Translate this page into:
Construction of three novel Co/Zn/Cd(II) coordination polymers based on the same ligands: Different spatial structures, electrocatalysis and photoluminescence properties
⁎Corresponding authors. liubo2017@iccas.ac.cn (Bo Liu), jiufulu@snut.edu.cn (Jiu-Fu Lu)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
Three new metal coordination polymers with different spatial structure, namely {[Co2(H2O)6(4-PDCA)2]2·3H2O}n (SNUT-12), {[Cd(H2O)2(4-PDCA)]·2H2O}n (SNUT-13) and [Zn2(4-PDCA)2]n (SNUT-14), where H2(4-PDCA) = 1-(4-carboxyphenyl)-5-methyl-4-oxo-1,4-dihydropyridazine-3-carboxylic acid, were synthesized by changing the central ion with the same ligand under solvothermal and hydrothermal conditions, which were further characterized by single crystal X-ray diffraction, powder XRD, FT-IR and TGA. Single crystal X-ray analysis revealed that SNUT-12 exhibits a 1D infinite zigzag chains which were constructed by Cobalt(II) ion and 4-PDCA2− ligand, SNUT-13 features a 2D ladder-like structure. Meanwhile, SNUT-14 displays 2-fold 3D → 3D parallel interpenetrating frameworks. In addition, SNUT-12 exhibited high electrocatalytic oxygen evolution reaction, and SNUT-13, SNUT-14 showed strong fluorescence at room temperature.
Keywords
Coordination polymers
Spatial structure
Electrocatalysis
Photoluminescence properties
1 Introduction
At present, industrial production is driven by a myriad of energy‐demanding electronic devices owing to the advancements of science and technology. The main energy sources are still based on fossil fuels that have limited reserves (Xu et al., 2017), a series of environmental problems caused by fossil energy seriously affect the survival of human beings, The key to solving the problems is to produce renewable energy in a green and environmental–friendly way (Wang et al., 2021; Zhang et al., 2021). Hydrogen energy has the most potential as a clean energy alternative to fossil energy due to its high heating value and non-polluting combustion product H2O. Hydrogen energy on the earth mainly exists in the form of water. The industry mainly uses water electrolysis to produce hydrogen. In this process, the oxygen evolution reaction (OER) has been bottlenecked for the sustainable hydrogen production in water electrolysis (Liang et al., 2020; Xiong et al., 2023). OER passes through a four-electron transfer process coupled with the breaking of O–H bond and the formation of O-O bond, which is kinetically sluggish and requires high overpotential to overcome the energy barrier (Lu et al., 2017; Dang et al., 2020).
In order to maximize the efficiency of the reaction and reduce the power consumption on the electrode, it is necessary to select unique composition and structure electrocatalyst which reduce the activation energy of electrocatalytic reactions and provide conduction sites for electrochemical reactions, improving the rate and selectivity of the reactions (Greeley et al., 2006; Zhu et al., 2016). Commercial noble metal catalysts such as RuO2 and IrO2 had been generally regarded as the one of highly efficient electrocatalyst for OER (Cheng et al., 2022; Zhang et al., 2020), but the limitations of expensive and scarce resources of precious metals, currently unavailable for practical application (Kumar et al., 2020; Zhang et al., 2021). Therefore, non-noble metal catalysts have become a hot field of electrocatalyst research, such as Co-based (Wang et al., 2019; Liu et al., 2022), Ni-based (Dao et al., 2022; Karuppasamy et al., 2019) and Fe-based (Nivetha et al.,2019; Liang et al., 2022).
Coordination polymers (CPs), which can be used to prepare highly efficient catalysts, selecting non-noble metals with electrocatalytic activity and coordinating with organic ligands (Wang et al., 2020), due to the bridging ligands, metal agglomeration was effectively avoided, resulting in electrocatalysts with high electron transfer efficiency and stability (Jin, 2018; Qian et al., 2017; Yang et al., 2021). In addition, as a branch of coordination polymers, Metal-Organic Frameworks (MOFs) construct an ordered network structure by coordinating metal ions and organic ligands, which had large surface area, uniform pores, controllable framework materials and other advantages (Kung et al., 2019), MOFs have been applied in electrocatalysts (Meng et al., 2023; Lu et al., 2022), sensitive detection(Zheng et al., 2021; Han et al., 2023; Wang et al., 2022), molecular probe (Liu et al., 2020; Han et al. 2022) and efficient removal (Lin et al., 2021).
In this paper, three new metal coordination polymers with different spatial structures had been constructed by H2(4-PDCA) (the synthetic method of the isomer of this ligand was recently reported by our another paper (Gao et al., 2023)), Co2+, Cd2+ and Zn2+ under solvothermal conditions, respectively: {[Co2(H2O)6(4-PDCA)2]2·3H2O}n (SNUT-12), {[Cd(H2O)2(4-PDCA)]·2H2O}n (SNUT-13) and [Zn2(4-PDCA)2]n (SNUT-14). OER performance (SNUT-12) and fluorescence emission properties (SNUT-13 and SNUT-14) had been in investigated at room temperature.
2 Experimental
2.1 Materials and physical measurements
The H2(4-PDCA) ligand was synthesized in our laboratory and all used chemical reagents are available from Shanghai Aladdin Biochemical Technology Co., Ltd. Crystal data was obtained on a Rigaku XtaLAB Synergy X-diffractometer. X-ray powder diffraction patterns was obtained on a Bruker D8 ADVANCE. IR spectra were recorded as KBr pellets on a Thermo scientific Nicolet™ iS20 FT-IR spectrometer. Electrochemical investigation data was obtained on a Shanghai Chenhua CHI660E workstation. Fluorescence spectra were recorded on EDINBURGH FLS-1000 at room temperature.
2.2 Synthesis of {[Co2(H2O)6(4-PDCA)2]2·3H2O}n (SNUT-12)
A mixture of Co(NO3)2·6 H2O (0.1 mmol), H2(4-PDCA) (0.1 mmol) 1,3-BIP (0.1 mmol) in DMF-H2O (4 mL) binary solvent was placed in a 25 mL teflon -lined stainless steel container, under heated to 150 ℃ for 72 h, and then cooled to room temperature over 2d, orange crystals of SNUT-12 was collected in 79.5 % yield based on Cobalt. Elemental analysis (%): calcd for C52H66Co4N8O37 (Mr = 1630.84): C 38.26, H 4.05, N 6.87; found: C 38.25, H 4.55, N 6.89. IR(v, cm−1): 3396(v), 1600(s), 1544(s), 1390(s), 1242(m), 786(m), 759(m).
2.3 Synthesis of {[Cd(H2O)2(4-PDCA)]·2H2O}n (SNUT-13)
A mixture of Cd(NO3)2·4H2O (0.2 mmol), H2(4-PDCA) (0.05 mmol), 4-Methylimidazole (0.2 mmol) and HNO3 (30 μL) in DMA-H2O (4 mL) binary solvent was placed in a 25 mL teflon-lined stainless steel container, under heated to 80 ℃ for 72 h, and then cooled to room temperature over 2d, light yellow crystals of SNUT-13 was collected in 74 % yield based on Cadmium. Elemental analysis (%): calcd for C13H16N2O9Cd (Mr = 456.68): C 34.16, H 1.31, N 6.13; found: C 34.19, H 1.35, N 6.10. IR(v, cm−1): 3433(v), 1639(s), 1611(s), 1451(m), 1395(s), 1255(m), 773(m).
2.4 Synthesis of [Zn2(4-PDCA)2]n (SNUT-14)
A mixture of Zn(NO3)2·6H2O (0.1 mmol), H2(4-PDCA) (0.05 mmol) and H2O (2 mL) was placed in a 25 mL teflon-lined stainless steel container, under heated to 140 ℃ for 72 h, and then cooled to room temperature over 2d, colorless crystals of SNUT-14 was collected in 89.5 % yield based on Zinc. Elemental analysis (%): calcd for C26H16N4O10Zn2 (Mr = 675.17): C 46.17, H 2.37, N 8.29; found: C 46.19, H 2.40, N 8.31. IR(v, cm−1): 1668(m), 1600(s), 1556(s), 1384(w), 1288(m), 781(m).
