Translate this page into:
Chitosan-polyvinyl alcohol membranes with improved antibacterial properties contained Calotropis procera extract as a robust wound healing agent
⁎Corresponding authors. szalshawwa@pnu.edu.sa (S.Z. Alshawwa), nasirmasood@cuivehari.edu.pk (N. Masood), bosalvee@yahoo.com (M. Iqbal)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
Due to excessive use of antibiotics, resistance against microorganisms is developed. An alternative use of antibiotics, natural remedies from plants have been used against infectious diseases. In the current study, bioactive compounds from Calotropis procera (C. procera) root were extracted and chitosan and polyvinyl alcohol (CS-PVA) were used asa carriers and for the delivery to treat induced infection. Different concentration of C. procera extracts (25–75 mg/mL) were loaded on CS-PVA membrane and applied on the induced wounds in rabbits. Wound reduction was recorded for 12 days. On 6th day, small tissue from healing area were collected and subject to histopathology for tissue regeneration. The antioxidant activity (DPPH, TPC and TFC) was also investigated of CS-PVA loaded C. procera root extract. The DPPH free radical inhibition for 75 mg/mL were recorded up to 66.37%. The TPC and TFC contents were recorded to be 36.52 ± 5.12 GAE mg/g of DW (dry weight) and 24.49 ± 6.27 CE mg/g of DW (dry weight), respectively. The antibacterial activity was evaluated against Escherichia coli and Staphylococcus aureus in comparison to control (Rifampicin). The zones of inhibition were recorded to be 18.50 ± 2.30 and 20.40 ± 4.20, respectively for CS-PVA membrane loaded with 75 mg extracts along with Rifampicin 28.50 ± 2.5 and 30.50 ± 1.38. The CS-PVA membranes were also studied for swelling and biodegradability. The biodegradability was increased, while swelling was decreased of CS-PVA membranes loaded with extract. The bioactive compounds from the CS-PVA loaded with extract released in controlled and sustainable way. Result revealed that CS-PVA loaded C. procera root extract has promising antimicrobial and antioxidant activity and could possibly be employed for the treatment of infectious diseases.
Keywords
Chitosan-polyvinyl alcohol
Biodegradability
Calotropis procera
Wound dressing
1 Introduction
The living organisms skin acts as a protection against environmental stress as well as attack of toxic agents. In case of loss in cellular tissue, a wound is produced and on the other hand, injury (physical or chemical) as well as microbial infections can also induce a wound. The healing of wounds significantly impacts the health of living organisms and material containing active agents are considered resourceful in this context (Iqbal et al., 2021; Kandra and Bajpai, 2020; Alminderej, 2020; Tamer et al., 2021; Hassan et al., 2021). The complex task of wound healing is multidimensional coordination of different cell types in different phases or in combination. The skin comprised of three layers (epidermis, hypodermis, dermis), the uppermost impermeable layer epidermis; then, dermis which further underlies subcutaneous adipose tissue. These three layers also contain immune cells that assess and respond to the damages (Yin and Xu, 2020; Sasmaz et al., 2017). Repair task of wounded skin is finished by different cell types from the three layers. The cell types bring together in specific precise manner to initiate the byzantine process in chronological sequence or overlap fashion. Hemostasis, angiogenesis, inflammation, growth, epithelial formation and restoration occurs to comprehend the efficiency (Yousefi et al., 2017; dos Santos et al., 2021). Different skin and wound healing treatments have been explored and different chemical treatment are in practice, which on the other hand may cause side effect and the wounds dressing based on eco-friendly and effective agents have various advantages over chemical treatments (Augustine et al., 2020; Zou et al., 2020; Chen et al., 2019; Colobatiu et al., 2019; Omer et al., 2021), i.e., an electrospun PVA membrane containing biosynthesized Ag NPs, which were fabricated using Mimosa pudica extracts. The PVA nanofiber membranes loaded with Ag NPs properties were improved significantly along with excellent antibacterial activity, compatibility and cytocompatibility (Augustine et al., 2018). Similarly, the CS and PVA based NFs loaded with ZnO were prepared and employed for wounds healing. The antibacterial potential of CS and PVA based NFs loaded with ZnO was studied for P. aeruginosa, B. subtilis, E. coli, and S. aureus and NFS based membranes showed auspicious antibacterial potential along with enhanced antioxidant potential versus CS and PVA NFs. The wound healing analysis revealed an accelerated wound healing of CS and PVA based NFs loaded with ZnO versus CS and PVA NFs (Ahmed et al., 2018). The bioactive compounds (antioxidant and antimicrobial) are present in abundant in plants, which can be employed for this purpose, which need to be investigated since plant extract may cause toxicity to the tissue (Friday et al., 2018; Ocheni and Clement, 2017; Hamid et al., 2017; Shahid et al., 2021; Munir et al., 2016; Sasmaz et al., 2018; Obek and Sasmaz, 2011; Abbasi et al., 2021).
The C. procera is indigenously well-known as “auk or akra”, which is a shrub, enormously forked, have dense latex system. It normally grown on dried lands, grubby and unproductive lands in a high temperature habitat (Awwad and Amer, 2020). Traditionally, in ethno-medication, the roots, leaf, flower and milk are used to treat flatulence, anorexia, indigestion and intestinal worm infestation (Chundattu et al., 2016; Ranjit et al., 2012; Amini et al., 2021). Rabid dog bite and paralysis of limbs has been cured by the root powder of C. procera mixed with butter. Wound healing effects and antibacterial activity have also been reported. In conventional medication practice, different parts are mixed with parts of other plants and applied as an anti-inflammatory and anesthetic treatments (Mali et al., 2020; Haider and Zhong, 2014). Biochemical analysis revealed a range of bioactive compounds in this plant, i.e., Calotropis phenylacetate, Calotropis friedelenyl, norditerpenyl organic compounds, calotropternyl, leonine triterpenes like calotropoleanyl, procerleanol, viscous glycosides calotropo genin, calotropin, uscharin, calotoxin calactin, cardenolides and anthocyanin have been reported in different parts of C. procera (Verma, 2019). Herbal preparation based on this plant include anthelmintic, anticancer, gastro protective, hypolipidemic, wound restorative and anticonvulsant properties (Yesmin et al., 2008).
Deacetylation of the naturally occurring biopolymer chitin, give rise to an amino polysaccharide, “chitosan”. The chitosan has various applications in medical and pharmaceutical fields (Iqbal et al., 2020). Antibacterial properties of CS and applications in wound healing have also been reported already, i.e., CS NFs loaded with curcumin (Dhurai et al., 2013), CS-polycaprolactone composite NFs scaffold for ferulic acid delivery (Poornima and Korrapati, 2017), CS-zein electrospun/polycaprolactone-hyaluronic acid bilayer NFs membrane (Figueira et al., 2016), Lysozyme-loaded CS-based NFs (Charernsriwilaiwat et al., 2012); bromelain-loaded CS NFs (Bayat et al., 2019) have been developed and applied for wound healing and promising efficiency have been reported.
In view of antioxidant and antibacterial potential of C. procera roots, present investigation was focused on the preparation of CS-PVA membrane, which were loaded with C. procera roots extracts and applied for induced wound healing in rabbits. The antioxidant and antibacterial was also evaluated. The CS-PVA membranes were loaded with different concentrations of the extracts and their wound healing potential were compared. Histological study was also conducted to monitor regeneration of connective tissues during wound healing process. The surface morphology, water uptake and biodegradability of CS-PVA membranes were also studied.
2 Material and methods
2.1 Chemicals
The PVA (87–89%) was obtained from Alfa Aesar Germany. The CS (deacetylation degree 93%) was bought from, Sigma Aldrich. Staining dyes, Eosin and Haematoxylin were purchased from, BDH Lab and Fluka, provided the tetraethylorthosilicate (TEOS) (density 0.933 g/mL). Glycerol (98%) was also obtained from, Sigma Aldrich. Nutrient media was purchased from Oxoid UK. The solvents like chloroform, ethanol, methanol was obtained from Merck, Salt like NaCl and NaOH were supplied by Merck. Hydrochloric acid (HCl) was purchased from, BDH Lab. To induce the local anesthesia, Xylocane injection were obtained from Barrett Hodgson, Pakistan.
