Translate this page into:
Chemical composition and potential bioactivities of essential oil from Quercus mongolica bark
⁎Corresponding authors. zhouhongli@jlict.edu.cn (Hongli Zhou), pengw@jlmu.edu.cn (Peng Wan)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
The chemical composition and potential bioactivities of essential oil from Quercus mongolica bark (EOQMB) were researched for value-added utilization processing by-product. The results of gas chromatography-mass spectrometry (GC–MS) analysis showed that 30 components accounting for 98.42% were identified in EOQMB, with pentadecanoic acid the most abundant compound accounting for 34.90%, which was further confirmed by the Fourier transform infrared observation. EOQMB exerts antioxidant activities, and the IC50 values for scavenging DPPH radical, ABTS radical, and hydroxyl radical were 8.48, 0.77, and 3.54 mg/mL, respectively. The effects of EOQMB on prolonging activated partial thromboplastin time and thrombin time and on decreasing fibrinogen are similar to those of heparin, and the promising anticoagulant activities of EOQMB could be largely contributed by pentadecanoic acid. Herein, the present study uncovered that the waste Q. mongolica bark can serve as a new potential material in pharmaceutical products.
Keywords
Quercus mongolica bark
Essential oil
Chemical composition
Antioxidant
Anticoagulant
1 Introduction
Quercus mongolica Fisch. ex Turcz (Q. mongolica), a member of the genus Quercus, is mainly distributed in Japan, South Korea, the Russian Far East, the Korean Peninsula, and northern and northeastern China (Zeng et al., 2016). Q. mongolica wood is used to make furniture. Q. mongolica acorns are utilized for the production of starch (Ning et al., 2018). Q. mongolica leaves are employed for the breeding of Antherea pernyi (Yang et al., 2010). Given the high nutritional value of A. pernyi, the demand for Q. mongolica in northern China is increasing, the planting area of Q. mongolica is increasing, and the amounts of side shoots pruned and cut in rotation are increasing annually. With the continuous degradation of Q. mongolica species (Kim et al., 2011), developing the valuable biological activities of its different parts is particularly important. A variety of chemical compositions have been extracted with ethanol and water from Q. mongolica leaves and showed antibacterial activity (Wang et al., 2016). Furan, essential phenol, and other ingredients are found in its heartwood, which has the effect of treating scald and trauma (Niu et al., 2021), Q. mongolica bark was extracted with absolute ethanol, and HPLC analysis showed that the bark contains dandelion ketone, ursolic acid ethyl ester, and other compounds (Wang et al., 2014). The bark of Q. mongolica was traditionally used as folk medicine to treat many diseases, including bacterial dysentery, acute gastroenteritis, dyspepsia in children, jaundice, bronchitis, hemorrhoids, burns, wounds, mild diarrhea, and minor oral mucosal inflammation (Deryabin and Tolmacheva, 2015). Although Q. mongolica bark is medicinally valuable, most of it is discarded as waste, and only the essential oil in Q. mongolica bark is rarely reported.
Pine needles as agricultural waste are steam-distilled to produce large quantities of pine needle oil, which is an important ingredient in pharmaceuticals, food and spices, and cosmetics (Antonella et al., 2017). Some essential oils can be used in the therapy of cardiovascular diseases (CDs) and are highly correlated with antioxidant and anticoagulant activities (Félix-Silva et al., 2014). At present, the mortality rate of CDs is higher than that of malignant tumors, accounting for >40% of disease-related deaths. Blood coagulation can induce thrombosis, which accelerates the progression of CDs (Lippi et al., 2011). Therefore, natural antioxidants play an important role in thrombosis (Saeidi et al., 2018). Anticoagulants have been widely used in the treatment of disseminated intravascular coagulation and the thrombosis of various diseases (Aboonabi and Singh, 2016; Wang et al., 2010). At present, heparin is limited by the supply and demand of pigs, and can cause a hemorrhage tendency (Dore et al., 2013). Some scholars have studied and developed the antithrombotic role of essential oils with reduced side effects and discernible effects (Xia et al., 2017).
In this study, we determined and analyzed the chemical composition of essential oil from Q. mongolica bark (EOQMB) by gas chromatography–mass spectrometry (GC–MS) and fourier transform infrared spectroscopy (FT-IR) by hydrodistillation. The potential biological activities, including antioxidant activities with DPPH radical, ABTS radical, and hydroxyl radical scavenging ability, and anticoagulant activities with activated partial thromboplastin time (APTT), thrombin time (TT), prothrombin time (PT), and fibrinogen (FIB), were explored to provide a scientific evidence for rationally improving the waste utilization of agricultural resources and developing its novel medicinal value.
2 Materials and methods
2.1 Plant material
Q. mongolica bark was collected from the scraped skin of large side shoots pruned on March 2018 at the Jilin Provincial Sericulture Institute, Jilin City, Jilin Province, China. Specimens were preserved in the Engineering Research Center for Agricultural Resources and Comprehensive Utilization of Jilin Province under the herbarium code QMB0601.
Fresh Q. mongolica bark powder (100 g) was placed on a 1000 mL two-necked flask equipped with a Clevenger-type apparatus and a thermometer. The power was added with 500 mL of distilled water, soaked for 2 h, and heated for 5 h. The mixture of essential oil and water was cooled to room temperature, dried with anhydrous Na2SO4, collected, and hermetically stored at 0 °C for GC–MS analysis.
