Translate this page into:
Co-pyrolysis of biomass and waste plastics for production of chemicals and liquid fuel: A review on the role of plastics and catalyst types
⁎Corresponding authors. hamizura179@uitm.edu.my (Hamizura Hassan), chazmier@usm.my (Mohd Azmier Ahmad)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
The increasing global fuel consumption and growing environmental concerns are the impetuses to explore alternative energy that is clean and renewable for fuel production. Converting biomass and plastic waste into high-value fuel and chemicals via co-pyrolysis technique may provide a sustainable remediation to this problem. This review critically discussed the influence of various types of plastic wastes as co-reactant in co-pyrolysis with biomass on the product distribution, synergistic effect, and quality of bio-oil. The outcome of this review revealed that the addition of plastic enhanced the yield and quality of bio-oil and inhibited the production of oxygenated compound and coke formation. Next, the critical role of zeolite-based catalyst (microporous, mesoporous, hierarchical, and metal modified zeolite) and low-cost mineral-based catalyst in upgrading the yield and quality of liquid fuel were compared and discussed. The characteristic, synthesis method, strength, and limitation of each catalyst in upgrading the products were summarized. Hierarchical zeolites can resolve the problems of mass transfer, and diffusion limitation of large molecules into active sites associated with conventional zeolite due to the combination of two levels of porosity. Finally, the potential challenges and future directions for this technique were also suggested.
Keywords
Biomass
Plastic waste
Co-pyrolysis
Zeolite
Low-cost mineral-based catalyst
Bio-oil
1 Introduction
Excessive consumption of fossil fuels and rapid population growth have led to several environmental problems, including greenhouse gas emission, SOx, NOx, acid rain, global warming, and urban smog (Abas et al., 2015; Abokyi et al., 2019; Zhang et al., 2018). Furthermore, the fluctuation of fossil fuels prices and the heavy reliance of energy and chemical sectors on fossil fuels have caused a dramatic increase in demand for alternative, renewable and sustainable energy. Biomasses stand out as a suitable renewable energy source to produce liquid fuels due to their environmental benefits, such as abundant availability, renewability, low cost and carbon neutral (Long et al., 2013). About 220 billion metric tons of lignocellulosic biomass are generated annually worldwide, making biomass the world's largest renewable source of energy (Hassan et al., 2016). Biomass-derived bio-oil can be an alternative to fossil fuels to produce value-added chemical, heat, electricity, and energy (Yaman et al., 2018). In 2016, lignocellulosic biomass constitutes about 70 % of the total primary energy supply, which was equivalent to 56.5 EJ as shown in Fig. 1 (Global Bioenergy Statistics, 2018). Currently, numerous countries have imposed strong policies on the utilization of renewable biofuels. For example, European Union (EU) Commission demands more than 20 % of the entire automotive fuel usage to be consisted of biofuels by 2020. The U.S governmental departments also have set an aim to achieve 25 % of oil-based chemicals and 20 % of transport energy with biofuel-based alternatives by 2030 (Liang et al., 2021).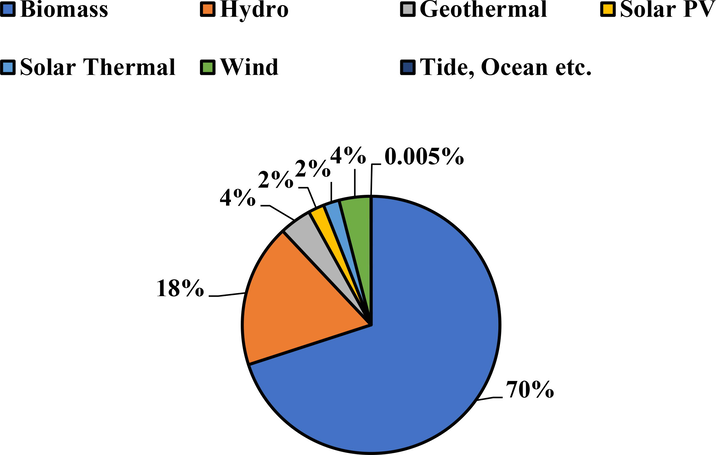
Total primary energy supply of all renewables in 2016 (Global Bioenergy Statistics, 2018).
Over the past two decades, increasing population and consumption have driven a massive increase in plastic demand due to its excellent characteristics of durability, light of weight, easy manufacturing, ease of use and resistance to corrosion. The global production of plastics is expected to expand from 300 million metric tons in 2015 to 1.8 billion metric in 2050 (Lee et al., 2021). In 2020, the global plastic production has reached 370 million tonnes, with Asian region contributing to about half of it (PlasticsEurope, 2020). Plastics including polypropylene (PP), polyethylene (PE), polyethylene terephthalate (PET), polystyrene (PS), and polyvinyl chloride (PVC) are extensively utilized in diverse areas, including packaging, construction, electronics, households, automobiles and others (Wang et al., 2021). The incessant growth of plastics demands has resulted in the increase of plastic solid waste (PSW) deposit every year. Municipal solid waste (MSW) accounts for around 30–35 % of the total plastic wastes in industrialized country (Tencati et al., 2016). At present, the traditional recycling methods, including incineration and landfills pose a serious threat to the environment via water resource pollution, air pollution and damages to marine ecosystems and terrestrial habitats (Ghayebzadeh et al., 2020). In addition, the natural degradation of plastic needs 400 to 1000 years, causing a major negative impact to the environment. Therefore, an alternative approach that can convert the abundant plastic waste into a more value-added product and protect the environment and human health needs to be explored.
Co-feeding hydrogen-rich materials to the oxygen-rich biomass has recently paved the way to upgrade bio-oil quality. The co-pyrolysis process is highly similar to pyrolysis because it can deliver high quality bio-oil, but it involves the combination of two or more feed materials. This technique can compensate the flaws of biomass-derived bio-oil, and provide safe and effective waste treatment (Chen et al., 2020). Hydrogen-rich materials such as plastics, tires and lubricant oil can act as hydrogen donor, increase the hydrogen-to-carbon ratio of feedstock and induce positive synergistic interaction with biomass to enhance the oil quality. The interactions between the intermediates of lignocellulosic biomass and synthetic polymers during co-pyrolysis can produce bio-oil with high carbon yields, high calorific value, aromatic selectivity and hydrocarbon (Dorado et al., 2015; Lu et al., 2018b). Furthermore, co-pyrolysis offers economic advantages since it requires less energy than the pyrolysis of biomass and plastic alone (Chen et al., 2020). Suriapparao and Vinu, (2021) investigated the synergistic effects between biomass (rice husk, groundnut shell, bagasse, mixed wood sawdust and Prosopis juliflora) and hydrogen-rich plastics (Polyisoprene (PIP) and low-density polyethylene (LDPE)). The study deduced that co-pyrolysis significantly boosted the calorific value of bio-oil. The heating value of co-pyrolysis oil varied from 38 to 42 MJ/kg as compared to the heating value of biomass pyrolysis oil of 20 to 28 MJ/kg. In addition, the deoxygenation degree also increased due to the synergistic effects. Rahman et al. (2021) carried out pyrolysis for mixtures of pine and HDPE in a double-column staged reactor and observed that the addition of HDPE to pine could increase the pyrolysis oil yield up to threefold compared to pyrolysis oil of pine alone. In addition, the oil produced was rich in hydrocarbon with 99 % selectivity. Adding the catalyst to the co-pyrolysis process could facilitate the cracking of pyrolysis vapor and deoxygenate the oxygenated compounds via dehydration, decarbonylation and decarboxylation reactions, improving selectivity towards the desired compounds, such as hydrocarbon (Dyer et al., 2021).
The integration of co-pyrolysis and microwave radiation could enhance the yield and properties of liquid fuel product with less energy input in a single step, and prevent the need of an additional upgrading reactor network. Microwave co-pyrolysis technique are advantageous compared to other pyrolysis techniques, including high heating efficiency, uniform heating, energy saving, fast response and better heat and mass transport even with large particle size (Chen et al., 2022; Suriapparao, et al., 2022). Xia et al. (2021) claimed that this technique could be one of the future trends in advanced pyrolysis technique. Microwave co-pyrolysis can resolve the limitations of conventional microwave pyrolysis of plastic, which produces highly viscous bio-oil consisting of heavy hydrocarbon (waxy paraffinic components) that results from the insufficient decomposition of long-chain hydrocarbon (Wan Mahari et al., 2022). The addition of microwave absorbent can enhance the pyrolysis efficiency since the plastic and biomass waste have weak microwave absorption capacity. The bio-oil obtained from microwave co-pyrolysis has high heating value and H/C ratio with low viscosity (Suriapparao, et al., 2020). This technique also accelerates the dehydration reactions with low energy utilization, resulting in bio-oil that has low moisture content and acidity. Nevertheless, the cracking of long-chain molecules into short-chain compounds and the conversion of oxygenated compounds which are achievable through catalytic up-gradation can enhance the product selectivity and properties.
Catalytic co-pyrolysis of biomass-plastic mixture can be a more reliable method compared to the catalytic pyrolysis of single biomass due to the catalyst deactivation resulted from hydrogen deficiency properties of biomass. Catalyst offers an alternative pathway with lower energy requirement for selective product generation. During pyrolysis, catalyst can accelerate the reactions, including cracking, hydrocracking, decarboxylation, alkylation, aromatization, decarboxylation, and Diels-Alder reactions for better product selectivity and quality (Suriapparao et al., 2022). It is critical to deeply understand the characteristics of the catalyst in order to select an appropriate catalyst for an effective co-pyrolysis process. Several comprehensive reviews have summarized the catalytic co-pyrolysis of lignocellulosic biomass and waste plastics. Gin et al. (2021) summarized and discussed the impact of heating systems, experimental conditions, and synergistic effects of the co-pyrolysis of plastic and biomass wastes. In addition, the reaction pathway and the kinetics of the catalytic co-pyrolysis version of the same feedstocks were exclusively presented. In another review, Ryu et al. (2020) summarized the latest progress in catalytic co-pyrolysis of biomass and plastic in terms of feedstock pre-treatment, properties of feedstock and catalyst on the production of the biofuels and desired chemicals, such as aromatic hydrocarbon. However, to the best of the author’s knowledge, a review on the influence of various types of plastic as co-reactant in co-pyrolysis with solid biomass to produce chemicals and liquid fuels is still lacking. The quality and product distribution of co-pyrolysis process depends on the biomass, plastic types and properties, and processing conditions, such as temperature, particle size, residence time, reactor type and catalyst addition. Therefore, this review paper focused on the influence of different types of plastic as the co-reactant in co-pyrolysis with solid biomass on the product distribution, synergistic effect, and quality of bio-oil. Furthermore, this review also provided concise information on the critical role of zeolite-based catalyst (microporous, mesoporous, hierarchical, and metal modified zeolite) and low-cost mineral-based catalyst in upgrading the yield and quality of liquid oil. The characteristics, synthesis methods, advantages, disadvantages, and performance of each catalyst in upgrading the bio-oil through the co-pyrolysis of biomass and plastic were compared in detail. Lastly, the potential challenges and future directions for this technique were also suggested.
2 Plastic as co-reactant in co-pyrolysis with solid biomass
2.1 High-density polyethylene
High density polyethylene (HDPE) is a common waste in municipal solid waste (MSW), with a H/Ceff ratio of 2 (He et al., 2021). HDPE is widely used to produce sturdy bottles, flexible pipes, toys, geomembranes, ropes, cutting boards and others. Due to its less fixed carbon with no oxygen, the addition of HDPE in co-pyrolysis of biomass can lower the formation of coke on catalyst and oxygenated compound in pyrolysis oil. Furthermore, HDPE is favourable for pyrolysis process since it possesses greater than 99 % volatile content with nil moisture (Rahman et al., 2021).
