Translate this page into:
Chemical composition variations, allelopathic, and antioxidant activities of Symphyotrichum squamatum (Spreng.) Nesom essential oils growing in heterogeneous habitats
⁎Corresponding author at: Plant Production Department, College of Food & Agriculture Sciences, King Saud University, P.O. Box 2460, Riyadh 11451, Saudi Arabia. dgawad84@mans.edu.eg (Ahmed M. Abd-ElGawad) aibrahim2@ksu.edu.sa (Ahmed M. Abd-ElGawad)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
The present study aimed to analyze the chemical composition of Symphyotrichum squamatum EOs growing in two different habitats to explore the ecological implication on the EOs production and evaluate their antioxidant and allelopathic potentialities. The EOs from the aerial parts collected from coastal Mediterranean belt and inland abandoned habitats in the Nile Delta of Egypt, were extracted and analyzed using gas chromatography-mass spectrometry. Sixty compounds were characterized as overall constituents of EOs from both samples. Sesquiterpenes were the main component and represented by 69.77% and 88.68% from coastal and inland sample, respectively. The coastal sample attained a relatively high content of monoterpenes compared to the inland sample. Major compounds from the EOs of the coastal habitat sample, were humulene epoxide, (-)-spathulenol, (-)-caryophyllene oxide, germacrene D, and α-humulene representing 59.72%. However, β-pinene, germacrene D, α-humulene, α-muurolene, humulene epoxide, (-)-caryophyllene oxide, and β-cadinene were the major compounds of EOs of the inland habitat sample, representing 63.70%. The correlation analysis revealed more correlation between the Egyptian inland S. squamatum and the Japanese ecospecies. However, the Egyptian coastal S. squamatum and Turkish ecospecies were more correlated to each other. The present data suggested that chemotypes of S. squamatum maintain their typical pattern despite ecological or climatic differences. The EOs of S. squamatum showed moderate antioxidant activity, wherein coastal and inland EOs have an IC50 value of 382.53 and 559.63 μL L−1, respectively. Also, the EOs from both habitats showed moderate allelopathic activity against the noxious weed Bidens pilosa. However, the activity of the coastal sample was more than inland one and could be attributed to the content of the major compounds, especially the oxygenated terpenes.
Keywords
Symphyotrichum squamatum
Essential oils
Environmental variables
PCA
Allelopathic potential
Antioxidant activity
1 Introduction
Essential oils (EOs) derived from different plant parts exhibit aromatic odors and are valuable resources of various secondary metabolites. In most of the cases, the major components of EOs are terpenes (Kim et al., 2014a). Essential oils have been commonly used in several applications in aromatherapy (Sharifi-Rad et al., 2018a, 2018b). Since ancient times, EOs have been used in folklore medicine due to their potent biological activities (Lang and Buchbauer, 2012). The pleasant fragrance or flavor of EOs have encouraged their use in significant amounts in cosmetic, perfume, pharmaceutical and food industries (Raut and Karuppayil, 2014; Salehi et al., 2018; Sharifi-Rad et al., 2018c).
Family Asteraceae represents about 10% of the overall of the flora around the world, with approximately 1535 genera and 23,000 plant species (Nakajima and Semir, 2001). Also, Asteraceae is one of the biggest family of the Egyptian flora (Barakat et al., 2014). The EOs from Asteraceae species include various components that might have several commercial applications in perfume and liquor industries. Also, several pharmacological potentials such as larvicide, nematicide, antispasmodic, genotoxic, antimicrobial, antifungal, and allelopathic properties were stated for the EOs of these plants (Dias et al., 2009).
The species of genus Aster (syn. Symphyotrichum) are widely distributed around the world, including Africa, Asia, North America, and Europe (Mabberley, 2008). The S. squamatum introduced to Egypt from Latin America, and the first record in Egypt back to early of the 1970s. Now it is a wide and common distributed plant in Egypt, and it is found with high biomass in different habitats such as wastelands, abandoned fields, fields of orchards, fields of crops, railways, high ways, edges of canals, drains, and lakes (Boulos and El-Hadidi, 1994).
It has been reported that several candidates of this genus are used in traditional medicines for the treatment of chronic bronchitis, pertussis, and pneumonia. Several biological activities associated with these plants and their metabolites were tested for antifungal, (Lanzotti, 2005), antitumor (Kim et al., 2014b), antioxidant and cytotoxicity properties (Bibi et al., 2011). Several bioactive metabolites were characterized from Aster species including diterpenes, monoterpene, sesquiterpenoids, flavones, coumarins, polyacetylenes, triterpene glycosides, phenolic compounds, saponins, peptides, benzofurans, sugars and esters (Choi, 2012; Dias et al., 2009; Kim et al., 2014b; Miyazawa and Kameoka, 1977; Nemanja et al., 2015; Shao et al., 1995a, 1995b).
Up to our knowledge, there are only two studies investigating the chemical composition of the EOs of Symphyotrichum squamatum (Spreng.) Nesom (syns: Aster subulatus Michx. & Aster squamatus (Spreng.) Hieron.) collected from Turkey (Ayaz et al., 2017) and Japan (Miyazawa and Kameoka, 1977). The results of these studies indicated that sesquiterpene, elemol, and the hydrocarbon, hexadecanoic acid, represented the main components of the Turkish ecospecies, while β-pinene and p-cymene were the main compounds of the Japanese one (Ayaz et al., 2017; Miyazawa and Kameoka, 1977). Various studies confirmed that the chemical composition of EOs is dependent on the plant organ, season, prevailing temperature, water availability, altitude, soil fertility, and genetic pool (Abd El-Gawad et al., 2019; Abd El-Gawad, 2016; Khazaie et al., 2008). Further, no study has examined the antioxidant or allelopathic activities of the EOs from S. squamatum.