2.2 Extract preparation
The C. procera roots were obtained from the field area of CVAS (College of Veterinary and Animal Sciences), Jhang, Pakistan, which were splashed with water to confiscate soil particles and any other solid material attached with root. Roots were dried under shady condition and then, chopped in to small pieces and ground to fine powder. C. procera roots powder (100 g) was added into 1000 mL volumetric flasks followed by the addition of respective 70% methanol. Afterwards, the flasks are placed shaker at 220 rpm at 25 °C for 48 h for the extraction of bioactive compounds from C. procera roots. Further, mixture was heated on magnetic stirrer for 30 min at 60 °C with continues stirrer at 120 rpm and then, allowed to cool at 25 °C. The contents was filtered and then, centrifuged at 300 rpm for 20 min. For the isolation of maximum bioactive compounds, same procedure was repeated twice, then whole contents were mixed. For removing solvents lyophilisation method were used for removing organic solvents from filtrate. Following concentration, 25–75 mg/ml were loaded on CS-PVA membrane and used for their biological screening as well as wound healing (induced dorsal skin wounds in rabbits) application (Gondwal and Pant, 2013).
2.3 Antioxidant activity
2.3.1 Diphenyl picrylhydrazyl (DPPH)
The antioxidant potential of C. procera root extract was evaluated by determining their scavenging for DPPH radical (Bozin et al., 2006). For this, C. procera root extract (25–75 mg/mL) were prepared scavenging potential analysis. From each extract, 100 µL transfer into sterilized test tube and then, a 0.004% solution of DPPH (5 mL of) was added. To avoid from photo reaction, all procedure were performed in dark and left for 30 min for reaction at 25 °C, after this, 400 µL sample from each test tube were transfer into disposable spectrophotometer cuvette and OD was recorded at 517 nm and by employing Eq. (1), the scavenging effect was estimated.
2.3.2 TPC (Total phenolic contents) analysis
The TPC was evaluated following reported method (Abbas et al., 2019), with slight amendment. Different concentration of C. procera extract (25–75 mg/mL) were prepared. A 300 µL of different concentration was transferred into test tube (sterilized) and then, 500 µL of Folin-Ciocalteu reagent was added in it and mixed well on vortex. Then, 1 mL of 800 mM Na2CO3 was supplemented and the mixture was left at 25 °C for 2 h. The contents are vortexed and 400 µL sample was taken for absorbance measurement at 765 nm. Gallic acid (standard) was used for TPC estimation and reported as GAE (Gallic acid equivalent) per dry matter.
2.3.3 TFC (Total flavonoids content) analysis
The TFC of C. procera extract were measured at different concentrations of C. procera extract (25–75 mg/mL). A freshly reaction reagents, 5% Na2NO3, 10% Al2Cl3 and 1 M NaOH were also prepared. Now, 1 mL of C. procera extract was transferred into a test tube and 400 µL of 5% Na2NO3 was added in it, mix well for homogenecity and 6 mL of distilled water was added. After 5 min of incubation, 700 µL of 10% Al2Cl3 and 3 mL of 1 M NaOH was added and the absorbance was determined at 510 nm. The TFC amounts were expressed as catechin equivalents per dry matter (Sakanaka et al., 2005).
2.4 Preparation of CS-PVA membranes
For CS-PVA membranes preparation, CS (80%) in acetic acid and PVA (20%) were mixed and then, 2% glycerol was poured and kept the contents at 60 °C for half an hour with continues stirring. Now, TEOS (4%) was mixed and mixtures were divided in to 4 equal parts. Then, roots extracts of C. procera were mixed with three parts at the concentration 25, 50 and 75 (mg/mL) and 4th part was used as control. Now, these mixtures are poured on glass petri plates and kept for membranes formation overnight. The membranes thus obtained used for further studies. Fig. 1 is showing the preparation of CS and PVA membrane loaded with C. procera extract and wound healing application in rabbit.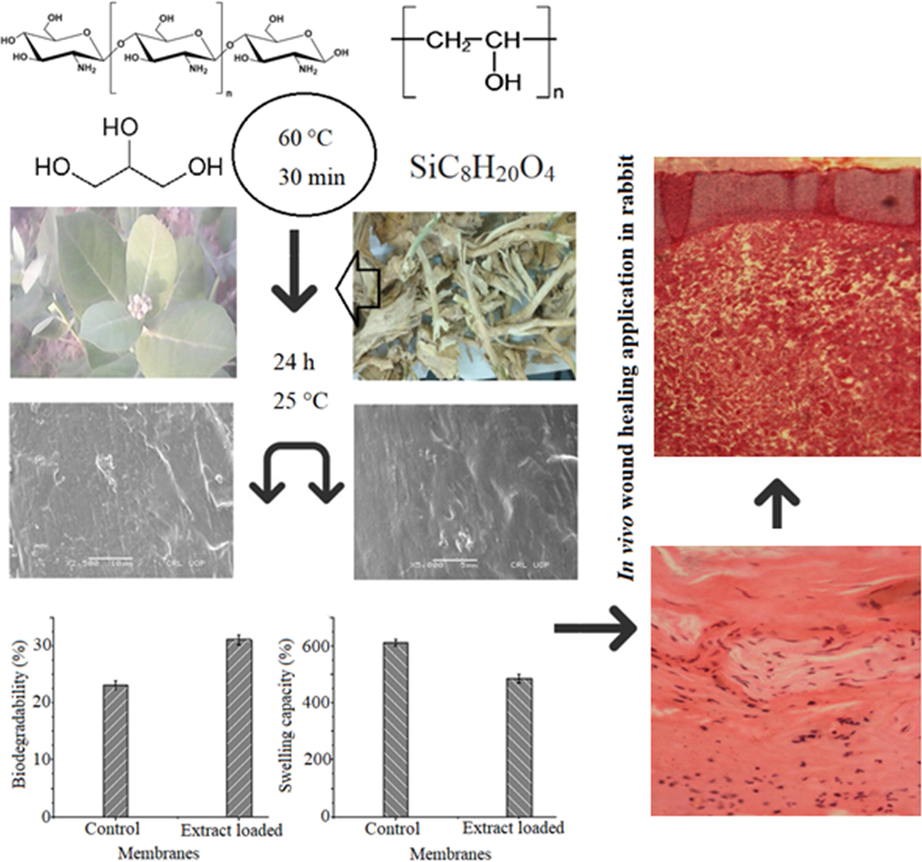
Schematic presentation of chitosan and polyvinyl alcohol membrane loaded with Calotropis procera extract and wound healing application in rabbit.
2.5 Antibacterial activity study
For antibacterial activity, different concentrations of C. procera extract loaded CS-PVA membranes was evaluated for S. aureus and E. coli. The bacterial culture was sustained on medium (nutrient agar). The inoculum with 1.2 × 108 CFU/mL was utilized for present investigation. The CS-PVA membrane loaded with different concentration (25–75 mg/mL) of C. procera extract were cut into 5 mm size. Rifampicin (1 mg/mL) was used as an antibiotic control. On wicks paper discs of size 5 mm, rifampicin solution (50 μL) was poured. Nutrient agar medium was autoclaved for 15 min at 121 °C. In each sterilized petri plate, 20 mL of nutrient agar (sterilized) was poured along with 100 μL of fresh bacterial culture and mix it well and left for 20 min at 25 °C for solidification. The CS-PVA membrane loaded with C. procera extract were laid flat on each petri plates and kept at 37 °C for 24 h. The zones thus formed were recorded in mm using zone reader (Bilal et al., 2020).
2.6 Surface morphology, swelling and biodegradation studies
The surface morphology of CS-PVA membrane loaded with C. procera extract was recorded using scanning electron microscopy (JEOL, Japan; Model: JSM5910). Before analysis by SEM, the samples were dried and coated (sputtered) with gold layer. The swelling of CS-PVA membranes were measured by dipping 0.1 g of membranes in distilled water for 24 h at 25 ± 2 °C. After stipulated time period, samples were collected, wiped (by pressing in filter paper) and weighed again. The swelling percentage was estimated based on weight of membranes before and after swelling (Iqbal et al., 2020). The biodegradability of CS-PVA membranes was studied in simulated body fluid (PBS, pH 7.4). For this, a 0.1 g of CS-PVA membrane was placed in 50 mL media for 30 days at 35 ± 2 °C. Then, membranes were collected, washed, wiped (by pressing in filter paper) and weighed again. The percentage loss in weight loss was estimated in comparison to control.
