Translate this page into:
Comprehensive analysis of resveratrol metabolites in rats using ultra high performance liquid chromatography coupled with high resolution mass spectrometry
⁎Corresponding authors. wangzhibin@tongrentang.com (Zhibin Wang), zhangjiayu0615@163.com (Jiayu Zhang)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
Resveratrol is an antitoxin secreted by plants such as Polygonum cuspidatum Sieb. et Zucc and Vitis vinifera L. when they are attacked by pathogens. In the present study, three methods were used to prepare biological samples, and then an efficient strategy based on ultra-high-performance liquid chromatography-linear ion trap-Orbitrap mass spectrometry (UHPLC-LTQ-Orbitrap MS) was developed to screen and identify resveratrol metabolites in rat urine, plasma and faeces. As a result, a total of 56 resveratrol metabolites were screened and characterized. Among them, 39 metabolites were found in rat urine, while 6 and 16 metabolites were characterized from rat plasma and faeces, respectively. In addition, 56, 12 and 15 metabolic products were screened by solid phase extraction (method I), methanol precipitation (method II) and acetonitrile precipitation (method III), respectively, indicating that method I could be adopted as the most acceptable method. The results also demonstrated that resveratrol mainly underwent glucuronidation, glucosylation, sulfation, hydroxylation, dehydrogenation, hydrogenation, methylation and their composite reactions. Moreover, these metabolic reactions occurred to form a possible metabolic network that is similar to a triangular pyramid model. In summary, this research provides an idea for the further study of drug metabolism.
Keywords
Resveratrol
Metablite
UHPLC-LTQ-Orbitrap MS
Metabolic network
1 Introduction
Resveratrol is a natural polyphenolic, non-flavonoid antioxidant produced by many herbal plants, such as Polygonum cuspidatum Sieb. et Zucc and Vitis vinifera L., during the process of reacting to a stimulus (Samarjit and Dipak, 2007). Resveratrol has been reported to have widespread biological activities, including antitumour (Patel et al., 2010), antioxidant (Xu et al., 2020), anti-inflammatory (Chen et al., 2020) and cardiovascular protection (Fremont, 2000). Similarly, resveratrol plays an important role in anti-inflammatory reactions by inhibiting the secretion of nitric oxides and TNF-α in N9 microglial cells and cortical microglia (Bergman et al., 2013). Resveratrol has also shown a positive effect on immune function, since it may be involved in the specific and non-specific immune response by directly regulating lymphocyte, macrophage and dendritic cell activation (Svajger and Jeras, 2012). Thus, resveratrol has been extensively adopted as a significant additive in food, cosmetics, medicine, health care and other fields.
Metabolism studies in vivo have been an important means to clarify the mechanism of action of the drug and provide an important basis to guide clinical medication recommendations (Sandermann, 1992; Wilkinson, 2005). M. Emília Juan et al. developed a methodology for the extraction and quantification of trans-resveratrol and its metabolites in the plasma, brain, testis, liver, lungs and kidney based on HPLC analysis (Emília Juan et al., 2010). A reversed-phase HPLC method proposed by David J et al. was applied to determine the levels of resveratrol and identify six major conjugated metabolites in the plasma and urine of human volunteers (Boocock et al., 2007). Nevertheless, there were shortages in resveratrol metabolite quantity and their related descriptions by the above methods. However, a problem that cannot be ignored in metabolism studies is that metabolites are often found in small quantities and hence are usually hard to detect (Shang et al., 2017). Therefore, it is necessary to obtain a complete chemical characterization of resveratrol metabolites and to develop sensitive, accurate and high-resolution methods for their qualitative analyses.
Over the last decade, establishing an efficient and powerful analytical method for identifying drug metabolites has been of great significance with the rapid development of ultra-high-performance liquid chromatography coupled with high-resolution mass spectrometry (UHPLC-HRMS). Moreover, the coverage of metabolite screening and the abundance of ESI-MS/MS information can increase by using the full scan-parent ion list-dynamic exclusion (FS-PIL-DE) method coupled to diagnostic product ion (DPI) analysis on a hybrid LTQ-Orbitrap mass spectrometer (Zhang et al., 2014; Xu et al., 2017; Dai et al., 2017). Two-step mass defect filtering-induced exclusion list-data dependent acquisition was established on the basis of two-dimensional liquid chromatography coupled with quadrupole-Orbitrap mass spectrometer, which further increased MS/MS coverage and selectivity (Pan et al., 2020). A multiplicate strategy, PIL in full MS/dd-MS2 to detect targeted components and “if idle-pick others” for distinguishing the MS2 information of those untargeted ones, developed on a high-resolution Q Exactive hybrid quadrupole-Orbitrap mass spectrometer coupled to offline comprehensive 2D-LC. It benefits the simultaneous identification and characterization of the targeted and untargeted metabolites (Fu et al., 2019). Herein, we established an UHPLC-LTQ-Orbitrap MS-based strategy to perform the comprehensive identification and characterization of resveratrol metabolites in the plasma, urine and faeces of Sprague-Dawley (SD) rats following intragastric administration of resveratrol.
2 Experiment
2.1 Chemicals and materials
Resveratrol and polydatin were purchased from Chengdu Must Biotechnology Co. Ltd (Sichuan, China). These reference standards with purities higher than 98% were applicable to UHPLC-LTQ-Orbitrap analysis.
HPLC grade acetonitrile, methanol and formic acid were purchased from Thermo Fisher Scientifific (Fair Lawn, NJ, USA). All the other chemicals of analytical grade were available at the work station, Beijing Chemical Works (Beijing, China). Deionized water used throughoutthe experiment was purifified by a Milli-Q Gradient Å 10 System (Millipore, Billerica, MA, USA). Grace Pure™ SPE C18-Low solid phase extraction cartridges (200 mg/3 mL, 59 μm, 70 Å) were purchased from Grace Davison Discovery Science (Deerfifield, IL, USA).
2.2 Animals
Eight male SD rats weighted 200 ± 20 g were obtained from Beijing Weitong Lihua Experimental Animals Company (Beijing, China). The rats were housed in a controlled room at standard temperature (24 ± 2 °C) and humidity (70 ± 5%), and kept on a 12 h light/12 h dark regime. After a week acclimation, rats were randomly divided into two groups: Drug Group (n = 4) for test plasma, urine and feces; Control Group (n = 4) for blank plasma, urine and feces. They were fasted for 12 h with free access to water prior to the experiment. The animal protocols were approved by the institutional Animal Care and Use Committee at Beijing University of Chinese Medicine. The animal facilities and protocols were complied with the Guide for the Care and Use of Laboratory Animals.
