Translate this page into:
Chemical composition and antimicrobial activity of the essential oils of Artemisia absinthium, Artemisia scoparia, and Artemisia sieberi grown in Saudi Arabia
⁎Corresponding author. hati@ksu.edu.sa (Hanan Y. Aati),
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Essential oils (EOs) are one of the most significant products of plant metabolites, the current research work was performed to determine and compare the chemical compositions of the EOs extracted from three different species of Artemisia (absinthium, sieberi, and scoparia) growing in Saudi Arabia and to test their antimicrobial potential against different bacterial and fungal strains. The EOs were isolated by hydro-distillation and analyzed by combining a gas chromatography–flame ionization detector (GC–FID) with the gas chromatography–mass spectroscopy (GC–MS) technique. Chemical analysis revealed that the three species had four compounds in common, i.e., limonene, camphor, terpinen-4-ol, and ethyl 2-methylbutyrate, while the main components identified in the EOs of A. absinthium and A. sieberi were cis-davanone (34.7% and 36.1%, respectively) and camphor (16.2% and 24.1%, respectively). In contrast, the keto compounds dominated in the oil of A. scoparia with 2-nonanone (55%) and 2-undecanone (24.5%) representing more than 80% of the total oil content. In addition, the antimicrobial activity of the isolated oils was evaluated by the broth microdilution method, revealing that all the EOs isolated from the examined Artemisia species displayed growth inhibiting actions in a concentration-dependent manner on selected tested microorganism species. The findings of the study also suggested that the tested EOs could be used to develop effective natural antimicrobial remedies with potential application in the fields of cosmetic industry, food manufacturing and medicine.
Keywords
Essential oils
Antimicrobial activity
A. absinthium
A. scoparia
A. sieberi
GC–FID
1 Introduction
Medicinal plants have been widely used by humans for centuries as part of traditional medicine to treat various diseases and infections. To date, many studies have focused on collecting information about the chemical content and activities of medicinal herbs, indicating that aromatic plants are important sources of secondary metabolites. Secondary metabolism in plants not only contributes to their survival, but also acts as a chemical defense against predators and diseases (Pandey and Tripathi, 2011). The family Asteraceae is particularly rich in plants that produce essential oils (EOs), among which the genus Artemisia holds a top position owing to its biological activities. This genus consists of small herbs and shrubs and comprises of about 500 species found in America, Asia, and several European countries (Abad et al., 2012).
The Kingdom of Saudi Arabia is a vast arid land with an area of about 2,250,000 km2 that covers most of the Arabian Peninsula, which is characterized by a wide diversity of plant species and different ecosystems (Hanan et al., 2019). The genus Artemisia is represented in Saudi Arabia by seven species (A. scoparia, A. monosperma, A. sieberi, A. judaica, A. abyssinica, A. herba-alba, and A. absinthium) and has been used worldwide in folk medicine since ancient times (Erel et al., 2012). The aromatic leaves of some Artemisia species are used for flavoring (as a culinary herbs). An in-depth literature survey revealed that different Artemisia species have been traditionally used as antidiabetic, anthelmintic, tonic, antimalarial, and antiulcer agents, as well as for the treatment of bronchitis, wounds inflammation, and tuberculosis. Many researchers have also explored the antioxidant, antipyretic, analgesic, antimicrobial, cytotoxic, and antifungal activities of different Artemisia species (Wright, 2002; Humara et al., 2014). The phytochemical studies confirmed that all classes of activity principles exist in this genus with particular reference to terpenoids and flavonoids. Moreover, it has been shown that the accumulation of EOs rich in sesquiterpene lactones and terpenoids results in strong aromatic odors and bitter tastes for most of the species, which may discourage herbivores and lead to a selective advantage (Bora and Sharma, 2011). An attractive application of essential oils and their constituents is in food products to prolong the shelf-life of foods by limiting growth or survival of microorganisms.
To the best of our knowledge, the aroma volatiles of A. absinthium grown in Saudi Arabia have not been explored in detail so far. Thus, herein, we performed a detailed gas chromatography–mass spectrometric (GC–MS) analysis for the EOs isolated from the aerial part of wild grown A. absinthium in the southwestern region of Saudi Arabia. Abou El-Hamd et al. (2010) has also confirmed that the various oil compositions observed in plants grown in different areas of the same country or different countries indicate the presence of several oil-dependent chemotypes in the same plant (Abou El-Hamd et al., 2010). Therefore, an additional aim of this study was to identify the chemical diversity of three different species from the genus Artemisia, i.e., A. absinthium, A. sieberi, and A. scoparia. Considering also that in recent decades, several antimicrobial herbal products have received considerable attention due to the rapid increase in the resistance of microorganisms to antibiotics, we tested the activity of the obtained EOs against Gram-positive and negative bacterial strains and a strain of filamentous fungi. The results of the study could be therefore used to develop of novel antimicrobial drugs isolated from a natural source or contributing in food manufacturing not only as flavorings but used as an interesting safe source of natural antimicrobials for food preservation.
2 Materials and methods
2.1 Plant material
The fresh aerial parts (each 500 g) of A. absinthium were collected from the city of Jazan and those of A. sieberi, and A. scoparia from the city of Riyadh, Saudi Arabia, in March 2018 during the flowering stage of plants. The plants were identified by the plant taxonomist Dr. Rajakrishnan Rajagopal, a botanist in the Science College Herbarium, King Saud University, Riyadh, Saudi Arabia.
