from ethanol. In case of 2c a fractional crystallized from EtOH and EtOH/H2O mixture afforded 5c and 6c.
4.2 2-(4-Methoxyphenyl)-1-(4-methoxyphenyl)methyl-1H-benzimidazole 5a
Yield 90%; m.p. 130–131 °C (lit Salehi et al., 2006 m.p 129–130 °C); IR (KBr, cm−1): υmax = 3060, 3049 (arom. CH), 2986 (aliph. CH), 1609 (C⚌C); 1H NMR δ, ppm = 7.70–6.84 (m, 12H, Ar-H), 5.49 (s, 2H, CH2), 3.83 (s, 3H, OCH3), 3.69 (s, 3H, OCH3); 13C NMR δ, ppm = 160.31, 158.45, 153.11, 142.64, 138.72 135.80, 130.47, 128.78, 127.37, 122.31, 121.99, 118.94, 114.21, 114.11, 110.97, 55.27, 54.97, 46.86; MS: m/z (%) 344.15 (34%); Anal. Calcd for C22H20N2O2: C, 76.72; H, 5.85; N, 8.13. Found: C, 76.66; H, 5.80; N, 8.11.
4.3 2-(3.4-Dimethoxyphenyl)-1-(3,4-dimethoxyphenyl)methyl-1H-benzimidazole 5b
Yield 89%; m.p. 170–172 °C; IR (KBr, cm−1): υmax = 3050 (arom. CH), 2999, 2963, 2838 (aliph. CH), 1611 (C⚌C); 1H NMR δ, ppm = 7.71–6.43 (m, 10H, Ar-H), 5.50 (s, 2H, CH2), 3.82, 3.71, 3.68, 3.64 (4 s, 4 OCH3); 13C NMR δ, ppm = 153.17, 149.98, 148.73, 148.58, 147.96, 142.55, 135.99, 129.31, 122.38, 122.35, 122.02, 121.55, 118.93, 117.91, 112.27, 111.74, 111.58, 110.92, 110.03, 55.52, 55.35, 55.31, 55.25, 47.19; MS: m/z (%) 404.17 (77%); Anal. Calcd for C24H24N2O4: C, 71.27; H, 5.98; N, 6.93. Found: C, 71.32; H, 5.88; N, 6.92.
4.4 2-(3-Nitrophenyl)-1-(3-nitrophenyl)methyl-1H-benzimidazole 5c
Yield 15%; m.p. 162–164 °C; IR (KBr, cm−1): υmax = 3054 (arom. CH), 2960 (aliph. CH), 1619 (C⚌C); 1H NMR δ, ppm = 8.30–7.20 (m, 12H, Ar-H), 5.80 (s, 2H, CH2); MS: m/z (%) 374.1 (100%); Anal. Calcd for C20H14N4O4: C, 64.17; H, 3.77; N, 14.97. Found: C, 64.15; H, 3.72; N, 14.86.
4.5 2-(3-Nitrophenyl)-1H-benzimidazole (6c) and 2-(4-chlorophenyl)-1H-benzimidazole (6d)
The compounds were obtained in 55% and 70% yields and were found identical with authentic specimens Nagawade and Shinde, 2006 (m.ps., IR, NMR).
4.6 2-(4-Nitrophenyl)-1H-benzimidazole 7
Yield 82%; m.p. 323–325 °C (lit (Deluca and Kerwin, 1997) m.p 327 °C); IR (KBr, cm−1): υmax = 3338 (NH), 3100 (arom. CH), 2830 (aliph. CH), 1655 (C⚌C); 1H NMR δ, ppm = 11.65 (S, 1H, NH), 7.91–7.74 (m, 8H, Ar-H); 13C NMR δ, ppm = 115.2;123.11; 124.42;127.33;135.12;141.70;147.82;152.22. MS: m/z (%) 239.07 (100%); Anal. Calcd for C13H9N3O2: C, 65.27; H, 3.79; N, 17.56. Found: C, 65.33; H, 3.69; N, 17.55.
Acknowledgements
This work was financed with the project fund [Kuwait, AAAET, TRANSFORM Grant TS-06-14] which is of great help in achievement this work.
References
- Synlett 2000:591.
- Synlett 2004:1832.
- Tetrahedron. 1997;53:457.
- Green Chem.. 2003;5:187.
- Molecules. 2008;13:2908.
- Tetrahedron. 2001;57:1793.
- Bioorg. Med. Chem. Lett.. 2001;11:187.
- J. Chem. Soc. B. 1971:2443.
- J .Med. Chem.. 1985;28:1925.
- J. Med. Chem.. 1996;4:992.
- Heterocycles. 2004;63:4154.
- J. Med. Chem.. 1996;39:5228.
- E-J. Chem.. 2007;4(4):606.
- Tetrahedron Lett.. 2006;47:2883.
- J. Med. Chem.. 1990;33:418.
- Bioorg. Med. Chem. Lett.. 2000;10:67.
- Green Chem.. 2008;10:592.
- Chinese Chem. Lett.. 2008;19:788.
- Clin. Cancer Res.. 2002;8:2963.
- J.Org. Chem.. 2006;42:453.
- Chem. Rev.. 2007;107:1862.
- Acc. Chem. Res.. 2004;37:21.
- J. Med. Chem.. 1999;42:3251.
- J. Med. Chem.. 1997;40:4199.
- Tetrahedron Lett.. 2006;47:2557.
- Tetrahedron Lett.. 2007;48:69.
- Tetrahedron Lett.. 2006;47:4823.
- Tetrahedron Lett.. 2000;41:9871.
- Bioorg. Med. Chem. Lett.. 1999;9:647.
- Bioorg. Med. Chem. Lett.. 2000;10:963.
- Angew. Chem. Int. Ed.. 2007;46:7509.







