Translate this page into:
Chemical speciation and contamination assessment of Pb and V by sequential extraction in surface sediment off Nile Delta, Egypt
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.

Abstract
Environmental pollution caused by lead and vanadium is almost entirely due to industrial activities, such as the production of steel, pigments, photographic materials and insecticides.
The concentration and chemical speciation of Pb and V were studied in surface sediments from 11 stations off Nile River Delta. Sequential extraction technique was applied to assess the four (exchangeable, acid-reducible, oxidizable-organic and residual) fractions in surface sediment, also to obtain an overall classification of lead and vanadium pollution in this area through its spatial distribution. This investigation was the first study on the basis of the chemical speciation of Pb and V in surface sediments off Nile Delta.
The total concentrations of metals were ranged (22.8–41.3 μg g−1) for Pb and (66.6–142.5 μg g−1) for V. The chemical speciation in most sampling stations was in the order of Residual > acid-reducible > oxidizable-organic > exchangeable for Pb and in the order of Residual > oxidizable-organic > exchangeable > acid-reducible for V. The results showed that the Pb in surface sediments off Nile River Delta existed in the nonresistant fractions while vanadium existed in the resistant fractions. The degree of surface sediments contamination was determined for individual contamination factors (ICF) and global contamination factor (GCF). The result of ICF and GCF values showed that those stations located in the vicinity of municipal area (especially Lake Burullus outlet) had high potential risk to fauna and flora of study area. Risk assessment code (RAC) analysis indicated that although Pb presented a moderate overall risk to the aquatic environment, vanadium showed a low risk (RAC < 10%) at six sites.
Keywords
Metals
Sediment
Speciation
Nile Delta coast
Individual contamination factors (ICF)
Global contamination factor (GCF)
Risk assessment code (RAC)
1 Introduction
The densely populated highly industrialized areas characterize the delta coasts of the world. This has discharged a large quantity of wastewaters into the river estuaries, leading to severe pollution of the wetlands. Heavy metals of anthropogenic origin are considered as serious inorganic pollutants because of their toxic effects on life in aquatic system, which are able to transfer hierarchically into human society through the food chain, and some of which, under certain circumstances, can be further transformed into more toxic compounds. Analyses of spatial and temporal distribution of heavy metals in the delta coasts’ sediments are useful to recognize the degradation processes of wetlands and trace sources of pollutants for better environmental assessment and management (Alloway and Ayres, 1993).
The Nile Delta is a fragile environment since it is subjected to erosion of its shores as a result of cease of sediment supply resulting from the construction of the High Dam at Aswan. After the construction of the High Dam no fresh water reaches the sea and the shelf is covered by typical Mediterranean water with salinity approaching 39‰. In addition, the transport of sediment to the Mediterranean shelf off the Nile Delta diminished as a result of damming the river. Both Rosetta and Damietta Cones are covered with mud and sandy mud that also cover the shore face around the main river mouths. Off the delta, the mud and sands of the inner and middle shelves contain very little biogenic carbonate (Rifaat, 2005).
Sediments are important sinks for various pollutants like pesticides and heavy metals and also play a significant role in the remobilization of contaminants in aquatic systems under favorable conditions (Ikem et al., 2003). Much concern has been focused on the investigation of the total metal contents in sediments. However, it cannot provide sufficient information about mobility, bioavailability and toxicity of metals. The speciation of metals in sediments is therefore a critical factor in assessing the potential environmental impacts (Yuan et al., 2004). Chemical speciation can be defined as the process of identification and quantification of different species, forms or phases of chemicals present in a material.
The form of metal in sediments (distribution among substrates) is important in determining metal ecotoxicological risk to biota. In this study, the procedure of sequential extraction technique consists of four (exchangeable, acid-reducible, oxidizable-organic and residual) fractions. This technique was applied to identify the amount of lithogenic metals in contrast with natural origin and can provide information about the identification of the main binding sites and the phase associations of trace elements in sediment. This could help us to understand the geochemical processes governing heavy metal mobilization and potential risks induced (Yuan et al., 2004).
To indicate the degree of risk of heavy metals to environment in relation with its retention time, the risk assessment code (RAC), the individual contamination factors (ICF) and the global contamination factor (GCF) have been used to assess environmental risks and estimate possible damage to benthic organisms caused by contaminated sediments, because, as mentioned before, metals are bound to both different sediment fractions and with different bonding strengths, with the latter influencing the bioavailability of the metal in the environment (Badri and Aston, 1983).
