Translate this page into:
Chemical reaction process and dynamic characteristics of urea pyrolysis products in inhibiting gas explosion
⁎Corresponding author at: College of Safety Science and Engineering, Liaoning Technical University, Huludao, Liaoning 125105, China. jiapengbom@gmail.com (Peng Jia)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Abstract
During the coal mining process, gas explosions pose significant hazards, causing casualties and property losses. In order to develop new types of inhibitors for gas explosions, this paper explores the reaction mechanisms of urea pyrolysis products NH3 and HNCO inhibiting gas explosions based on density functional theory (DFT), transition state theory, and grand canonical ensemble Monte Carlo method (GCMC). It analyzes the reaction processes and kinetic characteristics of urea pyrolysis products with key radicals and gas molecules involved in explosions from a microscopic dynamic perspective. The results show that urea pyrolysis products exhibit good inhibition effects on active radicals involved in explosion reactions·NH3 and HNCO show stronger van der Waals forces in electrostatic attraction towards O2 and *H, respectively, and faster rate advantages in reaction rate constants. The lower Gibbs free energy barrier indicates higher reaction activity of pyrolysis products, thereby diluting the concentration of key radicals involved in explosion reactions and effectively inhibiting explosion key elementary reactions (R32, R38, R53, R57, R156, and R170). This study provides new insights into the microscopic inhibition mechanism of urea pyrolysis products on gas explosions, offering a new approach for designing more targeted modified inhibitors, which helps reduce the hazards of gas explosions during coal mining and provides scientific support and technical guidance for safe coal mining production.
Keywords
Gas explosion
Urea
Explosion inhibition mechanism
Reaction rate constant
Electrostatic potential
1 Introduction
As a basic energy source and an important industrial raw material in China, coal provides a reliable energy guarantee for the national economy and social development. However, its complex mining environment makes the coal mining industry one of the high-risk industries (Liu et al., 2020; Li et al., 2021; Tu et al., 2016). Gas explosion is the most serious and destructive group casualty accidents in mining, and its occurrence is often accompanied by powerful shock waves, high temperature and pressure, as well as harmful gases (Nie et al., 2017), which poses a serious threat to the lives of miners. In addition, some gas explosions can also cause secondary accidents, such as coal dust explosion, which can produce harmful gases and oxygen scarcity caused by the harsh environment and then can lead to serious mass deaths and injuries, resulting in negative social effects (Nie et al., 2020). Therefore, gas explosion prevention and control has been the focus of researchers at home and abroad (Tang et al., 2022; Wen et al., 2015).
For the methods of gas explosion prevention and control, currently involves explosion suppression, explosion blocking, explosion relief and explosion isolation. The four methods have cross overlap part, and each method has its advantages and shortcomings, the coupling effect of the combination of different methods is better than its individual role (Chen et al., 2021). At present, explosion suppression is the main research direction of gas explosion prevention and control, the use of explosion suppression agent can effectively slow down and reduce the propagation speed of the explosion flame as well as the overpressure shock wave generated by the explosion. Research on gas explosion suppression by domestic and foreign researchers mainly focuses on inert gas explosion suppression technology (Wang et al., 2019; Mitu et al., 2017), water-based explosion suppression technology (Gu et al., 2021), inert separation explosion suppression technology (Meng et al., 2022) and so on, and the study is mostly from the perspective of experiment and numerical simulation.
Analyzed from an experimental point of view, the study of inert gas suppression is mostly focused on the materials based onCO2, N2 (Yu et al., 2020). Senecal et al (2005) found that the use of inert gas can effectively inhibit the combustion flame, thus established an explicit relationship between inert gas quenching concentration and heat capacity and fuel properties. Zhang et al (2017) compared the explosion suppression performance of N2 and CO2 and found that N2 does not participate in the explosive chain reaction, only play a physical explosion suppression effect, while CO2 plays a physical, chemical double explosion suppression effect, and the maximum reduction up to 96.2 %. In the study of the water spray explosion suppression technology, Gu (Gu et al., 2021) explored the chamber and atomized water on the pipeline gas explosion flame propagation, examined the cavity structure and water spray on the flame propagation speed and fire expansion, and it is demonstrated that the effect of gas explosion flame attenuation is better in a cavity with water mist than in an empty cavity. Thomas (2002) through the study of fine water mist on the methane laminar flame suppression ability got the conclusion that the smaller the particle size of the water mist; the higher its heat-absorption and evaporation efficiency, and the droplets in the 10 μm-30 μm particle size on the explosion flame suppression is more significant.
Analysis from the perspective of numerical simulation, for inert gas explosion suppression technology, Wen (2014) edited the CHEMKIN package in the SENKIN subroutine, established a gas explosion chemical reaction kinetic model of a restricted space, the study shows that the gas mixture filled with N2, CO2 and water vapor can effectively delay the time of the gas explosion, reduce the concentration of the center of the chemical reaction of the gas explosion, as well as reduce the explosion concentration of some causative gas. In the water mist explosion suppression research, Parra et al. (2004) used numerical simulation methods to study the performance of fine water mist quenching methane flames, and got the conclusion that the fine water droplets in the explosion will absorb a lot of heat and cracked into smaller water particles under the action of the explosion pressure wave, so that its heat absorption and evaporation ability will be strengthen, and the explosion intensity will be attenuated further.
Although the effect of inert gas to inhibit methane explosion is significant, it is easy to cause asphyxiation of trapped people downhole, less practical. Fine water mist suppressant needs to be prepared by high-pressure devices; it is difficult to be widely used in the complex environment of the underground. Many researchers carry out extensive research on the more practical and stable explosion suppression powder. Explosion suppression powder materials, including carbonates (such as NaHCO3, KHCO3, CaCO3), phosphates (such as NH4H2PO4, (NH4)2HPO4, CaHPO4), halides (such as KCl, NaCl), hydroxides (such as Al (OH)3, Mg (OH)2), as well as SiO2, urea, diatomaceous earth, kaolinite (Zhang et al., 2023).
Powder explosion suppression technology in the experimental aspect, Deng (Deng et al., 2012) et al. studied the gas explosion pressure and its ries rate with the addition of ABC ultrafine dry powder and diatomaceous earth in a 20 L spherical container after, found that ABC ultrafine dry powder explosion suppression effect is stronger than the diatomaceous earth. In 20 L spherical explosion containers and 5 L Plexiglas pipe, Wang (Wang et al., 2017) et al. used the solvent − anti-solvent method to prepare the NaHCO3/red mud composites for CH4 inhibition, and the results showed that the inhibition performance of NaHCO3/red mud composite powder was better than that of single powder.
From the perspective of numerical simulation on the powder explosion suppression, Qiao(Qiao et al., 2024) et al. used the quantum chemical calculation software Gaussian to analyze the gas–solid two-phase medium synergistic inhibition of gas explosion process, the cracking of NaHCO3 powder will absorb the heat in the reaction system, and its decomposition products will react preferentially with *OH, *H in the mixed system, impeding the production of *O, the chain process will inhibit the CH2O stage, play a role in inhibiting the chain reaction transfer process.
