Translate this page into:
Copolymerization of butadiene and styrene under the influence of n-butyllithium
⁎Corresponding author. os_kharitonova@mail.ru (Olga Kharitonova),
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
It is known that styrene-butadiene copolymers having an increased content of 1,2-links of butadiene give tires a unique combination of good grip properties and low rolling resistance. Under conditions of severe operation, heat generation, and, therefore, rolling resistance of this rubber turned out to be less than that of polymers with a conventional structure. Compared to other rubbers, SSBR rubber with a high content of carbon black (mire than 50%) is highly economical. Lower heat generation in SSBR tires is a significant advantage over other rubbers and is preferable for long-term safety of the run even in desert conditions. The growing requirements for energy saving, safety of road transport, ecology, as well as a sufficient level of scientific research in the field of ionic polymerization of monomers were prerequisites for the creation of technology for obtaining solution copolymers based on dienes and vinyl aromatic monomers with special properties based on the mathematical model of the isothermal process of copolymerization of styrene and butadiene in an aliphatic solvent on the n-BuLi/mod catalytic system, which includes four chain growth reactions, two chain transfer reactions to a transfer agent, the concentration of which is recalculated. The calculation of the copolymerization constants of styrene and butadiene in hexane in the presence of the tested catalytic system is presented and the parameters of the obtained mathematical model are determined. Compliance with the optimal mode of conducting the polymerization process makes it possible to obtain rubber with specified physical and mechanical properties. The models presented in this paper take into account the most complex mechanisms of the copolymerization process, taking into account the hydrodynamics and heat transfer in the system, as well as the features of the real technological process. For the first time, the universal approach to solving the problem of obtaining SSBR rubber with a predictable set of properties is proposed. Based on mathematical modeling, laboratory experiments and optimization of the copolymerization process, the possibility of directed synthesis of functionalized DSSC rubber with the required physical and mechanical characteristics has appeared.
Keywords
Copolymerization
Solution-polymerized styrene-butadiene rubber
Anionic polymerization
1 Introduction
In recent years, the top requirements for automobile tires have changed significantly. The importance of driving safety, fuel economy, and environmental safety has increased. In the first place, such requirements as high coupling properties (the traction with wet roads), low rolling losses, productive environmental factors were put forward.
One of the rubbers that meet these requirements is “environmentally friendly” butadiene-styrene rubber of the solution polymerization with medium and high content of vinyl links. Butadiene-styrene rubbers (divinyl-styrene rubbers, BSR, SSBR) are the synthetic rubbers, copolymerization products of butadiene (I) and styrene (II) of the general formula (Fig. 1).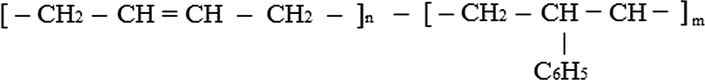
Formula of butadiene-styrene rubbers.
It is known that styrene-butadiene copolymers with an increased content of 1,2-butadiene links give tires a unique combination of the properties of good adhesion to the road surface and low rolling resistance. Under the conditions of hard operation, the heat generation, and, consequently, the rolling resistance of this rubber turned out to be less than that of polymers with a conventional structure.
According to the Michelin company, which has its own production of solution-polymerized styrene-butadiene rubber (SSBR), the tire sector is the main area of application of SSBR. The tire treads that are based on SSBR provide excellent traction on the road surface. With the same filling with technical carbon, tires made of dissolved SSBR have a lower rolling resistance, which affects fuel economy, since this factor alone accounts for 40% of the total energy loss when the tires are in motion.
Thus, the increasing requirements for the energy conservation, safety of motor transport, ecology, as well as a sufficient level of scientific research in the field of ion polymerization of monomers were suppositions for the creation of a technology for obtaining a copolymer solution based on dienes and vinylaromatic monomers with special properties.
Extensive research has been carried out on the polymerization of dienes and vinylaromatic compounds by the anionic polymerization (Balzamov et al., 2020a,b; Gizzatova and Spivak, 2012; Ignashina et al., 2020; Manuyko et al., 2015, 2020; McIntosh et al., 2007; Mueller et al., 2011; Qiang et al., 2007; Vagizov et al., 2017a,b; Van Beylen and Morita, 2011; Aminova et al., 2009; Hsieh and Quirk, 1996; Jose ́et al., 2009; Pliushchev et al., 2020; Tavtorkin et al., 2019). The works of Yerosalimsky, Korotkov and others (Balzamov et al., 2020; Kuznetsov et al., 2015; Tkachev and Sedykh, 2013; Akhmetov, 2010; Cooperman, 1994; Dolgoplosk, 1982; Erusalimskiy, 1974; Firsova et al., 2014; Korotkov, 1973; Kustov, 2004)are devoted to the mechanism of polymerization.
Anionic polymerization using lithium-organic initiators by the mechanism of “living” chains allows to obtain linear polymers with a narrow molecular weight distribution (MWD) and practically with any given molecular weight. The patterns of anionic polymerization in the presence of alkali metals and their compounds have been most widely studied.
In the conditions of modern industrial production of synthetic rubbers, methods of mathematical modeling of technological processes are increasingly being used to solve problems of forecasting and optimization of production (Balzamov et al., 2020; Ignashina et al., 2020; Kuznetsov et al., 2015; Tkachev and Sedykh, 2013; Dolgoplosk, 1982; Firsova et al., 2014; Glukhovskoy, 1986; Hsieh and Quirk, 1996; Kustov, 2004). The foundations of modeling of copolymerization processes were laid by foreign researchers Mayo F. and Lewis F., as well as Alfri T. and Goldfinger G., who independently proposed the simplest model of the eng group. Further complication of kinetic schemes and their calculation was carried out in the works of Russian scientists Academician of the Russian Academy of Sciences Berlin A. A., Wolfson S. A., Enikolopyan N. S., Semchikova Yu. D (Kovtunenko, 1991; Kovtunenko, 1992; Kovtunenko, 2001; Pat. 2175329 RF: IPC C 08 F 36 / 04, 2001; Synthetic rubber and chemicals for their production, 1998). and foreign researchers Mertz E., Melville X., Walling S. [40–43]. Since the copolymerization processes take place with the participation of molecules of several monomers, it is of great interest to study not only the size, but also the composition of the resulting macromolecules. The study of the compositional heterogeneity of copolymerization products was carried out in the works of Myagchenkov V. A., Frenkel S. Ya., Khokhlov A. R., Kuchanova S. I. (Cooperman, 1994; Cooperman, 1997; Dzhibera, 1997; Jose ́et al., 2009; Kirchevskaya, n.d.; Kovtunenko, 1992; Shakunova, 1991). When designing large-capacity productions, preference is given to continuous processes, for the mathematical description of which the kinetic approach is not enough. In the works of Academician of the Russian Academy of Sciences Kafarov V. V., Podvalny S. l. a modular principle of construction of a mathematical model is proposed, according to which the kinetic description of the process should be supplemented with a macrokinetic module that takes into account the hydrodynamic and energy patterns of the process under consideration.
The process of copolymerization of butadiene with styrene has been studied mainly experimentally. Attempts to describe the process quantitatively are few and have been made mainly to demonstrate computational methods for a simplified kinetic scheme, while physical and chemical modeling of the copolymerization process of butadiene with styrene in the production of synthetic rubber requires detailed consideration. In this connection, the problem of studying the copolymerization process of butadiene with styrene by numerical methods, taking into account the regularities of the process under consideration, is relevant today.
Works were organized as follows:
-
Analysis and investigation of the mechanism of the copolymerization process. Consideration of alternative kinetic schemes: from a two-center system to a system with a large number of active centers.
