Translate this page into:
Controlled drug release behavior of metformin hydrogen chloride from biodegradable films based on chitosan/poly(ethylene glycol) methyl ether blend
⁎Corresponding author. dratifislam@gmail.com (Atif Islam)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
Abstract
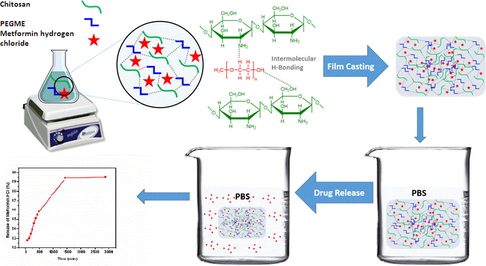
In this study, novel smart drug release films were prepared by blending chitosan with polyethylene glycol methyl ether (PEGME), also named as methoxy polyethylene glycol (mPEG), for controlled drug release applications. The polymeric films were characterized by Fourier transform infra-red for functional groups analysis, scanning electron microscopy for morphology and X-ray photoelectron spectroscopy for chemical and surface analysis followed by mechanical and thermal analysis. The mechanical properties showed that with the addition of PEGME (40%), the tensile strength and elongation break were increased up to 34.14 MPa and 26.40%, respectively as compared to the controlled sample (without PEGME). The developed biodegradable films were tested for Metformin hydrogen chloride release ability at a particular rate in phosphate buffer saline solution at pH 7.4. The results showed that chitosan/PEGME blends could be employed for controlled drug release and other biomedical applications.
Keywords
Chitosan
Biodegradable films
PEGME
Controlled drug release
PBS
Biomedical applications
1 Introduction
Recently, polymeric blend films are used as drug carrier in the field of agriculture and biotechnology. The functionality of a polymer could be increased by blending with other suitable polymers. Many biodegradable blend hydrogels have been widely studied for drug delivery, tissue engineering due to their nontoxic nature and biocompatibility and other superior characteristic properties (Kaewpirom and Boonsang, 2006; Teng et al., 2010; Tang et al., 2007; Islam et al., 2012; Saha et al., 2011; Das, 2013).
Chitosan, a natural polymer, is a biocompatible (Ma et al., 2010), biodegradable (Ghaffar et al., 2014), nontoxic and a cationic polymer obtained by the partial deacetylation of chitin (Gorochovceva et al., 2005; Yang et al., 2008; Ma et al., 2010). Chitin consists of N-acetyl glucosamine repeating units with 1,4-linkage and it is the second most abundant biopolymer in nature and can be easily obtained from the wastes in seafood processing. The properties of chitin can be enhanced in the form of chitosan by chemical modification. Chitosan can be modified into blend hydrogels, membranes and films as compared to chitin (Lee et al., 2000; Alsabagh et al., 2015). Being inexpensive, environment-friendly, hydrophilic, biodegradable, and of excellent adsorption capacity, chitosan has attracted much interest in the field of biomedicine. Due to its unique properties, chitosan and its derivatives are getting significantly used in biotechnology, pharmaceutics, textile, food, cosmetics, biomedical and other industries (Gorochovceva et al., 2004; Hu et al., 2005; Ito et al., 2013; Ma et al., 2008). It has been broadly investigated by numerous investigators in gene delivery, drug delivery and biomedical fields (Seo et al., 2009). However, raw chitosan has serious drawbacks, such as low mechanical strength, dissolution in acidic solutions, and the leaching of organics such as carbohydrates. Hence, various efforts have been made to stabilize chitosan, often by manipulating processing conditions (e.g., temperature), introducing a proper stabilizing cross-linking compound, developing chitosan blends with another polymer, or modifying the chitosan structure using chemical or ionic agents (Szymańska and Winnicka, 2015).
In recent times, polymer blends, rather than the single ones, are designed to achieve performances of their constituting ingredients synergistically (Boateng et al., 2008). In order to develop new biomaterials, blending of two or more polymeric materials shows a combination of properties that could not be achieved by applying each polymer (Chen et al., 2008). Blending of natural polymers with synthetic polymers can describe a wide range of physiochemical properties as well as biocompatibility and biological interactions of natural polymers with synthetic ones (Sarasam and Madihally, 2005). In the literature, there are many reports of blending chitosan with natural polymers as well as with synthetic polymers to improve various properties and biomedical applications such as konjac glucomannan (Ye et al., 2006), zein (Torres-Giner et al., 2009), and curdlan (Sun et al., 2011) as well as with synthetic ones such as poly (ethylene oxide) (Zivanovic et al., 2007), poly (vinyl pyrollidine) (Zeng et al., 2004), and poly (lactic acid) (Suyatma et al., 2004). Several blends have also been reported based on biomedical applications such as bone (Malafaya and Reis, 2009), cartilage (Alves da Silva et al., 2010), skin (Azad et al., 2004) and nerve (Haipeng et al., 2000) tissue engineering as well as drug delivery (Wang et al., 2007).
The in vitro drug release behavior of glutathione sensitive long-circulation polymeric micelles was developed through assembling in aqueous phase of a polymeric pro-drug which was synthesized by linkage of mPEG and 6-mercptopurine using propiolic acid as a joining limb (Shi et al., 2016). A thermosensitive gel composed of mPEG-PLGA-PLL-cRGD was developed as a nano drug delivery system which could prolong drug release time and overcome the drug resistance of pancreatic tumor cells (Shen et al., 2015). mPEG-PLA micelles and mPEG-PLA/TPGS mixed micelles were used to develop a highly efficient formulation for nimodipineb loaded micelles and mixed micelles for drug delivery application (Huang et al., 2014). A pH-responsive amphiphilic polymer (mPEG-hyd-TPE) with aggression induced emission nanoprobe micelles as a drug delivery system for bioimaging and cancer therapy was successfully designed (Wang et al., 2015). Nano particles based on mPEG-PLGA-PLL copolymer were prepared which are promising drug carriers and delivery performance (He et al., 2015). mPEG-PCL copolymers having great biocompatibility, amphiphilicity and controlled biodegradability with great potential applications in nano technology, pharmaceutics, tissue engineering and medicinal chemistry (Danafar, 2016). Both Folic acid– and mPEG–conjugated chitosan nanoparticles have been designed for prolong and targeted anticancer drug delivery system using Mitomycin C loaded to the mPEG–FA–NPs (Zhenqing et al. 2011). So far, no report on chitosan blends with mPEG loaded with Metformin hydrogen chloride and its controlled release was reported to the best of our knowledge.