2.2 Chemical composition of EOQMB
2.2.1 GC–MS analysis
A GCMS-QP2010 instrument with Rxi-5sil column was used to measure the chemical composition of EOQMB. The carrier gas was highly purified helium, and the flow rate was 1.0 mL/min. The collision energy for MS detection was 70 eV, and data were recorded within 40–450 amu. The vaporizer temperature and ion-source temperature were adjusted to 280 °C and 230 °C, respectively. Each chemical composition was identified by comparing the retention index obtained from a database (NIST05) with the retention indexes calculated on the basis of n-alkanes (C8–C40) and reported literature (Waterman, 2005).
An essential oil sample dissolved in ether (60 mg/mL) was injected automatically into a vaporizer at 250 °C with a split ratio of 1: 30, and the conditions of the programmed temperature were as follows: Start at 60 °C and maintain for 6 min; increase from 60 °C to 300 °C at 3 °C/min; maintain at 300 °C for 10 min.
2.2.2 FT-IR analysis
The FT-IR spectra of EOQMB and its main component (pentadecanoic acid) were determined by using an FTIR-650 spectrometer. Pentadecanoic acid and KBr were ground at a ratio of 1: 100 and then pressed into tablet. The obtained volatile oil was evenly spread on a KBr window, and the blank spectrum was scanned. The KBr film carrying the sample was detected for 20 times between 4000 cm−1 to 400 cm−1 by using an FT-IR spectrophotometer. Measurements were repeated every 3 min (Nie et al., 2018).
2.3 Antioxidant activity of EOQMB
The antioxidant activities of EOQMB were evaluated by detecting the scavenging ability of EOQMB on DPPH radical, ABTS radical, and hydroxyl radical. These methods are available for water-soluble and lipid-soluble antioxidants.
2.3.1 DPPH radical assay
Essential oil samples (2 mL) with different concentration gradients (0.5–20 mg/mL) and 2 mL of 0.5 mmol/L DPPH solution were mixed and shook thoroughly. Vc and ethanol were listed as a positive control and a negative control, respectively, and the absorbance was observed at 517 nm (Wang et al., 2010).
2.3.2 ABTS radical assay
The ABTS reserve liquid was prepared with 5 mL of 7 mmol/L ABTS liquid and 88 µL of 140 mmol/L K2S2O8 liquid, and the diluted solution of ABTS radical was a solution diluted with 50 times ethanol. Diluted solution (2 mL) was added into 2 mL of essential oil sample (0.1–2.0 mg/mL). The absorbance was assayed at 734 nm (Hou et al., 2012).
2.3.3 Hydroxyl radical assay
Phenanthroline solution (1 mL, 0.75 mmol/L) was added into 2 mL of PBS (pH 7.40) and 1 mL of essential oil (1.0–10 mg/mL) in a test tube. Subsequently, 1 mL of 0.75 mmoL ferrous sulfate was added to 1 mL of 0.12% H2O2, the mixture was thermostatically incubated at 37 °C for 1 h, and the absorbance was measured at 536 nm. Distilled water rather than H2O2 was used as the positive control, and 1 mL of distilled water rather than volatile oil was the negative control (Amir et al., 2011).
2.4 Assay of anticoagulant action
The anticoagulant activities of EOQMB and pentadecanoic acid were assayed through coagulation tests in a CL-2000BV coagulometer (Jiangsu Sinnowa Medical Technology Co., Ltd, China) with heparin (positive control) and 0.90% NaCl (negative control) as a reference. APTT, TT, PT, and FIB content were used to evaluate the anticoagulant activity (Wang et al., 2013; Yin et al., 2017).
Plasma pretreatment was conducted as follows: Sodium citrate (0.109 mol/L) was precisely mixed with fresh and healthy human plasma at a volume ratio of 1: 9 and jiggled several times. The mixture was centrifuged for 20 min at 3000 r/min, and the supernatant of platelet-poor plasma was collected, packed and sealed in plastic tubes, and maintained in frozen storage. The plasma preheating was conducted at 37 °C before the experiment. All the following experiments were completed at 37 °C within 2 h.
The contents of four parameters were measured by using assay kits (Jiangsu Sinnowa Medical Technology Co., Ltd). EOQMB was dissolved in 20% dimethyl sulfoxide at five different concentrations (1.87, 3.75, 7.5, 15, and 30 mg/mL). The clotting time was recorded.
2.4.1 APTT assay
In the APTT assays, 20 µL of EOQMB solution with the five abovementioned concentrations were incubated with 80 µL of plasma for 60 s. The mixtures were added with 100 µL of APTT reagent for 1 min, followed by 25 mmol/L CaCl2 (100 µL).
2.4.2 PT assay
In the PT assays, 20 µL of EOQMB solution with the five abovementioned concentrations were incubated with 80 µL of plasma for 30 s. The mixtures were then added with 200 µL of PT reagent.
2.4.3 TT assay
In the TT assays, 20 µL of EOQMB solution with the five abovementioned concentrations were incubated with 80 µL of plasma for 30 s. The mixtures were then added with 0.1 mL of preheated TT reagent.
2.4.4 FIB assay
In the FIB assays, a standard curve was first drawn. The fixed-value plasma of redissolved FIB was diluted in 20 mmol/L imidazole diluent at different proportions of 1: 5, 1: 10, 1: 15, 1: 20, and 1: 30. The fixed-value plasma of diluent at different concentrations was obtained at 200 µL, preheated at 37 °C for 30 s, and mixed with 100 µL of FIB thrombin to determine the clotting time. The plasma concentrations (mg/dL) with different solutions of FIB were used as the abscissa, and the corresponding clotting time (Antonella et al., 2017) was used as the ordinate. The standard curve was constructed in a double logarithmic coordinate system.