2.5 Crystal structure determination
Select suitable crystals of SNUT-12, SNUT-13 and SNUT-14 were selected, its X-ray diffraction analysis data collection which were performed at 293(2) K with a CCD four-circle diffractometer XtaLAB Synergy, Dualflex, HyPix, with mirror-monochromated Cu Ka radiation (λ = 1.54184 Å). The crystal structure was solved by direct methods with the SHELXT 2014/5 (Sheldrick, 2014) and refined with the SHELXL 2018/3 (Sheldrick, 2015) (Dolomanov et al., 2009; Sheldrick, 2015; Sheldrick, 2015), All non-hydrogen atoms were refined using anisotropic displacement parameters, and all hydrogen atoms were generated geometrically. Crystallographic data and experimental details of structural analyses for molecular was summarized in Table 1. The main bond lengths and angles of SNUT-12, SNUT-13 and SNUT-14 are listed in Table 2. R1 = Σ(||Fo|–|Fc||)/Σ|Fo|, wR2 = [Σ(||Fo|2–|Fc||2)2/Σw(Fo2)]1/2. Symmetry code: #1 (1 + x, −1 + y, z) #2 (1 + x, 1 + y, z) #3 (-1 + x, −1 + y, z) #4 (-1 + x, 1 + y, z) #5 (-1-x, 2-y, 2-z).
Crystal name
SNUT-12
SNUT-13
SNUT-14
Crystal size
0.32 × 0.30 × 0.25
0.25 × 0.18 × 0.12
0.23 × 0.21 × 0.18
Formula
C52H66Co4N8O37
C13H16CdN2O9
C26H16Zn2N4O10
Fw
1630.84
456.68
675.17
T/K
293(2)
293(2)
293(2)
Crystal system
orthorhombic
monoclinic
triclinic
Space group
Pccn
P21/n(14)
P1(2)
a/Å
37.377(4)
14.37834(19)
8.9441(9)
b/Å
7.0812(8)
6.70780(9)
9.1373(10)
c/Å
25.808(3)
16.1461(2)
14.8301(16)
α/°
90
90
72.3400(10)
β/°
90
98.0104(13)
87.208(2)
γ/°
90
90
87.383(2)
V/Å3
6830.7(13)
1542.05(4)
1152.9(2)
Z
4
4
2
Dc/g·cm−3
1.586
1.967
1.945
F(0 0 0)
3352
912
680
GOF on F2
1.176
1.076
1.030
Reflection/unique
5635/4721
2988/2881
4037/3593
R1, wR2 [I > 2σ(I)]
0.1225, 0.3296
0.0476, 0.1269
0.0292/0.0847
R1,wR2 (all data)
0.1353, 0.3353
0.0476, 0.1282
0.0338/0.0870
SNUT-12
Co(1)-O(1)#1
2.033(8)
Co(1)-O(11)#1
2.107(10)
Co(1)-O(1)
2.033(8)
Co(1)-O(12)#1
2.138(11)
Co(1)-O(11)
2.107(10)
Co(1)-O(12)
2.138(11)
O(1)#1-Co(1)-O(1)
180.0
O(1)#1-Co(1)-O(11)#1
87.0(4)
O(1)#1-Co(1)-O(11)
93.0(4)
O(1)–Co(1)-O(11)#1
93.0(4)
O(1)–Co(1)-O(11)
87.0(4)
O(15)#2-Co(3)-O(6)#2
91.0(4)
Symmetry code: #1 (1/2 + x, −1/2 + y, -z) #2 (1/2-x, −1/2-y, z)
SNUT-13
Cd(1)#1-O(3)
2.255(3)
Cd(1)-O(2)
2.296(3)
Cd(1)-O(1)
2.277(3)
Cd(1)-O(6)
2.347(3)
Cd(1)#2-O(4)
2.329(3)
Cd(1)-O(7)
2.310(3)
O(3)#1-Cd(1)-O(1)
172.23(10)
O(3)#1-Cd(1)-O(6)
84.18(12)
O(3)#1-Cd(1)#2-O(4)
86.70(11)
O(3)#1-Cd(1)-O(7)
102.74(12)
O(3)#1-Cd(1)-O(2)
98.50(10)
O(1)-Cd(1)#2-O(4)
90.72(10)
Symmetry code: #1 (1-x, 1-y, 1-z) #2 (3/2-x, −1/2 + y, 1/2-z)
SNUT-14
Zn(1)-O(1)
1.9890(19)
O(4)-Zn(3)#1
2.0523(19)
Zn(3)-O(2)
2.176(2)
O(5)-Zn(3)#1
2.0413(19)
O(3)-Zn(1)#1
1.9931(19)
O(6)-Zn(2)#2
2.0960(18)
O(7)#3-Zn(1)-O(3)#4
120.11(8)
O(1)-Zn(1)-O(8)
107.94(8)
O(1)-Zn(1)-O(3)#4
122.48(8)
O(3)#4-Zn(1)-O(8)
99.29(8)
O(7)#3-Zn(1)-O(8)
103.47(8)
O(10)-Zn(2)-O(10)#5
180.00(5)
2.6 Electrocatalytic properties
(Wang et al., 2022) To mixture of SNUT-12 (10 mg), H2O (500 μL), EtOH (490 μL) and Nafion (10 μL), Ultrasonic dispersion for 20 min, drop the suspension on the surface of the working electrode and air dry, this method will adhere SNUT-12 on the working electrode. Then, the carbon rod is used as the counter electrode, the saturated calomel electrode (SCE) is used as the reference electrode and KOH (1 mol/L) as electrolyte, Analysis of OER properties of the coordination polymer by cyclic voltammetry (CV) and linear sweep voltammetry (LSV). Meanwhile, according to electrochemical impedance spectroscopy (EIS), the ability of the material to transfer electrons can be obtained.
