Chemical modification of cellulose extracted from sugarcane bagasse: Preparation of hydroxyethyl cellulose
*Address: King Saud University, Saudi Arabia. Tel.: +966566186116 essamya@yahoo.com (E.S. Abdel-Halim)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Available online 29 May 2013
Peer review under responsibility of King Saud University.

Abstract
Cellulose was extracted from sugarcane bagasse by alkaline extraction with sodium hydroxide followed by delignification/bleaching using sodium chlorite/hexamethylenetetramine system. Factors affecting extraction process, including sodium hydroxide concentration, hexamethylenetetramine concentration and temperature were studied and optimum conditions for alkaline extraction were found to be boiling finely ground bagasse under reflux in 1 N sodium hydroxide solution and then carrying out the delignification/bleaching treatment at 95 °C using 5 g/l sodium chlorite together with 0.02 g/l hexamethylenetetramine. The extracted cellulose was used in the preparation of hydroxyethyl cellulose through reaction with ethylene oxide in alkaline medium. Factors affecting the hydroxyethylation reaction, like sodium hydroxide concentration during the alkali formation step, ethylene oxide concentration, reaction temperature and reaction duration were studied. Optimum conditions for hydroxyethylation reaction were using 20% NaOH solution and 200% ethylene oxide (based on weight of cellulose), carrying out the reaction at 100 °C for 60 min.
Keywords
Sugarcane bagasse
Extraction
Delignification
Cellulose
Hydroxyethyl cellulose
1 Introduction
The biopolymer cellulose is one of the most naturally occuring polymers, available worldwide. This polymer is characterized by being renewable and the annual natural production of cellulose is estimated to be 1.5 × 1011 tons. With this high annual production, cellulose is considered to be the major source of raw materials (Cao et al., 2009; Klemm et al., 2005). Cellulose has attracted much attention worldwide, for being a raw material for the production of many industrial products like essential chemicals, different paper products, varying panel products, as well as other industrial products. lignocellulosic materials such as grasses, bagasse and cereal or rice straw are all composed of single fiber cells having length ranging from 0.5 – 3.0 mm, whereas the family of bast fibers comprising flax and jute are characterized by single cells having length of about 77 mm (Reddy and Yang, 2005).
The main constituent of lignocellulosic plant’s cell wall is the cellulose polymer, where its content can reach up to 23 – 53% on a dry-weight basis from the total fiber composition. The percent of cellulose in bast fibers is less than its percent in cotton fiber, which is almost composed of pure cellulose (Yu et al., 2005). In such lignocellulosic materials, the cellulose fiber is embedded in a composite structure, which is composed of different noncellulosic cementing materials like, lignins, pectins, hemicelluloses, as well as other carbohydrate polymers (Knauf and Moniruzzaman, 2004; Hanley et al., 1997). The process of pure cellulose isolation has become the subject of extensive research work for many decades due to the cell wall structure complexity (Sun and Hughes, 1998; Brendel et al., 2000). Many papers in the literature reported the utilization of cellulose extracted from sugarcane bagasse for the production of different cellulose derivatives (Wang et al., 2009; Shaikh et al., 2009; Gurgel et al., 2008). On the other hand, our research group published a lot of research papers dealing with the feasibility of cellulosic agricultural and industrial wastes recycling by use of chemical techniques for the production of high-value products (Abdel-Halim et al., 2006, 2008a,b; Abdel-Mohdy et al., 2009; Hashem et al., 2005a,b, 2007a,b; Hebeish et al., 2009, 2010; Sokkar et al., 2004). Accumulation of huge amounts of sugarcane bagasse as a by-product resulting from the sugar industry presents a complicated waste problem, as so far very few commercial uses were developed for these large amounts of wastes. The situation is more or less the same regarding many other agricultural wastes like the straws of wheat, cereal and rice, in addition to other non-straw wastes like cotton stalks, etc. One of the most important research area dealing with agricultural wastes recycling is the agricultural biomass fractionation into its basic constituents, like, lignins, cellulose and hemicellulose, in a process known as bio-refinery. Each product from this fractions process can then be further separately industrially treated to produce important value-added derivatives. For instance, the obtained cellulosic fraction can be converted by simple chemical modifications into important cellulose derivatives like esters (Shaikh et al., 2009), which serve in wide range of applications, such as regenerated textile fibers and films, biologically degradable plastics, etc. and cellulose ethers (Vieira et al., 2009), which are widely used as thickeners in many industrial applications, including food industry, pharmaceutical industry and paints industry.
The main purpose of any bleaching treatment, whatever is the bleaching agent is to obtain white cellulosic fibers. This whiteness is to be achieved simply by removing the coloring matter present in the natural composition of cotton fiber with the help of the bleaching agents. According to the nature of the bleaching agents, it either oxidizes or reduces the coloring matter, thus destroying it to simpler compounds, which are dissolved and washed out resulting in permanent whiteness. Cellulosic fiber materials undergo several consecutive preparation and finishing operations with the aim of improving their performance properties and these processes comprise desizing preparation (Ahlawat et al., 2009; Bae et al., 2006; Peng et al., 2010), scouring pretreatment (Abdel-Halim et al., 2008, 2010; Abdel-Halim, 2012; Abdel-Halim et al., 2011) and bleaching process (Abdel-Halim and Al-Deyab, 2011; Abdel-Halim et al., 2010; Abdel-Halim, 2012; Abdel-Halim and Al-Deyab, 2012; Hashem et al., 2010; Hebeish et al., 2009; Hou et al., 2010; Ibrahim et al., 2008, 2010; Abdel-Halim et al., 2008a).