-
Determination of the type of the kinetic scheme, which is described taking into account the exchange between the centers. Identification of exchange schemes between the centers
-
Construction of mathematical models of the SSBR synthesis process. The feature of the description is the polycentricity of the catalytic system
-
Structural identification: the comparison of different kinetic schemes for the copolymerization of butadiene and styrene
-
Parametric identification: the calculation of molecular weight distribution according to various exchange schemes and subsequent comparison of theoretical results with experiment to identify the true mechanism of chain growth
-
Verification of the adequacy of the mathematical model of the SSBR rubber synthesis process
-
Development of a mathematical model of SSBR synthesis process Calculation of the combined process of hydrodynamics, heat transfer and chemical transformation in the cascade polymerizers, since the hydrodynamics and temperature are the determining factors in the displacement of the dynamic equilibrium between the active centers.
-
Analysis of the parametric sensitivity of the mathematical model and computational studies of the copolymerization process Investigation of the influence of the main control parameters of the process on the physical and mechanical characteristics of the polymer.
-
Based on the developed model, the most rational mode of the copolymerization process of butadiene and styrene is selected. Optimization of the SSBR rubber synthesis process.
The current level of scientific research in the field of anionic polymerization of monomers makes it possible to control the rate of copolymerization of dienes with styrene and the content of butadiene vinyl units by changing the polarity of the solvent and alkoxide metal ions in a randomizer.
The fundamentally new approach to the technology of obtaining SSBR is the use of new initiating systems n-butyllithium + modifier, the choice of the order of introducing the components of the catalytic system at an industrial plant, and the optimization of the technological process for obtaining rubber with stable properties that meet the requirements of consumers:
- the use of a new initiating system (based on n-butyllithium and modifiers of mixed alcoholates of alkali and alkaline earth metals) that ensures the production of rubber with a given set of properties;
- the prevention of gelation in the continuous process of monomer copolymerization, which ensures the exclusion of the expensive and laborious process of opening and cleaning polymerizers;
- the low consumption of expensive n-butyllithium 2.7–3.0 mol/t of monomers;
2 Preparation of solution butadiene-styrene rubbers
Copolymers of butadiene and styrene are obtained in the solution in the presence of organolithium catalysts with modifications (co-catalysts) that avoid the formation of polystyrene blocks. Due to the absence of low molecular fractions, they have better frost resistance, as well as the resistance to the growth of cuts and wear resistance.
The synthesis of solution styrene-butadiene rubber, proceeding according to Fig. 1a, gives an irregular copolymer with a statistical arrangement of links: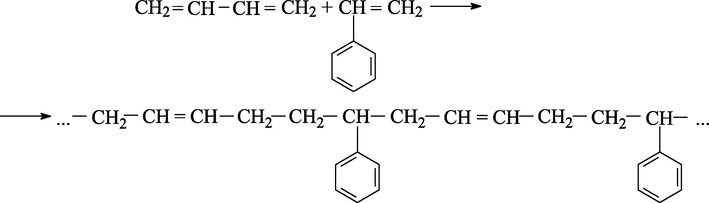
Chain growth reaction.
The main reasons for the growth in the production of solution butadiene-styrene rubbers is the ability to control the micro- and macrostructure of polymers due to the multistage of the polymerization process, obtaining materials that are suitable for the manufacture of a wide range of rubber products. When the polymerization is carried out in the solution, a smaller range of auxiliary materials is required, complete conversion of monomers is achieved.
The initial mixture is prepared by mixing monomers, styrene and butadiene with a solvent consisting of cyclohexane and gasoline in a ratio of 75:25 (Fig. 2).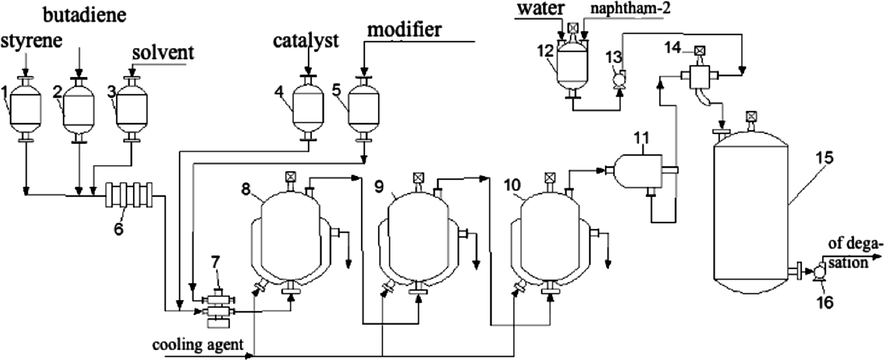
Polymerization scheme for obtaining SSBR-25: 1––5 - measuring instruments; 6 - diaphragm mixer; 7 - injection pump; 8––10- polymerizers; 11 - filter; 12 - apparatus for the preparation of stabilizer dispersion; 13,16 - pumps; 14 - intensive mixer; 15- averager.
Mass composition of the initial mixture (%), Butadiene: Styrene: Solvent(11.25:3.75:85).
The initial mixture of monomers and solvent enters the diaphragm mixer 6 from the measuring instruments 1–3 and is fed to the polymerization in the devices 8–10 by the dosing pump 7. Before the polymerizers, the feed is mixed with a solution of the catalyst and the modifier coming from the measuring instruments 4 and 5. The use of the modifier makes it possible to bring together the constants of the polymerization of butadiene and styrene and eliminate the formation of polystyrene blocks. The dosage of the catalyst and modifier is selected based on the binding of the micro-impurities in the initial charge and the polymerization process itself.
The polymerization process is carried out in a battery consisting of three or four polymerizers with a volume of 20 m3 with cooling jackets, with continuous mixture supply from below and output of the polymerization product from the upper part of the apparatus. The process is carried out at a temperature of 50–80 °C, at the exit from the last polymerization reactor, a complete conversion of monomers is achieved. The polymerization time is 5–6 h. The heat of the polymerization reaction is removed by industrial cold water through the jacket.
Upon exiting the last apparatus, the polymerizate enters the filter 11, where the insoluble polymer is separated, fed into the intensive mixer 14 for mixing with a 20% aqueous stabilizer solution which is prepared in the apparatus 12 and is supplied there with the help of a pump 13. Thus, the polymerizate filled with a stabilizer enters the neutralization in the apparatus 15, from where it is directed to water degassing by a pump 16. The degassing process is carried out according to the scheme of obtaining solution stereoregular rubbers at a temperature of 100–130 °C and a pressure of 0.15–0.30 MPa.
3 Polymerisation in the solution
Polymerization in the solution provides the heat transfer in the mass of the solution in which the reaction takes place. Therefore, the resulting polymer is homogeneous and it has the best set of properties. The use of organic solutions makes it possible to use various effective catalytic systems in the polymerization process, with the help of which it is possible to carry out directed synthesis of elastomers, to create high-molecular compounds with given structure and properties. The technological difficulty of such processes is the need to work with catalysts, many of which are highly reactive compounds that change properties during the storage. The use of such catalytic systems requires the careful preparation and purification of monomers and solvents that are used in synthesis. Polymerization in the solution implies the reaction system containing a monomer dissolved in a suitable solvent. This method has an advantage in terms of process control flexibility, increased reaction rates and heat extraction. The most common equipment for conducting the polymerization process in the solution is various types of mixing devices. The dilution of the monomer makes it relatively easy to regulate the process temperature regime in devices with an external heat extraction. At relatively low process speeds, the reaction is carried out either in the batch apparatuses or in a cascade mixing reactor of continuous action.
4 Properties and applications of styrene-butadiene rubbers
Statistical butadiene-styrene rubbers of SSBR obtained by the method of solution polymerization are superior to butadiene-styrene rubbers of emulsion polymerization in a number of properties (Table 1).