3 Results and discussion
3.1 Crystal structure of SNUT-12
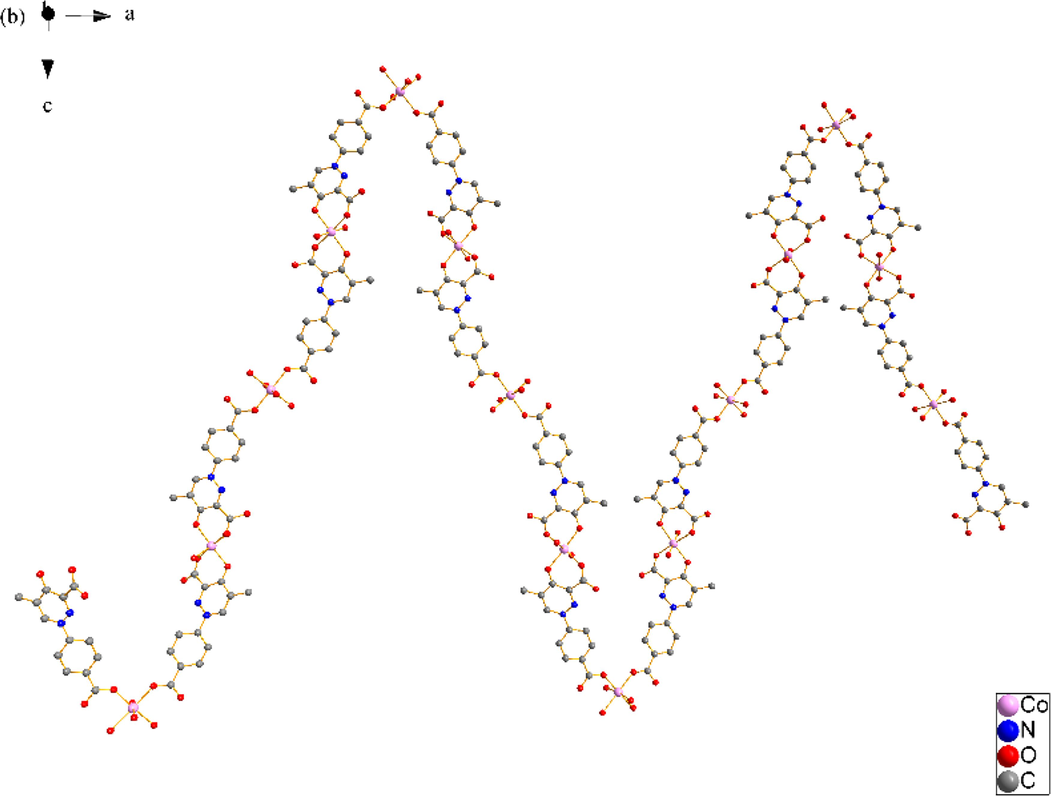
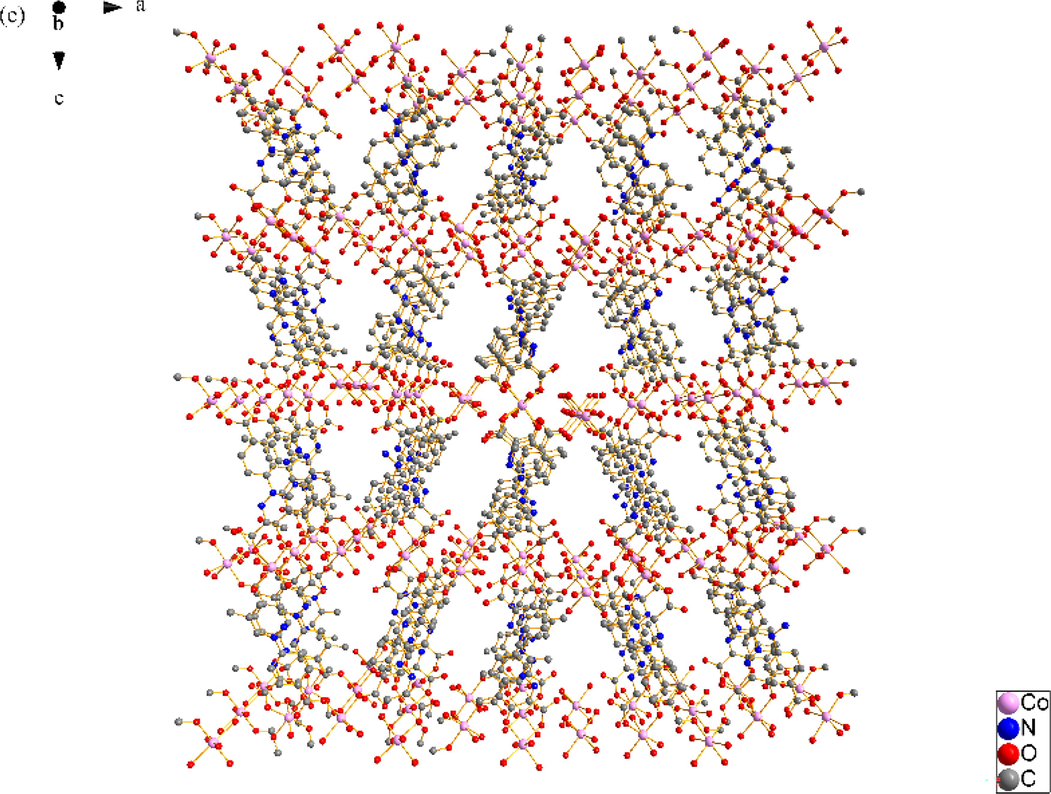
X-ray single crystal diffraction analysis reveals that SNUT-12 crystallizes in orthorhombic system, space group Pccn. The asymmetric unit of SNUT-12 consists of two crystallographically independent Co(II) ions, two deprotonated 4-PDCA2− ligands, six monodentate coordinated water molecules and three free water molecules (Fig. 1a). Two Co(Ⅱ) ions exhibit similar but different coordination geometry, Co1 is six-coordinated with four oxygen atoms from four monodentate coordinated water molecules and two oxygen atoms from two 4-PDCA2− ligands. Meanwhile, Co2 is also six-coordinated with two oxygen atoms from two monodentate coordinated water molecules, two carbonyl oxygen atoms from two 4-PDCA2− anions and two carboxyl oxygen atoms from two of the above 4-PDCA2− anions. Oxygen atoms from the same source the atoms are in opposite positions. The 4-PDCA2− is as a bidentate ligand bridging adjacent above-mentioned two crystallographically independent Co(II) ions to form a 1D zigzag chain (Fig. 1b), the chains are linked by intermolecular hydrogen bonding to form a 3D supermolecules structure (Fig. 1c).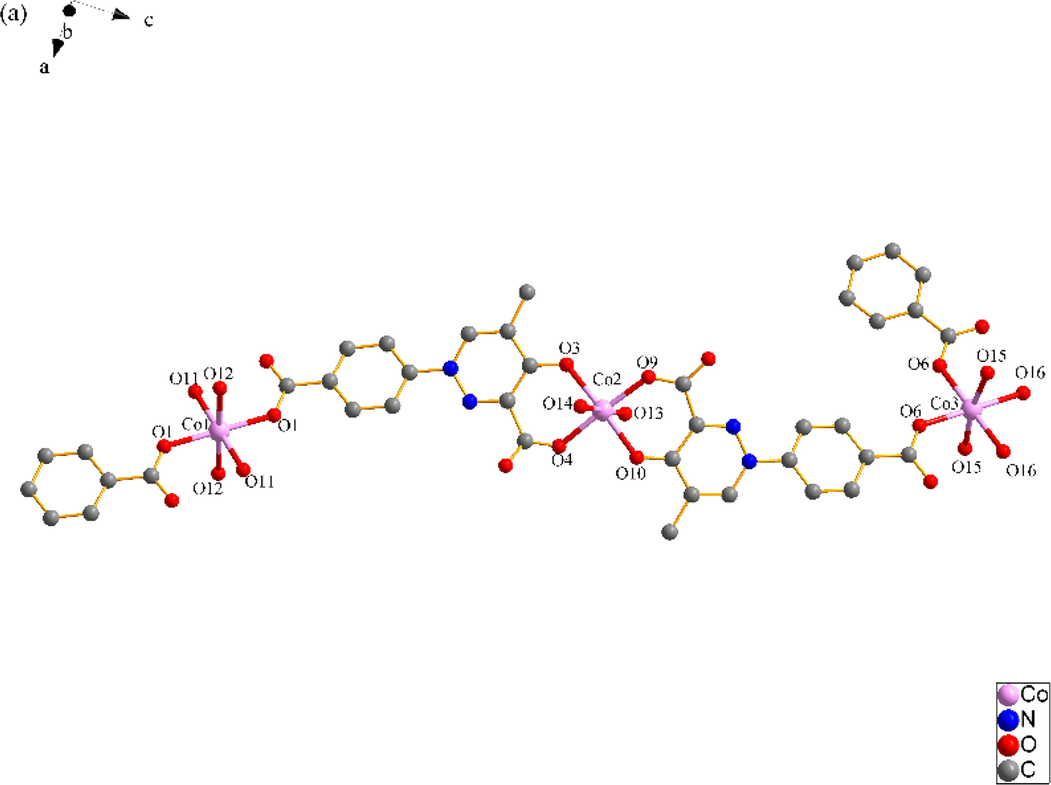
(a) Coordination environment of Co(II) ion in SNUT-12, in which the hydrogen atoms and free water molecules are omitted for clarity; (b) A View of 1D infinite chain of asymmetric unit was connected; (c) A View of 3D structure along the b axis in SNUT-12.
(a) Coordination environment of Co(II) ion in SNUT-12, in which the hydrogen atoms and free water molecules are omitted for clarity; (b) A View of 1D infinite chain of asymmetric unit was connected; (c) A View of 3D structure along the b axis in SNUT-12.
(a) Coordination environment of Co(II) ion in SNUT-12, in which the hydrogen atoms and free water molecules are omitted for clarity; (b) A View of 1D infinite chain of asymmetric unit was connected; (c) A View of 3D structure along the b axis in SNUT-12.
