Translate this page into:
Coordination complexes of rare earth metals with hydrazine and isomeric acetamidobenzoates as ligands– spectral, thermal and kinetic studies
⁎Corresponding author. drsvairam@rediffmail.com (Vairam Sundararajan)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
The isomeric acetamido benzoic acids (abbreviated as acambH) on reaction with hydrazine hydrate and lanthanides, La3+, Ce3+, Pr3+, Nd3+, Sm3+ and Gd3+ form complexes of formulae, [Ln{x-C6H4(CH3CONH)}3(N2H4)] where x = 2 (or) 3 (or) 4, at pH 3–4.5 in (1:1) aqueous ethanolic medium, which are insoluble in water and organic solvents. They are characterized by using elemental analysis, IR, UV, 13C, 1H NMR and mass spectroscopic, XRD, SEM-EDAX, thermal and conductance studies. The difference between IR bands of vC=O asym (acid) and vC=O sym(acid) range, 122–166 cm−1 supports the bidental coordination of carboxylate ions to metal. vN-N values of 955 to 980 cm−1, substantiate bridging bidentate coordination of hydrazine to metal. vC=O of amide group 1632 to1709 cm−1 indicates its non-coordination with metal. The thermal studies reveal that complexes undergo dehydrazination between 52 and 180 °C and exothermic degradation into phthalate intermediate between 172 and 496 °C and further degradation to form microsized metal oxide around 600 °C. The magnetic susceptibility measurements indicated that the presence of metals in the same electronic state and electronic spectral assignments suggested that the coordination number is eight for the complexes. The conductance measurement results in DMSO medium indicated that the complexes are neutral. The 13C – NMR, 1H- NMR and the LC-Mass techniques substantiated the composition of the complexes.
Keywords
Acetamidobenzoic acid
SEM-EDAX
Lanthanides
TG–DTA
Magnetic susceptibility
1 Introduction
Hydrazine, a versatile ligand with two basic sites, forms a variety of complexes with alkaline earth metals and with lanthanides and actinides (Schmidt et al., 1984). While studying these hydrazine complexes and their derivatives, more attention is given on their thermal behaviour. The thermal Chemistry of these complexes is more attractive since hydrazine is an exothermic compound and an enormous amount of heat energy is liberated due to the formation of nitrogen molecule, making metal oxide residues nanosized. Hence the metal complexes containing hydrazine have been utilized and are being exploited as precursors to metal oxides and mixed metal oxides such as ferrites, cobaltites, etc., (Ravindranathan and Patil, 1986, Ravindranathan et al., 1987) by their low temperature decomposition. Many complexes of inner transition metals with hydrazine and aliphatic and aromatic carboxylic acids have been explored (Govindarajan et al., 1986, Kuppusamy et al., 1996; Vairam and Govindarajan, 2006; Sivasankar et al., 2004, Bai and Vairam, 2013; Devipriya et al., 2013; Parimalagandhi and Vairam, 2014; Parimalagandhi et al., 2016). The transition metal complexes with acetamido substituted benzoic acids are also found in the literature (Mascarenhas et al., 1980; Li et al., 2007; Yang et al., 2009; Yang et al., 2009a; Manin et al., 2014; Manin et al., 2014a; Manin et al., 2014b; Almeida et al., 2015; Rakse et al., 2021). Additional- reports of 4-acambH lanthanides (Man, 2010; Yin et al., 2011; Wang et al., 2009; Gupta, 2019; TanTong et al., 2018) such as synthesis, crystal structures and photoluminescence of lanthanide coordination polymers with 4-acambH, coordination behavior of radii-dependent lanthanides with 4-acambH and 1, 10-phenanthroline, microbial neuraminidase inhibitors of derivatives of substituted 4-acambH, metallo-supramolecular Ti-based M4L4 cage assembly of 4-acambH are also available in the literature. Helen et al. has reported the synthesis, characterization and thermal degradation of transition metal complexes of isomeric acetamido benzoic acids (Bai and Vairam, 2013; Bai and Vairam, 2020). In this paper, synthesis, spectral and thermal studies of some lanthanide complexes of 2-acetamido, 3-acetamido and 4-acetamido benzoic acids (Fig. 1) with hydrazine are presented for the first time. Similarly self-assembled molecular structures of organic compounds and Recent advances in hybrid organic–inorganic materials with spatial architecture for state-of-the-art applications are carried out (Zoubi, et al., 2021; Zoubi, et al., 2020).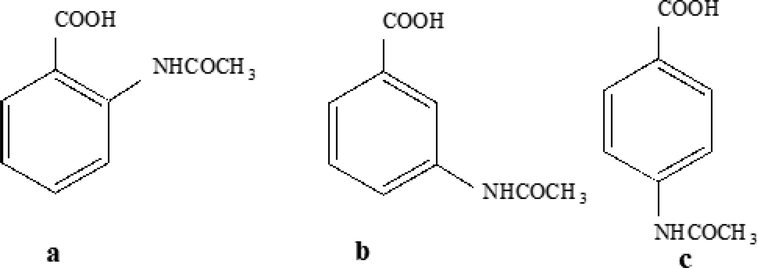
(a-c): Chemical structure of Ligands employed; 1(a): 2-acetamido benzoic acid (2-acambH); 1(b): 3-acetamido benzoic acid (3-acambH); 1(c): 4-acetamido benzoic acid (4-acambH).
2 Experimental
2.1 Preparation of [Ln{2/3/4-C6H4(NHCOCH3)3}.(N2H4)], where Ln = La, Ce, Pr, Nd, Sm& Gd
Lanthanum oxide (0.325 g, 1 mmol) was dissolved in a minimum quantity of 1:1 nitric acid (HNO3 Sp. gravity 1.42 g/mL), evaporated to eliminate excess of acid, and the residue was dissolved in 20 mL of ethanol. To a freshly prepared (1:1) ethanolic solution (40 mL) of the ligands containing 2-acetamido benzoic acid (1.075g, 6 mmol): hydrazine hydrate (0.912g, 8 mmol), the alcoholic solution of the metal was added stirring the reaction mixture vigorously. A pale yellow microcrystalline solid formed immediately at pH 3 and was kept over a hot water bath for 5 min at 90 °C. The product was cooled, filtered, and washed with ethanol and ether and then dried in a desiccator.
All the other complexes of 2, 3 & 4-acetamido benzoates were prepared by a similar procedure by adding the respective metal nitrate solution to the ligand solution in the mole ratios Metal:Acid:Base ≡ 1:6:8, 1:4:4 and 1:6:8 at pH 3, 4.5 respectively. The microcrystalline solid products formed were filtered, washed with alcohol and ether, and then they were dried over anhydrous CaCl2 in desiccators. The preparation scheme is described below.
2.2 Physicochemical methods
The hydrazine content in all complexes was determined volumetrically using 0.025 M Potassium iodate solution under Andrews’ conditions (Vogel, 2019). The metal content was determined by EDTA complexometric titration (Vogel, 2019) after decomposing a known weight of the sample with 1:1 HNO3. Magnetic susceptibility measurements of the complexes were carried out on a vibrating sample magnetometer, VSM EG & G model 155. The electronic spectra for solid-state complexes were obtained using a varian, Cary 5000 recording spectrophotometer. Infra-red spectra were recorded using KBR disc (4000–400 cm−1) on a Shimadzu FTIR-8201 (PC) S spectrophotometer. The simultaneous TG-DTA studies were done on a Perkin Elmer, Diamond TG/DTA analyzer and the curves were obtained in static air using 5–10 mg of the samples at the heating rate of 10 °C/min. The XRD patterns were recorded on a Bruker AXS D8 Advance diffractometer with an X-ray source Cu, wavelength 1.5406 Å using a Si (Li) PSD detector. The elemental analysis was carried out using a CHNS Elementar Vario EL- III Elemental Analyzer. The mass spectrum was obtained using TQD–WATERS liquid chromatography coupled mass spectrophotometer. The 13C and 1H NMR spectras were recorded for all the samples dissolved in DMSO using JEOL-NMR device with an operating frequency of 600 MHZ.
3 Results and discussion
All the complexes are insoluble in organic solvents like ethanol and ether. They are soluble in DMSO and sparingly soluble in water.
3.1 Magnetic susceptibility and electronic spectra measurements
The effective magnetic moment values of La, Ce, Pr, Nd, Sm, and Gd complexes were obtained from the VSM graph using the following formula μeff = 2.828 √χmT), where χm (magnetic moment of the lanthanide samples) = (Molecular Weight of the Sample / Sample Wt) X Slope (Bohr Magneton) and tabulated (Table 1), which are in good agreement with reported values (Want and Shah, 2016). Besides the observed values were compared with theoretical effective values calculated using the formula μeff = gJ √J(J + 1) μB, where μB - BM respectively.