Indicators
Solution-type rubber
Emulsion-type rubber
Mass content, %
data
data
polymer
98
91
organic acids
–
5.8
ash
0,05–0,1
0,4–0,6
The presence of side branches
No
Yes
Molecular weight distribution
Narrow
Wide
The most valuable properties of solution-type rubbers include the following:
-
the content of 1,2-links in the copolymer, which increases the strength, elasticity, frost resistance and wear resistance of vulcanizates filled with technical carbon;
-
a narrow molecular weight distribution, which provides the best dynamic properties of rubber;
-
low impurity content.
In addition, during mechanical processing, solution rubbers do not undergo degradation, they mix well with other general-purpose rubbers, such as polyisoprene, polybutadiene, etc.
Unlike emulsion-type butadiene-styrene rubbers, solution-type rubbers can be filled with a significantly large amount of carbon black, oil — while the physical and mechanical properties of rubbers do not deteriorate (Table 2). Note: These SSBR rubbers have the same structure and contain 40–50% cis-1,4 links, 32–47% trans-1,4 and 8–12 1,2 links.
Indicators
SSBR-10
SSBR-18
SSBR-25
SSBR-25LN
SSBR-45
SSBR-65
SSBR-86
Content, %
bound styrene
10
18
25
25
45
65
85
block styrene
0
0
0
6–8
15–30
15–40
45–60
volatile
0.4
0.4
0.4
0.4
0.4
0.4
0.8
ash
0.04–0.1 for all brands
residual styrene
0.01
0.01
0.01
0.01
0.05
0.05
0.05
Mooney Viscosity
40–55
40–55
40–55
30–35
40–60
60–80
–
The high linearity of polymer chains (34%) provides the rubber mixtures based on solution-type rubbers with less shrinkage, as well as a high value of Mooney Viscosity compared with emulsion-type ones with close values of plasticity.
However, it should be noted that an increase in the content of styrene in the copolymer increases the strength properties. The optimal content of bound styrene in the copolymer is considered to be 18–25% — in this case, the best combination of strength and elastic properties takes place.
Copolymers, in which the styrene content is more than 25% with its block distribution, serve as a good material for the shoe industry.
Butadiene-styrene rubbers of solution polymerization are used for the manufacture of rubber products for various purposes, cable products, rubber shoes, sports equipment, children's toys, as well as for food packaging.
In order to obtain statistical styrene-butadiene copolymers (SSBR), extensive patent information is considered. Here is only a part of the methods for obtaining SSBR, which differ only in the catalytic system, namely the n-butyllithium modifier, which are closest to the catalytic system implemented in industry.
The conditions of butadiene polymerization in the presence of n-butyllithium modified with sodium tert-butylate in various solvents were studied: toluene, heptane, cyclohexane. However, the indicated initiating system has a disadvantage - sodium tert-butylate is not soluble in hydrocarbons and is unsuitable for use in industrial conditions.
A method for producing diene rubbers with good physical, mechanical and technological properties is known. This method is used in the tire industry, polymerization or copolymerization of dienes with vinylaromatic monomers at 60 °C in a hydrocarbon medium with compounds of alkali metals (butyllithium in combination with N, N, N', N' -tetramethylethylenediamine) with the addition of 0.005 – 0.100 g per 100 g of monomer cross-linking agent (divinylbenzene) with following breakage of thepolymerization with a mixture of stannum halide (SnCl4) and organic compounds, containing the group C(=X)N, where X = 0 or S (e.g., aromatic amino (thio) aldehyde or ketone). The method allows to obtain copolymers with a high content of 1,2-links in the butadiene part (70%).
The disadvantage of this method is the need to maintain the copolymerization temperature within 55–65° C to ensure a high content of 1,2-links in the diene part, which complicates the heat removal of the reaction, especially in large-volume industrial apparatuses and suggests the use of cooling agents with low temperatures. To partially eliminate this disadvantage, it is necessary to reduce the monomers concentration in solution. However, this leads to a decrease in the copolymer yield, and, consequently, reduces the economic indicators of the process by reducing the productivity of the equipment.
It is known the method of producing diene polymers with controlled content of 1,2-links of diene part by the variability of the catalytic system used at the stage of copolymerization and consisting of lithium initiator (ethyllithium, isopropyl lithium, n-butyllithium, t-butyllithium), alkoxide sodium (formula NaOR, where R is an alkyl group containing 3 to 8 atoms of carbon (n-pentoxide Na)) and polar modificator (diethyl ether, di-n-propyl ether, dietilenglicol, tetrahydrofuran, dioxane, triethylene glycol, trimethylamine, N, N, N', N' - tetramethylethylenediamine, N-methylmorpholine andalkyltetrahydrofurin ethers) with the molar ratio of alkoxide sodium: polar modifier: lithium initiator that is equal 0.5–1.00: 0.40–3.00: 1, respectively, and holding the (co)polymerization in hydrocarbon solvent, representing one or more aromatic paraffin or cycloparaffin connection with 4–10 atom of carbon in the molecule.
The disadvantages of the proposed method are as follows:
-
the use of modifiers dissolved in water, which requires the development of the methods of sewage treatment, since when the polymer is separated from the solution by water degassing, part of the polar modifiers enters the sewage water;
-
a low level of maximum permissible concentrations of these polar modifiers that do not affect the operation of sewage treatment plants (no higher than 0.5 mg/l), which, with a minimal dosage of the polar modifier provided for in the method, will ensure its presence in sewage water of more than 1 mg/l;
-
the impossibility of obtaining a given number of 1,2-links (45–55%) in the diene part of the polymer at a temperature above 60° C entails difficulties in the heat extraction of the reaction in polymerization reactors and, as a consequence, the cost of obtaining a refrigerant.
Another way of obtaining statistical styrene-butadiene rubbers is the copolymerization of monomers in several reactors in the medium of hydrocarbon solvent in the presence of organolithium catalyst, for example, butyllithium and modifying additives as N, N, N', N' - Tetra(potassium hydroxypropyl) ethylenediamine, when the molar ratio of organolithium catalyst of 0.05–2.50 or a mixture of N, N, N', N' -Tetra (potassium hydroxypropyl) ethylenediamine with a compound selected from the group including ethers, dimethyl ether of diethylene glycol, tetrahydrofuran, potassium tetrahydrofurfurylate, derivatives of oxypropylated alcohols in the mole ratio of mixture components from organolithium catalyst (0.05–2.50): (0.025–1.000): 1 respectively.
This method makes it possible to increase the controllability and reproducibility of the process by equalizing the copolymerization constants of monomers, stabilize the Mooney viscosity of the rubber and reduce the yield of nonconditional rubber, ensure the formation of 1,2-links in the structure of the diene part of the polymer, contributing to an increase in the strength of the connection of tires with a wet road. However, the maximum content of 1,2-links in the diene part of the copolymer, achieved during the polymerization process by this method, is only 27.3%, which cannot allow obtaining the necessary high complex of properties of butadiene and statistical butadiene-styrene rubbers for their successful application in tire production.
A method of producing copolymers, in particular, the copolymerization of butadiene and styrene in their mass ratio 85–90: 15–10 respectively, in an inert organic solvent in the presence of catalytic systems, which is a product of interaction of n-butyllithium, isoprene and N, N, N', N' – Tetra (sodium hydroxypropyl) ethylenediamine, taken in a molar ratio butyllithium: N, N, N', N' - Tetra-(sodium hydroxypropyl)-ethylenediamine: isoprene 1: (0,07–0,15): (15–25), respectively. Moreover, the catalytic system is obtained by simultaneous mixing of n-butyllithium, isoprene and N, N, N', N' - tetra (sodium hydroxypropyl) ethylenediamine in an inert organic solvent and interaction at the reaction temperature.
