Translate this page into:
Comprehensive review in moisture retention mechanism of polysaccharides from algae, plants, bacteria and fungus
⁎Corresponding author. liuyang84@126.com (Yang Liu)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
Polysaccharides have attracted much attention due to their significant bio-activities. This review aims to summarize the main polysaccharides sources of related polysaccharides from algae, plants, fungus, and bacteria, and give insights into the structure–activity relationship between the moisture retention and structural characteristics of polysaccharides. The molecular weight, functional groups, polysaccharide modifications and apparent structure of polysaccharides are closely related to the moisturizing properties of polysaccharides in terms of moisturizing conformation. Based on recent moisturizing pieces of evidence, we propose a new framework focusing on the moisturizing intrinsic and extrinsic mechanisms. Polysaccharides molecular weight has different effects on moisturizing property. The extrinsic moisturization is mainly via the formation of hydrogen bonds between polysaccharides, the intrinsic moisturizing is mainly by regulating the production of some tight junction proteins. Accordingly, this review could further open the door for the production and application of polysaccharides as novel moisturizing agents in the cosmetic field.
Keywords
Polysaccharides
Moisture retention
Structure–activity relationship
1 Introduction
More and more consumers are paying attention to the use of cosmetics for their skincare effects, including moisturizing, antioxidants, and whitening. Among them, moisturization is not only a basic function of cosmetics to prevent skin moisture evaporation and improve cell activity, but also plays an essential role in skin care (Kircik, 2016; Razia et al., 2021). In general, there are two major sources of moisturizers, including traditional moisturizers from chemical production, and natural moisturizers from natural substances. As traditional moisturizing materials, polyols such as glycerol, propylene glycol, sorbitol, and polyethylene glycol are widely used in cosmetics owing to their low prices and high safety. They can also fill the stratum corneum space of the skin. However, polyol moisturizers containing multiple hydroxyl groups are highly hygroscopic and susceptible to environmental humidity, and have poor water retention. The skin, when exposed to hot and windy summer or cold environment, will absorb much water from its inner layer to be distributed in the outer layer. After a period of time, the skin will be abnormally dry and even damaged. Therefore, modern moisturizers are usually mixed from traditional moisturizers and natural moisturizers at certain ratio to avoid this shortcoming, and the effect is mild to some extent. Natural moisturizers including polysaccharides, peptides, and other active substances are mostly extracted from natural plants and animals. Particularly, polysaccharides are the more common moisturizers in cosmetics. The hydroxyl, carboxyl groups, and other polar groups commonly found in polysaccharides can form hydrogen bonds with water molecules. Hyaluronic acid (HA), an animal polysaccharide, was extracted for the first time from bovine vitreous in 1934 (Eleniet al., 2012). HA has excellent anti-aging and film-forming properties, especially moisturizing properties, so it is recognized as the best moisturizing factor in the cosmetics industry in recent years (Chen & Ling, 2010). However, because it mainly comes from some tissues and organs of cattle, its production cost and price are very high. In fact, it cannot be obtained in large quantities and is limited in the cosmetics industry (Bako et al., 1999; Ji et al., 2018). Fortunately, polysaccharides are polymers composed of various monosaccharide molecules, which are widely found in algae, bacteria, plants, animals and microorganisms. Due to abundant hydrophilic hydroxyl and carboxyl groups, polysaccharides exhibit some excellent physical and chemical properties, such as easy gel forming, high viscosity, and easy film forming (Hong, 2017). Reportedly, polysaccharides from seaweeds (e.g. altar nori, pine algae, green tube marsh), polysaccharides from some plants and mushrooms (e.g. Aloe barbadensis, Bletilla striata (Kong et al.,2015), Ginseng (Xu, 2020), Tremella fuciformis, and extracellular polysaccharides from certain microorganisms are widely used as moisturizing ingredients (Kanlayavattanakul et al., 2012) in moisturizing facial masks and cream (Fig. 1). These substances are used for food moisturizing (Torti et al., 2016) and cigarette moisturizing (Lei et al., 2020). It is unanimously agreed that the moisturizing effect of polysaccharides is mainly ascribed to the three-dimensional network structure formed from the interweaving of polysaccharide molecular chains, and the dense network has good film-forming properties that prevent moisture distribution from the skin. In addition, the polar groups on the polysaccharides form hydrogen bonds mutually, and are coupled with the dense network structure, which can tightly wrap the water molecules in the network chain to avoid water loss. Therefore, polysaccharides can be used as promising humectants to provide a continuously moist environment for the skin.
Source and application of moisturizing polysaccharide.
Despite some studies on the moisturizing activity of polysaccharides, their moisturizing abilities and action modes have not been systematically discussed. Therefore, a review of the structures and moisturizing abilities of polysaccharides will be of great significance for the future development of polysaccharides as natural moisturizers. Given the fact above and the obvious public need for developing the moisturizing function of polysaccharides, this review aims to introduce the main sources of relevant polysaccharides from algae, plants, fungus and bacteria, and give insights into their polysaccharide moisturizing constitutive relationships that concern with molecule weights, functional groups, modification and apparent structures.
2 Structural characteristic and conformational relationships of polysaccharides
In the nature, polysaccharides are polymeric carbohydrates with monosaccharides as their structural units, which are connected by glycosidic bonds. The common glycosidic bonds are α-1,4, β-1,3, β-1,4, and α-1,6-glycosidic bonds. The monosaccharide structural units are connected in a linear or branched form through glycosidic bonds (Cedric et al., 2011). These units usually contain more than 100 individual monosaccharides and can reach tens of thousands, or even millions of molecular weights (Qu et al., 2020). According to the composition, polysaccharides can be divided into homogeneous polysaccharides and heteropolysaccharides. From the arrangement of monosaccharides, straight chains are generally formed by α-1,4-glycosidic bonds and β-1,4-glycosidic bonds. The chain attachment point is often an α-1,6-glycosidic bond. Polysaccharides have a wide variety of monosaccharides, such as glucose, fructose, mannose, and uronic acid (Bao et al., 2009; Kamiryo et al., 2005). Various polysaccharides have a wide variety of components, mainly glucose, fructose, mannose, and glucuronic acid (He & Pan, 2010).
Polysaccharides are broadly divided into four structures. The current research focuses on the primary structures, including the way of polysaccharide linkage, chain length, and some functional groups (e.g. carboxyl and sulfate groups) attached to the polysaccharides. The secondary structures of polysaccharides are mainly represented by the polysaccharide chains mutually bonded through hydrogen bonds. Based on the secondary structures, polysaccharides are further coiled and folded to form spatial conformations with certain shapes and sizes, which are the tertiary structures, such as the three-stranded helical structure. Finally, the aggregates formed by the non-covalent bonds between polysaccharide chains are called quaternary structures (He & Pan, 2010).
In general, the various bioactivities of polysaccharides are often closely related to their chemical structures or conformational relationships. Most of the current studies also point to the conformational relationships between polysaccharides at the primary or higher levels of structures and activities. Along with the development of cellular and molecular biology and the advancement of polysaccharide separation and analysis, the bio-activity investigation of polysaccharides and the corresponding chemical structures become hot topics for researchers. Among them, the studies on the conformational relationships of polysaccharides mostly cover the types of glycosidic bonds, conformation of the main branched chains, composition of monosaccharides, three-dimensional conformations, types and numbers of groups, and molecular weights (He et al., 2014; Qu et al., 2020).
In terms of polysaccharide unit composition, some polysaccharides may inhibit human cell mutation and antioxidant activity owing to their unique monosaccharide composition, such as mannose isolated from yeast cell walls (Krizkova et al., 2001). The structural units of polysaccharides are connected by glycoside bonds, mainly including α- or, β-1,4-glycoside bond, and α- 1,6-glycoside bond. The polysaccharides linked by β-bonds are usually more active, mainly because polysaccharides linked by α-bonds are easily hydrolyzed by α-glycosidases in vivo (Wang et al., 2002). Studies confirm that Lentinan (Wasser, 2002) and Polyporus umbellatus polysaccharide (Liu et al., 2011) have significant antitumor activity, mainly owing to the main chain structures with β-(1 → 3)-d-glucan. In terms of substituents, the existence and quantity of sulfate play a very obvious role in anticoagulant and antiviral activities (Shang, Li, Zhou, & Huang, 2019). Compared with unmodified polysaccharides, the anticoagulant effect of some polysaccharides after sulfation modification is greatly enhanced (Zhou, Xie, & Fu, 2001). Mushroom polysaccharides and cleaved polysaccharides with three-helix advanced conformation are closely related to their antitumor activities. In terms of molecular weights, the antioxidant, anticoagulant, and antiviral activities of polysaccharides are improved with the increasing molecular weight. For example, dextran with larger molecular weight performs poorly in immune activity, and vice versa (Bimczok et al., 2009). The molecular weight of lysergic polysaccharides with strong anti-tumor activity is usually larger than 105 Da, but when its molecular weight is less than 5 × 104 Da, no significant biological activity is found(Bohn & Bemiller, 1995). Therefore, what is the relationship between the moisturizing activity and structure of nonanimal-derived polysaccharides? Thus, the structure–activity relationship of moisturizing with algae, plant and bacterial polysaccharides was explored here.
3 Moisturizing activity of polysaccharides from algae, plants, fungus, and bacteria
3.1 Moisturizing activity of polysaccharides from algae
As a biomass-rich and inexpensive resource, algae are generally divided into multicellular macroalgae and unicellular microalgae (microalgae). Macroalgae in the ocean include green algae, brown algae, and red algae, while microalgae include all ecosystems and phytoplankton found in ocean water (Ariede et al., 2017). Algae are often left on shore to decompose, causing waste problems. However, algae have fast growth rate, high photosynthetic efficiency, high CO2 fixation potential and low lignin ratio. They are excellent renewable resources for production of polymers and other products (Noreen et al., 2016). Seaweed polysaccharides, including cell wall polysaccharides and mucopolysaccharides (Murata & Nakazoe, 2001), are the research focus because of their high yields, sustainability and special chemical components. In particular, they contain abundant sulfurized polysaccharides (SPS), which have antioxidant, antiviral, and blood coagulation effects (Jiao et al., 2011), and are widely used in food, cosmetics, biotechnology, and other fields (Renn, 1997).
Some algal polysaccharides have good moisturizing effects (Table 1). From the sources of moisturizing polysaccharides, there are mainly green algae and brown algae as well as some freshwater algae and microalgae. The moisture absorption of various algae polysaccharides is slower than that of glycerin, but they have better moisture retention, especially marine green algae. In vitro moisturizing assays show the moisturizing rate of polysaccharides in E. prolifera (Li et al., 2017) can reach about 98 % (25 °C, 10 % RH, 96 h), which is closely related to the moisturizing component HA, and the polysaccharide in Enteromorpha linza (Wang et al.,2014). The moisture retention rate was 49.3 % (25 °C,43 % RH,96 h), which was slightly better than that of chitosan. As for brown algae, fucose was mostly present in its monosaccharide composition (Table 1) (Ning et al.,2020). As a component of glycoprotein, fucose widely exists in the plasma membranes of various cells. In addition to a certain moisturizing effect, it also has anti-aging effects, such as increasing skin thickness, accelerating skin tissue regeneration, reducing wrinkles and increasing skin elasticity by applying fucoidin on the skin. The polysacchariedes from Sargassum horneri (Shao et al., 2015), Sargassum vachellianum (Jesumani et al., 2020), Sargassum hemiphyllum (Wang et al., 2013) belonging to brown algae, have moisturizing retention rate over 94 % (25 °C, 43 % RH, 48 h). Reportedly, polysaccharide extraction was performed on three different brown algae of Sargassum and the moisturization rate was determined to be around 65 % (20 °C, 43 % RH,72 h) under the same conditions (Jesumani et al., 2019). In the sulfated polysaccharides obtained from red algae, porphyra, these polysaccharides can improve the maturation of the keratinized envelope of the stratum corneum and enhance the dermal-epidermal junction. Therefore, algal polysaccharides may be a good choice to improve dry or aging facial skin features and prolong moisturization when applied topically. Some polysaccharides produced by microalgae, which are used to produce high-value active substances (Nicoletti, 2016), have better moisturizing properties. For instance, the moisturizing rates of polysaccharides extracted and isolated from Sarcinochrysis marina Geitler (Wang et al., 2012), and Nostoc commune (Li et al., 2011) can reach 60 %-70 %. Currently, algal polysaccharides were more used as cosmetic moisturizing ingredients, such as sheep sorrel polysaccharide serum, and cosmetic patch compounded with seaweed polysaccharides as functional ingredients that are effective on skin moisturizing (Byeon et al., 2017; Yang et al., 2017). a “-” represents not studied. Different shapes represent different monosaccharides. Abbreviations: Mw, molecular weight; Glc, glucose; Gal, galactose; Ara, arabinose; Rha, rhamnose; Man, mannose; Xyl, xylose; Fuc, fucose; Glc, glucuronic acid.
source
parts
method
Mw(Da)
components
moisture absorption/retention
reference
E.prolifera
PEP
hot-water extraction,anion-exchange chromatography,gelfiltration chromatography
1.478 × 105
Rha:Glu:Gal:Xyl:Ara = 1.48:1.00:0.13:0.30:0.06
1)25 °C, 43 %RH, 96 h
Ra:HA > SPEP > SLEP > PEP ≈ LEP
2)25 °C, 81 %RH, 96 h
Ra:SPEP > SLEP ≈ PEP > LEP
3)25 °C, RH10%, 96 h,
Rr:HA > PEP > SPEP > SLPEP(Li etal.,2017)
LEP
(PEP)enzymatic degradation
4.48 × 104
Rha:Glu:Gal:Xyl:Ara = 1.49:1.00:0.16:0.85:0.07
SPEP
(PEP,LEP)sulfated modification
1.763 × 105
Rha:Glu:Gal:Xyl:Ara = 1.65:1.00:0.09:0.57:0.17
SLPEP
5.99 × 104
Rha:Glu:Gal:Xyl:Ara = 1.09:1.00:0.06:0.10:0.11
Enteromorpha linza
LEP
hot-water extraction,Ethanol precipitation,ascorbic acid,H2O2 degradation
–
–
1)20 °C , 43 %/81 %RH, 96 h
glycerol > chitosan > PHEP > LEP
2)25 °C , 43 %RH, 96 h
chitosan > PHEP > glycerol > LEP(Wang et al., 2014)
PHEP
Phthaloylation of LEP
–
Sargassum horneri.