Some studies were made on heavy metals in the two Nile branches and some others on the Egyptian coastal Mediterranean waters. This study, presents the distributions of the trace metals Pb and V in surface sediments of Nile Delta coast in Mediterranean Sea, north of Egypt in order to provide preliminary baseline data for control of pollution in this area and to appraise their anthropogenic discharge. The interpretation of results was based on the reduction of metal bioavailability or mobility at each successive extraction step. The first three fractions contained the most labile metals and the residual fraction the least bioavailable/mobile metals. The RAC was determined for each metal.
2 Materials and methods
2.1 Study area
Egyptian Mediterranean coast off Nile Delta is one of the most important estuaries in Mediterranean Sea (Fig. 1). It has approximately 132.83 km (82.54 miles) that extends from Damietta estuary to Rosetta estuary. The field work studies revealed that this area receives discharge from industrial area and urban area (e.g., fish farms) as well as agricultural activities (e.g., fertilizers and pesticides) from the two Nile branches in addition to the Lake Burullus outfall. Surface sediment samples were collected from 11 sites between January and April 2010 in four perpendicular sectors along the Nile Delta coast representing the sediment between the two Nile estuaries (Fig. 1).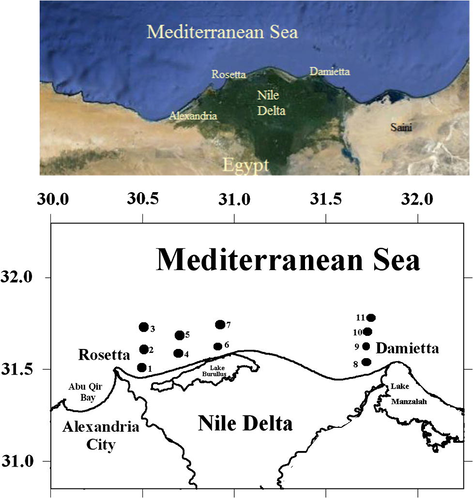
Location of sampling sites in Nile delta Estuary.
2.2 Sampling
Surface sediment samples were collected from these sites between January and April 2010 in four perpendicular sectors along the Nile Delta coast representing the sediments off the two Nile estuaries and off Burullus Lake (Fig. 1). Three samples were collected from Sector (1) off Rosetta Branch at distances of 10, 30 and 50 m from the coast line; two samples were collected from each of sector (2) and sector (3) at Abu Khashba area and off the outfall of Burullus Lake respectively at distances 10 and 30 m. Finally four samples were collected from sector (4) off Damietta Branch at distances 10, 30, 50 and 100 m. The VanVeen Grab sampler was used to collect a large amount of surface sediments; the samples (0–5 cm) were collected by using a clean plastic spoon to avoid contamination by the metallic parts of the sampler. The surface sediment samples were placed in polyethylene plastic bag and labeled and then kept in an ice box. In laboratory they were stored frozen at 4 °C until analysis. Unfixed samples for assessment were dried at 85 °C for 48 h in forced air oven, and were subsequently ground in an agate mortar, homogenized and sieved through 63 μm mesh size, and kept in an acid-washed container for future use.
2.3 Sequential extraction
Chemical speciation of Pb and V in surface sediments was analyzed by using the sequential extraction technique (Table 1) (Badri and Aston, 1983; Yap et al., 2002).
Step 1: Easily, freely or leachable and exchangeable (EFLE).
Step 2: Acid-reducible fraction (metals associated with oxides of Fe and Mn) (the residue from step1)
Step 3: Oxidizable-organic (oxidizable fraction—metals associated with organic matter and sulfides) (the residue from step2)
Step 4: Resistant (metals strongly associated with the crystalline structure of minerals) (the residue from step 3)
| Extraction step | Reagent(s) and time | Target phase (s) |
|---|---|---|
| 1 | NH4CH3COO 1 mol/L pH = 7 | Exchangeable |
| 2 | NH2OH.HCl 0.25 mol/L pH = 2 | Acid-reducible |
| 3 | H2O2 (30%), NH4CH3COO 1 mol/L pH = 2 | Oxidizable-organic |
| 4 | NHO3 (65%), HCLO4 (70%), HF | Resistant |
The residue of each step was rinsed with 20 ml double-distilled water (DDW) and filtered through Whatman® No.1 filter paper into precleaned 100 ml volumetric flasks. The supernatant liquid of each fractions after filtration were stored for metal determination, except fraction 1 that is sensitive and must be analyzed immediately.