M3+ ion
4fn
S
L
J
µeff (Anal.(Calcd.)) BM
[Ln(2-acamb)3(N2H4)]
[Ln(3-acamb)3(N2H4)]
[Ln(4-acamb)3(N2H4)]
La
0
0
0
0
–
–
–
Ce
1
½
3
5/2
2.5(2.54)
2.3(2.41)
2.4(2.48)
Pr
2
1
5
4
3.3(3.48)
3.2(3.51)
3.3(3.46)
Nd
3
3/2
6
9/2
3.6 (3.71)
3.4 (3.63)
3.5 (3.58)
Sm
5
5/2
5
5/2
1.6(0.98)
1.4(0.88)
1.5(0.94)
Gd
7
7/2
0
7/2
8.1(7.92)
8.0(7.78)
8.3(7.94)
The absorptions observed in electronic spectra of Pr and Nd complexes and the levels assigned are given in Table 2. The absorptions of Nd complex are comparable to that of Nd bis-bipyridyl complexes suggesting coordination number 8 in it (Petit et al., 2006).
Complexes
Observed bands (cm−1)
Assigned Levels
Parameters
[Nd(2- C6H4(CH3CONH)COO}3.(N2H4)]
17007, 12,407 & 11,025
2G 7/2, 4F5/2& 4F3/2
β = 1.0170
% δ = -1.671
[Nd(3- C6H4(CH3CONH)COO}3.(N2H4)]
25840, 17182, 13,459 & 12,392
2P3/2, 2G 7/2, 4F7/2& 4F5/2
β = 0.9974
% δ = 0.2606
[Nd(4- C6H4(CH3CONH)COO}3.(N2H4)]
26109, 17637, 16807, 13,578 & 12,403
2P3/2, 2G 7/2, 4G 5/2, 4F7/2& 4F5/2
β = 0.9974
% δ = 0.2606
[Pr(2- C6H4(CH3CONH)COO}3.(N2H4)]
22371, 20,704 & 11,038
3P2, 3P0&1G 4
β = 0.9987
% δ = 0.1301
[Pr(3- C6H4(CH3CONH)COO}3.(N2H4)]
22,422 & 21,186
3P2&1I6
β = 0.9829
% δ = 1.739
[Pr(4- C6H4(CH3CONH)COO}3.(N2H4)]
22487, 21368, 20747& 16,798
3P2,1I6, 3P0&1D2
β = 0.9816
% δ = 1.8744
On comparison of electronic spectral data of Pr and Nd complexes with their aquo complexes (Parimalagandhi et al., 2016), the shifts in band positions are observed compared to those of aquo lanthanide metal ions. It is observed in case of neodymium complexes, there is decrease in the wave number by 346–1757 cm−1, from its aquo complex, whereas there is no considerable change in wave number of praseodymium complex from its aquo complex. This shift, a measure of metal–ligand interaction, has been ascribed to nephelauxeatic effect (Jung et al., 2017). The involvement of f-orbital in M—O bonding is an important component of covalency (Berryman et al., 2019).
The Sinha’s covalency parameter (δ %) was calculated using the expression (Eq. (1)),
3.2 IR spectra of complexes
The analytical data and absorption frequencies of complexes are shown in Table 3 and the infrared spectra of all compounds are given in Figs. 2–4.
Complexes
Analytical data
IR data (cm−1) b- broad; s-sharp; m-medium;
Carbon
Hydrogen
Nitrogen
Hydrazine
Metal
vC=O asym
vC=Osym
vN-N
vOH
vN-H
vC=O(amido gp)
Anal.(Calcd.)
Anal.(Calcd.)
Anal.(Calcd.)
Anal.(Calcd.)
Anal.(Calcd.)
2-acetamido benzoate lanthanides[Ln(2-acamb)3(N2H4)] where Ln = La, Ce, pr, Nd, Sm and Gd
[La{2-C6H4(CH3CONH)COO}3(N2H4)]
45.90(45.93)
3.92(4.00)
9.73 (9.92)
4.49(4.54)
19.43(19.69)
1580b
1439 s
959 s
3385 b
3275 m
1674 s
[Ce{2-C6H4(CH3CONH)COO}3(N2H4)]
45.88(45.85)
3.78(3.99)
9.85(9.91)
4.50(4.53)
19.72(19.83)
1584 s
1439 s
955 s
3335 s
3275 m
1674 s
[Pr{2-C6H4(CH3CONH)COO}3 (N2H4)]
45.11(45.80)
3.78(3.99)
9.82(9.89)
4.53(4.52)
19.90(19.92)
1580 s
1441 s
961 s
3414 b
3150 m
1670 m
[Nd{2-C6H4(CH3CONH)COO}3(N2H4)]
45.43(45.58)
3.88(3.97)
9.91(9.85)
4.53(4.50)
19.87(20.29)
1584 s
1443 s
961 m
3389 b
3146 b
1676 m
[2-Sm{C6H4(CH3CONH)COO}3(N2H4)]
45.21(45.19)
3.79(3.94)
9.72(9.76)
4.54(4.46)
20.76 (20.97)
1583 m
1443 s
961 s
3431 b
3152 m
1676 s
[2-Gd{C6H4(CH3CONH)COO}3(N2H4)]
44.65(44.76)
3.93(3.90)
9.59(9.67)
4.36(4.42)
21.77 (21.73)
1593 s
1452 s
968 s
3360 s
3202b
1632 s
3-acetamido benzoate lanthanides [Ln(3- acamb)3(N2H4)] where Ln = La, Ce, pr, Nd, Sm and Gd
[La{3-C6H4(CH3CONH)COO}3(N2H4)]
45.72(45.93)
3.88(4.00)
9.89 (9.92)
4.41(4.54)
19.36(19.69)
1609 s
1489 s
980 s
3435 b
3337 m
1709 s
[Ce{3-C6H4(CH3CONH)COO}3(N2H4)]
45.79(45.85)
3.85(3.99)
9.76(9.91)
4.47(4.53)
19.80(19.83)
1555 m
1395 s
978 s
3416 m
3254 s
1655 s
[Pr{3-C6H4(CH3CONH)COO}3(N2H4)]
45.28(45.80)
3.84(3.99)
9.90(9.89)
4.43(4.52)
19.78(19.92)
1555 s
1393 s
978 s
3414 b
3252 m
1657 s
[Nd{3-C6H4(CH3CONH)COO}3(N2H4)]
45.02(45.58)
4.00(3.97)
9.80(9.85)
4.44(4.50)
20.00(20.29)
1553 m
1395 s
978 s
3416 b
3260 s
1659 s
[Sm{3-C6H4(CH3CONH)COO}3(N2H4)]
45.10(45.19)
3.87(3.94)
9.63(9.76)
4.41(4.46)
20.92(20.97)
1612 m
1485 s
980 s
3337 s
3273 b
1709 s
[Gd{3-C6H4(CH3CONH)COO}3(N2H4)]
44.38(44.76)
3.79(3.90)
9.59(9.67)
4.40(4.42)
21.67(21.73)
1559 m
1393 s
978 s
3431b
3202 m
1659 s
4-acetamido benzoate lanthanides [Ln(4acamb)3(N2H4)] where Ln = La, Ce, pr, Nd, Sm and Gd
[La{4-C6H4(CH3CONH)COO}3(N2H4)]
45.89 (45.93)
3.98(4.00)
9.90 (9.92)
4.56(4.52)
19.57(19.60)
1559b
1434 b
963 s
3313 b
3274
1675 b
[Ce{4-C6H4(CH3CONH)COO}3(N2H4)]
45.83(45.85)
4.00(3.99)
9.89(9.91)
4.53(4.51)
19.69(19.74)
1523 s
1401 s
966 s
3529 s
3302
1675 s
[Pr{4-C6H4(CH3CONH)COO}3(N2H4)]
45.78(45.80)
3.94(3.99)
9.90(9.89)
4.49(4.50)
19.85(19.83)
1553 s
1405 s
965 s
3309 s
3203
1674 s
[Nd{4-C6H4(CH3CONH)COO}3(N2H4)]
45.32(45.58)
4.02(3.97)
9.78(9.85)
4.45(4.48)
19.98(20.21)
1553 s
1403 s
965 s
3309 s
3206
1674 s
[Sm{4-C6H4(CH3CONH)COO}3(N2H4)]
45.32(45.19)
3.87(3.94)
9.63(9.76)
4.48(4.45)
20.87(20.89)
1556 s
1394 s
965 s
3304 s
3269
1675 s
[Gd{4-C6H4(CH3CONH)COO}3(N2H4)]
44.73(44.76)
3.85(3.90)
9.59(9.67)
4.39(4.40)
21.67(21.64)
1558 m
1414 s
966 s
3305 s
3271
1674 s
![FTIR spectra of [La{2-C6H4(CH3CONH)COO}3.(N2H4)].](/content/184/2022/15/9/img/10.1016_j.arabjc.2022.104009-fig2.png)
FTIR spectra of [La{2-C6H4(CH3CONH)COO}3.(N2H4)].