The objective of this work was to evaluate the blend characteristics of chitosan with mPEG as a controlled drug delivery material. In order to get rid of before mentioned problems with pristine chitosan films, it was blended with polyethylene glycol methyl ether (PEGME) which showed improvement in film formation, mechanical strength and thermal properties. In this study, the drug delivery applications and other improving biological properties were also examined. Metformin hydrogen chloride was chosen as a model drug molecule, loaded on suitable selected film and drug release was analyzed. Metformin hydrogen chloride is an oral antidiabetic drug and used to treat type II diabetes (Zarghi et al., 2003). The conventional form of Metformin hydrogen chloride tablets can cause gastrointestinal upset, cramps, nausea, diarrhea and increased flatulence (Adikwu et al., 2004). In order to reduce these side effects and to enhance patient compliance, controlled drug release formulations of Metformin hydrogen chloride were developed. A number of studies have been reported in the literature investigating the controlled drug release from different hydrogels (Bhavesh et al., 2007; Tiwari et al., 2003). To the best of our knowledge, there was no report in the literature to discuss about chitosan and PEGME blends with controlled release of Metformin hydrogen chloride and their properties as a potential biomaterial for drug delivery applications.
2 Experimental
2.1 Materials
Low molecular weight chitosan (200 MPa; molecular weight 17103.41 g mol−1 DDA = 90%, was obtained from Biotechnologie und Logistik Gmbh, (BioLog, Germany). Acetic acid was obtained from Merck Chemicals (Dusseldorf, Germany), and Metformin hydrogen chloride was collected from Merck Pharmaceuticals (pvt) Limited, PEGME (750 Da Aldrich, USA). All of the other chemicals and reagents were of analytical grade and used as received without further purification.
2.2 Methods
2.2.1 Film preparation
Chitosan/PEGME blends were prepared by solution casting of polymer solutions in different chitosan/PEGME ratios (100/0, 90/10, 80/20, 70/30, 60/40) and each film was coded as CHS-0, CHS-1, CHS-2, CHS-3, CHS-4, respectively. Firstly, chitosan was dissolved in 2% (v/v) acetic acid solution for 90 min. Secondly, PEGME was dissolved in 20 mL ethanol. The solutions of chitosan were blended with each solution of PEGME under continuous stirring at 65 °C for 60 min. The blended solutions were cast into polystyrene petri dishes and dried at room temperature. The prepared blend films were placed in air tight plastic bags and stored in refrigerator until further analysis. Film thickness (average 20–60 µm) was determined using a micrometer (Mitutoyo 156-101, Japan) by performing at least three measurements per sample.
2.2.2 Scanning electron microscopy
Blend films were examined using a FEI Quanta 200 FEG Scanning Electron Microscope with 4 nm resolution. The micrographs were taken at an accelerating voltage of 5 kV.
2.2.3 X-ray photoelectron spectroscopy
In order to investigate the chemical compositions of the samples, X-ray photoelectron spectroscopy analysis was carried out. Thermo Scientific K-Alpha was used for X-ray photoelectron spectroscopy (XPS) measurements. The Mg Kα (1253.6 eV) X-ray source was operated at 300 W. Pass energy of 117.40 eV was used for the survey scans.
2.2.4 Fourier transform infrared spectroscopy
Fourier transform infrared (FT-IR) spectra of the films were measured with a spectrophotometer (Bruker Alpha-P) using attenuated total reflection (ATR) mode. The spectra were obtained at a resolution of 4 cm−1 in the range of 4000–450 cm−1. The FTIR spectra were taken in a transmittance mode.
2.2.5 Mechanical properties
Mechanical properties were determined using TIRA Machinenbau GmbH. Tensile strength (TS) and % elongation at break were determined according to ASTM D882-09 (ASTM, 2009) procedure (ASTM D882-09, 2009). A double clamp at a crosshead speed of 1 mm s−1 with 20 mm as initial grip separation was used. TS was calculated by dividing the maximum load by the cross-sectional area and % elongation at break was determined by dividing the extension at break by the initial gauge length of the films and multiplying by 100.
2.2.6 Thermogravimetric analysis
Thermogravimetric analysis (TGA) was conducted using a TA Instruments (SDT Q600), over the heating range from 40 to 800 °C in nitrogen atmosphere with a flow rate of 20 mL/min. The heating rate was 10 °C/min.
2.2.7 Drug release
In order to study the controlled drug release, 20 mg of Metformin hydrogen chloride was dissolved in distilled water. The drug solution was mixed with blended solution containing 80% chitosan and 20% PEGME and stirred at 60 °C for 60 min. The solution was poured into glass petri dish for film formation at room temperature. Finally, the film loaded drug was stored in a refrigerator for further analysis.
The drug release was checked by cutting film from three different regions and put into 100 mL phosphate buffer saline solution (PBS) at temperature 37 °C. The average length, width and weight of sample of drug loaded film was 2.3 cm, 2 cm and 0.038 g, respectively. The drug loaded film thickness (average 30–50 µm) which was determined using a micrometer (Mitutoyo 156-101, Japan) by performing at least three to four measurements per sample. The pH of PBS solution was maintained as 7.4 using 0.1 M NaOH and 0.1 M HCl solution. The U.V. scans of solutions had done after every one hour at λmax (200 nm) using Labomed Inc. UVD-3200. The pH of all the solution was measured using InoLab pH 7110.