2.4 Determination of trace metal concentrations
Metal concentrations in the extracts obtained at each step were determined using an air-acetylene flame atomic absorption spectrophotometer SHIMADZU (Model AA-6800), D2 background correction and autosampler. All absorbance readings were made in triplicate. Instrument settings were as recommended in the manufacturer's manual, with wavelengths (nm) of 283.31 and 309.31 for (Pb) and (V) respectively.
2.5 Statistical analysis
All statistical analyses were computed by using Package for Social Science (SPSS) version 16. The graphs were performed with Microsoft Excel for Windows.
The accuracy of the analytical method was verified using a triplicate analysis of a certified reference material (BCSS-1) from the National Research Council of Canada and TM 23.2 for Pb and V respectively (Table 2). The recovery percentage values for the two metals were between 88.7% for V and 81% to 89.4% for Pb.
Concentration
V (TM 23.2)
Pb (BCSS-1)
Metalsa extracted
2.40 ± 0.6
22.70 ± 1.2
Metalsbextracted
2.13 ± 0.4
20.30 ± 1.8
Recovery%
88.7
89.4
2.6 Risk assessment code (RAC)
The risk assessment code, defined as the fraction of metal exchangeable and/or associated with carbonates (% F1), was determined for the two trace metals, and the values are interpreted in accordance with the RAC classifications. This classification is described by Perin et al. (1985).
3 Results and discussion
Chemical fractionation differentiates metals of natural origin from those derived from anthropogenic sources. Elevated concentrations of metals in the residual fraction indicate that sediments are relatively unpolluted, and that the elements derive mainly from lithogenic origins. Anthropogenic metals are predominantly found in the most labile sediment fractions, which are vulnerable to small changes in environmental conditions, such as those caused by human activity (Alves et al., 2007).
3.1 Lead speciation
The mean Pb concentrations of four chemical speciation fractions for each sampling station are shown in Table 3. The total Pb concentration ranged from 22.87 to 41.32 μg g−1 in stations 11 and 9, respectively off Damietta branch of Nile River. The EFLE fraction for Pb ranged from 2.61 to 11.41 μg g−1 with mean percentage of 14.6%. The acid-reducible fraction ranged from 2.93 to 17.17 μg g−1 with mean percentage of 22%. The oxidizable-organic fraction ranged from 2.07 to 10.21 μg g−1 with mean percentage of 46.9%. The resistant fraction ranged from 10.32 to 21.46 μg g−1 with mean percentage of 16.5%. The mathematical summation of EFLE, acid-reducible and oxidizable organic fractions constitutes the nonresistant fraction (non-lithogenous) (Badri and Aston, 1983). The comparison among resistant and nonresistant fractions of Pb in each sampling station of the surface sediment off Nile Delta is shown in Table 5 and Fig. 2.
Area
Site
Exchangeable F1
Acid-reducible-F2
Oxidizable-organic F3
Residual
Total
ΣF1 + F3 + F3 (%)
Rosetta
1
3.18 ± 0.2
3.31 ± 0.3
6.62 ± 1.9
16.71 ± 2.2
29.82
43.9
2
3.69 ± 0.8
5.54 ± 1.0
10.21 ± 2.2
15.40 ± 2.3
34.93
55.6
3
3.69 ± 0.8
6.19 ± 1.3
5.43 ± 2.0
21.46 ± 2.1
36.77
41.6
Abu
4
11.41 ± 1.7
5.43 ± 1.7
4.02 ± 1.5
14.81 ± 2.6
35.67
58.5
Khashba
5
4.67 ± 1.1
4.67 ± 1.1
5.65 ± 2.1
12.23 ± 2.0
27.22
42.0
Burullus
6
3.15 ± 0.6
17.17 ± 2.5
8.26 ± 2.0
10.32 ± 2.0
38.90
73.5
7
3.80 ± 1.0