2.2 Essential oils extraction
The freshly cut aerial parts of A. absinthium, A. sieberi, and A. scoparia were subjected to hydro-distillation (HD) for 4 h using a Clevenger apparatus and following the standard procedure described in the literature (European Pharmacopoeia, 2005). The recovered oils were dissolved in petroleum ether and then filtered over anhydrous sodium sulfate to give deep blue (A. absinthium), blue (for A. sieberi), and colorless Eos (for A. scoparia) in yields of 0.83% (v/w), 0.42% (v/w), and 0.34% (v/w), respectively. Finally, the obtained oils were stored in amber glass under N2 at 4 °C in the dark until their further testing and analysis. The isolated EOs were diluted in dichloromethane (1:20) and 1 μL of this solution was injected in the GC at a split ratio of 1:100.
2.3 GC–FID and GC–MS analysis
Gas chromatography–flame ionization detector (GC–FID) and GC–MS analyses were performed in one run using a gas chromatograph with a MS-FID-splitter consisting of a quartz Y-splitter, a short (ca. 20 cm) 0.1 mm id fused silica restrictor column as an inlet to the GC–MS interface, and a ca. 1 m × 0.25 mm deactivated fused silica column serving as a transfer line to the FID detector. The restrictor column was used to limit the flow in the MS vacuum and prevent the insertion of combustion gases from the FID, which operated at atmospheric pressure. Moreover, the flow in the analytical column had to be greater than the inflow to the MS detector, which was limited to about 1 mL/min by the restriction line. The GC column flow should also be constant, otherwise the FID/MS split ratio would change with temperature. This configuration yielded an FID and MS chromatogram with almost identical retention times (RT), thus facilitating the assignment of the FID peaks to each substance. For these measurements, we used a Thermo Fisher Scientific Trace GC Ultra with a split/split less injector heated at 250 °C and connected to a 50 m × 0.25 mm × 1.0 µm SE-52 (95% polydimethylsiloxane, 5% polydiphenylsiloxane) capillary column (prepared and tested for deactivation and separation efficiency in our lab (Kurt, 1986), an FID detector operating at 250 °C, and a TriPlus RSH Autosampler.
For the identification of the EO components, a Thermo Fisher Scientific ISQ mass spectrometer was used with a GC/MS interface heating at 250 °C, an ion source operating at 230 °C, an electron ionization mode at 70 eV, a filament with 50 µA, and a scan range of 40–500 amu. The oven temperature program involved heating at 60 °C for 1 min, then heating to 230 °C at a rate of 3 °C/min, and a 230 °C isotherm for 12.3 min. The carrier gas was helium 5.0 with a constant flow rate of 1.5 mL/min.
2.4 Identification of the essential oil components
The Thermo Xcalibur 2.2 software was used to identify the EO compounds by correlating the obtained mass spectra with the databases of the National Institute of Standards and Technology (NIST 08), Wiley 8th ed. (Wiley Registry™), Adams library (Adams, 2007), Mass Finder terpenoids library (König et al., 2001), and our own library. However, the simple comparison of the mass spectra is not sufficient for a clear identification, especially for sesquiterpenes. Thus, the position in the chromatogram was also considered as a second criterion determined by comparing the calculated retention indices (RI) of the peaks with the corresponding literature data, NIST 08, Adams (2007) and König et al. (2001) or reference compounds. The RI values were determined by measuring the RTs of a series of n-alkanes that were eluted across the entire chromatogram and were calculated according to the method of Van den Dool and Kratz (Van den Dool and Kratz, 1963; Gas Chromatographic Retention Data, NIST). Moreover, the EO components were quantified using normalized peak area calculations of the FID chromatogram without (by first approximation) relative FID-response factors.
2.5 Antimicrobial assay
The antimicrobial activity of the isolated EOs was evaluated by calculating their minimum inhibitory concentration (MIC) against Gram-positive [Staphylococcus aureus (CP011526.1), Bacillus licheniformis (KX785171.1), and Micrococcus luteus (NCTC 2665)] and Gram-negative [Enterobacter xiangfangensis (CP017183.1), Escherichia fergusonii (CU928158.2), and Pseudomonas aeruginosa (NR-117678.1)] bacterial strains. In addition, their activity against three pathogenic fungi, i.e., Candida albicans (MF942350), Candida parapsilosis (MF942354), and Aspergillus parasiticus (CBS 100926), was examined.
The MIC value for each strain was determined by the broth microdilution method following a previous reported procedure (Murthy et al., 2006). Each sample solution (10 µL) was loaded on wells and dissolved in 10% dimethyl sulfoxide (DMSO). The final concentration of the oil was 2500 mg/mL. To achieve a concentration range of 2500–5 mg/mL, a series of two-fold dilutions of the stock solution were prepared. Erythromycin (Saudi Pharmaceutical Industry, SPI), amikacin (Bristol Mayers Squibb, BMS), and itraconazole (SAJA) were used as the positive reference standards for the Gram-positive bacteria, Gram-negative bacteria, and fungi, respectively. Two-fold dilutions of the positive controls with concentrations of 1000–1 mg/mL were also prepared. The respective bacterial and fungal inoculums were of 104 CFU/mL and 103 CFU/mL, respectively, which were incubated in 96-well microplates at 37 °C for 24 and 48 h for bacteria and fungi, respectively. The experiments were performed in duplicate and repeated three times (n = 3). Accordingly, the lowest concentration of the tested oil that could inhibit the bacterial growth was expressed as MIC in μg/mL (Joshi, 2010).
