Translate this page into:
Comparative studies on ultrasound assisted treatment of tannery effluent using multiple oxy-catalysts using response surface methodology
⁎Corresponding author. kannank@kongu.ac.in (Kannan Kandasamy)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
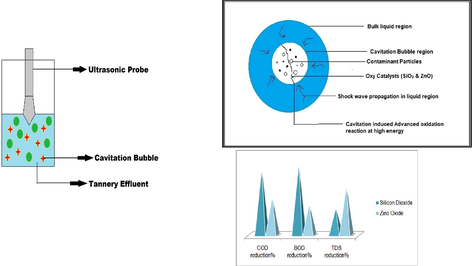
Abstract
Synergizing catalytic advanced oxidation with ultrasound. Zinc Oxide and Silicon Dioxide show equivalent activity as titanium dioxide. Surface changes on the catalysts after treatment. Response Surface methodology studies on the reduction of COD, BOD and TDS.
Abstract
Advanced oxidation of wastewater is a promising technique for tannery wastewater treatment, as it consumes less chemical addition and energy and it doesn’t liberate any secondary effluents. However, advanced oxidation can be improved by conjoining it with energy sources like ultraviolet radiation, ultrasound, etc. Catalysts capable of oxidation like titanium dioxide and iron oxide have been utilized for advanced oxidation of tannery effluent. The present work studies the synergic effect of ultrasound assisted advanced oxidation using two oxy-catalysts, namely zinc oxide and silicon dioxide. The effect of variables like time of treatment, catalyst loading, and power of ultrasound on the reduction of BOD, COD, and TDS were estimated and the results indicated a proficient reduction of contaminants. Upon treatment with silicon dioxide under ultrasound, the COD, BOD, and TDS reduction were found to be 88%, 89%, and 88% respectively, while zinc oxide catalyst indicated 89%, 85%, and 88% reduction. Response Surface Methodology has been utilized for derivation of a mathematical model for COD, BOD and TDS reduction. The spent catalysts were analyzed using Scanning Electron Microscopy and X-ray Diffraction to understand the changes in the characteristics of the spent catalyst. The deposition of contaminants on the catalysts and slight changes in the surface morphology were evident. Hence silicon dioxide and zinc oxide are promising catalysts for the treatment of tannery effluent combined with ultrasound.
Keywords
Tannery wastewater
Advanced oxidation
Silicon dioxide
Zinc oxide
Ultrasound
1 Introduction:
Leather is one of the fast moving commodities, which is utilized in products like footwear, bags, wallets, etc. The leather processing sector which involves tanning of leather plays a crucial role in determining international economy, contributing a business of approximately $100 billion per year. Oruko et al., 2019 Despite its economic potential, the leather industry holds a serious environmental threat due to generation of enormous liquid and solid wastes. The tanning process which is done to increase the shelf life of leather involves three stages namely, pre-tanning, tanning and post-tanning. Approximately 26 kilo litres of effluent are generated for a ton of skin/hide processed (Dixit et al., 2015).
The waste water from leather processing consists of physical, chemical and biological contaminants that evoke very high Biological Oxygen Demand(BOD), Chemical Oxygen Demand(COD) and Total Dissolved Solids(TDS) in the effluent generated (Suresh et al., 2001). It is identified that these pollutants present in the wastewater post serious health threats when disposed to water bodies like rivers, lakes, canals and sea. Tannery industries in Bangladesh are found to contaminate an entire river system affecting not only the river catchment area but also the soil and groundwater (Whitehead et al., 2019) The tannery effluent and the contaminants are phyto toxic and cytotoxic and hence it is evident that improper disposal of the effluent would cause death of flora-fauna and human usage would result in carcinogenic diseases like cancer (Shakir et al., 2012
Various approaches are researched to manage the solid and liquid wastes from tannery industries. Diversified methodologies like bio-leaching of the heavy metals, fuel preparation using the sludge, bio-char from sludge were studied in the earlier researches. But the presence of phyto toxic heavy metals, especially chromium disables the practical utilization of the wastewater and sludge for irrigation, manure preparation and other strategies (Alibardi and Cossu, 2016).
In industrial practice, a series of treatment techniques are applied and they are focused to reduce the concentration of physical, chemical and biological contaminants present in the wastewater. These include sedimentation, coagulation, precipitation, filtration, adsorption and microbial treatment which require further addition of chemicals. The utilization of eco-friendly adsorbents for the effluent treatment could be instrumental to improve the adsorption process. The possibilities of adsorption of wastewater in a continuous column, using low cost adsorbents like neem bark and cotton shell have been studied (Kannan et al., 2012)
Even though adsorption functions as a simple technique for removing the contaminants from the waste stream, the contaminants merely get t+ransferred from waste water to the adsorbents, which in turn put forth new problems of adsorbent regeneration and contaminant disposal. Unfortunately, regenerative adsorption process for wastewater treatment is still in the early stage of research.
Advanced oxidation is a familiar and promising technique for tannery effluent treatment, as it attempts to breakdown the contaminants without huge chemical addition. Fenton based advanced oxidation of industrial effluents is familiar. High organic load effluents can be effectively treated using Fenton reagent (Ebrahiem et al., 2017). Advanced oxidation carried out using strong oxidizers like hydrogen peroxide and catalysts like titanium dioxide are much effective, as the hydroxyl radical has high reactivity for degradation of contaminants (Farooq et al., 2013). However, advanced oxidation process requires integration with the other techniques to achieve the essential reduction in contamination and application in large scale (Dantas et al., 2003).