3.2 Crystal structure of SNUT-13
SNUT-13 crystallizes in monoclinic system with space group P21/n. The asymmetric unit of SNUT-13 consists of one Cd(II) ion, one deprotonated 4-PDCA2−ligand, two monodentate coordinated water molecules and two free water molecules. As shown in Fig. 2a, each Cd(II) ion is six-coordinated with two oxygen atoms from two monodentate coordinated water molecules and three carboxyl oxygen atoms from three different 4-PDCA2− anions and one carbonyl oxygen atom from one 4-PDCA2− ligand. Which shows a distorted octahedral geometry. Additionally, two adjacent Cd(II) ions are connected by 4-PDCA2− ligands to generate a 2D ladder-like plane (Fig. 2b). Due to the π-π stacking interaction between the aromatic rings, the stability of the structure is further enhanced (Fig. 2c). Also, these planes are further linked through intermolecular hydrogen bonding to form a 3D supermolecule structure (Fig. 2d).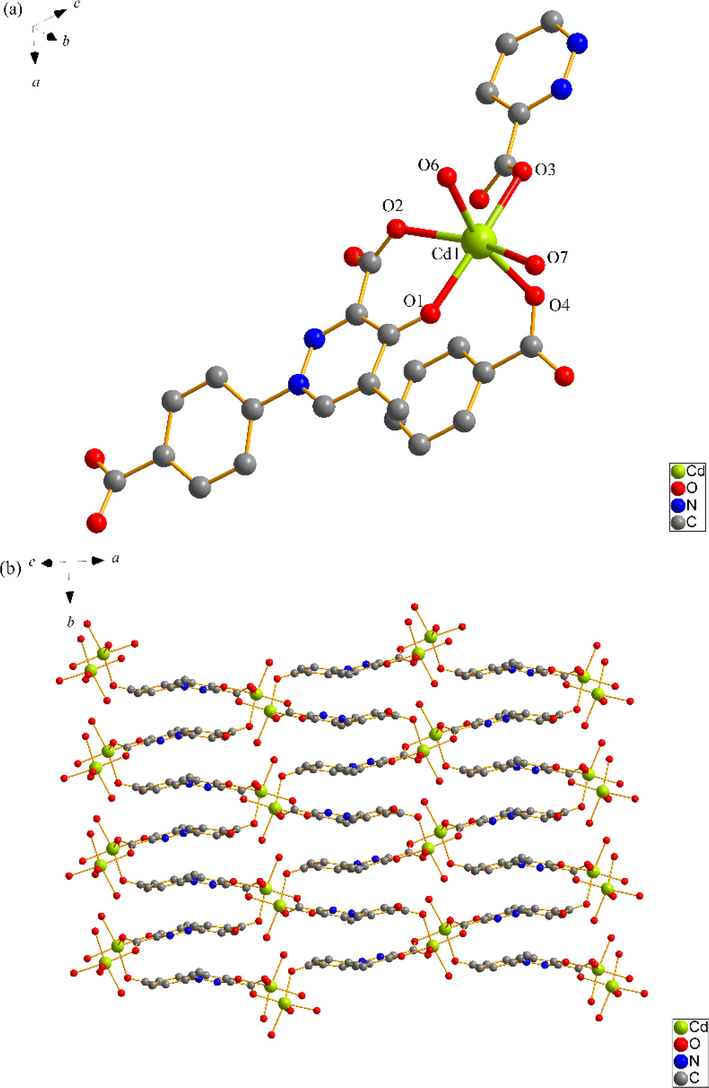
(a) Coordination environment of Cd(II) ion in SNUT-13, in which the hydrogen atoms are omitted for clarity; (b) A View of 2D infinite layered plane parallel to the b axis; (c) Schematic diagram of π-π stacking in SNUT-13; (d) A View of 3D structure along the b axis in SNUT-13.
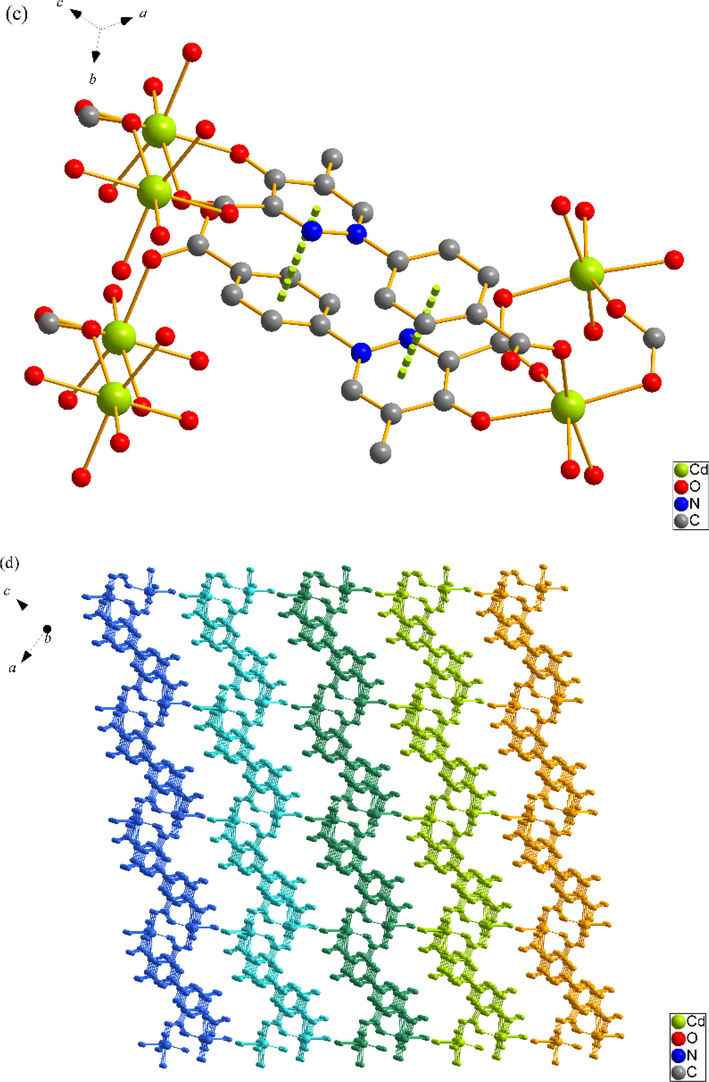
(a) Coordination environment of Cd(II) ion in SNUT-13, in which the hydrogen atoms are omitted for clarity; (b) A View of 2D infinite layered plane parallel to the b axis; (c) Schematic diagram of π-π stacking in SNUT-13; (d) A View of 3D structure along the b axis in SNUT-13.