This method makes it possible to obtain copolymers and tread rubbers that have high dynamic and fatigue properties, heat resistance, adhesion to wet road surfaces, low rolling resistance, which is due to the high content of 1,2-links in the diene part (51.5%). However, such a high content of 1,2-units in the diene part is achieved at a sufficiently low copolymerization temperature (30–60 °C), which causes the above-described technical difficulties in maintaining the temperature at this level.
A method of producing diene (co)polymers with a high content of 1,2 units, in particular, butadiene and styrene with styrene content in the copolymer 18–25 % by weight. in the presence of a catalyst including ethyllithium with subsequent introduction into the reaction mass of the monomer(s) (a mixture of butadiene with styrene) in a molar ratio of ethyllithium (active lithium): monomer(s) = 0,25–1,00: 1.0 and modifiers: reaction product of N, N, N', N' -tetraoxyethylene diamine with the dispersion of sodium in a molar ratio of 1: (4.05–4.10)respectively at 98–100 °C and the polar nitrogen compound of triethyleneheptamethyl-pentamine in a molar ratio of organolithium initiator for active lithium: alkoxide sodium: nitrogen-containing modifier equal to 1: (0.3–1.0): (0.3–1.0) respectively.
The proposed method allows to obtain (co) polymers of butadiene with styrene with a high content of 1,2-links with a static distribution of styrene in a polymer chain, at a polymerization temperature of up to 75 °C, that is, high productivity is ensured.
The disadvantage of this method is that with the continuous method of (co)polymerization of monomers, it is difficult to maintain the ratio of lithium-organic catalyst: sodium-organic modifier, resulting in a polymer with a wide spread in the content of 1,2-links from 32 to 47% instead of 45%.
The most acceptable method of producing high-vinyl rubber according to its technical essence is the method of obtaining carbon-chain polymers with an adjustable content of 1,2-links.
Complexes of the general formula Li(R1) are used as a catalyst R2R30Me, where R1 is butadienyl or isoprenyl, n is an integer 4–20; R2 and R3 are butyl, Me is sodium or potassium, with a ratio of Me: R equal to 0.05:1.5, respectively.
Disadvantages of this method are as follows:
-sodium butoxide or potassium butoxide used for the synthesis of catalyzers is insoluble in hydrocarbon solvents and precipitates;
-when storing the prepared complex, the catalyst activity is lost.
5 Kinetic modelling method
The kinetic method of modeling polymerization processes consists in the compilation and numerical solution of kinetic equations for the concentration of all types of particles involved in the process.
The process of copolymerization of butadiene with styrene has been studied mainly experimentally. Attempts to describe the process quantitatively are few and have been made mainly to demonstrate computational methods for a simplified kinetic scheme, while physical and chemical modeling of the process of the copolymerization of butadiene with styrene in the production of synthetic rubber requires detailed consideration. In this connection, the problem of studying the process of copolymerization of butadiene with styrene by numerical methods, taking into account the mechanism of the process under consideration, is relevant today.
During the copolymerization of butadiene and styrene in the medium of aliphatic and aromatic solvents on lithium-organic catalysts, polymerization of butadiene first occurs, and then, after the complete spend of butadiene, the polymerization of styrene begins. Under the separate polymerization, styrene is more active than butadiene. During copolymerization of butadiene with styrene in a hydrocarbon medium under the action of lithium alkyls (LiR), the lithium-polybutadiene active center is more stable than lithium-polystyrene. Accordingly, the higher reactivity of butadiene during anionic coordination copolymerization with styrene may be due to the greater stability of the active center formed after the addition of butadiene to the end of the growing chain (lithium polybutadiene) compared to the active centre, in which lithium is bound to the styrene link (lithium polystyrene).
It is known that organometallic compounds exist in an associated form, but a monomeric unassociated molecule is active in polymerization reactions. Dissociation (RMe)n → nRMe can be carried out in one or more stages: its speed depends on the nature of Me (alkali metal), the structure of R, and the properties of the solvent. There is no unambiguous opinion about the nature of the monomeric RMe involved in the initiation reaction. These can be ions, ion pairs or their agglomerates depending on many factors (Fig. 3).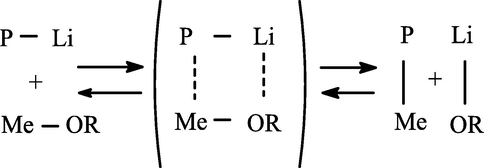
Ion pair.
Nevertheless, once formed, polystyrene carbanion interacts very quickly with butadiene, again forming polybutadiene carbanion. Thus, at the first stage of copolymerization, noticeable concentration of styrillithium is not formed, which means that the reaction rate of styrene-polystyrillithium can be neglected. This situation persists until almost all butadiene is exhausted, then the rate can be changed due to the interaction of styrene, which is in a significantly high concentration with polybutadiene lithium with the formation of polystyrene carbanion. The absorption rate of styrene increases to the usual homopolymerization rate.
Many papers present the constants of copolymerization of butadiene with styrene in the presence of lithium-organic initiators, their values vary widely.
During the transition from hydrocarbon solvents to electron-donating constants of copolymerization of butadiene and styrene noticeably converge and there is a change in the composition of copolymers both on the amount and on the type of ether added. According to the sources, the tendency to converge for the copolymerization constants increases in the series of triethylamine, diethyl ether, dioxane, tetrahydrofuran (THF). In the medium of pure tetrahydrofuran, styrene will be more active than butadiene, since in solvents with high solvating capacity due to solvation of the counterion and shielding of its field, a typically anionic process is observed, in which the active centers are ion pairs or free carbanions. With an increase in the electron-donor properties of the solvent, the proportion of vinyl 1,2-butadiene units, that is, typically anionic structures, increases in the series of amines, diethyl ether, tetrahydrofuran. The transition from polymerization on LiR to polymerization on NaR and KR in hydrocarbon medium acts in the same direction.
For the first time, polymerization initiated by lithiumalkyl in combination with alkali metal alkoxides (ROMe) was investigated by Hsich and Wofford. They showed that small additives of potassium, sodium, rubidium and cesium butylates dramatically increase the polymerization rate of butadiene and styrene, which depends on the temperature of the process, the type of monomer, the alkali metal, the molar ratio of metal butoxide: alkyllithium.
Authors supposed that as a result of the reaction of alkali metal alkoxide and alkyllithium, cross-associates are formed - complexes with special chemical properties that differ from the properties of mechanical mixtures of components. At the same time, there should be a dynamic tautomeric equilibrium between the metal–carbon and metal–acid bonds, resulting to completely different growth centers of the polymer chain than in the case of the use of a single alkyllithium. Additives of alkali metal alkoxides, along with a change in the polymerization rate, cause a change in the microstructure of polybutadiene, significantly increases (from 6 to 70%) the content of vinyl links, which depends on the temperature of limerization, the type of metal in the alkoxide and the ratio of metal alkoxide: alkyllithium.
It is more expedient to converge the copolymerization constants of butadiene and styrene by introducing Na, Rb or Cs ions into the reaction medium. Alkali metal alkoxides, along with esters, amines and other polar compounds, are effective modifiers of organo-lithium initiators. In the case of polymerization of diene monomers, a significant effect of alkali metal alkoxides on the molecular characteristics of the polybutadiens being formed was revealed. The published data show that the limiting values of the content of vinyl structures in polymers during the polymerization of dienes in carbohydrates and the use of polar compound modifiers (amines, THF, etc.) are found at the level of 40–60% by weight.
Compounds of the MeOR type, when added in small quantities to alkyllithium, provide the formation of a statistical copolymer, while the structure of the butadiene part changes slightly. The acceleration of the homopolymerization of styrene and butadiene was observed depending on the increase in the Me/Li ratio, and this acceleration is greater for styrene than for butadiene. Lithium alcoholates do not significantly change the homopolymerization rates of butadiene and styrene.