Ultraviolet radiation has been predominantly used for improving a broad class of chemical reactions, especially, catalysis. Photo catalytic treatment of di methyl formamide using titanium dioxide catalyst is found to be successful (Zhao et al., 2018). Advanced oxidation of tannery waste water with titanium dioxide and ozone has been carried out in the presence of UV radiation and the results indicate very limited extent of treatment. (Schrank et al., 2004). Titanium dioxide doped with silicon dioxide has been evaluated for photo catalytic activity (Gaidau et al., 2017).
Oxides of various metals can be purposive for improving the treatment of tannery effluent. Other than titanium dioxide, other catalysts like zinc oxide, silicon dioxide and iron oxide were also studied for catalytic degradation of pollutants. Zinc oxide microspheres have been studied for reduction of chromium in tannery effluent and the results show considerable reduction of chromium (Liu et al., 2016) Zinc oxide also indicates considerable photo catalytic activity and extended mechanical strength upon photo catalysis in a continuous column (Vaiano and Iervolino, 2018). Photo catalytic activity of silicon dioxide for degradation of organic pollutants is also found to be considerably good (Sivagami et al., 2017). Further research works for utilizing various catalysts for advanced oxidation could produce applicable results. Even though ultraviolet radiation is noticeable for tannery effluent treatment, its activity is restricted to energizing the catalyst. This phenomenon is not sufficient for breaking complex contaminants of tannery effluent.
The intervention of ultrasound in chemical reactions result in intensified processes with improved productivity, as the phenomena of cavitation is instrumental in energizing the species. Recent works indicate that cavitation can be induced by pressure fluctuations, known as hydrodynamic cavitation. This effect is utilized for treatment of tannery effluent along with coagulation, indicating the effectiveness of cavitation phenomena in the treatment of effluent (Wood et al., 2017). Ultrasound assisted catalytic degradation of tannery effluent has been attempted and the results obtained imply greater reduction in oxygen demands and dissolved solid concentration (Saxena et al., 2018). The treatment of tannery effluent with titanium dioxide, aided by ultrasound has been studied and promising results are obtained (Kandasamy et al., 2017).
The above findings implicate the effectiveness of ultrasound in intensification of catalytic degradation of tannery effluent. But the fate of catalysts during the treatment is not studied. Studies on the characteristics of the catalysts before and after the advanced oxidation treatment are essential and it would reveal the details about the feasibility of scaling up from batch to continuous and large scale treatment.
The above research works indicate various prospects for scientific exploration. Advanced oxidation using catalysts is superior to other methods and it can be attempted using multiple catalysts. The effectiveness of catalysts other than titanium dioxide for the tannery effluent treatment can be studied. Ultrasound assisted catalysis can be experimented to understand the influence of sono-catalytic degradation.
Studies on the fate of the catalysts on treatment would throw light upon possibilities of developing continuous regenerative catalytic treatment process. In this research work, zinc oxide and silicon dioxide catalysts are utilized for ultrasound assisted catalytic advanced oxidation of tannery effluent in batch mode. The effect of catalyst loading, power of ultrasound and time of treatment were evaluated. Response Surface Methodology has been applied to understand the influence of various factors on the treatment.
2 Materials and methods
2.1 Preparation of oxy catalysts
The oxy catalysts used for the present study are Zinc Oxide and Silicon Dioxide. The chemicals and reagents used for the synthesis are obtained from Sigma Aldrich India Ltd. For precipitation of Zinc oxide nano particles, zinc nitrate and an alkali were used as precursors at room temperature. (Ghorbani et al., 2015). In this work, sodium hydroxide is added to an aqueous solution of zinc nitrate in a conical flask and a precipitate is obtained. This precipitate contains zinc hydroxide, which is filtered using Whatman filter paper and dried in a hot air oven to remove the residual moisture. The powder obtained is calcined at 600 ℃, thus transforming the zinc hydroxide to zinc oxide. The following chemical reactions represent the formation of Zinc oxide nano particles. Eqn 2.1.1 and 2.1.2
Direct precipitation method is used with sodium silicate and hydrochloric acid for synthesizing silicon dioxide (Made et al., 2020). An aqueous solution of sodium silicate is added with hydrochloric acid. The pH of the solution is adjusted to 7 and room temperature is maintained. Silicon dioxide is obtained as precipitate and it is dried in a hot air oven. The residual impurities present in the sample are removed by calcination at 600 ℃. The following equation illustrates the synthesis of silicon dioxide Eqn 2.1.3
2.2 Analytical procedures
2.2.1 Characterization of tannery effluent
The tannery effluent utilized for the treatment was obtained from tannery industries located in Erode district, Tamilnadu, South India. Various physical and chemical characteristics of the effluent were measured. The effluent obtained was light yellow in color. The determination of pH, conductivity and turbidity were done using the Hanna India pH and Conductivity meters and Sigma 167 India Nephelometer respectively. The total suspended solids (TSS) and total dissolved solids (TDS) present in the effluent were determined by using standard procedures.
The reagents used throughout the study were lab grade. The determination of Biological Oxygen demand is done by a 5 day BOD incubation test and calculating the difference in the initial and final dissolved oxygen levels. For determination of Chemical and Biological Oxygen Demands, standard titrative procedures are followed. Tchobanoglous et al., 2013 The results obtained are given in the table below (see Table 2.1).
Parameter
Value
BOD (mg/l)
2,600
COD (mg/l)
7,700
pH
11.65
Turbidity (NTU)
1,486
Hardness (mPa)
917
Conductivity (Ω.m)
46.7
TS (mg/l)
17,233
TSS (mg/l)
1,533
TDS (mg/l)
15,700
2.2.2 Characterization of catalysts
Zinc oxide and Silicon dioxide catalysts were characterized by Scanning Electron Microscopy using QUANTA − 250 FEG, at Bharathiar University, Coimbatore, Tamilnadu to understand the morphology. X-ray diffraction studies were carried out to understand the crystalline nature of the catalysts, using Bruker XRD equipment at Bharathiar University, Coimbatore, Tamilnadu. The characterized catalysts were subjected to advanced oxidation process and the left out catalysts were filtered for further characterization. The pore size and surface area of the SiO2 and ZnO is determined by MicroTrac-Belsrop Max Surface Area Analyzer, Government College of Technology, Coimbatore, Tamilnadu.