2.3 Drug administration and biological sample preparation
Resveratrol was suspended in 0.5% carboxymethylcellulose sodium (CMC-Na) solution and given at a dose of 300 mg/kg body weight to rats in the drug group. A 0.5% CMC-Na aqueous solution (2 mL) was parallelly administered to rats in the control group. Blood samples (0.5 mL) were taken from the suborbital venous plexus of rats at 0.5, 1, 2 and 4 h post-administration. Each sample was centrifuged at 3 500 rpm for 10 min to obtain plasma samples. Urine and faecal samples were collected 0–24 h after intragastric administration. All homogeneous biological samples from the same group were finally merged into a collective sample.
Different processing methods were used to pretreat the collected blood, urine and faecal samples. The first method (method I) was performed to prepare biological samples by solid phase extraction (SPE). Plasma and urine samples (1 mL) were added to an SPE cartridge pretreated with methanol (5 mL) and deionized water (5 mL). Then, the SPE cartridges were successively washed with deionized water (5 mL) and methanol (3 mL). The methanol eluate was collected and evaporated by nitrogen at room temperature. The residue was redissolved in 100 μL of acetonitrile/water (10:90, v/v) and then centrifuged at 14,000 rpm for 15 min. Faecal samples (1.0 g) were ultrasonically extracted with deionized water (5.0 mL) for 15 min and then filtered. The supernatant (1 mL) was added to the pretreated SPE cartridge, and then the same process described above was conducted. The second method (method II) used methanol to precipitate the supernatants of the faeces, blood and urine samples. The proportion of methanol to these samples was 1:3. These samples were precipitated for 30 min and then centrifuged at 14,000 rpm for 15 min to obtain the solutions after treatment. The third method (method III) used acetonitrile to precipitate the above biological samples. The ratio of acetonitrile to sample was also one to three, and then the same process described in method II was carried out. All the supernatants were used for further instrumental analysis.
2.4 Instrument and conditions
LC analysis was performed on a DIONEX Ultimate 3000 UHPLC system (Thermo Electron, Bremen, Germany), equipped with a binary pump and an auto-sampler. The chromatographic separation was carried out using Waters ACQUITY BEH C18 column (2.1 × 100 mm i.d., 1.7 μm; Waters Corporation, Milford, MA, USA) at 35 °C. Methanol (solvent B) and 0.1% formic acid aqueous solution (solvent A) were used as mobile phase. The flow rate was set to 0.3 mL/min with a linear gradient as follows: 0–2 min, 25% B; 2–11 min, 25–55% B; 11–17 min, 55–95% B. The injection volume was 2 μL.
High resolution ESI-MS and MS/MS spectra were obtained using LTQ-Orbitrap MS (Thermo Electron, Bremen, Germany) connected to the UHPLC instrument via ESI interface. Samples were analyzed in negative ion mode with the tune method set as follows: sheath gas (nitrogen) flow rate of 40 arb, auxiliary gas (nitrogen) flow rate of 20 arb, capillary temperature of 350 °C, spray voltage of 4.0 kV, capillary voltage of 25 V, tube lens voltage of 110 V. Metabolites were detected using full-scan MS analysis from m/z 50-800 at a resolving power of 30,000 FWHM. The collision energy for collision induced dissociation (CID) was adjusted to 40% of maximum. In addition, the PIL-dependent acquisition mode was employed as a complementary tool to obtain the ESI-MS/MS dataset of potential metabolites.
2.5 Peak selections and data processing
Thermo Xcalibur 2.1 workstation was used for data acquisition and processing. In order to obtain as many ESI-MS/MS fragment ions of resveratrol metabolites as possible, the peaks detected with intensity over 10,000 were selected for identification. The accurate mass of chemical formulas attributed to all parent ions of the selected peaks were calculated using a formula predictor by setting the parameters as follows: C [0–35], H [0–40], O [0–16], S [0–1] and ring double bond (RDB) equivalent value [0–14]. Accurate mass measurements were set within mass error of ±5 ppm.
3 Results and discussion
3.1 The establishment of analytical strategy
In this study, all the collected biological samples (urine, plasma and faeces) were prepared using the three abovementioned methods, which included solid phase extraction, methanol precipitation and acetonitrile precipitation. Then the samples were injected into UHPLC-Q-Exactive Orbitrap MS to gain the HRMS data with full MS scanning acquisition. Then, data mining was processing based on common biotransformation reactions as well as the reported metabolites in literature. And subsequently, the ions which we were interested in were put into PIL to obtain more comprehensive MS2 information for structural identification. Among them, polydatin, dihydroresveratrol and pterostilbene were also regarded as pivotal candidate metabolites and filled into PIL. Finally, an efficient analysis based on UHPLC-LTQ-Orbitrap MS coupled with FS-PIL-DE was proposed to characterize and screen the metabolic products. Combining the chromatographic retention behaviours, accurate mass measurements, mass fragmentation patterns and previous relevant literature, was conducive to the comprehensive analysis of the metabolites of the resveratrol representative chemical components. In addition, a diagram that summarizes the presently developed analytical strategy and methodology for the detection and identification of resveratrol metabolites is shown in Fig. 1.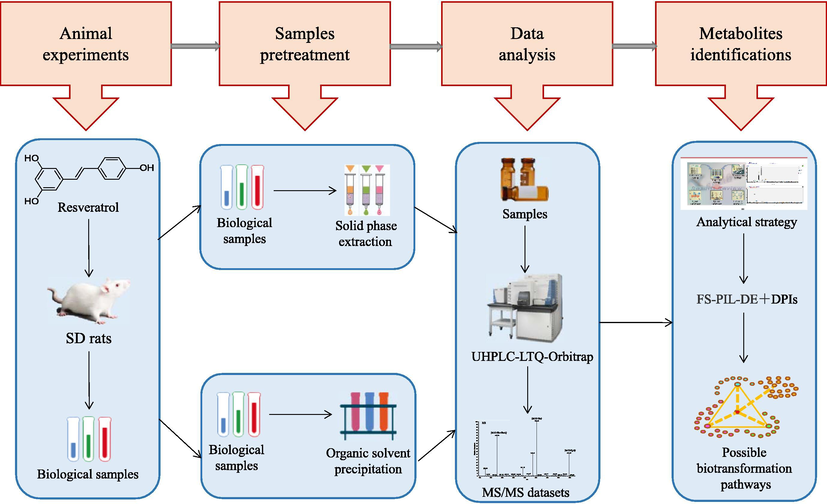
The summary diagram of analytical strategy and methodology.