2.7 Wound dressing application
The wound dressing efficiency of CS-PVA membrane loaded with C. procera extract was studied in rabbits for which was prior approval was obtained from the Animal Research Board, CVAS, Jhang, Pakistan. The average weight of the male rabbit was in 1.8–2 kg range, which were kept in animal house for 72 h with 12 h photoperiod and 25 ± 1 °C having unrestricted access to water and food. A total, 15 rabbits (angora breed) were divided into five groups. For animal trial, written approval was obtained from ethical committee, CVAS, Jhang (CVAS/11317/2019). Open skin wounds (3 × 3 cm) were shaped on dorsal surface of lumber region, under the supervision of Surgery Section, Department of Clinical Sciences, CVAS, Jhang via standard aseptic procedure. Before wound induction, the fur of each rabbit at dorsal surface of lumber region was shaved. The selected area was scrubbed with swab of methylated spirit. The wound area was locally anaesthetized by xylocaine through infiltration method; using a scalpel blade an open skin wound was created on dorsal surface of lumber region of rabbits (Patil and Makwana, 2015). The wounds induced were treated with CS-PVA membranes loaded with different concentrations of C. procera root extracts and control membrane (without extract). Group 1 was control, kept without any treatment; group 2 received CS-PVA membrane without any bioactive material loaded. Group 3, 4 and 5 treated with CS-PVA membranes loaded with 25 mg, 50 mg and 75 mg extracts. As a function of time, the reduction of wound size is recorded as per Eq. (2) (Phull et al., 2018).
2.8 Cytotoxicity evaluation
In vitro cytotoxicity study was performed as reported elsewhere (Powell et al., 2000), with some modifications. For cytotoxicity analysis, bovine blood (3 mL) was obtained in heparinized tube and isotonic BPS (pH 7.4) was added and centrifuged at 850g for 5 min. The upper layer was discarded and RBCs are rinsed with PBS (pH 7.4) three times. Erythrocytes are counted on haemacytometer and a concentration of 7.068 × 108 cells/mL was used in-vitro cytotoxicity assay (hemolytic assay). A 10 mg of membrane having different concentrations of C. procera root extract (25–75 mg) were treated with diluted blood cell suspension (180 µL), which are kept at 37 °C for 30 min with mixing after every 10 min. The tubes are kept in ice for 5 min and centrifuged at 1310g for 5 min. Now, a 100 µL supernatant was collected and diluted with chilled PBS (900 µL) and kept in ice after dilution. Now, mixture (200 µL) from each tube was poured in 96 well plates. The PBS and Triton x-100 were applied as a negative and positive controls. Finally, the OD was recorded at 576 nm (BioTek, Winooski, VT, USA). The lysis (%) of RBCs was calculated using Eq. (3).
2.9 Histological study
To track the healing process, histological studies were performed. Tissues were collected from healing wounds area and preserved in neutral buffered formalin for three days. The samples were washed and then, processed in different concentrations of alcohol and xylene. Each tissue sample was fixed into paraffin wax and kept in block. With the help of microtome, each block was sliced up to 0.5 µm thickness. The slides were prepared with Eosin and haemotoxylin dyes and examined under the microscope (Suvaneeth et al., 2018). The tissue samples are taken in 10% neutral buffered formalin and kept for 72 h. The samples were processed through paraffin embedding techniques and sectioned at 5 µm, which are stained with haemotoxylin and eosin and scored under microscope (Micros Austria).
2.10 Release of bio-actives CS-PVA membrane
The release of bioactive compounds from CS-PVA membrane loaded with C. procera root extract (25–75 mg/mL) was under simulated intestinal condition. From each concentration of CS-PVA loaded membrane, 25 mg was dispersed (separately) in 20 mL aqueous solution of PBS (pH = 7.4) at 37 °C with rotation at a speed of 100 rpm for 72 h. After specific intervals, the medium (1 mL) was collected for measuring of bioactive compounds from CS-PVA membrane; while an equivalent volume of the freshly prepared buffer was added to the medium. The cumulative bioactive compounds release percentage was measured at 429 nm.
2.11 Statistical analysis
The data reported as mean ± SE triplicates runs. The significant differences among mean was determined by ANOVA using LSD significant difference test at p ≤ 0.05.
3 Results and discussion
3.1 Antioxidant activity
Due to presence of the polyphenols in plants extracts, these are the main contributors of the antioxidant activity. The polyphenols act as an antioxidant, anti-inflammatory and antimicrobial agents, which are applied for skin damages (wounds and burns) treatments. At 25–75 mg/mL, the TPC and TFC in the root extract of C. procera were found to be 16.37 ± 2.3 GAE mg/g of DW, 27.42 ± 3.42 GAE mg/g of DW and 36.52 ± 5.12 GAE mg/g of DW and 10.46 ± 4.25 CE mg/g of DW, 18.28 ± 3.46 CE mg/g of DW and 24.49 ± 6.27 CE mg/g of DW, respectively (Table 1). Result revealed that as concentration of the extract was increased, the antioxidant activity was enhanced. Polyphenols scavenge the DPPH radicals by their hydrogen donating ability. The polyphenols in the extract has strong correlation with the antioxidant activity (Huang et al., 2005). Similar to TPC and TFC, as the concentration of C. procera root extract was increased from 25 mg/mL to 75 mg/mL, the free radical scavenging of DPPH was also boosted from 42.26 ± 4.53%, 53.42 ± 2.12% and 66.37 ± 3.27%, which revealed the activity to the extract concentration dependent. Thus, the polyphenolics in the extracts are the main components of the antioxidant activity, which scavenge the free radicals. Due to their strong free radical scavenging and antimicrobial potential, it is consider that the plants with significant antioxidant activity have potential for skin damages treatment like wounds and burns (Yates et al., 2007). The plants produce secondary metabolites, which are well known for their bioactivity, i.e., flavonoids inhibit the peroxidation of fats and aggregation of platelets (Murakami et al., 2010). The antioxidant prevents against oxidative damage from free radicals and antimicrobial compounds in plant preparations act as protective against the microbial attacks. Hence, C. procera extracts showed promising antioxidant potential and could possibly be a potential agent for wound dressing in combination with CS-PVA. Results are the average of their mean ± SD of triplicate samples (n = 3).