Among the many explosion suppression materials, urea is regarded as an excellent explosion suppression material, which has an important application potential in the prevention and control of gas explosions. Yu et al (2012) found that urea has an inhibitory effect on gas explosions in coal mines. Urea can reduce the pressure and explosion index of gas explosion, thus slowing down the explosion process and reducing the incidence of accidents. Meanwhile, Zhang et al (2016) studied the effect of urea addition on the indexes of methane explosion pressure, temperature and rate based on standard bottle experiments, and explored the mechanism of urea molecules on methane molecules through theoretical calculations. The results show that urea can effectively inhibit methane explosion, and the inhibition effect is enhanced with the increase of the amount of urea added. Fan et al (2017) used urea as a reagent to study the inhibition effect of urea pyrolysis products on the gas explosion, and explored the micro-mechanisms with infrared spectrometer, thermogravimetric analyzer. It was found that compounds such as NH3, CO and formaldehyde were generated in large quantities in the pyrolysis products of urea, which could react with the free radicals in the gas to play the role of inhibiting gas explosion, and at the same time, the N2 in the pyrolysis products of urea could also dilute the gas concentration.
However, the experimental research of the mechanism of urea explosion suppression is more, while the numerical simulation is less. Urea is prone to pyrolysis under high temperature environment, and the inhibition mechanism of its pyrolysis products on gas explosion needs to be studied further. Therefore, in this paper, based on the density-functional theory (Perriot et al., 2020; Li et al., 2006), we take the pyrolysis products of urea as the research object, optimize the molecular models of reactants, transition states and products, derive the key primitive reactions of urea for the inhibition of methane explosion and calculate the Gibbs free-energy barriers. The reaction rates of each reaction at different temperatures are discussed, and the electrophilic and nucleophilic abilities of the reactants in the reaction are analyzed by calculating the electrostatic potentials of the reactants in the key radical reaction of explosion suppression. The radial distribution function (g(r)) of urea pyrolysis products with gas explosion key radicals is also analyzed from the molecular dynamics point of view based on the giant regular system Monte Carlo calculation method. The aim of this paper is to reveal the action mechanism of urea on the free radicals and gas molecules involved in the reaction during the gas explosion process at the microscopic level, and to deeply understand the mechanism of urea's role in the prevention and control of gas explosions, to provide a strong support for further research and application in related fields. Through comprehensive theoretical analysis and computational simulations, new ideas are provided for the development of gas explosion inhibitors.
2 Simulation setup and calculation method
2.1 Simulation setup
In this paper, Dmol3 module of Materials Studio software was used to calculate the structure and energy of urea pyrolysis and its pyrolysis products, and the geometry optimization, annealing and kinetic relaxation of the model were completed by using the Forcite module (Zhang et al., 2023; Volkov et al., 2020; Moon et al., 2021).The transition states are calculated in the Dmol3 module using LST/QST, and the Perdew-Burke-Ernzerhof (PBE) level in Generalized-Gradient-Approximation (GGA) is chosen for spin calculations the double numerical plus polarization (DNP) basis set, RMS convergence is set to 0.01A, Basis set is selected as DND, base file is set to 3.5.Max.scf cycles is set to 500; NVT is selected for the system synthesis in the Forcite module, and time steps is set to 1 fs, total simulation times is set to 1 fs. 1 fs, total simulation times is set to 5 ps, frame output every is set to 5000 steps, energy deviation is set to 500 kcal/mol, k-space time step is set to 4 fs, 4steps, for non-bond option, the cutoff distance is set to 13 A, the line width is 1A, and the update every is 60 s. In this paper, we investigate the chemical inhibition of urea on gas, and its inhibition of micro-mechanism process is shown in Fig. 1: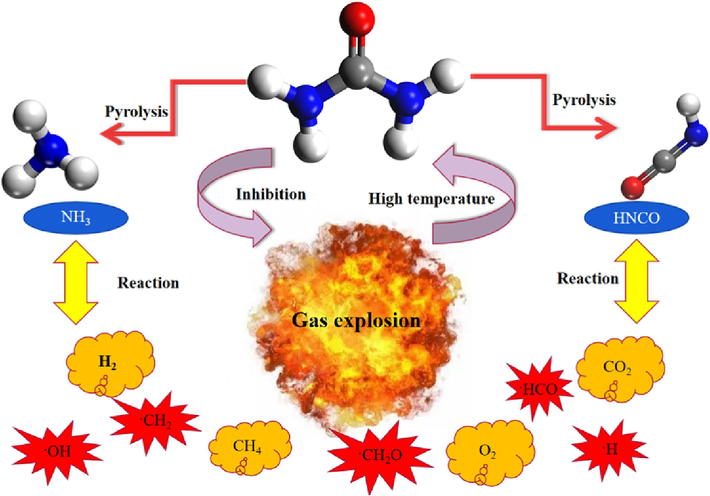
Schematic diagram of the inhibitory structure of urea on gas explosion.
2.2 Calculation methods
The process of methane oxidation chain reaction at the moment of gas explosion causes an increase in ambient temperature, which in turn affects the thermodynamics. The use of Materials studio simulation can realize the calculation of thermodynamics, combined with the transition state theory can be derived from the rate constant of each reaction with the temperature change. Comparing the magnitude of the rate constants, we can determine the influence of each reaction on each other.
In the simulation process, the temperature is 25 K ∼ 1000 K as the interval, every 25 K, the transition state theory reaction rate constant KTST calculation formula (He et al., 2022).
At low temperatures, the hydrogen transfer problem involved induces a strong tunneling effect, so the tunneling effect correction factor k needs to be calculated, and since the imaginary frequencies ω of the reactions involved in this paper are all less than 1000, the reaction rate constants are obtained from the following equations (SOBEREVA).
3 Results and discussion
3.1 Reaction mechanism of urea to inhibit gas explosion
Gas explosion is an extremely complex process, its explosion must have three basic conditions at the same time: first, the gas concentration in the explosion limit, generally 5 % to 16 %; second, the concentration of O2 in the mixture of gases is not less than 12 %; third, the high-temperature fire source of sufficient energy, generally 650 ℃ ∼ 750 ℃ (Dai et al., 2013). For the study of gas explosion mechanism and flame propagation law, the current recognized chemical kinetic mechanism of methane combustion is the GRI Mech 3.0 of Lawrence Livermore National Laboratory in the United States, which contains 53 components and 325 primitive reactions (Smith et al., 2019). To simplify the workload of numerical simulation studies, the more critical primitive reaction steps in the gas explosion reaction chain are used as a reference as shown in Table 1:
Primitive reaction number
equation of a chemical reaction
Primitive reaction number
equation of a chemical reaction
R32
O2 + CH2O==HO2+*HCO
R116
2HO2==H2O2 + O2
R38
O2+*H==*O+*OH
R119
HO2+*CH3== *OH + CH3O
R53
*H + CH4==*CH3 + H2
R156
*CH3 + O2==*OH + CH2O
R57
*H + CH2O(+M)== CH3O(+M)
R158
2*CH3(+M)==C2H6(+M)
R98
*OH + CH4==*CH3 + H2O
R161
*CH3 + CH2O==*HCO + CH4
R101
*OH + CH2O== HCO2 + H2O
R170
O2 + CH3O==HO2 + CH2O
It is observed that in addition to the free radicals such as *O and *OH, which are the elements triggering the chain reaction in the key radical reaction of methane explosion, the O2, *HCO, *H and *CH2 radicals as well as the H2 and CO2 gas molecules are also important factors involved in the reaction. Therefore, this paper mainly analyzes the micro-mechanism of gas explosion inhibition by urea pyrolysis products based on previous studies.