3 Results and discussion
3.1 Preliminary characterization
Chitosan/PEGME blends were prepared and according to the preliminary test results. Chitosan and its blend films were semi-clear and slightly yellowish in color. The proposed interactions between chitosan and PEGME are shown in Scheme 1.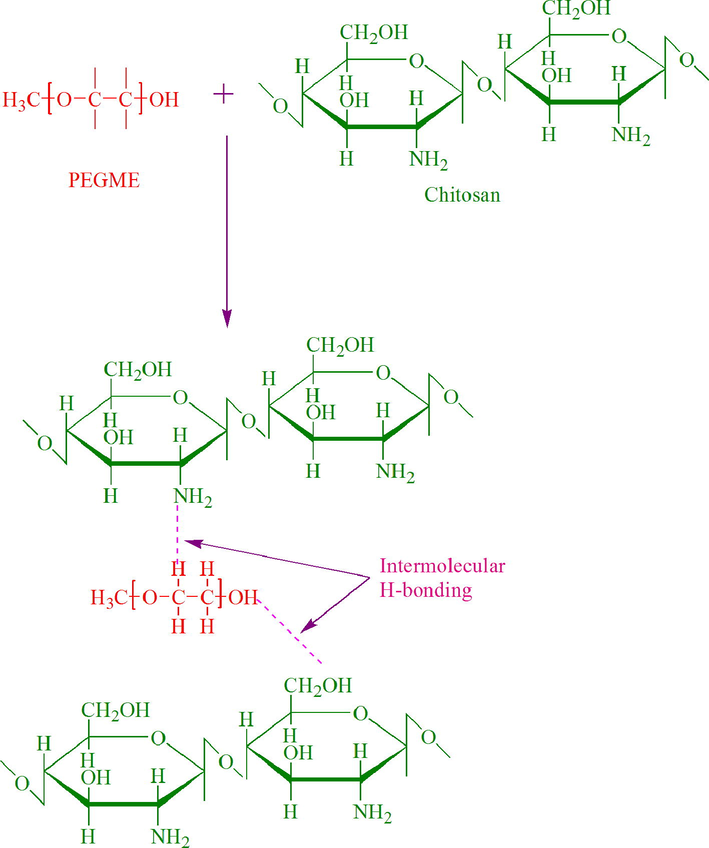
The proposed interactions between the incorporated components of the prepared hydrogels.
3.2 Scanning electron microscopy
Surface morphology of different chitosan/PEGME blend films was studied using SEM micrographs on top surface of the films as illustrated in Fig. 1. Microstructures by SEM showed that PEGME particles were well dispersed and the blends were homogeneous having 10 and 20% PEGME in the blends composition implying the partial miscibility of the components. As the percentage of PEGME in blend was increased up to 40% (w/w); the surface homogeneity of the films was decreased to some extent. Surfaces of the blend films were rougher than the blank chitosan film.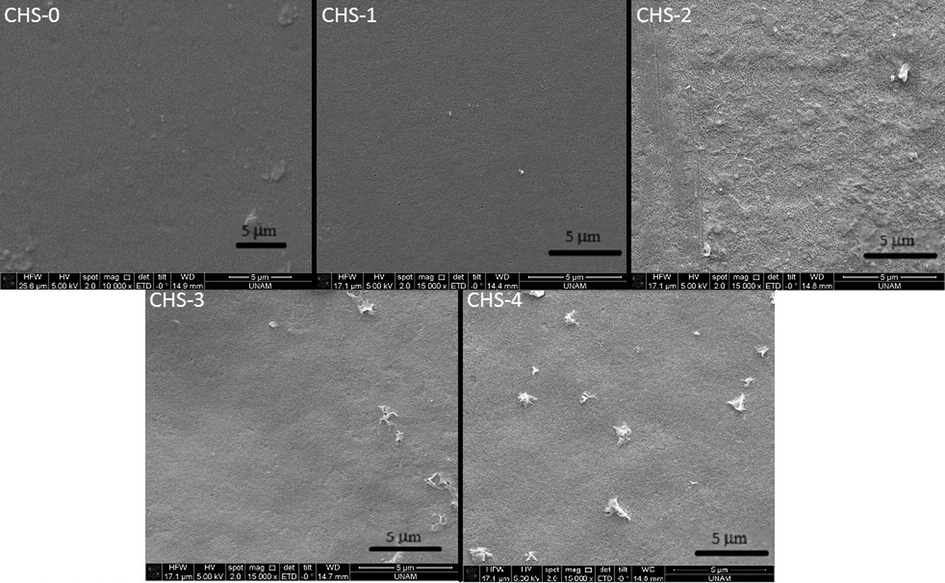
SEM micrographs of chitosan/PEGME blend films surface in different compositions consisting of 100/0 (CHS-0), 90/10 (CHS-1), 80/20 (CHS-2), 70/30 (CHS-3), and 60/40 (CHS-4).
3.3 X-ray photoelectron spectroscopy
For neat chitosan film and blend films, the spectra were recorded using a 60° take off angle relative to the surface normal.
Survey scan of CHS-0 (Fig. 2) identified chemical components of CHS-0 sample that contained chitosan only. These are C, O and N namely. In addition, Ca 2p peak was observed at 348.57 eV; however chitosan did not contain Ca in its chemical structure. The peak could be due to the chemicals used during the production of the films. The signal of N 1s orbital at 399.44 eV proved the –NH2 bond in chitosan structure.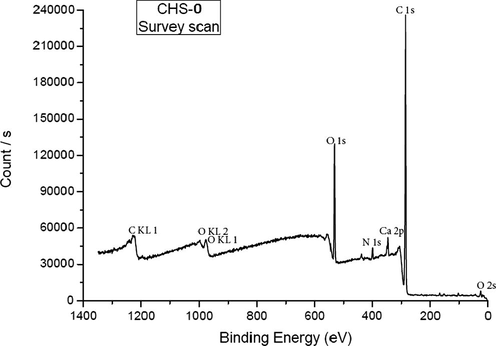
XPS survey-scan of pristine chitosan (CHS-0).
High resolution C 1s survey scan showed three distinct peaks after applying peak fitting (Fig. 3). The peak at 285.30 eV was assigned to C–C and C–N bonding in the chitosan structure. Peak at higher binding energy, namely 286.76 eV, corresponded to the binding of carbon atom to oxygen (C–O–C). The third peak with the highest binding energy (288.83 eV) was attributed to O–C–O chemical bonding (Amaral et al. 2005).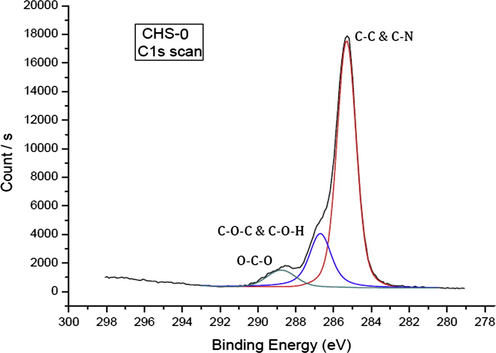
High resolution C 1s scan of pristine chitosan (CHS-0).
