Translate this page into:
Co-precipitation in aqueous solution synthesis of magnetite nanoparticles using iron(III) salts as precursors
*Tel.: +966 14675973; fax: +966 14675992 mkhalil@ksu.edu.sa (Mutasim I. Khalil)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.

Available online 23 February 2015
Abstract
An innovative quantitative synthetic method for preparing magnetite nanoparticles was achieved by co-precipitation in aqueous solution using only one single iron(III) salt as a precursor. A 2 Fe(III):1 Fe(II) mole ratio was first attained in solution by reducing iron(III) using KI solution, followed by filtering the iodine formed and hydrolyzing the filtrate by 25% ammonium hydroxide solution at pH 9–11. A high selectivity and atom economy percents were achieved indicating that the method is environmentally benign and green. The as-synthesized nanoparticles were characterized by fourier transform infrared spectroscopy (FTIR), X-ray diffraction (XRD), field emission scanning electron microscopy (FESEM), field emission transmission electron microscopy (FETEM), selected area electron diffraction (SAED), and 57Fe Mössbauer spectroscopy. Magnetite nanocrystals (d: 7.84 ± 0.05 nm) and nanorods (d: 6.3 ± 0.2 nm; l: 46.2 ± 0.9) formation was evident.
Keywords
Magnetite
Nanocubes
Nanorods
Co-precipitation
Hydrolysis
Selectivity
1 Introduction
Synthesis, characterization and applications of iron oxide nanocrystals have received tremendous attention in recent years due to their potentials for information storage devices, rotary shaft sealing, position sensing (Raj and Moskowitz, 1990), as well as medical and pharmaceutical applications (Xie et al., 2008). Several methods are known in the art for the synthesis of magnetite, Fe3O4. Cornwell and Schwertmann (Cornwell and Schwertmann, 2003) have reported several synthetic methods all of which require more than one iron component as precursors, several chemical reagents, inert atmosphere, special apparatus and/or restricting conditions. Exemplary methods are shown as follows:
-
(1)(David and Welch, 1956).
-
(2)(produces complicating side effect) (Schikorr, 1929).
-
Reduction of hematite at 400 °C in an atmosphere of 5% H2/95% Ar, saturated with water vapour free of O2 (Regazzoni et al., 1981).
-
Reaction of a 1:2 Fe(II)/Fe(III) solution, under alkaline conditions at 80 °C under N2 (Regazzoni et al., 1981).
-
Reaction at 85 °C of Fe(II) ammonium solution (buffered to pH 7 to 8 with sodium acetate) with hydroxylamine sulfate; the suspension is held under N2 gas (Ardizzone and Formaro, 1983), as shown thus
(3) -
Reductive transformation in a sealed ampoule of an akaganeite suspension in the presence of hydrazine at pH 9.5 to 11.5 and 100 °C (Blesa and Maroto, 1986).
(4) -
Decomposition of an alkaline (0.2–0.4 MOH) solution of Fe(III) NTA at 217 °C in an autoclave (Booy and Swaddle, 1978).
-
Heating of iron hydroxide acetate at 200–260 °C under N2 (Pinheiro et al., 1987).
-
Boiling a mixture of Fe(II) sulfate and bispyridoxylidene hydrazine phthalazine for 10 min at pH 7 (Sarel et al., 1989).
-
Thermal decomposition of Fe(II) sulfide in air at 500 °C, which is environmentally unfriendly (Robl, 1958).
(5) -
Holding a solution of Fe(III) acetylacetonate in 1-propanol under N2 in an autoclave at 300 °C for several hours (Kominami et al., 1999).
-
Reduction of nitrobenzene to aniline produces Fe3O4 (Laux, 1925) as shown thus:
Further, several Fe3O4 synthetic methods were patented (Michael et al., 2004; Shen, 2002; Yoshito et al., 1984; Tomio and Horoyuki, 1999; Shouheng, 2002; Maurice et al., 1978; Naoyoshi and Kenzo, 2000).
The most popular methods for the synthesis of the spinel structured Fe3O4 include co-precipitation in aqueous solution by adding hydroxides into iron salt solutions (Hui et al., 2008; Fried et al., 2001) and the thermal decomposition of iron organometallic compounds in high boiling organic solutions in the presence of stabilizers (Sun and Zeng, 2002; Xu et al., 2010).
Recently, Xu et al. (2010) reported on the organic phase synthesis of mono dispersed iron oxide nano crystals using iron chloride as a precursor.