![FTIR spectra of [Nd{3-C6H4(CH3CONH)COO}3.(N2H4)].](/content/184/2022/15/9/img/10.1016_j.arabjc.2022.104009-fig3.png)
FTIR spectra of [Nd{3-C6H4(CH3CONH)COO}3.(N2H4)].
![FTIR spectra of [Gd{4-C6H4(CH3CONH)COO}3.(N2H4)].](/content/184/2022/15/9/img/10.1016_j.arabjc.2022.104009-fig4.png)
FTIR spectra of [Gd{4-C6H4(CH3CONH)COO}3.(N2H4)].
3.2.1 FTIR spectra of [Ln {x-C6H4(CH3CONH)COO}3.(N2H4)] where x = 2 or 3 or 4)
The IR spectra of the complexes show vC=O asym (acid) between 1523 and 1612 cm−1 and vC=O sym(acid) between 1393 and 1489 cm−1. The difference of the two frequencies, 122–166 cm−1 supports the bidental coordination of carboxylate ions to metal (Nakamoto et al., 2009). The broad absorption peak in the range of 3336 – 3867 cm−1 corresponding to vOH of the acids was not observed in the spectra of the compounds because the carboxylate group was involved in the complex formation. The absorptions at 955–980 cm−1 observed in IR of complexes were assigned to vN-N of N2H4 present in complexes, which reveals the presence of hydrazine in the form of bridging bidentate coordination mode (Braibanti et al., 1968). The C⚌O of amide group of the complexes show their stretching at 1632–1709 cm−1 which is almost similar to the bands observed for acids, implying that these complexes have no indication of coordination of the amide group with metal. The N-H stretching of hydrazine and N-H of the amide groups are observed to be merging in the regions of 3146–3275 cm−1. Similarly, C⚌O stretching of the amide is also found to be overlapping that of C⚌O of carboxylate ion, which was observed in the region 1632–1709 cm−1 respectively, which could not be differentiated.
3.3 Thermal studies
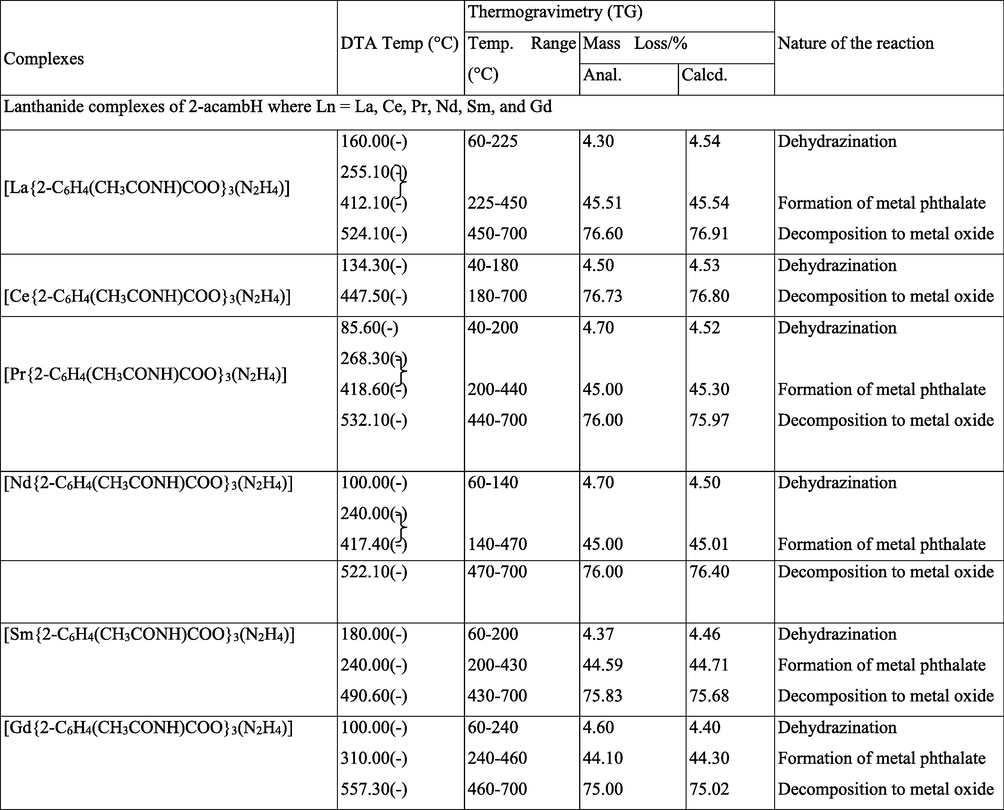
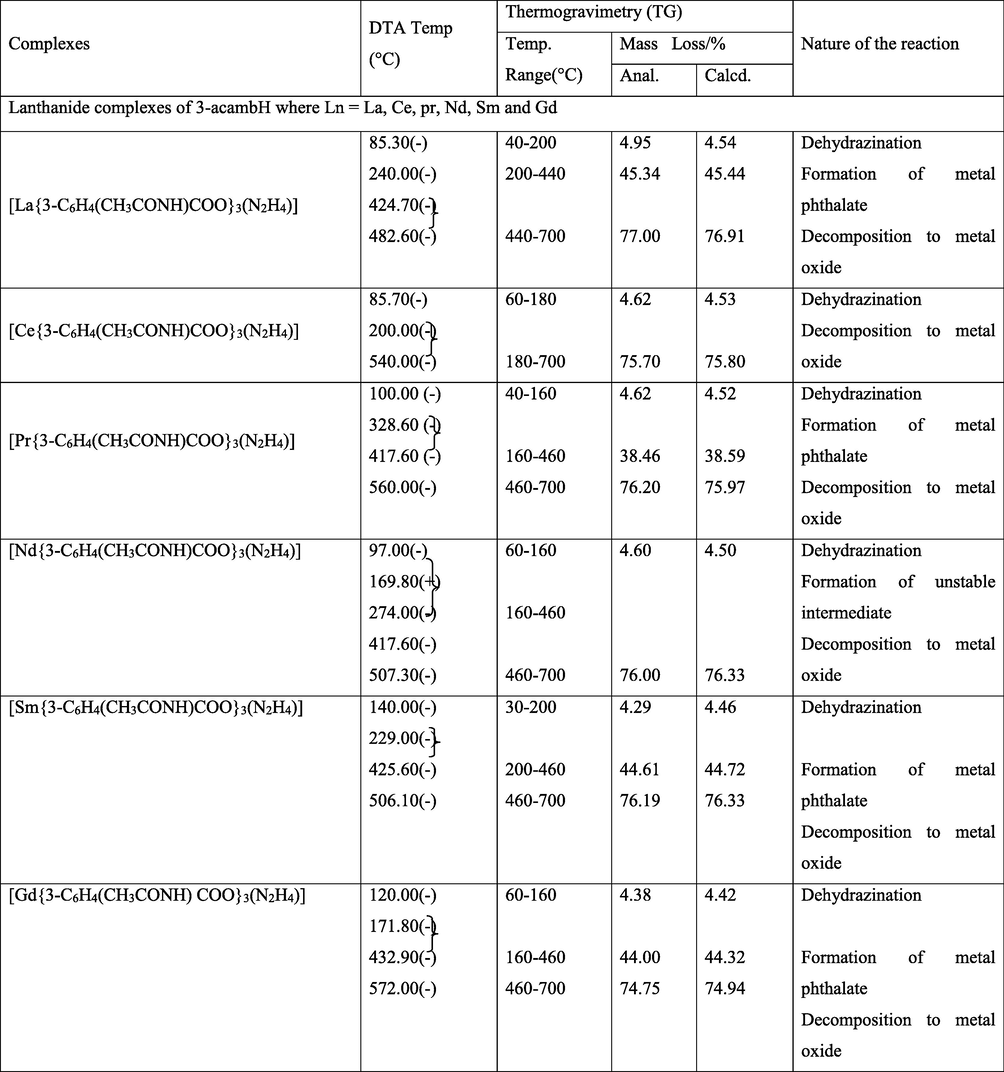
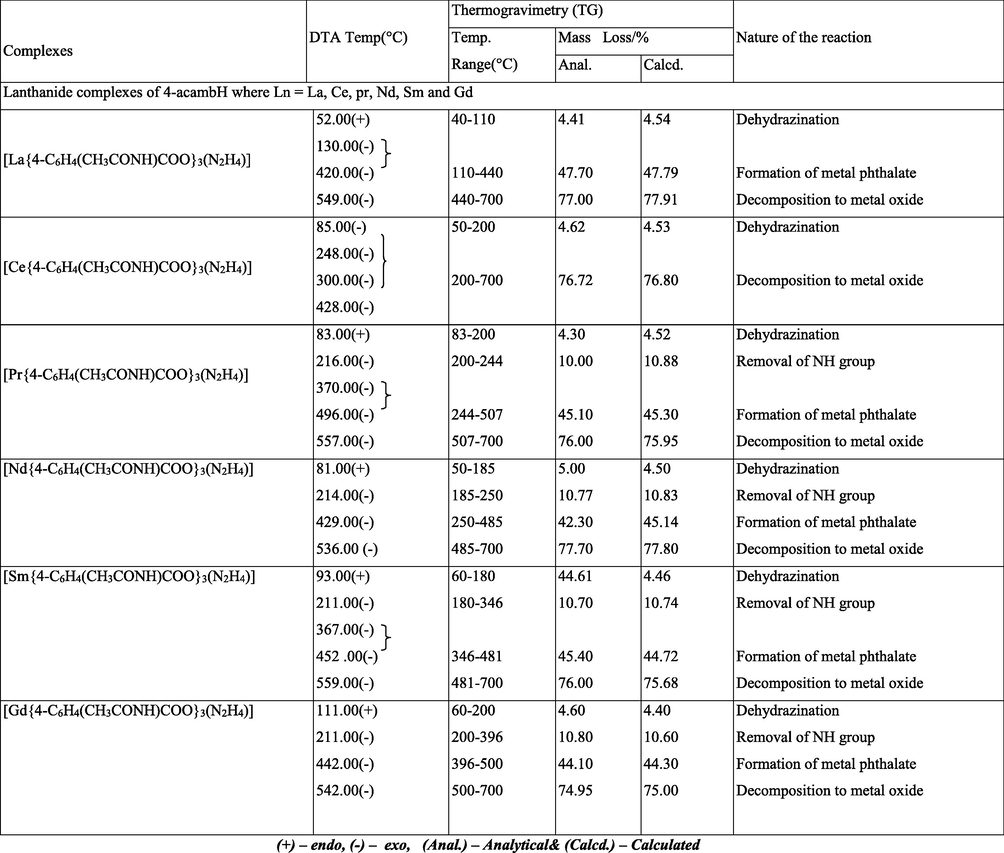
Simultaneous TG-DTA data of the complexes are summarized in Table 4. The compositions of the intermediates and the final products are those which best fit with the observed mass losses in the TG studies, and are in good agreement with the corresponding DTA data. The thermograms are shown in Figs. 5–7. (+) – endo, (−) – exo, (Anal.) – Analytical& (Calcd.) – Calculated.
![TG-DTA curves of Metal Hydrazine complexes: (a) [La{2-C6H4(CH3CONH)COO}3.(N2H4)]; (b) [Ce{2-C6H4(CH3CONH)COO}3.(N2H4)]; (c) [Pr{2-C6H4(CH3CONH)COO}3.(N2H4)]; (d)[Nd{2-C6H4(CH3CONH)COO}3.(N2H4)]; (e) [Sm{2-C6H4(CH3CONH)COO}3.(N2H4)]; (f)[Gd{2-C6H4(CH3CONH)COO}3.(N2H4).](/content/184/2022/15/9/img/10.1016_j.arabjc.2022.104009-fig5.png)
TG-DTA curves of Metal Hydrazine complexes: (a) [La{2-C6H4(CH3CONH)COO}3.(N2H4)]; (b) [Ce{2-C6H4(CH3CONH)COO}3.(N2H4)]; (c) [Pr{2-C6H4(CH3CONH)COO}3.(N2H4)]; (d)[Nd{2-C6H4(CH3CONH)COO}3.(N2H4)]; (e) [Sm{2-C6H4(CH3CONH)COO}3.(N2H4)]; (f)[Gd{2-C6H4(CH3CONH)COO}3.(N2H4).