The decomposition of sodium chlorite produces one of the strongest oxidizing gases, which is known as chlorine dioxide. Lowering the pH value and/or raising the bleaching bath temperature lead to speeding of sodium chlorite decomposition rate (Hubbell and Ragauskas, 2010; Abdel-Halim, 2012c). It is well known that aqueous sodium chlorite solutions are very stable in alkaline media and to make them active and effective as bleaching agents, the medium should be turned acidic. When activation is affected by use of strong acids, the toxic and corrosive chlorine dioxide gas is produced, whose rate of evolution is necessary to be controlled and in this regard, many technologies were developed to control the rate of chlorine dioxide evolution (Hirota et al., 2009). Practically this is achieved by different means, like controlling the bleaching bath temperature or adding some buffer solutions to control the pH of the bleach bath. Regulation of chlorine dioxide evolution can be achieved by addition of weak acids to form buffer with the alkali present in the chlorite solution, or by the addition of a mixture composed of weak acid together with its salt derived from a strong base.
Hydroxyethyl cellulose (HEC) is a water-soluble polymer, nonionic in nature. This polymer has excellent performance properties, that it is capable of thickening, binding, emulsifying, suspending, dispersing, stabilizing, this in addition to its ability to retain water and form film which provide good protective action. HEC is easily soluble in either hot or cold water to produce solutions having a wide range of viscosities. Hydroxyethyl cellulose finds applications in different industrial fields, like thickening paints (Dal-Bó et al., 2011), finishing of textile (Gorgieva and Kokol, 2011), thickener in cement mortar (Patural et al., 2011) and sizing agent in paper making (Kugge et al., 2004). In the industrial preparation of hydroxyethyl cellulose, pure cellulose (cellulose pulp) is treated with sodium hydroxide solution to swollen the cellulose and convert it to active alkali cellulose. When this reactive alkali cellulose is reacted with gaseous ethylene oxide, a series of etherification reactions occur and hydroxyethyl cellulose is thus produced. In this etherification reaction, each hydrogen atom in the cellulose hydroxyl group is to be replaced by hydroxyethyl group, which is the reason of the polymer’s water solubility. Fig. 1 represents the chemical structure of HEC.
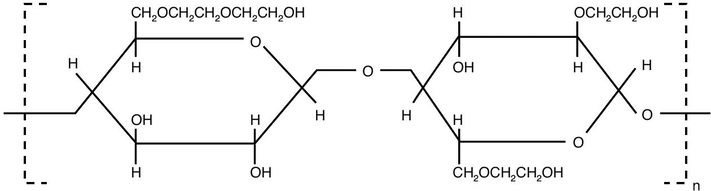
- Structure of hydroxyethyl cellulose.
The present work aims at increasing the economic value of sugarcane bagasse by simple recycling process which depends on extraction of the cellulosic component from the sugarcane bagasse through alkali treatment and sodium chlorite delignification. The so obtained cellulose is to be used in the preparation of hydroxyethyl cellulose, which has many important industrial applications and this will be done through reaction of the extracted cellulose with ethylene oxide in alkaline medium.
2 Experimental
2.1 Cellulosic raw material
All sugar residues were removed from freshly collected sugarcane bagasse by washing thoroughly in running water. This is done in order to minimize any microbial attack during drying the bagasse in sunny area until its complete dryness. The dry bagasse is collected and then grinded into fine particles using Fritsch mill, Type 15.302 (Germany). Before any chemical treatment, typical bagasse chemical compositions gives a moisture content of 9.19%, an ash content of 1.72%, a lignin content of 23.90%, a hemicelluloses content of 25 and a cellulose content of 40.21%.
2.1.1 Determination of moisture content (ASTM D 2216)
10 g sample of ground bagasse was weighed accurately in a dry Petri dish and dried at 105 °C for 3 h. The sample is allowed to cool in a desiccator, reweighed and the weight is recorded. The drying-cooling-reweighing procedure is repeated until the weight is constant. The moisture content is measured using the following equation
2.1.2 Determination of ash content (ASTM D2866)
Porcelain crucible was weighed accurately (A g). About 3 g of ground bagasse was put in the crucible and the crucible with the sample was weighed accurately (B g). The sample was ashed in an electric muffle furnace, which can maintain the temperature at 625 ± 25 °C overnight, cooled in the desiccator for 15 min and the crucible containing the ash was weighed (C g). Completeness of ashing was checked by shaking with a platinum wire to discover whether there exist any unburnt particles and if so, ashing is to be continued until constant weight. The ash content is calculated using the following equation
A = weight of empty crucible (gram)
B = weight of crucible and sample (gram)
C = weight crucible and ash (gram)
2.1.3 Determination of lignin
Lignin content, expressed as kalson lignin, was directly estimated according to the method of the institute of papaer chemistry, Appleton, Wisconsin. Ground bagasse was extracted at first with ethanol – benzene mixture (1:1) and dried. About 1 g (accurately weighed) was then treated with 20 ml 72% sulfuric acid, so that the acid is added to the sample drop-wise with constant stirring. After complete disintegration, the sample was allowed to stand covered with a watch glass and left overnight at room temperature. The sample was then transferred quantitatively to one-liter round flask, diluted to 3% sulfuric acid and boiled for 4 h under reflux. The lignin was filtered on a pre-weighed filter paper and washed with hot distilled water till neutrality. The lignin was then dried at 105 °C for 6 h and gravimetrically estimated according to the following equation
2.1.4 Determination of hemicelluloses
Accurately weighed ground bagasse sample (X) was extracted with 10% KOH using a material to liquor ratio of 1:20 for 10 h at 50 °C. At the end of the extraction process, the system was allowed to cool and filtered. The filtrate was made acidic by use of glacial acetic acid until pH 6 is attained. The filtrate is then mixed with a solution of 2 volumes ethanol. The formed precipitate was recovered by filtration and freeze-drying and was then weighed (Y). The hemicelluloses content (%) was calculated using the following equation
2.2 Chemicals
Sodium chlorite, sodium hydroxide, potassium hydroxide, potassium iodide and sodium thiosulfate were supplied by BDH. Hexamethylenetetramine (HMTA), red phosphorous and bromine solution were supplied by Riedel-de Haen. Isopropyl alcohol, acetic acid, acetone and sulfuric acid were supplied by Aldrich. Liquid ethylene oxide, in the form of 100-ml sealed ampules, hydroiodic acid and ammonium thiocyanate were supplied by Merck.