Different samples (20 μL, 1.87–30 mg/mL) were mixed with 80 μL of plasma. The mixtures were then diluted with 0.9 mL of 20 mmol/L imidazole and incubated at 37 °C for 30 s. The above diluted fixed-value plasma (0.1 mL) was added with 0.05 mL of the redissolved FIB thrombin. Subsequently, the content of FIB was computed by using the equation in accordance with the clotting time of the plasma to be tested.
2.5 Statistical analysis
The experiments were repeated in triplicate, and the results were analyzed on SPSS software. P < 0.05 and P < 0.01 indicated significant results and highly significant results, respectively.
3 Results and discussion
3.1 Essential oil characterization
The extraction yield of EOQMB was 0.08% ± 0.03%. EOQMB had an aromatic odor and pale-yellow to light-brown color. The essential oil was rich in fatty acids because it turned from liquid into solid when it was cooled to room temperature (Hao et al., 2018). GC–MS analysis successfully identified 30 compounds. These compounds accounted for 98.42% of the total composition. The qualitative and quantitative analyses of EOQMB are listed in Table 1. EOQMB is rich in acids (63.37%), esters (15.22%), aldehydes (8.53%), and alcohols (6.24%). The main constituents are pentadecanoic acid (34.90%), linoleic acid (19.80%), ethyl linoleate (4.87%), oleic acid (4.62%), octadecanal (4.62%), 2-pentylfuran (4.00%), phytol (2.85%), 1-octadecanol (2.25%), and hexadeca-1,4-lactone (2.24%). RIa:refers to the retention index identified by Database NIST 05; RIb:refers to the retention index calculated from the retention time relative to that of C8–C40 n-alkanes; RIc:refers to the retention index searched from the literature; EOQMB refers to the essential oils from the Quercus mongolica bark.
No.
RT
Compound
Molecular formula
RIa
RIb
RIc
References
Chemical
classificationContent/%
1.
9.425
2-pentylfuran
C9H14O
1040
988.26
956
(Xia et al., 2017)
Ester
4.00
2.
12.429
trans-2-Nonenal
C9H16O
1112
1057.97
1112
(Wang et al., 2015)
Aldehyde
0.52
3.
24.059
(E)-2,(E)-4-decadienal
C10H16O
1220
1278.22
1314
(Czigle et al., 2006)
Aldehyde
0.56
4.
43.416
phytone
C18H36O
1754
1822.75
1790
(El-Shamy et al., 2012)
Ketone
0.69
5.
46.56
valeric acid,undec-2-enyl ester
C16H30O2
1787
1819.43
–
–
Ester
0.84
6.
49.044
(Z)-7-hexadecenal
C16H30O
1808
1883.56
2157
(Zhao et al., 2016)
Aldehyde
1.66
7.
47.204
(Z)-11-hexadecenal
C16H30O
1808
1836.06
1812
(Uehara et al., 2015)
Aldehyde
1.17
8.
48.473
pentadecanoic acid
C15H30O2
1869
1854.43
1869
(Rahman et al., 2016)
Acid
34.90
9.
47.015
11-hexadecyn-1-ol
C16H30O
1872
1831.18
–
–
Alcohol
1.14
10.
45.995
methyl palmitate
C17H34O2
1878
1801.80
1825
(Sharma et al., 2010)
Ester
0.70
11.
45.685
hexahydrofarnesyl acetone
C18H30O
1902
1893.75
1905
(Zheng et al., 2006)
Ketone
1.00
12.
50.652
2-hexyl-decanoic acid
C16H32O2
1904
1925.06
–
–
Acid
0.45
13.
52.025
palmitic acid
C16H32O2
1968
1960.51
1966
(Sharma et al., 2010)
Acid
1.97
14.
51.155
hexadeca-1,4-lactone
C16H30O2
1980
1938.05
–
–
Ketone
2.24
15.
54.619
octadecanal
C18H36O
1999
1991.46
2030
(Pino and Quijano, 2012)
Aldehyde
4.62
16.
46.845
hexanoic acid, 6-tridecyl ester
C19H38O2
2013
2024.26
–
–
Ester
0.67
17.
51.336
phytol
C20H40O
2045
2168.37
2045
(Rahman et al., 2016)
Alcohol
2.85
18.
50.747
1-octadecanol
C18H38O
2053
2096.43
2070
(Boussaada et al., 2012)
Alcohol
2.25
19.
50.969
methyl linoleate
C19H34O2
2093
2132.40
2088
(Myazawa et al., 2005)
Ester
0.89
20.
49.277
stearic acid
C18H36O2
2167
2084.43
2170
(Khan et al., 2003)
Acid
0.98
21.
47.717
oleic acid
C18H34O2
2175
2079.86
2161
(Mehdi et al., 2010)
Acid
4.62
22.
53.166
linoleic acid
C18H32O2
2183
2115.54
2109
(Xiang et al., 2017)
Acid
19.80
23.
52.147
ethyl linoleate
C20H36O2
2193
2193.59
2531
(Zhao et al., 2011)
Ester
4.87
24.
56.057
8,11-Icosadienoic acid methyl ester
C22H38O2
2292
2307.28
–
–
Ester
0.70
25.
55.837
7,10,13-Icosatrienoic acid methyl ester
C21H36O2
2300
2300.69
–
–
Ester
0.77
26.