3.2 Fragmentation patterns analysis of resveratrol
In order to better characterize the in vivo metabolites, DPIs were essential for structural identification. Resveratrol was selected as the subject to determine its DPIs based on the comprehensive ESI-MSn information of the resveratrol standard via UHPLC-HRMS analysis. For example, resveratrol gave rise to deprotonated [M−H]− ions at m/z 227.07057, as shown in Fig. 2. Then, it would further afford a series of DPIs at m/z 185, 159 and 143 owing to the loss of C2H2O (42 Da), C2H2O + C2H2 (68 Da) and 2C2H2O (84 Da) (Wang et al., 2005; Mei et al., 2019). Therefore, the product ions of resveratrol metabolites at m/z 185 + X, m/z 159 + X and m/z 143 + X (X = molecular weight of the substituent groups), such as 14 (CH2), 80 (SO3), and 176 (GluA), could also be observed in the ESI-MS/MS spectra.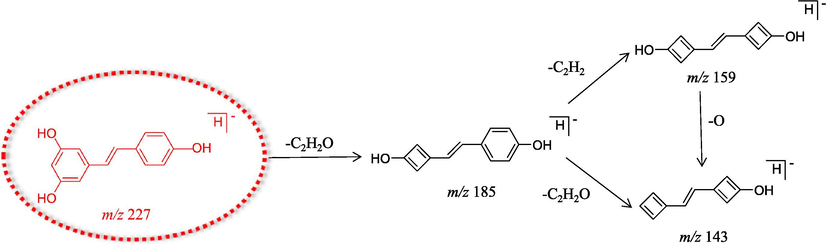
The proposed fragmentation pathways of resveratrol.
3.3 Identification of resveratrol metabolites
A total of 56 resveratrol metabolites (resveratrol included) were detected and characterized from rat urine, plasma and faecal samples by means of the UHPLC-LTQ-Orbitrap method coupled with the established strategy. Among them, 39 metabolites were found in rat urine, 6 metabolites were detected in rat plasma, and 16 metabolites were characterized from rat faeces. The correlative data in negative ion mode are summarized in Table 1. Note: tR: retention time; RDB: unsaturation; U: urine; P: plasm; F:feces; +: detected; *:unambiguously identification by comparing with the reference substances.
Peak
tR (min)
Formula [M + H]+
Theoretical Mass (m/z)
Experimental Mass (m/z)
RDB
Error (ppm)
MS/MS fragment ions
Identification/Reactions
U
P
F
N1
1.91
C14H11O4
243.06572
243.06580
9.5
2.529
173(100),201(56),212(47),199(18),145(14),198(14),225(11)
resveratrol hydroxylation/isomer
+
N2
2.74
C14H11O4
243.06636
243.06572
9.5
2.200
201(100),215(41),199(30),109(28),225(25),181(15),173(10)
resveratrol hydroxylation/isomer
+
N3
3.03
C7H5O3
137.02456
137.02394
5.5
4.521
93(100),137(55),109(8),95(5),117(3)
4-hydroxybenzoic acid
+
N4
3.59
C7H5O3
137.02456
137.02321
5.5
−0.807
137(100),93(91),119(19),95(18),109(14),106(14)
4-hydroxybenzoic acid
+
+
N5
3.61
C20H21O10
421.11399
421.11301
10.5
0.206
403(100),175(99),341(23),245(21),333(17),302(15)
dihydroresveratrol glucuronidation, hydroxylation
+
N6
3.65
C14H11O7S
323.02306
323.02252
9.5
1.610
243(100),244(5),241(3),267(2),278(1)
resveratrol sulfation, hydroxylation/isomer
+
N7
3.71
C26H29O14
565.15629
565.15515
12.5
−0.056
403(100),227(74),547(65),175(56),341(38),389(37)
resveratrol glucosylation, glucuronidation
+
N8
3.91
C26H29O14
565.15629
565.15533
12.5
0.262
403(100),227(73),547(71),385(41),341(14),175(12)
resveratrol glucosylation, glucuronidation
+
N9
3.95
C26H27O15
579.13556
579.13428
13.5
−0.287
403(100),175(1)
resveratrol bis-glucuronidation
+
N10
3.96
C26H29O15
581.15119
581.15100
12.5
1.555
403(100),534(2),175(2),535(1)
dihydroresveratrol bis-glucuronidation
+
N11
4.05
C20H21O11S
469.08099
469.08044
10.5
1.133
227(100),389(29),307(9),306(8),405(8),243(5)
resveratrol glucosylation, sulfation
+
N12
4.11
C14H11O7S
323.02306
323.02237
9.5
1.146
243(100),306(10),189(6),203(6)
resveratrol sulfation, hydroxylation/isomer
+
N13
4.22
C14H11O7S
323.02306
323.02264
9.5
1.982
243(100),244(8)0.295(7),241(6),189(4)
resveratrol sulfation, hydroxylation/isomer
+
N14
4.28
C20H21O11S
469.08099
469.07977
10.5
−0.295
227(100),307(18),241(10),269(6)
resveratrol glucosylation, sulfation
+
N15
4.45
C20H19O9
403.10339
403.10275
11.5
0.971
384(100),385(71),374(12),227(12),375(11),175(9)
resveratrol glucuronidation/isomer
+
N16
4.67
C20H19O10
419.09836
419.09790
11.5
1.496
243(100),175(22),401(3)
resveratrol hydroxylation, glucuronidation
+
N17
4.70
C14H11O7S
323.02306
323.02255
9.5
1.703
243(100),295(16),241(12),244(9),225(4)
resveratrol sulfation, hydroxylation/isomer
+
N18
4.78
C20H21O11S
469.08099
469.08044
10.5
1.133
227(100),307(16),269(3),185(2)
resveratrol glucosylation, sulfation
+
N19
4.78
C26H29O14
565.15629
565.15515
12.5
−0.056
337(100),385(26),179(17),227(16),175(8),237(7)
resveratrol glucosylation, glucuronidation
+
N20*
4.80
C20H21O8
389.12416
389.12402
10.5
2.380
175(100),385(28),227(21),323(10)
polydatin
+
N21
4.87
C20H19O9
403.10346
403.10269
11.5
0.822
175(100),227(36),385(20),341(4)
resveratrol glucuronidation/isomer
+
+
N22
5.01
C14H11O7S
323.02306
323.02261
9.5
1.889
243(100),259(2),241(1)
resveratrol sulfation, hydroxylation/isomer
+
N23
5.10
C14H11O6S
307.02816
307.02789
9.5
2.621
227(100),228(1)
resveratrol sulfation/isomer
+
N24
5.24
C14H11O6S
307.02816
307.02713
9.5
0.146