2.3 Alkali treatment
Sodium hydroxide solution was used to extract noncellulosic binding materials like hemicelluloses/lignin complexes from sugarcane bagasse composition. For this purpose, finely ground sugarcane bagasse was treated with sodium hydroxide solution (0.1–2.5 N) and non ionic wetting agent (Lavotan DSU) (2 g/l) using material to liquor ratio (1:20) at the boil under reflux for one hour. After the desired reaction time, the samples were filtered, washed twice with hot water, rinsed twice in cold water and finally dried at ambient conditions.
2.4 Delignification/bleaching
Delignification of sugarcane bagasse was carried out by introducing the finely ground samples in a solution containing NaClO2 (5 g/l), hexamethylene tetramine (0–0.5 g/l) and non ionic wetting agent (Lavotan DSU) (2 g/l) using material to liquor ratio (1:30) and boiling under reflux. The progress of the bleaching reaction was followed by measuring the percent decomposed NaClO2 at different reaction time intervals (Vogel, 1961). After bleaching duration is vanished, the bleached samples were removed from the bleaching bath, washed twice with hot water and twice with cold water and finally dried in open air.
2.5 FTIR spectral analysis
The FTIR spectra of bagasse cellulose were recorded by means of Bruker, TENSOR Series FTIR Spectrophotometer, Germany. 100 mg samples of potassium bromide containing about 2% of cellulose powder were prepared, finely grinded and subjected to analysis.
2.6 Hydroxyethylation
Hydroxyethylation of the residual cellulose extracted from sugarcane bagasse was carried out by a two-step method, namely alkali cellulose formation and hydroxyethylation (Zahran et al., 1998). In the first step, cellulose (5 g) was immersed in sodium hydroxide solution (different concentrations from sodium hydroxide were used) for 1 h at room temperature. A material to liquor ratio of 1:20 was used and the formed alkali cellulose was filtered under suction until the ratio between the cellulose and sodium hydroxide solution reaches 1:3 (wt/wt). Liquid ethylene oxide, supplied in the form of 100-ml sealed ampules was cooled to −5 °C and then transferred to a −5 °C-cold bottle and stored in a freezer, as long as it is not in use. In the second step, cold isopropyl alcohol is added to the formed alkali cellulose (4 ml isopropyl alcohol for 1 g dry cellulose powder), at last ethylene oxide cooled at −5 °C was added to the reaction cup, which was tightly closed. The cups are shaken well to homogenize the reaction mixture and then they are put in a thermostatic water bath at the predetermined reaction temperature. After the reaction duration is completed, the formed product is neutralized with acetic acid then washed several times with acetone in order to be precipitated, and finally dried and crushed. The yield of the hydroxyethyl cellulose (based on the initial weight of cellulose incorporated in the hydroxyethylation reaction) was calculated using the following equation and the results are given in Table 1.
| [EO] | 50 | 75 | 100 | 125 | 150 | 175 | 200 | 225 | 250 | 275 | 300 |
| Yield of HEC (%) | 108 | 120 | 155 | 180 | 210 | 235 | 260 | 275 | 290 | 305 | 320 |
• 20% NaOH solution (based on weight of cellulose) in the alkali cellulose formation step, carrying out the hydroxyethylation reaction at 100 °C for 60 min.
• [EO] is ethylene oxide concentration in percent (based on weight of cellulose).
2.7 Determination of ethoxyl content
The ethoxyl content and accordingly molar substitution were measured according to a previously reported method (Morgan, 1946). The apparatus for the analysis is shown in Fig. 2.
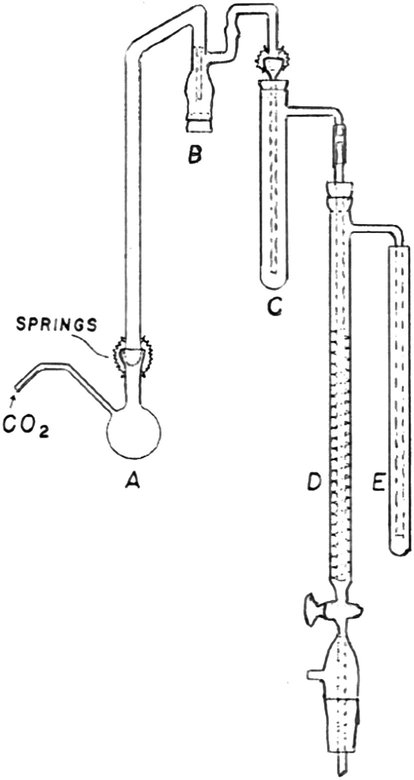
- Diagram for ethoxyl content measurement apparatus.