S. no
C. procera extracts conc.
Total phenolic contents (mg GAE/g DW)
Total flavonoids contents (mg CE/g DW)
DPPH scavenging (%)
2
25 mg/mL
16.37 ± 2.36
10.46 ± 4.25
42.26 ± 4.53
3
50 mg/mL
27.42 ± 3.42
18.28 ± 3.46
53.42 ± 2.12
4
75 mg/mL
36.52 ± 5.12
24.49 ± 6.27
66.37 ± 3.27
3.2 Characteristics of CS-PVA membrane
The surface morphology of CS-PVA membranes was analyzed by SEM analysis (Awwad and Amer, 2020; Al Banna et al., 2020; Awwad et al., 2020; Iroha and Maduelosi, 2020; Alaqarbeh et al., 2020) and responses are presented in Fig. 2. Fig. 2A is an image of CS-PVA membrane (control), while Fig. 2B is the CS-PVA loaded with C. procera extract. A rough morphology of CS-PVA membranes was observed in case of control membrane, which was uniform and smooth after loading of C. procera extract. The surface morphology analysis revealed the successful incorporation of plant extract in the CS-PVA membrane since the surface structure in loaded membrane is changed to more dense color. Also, small pores are observed in the structure of membrane, which accommodates the bioactive in these pores, and the porous nature enables the membrane to accommodate bioactive and release them easily on swelling. These observations are in line with the biodegradability and swelling analysis of the membrane. The biodegradability of the CS-PVA membrane was increase in loaded membrane, while the swelling capacity decreased of extract loaded CS-PVA membrane, which is due to change the porosity swelling and entrapment of the bioactive agents in the loaded CS-PVA membrane. The rough surface can be supportive for accommodating the bioactive agents. The biodegradation of CS-PVA membranes was measured in SBF and responses are presented in Fig. 3. The CS-PVA membrane loaded (75 mg of C. procera extract) with bioactive and control (without extract) showed the degraded up to 31% and 23%, respectively, which could be due to hydrolytic bond cleavage of soft segments of the membranes. The CS-PVA membrane loaded with bioactive showed higher weight loss versus control CS-PVA membrane, which is due to leaching of bioactive compounds from the CS-PVA membrane to the medium. This weight loss is due to leaching of bioactive from the CS-PVA membranes. The bioactive release from the membranes is dependent to the structure of the CS-PVA membrane, which forms cross-linking based the on the interactions among the the individual counterparts of the membranes. As the membranes are immersed in the medium, the bioactive release slowly on swelling of the CS-PVA membrane. The swelling of membranes is also important for membranes used in biological application, which is tested by immersing CS-PVA membranes in water and responses thus obtained are shown in Fig. 4. The medium absorption efficiency is one of prime characteristics of the materials to be employed as a wound dressing agent because by this process, the membrane releases the bioactive by absorption of medium. The CS-PVA membrane showed swelling behavior dependent to the contents loaded of C. procera root extracts. The control CS-PVA membrane showed the higher swelling of 610%, while it was 450% for loaded CS-PVA membrane (75 mg of C. procera extract) in 24 h, which is an indication of water uptake ability of the membranes.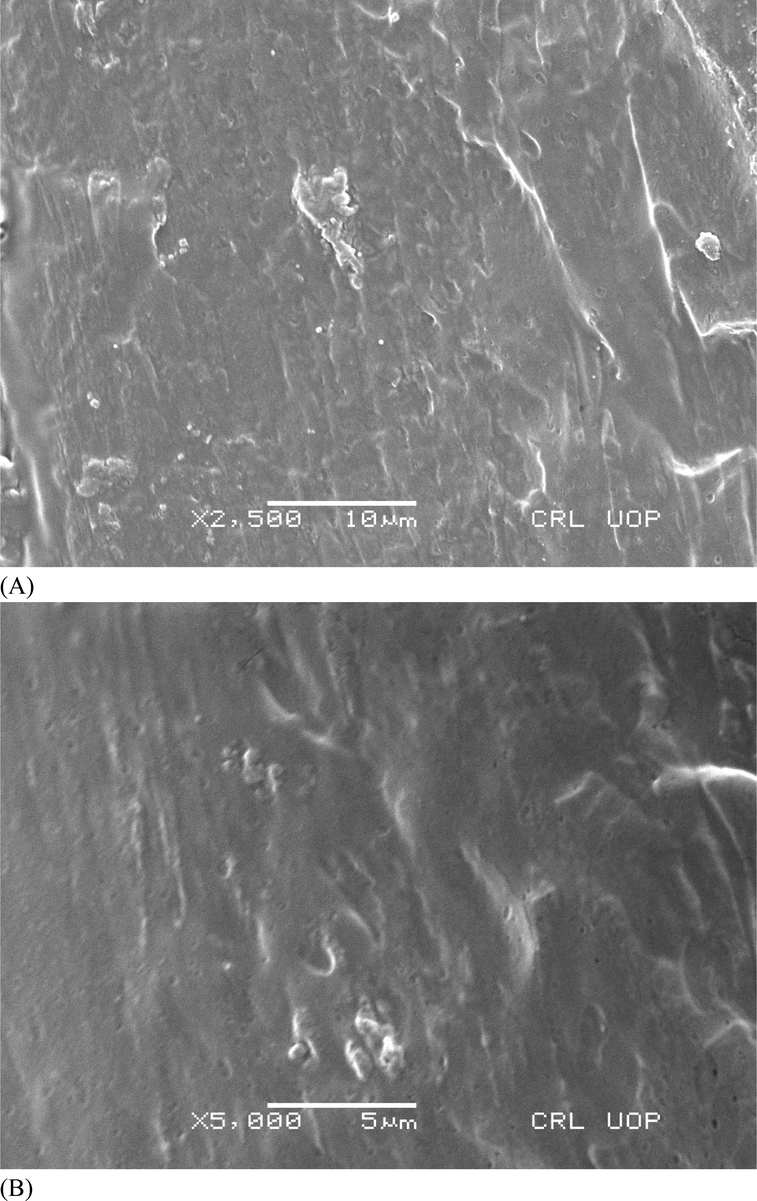
SEM images of chitosan and polyvinyl alcohol membranes, (A) without extract and (b) loaded with Calotropis procera extract.
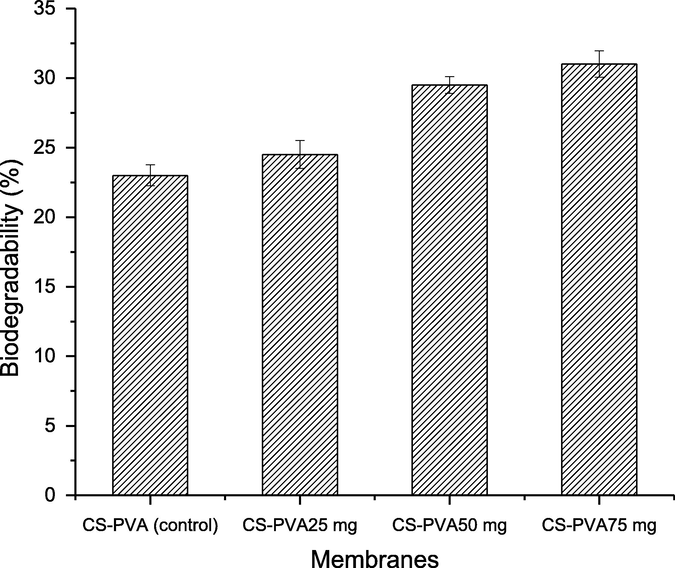
Biodegradability of chitosan and polyvinyl alcohol (CS-PVA) membranes in BPS medium.
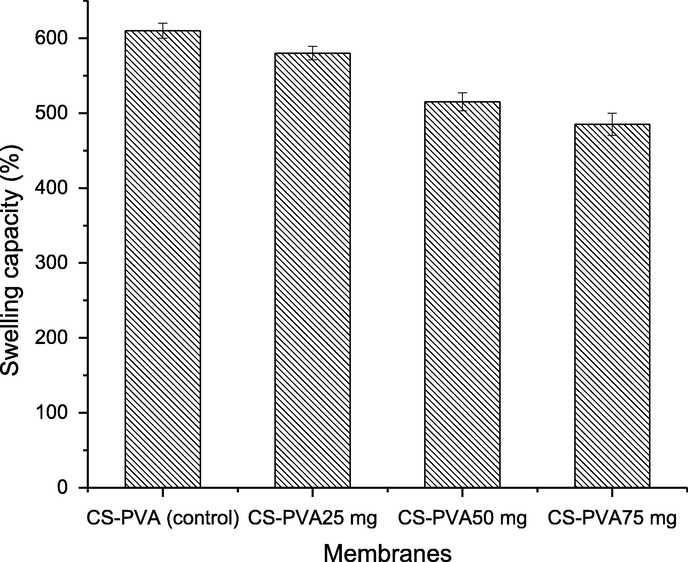
Swelling behavior of chitosan and polyvinyl alcohol (CS-PVA) membranes in aqueous medium.
3.3 Antimicrobial activity of CS-PVA membrane
Natural products put down the foundations of conventional medicine (Hamid et al., 2017; Muhammad et al., 2020; Yeshiwas and Mekonnen, 2018; Abate and Yayinie, 2018), which are used against different diseases (Sorg et al., 2017). The antimicrobial activity of CS-PVA membranes loaded with different concentrations of C. procera root extracts were performed against E. coli and S. aureus. The concentrations of extracts tested, showed promising antimicrobial activity and inhibition zones against the bacterial strains are shown in Table 2. The zone of inhibition of CS-PVA membranes loaded with 75 mg C. procera root extracts were recorded to be 18.50 ± 2.30 and 20.40 ± 4.20 (mm) against E. coli and S. aureus, respectively. The zone of inhibition of CS-PVA membranes without C. procera root extracts were recorded to be 7.42 ± 1.57 and 10.36 ± 2.51 (mm) against E. coli and S. aureus, respectively. The microbial activities impair the healing process of wounds, which is the main reason in the delaying of wound healing. Also, the antibacterial activity of CS is well reported (Bano et al., 2017). Hence, the CS-PVA membrane loaded with C. procera root extracts have potential for infectious wound healing. The values are mean ± SD of triplicate samples (n = 3); the standard drug used as control at a conc. of 1 mg/mL.