Therefore, our study is aimed to (i) determine the chemical composition of the EOs of S. squamatum collected from two different habitats in Egypt, (ii) study the correlation between the chemical composition of the EOs and the soil variables of these habitats, (iii) correlate the EOs of the Egyptian ecospecies of S. squamatum from different habitats with other reported ecospecies, (iv) asses the antioxidant activity of the EOs, and (v) evaluate the allelopathic effect of the EOs against the noxious weed Bidens pilosa as potential green eco-friendly bioherbicide.
2 Material and methods
2.1 Plant materials
The aerial parts of S. squamatum were collected during March (flowering stage) from two different habitats in Egypt. The first sample was collected from the Mediterranean coastal belt at Gamsa City, Al-Dakahlia Governorate, Egypt (31°26′35.4″N 31°33′35.5″E), and was called coastal sample. The second sample was collected at the same time from an abandoned area near Mansoura University, Mansoura, Egypt (31°02′22.2″N 31°21′09.7″E). This location is in the middle of the Nile Delta and was called inland sample. The collected samples were cleaned from dust and dried at room temperature (25 °C ± 2). The dried plant materials were ground into a fine powder using a grinder (IKA® MF 10 Basic Microfine Grinder Drive, Breisgau, Germany) and stored in paper bags until further analyses. Voucher specimen with code: Mans.0010272703 was added in the herbarium of Botany Department, Faculty of Science, Mansoura University, Egypt.
2.2 Extraction and analysis of the EOs
The EOs were separately extracted from 300 g of aerial parts of S. squamatum from each sample/habitat (coastal and inland) immediately after preparation by hydrodistillation using a Clevenger-type apparatus for three h. The oil layer of the oil was separated using diethyl ether and dried with anhydrous sodium sulfate (0.5 g). This extraction was repeated twice afforded two samples of EOs for each location. The extracted EOs were stored in sealed air-tight glass vials at 4 °C until further analysis.
The EOs components of the extracted samples were analyzed separately and identified following GC-MS analysis. The GC-MS analysis was performed via chromatography-mass spectrometry instrument at the Department of Medicinal and Aromatic Plants Research, National Research Center, Egypt. The GC-MS systems consist of TRACE GC Ultra Gas Chromatographs (THERMO Scientific Corp., USA), accompanied by thermo mass spectrometer detector (ISQ Single Quadrupole Mass Spectrometer; Model ISQ spectrometer). The GC-MS system contained a TR-5 MS column with a dimension of 30 m × 0.32 mm ID × 0.25 μm. Helium gas was used as a carrier gas with a flow rate of 1.0 mL min−1 and a split ratio of 1:10. The temperature cycle was as follows: 60 °C for one min and then rising at 4.0 °C per min until 240 °C and held for one min. The detector and injector were held at 210 °C. An aliquot of one μL of the diluted samples (1:10 hexane, v/v) was always injected. Mass spectra were derived by electron ionization (EI) at 70 eV, using a spectral range of m/z 40–450.
2.3 Identification of EOs constituents
The chemical compounds were identified according to their retention indices (relative to n-alkanes C8-C22), comparison with the authentic constituents available in our laboratories, and comparison of their mass spectra with that of Wiley spectral libraries databases (NIST AMDIS). The compound percentage was calculated based on the peak area derived from gas chromatography.
2.4 Antioxidant activity
The antioxidant scavenging activity of the extracted EOs from coastal and inland samples was evaluated according to their ability to scavenge the 1,1-Diphenyl-2-picrylhydrazyl (DPPH) free radical. In accordance with Sharma and Bhat (2009), a reaction mixture of two mL of DPPH (0.15 mM) and equal amount of various concentrations (50, 100, 200, 300, 400, 500, and 600 μL L−1) of either EOs, in methanol, or ascorbic acid (standard antioxidant) was prepared in test tubes. The test tubes were shaken vigorously and incubated in dark condition at 25 °C for 30 min. The absorbance was measured by a spectrophotometer (Spectronic® 21D model) at 517 nm. The antioxidant scavenging activity was expressed in percentage as follows:
The IC50 (the concentration of the EOs required to scavenge 50% of the initial concentration of DPPH) was also calculated graphically.
2.5 Allelopathic bioassay
The allelopathic activity of the extracted EOs was evaluated against B. pilosa as one of the dangerous noxious weeds. Ripe seeds of B. pilosa weed were collected in June from the garden of Mansoura University, Mansoura, Egypt (30°38′33.5″N 31°59′50.4″E). The collected seeds were surface sterilized by 0.3% NaOCl for three min. To examine the allelopathic effect of the EOs, various concentrations (100, 200, 400, 600, 800, and 1000 µL L−1) were prepared in Tween® 80 (Sigma–Aldrich, Germany) as an emulsifier. Twenty B. pilosa seeds were placed in Petri dishes (9 cm) lined with a filter paper Whatman No. 1. and five mL of each EOs concentration or tween (as control treatment) was added. The Petri dishes were sealed with Parafilm® tape and incubated in a growth chamber at 27 °C light regime conditions of 16 h/8h light/dark for five days (Abd El-Gawad, 2016). The shoot and root lengths of all seedlings per each plate were measured, and the allelopathic inhibition of root and shoot growth was calculated, with respect to control, as follows:
2.6 Statistical analysis
The data of the allelopathic bioassay of the EOs were expressed as the percentage of inhibition with respect to control and were subjected to Generalized Linear Models (GLM) analysis, using the STATISTICA (version 7) software system (StatSoft, Inc. Tulsa, Oklahoma, USA, www.statsoft.com). The data of antioxidant, in triplicates, were subjected to one-way ANOVA followed by Duncan's test at probability level 0.05 using CoStat (version 6.311, CoHort Software, USA, www.cohort.com). The data of the EOs composition derived from GC-MS analysis of the coastal and inland samples from the Egyptian ecospecies as well as the data of the EOs reported from the Japanese and Turkish ecospecies were subjected to Pearson similarity correlation. The dataset consists of the concentrations (%) of 99 chemical compounds that were identified in the three investigated ecospecies. Also, the dataset was submitted to principal component analysis (PCA) to determine whether a significant difference exists between different ecospecies, based on the EOs composition. The software XLSTAT (version 2018, Addinsoft, NY, USA, www.xlstat.com) was used in the analysis of Pearson correlation and PCA.