In this paper, a report was made on an innovative invention (Khalil, 2012) that relates to a process of preparing magnetite nanocrystals by the co-precipitation method using iron(III) chloride or nitrate salts as precursors. The objective of this invention was to provide a process of preparing magnetite (Fe3O4) and derivatives thereof, not reported herein, which overcomes the drawbacks of the prior art, especially a process which only requires one iron compound as the starting precursor, a limited number of additional chemical reagents, and a process which can be carried out under simple reaction conditions, preferably at room temperature, with easy work-up of the product obtained.
2 Experimental
2.1 Starting material
Iron chlorides (FeCl3 and FeCl3·6H2O) were purchased from WINLAB, Leicestershire, England. Iron nitrate (Fe(NO3)3·9H2O) sodium hydroxide (NaOH) and 25% ammonia solution were all purchased from BDH, Poole, England. And potassium iodide (KI) and polyvinyl alcohol (PVA 72000) were purchased from MERCK, Darmstadt, F.R. Germany.
2.2 Characterization
The FTIR spectrum was recorded on a Shimadzu FTIR-8400S, Prestige-21 spectrophotometer in a KBr matrix. Powder X-ray diffraction (XRD) pattern was obtained with an Ultima IV X-ray diffractometer using copper-monochromatized Cu Kα 1 radiation under the acceleration voltage of 40 kV and a current of 40 mA. The morphology of the Fe3O4 nanocrystals was examined by a JEOL JSM-6380 LA scanning electron microscope and a JEOL, TEM-2100F transmission electron microscope with an acceleration voltage of 200 kV. 57Fe Mössbauer spectra were recorded in the Institute des Molecules et Matériaux du Mans (IMMM), Université du Maine, France.
2.3 Synthesis of magnetite nanoparticles
The work-up consists of mixing iron(III) salt solution with potassium iodide aqueous solution in a 3:1 mol ratio, filtering out the iodine formed, hydrolyzing the filtrate with either sodium hydroxide or ammonia solution, filtering the black magnetite precipitate, washing with water and drying at 250 °C.
An exemplary experiment is carried out as follows:
19.46 g (0.119 mol) of anhydrous FeCl3 was completely dissolved in 150 ml distilled water to prepare aqueous solution A. Further, 6.584 g (0.0396 mol) of potassium iodide was dissolved in 50.0 ml of distilled water to prepare aqueous solution B. Solutions A and B were then mixed together at room temperature, stirred and allowed to reach equilibrium for one hour. The precipitate of iodine was filtered out, washed with distilled water, dried at 100 °C and weighed (yield: 5.076 g, 86.6%). The washing was added to the filtrate. The whole volume of filtrate was then hydrolyzed using 25% ammonia solution which was added drop-wise with continuous stirring until complete precipitation of the black magnetite was achieved (pH 9–11). The set up was then left to settle, filtered, washed with distilled water, dried at 250 °C and weighed (yield: 9.2 g, 99.0%).
The same procedure was repeated using FeCl3.6H2O as a precursor. 27.03 g FeCl3·6H2O dissolved in 150.0 ml distilled water was reacted with 5.533 g (0.033 mol) KI aqueous solution. Iodine yield was 3.15 g, 75.53%. Magnetite was precipitated from three 50.0 ml portions of the filtrate. The average yield was 1.40 g, 91.61%.
The yield of magnetite from Fe(NO3)3.9H2O precursor was low (52.8%) when a 3Fe(III): 1.0 KI mole ratio was used. Increasing the ratio to 1:1.325 yielded a 97.0% I2 and a 96.85% Fe3O4.
3 Results and discussion
Instead of using two iron precursors, that is Fe(III) and Fe(II) mixed in a 2:1 mol ratio for the preparation of magnetite, one can start with only an aqueous iron(III) salt solution and reduce it by potassium iodide to maintain the appropriate mole ratio according to Eq. (7):
Thereafter, filtering out the iodine precipitate and then hydrolyzing the filtrate by 25% ammonium or sodium hydroxide solution at pH 9–11 were used to obtain black magnetite nanoparticles. The percentage yield of magnetite and iodine was 99% and 86%, respectively.
This innovative method for the preparation of magnetite is economical and green, achieving a high selectivity and atom economy percents (Lancaster, 2002). Eqs. (8) and (9) imply that both magnetite and iodine are desired products.