3.1.1 Urea pyrolysis reaction mechanism
Urea (H2N-CO-NH2) is a symmetric molecule composed of C, N, O and H. Its molecular structure is shown in Fig. 2. The C atom in urea is in the middle, and the O atom and two N atoms are connected on both sides. During the pyrolysis reaction, the radical reaction mainly occurs on the C = O double bond and C-N single bond.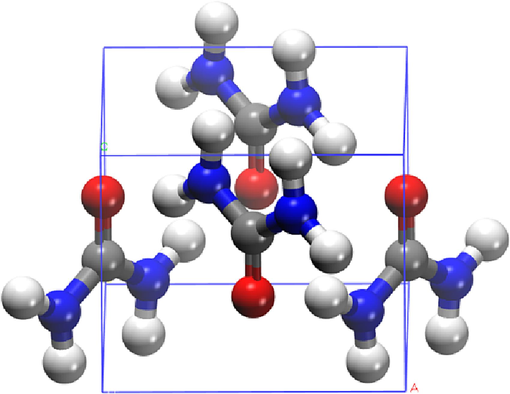
Molecular structure of urea.
Currently, based on relevant studies, it is concluded that the main urea pyrolysis reactions are (Alzueta et al., 2000):
In the reaction process, urea in a C-N bond breakage, at the same time another N on the H transfer, generating NH3 and HNCO. HNCO in the high temperature hydrolysis of H-N bond breakage to generate NH3, CO2 and HNCNH respectively·NH3 is the main gas product in the process of urea pyrolysis, it has a high degree of electrophilicity and nucleophilicity, easy to chemical reaction. HNCO is another important product of urea pyrolysis, it has a special molecular structure and chemical properties, easy to participate in the electrophilic-nucleophilic reaction·NH3, HNCO is the existence of a more stable in the process of urea pyrolysis is the main pyrolysis product. This paper mainly focuses on NH3, HNCO, conducts in-depth analysis of the two on the free radicals generated in the process of gas explosion as well as the impact of the gas, and optimize the structure of the reactants, transition states and products on the basis of the reaction to confirm the authenticity of the reaction as well as the reliability of the pathway, and ultimately determined that the pyrolysis process of urea is shown in Fig. 3.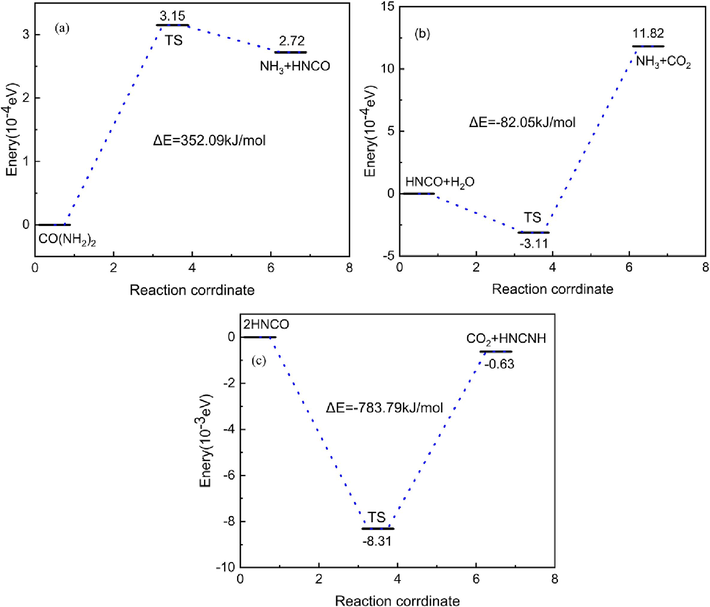
Mechanism of urea thermal decomposition reaction.
As shown in Fig. 3, urea pyrolysis reaction energy barrier of 352.08 kJ/mol, the reaction is an adsorptive reaction, HNCO reaction with water to generate NH3 and CO2 energy barrier of −82.05 kJ/mol, an exothermic reaction, and further pyrolysis energy barrier of −783.79 kJ/mol, the reaction is an exothermic reaction. As the pyrolysis of urea did not undergo too many intermediate processes, the product is mainly NH3 and HNCO-based, NH3 and HNCO will interfere with the free radicals in the key reaction of the methane explosion, thus playing a role in suppressing the explosion.
3.1.2 Explosion suppression mechanism of urea pyrolysis product NH3
Combined with the molecules and radicals involved in the detailed mechanism of the gas explosion chemical reaction, the possible reactions of NH3 in the methane explosion chain reaction are shown in Table 2:
working condition
pyrolysis product
Free radicals (Rea)
Transition State (TS)
Product (Pro)
Reaction (1)
NH3
HCO
TS1
NH2 + H2O + CO2
Reaction (2)
NH3
H2
TS2
NH4+
Reaction (3)
NH3
*H
TS3
*NH2 + H2
Reaction (4)
NH3
CH2O
TS4
N2 + CO + H2
Reaction (5)
NH3
CH4
TS5
CO+*NH2+*H
Reaction (6)
NH3
CO2
TS6
NH4++NH2COO–
Reaction (7)
NH3
*OH
TS7
*NH2 + H2O
Reaction (8)
NH3
O2
TS8
*NH2+*HO2
When the transition state search was performed using the Dmol3 module, the reactions (1), 4, and 5 had too many reactions false frequencies, the reactions were not valid. While reaction (2), 3, 6, 7 and 8 have only 1 reaction false frequency, the validation yields the following reactions for NH3 involvement in suppressing methane explosion:
The reaction pathways involving NH3 are depicted in Fig. 4. In reaction (2), NH3 successfully abstracts a *O from O2, generating *NH4. In reaction (3), NH3 breaks a H bond and combines with the free state *H, producing H2 and *NH2. In reaction (6), NH3 combines with a hydrogen atom generated from its own thermal decomposition at high temperatures, and the resulting *NH2 further reacts with CO2 generated in gas explosions to produce *NH4 and *NH2COO. In reaction (7), *OH abstracts a hydrogen atom from NH3, generating H2O. In reaction (8), an O2 atom reacts with an H atom on NH3, producing *HO2 and *NH2. Transition states were searched for, with reaction barriers of 101.58 kJ/mol for reaction (2), 31.54 kJ/mol for reaction (3), −187.43 kJ/mol for reaction (6), −68.53 kJ/mol for reaction (7), and 50.05 kJ/mol for reaction (8). Among these, reactions (2), 3, and 8 are endothermic, while reactions (6) and (7) are exothermic. Comparatively, reaction (3) exhibits a lower energy barrier, indicating a higher likelihood of occurrence.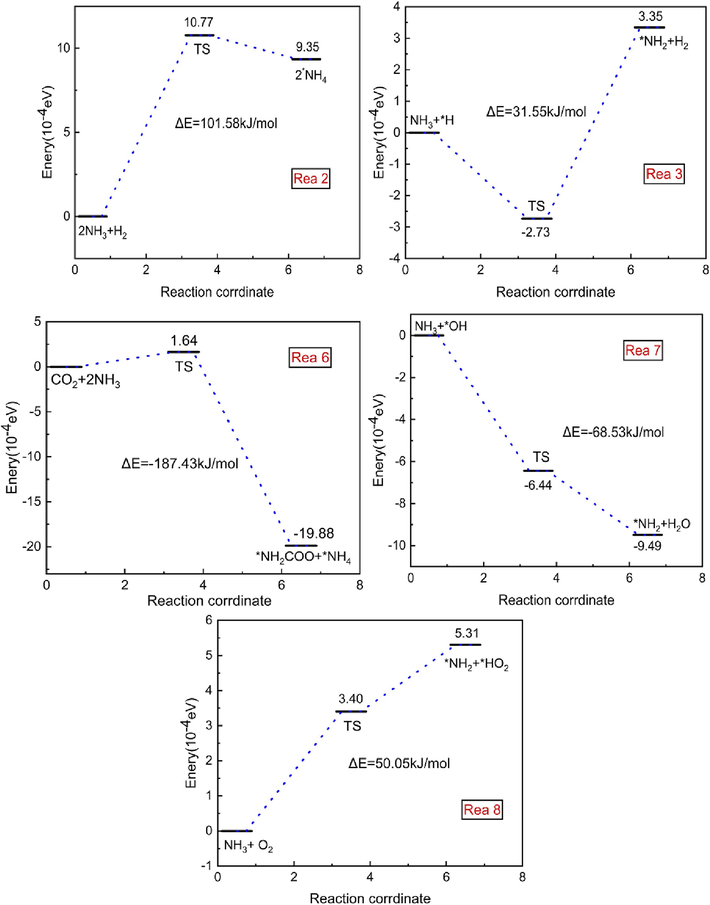