![TG-DTA curves of Metal Hydrazine complexes.(a) [La{3-C6H4(CH3CONH)COO}3.(N2H4)]; (b) [Ce{3-C6H4(CH3CONH)COO}3.(N2H4)]; (c) [Pr{3-C6H4(CH3CONH)COO}3.(N2H4)]; (d) [Nd{3-C6H4(CH3CONH)COO}3.(N2H4)]; (e) [Sm{3-C6H4(CH3CONH)COO}3.(N2H4)]; (f) [Gd{3-C6H4(CH3CONH)COO}3.(N2H4)].](/content/184/2022/15/9/img/10.1016_j.arabjc.2022.104009-fig6.png)
TG-DTA curves of Metal Hydrazine complexes.(a) [La{3-C6H4(CH3CONH)COO}3.(N2H4)]; (b) [Ce{3-C6H4(CH3CONH)COO}3.(N2H4)]; (c) [Pr{3-C6H4(CH3CONH)COO}3.(N2H4)]; (d) [Nd{3-C6H4(CH3CONH)COO}3.(N2H4)]; (e) [Sm{3-C6H4(CH3CONH)COO}3.(N2H4)]; (f) [Gd{3-C6H4(CH3CONH)COO}3.(N2H4)].
![TG-DTA curves of Metal Hydrazine complexes: (a)[La{4-C6H4(CH3CONH)COO}3.(N2H4)]; (b) [Ce{4-C6H4(CH3CONH)COO}3.(N2H4)]; (c) [Pr{4-C6H4(CH3CONH)COO}3.(N2H4)]; (d) [Nd{4-C6H4(CH3CONH)COO}3.(N2H4)]; (e) [Sm{4-C6H4(CH3CONH)COO}3.(N2H4)]; (f) [Gd{4-C6H4(CH3CONH)COO}3.(N2H4)].](/content/184/2022/15/9/img/10.1016_j.arabjc.2022.104009-fig7.png)
TG-DTA curves of Metal Hydrazine complexes: (a)[La{4-C6H4(CH3CONH)COO}3.(N2H4)]; (b) [Ce{4-C6H4(CH3CONH)COO}3.(N2H4)]; (c) [Pr{4-C6H4(CH3CONH)COO}3.(N2H4)]; (d) [Nd{4-C6H4(CH3CONH)COO}3.(N2H4)]; (e) [Sm{4-C6H4(CH3CONH)COO}3.(N2H4)]; (f) [Gd{4-C6H4(CH3CONH)COO}3.(N2H4)].
All the complexes undergo almost similar type of degradation pattern. The complexes show three step decompositions as recorded by TG, namely, dehydrazination, decomposition of lanthanide carboxylate to phthalate intermediate and decomposition of phthalate intermediate to metal oxides. Though the thermograms of Cerium complexes show similar pattern of decomposition, the intermediate was unstable and unidentifiable. Dehydrazination occur showing endotherms in the range of 52 to 111 °C in case of 4-acambH complexes. Exothermic dehydrazination is observed for 2-acambH and 3-acambH complexes in the range 85 to 180 °C. This may be due to stability of the formation of the former than the latter two. In case of lanthanum complexes of 4-acambH the dehydrazinated metal carboxylates undergo exothermic decomposition showing exothermic peaks in the range of 172 to 370 °C and 412 to 496 °C to metal phthalates. The mass loss percentage indicates phthalates formation. The decomposition temperature of these intermediates more or less going along with the values reported (Gorgola and Brzyska, 1999). The Neodymium complex of 3-acetamido benzoate decomposes directly into its metal oxide, whose intermediate is found to be unstable, which could not be identified. Metal phthalates finally undergo oxidative decomposition showing broad exotherms in the range of 428 to 572 °C to the metal oxide residues.
All cerium complexes show dehydrazination in the first step. As in the other cases, 4-acambH complexes show endothermic dehydrazination. After dehydrazination, cerium complexes decompose to their corresponding metal oxide with no stable intermediate formation.