57.934
hexanoic acid,4-hexadecyl ester
C22H44O2
2311
2370.72
–
–
Ester
1.31
27.
56.393
cis-9-tricosene
C23H46
2315
2323.51
2269
(Dhief et al., 2011)
Alkene
0.69
28.
56.659
tetracosane
C24H50
2407
2329.22
2300
(Xiang et al., 2017)
Alkane
0.44
29.
59.201
bis(2-ethylhexyl) adipate
C22H42O4
2414
2410.35
–
–
Ester
0.47
30.
45.196
tetracosanoic acid
C25H50O2
2763
2735.33
2827
–
Acid
0.65
Total
–
–
–
–
–
–
98.42
In the FT-IR spectrum of the functional groups, the characteristic absorption peaks in all chemical components were overlapped. In accordance with the IR spectra in Fig. 1, EOQMB and pentadecanoic acid had mostly similar absorption peaks of functional groups, and their IR spectra were consistent.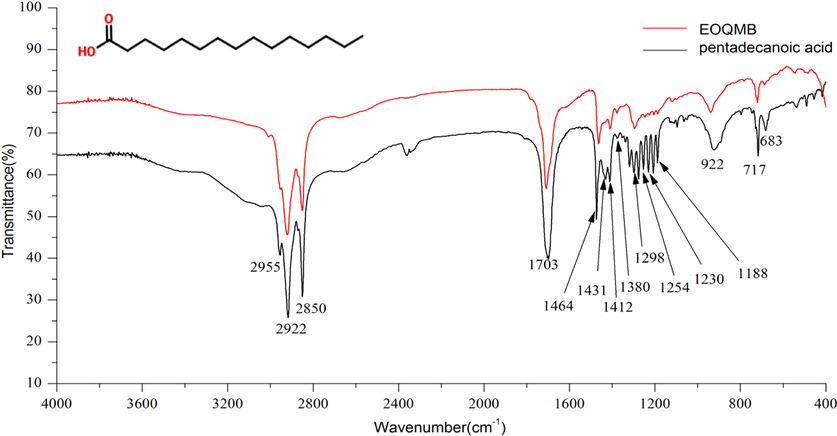
The infrared spectrum of EOQMB and pentadecanoic acid.
The characteristic peaks at 2922, 2850, and 1464 cm−1 showed asymmetrical, symmetric stretching vibration peaks, and in-plane scissoring vibration peak of —CH2, respectively. The absorption peaks at 2955, 1431, and 1380 cm−1 proved the presence of asymmetric stretching vibration peaks and bending vibration of —CH3. The peak at 1703 cm−1 was caused by the stretching vibration of C⚌O among the carboxyl group (Hosseini et al., 2013). The peaks at 1412 and 1254 cm−1 were the bending vibrations of O—H and asymmetric stretching vibrations of C—O (δ—CH2—COOH) (Lu and Deng, 1989). The strong broad peaks around 922 cm−1 were the rocking vibration of —OH (carboxylic acid). The adsorption bands at 1318, 1298, 1275, 1230, 1203, and 1188 cm−1 were the characteristic peaks of —(CH2)n— in-plane rocking vibrations, and the peak at 717 cm−1 confirmed the long chains of n ≥ 4. The above IR analysis proves that the peak shape of pentadecanoic acid is complete, and the peak position is correct.
In the IR spectra, the functional groups of EOQMB and pentadecanoic acid were consistent. The IR analysis results further demonstrated that the main components of EOQMB were consistent with those of GC–MS.
3.2 Antioxidant activity
As shown in Fig. 2A, the ability of EOQMB to scavenge DPPH radical increased with concentration within a certain range. The IC50 value of EOQMB for DPPH radical was 8.48 mg/mL, and that of Vc for DPPH radical was 0.01 mg/mL.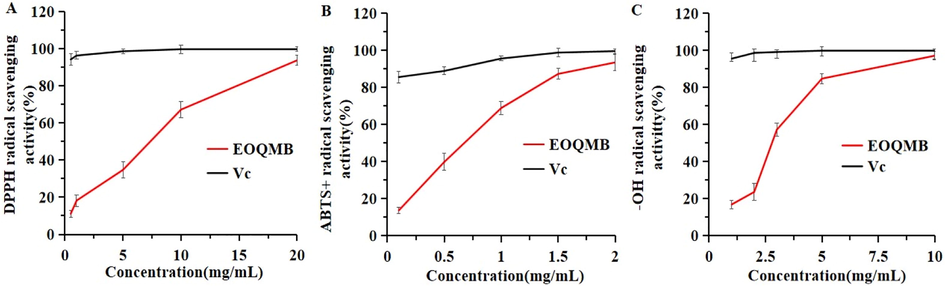
Radical scavenging activity (%) of Q. mongolica bark essential oil; A, DPPH: Diphenyl picryl hydrazinyl radical; B, ABTS+: 2,2-azinobis 3-ethylben-zothiazoline-6-sulfonic acid; C,OH–: Hydroxyl radical.
In Fig. 2B, the ABTS radical scavenging ability of EOQMB increased with concentration within a certain range. The IC50 value (0.77 mg/mL) of EOQMB was second only to that of Vc (0.05 mg/mL).
The hydroxyl radical scavenging was another experiment used to evaluate the antioxidant activity. As shown in Fig. 2C, the ability of EOQMB to scavenge hydroxyl radicals increased with concentration within a certain range. The IC50 value (3.54 mg/mL) of essential oil was larger than the IC50 value (0.03 mg/mL) of Vc.