The apparatus must be cleaned and dried before filling Trap B with a suspension of minor amount of red phosphorous in large excess of water to cover the inlet. 10 ml of silver nitrate solution is placed in the first absorption tube, C, 15 ml of bromine solution is placed in the spiral absorption tube, D, and 10 ml of 10% potassium iodide solution is placed in the final tube, E. A pre-weighed sample of dry hydroxyethyl cellulose (0.05–1.2 g) is placed in the reaction flask A, together with hanger boiling granules and 10 ml of hydroiodic acid. The flask is connected to the apparatus, a slow stream of nitrogen is passed through, and the flask is heated slowly for a minimum of 40 min. At the completion of the decomposition, tubes D and C are disconnected and then the nitrogen source is disconnected and the heating source is removed from flask A. The contents of the absorption tube, D, is then added to 500-ml titration flask containing 10% potassium iodide solution (10 ml) and 150 ml of water. The potassium iodide tube, E, is removed and the side arm rinsed into it. Its contents are transferred into a titration flask, which is stoppered and stand for 5 minutes before titration. Five ml of 10% sulfuric acid is then added and the solution is titrated immediately against 0.05 N sodium thiosulphate solution, using 2 ml of starch solution as an indicator (blank titration is carried out in the same way for external 15 ml from the same bromine solution). The content of silver nitrate trap is to be rinsed into a flask and diluted to 150 ml with distilled water, boiled, cooled to room temperature and finally titrated against 0.05 N ammonium thiocyanate solution using 3 ml of ferric ammonium sulfate solution as an indicator (blank titration is carried out in the same way for external 10 ml from the same silver nitrate solution).
2.7.1 Calculations
The total ethoxyl content is
3 Results and discussion
3.1 Extraction by alkali treatment
Fig. 3 shows the effect of sodium hydroxide concentration used in the extraction treatment on the extent of hemicelluloses removal expressed as percent loss in weight. It is well known that the non-cellulosic materials like hemicelluloses and lignin are present in the form of network binding the fiber bundles in a composite-like structure. The removal of hemicelluloses during the alkali treatment weakens this network and some lignins become loose and removed due to this damaged network. It is clear from the figure that the amount of hemicelluloses removed increases gradually with increasing the strength of the sodium hydroxide solution used in the extraction treatment. The extent of hemicelluloses removal reaches a high value (24%) at sodium hydroxide concentration of 1 N. Increasing the strength of the alkali solution over this value until 2.5 N is accompanied by a negligible increase in the percent loss in weight, which means that there is no more hemicelluloses to be removed and that 1 N is enough alkali concentration to get rid of almost all hemicelluloses.
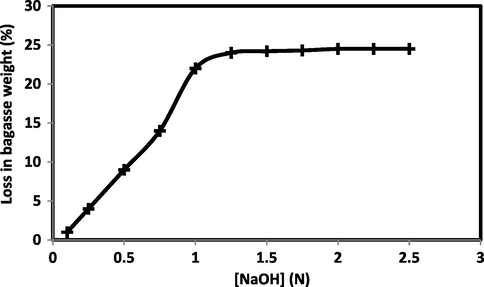
- Effect of sodium hydroxide concentration on percent loss in weight. M/L Ratio, 1:20; wetting agent, 2 g/l; treatment temperature, boil under reflux; treatment duration, 60 min.
3.2 Catalytic effect of hexamethylenetetramine in sodium chlorite deliginification/bleaching
Hexamethylenetetramine is a heterocyclic organic compound having the chemical formula (CH2)6N4. Hexamethylenetetramine is explained as follows: HMTA decomposes to give 4 molecules of ammonia and 6 molecules of formaldehyde (Eq. (1)).
The liberated HCHO will accelerate NaClO2 decomposition according to the redox reaction suggested by Eq. (2) to produce sodium hypochlorite and formic acid.
The formed hypochlorite is readily hydrolyzed to form hypochlorous acid. This product is known to be unstable and decomposes to its components, HCl and oxygen (Eq. (3)). The hypochlorite ion decomposes also to liberate oxygen atoms (Eq. (4)) or alternatively reacts with HOCl to form different active free radical species (Eqs. (5) and (6)):
In the mechanism suggested by Eqs. (5) and (6), the formed hydroxyl radicals react with the hypochlorite and/or chlorite ions, thereby, starting a chain reaction (Eqs. (7) and (8)).
The liberated NH3 molecules (Eq. (1)) keep the alkalinity of the bleaching medium, particularly at the initial stage of the treatment, this is in addition to the role of the liberated ammonia in improving the delignification efficiency (Rodríguez et al., 2011). On the other hand, due to the formic acid formation, as the bleaching reaction proceeds, the medium turns acidic (Eq. (2)).