MeOR-type compounds and organolithium compounds form complexes whose exact stoichiometry is unknown. There is a tautomeric dynamic equilibrium between the C-Me and O-Me bond, which leads to completely different growth centres other than one lithium alkyl.
The flow of the process directly on the four-center active complex provides a statistical distribution of links along the chain.
Despite the effectiveness of the initiating system of lithium-organic compounds with various rendomizers and polar additives, it has a number of disadvantages: the deficiency of organolithium compounds, the complexity of sewage treatment from lithium, the toxicity of lithium.
Thus, the current level of scientific research in the field of ionic polymerization of monomers makes it possible to regulate the rate of copolymerization of dienes with styrene and the content of vinyl butadiene links by changing the polarity of the solvent and metal ions of alkoxides in the rendomizer. At the same time, there is no data on the effect of the catalyst composition and synthesis conditions on the molecular weight characteristics, the viscosity of the copolymer and the physical and mechanical properties of vulcanizates based on them.
The complex mathematical model for ion polymerization processes is based on the method of moments and calculates the degree of polymerization, average mass and average number molecular weights. The model takes into account all important reactions, such as reversible initiator bonds, chain initiation, growth, bonds of growing polymer chains, equilibrium between free ions and ion pairs, etc. An ionic kinetic scheme was used to simulate the copolymerization of styrene and butadiene in a semi-continuous reactor. The results of modeling the conversion of the monomer, the average molecular weight of the polymer, the fraction of the living polymer, copolymer and other were compared with the available literature data.
Styrene-butadiene is obtained by ion copolymerization. The ion mechanism is the best tool for the production pf polymers with a controlled microstructure. In ionic polymerization, negatively and positively charged free ions and ion pairs, which are often in equilibrium, are actively growing species. These processes are complex due to the presence of many growing particles and other complex reactions. In addition, the solvent affects not only the reaction rate, but also the molecular weight distribution (MWD). Therefore, modeling of ion polymerization is a difficult task. Styrene and butadiene can be polymerized in a wide temperature range (from room temperature to 100 °C) using the initiator of n- or tert-butyllithium.
Based on the reference data (under the assumption of instantaneous initiation) the kinetic scheme of binary copolymerization, which includes 4 growth reactions, is considered (Equation (1) and two transfer reactions to the solvent with the reinitiation (Equation (2).
Here is the i-type monomer, is the growing polymer chain with the i-type end group, is the dead polymer chain, is the number of monomer links in the chain.
Index 1 will be attributed to styrene, index 2 to butadiene in the context of the butadiene and styrene copolymerization.
According to the kinetic scheme, the time change in the total concentration of monomers
and the distribution of active and dead chains by length is described by the following equations:
In equations (3–6), is the total concentration of i-type active centres. In the derivation of equation (3), the assumption of rapid reinitiation ( ) was used.
The principle of quasi-stationarity is used for
and
:
Since there is no kinetic breakage of the chain, the total concentration of active centers is constant and equal to the initial concentration of the initiator, i.e.:
Using the equations (7) and (8) the following equation are obtained:
Equation (9) are derived under the assumption of the first order of all reactions by active centers.
Using the ratio (9), equation (3) is more convenient to rewrite in terms of conversion
:
6 Instantaneous transmission intensity
The nature of the dependence on the ratio of monomers was analyzed. Two limited cases are as follows:
-
the partial intensities of chain transfer during the homopolymerization of monomers and are equal ;
-
the polymerization of one of monomers proceeds by the mechanism of living chains ( ) and the polymerization of the second monomer is accompanied by intensive transfer. Obviously, all other cases are intermediate.
In this case,
should not depend on the composition of the monomer mixture. Anyway, the analysis of the equation (12) shows that this confirmation is true only for
(and
); for all other values of
and
the dependence
on the composition passes through a maximum or minimum, as shown in Fig. 4, at
and the extreme value
is equal as follows:
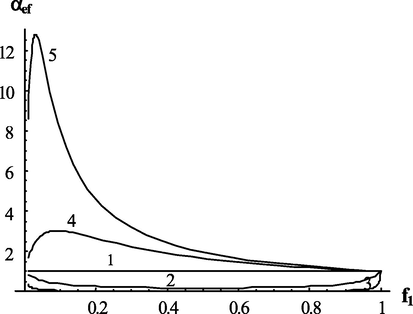
Dependence of the effective transfer intensity parameter on the composition of monomers at different values of copolymerization constants.
;
(1);
(2);
(3);
(4);
(5).
when , the effective intensity of chain transfer in the whole area of composition changes (except the extreme points) is less than of single component and when and are very small, i.e. with a high contribution of cross-growth, can be very low.
On the other hand, when , the effective intensity of chain transfer during the copolymerization will be higher than during the homopolymerization and, accordingly, the average degrees of polymerization are lower.
The dependences of the effective transfer intensity parameter on the composition of monomers and the copolymerization constant
at different copolymerization constants are shown in Figs. 5–8.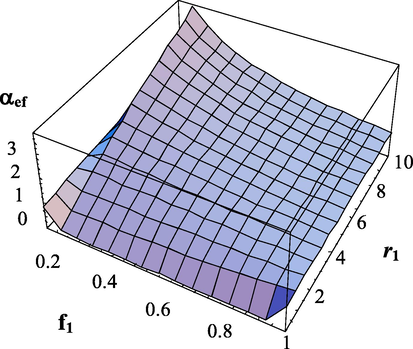
Dependence of the effective transfer intensity parameter on the composition of monomers and the copolymerization constant
.
. The copolymerization constant -
.
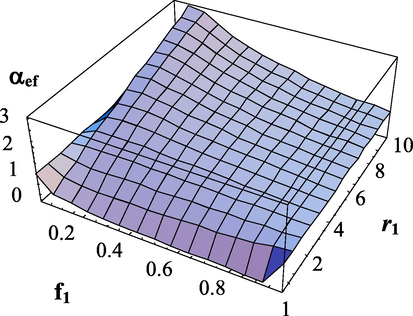
Dependence of the effective transfer intensity parameter on the composition of monomers and the copolymerization constant
.
. The copolymerization constant -
.
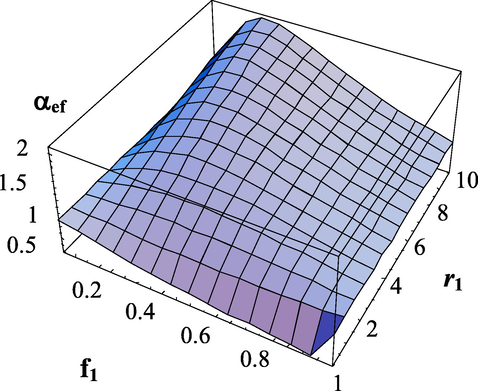
Dependence of the effective transfer intensity parameter on the composition of monomers and the copolymerization constant
.
. The copolymerization constant -
.
Dependence of the effective transfer intensity parameter on the composition of monomers and the copolymerization constant
.
. The copolymerization constant -
.
When
, the dependences
on the initial composition of monomers for this case are shown in Fig. 9. The analysis of the equation (12) shows that when
effective intensity of chain transfer monotonically decreases with an increase in the content of the non-transmitting monomer. More interesting is the case of
(curve 4 in Fig. 9), when the proportion of the non-transmitting monomer increases with an increase. Comparison with the kinetics of the process shows that the maximum of the
curve corresponds to the minimum on the velocity - composition curve.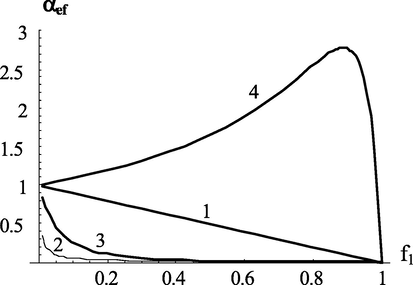
Dependence of the effective transfer intensity parameter on the composition of monomers at different values of copolymerization constants. Values of copolymerization constants:
(1);
(2);
(3);
(4).