3 Results and discussion
3.1 Chemical constituents of S. squamatum EOs
The hydro-distillation extraction of the aerial plant parts of coastal and inland S. squamatum yielded 0.017% and 0.014% (v/w) of the EOs, respectively. The Japanese ecospecies of S. squamatum yielded more EOs (0.03%) than that of the Egyptian ecospecies (Miyazawa and Kameoka, 1977). The yield was more pronounced in the coastal sample, where the habitat is affected by salinity from the Mediterranean Sea. Bourgou et al. (2010) reported an increase of the EOs yield in Nigella sativa by increasing the salinity.
An overall of sixty compounds of EOs from both coastal and inland samples, representing 100% of the total mass, were characterized based on GC-MS analysis. Forty-six compounds were identified from the coastal sample and forty-nine from the inland sample of S. squamatum (Table 1). Among the identified compounds, sesquiterpenes represented the main component comprising 69.77% and 88.68% of the total EOs from the coastal and inland sample, respectively (Fig. 1). Sesquiterpene hydrocarbons were characterized as major components of EOs of the inland sample (47.39%), while the EOs of the coastal sample has 27.13% as sesquiterpene hydrocarbons of the total mass. On the other hand, the oxygenated sesquiterpenes were the major components in the coastal sample (61.55%) while they were represented by 22.38% in the inland sample (Fig. 1). These findings were in consonance with that of the Turkish ecospecies which attained high content of sesquiterpenes in its EOs (Ayaz et al., 2017). It is pertinent to mention here that sesquiterpenes have been reported as dominant group of the EOs of other related species of Aster such as A. albanicus (Nemanja et al., 2015), A. handelii (Xiao-ping and Xiaoping, 2006), A. lanceolatus (Dias et al., 2009), and A. tataricus (Choi, 2012).
No
Rta
KILitb
KIExpc
Compound
Area %
Identificatione
Inland
Coastal
Monoterpene hydrocarbons
1
4.44
933
939
(-)-α-Pinene
1.07d ± 0.02
–
MS; KI
2
5.43
1005
1001
α-Phellandrene
0.28 ± 0.01
–
MS; KI
3
5.58
974
975
β-Pinene
24.16 ± 0.04
1.99 ± 0.02
MS; KI
4
5.92
991
993
α-Myrcene
0.25 ± 0.01
–
MS; KI
5
7.12
1024
1028
D-Limonene
0.63 ± 0.01
–
MS; KI
Oxygenated monoterpenes
6
11.33
1183
1180
Pinocarveol
0.52 ± 0.01
1.15 ± 0.01
MS; KI
7
12.25
1162
1161
Pinocarvone
1.09 ± 0.02
1.53 ± 0.02
MS; KI
8
13.68
1193
1190
(1R)-(-)-Myrtenal
0.96 ± 0.01
2.28 ± 0.02
MS; KI
9
17.12
1285
1287
Bornyl acetate
0.21 ± 0.01
0.70 ± 0.01
MS; KI
Sesquiterpene hydrocarbons
10
19.29
1432
1429
β-Gurjunene
0.48 ± 0.01
–
MS; KI
11
20.67
1376
1379
α-Copaene
1.38 ± 0.01
0.50 ± 0.01
MS; KI
12
21.00
1505
1509
(-)-α-Bourbonene
0.90 ± 0.01
0.43 ± 0.01
MS; KI
13
21.30
1386
1383
α-Elemene
2.83 ± 0.02
2.55 ± 0.03
MS; KI
14
21.50
1402
1398
Longifolene
0.31 ± 0.01
–
MS; KI
15
22.48
1337
1331
trans-Caryophyllene
4.71 ± 0.03
1.66 ± 0.02
MS; KI
16
22.95
1351
1356
α-Cubebene
2.13 ± 0.02
0.52 ± 0.01
MS; KI
17
23.07
1436
1439
trans-α-Bergamotene
–
0.22 ± 0.01
MS; KI
18
24.15
1439
1434
Aromadendrene
–
0.44 ± 0.01
MS; KI
19
24.00
1454
1450
α-Humulene
7.43 ± 0.04
5.46 ± 0.03
MS; KI
20
24.84
1477
1472
γ-Muurolene
3.43 ± 0.02
0.84 ± 0.02
MS; KI
21
25.06
1480
1483
Germacrene D
8.59 ± 0.05
5.87 ± 0.03
MS; KI
22
25.42
1494
1490
α-Selinene
1.14 ± 0.01
1.31 ± 0.01
MS; KI
23
25.65
1494
1492
Bicyclogermacrene
0.32 ± 0.01
2.30 ± 0.02
MS; KI
24
25.82
1499
1502
α-Muurolene
6.84 ± 0.04
1.60 ± 0.01