Sr#
CS-PVA membrane loaded with and without C. procera extracts
E. coli
S. aureus
1
CS-PVA
7.42 ± 1.57
10.36 ± 2.51
2
CS-PVA@25 mg extract
10.50 ± 2.70
12.60 ± 3.10
3
CS-PVA@50 mg extract
13.15 ± 1.20
15.30 ± 2.60
4
CS-PVA@75 mg extract
18.50 ± 2.30
20.40 ± 4.20
5
Rifampicin
28.50 ± 2.5
30.50 ± 1.38
3.4 Wound healing potential of CS-PVA membrane
Induced wounds in rabbits were treated by CS-PVA membrane loaded with three different concentrations (25–75 mg) of C. procera extracts and their wound healing potential was compared with control and CS-PVA membrane without extract (Table 3). Wound sizes were measured on 6th and 12th day for all groups and compared with control. The data showed that group 5 showed maximum reduction in wound size on day 6th and 12th day versus other groups (Table 3). The percentage of wound healing in group 3, treated with CS-PVA loaded membrane with 25 mg of C. procera root extracts revealed the wound reduction (6th day) was up to 25.33% and on the 12th day of treatment, the recovery was recorded up to 52.33%. Group (G4) treated with CS-PVA loaded with 50 mg C. procera root extracts showed wound reductions of 29.67% and 59% on day 6th and 12th day, respectively. In group (G5), the reduction in wounds was 32.33% on 6th day of treatment and on 12th day, the wound reduction recorded to be 63.37%. The control group (G1) showed slow healing that of 14.67% on 6th day and 40.66% on 12th day. CS-PVA treated group (G2) reduction in wounds was 21.67% on 6th day and on 12th day, the reduction was 44.67%. The skin tissues from wounds were collected on 6th day of treatment and subjected to histological study and it was observed that all regeneration process was in line with wound reduction data. Wound size and wound reduction were documented on 6th and 12th. In each group three (03) experimental animals (Rabbits) were selected and their results are the average of their values in term of their mean ± SD of triplicate samples (n = 3).
Group no
No. of animal
Wound size
Wound reduction
6th day
12th day
6th day
12th day
Control
3
2.57 ± 0.12
1.78 ± 0.10
14.67%
40.66%
CS-PVA membranes
3
2.35 ± 0.50
1.66 ± 1.20
21.67%
44.67%
CS-PVA@25 mg extract
3
2.24 ± 1.30
1.43 ± 0.60
25.33%
52.33%
CS-PVA@50 mg extract
3
2.11 ± 1.20
1.23 ± 0.70
29.67%
59.00%
CS-PVA@75 mg extract
3
2.03 ± 0.85
1.099 ± 0.46
32.33%
63.37%
In comparison control, the CS-PVA membrane loaded with bioactive accelerated the wound healing process. Regeneration phase was initiated earlier with the development of connective tissues in group 5 that was treated with 75 mg extract loaded CS-PVA membrane. On 12th day of treatment, the rabbits treated with CS-PVA membrane without containing any bioactive compounds showed higher wound size versus group 5 rabbits. The establishment of final phase of wound healing and end of regeneration phase was also observed better in group 5 versus control and CS-PVA membrane treated group. At this stage, the negative control group was at the middle of healing phase, although, the wound area was reduced. The CS/PVA membrane loaded with C. procera extracts proved to be highly efficient for the wound healing, which are also in accordance with already documented studies that material loaded with bioactive materials showed higher wound healing capability versus their individual counterparts other (Ahmed et al., 2018; Sun et al., 2018). A different material has been prepared and loaded with bioactive agents form different sources (cellulose acetate, chitosan, polycaprolactone, polyvinyl alcohol, polypropylene carbonate, polycaprolactone, hyaluronic acid, zein, poly-ε-caprolactone, carbopol, polycaprolactone, polyvinyl alcohol) loaded with bioactive agents showed promising wound healing potential (Table 4), i.e., a PVA/CS NFs with carboxymethyl CS encapsulating the antibacterial peptide OH-CATH30 (OH-30) were prepared, which showed excellent efficiency for skin wound healing along with antibacterial activity against E. coli and S. aureus (Zou et al., 2020). In another study, nanobioglass incorporated CS-PVA trilayer NFs membrane was fabricated, which were biocompatible and active against panel of microbes. The membrane also applied in the treatment of mice diabetic chronic wound and positive response was reported for wound dressings ability (Chen et al., 2019). Similarly, Colobatiu et al. (Colobatiu et al., 2019) prepared the bioactive compounds-loaded CS film as a wound dressing material. The bioactive compounds-loaded CS film showed excellent antioxidant capacity and was also biocompatible. The in vivo performance of the films was evaluated in streptozotocin-induced diabetic rat model and CS film efficiency was promising for wound healing. In another study, curcumin loaded CS/PLA NFs were prepared and tested for antioxidant, drug release and biocompatibility along with wound healing potential on rat model. A significant reduction of wound area was observed, which was correlated with curcumin and CS contents (Dhurai et al., 2013). Also, poly(ε-caprolactone) (PCL) composite was prepared with CS and loaded with NO containing biomaterials and was tested for wound healing and PCL/CS-NO dressing was promising for wound treatment, especially for the chronic wound caused by the ischemia (Zhou et al., 2017).
S. no
Materials
loading agents
Bioactivity
Applications
References
1
CS
Henna leaves extract
Antibacterial
Wound healing
(Yousefi et al., 2017)
2
Cellulose acetate
Annatto extract
Biocompatible
Wound healing
(dos Santos et al., 2021)
3
Polycaprolactone/CS
Aloe vera
Antibacterial, biocompatible
Wound dressing
(Yin and Xu, 2020)
4
PCA/CS
CMCS-OH30 NPs
Antimicrobial
Wound dressing in mouse
(Zou et al., 2020)
5
CS/PVA
Nanobioglass
Biocompatible, antibacterial
Wound dressing in mice
(Chen et al., 2019)
6
CS
Plantago lanceolata, Tagetes patula, Symphytum officinale, Calendula officinalis and Geum urbanum
Antioxidant, biocompatible
Wound dressing
(Colobatiu et al., 2019)
7
CS
Curcumin
Antioxidant, biocompatible
Wound dressing in rats
(Dhurai et al., 2013)
8
CS/poly(propylene carbonate)
Curcumin
Antioxidant, biocompatible
Wound healing in rats
(Mei et al., 2017)
9
Chitosan-Polycaprolactone nanofiber
Ferulic acid and resveratrol
Antioxidant, biocompatible
Wound healing in rats
(Poornima and Korrapati, 2017)
10
Polycaprolactone- hyaluronic acid/chitosan- zein electrospun
salicylic acid
Antimicrobial
Wound dressing
(Figueira et al., 2016)
11
CS/PVA
Lysozyme
Biocompatible
Wound healing in rats
(Charernsriwilaiwat et al., 2012)
12
CS
Bromelain
Biocompatible
Wound healing in rats
(Bayat et al., 2019)
13
Poly (ε-caprolactone/CS
Nitric oxide
Wound healing in mouse
(Zhou et al., 2017)
14
CS/PVA
Tetracycline hydrochloride
Biocompatible, antimicrobial
Wound healing in rabbit
(Alavarse et al., 2017)
15
PVA/CS/ZnO
Heparin
Biocompatible, antimicrobial
Wound dressing
(Khorasani et al., 2018)
16
PVA/CS
Azadirachta indica (neem)
Antibacterial
Wound dressing
(Ali et al., 2019)
17
PVA
Schizophyllan
Biocompatible
Wound dressing
(Safaee-Ardakani et al., 2019)
18
CS/PCA/carbopol/polycaprolactone
Curcumin
Biocompatible
Wound healing in mouse
(Golchin et al., 2020)
19
CS/polyvinyl alcohol
Curcumin
Antibacterial
Wound healing in rabbits
(Abbas et al., 2019)
20
CS/polyvinyl alcohol
C. procera extract
Antibacterial, antioxidant, biodegradable
Wound healing in rabbits
Present study
Also, PVA/CS/ZnO was fabricated and tested for their biocompatibility, antibacterial activity along with wound healing efficiency. Cell viability of analysis showed no toxicity and was active against panel of microbes. The PVA/CS/ZnO effectively protected the wounds and are effective for robust wound dressings (Khorasani et al., 2018). Also, PVA/CS loaded with tetracycline hydrochloride was used for wound dressing, which showed promising antibacterial activity against E. coli, S. epidermidis and S. aureus. Also, the composite was biocompatible and have potential for wound dressing for healing promotion (Alavarse et al., 2017). Similarly, CS/poly(propylene carbonate) loaded with curcumin showed excellent free-radical scavenging capabilities along with wound healing efficacy. The CS and curcumin improved the wound healing process and showed potential for wound repair applications (Mei et al., 2017). Besides, bromelain was gummed with CS NFs and resultant material was biocompatible and active to reduce the burn wound. The CS NFs loaded with bromelain possesses excellent wound healing activity and is considered as an effective natural topical burn wound healing treatment (Bayat et al., 2019). Also, CS–ethylenediaminetetraacetic acid-PVA blends loaded lysozyme were prepared and applied as a wound healing agent. The lysozyme loaded CS–EDTA NFs have a potential for wound healing applications (Charernsriwilaiwat et al., 2012). Similarly, CS-polycaprolactone (CS-CPL) NF was prepared for wound dressing and drug delivery application. Ferulic acid and resveratrol were incorporated in CS-CPL NFs and tested for their biocompatibility and wound healing efficiency, which are considered viable for sustained drug delivery and wound therapeutics (Poornima and Korrapati, 2017). Figueira et al. (Figueira et al., 2016) also fabricated CS and zein composite and salicylic acid was incorporated. The prepared composite showed suitability for wound dressing application. Hence, the findings revealed that the CS-PVA membranes loaded with bioactive agents from the C. procera root extract is efficient for the wound healing. The plant extract contains bioactive compounds, which act as an antimicrobial and antioxidant (Awwad et al., 2020; Shadrach et al., 2020; Mekonnen et al., 2020; Awwad et al., 2020; Abate, 2019), that have potential to treat the infections, which is more eco-benign versus synthetic pharmaceutical agents.