3.3 Crystal structure of SNUT-14
SNUT-14 crystallizes in triclinic system with space group P-1. The asymmetric unit of SNUT-14 consists of two crystallographically independent Zn(II) ions and two 4-PDCA2− ligands. As shown in Fig. 3a, two Zn(II) ions exhibit different coordination mode, which each Zn1 is four-coordinated with four carboxyl oxygen atoms from four different 4-PDCA2− anions (Zn-O = 1.9619(2)-1.9991(2) Å). Meanwhile, each Zn2 exhibit distorted octahedral coordination geometry, which is six-coordinated with two carbonyl oxygen atoms from two different 4-PDCA2− ligands, four carbonyl oxygen atom from four different 4-PDCA2− ligands. Additionally, the carboxyl groups of L act as μ2-bridgings to connect adjacent Zn(II) ions to generate a 1D ⋯Zn–OCO–Zn–OCO⋯ zigzag chain along the c-axis direction (Fig. 3b). Then, this chain acts as an inter-connector of countless parallel chains further connects near four neighbors by 4-PDCA2− ligands to form a porous 3D architecture (Fig. 3c). As illustrated in Fig. 3d, because there is a large void space, the individual 3D frameworks are interpenetrated into each other, and a 2-fold 3D → 3D interpenetrating architecture is thus generated.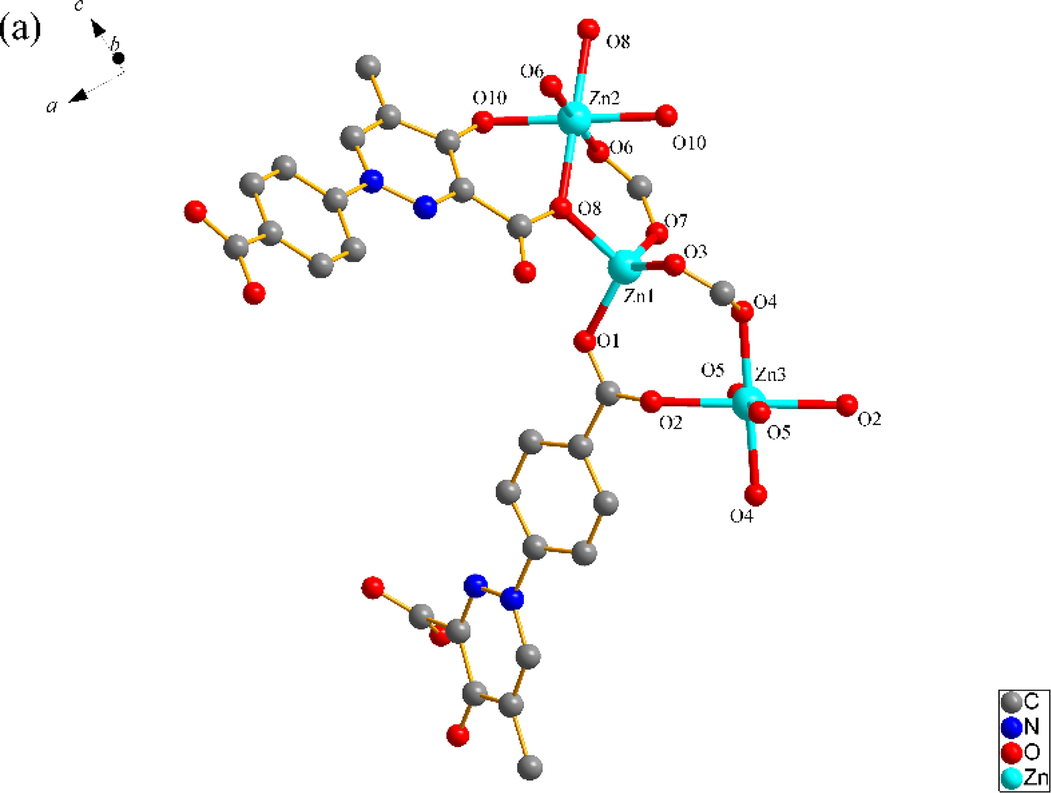
(a) Coordination environment of Zn(II) ion in SNUT-14, in which the hydrogen atoms are omitted for clarity; (b) View of a 1D zigzag chain along the c-axis direction; (c) View of 3D structure along the c axis in SNUT-14; (d) 2-fold 3D → 3D parallel interpenetrating structure of SNUT-14.
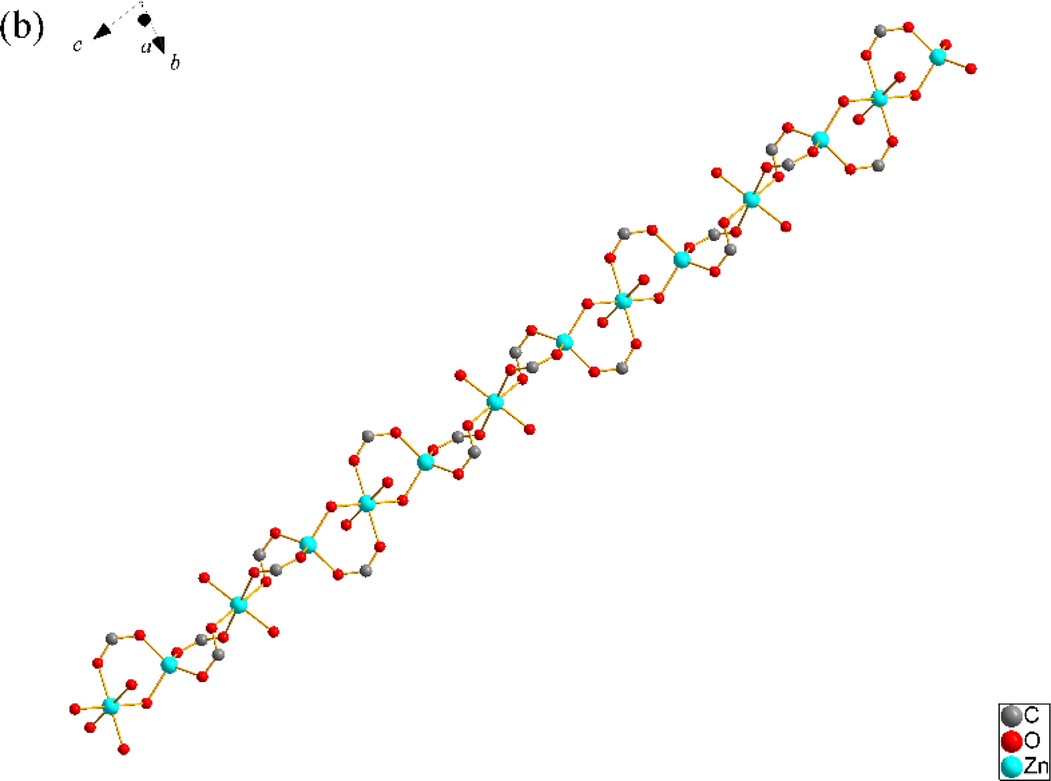
(a) Coordination environment of Zn(II) ion in SNUT-14, in which the hydrogen atoms are omitted for clarity; (b) View of a 1D zigzag chain along the c-axis direction; (c) View of 3D structure along the c axis in SNUT-14; (d) 2-fold 3D → 3D parallel interpenetrating structure of SNUT-14.
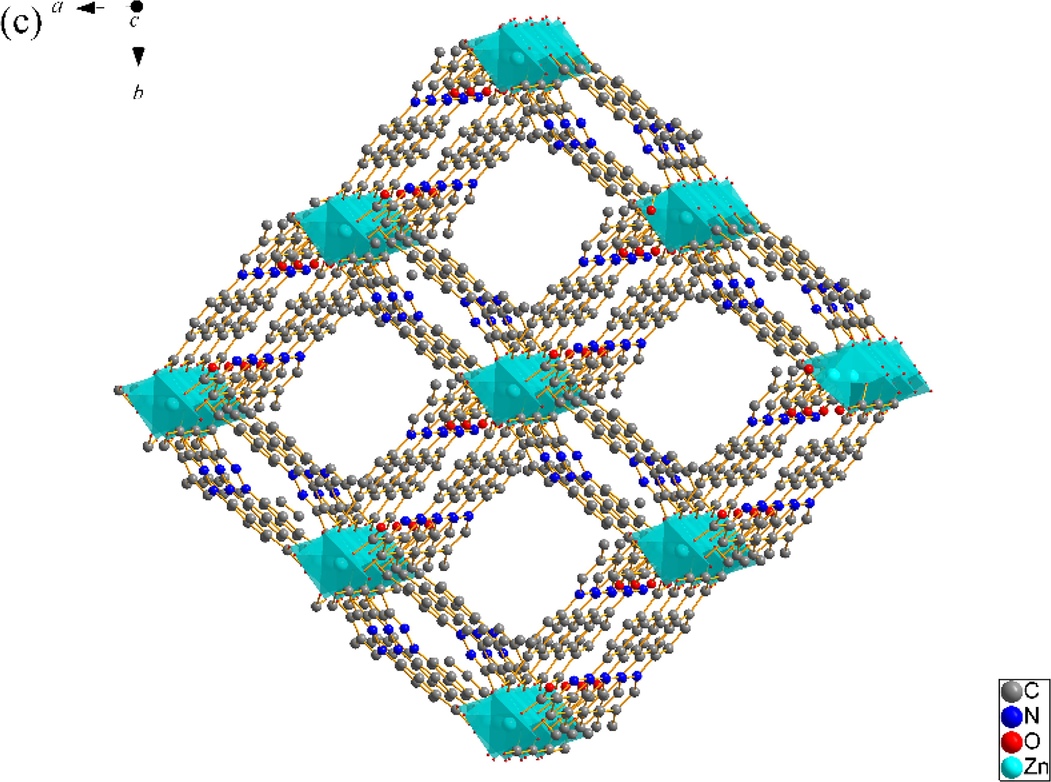
(a) Coordination environment of Zn(II) ion in SNUT-14, in which the hydrogen atoms are omitted for clarity; (b) View of a 1D zigzag chain along the c-axis direction; (c) View of 3D structure along the c axis in SNUT-14; (d) 2-fold 3D → 3D parallel interpenetrating structure of SNUT-14.