Chitosan has two different oxygen charge states due to the C–O–H and C–O–C bonding. However, high resolution O 1s scan in Fig. 4 shows only one peak at 532.58 eV corresponding to both bindings. These were very close to each other and could not be resolved in this spectrum (Jiang et al., 1997).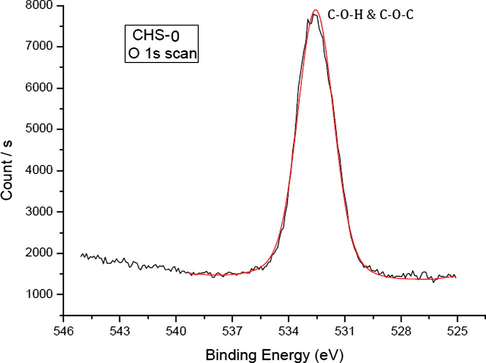
High resolution O 1s scan of pristine chitosan (CHS-0).
In the high resolution N 1s spectrum as shown in Fig. 5, two peaks were identified. The peak at 399.44 eV was assigned to –NH2 chemical binding, while the other peak at 401.36 eV was thought belonged to NH3+ ion. –NH2 and NH3+ forms were present in chitosan structure because the polymer was immersed in the distilled water before it was dried (Amaral et al. 2005).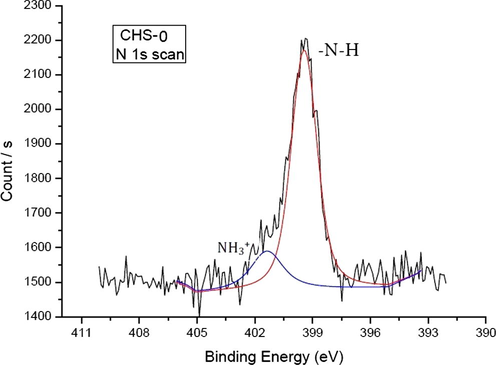
High resolution N 1s scan of CHS-0.
The addition of PEGME in chitosan did not affect the survey scans significantly, because PEGME consisted of C, H and O in its structure (see Figs. 6–9).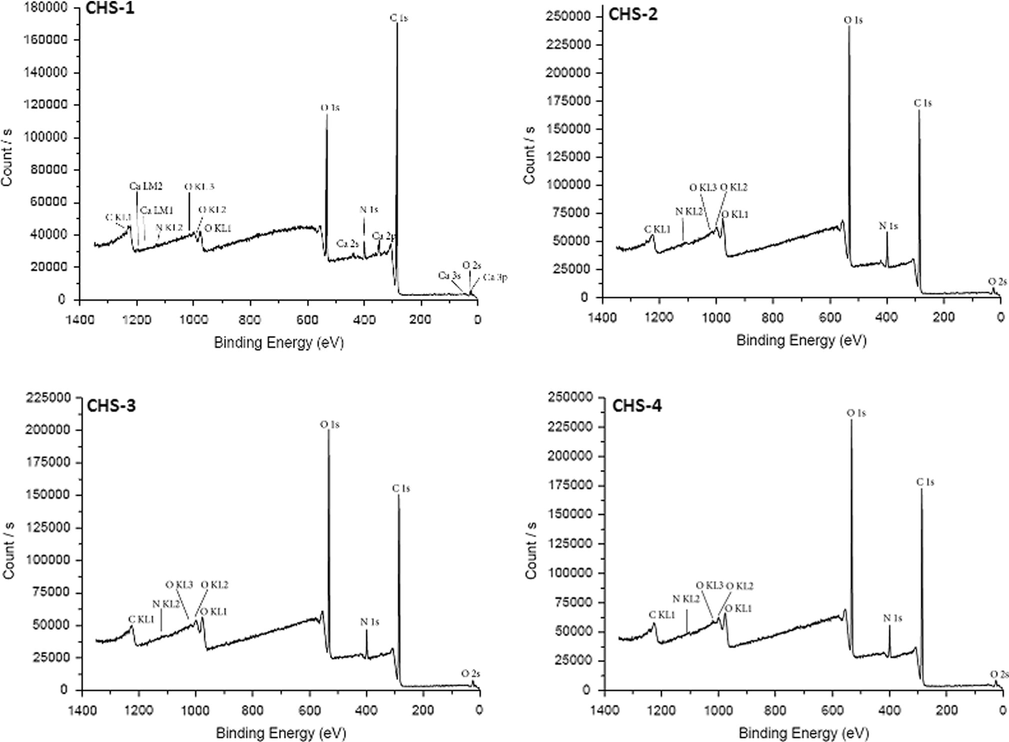
Survey scans of chitosan/PEGME blend films in different compositions consisting of 90/10 (CHS-1), 80/20 (CHS-2), 70/30 (CHS-3), and 60/40 (CHS-4).
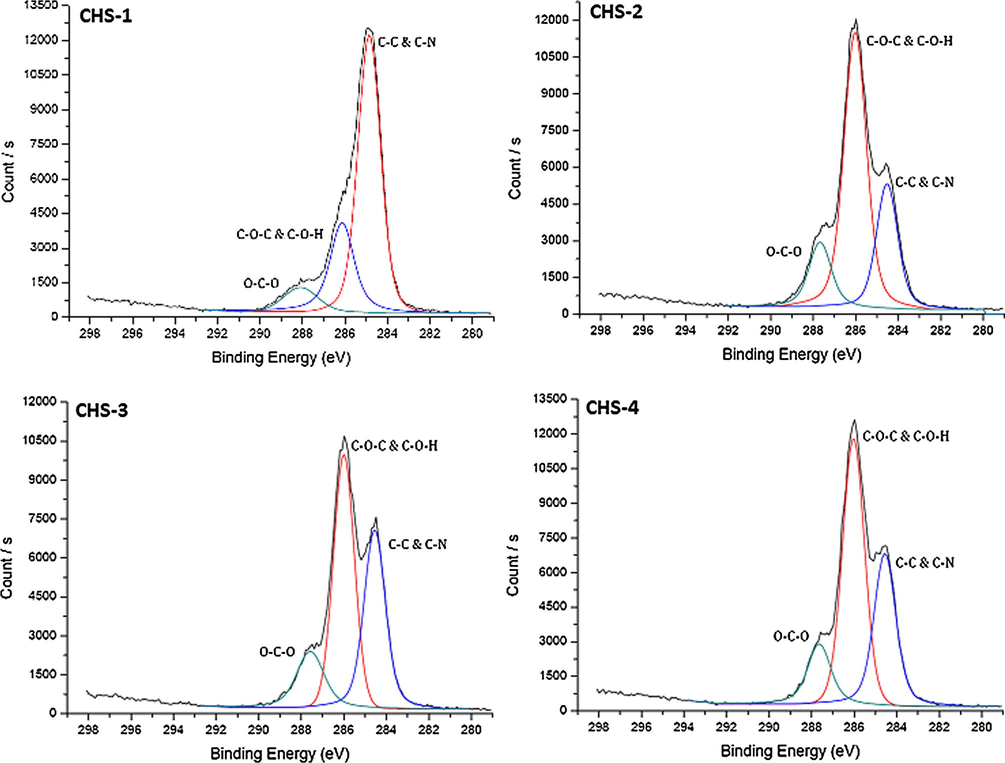
High resolution C 1s scans of chitosan/PEGME blend films in different compositions consisting of 90/10 (CHS-1), 80/20 (CHS-2), 70/30 (CHS-3), and 60/40 (CHS-4).