The experimental results showed that EOQMB had an antioxidant effect on DPPH radical, ABTS radical, and hydroxyl radical, and had the strongest effect on ABTS radical. Different methods for determining antioxidant activity are available, and each method depends on lipophilic/hydrophilic balance and oxidant/antioxidant models used. Multiple detection is highly desirable when performing antioxidant testing on essential oils (Ray et al., 2018). The whole antioxidant effect can be related to fatty acids in EOQMB. Pentadecanoic acid (Sharma et al., 2016), linoleic acid (Santos et al., 2017), oleic acid (Xiang et al., 2017), and palmitic acid (Lv et al., 2014) have considerable antioxidant activity. Given that the GC–MS analysis showed that >60% of EOQMB components were fatty acids, the antioxidant activity of EOQMB may be due to fatty acids.
As shown in Fig. 3, the oxidative stress produced by vascular endothelial cells accelerated the release of tissue factor and interleukin-6, thereby promoting fibrin formation and initiating coagulation (Aizawa et al., 2015). Antioxidants inhibit the release of reactive oxygen species to protect vascular endothelial cells from oxidative damage (Fre, 1999).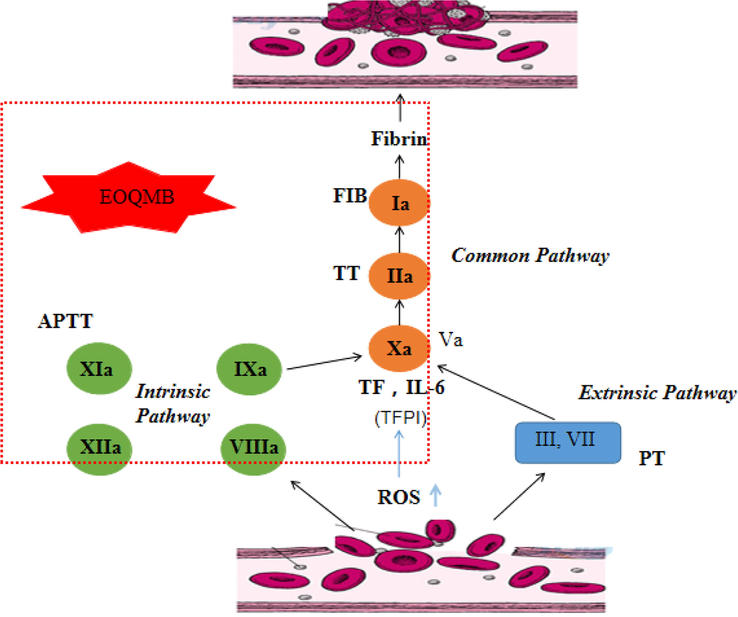
Schematic diagram of anticoagulation mechanism.
3.3 Anticoagulant action of EOQMB
As shown in Fig. 3, coagulation is a series of enzymatic reactions that are activated by its former related factor and ultimately produces thrombin and fibrin. The results of the anticoagulation experiments of EOQMB and pentadecanoic acid are shown in Fig. 4.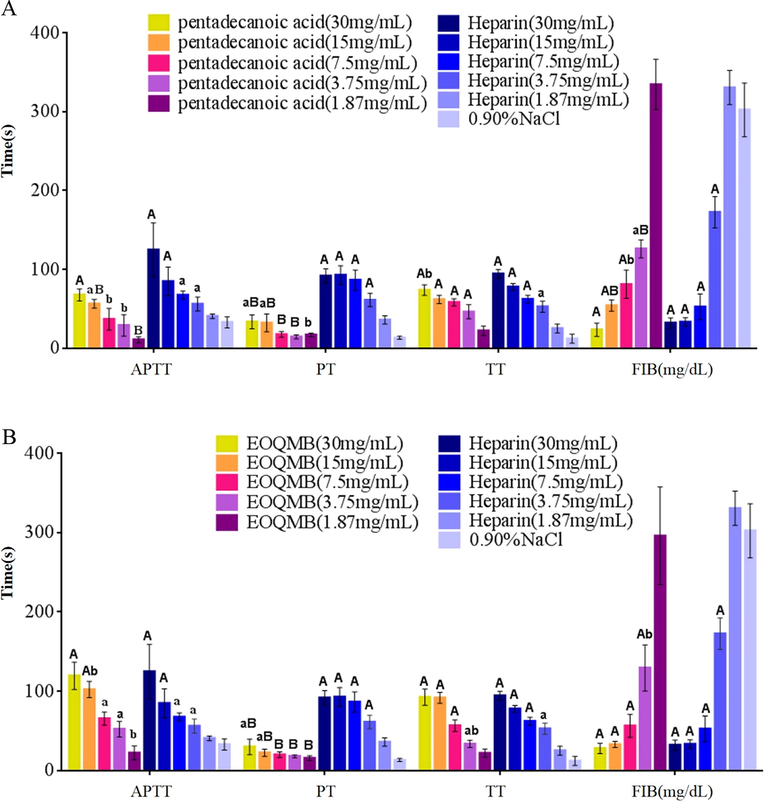
Effects of EOQMB(Zeng et al.) and pentadecanoic acid(Deryabin and Tolmacheva) on plasma coagulation time measured by APTT, TT, PT and, FIB in vitro; A P < 0.01,or,a P < 0.05 vs. Control group;B P < 0.01,or, b P < 0.05 vs. Same concentration Heparin.