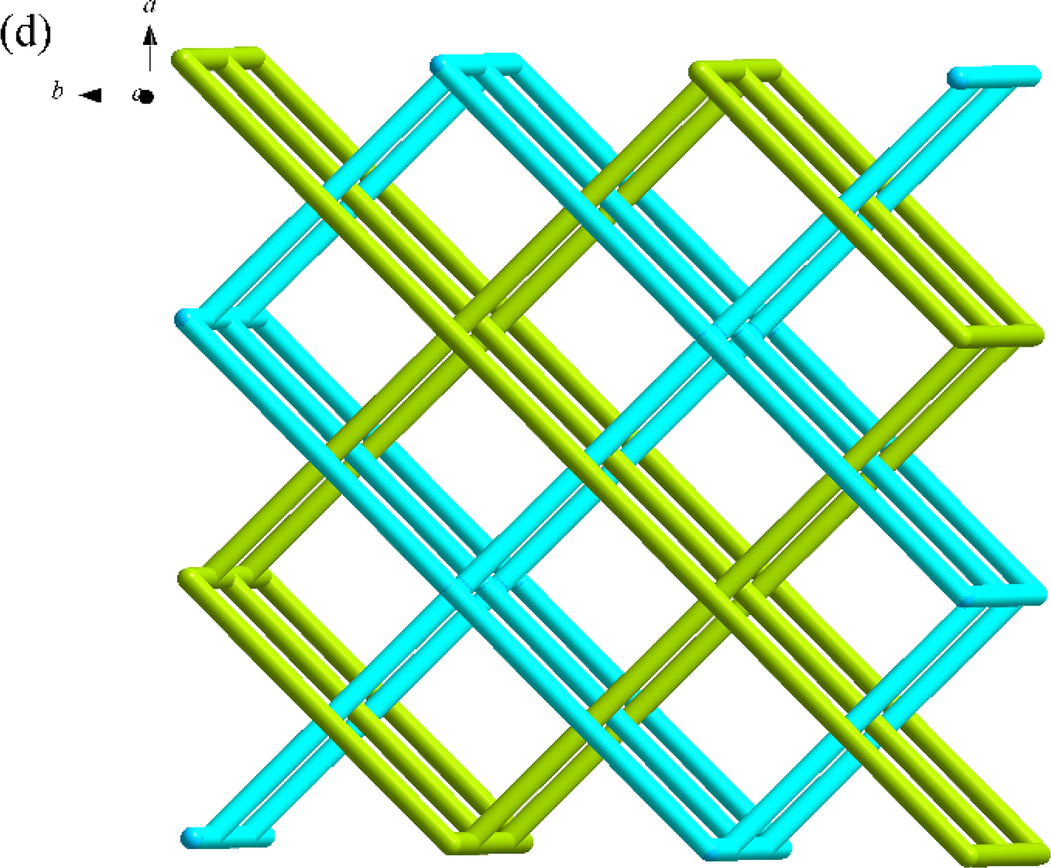
(a) Coordination environment of Zn(II) ion in SNUT-14, in which the hydrogen atoms are omitted for clarity; (b) View of a 1D zigzag chain along the c-axis direction; (c) View of 3D structure along the c axis in SNUT-14; (d) 2-fold 3D → 3D parallel interpenetrating structure of SNUT-14.
3.4 X-ray diffraction(XRD) patterns and FT-IR spectra
The powder XRD patterns of as-synthesized compounds and the simulated patterns on the basis of single-crystal structures of SNUT-12 (Fig. 4), SNUT-13 (Fig. 5) and SNUT-14 (Fig. 6), respectively. The diffraction peaks on patterns correspond well in position, indicating the phase purity of the as-synthesized samples.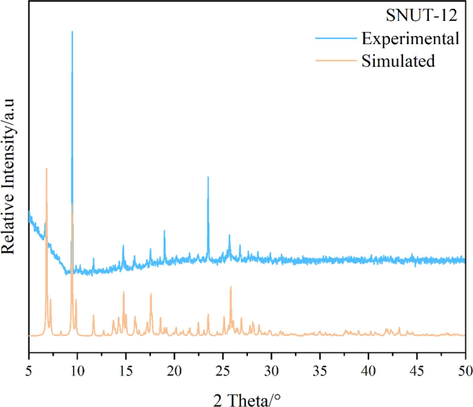
XRD pattern of SNUT-12.
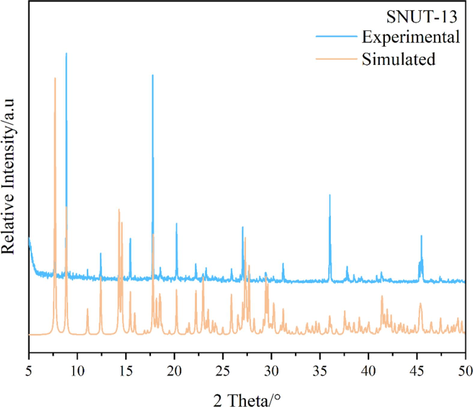
XRD pattern of SNUT-13.
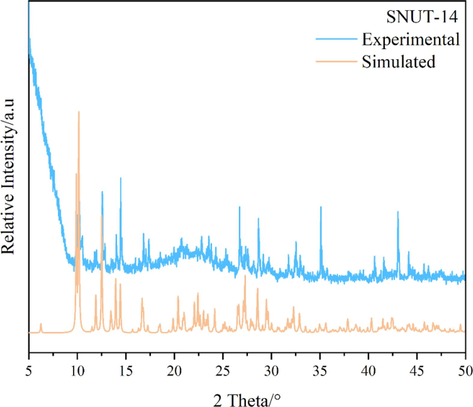
XRD pattern of SNUT-14.
As shown in Fig. 7, due to same ligand was selected for the three novel coordination polymers reported, so they exhibit the similar IR spectrum. The broad bands at 3430 cm−1 for SNUT-12 and SNUT-13 are the stretching mode of O–H, but SNUT-14 does not show the band, which indicates there exists coordinated and free water molecules. Furthermore, the C = O vibration peaks of carboxylate groups from titled MOFs can be observed at 1606 and 1400 cm−1, respectively.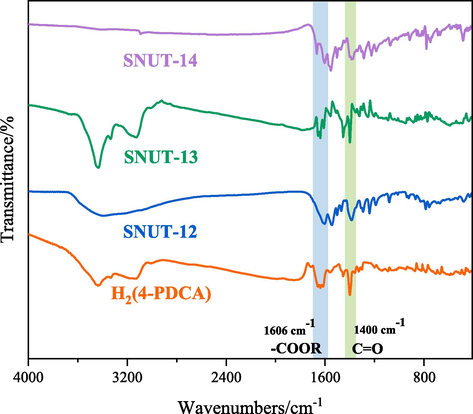
FT-IR spectra of SNUT-12, SNIT-13 and SNUT-14.
3.5 Thermal properties
Thermogravimetric (TG) measurements were carried out from 25 to 800 ℃ under N2 atmosphere flow with a heating rate of 10 °C·min−1 (Fig. 8). For SNUT-12 and SNUT-13, the first weight loss at 50∼200 ℃ may be attributed to the removal of free water molecules and monodentate coordinated water molecules, SNUT-12 exhibits a weight loss of 19.44 % (calcd 20.93 %) and SNUT-13 exhibits 15.54 % (calcd 15.72 %). Afterwards, the weight loss after 300 ℃ corresponds to the collapse of the skeleton. However, for SNUT-14, only one weight loss occurred within 425 ∼ 500 ℃, corresponding to the loss of collapse of the skeleton.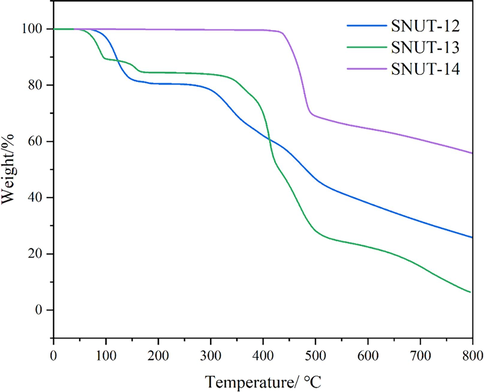
TG curves for SNUT-12, SNUT-13 and SNUT-14.