Reaction Mechanism of NH3 with Various Molecules and Free Radicals.
3.1.3 Explosion suppression mechanism of urea pyrolysis product HNCO
Analyze the detailed mechanism of gas explosion chemical reaction involved in the reaction of molecules and free radicals, combined with the properties of HNCO itself, HNCO in the methane explosion chain reaction may occur in the reaction shown in Table 3:
working condition
pyrolysis product
Free radicals (Rea)
Transition State (TS)
Product (Pro)
Reaction (9)
HNCO
*HCO
TS9
NCO + CH2O
Reaction (10)
HNCO
O2
TS10
*HNO + CO2
Reaction (11)
HNCO
*H
TS11
*NCO + H2
Reaction (12)
HNCO
*H
TS12
*NH2 + CO
Reaction (13)
HNCO
*OH
TS13
*NCO + H2O
Reaction (14)
HNCO
CO2
TS15
CH2O + NCO
Reaction 15
HNCO
CH4
TS16
HNC + CO2 + H2O
Reaction 16
HNCO
CH2O
TS17
CH3 + NCO
After optimizing the reactant and product structures, transition state search was carried out using Dmol3 software, and the transition state did not exist due to too many reactions false frequencies for reactions (10), 14, 15, and 16, the reaction could not occur. Reactions (9), 11, 12, and 13 have only 1 reaction imaginary frequency, the participation of HNCO in the inhibition of methane explosion was verified as follows:
The reaction paths of each reaction are shown in Fig. 5. In reaction (9), HNCO decomposes and the cleaved *H and *HCO combine rapidly to form *NCO radicals and CH2O; in both reaction (11) and reaction (12), the reaction is between HNCO and *H, but the products are not the same. HNCO pyrolytically breaks off *H at high temperature and combines it with free radicals to form *NCO and H2; in reaction (12), the C on the HNCO atom breaks the bonds *H and *N to produce *NH2 and CO. In reaction (13), *OH captures an H atom on NH3 to form H2O, which ultimately gives *NCO and H2O. reaction (9) has a reaction energy barrier of 67.06 kJ/mol, reaction (11) has a barrier of −17.92 kJ/mol, reaction (12) has a barrier of –33.61 kJ/mol, and reaction (13) has a barrier of 27.21 kJ/mol. Reactions (9) and (13) are adsorptive, reaction (11) and (12) are exothermic. Reactions (9) and (13) are heat-absorbing and reactions (11) and (12) are exothermic. Combined with the energy barrier data, the energy barrier for reaction (11) is lower and the reaction is more likely to occur.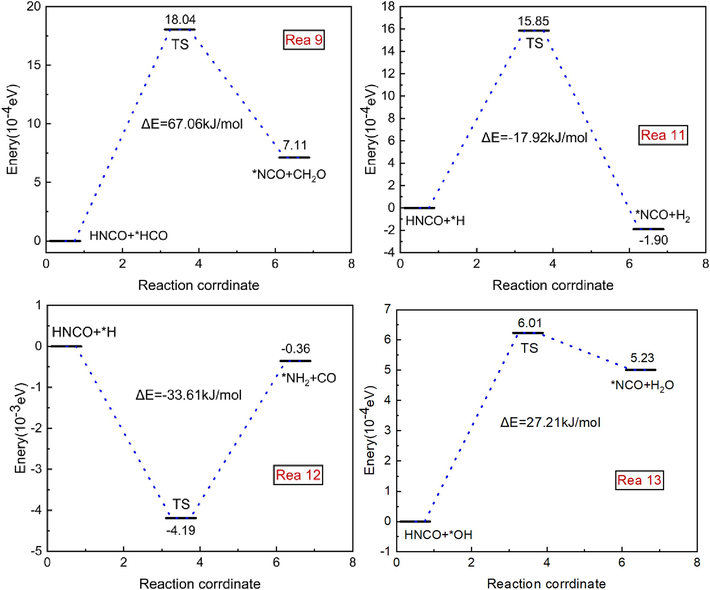
Reaction Mechanism of HNCO with Various Molecules and Free Radicals.
When transition state searches were performed using the Dmol3 software, no transition states were found for some of the reactions. This may be attributed to the influence of kinetic factors, i.e., the rate of formation of some transition states is much lower than their decomposition rate, resulting in their instability during the reaction; on the other hand, in some reactions, reactive reactants or free radicals in the explosion make it difficult for intermediates to be formed.
The process of gas explosion generates a large amount of heat, which in turn contributes to urea pyrolysis. This is consistent with the results reported in the literature (Zhang et al., 2022). The pyrolysis products of urea, NH3 and HNCO, can influence the molecules and free radicals involved in the gas explosion process. However, differences in the choice of simulation paths and parameter settings lead to a slight deviation in the simulation results of the reaction energy barriers compared to the values reported in the literature. For example, the reaction energy barrier for the reaction of HNCO with *OH to form *NCO with H2O in the literature(Zhang et al., 2022) is 12.44 kJ/mol, whereas in this study, the value of this reaction energy barrier is 27.21 kJ/mol.