Among the praseodymium complexes, thermogram of 4-isomer has registered an additional exotherm at 216 °C indicating the removal of NH group prior to the formation of the phthalate intermediate. This trend is also observed in other complexes like Nd, Sm and Gd of the same isomeric benzoate. The decomposition steps involved were given in equations 2–5. The decomposition path was followed by analyzing the decompositions of 4-acetamido samarium complex at different steps using IR spectra. The IR spectra of the intermediates of 4 - acmbH of samarium complex are shown in Figs. 8–11. The IR spectra of the intermediate (1) after heating the sample to 93 °C, intermediate (2) after heating the sample to 211 °C, intermediate (3) after heating the sample to 367 °C and the intermediate (4) after heating the sample to 452 °C were analyzed for their characteristic peaks. The spectra of the intermediates reveal that N-N stretching of the NH moiety and the NH frequency of the amide group disappear gradually by forming broad peaks. The IR results of intermediate (4) shows vC=O(asym) band at 1582 cm−1 and vC=O(sym) band at 1495 cm−1, which are the characteristic frequencies of phthalate ion (Nakamoto et al., 2009; Braibanti et al., 1968). The decomposition steps involved are given below.![FTIR analysis of intermediate −1 of [Sm{4-C6H4(CH3CONH)COO}3.(N2H4)].](/content/184/2022/15/9/img/10.1016_j.arabjc.2022.104009-fig8.png)
FTIR analysis of intermediate −1 of [Sm{4-C6H4(CH3CONH)COO}3.(N2H4)].
![FTIR analysis of intermediate-2 of [Sm{4-C6H4(CH3CONH)COO}3.(N2H4)].](/content/184/2022/15/9/img/10.1016_j.arabjc.2022.104009-fig9.png)
FTIR analysis of intermediate-2 of [Sm{4-C6H4(CH3CONH)COO}3.(N2H4)].
![FTIR analysis of intermediate- 3 of [Sm{4-C6H4(CH3CONH)COO}3.(N2H4)].](/content/184/2022/15/9/img/10.1016_j.arabjc.2022.104009-fig10.png)
FTIR analysis of intermediate- 3 of [Sm{4-C6H4(CH3CONH)COO}3.(N2H4)].
![FTIR analysis of intermediate- 4 of [Sm{4-C6H4(CH3CONH)COO}3.(N2H4)].](/content/184/2022/15/9/img/10.1016_j.arabjc.2022.104009-fig11.png)
FTIR analysis of intermediate- 4 of [Sm{4-C6H4(CH3CONH)COO}3.(N2H4)].
Reaction scheme 1:
Reaction scheme 2:
The remaining IR spectras of 15 isomeric complexes are presented in the supplement index.
3.4 X-Ray diffraction studies
The powder XRD patterns along with their d-spacings are given in Table 5. The comparison of XRD patterns of the lanthanides are shown in Figs. 12–14. The XRD patterns of acetamidobenzoate lanthanides series reveal that each set of acetamidobenzoic acid complexes has similarities in their structures implying similar compositions. The powder XRD patterns of the 2 and 3-isomeric complexes exhibit more or less sharp peaks indicating polycrystalline nature whereas the X-ray pattern of the 4-isomeric lanthanide complexes display wide and weak intensity peaks may be due to smaller particle sizes. All substances are found to be crystalline and complexes of 2, 3 isomeric acids show more crystallinity. Correlation studies of the X-ray patterns were subsequently used to confirm that the above products were single-phase materials. However, they display similar patterns implying isomorphism. Their particle sizes were substantiated by SEM- EDAX analysis (Gaye, et al., 2003). With the justification of analytical, magnetic, spectral, thermal and XRD characterizations the eight coordination sites were ascribed around the Ln3+ ions. Among the eight coordinated sites six were occupied by three bidentate acetamido benzoate moieties and the rest of two sites were inhabited by two bridging bidentate hydrazine groups.
[Ln{2-C6H4(CH3CONH)COO}3(N2H4)]where Ln = La, Ce, Pr, Nd, Sm and Gd for 2-acambH
La
Ce
Pr
Nd
Sm
Gd
12.78(100.00)
12.90(100.00)
12.86(49.30)
17.90(100.00)
13.05(100.00)
11.54(91.30)
8.05(10.30)
7.54(19.20)
7.40(32.80)
8.07 (8.30)
8.11(6.70)
10.07(78.30)
7.51 (14.70)
6.56(9.60)
6.44(23.90)
7.53(21.70)
7.59(15.00)
7.83(26.10)
6.54(7.40)
5.70(11.50)
5.61(29.90)
6.54(11.70)
6.59(6.70)
7.29(32.60)
5.69(8.80)
5.24(9.60)
4.91(100.00)
5.69(11.70)
5.72(6.70)
6.35(19.60)
5.23((5.80)
4.97(36.50)
4.62(16.40)
5.22(6.70)
4.99(20.00)
5.82(26.10)
4.97(26.50)
4.39(15.38)
4.35(37.30)
4.96(40.00)
4.41(10.00)
5.19((4.60)
4.39(17.60)
3.82(26.90)
3.79(55.20)
4.68(8.30)
4.06(5.00)
4.71(52.20)
4.15(5.80)
3.47(23.10)
3.43(38.80)
4.38(21.70)
3.83(15.00)
4.49(56.50)
3.92(8.80)
2.89(7.70)
3.19(26.90)
3.82(23.30)
3.47(6.70)
4.24(52.20)
3.84(23.50)
2.73(9.60)
3.11(23.90)
3.37(10.00)
3.15(6.70)
4.04(45.70)
3.34(7.40)
2.60(19.60)
2.87(19.40)
3.13(11.70)
2.89(6.70)
3.87(43.50)
3.14(16.20)
2.70(20.90)
2.88(8.30)
2.73(6.70)
3.58(37.00)
2.90(5.80)
2.59(23.90)
2.72(8.30)
2.61(6.70)
3.32(67.40)
2.51(5.80)
2.48(14.90)
2.60(8.30)
2.50(5.00)
3.14(28.30)
1.95(1.50)
2.30(17.90)
2.30(6.70)
2.32(5.00)
2.48(23.90)
2.24(11.90)
2.07(5.00)
2.21(23.90)
2.04(17.90)
2.03(15.20)
2.00(19.60)
[Ln{3-C6H4(CH3CONH)COO}3(N2H4)]where Ln = La,Ce, Pr, Nd, Sm and Gd for 3-acambH
La
Ce
Pr
Nd
Sm
Gd
13.71(45.50)
14.03(17.50)
9.99(14.75)
13.60(23.53)
9.73(9.09)
9.26(24.59)
12.29(100.00)
12.63(49.12)
9.42(29.51)
12.19(100.00)
9.15(14.55)
7.21(4.92)
9.22(7.27)
11.57(3.50)
7.33(6.56)
9.81(7.84)
7.15(7.27)
5.57(14.75)
7.71(25.50)
10.02(10.50)
5.67(19.67)
9.22(11.76)
5.58(18.18)
5.28(100.00)
7.01(18.10)
9.43(22.80)
5.35(100.00)
7.68(13.70)
5.25(100.00)
5.11(34.43)
6.19(3.64)
7.85(22.80)
4.73(29.50)
7.18(7.84)
5.08 (29.09)
4.66(32.79)
5.62(10.91)
7.28(7.02)
4.17(21.31)
6.96(15.69)
4.65(25.45)
4.13(19.67)
5.26(38.18)
5.67(17.50)
3.83(14.75)
6.18(3.92)
4.12(23.64)
3.65(29.50)
4.90(54.50)
5.36(100.00)
3.68(83.61)
5.59(17.65)
3.78(9.09)
3.39(21.31)
4.21(25.50)
5.18(38.60)
3.41(54.09)
5.27(56.86)
3.62(52.73)
3.32(24.59)
3.89(14.50)
4.97(29.82)
3.17(37.70)
4.86(31.37)
3.38(45.45)
3.15(22.95)
3.63(60.00)
4.73(35.09)
3.11(42.62)
4.66(27.45)
3.32(32.73)
2.50(1.48)
3.39(38.18)
3.93(5.26)
2.60(9.84)
4.19(27.45)
3.08(40.00)
2.46(6.56)
3.08(32.70)
3.67(31.58)
2.52(13.11)
4.04(19.60)
2.49(23.64)
2.03(8.20)
2.87(7.27)
3.42(26.32)
2.48(8.20)
3.88(9.80)
2.46(9.09)
2.70(9.09)
3.36(26.30)
2.04(8.20)
3.62(50.98)
2.05(10.91)
2.55(10.91)
3.17(15.79)
3.49(17.65)
2.50(10.91)
3.11)21.05)
3.38(45.10)
2.42(10.91)
2.89(21.00)
3.29(19.60)
2.35(14.50)
2.52(15.79)
3.09(2.94)
2.28(12.73)
2.64(8.77)
2.99(7.84)
2.23(9.09)
2.69(11.76)
2.12(9.09)
2.60(3.92)
2.06(10.90)
2.53(9.80)
2.46(15.69)
2.41(9.80)
[Ln{4-C6H4(CH3CONH)COO}3(N2H4)]where Ln = La,Ce, Pr, Nd, Sm and Gd for 4-acambH
La
Ce
Pr
Nd
Sm
Gd
15.45(100.00)
21.75(57.70)
15.48(100.00)
15.35(100.00)
15.48(100.00)
16.64(100.00)
8.38(16.20)
15.50(96.10)
8.35(22.60)
8.23(20.00)
10.30(4.80)
9.93(8.70)
8.56(26.90)
7.03(16.10)
8.44(23.80)
8.47(56.50)
4.87(100.00)
5.48(22.60)
7.30(28.60)
6.62(28.30)
3.66(57.70)
4.89(25.80)
3.65(16.10)
6.65(28.60)
5.65(23.90)
3.41(53.80)
3.66(16.10)
5.50(33.30)
4.61(26.10)
4.70(28.60)
3.65(15.20))
![XRD patterns of Metal Hydrazine complexes: (a) [La{2-C6H4(CH3CONH)COO}3.(N2H4)]; (b) [Ce{2-C6H4(CH3CONH)COO}3.(N2H4)]; (c) [Pr{2-C6H4(CH3CONH)COO}3.(N2H4)]; (d)[Nd{2-C6H4(CH3CONH)COO}3.(N2H4)]; (e) [Sm{2-C6H4(CH3CONH)COO}3.(N2H4)]; (f) [Gd{2-C6H4(CH3CONH)COO}3.(N2H4)].](/content/184/2022/15/9/img/10.1016_j.arabjc.2022.104009-fig12.png)
XRD patterns of Metal Hydrazine complexes: (a) [La{2-C6H4(CH3CONH)COO}3.(N2H4)]; (b) [Ce{2-C6H4(CH3CONH)COO}3.(N2H4)]; (c) [Pr{2-C6H4(CH3CONH)COO}3.(N2H4)]; (d)[Nd{2-C6H4(CH3CONH)COO}3.(N2H4)]; (e) [Sm{2-C6H4(CH3CONH)COO}3.(N2H4)]; (f) [Gd{2-C6H4(CH3CONH)COO}3.(N2H4)].