227(100),243(8)
resveratrol sulfation/isomer
+
N25
5.29
C14H11O4
243.06572
243.06593
9.5
3.064
225(100),201(63),199(38),175(31),200(18),159(16)
resveratrol hydroxylation/isomer
+
N26
5.46
C14H11O6S
307.02816
307.02695
9.5
−0.440
227(100),243(10)
resveratrol sulfation/isomer
+
N27
5.54
C20H21O8
389.12416
389.12427
10.5
3.022
175(100),385(34),227(14),359(11)
polydatin/isomer
+
N28
5.55
C20H19O9
403.10346
403.10364
11.5
3.179
175(100),385(34),227(14),359(11)
resveratrol glucuronidation/isomer
+
N29
5.56
C14H11O4
243.06572
243.06587
9.5
2.817
225(100),201(65),199(34),175(33),200(17),215(16)
resveratrol hydroxylation/isomer
+
N30
5.59
C14H11O6S
307.02816
307.02771
9.5
2.035
227(100),243(8),289(1)
resveratrol sulfation/isomer
+
N31
5.64
C14H11O6S
307.02816
307.02800
9.5
2.979
227(100),243(13),228(2)
resveratrol sulfation/isomer
+
N32
5.81
C15H13O4
257.08186
257.08115
9.5
1.224
241(100),242(49),224(17),172(11),147(9)
resveratrol methylation, hydroxylation/isomer
+
N33
5.81
C20H19O9
403.10346
403.10233
11.5
−0.071
175(100),227(23),385(17),244(4),384(3)
resveratrol glucuronidation/isomer
+
N34
5.85
C14H11O4
243.06572
243.06598
9.5
3.269
225(100),201(63),199(38),175(32),200(18),215(16)
resveratrol hydroxylation/isomer
+
N35
6.03
C20H19O9
403.10346
403.10248
11.5
0.301
175(100),227(28),385(22),341(4),345(4)
resveratrol glucuronidation/isomer
+
N36
6.09
C14H11O3
227.07136
227.07103
9.5
3.344
185(100),183(42),159(32),157(27),143(16),227(14),209(8)
resveratrol/isomer
+
N37
6.09
C20H21O8
389.12416
389.12375
10.5
1.686
345(100),289(72),307(46),371(21),306(11)
polydatin/isomer
+
N38
6.25
C14H11O3
227.07146
227.07065
9.5
1.670
185(100),183(40),159(31),157(25),143(15),227(15),141(5)
resveratrol/isomer
+
N39
6.30
C14H11O6S
307.02816
307.02747
9.5
1.253
227(100),243(7),289(2),225(1)
resveratrol sulfation/isomer
+
+
N40
6.39
C14H9O4
241.05056
241.05030
10.5
3.172
213(100),223(27),199(17),184(11),173(10),197(5)
resveratrol hydroxylation, dehydrogenation
+
N41
6.57
C15H13O4
257.08186
257.08112
9.5
1.107
135(100),121(58),239(29),109(24),147(14),242(11)
resveratrol methylation, hydroxylation/isomer
+
+
N42
6.65
C15H13O4
257.08186
257.08136
9.5
2.041
135(100),121(56),109(54),242(37),239(35),241(33),147(22)
resveratrol methylation, hydroxylation/isomer
+
N43
6.65
C14H11O6S
307.02816
307.02747
9.5
1.253
227(100),243(11)
resveratrol sulfation/isomer
+
N44*
6.68
C14H11O3
227.07136
227.07057
9.5
1.318
185(100),183(40),159(33),157(24),143(13),227(12)
resveratrol
+
N45
6.68
C20H19O9
403.10339
403.10272
11.5
0.897
385(100),384(27),367(19),387(6)
resveratrol glucuronidation/isomer
+
N46
7.64
C14H11O3
227.07136
227.07083
9.5
2.463
185(100),183(39),159(29),157(24),143(15),227(13)
resveratrol/isomer
+
+
N47
7.84
C20H19O9
403.10346
403.10287
11.5
1.269
175(100),385(72),323(57),322(32),324(26),285(25),359(24)
resveratrol glucuronidation/isomer
+
N48
7.99
C14H11O7S
323.02306
323.02136
9.5
−1.981
149(100),279(45),243(43),261(20),305(19),251(14)
resveratrol sulfation, hydroxylation/isomer
+
N49
8.06
C14H11O3
227.07136
227.07089
9.5
2.727
185(100),183(46),159(31),157(23),143(17),227(12),181(5)
resveratrol/isomer
+
N50
8.70
C14H11O3
227.07136
227.07088
9.5
2.683
185(100),183(46),159(29),157(27),143(17),227(14),141(6)
resveratrol/isomer
+
N51
9.64
C15H13O4
257.08186
257.08142
9.5
2.274
109(100),239(95),213(29),242(25),147(15),148(14),136(13)
resveratrol methylation, hydroxylation/isomer
+
N52
9.85
C15H13O4
257.08186
257.08072
9.5
−0.449
239(100),221(10),195(7),147(7),213(6),237(5),242(5)
resveratrol methylation, hydroxylation/isomer
+
N53
9.86
C20H19O9
403.10346
403.10260
11.5
0.599
175(100),385(88),323(82),293(71),335(65),359(56)
resveratrol glucuronidation/isomer
+
N54
9.92
C14H11O3
227.07136
227.07092
9.5
2.859
185(100),183(42),159(32),157(27),143(14),207(12),212(12)
resveratrol/isomer
+
N55
10.74
C14H11O3
227.07136
227.07086
9.5
2.595
185(100),183(58),159(43),157(36),143(13),227(12),199(9)
resveratrol/isomer
+
N56
12.18
C14H11O3
227.07136
227.07088
9.5
2.683
185(100),183(60),157(42),143(33),159(32),207(20)
resveratrol/isomer
+
Metabolites N1, N2, N25, N29 and N34 possessed deprotonated molecular ions at m/z 243.06580, m/z 243.06572, m/z 243.06593, m/z 243.06587, and m/z 243.06572 (C14H11O4) with mass errors of 2.529, 2.200, 3.064, 2.817 and 3.269 ppm, respectively. Fragment ions at m/z 201 [M−H−C2H2O]−, m/z 175 [M−H−C2H2O−C2H2]− and m/z 159 [M−H−2C2H2O]− were all observed in the ESI-MS2 spectra. In addition, their [M−H]− ions at m/z 243 were 16 Da more than that of resveratrol, which implied the occurrence of a hydroxylation reaction. Thus, N1, N2, N25, N29 and N34 were plausibly assigned as hydroxylated resveratrol.