Urea is heated to generate NH3, HNCO, and HNCO in contact with water to generate NH3 and CO2. As the temperature continues to increase, HNCO further decomposition of CO2 and HNCNH, generated CO2 will reduce the probability of the collision of CH4 and O2 in the mixed system, effectively reducing the concentration of reactants, oxygen and oxidation of the reaction of the free radicals, to achieve the inhibition effect. Combined with Fig. 4, due to the low reaction energy barrier, NH3 at high temperatures will spontaneously first react with the *OH and *H generated in the explosive chain reaction, hindering the normal progress of R38, R53, R57, R98, R101. The product *NH2 can further participate in the reaction to consume *H, reducing its concentration and thus interrupting or delaying the chain reaction. In addition, the *OH consumed by Reaction7 reacts with NH3 to form H2O, which further reacts with HNCO to form CO2, thereby diluting CH4. The reaction of NH3 with O2 inhibits the forward progress of R32, R156, and R170, resulting in an interruption or delay of the explosive chain reaction. This effect will reduce the heat released in the explosion and lower the pressure generated during the explosion. In conjunction with Fig. 5, the urea pyrolysis product, HNCO, primarily consumes the *OH and *H produced in the explosive chain reaction, further affecting R38, R53, R57, R98, and R101 by reducing the free radicals involved in the main gas chain reaction, thereby hindering the methane explosion process.
3.1.4 Reaction rate constants
(1) Rate constants for urea pyrolysis.
Fig. 6 shows the reaction rate constants for the three reactions of urea pyrolysis at different temperatures. From the figure, it can be seen that the rate constants of the three reactions increase with increase in temperature and hence the higher the rate of reaction. The value of rate constants for reaction (3) varies more with temperature compared to reaction (1) and (2) in temperatures ranging from 25 K to 1000 K. When the temperature is 25 K to 125 K, the reaction rate constant of reaction (1) is higher than that of reaction (2) and reaction (3) and the reaction rate is the fastest, and the reaction rate of reaction (3) is the slowest at this temperature; at a temperature of 125 K, the values of the reaction rate constants of reaction (1) and reaction (2) are equal at 6.71; at a temperature of 125 K to 450 K, the reaction rate constant of reaction (1) is higher than the reaction (3) The rate constant of reaction (1) is greater than that of reaction (3), but the rate of both reactions is still lower than that of reaction (2); at a temperature of 450 K, the rate constants of reaction (1) and reaction (3) have equal values of 44.53; at a temperature of 450 K to 975 K, the rate constant of reaction (1) is greater than that of reaction (1) and reaction (3), and the reaction rate is the fastest. The pyrolysis rate of HNCO is the fastest after the temperature is higher than 1000 K. Through the above analysis, it can be obtained that after the occurrence of gas explosion, urea pyrolysis reaction in the pyrolysis of HNCO pyrolysis reaction rate is the fastest, indicating that with the increase in temperature the pyrolysis reaction of HNCO is more likely to occur.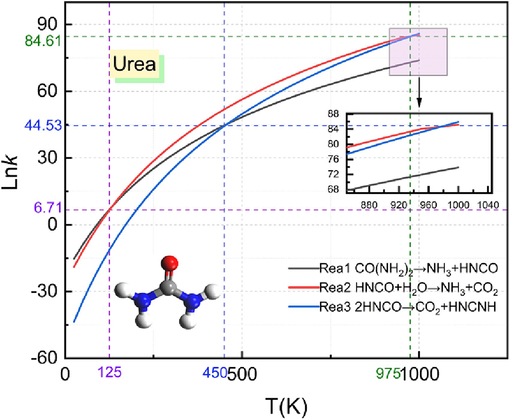
Urea reaction rate constant.
(2) NH3 explosion suppression reaction rate constants.
The reaction rate constants of urea pyrolysis product NH3 involved in the inhibition of methane explosion reaction at different temperatures are shown in Fig. 7. It is obvious from the figure that the reaction rate accelerates gradually with the increase of temperature. Temperature at 25 K ∼ 575 K, reaction rate constant of reaction (2) is higher than the reaction rate constant of reaction (3), reaction (6), reaction (7) and reaction (8), the fastest reaction rate, reaction rate of reaction (6) is the slowest; in the temperature of 575 K, reaction (2) and reaction (8) rate constant value is equal to 62.36. Temperature of 575 K ∼ 1000 K, the rate constant value of reaction (8) with the increase of temperature and increases with increasing temperature. Combining the reaction rate constants for each reaction shows that reaction (8) has the fastest reaction rate for the reaction of NH3 with O2, indicating that this reaction is more likely to occur.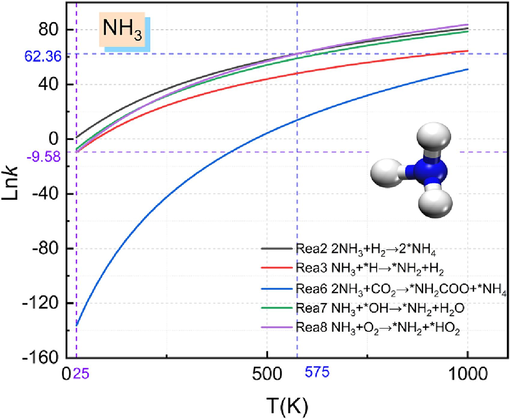
Comparison of NH3 reaction rate constants.
(3) HNCO explosion suppression reaction rate constants.
Fig. 8 shows the reaction rate constants of the urea pyrolysis product HNCO involved in the inhibition of methane explosion at temperatures from 25 K to 1000 K. The reaction rate constants are shown in Fig. 8. From the figure, it is clear that the reaction rate constant with the increase in temperature tends to increase, that is, the reaction rate increases.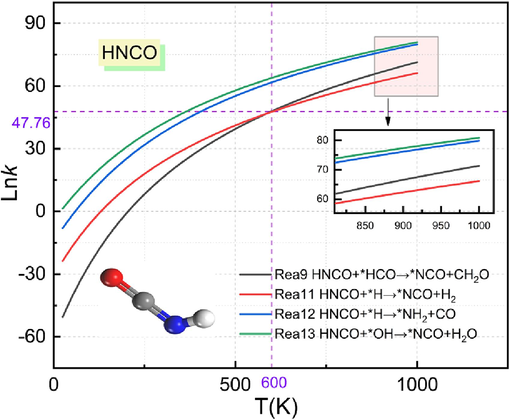
Comparison of HNCO reaction rate constants.
When the temperature is 25 K ∼ 600 K, the reaction rate constant of reaction (13) is higher than the reaction rate constant of reaction (9), reaction (11) and reaction (12), the fastest reaction rate, the reaction rate of reaction (9) is the slowest; in the temperature of 600 K, reaction (9) and reaction (11) rate constant value is equal to 47.76; in the temperature of 600 K ∼ 1000 K, reaction (13) reaction rate is still the fastest at 600 K to 1000 K, the reaction rate of reaction (13) is still the fastest, and the reaction rate of reaction (11) slows down. Through the above analysis can be obtained, after the gas explosion, urea pyrolysis reaction of HNCO and *OH reaction rate is the fastest. This indicates that as the temperature increases, the reaction of HNCO with *OH is more likely to occur.