The FTIR spectrum of the as-synthesized magnetite from anhydrous FeCl3 (Fig. 1) exhibited vibrational bands at 634 sh., 582 s, 397 m cm−1 characteristic of magnetite (Cornwell and Schwertmann, 2003; Zheng et al., 2011; Liese, 1967). The intense band at 582 cm−1 could be assigned to the IR active T1u mode corresponding to the vibration of the Fe2+–O2- functional group. The splitting-up peak at 634 cm−1 is due to the symmetry degeneration on octahedral B sites (Zheng et al., 2011). Rowan and Patterson (Rowan and Patterson, 2009) have reported that for monoclinic unit cell at low temperature, the IR modes in the Fe2+–O2− stretching vibrational band would be induced as obvious split pairs for fields along the x and y directions.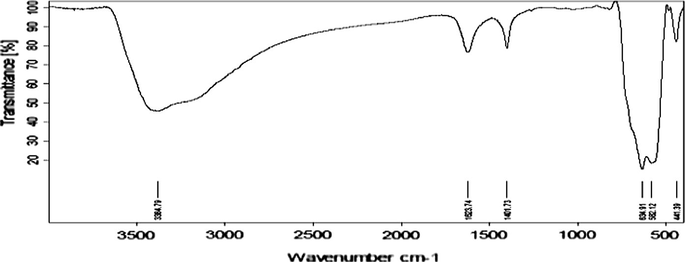
FTIR spectrum of the as-synthesized magnetite.
The EDS intensity peaks (Fig. 2) show the exact iron percent in Fe3O4. The Cu signals were from the Cu grid. No other signal was detected within the detection limits of EDS which confirms the purity of the Fe3O4 nanoparticles.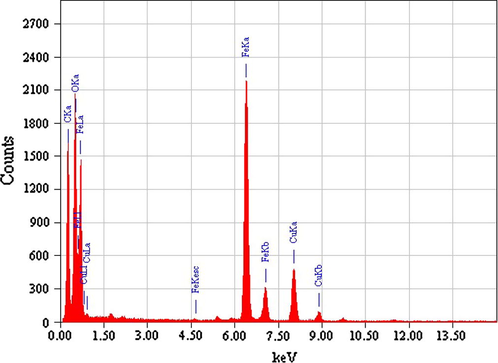
EDS pattern of the as-synthesized magnetite.
The crystallinity of the magnetite sample was investigated by XRD as shown in Fig. 3. The results show that the sample has six peaks at 2θ of 30.36°, 35.74°, 43.52°, 53.95°, 57.34° and 63.0° representing the corresponding indices of (2 2 0), (3 1 1), (4 0 0), (4 2 2), (5 1 1), and (4 4 0) respectively Cornwell and Schwertmann, 2003; Xu et al., 2010.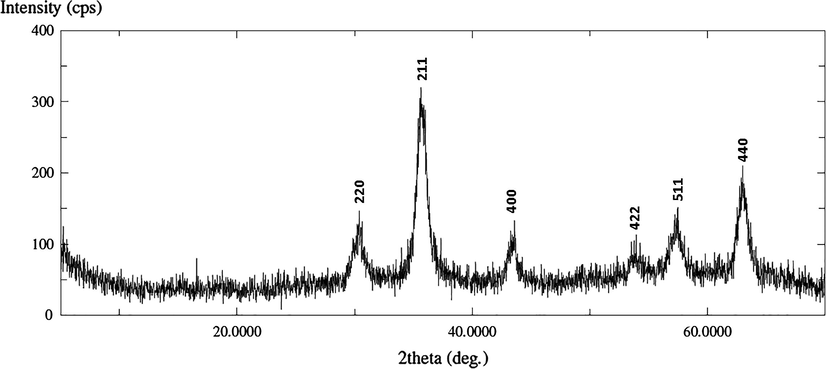
XRD pattern of the as-synthesized magnetite.
The crystal system is cubic with Z value of 8.0, a = b = c = 8.3199 Å, α = β = y = 90° belonging to space group Fd-3m.
A typical scanning electron microscopic (SEM) image of the as-synthesized iron oxide nanoparticles is shown in Fig. 4. It is evident from the picture that the particles are in a nano scale.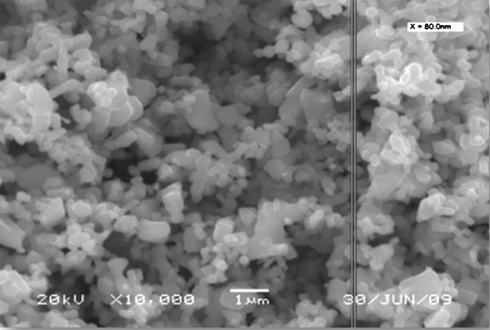
SEM image of the as-synthesized magnetite.