The dependences of the effective transfer intensity parameter on the composition of monomers and the copolymerization constant
for different copolymerization constants for the case
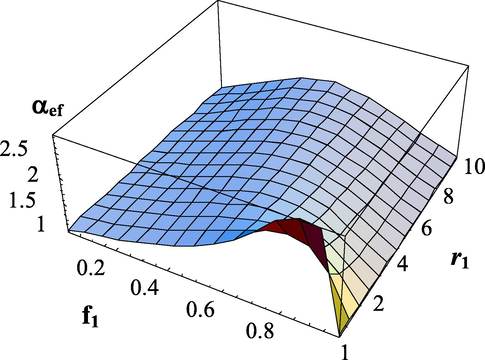
are shown in Figs. 9–13.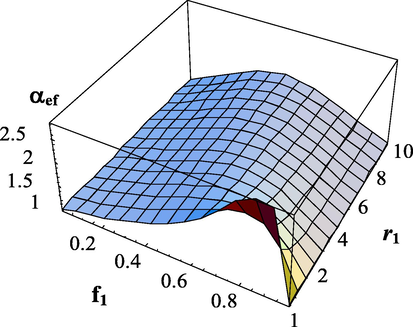
Dependence of the effective transfer intensity parameter on the composition of monomers and the copolymerization constant
.
. The copolymerization constant -
.
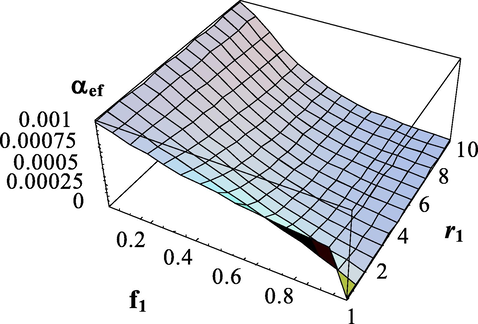
Dependence of the effective transfer intensity parameter on the composition of monomers and the copolymerization constant
.
. The copolymerization constant -
.
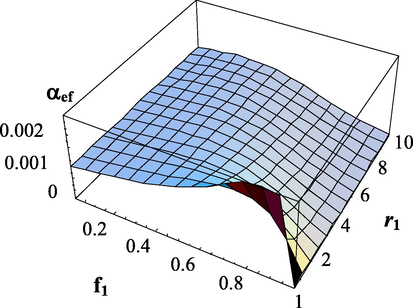
Dependence of the effective transfer intensity parameter on the composition of monomers and the copolymerization constant
.
. The copolymerization constant -
.
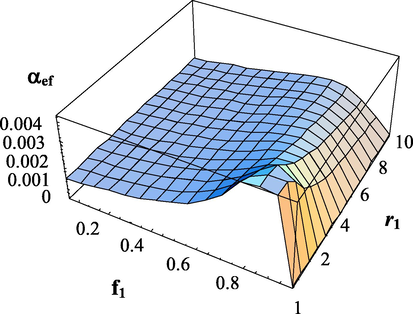
Dependence of the effective transfer intensity parameter on the composition of monomers and the copolymerization constant
.
. The copolymerization constant -
.
It is worth noting that the case when both constants exceed one is extremely rare during the copolymerization. During the polymerization under the action of an organic sodium initiator (1,1,3-triphenylpropyl sodium) in toluene medium for butadiene
, and for styrene
. Therefore, this case is close to the theoretical case, when polymerization of one of the monomers proceeds without the chain transfer
. For the initial stages of the process, when it is possible to neglect the change in the composition of the monomer mixture, as well as the consumption of the monomer in the reinitiation process, the effective constant of the first order, that is experimentally determined
, describes the change in the total concentration of monomers over time. As far as
, it means that the effective constant is as follows:
It follows from equation (10) that
. Then for
, the following equation is obtained:
Depending on the ratio between
,
and
the initial copolymerization rate can change with increasing
either monotonically, or pass through a maximum or minimum (Fig. 14). When
, the minimum is reached at
, the maximum - at
, in all other cases
monotonically changes with an increase in the proportion of the monomer polymerizing at a higher rate. Obviously, under
for all case, except for
, the change in the effective constant occurs nonmonotonically:
has maximum at
and minimum at
.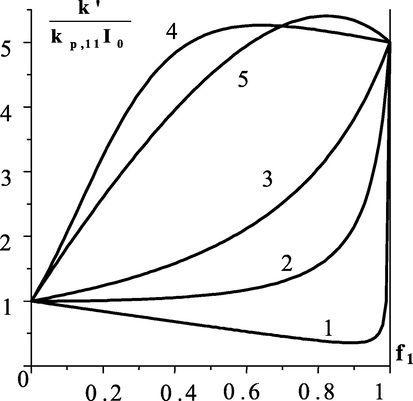
Dependence of the effective initial rate constant of the first order on the composition of the monomer mixture. For curse 1–4
. Values of copolymerization constants:
(1);
(2);
(3);
(4);
(5).
It is interesting to note that the extreme value has exactly the same form as the dependence of the value, the inverse copolymerization rate ( , on the composition.
Thus, with the same values of the absolute constants of the chain transfer rates on both types of active centers , the minimum corresponds to the maximum polymerization rate. The role of cross-growth actions are essential: the greater the contribution of these acts, the higher the copolymerization rate and the smaller the role of chain transfer to the solvent.
7 Determination of copolymerization constants
In order to determine the copolymerization constants, it is essential to carry out copolymerization of mixtures of monomers and several compositions at initial conversions (usually less than 10% to exclude the effect of changes in the composition of the reaction mixture during the reaction), isolate the formed copolymers, analyze their composition for the content of both components and .
It is important to denote the ratio of molar concentrations of comonomers in the initial mixture by , and the corresponding composition of the copolymer by . The copolymerization constants are determined after processing the experimental data in the following ways:
-
The Mayo-Lewis method
The equation of the composition of the copolymer can be written as follows:
For arbitrary values, the corresponding values are calculated for each pair of experimental values and . By constructing rectilinear dependencies on , for each pair and get a series of lines intersecting ideally at one point. According to the coordinates of the intersection point of these lines, the values and are found.
-
The Fayiman-Ross method
The Fayiman-Ross method is based on the following modification of the copolymer composition equation:
When building a dependency on the straight line is obtained from; the segment cut off on the ordinate axis gives - , and the angle of inclination - .
8 Compilation of a mathematical model of the binary copolymerization process taking into account the transfer of the chain to the transfer agent
To describe the process under consideration, the mathematical model of binary copolymerization, which includes four chain growth reactions, it is important to supplement by the chain transfer reaction to the transfer agent A:
The following system of equations is obtained:
In equations (18)-(23)
is the full concentration of active centres of i-th type. When writing equations (18)-(20), the usual assumption of rapid reinitiation (k r,i≫>≫>>k ai) was used. Using the principle of quasi-stationarity (
and the absence of kinetic breakage (when the total concentration of active centers (
) is constant and equal to the initial concentration of the initiator I 0), allows to get the following expressions for I 1 and I 2
The relations (8) are obtained under the assumption of the first order of all reactions by active centers.