3.6 Electrochemical performance
To evaluate the OER activity of SNUT-12, the linear sweep voltammetry (LSV) polarization plots were first carried out at a sweeping rate of 5 mV·s−1 in 1.0 M KOH solutions on a three-electrode system.
It was worth noting that an overpotential of 463 mV was required to allow a current density of 20 mA cm−2, which was close to that of RuO2 (E20 mA cm−2 = 330 mV), as shown in Fig. 9. Also, SNUT-12 showed a lower Tafel slope of 68 mV dec−1, in comparison with RuO2 (103 mV dec−1) (Du et al., 2021; Lyn et al. 2022).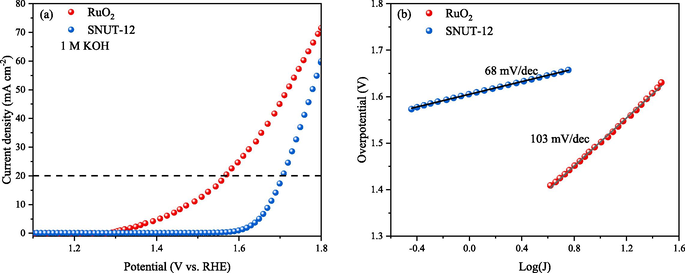
OER activity in 1 M KOH electrolyte solution, (a) the Polarization curves and (b) Tafel plots of RuO2 and SNUT-12.
The CV patterns of a series of scan rates were conducted to extract the linear relationship of current density diference (Δj/2) against scan rate (Fig. 10a). Double-layer capacitance (Cdl) was carried out to estimate the electrochemically active surface area (ECSA) (Li et al., 2023), from Fig. 1, it can be seen that SNUT-12 exhibit the Cdl of 61 mF cm−2 (Fig. 10b), which demonstrate the potential of this material as an available candidate toward OER (Kang et al., 2021).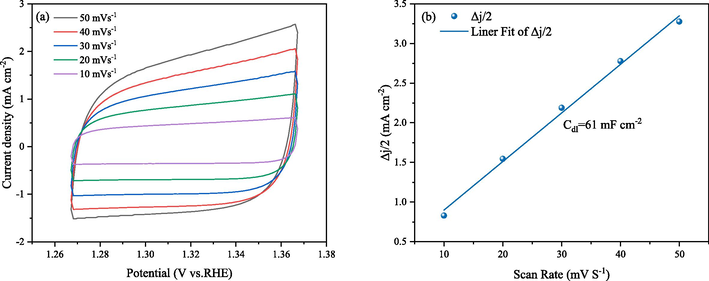
Investigation of the electrochemical active surface areas. (a) The cyclic voltammetry (CV) curves of SNUT-12; (b) estimation of Cdl by plotting the current density variation against scan rate to fit a linear regression.
These data illustrate SNUT-12 had the smaller overpotential at a current density of 20 mA⋅cm−2, which can effectively reduce the energy consumption required for the electrolytic water reaction. Tafel slope and Cdl can also well characterize the electrocatalytic performance of the catalyst.
As shown in Fig. 11, Electrochemical impedance spectroscopy (EIS) was used to characterize the electrode kinetics of the interface reactions during the OER process, through this method, the size of the charge-transfer resistances (Rct) of SNUT-12 will be obtained in EIS.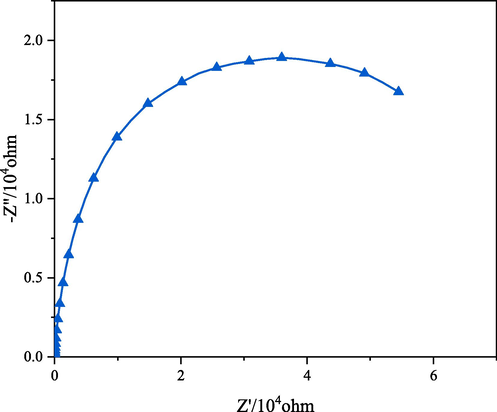
Nyquist plots of electrochemical impedance spectra (EIS, 5 mV) for SNUT-12.
3.7 Fluorescence properties
The solid-state luminescence properties of the H2(4-PDCA) ligand as well as coordination polymers SNUT-13 and SNUT-14 were investigated at room temperature. H2(4-PDCA) shows emission with a maximum at 448 nm upon excitation at 380 nm in solid state, the maximum emission peaks for SNUT-13 and SNUT-14 are observed at 478 nm and 423 nm upon excitation at 410 nm and 360 nm, shown in Fig. 12, fluorescence intensity was enhanced compared to free H2(4-PDCA) ligands. In addition, in order to examine all the possible colors by combining three primary colors, the CIE-1931 (Commission International de L’Elairage 1931) chromaticity coordinates for SNUT-13 and SNUT-14 were determined based on matching their emission spectra and were calculated as (0.22, 0.32) for SNUT-13, and (0.17, 0.12) for SNUT-14 (Fig. 13). At room temperature, the emissions of the two d10 MCPs are located in the blue region, which is consistent with their visible color.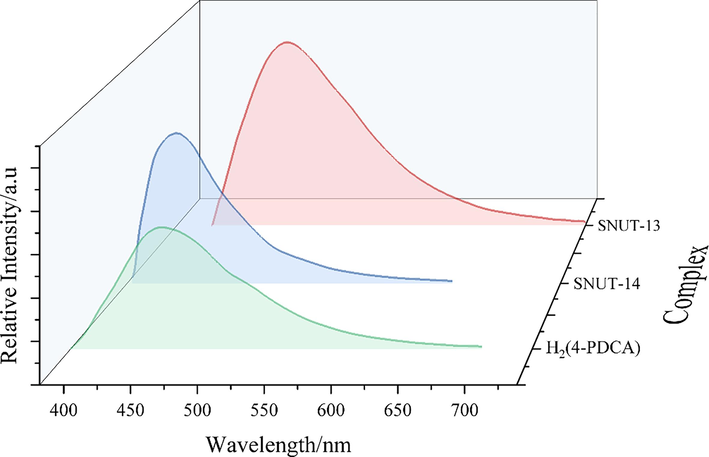
Solid-state emission spectra of the H2(4-PDCA), SNUT-13 and SNUT-14.
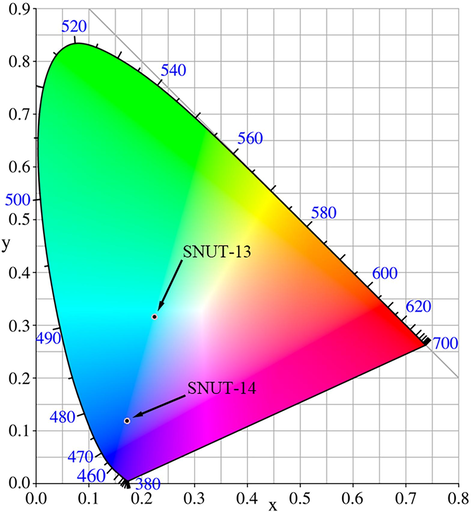
CIE-1931 chromaticity diagram for SNUT-13 and SNUT-14 at room temperature.
4 Conclusions
In summary, three novel metal coordination polymers had been constructed by 4-PDCA ligand under solvothermal or water conditions. By changing the central ion, three new coordination polymers exhibit different steric configuration. SNUT-12 features a 1D infinite zigzag chain, SNUT-13 features an unusual 2D layered structure, and, SNUT-14 exhibits a porous 2-fold 3D → 3D architecture. In addition, SNUT-12 exhibit excellent catalytic activity for OER in electrochemical performance, SNUT-13 and SNUT-14 exhibits different fluorescence properties due to their different coordination structures.
5 Supplementary materials
Crystallographic data for the structural analysis have been deposited with the Cambridge Crystallographic Data Center, and CCDC No. 2,232,541 for SNUT-12, 2,232,542 for SNUT-13 and 2,232,543 for SNUT-14. Copies of the data can be obtained free of charge on application to the Director, CCDC, 12 Union Road, Cambridge, CB2 1EZ, UK (Fax: +44–1223–336033; e-mail: deposit@ccdc.cam.ac.uk or https://www.ccdc.cam.ac.uk).