2.6 Statistical analysis
All data were expressed as mean ± standard deviation (SD) values of three replicates. Where applicable, the data were subjected to one-way analysis of variance. Based on the statistical package analysis of Microsoft Excel 2010, the differences were considered statistically significant for p values of <0.05.
3 Results
The application of HD to the aerial parts of the three Artemisia species (A. absinthium, A. sieberi, and A. scoparia) provided deep blue, blue, and colorless EOs in yields of 0.83% (v/w), 0.42% (v/w), and 0.34% (v/w), respectively, depending on the fresh weight. The complexity of the chemical composition of the EOs isolated from the aerial parts of A. absinthium, A. sieberi, and A. scoparia, as determined by GC–MS analysis, is demonstrated in Table 1. Rt = Retention time (min.), RI = Retention indices.
No.
Compounds*
RT (min)
A. absinthium
A. sieberi
A. scoparia
RI
1
Ethyl 2-methylbutyrate
10.83
1.0
1.1
tr.
844
2
Ethyl isovalerate
10.94
0.2
0.1
–
846
3
α-Thujene
14.67
0.2
0.2
–
930
4
α-Pinene
15.12
2.1
2.1
–
940
5
Propyl 2-methylbutyrate
15.20
0.6
1.0
–
941
6
Camphene
15.90
2.0
2.0
–
956
7
2-Octanone
17.37
–
–
0.4
986
8
Myrcene
17.55
1.1
0.6
–
990
9
α-Terpinene
19.08
0.8
0.6
–
1021
10
p-Cymene
19.42
0.3
0.5
–
1028
11
Limonene
19.70
0.3
0.4
0.1
1034
12
γ-Terpinene
21.14
1.4
1.2
–
1062
13
2-Nonanone
22.48
–
0.6
55.0
1088
14
Terpinolene
22.67
0.4
0.3
–
1093
15
2-Nonanol
22.84
–
–
2.6
1097
16
Linalool
22.93
3.2
3.6
–
1098
17
Nonanal
23.07
–
–
0.8
1101
18
trans-Sabinene hydrate
23.14
0.2
0.2
–
1103
19
cis-p-Menth-2-en-1-ol
24.34
0.1
0.2
–
1127
20
2-Octanol acetate
24.66
–
–
0.6
1134
21
trans-p-Menth-2-en-1-ol
25.19
0.1
0.2
–
1145
22
Camphor
25.63
16.2
24.1
1.1
1154
23
Nonanol
26.31
–
–
0.3
1168
24
Borneol
26.66
0.4
0.8
–
1175
25
Terpinen-4-ol
27.15
3.4
5.4
0.1
1185
26
2-Decanone
27.39
–
–
1.7
1190
27
α-Terpineol
27.71
0.3
0.4
–
1196
28
2-Nonanol acetate
29.38
–
–
9.3
1232
29
Bornyl acetate
32.26
2.0
3.6
0.0
1293
30
2-Undecanone
32.20
–
–
24.5
1292
31
Nonyl acetate
32.50
–
–
0.6
1298
32
Myrtanol acetate
33.01
0.4
0.4
–
1309
33
Ethyl hydrocinnamate
34.85
0.3
0.4
–
1351
34
6-Dodecanone
35.44
–
–
0.5
1364
35
Z-Ethyl cinnamate
36.07
0.6
0.6
–
1378
36
Limonen-10-yl acetate
37.61
0.2
0.3
–
1413
37
2-Acetoxyundecane
38.22
–
–
0.8
1428
38
E-β-Caryophyllene
38.77
0.3
0.1
–
1441
39
E-Ethyl cinnamate
39.98
2.5
1.6
–
1469
40
Davana ether isomer 1
40.96
0.3
–
–
1493
41
β-Selinene
41.59
0.2
0.1
–
1508
42
Davana ether isomer 2
41.78
1.6
tr.