MS; KI
25
26.13
1503
1503
Germacrene A
1.77 ± 0.01
1.31 ± 0.01
MS; KI
26
26.41
1506
1508
α-Amorphene
–
0.22 ± 0.01
MS; KI
27
26.58
1473
1470
β-Cadinene
4.78 ± 0.03
1.90 ± 0.02
MS; KI
28
27.65
1542
1541
α-Calacorene
0.35 ± 0.01
–
MS; KI
Oxygenated sesquiterpenes
29
24.94
1659
1662
Patchouli alcohol
1.18 ± 0.02
0.31 ± 0.01
MS; KI
30
26.78
1646
1644
Isolongifolan-8-ol
–
0.31 ± 0.01
MS; KI
31
28.60
1482
1481
1,5-epoxysalvial-4(14)-ene
–
0.52 ± 0.01
MS; KI
32
28.90
1580
1583
Cubedol
0.22 ± 0.01
–
MS; KI
33
28.99
1578
1580
(-)-Spathulenol
0.43 ± 0.01
15.49 ± 0.04
MS; KI
34
29.13
1580
1577
(-)-Caryophyllene oxide
6.00 ± 0.02
14.42 ± 0.04
MS; KI
35
29.65
1625
1631
Aromadendrene oxide-1
0.47 ± 0.01
0.39 ± 0.01
MS; KI
36
30.25
1605
1601
Humulene epoxide
6.28 ± 0.03
18.48 ± 0.05
MS; KI
37
30.65
1794
1799
Verrucarol
0.81 ± 0.01
0.67 ± 0.01
MS; KI
38
30.94
1642
1637
Cubenol
0.51 ± 0.01
0.67 ± 0.01
MS; KI
39
31.17
1625
1628
Isospathulenol
–
0.94 ± 0.01
MS; KI
40
31.64
1653
1656
α-Cadinol
1.58 ± 0.03
1.03 ± 0.02
MS; KI
41
31.74
1645
1649
Torreyol
1.11 ± 0.02
0.59 ± 0.01
MS; KI
42
31.87
1633
1630
Alloaromadendrene oxide-2
–
0.29 ± 0.01
MS; KI
43
32.08
1636
1631
.tau.Muurolol
0.84 ± 0.01
1.44 ± 0.02
MS; KI
44
32.22
1620
1626
Calarene epoxide
0.53 ± 0.02
1.20 ± 0.01
MS; KI
45
32.63
1591
1596
(+) spathulenol
0.61 ± 0.01
1.47 ± 0.03
MS; KI
46
33.02
1613
1618
Geranyl isovalerate
0.59 ± 0.01
0.67 ± 0.01
MS; KI
47
33.34
1678
1673
Z-Nerolidol-epoxyacetate
–
0.22 ± 0.01
MS; KI
48
33.23
1584
1589
Isoaromadendrene epoxide
0.35 ± 0.01
–
MS; KI
49
33.44
1634
1636
Ledene oxide II
0.25 ± 0.01
–
MS; KI
50
34.07
1631
1637
8-Cedren-13-ol
0.28 ± 0.01
0.59 ± 0.01
MS; KI
51
34.39
1666
1669
Alloaromadendrene oxide-1
–
0.36 ± 0.01
MS; KI
52
34.71
1634
1632
trans-Longipinocarveol
0.34 ± 0.01
0.75 ± 0.01
MS; KI
53
35.55
1763
1758
Aristolene epoxide
–
0.29 ± 0.01
MS; KI
54
38.73
1845
1848
Hexahydrofarnesyl acetone
–
0.45 ± 0.01
MS; KI
Diterpenoids
55
38.26
1811
1808
Phytane
–
0.25 ± 0.01
MS; KI
56
38.80
1845
1839
Phytone
0.25 ± 0.01
–
MS; KI
57
40.25
2218
2215
Phytol, acetate
–
0.34 ± 0.01
MS; KI
Oxygenated hydrocarbons
58
28.19
1623
1629
2-Hydroxy-2,4,4-trimethyl-3-(3-methylbuta-1,3-dienyl)cyclohexanone
–
0.56 ± 0.01
MS; KI
59
31.06
1655
1650
Acetic Acid 1-[2-(2,2,6-trimethylbicyclo[4.1.0]hept-1-yl)-ethyl]-vinyl ester
0.33 ± 0.01
0.88 ± 0.01
MS; KI
60
36.17
2139
2144
Ethyl linoleate
0.48 ± 0.01
1.64 ± 0.02
MS; KI
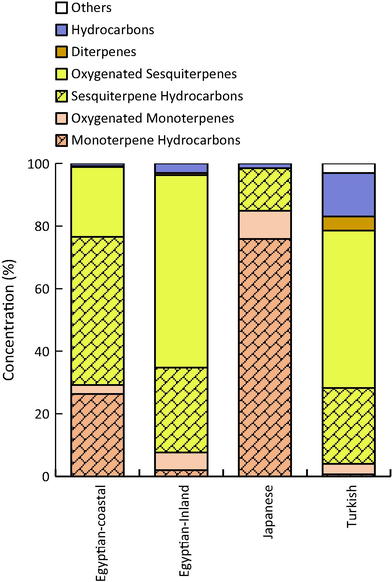
Percentage of various classes of the chemical compounds of EOs from coastal and inland samples of Egyptian S. squamatum as well as Japanese (Miyazawa and Kameoka, 1977) and Turkish ecospecies (Ayaz et al., 2017).
Contrarily, the EOs of the Japanese ecospecies was characterized by the dominance of monoterpenes (Miyazawa and Kameoka, 1977) which represented 84.88% of the total mass. Also, our results showed that the Egyptian ecospecies have a relatively high content of monoterpenes, but the coastal habitat sample attained higher content than that of the inland sample. Moreover, other species of Aster have been reported to contain monoterpenes as the major class of their EOs such as A. ageratoides (Miyazawa et al., 2008), A. poliothamnus (Tu et al., 2006), A. spathulifolius (Kim et al., 2014a), and A. scaber (Lee et al., 2012). In the present study, diterpenoids and hydrocarbons were determined as minor classes of the EOs in both inland and coastal samples.