N3 and N4 afforded [M−H]− ions at m/z 137.02394 and m/z 137.02321 (C7H5O3, mass errors 4.521 ppm and −0.807 ppm), respectively. They generated ESI-MS2 DPIs at m/z 93 and m/z 109 due to the neutral loss of CO2 and CO, which indicated the presence of carboxyl groups. Therefore, N3 and N4 could be deduced as isomeric 4-hydroxybenzoic acid.
N5 was 194 Da greater than that of dihydroresveratrol, which displayed an [M−H]− ion at m/z 421.11301 with a mass error of 0.206 ppm. In its ESI-MS2 spectrum, the deprotonated molecular [M−H]− ion at m/z 421 further generated several product ions at m/z 245 [M−H−GluA]− and m/z 175 [GluA-H]−. Based on this, N5 was identified as glucuronidation and hydroxylation product of dihydroresveratrol.
Six isomeric constituents, N6, N12, N13, N17, N22 and N48, gave rise to [M−H]− ions at m/z 323.02252, m/z 323.02237, m/z 323.02264, m/z 323.02255, m/z 323.02261 and m/z 323.02136 (C14H11O7S) with mass errors of 1.610, 1.146, 1.982, 1.703, 1.889 and −1.981 ppm, respectively. In the ESI-MS2 spectra, the characteristic product ion at m/z 243 was generated by the loss of 80 (SO3) from the deprotonated molecular [M−H]− ion at m/z 323. Moreover, N6, N12, N13, N17, N22 and N48 were 96 Da more than that of resveratrol. Therefore, N6, N12, N13, N17, N22 and N48 were isomeric sulfated and hydroxylated metabolites of resveratrol.
N7, N8 and N19 showed [M−H]− ions at m/z 565.15515, m/z 565.15533 and m/z 565.15515 (C26H29O14, mass error −0.056, 0.262 and −0.056 ppm), respectively.
These peaks were 338 Da more than that of resveratrol, indicating that they could be deduced as glucosylated and glucuronidated metabolites of resveratrol. In addition, the fragment ions at m/z 547 [M−H−H2O]−, DPIs at m/z 403 [M−H−Glc]− and m/z 227 [M−H−Glc−GluA]− were all observed in the ESI-MS2 spectra. Based on this, N7, N8 and N19 were tentatively identified as isomeric resveratrol glucosylated and glucuronidated metabolites.
N9 produced the theoretical [M−H]− ion at m/z 579.13428 (C26H27O15, mass error −0.287 ppm) with the mass being 352 Da more than that of resveratrol and was formed by the bis-glucuronidation of resveratrol in vivo. Owing to the successive loss of GluA, the [M−H]− ion generated an ion at m/z 403 in the ESI-MS2 spectrum. Therefore, N9 could be deduced as a resveratrol bis-glucuronidated metabolite. Additionally, N10 generated an [M−H]− ion at m/z 581.15100 (C26H29O15, mass error 1.555 ppm). The ESI-MS2 characteristic product ion [GluA-H]− at m/z 175 showed the loss of 176 (GluA). Moreover, its [M−H]− ions were 2 Da more than that of N9, revealing that the bis-glucuronidation reaction most likely occurred. Thus, N10 was speculated to be a dihydroresveratrol bis-glucuronidation metabolite.
In negative ion mode, N11, N14 and N18 produced [M−H]− ions at m/z 469.08044, m/z 469.07977 and m/z 469.08044 with mass errors of 1.133, −0.295 and 1.133 ppm (C20H21O11S), respectively. In the ESI-MS2 spectra, the fragment ion at m/z 389 [M−H−SO3]− indicated that a sulfation reaction occurred. In addition, the neutral loss of 162 Da (m/z 389 → m/z 227) demonstrated the existence of a glucosyl group. Therefore, N11, N14 and N18 were tentatively characterized as isomeric glucosylated and sulfated metabolites of resveratrol.
Eight isomers, N15, N21, N28, N33, N35, N45, N47 and N53, were 176 Da more than that of resveratrol, which respectively afforded [M−H]− ions at m/z 403.10275, m/z 403.10269, m/z 403.10364, m/z 403.10233, m/z 403.10248, m/z 403.10272, m/z 403.10287, and m/z 403.10260 (C20H19O9, mass errors 0.971, 0.822, 3.179, −0.071, 0.301, 0.897, 1.269 and 0.599 ppm), respectively. In their ESI-MS2 spectra, the characteristic product ions at m/z 227 [M−H−GluA]− and m/z 175 [GluA-H]− were detected. Therefore, N15, N21, N28, N33, N35, N45, N47 and N53 were tentatively identified as glucuronidated resveratrol or its isomers (Zhou et al., 2009; Zhang et al., 2008).
N16 possessed theoretical [M−H]− ions at m/z 419.09790 (C20H19O10, mass error 1.496 ppm). In the ESI-MS2 spectra, a battery of fragment ions at m/z 243 [M−H−GluA]−, m/z 175 [GluA-H]− and m/z 401 [M−H−H2O]− provided substantial evidence for this deduction. From the abovementioned analysis, N16 was tentatively judged as isomeric hydroxylation and glucuronidation metabolite of resveratrol.
N20, N27 and N37 displayed significant [M−H]− ions at m/z 389.12402, m/z 389.12427 and m/z 389.12375 (C20H21O8, mass errors 2.380, 3.022 and 1.686 ppm), respectively. They generated a DPI ion at m/z 227 in their ESI-MS2 spectra. In addition, their [M−H]− ions were 162 Da more than that of resveratrol, which indicated the occurrence of a glucosylation reaction. Based upon comparison of their ESI-MS/MS spectra and retention time with the corresponding reference standard, N20 was characterized as polydatin, while N27 and N37 were identified as polydatin isomers (Fu et al., 2018).