Few studies have reported that the incorporation of HDPE can considerably improve the quality of pyrolysis oil in terms of olefins and monoaromatic hydrocarbon while reducing the undesired product of polycyclic aromatic hydrocarbons (PAHs), which are toxic, carcinogenic, and mutagenic (Chen et al., 2016). He et al. (2021) investigated the co-pyrolysis of HDPE and corn stalk over HZSM-5 using Py-GC/MS in the temperature range and biomass-to-HDPE ratio of 550–800 °C and 1.0–0:1, respectively. The result revealed that the addition of HDPE to corn stalk sharply reduced the oxygenated compounds from 97.02 % to 42.03 %. In addition, significant synergistic effect on condensable volatile organic products (CVOPs) and hydrocarbon was observed as the experimental value of CVOPs and hydrocarbon was higher than the calculated value. During co-pyrolysis, the corn stalk-derived oxygenates could interact with HDPE-derived olefins to form hydrocarbon via Diels-Alder reactions, enhancing the hydrocarbon production while reducing the oxygenated compounds. In addition, the hydrogen atoms transferred from HDPE could promote the hydrocarbon production by enhancing the cracking and deoxidation (decarbonylation, decarboxylation and dehydration) reaction of corn stalk-derived oxygenates to hydrocarbon. Furthermore, the addition of HDPE to corn stalk as co-reactant could inhibit the coke formation by stabilizing the corn stalk-derived oxygenates (lignin-derived phenolic compound) and preventing it from undergoing polymerization on the surface of HZSM-5. A comparable trend was observed by Rahman et al. (2021) who attributed the improvement in gasoline range hydrocarbon to proton supplement provided by HDPE. The highest selectivity of gasoline range hydrocarbon (77 %) was found at pine-to-HDPE ratio and temperature of 50:50 and 550 °C, respectively. The authors also highlighted that the amount of oxygenated compounds reduced from 31.11 % to 0 as the pine-to-HDPE ratio surged from 100/0 to 0/100 because H/Ceff ratio increased as the HDPE ratio in the feedstock increased, promoting the cracking of oxygenated compounds such as phenolic to gasoline-equivalent hydrocarbons (C6-C12).
Yuan et al. (2018) carried out the co-pyrolysis of cellulose and HDPE at different ratios and reported that the synergistic effects in the co-pyrolysis accelerated the generation of small molecule volatiles, including H2O, CO/C2H4, and CO2. The decomposition of HDPE via chain-end and random scission can transfer hydrogen for the decomposition of cellulose-derived anhydrosugars to aldehyde and ketone while cellulose-derived oxygenated compounds, which act as acceptor, promote the scission of HDPE to alkane and alkene groups. During co-pyrolysis, aldehyde and ketone can be further decomposed to hydrocarbon. Fig. 2 shows the reaction mechanism between cellulose and HDPE at different biomass-to-plastic ratio. In co-pyrolysis of biomass with HDPE, HDPE generally provides positive synergistic effect on bio-oil yield (Hassan et al., 2020; Önal et al., 2014; Rahman et al., 2021). Co-pyrolysis of discarded newspaper and HDPE produced more bio-oil and less gas than the theoretical value due to cross reaction between newspaper and HDPE which interfered with the degradation of functional groups attached to the cellulose structure of WP (Chen et al., 2016). This condition inhibited the production of gases of low molecular weight and favoured the production of oil. The highest oil yield of 68.43 wt% was achieved at newspaper-to-HDPE blend ratio of 1:2, and it was 31.59 % higher than the theoretical data based on weighted averages. Positive synergistic effects on fuel properties were also observed in terms of significant reduction of total acid number and viscosity by 216 % and 76 %, respectively. In addition, the quality of bio-oil was also enhanced with maximum hydrocarbon and alcohol yield of 85.88 %, which was obtained at WP:HDPE ratio of 50:50.
Chemical structure of lignocellulosic biomass (a) Cellulose; (b) Hemicellulose; (c) Lignin..
Reproduced with permission from (Hansen and Plackett, 2008; Shahzadi et al., 2014)
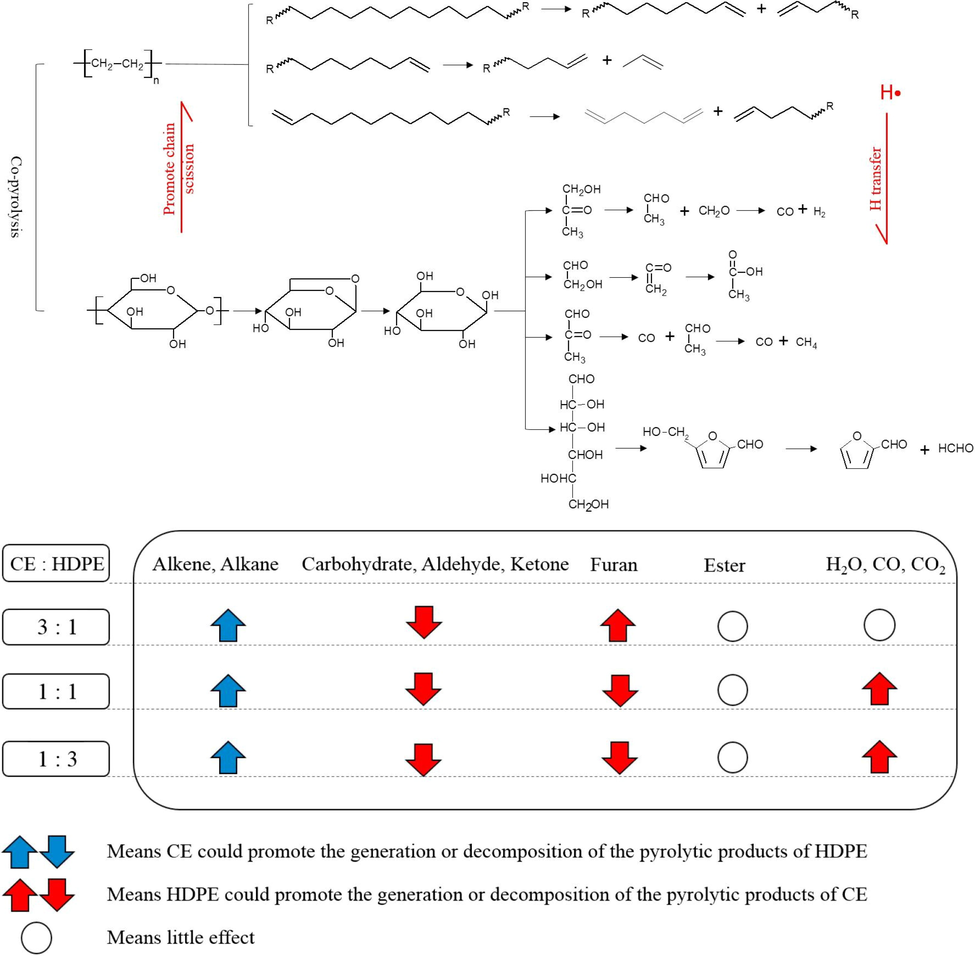
Reaction mechanism between cellulose and HDPE at different biomass-to-plastic ratio.
Reproduced with permission from (Yuan et al., 2018)
2.2 Low density polyethylene (LDPE)
LDPE represents the second biggest portion of plastic waste with the approximate consumption of 415 million in 2015. This value is expected to increase by 4 % in the following years. LDPE is widely used as plastic bags and packaging due to its excellent characteristics of flexibility, ease of processing and low cost (Duan et al., 2021). Compared to HDPE, LDPE has more branching (2 % of carbon atom) and weaker intermolecular forces. Suriapparao and Vinu, (2021) examined the co-pyrolysis of LDPE with five different biomass and found that the experimental bio-oil yield (13.2 – 32.3 wt%) was less than the theoretical value (42–47.5 wt%). Excessive cracking of heavier molecules into lighter gases contributed to low bio-oil yield. Although the yield of bio-oil was low, the heating value of bio-oil (37.6–41 MJ/kg) was better than the theoretical value (32.6–37.8 MJ/kg) due to the interaction between oxygen transfer from condensable phase to gas phase and hydrogen release from LDPE vapours. Substantial progress has been observed in the selectivity of naphthalene derivatives, including methylnaphthalene and 2-methylnaphthalene produced from catalytic co-pyrolysis of LDPE, and cellulose and pine wood. LDPE was a high molecular weight polyolefin, and its pyrolysis and chain scission were incomplete, resulting in the production of larger molecules. Furthermore, the addition of LDPE could inhibit the coke formation during pyrolysis of biomass due to the breakdown of LDPE and biomass via free radical. Compared to LDPE, biomass could decompose earlier due to its poor thermal stability to produce primary radicals. As the temperature increased, the LDPE started to decompose to hydrocarbon that was rich in hydrogen (H) radical. The LDPE-derived H radical could promote secondary decomposition of biomass to generate volatile substances. These volatile substances prevented the LDPE from covering the biomass by melting down at high temperature. Furthermore, the coke growth was also hindered due to the inhibition of free radical polymerization by the precipitation of volatile substances (Zheng et al., 2018).
Al-Maari et al. (2021) studied the co-pyrolysis of empty fruit bunch (EFB) and oil palm frond (PF) with LDPE for bio-oil production. The results showed positive synergistic interaction on the production of aliphatic hydrocarbons and inhibition of oxygenated compounds. The hydrogen released from LDPE enhanced the decarbonylation of carbonyls and sugar, and decarboxylation of acid to hydrocarbon due to oxygen removal via CO and CO2, respectively. In addition, significant synergistic interaction between EFB and PF with LDPE on the production of bio-oil has also been observed. The positive synergistic effect could be attributed to the secondary radical reaction, leading to the condensation of non-condensable fragments. Furthermore, LDPE that acted as the hydrogenation medium for biomass could inhibit the cross-linking reactions and polymerization of biomass, leading to greater biomass weight loss (Aboulkas et al., 2012; Yuan et al., 2018). Co-feeding LDPE and sugarcane bagasse yielded pyrolysis oil which mainly consisted of aliphatic compounds with fewer aromatic compounds as compared to individual biomass with high calorific value of 40 MJ/kg. The addition of LDPE to sugarcane bagasse enhanced the H/C ratio from 1.25 to 1.47 and boosted the formation of saturated hydrocarbon in the range of C6 – C25 (Dewangan et al., 2016).
2.3 Polyethylene terephthalate (PET)
PET is the third largest thermoplastic consumed in Europe after polypropylene and LDPE. PET is usually used in a variety of consumer goods, including synthetic polyester fibres, bottles and films due to its characteristics of clear and strong thermoplastic (Choi et al., 2021; Dhahak et al., 2020). Özsin and Pütün, (2018) investigated the co-pyrolysis of PET blended with peach stones and walnut shells using a fixed bed reactor, and observed increased ester and acid compounds and decreased phenolic compound. Maximum acid and ester yield of 65.87 % and 63.11 % were achieved in co-pyrolysis of PET with walnut shells and peach stones, respectively. The liquid was dominated by benzenecarboxylic acid with more than 40 % yield for both co-pyrolysis blend. Benzenecarboxylic acid and vinyl benzoate were formed when the ester link of carboxylic group was broken via beta scission, initiating the decomposition of PET. One of the biggest challenges regarding pyrolysis oil from PET is the high acid content such as benzoic acid. The acidic characteristic of pyrolysis oil can lead to corrosiveness, depreciating the fuel quality. In addition, benzoic acid can clog the pipelines and heat exchanger, triggering issues during operation at industrial scale (Lee et al., 2017). Despite the disadvantages, it is noteworthy that benzoic acid is a valuable precursor/feedstock for various industries (Çepelioǧullar and Pütün., 2014).
Chen et al. (2017) investigated the synergistic interaction effects on char morphology and thermal behaviour during co-pyrolysis of PET with paulownia wood (PAW) using TGA. Their result showed a remarkable deviation between the experimental and calculated value on volatile release. Higher char yield was obtained from the PAW/PET blends at final decomposition temperature of 530 °C with ΔW above zero. In addition, the char yield increased as the PET blending ratio increased. With the increment of PET ratio in the feedstock, more cross-link reaction between PET-derived products and PAW-derived char occurred, leading to greater char production. The PET decomposition played a role as a limiting factor for the cross-linking reaction. Meanwhile, the addition of PET to PAW resulted in the agglomeration morphology of char. Ablative surface and granule cohesion were observed on the char topography as the PET blending ratio increased due to the reaction between PET decomposition products and PAW-derived initial char. PET typically decomposed at temperature between 370 °C and 460 °C. Non-catalytic pyrolysis of PET produced a liquid containing terephthalic acid and benzoic acid along with CO and CO2 gas whereas co-pyrolysis of PET and biomass formed mainly acid and esters. The upgraded bio-oil from catalytic co-pyrolysis of biomass and PET demonstrated high content of aromatic compounds in the range of C5-C12 of carbon number fuel range (Dyer et al., 2021).
2.4 Polycarbonate (PC)
Polycarbonates (PC) are a group of thermoplastic polymers that contain carbonate groups in their chemical structures. PC is a prominent engineering plastic due to its characteristics, such as high impact strength, superb thermal resistance, and exceptional electrical insulation properties; and it is widely used in automobile industry, building and construction, and data storage devices such as compact disc and DVDs (Antonakou et al., 2014; Bai et al., 2020). In 2017, the global PC production has reached 6 million metric tons (Do et al., 2018). PC is unrecyclable due to its superior opposition to chemical attacks and difficulty to be extracted from the waste stream. Landfilling PC could pose environment threats due to the leaching of bisphenol. Bisphenol A (BPA) and diphenyl carbonate (DPC) substance contained in PC are regarded as endocrine disruptors that cause serious illnesses, including cancer, threaten adult health and interfere with infant hormones (Bai et al., 2020).