In the EOs of the coastal sample, humulene epoxide, (-)-spathulenol, (-)-caryophyllene oxide, germacrene D, and α-humulene were identified as major compounds representing 59.72% of the EOs total mass. However, β-pinene, germacrene D, α-humulene, α-muurolene, humulene epoxide, (-)-caryophyllene oxide, and β-cadinene were the major compounds of the inland sample of the Egyptian S. squamatum ecospecies, where they represented 63.70% of its EOs (Table 1). Other minor compounds are listed in detail in Table 1.
In a general overview of the chemical composition of EOs of the two samples, we observed that the two samples include most of the components with different percentages. All the major components of the two samples especially germacrene D, β-pinene, spathulenol, and caryophyllene oxide were already reported as major compounds in the EOs of other species of Aster such as A. subulatus, A. albanicus, A. spathulifolius and A. lanceolatus (Ayaz et al., 2017; Dias et al., 2009; Kim et al., 2014a; Nemanja et al., 2015).
3.2 Correlation between S. squamatum ecospecies based on EOs composition
The analysis of PCA showed that the two samples of the Egyptian S. squamatum, as well as the Turkish and Japanese plants, were more comparable in the composition of the EOs (Fig. 2). Specifically, more correlation was found between the Egyptian inland S. squamatum of the present study and the Japanese ecospecies. These two samples were also correlated to the β-pinene, D-limonene, β-cadinene, and trans-caryophyllene. Also, β-pinene represented 40.9% and 24.2% of the total EOs from the Japanese and Egyptian inland S. squamatum, respectively.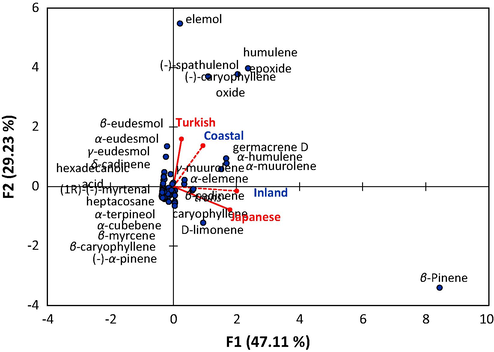
Principal component analysis (PCA) based on the chemical composition of the EO derived from Egyptian ecospecies of S. squamatum collected from Mediterranean coastal desert (coastal) and inland abandoned habitat (inland) as well as Turkish and Japanese ecospecies.
On the other hand, the other two samples (Egyptian coastal and Turkish S. squamatum) were more correlated to each other, and showed a close relation to elemol, (-)-caryophyllene oxide, (-)-spathulenol, humulene epoxide, α-humulene, α-muurolene, and germacrene D. This correlation could be ascribed to the similarity in the climate of both geographical regions of the Mediterranean climate (Ayaz et al., 2017). The formation of EOs in plants is profoundly affected by climatic conditions, particularly temperature, solar radiation, day length, and water supply (Baser and Buchbauer, 2015). Moreover, the variation in the EOs can be attributed to the habitat structure, population genetics, soil type, and stress condition (Abd El-Gawad, 2016; Khazaie et al., 2008).
Pearson correlation coefficient analysis revealed a significant positive correlation between the inland sample and the Japanese ecospecies (0.786) as well as the coastal sample (0.376) (Table S1). However, the Japanese ecospecies showed a negative correlation with the Turkish ecospecies (−0.023).
Generally, the rest of major compounds didn’t show a specific correlation to any samples (Fig. 2). The present data revealed that the chemotypes of S. squamatum maintain their typical pattern despite ecological or climatic differences. Environmental factors induce the production of specific chemicals pattern by activation of the gene expression. This means that the potential to produce bioactive compounds is genetically coded (Franz, 1993).
On the other hand, our results of soil analysis revealed a significant variation in the organic carbon and salinity between the two locations (Table S2). Salinity stress is one of the most important factors that can affect the composition of EOs in plants. Changes in the composition of the EOs in response to salinity have been reported in various plants such as Salvia officinalis (Taarit et al., 2010), Nigella sativa (Bourgou et al., 2010), and Ocimum basilicum (Tarchoune et al., 2013). Germacrene D and caryophyllene oxide (major compounds in the present study) were induced in Ocimum basilicum due to salinity (Hassanpouraghdam et al., 2011).
3.3 Antioxidant activity of S. squamatum EOs
The EOs from the aerial parts of S. squamatum showed moderate radical scavenging activity of the DPPH in a concentration-dependent manner (Table 2). The EOs from the coastal sample of S. squamatum revealed more antioxidant activity than that of the inland sample, where they attained an IC50 value of 382.53 μL L−1 and 559.63 μL L−1, respectively. However, the IC50 value for ascorbic acid (standard antioxidant) was 107.81 μL L−1 (Table 2). The significant variation in the antioxidant activity between the two samples could be ascribed to the variation in the composition of the EOs.