TEM image (Fig. 5) of the synthesized magnetite indicates the formation of nanocubes and nanorods composite.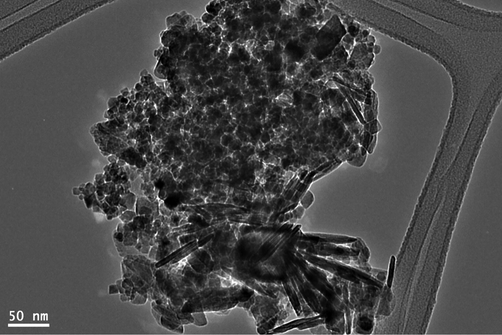
TEM image of the as-synthesized magnetite.
The selected area electron diffraction (SAED) pattern of the as-synthesized iron oxide is shown in Fig. 6(a). The high resolution TEM image of a single iron oxide cube (Fig. 6b) shows lattice fringes with an interfringe distance of 0.292 nm, which is close to the interplane distance (2 2 0) of the planes in the cubic spinel structured iron oxide (Xu et al., 2010).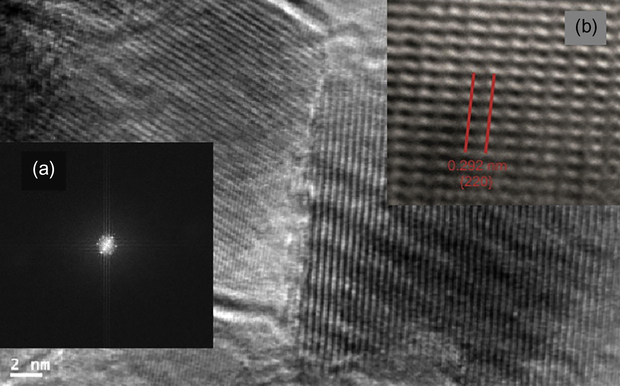
HRTEM image of a single magnetite cube; (a) SAED pattern and (b) Lattice fringes.
Information of the mean size and standard deviation (SD) was calculated from measurements of the nano cubes and nanorods in random fields of view and presented in Fig. 7. The mean ± SD of the nanocubes was 7.8 ± 0.05 nm while the mean ± SD of the nanorods was 6.3 ± 0.2 nm in diameter and 46.2 ± 0.9 nm in length.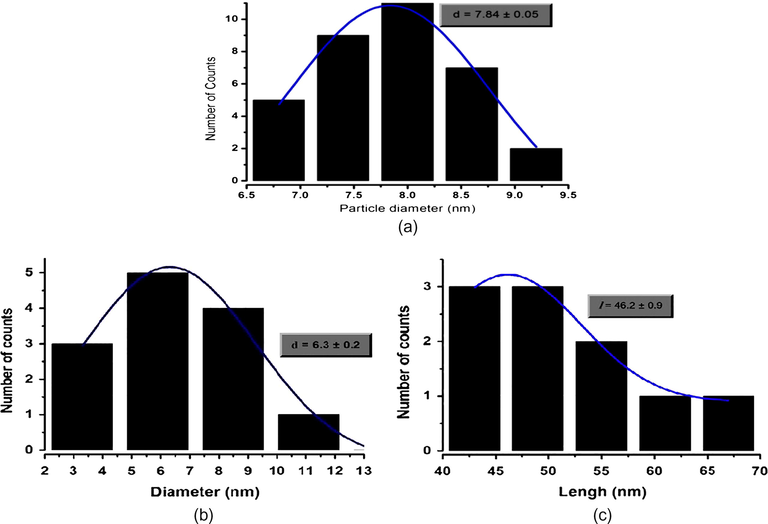
Mean size and standard deviation of: (a) Fe3O4 nano cubes; (b and c) Fe3O4 nano rod.
Liquid nitrogen temperature (77 K) and room temperature (300 K) 57Fe Mössbauer spectra of one magnetite-loaded PVA dry hydrogel sample are presented in Fig. 8(a) and (b). The 77 K recorded spectrum, Fig. 8(a), is similar to that of an assembly of 8–10-nm noninteracting magnetite particles published by Morup et al. (1980) and Szabó et al. (2000). The RT 57Fe Mössbauer spectrum consists of two sextets with isomer shifts of 0.15 mm/s and a hyperfine field of 48.6T for Fe3+ in the A sites and 0.56 mm/s and a hyperfine field of 45.7T for the Fe2.5+ average valence on the B sites (Dzési et al., 2008). A quadrupole splitting of 0.00 and −0.01 mm/s is indicative of cubic symmetry. The absence of a paramagnetic component indicates that the transition to paramagnet occurs above 300 K.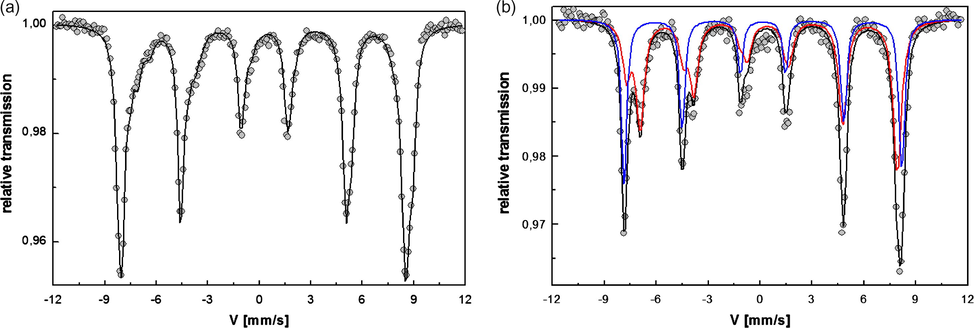
Mössbauer spectra of a disk-shaped dried ferrogel at 77 K (a) and 300 K (b).