–
1513
43
Davana ether isomer 3
42.53
0.6
–
–
1532
44
Davanone B (allo-Davanone)
43.82
1.0
–
–
1564
45
E-Nerolidol
43.97
4.0
5.0
–
1568
46
Davanone
44.08
1.8
–
–
1570
47
iso-Davanone
44.34
0.6
–
–
1577
48
cis-Davanone
45.01
34.7
36.1
–
1594
49
Davanol
46.01
0.3
0.2
–
1620
50
Methyl jasmonate
47.25
0.6
0.1
–
1652
51
Chamazulene
51.09
8.2
2.9
–
1756
Oxygenated monoterpenes
26.7
39.5
1.2
–
Monoterpene hydrocarbons
8.7
8.1
0.1
–
Oxygenated sesquiterpenes
44.9
41.3
–
–
Sesquiterpene hydrocarbons
8.8
3.2
–
–
Saturated fatty acids
1.9
2.3
0.4
–
Miscellaneous
4.1
3.4
96.9
–
Total (%)
95.1
97.8
98.6
–
The volatile constituents of the three EOs were identified based on the MS libraries (NIST 08, Wiley 8th ed., Adams library, Mass Finder terpenoids library) and by comparing the obtained data with the corresponding MS literature findings. The relative percentages of the individual components were calculated based on the GC peak areas. Hence, GC–MS analysis revealed the presence of 57 constituents in the A. absinthium oil, which represented 95.1% of the total oil. The major constituents of this oil were cis-davanone (34.7%), camphor (16.2%), chamazulene (8.2%), E-nerolidol (4%), and terpinen-4-ol (3.4%). The A. sieberi oil consisted of 54 volatile components, which represented 97.8% of the total oil content, while the main volatile compounds were cis-davanone (36.1%), camphor (24.1%), terpinen-4-ol (5.4%), E-nerolidol (5%), linalool (3.6%), bornyl acetate (3.6%), and chamazulene (2.9%). Furthermore, the GC analysis of the A. scoparia oil indicated the presence of 23 aroma constituents, among which 2-nonanone (55%) and 2-undecanone (24.5%) represented more than 80% of the total oil content. A comparison of the findings for the three plant species indicated that the oxygenated sesquiterpenes and monoterpenes were the major chemical classes in the EOs obtained from A. absinthium and A. sieberi, while the keto compounds dominated in the A. scoparia oil (Fig. 1). The GC–MS total ion chromatogram of the oils is shown in Figs. 2-4.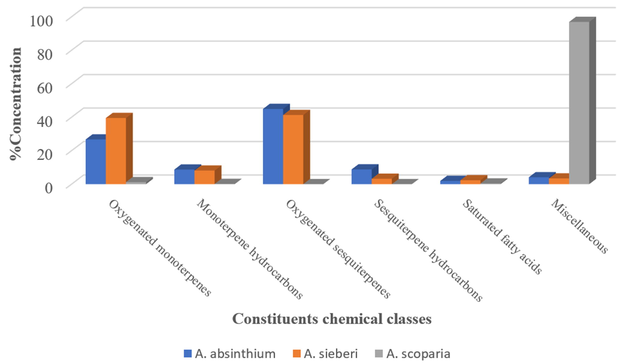
Keto compounds classes identified in the essential oils of A. absinthium, A. sieberi, and A. scoparia.
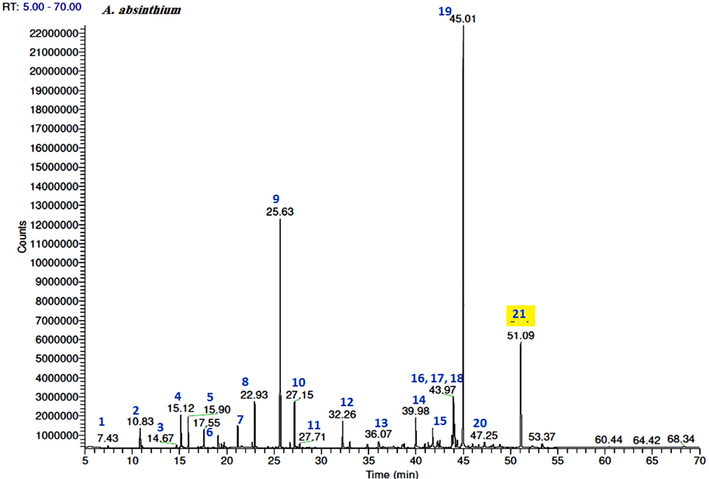
GC–FID chromatograms of the volatile compounds in the A. absinthium essential oil. The numbers represent the peaks of (1) ethyl isobutyrate, (2) ethyl 2-methylbutyrate, (3) α-thujene, (4) α-pinene, (5) camphene, (6) myrcene, (7) γ-terpinene, (8) linalool, (9) camphor, (10) terpinen-4-ol, (11) α-terpineol, (12) bornyl acetate, (13) Z-ethyl cinnamate, (14) E-ethyl cinnamate, (15) davana ether isomer 2, (16) davanone B (allo-davanone), (17) E-nerolidol, (18) davanone, (19) cis-davanone, (20) methyl jasmonate, and (21) chamazulene.
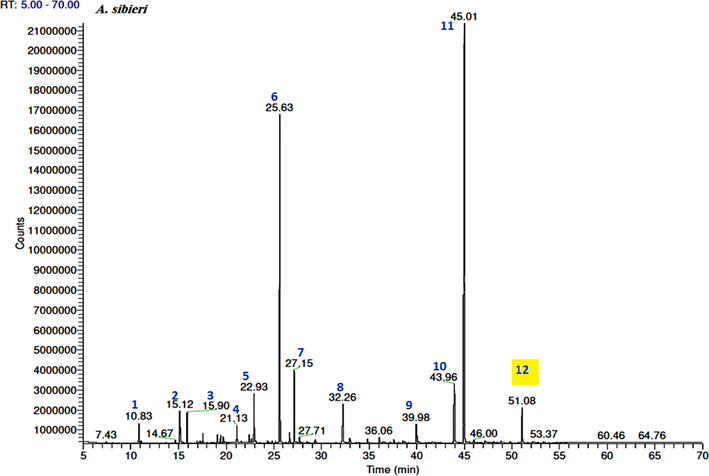
GC–FID chromatograms of the volatile compounds in the essential oil obtained from A. sieberi. The numbers represent the peaks of (1) ethyl 2-methylbutyrate, (2) α-pinene, (3) camphene, (4) γ-terpinene, (5) linalool, (6) camphor, (7) terpinen-4-ol, (8) bornyl acetate, (9) E-ethyl cinnamate, (10) E-nerolidol, (11) cis-davanone, and (12) chamazulene.