3.2.1 Effect of hexamethylenetetramine concentration on NaClO2 decomposition
It is well established that NaClO2 decomposes in acidic medium and liberates chlorine dioxide gas rather than chlorine gas and the efficiency of the bleaching bath is determined by the rate of chlorine dioxide liberation. Sugarcane bagasse treated with the optimum sodium hydroxide concentration (1 N) during the alkali treatment step was further subjected to delignification/bleaching treatment at the boil using 5 g/l NaClO2, 2 g/l nonionic wetting agent and different concentrations of hexamethylenetetramine were introduced to the bleaching medium to activate sodium chlorite decomposition. Fig. 4 shows the dependence of the percent decomposed NaClO2 during delignifying/bleaching sugarcane bagasse on the hexamethylenetetramine concentration incorporated to the bleaching bath. The results show that the higher the concentration of hexamethylenetetramine added to the bleaching medium, the higher will be the decomposition percent of NaClO2 at a given bleaching time. It was also found that at a given hexamethylenetetramine concentration, prolonging the bleaching time will lead to increase in the percent decomposed NaClO2. When high concentration of hexamethylenetetramine is used, 100% decomposition of NaClO2 will take place instantly without imparting noticeable bleaching effect to the bagasse. On the other hand, the evolved amount of ClO2 during bleaching was found to be negligible whatever is the reaction duration or the incorporated amount of hexamethylenetetramine to the bleaching medium. This proves that when hexamethylenetetramine is used to activate NaClO2 decomposition, the active species released will be mainly oxygen rather than chlorine dioxide. Samples obtained after bleaching were evaluated from the loss in weight point of view and also from the sodium chlorite decomposition point of view and it was found that using hexamethylenetetramine concentration of 0.02 g/l is optimum condition that it leads to gradual decomposition of sodium chlorite within reasonable time of two hours and gave 30.6% loss in weight which is found to be equivalent to the amount of lignin present originally in the untreated bagasse.
![Effect of hexamethylenetetramine concentration on sodium chlorite decomposition. [Sodium chlorite], 5 g/l; M/L Ratio, 1:30; wetting agent, 2 g/l; treatment temperature, boil under reflux; treatment duration, 240 min.](/content/184/2014/7/3/img/10.1016_j.arabjc.2013.05.006-fig4.png)
- Effect of hexamethylenetetramine concentration on sodium chlorite decomposition. [Sodium chlorite], 5 g/l; M/L Ratio, 1:30; wetting agent, 2 g/l; treatment temperature, boil under reflux; treatment duration, 240 min.
3.2.2 pH change during the bleaching reaction
According to the data obtained from studying the effect of hexamethylenetetramine concentration it was found that 0.02 g/l is the optimum concentration. Based on that, a separate run of bleaching/delignification was carried out using this optimum condition and the change in the bleaching bath pH was followed at different time intervals to understand the role of pH in controlling the rate of sodium chlorite decomposition. Parallel to this run another run was carried out by using higher hexamethylenetetramine concentrations, namely, 0.2 g/l and the pH was followed just for comparison. Controlling the pH by the buffering effect of gradually liberated ammonia leads to gradual sodium chlorite decomposition to its oxidizing species and thus increasing their efficiency in removal of lignins, rather than fast sodium chlorite decomposition without imparting any delignification effect to the bagasse. Fig. 5 presents the change in the pH of the bleaching bath with time, carrying out the bleaching reaction at 95 °C and using 0.02 and 0.2 g/l, separately, as initial concentrations of hexamethylenetetramine. The data show that for 0.02 g/l hexamethylenetetramine concentration, by prolonging the bleaching duration, the pH value decreases to reach the value of 4.2 after 120 min. At the high hexamethylenetetramine concentrations, formic acid is formed in large amounts and causes sudden decrease in the pH within the first 30 min, which in turn results in uncontrollable fast sodium chlorite decomposition at an early stage from the bleaching process without being useful in actual delignification/bleaching of bagasse.
![Change in pH during the bleaching reaction. [Sodium chlorite], 5 g/l; M/L Ratio, 1:30; wetting agent, 2 g/l; treatment temperature, boil under reflux; treatment duration, 120 min.](/content/184/2014/7/3/img/10.1016_j.arabjc.2013.05.006-fig5.png)
- Change in pH during the bleaching reaction. [Sodium chlorite], 5 g/l; M/L Ratio, 1:30; wetting agent, 2 g/l; treatment temperature, boil under reflux; treatment duration, 120 min.
3.2.3 Effect of bleaching temperature on delignification/bleaching efficiency
Delignification/bleaching reactions were carried out using the optimum hexamethylenetetramine concentration, 0.02 g/l but at different temperatures to show the effect of temperature on the efficiency of delignification/bleaching reaction, expressed in terms of percent loss in bagasse weight. Fig. 6 shows the dependence of the percent loss in bagasse weight on the delignification/bleaching medium temperature. It is clear from the figure that carrying the treatment at 30 °C is almost useless that it causes no remarkable effect in delignification of the bagasse (only 2.1% loss in weight). Raising the delignification temperature to 50 and 70 °C led to increase in the percent loss in weight to some extent and maximum impurities’ removal was achieved on raising the delignification/bleaching temperature to 95 °C. The improvement in impurities removal on raising the temperature is due to the fact that sodium chlorite decomposition is proportionally related to the temperature of the delignification/bleaching medium. When the bleaching reaction is carried out at 95 °C, sodium chlorite is decomposed completely in 2 hours, while when the bleaching reaction is carried out at lower temperatures, namely 70 °C, 8 hours are needed to attain 100% sodium chlorite decomposition. When the bleaching reaction is carried out at 50 °C and 30 °C, complete decomposition of sodium chlorite was noticed not to be attained even after 24 h. The improvement in activation of sodium chlorite at high bleaching temperatures (95 °C) can be explained in terms of the favorable effect of high temperature on hexamethylenetetramine decomposition to formaldehyde, which accordingly activates decomposition of sodium chlorite, in addition to giving the required energy for decomposing of sodium chlorite itself to the bleaching species shown in Eqs. (1)–(8). Also one decomposition product of hexamethylenetetramine is ammonia which acts efficiently as a buffer to keep the pH of bleaching medium almost constant during the reaction course. In addition to the previously mentioned discussion, high temperature helps in improving the swelling and accessibility of the bagasse particles themselves, resulting in faster diffusion of the bleaching agent inside the bulk of the particles.