15.54 ± 1.8
4.67 ± 1.6
14.94 ± 1.8
38.95
61.6
Damietta
8
7.39 ± 1.4
2.28 ± 0.9
5.87 ± 2.0
18.20 ± 2.1
33.74
46.1
9
6.95 ± 1.8
13.69 ± 2.2
2.07 ± 1.0
18.61 ± 2.5
41.32
54.9
10
2.61 ± 0.7
2.93 ± 0.9
4.89 ± 2.0
19.83 ± 2.4
30.26
34.5
11
3.69 ± 1.2
4.78 ± 1.1
3.26 ± 1.4
11.14 ± 2.1
22.87
51.3
Mean %
14.6
22.0
16.5
46.9
51.2
Site
Pb
V
GCF
Non resistant percentage
Resistant percentage
ICF
Non resistant percentage
Resistant percentage
ICF
1
44.0
56.0
0.78
14.9
85.1
0.17
0.95
2
55.7
44.3
1.25
6.2
93.8
0.06
1.31
3
41.6
58.4
0.71
22.5
77.5
0.29
1.0
4
58.5
41.5
1.41
36.9
63.1
0.58
1.99
5
55.1
44.9
1.23
40.3
59.7
0.67
1.90
6
73.5
26.5
2.77
21.3
78.7
0.27
3.04
7
61.6
38.4
1.60
65.8
34.2
1.92
3.52
8
46.1
53.9
0.86
34.9
65.1
0.53
1.39
9
55.0
45.0
1.22
40.7
59.3
0.68
1.90
10
34.5
65.5
0.52
35.3
64.7
0.55
1.07
11
51.3
48.7
1.05
52.0
48.0
1.08
2.13
Mean
52.4
47.6
1.22
33.7
66.3
0.62
1.83
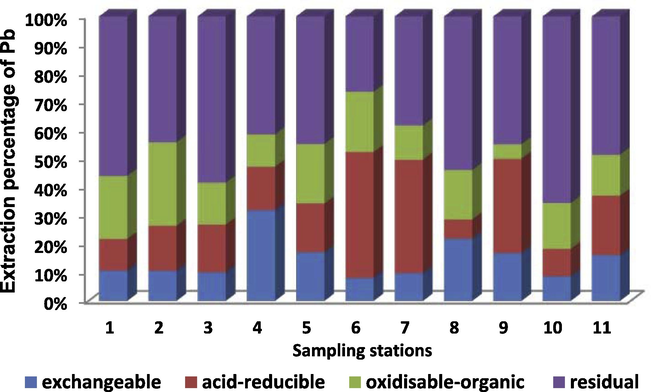
Extraction percentage of Pb in sampling sites off Nile Delta sediments.
The main speciation of Pb in the surface sediments off Nile Delta was in the order of residual > acid-reducible > oxidizable organic > exchangeable. The nonresistant fractions ranged from 34.5% to 73.5% in stations 10 and 6 respectively with mean value of 52.4% (Table 5).
On the basis of the above statement, the Pb concentration in most sampling stations was dominated by the resistant fraction. Stations (2, 4, 5, 6, 7, 8, 9 and 11) showed that the concentration of nonresistant fraction was greater than resistant fraction. The resistant fraction of this metal in sediments was probably due to natural sources such as chemical weathering of igneous and metamorphic rocks, as well as decomposition of biota detritus (Badri and Aston, 1983).
Considering that bioavailability is related to solubility, then metal bioavailability decreases in the order of exchangeable forms > acid reduction forms > organic forms > residual forms (Xiangdong et al., 2000).
The residual phase represents metals largely embedded in the crystal lattice of the sediment fraction and should not be available for remobilization or dissociation except under very hard condition (Yuan et al., 2004). The exchangeable (EFLE) step found in the present study ranged from 8.1% to 32.0%. This fraction presents a high bioavailability of the associated metals because metal adsorption is related to changes in the ionic composition of the water, which may affect the processes of adsorption–desorption and the mobility of metals on the ground and it has great adverse effect on the aquatic environment in comparison to other fractions (Fuentes et al., 2008). The high percentage of acid-reducible fraction of Pb (6.8–44.1%) with other nonresistant fractions determined that the affinity for this metal in the acid-reducible fraction of the surface sediments was high. The acid-reducible fraction includes metals associated with manganese and iron oxides and hydroxides and possibly also with carbonates. Iron and manganese-oxides bind the trace metals since they have high scavenging efficiencies for trace metals from solution through processes such as adsorption and co-precipitation. The study area receives inputs of urban and industrial effluents; hence, the significant amount of Pb present in the most bioavailable fraction is likely to be due to the presence of anthropogenic material.