Liu et al. (2021) researched the co-pyrolysis of pinewood blended with PC to determine the synergistic effect. The extent of synergistic effect was determined via comparison between the experimental result of co-pyrolysis of pinewood-PC mixture with the weighted average values from individual feedstock pyrolysis. Positive synergy between pinewood and PC was obtained due to the enhancement of H2, CO and total syngas yield of 33 %, 36 % and 19 %, respectively, compared to the theoretical value from individual pyrolysis. However, negative synergistic effect was noticed in the formation of CnHm. The variation in synergistic behaviour of different gas components could be attributed to the interactions between PW and PC intermediates during co-pyrolysis, producing more oxygenated compounds (alcohols, carboxylic acids, and aldehydes) with less hydrocarbons. In addition, co-pyrolysis of PW and PC remarkably enhanced the gas production yield (from 67.6 wt% to 77.2 wt%) but reduced the tar and char yield compared to the theoretical values from individual feedstock pyrolysis. This phenomenon suggested that the synergistic effects of co-pyrolysis of PW and PC involved both mutual interaction of volatile in gas phase and volatile-solid interaction which enhanced the total conversion of solid feedstock to gases. The pyrolysis of PC tends to generate more phenol via oxygen removal as CO and CO2 (Burra and Gupta, 2018). Decomposition of PC mainly occurs via chain scission mechanism which can be divided into two main reactions: primary step in cyclic oligomers production by an intramolecular exchange reaction and hydrolytic cleavage of the carbonate group, generating hydroxyl-terminated oligomers and CO2 at 400 to 500 °C temperature (Jin et al., 2016). Blending pinewood (PW) with PC could enhance this pathway, and stable phenolic intermediates could be formed with the lignin portion, enhancing the breakdown and conversion of PW to low molecular weight aromatics that exist as volatiles, and decreasing the char formation at about 10 % (Burra and Gupta, 2018). On the other hand, addition of lignin to PC pyrolysis can escalate the decomposition of PC to phenolic type compounds by enhancing the release of C⚌O during co-pyrolysis while inhibiting the aromatic compound (Jin et al., 2016).
2.5 Polyvinyl chloride (PVC)
Polyvinyl chloride (PVC) is widely used in the production of cable and wire insulation, fashion and footwear, packaging, window frames, and water pipes. PVC has a longer lifespan than other packaging plastics. About 44.3 million metric tons of PVC was produced globally in 2018, and by 2025 the world’s market size of PVC is expected to grow to nearly 60 million metric tons (Statistica.com, 2021). PVC is the main source of chlorine in municipal solid waste (MSW) and one of the problematic plastics in the feed. Its presence in the feedstock is limited to less than 5 % and generally around 1 to 2 %. The release of chlorinated hydrocarbons and HCl in PVC results in corrosion in the reactor and renders the oil halogenated (Qureshi et al., 2020). As there is no public recycling system for PVC, the proportion of the recovered PVC is relatively low. Moreover, PVC needs to be treated using hydrochloride scrubber for PVC cracking as chloride is not desired in the fuels (Xue et al., 2017).
Özsin and Pütün, (2018) analysed the synergistic effects during co-pyrolysis of PVC with two solid biomasses (walnut shell and peach stones). Negative synergistic interaction on the liquid yields were observed as the liquid yield during the co-pyrolysis (14.70 – 17.60 wt%) was lower than the aggregate values (17.21 – 18.64 wt%). On the other hand, positive synergistic effect was observed with higher aromaticity of tars in co-pyrolysis yields than biomass alone. 1H NMR result showed that both aromatic protons comprised of guaiacyl units (ArH and HC = C-(conjugated)); and ɑ-hydrogen atoms of the branched chain of aromatic ring carbons, methoxy and aliphatic hydroxyl were increased when PVC was added into the biomasses. Polyenes condensation and aromatization during PVC decomposition contributed to the enhanced formation of tars aromatic. It has been well established that chlorine radicals generated during PVC decomposition could initiate condensation reaction, cyclization and aromatization. In addition, considerable value of PAHs was observed during co-pyrolysis of PVC with walnut shell (64.40 %) and peach stones (59.06 %). The decomposition of PVC favoured aromatization reaction and creation of heavier tar compounds via dichlorination, followed by inter-molecular chain transfer; the aromatic chain scission generated two or three aromatic-ring side chain before the coke deposition. HCl release during co-pyrolysis of PVC blends escalated the progression of light tar portions to heavy portions, resulting in the generation of higher molecular weight substances, such as PAHs (Tang et al., 2018).
The addition of PVC could instigate the decomposition of pinewood (PW) at lower temperature range due to the acceleration of PW decomposition by HCl from the dehydrochlorination of PVC. In addition, the co-pyrolysis of PW and PVC yielded more char and less liquid compared to the theoretical data. HCl generation from PVC at lower temperature range (230–300 °C) promoted the dehydration of cellulose to aldehyde compound which was confirmed from the cleavage of glycosidic units. The hydrogen and oxygen atoms in cellulose were lessened due to the dehydration at low temperature, leading to higher char yield. Furthermore, the dehydration also reduced the tendency of depolymerization, consequently reducing the liquid yield. Furthermore, the PW-derived solid char could also act as a catalyst owing to the presence of some inorganics, such as Cao, K2O and NaO that promoted the secondary cracking of PVC oil to generate more char and gas (Lu et al., 2018a). The presence of PVC could influence the reactivity and activation energy of lignocellulosic biomass. The magnitude of reactivity of co-pyrolysis of cherry seed (CS) and PVC was nearly-two orders higher than the pyrolysis of CS at all heating rates. This observation was credited to the chemical structure of PVC which contained high electronegative chloride ions. The activation energy of co-pyrolysis of CS/PVC fell between CS and PVC value. The deviation between theoretical and experimental value of activation energy signifies the occurrence of synergistic effect between CS and PCV during co-pyrolysis (Özsin and Pütün, 2019).
2.6 Polystyrene
Generally, the addition of PS to biomass can enhance the liquid yield while decreasing the gas and char yield. (Stančin et al., 2021) reported that an addition of 25 % of PS to sawdust (SD) could double the yield of pyrolysis oil from 31 % to 62 %, specifically on the expense of gas formation, indicating the occurrence of synergistic effect in the process. Moreover, blending 25 % of PS with SD could enhance the quality of bio-oil in terms of reduction of oxygenates and PAHs while promoting the aromatic hydrocarbon. However, when the ratio of PS exceeded 25 %, a higher generation of undesired benzene derivatives and toxic PAHs became noticeable due to the secondary cracking of PS-derived styrene monomer accelerated by the interaction with biomass feedstock. Benzene derivatives in bio-oil limit its further utilization since such compounds are categorized as carcinogenic.
Samal et al. (2021) examined the co-pyrolysis of eucalyptus biomass and polystyrene waste on the physiochemical and thermal characteristic of the solid char. Two distinct physiochemical and thermal characteristics of char have been observed basically at temperature below and above 450 °C. The char generated below 450 °C has high heating value and volatile content with low fixed carbon because of the polystyrene coating on the char surface. The melting polystyrene waste could deposit over biomass at temperature below 450 °C, go through volatilization with additional increase in temperature, and be transformed to liquid oil and syngas. Solid fuels with high volatile content and low fixed carbon generally possess low ignition and burnout temperatures and a higher mass-loss rate, making them unstable. However, the increased high heating value due to the existence of waste plastic coating could ease in enhancing the combustion efficiency of the fuel. In contrast, the produced chars at temperature 450 °C and above possessed more high heating value and fixed carbon with low volatile content. This kind of solid fuel demonstrates superior combustible behaviour with broader temperature range and longer time for complete combustion, all of which signify an excellent solid fuel.
The addition of PS enhances the yield and property of pyrolysis oil. In contrast to pyrolysis oil from biomass (Mahua seeds) alone, the addition of 20 wt% of PS in co-pyrolysis enhanced the liquid yield from 39.26 wt% to 45.89 wt%(Mishra and Mohanty, 2020). At 20 % blending ratio, the plastic could have a maximum synergistic interaction between particles which subsequently maximize the generation of hot volatiles that could be further transformed to liquid form. At this state, greater heat and mass interaction happened between biomass and plastic particles. However, at 10 wt% and 30 wt% blending ratios, the interaction between biomass and plastic particles created negative synergistic effect, reducing the formation of hot volatiles and the production of liquid oil. Furthermore, the higher plastic ratio in the feedstock could cause the plastic melting, which would coat the biomass surface, eventually creating resistance for the discharge of hot volatiles and reducing the liquid yield. The NMR study showed the increment of aromatic and olefinic percentage in the co-pyrolysis oil (as confirmed in the FTIR diagnostics showing the peak of 1650 cm−1 –1580 cm−1 attributed to C⚌C stretching vibration). Meanwhile, the GC–MS results revealed that an addition of 20 wt% of PS as co-reactant substantially enhanced the hydrocarbon compounds and reduced the oxygenate derivatives such as acid, making it attractive compared to thermal pyrolysis oil. However, further upgrading technique is needed due to higher viscosity value than diesel fraction. Van Nguyen et al. (2021) examined the co-pyrolysis of waste PS and coffee-grounds at various blending ratio of 75:25, 50:50, and 25:75. The results revealed that co-pyrolysis could accelerate the deoxygenation reaction, causing a reduction of oxygenated compounds and enhancement in carbon content. The effect was strongest at the PS ratio of 75 % with reduction of oxygen content to 5.68 wt%. This condition contributed to an improvement in the calorific value (39.66 MJ/kg) of pyrolysis oil which was comparable to the heating value of conventional fuel. Table 1 shows the yields and quality of bio-oil obtained from co-pyrolysis of various biomasses and plastics.
Biomass
Plastic
Temperature (°C)
Biomass to plastic ratio
Bio-oil Yield (%)
Remarks
Reference
Pine sawdust (PS)
HDPE
450, 500, 550
0:100, 25:75, 50:50, 75:25, and 100:0
16.7 % to 30.5 %
At 500 °C and pine-to-HDPE ratio of 25:75, the bio-oil yield was 22.5 % which was more than threefold of pine pyrolysis. The selectivity of gasoline range hydrocarbon is increased as the pine-to-HDPE decreased from 100:0 to 0:100. As the HDPE fraction increased from 0:100 to 100:0, the selectivity of hydrocarbon is increased from 68.9 % to 100 % while the selectivity of oxygenated decreased from 31.10 % to 0 %.
(Rahman et al., 2021)
Sugarcane Bagasse (SCB)
LDPE
350 to 600
9:1, 3:1, 1:1 and 1:3
The maximum liquid yield was 52.75 % at 500 °C with 1:1 blending ratio
The increase in H/C ratio from 1.25 to 1.47 due to addition of LDPE enhance the caloric value of bio-oil from 35 to 40 MJ/kg while decreased the oxygen content from 26.15 % to 15.50 %.
(Dewangan et al., 2016)
Empty fruit bunch (EFB) and Oil palm frond (PF)
LDPE and PP
PF: LDPE = (5 1 0), EFB: LDPE = (5 2 0), EFB: PP = (5 4 0) and PF: PP = (5 4 0)
1:1
EFB: LDPE = 67.10 PF: LDPE = 65.00 PF: PP = 54.70 EFB: PP = 59.80
The quality and quantity of bio-oil is highly depended on the biomass-plastic pairs. All pairs decreased the oxygenated compounds. The hydrogen supplement from plastic promoted the decarboxylation of acids and decarbonylation reactions of carbonyls and sugars to hydrocarbon.
(Al-Maari et al., 2021)
Lignin (L)
PE, PP, PS and PC
500
1:1
L/PE = 43.6 L/PS = 51.6 L/PP = 42.7 L/PC = 38.8
Co-pyrolysis of lignin with PE and PP favour the formation of water as compared to PC [(L/PE = 15.9 %), (L/PP = 20.1 %), (L/PC = 11.8 %), (L/PS = 6.4 %)]. Pyrolysis of PE and PP involves hydrogen transfer reaction that impede the decomposition of oxygen-containing functional group linked to the aromatic structures in lignin which favoured the formation of water.