![Effect of bleaching temperature on percent loss in bagasse weight. [Sodium chlorite], 5 g/l; M/L Ratio, 1:30; wetting agent, 2 g/l; treatment duration, 120 min.](/content/184/2014/7/3/img/10.1016_j.arabjc.2013.05.006-fig6.png)
- Effect of bleaching temperature on percent loss in bagasse weight. [Sodium chlorite], 5 g/l; M/L Ratio, 1:30; wetting agent, 2 g/l; treatment duration, 120 min.
3.2.4 FTIR spectroscopy
FTIR spectroscopic investigations gave different absorption bands which are characteristic for cellulose (Fig. 7). There is a broad band at 3346 cm−1, which is characteristic for the OH-stretching vibration and this band gives good information about the hydrogen bonds formation. The band at 2900 cm−1 represents the C–H stretching vibration. In addition, the absorption band at 1430 cm−1, represents the symmetric CH2 bending vibration, the absorption band at 898 cm−1, represents the C–O–C stretching at β-(1 → 4)-glycosidic linkages, the absorption band at 1642 cm−1, assigned to absorbed H2O, the absorption band at 1059 cm−1, represents the C–O vibration, the absorption band at 1430 cm−1, represents the CH2 and the absorption band at 1372 cm−1, assigned to CH.
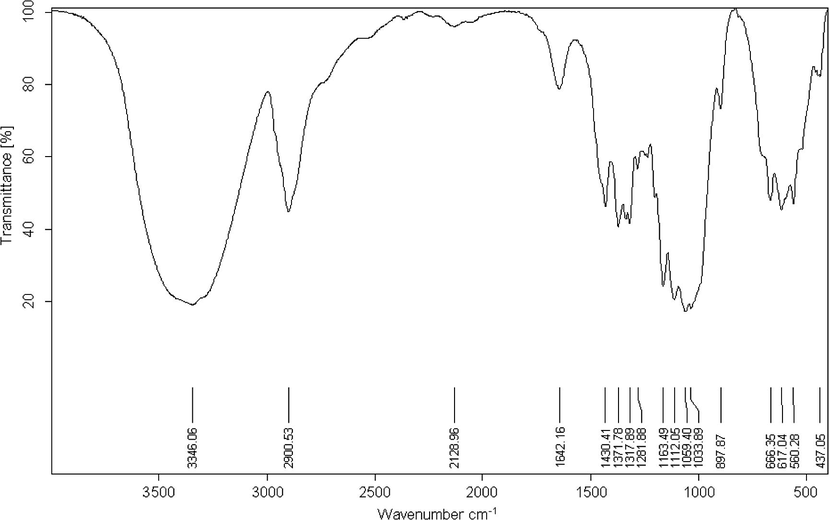
- FTIR Spectra of bagasse cellulose.
3.3 Hydroxyethylation reaction
Hydroxyethyl cellulose is one of the most important cellulose ethers having many industrial applications. The hydroxyethylation process was carried out using different reaction conditions including varying alkali concentrations, varying ethylene oxide concentrations and different temperatures and reaction durations. The way by which ethylene oxide can be added to cellulose is described in two terms, molar substitution (M.S.) and Degree of substitution (D.S.). Degree of substitution represents the average number of hydroxyl groups in an anhydroglucose unit which undergone etherification reaction with ethylene oxide. The D.S. value will not exceed three, since each anhydroglucose unit has only three hydroxyl groups. Molar substitution, on the other hand is an average value which represents the number of ethylene oxide molecules that reacted with an anhydroglucose unit. Once a hydroxyethyl group is attached to a hydroxyl group of an anhydroglucose unit, it can undergo further propagation and react with more ethylene oxide molecules. Theoretically, as long as there are ethylene oxide molecules in the reaction medium, this propagation reaction can continue without limit. The mechanism of hydroxyethylation reaction can be represented by the following equations.
3.3.1 Effect of alkali treatment on the molar substitution of hydroxyethyl cellulose
Fig 8 demonstrates the role of NaOH concentration during the alkaline pretreatment step, prior to the hydroxyethylation reaction, on the resultant hydroxyethyl cellulose molar substitution. The alkali treatment step was carried out at different sodium hydroxide concentrations, namely 5–40% (based on weight of bagasse cellulose), keeping fixed ethylene oxide concentration for the hydroxyethylation step (200% based on weight of bagasse cellulose) and carrying out the hydroxyethylation reaction at 100 °C for 60 min. The obtained data indicate that the resultant hydroxyethyl cellulose shows noticeable enhancement in the molar substitution upon increasing the amount of NaOH during the alkali pretreatment step up to 20%, based on the weight of bagasse cellulose. On the other hand, it was found that increasing sodium hydroxide concentration above this limit results in decrease in the hydroxyethyl cellulose molar substitution. The improvement in the molar substitution upon increasing the concentration of NaOH during the alkaline pretreatment step could be understood in terms of the increased cellulose swellability in such high alkali concentration and accordingly the increase in the affinity of the cellulose hydroxyls toward the hydroxyethylation reaction. The opposite trend of the molar substitution, upon using higher alkali concentration indicates the occurrence of other different reactions other than the hydroxyethylation reaction.