3.2 Vanadium speciation
The mean V concentrations and percentage of four chemical speciation fractions for each sampling station are listed in Table 4. The total V concentration ranged from 73.6 to 154.8 μg g−1 in stations 6 and 7, respectively off Burullus Lake outfall. The EFLE fraction for V ranged from not detectable to 14.1 μg g−1 with mean percentage of 7.2%. The acid-reducible fraction ranged from not detectable to 12.33 μg g−1 with mean percentage of 1.6%. The oxidizable-organic fraction ranged from not detectable to 89.58 μg g−1 with mean percentage of 63.8%. The resistant fraction ranged from 43.2 to 83.86 μg g−1with mean percentage of 27.4%.
Area
Site
Exchangeable F1
Acid-reducible-F2
Oxidizable-organic F3
Residual
Total
ΣF1 + F3 + F3 (%)
Rosetta
1
N.D
12.33 ± 2.1
N.D
70.64 ± 4.7
82.97
14.8
2
N.D
5.22 ± 2.2
N.D
78.51 ± 5.5
83.73
6.2
3
12.30 ± 1.9
1.73 ± 0.8
8.74 ± 2.4
78.51 ± 7.1
101.28
22.5
Abu
4
10.52 ± 2.1
N.D
38.43 ± 3.6
83.86 ± 3.0
132.81
36.8
Khashba
5
6.97 ± 1.8
N.D
44.34 ± 4.1
75.87 ± 4.5
127.18
40.3
Burullus
6
6.97 ± 2.0
N.D
8.74 ± 2.6
57.91 ± 4.8
73.62
21.3
7
12.30 ± 2.1
N.D
89.58 ± 6.1
52.94 ± 2.7
154.82
65.8
Damietta
8
6.96 ± 2.6
N.D
36.49 ± 3.6
81.17 ± 3.7
124.62
34.8
9
6.94 ± 1.8
N.D
34.56 ± 3.1
60.42 ± 3.2
101.92
40.7
10
8.74 ± 1.6
N.D
32.64 ± 3.9
75.87 ± 2.9
117.25
35.3
11
14.10 ± 1.9
N.D
32.63 ± 5.2
43.20 ± 3.0
89.93
52.0
Mean %
7.2
1.6
27.4
63.8
33.7
The comparison among resistant and nonresistant fractions of V in each sampling station of the surface sediments off Nile delta is shown in Fig. 3 and Table 5. The speciation of V in the surface sediments off Nile Delta was in the order of residual > oxidizable-organic > exchangeable acid reducible. The nonresistant fractions ranged from 6.2% in station 2 and 65.8% in station 7 with mean value of 33.7%. Among the nonresistant fractions, EFLE, acid-reducible and oxidizable-organic constituted 7.2%, 1.6% and 27.4%, respectively. Higher percentage of V in oxidation-organic fraction may be related to different types of organic matter discharged from direct influx of domestic wastes and insufficiently treated industrial wastes in vicinity of the two branches of Nile River, as has been reported in other studies (Cuong and Obbard, 2006; Hanson et al., 1993) stated that nonresistant steps are closely related to the anthropogenic origin.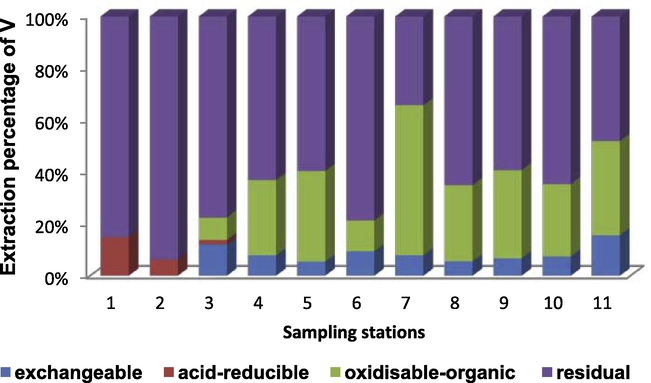
Extraction percentage of V in sampling sites off Nile Delta sediments.
On the basis of above statement, the V concentration in most sampling stations was dominated by the resistant step. The nonresistant fractions (the sum of fractions 1, 2 and 3) are potentially toxic for organisms because it is easily removed and used by organisms (fraction 1), while fractions 2 and 3 can be solubilized depending upon physical and chemical parameters, such as oxygen content, redox potential changes, and bacterial activity (Ramirez et al., 2005; Yap et al., 2006).