3.5 Cytotoxicity studies
The hemolytic assay of CS-PVA membrane loaded with C. procera extracts were performed against bovine red blood cells (RBCs). It was observed that the CS-PVA membrane loaded with C. procera extracts were non-toxic to bovine RBCs. The CS-PVA membrane loaded with C. procera extracts (25–75 mg) showed the RBCs lysis of 2.55 ± 0.7, 3.60 ± 0.8 and 4.90 ± 0.6, respectively (Fig. 4). In the controls, phosphate buffer saline and triton (X-100) (0.1%) showed the lysis of RBCs of 0.0 and 100 (%), respectively. The finding revealed that CS-PVA membrane loaded with C. procera extracts can be applied for wound healing since no toxic signs are observed.
3.6 Histopathology studies
Wound healing is a complex process involving interaction of various cellular and extracellular elements leading to restoration of tissue architecture as close to normal as possible (Sun et al., 2018). In the current study, on the 6th day of experiment, no difference was observed between the control group and CS-PVA membrane group as the tissues showed mild angiogenesis and infiltration of a hetrogenous population of inflammatory cells predominantly, neutrophils. Indicative of inflammatory phase with formation of small amount of loosely arranged immature granulation tissue. The tissue healing is divided into inflammatory phase, proliferative phase, deposition of extracellular matrix and remodeling phase (Zhou et al., 2017). Inflammatory phase involves hemostasis, infiltration of polymorph cellular infiltrates, and edema in the injured tissue. Groups loaded with 25 and 50 mg C. procera extracts showed no difference with each other, but as compare to control groups, angiogenesis, epithelial cells proliferation at the wound edges were more prominent (Figs. 5 and 6). The group treated with 75 mg of C. procera showed marked epithelial hyperplasia, angiogenesis, fibroblasts proliferation and replacement of neutrophils with macrophages. C. procera has anti-bacterial properties due to calo-protein and its extracts has shown considerable therapeutic efficiency in wound healing (Khorasani et al., 2018).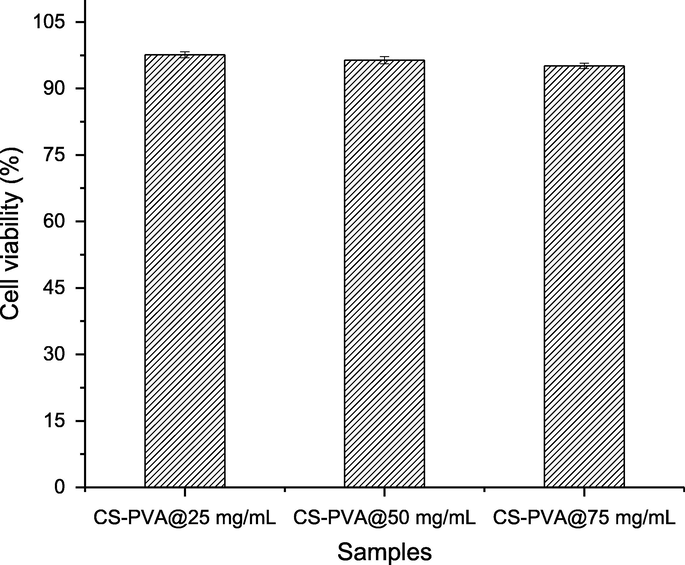
Cytotoxic activity of CS-PVA membranes loaded with different concentrations of C. procera extracts (25–75 mg).
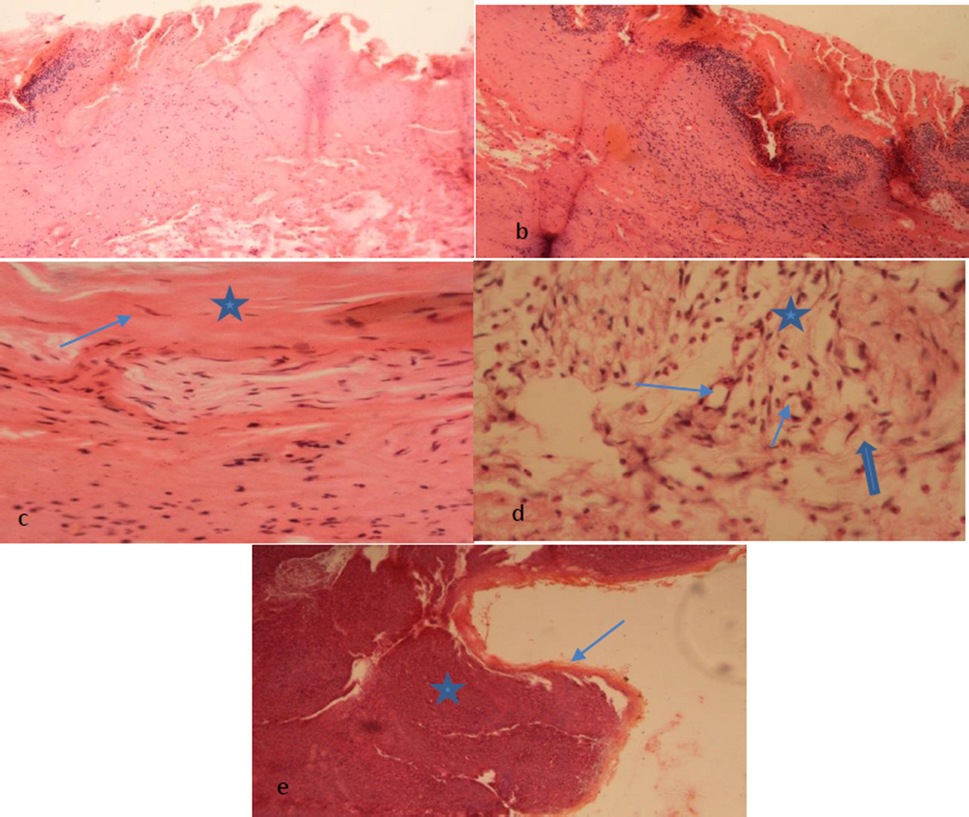
(a) Control group showing edematous reaction indicated by Eosinophillic homogenous masses admixed with mononuclear cellular infiltrate, (b) partial formation of surface skin layer epidermis with less deposition of keratinized tissue seen, (c) fibrocytes are seen (Blue arrow) admixed collagen fibers (star) arranged underneath epidermis layer. Some inflammatory cells masses are also seen, (d) granulation mass is seen underneath epithelium showing formation of thin-walled capillaries (Angiogenesis) indicated by blue stars in lumen. Infiltration of mononuclear cells especially neutrophils indicated by star representing host inflammatory response. Mature connective tissue cells are deposited in the form of fibrocytes and collagen fibers (Thick blue arrow) evidence of healing (Proliferation Phase) and (e) re-epithelisation is seen (thin blue arrow shows epidermis layer) clearly indicated by thin arrow a star indicates dermal region.