The reaction rate constants of the urea pyrolysis products increased with increasing temperature, which agrees with the results reported in the literature (He et al., 2022; Baradyn and Ratkiewicz, 2022), all the rates showed an increasing trend. It is noteworthy that there is a significant variability in the reaction rate of urea pyrolysis products at T < 600 K stage, which is related to the electrostatic interaction of its internal groups, reactive sites, which causes the formation of rate gap.
3.2 Surface electrostatic potential analysis of different reactions
Molecules mostly shorten the distance at the beginning of a chemical reaction by electrostatic interaction, which makes the reaction rate faster. Therefore, the electrostatic potential is important in the study of electrostatic interactions between molecules and the prediction of reaction active sites (Wu et al., 2023; Alenaizana et al., 2020; Fu et al., 2014). Combined with the study of urea pyrolysis products in the paper, the surface electrostatic potential distribution of different molecules is shown in Fig. 9. Before reacting with free radicals and gas molecules, the maximum positive value of ESP of urea molecules is 0.09 a.u., distributed in the H region, and the maximum negative value is −0.08 a.u., distributed in the O region. Combined with the legend to analyze urea susceptible to nucleophilic reaction; urea pyrolysis of NH3 surface H of the electrostatic potential of positive, ESP maximum value of 0.05 a.u., N of the potential of negative, the maximum value of −0.08a.u., susceptible to electrophilic reaction; H in the HNCO surface of the electrostatic potential of positive value, ESP maximum value of 0.01a.u., the electrostatic potential of the N of the negative, ESP maximum value of − 0.04a.u., prone to electrophilic reactions; O2 in the air at both ends of the electrostatic potential of the molecules is negative, the maximum value of −0.01 a.u., the center of the region of the electrostatic potential is positive, showing a “negative inside and outside the distribution of positive” characteristics, susceptible to nucleophilic reactions; H on the surface of the CH4 electrostatic potential is positive, the maximum positive ESP value of 0.02 a.u., the electrostatic potential of C is negative, of which the maximum negative value of ESP is −0.06 a.u., by the electrophilic reaction is obvious; CO2 gas molecules in the electrostatic potential of C is positive, the maximum value of 0.05 a.u., the electrostatic potential of O atoms is negative, the maximum value of ESP is −0.06 a.u.; the electrostatic potential of the molecules of the two ends of the H2 positive, the maximum value of ESP for the 0.02a. u., and the electrostatic potential in the center region is negative with an ESP maximum of −0.03 a.u.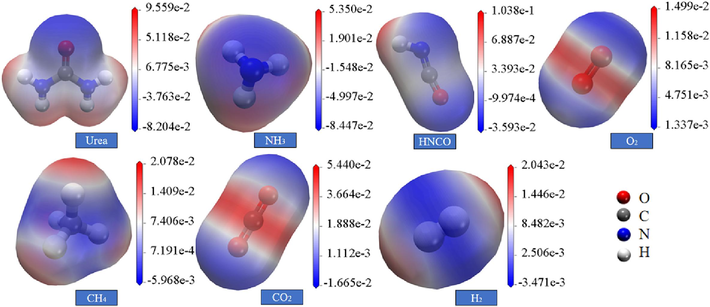
Electrostatic potential distribution of pyrolysis products and gas molecules.
Comparing urea and its pyrolysis products, urea is more susceptible to nucleophilic reactions, and the pyrolysis products NH3 and HNCO are more susceptible to electrophilic reactions. Taken together, the surface electrostatic potentials of the key gases involved in the gas explosion, urea and its pyrolysis products show an inhomogeneous distribution due to the electrophilic and nucleophilic nature of the respective atoms.
The electrostatic interaction of NH3 with gas molecules and free radicals in the system during the reaction is shown in Fig. 10. In reaction (2), both the electrostatic potential of NH3 and the electrostatic potential of H2 involve the distribution of electrostatic potential within the molecules. When the positive charge of NH3 and the negative charge of H2 are near each other, an electrostatic field, i.e., an intermolecular electrostatic interaction, is generated between them. In this case, the electrostatic interaction between NH3 and H2 causes the formation of hydrogen bonding, which leads to the production of *NH4 as a hydrogen donor; similarly in reaction (3), the electrostatic interaction between NH3 and *H causes the formation of hydrogen bonding, which leads to the production of NH2 and H2. the force between the positive and negative charges between the molecules is clearly seen in reaction (6) and (7). In comparison, NH3 is more susceptible to electrophilic reactions with H2 and NH3 is more susceptible to nucleophilic reactions with CO2.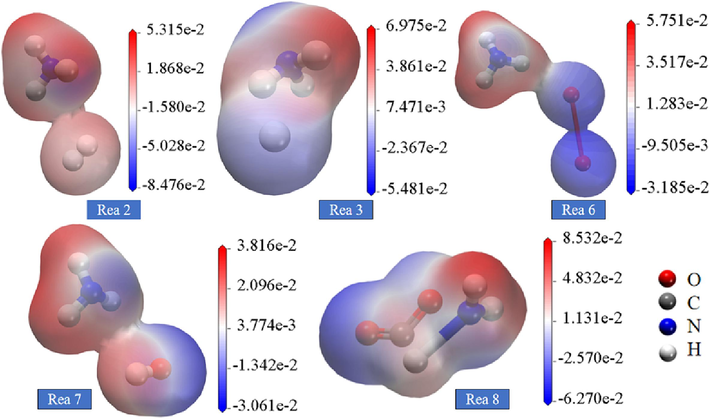
Electrostatic potential distribution of NH3 reacting with various gas molecules and radicals.
As shown in Fig. 11, HNCO is attracted to the positive and negative structural radicals as well as the electrostatic potential of the gas molecules during the gas explosion. In reaction (9), hydrogen bonds are formed between HNCO and *HCO, creating a transition state gap. In the transition state gap, the atoms in the hydrogen bond undergo processes such as rearrangement, sharing electrons, and transferring electrons to form a new chemical bond. In reaction (11), electrostatic forces are generated between the C in HNCO and *H apparently. In reaction (13), HNCO exhibits electrostatic attraction with *OH. In contrast to the other reactions, HNCO and *HCO are more obviously affected by electrophilic nucleophilicity.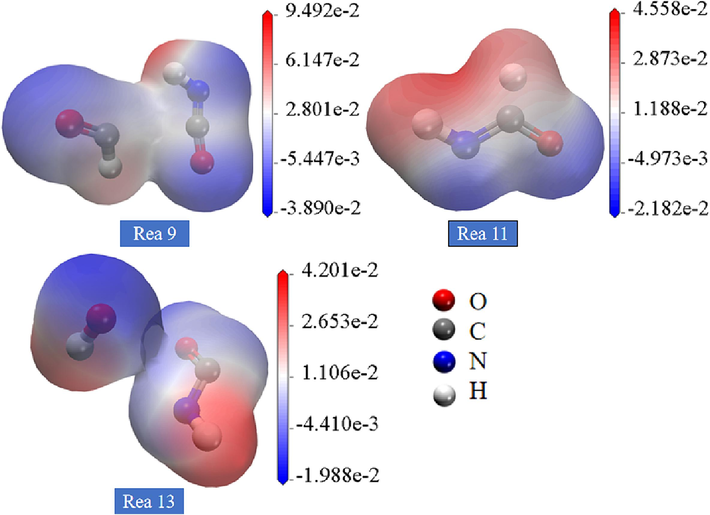
Electrostatic potential distribution of HNCO reacting with various gas molecules and radicals.