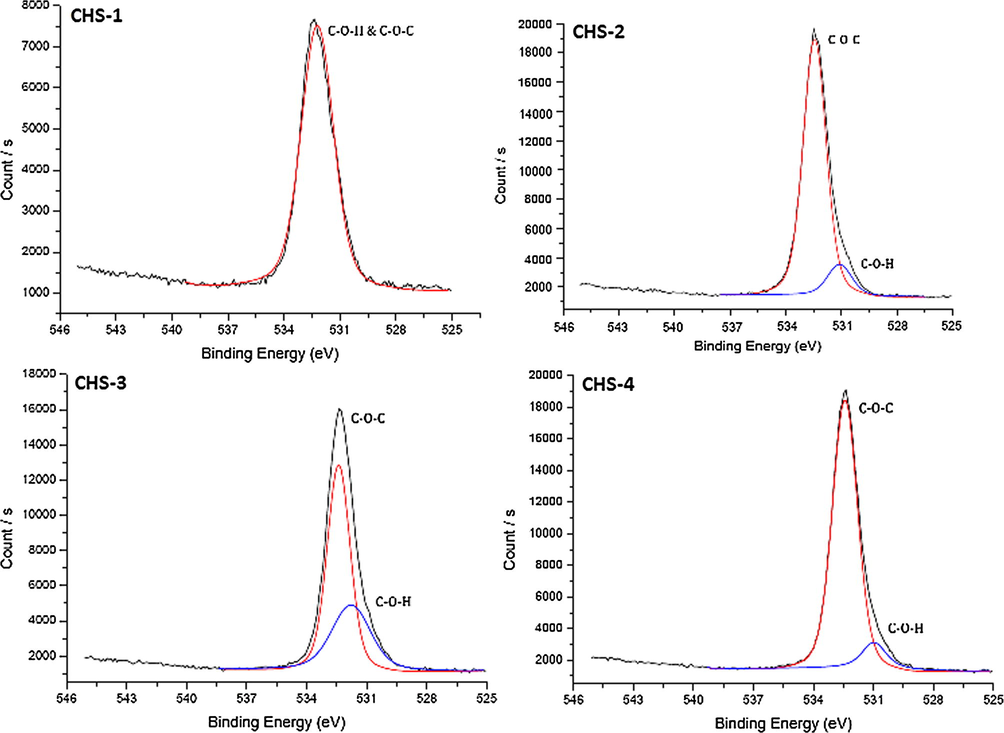
High resolution O 1s scans of chitosan/PEGME blend films in different compositions consisting of 90/10 (CHS-1), 80/20 (CHS-2), 70/30 (CHS-3), and 60/40 (CHS-4).
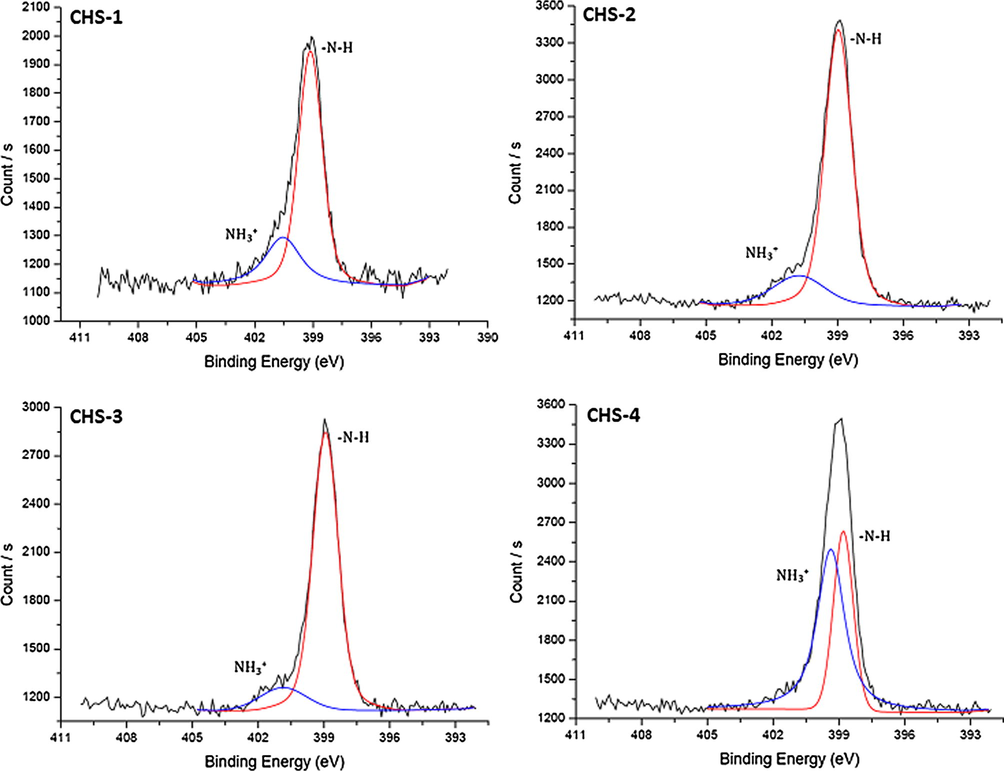
High resolution N 1s scans of chitosan/PEGME blend films in different compositions consisting of 90/10 (CHS-1), 80/20 (CHS-2), 70/30 (CHS-3), and 60/40 (CHS-4).
Examining the high resolution C 1s scans of the samples, an increase in counts of C–O–C and C–O–H peak was observed while with a decrease in C–N signal. This also happened because of the chemical structure of PEGME, as the ratio of PEGME was increased in the blend, count of C–O–H/C increased having oxygen in the backbone of the polymer. On the other hand, C–N signal became lower since chitosan ratio was decreased in the blend.