SHP
Dextranase hydrolysis
1.79 × 106
Glu:Rha:Man:Gal:Gyl = 48.04:32.19:6.93:10.01:2.83
1)25 °C, 43 %RH, 48 h
Ra:SHPH2 > SHPH1 > SHP
2)25 °C, 81 %RH, 48 h
Ra:SHPH2 > SHPH1 > SHP
3)25 °C, RH43%, 96 h
Rr:SHPH2 > SHPH1 > SHP > glycerin > propanedol(Shao et al., 2015)
SHPH1
(SHP)ion-exchange chromatography of DEAE-cellulose,and gel permeation chromatography of Sephadex G-25,hydrolyzates
7.831 × 104
Glu:Rha:Man:Gal:Gyl = 69.04:16.34:3.17:11.45:0
SHPH2
2.142 × 104
Glu:Rha:Man:Gal:Gyl = 70.11:16.09:3.25:10.55:0
papaya
P
hot-water extraction
2.65 × 106
Gal:Glc:Ara:Rha:Xyl:Man = 42.5:0.1:41.2:15.4:0.1:0.1
1)25 °C, 43 %RH, 96 h
Ra:p2 > P > P1
2)25 °C, 81 %RH, 48 h
Ra:p > p1 > P2
3)25 °C, RH43 %, 96 h
Rr:HA > P ≈ P2(Zhang et al., 2012)
P1
(P) fractionated by chromatography
2.51 × 106
Gal:Glc:Ara:Rha:Xyl:Man = 45.3:9.34:30.9:11.7:3.28:1.51
P2
2.54 × 106
Gal:Glc:Ara:Rha:Xyl:Man = 52.0:11.0:21.6:10.2:0.1:3.04
Sargassum vachellianum
SPS
Ethanol precipitation
–
Fuc(49.5 %),Glu(2.2 %),Gal(9.3 %),Xyl(3.5 %),Man(11.2 %),Glucuronicacid(1.01 %)
1)25 °C, 80 %RH, 72 h
Glycerol > SPS > SPP
2)25 °C, 43 %RH, 72 h
SPS > Glycerol > SPP(Jesumani et al., 2020)
SPP
hot-water extraction, CaCl2precipitation, Ethanol precipitation
–
Fuc(-),Glu(1.96),Gal(1.05),Xyl(-),Man(-),Glucuronicacid(-)
source
parts
method
Mw(Da)
components
moisture absorption/retention
reference
Saccharina japonica
L
hot-water extraction,Ethanol precipitation
8.7 × 104
Fuc:Gal:Man:Glc:Rha:Xyl:Arb = 1.00:0.36:0.09:0.03:0.00:0.10:0.00
1)20 °C, 43 %RH, 24h
Ra:DL > HA > P > DP > L > E > B > C
2)20 °C, 81 %RH, 24h
Ra:DL > HA > P,DP > L > C > B > E
3)20 °C, 43 %RH, 24h
Rr:DL > HA > P > DP > C > E > L > B(Wang et al., 2013)
DL
(L)free radical degradation
8 × 103
Fuc:Gal:Man:Glc:Rha:Xyl:Arb = 1.00:0.17:0.02:0.02:0.00:0.00:0.00
Porphyra haitanensis
P
hot-water extraction,Ethanol precipitation
2.77 × 105
–
DP
(P)free radical degradation
1.4 × 104
–
Bryopsis plumose
B
hot-water extraction,Ethanol precipitation
1.5 × 105
Fuc:Gal:Man:Glc:Rha:Xyl:Arb = 0.25:1.00:0.17:0.11:0.10:0.00:0.87
Codium fragile
C
hot-water extraction,Ethanol precipitation
2.82 × 105
Fuc:Gal:Man:Glc:Rha:Xyl:Arb = 0.01:1.00:0.03:0.00:0.01:0.00:0.24
Enteromorpha linza
E
hot-water extraction,Ethanol precipitation
2 × 105
Fuc:Gal:Man:Glc:Rha:Xyl:Arb = 0.00:0.04:0.04:0.03:1.00:0.44:0.00
Sargassum vachellianum
SvP
hot-water extraction,Ethanol precipitation
–
Fuc(25.14 %),Rha(1.81 %),Man(19.25 %),Gal(6.75 %),Xyl(3.12 %)
1)20 °C, 81 %RH, 72h
Ra:glycerol > ShoP > SheP > SvP
2)20 °C, 81 %RH, 72h
Rr:ShoP > SvP > SheP > glycerol(Jesumani et al., 2019)
Sargassum horneri
ShoP
hot-water extraction,Ethanol precipitation
–
Fuc(22.56 %),Rha(2.84 %),Man(8.28 %),Gal(10.46 %),Xyl(2.49 %)
Sargassum hemiphyllum
SheP
hot-water extraction,Ethanol precipitation
–
Fuc(15.28 %),Rha(3.93 %),Man(10.71 %),Gal(8.87 %),Xyl(10.98 %)
Sarcinochrysis marina Geitler
SMP-0
(crude sample)Sephadex-G150
3.64 × 106
Ara:Fru:Glc = 4.834:1.000:5.513
1)25 °C, 43 %RH, 176h
Ra:glycerol > SMP-0 > SMP-1 > HA ≈ SMP-2 > SMP-3 > chitosan
2)20 °C, 50h
Rr:HA > chitosan > SMP-0 > SMP-1 > HA ≈ SMP-2 > SMP-3(Wang et al., 2012)
SMP-1
(SMP-0)ultrasound assisted H2O2-VC system degradation, Fractionation by Sepharose 6B
4.53 × 105
Ara:Fru:Glc = 2.897:1.000:4.830
SMP-2
1.69 × 105
Ara:Fru:Glc = 1.000:2.506:4.113
SMP-3
8.69 × 103
Ara:Fru:Glc = 1.000:1.606:2.070
Ulva fasciata
UFP31
hot-water extraction,Ethanol precipitation, radial flow column chromatography method, ultrafiltration
–
rha:xyl:glu = 1:0.46:0.27
1)25 °C, 80 %RH, 96h
Ra:UFP31 > glycerol
2)25 °C, RH43 %, 12h,
Rr:UFP31 > glycerol(Shao et al., 2015)
source
parts
method
Mw(Da)
components
moisture absorption/retention
reference
Porphyridium cruentum
ESPS0
–
3040.88ku
–
1)20 °C, 70 %RH
Ra:Gly > ESPS0 > CTS > HA
1)20 °C, RH70 %
Rr:HA > CTS > EPS0 > EPS1 > EPS2 > EPS3 > Gly(Liu et al., 2014)
ESPS1
1807.59ku
–
ESPS2
ESPS0)ultrasound assisted H2O2-VC system degradation,Sepharose-6B
374.97ku
–
ESPS3
33.73ku
–
Microalga Nostoc sphaeroides
nostoglycan
hot water extraction,papain treatment,Ethanol precipitation
1.99 × 106
Man(34.5 %),Fru(21.8 %),Gal(14.6 %),Glu(17.7 %),Xyl(6.1 %),Rha(2.2 %),Galacturonic acid(3.1 %).
1)25 °C, 43 %/81 %RH, 24h
Ra:nostoglycan > chitosan
1)25 °C, 43 %RH, 24h
Rr:nostoglycan > chitosan(Li et al., 2018)
Pavlova viridi
PPS0
ultrasound assisted hot water extraction,Ethanol precipitation
2.6 × 106
–
1)20 °C, 70 %RH, 24h
Ra:Gly > PPS0 > PPS2 > HA > PPS1 > CTS
1)20 °C, 70 %RH, 48h
Rr:PPS0 > PPS1 > PPS2 > Gly(Wang and Sun, 2012)
PPS1
(PPS0)ultrasound assisted H2O2-VC system,Sepherose 6B
3.9 × 105
–
PPS2
5.5 × 104
–
Nostoc commune
–
hot water extraction,Ethanol precipitation
–
–
1)25 °C, 43 %/81 %RH, 24h
Ra:Nostoc > chitosan > urea
Rr:urea > Nostoc > chitosan(Li et al., 2011)
3.2 Moisturizing activity of polysaccharides from plants
Plant polysaccharides are derived from the flowers, stems, leaves, and fruits of plants. The polysaccharides in natural plant products have anti-oxidation, whitening, and anti-aging effects as well as good moisturizing activity, and are widely used in cosmetics (Feng et al., 2021; Li et al., 2012; Liao et al., 2017; Liu et al., 2018). Generally, products containing plant polysaccharides can relieve dryness and provide a soothing film covering human skin (Femenia, 2007). Even at low concentrations, these products can improve the moisturizing effect of the skin and reduce water loss (Damasceno et al., 2016).
Among plants, Aloe vera extract is widely used as a natural raw material in the cosmetic field, where polysaccharides as the main active ingredients are mainly composed of monosaccharides, such as glucose and mannose polymers (Nema, 2012), and are representative of plant-derived moisturizing polysaccharides with excellent moisturizing activity and some whitening and antioxidant properties. Dal'Belo et al. (2010) applied freeze-dried aloe extracts containing polysaccharides at different concentrations in cosmetic formulations to explore their effects on skin hydration. Aloe extract containing polysaccharides is a natural effective ingredient that improves skin hydration, and can be used in moisturizing cosmetic formulations and as a supplement for the treatment of dry skin. Active polysaccharide from Aloe vera has a significant moisturizing effect and strong skin penetration and high security on the human skin (Ren et al., 2012). In addition, the moisturizing effect of aloe polysaccharides is endogenous to certain extent. Aloe polysaccharides have the effect of moisture absorption and moisturizing on the surface of the skin, and make human fibroblasts proliferate actively, promoting the expression and synthesis of type I and type III collagens, hyaluronic acid and hydroxyproline. The secretion of hyaluronic acid and hydroxyproline can nourish and protect the skin, and improve the dry skin from the inside (Liu et al., 2010).
Some other polysaccharides from different parts of other plants have been gradually discovered in recent years to explore the feasibility and utilization as moisturizing components, and some of them have better moisturizing effects. Polysaccharide fractions obtained from Eragrostis Teff show a moisture retention rate of 103.29 % (20 °C, 81 %RH, 24 h) (Table 2), which is significantly higher than those of glycerol, polyethylene glycol 400, and chitosan (Zhu & Sun, 2013). The four polysaccharide fractions obtained from onion show less variation in moisturization rate, which are all-around 55 % (20 °C, 48 h) (Zhu et al., 2017). Some polysaccharides from plant petals, such as RRPS-2 isolated from Rosa rugosa petal (Zhang et al., 2019), and NHP-2 extracted and purified from Nymphaea hybrid (Cong, 2018), show both outstanding moisturizing properties and excellent antioxidant activity. In addition, polysaccharides extracted from the fruits of Litchi chinensis (Jing et al., 2016), Blackcurrant (Xu et al., 2021), although not outstanding in moisturizing properties, have great potential for application because of their antioxidant and hypoglycemic effects. a“-” represents not studied. Different shapes represent different monosaccharides. Abbreviations: Mw, molecular weight; Glc, glucose; Gal, galactose; Ara, arabinose; Rha, rhamnose; Man, mannose; Xyl, xylose; Fuc, fucose; GlcA, glucuronic acid.