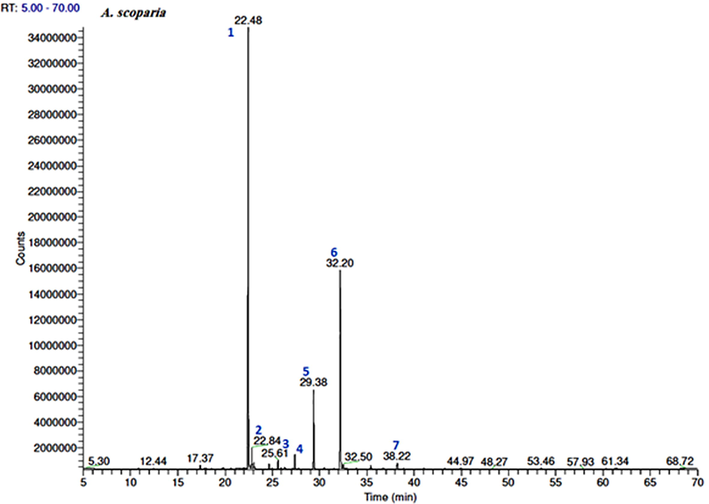
GC–FID chromatograms of the volatile compounds in the essential oil obtained from A. scoparia. The numbers represent the peaks of (1) 2-nonanone, (2) 2-nonanol, (3) camphor, (4) 2-decanone, (5) 2-nonanol acetate, (6) 2-undecanone, and (7) 2-acetoxyundecane.
Traditionally, A. absinthium, A. sieberi and A. scoparia used an anthelmintic, antiparasitic, antihypertensive, relieve tendonitis, earache, bronchitis, wound burns infection and to cure different type of infections. For that, we assessed quantitatively the antimicrobial and antifungal activities of the three tested EOs by determining their MIC values to validate the folkloric use for these plants. As shown in Table 2, the EOs of A. sieberi and A. scoparia showed the most significant activities against both Gram-negative and -positive bacteria. Especially the EO of A. scoparia exhibited the lowest MIC against Staphylococcus aureus, Enterobacter xiangfangensis, and Candida albicans (0.2 ± 0.3, 3.0 ± 0.1 and 0.1 ± 0.5 μg/mL, respectively) with higher inhibition values than the positive control broad-spectrum standard antimicrobial drugs erythromycin, amikacin, and itraconazole, 2.0 ± 1.0, 9.0 ± 4.0 and 0.29 ± 0.6 μg/mL, respectively. Moreover, the EO isolated from A. sieberi was the most active against Micrococcus luteus with a MIC value of 0.5 ± 0.1 μg/mL, whereas the EO of A. absinthium was more sensitive to Gram-positive bacteria and fungi and completely inactive against the selected Gram-negative bacteria (Table 2, Fig. 5).
Microbial strains
MIC [mean ± SD (µg/mL)]*
A. absinthium
A. sieberi
A. scoparia
Erythromycin
Amikacin
Itraconazole
Gram-positive
S. aureus CP011526
5 ± 0.7
2 ± 0.6
0.2 ± 0.3
2.0 ± 1.0
–
–
B. licheniformis KX785171
11 ± 0.8
4 ± 0.3
2 ± 0.2
1.0 ± 1.0
–
–
M. luteus NCTC2665
8 ± 0.4
0.5 ± 0.1
4 ± 0.1
1.0 ± 1.0
–
–
Gram-negative
E. xiangfangensis CP017183
>25
16 ± 0.2
3 ± 0.1
–
9.0 ± 4.0
–
E. fergusonii CU928158
>25
12 ± 0.5
> 25
–
7.0 ± 8.0
–
P. aeruginosa NR117678
>25
17 ± 0.1
>25
–
4.0 ± 3.0
–
Fungi
C. albicans MF942350
4 ± 0.1
2 ± 0.0
0.1 ± 0.5
–
–
0.29 ± 0.6
C. parapsilosis MF942354
5 ± 0.6
3 ± 0.2
2 ± 0.3
–
–
0.33 ± 0.4
A. parasiticus CBS 100926
2.5 ± 0.5
1 ± 0.1
4 ± 0.1
–
–
0.28 ± 1.3
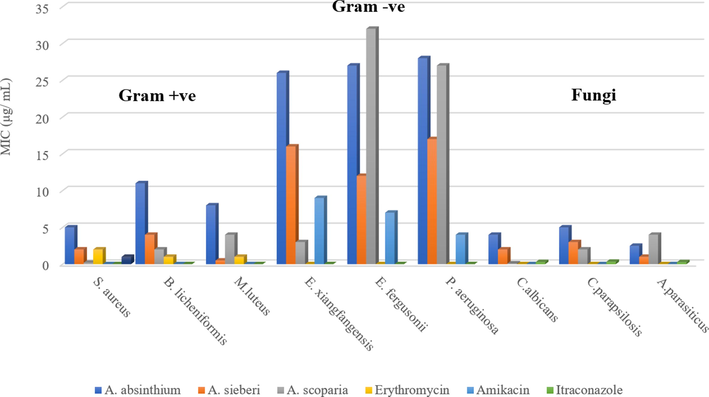
MIC (µg/mL) of the essential oils obtained from A. absinthium, A. sieberi, and A. scoparia.