Vanadium showed highest contents (27.4%) in the oxidizable fraction. This metal is strongly complexed in sediments, and is released following degradation of the organic matter or oxidation of sulfides to sulfates. The observed behavior was probably due to the affinity of the metal for the OM present in water, given the high anthropogenic loadings in the study region (Alves et al., 2007). Reports in the literature for regions where there are high inputs from human activity are indicative of the presence of significant percentages of V in this sediment fraction.
3.3 Definition of natural and anthropogenic sources
In regions where there are heavy anthropogenic inputs, significant proportions of trace metals have been found in the first three extraction fractions (soluble in acid, associated with Fe and Mn oxides, and associated with organic matter and sulfides) (Passos et al., 2010), indicating that the sediments were polluted. Meanwhile, different relative behaviors of the metals in the different phases (carbonates, Fe and Mn oxides, OM and sulfides) are probably reflective of different origins of pollution. Regions where the metals are weakly associated with sediments, and show an affinity for carbonate, have experienced more recent pollution. Locations where metals are significantly associated with the oxidizable or reducible sediment fractions are influenced by pollution that is less recent. Considering the percentage of metals extracted in the most labile fractions (F1 + F2 + F3), the order of mobility (from most to least bioavailable) was: Pb (51%) > V (33.7%) (Tables 2 and 3).
Pb showed the greatest amount in the bioavailable fractions, while V showed lower percentages in the labile fractions. It has been found previously that V is weakly adsorbed (retained on the sediment surface by weak electrostatic interaction), that the oxides of Fe and Mn are important scavengers of Pb (Li et al., 2007; Pertsemli and Voutsa, 2007) the refining activities in the Bay are an important source of V inputs (FAO, 1992).
3.4 Contamination assessment
3.4.1 Individual and global contamination factor
The determination of contamination factor of heavy metals is an important aspect that indicates the degree of risk of heavy metals to environment in relation with its retention time (Nemati et al., 2009). The individual contamination factors (ICF) for the various sampling sites were calculated from the result of the fractionation study by dividing the sum of the first three extractions (i.e. exchangeable, acid-reducible and oxidizable-organic forms) by the residual fraction for each site (Table 5). The global contamination factor (GCF) for each site was calculated by summing the ICF for Cd and Zn obtained for a site (Ikem et al., 2003). The ICF and GCF were computed for each station as per the following equation: The highest level of individual contamination factor for Pb was analyzed at site 6 (off Burullus Lake) with a value of 2.769, while the lowest level of it was analyzed at site 10 with a value of 0.526 (off Damietta branch). The highest level of individual contamination factor for V was computed at site 7 (off Burullus Lake) with a value of 1.924, while the lowest level of it was computed at site 2 (off Rosetta branch) with a value of 0.066. ICF reflects the risk of contamination of a water body by a pollutant (Ikem et al., 2003). Therefore, highest risks of Pb and V were computed at sites 6 and 7. Conversely, lowest risks of them were calculated at Stations 10 and 2. The average individual contamination factors in the surface sediment of sampling stations were ranged in the order of Pb > V.
The global contamination factor analyzed from ICF values showed that sediments located in the vicinity of Burullus Lake were highly impacted by metal pollutants. The tendency of trace metals is to accumulate in sediments, contamination from each source tends to be localized in a hotspot near the input, and then dispersed regionally in lower concentrations (Luoma and Rainbow, 2008).
Hence, the results obtained in this investigation in accordance with Pb and V showed that those stations, especially Stations 6 and 7, located in the vicinity of polluted lake had high potential risk to fauna and flora of River Nile Delta coast (Table 5). Since there is no guidelines compatible with GCF value it cannot assay comprehensively the effects of the two metals contamination.