2.3 Ultrasonic – catalytic treatment
The catalytic degradation of tannery effluent was carried out in a reactor fitted with Ultrasonic Probe – Heilscher UP 200 HT, Germany. A schematic representation of the experimental set up is shown below Fig. 2.3.1.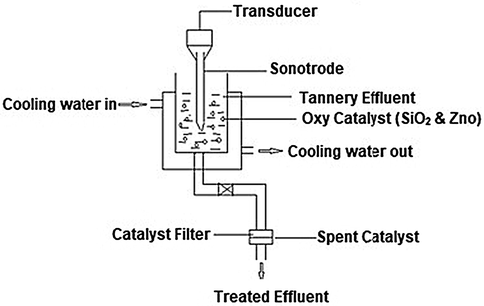
Schematic representation of experimental set up for sono-catalytic treatment of tannery effluent.
About 250 ml of effluent to be treated was fed into the reactor. The catalysts namely zinc oxide and silicon dioxide were calcined and fed at different loading rates from 0.05 to 0.25 g. The waste water and catalyst mixture was energized by ultrasound at different powers, ranging from 20 W to 100 W. The frequency was maintained at 26 kHz. The reaction time was varied from 40 to 120 min. The catalytic treatment was carried out in room temperature and the increase in temperature due to ultrasonic cavitation is removed by circulating water through a jacket around the vessel. The following table summarizes the operating conditions employed for the ultrasound assisted catalytic treatment of tannery effluent (see Table 2.2).
S.No
Parameter Maintained
Range/Value
1.
Quantity of Effluent (ml)
250
2.
Catalyst Loading (g)
0.05 to 0.25
3.
Power of Ultrasound (W)
20 to 100
4.
Frequency of Ultrasound (kHz)
26
5.
Time of treatment (Mins)
40 to 120
6.
Temperature (°C)
25 to 35
3 Results and discussions
3.1 Morphology of catalysts
Zinc oxide and Silicon dioxide catalysts prepared by direct precipitation were characterized by Scanning Electron Microscopy. The SEM images of zinc oxide before treatment are given in Fig. 3.1.1 Zinc oxide catalyst shows distinct three dimensional structures with ridges and cavities. The particles appear like cluster of non-uniform sizes as shown in Fig. 3.1.1a. Closer examination of these particles indicates the presence of needle like structures, as indicated in Fig. 3.1.1b. The morphology of zinc oxide nano catalyst shows resemblance with the zinc oxide nano particles synthesized by co-precipitation method (Adam et al., 2018). Fig. 3.1.2a & b represent the SEM image of Silicon dioxide. The surface of silicon dioxide has irregular clusters, but upon magnification, it is witnessed that the clusters have tiny particles that are of uniform size. The SEM images indicate identical morphology with the silicon dioxide nano particles prepared by sol-gel method. (Kao et al., 2014) The clusters of silicon dioxide catalysts are smaller than that of zinc oxide.
(a) & (b) SEM images of Zinc oxide catalyst before advanced oxidation process.
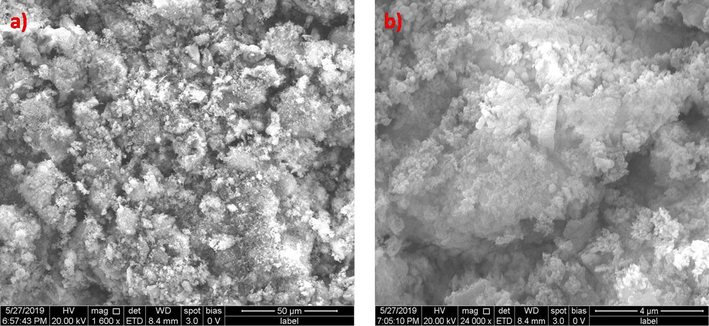
(a) & (b) SEM images of Silicon dioxide catalyst before advanced oxidation process.
The morphology of catalysts after treatment indicates significant dissimilarities with the characteristics of pure catalysts. The SEM images of the utilized zinc oxide catalyst are shown in Fig. 3.1.3a & b. The images show two distinct layers indicating the deposition of foreign substances. Upon magnification, the needles like structures are witnessed with presence of foreign matter which confirms the deposition on the catalyst surface. Fig. 3.1.4a & b show the SEM images of utilized silicon dioxide catalyst. The silicon dioxide catalysts show agglomeration and formation of clusters with larger sizes. Distinct zones of deposition are clearly evident on the surface.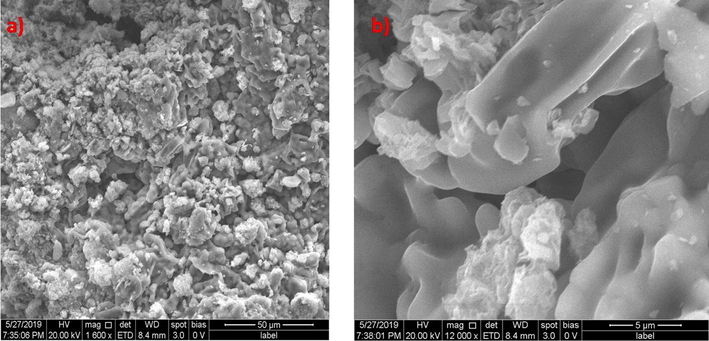
(a) & (b) SEM images of Zinc oxide catalyst after advanced oxidation process.