(Brebu and Spiridon, 2012)
Cotton stalks (CS), hazelnut shells (HS), sunflower residue (SFR)
PVC and PET
500
1:1
CS: PET = 25.78 SFR: PET = 25.56 HS: PET = 29.89 The bio-oil for biomass-PVC blend were lower that biomass-PET blends.
Addition of PET to biomass enhanced the bio-oil yield as compared to pyrolysis yield of individual material while opposite trends were found for biomass-PVC mixture. The structure of PET which is based on the benzene ring could induce greater synergistic effect for bio-oil production. However, PVC decompose mainly as gas at high temperature which resulted in a decline in the oil yields but increase in the gas yields of biomass-PVC mixtures.
(Çepelioǧullar and Pütün., 2014)
Sawdust (SD) (oak, poplar, and fir wood)
PS
100–600
25:75; 50:50. 75:25,
The highest liquid yield of 83.86 was obtained at 25:75 blending ratio.
Addition of PS significantly enhanced the quality and quantity of bio-oil. 25 % of PS could increase the bio-oil yield from 32 % to 62 %, reduced the oxygenated compounds and promoted the formation of hydrocarbon. The bio-oil produced composed of 69.10 % of gasoline range compounds (C4 – C12).
(Stančin et al., 2021)
3 Catalyst
Employing suitable catalyst in co-pyrolysis is beneficial to the thermochemical decomposition of biomass and plastic by tailoring the products composition and lowering the activation energy of the reaction. The benefits of catalyst addition in the degradation process include shortening the reaction time, lowering the degradation temperature, promoting the extend of degradation, reducing the amount of solid residue in final products and narrowing the product distribution (Antonakou et al., 2014). In addition, the catalyst helps to direct the reaction toward the desired products via interactions between its structure, and the reaction pyrolyzates and products (Rocha et al., 2020). The effectiveness of a catalyst depends on its acidic characteristics, redox properties, and porosity. Tuning the catalyst acidity based on its density, strength, and type is vital in designing the catalyst as each of these elements have particular influence on the activity, product selectivity and reaction pathway (Antonakou et al., 2014).
3.1 Microporous zeolite
Zeolite is recognized as the most efficient catalyst to produce high-value chemicals because of its high acidity, high specific surface area, high adsorption ability and shape selectivity (Han et al., 2020; Ryu et al., 2020). Its unique pore structure with strong acidity favours aromatic selectivity with excellent cracking and deoxygenation ability (Hassan et al., 2016). The acidity of zeolite which is expressed by the Si/Al ratio determines their reactivity and affects the end products of pyrolysis process with low ratio, indicating high acidity (Chi et al., 2018). Generally, the introduction of microporous zeolite in the pyrolysis is usually favourable to enhance the aromatic production.
It is well established that the introduction of microporous zeolite in the pyrolysis is favourable to enhance the aromatic production. Park et al. (2019b) investigated the co-pyrolysis of Quercus variabilis (Q. variabilis) and waste plastic films (PFs) over two microporous zeolites (HZSM-5 and HY) of different acidity and surface area. The acidity (SiO2/Al2O3) of HZSM-5 and HY zeolite was adjusted to 30 and 23, respectively. The result showed that HZSM-5 with higher and stronger acidity could enhance the aromatics production than HY catalyst during the co-pyrolysis at 600 °C due to higher cracking efficiency of pyrolyzates. In addition, more appropriate shape selectivity of HZSM-5 which has medium pore size, appropriate pore window size and internal pore volume together with steric hindrance characteristic could favour the production of aromatics (Jae et al., 2011). On the other hand, higher formation of coke was observed for HY catalyst due to the more space provided as it had higher surface area (780 m2/g) than HZSM-5 (425 m2/g). In contrast, Kim et al. (2016) observed greater aromatic production over HZSM-5 catalyst at high temperature and catalyst-to-reactant ratio compared to HY catalyst during catalytic co-pyrolysis of cellulose-PP/LDPE mixture. HZSM-5 which had strong acidity was advantageous for aromatic production while high catalyst-to-reactant ratio of 1:10 could provide a large number of active sites for aromatization reaction. The authors emphasized that the properties of catalyst, specifically acidity and pore size, are crucial in determining the aromatic production efficacy during catalytic co-pyrolysis reaction. On the other hand, low temperature and less catalyst-to-reactant ratio were applied for HY catalyst since the reaction intermediates could diffuse easily into its pore and make intimate contact with active sites to undergo further reaction to form aromatic.
Coke deposition and limitation of mass transfer and reactant flow diffusion are among the major challenges of pyrolysis over microporous zeolite (Kim et al., 2017b). The small pore size (less than2 nm) of microporous HZSM-5 zeolite inhibits the diffusion of large biomass and plastic reaction intermediates produced during the initial stage of pyrolysis into its internal acidic sites. The large molecules of biomass and plastic pyrolysis intermediates formed during the initial stage of pyrolysis cannot pass through the inner pores and contact the active sites of HZSM-5 since their kinetic diameter is greater than the pore size of ZSM-5 (Hassan et al., 2019). Furthermore, pore blockage from polymerization and polycondensation reactions due to acidic properties of zeolite causes deactivation of the catalyst and reduces the catalyst lifetime. Shao et al. (2017) reported that the parallel side reactions of anhydrosugars, furans, and other organic molecules in the hydrocarbon pool could lead to the coke formation on the interior surface of zeolite while Custodis et al. (2014) stated that the competing side reaction of phenol repolymerization and lignin polycondensation could cause the coke deposition.
3.2 Mesoporous zeolite
Mesoporous zeolite catalysis has been recognized as an efficient approach to attenuate the diffusion restriction of bulky biomass and plastic molecules and expand the production of aromatic hydrocarbon through the larger pore size. High surface area of mesoporous zeolite provides greater access to active sites and enhance the catalytic interaction between the co-pyrolyzed reactants, causing higher conversion rate of oxygenates to aromatic hydrocarbon. Hong et al. (2017) reported the influence of microporous and mesoporous HZSM-5 during co-pyrolysis of cellulose and polypropylene on the aromatic formation efficiency. The result showed that mesoporous HZSM-5 by ZSM-5 desilication could offer better catalytic activity than microporous HZSM-5 in term of aromatic yield. Larger pore opening obtained by desilication can enhance the diffusion of bulky intermediates to active sites of catalyst to undergo further reactions to aromatics. In addition, mesoporosity can be allocated into the zeolite core-structure via post-synthesis treatments, such as steaming and leaching with acidic or basic media (Zhu et al., 2013).
MCM-41 is a type of mesoporous zeolite that has bigger pore size, making it suitable for adsorption, separation and macromolecular catalysis. Its larger pore size could ease the diffusion limitation in pores. MCM-41 could provide enough active sites for adsorption and catalytic reaction due to its high specific surface area greater than 1000 m2/g. Chi et al. (2018) conducted co-pyrolysis of cellulose and PP in the presence of MCM-41 and Al-MCM-41. The cracking of oxygenated compounds was heightened by the strong acidity originated from the inclusion of Al onto the mesoporous MCM-41. The results indicated that the production of olefins and aromatics were enhanced by using Al-MCM-41, inferring that Al-MCM-41 had superior cracking and deoxygenation effect. The aromatic formation during the co-pyrolysis was governed by internal acid sites, hydrocarbon pool, and Diels-Alder reaction (Fig. 3). Cellulose was decomposed earlier compared to polypropylene as it had lower decomposition temperature. Numerous oxygenated compounds and penta heterocyclic furans were produced via ring cleavage and catalytic cracking to break its hexa heterocyclic, followed by dehydration and cyclization. During the catalytic co-pyrolysis, olefin was produced from direct cracking of polypropylene via carbonium ion and β-scission and deoxygenation of oxygenated compounds at acid sites via dehydration, decarbonylation, and decarboxylation reactions. These intermediates (olefins and oxygenates) participated in deoxygenation and oligomerization to form carbocation hydrocarbon pool where the aromatic and olefins were formed. Along with hydrocarbon pool mechanism, the monocyclic aromatic hydrocarbon can be formed via Diels-Alder reaction between cellulose-derived furans and polypropylene-derived olefin.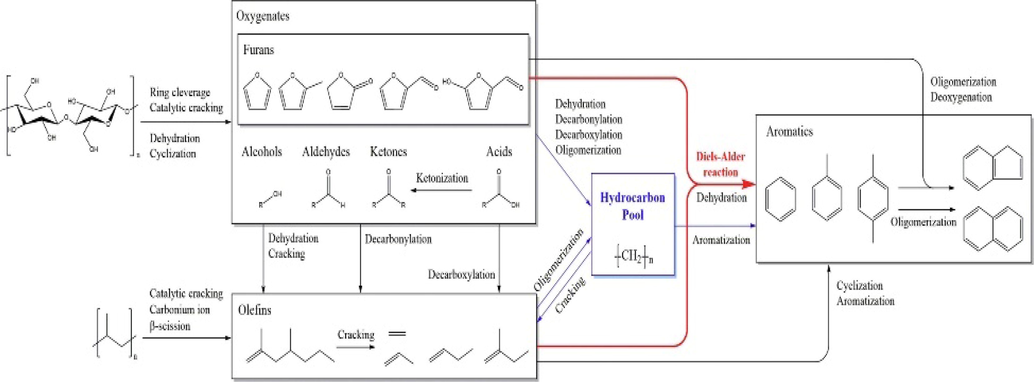
Chemical mechanism of catalytic co-pyrolysis of cellulose and polypropylene over Al-MCM-41.
Reproduced with permission from (Park et al., 2019)
Kim et al. (2017c) investigated the impact of acidity and molecular diameter on the formation of aromatic hydrocarbon in co-pyrolysis of carbohydrates with linear LDPE. They assessed the catalytic activity of microporous and mesoporous ZSM-5 with high mesoporosity and poor acidity Al-SBA-15. Higher yield of monoaromatic hydrocarbons was obtained under catalysis of ZSM-5 due to the combination of micropores and mesopores structure. This framework is suitable for the shape selectivity of aromatic production and to improve diffusivity of bulky molecular pyrolysis intermediates into the catalyst pore. The finding of this study indicated that catalyst with higher acidity together with an appropriate structure and pore diameter was an ideal catalyst for aromatic formation in co-pyrolysis reaction. Similar trend was found in the catalytic co-pyrolysis of yellow poplar and HDPE over three types of mesoporous catalysts, including hierarchical mesoporous MFI, hierarchical mesoporous Y, and Al-SBA-15 (Rezaei et al., 2017). Hierarchical mesoporous MFI which had large mesopores and strong acidity delivered the highest yield of olefins and aromatic hydrocarbons attributable to the efficient hydrocarbon pool mechanism. The yield of solid residue (char/coke) decreased for all three types of mesoporous catalyst. The lifespan of catalyst could be enhanced by reducing the coke deposition.
3.3 Metal modified zeolite
Metal addition could modify the textural characteristics and acid sites, and enhance thermal stability of the catalyst. This process aids in decreasing the rate of coke growth over the catalyst and enhancing the liquid production (Botas et al., 2014; Iliopoulou et al., 2012). Razzaq et al. (2019) observed that anchoring of Ni, Co, Zn and Fe oxides onto the HZSM-5 framework by wet impregnation technique could reduce the coke yield by 50 % compared to intrinsic HZSM-5 during the co-pyrolysis of wheat straw and polystyrene. This was due to the moderate acidic strength of metal-modified zeolite which was helpful in decreasing the coke formation over the zeolite. In addition, pyrolytic oil catalysed over metal-modified zeolite contained relatively higher organic phase yield instead of aqueous phase as compared to unmodified HZSM-5. The presence of metal-modified zeolite could enhance the decarboxylation and decarbonylation while inhibiting the dehydration reaction. French and Czernik, (2010) reported that incorporation of metal sites onto the zeolite framework could alter the deoxygenation pathway so that it favourably released more oxygen in the form of carbon monoxide instead of carbon dioxide and water, thereby offering more hydrogen available for aromatic production. The presence of metals boosted the aromatic selectivity towards high value mono-aromatic hydrocarbon (MAHs) and supressed the formation of oxygenated compounds.
Kim et al. (2017b) investigated HZSM-5, mesoporous MFI, Pt/mesoporous MFI and Al-SBA-16 catalyst effect for the Laminaria Japonica and PP co-pyrolysis. Pt/mesoporous MFI showed higher aromatic yield and oxygenate removal efficiency than the other catalysts due to the strong Brönsted acid sites and large pore size as well as catalytic effect resulted from the incorporation of Pt. Pt promoted the cracking and deoxygenation of oxygenated compounds to aromatic. The authors also highlighted that the strength of acidity played more essential role than the pore size in the production of aromatic hydrocarbon. Coupling of weak acid sites and large mesopores lowered the catalytic performance of Al-SBA-16. Conversely, mesoporous Al-SBA-15 with weak acidity showed a better oxygen removal efficiency than HZSM-5, concluding that the pore size played an important role during the cracking of large oxygenate molecules.