Source
Parts
Method
Mw(Da)
Components
Moisture absorption/retention
Reference
Rosa rugosa petals
RRPS
hot water extraction,Ethanol precipitation
–
–
1) 25 °C, 43 %RH, 96 h
Ra:glycerol > RRPS-2≈chitosan > RRPS-1
2) 25 °C, 81 %RH, 96 h
Ra:glycerol > chitosan > RRPS-2 > RRPS-1
3) 25 °C, RH43%, 96 h
Rr:chitosan > RRPS-2 > glycerol > RRPS-1(Zhang et al.,2019)
RRPS-1
(RRPS)DEAE-52 column,gel filtration column of Sephadex G-100,
8.8 × 103
Rha(-%),Fuc(1.79 %),Ara(6.14 %),Xyl(5.64 %),Man(2.69 %),Glc(65.47 %),Gal(18.26 %)
RRPS-2
4.438 × 105
Rha(10.31 %),Fuc(-%),Ara(35.53 %),Xyl(-%),Man(-%),Glc(14.02 %),Gal(40.14 %)
Eragrostis Teff
TPS1
cellulase-ultrasound assisted hot water extraction,Ethanolprecipitation,ion-exchange chromatography of DEAE-cellulose,and gel chromatography of Sephadex G-50
7.886 × 103
Fru
1) 25 °C, 43 %/81 %RH, 12 h
Ra:TPS2 > TPS3 > TPS1
2) 25 °C, RH43%, 12 h
Rr:TPS2 > TPS3 > TPS1(Zhu & Sun, 2013)
TPS2
8.467 × 103
Ara,Gal,Glu(44 %),Xyl,Man,Rib,Fru
TPS3
6.366 × 103
Ara,Gal,Glu,Xyl,Man,Rib,Fru(45.5 %)
Blackcurrant
DP-1
microwave-assisted solvent method,Fe2+ -Vc-H2O2degradation,Sepharose 6B
1.29 × 106
Ara(44 %),Rha(22.86 %),Gal(11.98 %),Galacturonic acid(7.98 %),Man(9.37 %),Glc(6.56 %)
1) 20 °C, 81 %RH, 24 h
Ra:glycerol > DP-2 > DP-1
2) 25 °C, 81 %RH, 64 h
Rr:glycerol > DP-2 > DP-1(Xu et al.,2020)
DP-2
1.07 × 106
Ara(39.95 %),Rha(21.54 %),Gal(13.67 %),Galacturonic acid(9.29 %),Man(8.24 %),Glc(7.31 %)
Dalbergia sissoo
DSLP
hot water extraction,Ethanol precipitation,ion exchange chromatography
–
–
1) 25 °C, 43 %/81 %
RH, 144 h Ra:glycerol > HA > MDSP > TGBP > DSLP
2) 25 °C, RH43%, 96 h
Rr:MDSP > TGBP > DSLP
3) 25 °C, RH81%, 144 h
Rr:MDSP > DSLP > TGBP(Rana et al.,2014)
Tectona grandis
TGBP
–
–
Mimosa diplotricha
DSLP
–
–
Litchi chinensis
PLC-1
hot water extraction,Ethanol precipitation,DEAE-cellulose 52,Sephadex G-100
2.35 × 104
Gal:Rha:Glu = 1.00:3.52:5.89
1)20 °C, 80 %RH, 32 h
Ra:glycerol > PLC-1 Rr:PLC-1 > glycerol(Jing et al.,2016)
Nymphaea hybrid
NHP-2
hot water extraction,Ethanol precipitation,DEAE-cellulose 52,Sephadex G-150
3.3 × 104
–
1) 25 °C,43 %/81 %RH,144 h
Ra:glycerol > NHP-2 > HA > chitosan
2) 25 °C, RH43%,96 h
Rr:HA > NHP-2≫chitosan(Cong 2018)
onion
HBSS
dissolved in sodium acetate buffer,water bath
7.702 × 106
Rha(0.94 %),Ara(3.56 %),Xyl(1.18 %),Man(81.68 %),Glu(5.56 %),Gal(7.08 %)
1)20 °C, 50 h
Ra:glycerol > DASS > HBSS > CASS > CHSS
2)20 °C, 50 h
Rr:CHSS > HBSS > CASS > DASS > glycerol(Zhu et al.,2017)
CHSS
(HBSS)mixed with chelating agent,water bath
4.690 × 106
Rha(6.68 %),Ara(16.73 %),Xyl(7.88 %),Man(25.80 %),Glu(11.54 %),Gal(31.37 %)
DASS
(HBSS)mixed with sodium hydroxideNaBH4,water bath
4.943 × 106
Rha(13.53 %),Ara(15.82 %),Xyl(8.99 %),Man(20.33 %),Glu(7.37 %),Gal(33.96 %)
CASS
(HBSS)mixed with water sodium hydroxide NaBH4,water bath
1.390 × 106
Rha(6.98 %),Ara(19.40 %),Xyl(2.57 %),Man(2.35 %),Glu(1.11 %),Gal(67.59 %)
Mentha haplocalyx
MHP
a high-pressure ultrasound assisted extraction (HUAE) method
5.958 × 104
Man(2.13 %), Rha(0.89 %), glucuronic acid(1.14 %), Glu(39.3 %),Gal(44.85 %),galacturonicacid(3.79 %),Ara(7.89 %)
1) 20 °C, 48 h
Ra:glycerol > MHP
2) 20 °C, 50 h
Rr:glycerol > MHP(Chen et al., 2019)
Tea
TPP
hot water extraction, extracted with ethylacetate, citric acid buffer solution, butyl acetate
–
polyphenols(98.25 %)
1) 43 %RH, 70 h
Ra:glycerol > TPS1 > TPP > TPS2
2) 81 %RH, 70 h
Ra:glycerol > TPS1 > TPP > TPS2
3) 43 %RH, 48 h Rr:glycerol > TPS1 > TPP > TPS
4) 81 %RH, 48 h Rr:glycerol > TPS1 > TPP > TPS(Wei et al.,2009)
TPS1
extracted with plant hydrolase Ethanol precipitation
–
Rha:Rib:Ara:Man:Glu:Gal:Galacturonic Acid = 4.8:1.6:15.4:7.3:6.6:44.9:19.4.
TPS2
Ethanol refluxing, extracted with plant hydrolase, Ethanol precipitation, washed sequentially with ethanol, acetone, ether
–
–
Acanthopanax gracilistylus
AGSL-P
smashing tissue extraction
–
Glu,Rha,Gal
1) 20 °C, 43 %RH, 48 h
Ra:AGSL-P-2 > AGSL-P-1 > glycerol > AGSL-P
2) 20 °C, 75 %RH, 48 h Ra:AGSL-P-2 > glycerol > AGSL-P-1 > AGSL-P1
3) 20 °C, 43 %RH, 48 h Rr:glycerol > AGSL-P-2 > AGSL-P > AGSL-P-1(Liu et al.,2015)
AGSL-P-1
DEAE-Sepharose Fast Flow
–
Glu,Rha,Gal
AGSL-P-2
–
The water extracts of polysaccharides from Dalbergia sissoo, Tectona grandis and Mimosa diplotricha show effective moisture retention properties in comparison with hyaluronic acid and glycerol. The polysaccharides from Dalbergia sissoo in all antioxidant assays are more active than the other two polysaccharides (Rana et al., 2014). The polysaccharides from Mentha haplocalyx (MHP) by a high-pressure ultrasound-assisted extraction method exhibits substantial hygroscopicity and a relatively strong moisture retention capacity and may be used as a supplement in cosmetics, although the hygroscopicity and moisture retention of MHP (28.11 % and 35.14 %) are lower than glycerin (57.96 % and 41.97 %) (Chen et al., 2019). The protective effects of green tea polysaccharides (TPS) and polyphenols (TPP) on skin were investigated (Wei et al., 2009). TPS and TPP have good moisture absorption and water retention properties. TPS can hardly protect the skin from solar ultraviolet (UV), but TPP can. TPP and TPS have certain complementary advantages, and can be used in combination as cosmetic active ingredients. Besides, the properties of the moisture retention and moisture absorption of polysaccharides from Acanthopanax gracilistylus were studied and compared with common humectants in relative humidity 43 %and 75 %. Results show the polysaccharides of AGSL-P-2 are excellent moisturizers (Liu et al., 2015).
3.3 Moisturizing activity of polysaccharides from bacteria and fungus
Extracellular polysaccharides gradually become effective alternatives to polysaccharide products from plants and animals owing to their non-toxicity, safety, short production cycle, easy isolation and purification, excellent physical and chemical properties and bioactivity, and the demand for EPSs is increasing (Liu et al., 2016; Reitas et al., 2011). For some fungal polysaccharides, research on moisturizing is less, and polysaccharides from Tremella fuciformis are the most studied.
Microbial polysaccharides have great potential in cosmetics, pharmaceuticals, and biomedicine because they meet the required degree of purity and certain specific functional properties compared to traditional polymers (Liu et al., 2016). Microorganisms are more suitable for polysaccharide production than plants or algae because of their faster growth rate and easier technical manipulation (Raza et al., 2012). Microbial polysaccharides are divided into extracellular polysaccharides, cell wall polysaccharides, and intracellular polysaccharides (Zhu & Tong, 2012). Common microbial polysaccharides such as Xanthan gum, Gellan gum, and Levan are often used as gelling agents, flocculants, and film-forming agents in many fields, such as food and pharmaceuticals because of their water solubility, low viscosity, and skin protection and moisturizing properties (Freitas et al., 2015).
The polysaccharides with good moisturizing effect obtained from microorganisms are all extracellular polysaccharides, which are easily separated from bacteria and secreted outside of cells during the growth and metabolism of microorganisms (Table 3). Microbial extracellular polysaccharides are essential for biofilm formation and are involved in bacteria protection from harmful environments and adhesion. High viscosity is formed by the accumulation of various polymeric substances in EPS (exopolysaccharides) layers. They tend to be hygroscopic and aerobic bacteria and usually contain more water than the surrounding environment (Helm et al., 2000). The moisturizing retention of EPS plays an important role in cosmetics and clinical medicine. In terms of the in vitro moisturizing effect, all these reported polysaccharides have excellent moisturizing properties. The moisturizing rate of Lachnum YM262 is up to 79.6 % (25 °C, RH43 %, 72 h) (Chen et al., 2017). The extracellular polysaccharide obtained from Zunongwangia profunda SM-A87 outperforms sodium alginate (25 °C, RH81 %,70 h) (Sun et al., 2014), P. fluorescens PGM37 extracellular polysaccharides are close to hyaluronic acid (Zhao et al., 2013). Interestingly, some bacterial strains produce extracellular polysaccharides with moisturizing effects even better than hyaluronic acid. The moisturizing rates of extracellular polysaccharides produced by Phyllobacterium sp.921f (Liet al., 2017a) and Polaribacter sp.sm1127 (Sun et al., 2015) are all about 93.4 % (25 °C, 43 % relative humidity), which is higher than that of hyaluronic acid. Similarly, the extracellular polysaccharide from Rhodococcus SM-1strain (Urai et al., 2002) is more hygroscopic than glycerol (a strong hygroscopic polyol), and is better than hyaluronic acid in moisturizing performance. a“-” represents not studied. Different shapes represent different monosaccharides. Abbreviations: Mw, molecular weight; Glc, glucose; Gal, galactose; Ara, arabinose; Rha, rhamnose; Man, mannose; Xyl, xylose; Fuc, fucose; GlcA, glucuronic acid.
Source
Parts
Method
Mw(Da)
Components
Moisture absorption/retention
Reference
Lachnum YM262
LEP-2a
(LEP)DEAE-Cellulose 52 and Sepharose CL-6B chromatographic column
1.52 × 105
Man:Gal = 20.6:1.0
1) 25 °C, 43 %/81 %RH, 72 h
Ra:glycerol > LEP-2a > chitosan
2) 25 °C, RH43%, 72 h
Rr:LEP-2a > glycerol > chitosan(Chen et al., 2017)
P.fluorescens PGM37
EPS
–
3.676 × 105
Glu: Man = 1:1
1) 25 °C, 43 %RH, 24 h
Ra:glycerol > HA > EPS
2) 25 °C, 43 %RH, 24 h
Rr:HA > EPS≈glycerol(Zhao et al., 2013)
Phyllobacterium sp.921F
EPS
–
1.082 × 105
Glu:Gal = 1:1.07
1) 25 °C, 43 %/81 %RH, 7d
Ra:glycerol > EPS > HA > chitosan
2) 25 °C, 43 %RH, 4d
Rr:EPS > HA > glycerol > chitosan(Li et al., 2017a)
Aerococcus Uriaeequi
EPS-A
Fermentation,(DEAE) ion exchange chromatography,gel filtration chromatography
2.84 × 105
Man(10.71 %),Glu(66.99 %)
Ra:sodiualginate > EPS-A > chitosan
Rr:sodiualginate > chitosan > EPS-A(Wang et al., 2018)
Polaribacter sp.SM1127
EPS
Ethanol precipitation,DEAE-Sepharose Fast Flow column,gel-filtration chromatography
2.20 × 105
Rha(0.8 %),Fuc(7.4 %),GlcA(21.4 %),Man(23.4 %),Gal(17.3 %),Glc(1.6 %),GlcNA(28.0 %)
1) 25 °C, 43 %/81 %RH, 96 h
Ra:Gly > HA > sodiumalginate > EPS > chitosan
2) 25 °C, 43 %RH Rr:EPS > sodiumalginate > HA > chitosan > Gly(Sun et al.,2015)
Source
Parts
Method
Mw(Da)
Components
Moisture absorption/retention
Reference
Zunongwangia profunda SM-A87
EPS
Ethanol precipitation
–
–
1) 25 °C, 43 %/81 %RH, 70 h Ra:glycerol > HA > EPS > sodiualginate > chitosan
2) 25 °C, RH81%, 70 h, Rr:chitosan > HA > sodiualginate > EPS > glycerol(Mezouari et al.,2010)
Rhodococcusrhodochrous SM-1
Ethanol precipitation
2 × 106
Gal:Glu:Fuc:GluA = 6:3:2:4
1) 37 °C, 32 %RH, 24 h Ra:SM-1 EPS > Hyaluronic acid > Glycerol
2) 37 °C, 32 %RH, 24 h Rr:SM-1 EPS > Hyaluronic acid > Glycerol(Sunet al.,2014)
Paenibacillus sp. ZX1905
lubcan
ethanol precipitation,DEAE cellulose-32 anion-exchange chromatography column,Sepharose CL-4B column
–
GluA:Glu:Man:Gal:Rha = 2:3:1:2:2
1)25 °C, 43 %RH, 24 h Ra:HA≈lubcan > Glycerol
2) 25 °C, 81 %RH, 24 h Ra:Glycerol > HA≈lubcan
3) 25 °C, 43 %RH, 24 h Rr:lubcan > HA > Gly(Sun et al.,2020)
Pseudoalteromonas agarivorans
EPS
DEAE-52 ion-exchange chromatography and gel fifiltration chromatography
2.84 × 105
Man(6.66 %),Glu(90.28 %)
1) 25 °C, 32 %RH, 160 h Chitosan > EPS > Sodium hyaluronate
(Hao et al.,2019)
Haloterrigena turkmenica
EPS
–
–
Glc:Glucosamine:GlcA:Gal:Galactosamine = 1:0.65:0.24:0.22:0.02
1) 25 °C, 81 %RH, 72 h Ra:HA > sodium alginate > EPS > chitosan
2) 25 °C, 72 h Rr:chitosan > EPS > sodium alginate > HA(Squillaci et al., 2016)
Source
Parts
Method
Mw(Da)
Components
Moisture absorption/retention
Reference
Fruit Bodies of Tremella fuciformis
TFP
hot water extraction,Ethanol precipitation,DEAE Fast Flow column
2.349 × 106
Fuc(12.78 ± 0.65 %),Xyl(25.84 ± 0.42 %),Man(35.03 ± 0.31 %),Gal(1.40 ± 0.22 %),Glu(12.60 ± 0.10 %),GluA(12.23 ± 0.35 %)
1) 25 °C, 43 %/81 %RH, 96 h Ra:35.04 %/42.00 %
2) 25 °C, RH43%, 72 h, Rr:79.55 %(Xu et al.,2020)
Tremella
TP1
wet mashing extraction,DEAE-52 ion-exchange column chromatography, controlled degrading
1.8696 × 106
–
1) 25 °C, 43 %RH, 60 h
Ra:TP4 > TP3 > glycerol > TP2 > TP1
2) 25 °C, 2)81 %RH, 64 h
Ra:glycerol > TP4 > TP3 > TP2 > TP1
3) 25 °C, 3)0 %RH, 64 h
Rr:TP1 > TP2 > TP3 > glycerol > TP4(Chen 2017)
TP2
1.231 × 105
TP3
3.1 × 104
TP4
4.3 × 103
Tremella fuciformis
TP1
Acid assisted methods
3.55 × 106
–
1) 25 °C, 43 %RH, 60 h
Ra:glycerol > TP3 > TP2 > TP1 > XG
2) 25 °C, 10 %RH, 60 h
Rr:glycerol > TP3 > TP1 > TP2 > XG(Wang et al., 2019)
TP2
alkali assisted methods
6.35 × 106
TP3
enzymeassisted methods
1.16 × 106
Pholiota nameko
PNP-40
fractional precipitation(40 %, 60 %,80 %(v/v))
3.33487 × 105
–
1)25 °C, 81 %RH, 96 h,
Ra: glycerol > PNP-60 > PNP-80≈PNP-40
2) 25 °C, RH43%, 96 h
Rr:PNP-80 > PNP-60≈PNP-40 > glycerol(Chou et al.,2019)
PNP-60
2.157 × 104
PNP-80
4.40 × 103
Cordyceps sinensis Cs-HK1
EPS1
ethanol precipitation
2.7 × 106
Glc(81.3 %),Man(13.8 %), Gal(4.9 %)
1) 25 °C, 43 %RH, 70 h Ra:EPS1 > EPS1U > chitosan > urea
2) 25 °C, 81 %RH, 24 h Ra:EPS1U > EPS1 > chitosan > urea
3) 25 °C, 43 %RH, 70 h Rr:EPS1 > EPS1U > chitosan > urea(Chen et al.,2014)
EPS1U
Ultrasonic treatment of EPS1
7.3 × 105
Glc(98.5 %),Man(1.3 %), Gal(0.2 %)
Some fungal polysaccharides also have good moisturizing activity. The moisturizing activity of fungal polysaccharides is mainly concentrated in Tremella (Chen, 2017), Tremella fuciformis (Wang et al., 2019; Xu et al., 2020), Pholiota nameko (Chou et al., 2019), and Cordyceps sinensis (Chen et al., 2014). The polysaccharides from Tremella fuciformis and its fruiting bodies show good moisturizing activity.