![Effect of alkali pretreatment on the molar substitution of hydroxyethyl cellulose. Cellulose/NaOH solution during alkali formation step, 1:20; soaking time 1 hour at 30 °C; M/L ratio after filtration and pressing, 1:3; [ethylene oxide], 200% (based on weight of bagasse cellulose); hydroxyethylation temperature, 100 °C; hydroxyethylation duration, 60 min.](/content/184/2014/7/3/img/10.1016_j.arabjc.2013.05.006-fig8.png)
- Effect of alkali pretreatment on the molar substitution of hydroxyethyl cellulose. Cellulose/NaOH solution during alkali formation step, 1:20; soaking time 1 hour at 30 °C; M/L ratio after filtration and pressing, 1:3; [ethylene oxide], 200% (based on weight of bagasse cellulose); hydroxyethylation temperature, 100 °C; hydroxyethylation duration, 60 min.
3.3.2 Effect of ethylene oxide concentration on the molar substitution
Fig. 9 shows the enhancement in the molar substitution of the prepared hydroxyethyl cellulose upon incorporating increasing amounts of ethylene oxide to the reaction medium. The alkali treatment step was carried out using the optimum sodium hydroxide concentration, 20% NaOH (based on weight of bagasse cellulose) and hydroxyethylation reaction was carried out using different ethylene oxide concentrations, namely, (50–300%) (based on weight of bagasse cellulose), and carrying out the hydroxyethylation reaction at 100 °C for 60 min. The data in Fig. 9 show that increasing the incorporated amount of ethylene oxide to the reaction medium from 50% to 300% is accompanied by continuous increase in the molar substitution of the resulting hydroxyethyl cellulose. This is in accordance with the proposed mechanism of hydroxyethylation (Eqs. ()()()()(9)–(12)). The cellulose hydroxyls are known to be immobile and accordingly the reaction of ethylene oxide molecules with the unhydroglucose hydroxyl groups depends basically on the availability of ethylene oxide molecules in the vicinity of the hydroxyl groups. Also the more the ethylene oxide molecules available in the reaction medium, the higher will be the chance for the polyethylene oxide side chains to grow and propagate (Eq. (10)).
![Effect of ethylene oxide concentration on the molar substitution of hydroxyethyl cellulose. [NaOH], 20% (based on weight of bagasse cellulose); cellulose/NaOH solution during alkali formation step, 1:20; soaking time 1 h at 30 °C; M/L ratio after filtration and pressing, 1:3; hydroxyethylation temperature, 100 °C; hydroxyethylation duration, 60 min.](/content/184/2014/7/3/img/10.1016_j.arabjc.2013.05.006-fig9.png)
- Effect of ethylene oxide concentration on the molar substitution of hydroxyethyl cellulose. [NaOH], 20% (based on weight of bagasse cellulose); cellulose/NaOH solution during alkali formation step, 1:20; soaking time 1 h at 30 °C; M/L ratio after filtration and pressing, 1:3; hydroxyethylation temperature, 100 °C; hydroxyethylation duration, 60 min.
3.3.3 Effect of hydroxyethylation temperature on the molar substitution
Fig. 10 reflects the effect of hydroxyethylation reaction temperature on the molar substitution of the prepared hydroxyethyl cellulose. The alkali treatment step was carried out by use of the optimum sodium hydroxide concentration, 20% NaOH (on weight of bagasse) and the hydroxyethylation reaction was carried out using the optimum ethylene oxide concentrations, 200% (based on weight of bagasse cellulose). The hydroxyethylation reaction was affected at various temperature conditions, namely, 30–120 °C for 60 min. The data indicate that increasing the hydroxyethylation reaction temperature from 30 to 120 °C is accompanied by noticeable enhancement in the molar substitution of the prepared hydroxyethyl cellulose. This improvement in the molar substitution upon raising the reaction temperature could be attributed to enhancement in the swellability of the alkali cellulose at high temperatures. Also as the reaction is carried out in closed system, as the temperature increases, the pressure inside the reaction vessel increases as well and ethylene oxide molecules will be forced to penetrate to the bulk of the alkali cellulose and increase the efficiency of the etherification reaction, this is in addition to enhancement the kinetic energy of ethylene oxide molecules at high temperatures.
![Effect of hydroxyethylation temperature on the molar substitution. [NaOH], 20% (based on weight of bagasse cellulose); cellulose/NaOH solution during alkali formation step, 1:20; soaking time 1 h at 30 °C; M/L ratio after filtration and pressing, 1:3; [ethylene oxide], 200% (based on weight of bagasse cellulose); hydroxyethylation duration, 60 min.](/content/184/2014/7/3/img/10.1016_j.arabjc.2013.05.006-fig10.png)
- Effect of hydroxyethylation temperature on the molar substitution. [NaOH], 20% (based on weight of bagasse cellulose); cellulose/NaOH solution during alkali formation step, 1:20; soaking time 1 h at 30 °C; M/L ratio after filtration and pressing, 1:3; [ethylene oxide], 200% (based on weight of bagasse cellulose); hydroxyethylation duration, 60 min.