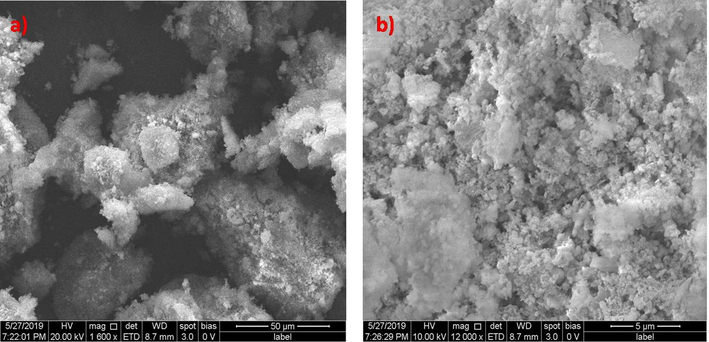
(a) & (b) SEM images of Silicon dioxide catalyst after advanced oxidation process.
3.2 X-ray diffraction Studies:
The prepared catalysts were analyzed by x-ray diffraction to understand the crystallographic properties. Fig. 3.2.1a shows the x-ray diffraction pattern for zinc oxide catalyst. The diffraction pattern has peaks that are not very sharp and this indicates that the catalysts are semi-crystalline in nature. Fig. 3.2.2a shows the X-ray diffraction pattern of the silicon dioxide catalyst, which shows sharp peaks. This is because of the crystalline nature of silicon dioxide. X-ray diffraction studies were carried out for catalysts obtained after the advanced oxidation treatment process and interesting results were obtained. Fig. 3.2.1b shows the X-ray diffraction pattern of utilized zinc oxide catalyst. New peaks are witnessed in the catalyst indicating the introduction of foreign matter on the catalyst during the treatment. Fig. 3.2.2b shows the X-ray diffraction pattern of utilized silicon dioxide catalyst. Here the peaks which were already present are absent. This is because of agglomeration of cluster of silicon dioxide upon treatment. Both observations are in accordance with the findings from SEM images. Hence it can be confirmed that zinc oxide catalyst is deposited with the contaminants from tannery effluent while silicon dioxide catalyst undergoes agglomeration upon advanced oxidation treatment.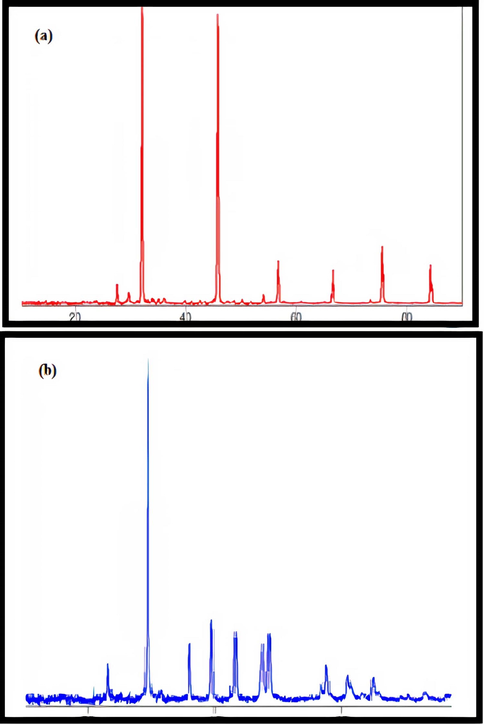
(a) & (b) XRD patterns of Zinc oxide catalyst before and after advanced oxidation process.
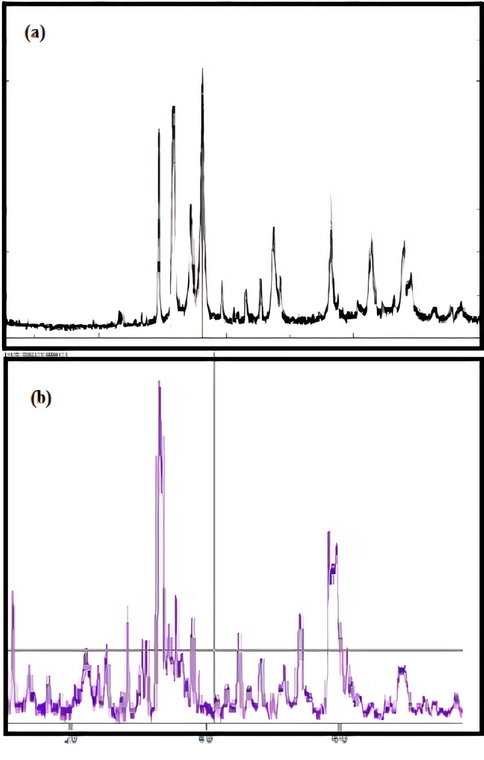
(a) & (b) XRD patterns of Silicon dioxide catalyst before and after advanced oxidation process.