Impregnation of phosphorous onto the zeolite framework could enhance the hydrothermal stability and anti-coking properties of zeolite and ease the transformation of alkane to olefin, which was subsequently converted to aromatic. Yao et al. (2015) found that the modification of ZSM-5 with phosphorous (P) and nickel (Ni) increased the production of valuable aromatic hydrocarbons and olefins in the catalytic fast co-pyrolysis of pine wood and LDPE due to the enhanced zeolite’s Lewis acid sites which acted as electron pair acceptor and which had a high tendency to accept the hydride ions generated during the conversion of alkanes to olefins. Higher content of aromatic hydrocarbon boosts the commercial value of bio-oil as the aromatic compound is vastly used as additives in transportation fuel and feedstock materials in the petrochemical industry (Kim et al., 2017a). In addition, the rate of coke-induced catalyst deactivation, which is the main concern in catalytic fast pyrolysis, has also been reduced due to the impregnation of ZSM-5 with P and P/Ni cation. The incorporation of P and Ni onto the ZSM-5 significantly decreased the strong Bronsted acid sites of zeolite, in turn reducing the coke deposition. Gallium (Ga) altered the texture characteristic and acidity of zeolite by reducing the pore volume and surface area of zeolite (Li et al., 2015). Ga was introduced into the zeolite framework via incipient wetness impregnation. The Ga decreased the density of Brönsted acid sites due to the replacement of some Brönsted acid sites by Ga. Ga-containing zeolite substantially increased the production of olefin and/or monoaromatic hydrocarbons at the expense of less valuable alkane during the catalytic co-pyrolysis of pine wood and LDPE. Non-framework Ga provided a new route for dehydrogenation of alkane to olefin, which is subsequently converted to aromatic.
3.4 Hierarchical zeolite
In an effort to enhance the catalytic activity of the zeolite catalysts, the incorporation of hierarchical porosity or alteration through metals and oxide supplement has been frequently reported (Han et al., 2020; Jin et al., 2016; W. Wang et al., 2019). Although the mesoporous materials are synthesized to solve the problem of diffusion limitations, it has poor surface acidity and unstable structure property, bringing about unsatisfactory activity in acid-catalysed reactions. To solve this shortcoming, researchers have combined the advantages of microporous molecular sieve and mesoporous material, producing zeolites with hierarchical micro-mesoporous composite (Talebian-Kiakalaieh and Tarighi, 2020). It works in the way that the external mesopores capture molecules in several directions and concentrate them towards the zeolite micropores. The mesoporous structure could enhance mass transfer and cracking of large molecular pyrolysis vapours, which are hard to diffuse into the microporous zeolite (Kim et al., 2019). Furthermore, every mesopore behaves as a funnel and enables the effective penetration of molecules within the narrow one-dimensional micropore system. Such a combination of the properties of both porous systems would make the hierarchical aluminosilicates a versatile material for many applications. Five different approaches to synthesize hierarchical micro-mesoporous include recrystallization of ordered mesoporous silicas, zeolite-seeding, mesoporous carbon templating during crystallization, alkaline extraction of zeolites, and combining mesostructure and microstructure-directing agents (Enterría et al., 2014).
Several studies reported that the hierarchical zeolites could substantially resolve the limitations of the conventional zeolite, such as low mass transfer problem, deactivation of catalytic activity and low activity to bulky substrates in different chemical reactions due to significant deoxygenation and excellent aromatic selectivity (Ahmed et al., 2020; Chi et al., 2018). Moreover, Song et al. (2018) mentioned that more advantages from hierarchical zeolites could be observed, such as shortened diffusion path length, abundant external acid sites and surface area, and excellent hydrothermal stability. Combination of mesoporosity and traditional zeolites of hierarchical zeolite could broaden its application in catalysis.
The catalytic activity of hierarchical zeolite is mainly dependent on the synthesis method. Desilication (removing silica) and dealumination (removing aluminum) are an efficient approach to generate mesoporosity though it may result in a considerable shift in acidic properties (Ahmed et al., 2020). The alteration of zeolite structure during desilication and dealumination of zeolite is shown in Fig. 4. Proper acid sites distribution and mesoporosity resulted from the alteration in acidity could benefit the reaction pathway and intermediates stabilization as reported by (Hong et al., 2017). The desilicated ZSM-5 showed superior catalytic activity in term of aromatic selectivity (33.50 wt%) compared to parent ZSM-5 during co-pyrolysis of cellulose and polypropylene. The treatment enlarged the pore for better diffusion while retaining its strong acidity. The desilication enhanced the weak acid sites, thus improving the liquid products yield. In addition, the weak acid sites also fostered the deoxygenation of furan via reaction with olefins to produce more aromatics. Hierarchical zeolite has great potential in catalytic reactions related to bulky molecules due to the presence of microporous and mesoporous structure (Lv et al., 2020). A hierarchical pore structure could be created in ZSM-5 by including larger pore structures namely mesopore linked to the core microporous framework as an endeavour to inhibit the coke deposition and attain higher transformation of bulky oxygenates (Feliczak-Guzik, 2018). The mesoporous structure could enhance mass transfer and cracking of large molecular pyrolysis vapours, which were hard to be diffused into the microporous zeolite (Kim et al., 2019). Alkaline treatment is well-known and established as a post-synthetic technique comprised of fractional desilication of the zeolite structure to create secondary mesopores in ZSM-5 with bigger pore opening and outer surface area (Li et al., 2014). Apart from alkaline treatment, re-assembly aided with organic templating agent permits restructuring and redeposition of silicate and aluminosilicate fragment into the mesoporous material while maintaining the weight and/or acidity in basic medium (Chen et al., 2018).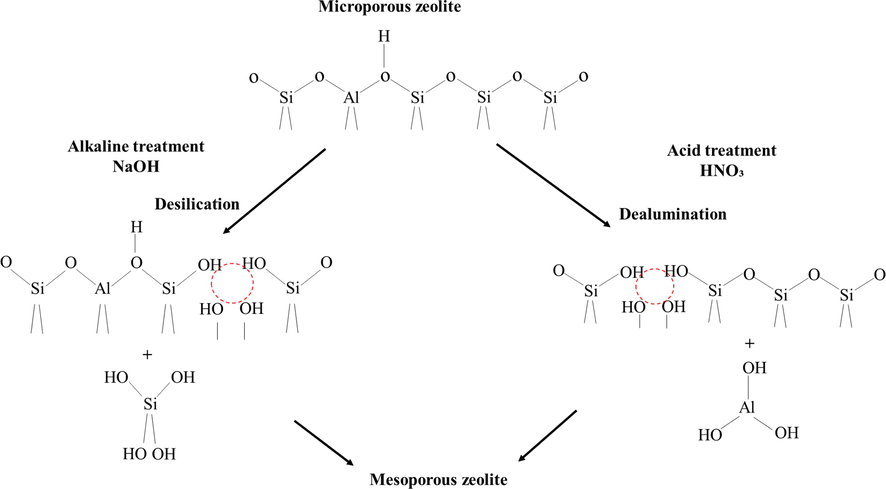
Desilication and dealumination method.
Reproduced with permission from (Feliczak-Guzik, 2018)
Lin et al. (2021) developed a series of hierarchical HZSM-5 with various alkaline solutions ranging from 0.2 to 0.4 mol/L and found that low alkaline solution (≤0.3 mol/L) accelerated the formation of monoaromatics from 63.79 % catalysed by HZSM-5 to 71.75 % for 0.3-HZSM-5 while higher alkaline solution diminished the framework of HZSM-5, leading to reduction of aromatic production. The alkaline treatment enhanced the mesoporosity of the zeolite so that the larger intermediates including oxygenated compounds and aliphatic hydrocarbons could effortlessly access the acid sites of hierarchical zeolite to form aromatics. Furthermore, the alkaline treatment reduced the polyaromatic hydrocarbons (PAHs) formation due to shorter diffusion path distance of molecules in the hierarchical HZSM-5 zeolites, retarding the secondary polymerization reactions of mono-aromatics inside the catalyst channel. Conversely, the selectivity of aliphatic hydrocarbons and oxygenated compounds were reduced as the alkaline concentration reached 0.3 mol/L, probably due to the conversion to aromatics at the catalyst pores via a series of reactions.
Li et al. (2020) investigated the catalytic fast co-pyrolysis of waste greenhouse plastic films and rice husk over hierarchical micro-mesoporous zeolite with HZSM-5 as core and MCM-41 as shell (HZSM-5/MCM-41). The result showed that the relative content of hydrocarbons and CO2 were higher than for the non-catalytic pyrolysis, suggesting that HZSM-5/MCM-41 promoted the conversion of pyrolyzates to aromatic and decarboxylation becoming one of the routes that governed the conversion. The addition of MCM-41 mesopore around the HZSM-5 crystal particles assisted in cracking the large-molecular weight volatile to small molecular compound (Lin et al., 2021). Zhang et al. (2018) reported that hierarchical HZSM-5/MCM-41 which contained a moderate amount of mesopore was effective for pyrolysis intermediate upgrading while reducing the coke formation simultaneously.
Qian et al. (2021) synthesized a novel hierarchical zeolite with the aid of alkaline lignin in the re-organization of alkaline treatment core material. The result revealed that the yield of bio-oil and gas was enhanced at the expense of solid residue. Higher transformation of main pyrolyzates derived from co-reactant and inhibition of char was observed due to higher acidity and hierarchical pore system of the catalyst. In addition, coke yield also decreased due to the enhanced diffusion capability of the feedstock and coke precursor, and shorter diffusion path length in the ZSM-5 structure. Deactivation rate could be reduced as no secondary reaction was produced resulting from the short residence time (Serrano et al., 2013). More particularly, the abundant reactive species of pyrolyzates from biomass-plastic mixture rapidly traverse the catalyst layer by hierarchical pore structure prior to absorption, producing solid residue. Diffusion through hierarchical zeolite crystals is faster in a manner that is closely related to Knudsen regime since the diffusion through mesoporous materials proceeds by molecule-to-molecule interaction as well as molecule-to-pore interaction.
3.5 Low-cost, mineral-based catalyst
Extensive efforts are being made to develop new catalyst with low-cost, good catalytic performance and environmental friendliness. The utilization of natural ore and industrial waste as low-cost and high-activity catalyst in the production of value-added bio-oil can pave ways for recycling and reusing those mineral and waste. Red mud (RM) is a waste residue generated from aluminium industries by the Bayer process of alumina production from bauxite (Wang et al., 2019). It comprises a complex mixture of metal oxides, notably iron oxides and small amounts of alkali earth metals (Das and Mohanty, 2019). Recently, there are significant interest in making use of red mud as a catalyst in pyrolysis of biomass due to its compositional properties containing metal oxides, including CaO, TiO2, Fe2O3, Al2O3, MgO and SiO2. (Chang et al., 2020) investigated the catalytic pyrolysis of palm kernel shell over red mud using a bench scale fixed bed reactor. The result indicated that the presence of RM could enhance the cleavage of oxygen-containing double bonds and functional groups in-side chains on benzene ring to phenol and aromatic. Duman et al. (2013) studied the catalytic pyrolysis of safflower oil cake over RM in a dual reactor system and found that the RM was an effective catalyst in deoxygenation reaction, enhancing the aromatic selectivity. Although the base property of RM could provide the additional cracking efficiency, high production of aromatic could not be achieved since RM did not possess strong acid sites and shape selectivity that were able to limit the diffusivity of longer chain intermediates into the active sites and to foster the secondary reactions including isomerization and aromatization to produce aromatic hydrocarbon (Kelkar et al., 2015). Therefore, the combination of low-cost alkaline catalyst and acidic catalyst is regarded as an ideal approach to achieve higher formation of aromatic hydrocarbon and enhance the zeolite lifetime. Yathavan and Agblevor, (2013) pyrolyzed pinyon − juniper (PJ) woody biomass over HZSM-5 and RM catalyst. The addition of RM as fractional catalyst could enhance the deoxygenation process in which the oxygen was rejected via decarboxylation (CO2) process instead of decarbonylation (CO) and dehydration (H2O) process. This process could enhance the overall carbon and hydrogen efficiency and thus, more hydrogens are available for aromatic production. Furthermore, the pyrolysis oil catalysed by RM has relatively lower viscosities than HZSM-5 catalyst. In another study, in-situ RM was used in the catalytic fast co-pyrolysis (CFCP) of organosolv lignin (OL) and polypropylene (PP) over ex-situ HZSM-5. The authors reported an increase in the cracking efficiency of OL/OP intermediates as well as an enhancement of aromatic selectivity due to the effective interaction between pyrolyzates. The presence of RM in the in-situ catalytic reactor could improve the formation of selected hydrocarbon that acted as precursor to produce aromatics over ex-situ HZSM-5 in second reactor (Ryu et al., 2020a).