High resolution O 1s scans were all similar, but as the PEGME ratio increased, resolution of C–O–H and C–O–C signals become clear. There were two distinct peaks at 532.4 eV and 531.1 eV, former belonging to C–O–C binding while the latter represented C–O–H chemical bonding. In CHS-2, this resolution could not be obtained due to lower PEGME ratio.
Nitrogen occurred only in chitosan structure, so decrease in chitosan ratio in the blend did not cause a significant change in high resolution N 1s scans. In CHS-5 sample, the widths of the fitted peaks were very close to each other. NH3+ peak at 399.39 eV was dissolved form of NH2 and the increase in counts of this peak might be due to the lack of sample drying before analysis.
The experimental and theoretical atomic percentages of the samples did not match perfectly which was the general case for XPS analysis, but generally the existence of elements (Table 1). The variation of the percentages might be due to adsorbed air which also contains C, O and N extensively.
Sr. No.
Experimental atomic %
Theoretical atomic %
C
O
N
C
O
N
CHS-0
81.26
16.21
2.54
48.00
42.67
9.33
CHS-1
78.14
18.23
3.63
48.35
42.59
9.06
CHS-2
62.95
30.41
6.63
48.75
42.50
8.75
CHS-3
66.47
28.11
5.42
49.23
42.39
8.38
CHS-4
65.86
28.52
5.63
49.81
42.26
7.92
3.4 FT-IR spectroscopy
FT-IR spectroscopy was used to determine the interactions between chitosan and PEGME. The spectra of chitosan, PEGME and their blend are shown in Fig. 10. A band at 1652 cm−1 and band ranges from 1540 to 1570 cm−1 correspond to amide-I and amide-II groups, respectively. Cis-amide III band is also observed at 1340 cm−1. All of these bands correspond to chitosan without PEGME. The glycosidic linkages are showed by the bands 1150 and 890 cm−1 (Saha et al., 2011; Islam and Yasin, 2012; Kammoun et al., 2013; Sachdev et al., 2013). The stretching vibrations of –CH3 and –CH2 are assigned to the bands at 2880 cm−1 and at 2920 cm−1, respectively. A stretching band at 1110 cm−1 confirms acyclic C–O–C (PEGME) and another band is observed at 1230 cm−1 which is due to cyclic C–O–C (chitosan) (Mahatmanti et al., 2014). A band ranges 3470–3200 cm−1 is assigned to O–H stretching. As the concentration of PEGME increases, the intensity of band is also increased.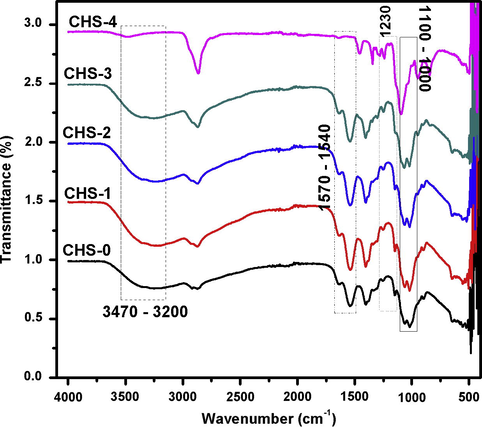
FT-IR study of chitosan/PEGME blend films in different compositions.
In the typical spectrum of chitosan-PEGME composite film, the amino peak of chitosan shifts from 1541 to 1535 cm−1 with the addition of PEGME which indicated the interactions between the hydroxyl groups of PEGME and the amino groups of chitosan (Tuhin et al., 2012) which are consistent with our results (Fig. 10).
3.5 Mechanical properties
The mechanical properties of the blend films, elongation at break (%) and tensile strength (MPa), were analyzed and compared with the blank chitosan films. Table 2, shows mechanical properties such as Tensile strength (MPa) and elongation at break (%) of chitosan and blend films including mean ± SD. Neat chitosan film had tensile strength of 20.23 ± 2 MPa and % elongation of 4.18 ± 1%. In fact, blank chitosan film had low TS as well as low % elongation in comparison with the blend films. The plot of % age of PEGME in blend films and TS (MPa), Fig. 11, shows that blend films have increment of TS and % elongation with increase in PEGME content. It could be evidence on more elasticity of the samples as a function of more PEGME content while other essential properties remained approximately same as a drug release material. Considering the rigid structure of chitosan, originated from weak but high number of intramolecular bond forces, such as H-bonds, introducing PEGME might seriously affected the crystallinity of chitosan, thereby increased the amorphous phase. In the other words, adding PEGME to the film composition caused it to become ductile. The 60/40 chitosan to PEGME ratio was an optimum choice in the mechanical properties of the blends since the introduction of PEGME to the chitosan made the films to be more flexible and ductile. This sample possessed both the relatively high TS and the good flexibility.
Sr. No.
Tensile strength (MPa)
Elongation at break (%)
CHS-0
20.23 ± 2
4.18 ± 1
CHS-1
28.28 ± 2
10.07 ± 6
CHS-2
32.83 ± 4
12.54 ± 1
CHS-3
33.55 ± 3
13.74 ± 1
CHS-4
34.14 ± 2
26.40 ± 1
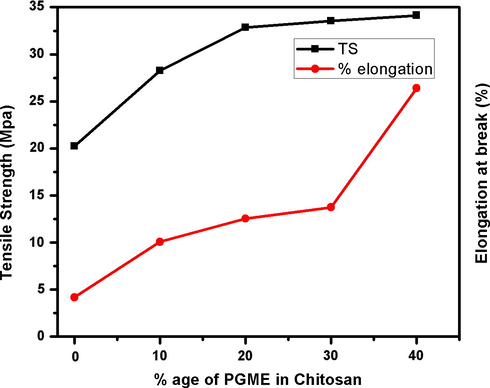
The plot of % age of PEGME in blend films and TS (MPa).
3.6 Thermogravimetric analysis
Thermogravimetric analysis (TGA) was performed on pure chitosan and chitosan-PEGME films (Fig. 12). The mass loss before the onset degradation temperature was related to the volatilization of water (Ma et al., 2007). The introduction of PEGME increased the thermal stability attributed to the better thermal stability provided by PEGME, and the better interfacial interaction between PEGME and chitosan.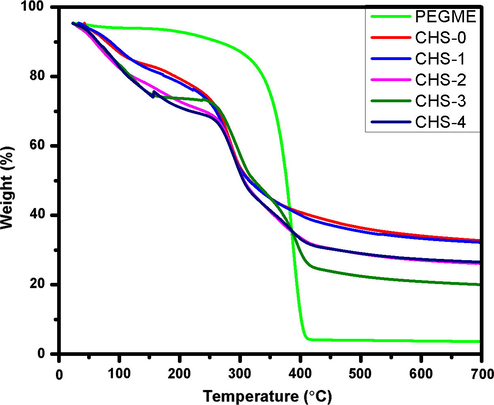
TGA of chitosan/PEGME blend films.