4 Relationship between polysaccharide structure and moisturizing activity
From the perspective of moisturizing effect as stated above, algae, plants and microorganisms are the main sources of natural polysaccharides with good moisturizing effect. Among them, the moisturizing rate of microbial polysaccharides is similar to or better than hyaluronic acid, so it may be the best source of moisturizing polysaccharides. These natural polysaccharides are almost inferior to polyols such as glycerol in terms of moisture absorption speed and effect. The reason may be that glycerol is a small-molecule adsorbate. In the early stage of water absorption, the water molecules bound to the surface transfer rapidly to the interior with the adsorbate, maintaining a low water vapor partial pressure on the surface. Therefore, it can continuously absorb water in the external environment, with fast absorption rate (Yan et al., 2011). Fortunately, natural polysaccharides have relatively good moisturizing effect. Polysaccharides are large molecules rich in hydroxyl and carboxyl groups and other hydrophilic substances, and the molecular chains contain abundant strong hydrophilic groups, and carboxyl groups in addition to hydroxyl. The number of hydrogen bonds formed with water molecules is much larger than that of small-molecule moisturizers, such as glycerol. Polyols have a significant moisture absorption effect, but do not have a long-lasting moisturizing effect. Polyols are small molecules and mainly rely on the hydrogen bonding formed by hydroxyl groups and water molecules to hinder water dissipation and maintain water stability in the system. Besides, the spatial three-dimensional network structure of polysaccharide molecules can wrap water in. This structure further increases the obstruction against water molecules, making the polysaccharide and water combination very firm (Sivam et al., 2013). Moreover, the bioactivity of polysaccharides is related to their structural characteristics, including molecular weight and concentration, composition, branching structure, and type of glycosyl bonds(He et al., 2014). What is the relationship between the moisturizing activity and structure of polysaccharides? We will also discuss several aspects below.
4.1 Effect of molecular weight of polysaccharides on moisturizing activity
Based on Tables 1 to 3, the molecular weights of polysaccharides obtained from algae, plants, and microorganisms are 5.54 × 103 –3.64 × 106 Da, 6.366 × 103 –7.702 × 106 Da, and 4.3 × 103 –6.35 × 106 (mostly 105 –3 × 105) Da respectively. This is in agreement with another report (Sandford & Laskin, 1977). As for the separation and purification of the above polysaccharides, crude polysaccharides extracted from algae or plants often yield multiple fractions after pure characterization, and the molecular weights of the polysaccharides were further reduced by the impurity removal after purification, whereas microbial polysaccharides often exhibit single components after purification.
However, the moisture retention of polysaccharides extracted by different methods from Codium fragil (Song, 2010), onion (Zhu et al., 2017), Bryopsis Plumosa (Song, 2010), and Tremella fuciformisonion (Wang et al., 2019) is not significantly related to the molecular weight. This may be because the influence of the extraction method on the structure of polysaccharide results in great differences in the chemical composition and physical properties.
On the one hand, studies confirm that polysaccharides with larger molecular weights have better moisturizing effect. For the polysaccharide fractions obtained from the isolated and purified from papaya (Zhang et al., 2012) and Pholiota nameko (Chou et al., 2019) by graded alcoholic precipitation, their moisturizing and hygroscopic properties were further enhanced with the increase of molecular weight. On the other hand, other studies confirm that the degradation of large-molecular-weight polysaccharides into small-molecular-weight polysaccharides helps improve the moisturizing activity. Noticeably, the bioactivity of Ganoderma lucidum polysaccharides can usually be improved by reducing the molecular weight of polysaccharides through degradation (Xuet al., 2019). The crude polysaccharides extracted from Sargassum horneri (Shao et al., 2015), Rosa rugosa petals (Zhang et al., 2019), or Blackcurrant (Xu et al., 2021) were purified and degraded to small-molecular-weight polysaccharides, which significantly increased moisture retention and absorption.
The method of polysaccharide degradation may also affect moisturizing activity. Unlike acid digestion, H2O2 oxidative degradation, ultrasonic degradation, and enzymatic digestion have high efficiency and mild reaction conditions. Especially, ultrasound-assisted oxidative degradation can reduce the molecular weight of polysaccharides without changing their primary structure (Huang et al., 2020). The crude polysaccharides extracted from Sarcinochrysis marina Geitle (Wang et al., 2012), or Pavlova viridi (Wang & Sun, 2012) were degraded using an ultrasound-assisted H2O2-VC system. The moisture retention effect of the polysaccharide fractions of Ulva pertusa after H2O2 degradation was improved with an increase in the molecular weight. However, after degradation, the hygroscopicity of Tremella polysaccharides (Chen, 2017) increases with the decrease of molecular weight. Moreover, Saccharina japonica (Wang et al., 2013), Porphyra haitanensis (Wang et al., 2013), and E. prolifera (Li et al., 2017) all have better moisture absorption and moisturizing properties of polysaccharides after degradation with decreasing molecular weight. Huang et al. (2014) separated polysaccharides from Gracilaria verrucosa into three fractions: less than 104 Da, 104 –105 Da, and more than 105 Da by using radial flow coupled ultrafiltration. The polysaccharide fractions below 104 Da were improved in terms of preventing desorption and moisture absorption. Qin et al. (2002) found the hygroscopic activity of modified chitosan increased with decreasing molecular weight and increased rapidly when the molecular weight varied from 1.0 × 104 to 0.4 × 104 Da.
When the polysaccharides are in a certain molecular weight range, the moisturizing activity can be significantly displayed. As the polysaccharide molecules are a linear long-chain structure, the larger relative molecular weight of polysaccharides within a certain range indicates the presence of more polar groups. The formation of more hydrogen bonds makes it easier to form a network structure and avoids water loss, so the moisture retention is better. Research on the antioxidant activity of related polysaccharides confirms that the degraded polysaccharides have better water solubility. The smaller molecular weight makes it easier to expose the active groups and trap free radicals. Hence, the contact area between active sites and free radicals is larger(Ma et al., 2005; Zhao et al., 2006).
Therefore, the above results indicate that the molecular weight of polysaccharides does not regularly change with the moisturizing activity. However, it can also be inferred that the reduction of polysaccharide molecular weight may lead to a loose structure, enlarge the contact area between water molecules and polysaccharides, and increase the exposure opportunity of hydrophilic groups (such as carboxyl and hydroxyl). The hydrophilic groups on these newly-added sites easily associate with water molecules in the surrounding environment to form hydrogen bonds, which strengthen the binding of water molecules and improve their moisture retention. Meanwhile, the molecular chain becomes shorter and a more complex three-dimensional network structure is gradually formed. When the water molecules enter, the water molecules become less movable due to the hindrance of the grid structure, and can hardly escape. As a result, the moisturizing effect is further improved. The above research indicates that most polysaccharides with a molecular weight of about 104 Da show good moisture retention. Nevertheless, if the molecular weight of the polysaccharides is too large, the water solubility is poor, which often limits their biological effects. If the molecular weight of the polysaccharides is too small, the network structure may be destroyed, and no active polymerization structure space can be formed, weakening the inhibitory effect on water and significantly reducing the moisture retention.
4.2 Effect of monosaccharide composition on moisturizing activity
The different structures of polysaccharides may be caused by different monosaccharide compositions, and are important reasons for the differences in the biological activity of polysaccharides (Yi et al., 2017). Among the polysaccharides from different sources, the monosaccharide composition of galactose, mannose, glucose appears more frequently (Table 1-3). Algae and plant polysaccharides contain many types of monosaccharides. On the contrary, the types of monosaccharides contained in bacteria and fungus are relatively single, mainly glucose, galactose, mannose, and some neutral monosaccharides such as rhamnose. This result is consistent with related literature (Freitas et al., 2021). For algae polysaccharides, studies on the monosaccharide composition confirm that the frequency of rhamnose is higher. For brown algae, there is more fucose in monosaccharide composition (Table 1). In addition to a certain moisturizing effect, fucose also has anti-aging effects, such as increasing skin thickness and accelerating skin tissue regeneration. Polysaccharides are affected not only by the composition of monosaccharides but also by various other factors. Only certain speculations are made about the relationship between the moisturizing activity of polysaccharides and the composition of monosaccharides. Some microbial polysaccharides are even mainly composed of two monosaccharides. In particular, glucose occurs most frequently, which may be the underlying reason for its moisturizing effect.
4.3 Effect of functional groups of polysaccharides on moisturizing activity
The activity of polysaccharides is not only affected by molecular weight and monosaccharide composition, but also is closely related to functional groups such as carboxyl, hydroxyl, amino, ethyl, acetyl, and sulfate groups (Ramesh & Tharanathan, 2003). Similarly, the reason why some polysaccharides have strong moisturizing ability may be due to the strong interaction between water molecules and the hydrophilic groups of polysaccharides (Chen et al., 2003, 2017).That is, polysaccharides contain polar groups such as polyhydroxy and carboxyl groups, which endow them strong electronegativity (Chou et al., 2019), allowing the polysaccharides to form hydrogen bonds with water. For example, HA is widely used as a humectant component in biochemical products and cosmetics, because the carboxyl and acetamide groups on the HA polysaccharide chain may attract water molecules through intermolecular hydrogen bonds (Zhang, et al., 2013). Another reason is that its relatively high viscosity (Li et al., 2018) is conducive to the moisturizing of polysaccharides (Chen et al., 2017; Helm et al., 2000).
The existence of some special peripheral groups may also help adjust the rheological properties of polysaccharides, thereby affecting their water retention capacity and application potential. Sulfate content is very positively correlated with the antioxidant activity of sulfated polysaccharides(Cui, Liu, Li, Hao, & Lu, 2018). The moisturizing activity of polysaccharides is also closely related to sulfate content of polysaccharides. Besides sulfated polysaccharides from plants with good moisture retention, sulfate components are also found in microbial polysaccharides, especially algae polysaccharides that generally contain sulfate groups(Percival, 1979). The sulfated polysaccharide extracted in Ulva fasciata has higher viscosity and hydroxyl content, and sulfation can improve water solubility, which positively affects its hygroscopicity and moisture retention (Shao et al., 2015). Research on the polysaccharide components extracted from E.prolifera (Li et al., 2017), Sargassum vachellianum (Jesumani et al., 2020), and Sargassum (Jesumani et al., 2019) confirms that the moisture retention of polysaccharides slightly rises with the increase of sulfate content. When investigating the relationship of water absorption and retention capacity with the content of sulfate groups, Wang et al. (2013) found that the sulfate contents of the five algae polysaccharides had inconsistent effects on the moisture absorption capacity. The sample with the highest sulfuric acid content exhibited the highest moisture absorption capacity, while the sample with the second-highest sulfuric acid content exhibited extremely low moisture absorption capacity. As the sulfate content decreased, the moisturizing retention of polysaccharides was weakened. This result may be affected by factors such as the molecular weight of polysaccharides and the complexity of monosaccharide structure. Because the chemical composition of the sulfated polysaccharides of the seaweed family is specific, and the degree and distribution of sulfate groups are different, it may also have a certain relationship with the algae species.
The glyoxylate group significantly influences the physiological, biological, and pharmacological properties of polysaccharides. This is because changes in the basic structure of biopolymers alter their physical and chemical properties, resulting in characteristic bioactive and solubility behavior (Chen et al., 2004). Reportedly, polysaccharides with a larger proportion of glyoxalate are mostly negatively charged, leading to a smaller spatial site resistance that allows a simple way to react with free radicals. Therefore, polysaccharides with a higher content of aldehyde acids have better free radical scavenging activity (Ye et al., 2012). Some recent reports also suggest a link between the glyoxalic acid contained in polysaccharides and the moisturizing activity of polysaccharides. Studies on polysaccharides extracted from Onion (Zhu et al., 2017), Tremella fuciform (Wang et al., 2015), Rosa rugosa petals (Zhang et al., 2019), and Blackcurrant (Xu et al., 2021) found an increase in water retention with increasing glyoxylate content, which may be because the presence of glyoxylate can alter the rheological properties of polysaccharides by imparting charge and interfering with the conformation. Among polysaccharide molecules, uronic acid can also form intermolecular hydrogen bonds with other polar groups on the polysaccharide chain, which further strengthens the network structure and facilitates water preservation (Chou et al., 2019; Xu et al., 2020).
In addition to the above sulfuric acid and uronic acid groups, some microbial polysaccharides also contain certain amounts of pyruvate groups (Freitas et al., 2021). The presence of pyruvate substituents in some bacterial extracellular polysaccharides can greatly change their properties in solutions by imparting charges and interfering with confirmation(Cesàro et al., 1992). Li et al.(2017a) analyzed the structure and moisturizing activity of the extracellular polysaccharide produced by Phyllobacterium sp. 921F, and speculated that the pyruvate substituent can enhance its moisturizing properties.