Conc. (μL L−1)
Scavenging activity %
LSD0.05
Coastal sample
Inland sample
Ascorbic acid
50
17.77 ± 0.74
5.00 ± 0.39
44.02 ± 1.21
2.00
100
24.88 ± 0.51
13.32 ± 0.82
50.94 ± 1.33
3.89
200
39.30 ± 0.86
23.44 ± 0.80
58.95 ± 1.29
4.15
300
48.83 ± 0.78
28.32 ± 0.82
64.22 ± 0.62
3.62
400
51.05 ± 1.13
39.14 ± 0.78
82.77 ± 0.66
3.96
500
59.77 ± 1.33
45.00 ± 1.17
88.87 ± 0.82
5.86
600
64.57 ± 1.76
51.37 ± 1.29
98.13 ± 1.48
7.38
IC50 (μL L−1)
382.53
559.63
107.81
The EOs are considered as an important class of the allelochemicals that are enhanced in the plant under stress conditions such as salinity stress. Thus, as we expect, the extracted EOs sample from the coastal area of the Mediterranean Sea to show more antioxidant activity. Under salt stress condition, the reactive oxygen species (ROS) are generated in plant cells, and in consequence, the antioxidant defense systems (enzymatic and non-enzymatic) are triggered (El-Shora and Abd El-Gawad, 2015a, 2015b). It is worth mentioning here that plants produce a vast array of chemical compounds specific to their habitats. These compounds play a significant role in stress amelioration, enhancement of the defense system, as well as communication with other organisms including herbivores, insects, pathogens, and other plants (Jones and Dangl, 2006).
However, the general profile of the EOs of the two samples of S. squamatum was comparable, though the composition is so different. The sample of the coastal location has a high content of oxygenated compounds, particularly sesquiterpenes, whereas it was triple fold for the inland sample (Fig. 1). This explains the more antioxidant activity of the coastal sample compared to the inland one. Therefore, the antioxidant activity of the coastal sample of S. squamatum might be attributed to the synergistic or singular effect of the major compound(s) identified in this sample such as humulene epoxide, (-)-spathulenol, (-)-caryophyllene oxide, germacrene D, and α-humulene. The synergistic activity of the terpenoid compounds was reported for other terpenoid compounds in other plants (Chaubey, 2012; Mitić et al., 2018).
On the other hand, the antioxidant activity of the inland sample could be attributed to β-pinene, germacrene D, α-humulene, α-muurolene, humulene epoxide, (-)-caryophyllene oxide, and β-cadinene. Nevertheless, the other minor compounds could play a significant role in antioxidant activity, even at low concentration (Carrillo and Tena, 2006). It is possible that the minor constituents may be implicated in synergism with the other active compounds (Hou et al., 2007; Lattaoui and Tantaoui-Elaraki, 1994).
Caryophyllene oxide was reported as an antioxidant compound in the EOs of Cullen plicata and Allophylus africanus (Abd El-Gawad, 2016; Balogun et al., 2014). Usually, the EOs rich in caryophyllene and its isomers α-humulene and β-caryophyllene possess biological activity (Sabulal et al., 2006). The α-humulene and β-pinene have been reported as antimicrobial agents (Jirovetz et al., 2006), while α-humulene, caryophyllene oxide were showed cytotoxic activity (Hou et al., 2007). Therefore, these major compounds could be responsible for the observed antioxidant activity of the EOs of S. squamatum.
3.4 Allelopathic activity of S. squamatum EOs
The EOs of S. squamatum aerial parts showed significant allelopathic activity against the root and shoot growth of the noxious weed Bidens pilosa (Fig. 3 & Table S3). The inhibition was dose-dependent. The root system of B. pilosa was inhibited more than shoot system under the effect of the EOs. This inhibition could be ascribed to the direct contact of the radicle with the EOs or even due to the higher permeability of the root cells (Abd El-Gawad et al., 2018). The present results showed significant variation in the allelopathic activity between the coastal and inland samples of S. squamatum (Fig. 3). This variation could be attributed to variation in the content of the EOs which is affected indirectly either by the soil properties (Table S2) or the climatic conditions. Soil analysis of the coastal desert sample revealed significantly high salinity compared to the inland sample due to the effect of the Mediterranean Sea. Also, salinity has been reported as a stress factor responsible for the induction of the EOs in plants (Hassanpouraghdam et al., 2011).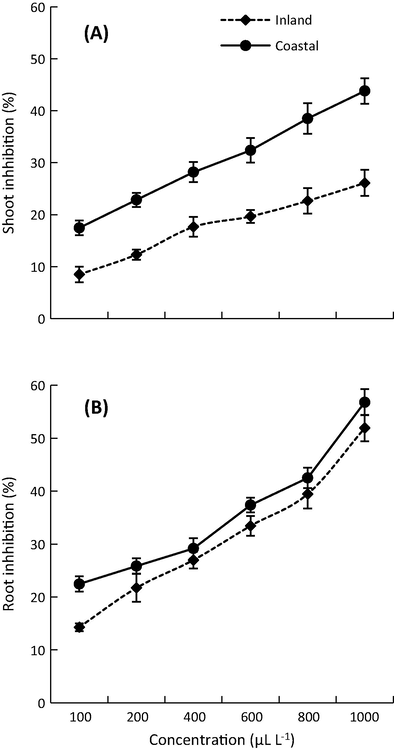
Inhibitory effect of the EOs from the aerial parts of S. squamatum collected from inland and costal deserts on the shoot (A), and root (B) growth of B. pilosa.
The variation in the allelopathic activity is consonant with the antioxidant activity, and it might be correlated to the variation in composition of the EOs between the two samples. The variations in soil factors, light, temperature, nutrients, water content, daylength, genetic pool, and harvesting time have been reported to influence the EOs composition and hence affect biological activities such as allelopathy (Abd El-Gawad et al., 2019).
The allelopathic activity of the EOs against B. pilosa was studied for other donor species such as Xanthium strumarium (Abd El-Gawad et al., 2019), Cullen plicata (Abd El-Gawad, 2016), Artemisia scoparia (Kaur and Batish, 2010), Tagetes minuta (Arora et al., 2017), Eucalyptus citriodora (Setia et al., 2007). The activity of the EOs from these plants against B. pilosa could be ranked in the following order: E. citriodora > C. plicata > X. strumarium > T. minuta > S. squamatum (present study) > A. scoparia. This variation in the allelopathic activity might be ascribed to the variation in the EOs composition.