3.3 Surface area of catalyst determination
The Table 3.3.1 shows the surface area, pore volume and pore diameter of zinc oxide and silicon dioxide catalysts. The adsorption isotherms of ZnO and SiO2 show type IV isotherm curve with Hysteresis H3. The type IV isotherm indicates that the catalysts are mesoporous in nature and the pores are in nano scale. The hysteresis in adsorption-desorption curve occurs due to presence of multi-layers and the phenomenon of capillary condensation. The silicon dioxide catalyst is characterized by larger surface area and pore volume than zinc oxide (Salvador et al., 2002)
Catalyst
Surface area (m2/g)
Pore volume (cm3/g)
Pore diameter (nm)
SiO2
180
1.3
28.02
ZnO
25
0.02
3.6
3.4 Mechanism of ultrasonic cavitation on catalytic Treatment:
The objective of ultrasound assisted catalytic treatment is to intensify the breakdown of contaminants present in the wastewater. When a liquid medium is exposed to ultrasound, cavitation is induced. This cavitation causes shock wave propagation through the liquid medium. When the zinc oxide and silicon dioxide catalysts are added to the liquid it becomes a heterogeneous system and the shock wave causes generation of bubbles that collapse symmetrically and asymmetrically (Sancheti and Gogate, 2017). As a result of this bubble collapse, the nodal pressure and temperature at the bubble-solid interface increases, this reduces the mass transfer resistance between the catalyst and effluent, instigating the advanced oxidation of the contaminants. As a result the breakdown of contaminants is escalated as illustrated in Fig. 3.4.1.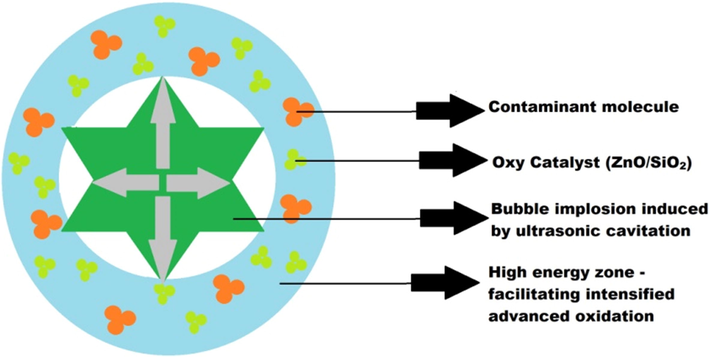
Mechanism of Ultrasound induced Cavitation – Catalytic Treatment.
3.5 RSM studies on COD reduction by sono Catalysis:
The tannery effluent which had 7700 mg/l COD was subjected to sono-catalytic degradation using zinc oxide and silicon dioxide separately. Different loading rates of catalyst were utilized varying the power of ultrasound and time of treatment. Upon sono-catalysis using zinc oxide, the COD reduction achieved was found to be approximately 89% with maximum catalyst loading of 0.25 g. Silicon dioxide showcases approximately 91% of COD reduction under maximum catalyst dosage of 0.25 g. All the three variables, namely catalyst loading, time of treatment and power of ultrasound directly influence the degradation. Zinc oxide indicates better catalytic activity in association with ultrasound than under ultraviolet environment (Vaiano et al., 2018). The results obtained for both zinc oxide and silicon dioxide is better than the reduction obtained by photocatalytic advanced oxidation using titanium dioxide and hydrogen peroxide. Lofrano et al., 2013 Graphitic carbon tri-nitride – titanium dioxide composites also shows lesser degradation (Tripathi and Narayanan, 2018). The reduction of COD is considerably equal to that obtained by alkali assisted ozonation (Preethi et al., 2009; Houshyar et al., 2012). However, the ultrasound assisted catalysis is superior to pH adjusted ozonation, because it doesn’t require any chemical addition. Ultrasound assisted catalytic degradation of tannery effluent using titanium dioxide also indicates approximately 89% reduction of COD (Kannan et al., 2018; Mandal et al., 2019). The comparative analysis substantiates the superiority of ultrasound exploitation for catalytic degradation due to cavitation phenomena. The 3D contour for reduction of COD using zinc oxide is shown in Fig. 3.5.1. The model developed for COD reduction using zinc oxide is given below. The “Predicted R-Squared” of 0.9029 is in reasonable agreement with the “Adjusted R-Squared” of 0.9861.
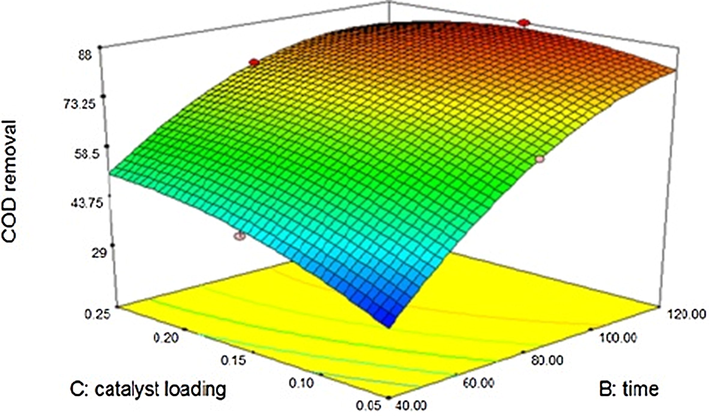
Effect of Zinc oxide – sono catalytic treatment on COD.
The 3D contour for reduction of COD upon silicon dioxide sono catalysis is shown in Fig. 3.5.2. The model developed with “Predicted R-Squared” of 0.8874 is in reasonable agreement with the “Adjusted R-Squared” of 0.9839. All the three variables are found to be significant.
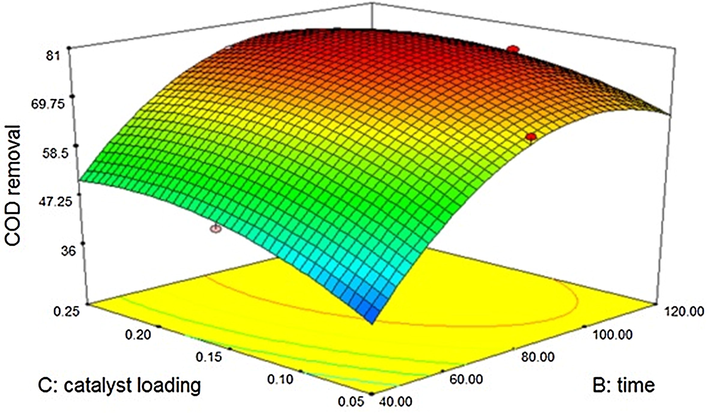
Effect of Silicon dioxide – sono catalytic treatment on COD.