3.3 Kinetic process of gas explosion inhibition by urea pyrolysis products
The kinetic processes of the free radicals as well as gas molecules critical in the NH3 to gas explosion are shown in Fig. 12. The kinetic analysis of the urea pyrolysis product NH3 radicals as well as gas molecules was performed using the Forcite module of Materials studio. The temperature was gradually adjusted during the simulation to simulate the temperature change during the gas explosion. The temperature was set in the range of 650 K to 750 K and appropriate time evolution was performed to observe the kinetic behavior before the reaction. The peaks and shapes of the radial distribution functions were used to analyze the strength of the interactions between the NH3 radicals as well as the gas molecules. Analyzing the radial distribution function, the radial distribution of O2 is significantly higher than that of other radicals or gas molecules when the radii r is the same, indicating that O2 is more likely to approach NH3 and then react.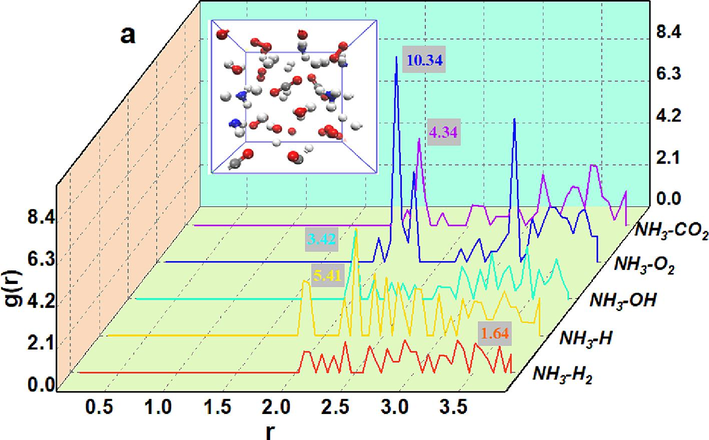
Dynamics of NH3 Inhibition of Free Radicals and Gas Molecules in Gas Explosion.
The kinetic process of HNCO on the key radicals as well as gas molecules in the gas explosion is shown in Fig. 13. In the kinetic process of inhibition of gas explosion by urea pyrolysis product, analyzed by radial distribution function, *H shows a clear peak in the radial distribution function plot when the radius r is the same, which indicates that *H is more likely to be close to HNCO and more likely to react with it relative to other free radicals or gas molecules. It suggests that the urea pyrolysis product HNCO plays a key role in the inhibition of *H during gas explosions.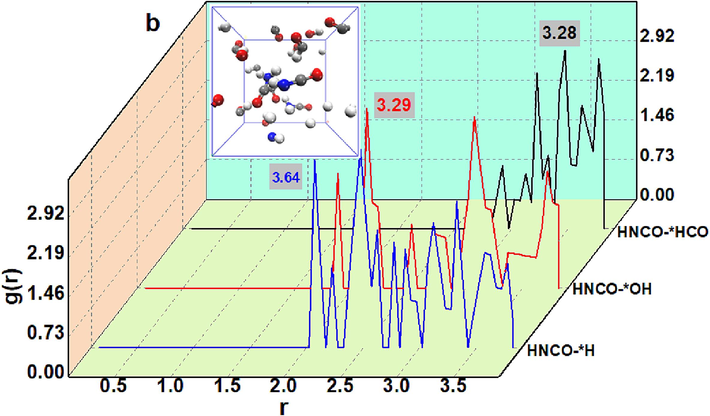
Dynamics of HNCO Inhibition of Free Radicals and Gas Molecules in Gas Explosion.
Observing the reaction rate, electrostatic potential and radial distribution function, the fastest reaction rate was observed between NH3 and O2, which was related to the electrophilicity of NH3, making it easier to be oxidized, thus accelerating the reaction rate. The higher radial distribution of O2 and *H indicated that they interacted more strongly with NH3 and HNCO, clarifying the key role of NH3 and HNCO in the process of inhibiting the gas explosions. It indicates that there is a certain inhibition effect of urea pyrolysis products NH3 and HNCO on gas explosion.
4 Discussion
This paper delves into the inhibition process of urea detonation in-depth using DFT, transition state theory, and GCMC methods, investigating reaction barriers, reaction rate constants, electrostatic distributions, and radial distribution functions. The thermal decomposition products of urea, NH3, and HNCO, exhibit a certain inhibitory effect on gas explosions with relatively low Gibbs free energy barriers. The reaction rates of urea and its constituents increase with rising temperatures. Particularly, the thermal decomposition reaction of HNCO is more prone to occur, NH3 exhibits the fastest reaction rate with O2 during the explosion process, and the reaction rate of HNCO's molecular model is most influenced by *H. The molecular surface electrostatic potential reveals that the distribution of surface electrostatic potential of urea's thermal decomposition products affects the reaction rate. Specifically, urea molecules are significantly affected by nucleophilic reactions, where NH3 and CO2 in its thermal decomposition products are more susceptible to nucleophilic reactions, while H2 is more prone to electrophilic reactions. HNCO and *HCO are notably affected by both electrophilic and nucleophilic reactions. Analysis of radial distribution functions indicates that the thermal decomposition products of urea tend to react with O2 and *H in the gas explosion process, thus exerting inhibitory effects on detonation.
5 Conclusion
This study employs density functional theory (DFT), transition state theory, and grand canonical ensemble Monte Carlo (GCMC) methods to comprehensively elucidate the inhibitory effects of urea thermal decomposition products (NH3 and HNCO) on gas explosions at a microscopic level. The results indicate low energy barriers and easy occurrence of reactions, providing a new theoretical basis for gas explosion inhibitors. Analysis of molecular surface electrostatic potential reveals the non-uniform charge distribution characteristics of urea and its thermal decomposition products at the atomic level, further elucidating their mechanisms in reactions. Dynamics analysis demonstrates that urea thermal decomposition products exhibit a strong tendency to react with key reactive species O2 and *H in the explosion process, diluting the concentration of critical reactive radicals involved in the explosion reaction, thereby effectively inhibiting gas explosion progression.
This study holds significant scientific and practical value in delving into the inhibition mechanisms of gas explosions and developing innovative gas explosion inhibitors. It provides a new direction for future research in related fields, offering important guidance for mechanistic analysis of gas explosions, inhibitor design and optimization, as well as industrial applications.
CRediT authorship contribution statement
Jinzhang Jia: Funding acquisition, Methodology, Writing – review & editing, Resources, Supervision. Shiwen Shan: Investigation, Visualization, Writing – original draft, Writing – review & editing. Peng Jia: Formal analysis, Funding acquisition, Supervision, Validation, Writing – review & editing. Hailong Song: Methodology, Software, Validation, Visualization, Writing – review & editing.