3.5 Environmental implications
Metals are bound to different sediment fractions, with the strength of the binding determining their bioavailability and the risk associated with their presence in aquatic systems. The risk assessment code (RAC) was determined based on the percentage of the total metal content that was present in the first sediment fraction (% F1), where binding is weak and the metals pose a greater risk to the aquatic environment (Jain, 2004). When this percentage is less than 1%, the sediment is of no risk to the aquatic environment. Percentages of 1–10% reflect low risk, 11–30% medium risk, and 31–50% high risk. Above 50%, the sediment poses a very high risk, and is considered dangerous, with metals easily able to enter the food chain (Jain, 2004). Fig. 4 illustrates the results of the risk factor analysis, with values given as percentages of the fraction soluble in acid (% F1) for the two trace metals. In general, the sediments show a low risk for V and low to medium risk for all Pb, with RAC values greater than 11% indicating a substantial risk of metal mobilization from sediments across the entire study region. Lead showed a medium to high risk (RAC > 30%) in sediments from sites 5, 8, 9 and 11. While site No. 4 shows high risk V it showed a low risk at all sites (RAC < 10%) except stations 3 and 11 that showed medium risk (RAC > 10).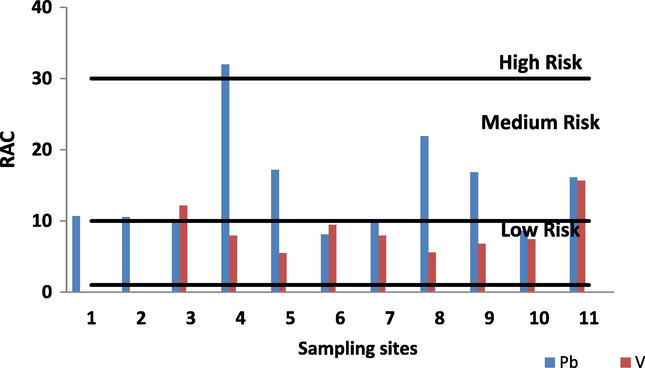
Risk assessment code (RAC) for Pb and V in surface sediments off Nile delta.
Small variations in environmental conditions, such as pH or salinity, could therefore increase the availability of the elements to the aquatic system. In an earlier study in the same region (Chen et al., 2010), it was shown that sediments from sites 6 and 7, located in area directly influenced by effluent inputs from Lake Burullus, were vulnerable to enrichment by anthropogenic metals. The total content of Pb was indicative of probable enrichment at these sites, with concentrations exceeding the natural limit of the region. No contamination was observed for vanadium; however in the earlier work total elemental concentrations were used to calculate possible enrichment. It is acknowledged that the total concentration of an element only reflects the amount stored in the system, and do not provide any information concerning the availability of the metal.
Similar studies conducted by (Passos et al., 2010; Erol, 2008), using sediments from Poxim river (Brazil) and Nilüfer Stream (Turkey), highly polluted by contaminants contained in domestic and industrial effluents, showed that 18% of Pb were present in the first sediment fraction, resulting in a medium risk to the environment. In work using sediments from the Egyptian coast of Mediterranean Sea (El-Mex Bay), having several industrial plants that directly discharge their effluents into it, (Abdallah, 2007; Abdallah et al., 2007) showed that Cu and Cd presented a medium risk (10–13%) and could be readily released to the water column.
4 Conclusion
The sequential extraction technique was applied to identify the amount of anthropogenic metals in contrast with natural origin; also it can be used as a valuable tool to provide information on the mobility, bioavailability and potential toxicity of trace metals in the environment. The chemical speciation of Pb and V in majority of stations was in the order of Residual > acid-reducible > oxidizable-organic > exchangeable for Pb and in the order of Residual > oxidizable-organic > exchangeable > acid-reducible for V. It determined that the Pb in Nile River delta coast surface sediments existed in the nonresistant fractions, while V existed in the resistant fractions (sedimentary matrix). It, therefore, showed that lithogenic discharge of Pb is greater than V. Chemical speciation of Pb and V fractions was different from each other. The mean exchangeable concentration of Pb and V was less than the rest of the fractions since, this fraction has adverse effect on the aquatic environment in contrast with other fractions. It, therefore, reflected the high and continuous discharge or sufficient time for redistribution of Pb and V in surface sediments of Nile River delta coast. Risk assessment code (RAC) values were reflective of average risks to the environment for the two metals in most samples. The results suggest that fluctuations in pH or salinity could mobilize the metals from sediments to the water column. All of the sediment samples presented evidence of anthropogenic enrichment, so that metal concentrations were not representative of natural conditions in this estuarine region. Around 45% of the metal content was present in the residual fraction, similar to the proportion found for contaminated regions worldwide. Although the level of Pb and V was not extremely enriched in these surface sediments and did not present a serious threat to the local fauna and flora, there is a need to reduce anthropogenic sources of pollution in the area.
Acknowledgements
The author would like to thank the National Institute of Oceanography and Fisheries for providing the samples used in this research which was part of a plan of work under title of sustainable development of fisheries in areas exposed to excessive exploitation.