3.7 Release of bioactive compounds
The release of bioactive components in C. procera root extract loaded in CS-PVA membrane were investigated under simulated intestinal condition and results are depicted in Fig. 7. Results shows that the CS-PVA membrane loaded with C. procera root extract (25 and 50 mg/mL) released up to 16% and 16% bioactive in 20 h. The data analysis revealed a slow release of bioactive components form the CS-PVA membrane loaded with C. procera root extract. This slow release of bioactive indicates the potential of CS-PVA membrane for the treatment of infections, which need the presence of bioactive for longer time. Hence, the slow release of bioactive from CS-PVA membrane loaded with C. procera has potential for the provision of bioactive slowly for extended period of time. The active secondary metabolites in C. procera root extract are trapped in the matrix of CS-PVA. The CS-PVA membrane loaded with C. procera root extract (75 mg/mL) provides sustained release of bioactive compounds, which releases 15% bioactive in last 30 h. In the same duration, 25 and 50 (mg/mL) formulations released 12% and 13% bioactive. Due to this slow release of bioactive compounds from CS-PVA loaded membrane, it provides a significant for extended period of time, which have potential for the treatment of wounds.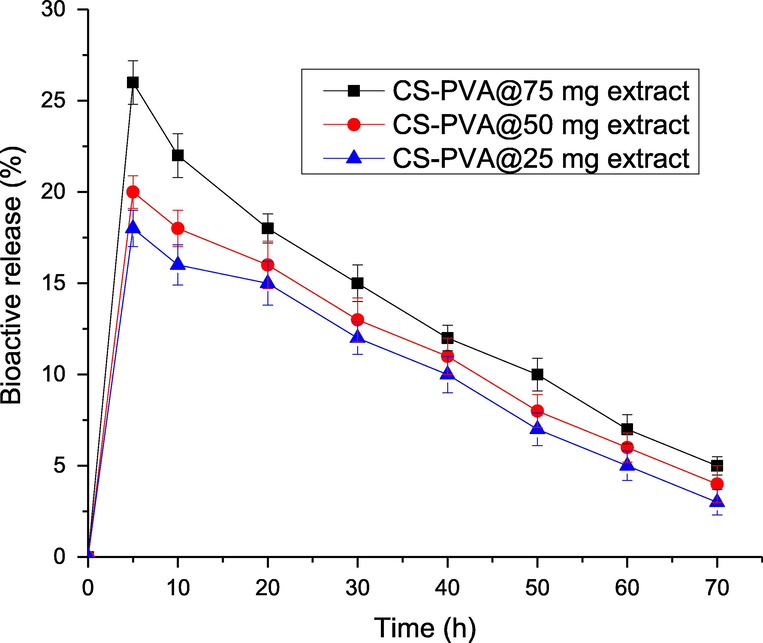
Percentage release of bioactive compounds from CS-PVA membranes loaded with different concentrations of C. procera extracts.
4 Conclusions
The CS-PVA membranes was prepared and loaded with bioactive agents from the C. procera root extract and employed for wound healing in rabbits. The CS-PVA membranes swelling, biodegradability and surface properties were found dependent to the concentration of extracts (25–75%) loaded with membrane. The extracts showed excellent antioxidant activity evaluated in term of DPPH radical scavenging, TPC and TFC. The CS-PVA loaded with extract were applied on induced wounds in rabbits and efficiency was compared with control. The reduction in wound size was promising and was dependent to concentration of extracts used for the preparation of membrane. The CS-PVA membranes loaded with C. procera root extract are effective to treat wounds since the regeneration and formation of fibrous connective tissues was fast in wounds treated with CS-PVA membrane loaded with C. procera root extract and CS-PVA membrane effect was more promising loaded with higher concentration of C. procera root extract. Based on findings, the CS-PVA membranes loaded with C. procera root extract have potential as a robust wound healing agent. In future, the biocompatibility of CS-PVA membranes loaded with C. procera root extract can be studied using cell lines.
Funding
This research was funded by Princess Nourah bint Abdulrahman University Researchers Supporting Project (Grant No. PNURSP2022R165), Princess Nourah bint Abdulrahman University, Riyadh, Saudi Arabia. Taif University Researchers Supporting Project number (TURSP-2020/243), Taif University, Taif, Saudi Arabia.
Acknowledgments
The authors express their gratitude to Princess Nourah bint Abdulrahman University Researchers Supporting Project (Grant No. PNURSP2022R165), Princess Nourah bint Abdulrahman University, Riyadh, Saudi Arabia. Taif University Researchers Supporting Project number (TURSP-2020/243), Taif University, Taif, Saudi Arabia.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Bioactivity profiling of four traditional medicinal plants leave extracts native to Alemsaga Forest, Ethiopia. Chem. Int.. 2019;5(4):281-290.
- [Google Scholar]
- Effect of solvent on antioxidant activity of crude extracts of Otostegia integrifolia leave. Chem. Int.. 2018;4(3):183-188.
- [Google Scholar]
- Wound healing potential of curcumin cross-linked chitosan/polyvinyl alcohol. Int. J. Biol. Macromol.. 2019;140:871-876.
- [Google Scholar]
- Formulation and characterization of a novel cutaneous wound healing ointment by silver nanoparticles containing Citrus lemon leaf: a chemobiological study. Arabian J. Chem.. 2021;14(7):103246.
- [Google Scholar]
- Novel electrospun chitosan/polyvinyl alcohol/zinc oxide nanofibrous mats with antibacterial and antioxidant properties for diabetic wound healing. Int. J. Biol. Macromol.. 2018;120:385-393.
- [Google Scholar]
- Green synthesis of sulfur nanoparticles using Rosmarinus officinalis leaves extract and nematicidal activity against Meloidogyne javanica. Chem. Int.. 2020;6(3):137-143.
- [Google Scholar]
- Nano platelets kaolinite for the adsorption of toxic metal ions in the environment. Chem. Int.. 2020;6:49-55.
- [Google Scholar]
- Tetracycline hydrochloride-loaded electrospun nanofibers mats based on PVA and chitosan for wound dressing. Mater. Sci. Eng., C. 2017;77:271-281.
- [Google Scholar]
- Antibacterial bi-layered polyvinyl alcohol (PVA)-chitosan blend nanofibrous mat loaded with Azadirachta indica (neem) extract. Int. J. Biol. Macromol.. 2019;138:13-20.
- [Google Scholar]
- Study of new cellulosic dressing with enhanced antibacterial performance grafted with a biopolymer of chitosan and myrrh polysaccharide extract. Arabian J. Chem.. 2020;13(2):3672-3681.
- [Google Scholar]
- Important insights from the antimicrobial activity of Calotropis procera. Arabian J. Chem.. 2021;14(7):103181.
- [Google Scholar]
- Electrospun polyvinyl alcohol membranes incorporated with green synthesized silver nanoparticles for wound dressing applications. J. Mater. Sci. - Mater. Med.. 2018;29(11):163.
- [Google Scholar]
- Electrospun chitosan membranes containing bioactive and therapeutic agents for enhanced wound healing. Int. J. Biol. Macromol.. 2020;156:153-170.
- [Google Scholar]
- Green synthesis of zinc oxide nanoparticles (ZnO-NPs) using Ailanthus altissima fruit extracts and antibacterial activity. Chem. Int.. 2020;6(3):151-159.
- [Google Scholar]
- Biosynthesis of copper oxide nanoparticles using Ailanthus altissima leaf extract and antibacterial activity. Chem. Int.. 2020;6(4):210-217.
- [Google Scholar]
- Green synthesis, characterization of silver sulfide nanoparticles and antibacterial activity evaluation. Chem. Int.. 2020;6(1):42-48.
- [Google Scholar]
- Bioactivity of variant molecular weight chitosan against drug-resistant bacteria isolated from human wounds. Microbial. Drug Resist.. 2017;23(8):958-965.
- [Google Scholar]
- Bromelain-loaded chitosan nanofibers prepared by electrospinning method for burn wound healing in animal models. Life Sci.. 2019;229:57-66.
- [Google Scholar]
- Biological evaluation of antimicrobial activity of Calotropis procera against a range of bacteria. J. Pharmacog. Phytochem.. 2020;9(1):31-35.
- [Google Scholar]
- Characterization of the volatile composition of essential oils of some Lamiaceae spices and the antimicrobial and antioxidant activities of the entire oils. J. Agric. Food Chem.. 2006;54(5):1822-1828.
- [Google Scholar]
- Lysozyme-loaded, electrospun chitosan-based nanofiber mats for wound healing. Int. J. Pharm.. 2012;427(2):379-384.
- [Google Scholar]
- Electrospun chitosan/PVA/bioglass Nanofibrous membrane with spatially designed structure for accelerating chronic wound healing. Mater. Sci. Eng., C. 2019;105:110083.
- [Google Scholar]
- Phytochemical investigation of Calotropis procera. Arabian J. Chem.. 2016;9:S230-S234.
- [Google Scholar]
- Evaluation of bioactive compounds-loaded chitosan films as a novel and potential diabetic wound dressing material. React. Funct. Polym.. 2019;145:104369.
- [Google Scholar]
- Electrospinning of chitosan nanofibres loaded with curcumin for wound healing. J. Polym. Mater.. 2013;30(4)
- [Google Scholar]
- Cellulose acetate nanofibers loaded with crude annatto extract: Preparation, characterization, and in vivo evaluation for potential wound healing applications. Mater. Sci. Eng., C. 2021;118:111322.