Detailed 57Fe Mössbauer effect study will be conducted and published elsewhere.
Acknowledgement
This project was supported by the Deanship of Scientific Research, College of Science Research Center, King Saud University, Saudi Arabia.
We would like to thank Prof. Jean-Marc Greneche, Institute des Molecules et Matériaux du Mans (IMMM), Université du Maine, France, for recording the 57Fe Mössbauer spectra.
References
- Mat. Chem. Phys.. 1983;8:125-133.
- J. Chem. Phys.. 1986;83:757-764.
- Can. J. Chem.. 1978;56:402-403.
- The Iron Oxides. Structure, Properties, Reactions, Occurrences and Uses. Wiley-VCHGmbh&Co.; 2003. Sed. Edition
- Trans. Faraday Soc.. 1956;52:1642-1650.
- J. Appl. Phys.. 2008;103:104312.
- Adv. Mater.. 2001;13:1158.
- J. Phys. Chem. B. 2008;112:11336.
- Khalil, M. I., October 2012. Process for preparing magnetite (Fe3O4) and derivatives thereof. EP 2 505 558 A 1.
- J. Am. Ceram. Soc.. 1999;82:1937-1940.
- Green Chemistry. An Introduction Text; Royal Society of Chemistry. Thomas Graham House; 2002.
- Laux, J.I.G., 1925, Farbenindustrie DE. pp. 463–773.
- Am. Mineral.. 1967;52:1198-1205.
- Maurice, G.F., Charles, B.W., Paul, E.M., Francis, J. Harvey, 31 January, 1978. Process for the production of magnetite spheres with an arc heater, U.S. Patent 4071588.
- Michael, B., Dimitri, B., Norbert, B., Joachim, C., Peter, G., Klaus, H., Kay-Oliver, K., Thomas, K., Matthias, S., Sebastian, V., Kerstin, W., Christian, G., July 27, 2004, Magnetic Nanoparticles having biochemical activity. Method for the production thereof and their use, U.S. Patent 6, 767, 635 B1.
- Morup, S., Dumesic, J.A., Topsoe, H., 1980. Magnetic microcrystals in application of Mössbauer spectroscopy, in: Cohen, R.L. (Ed.), vol. II, p. 1.
- Naoyoshi, M., Kenzo, H., 4 July, 2000, Black ultrafine magnetite particles and process for preparing the same, U.S. Patent 6083476.
- Magn. Magn. Mater.. 1990;85:233.
- J. Inorg. Nucl. Chem.. 1981;43:1481-1493.
- Angew. Chem.. 1958;12:367-371.
- Phys. Rev. B. 2009;79:205103.
- Inorg. Chem.. 1989;28:4187-4189.
- Z. Elektrochem.. 1929;35:65.
- Shen, H., August 28, 2002, Process for preparing dispersive nano Fe3O4 particles. Publication number: CN 1365951 (A).
- Shouheng, S., November, 2002. Synthesis of magnetite nanoparticles and the process of forming Fe-based, U.S. Patent 6962685 17.
- J. Am. Chem. Soc.. 2002;124:8204.
- J. Colloid Interface Sci.. 2000;221:166-172.
- Tomio, H., Hiroyuki, S., March 23, 1999, Magnetite particles U.S. Patent 5885740.
- J. Am. Chem. Soc.. 2008;130:7542.
- Nanoscale. 2010;2:1027-1032.
- Yoshito, N., Haoren, Z., Takeshi, N., March 13, 1984, Method for preparing magnetite magnetic powder, U.S. Patent 4436681.
- Nanotechnology. 2011;22:485706.