As illustrated by the results of APTT assay in Fig. 4, anticoagulant activity was positively correlated with sample concentration. The APTTs of EOQMB and pentadecanoic acid groups were longer than the APTT of heparin at 3.75 mg/mL concentration (P < 0.01 and P < 0.05). Compared with the 0.9% NaCl group, EOQMB prolonged the time by threefold and showed excellent activity among the three samples at 30 mg/mL. The effect of EOQMB on APTT was attributed to pentadecanoic acid as the main component of EOQMB, which considerably prolonged APTT at 30 mg/mL and was twice that of the 0.9% NaCl group. The samples were potent anticoagulants. The mechanism of APTT prolongation may be attributed to the inhibition of endogenous factors, such as VIII, IX, XI, XII, and/or common pathways (Wang et al., 2010).
PT mainly reflects the status of an exogenous coagulation system and prolongs when congenital coagulation factors III, V, VII, and X are absent (Wei et al., 2014). Compared with the NaCl group, the PTs of EOQMB and pentadecanoic acid showed significantly differences only when the concentration was higher than 15 mg/mL, and heparin concentration showed significant differences within the range of 3.75–30 mg/mL (P < 0.01). The PTs of EOQMB and pentadecanoic acid significantly differed from that of heparin within the concentration range of 1.87–30 mg/mL (P < 0.05 or P < 0.01), indicating that the inhibitions of EOQMB and pentadecanoic acid on the external coagulation pathway were weak. The fibrin clot formation activated by extrinsic pathway is considered to be a response of tissue injury (Qi et al., 2012). Several side effects, such as uncontrolled bleeding symptoms, are associated with the inhibition of coagulation by the extrinsic pathway (Pawlaczyk-Graja, 2018). Thus, the weak anticoagulation effect of EOQMB on the PT group may be considered an advantage to reduce the side effects.
The TT assay showed that the anticoagulant activities of EOQMB, pentadecanoic acid, and heparin increased with concentration. Compared with the 0.90% NaCl group, the TTs began to remarkably prolong when the concentrations of the above three samples were 3.75 mg/mL, and the results were statistically significant (P < 0.05, P < 0.01, and P < 0.05). The TTs of EOQMB and pentadecanoic acid were longer than the TT of heparin at 1.87 mg/mL but were statistically insignificant (P > 0.05). The above effects suggested that pentadecanoic acid might become an important anticoagulant ingredient in EOQMB. The TT of EOQMB at 30 mg/mL concentration was 92.32 s, and no significant difference was observed on the TT between EOQMB and heparin (P > 0.05, Fig. 4). TT mainly reflected the results of the time when FIB was changed to fibrin by thrombin (Wei et al., 2014), and the increments in TT suggested either impaired fibrin polymerization or thrombin inhibition (Qiu et al., 2017). The results indicated that the activities of EOQMB and pentadecanoic acid on common coagulation pathway were obvious at every concentration.
FIB mainly reflects the FIB content and plays a vital physiological action in coagulation. The standard curve of FIB was: lgT = −0.821lgC + 3.3316, R2 = 0.9865; the FIB value of EOQMB group at 30 mg/mL was one-tenth of that of the 0.90% NaCl group. Compared with the 0.90% NaCl group, the FIB values of EOQMB, pentadecanoic acid, and heparin group significantly decreased (P < 0.01 or P < 0.05). The results showed a dose-dependent manner within the concentration ranging from 1.87 mg/mL to 30 mg/mL. The FIB contents of EOQMB, pentadecanoic acid, and heparin at 30 mg/mL were 27.89 ± 6.45, 23.39 ± 8.45, and 31.96 ± 6.49 mg/dL, respectively, and the anticoagulant activities of EOQMB and pentadecanoic acid were better than the anticoagulant activity of heparin, but no statistical differences was observed (P > 0.05). Accordingly, the experimental results showed that EOQMB and pentadecanoic acid had a certain effect on the anticoagulation and fibrinolytic systems.
Given that the above effects of EOQMB on APTT, TT, and FIB are similar to those of heparin, EOQMB and its main component pentadecanoic acid have anticoagulant activity. Pentadecanoic acid and linoleic acid with other components in essential oil may have a synergistic anticoagulant effect (Benjamin et al., 2015; Jung-Hee et al., 2019) because a variety of fatty acids play an active role in human health (Itakura et al., 2011). The research results preliminarily demonstrated that pentadecanoic acid and EOQMB may inhibit the intrinsic pathways and/or common pathway or inhibit the conversion of FIB into fibrin or both.
4 Conclusions
The extraction yield of EOQMB was 0.08% ± 0.03%. Thirty components accounted were analyzed by GC–MS, and pentadecanoic acid was one of the main components accounting for 34.90%, which was further confirmed by the FT-IR observation. The IC50 values of EOQMB with DPPH radical, ABTS radical, and hydroxyl radical scavenging ability showed that it has good antioxidant activity but not as good as Vc. The effects of EOQMB on APTT, TT, and FIB are similar to those of heparin in vitro, and the fatty acids played a chief role in anticoagulant activity. This study proves the structure–activity relationship of EOQMB and its main components in the direction of antioxidation and anticoagulation.These findings indicate the EOQMB may act as a potential natural anticoagulant agent and provide a new idea for the medicinal development of Q. mongolica bark.
Acknowledgements
This work was supported by the programs of Jilin Province Development and Reform Commission (Grant No. 2019C045-4) and the Science and Technology Department of Jilin Province (Grant No. 20190101006JH and Grant No. 20190304102YY).