References
- Speciation of Trace Metals in coastal sediments of El-Mex Bay South Mediterranean Sea-west of Alexandria (Egypt) Environ. Monito. Assess.. 2007;13:111.
- [Google Scholar]
- Distribution and enrichment evaluation of Heavy metals in El-Mex Bay using Normalization models. Fresen. Environ. Bull.. 2007;16(7):719.
- [Google Scholar]
- Chemical principles of Environmental pollution. Oxford, UK: Blackie Academic and professional. An imprint of Chapman and Hall; 1993. p. 291
- Metals and acid volatile sulfide in sediment cores from the Sergipe River Estuary, Northeast, Brazil. J. Braz. Chem. Soc.. 2007;18:758.
- [Google Scholar]
- Observation on heavy metal geochemical associations in polluted and nonpolluted estuarine sediments. Environ. Pollut. (Series B). 1983;6:181.
- [Google Scholar]
- Ecological implications of heavy metal concentrations in the sediments of Burullus Lagoon of Nile Delta, Egypt. Estuar, Coast. and Shel Scienc.. 2010;86:491.
- [Google Scholar]
- Metal speciation in coastal marine sediments from Singapore using a modified BCR-sequential extraction procedure. Appl. Geochem.. 2006;21:1335.
- [Google Scholar]
- Sources and Distribution of Heavy Metals in River Sediments from the southern drainage basin of the Sea of Marmara, Turkey. Fresen. Environ. Bull.. 2008;17(21a):2007.
- [Google Scholar]
- FAO, 1992. Committee for inland fisheries of Africa. Report of the third session of the working party on pollution and fisheries. FAO Fisheries report No. 471.
- Comparative study of six different sludges by sequential speciation of heavy metals. Bioresour Technol.. 2008;99:517.
- [Google Scholar]
- Assessment of elemental contamination in estuarine and coastal environments based on geochemical and statistical modeling of sediments. Mar. Environ. Resear.. 1993;36:237.
- [Google Scholar]
- Trace elements in water, fish and sediment from Tuskegee Lake, southern USA. Water Air Soil Pollut.. 2003;149:51.
- [Google Scholar]
- Metal fractionation study on bed sediments of River Yamuna, India. Water Res.. 2004;38:569.
- [Google Scholar]
- Heavy metals in coastal wetland sediments of the Pearl River Estuary, China. Environ. Pollut.. 2007;149:158.
- [Google Scholar]
- Metal Concentration in Aquatic Environments. New York: Cambridge University Press; 2008.
- Investigation of heavy metals mobility in shrimp aquaculture sludge—comparison of two sequential extraction procedures. Microchem. J.. 2009;91:227.
- [Google Scholar]
- Assessment of trace metals contamination in estuarine sediments using a sequential extraction technique and principal component analysis. Microchem. J.. 2010;96:50.
- [Google Scholar]
- Perin, G., Craboledda, L., Lucchese, M., Cirillo, R., Dotta, L., Zanette, M.L., Orio, A.A., 1985. Heavy metal speciation in the sediments of Northern Adriatic sea — a new approach for environmental toxicity determination, in: T.D. Lekkas (Ed.), Heavy Metal in the Environment. 2, 454.
- Distribution of heavy metals in lakes doirani and kerkini, northern Greece. J. Hazard. Mater.. 2007;148:529.
- [Google Scholar]
- Metal speciation and environmental impact on sandy beaches due to El Salvador copper mine, Chile. Mar. Pollut. Bullet.. 2005;50:62.
- [Google Scholar]
- Major Controls of Metals' Distribution in Sediments off the Nile Delta. Egypt. Egyp. J. Aqua. Resea.. 2005;31:16.
- [Google Scholar]
- Chemical partitioning of heavy metal contaminants in sediments of the Pearl River Estuary. Chem. Speciat. Bioavailab.. 2000;12:17.
- [Google Scholar]
- Concentrations of Cu and Pb in the offshore and intertidal sediments of the west coast of Peninsular Malaysia. Environ. Int.. 2002;28:467.
- [Google Scholar]
- Comparison of heavy metal concentrations in surface sediment of Tajung Piai wetland with other sites receiving anthropogenic inputs along the southwestern coast of Peninsular Malaysia. Wetland Sci.. 2006;4:48.
- [Google Scholar]
- Speciation of heavy metals in marine sediments from the East China Sea by ICP-MS with sequential extraction. Environ. Int.. 2004;30:769.
- [Google Scholar]