3.6 RSM studies on BOD reduction by sono Catalysis:
The reduction of BOD upon sono catalysis was evaluated against catalyst dosage, time of treatment and power of ultrasound. Both zinc oxide and silicon dioxide indicated considerable BOD reduction. The reduction of BOD with silicon dioxide was found to be 92%, whereas zinc oxide indicated 85% reduction. Silicon dioxide indicates better degradation than zinc oxide, due to the fact that it has got two oxygen atoms. Zinc oxide shows better catalytic activity in ultrasound than ultraviolet radiation (Vaiano et al., 2018). The results obtained are slightly lesser than photo catalytic advanced oxidation using TiO2 and hydrogen peroxide (Kannan et al., 2018; Mandal et al., 2019) Ozone assisted advanced oxidation with pH adjustment also proves to be superior with respect to COD reduction (Preethi et al., 2009). This could be because of the destructive effects of ozone and ultraviolet radiation on biological contaminants. But the results obtained confirm its accordance with the sono catalytic treatment using titanium dioxide (Tripathi and Narayanan, 2018). Hence, the influence of ultrasound is strong for carrying out catalytic degradation. Contour plots indicating the effects of the variables upon treatment for zinc oxide is depicted in Fig. 3.6.1. A mathematical model is generated with “Predicted R-Squared” value of 0.8977 is in agreement with the “Adjusted R-Squared” value of 0.9854 and the same is given below. The variables namely catalyst dosage, time of treatment and power of ultrasound are found to be significant.
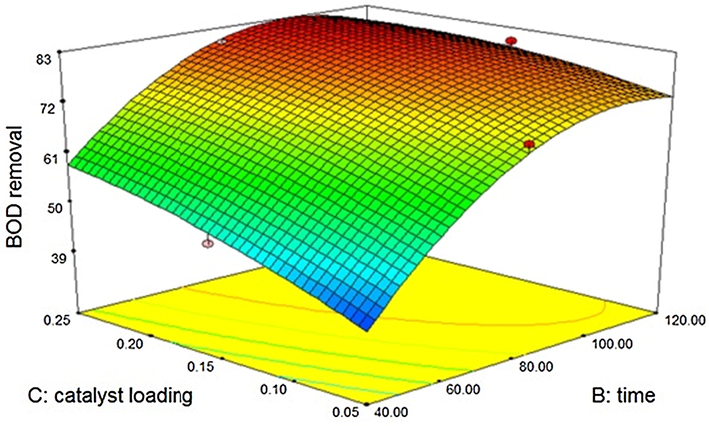
Effect of Zinc oxide – sono catalytic treatment on BOD.
The effect of silicon dioxide catalysis on the treatment is shown through contour plot in Fig. 3.6.2. A mathematical model is generated and the “Predicted R-Squared” of 0.8977 is in reasonable agreement with the “Adjusted R-Squared” of 0.9854.
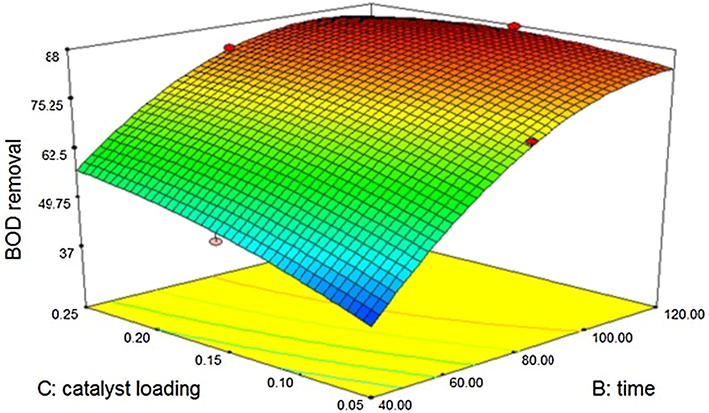
Effect of Silicon dioxide – sono catalytic treatment on BOD.
3.7 RSM studies on TDS reduction by sono catalysis
Both catalysts show considerable reduction of TDS and the reduction of TDS is directly related to the power of ultrasound, time of treatment and catalyst dosage. Zinc oxide shows 88% of TDS reduction at 100 W power with 120 min duration and with maximum catalyst loading (0.25 g) and Silicon dioxide shows 85% reduction. The reduction in TDS is slightly less when compared with that of titanium dioxide sono catalysis (Kannan et al., 2018) This indicates partial dissolution of catalysts with the wastewater. However, the activity of catalyst is considerably good and hence it can be supported with a binder to reduce the dissolution. A contour of the reduction of TDS using zinc oxide is shown in Fig. 3.7.1. The mathematical model is generated for zinc oxide catalysis with “Predicted R-Squared” of 0.9738 and the “Adjusted R-Squared” of 0.9963. The model is given below
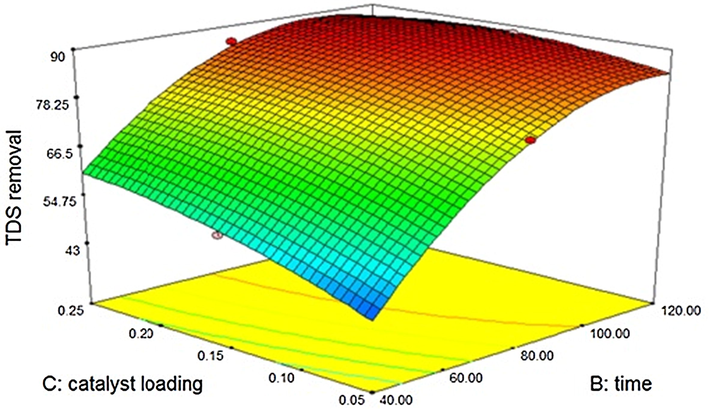
Effect of Zinc oxide – sono catalytic treatment on TDS.