N23, N24, N26, N30, N31, N39 and N43 showed [M−H]− ions at m/z 307.02789, m/z 307.02713, m/z 307.02695, m/z 307.02771, m/z 307.02800, m/z 307.02747, and m/z 307.02747 with mass errors of 2.621, 0.146, −0.440, 2.035, 2.979, 1.253 and 1.253 ppm, respectively. They were 80 Da more than that of resveratrol, which illustrated the existence of a sulfation reaction. Based upon the obtained high-resolution mass spectrometry data, they yielded DPIs at m/z 227 due to the loss of SO3. Thus, N23, N24, N26, N30, N31, N39 and N43 were tentatively interpreted as isomeric sulfated metabolites of resveratrol.
N32, N41, N42, N51 and N52 yielded significant [M−H]− ions at m/z 257.08115, m/z 257.08112, m/z 257.08136, m/z 257.08142 and m/z 257.08072 (C15H13O4, mass error 1.224, 1.107, 2.041, 2.274 and −0.449 ppm), respectively. They were 30 Da more than that of resveratrol, indicating that they could be methylated and hydroxylated metabolites of resveratrol. Moreover, they afforded DPIs at m/z 242 [M−H−CH3]− and 147 [M−H−2C2H2O−C2H2]−. According to the above deduction, N32, N41, N42, N51 and N52 were predicted to be methylated and hydroxylated metabolites of resveratrol.
Nine isomers, N36, N38, N44, N46, N49, N50, N54, N55 and N56, gave rise to theoretical [M−H]− ions at m/z 227.07103, m/z 227.07065, m/z 227.07057, m/z 227.07083, m/z 227.07089, m/z 227.07088, m/z 227.07092, m/z 227.07086, m/z 227.07088 with mass errors of 3.344, 1.670, 1.318, 2.463, 2.727, 2.683, 2.859, 2.595 and 2.683 ppm, respectively. In the ESI-MS2 spectra, there were many observed characteristic product ions at m/z 185, m/z 159 and m/z 143. Among them, the ion at m/z 185 [M−H−C2H2O]− was further fragmented to generate m/z 159 [M−H−C2H2O−C2H2]− and m/z 143 [M−H−2C2H2O]− after the loss of 26 Da and 42 Da, which were consistent with those documented in a previous report. Compared with the standard substance, N44 was positively identified as resveratrol. From the above data, N36, N38, N46, N49, N50, N54, N55 and N56 were tentatively characterized as resveratrol isomers.
N40 generated a deprotonated molecular [M−H]− ion at m/z 241.05030 (C14H9O4, mass error 3.172 ppm). It was 14 Da more than that of resveratrol, revealing that hydroxylation and dehydrogenation in vivo might have occurred. In its ESI-MS2 spectra, DPIs at m/z 199 and m/z 173 were observed from the loss of C2H2O and C2H2O + C2H2. Therefore, N40 was tentatively identified as an isomeric hydroxylated and dehydrogenated metabolite of resveratrol. In addition, the ESI-MS2 spectra of N8, N11 and N16 are illustrated in Fig. 3.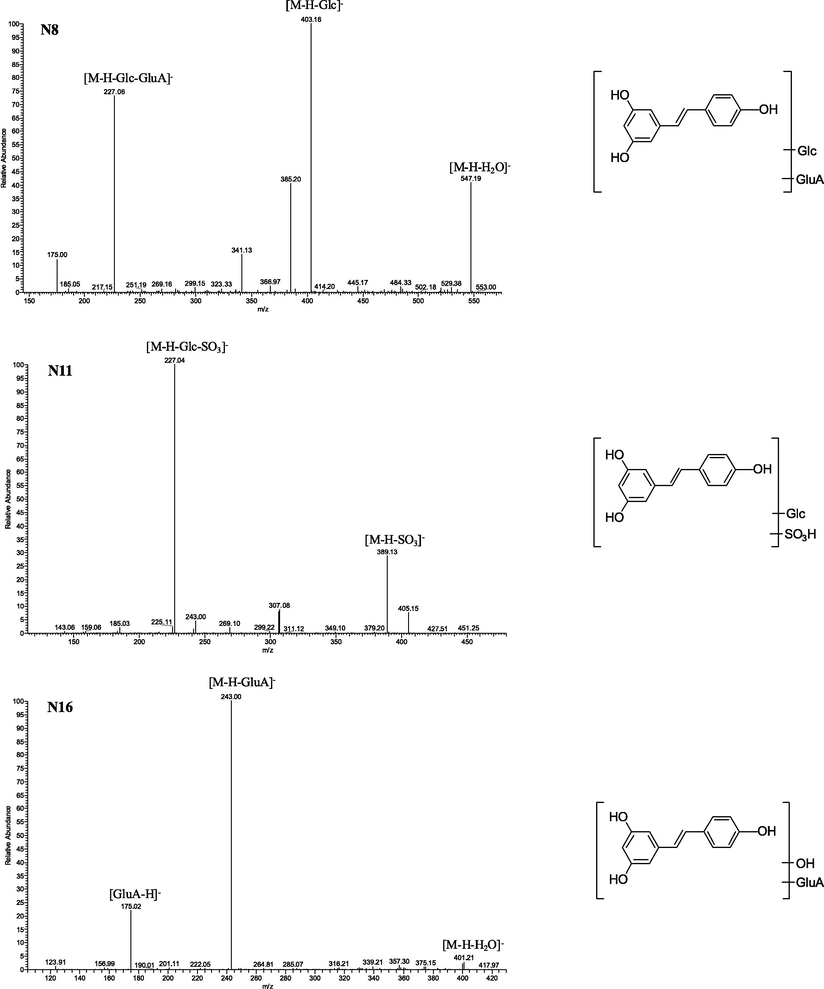
The ESI-MS2 spectra and chemical structures of N8, N11 and N16.