The allelopathic activity of the EOs from S. squamatum could be ascribed to the activity of the major compounds which act either singular or synergistic. Most of the major compounds of the EOs were reported as allelochemicals such as germacrene D (Dali and Xinru, 1996), spathulenol (Nishimura and Mizutani, 1995), caryophyllene oxide (Abd El-Gawad, 2016), humulene epoxide (Tellez et al., 2000), and pinene (Wang and Zhu, 1996).
Although S. squamatum produce a little amount of the EOs, it showed promising biological activities (Antioxidant and allelopathic). Also, this plant has a wide range of distribution in Egypt, so significant biomass can be obtained easily and could be integrated in EOs production. As a weed, this may be a potential way to control this plant as well as to become a good resource for bioactive compounds.
4 Conclusion
The EOs of the Egyptian S. squamatum is richer than the Turkish or Japanese ecotypes in the chemical compounds where it contained 60 compounds with the predominance of sesquiterpenes. A substantial variation was observed in the compound diversity in the EOs between the coastal and inland samples of Egyptian S. squamatum, while the variation in the concentration was little different. This might be attributed to the soil factors particularly the salinity and organic matter content. Humulene epoxide, (-)-spathulenol, (-)-caryophyllene oxide, germacrene D, and α-humulene were identified as major compounds in the coastal sample. However, β-pinene, germacrene D, α-humulene, α-muurolene, humulene epoxide, (-)-caryophyllene oxide, and β-cadinene were the major compounds of the inland sample of the Egyptian S. squamatum ecospecies. The PCA showed more correlation between the Egyptian inland S. squamatum and the Japanese ecospecies, while the Egyptian coastal S. squamatum and Turkish were more correlated to each other, reflecting the effect of the climatic factor. The present data revealed that the chemotypes of S. squamatum keep their typical pattern in spite of the ecological or climatic difference. The EOs of S. squamatum showed moderate antioxidant and allelopathic activities. However, the coastal sample had more allelopathic potential than that of the inland one and may be attributed to the oxygenated terpenes content. Further study is needed for the separation, characterization, and evaluation of the modes of action of the identified major compounds either singular or in combination.
Acknowledgements
The authors express their appreciation to the Deanship of Scientific Research at King Saud University for funding this work through research group No (RG-1440-112). In addition, authors sincerely thank the Department of Botany, Faculty of Science, Mansoura University, and National Research Centre, Egypt.
Declaration of Competing Interest
The authors declare that there is no conflict of interest.
References
- Volatiles profiling, allelopathic activity, and antioxidant potentiality of Xanthium strumarium leaves essential oil from Egypt: evidence from chemometrics analysis. Molecules. 2019;24:584.
- [Google Scholar]
- Chemical constituents, antioxidant and potential allelopathic effect of the essential oil from the aerial parts of Cullen plicata. Ind. Crops Prod.. 2016;80:36-41.
- [Google Scholar]
- Essential oil composition, antioxidant and allelopathic activities of Cleome droserifolia (Forssk.) Delile. Chem. Biodivers.. 2018;15:e1800392
- [Google Scholar]
- Allelopathic impact of essential oil of Tagetes minuta on common agricultural and wastedland weeds. Innovare J. Agric. Sci.. 2017;5:1-4.
- [Google Scholar]
- Essential oil composition and antimicrobial activity of Aster subulatus Michx. from Turkey. Rec. Nat. Prod.. 2017;11:389-394.
- [Google Scholar]
- Chemical compositions and antioxidant potential of essential oils from leaves and flowers of Allophylus africanus. J. Essent. Oil Bear. Plants. 2014;17:769-775.
- [Google Scholar]
- A contribution to the ecology and floristic markers of plant associations in different habitats of Sinai Peninsula. Egypt. Rend. Fis. Acc. Lincei. 2014;25:479-490.
- [Google Scholar]
- Handbook of Essential Oils: Science, Technology, and Applications (second ed.). New York: CRC Press; 2015.
- Antitumor, cytotoxic and antioxidant potential of Aster thomsonii extracts. Afr. J. Pharm. Pharmacol.. 2011;5:252-258.
- [Google Scholar]
- The Weed Flora of Egypt. Cairo, Egypt: The American University Press; 1994.
- Fatty acids, essential oil, and phenolics modifications of black cumin fruit under NaCl stress conditions. J. Agric. Food Chem.. 2010;58:12399-12406.
- [Google Scholar]
- Determination of volatile compounds in antioxidant rosemary extracts by multiple headspace solid-phase microextraction and gas chromatography. Flavour Frag. J.. 2006;21:626-633.
- [Google Scholar]
- Acute, lethal and synergistic effects of some terpenes against Tribolium castaneum Herbst (Coleoptera: Tenebrionidae) Ecologia Balkanica. 2012;4:53-62.
- [Google Scholar]
- Comparison of the essential oil composition between Aster tataricus and A. koraiensis. Anal. Chem. Lett.. 2012;2:138-151.
- [Google Scholar]
- GC and GC/MS analysis of volatile of Ambrosia artemissifolia and A. trifida. J. Chin. Mass Spectrom. Soc.. 1996;17:37-40.
- [Google Scholar]
- Composition of essential oil and allelopathic activity of aromatic water of Aster lanceolatus Willd: (Asteraceae) Braz. J. Pharm. Sci.. 2009;45:469-474.
- [Google Scholar]
- Physiological and biochemical responses of Cucurbita pepo L. mediated by Portulaca oleracea L. allelopathy. Fresen. Environ. Bull.. 2015;24:386-393.
- [Google Scholar]
- Response of Cicer arietinum L. to allelopathic effect of Portulaca oleracea L. root extract. Phyton Ann. Rei Bot.. 2015;55:215-232.