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- The effectiveness of antioxidant therapy in aspirin resistance, diabetes population for prevention of thrombosis. Biomed. Pharmacother.. 2016;83:277-282.
- [Google Scholar]
- In vitro evaluation of the antioxidant, cytoprotective, and antimicrobial properties of essential oil from Pistacia vera L. variety bronte hull. Int. J. Mole. Sci.. 2017;18:1212.
- [Google Scholar]
- Phytochemical analysis and in vitro antioxidant activity of Zingiber officinale. Free Radicals Antioxid.. 2011;1:75-81.
- [Google Scholar]
- Nicorandil prevents sirolimus-induced production of reactive oxygen species, endothelial dysfunction, and thrombus formation. J. Pharmacol. Sci.. 2015;127:284-291.
- [CrossRef] [Google Scholar]
- A review of odd-chain fatty acid metabolism and the role of pentadecanoic Acid (c15:0) and heptadecanoic Acid (c17:0) in health and disease. Molecules. 2015;20:2425-2444.
- [Google Scholar]
- Chemical composition and antimicrobial activity of volatile components from capitula and aerial parts of Rhaponticum acaule DC growing wild in Tunisia. Microbiol. Res.. 2012;163:87-95.
- [Google Scholar]
- Identification of the Components of Philadelphus coronarius L. Essential Oil. J. Essent. Oil Res.. 2006;18:423-426.
- [Google Scholar]
- Antibacterial and anti-quorum sensing molecular composition derived from Quercus cortex (Oak bark) Extract. Mole. 2015
- [CrossRef] [Google Scholar]
- Comparative study of chemical composition of the essential oils from three calligonum species growing-wild in Tunisian desert. J. Essential Oil Bear. Plants. 2011;14:11-22.
- [Google Scholar]
- A sulfated polysaccharide, fucans, isolated from brown algae Sargassum vulgare with anticoagulant, antithrombotic, antioxidant and anti-inflammatory effects. Carbohydr. Polym.. 2013;91:467-475.
- [Google Scholar]
- Composition and antimicrobial activity of essential oil of Kochia scoparia (L.) schrad. J. Essential Oil Bear. Plants 2012:15,5.
- [Google Scholar]
- In vitro anticoagulant and antioxidant activities of Jatropha gossypiifolia L. (Euphorbiaceae) leaves aiming therapeutical applications. Bmc Comp. Alternat. Med.. 2014;14(405):2-13.
- [Google Scholar]
- The ethanol extract of holotrichia diomphalia larvae, containing fatty acids and amino acids, exerts anti-asthmatic effects through inhibition of the GATA-3/Th2 signaling pathway in asthmatic mice. Molecules. 2019;852:1-22.
- [Google Scholar]
- On the role of vitamin C and other antioxidants in atherogenesis and vascular dysfunction. Proc. Soc. Exp. Biol. Med.. 1999;222:196-204.
- [Google Scholar]
- Essential oils from Carex meyeriana Kunth: Optimization of hydrodistillation extraction by response surface methodology and evaluation of its antioxidant and antimicrobial activities. Ind. Crops Prod.. 2018;124:669-676.
- [Google Scholar]
- Two-step method for encapsulation of oregano essential oil in chitosan nanoparticles: preparation, characterization and in vitro release study. Carbohydrate Polym.. 2013;95:50-56.
- [Google Scholar]
- Isolation of some compounds from nutmeg and their antioxidant activities. Czech J. Food Sci.. 2012;30:164-170.
- [Google Scholar]
- Chemical composition of fruit and stem essential oils of Lantana camara from northern India. Flav. Fragrance J.. 2003;18:376-379.
- [Google Scholar]
- Relationships between plasma fatty acid composition and coronary artery disease. J. Atheros. Thromb.. 2011;18:99-107.
- [CrossRef] [Google Scholar]
- Ecological comparison of Mongolian oak (Quercus mongolica Fisch. ex Ledeb.) community between Mt. Nam and Mt. Jeombong as a Long Term Ecological Research (LTER) site. J. Ecol. Environ.. 2011;34:75-85.
- [CrossRef] [Google Scholar]
- Arterial thrombus formation in cardiovascular disease. Nat. Rev. Cardiol.. 2011;8:502-512.
- [Google Scholar]
- Antioxidant Activity and Chemical Constituents of Microalgae Oil of Schizochytrium aggregatum. Adv. Mater. Res.. 2014;919:2022-2029.
- [Google Scholar]
- Lu, Y.Q., Deng, Z.H., 1989. Practical Infrared Spectrum Analysis, first ed. China: Publishing House of Electronics Industry, pp.145–146. ISBN 7-5053-0189-6/TN96.
- Essential oils from stem and leaves of Angelica urumiensis (Mozaffarian) from Iran. Natl. Prod. Res.. 2010;24:1347-1351.
- [CrossRef] [Google Scholar]
- Composition of the essential oil from Nuphar pumilum (Timm.) DC. Growing in Russia. J. Essent. Oil Res.. 2005;17:619-621.
- [CrossRef] [Google Scholar]
- Purification, characterization and immunomodulatory activity of polysaccharides from stem lettuce. Carbohydr. Polym.. 2018;188:236.
- [Google Scholar]
- Ethanol production form acorn starch by tannin tolerance mutant Pachysolen tannophilus. Energy Sourc. Part A Recov. Utiliz. Environ. Effects. 2018;40:1-7.