The catalytic activity of silicon dioxide is represented in Fig. 3.7.2. The mathematical model generated has “Predicted R-Squared” of 0.7949 which is in agreement with the “Adjusted R-Squared” of 0.9707.
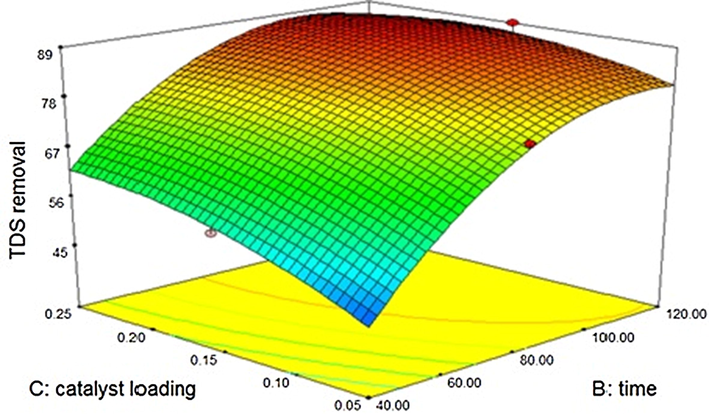
Effect of Silicon dioxide – sono catalytic treatment on TDS.
3.8 Comparison of Anti-Oxidation activity of silicon dioxide and zinc Oxide:
The effluent degradation studies were carried out at constant catalyst loading rates, reaction time and power of ultrasound. For reduction of Chemical Oxygen Demand, silicon dioxide shows more activity than zinc oxide, as shown in Fig. 3.8.1a. This improved activity of silicon dioxide is because of two reasons. Silicon dioxide consists of two oxygen atoms where as zinc oxide has only one oxygen atom. Therefore, silicon dioxide catalyst has higher oxidation potential than zinc oxide. Also, the surface area, pore volume and pore diameter of silicon dioxide is higher than zinc oxide. Thus the catalyst has larger exposure to effluent. Fig. 3.8.1b shows that the Biological Oxygen Demand reduction activity of silicon dioxide is also better than zinc oxide. This stronger activity is also because of the above factors. The Total Dissolved Solids reduction by the two oxy catalysts is shown in Fig. 3.8.1c. Silicon dioxide is found to dissolve in the effluent stream and because of this the reduction of TDS is slightly less than that of zinc oxide. At the outset, both catalysts showcase remarkable catalytic activity for degradation of tannery effluent.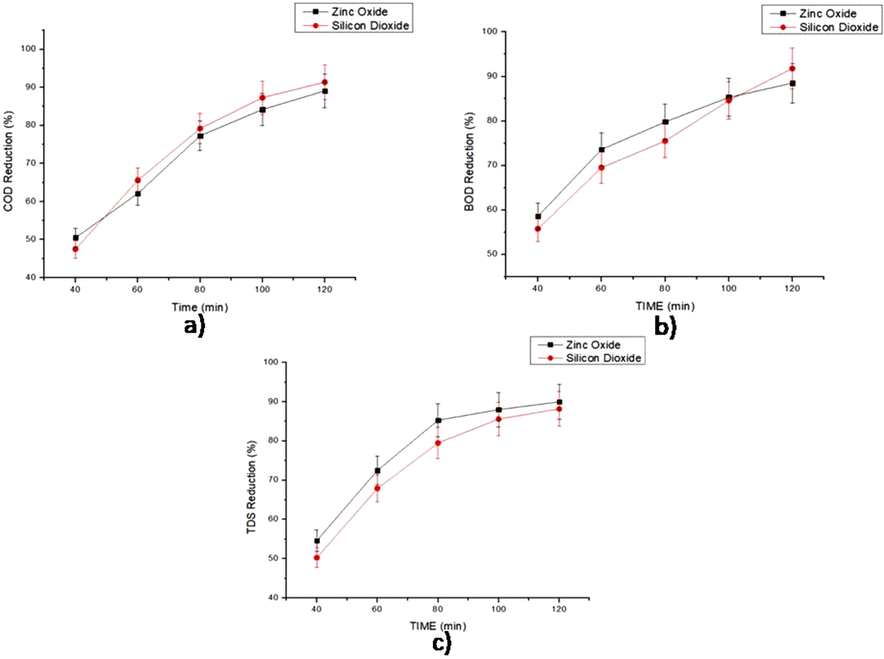
Comparison of Sono catalytic activity of Oxy catalysts.
4 Conclusion
Thus, in the present work, zinc oxide, and silicon dioxide prepared by direct precipitation were utilized for ultrasound-assisted advanced oxidation treatment of tannery effluent. The physical characterization of the catalysts shows desirable morphology with clusters and particles with pores. Hence, zinc oxide and silicon dioxide are potential catalysts for advanced oxidation treatment. The COD, BOD, and TDS reduction were found to be 88%, 89%, and 88% for silicon dioxide respectively and zinc oxide catalyst indicated 89%, 85%, and 88% reduction. Upon comparison with photo-catalytic degradation and hydrodynamic cavitation, sono-catalytic treatment is also a potent technique for treatment of tannery effluent. And, the catalytic activity of Zinc oxide and silicon dioxide are equivalent with other oxy-catalysts like Titanium dioxide. These catalysts also possess good durability and hence they showcase good scope to be used as composite catalysts along with other catalysts. The effectiveness of ultrasound is clearly evident from the extent of degradation. Hence, ultrasound assistance can provide a promising reduction in contamination of tannery effluent. Hybridization of the available techniques has the potential to provide viable solutions for tannery effluent decomposition that finds application in real-time.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Synthesis of ZnO nanoparticles by co-precipitation method for solar driven photodegradation of Congo red dye at different pH. PNFA. 2018;08
- [CrossRef] [Google Scholar]
- Pre-treatment of tannery sludge for sustainable landfilling. J. Waste Manag. 2016
- [CrossRef] [Google Scholar]
- Fenton and Photo-Fenton oxidation of tannery wastewater. Maringa. 2003;25(1):91-95.