![XRD patterns of Metal Hydrazine complexes. (a) [La{3-C6H4(CH3CONH)COO}3.(N2H4)]; (b) [Ce{3-C6H4(CH3CONH)COO}3.(N2H4)]; (c) [Pr{3-C6H4(CH3CONH)COO}3.(N2H4)]; (d)[Nd{3-C6H4(CH3CONH)COO}3.(N2H4)]; (e) [Sm{3-C6H4(CH3CONH)COO}3.(N2H4)]; (f) [Gd{3-C6H4(CH3CONH)COO}3.(N2H4).](/content/184/2022/15/9/img/10.1016_j.arabjc.2022.104009-fig13.png)
XRD patterns of Metal Hydrazine complexes. (a) [La{3-C6H4(CH3CONH)COO}3.(N2H4)]; (b) [Ce{3-C6H4(CH3CONH)COO}3.(N2H4)]; (c) [Pr{3-C6H4(CH3CONH)COO}3.(N2H4)]; (d)[Nd{3-C6H4(CH3CONH)COO}3.(N2H4)]; (e) [Sm{3-C6H4(CH3CONH)COO}3.(N2H4)]; (f) [Gd{3-C6H4(CH3CONH)COO}3.(N2H4).
![XRD patterns of Metal Hydrazine complexes: (a) [La{4-C6H4(CH3CONH)COO}3.(N2H4)]; (b) [Ce{4-C6H4(CH3CONH)COO}3.(N2H4)]; (c) [Pr{4-C6H4(CH3CONH)COO}3.(N2H4)]; (d) [Nd{4-C6H4(CH3CONH)COO}3.(N2H4)]; (e) [Sm{4-C6H4(CH3CONH)COO}3.(N2H4)]; (f) [Gd{4-C6H4(CH3CONH)COO}3.(N2H4)].](/content/184/2022/15/9/img/10.1016_j.arabjc.2022.104009-fig14.png)
XRD patterns of Metal Hydrazine complexes: (a) [La{4-C6H4(CH3CONH)COO}3.(N2H4)]; (b) [Ce{4-C6H4(CH3CONH)COO}3.(N2H4)]; (c) [Pr{4-C6H4(CH3CONH)COO}3.(N2H4)]; (d) [Nd{4-C6H4(CH3CONH)COO}3.(N2H4)]; (e) [Sm{4-C6H4(CH3CONH)COO}3.(N2H4)]; (f) [Gd{4-C6H4(CH3CONH)COO}3.(N2H4)].
3.5 SEM–EDAX studies
It is generally observed that the hydrazine complexes yield metal oxides of nano scale on decomposition, due to their explosive nature (ref). Similar study was undertaken by decomposing the complexes in muffle furnace at their decomposition temperatures (observed from thermal analysis), keeping them at the same temperature for 30 min., and analyzing for their morphology and particle size of the residual oxides using SEM EDAX studies. Owing to the breaking of hydrazine and exothermic decomposition of organic moiety, the residues were of irregular shapes as evidenced by their SEM images. The SEM–EDAX pictures of Nd2O3 and Pr6O11 confirmed the existence of corresponding metals presented in Figs. 15–20. A comparison between the SEM–EDAX pictures of residues with the reported nano-neodymium and praseodymium oxides (Figs. 16, 18), shows that they display similar spectra, supporting the existence of the corresponding rare earth metal in the compounds as reported (Lembang et al., 2018; Pourmortazavi et al., 2017). The size of metal oxide particles lie in the range of 1–10 µm due to the insufficient quantity of hydrazine in the complex to decompose to oxides of nano scale.![SEM images of Nd2O3 residue of [Nd{2-C6H4(CH3CONH)COO}3.(N2H4)].](/content/184/2022/15/9/img/10.1016_j.arabjc.2022.104009-fig15.png)
SEM images of Nd2O3 residue of [Nd{2-C6H4(CH3CONH)COO}3.(N2H4)].
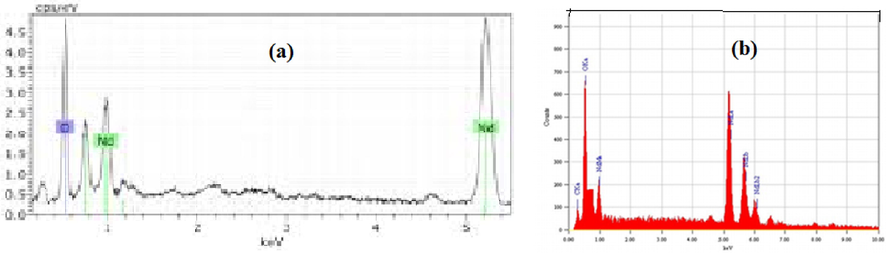
SEM-EDAX image of (a) Nd2O3 nanoparticles (Reported); (b): Nd2O3 residue of [Nd{2-C6H4(CH3CONH)COO}3.(N2H4).
![SEM images of Pr6O11 residue of [Pr{3-C6H4(CH3CONH)COO}3.(N2H4)].](/content/184/2022/15/9/img/10.1016_j.arabjc.2022.104009-fig17.png)
SEM images of Pr6O11 residue of [Pr{3-C6H4(CH3CONH)COO}3.(N2H4)].
![SEM-EDAX image of (a)Pr2(WO4)3Nanoparticles (reported; (b)Pr6O11 residue of [Pr{3-C6H4(CH3CONH)COO}3.(N2H4)].](/content/184/2022/15/9/img/10.1016_j.arabjc.2022.104009-fig18.png)
SEM-EDAX image of (a)Pr2(WO4)3Nanoparticles (reported; (b)Pr6O11 residue of [Pr{3-C6H4(CH3CONH)COO}3.(N2H4)].
![SEM images of Pr6O11 residue of[Pr{4-C6H4(CH3CONH)COO}3(N2H4)].](/content/184/2022/15/9/img/10.1016_j.arabjc.2022.104009-fig19.png)
SEM images of Pr6O11 residue of[Pr{4-C6H4(CH3CONH)COO}3(N2H4)].
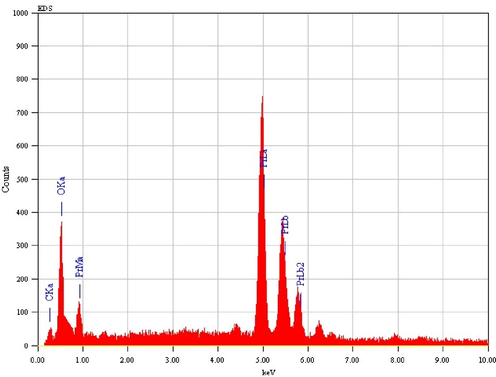
SEM-EDAX image of Pr6O11 residue.