Fig. 12 shows that in blend films and neat chitosan, take place mass from 40 °C to 260 °C loss owing to the moisture removal, loss of solvent and dehydration. Thermal degradation of neat chitosan starts approximately at 260 °C which corresponds to the onset of degradation and degradation was completed at temperature around 350 °C which corresponds to the offset of degradation. However, in blend films, the onset of degradation is appeared at temperature around 270 °C and offset degradation is observed at temperature around 420 °C which is varied due to the presence of PEGME in films. It can be inferred from the TG curves that PEGME increases the thermal stability of chitosan hence more temperature is required for thermal degradation of chitosan/PEGME blend films as compared to the chitosan film without PEGME. It is evident from the graph that CHS-3 film is more stable than other films while CHS-4 shows less thermal stability which may be due to weak polymeric interaction.
3.7 Drug release
Model drug Metformin hydrogen chloride was loaded in CHS-2 and its controlled release was studied in PBS solution (pH = 7.4) as function of time as shown in Fig. 13. The graph shows that model drug was released in a controlled manner and most of the drug was released around 22 h. The remaining amount of drug could not be easily determined because blend film is broken into large fragments. It is evident from the Fig. 13 that Metformin hydrogen chloride can be used for intravenous (IV) medication.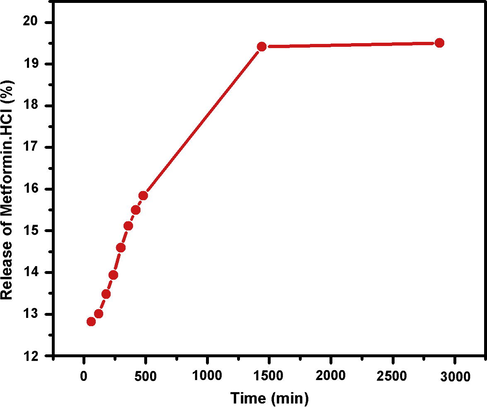
The absorbance of PBS solution containing film loaded with drug.
4 Conclusions
In this study, blend films of chitosan/PEGME with different compositions were prepared. The relationship between physicochemical characteristics of the blend films and blend ratios was assessed. Blending of chitosan with PEGME not only enhanced mechanical properties but also affected the thermal stability. The mechanical properties showed that with the addition of PEGME up to 40%, the tensile strength, elongation break and thermal stability of blend films were considerably increased as compared to the controlled sample (without PEGME). Metformin hydrogen chloride was loaded in chitosan/PEGME blend films and results indicated that there was good compatibility between drug and film matrix. The release analysis showed that the prepared blends are suitable candidates and can be employed for controlled drug release and other biomedical applications.
References
- Chemical composition, antimicrobial activity, proximate analysis and mineral content of the seed of Detarium senegalense JF Gmelin. J. Biomater.. 2004;25:3041-3048.
- [Google Scholar]
- Preparation and characterization of chitosan/silver nano particle/copper nanoparticle/carbon nanotube multi-functional nano-composite for water treatment: heavy metals removal; kinetics, isotherms and competitive studies. RSC Adv.. 2015;5:55774-55783.
- [Google Scholar]
- Chitosan/polyester-based scaffolds for cartilage tissue engineering: assessment of extracellular matrix formation. J. Act. Biomater.. 2010;6:1149-1157.
- [Google Scholar]
- Chemical modification of chitosan by phosphorylation: an XPS, FT-IR and SEM study. J. Biomater. Polym. Sci. Ed.. 2005;16:1575-1593.
- [Google Scholar]
- ASTM Standards, D882-09, 2009. Standard test method for tensile properties of thin plastic sheeting. West Conshohocken, PA: ASTM International. http://dx.doi.org/10.1520/D0882-09, www.astm.org (ASTM Standards, E96/E96M-10, 2010).
- Chitosan membrane as a wound-healing dressing: characterization and clinical application. J. Biomed. Mater. Research Part B: Appl. Biomater.. 2004;69:216-222.
- [Google Scholar]
- Estimation and pharmacokinetics of metformin in human volunteers. J. Pharm. Educ. Res.. 2007;41:135-139.
- [Google Scholar]
- Wound healing dressings and drug delivery systems. J. Pharm. Sci.. 2008;97:2892-2923.
- [Google Scholar]
- Studies of chitosan. II. Preparation and characterization of chitosan/poly(vinyl alcohol)/gelatin ternary blend films. Int. J. Biol. Macromol.. 2008;43:37-42.
- [Google Scholar]
- MPEG-PCL co-polymeric nanoparticles in drug delivery systems. Cogen. Med.. 2016;3:1142411.
- [Google Scholar]
- Preparation methods and properties of hydrogel: a review. Int. J. Pharm. Pharm. Sci.. 2013;5:112-117.
- [Google Scholar]
- Methods for the chemical analysis of degradable synthetic polymeric biomaterials. Crit. Rev. Anal. Chem.. 2014;44(1):23-40.
- [Google Scholar]
- Chitosan-N-poly(ethylene glycol) brush copolymers: synthesis and adsorption on silica surface. J. Eur. Polym.. 2005;41:2653-2662.
- [Google Scholar]
- Synthesis and study of water-soluble chitosan-O-poly(ethylene glycol) graft co-polymer. Eur. Polym. J.. 2004;40:685-691.
- [Google Scholar]
- Studies on nerve cell affinity of chitosan-derived materials. J. Biomed. Mater. Res. Part A.. 2000;52:285-295.
- [Google Scholar]
- Degradation and bio-safety evaluation of mPEG-PLGA-PLL copolymer-prepared nanoparticles. J. Phys. Chem. C. 2015;119(6):3348-3362.
- [Google Scholar]
- Preparation and characterization of poly(ethylene glycol)-g-chitosan with water and organosolubility. Carbohyd. Polym.. 2005;61:472-479.
- [Google Scholar]
- The efficacy of nimodipine drug delivery using mPEG-PLA micelles and mPEG-PLA/TPGS mixed micelles. Eur. J. Pharm. Sci.. 2014;63:187-198.