- [CrossRef] [Google Scholar]
- Toxic hazards of leather industry and technologies to combat threat: a review. J. Clean Prod. 2015;87:39-49.
- [CrossRef] [Google Scholar]
- Removal of organic pollutants from industrial wastewater by applying photo-Fenton oxidation technology. Arab. J. Chem.. 2017;10:S1674-S1679.
- [CrossRef] [Google Scholar]
- Treatment of tanneries waste water by ultrasound assisted electrolysis process. J. Chem. Soc. Pakistan. 2013;35:3.
- [Google Scholar]
- Preparation of silica doped titania nanoparticles with thermal stability and photocatalytic properties and their application for leather surface functionalization. Arab. J. Chem.. 2017;10:985-1000.
- [CrossRef] [Google Scholar]
- Synthesis of ZnO nanoparticles by precipitation method. OrientJchem. 2015;31(2):1219-1221.
- [CrossRef] [Google Scholar]
- Inflfluence of ozonation process on characteristics of pre-alkalized tannery effluents. Chem. Eng. J.. 2012;191(2012):59-65.
- [CrossRef] [Google Scholar]
- Treatment of tannery effluent using sono catalytic reactor. J. Water Process Eng.. 2017;15:72-77.
- [CrossRef] [Google Scholar]
- Studies on effectiveness of low cost adsorbents in continuous column for textile effluents. IJBBB. 2012;2:6.
- [CrossRef] [Google Scholar]
- Synthesis and characterization of SiO2 nanoparticles and their efficacy in chemical mechanical polishing steel substrate. Adv. Mater. Sci. Eng.. 2014;69167
- [CrossRef] [Google Scholar]
- Flower-like ZnO hollow microspheres on ceramic mesh substrate for photocatalytic reduction of Cr(VI) in tannery wastewater. Ceramics International 2016
- [CrossRef] [Google Scholar]
- Chemical and biological treatment technologies for leather tannery chemicals and wastewaters: a review. Sci. Total Environ. 2013;461–462(2013):265-281.
- [CrossRef] [Google Scholar]
- Synthesis of Silica Particles by Precipitation method of sodium silicate: effect of temperature, pH and mixing technique. AIP Conf. Proc. 2020;2219:080018
- [CrossRef] [Google Scholar]
- Interactive Fe2O3/porous SiO2 nanospheres for photocatalytic degradation of organic pollutants: Kinetic and mechanistic approach. Chemosphere. 2019;234:596-607.
- [CrossRef] [Google Scholar]
- Contemporary and future direction of chromium tanning and management in sub Saharan Africa tanneries. Process Saf. Environ. 2019
- [CrossRef] [Google Scholar]
- Ozonation of tannery efflfluent for removal of cod and color. J. Hazard. Mater. 2009;166(2009):150-154.
- [CrossRef] [Google Scholar]
- A review on application of BET equation to experimental data: the C parameter. Stud. Surd Sci. Catal.. 2002;144:379-386.
- [CrossRef] [Google Scholar]
- A review of engineering aspects of intensification of chemical synthesis using ultrasound. J. Ultrasonch.. 2017;36:527-543.
- [CrossRef] [Google Scholar]
- An advanced pretreatment strategy involving hydrodynamic and acoustic cavitation along with alum coagulation for the mineralization and biodegradability enhancement of tannery waste effluent. Ultrason Sonochem. 2018;44:299-309.
- [CrossRef] [Google Scholar]
- Elucidation of the behavior of tannery wastewater under advanced oxidation conditions. Chemosphere. 2004;56:411-423.
- [CrossRef] [Google Scholar]
- Ecotoxicological risks associated with tannery effluent wastewater. Environ. Toxicol. Pharmacol.. 2012;34:180-191.
- [CrossRef] [Google Scholar]
- Advanced oxidation processes for the treatment of tannery wastewater. J. Environ. Chem. Eng.. 2017;6(2018):3656-3663.
- [CrossRef] [Google Scholar]
- An improved product-process for cleaner chrome tanning in leather processing. J. Clean Prod. 2001;9:483-491.
- [CrossRef] [Google Scholar]
- Wastewater Engineering: Treatment and Resource Recovery (fifth edition). New York: McGraw Hill Education; 2013. https://doi.org/10.1002/9780470168219.ch8
- Impact of TiO2 and TiO2/g-C3N4 Nanocomposite to Treat Industrial Wastewater. Environ. Nanotechnol. Monit. 2018;10:280-291.
- [Google Scholar]
- Facile method to immobilize ZnO particles on glass spheres for the photocatalytic treatment of tannery waste water. J. Colloid Interface Sci.. 2018;518:192-199.
- [CrossRef] [Google Scholar]
- Modelling heavy metals in the Buriganga River System, Dhaka, Bangladesh: Impacts of tannery pollution control. Sci. Total Environ.. 2019;697:134090
- [CrossRef] [Google Scholar]
- A parametric review of sonochemistry: Control and augmentation of sonochemical activity in aqueous solutions. Ultrasonics Sonochemistry. 2017;38:351-370.
- [CrossRef] [Google Scholar]
- Efficient photocatalytic degradation of gaseous N, N-dimethylformamide in tannery waste gas using doubly open-ended Ag/TiO2 nanotube array membranes. Surf. Sci. Appl. 2018
- [CrossRef] [Google Scholar]