4.4 Effect of modificationof polysaccharides on moisturizing activity
Although natural polysaccharides exhibit strong bioactivity, the existence of various functional groups provides a broad space for chemical structure modification. In addition, modification is of great significance for studies on the relationship between polysaccharide structure and activity. Therefore, in-depth study of structural modification methods and mechanisms can promote the application of polysaccharides (Lee et al., 2010; Liu et al., 2012; Silva et al., 2004). In fact, there are many studies on sulfated, acetylated, glyoxylated, and carboxymethylated derivatives of polysaccharides. Most studies demosntrate that the moisturizing effect of polysaccharides plays a significant role through the modification of polysaccharides.
The moisture absorption and moisturizing retention of polysaccharides are generally enhanced after modification, and are generally superior over the traditional moisturizing component HA, achieving significant effects (Table 4). For example, extracellular polysaccharides of Phomopsisliquidambari NJUSTb1(Liu et al., 2021) outperform unmodified polysaccharides in terms of moisture absorption and moisturizing ability after sulfation modification. When the polysaccharides obtained from Actinidia chinensis roots were subjected to a SO3-pyridine procedure to prepare sulfated polysaccharides with different degrees of substitution, the moisturizing retention increased with the rise of substitution (Lin et al.,2014). This may be because sulfation of polysaccharides can increase their solubility (Shao et al., 2015) and change the conformation of the chains, which leads to changes in their bioactivity. Yang et al. (2009) prepared hyaluronic acid quaternary ammonium salt (QHA), starch quaternary ammonium salt (QS) and chitosan quaternary ammonium salt (QCTS), and measured their moisturizing activities. The moisture absorption of QHA was superior over HA at both 43 % and 81 % RH. The same trend was observed for QS and QCTS. In addition, the moisturizing retention of all three quaternate polysaccharides was better than the corresponding polysaccharides at 81 % RH, 43 % RH and dry conditions. The N+(CH3)3 critically affects the moisture absorption and moisturizing retention of quaternate polysaccharides. QCTS and QHA have comparable to or better moisture absorption and moisturization than HA and thus can be used as moisturizing ingredients in cosmetics. There are also modifications of polysaccharides such as carboxymethylation and acetylation (Chen et al., 2021). The transverse order and orientation of polysaccharide molecules are changed by introducing acetyl groups, exposing polar groups such as hydroxyl and carboxyl groups, which improve the water solubility of polysaccharides. The introduction of carboxymethyl improve the electronegativity and water solubility of polysaccharides, which in turn enhances the polysaccharide activity or generates new activity. The carboxymethyl substitution at the OH-3 site on chitosan is beneficial to increasing moisturizing property, and a larger molecular weight of polysaccharides makes it easier to form a net-like structure to prevent water loss, and leads to the better moisturizing retention (Chen et al., 2003). a“-” represents not studied. Different shapes represent different monosaccharides. Abbreviations: Mw, molecular weight;DS, degree of substitution.
Source
Parts
Method
Mw(Da)
DS
Moisture absorption/retention
Reference
Phomopsisliquidambari NJUSTb1
PLN
ethanol precipitation
–
–
1) 20 °C, 43 %RH, 72 h
Ra:glycerol > HA > S-PLN-1 > C-PLN-1 > PLN-1 > PLN Ra:
2) 20 °C, 81 %RH, 72 h
glycerol > S-PLN-1 > HA > PLN > C-PLN-1 > PLN-1
3) 20 °C, 43 %RH, 72 h
Rr:S-PLN-1 > HA > glycerol > PLN > C-PLN-1 > PLN-1(Liu et al.,2021)
PLN-1
SephadexG-200
3.43 × 105
–
S-PLN-1
Sulfation
–
1.228
C-PLN-1
Carboxymethylation
–
0.903
Actinidia chinensis roots
ACPA1
hot water extraction,Ethanol precipitation
5.5 × 103
1) 20 °C, 43 %RH, 96 h
Ra:HA > SA1 > SA2 > SA3 > ACPA1
2) 25 °C, 81 %RH, 96 h
Ra:HA > SA1 > SA2 > SA3 > ACPA1
3) 25 °C, 43 %RH, 64 h
Rr:HA > SA1 > SA2 > SA3 > ACPA1(Lin et al.,2014)
SA1
ACPA1.Sulfation (SO3-pyridine procedure)
–
2.23
SA2
–
2.10
SA3
–
1.78
Tremella fuciformis
ATP
hot water extraction,Ethanol precipitation
–
–
1) 20 °C, 43 %RH, 96 h Ra:CATP4 > chitosan > CATP3 > CATP2 > CATP1 > ATP
2) 20 °C, 81 %RH, 96 h
Ra:CATP4 > chitosan > CATP3 > CATP2 > CATP1 > ATP
3) 20 °C, 43 %RH, 96
Rr:chitosan > CATP4 > CATP3 > CATP2 > CATP1 > ATP(Wang et al.,2015)
CATP1
Carboxymethylation of ATP
–
0.36
CATP2
–
0.47
CATP3
–
0.54
CATP4
–
0.70
schizophyllan
SPG
Ethanol precipitation
2.27 × 104
1)25 °C, 43 %RH, 60 h
Ra:CM-SPG1 > CM-SPG3 > CM-SPG2 > Gl > HA > SPG
2) 25 °C, 81 %RH, 60 h Ra:CM-SPG1 > CM-SPG3 > CM-SPG2 > Gl > HA > SPG
3) 25 °C, RH43 %, 60 h Rr:glycerol > CM-SPG1 > CM-SPG3 > CM-SPG2 > HA > SPG(Zheng et al.,2017)
CM-SPG1
(SPG) carboxymethyl modification (sodium hydroxide, chloroacetic acid)
1.19 × 105
0.52
CM-SPG2
1.21 × 104
0.45
CM-SPG3
1.05 × 104
0.37
In fact, the moisture retention of polysaccharides and the substitution degree of polysaccharide modification may only show a direct positive correlation in a certain range. The moisturizing retention may increase with the increment of carboxymethyl substitution, but when the substitution degree increases further, the moisturizing property will decrease instead. Guo et al. (2007) studied the relationship between the molecular structure of succinyl chitosan and moisturizing retention, and found 50 % deacetylation showed the greatest moisture absorption under high humidity environments. When the degree of substitution is between 0.65 and 0.79, the moisture retention of succinyl chitosan is better than that of HA. Skin hydration in the moisturizing assay coated area increased first and then decreased with increasing degree of substitution, and the same trend was observed for transcutaneous water loss, with a higher moisturizing property at a degree of substitution of 0.45 (Zheng et al., 2017). Based on the above polysaccharide modification effects, we further know that most microbial polysaccharides have varying degrees of acetyl substitution. For example, the naturally occurring gellan gum has acetyl and glyceryl groups substituted on its glucose monomer (Zhu & Tong, 2012), which may be another reason why microbial polysaccharides show better moisture retention.
4.5 Relationship between apparent structure of polysaccharides and moisturizing activity
The surface morphology of the substance is closely related to its physicochemical properties (Fig. 2), which may affect its biological function (Emanuele et al., 2010). Some natural polysaccharides can form helical structures such as β-(1 → 3)-d-glucan, which has high viscosity and gel formation ability (Laroche & Michaud, 2007). In addition, the straight chain and branched chain structures of polysaccharides are related to the moisturizing of polysaccharides (Chen et al., 2017). In contrast to modern instruments for detecting the structure of polysaccharides, such as infrared spectroscopy and nuclear magnetic resonance, scanning electron microscopy (SEM) allows to understand polysaccharide wholeness, obtain relatively intuitive information and reveal the apparent structure of polysaccharides. Atomic force microscopy (AFM) is a powerful tool for imaging single-molecule chains and polysaccharide aggregates under “near-native” conditions, providing information on the size and morphology of individual polysaccharide chains.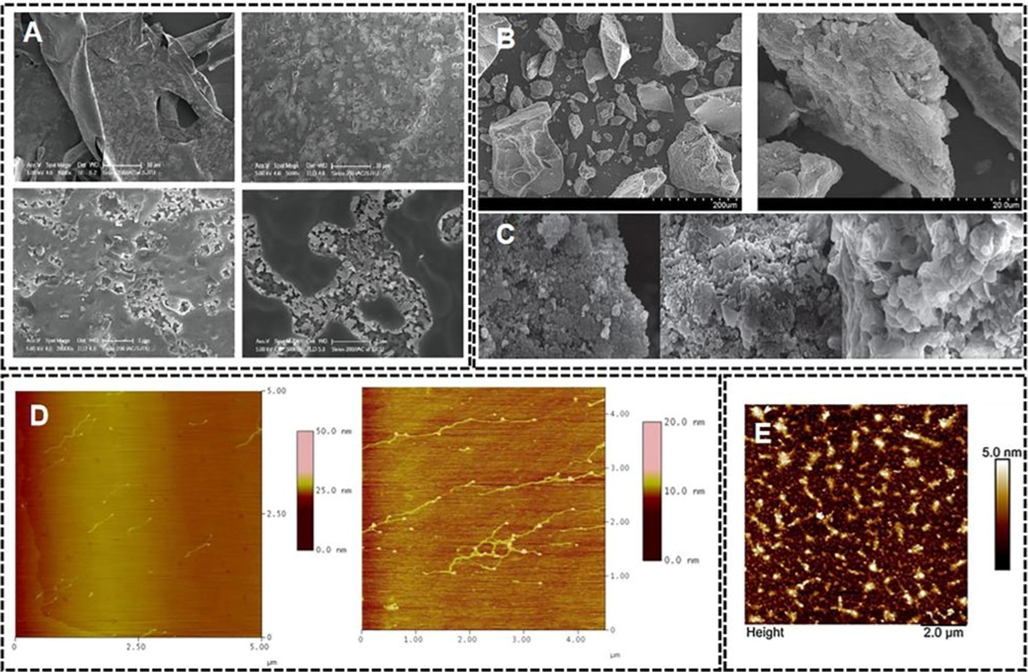
(A) Scanning electron micrographs of Nymphaea hybrid polysaccharide (at manification of 1000, 5000, 20,000 and 50000)(Cong, 2018); (B) Surface morphology of nostoglycan by scanning electron microscopy(at manification of 200, 2000)(Li et al., 2011); (C) SEM images of three Laminaria japonica polysaccharide samples (at manification of 5000)(Cai et al., 2019); (D) Tapping mode AFM images of the EPS from Phyllobacterium sp. 921F solution (5 mg/L;10 mg/L)(Chi et al., 2019); (E) The molecular morphology of TFP at the concentration of 10 μg/mL via AFM(Xu et al., 2020).
In the SEM of extracted crude polysaccharide samples of Lotus fragrance (Fig. 2A-B), Cong (2018) and Li (2011) found a flaky or crumbly accumulation with cotton wool-like fibers and pore channels of different sizes on the surface, with good adsorption to water. Cai et al. (2019) found the surface of acetylated degraded polysaccharides from Laminaria japonica was rough and showed a certain pore-like structure at a magnification of 5000 times (Fig. 2C), which resulted in its better water solubility and larger specific surface area. The unmodified polysaccharide of Laminaria japonica had a smoother surface and no porous structure, which are consistent with its lower water solubility and higher relative viscosity. The microalgae polysaccharide with better moisture retention (Li et al., 2018) had a rough surface under 200 × magnification, indicating the polysaccharide is amorphous. The 2000 × magnification showed its multilayered and lamellar morphology, similar to its reported porous polysaccharide of a drought-tolerant plant. Therefore, we also speculate that polysaccharides with better moisture-absorbing ability at the polysaccharide phenotype level may usually contain more pore-like structures.
Chi et al. (2019) similarly used AFM to obtain single-molecule chain images of exopolysaccharides (EPS) at a concentration of 5 mg/L (Fig. 2D). The characteristics of EPS are consistent with a hard elongated molecule without branches. The average length of polysaccharides in an aqueous solution was about 600 nm. As the concentration increased to 10 mg/L, EPS clustered and the chain height observed in the AFM image increased. This also shows the high molecular weight, linear chain, and polyhydroxylation, which explain the strong hydrogen bonds between molecules and subunits. Xu et al. (2020) used AMF to observe the chain characteristics and thickness of TFP molecules dispersed on the surface of mica matrix, and revealed the existence of TFP molecules as flexible chains from morphological analysis, with an average thickness of 1.1 nm (Fig. 2E). The TFP is higher than that of a single polysaccharide chain, and the average length is 0.1–1 nm, indicating the branches in the TFP structure are intertwined. Some aggregation between TFP chains may be due to strong intramolecular and intermolecular interactions.
5 Analysis of moisturizing mechanism of polysaccharides
A few studies are available on the moisturizing mechanism of polysaccharides. For skin moisturizing, there are mainly-two kinds of determination methods in vitro and in vivo (Table 5). Therefore, according to the summary and speculation of related reports, the moisturizing mechanism of polysaccharides can be discussed from two ways: extrinsic and intrinsic ways (Fig. 3).
Types
Method
Determination indices
Characteristic
Reference
Evaluation in Vitro
Weighing method
–
Simulating the human environment, by controlling the temperature and humidity of the environment, the weight of the sample is measured in vitro to evaluate the strength of the sample's ability to absorb water or lose water.
(Wang et al., 1999)
Biological cell method
Cell proliferation test
By establishing a model that simulates cell drying damage, the cell proliferation test (MTT) is used to calculate the cell drying mortality and protection rate, so as to detect the cell's resistance to drying and the ability to prevent dehydration.
(Chen et al., 2015)
Expression of moisturizing protein
To detect the expression of related skin hydration genes such as AQP3, aggregated silk fibroin, mediator protein and hyaluronic acid synthase in cultured cells.
(Tito et al., 2015)
Evaluation in Vivo
Subjective evaluation method
–
Experts conduct qualitative or hierarchical evaluation of the subjects’ skin condition such as skin elasticity, skin transparency;subject self-assessment is mostly conducted in the form of questionnaires, indicators include skin moisturization, non-dryness, etc.
(Chou et al., 2019)
Stratum corneum water content
Electrical parameters (capacitance,conductance)
The method of indirectly measuring the water content of the stratum corneum is simple and easy to implement. It is closely related to the water content of the stratum corneum but is not completely equal to each other.