Coal fly ash (CFA), a by-product of coal-fired thermal power plants (TPP) is often disposed in the landfill, causing environmental and economic issues. One of the key features of CFA is that it consists of aluminosilicates, such as SiO2 and Al2O3, making it appealing as a precursor to produce zeolite-based catalysts (Supelano et al., 2020). Vichaphund et al. (2019) successfully synthesized ZSM-5 from CFA (HZSM5-FA) via consecutive alkaline fusion and hydrothermal treatment (Fig. 5). The zeolite crystallization time was varied at 24 hr (HZSM5-FA −24) and 72 hr (HZSM5-FA-72). The catalytic activity of the HZSM5-FA was determined in catalytic fast pyrolysis of Jatropha waste at the temperature of 500 °C and Jatropha-to-catalyst ratio of 1:1–1:10. The addition of HZSM5-FA considerably enhanced the aromatic selectivity up to 97.2 % and reduced the undesired oxygenated and N-containing compounds via deoxygenation and denitrogenation reactions. HZSM5-FA promoted the cracking of large oxygenates and nitrogenated species and further converted them to olefins and aromatic via a series of reactions, including decarbonylation, decarboxylation, dehydration, cyclization, aromatization, dehydronitration, deamination, and hydrogenation. On the other hand, HZSM5-FA-72 produced low amounts of aromatics compared to HZSM5-FA-24 due to both low acidity and high mesopore volume. Low acidity zeolite had low number of active sites (Bronsted acid sites) which were responsible to convert oxygenated compounds to aromatic compounds within the framework of zeolite catalyst while high mesopore volume could limit the molecular diffusion of pyrolyzates to inner pore of zeolite to further undergo the series of reactions for aromatic formation. Based on this result, it can be concluded that the pore structure and type of acidity play an important role for aromatic formation.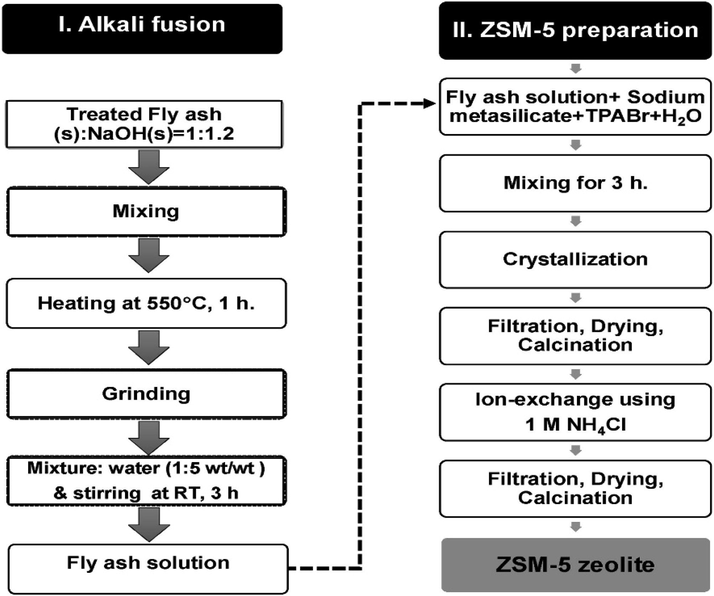
The schematic HZSM- 5 derived from fly ash by alkali fusion followed by hydrothermal treatment.
Reproduced with permission from (Vichaphund et al., 2019).
Steel-slag is a waste by-product derived from steel-making process which accounts about 15 % of the total crude steel output. Most of the steel slags are accumulated heavily in landfill, becoming environmental hazards due to the leaching of heavy metals, particularly mercury (Hg), lead (Pb), chromium (Cr), cadmium (Cd), and arsenic (As) (Song et al., 2021). The higher activity of Faujasite zeolite derived from steel slag in hydrocarbon production was described by Hassan et al. (2019) in the co-pyrolysis of sugarcane bagasse and HDPE. The origin of Faujasite zeolite influenced the formation of mesopore with an average size of 45 nm and a modest surface area of 39.6 m2/g. Even though the surface area of the zeolite is quite low, the NH3-TPD measurement showed a relatively strong acidity. Promotion to the hydrocarbon pool and deoxygenation reaction takes place due to the fact that the microporous structure enables intimate contact to the strong acid sites. Due to the lack of weak acidity in the catalyst, thermal condition is thought to be responsible for the decomposition and cracking of biomass and HDPE molecules. In increasing the pyrolysis temperature, they have been able to compensate the inadequacy of weak acidity while the strong acidity contributes to the upgrading of bio-oil pyrolyzates through a succession of dehydration, decarbonylation, decarboxylation, and oligomerization reactions. Nonetheless, at the reaction temperature of 500 °C and above, reverse Diels-Alder reaction would begin to occur, hindering further upgrading of the product by favouring the generation of olefins in place of aromatics.
3.6 Summary on catalyst types
With its distinctive pore structure and high acidity, microporous zeolite has exceptional cracking and deoxygenation abilities which favour the aromatic selectivity. However, a very small pore size (0.54–0.56 nm) of microporous zeolite resulted to coke formation, mass transfer limitation, and slow diffusion of large molecules into its inner active sites, preventing further reactions of macromolecules to valuable aromatic. Acidity and porosity are two paramount factors that influence the catalytic activity of zeolite catalyst. Mesoporous zeolite has been recognised as an effective method for reducing the diffusion restriction of bulky biomass and plastic molecules, and increasing aromatic hydrocarbon production due to the larger pore size. Although mesoporous materials are synthesised to address the issue of diffusion limitations, they have poor surface acidity and an unstable structure, resulting in inadequate activity in acid-catalysed reactions. To solve this challenge, introduction of new mesoporosity in the micropore of zeolite produces zeolites with hierarchical micro-mesoporous composite. Hierarchical zeolites can substantially resolve the limitations of the conventional zeolite, such as low mass transfer problem, deactivation of catalytic activity and low activity to bulky substrates in different chemical reactions due to the combination of two levels of porosity. Desilication and dealumination are the effective methods to create mesoporous structure with large pore size for better diffusion of bulky intermediates to active sites of catalyst to undergo further reactions to aromatic. In addition, the acidity could also be altered to foster the deoxygenation reaction. Incorporation of metal into the zeolite could alter the pore size and total number of acidic sites, and enhance the thermal stability of the catalyst, all of which are helpful to decrease the coke formation of zeolite catalyst. With the addition of metal, new enhanced Lewis acid sites of zeolite were generated, which boosted the aromatic production. Ubiquitous, low cost and a complex mixture of metal oxides, natural ores such as red mud could be utilized as a catalyst in pyrolysis of biomass. The base properties of red mud could provide additional cracking and deoxygenation for aromatic production. However, the result was still unsatisfactory due to the lack of strong acid sites and shape selectivity as compared to the conventional zeolite catalyst. Therefore, it is advisable to combine low-cost base catalyst with acidic catalyst to achieve higher production of aromatic. Natural mineral wastes including coal fly ash and steel slag consisting of aluminosilicates, such as SiO2 and Al2O3 could be exploited as precursors for the synthesis of zeolite. However, two paramount factors that need to be considered to ensure high production of aromatics are pore structure and type of acidity. A zeolite catalyst with high acidity with an appropriate pore structure needs to be tailored to obtain high cracking and deoxygenation efficiency for the production of aromatics. The performance of different types of catalysts in the catalytic co-pyrolysis of biomass and plastic is summarized in Table 2.
Catalyst
Preparation method
Feedstock
Summary
References
Microporous zeolite
Wet impregnation
Cellulose with HDPE and PP
- ZSM-5 exhibited better results in terms of aromatics yield
- Enhanced coke production.(Kim et al., 2016)
Mesoporous zeolite
By post-synthesis treatments including steaming and leaching method with acidic or basic media.
Cellulose with PP
- Increase olefins and aromatic
- Coke reduction- Mono-aromatic hydrocarbons (MAHs)
increased.(Chi et al., 2018)
Metal modified zeolite
Wet impregnation
(Metal: phosphorous and nickel)Pine wood and LDPE
- Decrease in strong Bronsted acidity and an increase in Lewis’s acidity was observed after dual impregnation.
(Yao et al., 2015)
Metal modified zeolite
(Pt modified /mesoporous ZSM-5 and SBA-16)Wet impregnation
Laminaria japonica and PP
- Reduced oxygenates
- Aromatic and light hydrocarbon increased.(Kim et al., 2017b)
Hierarchical zeolite
(Micro-mesoporous zeolite HZM-5/AL-MCM-41)Alkaline extraction of zeolite
Torrefied yellow poplar and HDPE
- Aromatic hydrocarbons yield from the catalytic co-pyrolysis were higher than that of aggregate values.
-Amounts of aromatics yield using HZSM-5 was larger than that using AL-MCM-41.(Kim et al., 2017a)
Mesoporous hierarchical zeolite
Top-Down method: Desilication
Cellulose and PP over ZSM-5, desilicated ZSM-5, and Al-SBA-15
- The results of desilicated ZSM-5 showed better catalytic performance with highest yield 33.50 wt% of aromatic hydrocarbon
- The desilication had only increased the amount of weak acid sites enhancing the yield of liquid products.
- Promoted the deoxygenation of furan compound.2(Hong et al., 2017)
Industrial wastes
(Red mud)Acid-Treated
Organosolv lignin (OL) and polypropylene (PP)
- Further increase in the cracking efficiency
- Increase in the aromatic formation efficiency(Ryu et al., 2020a)
Industrial wastes (Fly Ash)
Alkali Treatment
Polypropylene (PP)
- The yield of the liquid products decreases with increasing feedstock ratio.
- Producing more gas than liquid.(Na et al., 2006)
Industrial waste (Steel waste -Mesoporous Faujasite zeolite)
Synthesized mesoporous catalyst
Sugarcane bagasse and HDPE
- Enhanced the cracking and deoxygenation of oxygenates to hydrocarbon. The maximum pyrolysis oil yield reached 68.56 wt%, and the pyrolysis oil contained mainly hydrocarbons (74.55 %) with minimal amounts of oxygenated compounds.
(Hassan et al., 2019)
4 Challenges and future research needs
Co-feeding hydrogen-rich plastic to the oxygen-rich biomass offers a promising technique for the production of chemicals and bio-oil. Utilization of biomass and plastic waste in co-pyrolysis process could bring a positive impact to the environment and human being since a large amount of waste polymer could be reduced and value-added fuels and chemicals could be produced. However, to be able to fully exploit this technique, further research and development are required.
Co-pyrolysis of biomass and various plastic mixture needs to be considered as a feedstock in the future as the waste materials generally are not collected separately according to their criterion. According to waste management situation, the separation of biomass and plastic waste from each other during the recycling stage is not feasible and uneconomical. Many studies have focused on the co-pyrolysis of binary mixtures instead of multi-component mixtures. Therefore, multi-component feedstocks with the optimal reaction conditions needs to be investigated Furthermore, the gas emission associated with the multi-component pyrolysis needs to be examined to fully optimize the pyrolysis technology to achieve high quantity and quality of bio-oil.
Although co-pyrolysis of biomass with plastic remarkably supresses the coke formation as compared to pyrolysis of individual biomass, catalyst deactivation remains a great challenge. Selecting suitable catalysts that have high catalytic activity as well as stability is of a great importance. Acidity/basicity, shape selectivity, porous structure and number of active sites are paramount factors that need to be considered when designing a catalyst. Bifunctional catalyst that possesses acid and base properties should be developed. Base catalyst promotes the fragmentation of oxygenates which can easily diffuse into the pores of acidic zeolite. The oxygenates will then be converted to aromatic hydrocarbon via cracking and deoxygenation reaction induced by acid catalyst. Furthermore, the detailed catalytic pyrolysis and catalytic co-pyrolysis reaction mechanisms of pyrolyzates on the external surface and inner pores of catalyst also need to be understood.