By definition, the average number and average mass degrees of polymerization and the polydispersity coefficient are equal:
The zero moment MWD μ0 = μ0 + μd,0 is the total concentration of circuits (active and dead) in the system. As a result of the chain transfer reaction to the transfer agent A μ0 increases with conversion at a rate of:
The change of moleculat mixture composition with the conversion × is described by the following proportion:
For the rates of change μ1 and μ2, the equations are obtained:
As it is known, μ1 is the concentration of the formed copolymer (μ1≈M0 x).
The numbers of styrene and butadiene links per one copolymer molecule on average were determined by the formulas:
The average number molecular weight and mass fraction of styrene in the copolymer were calculated using the formulas:
9 Identification of mathematical model parameters (or identification of kinetic constants and adequacy assessment of mathematical model)
Experiments were carried out to identify the kinetic constants of the polymerization process model. The relative deviations of the experimental indicators y(e) from the calculated y(c) were estimated: where i is the number of the observed indicator. , where i is the number of the observed indicator. At the same time, an experimental data set was used . Model identification was performed by varying the parameters . Parameters of periodic process model according to the experimental data obtained the following values: ; ; ; ; ; .
Comparison of calculated and experimental data showed that the mathematical model adequately describes the process. The calculation results are presented in Table 3. Experimental value of average calculated molecular weights of copolymers
, obtained with different initial compositions of monomers that are presented in Table 3.
I0, mmol/l
S, mmol/l
M0, mmol/l
f10
10-3
10-3
exp.
calc.
exp.
calc.
5.85
6.53
2.66
0
0.34
0.34
0.72
0.66
2.21
5.76
3.11
0.17
0.50
0.51
0.9
1.1
2.50
6.01
2.52
0.22
0.70
0.48
0.8
0.8
2.13
5.77
2.84
0.50
1.50
1.09
2.50
2.9
2.16
5.81
2.92
0.68
3.70
3.47
5.70
4.74
2.90
7.80
0.79
1.00
16.2
16.2
25.2
25.0
The values of the average calculated molecular weights calculated using the equations (26)-(27) are also given there. The calculations took into account the contribution of the solvent molecule residue to the molecular weight of macromolecules. The experimentally determined average calculated molecular weight at full conversion is related to the calculated : , where is molecular weight of i-type monomer, is molecular weight of the solvent fragment (toluene). This correction is most significant when the styrene content is low, when the average polymerization degree is small.
In this case, a more visual comparison in the form of a graph is impossible, since the experimental values in different experiments were obtained at different ratios of and the initial compositions of the monomer mixture.
The initial concentrations of monomers M0 are calculated from experimental data (1.51 mol/l) and initiator , , .
According to the conversion curve of homopolymerization of butadiene in hexane in the presence of the considered catalytic system, it was found that k p22 I 0 = 0.034 min−1 (Fig. 17), →k p22 = 51.0128 l/(mol min).
There are no experimental data on the polymerization of styrene in hexane in the presence of the studied catalytic system, therefore, was given in numerical calculations using , where a is the desired parameter.
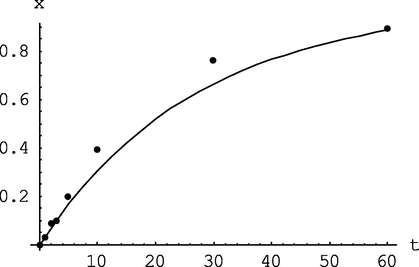
The calculation results are shown in Figs. 15-19.![Dependence of butadiene conversion on polymerization time for two experiments: points - experimental data (mod/n-BuLi = 1, T = 50 °C); dotted line- calculation by the formula x = 1-exp[-(kpi) t] (w p = kpI = 0.034 min−1 and w p = 0.03 min−1).](/content/184/2023/16/11/img/10.1016_j.arabjc.2023.105205-fig16.png)
Dependence of butadiene conversion on polymerization time for two experiments: points - experimental data (mod/n-BuLi = 1, T = 50 °C); dotted line- calculation by the formula x = 1-exp[-(kpi) t] (w p = kpI = 0.034 min−1 and w p = 0.03 min−1).
Change of monomer conversion with time of copolymerization process: points – experimental data (mod/n-BuLi = 1, T = 50 °C), curve – calculation model at I0 = 1.333 10-3mol/l, M0 = 1.51 mol/l (error Δ1 = 0.197, Δ 2 = 0.217, Δ 3 = 0.038, Δ 4 = 0.165, Δ 5 = 0.216, Δ 6 = 0.127, Δ 7 = 0.005; ψx = 0.138).
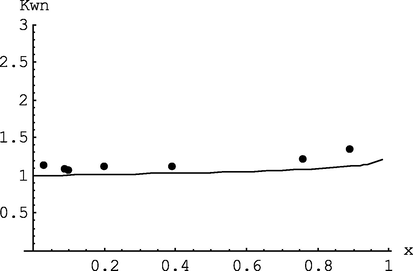
Change in the coefficient of polydispersity with conversion of monomers: dots – experimental data (mod/n-BuLi = 1, T = 50 °C), curve – calculation model at I0 = 1.333 10-3mol/l, M0 = 1.51 mol/l (error Δ 1 = 0.113, Δ 2 = 0.069, Δ 3 = 0.051, Δ 4 = 0.096, Δ 5 = 0.083, Δ 6 = 0.117, Δ 7 = 0.166; ψwn = 0.099).
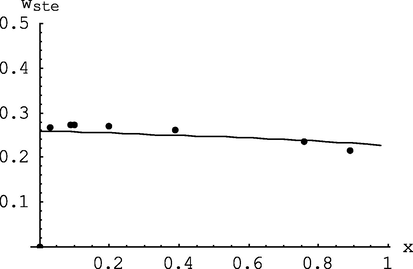
Change in the proportion of styrene in the copolymer with monomer conversion: points – experimental data (mod/n-BuLi = 1, T = 50 °C), curve – calculation model at I0 = 1.333 ·10-3mol/l, M0 = 1.51 mol/l (error Δ 1 = 0.033, Δ 2 = 0.059, Δ 3 = 0.056, Δ 4 = 0.054, Δ 5 = 0.040, Δ 6 = 0.013, Δ 7 = 0.076; ψws = 0.047).
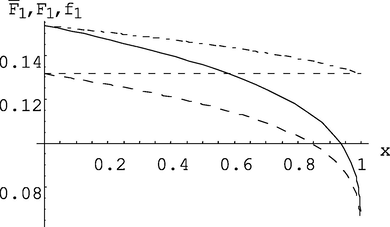
Dependence of the composition of the monomer mixture f1 (dotted line), instantaneous F1 (solid line) and average
(dash-dotted line) of the compositions of the copolymer from the conversion (calculation model at I0 = 1.333·10-3mol/l, M0 = 1.51 mol/l).
The proposed model with the chain growth and transfer constants satisfactorily describes the available experimental data when the initial mole fraction of styrene in the monomer mixture changes from 0.0615 to 0.261, while the efficiency of the catalytic system varies from 0.5 to 0.65, which may be due to different initiation rates of styrene and butadiene active centers.
10 Conclusion
During the copolymerization process in hydrocarbon solvents on organolithium compounds without the introduction of an electron donor, the reactivity of butadiene is higher than styrene, so the copolymer is enriched mainly with diene. During the formation of the active center, the conformation of the monomer molecule is fixed by the formation of πan allyl complex, which ensures the stereospecificity of the addition of the monomer to the negatively polarized end of the chain (anionic coordination polymerization process). With the introduction of a small amount of electron donor, the nature of the process changes due to the solvation of the counterion; the reactivity of styrene becomes higher than butadiene; the copolymerization process becomes anionic. With an increase in the electron donor content, the speed of the copolymerization process increases. Thus, it is known from reference that for the copolymerization of styrene and butadiene on similar catalytic systems Copolymerization constants for the considered polymerization system are determined by the Mayo-Lewis method , . When calculating the cross-growth rate constants were determined by the formulas , . The chain transfer rate constants are expressed as fractions of the corresponding chain growth rate constants: , .