Funding
This work is supported by the National Natural Science Foundation of China (No.52003148), the Shaanxi Provincial Department of Education Project (22JK 0319), the Key Research and Development Project of Shaanxi province (No. 2023-YBGY-475), the Key Scientific Research and Project of Education Department of Shaanxi province (No. 2022JS003), the Industrialization Project of the State Key Laboratory of Biological Resources and Ecological Environment (Cultivation) of Qinba Region (No. SXC-2310), the Shaanxi provincial of Scientific Research and Project of Education Department (No. 2022JK0311) and the doctor research start foundation of Shaanxi University of Technology (SLGRCQD2108).
CRediT authorship contribution statement
Jia-Hao Gao: Conceptualization, Methodology, Software, Investigation, Writing – original draft. Pei-Pei Huang: Resources, Writing – review & editing, Supervision, Data curation. Jie Liu: Validation, Formal analysis, Visualization, Software. Nan Zheng: Software, Data curation. Dong Wang: Resources, Writing – review & editing, Supervision, Data curation. Si-Yu Yue: Data curation, Formal analysis, Methodology, Investigation. Er-Nu Liu: Validation, Formal analysis, Visualization. Quan Liu: Writing – review & editing. Bo Liu: Validation, Formal analysis, Visualization. Jiu-Fu Lu: Writing – review & editing.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Morphology-dependent electrocatalytic performance of a two-dimensional nickel-iron MOF for oxygen evolution reaction. Inorg. Chem.. 2022;61(18):7095-7102.
- [Google Scholar]
- Low-crystalline mixed Fe-Co-MOFs for efficient oxygen evolution electrocatalysis. J. Mater. Sci.. 2020;55:13951-13963.
- [Google Scholar]
- Electrochemical performance of metal-organic framework MOF(Ni) doped graphene. Int. J. Hydrogen Energ.. 2022;47(38):16741-16749.
- [Google Scholar]
- OLEX2: a complete structure solution, refinement and analysis program. J. Appl. Cryst.. 2009;42:339-341.
- [Google Scholar]
- Co-heteroatom-based MOFs for bifunctional electrocatalysts for oxygen and hydrogen evolution reactions. Inorg. Chem.. 2021;60(17):13434-13439.
- [Google Scholar]
- A new pyrazine carboxyl derivative and its two d10 metal coordination polymers: Syntheses, characterization, DFT and Property. J. Mol. Struct.. 2023;1290:135935
- [Google Scholar]
- Computational high-throughput screening of electrocatalytic materials for hydrogen evolution. Nat. Mater.. 2006;5:909-913.
- [Google Scholar]
- A multicenter metal-organic framework for quantitative detection of multicomponent organic mixtures. CCS Chem.. 2022;4:3238-3245.
- [Google Scholar]
- Preparation and quantitative analysis of multicenter luminescence materials for sensing function. Nat. Protoc.. 2023;18:1621-1640.
- [Google Scholar]
- Optimal cobalt-based catalyst containing high-ratio of oxygen vacancy synthesized from metal-organic-framework (MOF) for oxygen evolution reaction (OER) enhancement. Appl. Surf. Sci.. 2021;560:150035
- [Google Scholar]
- Metal-organic framework derived NiMo polyhedron as an efficient hydrogen evolution reaction electrocatalyst. Appl. Surf. Sci.. 2019;478:916-923.
- [Google Scholar]
- Insight into the catalytic performance of HER catalysis of noble metal/3D-G nanocomposites. Electrochim. Acta. 2020;333:135467
- [Google Scholar]
- Electronically conductive metal–organic framework-based materials. APL Mater.. 2019;7:110902
- [Google Scholar]
- Recent progress in metal–organic frameworks (MOFs) for electrocatalysis. Ind. Chem. Mater.. 2023;122(3):2755-12608.
- [Google Scholar]
- Transition metal-based metal-organic frameworks for oxygen evolution reaction. Coordin. Chem. Rev.. 2020;424:213488
- [Google Scholar]
- Co porphyrin-based metal-organic framework for hydrogen evolution reaction and oxygen reduction reaction. Chinese Chem. Lett.. 2022;33(8):3999-4002.
- [Google Scholar]
- A dynamic MOF for efficient purification of propylene. Sci. China Chem.. 2021;64:2053-2054.
- [Google Scholar]
- Multi-functional fluorescent responses of cobalt complexes derived from functionalized amide-bridged ligand. Dyes Pigm.. 2020;174:108064
- [Google Scholar]
- Two isostructural Ni/Co(II) MOFs based on nitrogen heterocyclic ligands and their derived carbon materials for HER performance. J. Mol. Struct.. 2022;1252:132184
- [Google Scholar]
- Highly-stable cobalt metal organic framework with sheet-like structure for ultra-efficient water oxidation at high current density. J Colloid. Interf. Sci.. 2022;611:599-608.
- [Google Scholar]
- First-row transition metal based catalysts for the oxygen evolution reaction under alkaline conditions: Basic principles and recent advances. Small. 2017;13:1701931.
- [Google Scholar]
- Rare earth-based MOFs for photo/electrocatalysis. Mater. Chem. Front.. 2023;7:806-827.
- [Google Scholar]
- Role of MIL-53(Fe)/hydrated–dehydrated MOF catalyst for electrochemical hydrogen evolution reaction (HER) in alkaline medium and photocatalysis. RSC Adv.. 2019;9:3215-3223.
- [Google Scholar]
- Electrocatalysts derived from metal-organic frameworks for oxygen reduction and evolution reactions in aqueous media. Small. 2017;13(37):1701143.
- [Google Scholar]
- SHELXT - Integrated space-group and crystal-structure determination. Acta Cryst.. 2015;A71:3-8.
- [Google Scholar]
- MOF-derived electrocatalysts for oxygen reduction, oxygen evolution and hydrogen evolution reactions. Chem. Soc. Rev.. 2020;49:1414-1448.
- [Google Scholar]
- New 2D layered binary Cd(II) coordination polymer applied as luminescent sensor for the detection of acac. J. Mol. Struct.. 2022;1264:133323
- [Google Scholar]
- Solvent-free assembly of Co/Fe-containing MOFs derived N-doped mesoporous carbon nanosheets for ORR and HER. Carbon. 2019;146:671-679.
- [Google Scholar]
- Porous Co3O4 stabilized VS2 nanosheets obtained with a MOF template for the efficient HER. CrstEngComm. 2021;23:5097.
- [Google Scholar]
- Ultraviolet/ozone treatment for boosting OER activity of MOF nanoneedle arrays. Chem. Eng. J.. 2022;427:131498-131507.
- [Google Scholar]
- A review on electrocatalysis for alkaline oxygen evolution reaction (OER) by Fe-based catalysts. J. Mater. Sci.. 2023;58:2041-2067.
- [Google Scholar]
- Earth-abundant amorphous catalysts for electrolysis of water. Chinese J. Catal.. 2017;38:991-1005.
- [Google Scholar]
- Organic carboxylate-based MOFs and derivatives for electrocatalytic water oxidation. Coordin. Chem. Rev.. 2021;428:213619
- [Google Scholar]
- Recent advances in cobalt-based electrocatalysts for hydrogen and oxygen evolution reactions. J. Alloy. Compd.. 2020;821:153542
- [Google Scholar]
- Noble-metal single-atoms in thermocatalysis, electrocatalysis, and photocatalysis. Energ. Environ. Sci.. 2021;14:2954-3009.
- [Google Scholar]
- Advanced transition metal-based OER electrocatalysts: current status, opportunities, and challenges. Small 20212100129
- [Google Scholar]
- Construction of zinc(II) coordination polymers based on 3-(3-carboxypropoxy)benzoic acid and N-containing ligands for highly sensitive detection of picric acid. Inorg. Chem. Acta. 2021;516:120111
- [Google Scholar]
- Construction of a cobalt-embedded nitrogen-doped carbon material with the desired porosity derived from the confined growth of MOFs within graphene aerogels as a superior catalyst towards HER and ORR. J. Mater. Chem. A. 2016;4:15536-15545.
- [Google Scholar]