Pre-treatment of biomass such as torrefaction and hydrothermal could be a solution to enhance the physicochemical characteristics of the biomass which could lead to the enhancement of conversion efficiency, reduction of the coke formation and improvement to the aromatic production during the catalytic pyrolysis of biomass. For example, torrefaction can enhance the cellulose content and physicochemical properties of biomass including less oxygenated compounds and high heating value to produce bio-oil with low oxygenated compounds, low acidity, high energy content and high monoaromatic hydrocarbons (Boateng and Mullen, 2013; Ryu et al., 2020). Hydrothermal treatment can produce crystalline cellulose and remove the alkali and alkaline metals, especially the K and Na metals, which provide a suitable medium for aromatic formation (Wang et al., 2021).
Synergistic effect mechanism in catalytic co-pyrolysis of biomass and plastic is extremely complex reaction pathway, dominated by free radical fragments at high temperature. During catalytic co-pyrolysis, different free radicals that act as reaction intermediates are participating in hundreds of parallel or continuous reaction pathways. However, the detailed knowledge on the evolution of free radicals as reaction intermediates during catalytic co-pyrolysis is limited and unclear as it is hard to be obtained by the conventional experimental methods alone. Most researchers propose the synergistic mechanism based on the weight loss and final product obtained via TGA and GC–MS instead of reaction intermediate verification. Until now, there has been no solid and unequivocal hypothesis explaining the synergistic effect mechanism involved in radical-induced catalytic co-pyrolysis of biomass and plastic. Therefore, it is important to identify the type and composition of free radicals present during catalytic co-pyrolysis of biomass and plastic.
Funding
The authors thankfully acknowledge the support obtained from Lotte Chemical Titan (M) Sdn. Bhd. and Universiti Sains Malaysia (Grant No: 304/PJKIMIA/6050422/L128), in the form of research grant and facilities which brought forth this article. The first and second authors also acknowledge the research grant provided by Universiti Teknologi MARA, under Research Incentive Grant (Grant No: 600-RMC/GIP 5/3 (045/2021)) that has resulted in this article.
Data availability
The authors confirm that the data supporting the findings of this study are available within the article. Raw data that support the findings of this study are available from the corresponding author, upon reasonable request.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Review of fossil fuels and future energy technologies. Futures. 2015;69:31-49.
- [CrossRef] [Google Scholar]
- Industrial growth and emissions of CO2 in Ghana: the role of financial development and fossil fuel consumption. Energy Rep.. 2019;5:1339-1353.
- [CrossRef] [Google Scholar]
- Co-pyrolysis of oil shale and plastics: influence of pyrolysis parameters on the product yields. Fuel Process. Technol.. 2012;96:209-213.
- [CrossRef] [Google Scholar]
- A review on advanced catalytic co-pyrolysis of biomass and hydrogen-rich feedstock : insights into synergistic effect, catalyst development and reaction mechanism. Bioresour. Technol.. 2020;310:123457
- [CrossRef] [Google Scholar]
- Co-pyrolysis of oil palm empty fruit bunch and oil palm frond with low-density polyethylene and polypropylene for bio-oil production. Arabian J. Chem.. 2021;14:103282
- [CrossRef] [Google Scholar]
- Catalytic and thermal pyrolysis of polycarbonate in a fixed-bed reactor: the effect of catalysts on products yields and composition. Polym. Degrad. Stab.. 2014;110:482-491.
- [CrossRef] [Google Scholar]
- Experimental investigation on gasification characteristics of polyethylene terephthalate (PET) microplastics in supercritical water. Fuel. 2020;262:624-633.
- [CrossRef] [Google Scholar]
- Fast pyrolysis of biomass thermally pretreated by torrefaction. J. Anal. Appl. Pyrol.. 2013;100:95-102.
- [CrossRef] [Google Scholar]
- Catalytic conversion of rapeseed oil for the production of raw chemicals, fuels and carbon nanotubes over Ni-modified nanocrystalline and hierarchical ZSM-5. Appl. Catal. B: Environ.. 2014;145:205-215.
- [CrossRef] [Google Scholar]
- Co-pyrolysis of LignoBoost® lignin with synthetic polymers. Polym. Degrad. Stab.. 2012;97:2104-2109.
- [CrossRef] [Google Scholar]
- Kinetics of synergistic effects in co-pyrolysis of biomass with plastic wastes. Appl. Energy. 2018;220:408-418.
- [CrossRef] [Google Scholar]
- Products characterization study of a slow pyrolysis of biomass-plastic mixtures in a fixed-bed reactor. J. Anal. Appl. Pyrol.. 2014;110:363-374.
- [CrossRef] [Google Scholar]
- Enhanced pyrolysis of palm kernel shell wastes to bio-based chemicals and syngas using red mud as an additive. J. Clean. Prod.. 2020;272
- [CrossRef] [Google Scholar]
- Study on microwave-assisted co-pyrolysis and bio-oil of Chlorella vulgaris with high-density polyethylene under activated carbon. Energy. 2022;247:123508.
- [CrossRef] [Google Scholar]
- Independent parallel pyrolysis kinetics of cellulose, hemicelluloses and lignin at various heating rates analyzed by evolutionary computation. Energy Convers. Manage.. 2020;221
- [CrossRef] [Google Scholar]
- Co-pyrolysis of waste newspaper with high-density polyethylene: synergistic effect and oil characterization. Energy Convers. Manage.. 2016;112:41-48.
- [CrossRef] [Google Scholar]
- Controlled synthesis of hierarchical ZSM-5 for catalytic fast pyrolysis of cellulose to aromatics. J. Mater. Chem. A. 2018;6:21178-21185.
- [CrossRef] [Google Scholar]
- Synergistic effect on thermal behavior and char morphology analysis during co-pyrolysis of paulownia wood blended with different plastics waste. Appl. Therm. Eng.. 2017;111:834-846.
- [CrossRef] [Google Scholar]
- Insight into synergistic effects of biomass-polypropylene co-pyrolysis using representative biomass constituents. Bioresour. Technol.. 2020;307
- [CrossRef] [Google Scholar]
- Catalytic co-pyrolysis of cellulose and polypropylene over all-silica mesoporous catalyst MCM-41 and Al-MCM-41. Sci. Total Environ.. 2018;633:1105-1113.
- [CrossRef] [Google Scholar]
- Air gasification of polyethylene terephthalate using a two-stage gasifier with active carbon for the production of H2 and CO. Energy. 2021;223:120122
- [CrossRef] [Google Scholar]
- Mechanism of fast pyrolysis of lignin: studying model compounds. J. Phys. Chem. B. 2014;118:8524-8531.
- [CrossRef] [Google Scholar]
- A review on advances in sustainable energy production through various catalytic processes by using catalysts derived from waste red mud. Renew. Energy. 2019;143:1791-1811.
- [CrossRef] [Google Scholar]
- Co-pyrolysis of sugarcane bagasse and low-density polyethylene: influence of plastic on pyrolysis product yield. Fuel. 2016;185:508-516.
- [CrossRef] [Google Scholar]
- Real time monitoring of slow pyrolysis of polyethylene terephthalate (PET) by different mass spectrometric techniques. Waste Manage.. 2020;106:226-239.
- [CrossRef] [Google Scholar]
- Chemical recycling of poly(bisphenol A carbonate): 1,5,7-Triazabicyclo[4.4.0]-dec-5-ene catalyzed alcoholysis for highly efficient bisphenol A and organic carbonate recovery. Polymer. 2018;143:106-114.
- [CrossRef] [Google Scholar]
- Origin of carbon in aromatic and olefin products derived from HZSM-5 catalyzed co-pyrolysis of cellulose and plastics via isotopic labeling. Appl. Catal. B: Environ.. 2015;162:338-345.
- [CrossRef] [Google Scholar]
- Improving bio-oil quality from low-density polyethylene pyrolysis: effects of varying activation and pyrolysis parameters. Energy. 2021;232:121090
- [CrossRef] [Google Scholar]
- Two-step pyrolysis of safflower oil cake. J. Anal. Appl. Pyrol.. 2013;103:352-361.
- [CrossRef] [Google Scholar]
- Catalytic co-pyrolysis of biomass and waste plastics as a route to upgraded bio-oil. J. Energy Inst.. 2021;97:27-36.
- [CrossRef] [Google Scholar]
- Preparation of hierarchical micro-mesoporous aluminosilicate composites by simple y zeolite/MCM-48 silica assembly. J. Alloy. Compd.. 2014;583:60-69.
- [CrossRef] [Google Scholar]
- Hierarchical zeolites: synthesis and catalytic properties. Micropor. Mesopor. Mater.. 2018;259:33-45.
- [CrossRef] [Google Scholar]
- Catalytic pyrolysis of biomass for biofuels production. Fuel Process. Technol.. 2010;91:25-32.
- [CrossRef] [Google Scholar]
- Estimation of plastic waste inputs from land into the Persian Gulf and the Gulf of Oman: an environmental disaster, scientific and social concerns. Sci. Total Environ. 2020
- [CrossRef] [Google Scholar]
- Recent progress on catalytic co-pyrolysis of plastic waste and lignocellulosic biomass to liquid fuel: the influence of technical and reaction kinetic parameters. Arabian J. Chem.. 2021;14:103035
- [CrossRef] [Google Scholar]
- Global Bioenergy Statistics, (2018). WBA Global Bioenergy Statistics 2018. World Bioenergy Association 43. <https://www.worldbioenergy.org/uploads/181017%20WBA%20GBS%202018_Summary_hq.pdf>Accessed 20 January 2022
- The synergistic effects of polyvinyl chloride and biomass during combustible solid waste pyrolysis: experimental investigation and modeling. Energy Convers. Manage.. 2020;222:113237
- [CrossRef] [Google Scholar]
- Sustainable films and coatings from hemicelluloses: a review. Biomacromolecules. 2008;9:1493-1505.
- [CrossRef] [Google Scholar]
- Recent progress on biomass co-pyrolysis conversion into high-quality bio-oil. Bioresour. Technol.. 2016;221:645-655.
- [CrossRef] [Google Scholar]
- Catalytic co-pyrolysis of sugarcane bagasse and waste high-density polyethylene over faujasite-type zeolite. Bioresour. Technol.. 2019;284:406-414.
- [CrossRef] [Google Scholar]
- Co-pyrolysis of sugarcane bagasse and waste high-density polyethylene: synergistic effect and product distributions. Energy. 2020;191:116545
- [CrossRef] [Google Scholar]
- Enhancing hydrocarbon production via ex-situ catalytic co-pyrolysis of biomass and high-density polyethylene: study of synergistic effect and aromatics selectivity. Waste Manage.. 2021;128:189-199.
- [CrossRef] [Google Scholar]
- In-situ catalytic copyrolysis of cellulose and polypropylene over desilicated ZSM-5. Catal. Today. 2017;293–294:151-158.
- [CrossRef] [Google Scholar]
- Catalytic upgrading of biomass pyrolysis vapors using transition metal-modified ZSM-5 zeolite. Appl. Catal. B: Environ.. 2012;127:281-290.
- [CrossRef] [Google Scholar]
- Investigation into the shape selectivity of zeolite catalysts for biomass conversion. J. Catal.. 2011;279:257-268.
- [CrossRef] [Google Scholar]
- Evaluation of the co-pyrolysis of lignin with plastic polymers by TG-FTIR and Py-GC/MS. Polym. Degrad. Stab.. 2016;133:65-74.
- [CrossRef] [Google Scholar]
- A survey of catalysts for aromatics from fast pyrolysis of biomass. Appl. Catal. B: Environ.. 2015;174–175:85-95.
- [CrossRef] [Google Scholar]
- Catalytic co-pyrolysis of torrefied yellow poplar and high-density polyethylene using microporous HZSM-5 and mesoporous Al-MCM-41 catalysts. Energy Convers. Manage.. 2017;149:966-973.
- [CrossRef] [Google Scholar]
- Catalytic copyrolysis of cellulose and thermoplastics over HZSM-5 and HY. ACS Sustainable Chem. Eng.. 2016;4:1354-1363.
- [CrossRef] [Google Scholar]
- Catalytic co-pyrolysis of polypropylene and Laminaria japonica over zeolitic materials. Int. J. Hydrogen Energy. 2017;42:18434-18441.
- [CrossRef] [Google Scholar]
- Catalytic co-pyrolysis of biomass carbohydrates with LLDPE over Al-SBA-15 and mesoporous ZSM-5. Catal. Today. 2017;298:46-52.
- [CrossRef] [Google Scholar]
- Catalytic pyrolysis of wood polymer composites over hierarchical mesoporous zeolites. Energy Convers. Manage.. 2019;195:727-737.
- [CrossRef] [Google Scholar]
- Enhanced energy recovery from polyethylene terephthalate via pyrolysis in CO2 atmosphere while suppressing acidic chemical species. Energy Convers. Manage.. 2017;148:456-460.