3.3.4 Effect of hydroxyethylation duration on the molar substitution
Fig. 11 shows the effect of hydroxyethylation duration on the molar substitution of the prepared hydroxyethyl cellulose. The alkali treatment step was carried out by use of the optimum sodium hydroxide concentration, 20% NaOH (based on weight of bagasse cellulose) and the hydroxyethylation reaction was carried out using the optimum ethylene oxide concentrations, 200% (on weight of bagasse). The hydroxyethylation reaction was carried out at the optimum temperature, namely, 100 °C and the reaction proceeds for varying duration from 30 to 210 min. The data in Fig. 11 show tremendous improvement in the molar substitution upon increasing the reaction duration up to 60 min. prolonging the hydroxyethylation duration beyond this limit is found to be accompanied by very small increase in the molar substitution of the prepared hydroxyethyl cellulose. This behavior suggests that maximum propagation in the polyethylene oxide side chains occurs in an early stage of the hydroxyethylation reaction and thus consuming most of the ethylene oxide molecules in the first 60 min. After this duration very low concentrations of ethylene oxide will be available in the reaction medium and accordingly little increase in the molar substitution is observed after 60 min.
![Effect of duration on the molar substitution of hydroxyethyl cellulose. [NaOH], 20% (based on weight of bagasse cellulose); cellulose/NaOH solution during alkali formation step, 1:20; soaking time 1 h at 30 °C; M/L ratio after filtration and pressing, 1:3; [ethylene oxide], 200% (based on weight of bagasse cellulose); hydroxyethylation temperature, 90 °C.](/content/184/2014/7/3/img/10.1016_j.arabjc.2013.05.006-fig11.png)
- Effect of duration on the molar substitution of hydroxyethyl cellulose. [NaOH], 20% (based on weight of bagasse cellulose); cellulose/NaOH solution during alkali formation step, 1:20; soaking time 1 h at 30 °C; M/L ratio after filtration and pressing, 1:3; [ethylene oxide], 200% (based on weight of bagasse cellulose); hydroxyethylation temperature, 90 °C.
Upon trying the solubility of the prepared hydroxyethyl cellulose it was found that completely water soluble hydroxyethyl cellulose is obtained only when the value of the molar substitution exceeds 0.43. As a result of all the above mentioned reaction parameters it could be concluded that the optimum conditions for the preparation of water-soluble hydroxyethyl cellulose from bagasse are carrying out the alkaline pretreatment step at NaOH concentration of 20% (based on weight of bagasse cellulose) in a M/L ratio of 1:20, and carrying out the alkali treatment step at temperature of 30 °C for a duration of 60 min. After filtering the alkali cellulose to M/L ratio of 1:3, the hydroxyethylation step should be carried out by mixing the alkali cellulose with isopropyl alcohol at a M/L ratio of 1:4 and adding ice cold ethylene oxide 200% (based on weight of bagasse cellulose) and carrying out the hydroxyethylation reaction at 100 °C for 60 min.
Acknowledgement
The authors extend their appreciation to the Deanship of Scientific Research at King Saud University for funding the work through the research group project No. RGP-VPP-029.
References
- Carbohydr. Polym.. 2012;88:1233.
- Carbohydr. Polym.. 2012;88:1201.
- Carbohydr. Polym.. 2012;90:316.
- Carbohydr. Polym.. 2011;84:454.
- Carbohydr. Polym.. 2011;86:988.
- Polym. Plast. Technol. Eng.. 2006;45:71.
- Polym. Plast. Technol. Eng.. 2008;47:58.
- Carbohydr. Polym.. 2008;74:707.
- Carbohydr. Polym.. 2010;82:195.
- Carbohydr. Polym.. 2010;82:202.
- Carbohydr. Polym.. 2009;75:44.
- Process Biochem.. 2009;44:521.
- Chemosphere. 2006;63:1041.
- Phytochem. Anal.. 2000;11:7.
- Chem. Eng. J.. 2009;147:13.
- Colloid. Surfaces A: Physicochem. Eng. Aspects. 2011;380:100.
- Carbohydr. Polym.. 2011;85:664.
- Carbohydr. Polym.. 2008;74:922.
- Cellulose. 1997;4:209.
- Adsorpt. Sci. Technol.. 2005;23:367.
- Adsorpt. Sci. Technol.. 2005;23:455.
- Polym. Plast. Technol. Eng.. 2007;46:71.
- Energy Edu. Sci. Technol.. 2007;19:45.
- Carbohydr. Polym.. 2010;79:533.
- Carbohydr. Polym.. 2010;82:933.
- Carbohydr. Polym.. 2009;78:961.
- Carbohydr. Polym.. 2009;78:330.
- Carbohydr. Polym.. 2010;82:618.
- Bioresour. Technol.. 2010;101:7410.
- Carbohydr. Polym.. 2010;82:1248.
- J. Clean Prod.. 2008;16:1321.
- Angew. Chem. Int. Ed.. 2005;44:3358.
- Int. Sugar J.. 2004;106:147.
- Colloid. Surfaces A: Physicochem. Eng. Aspects. 2004;238:1.
- Ind. Eng. Chem. Anal. Ed.. 1946;18:500.
- Cement Concrete Res.. 2011;41:46.
- Appl. Surf. Sci.. 2010;256:4103.
- Trends Biotechnol.. 2005;23:22.
- Bioresour. Technol.. 2011;102:7946.
- Carbohydr. Polym.. 2009;76:23.
- Adsorpt. Sci. Technol.. 2004;22:679.
- Carbohydr. Polym.. 1998;36:293.
- Carbohydr. Polym.. 2009;78:779.
- Quantitative Inorganic Analysis (third ed.). London: Longman Group Limited; 1961.
- Bioresour. Technol.. 2009;100:1687.
- Polymer. 2005;46:5689.
- Colourage. 1998;45:19.