- [Google Scholar]
- Controlled delivery of drug from pH sensitive chitosan/poly(vinyl alcohol) blend. Carbohyd. Polym.. 2012;88:1055-1060.
- [Google Scholar]
- Controlled release of aspirin from pH sensitive chitosan/poly(vinyl alcohol) hydrogel. J. Appl. Polym. Sci.. 2012;124:4184-4192.
- [Google Scholar]
- Designing of novel sheet-shaped chitosan hydrogel for wound healing: a hybrid biomaterial consisting of both PEG-grafted chitosan and crosslinkable polymeric micelles acting as drug containers. Mater. Sci. Eng., C. 2013;33:3697-3703.
- [Google Scholar]
- Characterization of chitosan and rare-earth-metal-ion doped chitosan films. Macromol. Chem. Phys.. 1997;198:1561-1578.
- [Google Scholar]
- Electrical response characterization of poly(ethylene glycol) macromer (PEGM)/chitosan hydrogels in NaCl solution. J. Eur. Polym.. 2006;42:1609-1616.
- [Google Scholar]
- Int. J. Biol. Macromol.. 2013;62:433-438.
- Interpenetrating polymer network hydrogels based on poly(ethylene glycol) macromer and chitosan. Carbohyd. Polym.. 2000;41:197-205.
- [Google Scholar]
- Adsorption of Ca(II), Mg(II), Zn(II) and Cd(II) on chitosan membrane blended with rice hull ash silica and polyethylene glycol. Indo. J. Chem.. 2014;14:131-137.
- [Google Scholar]
- Preparation and characterization of water-soluble N-alkylated chitosan. J. Nie. Carbohydr. Polym.. 2008;74:121-126.
- [Google Scholar]
- Preparation and properties of water-soluble chitosan and polyvinyl alcohol blend films as potential bone tissue engineering matrix. Polym. Adv. Technol.. 2010;21:189-195.
- [Google Scholar]
- Bilayered chitosan-based scaffolds for osteochondral tissue engineering: influence of hydroxyapatite on in vitro cytotoxicity and dynamic bioactivity studies in a specific double-chamber bioreactor. J. Act. Biomater.. 2009;5:644-660.
- [Google Scholar]
- Production of thermoplastic starch/MMT sorbitol nano composites by dual-melt extrusion processing. J. Macromol. Mater. Eng.. 2007;292:723-728.
- [Google Scholar]
- A novel one step synthesis of PEG passivated multicolour fluorescent carbon dots for potential biolabeling application. RSC Adv.. 2013;3:16958-16961.
- [Google Scholar]
- Polymeric biomaterials based hydrogels for biomedical applications. J. Biomater. Nanobiotechnol.. 2011;2:85-90.
- [Google Scholar]
- Characterization of chitosan-polycaprolactone blends for tissue engineering applications. Biomaterials. 2005;26:5500-5508.
- [Google Scholar]
- Methotrexate-incorporated nanoparticles of methoxy poly(ethylene glycol)-grafted chitosan. Colloids Surf. B. 2009;69:157-163.
- [Google Scholar]
- Preparation of a thermosensitive gel composed of a mPEG-PLGA-PLL-cRGD nano drug delivery system for pancreatic tumor therapy. ACS Appl. Mater. Interfaces. 2015;7(37):20530-20537.
- [Google Scholar]
- Preparation, characterization, and in-vitro drug release behavior of glutathione sensitive long-circulation micelles based on polyethylene glycol prodrug. J. Biomater. Sci. Polym. Ed.. 2016;27:472-489.
- [Google Scholar]
- Preparation and characterization of novel curdlan/chitosan blending membranes for antibacterial applications. Carbohyd. Polym.. 2011;84:952-959.
- [Google Scholar]
- Mechanical and barrier properties of biodegradable films made from chitosan and poly (lactic acid) blends. J. Polym. Environ.. 2004;12:1-6.
- [Google Scholar]
- Stability of chitosan-A challenge for pharmaceutical and biomedical applications. Mar. Drug. 2015;13:1819-1846.
- [Google Scholar]
- Rheological characterization of a novel thermosensitive chitosan/poly(vinyl alcohol) blend hydrogel. Carbohyd. Polym.. 2007;67:491-499.
- [Google Scholar]
- Synthesis and characterization of in situ cross-linked hydrogel based on self-assembly of thiol-modified chitosan with PEG diacrylate using Michael type addition. Polymer. 2010;51:639-646.
- [Google Scholar]
- Controlled release formulation of tramadol hydrogen chloride using hydrophilic and hydrophobic matrix system. AAPS Pharm. Sci. Technol.. 2003;4:E31.
- [Google Scholar]
- Novel antimicrobial ultrathin structures of zein/chitosan blends obtained by electrospinning. Carbohyd. Polym.. 2009;77:261-266.
- [Google Scholar]
- Biodegradable polymer blends on corn starch and thermoplastic chitosan processed by extrusion. Radiat. Phys. Chem.. 2012;81:1659-1668.
- [Google Scholar]
- Controlled release of ciprofloxacin hydrochloride from chitosan/polyethylene glycol blend films. Carbohyd. Polym.. 2007;69:336-343.
- [Google Scholar]
- A pH-responsive AIE nano probe as a drug delivery system for bioimaging and cancer therapy. J. Mater. Chem. B. 2015;3(37):7401-7407.
- [Google Scholar]
- Self-aggregated nanoparticles from methoxy (polyethylene glycol)-modified chitosan: synthesis; characterization; aggregation and methotrexate release in vitro. Colloids Surf. B. 2008;61:125-131.
- [Google Scholar]
- Condensed state structure and bio-compatibility of the konjacglucomannan/chitosan blend films. Carbohyd. Polym.. 2006;64:532-538.
- [Google Scholar]
- Rapid determination of metformin in human plasma using ion-pair HPLC. J. Pharm. Biomed. Anal.. 2003;31:197-200.
- [Google Scholar]
- Effect of compatibility on the structure of the microporous membrane prepared by selective dissolution of chitosan/synthetic polymer blend membrane. J. Membr. Sci.. 2004;230:175-181.
- [Google Scholar]
- Both FA- and mPEG-conjugated chitosan nanoparticles for targeted cellular uptake and enhanced tumor tissue distribution. Nanoscale Res. Lett.. 2011;6:563.
- [Google Scholar]
- Physical, mechanical, and antibacterial properties of chitosan/PEO blend films. Biomacromol. 2007;8:1505-1510.
- [Google Scholar]