3.6 Conductance studies
Conductance of hydrazine complexes lanthanide metal complexes of isomeric acetamido benzoic acids was measured by solution method using DMSO as solvent. Solutions of complexes were prepared in DMSO and specific conductance was measured first and then molar conductance was calculated as in the case of transition metal complexes the values are given in Table 6. The molar conductance values are found to be in the range of 8–20 O−1cm2mol−1 indicating that these compounds are neutral complexes (Geary, 1971; Ramalingam and Soundararajan, 1967).
S.No
Complexes
Specific Conductance
(mmhos)Molar Conductance
(ohm−1 cm2mol−1)
1
[La{2-C6H4(CH3CONH)COO}3(N2H4)]
0.30 × 10−3
30
2
[Pr{2-C6H4(CH3CONH)COO}3(N2H4)]
0.24 × 10−3
24
3
[Gd{2-C6H4(CH3CONH)COO}3(N2H4)]
0.1 × 10−3
10
4
[La{4-C6H4(CH3CONH)COO}3(N2H4)]
0.13 × 10−3
13
5
[Ce{4-C6H4(CH3CONH)COO}3(N2H4)]
0.12 × 10−3
12
6
[Gd{4-C6H4(CH3CONH)COO}3(N2H4)]
0.08 × 10−3
8
3.7 13C and 1H - NMR spectral studies
To ascertain the presence acid moiety in the complexes, 13C – NMR spectra were recorded and given in Fig. 22. In the spectra of the complexes, [La{2-C6H4(NHCOCH3)COO}3(N2H4)], [Gd{3-C6H4(NHCOCH3)COO}3(N2H4)] & [La{4-C6H4(NHCOCH3)COO}3(N2H4)], the important features noticed are, 13C- NMR (600 MHz, DMSO) On analyzing 13C - NMR spectra, the important characteristic peaks exhibited by the complexes are listed in Table 7 which are similar to that shown by the 4-acetamido benzoic acid Fig. 21 (SDBS No.6318 CDS-01-576,). The observations indicate that the complexes contain acid moiety, which has not undergone any degradation during the preparation of complexes (Abraham and Loftus, 1980).
S. No.
Peak Value(m/z)
Fragmented Species (Ions & neutral)
1
59
CH3CONH2
2
121
C6H5COO−
3
136
La3+
4
166
C6H4(COO)2)−
5
303
La{C6H4(COO)2)
6
392 (Base Peak)
(C7H5O2)3.N2H4
7
707
[La{C6H4 (CH3CONH)COO}3]
8
1380
2[La{C6H4 (CH3CONH)COO}3.N2H4]
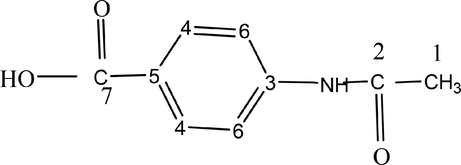
4-acetamido benzoic acid with carbon numbering.
![13C – NMR Spectra of (a): [La{2-C6H4(NHCOCH3)COO}3(N2H4)]; (b): [Gd{3-C6H4(NHCOCH3)COO}3(N2H4)]; (c): [La{4-C6H4(NHCOCH3)COO}3(N2H4)].](/content/184/2022/15/9/img/10.1016_j.arabjc.2022.104009-fig22.png)
13C – NMR Spectra of (a): [La{2-C6H4(NHCOCH3)COO}3(N2H4)]; (b): [Gd{3-C6H4(NHCOCH3)COO}3(N2H4)]; (c): [La{4-C6H4(NHCOCH3)COO}3(N2H4)].
On evaluating the 1H - NMR(SDBS No.6318 HSP- 47–33) spectrum of 4-acetamido benzoic acid, majority of the peaks observed are found to be compatible with those of the complexes, which are given in Table 7. 1H -NMR (600 MHz, DMSO. Since the value of δ 12.8 is ascribed to C1H in the 4-acetamido benzoic acid is vanishes in the complexes implying that the acid moiety is involved in complexation (Milenkovic et al., 2012).
3.8 Mass spectral studies
The mass Spectrum of 4 - acetamido complex was recorded to find out the molecular mass of the complex, as a representative example. Since the attempt made to obtain the single crystals of the synthesized compounds was not fruitful, the mass spectroscopic characterization was carried out to find the molecular mass of the complexes (Pavia et al., 2015).
The mass Spectrum of 4 - acetamido benzoic acid (NIST number 375119) is correlated with that of the corresponding complex [La{x-C6H4(NHCOCH3)COO}3(N2H4)] shown in Fig. 23. The prominent peaks identified in 4- acetamido benzoic acid with m/z values are 43, 120 and 135 representing the fragments of acyl radical, benzoate radical and phenyl acetamido moieties respectively. The various characteristic fragments of the above-mentioned complex, such as anions, cations and neutral species are listed in Table 7. The prominent peak identified in the spectrum at m/z value of 673, which represents the formula La{C6H4(CH3CONH)COO}3 is corresponding to M+ ion. The m/z value of 59 represents the acetamide (CH3CONH2) moiety. The benzoate radical (C6H5COO) is shown by the peak at 121(m/z). Lanthanum metal cation is observed at m/z of 136. The phthalate radical {C6H4(COO)2} ascribed for the m/z value of 166 in the spectrum. A fragment of {La(C6H4(COO)2}is observed at m/z 303. Besides, the base peak (most abundant) is corresponding to [(C7H4O2)3.N2H4] moiety is exhibited at m/z 392. As per the spectrum, the molecular ion peak at m/z 1380 accounts for the molecular formula [La2{C6H4(NHCOCH3)COO}6] which was the dimer of the target compound, due its instability the abundance is found to be minimum.![1H – NMR Spectra of (a): [La{2-C6H4(NHCOCH3)COO}3(N2H4)]; (b): [Gd{3-C6H4(NHCOCH3)COO}3(N2H4)]; (c): [La{4-C6H4(NHCOCH3)COO}3(N2H4)].](/content/184/2022/15/9/img/10.1016_j.arabjc.2022.104009-fig23.png)
1H – NMR Spectra of (a): [La{2-C6H4(NHCOCH3)COO}3(N2H4)]; (b): [Gd{3-C6H4(NHCOCH3)COO}3(N2H4)]; (c): [La{4-C6H4(NHCOCH3)COO}3(N2H4)].
4 Conclusion
The isomeric acetamido benzoic acids undergo reaction with hydrazine hydrate and trivalent lanthanides La3+, Ce3+, Pr3+, Nd3+, Sm3+ and Gd3+ form complexes of formulae, [Ln{x-C6H4(CH3CONH)}3(N2H4)] at pH 3 and 4.5 (1: 1) ethanolic medium and x = 2, 3, & 4 isomers. The sparingly soluble nature of the formed complexes suggests a polymeric nature through hydrazine bridging substantiated by N-N stretching observed for complexes. All the complexes decompose to 1–10 µm sized metal oxides via metal phthalates intermediates as confirmed by their IR spectra, eliminating hydrazine below 200 °C. Cerium complexes decompose directly to their metal oxides via high unstable intermediates, showing difference in thermal behaviour. The NMR studies of the complexes substantiate the formation of complexes by showing the characteristic peaks of the acid moiety. The mass spectral data of the complex [La{x-C6H4(NHCOCH3)COO}3(N2H4)] where x = 2, 3 and 4, confirms the formation of the complex by showing the molecular ion peak. Based on the analytical and spectroscopic data, the structures of the complexes are proposed as depicted in the Fig. 24. The electronic spectral values indicate that the complexes may have coordination number 8. However, these structures can be confirmed by their single crystal analysis (see Fig. 25).![Mass Spectrum of [La{C6H4(NHCOCH3)COO}3(N2H4)].](/content/184/2022/15/9/img/10.1016_j.arabjc.2022.104009-fig24.png)
Mass Spectrum of [La{C6H4(NHCOCH3)COO}3(N2H4)].
![Proposed structure of [Ln{2 / 3 & 4-C6H4(CH3CONH)COO}3(N2H4)] where Ln = La, Ce Pr, Nd, Sm& Gd.](/content/184/2022/15/9/img/10.1016_j.arabjc.2022.104009-fig25.png)
Proposed structure of [Ln{2 / 3 & 4-C6H4(CH3CONH)COO}3(N2H4)] where Ln = La, Ce Pr, Nd, Sm& Gd.
Acknowledgment
The current research work was the grant-in-aid of All India council of Technical education, New Delhi, [8023/BOR/RID/RS, 2008- 2009]. The authors of the manuscript are thankful to the management and SITRA for helping in the successful completion of work.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Proton and carbon -13 NMR spectroscopy an integrated approach (3rd edition). London: Heyden & Son Ltd.; 1980.