4 Discussion
The A. absinthium oil was also characterized by a high content of oxygenated compounds including both sesquiterpenes (44.9%) and monoterpenes (26.7%) (Table 1, Fig. 1). More specifically, davanone and its derivatives were the most common sesquiterpenes, accounting for approximately 40.8% of the total detected compounds, while camphor was the predominant monoterpene with a concentration of 16.2% in a total monoterpene concentration of 26.7% (Fig. 2).
Furthermore, the main compounds isolated from the A. sieberi distilled oil were cis-davanone, camphor, terpinen-4-ol, E-nerolidol, linalool, bornyl acetate, and chamazulene, which accounted for approximately 97.8% of the total oil content (Table 1). Moreover, the GC–MS and GC–FID analysis indicated that the main chemical classes of components were oxygenated sesquiterpenes (41.3%), which were mainly represented by davanone, its alcohol derivative, and camphor, and monoterpenes (39.5%), including terpinen-4-ol, linalool, and bornyl acetate (Fig. 3).
In contrast to the other two species, the composition of the A. scoparia EO was dominated by the ketones, 2-nonanone and 2-undecanone, Table 1 and Fig. 4 which represented more than 80% of the total oil content (98.6%).
The chamazulene content in the A. absinthium and A. sieberi species was 8.2% and 2.9%, respectively, giving the corresponding oils a blue color with varying intensity depending on the chamazulene percent concentrations (Rezaeinodehi and Khangholi, 2008) (Figs. 2 and 3).
Davanone is a sesquiterpene lactone found in various Artemisia species (Luigi et al., 2016) and has a chemotaxonomic value. The davana (A. pallens) oil contains davanone as major constituent and exhibits antispasmodic, antibacterial, antifungal properties, as well as wound healing, antihelmintic, hypoglycemic, and antidepressant activities also, it used as food flavoring (Shreyas et al., 2018). As previously mentioned, the EOs isolated from the A. absinthium and A. sieberi Saudi species were dominated by cis-davanone (34.7% and 36.1%, respectively). Thus, the present study could also set the basis for the isolation of davanone from a natural source that is widely used in the medical field as well in food industry. Furthermore, davanone has been reported to be odorless, therefore, the odoriferous constituents of oils containing davanone were attributed to the presence of davana ethers (Thomas and Dubini, 1971). Herein, three out of four natural davana ether stereoisomers (Garneau et al., 2013) were detected in the EO isolated from A. absinthium, while traces from davana isomer 2 were identified in the A. sieberi EO. In contrast, no davana ethers were detected in the A. scoparia oil.
A previous study on the leaf extract of A. sieberi growing in Saudi Arabia led to the isolation of davanone (Lamya et al., 2020), but there was no reference to the content of Saudi A. sieberi EOs in davanone. Instead, an Iranian report confirmed that the EO isolated from A. sieberi contains davanone in 15%v/w, which is, significantly lower that the value determined in this study for the Saudi species (36.1%v/w). Arbi et al. also reported that the EO isolated from the A. sieberi Saudi species collected from the northern region of the Kingdom was dominated by spathulenol (30.4%v/w) (Arbi et al., 2017).
In addition to the A. sieberi species, the EO isolated from the A. absinthium Ethiopian species included 26.5% davanone (Asfaw and Demissew 2015), while the davanone content in the currently investigated A. absinthium Saudi species was 34.7%v/w out of the total oil content. Given that, to the best of our knowledge, no studies have so far explored the EO of the A. absinthium Saudi species, our study can be considered the first report. Nevertheless, several studies on the EOs of different Artemisia species have attributed their antimicrobial activity to their davanone-type sesquiterpenes content (Vlatka et al., 2004; Stefanie et al., 2008).
In contrast to the other two species, the EOs isolated from the aerial part of A. scoparia that was collected from the central region of Saudi Arabia consisted mainly of 2-nonanone and 2-undecanone. However, the oil isolated from the same species growing in the same country (Saudi Arabia) but collected from the northern region of Saudi Arabia was dominated by acenaphthene (83.2%v/w) (Arbi et al., 2017). Previous studies have also shown that the insecticidal and antimicrobial activities of EOs isolated from A. herba-alba are mainly due to their ketone content (Herrera et al., 2015; Nassiba et al., 2017). Therefore, since A. scoparia contains the highest percent concentration of ketones, we assumed that the presence of ketone groups may increase the toxicity against microbes.