- [Google Scholar]
- Genetics. In: Hay R.K.M., Waterman P.G., eds. Volatile Oil Crops. Harlow, England: Longman Scientific and Technical; 1993. p. :63-96.
- [Google Scholar]
- NaCl salinity and Zn foliar application influence essential oil composition of basil (Ocimum basilicum L.) Acta Agric. Slov.. 2011;97:93-98.
- [Google Scholar]
- Chemical composition, cytotoxic and antioxidant activity of the leaf essential oil of Photinia serrulata. Food Chem.. 2007;103:355-358.
- [Google Scholar]
- Antimicrobial testings, gas chromatographic analysis and olfactory evaluation of an essential oil of hop cones (Humulus lupulus L.) from Bavaria and some of its main compounds. Sci. Pharm.. 2006;74:189-201.
- [Google Scholar]
- Assessment of allelopathic potential of Artemisia scoparia against some plants. Bioscan. 2010;5:411-414.
- [Google Scholar]
- Effect of irrigation frequency and planting density on herbage biomass and oil production of thyme (Thymus vulgaris) and hyssop (Hyssopus officinalis) Ind. Crops Prod.. 2008;27:315-321.
- [Google Scholar]
- Chemical compositions and anti-inflammatory activities of essential oils from Aster spathulifolius and Vitex rotundifolia maxim. J. Appl. Pharm. Sci.. 2014;4:12-15.
- [Google Scholar]
- Phenolic compounds with IL-6 inhibitory activity from Aster yomena. Arch. Pharm. Res.. 2014;37:845-851.
- [Google Scholar]
- A review on recent research results (2008–2010) on essential oils as antimicrobials and antifungals. A review. Flavour Frag. J.. 2012;27:13-39.
- [Google Scholar]
- Comparison of volatile aroma compounds between Synurus deltoides and Aster scaber leaves. Korean J. Med. Crop Sci.. 2012;20:54-62.
- [Google Scholar]
- Mabberley's Plant-Book: A Portable Dictionary of Plants, their Classifications and Uses. UK: Cambridge University Press; 2008.
- Comparative study of the essential oils of four Pinus species: Chemical composition, antimicrobial and insect larvicidal activity. Ind. Crops Prod.. 2018;111:55-62.
- [Google Scholar]
- The constituents of the essential oil from Aster subulatus Michx. J. Agric. Chem. Soc. Japan. 1977;51:23-26.
- [Google Scholar]
- Essential oil and headspace constituents from the aerial parts of Aster ageratoides Turcz. var. ovatus Nakai. J. Essent. Oil Res.. 2008;20:9-11.
- [Google Scholar]
- Asteraceae do Parque Nacional da Serra da Canastra, Minas Gerais, Brazil. Braz. J. Bot.. 2001;24:471-478.
- [Google Scholar]
- Chemical composition of Aster albanicus Deg. (Asteraceae) essential oil: taxonomical implications. Arch. Biol. Sci.. 2015;67:1055-1061.
- [Google Scholar]
- Identification of allelochemicals in Eucalyptus citriodora and Polygonum sachalinense. In: Inderjit, Dakishini K.M.M., Einhellig F.A., eds. Allelopathy: Organisms, Processes, and Applications. Washington, DC: American Chemical Society; 1995. p. :74-85.
- [Google Scholar]
- A status review on the medicinal properties of essential oils. Ind. Crops Prod.. 2014;62:250-264.
- [Google Scholar]
- Caryophyllene-rich rhizome oil of Zingiber nimmonii from South India: chemical characterization and antimicrobial activity. Phytochemistry. 2006;67:2469-2473.
- [Google Scholar]
- Nepeta species: from farm to food applications and phytotherapy. Trends Food Sci. Technol.. 2018;80:104-122.
- [Google Scholar]
- Phytotoxicity of volatile oil from Eucalyptus citriodora against some weedy species. J. Environ. Biol.. 2007;28:63-66.
- [Google Scholar]
- Antiulcer agents: from plant extracts to phytochemicals in healing promotion. Molecules. 2018;23:1751.
- [Google Scholar]
- Matricaria genus as a source of antimicrobial agents: from farm to pharmacy and food applications. Microbiol. Res.. 2018;215:76-88.
- [Google Scholar]
- Salvia spp. plants-from farm to food applications and phytopharmacotherapy. Trends Food Sci. Technol.. 2018;80:242-263.
- [Google Scholar]
- Changes in fatty acid and essential oil composition of sage (Salvia officinalis L.) leaves under NaCl stress. Food Chem.. 2010;119:951-956.
- [Google Scholar]
- Essential oil and volatile emissions of basil (Ocimum basilicum) leaves exposed to NaCl or Na2SO4 salinity. J. Plant Nutr. Soil Sci.. 2013;176:748-755.
- [Google Scholar]
- Composition and some biological activities of the essential oil of Callicarpa americana (L.) J. Agric. Food Chem.. 2000;48:3008-3012.
- [Google Scholar]
- Chemical component analysis of essential oil from Aster poliothamnus Diels. by GC-MS. West China J. Pharm. Sci.. 2006;21:445-447.
- [Google Scholar]
- Research on allelopathy of Ambrosia artemisiifolia. Acta Ecol. Sin.. 1996;16:11-19.
- [Google Scholar]
- Chemical components of essential oils from the herb of Aster handelii Onno. J. Chengdu Univ. Tradit. Chin. Med.. 2006;1:021.
- [Google Scholar]
Appendix A
Supplementary material
Supplementary data to this article can be found online at https://doi.org/10.1016/j.arabjc.2019.07.005.
Appendix A
Supplementary material
The following are the Supplementary data to this article:Supplementary data 1
Supplementary data 1