Acknowledgement
This research is conducted with financial support from the National Natural Science Foundation of China (No.52174183 and 52374203) and Liaoning Province Doctoral Research Start-up Fund Project (No.2023-BS-203).
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Python Implementation of the Restrained Electrostatic Potential Charge Model. Int. J. Quantum Chem. 2020;120(2):e26035.
- [Google Scholar]
- Impact of new findings concerning urea thermal decomposition on the modeling of the urea-SNCR process[J] Energy Fuel. 2000;14(2):509-510.
- [Google Scholar]
- On-The-Fly Kinetics of the Hydrogen Abstraction by Hydroperoxyl Radical: An Application of the Reaction Class Transition State Theory. Front. Chem.. 2022;9:806873
- [Google Scholar]
- Summary and outlook on the development law and prevention of coal mine gas explosion. Fire Science. 2021;30(02):63-79.
- [Google Scholar]
- Experimental Study on the Conditions and Mechanism of Gas Explosion in Underground Coal Mines. Fuel. 2013;108:626-632.
- [Google Scholar]
- Comparative experimental study on inhibiting gas explosion using ABC dry powder and diatomite powder. Journal of Coal Science and Engineering (china). 2012;18:138-142.
- [Google Scholar]
- Inhibitory effect and mechanism of urea on coal dust explosion. J. Loss Prev. Process Ind.. 2017;49:408-413.
- [Google Scholar]
- Comparing Methods for Predicting the Reactive Site of Electrophilic Substitution. Acta Physico-Chimica Sinica. 2014;30:628.
- [Google Scholar]
- Study on Influence of Cavity and Water Mist on Flame Propagation of Gas Explosion in a Pipeline. Geofluids. 2021;2021(2021):1-8.
- [Google Scholar]
- Microscopic Mechanism Analysis of Gas Explosion Inhibition by Diatomite. Journal of Coal Science. 2022;47(10):3695-3703.
- [Google Scholar]
- Characteristics and trends of coal mine safety development. Energy Sources, Part A: Recovery, Utilization, and Environmental Effects; 2021. p. :1-19.
- Theoretical study on the reaction mechanism of CH2F radical with HNCO. Chin. J. Chem. Phys.. 2006;19(5):451.
- [Google Scholar]
- An empirical study of early warning model on the number of coal mine accidents in China. Saf. Sci.. 2020;123:104559
- [Google Scholar]
- Study on mechanism and dynamics of inert powder explosion inhibitor inhibiting aluminum powder explosion. Adv. Powder Technol.. 2022;33(11):103773
- [Google Scholar]
- Inert gas influence on the laminar burning velocity of methane-air mixtures. J. Hazard. Mater.. 2017;321:440-448.
- [Google Scholar]
- Simulation of indoor fire dynamics of residential buildings with full-scale fire test. Sustainability. 2021;13(9):4897.
- [Google Scholar]
- Chemical kinetic characteristics of methane/air mixture explosion and its affecting factors. J. Loss Prev. Process Ind.. 2017;49:675-682.
- [Google Scholar]
- Experimental analysis on gas and solid residues of pre- and post-explosion coal dust. Energy Fuel. 2020;35(2):1727-1740.
- [Google Scholar]
- Extinction of premixed methane–air flames by water mist. Fire Saf. J.. 2004;39(7):581-600.
- [Google Scholar]
- Reaction rates in nitromethane under high pressure from density functional tight binding molecular dynamics simulations. Chem. A Eur. J.. 2020;124(17):3314-3328.
- [Google Scholar]
- Experimental and Molecular Dynamics Study on Cooperative Inhibition of Gas Explosion by Gas-Solid Two-Phase Medium. Explosion and Shock Waves 2024:1-13.
- [Google Scholar]
- Flame extinguishing in the cup-burner by inert gases. Fire Saf. J.. 2005;40(6):579-591.
- [Google Scholar]
- Smith, G.P., Golden, D.M., Frenklach, M., et al. (2019-12-05). Gri-Mech 3.0 [EB/OL]. Available from.
- SOBEREVA. Excel Spreadsheet for Calculating Reaction Rate Constants Based on Transition State Theory [EB/OL]. Available from: http://sobereva.com/310.
- Synergistic Inhibiting and Sealing Material for Preventing the Compound Disaster of Gas and Coal Spontaneous Combustion: Ratio Optimization and Inhibition Test. Combust. Sci. Technol. 2022:1-21.
- [Google Scholar]
- The quenching of laminar methane-air flames by water mists. Combust. Flame. 2002;130(1–2):147-160.
- [Google Scholar]
- Experimental Study of Coal and Gas Outbursts Related to Gas-Enriched Areas. Rock Mech. Rock Eng.. 2016;49(9):113.
- [Google Scholar]
- Calculation of chemical potential of a molecule on the basis of radial distribution functions. Colloid J.. 2020;82:634-640.
- [Google Scholar]
- Methane explosion suppression characteristics based on the NaHCO3/red-mud composite powders with core-shell structure. J. Hazard. Mater.. 2017;335:84-91.
- [Google Scholar]
- The inhibition effect of gas–solid two-phase inhibitors on methane explosion. Energies. 2019;12(3):398.
- [Google Scholar]
- Numerical Simulation Study on Inhibition of Methane Explosion in Confined Space by N2, CO2 and H2O. Liaoning Technical University); 2014. Master's thesis
- Methane–air explosion characteristics with different obstacle configurations. Int. J. Min. Sci. Technol.. 2015;25(2):213-218.
- [Google Scholar]
- Adsorption and Competition Mechanism of Tetracycline and Erythromycin on Montmorillonite: Experimental and Theoretical Investigation. J. Mol. Liq.. 2023;370:121037
- [Google Scholar]
- Study on gas explosion suppression influence of thermal properties of powder. J. China Coal Soc.. 2012;37(5):830-835.
- [Google Scholar]
- Research Progress and Development Trend of Gas Explosion Suppression and Disaster Reduction Technology in Coal Mines in China. J. China Coal Soc.. 2020;45(01):168-188.
- [Google Scholar]
- Explosive characteristics and kinetic mechanism of methane–air mixtures under high-temperature conditions. ACS Omega. 2023;8(4):4251-4260.
- [Google Scholar]
- Experimental Investigation on the Inhibitory Effect of Urea on Methane Explosion. Fuel Process. Technol.. 2016;145:136-142.
- [Google Scholar]
- Study on the Key Elementary Reaction Mechanism of Methane Explosion Inhibited by Urea. Fire Science and Technology. 2022;41(06):732-735.
- [Google Scholar]
- Research Progress and Prospect of Multi-phase Synergistic Explosion Inhibition in Coal Mine Gas. China Safety Science Journal. 2023;33(S1):97-104.
- [Google Scholar]
- Experimental study on inhibition of methane explosion by inert gas N2/CO2. Explosion and Shock Waves. 2017;37(05):906-912.
- [Google Scholar]