- [Google Scholar]
- Production and characterization of polycaprolactone-hyaluronic acid/chitosan-zein electrospun bilayer nanofibrous membrane for tissue regeneration. Int. J. Biol. Macromol.. 2016;93:1100-1110.
- [Google Scholar]
- Phytochemical screening and antimicrobial studies of afzelia africana and detarium microcarpum seeds. Chem. Int.. 2018;4(3):170-176.
- [Google Scholar]
- Wound healing improvement by curcumin-loaded electrospun nanofibers and BFP-MSCs as a bioactive dressing. Polym. Adv. Technol.. 2020;31(7):1519-1531.
- [Google Scholar]
- Biological evaluation and green synthesis of silver nanoparticles using aqueous extract of Calotropis procera. Int. J. Pharma Bio Sci.. 2013;4(4):635-643.
- [Google Scholar]
- Ethno-medicinal uses of plants from district Bahawalpur, Pakistan. Curr. Res. J. Biol. Sci.. 2014;6:183-190.
- [Google Scholar]
- Chemical constituents, antimicrobial and antioxidant properties of the aerial parts of Coccinia barteri. Chem. Int.. 2017;3:428-441.
- [Google Scholar]
- Antioxidant and antibacterial polyelectrolyte wound dressing based on chitosan/hyaluronan/phosphatidylcholine dihydroquercetin. Int. J. Biol. Macromol.. 2021;166:18-31.
- [Google Scholar]
- The chemistry behind antioxidant capacity assays. J. Agric. Food. Chem.. 2005;53(6):1841-1856.
- [Google Scholar]
- Synthesis and characterization of chitosan and guar gum based ternary blends with polyvinyl alcohol. Int. J. Biol. Macromol.. 2020;143:546-554.
- [Google Scholar]
- Enhanced antibacterial activity of chitosan, guar gum and polyvinyl alcohol blend matrix loaded with amoxicillin and doxycycline hyclate drugs. Arabian J. Chem.. 2021;14(6):103156.
- [Google Scholar]
- Pipeline steel protection in oil well acidizing fluids using expired pharmaceutical agent. Chem. Int.. 2020;6(4):267-276.
- [Google Scholar]
- Synthesis, mechanical properties of fluorescent carbon dots loaded nanocomposites chitosan film for wound healing and drug delivery. Arabian J. Chem.. 2020;13(4):4882-4894.
- [Google Scholar]
- Incorporation of ZnO nanoparticles into heparinised polyvinyl alcohol/chitosan hydrogels for wound dressing application. Int. J. Biol. Macromol.. 2018;114:1203-1215.
- [Google Scholar]
- Wound healing activity of calotropis procera root bark on diabetic rats. J. Drug Deliv. Therapeut.. 2020;10(2–s):86-89.
- [Google Scholar]
- Nanofibers for improving the wound repair process: the combination of a grafted chitosan and an antioxidant agent. Polym. Chem.. 2017;8(10):1664-1671.
- [Google Scholar]
- Appraisal of solvent system effect on bioactivity profiling of Cordia africana stem bark extracts. Chem. Int.. 2020;6(1):1-10.
- [Google Scholar]
- Antibacterial and antioxidant activity of p-quinone methide derivative synthesized from 2, 6-di-tert-butylphenol. Chem. Int.. 2020;6(4):260-266.
- [Google Scholar]
- Application of Acacia modesta and Dalbergia sissoo gums as green matrix for silver nanoparticle binding. Green Process. Synth.. 2016;5(1):101-106.
- [Google Scholar]
- Hydrogel blends of chitin/chitosan, fucoidan and alginate as healing-impaired wound dressings. Biomaterials. 2010;31(1):83-90.
- [Google Scholar]
- Bioaccumulation of aluminum by Lemna gibba L. from secondary treated municipal wastewater effluents. Bull. Environ. Contam. Toxicol.. 2011;86(2):217-220.
- [Google Scholar]
- Synthesis, characterization and antimicrobial activities of 1, 5-dimethyl-2-phenyl-4-(pyrolidin-2-ylideneamino)-pyrazolidin-3-one and complex with iron (II) Chem. Int.. 2017;3(3):244-249.
- [Google Scholar]
- Formulation of quaternized aminated chitosan nanoparticles for efficient encapsulation and slow release of curcumin. Molecules. 2021;26(2):449.
- [Google Scholar]
- Anti-hyperbilirubinemic and wound healing activity of aqueous extract of Calotropis procera leaves in Wistar rats. Ind. J. Pharmacol.. 2015;47(4):398-402.
- [Google Scholar]
- Wound healing effects of bentonite: a rabbit model experimental study. Biomed. J. Sci. Tech. Res.. 2018;10(2):7683-7686.
- [Google Scholar]
- Fabrication of chitosan-polycaprolactone composite nanofibrous scaffold for simultaneous delivery of ferulic acid and resveratrol. Carbohydr. Polym.. 2017;157:1741-1749.
- [Google Scholar]
- Design of self-processing antimicrobial peptides for plant protection. Lett. Appl. Microbiol.. 2000;31(2):163-168.
- [Google Scholar]
- An overview of phytochemical and pharmacological activities of Calotropis procera. Fs J. Pharm. Res.. 2012;1(2):18-25.
- [Google Scholar]
- Electrospun Schizophyllan/polyvinyl alcohol blend nanofibrous scaffold as potential wound healing. Int. J. Biol. Macromol.. 2019;127:27-38.
- [Google Scholar]
- Preparation and antioxidant properties of extracts of Japanese persimmon leaf tea (kakinoha-cha) Food Chem.. 2005;89(4):569-575.
- [Google Scholar]
- The accumulation of La, Ce and Y by Lemna minor and Lemna gibba in the Keban gallery water, Elazig Turkey. Water Environ. J.. 2018;32(1):75-83.
- [Google Scholar]
- The hematological and biochemical changes in rats exposed to britholite mineral. Appl. Radiat. Isot.. 2017;129:185-188.
- [Google Scholar]
- Nutraceutical potential of ripe and unripe plantain peels: a comparative study. Chem. Int.. 2020;6(2):83-90.
- [Google Scholar]
- Nanoparticles encapsulation of Phoenix dactylifera (date palm) mucilage for colonic drug delivery. Int. J. Biol. Macromol.. 2021;191:861-871.
- [Google Scholar]
- Skin wound healing: an update on the current knowledge and concepts. Eur. Surg. Res.. 2017;58(1–2):81-94.
- [Google Scholar]
- Carboxymethyl chitosan nanoparticles loaded with bioactive peptide OH-CATH30 benefit nonscar wound healing. Int. J. Nanomed.. 2018;13:5771.
- [Google Scholar]
- Gross and histopathologic changes of porcine cholecyst assisted full thickness skin wound healing in rabbits. J. Livestock Sci. (ISSN online 2277–6214). 2018;9:75-80.
- [Google Scholar]
- Hemostatic and antibacterial PVA/Kaolin composite sponges loaded with penicillin–streptomycin for wound dressing applications. Sci. Rep.. 2021;11(1):1-15.
- [Google Scholar]
- Phytochemical and pharmacological review of calotropis procera. J. Gujarat Res. Soc.. 2019;21(8):1366-1379.
- [Google Scholar]
- The effect of multifunctional polymer-based gels on wound healing in full thickness bacteria-contaminated mouse skin wound models. Biomaterials. 2007;28(27):3977-3986.
- [Google Scholar]
- Comparative study of the antioxidant and antibacterial activities of two guava (Psidium guajava) fruit varieties cultivated in Andasa Horticulture Site, Ethiopia. Chem. Int.. 2018;4(3):154-162.
- [Google Scholar]
- Antioxidant and antibacterial activities of Calotropis procera Linn. Am.-Eurasian J. Agric. Environ. Sci.. 2008;4(5):550-553.
- [Google Scholar]
- Batch preparation of electrospun polycaprolactone/chitosan/aloe vera blended nanofiber membranes for novel wound dressing. Int. J. Biol. Macromol.. 2020;160:352-363.
- [Google Scholar]
- An investigation of electrospun Henna leaves extract-loaded chitosan based nanofibrous mats for skin tissue engineering. Mater. Sci. Eng., C. 2017;75:433-444.
- [Google Scholar]
- Functional poly (ε-caprolactone)/chitosan dressings with nitric oxide-releasing property improve wound healing. Acta Biomater.. 2017;54:128-137.
- [Google Scholar]
- Wound dressing from polyvinyl alcohol/chitosan electrospun fiber membrane loaded with OH-CATH30 nanoparticles. Carbohydr. Polym.. 2020;232:115786.
- [Google Scholar]