3.4 Comparison of the different biological treatment methods
It should be noted that three methods were used to prepare the biological samples, and then the same analytical method was applied to detect the signals. According to the accurate mass measurements, fragmentation patterns, diagnostic product ions and literature reports, a total of 56 resveratrol phase I and phase II metabolites were screened and identified in rats by using the UHPLC-LTQ-Orbitrap method. Among them, 56 metabolic products were obtained by solid phase extraction, which was also named method I. Twelve and 15 metabolites were screened by methanol precipitation (method II) and acetonitrile precipitation (method III), respectively, as shown in Fig. 4.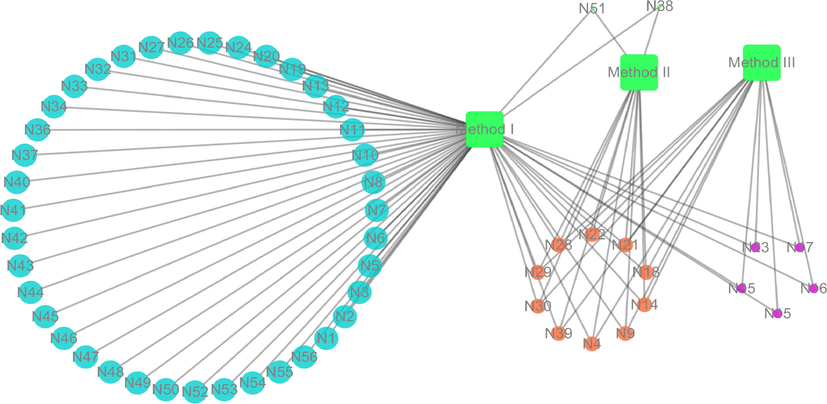
The metabolic prodycts distribution of three biological treatment methods.
As far as we know, the above three methods which bear a great significance in the pretreatment of biological materials are widely applied in various laboratories at present. On the basis of the results, it could be inferred that the majority of the metabolites were obtained by method I after the same detection analysis. It showed that the SPE cartridge was the most suitable method with the functions of enrichment and purification. In the preparation process, the target substance could be enriched, and then the impurities could be removed before instrumental analysis to improve analytical sensitivity and reduce damage to the instrument (Jordan et al., 2009; Nema et al., 2010). It might be deduced that SPE cartridge could provide much more effective and reliable support for removing matrix effect. After the sample was concentrated by SPE cartridge, more response signals could be detected in mass spectrometry. However, methanol and acetonitrile as organic solvents played the role of extraction and dilution in the process of sample precipitation, which might also indirectly lead to the concentration difference and undetection phenomenon. Furthermore, products of metabolism afforded by acetonitrile precipitation were slightly more abundant than those obtained by methanol precipitation, which indicated that acetonitrile might be more adaptive for resveratrol as a precipitation solvent than methanol. These differences in metabolites quantity might be due to the different separation selectivities of organic solvents, which lead to signal peaks with diverse intensity and quantity concerning resveratrol metabolites after UHPLC-HRMS detection.
3.5 Possible biotransformation pathways of resveratrol in rats
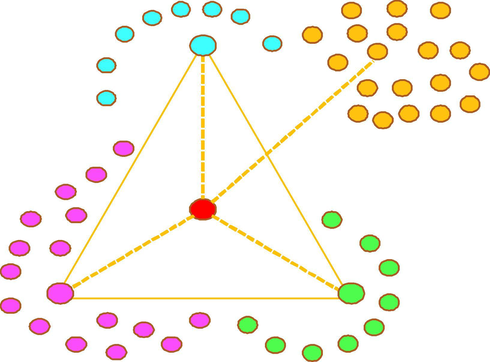
As“metabolite clusters” mentioned by Mei (Mei et al., 2019), resveratrol was regarded as the parent drug. When the parent drug was taken into the rat body, a series of metabolic reactions could occur to form a metabolic network similar to a triangular pyramid model (as shown in Figs. 5 and 6). These reactions could be roughly summarized as follows: the parent drug acted as the metabolic centre to generate secondary metabolic centres such as polydatin, dihydroresveratrol and hydroxylated resveratrol, and a great deal of final metabolites were derived from the secondary metabolic centres step by step. Among them, resveratrol mainly underwent glucuronidation, glucosylation, sulfation, hydroxylation, dehydrogenation, hydrogenation, methylation and their composite reactions. Phase II metabolism and its complicated reactions occupied the main status of these reactions. Of course, there were differences between the metabolic products and metabolic rates. More water-soluble metabolites, such as glucuronidated resveratrol and sulfated resveratrol, are generally produced through phase II reactions in order to prepare the drug for excretion (Wilkinson, 2005; Xu et al., 2005). It is also worth noting that the metabolic centres or the final metabolism products were not isolated. There might be some transformation relationships among these products. With the development of the reactions in vivo, drug metabolites were also enriched step by step. This “metabolite clusters” theory could better guide the screening of metabolites and reveal the metabolic process. However, these metabolites were also difficult to detect due to their low content, and many isomers were easily yielded in the biotransformation process (Wang et al., 2019). Therefore, it is urgent to establish high performance testing instruments and comprehensive and accurate analysis methods to help solve such problems.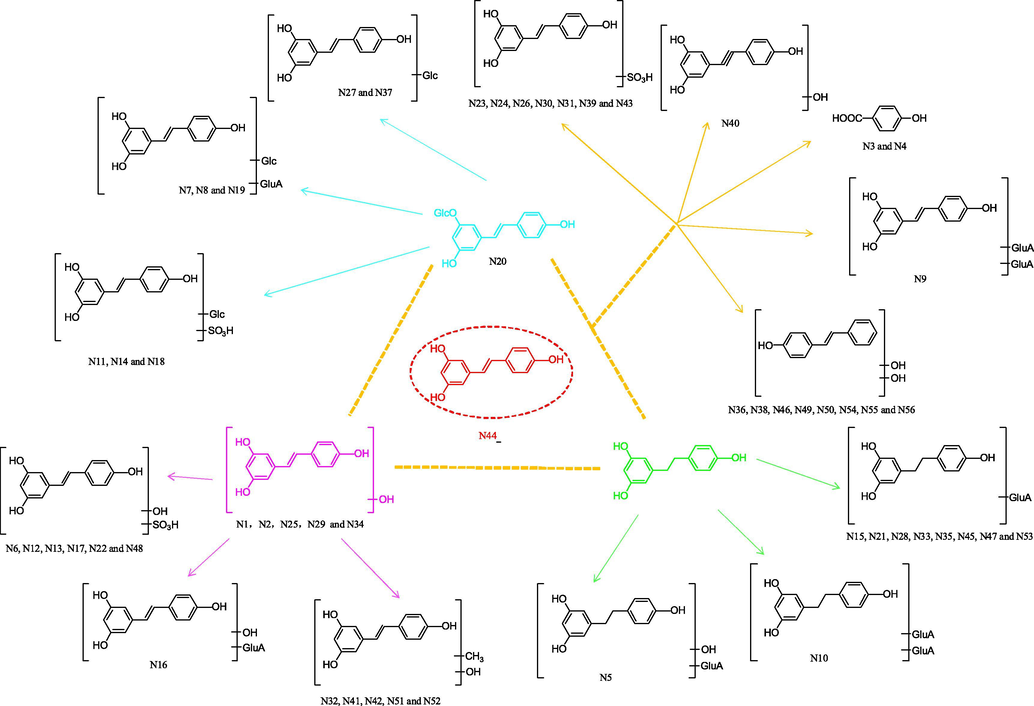
The possible resveratrol metabolic patterns in rats in vivo.