(Clarys et al., 2012; Fluhr et al., 1999)
ATR-FTIR
The ratio of (AamideI/AamideII) can be used as an index to measure the relative water content of the stratum corneum, and to determine the composition of the skin and transdermal absorption of chemical substances.
(Bello et al., 2006; Gloor et al., 1981; Kazarian & Chan, 2006)
NIR
In NIR, water molecules show two absorption bands at 1450 nm and 1920 nm, which directly detect water molecules.
(Kilpatrick-Liverman et al., 2006)
IR
The peak at 2100 cm-1 can be used as the characteristic absorption peak of water molecules. The strength of the peak is directly proportional to the moisture content of the skin, and the moisture content of the skin surface can be obtained by analyzing the changes in the intensity of the characteristic absorption peaks.
(Gloor et al., 1981)
Confocal Raman spectroscopy
molecular composition and the structure of skin, for example, water content,moisturization and changes in the skin barrier function can all be observed.
(Förster et al., 2011)
Water retention capacity of the stratum corneum
TEWL
TEWL indicates the loss of water in the stratum corneum.A high TEWL value indicates that much water is lost through the skin, and the stratum corneum has a poor barrier effect on water.
(Vergou et al., 2012)
Corneal sampling method
Stratum corneum adhesion
By sampling the stratum corneum and detecting the adhesion of the stratum corneum and serine protease activity, as well as measuring the NMF in the stratum corneum, the water content of the stratum corneum can be characterized.
(Wang & Zhao, 2018)
Serine protease activity
NMF components
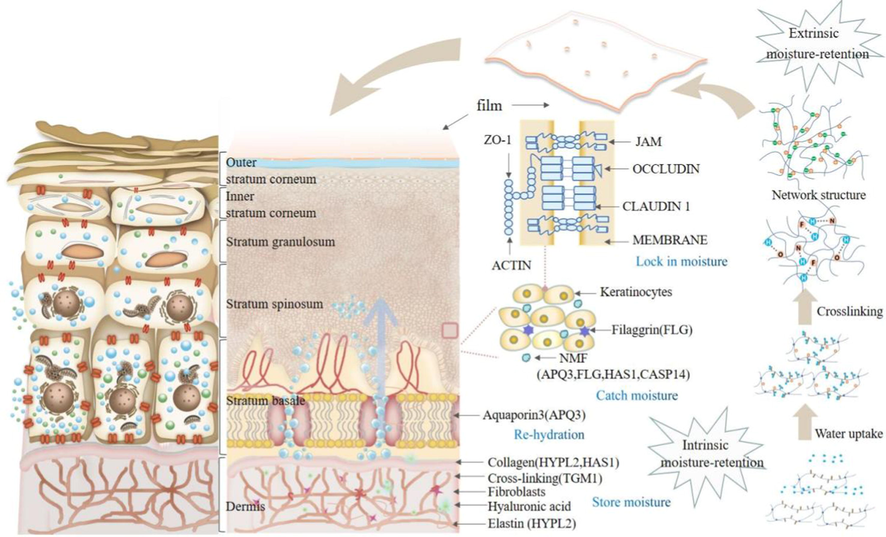
Polysaccharide intrinsic and extrinsic moisturizing mechanism.
5.1 Extrinsic moisurizing retention
Hydroxyl and carboxyl groups and some other polar groups hydrate with water molecules through hydrogen bonds. After a certain amount of water is absorbed by hygroscopicity, the polysaccharide molecular chain network expands, allowing more polar groups to contact water molecules and combine. Under the action of intermolecular hydrogen bonds and electric charges, polysaccharide molecular chains are cross-linked to form a three-dimensional grid structure, so that water molecules are bound in the grid, limiting the escape of water molecules, and further forming a hydration film on the skin surface to prevent water evaporation. In this process, hydrogen bonding, network structure, and film formation are important links, which will be described in detail below.
5.1.1 Hydrogen bonding
Hydrogen bonding is a strong intermolecular force. When an X-atom with a small atomic radius and strong electronegativity and unshared electron pair is combined with a hydrogen atom, the strong electron absorption of X-atom makes itself partially negatively charged. When water molecules and polysaccharides are mutually close, protons will form hydrogen bonds with oxygen on polysaccharides and aldehyde groups. The polysaccharide contains abundant polar groups such as - OH and - COOH as moisture absorption sites, which can attract water molecules by forming hydrogen bonds. The polysaccharide chains form aggregate through hydrogen bonds and further cross-link with each other (Wei et al., 2016).
5.1.2 Network structure
In the outer layer of the skin, because of hydrogen bonding between polysaccharide molecules, the structure of polysaccharides is interwoven to form a dense network structure. With the cooperation of hydrogen bonds, water molecules can be wrapped between the polysaccharide network chains. It can inhibit the loss of water molecules in a low-humidity environment, and avoid excessive water absorption like glycerin under high humidity, which can effectively create a continuously moist environment for the skin (Shaw et al., 2003; Yi et al., 2017). The polysaccharide components and fibrous proteins in the inner dermis layer form an extracellular gel-like matrix containing abundant water, and this water-containing gel-like matrix constitutes a moisture reservoir for the entire skin. Therefore, the three-dimensional network of polysaccharide chemical structures may be beneficial to moisture absorption and retention capacity.
5.1.3 Film formation
The epidermal layer of human skin is located in the outermost layer, which is equivalent to the first barrier of the human body. The moisture content of the epidermis is the key point to skin moisturizing. The amount of water determines the appearance of the skin. The basal layer located in the deeper layer of the skin is rich in water content and is the water source of the entire moisturizing system. Reportedly, these polysaccharides have good film-forming properties and easily form when they are on the skin surface. After a while, the film layer can become more uniform. At this time, moisture is not easy to lose, and skin moisture is maintained (Sebti & Coma, 2002). After high-molecular-weight hyaluronic acid is evenly applied to the skin, it can form a sponge-like transparent hydration film on the epidermal layer, preventing water evaporation and absorbing the deepwater under the skin. At the same time, the epithelial stratum corneum receives the moisture diffused from the basal tissue, so the sebum layer is filled with numerous moisture to moisturize the skin (Mao et al., 2012). Polysaccharides with larger molecular weights (e.g. polysaccharides from Tremella (Chen, 2017) tend to have a greater moisturizing rate, because of their higher viscosity and easiness to attach to the skin surface to form films.
5.2 Intrinsic moisturizing retention
In addition to in vitro moisturizing effects, polysaccharides also have endogenous moisturizing effects. The epidermis, dermis, and subcutaneous tissue in the skin are the three-layer structure of human skin, and each layer influences the skin nourishing and moisturizing effects to varying degrees. Keratin fibers are concentrated in the epidermal layer. At this time, the skin can prevent both the penetration of chemical substances and the extravasation of body fluids. The moisture content of the stratum corneum is certainly the key point to skin moisturizing, but in fact, the moisture content of the skin depends on the moisture content of each layer. Moisturizing is the process of moisture transportation from the dermis to the stratum corneum for dynamic distribution. From the inside to outside of the skin, the moisturizing functions can be summarized as water capturing, water locking, water activating, and water storing (Yun et al., 2017) (Table 6). Polysaccharides can promote the skin's moisturizing system.
source
Internal regulation mechanism
reference
Tremella aurantialb
Promoting AQP3 protein and tight junction protein Claudin-1 expression to regulate keratinocyte moisturization and barrier function.
(Feng et al., 2015)
Nymphaea hybrid
It can promote the up regulation of AQP3 protein expression in HaCaT cells, make water diffuse from dermis to epidermis, and increase the water content in epidermis.
(Cong 2018)
Tremella fuciformis
The expression of occludin, claudin-1, ZO-1 and JAM-1 was significantly increased, and the skin barrier was strengthened
(Cao et al., 2020)
Tremella
Increasing the content of NMF in keratinocytes,increasing the elasticity and stability of the the dermal layer, and improving the ability to transport water from the dermal layer to the keratinocytes.
(Yang et al., 2021)
Aloe
It is helpful for the formation of human fibroblasts and promotes the expression of collagen I, III, hyaluronic acid and other important extracellular matrix
(Liu et al., 2010)
Aloe barbadensis Miller
At low concentration, AQP-3 and ZO-1 protein expression related to active moisturizing and water locking barrier in keratinocytes were also increased
(Zhang et al., 2020)
Dendrobium candidum
The tendency to increase the expression of water channel proteins, intermediate filament aggregation proteins and tropomyosin may have a role in the maintenance of epidermal barrier structure and hydration function
(Chen et al., 2015)
5.2.1 Skin water storage
There are many fibers in the dermis of the human body, including reticular fibers, elastic fibers, and collagen fibers. These fibers can make the skin maintain normal toughness and full elasticity for long time, and determine the degree of skin filling. Large amounts of collagen and various monopolysaccharides such as HA contained in the dermis can form a composite grid and combine with abundant water to form a gel-like matrix that constitutes the entire skin's moisture reservoir. It also fills the dermis layer, making the dermis layer structure more complete. Exogenous HA supplementation can replenish HA in the skin and thus enhance the water storage capacity. Aloe polysaccharides and oat β-glucan can not only promote the proliferation of human fibroblasts, but also regulate the synthesis of collagen, enhance the secretion of hyaluronic acid (Meng et al., 2006), and promote water storage in colloidal matrix reservoirs (Liu et al., 2010).
5.2.2 Skin active water
Aquaporin exists on the cell membrane between the epidermal cells of the human body. It not only transports water and participates in the hydration of the epidermis, but also controls the water flow in and out of cells. This process is called living water (Boury-Jamot, Daraspe, Bonté, Perrier, & Verbavatz, 2009). So far, 13 aquaporins have been found in the human body. Aquaporin-3 (AQP3) is the most abundant aquaporin subtype in human skin. Enhancing the expression of AQP3 can promote water diffusion from the dermis to the epidermis and increase the water content of the epidermis (Glaser & Mattox, 2013; Hwang et al., 2012). Cong (2018) found the expression of AQP3 protein in HaCaT cells was also further up-regulated with the increase in the concentration of Perfume lotus polysaccharide, the endogenous moisture retention was enhanced. Zhang et al. (2020) also reported that Aloe vera polysaccharide gel can also increase the expression of aquaporins related to active moisturizing and water-locking barriers in keratinocytes at low concentrations.
5.2.3 Skin water locking
Tight junction proteins composed of many tight junction transmembrane proteins can close the gap among cells, prevent excessive water volatilization in the cell bypass, effectively maintain the skin barrier and water content in the epidermis. Tight junction proteins include Claudin protein, Occludin closure protein, and junction adhesion molecule protein (Brandner et al., 2002; Verdier-Sévrain & Bonté, 2010). The commonly-studied proteins include a series of MAGUK proteins (ZO-1, ZO-2, and ZO-3) as well as cingulin. In the epidermis, the expression amounts and sites of tight junction proteins vary depending on their species. For example, the closure and cingulin proteins are only found in the granular layer. ZO-1 and Cldn-4 are mainly distributed in the basal layer of the skin, while Cldn-1 and MUPP-1 proteins can be found in all layers of the epidermis. Claudin proteins are important functional factors that constitute tight junction proteins, and are related to the barrier function of tissues. Feng et al. (2015) used cellular immunofluorescence assay to find that acid-degraded golden ear polysaccharide up-regulated Claudin-1 expression in keratinocytes and promoted the synthesis of more tight junction proteins in epidermal keratinocytes. Cao et al. (2020) found that polysaccharides from Tremella fuciformis strengthened the gene expressions of tight linkage-related proteins Occludin, Claudin-1, ZO-1, and JAM-1 to strengthen the skin barrier and played a significant role in promoting and repairing. Skin water capture Filaggrin, derived from profilaggrin, is a protein that can aggregate keratin filaments, contributing to the flattering corneocytes (Verdier-Sévrain & Bonté, 2010). In the deep skin, it can eventually form a natural moisturizing factor that helps cells to obtain a certain amount of water by capturing water from outside. Yang et al. (2021) found that polysaccharides from Tremella can promote the production of filamentous polyproteins, which further formed the moisturizing factor. Katekawa et al. (2020) also reported that a colubrina polysaccharide-rich phytopharmaceutical preparation is an effective additive product to skin hydration that can modulate filaggrin in human skin explants by immunofluorescence assay.
6 Summary and prospect
The structure–activity relationships between the moisture retention and structural characteristics of polysaccharides from algae, plants, bacteria and fungus were systematically described in detail. Compared to traditional polyols, polysaccharides generally exhibit superior moisturizing retention, although they are slightly less hygroscopic. In particular, algal and microbial polysaccharides and their derivatives have superior moisturizing retention that is mostly even better than HA. The molecular weight, functional groups, polysaccharide modifications, and apparent structure of polysaccharides are closely related to the moisturizing retention. Most polysaccharides with a molecular weight of about 104 Da show good moisture retention. Functional groups of polysaccharides, including sulfate content, glyoxylate groups, uronic acid groups, and pyruvate groups, are potential factors for the moisturizing retention. The modified polysaccharide derivatives all exhibit better moisturizing properties, and a porous structure that facilitates water retention in the apparent structure.
The mechanism of moisturization of polysaccharides can be inferred from extrinsic and intrinsic aspects. Extrinsic moisturization is mainly through hydrogen bonding between polysaccharides and water molecules to absorb water, the formation of a three-dimensional network structure to prevent water from escaping, and the generation of hydration films on the skin surface to prevent water evaporation. For intrinsic moisturizing, some polysaccharides can enhance skin barrier function by regulating the production of some tight junction proteins, barrier proteins, and the expression of barrier genes, and by promoting the synthesis of water channel proteins and moisture-related proteins as well as the expression of hydration-related genes to enhance skin internal moisturizing.
However, the mechanism of moisturization, especially intrinsic moisturization, needs to be further studied. The moisturizing effects of polysaccharides need to be further developed and studied, and their rich sources and excellent moisturizing effects have a broad application prospect in the fields of cosmetic and food products.
Acknowledgements
The authors express gratitude to Changshu Institute of Technology for data and refereces collection.
Funding
This study was funded by Science and technology planning project of 2021, Suzhou science and Technology Bureau (Grant No. SNG2021017) of China, and Scientific Research Foundation for Advanced Talents, Changshu Institute of Technology, China (Grant No. KYZ2020045Q).
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Hydroxyapatite–collagen–hyaluronic acid composite. Biomaterials. 1999;20(2):191-195.