- [CrossRef] [Google Scholar]
- Characteristics of fractionated drop-in liquid fuel of plastic wastes from a commercial pyrolysis plant. Waste Manage.. 2021;126:411-422.
- [CrossRef] [Google Scholar]
- Catalytic fast pyrolysis of biomass with mesoporous ZSM-5 zeolites prepared by desilication with NaOH solutions. Appl. Catal. A: General. 2014;470:115-122.
- [CrossRef] [Google Scholar]
- Maximizing carbon efficiency of petrochemical production from catalytic co-pyrolysis of biomass and plastics using gallium-containing MFI zeolites. Appl. Catal. B: Environ.. 2015;172–173:154-164.
- [CrossRef] [Google Scholar]
- Catalytic fast co-pyrolysis of waste greenhouse plastic films and rice husk using hierarchical micro-mesoporous composite molecular sieve. Waste Manage.. 2020;102:561-568.
- [CrossRef] [Google Scholar]
- Catalytic fast pyrolysis of lignocellulosic biomass: critical role of zeolite catalysts. Renew. Sustain. Energy Rev.. 2021;139:110707
- [CrossRef] [Google Scholar]
- Catalytic co-pyrolysis of torrefied poplar wood and high-density polyethylene over hierarchical HZSM-5 for mono-aromatics production. Renew. Energy. 2021;164:87-95.
- [CrossRef] [Google Scholar]
- Towards enhanced understanding of synergistic effects in co-pyrolysis of pinewood and polycarbonate. Appl. Energy. 2021;289
- [CrossRef] [Google Scholar]
- Biomass resources and their bioenergy potential estimation: a review. Renew. Sustain. Energy Rev.. 2013;26:344-352.
- [CrossRef] [Google Scholar]
- Lu, P., Huang, Q., (Thanos) Bourtsalas, A.C., Chi, Y., Yan, J., 2018a. Synergistic effects on char and oil produced by the co-pyrolysis of pine wood, polyethylene and polyvinyl chloride. Fuel 230, 359–367. https://doi.org/10.1016/j.fuel.2018.05.072
- Lu, P., Huang, Q., Bourtsalas, A.C. (Thanos), Chi, Y., Yan, J., 2018b. Synergistic effects on char and oil produced by the co-pyrolysis of pine-wood, polyethylene and polyvinyl chloride. Fuel 230, 359–367
- Catalytic conversion of coal pyrolysis vapors to light aromatics over hierarchical Y-type zeolites. J. Energy Inst.. 2020;93:1354-1363.
- [CrossRef] [Google Scholar]
- Co-pyrolysis of waste biomass and waste plastics (polystyrene and waste nitrile gloves) into renewable fuel and value-added chemicals. Carbon Resour. Convers.. 2020;3:145-155.
- [CrossRef] [Google Scholar]
- Pyrolysis of low-density polyethylene using synthetic catalysts produced from fly ash. J. Mater. Cycles Waste Manage.. 2006;8:126-132.
- [CrossRef] [Google Scholar]
- Önal, E., Uzun, Burcu, B., Pütün, A.A., 2014. Bio-oil production via co-pyrolysis of almond shell as biomass and high density polyethylene. Energy Conversion and Management 78, 704–710. https://doi.org/10.1016/j.enconman.2013.11.022
- A comparative study on co-pyrolysis of lignocellulosic biomass with polyethylene terephthalate, polystyrene, and polyvinyl chloride: synergistic effects and product characteristics. J. Clean. Prod.. 2018;205:1127-1138.
- [CrossRef] [Google Scholar]
- TGA/MS/FT-IR study for kinetic evaluation and evolved gas analysis of a biomass/PVC co-pyrolysis process. Energy Convers. Manage.. 2019;182:143-153.
- [CrossRef] [Google Scholar]
- Co-feeding effect of waste plastic films on the catalytic pyrolysis of Quercus variabilis over microporous HZSM-5 and HY catalysts. Chem. Eng. J.. 2019;378:122151
- [CrossRef] [Google Scholar]
- PlasticsEurope, 2020. Plastics - the Facts 2020 [Online]. Available: https://plasticseurope.org/knowledge-hub/plastics-the-facts-2020/Accessesd 5 March 2021.
- Enhanced production of renewable aromatic hydrocarbons for jet-fuel from softwood biomass and plastic waste using hierarchical ZSM-5 modified with lignin-assisted re-assembly. Energy Convers. Manage.. 2021;236
- [CrossRef] [Google Scholar]
- Pyrolysis of plastic waste: opportunities and challenges. J. Anal. Appl. Pyrol.. 2020;152:104804
- [CrossRef] [Google Scholar]
- Thermo-catalytic co-pyrolysis of biomass and high-density polyethylene for improving the yield and quality of pyrolysis liquid. Energy. 2021;225:120231.
- [CrossRef] [Google Scholar]
- Investigating use of metal-modified HZSM-5 catalyst to upgrade liquid yield in co-pyrolysis of wheat straw and polystyrene. Fuel. 2019;257:116119
- [CrossRef] [Google Scholar]
- In-situ catalytic co-pyrolysis of yellow poplar and high-density polyethylene over mesoporous catalysts. Energy Convers. Manage.. 2017;151:116-122.
- [CrossRef] [Google Scholar]
- Enhancement of bio-oil obtained from co-pyrolysis of lignocellulose biomass and LDPE by using a natural zeolite. Therm. Sci. Eng. Prog.. 2020;19:100654
- [CrossRef] [Google Scholar]
- Recent advances in catalytic co-pyrolysis of biomass and plastic waste for the production of petroleum-like hydrocarbons. Bioresour. Technol.. 2020;310:123473
- [Google Scholar]
- Catalytic fast co-pyrolysis of organosolv lignin and polypropylene over in-situ red mud and ex-situ HZSM-5 in two-step catalytic micro reactor. Appl. Surf. Sci.. 2020;511:1-10.
- [CrossRef] [Google Scholar]
- Influence of process parameters on thermal characteristics of char from co-pyrolysis of eucalyptus biomass and polystyrene: Its prospects as a solid fuel. Energy. 2021;232
- [CrossRef] [Google Scholar]
- Synthesis strategies in the search for hierarchical zeolites. Chem. Soc. Rev.. 2013;42:4004-4035.
- [CrossRef] [Google Scholar]
- Advances in lignocellulosic biotechnology: a brief review on lignocellulosic biomass and cellulases. Adv. Biosci. Biotechnol.. 2014;5:246-251.
- [CrossRef] [Google Scholar]
- Catalytic Conversion of Furan to Hydrocarbons using HZSM-5: Coking Behavior and Kinetic Modeling including Coke Deposition. Energy Technology. 2017;5:111-118.
- [CrossRef] [Google Scholar]
- Synthesis and characterization of hierarchical ZSM-5 zeolites with outstanding mesoporosity and excellent catalytic properties. Nanoscale Res. Lett.. 2018;13:364.
- [CrossRef] [Google Scholar]
- Use of steel slag as sustainable construction materials: a review of accelerated carbonation treatment. Resour. Conserv. Recycl.. 2021;173
- [CrossRef] [Google Scholar]
- Co-pyrolysis and synergistic effect analysis of biomass sawdust and polystyrene mixtures for production of high-quality bio-oils. Process Saf. Environ. Prot.. 2021;145:1-11.
- [CrossRef] [Google Scholar]
- Statistica.com, 2021. PVC production volume worldwide.[Online]Available: https://www.statista.com/statistics/720296/global-polyvinyl-chloride-market-size-in-tons/#:∼:text=The%20total%20global%20production%20volume,million%20metric%20tons%20in%202025.Accessed: 7 September 2021.
- Synthesis of magnetic zeolites from recycled fly ash for adsorption of methylene blue. Fuel. 2020;263:116800
- [CrossRef] [Google Scholar]
- Analysis of pyrolysis index and reaction mechanism in microwave-assisted ex-situ catalytic co-pyrolysis of agro-residual and plastic wastes. Bioresour. Technol.. 2022;357:127357.
- [CrossRef] [Google Scholar]
- Effective deoxygenation for the production of liquid biofuels via microwave assisted co-pyrolysis of agro residues and waste plastics combined with catalytic upgradation. Bioresour. Technol.. 2020;302:122775.
- [CrossRef] [Google Scholar]
- Biomass waste conversion into value-added products via microwave-assisted Co-Pyrolysis platform. Renew. Energy. 2021;170:400-409.
- [CrossRef] [Google Scholar]
- Synthesis of hierarchical Y and ZSM-5 zeolites using post-treatment approach to maximize catalytic cracking performance. J. Indus. Eng. Chem.. 2020;88:167-177.
- [CrossRef] [Google Scholar]
- Co-pyrolysis characteristics and kinetic analysis of organic food waste and plastic. Bioresour. Technol.. 2018;249:16-23.
- [CrossRef] [Google Scholar]
- Prevention policies addressing packaging and packaging waste: Some emerging trends. Waste Manage.. 2016;56:35-45.
- [CrossRef] [Google Scholar]
- Co-pyrolysis of coffee-grounds and waste polystyrene foam: synergistic effect and product characteristics analysis. Fuel. 2021;292:120375
- [CrossRef] [Google Scholar]
- In situ catalytic pyrolysis of Jatropha wastes using ZSM-5 from hydrothermal alkaline fusion of fly ash. J. Anal. Appl. Pyrol.. 2019;139:156-166.
- [CrossRef] [Google Scholar]
- Generating alternative fuel and bioplastics from medical plastic waste and waste frying oil using microwave co-pyrolysis combined with microbial fermentation. Renew. Sust. Energ. 2022;153:111790.
- [CrossRef] [Google Scholar]
- Co-pyrolysis of waste plastic and solid biomass for synergistic production of biofuels and chemicals-a review. Prog. Energy Combust. Sci.. 2021;84:100899
- [CrossRef] [Google Scholar]
- Catalytic pyrolysis of lignin in a cascade dual-catalyst system of modified red mud and HZSM-5 for aromatic hydrocarbon production. Bioresour. Technol.. 2019;278:66-72.
- [CrossRef] [Google Scholar]
- Copyrolysis behavior of xylan and polyvinyl chloride plastic. Energy Fuels. 2019;33:8727-8734.
- [CrossRef] [Google Scholar]
- Effect of hydrothermal treatment on biomass structure with evaluation of post-pyrolysis process for wood vinegar preparation. Fuel. 2021;305:121513
- [CrossRef] [Google Scholar]
- A review on the modeling and validation of biomass pyrolysis with a focus on product yield and composition. Biofuel Res. J. 2021;8:1296-1315.
- [CrossRef] [Google Scholar]
- Synergetic effect of co-pyrolysis of cellulose and polypropylene over an all-silica mesoporous catalyst MCM-41 using thermogravimetry-fourier transform infrared spectroscopy and pyrolysis-gas chromatography–mass spectrometry. Energy Fuels. 2017;31:9576-9584.
- [CrossRef] [Google Scholar]
- Catalytic upgrading of pyrolysis vapours: effect of catalyst support and metal type on phenolic content of bio-oil. J. Cleaner Prod.. 2018;185:52-61.
- [CrossRef] [Google Scholar]
- Thermally stable phosphorus and nickel modified ZSM-5 zeolites for catalytic co-pyrolysis of biomass and plastics. RSC Advances. 2015;5:30485-30494.
- [CrossRef] [Google Scholar]
- Catalytic pyrolysis of pinyon-juniper using red mud and HZSM-5. Energy Fuels. 2013;27:6858-6865.
- [CrossRef] [Google Scholar]
- Study of synergistic effects during co-pyrolysis of cellulose and high-density polyethylene at various ratios. Energy Convers. Manage.. 2018;157:517-526.
- [CrossRef] [Google Scholar]
- Catalytic pyrolysis of biomass and polymer wastes. Catalysts. 2018;8:2-45.
- [CrossRef] [Google Scholar]
- Bio-oil production from sequential two-step microwave-assisted catalytic fast pyrolysis of water hyacinth using Ce-doped Γ-Al2O3/ZrO2 composite mesoporous catalyst. J. Anal. Appl. Pyrol.. 2018;132:143-150.
- [CrossRef] [Google Scholar]
- Study of the thermal behavior, kinetics, and product characterization of biomass and low-density polyethylene co-pyrolysis by thermogravimetric analysis and pyrolysis-GC/MS. J. Anal. Appl. Pyrol.. 2018;133:185-197.
- [CrossRef] [Google Scholar]
- Mesoporous zeolites as efficient catalysts for oil refining and natural gas conversion. Front. Chem. Sci. Eng.. 2013;7:233-248.
- [CrossRef] [Google Scholar]