4 Conclusion
In this study, three methods of biological sample preparation were applied to analyse the in vivo metabolism of resveratrol in rats. It’s noted that SPE cartridge could remove matrix effect better. Meanwhile, the automation of SPE cartridge was helpful to process analysis of massive biological samples, which presented some advantages in saving time and working efficiently. Finally, a total of 56 resveratrol metabolites were detected and identified by the UHPLC-HRMS method. Among them, 39 metabolites were found in rat urine, 6 metabolites were detected in rat plasma, and 16 metabolites were characterized from rat faeces. The discrepancy in the number of metabolites detected might be due to the different metabolic transformation ability of phase II in plasma, urine and feces. At the same time, the intermediate and final metabolites formed a metabolite cluster, which was set in the triangular pyramid model, which revealed the potential pharmacodynamics forms of resveratrol.
In addition, the distinguishment of some isomers is still a challenge, which needs to pay more attention in the following researches. The ESI-MS/MS spectra fragmentation information with some other information such as the ClogP value to determine the possible position of substituents are much more logical technique. In a word, the present results not only supplied useful data for a better understanding of further research on resveratrol but also provided ideas and analysis for metabolites of other compounds.
Acknowledgments
This work has been financially supported by Young and Creative Team for Talent Introduction of Shandong Province, Binzhou Medical University Scientific Research Fund for High-level Talents (2019KYQD06), Locality-University Cooperation Project of Yantai City (2019XDRHXMPT18).
Declaration of Competing Interest
There are no conflicts of interest to declare.
References
- Resveratrol affects the cross talk between immune and colon cancer cells. Biomed. Pharmacother.. 2013;67:43-47.
- [Google Scholar]
- Quantitation of trans-resveratrol and detection of its metabolites in human plasma and urine by high performance liquid chromatography. J. Chromatogr. B. 2007;848:182-187.
- [Google Scholar]
- Preparation of resveratrol dry suspension and its immunomodulatory and anti-inflammatory activity in mice. Pharm Bio.. 2020;58:8-15.
- [Google Scholar]
- Novelty application of multi-omics correlation in the discrimination of sulfur-fumigation and non-sulfur-fumigation Ophiopogonis Radix. Sci. Rep.-UK. 2017;7:9971.
- [Google Scholar]
- Planas. Quantification of trans-resveratrol and its metabolites in rat plasma and tissues by HPLC. J. Pharm. Biomed.. 2010;70:391-398.
- [Google Scholar]
- Simultaneously targeted and untargeted multicomponent characterization of Erzhi Pill by offline two-dimensional liquid chromatography/quadrupole-Orbitrap mass spectrometry. J. Chromatogr. A. 2019;1584:87-96.
- [Google Scholar]
- Intestinal Metabolism of Polygonum Cuspidatum in vitro and in vivo. Biomed. Chromatogr.. 2018;32:4190.
- [Google Scholar]
- Selection of SPE cartridge for automated solid-phase extraction of pesticides from water followed by liquid chromatography-tandem mass spectrometry. Anal. Bioanal. Chem.. 2009;394:2257-2266.
- [Google Scholar]
- Comprehensive metabolism study of polydatin in rat plasma and urine using ultra-high performance liquid chromatography coupled with high-resolution mass spectrometry. J. Chromatogr. B. 2019;1117:22-35.
- [Google Scholar]
- Application of silica-based monolith as solid phase extraction cartridge for extracting polar compounds from urine. Talanta. 2010;82
- [Google Scholar]
- An integrated approach for global profiling of multi-type constituents: Comprehensive chemical characterization of Lonicerae Japonicae Flos as a case study. J. Chromatogr. A. 2020;1613:460674.
- [Google Scholar]
- Clinical pharmacology of resveratrol and its metabolites in colorectal cancer patients. Cancer Res.. 2010;70:7392-7399.
- [Google Scholar]
- An integrated strategy for rapid discovery and identification of the sequential piperine metabolites in rats using ultra high-performance liquid chromatography/high resolution mass spectrometery. J. Pharmaceut. Biomed.. 2017;146:387.
- [Google Scholar]
- Anti-inflammatory effects of resveratrol and its potential use in therapy of immune-mediated diseases. Int. Rev. Immunol.. 2012;31:202-222.
- [Google Scholar]
- Identification of the major metabolites of resveratrol in rat urine by HPLC-MS/MS. J. Chromatogr. B. 2005;829:97-106.
- [Google Scholar]
- Drug metabolite cluster-Based data-mining method for comprehensive metabolism study of 5-hydroxy-6,7,3′,4′-tetramethoxyflavone in Rats. Molecules. 2019;2:3278.
- [Google Scholar]
- Drug metabolism and variability among patients in drug response. N. Engl. J. Med.. 2005;352:2211-2221.
- [Google Scholar]
- Neurological recovery and antioxidant effects of resveratrol in rats with spinal cord injury: a meta-analysis. Neural. Regen. Res.. 2020;3
- [Google Scholar]
- Induction of phase I, II and III drug metabolism/transport by xenobiotics. Arch. Pharm. Res.. 2005;28:49-268.
- [Google Scholar]
- Rapid screening and identification of diterpenoids in tinospora sinensis based on high-performance liquid chromatography coupled with linear ion trap-orbitrap mass spectrometry. Molecules. 2017;22:912.
- [Google Scholar]
- Rapid screening and identification of target constituents using full scan-parent ions list-dynamic exclusion acquisition coupled to diagnostic product ions analysis on a hybrid LTQ-Orbitrap mass spectrometer. Talanta. 2014;124:111-122.
- [Google Scholar]
- Direct determination of polydatin and its metabolite in rat excrement samples by high-performance liquid chromatography. Chem. Pharm. Bull.. 2008;56:1592-1595.
- [Google Scholar]
- Dose-dependent absorption and metabolism of trans-polydatin in rats. J. Agric. Food Chem.. 2009;57:4572-4579.
- [Google Scholar]