- Thermodynamic properties of sublimation of the ortho and meta isomers of acetoxy and acetamido benzoic acids. J. Chem. Thermodyn.. 2015;86:6-12.
- [Google Scholar]
- Spectral and thermal studies of transition metal complexes of acetamido benzoic acids with hydrazine. Asian J. Chem.. 2013;25:209-216.
- [Google Scholar]
- Hydrazine complexes of Lanthanides with 3-acetoxy and 4-acetoxybenzoic acids: Spectroscopic, thermal and XRD studies. J. Chem.. 2013;2013:1-10.
- [Google Scholar]
- Bai, E.H.P., Vairam, S., 2020. A Study on Thermal Degradation of hydrazinated transition metal acetamido benzoates. Current perspectives on chemical sciences, 1st edition, India &UK. 2, 90–106.
- Computational analysis of M-O covalency in M(OC6H5)4 (M=Ti, Zr, Hf, Ce, Th, U) Dalton Trans.. 2019;48:2939-2947.
- [Google Scholar]
- The nitrogen-nitrogen stretching band in hydrazine derivatives and complexes. Inorg. Chem.. 1968;7:1430-1433.
- [Google Scholar]
- Synthesis and Thermal Characterization of Lanthanide(III) Complexes with Mercaptosuccinic Acid and Hydrazine as Ligands. J. Chem.. 2013;2013:1-10.
- [Google Scholar]
- Spectroscopic studies of some lanthanide (III) nitrate complexes synthesized from a new ligand 2,6-bis-(salicylaldehyde hydrazone)-4-chlorophenol. Bull. Chem. Soc. Ethiop.. 2003;17:27-34.
- [Google Scholar]
- The use of conductivity measurements in organic solvents for the characterization of coordination compounds. Coord. Chem. Rev.. 1971;7:81-122.
- [Google Scholar]
- Preparation and properties of rare earth 4-nitrophthalates. Croat. Chem. Acta.. 1999;72:77-86.
- [Google Scholar]
- Hydrazinium as a ligand – structural, thermal, spectroscopic, and magnetic studies of hydrazinium lanthanide di-sulfate monohydrates – crystal structure of the neodymium compound. J. Chem. Dalton Trans.. 1986;1:119-123.
- [Google Scholar]
- Synthesis, antimicrobial evaluation and docking studies of novel 4-acetamido-3-aminobenzoic acid derivatives as microbial neuraminidase inhibitors. Research J. Pharm. and Tech.. 2019;12:303-313.
- [Google Scholar]
- Ab initio ligand-field theory analysis and covalency trends in actinide and lanthanide free ions and octahedral complexes. Inorg. Chem.. 2017;56:8802-8816.
- [Google Scholar]
- Preparation and thermal reactivity of hydrazinium uranyl carboxylates. Thermochim. Acta.. 1996;274:139-148.
- [Google Scholar]
- A facile method for green synthesis of Nd2O3 nanoparticles using aqueous extract of terminalia catappa leaf. AIP Conf. Proc.. 2018;020093:1-6.
- [Google Scholar]
- Hydrothermal synthesis, crystal structure and thermal stability of the complex [Zn(p-ABA)2(phen0. (H2O)].H2O. Chinese J. Inorg. Chem.. 2007;23:2023-2027.
- [Google Scholar]
- Transition Metal and Lanthanide Complexes for Catalysis and Protein Structure Determination PhD Thesis, submitted to. The University of New South Wales School of Chemistry; 2010.
- Cocrystal screening of hydroxybenzamides with benzoic acid derivatives: a comparative study of thermal and solution-based methods. Eur. J. Pharm. Sci.. 2014(A).;65:56-64.
- [Google Scholar]
- Salicylamide Cocrystals: Screening, Crystal Structure, Sublimation Thermodynamics, Dissolution, and Solid-State DFT Calculations. J. Phys. Chem. B. 2014;118:6803-6814.
- [Google Scholar]
- Acetamidobenzoic acid isomers: Studying sublimation and fusion processes and their relation with crystal structures. Thermochim. Acta.. 2014(B).;583:72-77.
- [Google Scholar]
- N-Acetylanthranilic acid (O-acetamidobenzoic acid) a strongly triboluminescent material. Acta. Crystallogr. B. Struct. Sci. Cryst. Eng. Mater.. 1980;36:502-504.
- [Google Scholar]
- Synthesis, Spectral and solid-State Characterization of a new Bioactive Hydrazine bridged Cyclic Diphosphonium Compound. Molecules.. 2012;17:2567-2578.
- [Google Scholar]
- Infrared and Raman Spectra of Inorganic and Co-ordination Compounds, 6thedition. New York, USA: Wiley Interscience Co; 2009.
- A general method for preparing lanthanide oxide nanoparticles via thermal decomposition of lanthanide(III) complexes with 1-hydroxy-2-naphthoic acid and hydrazine ligands. J. Phys. Chem. Solids. 2016;96:60-67.
- [Google Scholar]
- Kinetics and thermal decomposition of Tetrahydrazine lanthanum 2-hydroxy-1- naphthoate. Orient. J. Chem.. 2014;30:1957-1963.
- [Google Scholar]
- Pavia, L., Lampman, G.M., krizand, G.A., Vyvyan, J.R., 2015. Introduction to spectroscopy, 5th edition, Cengage Learning, USA.
- A theoretical characterization of covalency in rare earth complexes through their absorption electronic properties:f-f transition. Inorg. Chem.. 2006;45:7382-7388.
- [Google Scholar]
- Facile and effective synthesis of praseodymium tungstate nanoparticles through an optimized procedure and investigation of photocatalytic activity. Open Chem.. 2017;15:129-138.
- [Google Scholar]
- Design, Synthesis and Biological Evaluation of 3-(2-(benzo[d]thiazol-2- ylthio)acetamido)benzoic Acid Derivatives as Inhibitors of Protein Tyrosine Phosphatase 1B. Lett. Drug Des. Discov.. 2021;18:46-56.
- [Google Scholar]
- Dimethyl sulphoxide complexes of lanthanide and yttrium nitrates. J. Inorg. Nucl. Chem.. 1967;29:1763-1768.
- [Google Scholar]
- Low-temperature preparation of fine-particle cobaltites. J. Solid State Chem.. 1987;66:20-25.
- [Google Scholar]
- A one-step process for the preparation of γ-Fe2O3. J. Mat. Sci. Lett.. 1986;5:221-222.
- [Google Scholar]
- Hydrazine and its Derivatives - Preparation, Properties and Applications. NY, USA: Wiley Inter. Sci.; 1984.
- Preparation and thermal reactivity of some rare earth and uranyl hydrazine sulfinates and sulfite hydrazinates. Thermochim. Acta.. 2004;417:107-113.
- [Google Scholar]
- Cascade covalent and coordination bond formation for Ti-based cage assembly: catalysis and coordination bifunctionality of TiCl4. Dalton Trans.. 2018;47:3239-3242.
- [Google Scholar]
- Hydrazinium complexes of Lanthanide and Transition metal squarates. Polish J. Chem.. 2006;80:1601-1614.
- [Google Scholar]
- A Text Book of Quantitative Inorganic Analysis Including Elemental Analysis (5th ed.). London, UK: Longmans; 2019.
- Studies of radii-dependent lanthanide coordination behavior with 4-acetamidobenzoate and 1,10-phenanthroline. Z. Anorg. Allg. Chem.. 2009;635:2333-2339.
- [Google Scholar]
- Magnetic susceptibility measurements of pure and mixed gadolinium-terbium formate heptahydrate crystals. J. Magn. Magn. Mater.. 2016;401:391-393.
- [Google Scholar]
- Solvothermal Synthesis, Crystal Structure and Electrochemical Properties of the Complex Cd-2(2,4-DAA)(4)(phen)(2) Chinese J. Inorg. Chem.. 2009;25:1120-1123.
- [Google Scholar]
- Synthesis, crystal structure and fluorescence characterization of cadmium(II) coordination polymer with 4-acetamidobenzoic acid and 4, 4'-bipyridine. Chinese J. Inorg. Chem.. 2009;25:1304-1307.
- [Google Scholar]
- Synthesis, crystal structures, and photoluminescence of Lanthanide coordination polymers with 4-acetamidobenzoate. Z. Anorg. Allg. Chem.. 2011;637:773-777.
- [Google Scholar]
- Self-assembled molecular network formed by controlling molecular deposition of organic compounds. Flat Chem.. 2021;29
- [Google Scholar]
- Recent advances in hybrid organic-inorganic materials with spatial architecture for state-of-the-art applications. Prog. Mat. Sci.. 2020;112
- [Google Scholar]