Composition and yield of secondary metabolites in plants like EOs, can be affected in a number of ways, from their formation in the plant to their isolation. Several factors have been studied, in particular for commercially important crops, to optimize the cultivation conditions and time of harvest and to obtain higher yields of high-quality essential oils. In addition to the commercial importance of the variability in yield and composition, the possible changes are also important when the essential oils and volatiles are used as chemotaxonomic tools. Knowledge of the factors that determine the chemical variability and yield for each species are thus very important. These include: physiological variations (organ development, pollinator activity cycle, type of plant material [leaves, flowers, etc.], type of secretory structure, seasonal variation and mechanical or chemical injuries); environmental conditions (climate, pollution, diseases and pests and edaphic factors); geographic variations; genetic factors and evolution; storage; political/social conditions; and also amount of plant material needs (Cristina et al., 2008).
Table 1 showed some similar compounds could be detected in all three Artemisia species EOs (limonene, camphor, and terpinen-4-ol), but their quantities were different. Although the plant aerial parts were collected from the same country and belonged to the same family and genus, the Artemisia species exhibited chemical polymorphism, which was attributed to the chemovariation of EOs due to the different genetic and environmental (temperature, moisture, and chemical composition of soil) factors that affected the EO compositions. Therefore, by comparing the current results with relevant data from previous studies we concluded that the geographic origin considerably affects the chemical composition of the Artemisia species.
In recent years, the resistant isolates of microorganisms have significantly increased (Bingyun and Thomas, 2018) due to the increasing use of antibiotics. Taking also into account the adverse effects caused by chemical agents used for the treatment of infectious diseases, the research community has also focused on the discovery of new natural compounds. In this study, it was revealed that the examined Gram-positive bacteria were more susceptible to the antibacterial activity of EOs than the Gram-negative bacteria. This higher resistance of the Gram-negative bacteria could be attributed to their external membrane, which surrounds their cell wall and inhibits the penetration of the hydrophobic EOs through its lipopolysaccharide membrane that would affect the cell structure and functionality. Additionally, the external capsule of some Gram-negative bacteria limits or prevents the diffusion of EOs into the bacterial cell (Ratledge and Wilkinson, 1988). A previous study has demonstrated that the most abundant chemical groups in the A. absinthium and A. sieberi oils, i.e., oxygenated monoterpenes, such as 1,8-cineole, camphor, 1-terpinen-4-ol, linalool, α-terpineol, borneol, and bornyl acetate, have potent antimicrobial activities (Carson and Riley 1995). Moreover, the antibacterial and antifungal activities of the oxygenated sesquiterpenes, which were the main constituents of the A. absinthium and A. sieberi oils accounting for 44.9% and 41.3%, respectively, of the total EOs content and consisted of a high amount of davanone and its derivatives, have also been established (Shreyas et al., 2018). In addition, the antimicrobial properties of the hydrocarbons of monoterpenes and sesquiterpenes that were also identified in high concentrations in these EOs have also been recognized (Adorjan and Buchbauer, 2010). Especially chamazulene has been identified as a fungicidal agent against 34 species of fungi at a dose of 20 µL (Kordali et al., 2005). Minor components should be considered as well as, they may also play a critical role in the EOs antibacterial activity through their synergistic effect with other oil components (Lopes-Lutz et al., 2008).
5 Conclusions
The present study investigated the chemical compositions and tested the antimicrobial activity of three Artemisia species by isolating volatile EOs from plants growing in different regions of Saudi Arabia. The results indicated that the chemotype of the studied Artemisia EOs was specific and different from other already reported Artemisia EOs chemotypes. In addition, the tested Artemisia EOs showed good antimicrobial activities, which indicated their effectiveness against diseases caused by the overproduction of microorganisms. Thus, these species could be further investigated to develop new antimicrobial agents, can be used as natural safe additives in food products to prolong the shelf-life of foods by limiting growth or survival of microorganisms. However, additional studies are required regarding the safety and toxicity of the individual major compounds, as well as the effectiveness of the EOs as a source of active biological compounds in animal models.
Funding
This work was supported by the Deanship of Scientific Research at the King Saud University through the Research Group Project no RGP-221.
Authors' contributions
HYA and SP conceived and designed the experiments; HYA, SP, RO and JW performed the experiments; HYA and SP analyzed the data and wrote the primary draft of the manuscript; SY participated in the sample collections and manuscript writing and editing; All authors have read and agreed to the published version of the manuscript.
References
- The Artemisia L. genus: A review of bioactive essential oils. Molecules. 2012;17:2542-2566.
- [Google Scholar]
- Chemical constituents and biological activities of Artemisia herba-alba. Rec. Nat. Prod.. 2010;4:1-25.
- [Google Scholar]
- Identification of Essential Oil Components by Gas Chromatography/Mass Spectrometry (fourth ed.). Illinois, USA: Allured Publishing Corp. Carol Stream; 2007.
- Biological properties of essential oils: an updated review. Flavour Frag. J.. 2010;25:407-426.
- [Google Scholar]
- The genus Artemisia L.in the northern region of Saudi Arabia: essential oil variability and antibacterial activities. Nat. Prod. Res.. 2017;31:598-603.
- [Google Scholar]
- Essential oil composition of four Artemisia species from Ethiopia. Bull. Chem. Soc. Ethiop.. 2015;29:123-128.
- [Google Scholar]
- Bacteria antibiotic resistance: New challenges and opportunities for implant-associated orthopaedic infections. J. Orthop. Res.. 2018;36:22-32.