- [Google Scholar]
- Structural characterisation of polysaccharides purified from longan (Dimocarpus longan Lour.) fruit pericarp. Food Chem.. 2009;115(2):609-614.
- [Google Scholar]
- An FTIR investigation of isocyanate skin absorption using in vitro guinea pig skin. J. Environ. Monitor. Jem.. 2006;8(5):523-529.
- [Google Scholar]
- Short chain regioselectively hydrolyzed scleroglucans induce maturation of porcine.dendritic cells. Appl. Microbiol. Biotechnol.. 2009;82(2):321-331.
- [Google Scholar]
- (1→3)-β-d-Glucans as biological response modifiers: a review of structure-functional activity relationships. Carbohyd. Polym.. 1995;28(1):3-14.
- [Google Scholar]
- Skin aquaporins: function in hydration, wound healing, and skin epidermis homeostasis. Handbk. Exp. Pharmacol.. 2009;190(190):205-217.
- [Google Scholar]
- Organization and formation of the tight junction system in human epidermis and cultured keratinocytes. Eur. J. Cell Biol.. 2002;81(5):253-263.
- [Google Scholar]
- Byeon, S. Y., Cho, M. K., Shim, K. H., Kim, H. J., Shin, H. S., & Biotechnology., 2017. Development of a Spirulina Extract/Alginate-Imbedded PCL Nanofibrous Cosmetic Patch. J. Microbiol. 27(9), 1657-1663.
- Cai, W. J., Liu, S. W., Ran, L. I., Qin, T., & Shi, X. Y., 2019. Antioxidant and Absorption/Retention Activities of the Acetylated Degraded Laminaria japonica Polysaccharides. Sci. Technol. Food Ind. 40(10):109-114+121.
- Cao, M., Jia, W. C., & Yang., S. P., 2020. The Sdudy of WSK in Enhancing Skin Barrier. J. Med. Chem. Res. 106-108.
- Galactans: an overview of their most important sourcing and applications as natural polysaccharides. Braz. Arch. Biol. Techn.. 2011;54(6):1075-1092.
- [Google Scholar]
- Solution conformation and properties of the galactoglucan from Rhizobium meliloti strain YE-2(S1) Carbohydr. Res.. 1992;231:117-135.
- [Google Scholar]
- Research on Fast Extraction and Controlled Degradation of Polysaccharides from Tremella. Guangzhou, China: South China University of Technology; 2017. Master thesis
- Relationships between the molecular structure and moisture-absorption and moisture-retention abilities of carboxymethyl chitosan - II. effect of degree of deacetylation and carboxymethylation. J. Agr. Food Chem.. 2003;338(4):333-340.
- [Google Scholar]
- Chen, G., Fang, C. X., Chen, Z., Wang, Liu, M., & Kan, J., 2019. High-pressure ultrasonic-assisted extraction of polysaccharides from Mentha haplocalyx: Structure, functional and biological activities. Ind. Crops. Prod. 130, 273-284.
- Structure and properties of a (1→3)-β-d-glucan from ultrasound-degraded exopolysaccharides of a medicinal fungus. Carbohyd. Polym.. 2014;106:270-275.
- [Google Scholar]
- Study on the Moisturizing Effect of Dendrobium officinale. Acta Universitatis Tradit. Med. Sin. Pharmacol.. 2015;29(6):70-73.
- [Google Scholar]
- Purification, structural features, antioxidant and moisture-preserving activities of an exopolysaccharide from Lachnum YM262. Bioorg. Med. Chem. Lett.. 2017;27(5):1225-1232.
- [Google Scholar]
- Carboxymethylation of polysaccharide isolated from alkaline peroxide mechanical pulping (APMP) waste liquor and its bioactivity. Int. J. Biol. Macromol.. 2021;181(4):211-220.
- [Google Scholar]
- Quantification of uronic acids in tea polysaccharide conjugates and their antioxidant properties. J. Agr. Food Chem.. 2004;52(11):3333.
- [Google Scholar]
- Structure and molecular morphology of a novel moisturizing exopolysaccharide produced by Phyllobacterium sp. 921F. Int. J. Biol. Macromol.. 2019;135:998-1005.
- [Google Scholar]
- Chemical analysis, moisture-preserving, and antioxidant activities of polysaccharides from Pholiota nameko by fractional precipitation. Int. J. Biol. Macromol.. 2019;131:1021-1031.
- [Google Scholar]
- Clarys, P., Clijsen, R., Taeymans, J., & André O, B., 2012. Hydration measurements of the stratum corneum: comparison between the capacitance method (digital version of the Corneometer CM 825) and the impedance method (Skicon‐200EX). Skin. Res. Technol. 18(3), 316-23.
- Cong, H. X., 2018. The studies on extration technology and moisturizing efficacy of nymphaea hybrid polysaccharide. Master thesis, Shanghai Jiao Tong University.
- Extraction, characterization and biological activity of sulfated polysaccharides from seaweed Dictyopteris divaricata. Int. J. Biol. Macromol.. 2018;117:256-263.
- [Google Scholar]
- Dal'Belo, S. E., Gaspar, L. R., & Berardo Gonçalves Maia Campos, P. M., 2010. Moisturizing effect of cosmetic formulations containing Aloe vera extract in different concentrations assessed by skin bioengineering techniques. Skin Res. 12(4), 241-246.
- Use of Opuntia ficus-indica (L.) Mill extracts from Brazilian Caatinga as an alternative of natural moisturizer in cosmetic formulations. Braz. J. Pharm. Sci.. 2016;52(3):459-470.
- [Google Scholar]
- Eleni, Papakonstantinou, Michael, Roth, George, & Karakiulakis., 2012. Hyaluronic acid: A key molecule in skin aging. Derm. Endocrinology. 4, 253-258.
- The effect of surface nanometre-scale morphology on protein adsorption. PLoS.. 2010;5(7):e11862.
- [Google Scholar]
- High-value co-products from plant foods: cosmetics and pharmaceuticals - ScienceDirect. Handbk. Waste Manage. Co-Prod. Recov. Food Process.. 2007;36(7):470-501.
- [Google Scholar]
- Study of moisturizing efficacy of acidolysis products of Tremella aurantialba polysaccharides. Deterg. Cosmet. 2015:19-21.
- [Google Scholar]
- Research progress on the potential delaying skin aging effect and mechanism of tea for oral and external use. Food Funct.. 2021;12:2814-2828.
- [Google Scholar]
- Comparative study of five instruments measuring stratum corneum hydration (Corneometer CM 820 and CM 825, Skicon 200, Nova DPM 9003, DermaLab). Part I. In vitro. Skin. Res. Technol.. 1999;5:171-178.
- [Google Scholar]
- Confocal Raman microspectroscopy of the skin. Eur. J. Dermatol. Ejd.. 2011;21(6):851.
- [Google Scholar]
- Freitas, F, Alves, V. D., & Reis, M., 2015. Bacterial Polysaccharides: Production and Applications in Cosmetic Industry: Bacterial Polysaccharides: Prod. Appl. Cosmet. Ind. 1-24
- Freitas, F., Torres, C. A. V., Araújo, D. Farinha, I., Pereira, J. R., Concórdio-Reis, P., & Reis., M. A. M., 2021. Advanced Microbial Polysaccharides. Biopoly. Biomed. Biotechnol. Appl.19-62.
- D. Glaser, & A. Mattox., 2013. Cutaneous Barrier Function, Moisturizer Effects and Formulation. Wiley-Blackwell. 55-65.
- On the use of infrared spectroscopy for the in vivo measurement of the water content of the horny layer after application of dermatologic ointments. J. Arch. Dermatol. Res.. 1981;271(3):305-313.
- [Google Scholar]
- Relationships between the molecular structure and moisture-absorption and moisture-retention abilities of succinyl chitosan. Polym. Bull.. 2007;59(4):509-516.
- [Google Scholar]
- Hao, L., Liu, W., Liu, K., Shan, K., Wang, C., Xi, C., Liu, J.,…Cui, B., 2019. Isolation, Optimization of Fermentation Conditions, and Characterization of an Exopolysaccharide from Pseudoalteromonas agarivorans Hao 2018. Mar. Drugs. 17(12), 703.
- Biological activity and structure of plant polysaccharides. Food Sci.. 2010;17:493-496.
- [Google Scholar]
- Structural characterization of a water-soluble polysaccharide from the fruiting bodies of Agaricus bisporus. Int. J. Mol. Sci.. 2014;15:787-797.
- [Google Scholar]
- Structural characterization of the released polysaccharide of desiccation-tolerant Nostoc commune DRH-1. J. Bacteriol.. 2000;182(4):974-982.
- [Google Scholar]
- The application of natural plant products in cosmetics. Subtrop. Plant. Sci.. 2017;46(3):297-300.
- [Google Scholar]
- Degradation of Porphyra haitanensis polysaccharide by ultrasonic assisted hydrogen peroxide method and its antioxidant activity analysis. South China Fish. Sci.. 2020;16:110-119.
- [Google Scholar]
- Microwave-assisted extracting combined with ultrafiltration technology to purify Gracilaria verrucosa polysaccharide and its moisture retentive research. Food. Ind.. 2014;46:297-300.
- [Google Scholar]
- Expression and localization of aquaporins in benign prostate hyperplasia and prostate cancer. Chonnam. Med. J.. 2012;48(3):174-178.
- [Google Scholar]
- Unravelling property of polysaccharides from Sargassum sp. as an anti-wrinkle and skin whitening property. Int. J. Biol. Macromol.. 2019;140:216-224.
- [Google Scholar]
- Comparative study on skin protection activity of polyphenol-rich extract and polysaccharide-rich extract from Sargassum vachellianum. PLoS ONE. 2020;15(1):e0227308.
- [Google Scholar]
- Ji,X. L.,I., Zhao, G. Q., & Liu, J. L., 2018. Physicochemical Properties, Hygroscopicity and Moisturizing Performance of Mannan from Saccharomyces cerevisiae. Fine. Chem. 35(3), 284-290.
- Chemical structures and bioactivities of sulfated polysaccharides from marine algae. Mar. Drugs. 2011;9(2):196-223.
- [Google Scholar]
- Purification, antioxidant, hygroscopicity and moisture retention activity of low molecular weight polysaccharide from Litchi chinensis. Trans. Chin. Soc. Agric. Eng.. 2016;32(9):277-283.
- [Google Scholar]
- Soluble branched (1,4)-beta-D-glucans from Acetobacter species enhance antitumor activities against MHC class I-negative and -positive malignant melanoma through augmented NK activity and cytotoxic T-cell response. Int. J. Cancer. 2005;115(5):769-776.
- [Google Scholar]
- Moisturizing effect of alcohol-based hand rub containing okra polysaccharide. Int. J. Cosmet. Sci.. 2012;34(3):280-283.
- [Google Scholar]
- Novel topical skin hydration agent containing Anadenanthera colubrina polysaccharide-standardized herbal preparation. J. Cosmet. Dermatol.. 2020;19(7):1691-1698.
- [Google Scholar]
- Applications of ATR-FTIR spectroscopic imaging to biomedical samples. Biochim. Biophys. Acta. 2006;1758(7):858-867.
- [Google Scholar]
- The use of near-infrared spectroscopy in skin care applications. Skin Res. Technol.. 2006;12(3):162-169.
- [Google Scholar]
- Evidence-Based Skincare: The Importance of Offering Moisturization, Relief, and Protection in Common Skin Disorders. J. Drugs. Dermatol.. 2016;15(11):s76
- [Google Scholar]
- Molecular weight, hygroscopicity and moisturizing performance of polysaccharide from Rhizoma Bletillae. Chin. Surfactant. Deterg. Cosmet.. 2015;45:94-98.
- [Google Scholar]
- Antioxidative and antimutagenic activity of yeast cell wall mannans in vitro. Mutat. Res.. 2001;497:213-222.
- [Google Scholar]
- New developments and prospective applications for β (1,3) glucans. Recent. Pat. Biotechnol.. 2007;1(1):59-73.
- [Google Scholar]
- Structure of rhamnan sulfate from the green alga Monostroma nitidum and its anti-herpetic effect. Carbohyd. Polym.. 2010;81(3):572-577.
- [Google Scholar]
- Effect of natural polysaccharide on moisture retention capacity and moisture kinetic of tobacco. Food Mach.. 2020;36:28-32.
- [Google Scholar]
- Sulfated modification, characterization, and antioxidant and moisture absorption/retention activities of a soluble neutral polysaccharide from Enteromorpha prolifera. Int. J. Biol. Macromol.. 2017;105:1544-1553.
- [Google Scholar]
- Antioxidant and moisture-retention activities of the polysaccharide from Nostoc commune. Carbohyd. Polym.. 2011;83:1821-1827.
- [Google Scholar]
- Physicochemical characterization and functional analysis of the polysaccharide from the edible microalga Nostoc sphaeroides. Molecules. 2018;23(2):508.
- [Google Scholar]
- Mitochondrial protection and anti-aging activity of astragalus polysaccharides and their potential mechanism. Int. J. Mol. Sci.. 2012;13(2):1747-1761.
- [Google Scholar]
- Characterization of high yield exopolysaccharide produced by Phyllobacterium sp. 921F exhibiting moisture preserving properties. Int. J. Biol. Macromol.. 2017;101:562-568.
- [Google Scholar]
- Thermal and antioxidant properties of polysaccharides sequentially extracted from mulberry leaves (Morus alba L.) Int. J. Biol. Macromol.. 2017;22(12):2271.
- [Google Scholar]
- Purification, antioxidant and immunological activities of polysaccharides from Actinidia Chinensis roots. Int. J. Biol. Macromol.. 2014;72:975-983.
- [Google Scholar]
- Progress on antitumor mechanisms of polyporus polysaccharide. J. Henan Univ. Sci. Technol. Nat. Sci.. 2011;29(3):236-238.
- [Google Scholar]
- Influence of Aloe polysaccharide on proliferation and hyaluronic acid and hydroxyproline secretion of human fibroblasts in vitro. J. Chin. Integr Med.. 2010;8(3):256.
- [Google Scholar]
- Antioxidation and moisture retention activity of extracellular polysaccharides from Porphyridium cruentum. Food. Res. Dev.. 2014;5:1-6.
- [Google Scholar]
- Preparation and antioxidant activities of important traditional plant polysaccharides. Int. J. Biol. Macromol.. 2018;111:780-786.