- [CrossRef] [Google Scholar]
- Analysis of Volatile Components in the Heartwood of Quercus liaotungensis Koidz and Q. mongolica Fisch. J. Food Sci.. 2021;42(06):265-272.
- [CrossRef] [Google Scholar]
- Polyphenolic-polysaccharide conjugates from flowers and fruits of single-seeded hawthorn (Crataegus monogyna Jacq.): chemical profiles and mechanisms of anticoagulant activity. Int. J. Biol. Macromol.. 2018;29:50-370.
- [CrossRef] [Google Scholar]
- Study of the volatile compounds from plum (Prunus domestica L. cv. Horvin) and estimation of their contribution to the fruit aroma. Ciência E Tecnologia De Alimentos. 2012;32:76-83.
- [Google Scholar]
- Chemical characteristic of an anticoagulant-active sulfated polysaccharide from Enteromorpha clathrata. Carbohydr. Polym.. 2012;90:1804-1810.
- [Google Scholar]
- Capture of anti-coagulant active ingredients from Moutan Cortex by platelet immobilized chromatography and evaluation of anticoagulant activity in rats. Biomed. Pharmac.. 2017;95:235-244.
- [CrossRef] [Google Scholar]
- In vitro antibacterial properties of essential oil and organic extracts of Premna integrifolia Linn. Arab. J. Chem.. 2016;9:S475-S479.
- [Google Scholar]
- Chemical diversity, antioxidant and antimicrobial activities of the essential oils from Indian populations of Hedychium coronarium Koen. Ind. Crops Prod.. 2018;112:353-362.
- [Google Scholar]
- Phytochemical evaluation, antioxidant assay, antibacterial activity and determination of cell viability (J774 and THP1 alpha cell lines) of P. sylvestris leaf crude and methanol purified fractions. Excli J.. 2016;15:85-94.
- [Google Scholar]
- Antimutagenic extract from Tinospora cordifolia and its chemical composition. J. Med. Plants Res.. 2010;4:2488-2494.
- [Google Scholar]
- Chemical characterization of the essential oil compositions and antioxidant activity from Iranian populations of Achillea wilhelmsii K.Koch. Ind. Crops Prod.. 2018;112:274-280.
- [Google Scholar]
- Chemical composition, antioxidant activity and thermal analysis of oil extracted from favela (Cnidoscolus quercifolius) seeds. Ind. Crops Prod.. 2017;97:368-373.
- [Google Scholar]
- Identification of the Sex Pheromone of the Diurnal Hawk Moth, Hemaris affinis. J. Chem. Ecol.. 2015;41:9-14.
- [Google Scholar]
- Potential antioxidant and anticoagulant capacity of low molecular weight fucoidan fractions extracted from Laminaria japonica. Int. J. Biol. Macromol.. 2010;46:6-12.
- [Google Scholar]
- Sulfation, anticoagulant and antioxidant activities of polysaccharide from green algae Enteromorpha linza. Int. J. Biol. Macromol.. 2013;58:225-230.
- [Google Scholar]
- Chemical constituents from barks of Quercus mongolica. Chin. Herbal Med.. 2014;45(21):3062-3066.
- [Google Scholar]
- Analysis of volatile components of lablab(Dolichos lablab L.) from Northeast China using HS-SPME/GC-MS. J. Northwest A & F Univ.. 2015;43:79-84.
- [Google Scholar]
- In vitro antibacterial activity test of the extract of tussah leaves. Sci. Sericulture. 2016;42(2):5-9.
- [Google Scholar]
- Effects of chitin and sepia ink hybrid hemostatic sponge on the blood parameters of mice. Mar. Drugs. 2014;12:2269-2281.
- [Google Scholar]
- Identification of essential oil components by gas chromatography/mass spectroscopy : Robert P. Adams, Allured Publishing Corporation, Illinois. Biochem. Syst. Ecol.. 2005;24:1902-1903.
- [Google Scholar]
- Quality, composition, and antioxidant activity of virgin olive oil from introduced varieties at Liangshan. LWT - Food Sci. Technol.. 2017;78:226-234.
- [Google Scholar]
- High hydrostatic pressure treatments enhance volatile components of pre-germinated brown rice revealed by aromatic fingerprinting based on HS-SPME/GC–MS and chemometric methods. Food Res. Int.. 2017;91:103-114.
- [CrossRef] [Google Scholar]
- Isolation, purification, structural analysis and coagulatory activity of water-soluble polysaccharides from Ligustrum lucidum Ait flowers. Chem. Cent. J.. 2017;11:98.
- [Google Scholar]
- Optimization for ultrasound extraction of polyphenol from Quercus mongolica leaves and its antioxidant activity. J. Northeast Forest. Univ.. 2010;39:70-73.
- [Google Scholar]
- Multiple glacial refugia for cool-temperate deciduous trees in northern East Asia: the Mongolian oak as a case study. Mol. Ecol.. 2016;24:5676-5691.
- [Google Scholar]
- Comparison of volatile compounds in two brandies using HS-SPME coupled with GC-O, GC-MS and sensory evaluation. South African J. Enol. Viticult.. 2011;32:9-20.
- [Google Scholar]
- Characterization of the aroma compounds from stewing lamb. Sci. Technol. Food Ind.. 2016;37:284-293.
- [Google Scholar]
- Chemical composition and antimicrobial activity of the essential oil of Sagittaria trifolia. Chem. Nat. Compd.. 2006;42:520-522.
- [Google Scholar]