- [Google Scholar]
- Antimicrobial activity of the major components of the essential oil of Melaleuka alternifolia. J. Appl. Bacteriol.. 1995;78:264-269.
- [Google Scholar]
- Factors affecting secondary metabolite production in plants: volatile components and essential oils. Flavour Fragr. J.. 2008;23:213-226.
- [Google Scholar]
- Antimicrobial and antibacterial properties of Artemisia L. species from western Anatolia. Turk J Biol.. 2012;36:75-84.
- [Google Scholar]
- European Pharmacopoeia, 2005. Council of Europe, fifth ed., vol. 2, Strasbourg, pp. 2710–2711.
- Essential oils from Bolivia. Asteraceae: Ophryosporus piquerioides (D.C.) Benth. ex Baker. J. Essent. Oil Res.. 2013;25:388-394.
- [Google Scholar]
- Gas Chromatographic Retention Data, http://webbook.nist.gov/chemistry/gc-ri/.
- Traditional use of ethnomedicinal native plants in the Kingdom of Saudi Arabia. J. Ethnobiol. Ethnomed.. 2019;15(2)
- [Google Scholar]
- Terpene ketones as natural insecticides against Sitophilus zeamais. Ind. Crops Prod.. 2015;70:435-442.
- [Google Scholar]
- Chemical composition and antioxidant activity of the leaf essential oil of Artemisia absinthium growing wild in Kashmir. India. J. Phytopharmacol.. 2014;3:90-94.
- [Google Scholar]
- In vitro antimicrobial and antioxidant activities of the essential oil of Craniotome furcata. J. Appl. Nat. Sci.. 2010;2:57-62.
- [Google Scholar]
- Terpenoids and Related Constituents of Essential Oils. Library of Mass Finder 2.1. Hamburg: Institute of Organic Chemistry; 2001.
- Determination of the chemical composition and antioxidant activity of the essential oil of Artemisia dracunculus and of the antifungal and antibacterial activities of Turkish Artemisia absinthium, A. dracunculus, Artemisia santonicum, and Artemisia spicigera essential oils. J. Agric. Food Chem.. 2005;53:9452-9458.
- [Google Scholar]
- Making and Manipulating Capillary Columns for Gas Chromatography. Heidelberg: Dr. Alfred Huethig Verlag; 1986. ISBN 3-7785-1312-5
- Comparative study on the essential oils of Artemisia judaica and A. herba-alba from Saudi Arabia. Arab. J. Chem.. 2020;13:2053-2065.
- [Google Scholar]
- Screening of chemical composition, antimicrobial and antioxidant activities of Artemisia essential oils. Phytochemistry. 2008;69:1732-1738.
- [Google Scholar]
- Essential oil composition and biological activity from Artemisia caerulescens subsp. densiflora (Viv.) Gamisans ex Kerguélen & Lambinon (Asteraceae), an endemic species in the habitat of La Maddalena Archipelago. Nat. Prod. Res.. 2016;30:1802-1809.
- [Google Scholar]
- Antimicrobial activities of bharangin from Premna herbaceae Roxb. and Bharangin monoacetate. J. Ethnopharmacol.. 2006;104:290-292.
- [Google Scholar]
- Thionation of essential oils from Algerian Artemisia herba-alba l. and Ruta montana l.: impact on their antimicrobial and insecticidal activities. Chem. J. Mol.. 2017;12:50-57.
- [Google Scholar]
- Aromatic plants of Gorakhpur forest division: Their antimycotic property and medicinal value. Int. J. Pharm. Sci. Rev. Res.. 2011;7:142-147.
- [Google Scholar]
- An overview of microbial lipids. In: Ratledge C., Wilkinson S.G., eds. Microbial Lipids 1. London: Academic Press; 1988. p. :3-22.
- [Google Scholar]
- Chemical composition of the essential oil of Artemisia absinthium growing wild in Iran. Pak. J. Biolo. Sci.. 2008;11:946-949.
- [Google Scholar]
- A review on phytoconstituents and medicinal uses of Dhavana. Asian J. Res. Chem. Pharm. Sci.. 2018;6:202-205.
- [Google Scholar]
- GC-MS-analysis, antimicrobial activities and olfactory evaluation of essential Davana (Artemisia pallens Wall. ex DC) Oil from India. NPC. Nat. Prod. Comm.. 2008;3:1057-1062.
- [Google Scholar]
- The NIST 08 Mass Spectrometer Database, Scientific Instrument Services Inc., New Jersey, http://www.sisweb.com/software/ms/nist.htm.
- The isolation, structure, and synthesis of davana ether, an odoriferous compound of the oil of Artemisia pallens Wall. Helv. Chim. Acta. 1971;54:1890-1891.
- [Google Scholar]
- A generalization of the retention index system including linear temperature programmed gas-liquid partition chromatography. J. Chromatogr.. 1963;11:463-471.
- [Google Scholar]
- Antifungal activity of davanone-type sesquiterpenes from Artemisia lobelii var. conescens. J. Serb. Chem. Soc.. 2004;69:969-972.
- [Google Scholar]
- Wiley Registry™ of Mass Spectral Data, eighth ed., Scientific Instrument Services Inc., New Jersey, http://www.sisweb.com/software/ms/wiley.htm.
- Artemisia. London-New York: Taylor & Francis; 2002.