- [Google Scholar]
- Controlled acetylation of water-soluble glucomannan from Bletilla striata. Carbohyd. Polym.. 2012;89(1):158-162.
- [Google Scholar]
- Recent advances in endophytic exopolysaccharides: production, structural characterization, physiological role and biological activity. Carbohyd. Polym.. 2016;157:1113-1124.
- [Google Scholar]
- Characterization and chemical modification of PLN-1, an exopolysaccharide from Phomopsis liquidambari NJUSTb1. Carbohyd. Polym.. 2021;253:117197
- [Google Scholar]
- Extraction, isolation, and purification of polysaccharides from leaves of Acanthopanax gracilistylus and property of their moisture retention. Chin. Tradit. Herb. Drugs. 2015;46:1167-1173.
- [Google Scholar]
- Preparation of low molecular weight Eucheuma striatum sulfated polysaccharides by oxidative degradation with H2O2 under the ultrasonic wave. Chin. J. Mar. Drugs. 2005;20(4):10-13.
- [Google Scholar]
- Mao, H., H.Y. Wang, Luan, Y. H., & Guo, X. P., 2012. Evaluation of moisturizing effect of sodium hyaluronate with different molecular weights. In Proceedings of the Ninth Chinese Cosmetic Symposium, Shanghi, China. Ninth China Cosmetic Symposium. 19-22.
- Meng, H., Dong, Y. M., & Qiu, X. R., 2006. Effect of oat β-glucan on skin moisturization in experimental rats, In Proceedings of the 2006 Chinese Cosmetic Symposium. 301-304.
- A novel exopolysaccharide from deep-sea bacterium Zunongwangia profunda SM-A87: low-cost fermentation, moisture retention, and antioxidant activities. Appl. Microbiol. Biot.. 2010;108(8):679-686.
- [Google Scholar]
- Physicochemical study of acemannan polysaccharide in Aloe species under the influence of soil reaction (pH) and moisture application. Afr. J. Pure. App. Chem.. 2012;6(9):132-136.
- [Google Scholar]
- Ning, X. Y., Zhang, M. C., Sun, Z. Y., Z. p. Z., & Shen, P. L., 2020. Advances in the Efficacy Application of Fucoidan in Cosmetics. China. Clean. Ind. (8), 73-80.
- A critical review of algal biomass: a versatile platform of bio-based polyesters from renewable resources. Int. J. Biol. Macromol.. 2016;86:937-949.
- [Google Scholar]
- The polysaccharides of green, red and brown seaweeds: their basic structure, biosynthesis and function. Brit. Phycol. J.. 1979;14:103-117.
- [Google Scholar]
- Moisture retention and antibacterial activity of modified chitosan by hydrogen peroxide. J. Appl. Polym. Sci.. 2002;86(7):1724-1730.
- [Google Scholar]
- Hepatoprotective effect of plant polysaccharides from natural resources: a review of the mechanisms and structure-activity relationship. Int. J. Biol. Macromol.. 2020;161:24-34.
- [Google Scholar]
- Carbohydrates –- the renewable raw materials of high biotechnological value. Crit. Rev. Biotechnol.. 2003;23:149-173.
- [Google Scholar]
- Multifunctional properties of polysaccharides from Dalbergia sissoo, Tectona grandis and Mimosa diplotricha. Carbohydr. Polym.. 2014;102:341-350.
- [Google Scholar]
- Optimization and characterization of a polysaccharide produced by Pseudomonas fluorescens WR-1 and its antioxidant activity - ScienceDirect. Carbohyd. Polym.. 2012;90(2):921-929.
- [Google Scholar]
- Effects of aloe vera flower extract and its active constituent isoorientin on skin moisturization via regulating involucrin expression. in vitro and molecular docking studies. Molecules. 2021;26(9):2626.
- [Google Scholar]
- Advances in bacterial exopolysaccharides: from production to biotechnological applications. Trends Biotechnol.. 2011;29(8):388-398.
- [Google Scholar]
- Skincare characteristic of active polysaccharide from Aloe vera. Drug Evaluat. Res.. 2012;35:431-434.
- [Google Scholar]
- Biotechnology and the red seaweed polysaccharide industry: status, needs and prospects. Trends Biotechnol.. 1997;15(1):9-14.
- [Google Scholar]
- [ACS Symposium Series] extracellular microbial polysaccharides. Eds. Am. Chem. Soc.. 1977;45:40-57.
- [Google Scholar]
- Active edible polysaccharide coating and interactions between solution coating compounds. Carbohyd. Polym.. 2002;49(2):139-144.
- [Google Scholar]
- Advances in research on structure-activity relationship of plant polysaccharides. Carbohyd. Polym.. 2019;46:99-100.
- [Google Scholar]
- Improvement of antioxidant and moisture-preserving activities of Sargassum horneri polysaccharide enzymatic hydrolyzates. Int. J. Biol. Macromol.. 2015;74:420-427.
- [Google Scholar]
- Separation, preliminary characterization, and moisture-preserving activity of polysaccharides from Ulva fasciata. Int. J. Biol. Macromol.. 2015;72:924-930.
- [Google Scholar]
- Unusual water flux in the extracellular polysaccharide of the cyanobacterium Nostoc commune. Appl. Environ. Microb.. 2003;69(9):5679-5684.
- [Google Scholar]
- Carboxymethylation of cashew tree exudate polysaccharide. Carbohyd. Polym.. 2004;58(2):163-171.
- [Google Scholar]
- Structure and dynamics of wheat starch in breads fortified with polyphenols and pectin: an ESEM and solid-state CP/MAS13C NMR spectroscopic study. Food Bioproc. Tech.. 2013;6(1):110-123.
- [Google Scholar]
- Investigation of Chemical Composition and Biological activities of Polysaccharides from Bryopsidales. Master, Graduate School of Chinese Academy of Sciences; 2010.
- Production and properties of an exopolysaccharide synthesized by the extreme halophilic archaeon Haloterrigena turkmenica. Food Bioproc. Tech.. 2016;100(2):613-623.
- [Google Scholar]
- A novel exopolysaccharide from deep-sea bacterium Zunongwangia profunda SM-A87: low-cost fermentation, moisture retention, and antioxidant activities. Appl. Microbiol. Biot.. 2014;98(17):7437-7445.
- [Google Scholar]
- The chemical properties and hygroscopic activity of the exopolysaccharide lubcan from Paenibacillus sp. ZX1905. Int. J. Biol. Macromol.. 2020;164(11):2641-2650.
- [Google Scholar]
- characterization and biotechnological potential analysis of a new exopolysaccharide from the arctic marine bacterium polaribacter sp SM1127. Sci. Rep.. 2015;5(1):18435.
- [Google Scholar]
- Characterization and biotechnological potential analysis of a new exopolysaccharide from the arctic marine bacterium Polaribacter sp. SM1127. Sci. Rep.. 2015;5(1):18435.
- [Google Scholar]
- An oil-soluble extract of Rubus idaeus cells enhances hydration and water homeostasis in skin cells. Int. J. Cosmet. Sci.. 2015;37(6):588-594.
- [Google Scholar]
- Yang, Shaoli.,Guo, Zhanyong., Xue, Qinzhao.,Miao, Fengping., Wang, Yan., Qin, Song., 2009. The moisture absorption and retention abilities of hyaluronan, chitosan, starch and their quaternary derivatives. In The 3rd International Conference on Bioinformatics and Biomedical Engineering(ICBBE 2009), Beijing(CN), 1-4.
- Torti, M. J., Sims, C. A., Adams, C. M., & Sarnoski, P. J., 2016. Polysaccharides as Alternative Moisture Retention Agents for Shrimp. J. Food Sci. 81(1-3), S728-S735.
- A novel moisture-absorbing extracellular polysaccharide from Rhodococcus rhodochrous SM-1. Actinomycetologica. 2002;16(2):26-31.
- [Google Scholar]
- Skin hydration: a review on its molecular mechanisms. Carbohydr. Res.. 2010;6(2):75-82.
- [Google Scholar]
- Comparison between TEWL and laser scanning microscopy measurements for the in vivo characterization of the human epidermal barrier. J. Biophotonics. 2012;5(2):152-158.
- [Google Scholar]
- Isolation, Characterization, and Pharmaceutical Applications of an Exopolysaccharide from Aerococcus Uriaeequi. Mar Drugs. 2018;16(9):337
- [Google Scholar]
- Chemical composition and moisture-absorption/retention ability of polysaccharides extracted from five algae. Int. J. Biol. Macromol.. 2013;57:26-29.
- [Google Scholar]
- Review on structure-activity relationship of active polysaccharides. Mod. Chem. Ind.. 2002;22(5):18-23.
- [Google Scholar]
- Properties of crude polysaccharides extracted from Tremella fuciformis by acid, alkali and enzyme-assisted methods. Food Sci. Technol.. 2019;44(04):200-204.
- [Google Scholar]
- Antioxidant activity, hygroscopicity and water retention of natural and degraded polysaccharides from pavlova viridi. Food Sci.. 2012;33:87-90.
- [Google Scholar]
- Physicochemical property analysis of polysaccharides from marine microalgae and their antioxidation, hygroscopicity and moisture retention activities. Fine. Chem.. 2012;29(1):20-88.
- [Google Scholar]
- Novel method for measuring moisture holding capacity of cosmetics. J. Dalian. Inst. Light Ind.. 1999;18:20-22.
- [Google Scholar]
- Effect of phthaloylation on radical-scavenging and moisture-preserving activities of polysaccharide from Enteromorpha linza. Carbohyd. Polym.. 2014;111(1):729-733.
- [Google Scholar]
- Carboxymethylation of polysaccharides from Tremella fuciformis for antioxidant and moisture-preserving activities. Int. J. Biol. Macromol.. 2015;72:526-530.
- [Google Scholar]
- Efficacy evaluation of cosmetics(Ⅱ)Scientific support for moisturizing cosmetic efficacy claims. China Surf. Deterg. Cosmet.. 2018;48:67-72.
- [Google Scholar]
- Medicinal mushrooms as a source of antitumor and immunomodulating polysaccharides. Appl. J. Microbiol. Biotechnol.. 2002;60(3):258-274.
- [Google Scholar]
- Structure and chain conformation of a neutral intracellular heteropolysaccharide from mycelium of Paecilomyces cicadae. Carbohyd. Polym.. 2016;136(4):728.
- [Google Scholar]
- Protective effects of tea polysaccharides and polyphenols on skin. Agri. Food Chem.. 2009;57(17):7757-7762.
- [Google Scholar]
- Research on the application of ginseng polysaccharide in cosmetics. Mod. Chem.. 2020;9:97-98.
- [Google Scholar]
- Chain conformation and physicochemical properties of polysaccharide (glucuronoxylomannan) from Fruit Bodies of Tremella fuciformis. Carbohyd Polym.. 2020;245(6):116354
- [Google Scholar]
- Physicochemical properties, structural characterization and hypoglycemic activity in vitro of degraded polysaccharides from blackcurrant. Food Sci.. 2021;42(15):37-43.
- [Google Scholar]
- Characterization, hypolipidemic and antioxidant activities of degraded polysaccharides from Ganoderma lucidum. Int. J. Biol. Macromol.. 2019;135:706-716.
- [Google Scholar]
- Yang, A. N., Chai, Y. C., Zhao, T. D., Song, S. L., Guo, J. A., & Center, D., 2017. The Extraction of Polysaccharides from Sargassum fusiforme and Its Application in Cosmetics. Flavour. Frag Cosmet. (1), 58-61+65.
- Moisture Retention Characteristics of Konjac Glucomannan and Its Derivatives. Food Sci. (Beijing, China). 2011;32(3):46-50.
- [Google Scholar]
- Study on the structure characterization and moisturizing effect of Tremella polysaccharide fermented from GCMCC5.39. Food Sci. Hum. Well.. 2021;10(4):471-479.
- [Google Scholar]
- Antioxidant activities of an exopolysaccharide isolated and purified from marine Pseudomonas PF-6. Carbohydr. Polym.. 2012;87(1):764-770.
- [Google Scholar]
- Activity diversity structure-activity relationship of polysaccharides from lotus root varieties. Carbohyd. Polym.. 2017;190(8):67-76.
- [Google Scholar]
- Tetralogy of moisturization: from skin physiology to solution. Deterg. Cosmet.. 2017;40(10):48-52.
- [Google Scholar]
- A decrease in moisture absorption–retention capacity of N-deacetylation of hyaluronic acid. Glycoconj. J.. 2013;30(6):577-583.
- [Google Scholar]
- Purification, antioxidant and moisture-preserving activities of polysaccharides from papaya. Carbohydr. Polym.. 2012;87(3):2332-2337.
- [Google Scholar]
- Study on moisturizing effect of aloe vera macromolecule gel. Flavour. Frag. Cosmetic 2020:31-35.
- [Google Scholar]
- Purification, characterization, antioxidant and moisture-preserving activities of polysaccharides from Rosa rugosa petals. Int. J. Biol. Macromol.. 2019;124(3):938-945.
- [Google Scholar]
- Culture medium optimization of a new bacterial extracellular polysaccharide with excellent moisture retention activity. J. Appl. Microbiol. Biot.. 2013;97(7):2841-2850.
- [Google Scholar]
- Degradation of porphyran from Porphyra haitanensis and the antioxidant activities of the degraded porphyrans with different molecular weight. Int. J. Biol. Macromol.. 2006;38(1):45-50.
- [Google Scholar]
- Synthesis and moisture retention activity of carboxymethyl Schizophyllan. Mod. Food Sci. Technol.. 2017;33(8):254-261.
- [Google Scholar]
- A review of the studies on the polysaccharide structure. Nat. Nanchang Univ. Sci.. 2001;21(25):197-204.
- [Google Scholar]
- Zhu, S. H., & Sun, P. D., 2013. Extraction and Moisture Retention of Polysaccharides from Eragrostis Teff. Nat. Prod. Res. Development. 25(1), 83-70.
- Insights into physicochemical and functional properties of polysaccharides sequentially extracted from onion (Allium cepa L.) Int. J. Biol. Macromol.. 2017;105(Part 1):1192-1201.
- [Google Scholar]
- Research progress on microbial polysaccharide. Sci. Technol. Food Ind.. 2012;33(6):444-448.
- [Google Scholar]