The parameters of the model a, , were determined from numerical experiments. As a criterion for the correspondence of the calculated and experimental values of the copolymer characteristics, a system of inequalities was used, which assumes a change in the experimental data within the limits of the error of their measurements.
Modeling of the SSBR rubber synthesis process is carried out:
-
a mathematical model of the periodic isothermal process of copolymerization of styrene and butadiene in an aliphatic solvent on an n-BuLi/mod catalytic system has been compiled, including four chain growth reactions, two chain transfer reactions to a transfer agent whose concentration is recalculated;
-
the copolymerization constants of styrene and butadiene in hexane in the presence of the tested catalytic system are calculated;
-
the parameters of the obtained mathematical model are identified.
The results were obtained using modern methods of mathematical modeling of polymerization processes.
The reliability of the obtained results is confirmed by their consistency with the main theoretical concepts in the field of anionic copolymerization.
Based on mathematical modeling, laboratory experiments and optimization of the copolymerization process, the possibility of directed synthesis of functionalized SSBR rubber with the required physical and mechanical characteristics has appeared.
This study was carried out using the equipment of the Center for Collective Use “Nanomaterials and Nanotechnology” of the Kazan National Research Technological University, Russian Federation.
Acknowledgement
This paper has been supported by the Kazan Federal University Strategic Academic Leadership Program (“PRIORITY-2030”), Russian Federation.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Akhmetov, I.G. Kinetics of polymerization and the molecular characteristics of lithium polybutadiene impact modifier concentration [Text] / I.G. Akhmetov // Rubber. - 2010. - ʋ 4. - P. 2-5.
- Aminova, G.A., Manuiko, G.V., Bronskaya, V.V., Ignashina T.V.a, D'Yakonov, G.S., Bashkirov, D.V. Controlling the characteristics of butadiene rubber on the basis of molecular weight distribution. Theoretical Foundations of Chemical Engineering 2009, 43(2), pp. 206–211.
- Beneficial use of thermal secondary energy resources in the rectification cycle at ethylene glycol production unit. IOP Conf. Ser.: Mater. Sci. Eng.. 2020;919(6):062027
- [Google Scholar]
- Analysis of the possibility of modernization of the state district power station by building the combined cycle plant. J. Phys. Conf. Ser.. 2020;1515(4):042100
- [Google Scholar]
- Cooperman, F.E. Effect of 1,2- butadiene in the BSC on the properties of rubber [Text] / F.E. Cooperman // Rubber. - 1994. - ʋ 2. - P. 8-12.
- Cooperman, F.E. Status and prospects of work on the new rubber for tires [Text] / F.E. Cooperman // Production and use of elastomers. - 1997. - ʋ 10, 11. - P. 5-19.
- Dolgoplosk, B.A. Organometallic catalysis in polymerization processes [Text] / B.A. Dolgoplosk, E.I. Tinyakova. – M.: Nauka, 1982. - 511 p.
- Dzhibera, S.J. Technology and sketches the future of our polymers. Paper presented at the 38th meeting of the Board of Directors IISRP [Text] / C.J. Dzhibera. - Goodyear Tire and Rubber Co., 1997. - 8.
- Erusalimskiy, B.A. The processes of anion - polymerisation [Text] / B.A. Erusalimskiy, S.G. Lyubetsky. - L : Himiya, 1974. - 140 p.
- Firsova A.V., Karmanova O.V., Glukhovskoy V.S., Zemsky D.N. Study of the influence of mixed alcoholates of hydroxypropylated aromatic secondary amines on the structure of dienes. Vestnik VGUIT 2014, 4, 147-150.
- Modeling of copolymerization of butadiene and isoprene on a Ziegler-Natt catalyst. Bull. SSTU. 2012;1:66-69.
- [Google Scholar]
- Glukhovskoy, V.S. The technology of the dispersion of anionic polymerization of styrene and synthesis of anionic catalysts [Text ]: abstr. dis. ... PhD. - Voronezh, 1986. - 28 p.
- Hsieh, L., Quirk, P. Anionic Polymerization. Princip lesand Practical Applations; Marcel Dekker Inc.: New York, 1996, 37 p.
- Calculation of permissible monomer conversion in cascade polymerizers during the synthesis of general-purpose rubbers. IOP Conf. Ser.: Mater. Sci. Eng.. 2020;734(1):012203
- [Google Scholar]
- Jose ́. A. Tenorio Lopez, Juan. J. Benvenuta Tapia, Marı́a del Carmen Cuevas Dı́az, Kinetics Approximation Considering Different Reactivities of the Structural Units Formed by the Anionic Copolymerization of 1,3-Butadiene and Styrene Using Al/Li/Ba as Initiator. Macromol. React. Eng. 2009, 3, pp. 473–485.
- Kirchevskaya, I.Y. New ways of obtaining and use of polymers and latexes [Text] / I.Y. Kirchevskaya, N.P. Poluektova. – M.: CSRNeftehim. - 78 p.
- Korotkov, A.A. The catalytic polymerization of vinyl monomers [Text] / A.A. Korotkov, A.F. Podolsky. – L.: Nauka, 1973. -159 p.
- Kovtunenko, L.V. Copolymers of butadiene with styrene , obtained by anionic polymerization [Text] / L.V. Kovtunenko // Production and use of elastomers: IP. - 1991. - ʋ 6. - P. 15-18.
- Kovtunenko, L.V. Mortar properties of styrene-butadiene rubber SBR-type [Text] / L.V. Kovtunenko // Abstracts of the Scientific and Technical Symposium of the international exhibition “Chemistry 92”, Moscow, 15-23 September 1992. – M.: CSRITEneftehim, 1992. – P. 14-15.
- Kovtunenko, L.V The study of statistical styrene-butadiene rubber [Text] / L.V. Kovtunenko // Proceedings of the Eighth Scientific Conference “Rubber Industry”, Moscow, 14-18 May 2001. - P. 60-61.
- Kustov, L.M. “Green Chemistry”- a new way of thinking [Text] / L.M. Kustov, I.P. Beletskaya // Russian Journal of Chemistry. - 2004. - ʋ 6. - P. 3-7.
- Self-organizing systems poly-N-vinylpyrrolidone - sulfanol in dilute aqueous solutions. Bull. VSU, Ser.: Chem. Biol. Pharm.. 2015;1:10-15.
- [Google Scholar]
- Kinetic heterogeneity of neodymium catalyst systems modified with methylaluminoxane. Theor. Found. Chem. Eng.. 2015;49(3):246-251.
- [Google Scholar]
- Creating molecular weight distribution of butadiene rubber on neodymium-based catalytic system. IOP Conf. Ser.: Mater. Sci. Eng.. 2020;734(1):012066
- [Google Scholar]
- Estimation of kinetic parameters for the polar-modified anionic solution copolymerization of 1,3-butadiene and styrene. Macromol. Symp.. 2007;259:102-109.
- [Google Scholar]
- Polymerization reactor modeling in industry. Macromol. React. Eng.. 2011;5:261-277.
- [Google Scholar]
- Pat. 2175329 RF: IPC C 08 F 36 / 04. A method for producing diene (co) polymers with a high content of 1,2 -polybutadiene [Text] / V.S. Glukhovskoy. - Appl. 25.01.2001, publ. 27.10.2001, Bull. ʋ 30.
- Pliushchev, V.V., Kharitonova, O.S., Nazarova, M.A. Bronskaya V.V.a,Manuyko G.V.a, Shaikhetdinova R.S.a, .Aminova, G.A., Balzamov, D.S. Heat transfer efficiency in PEBC synthesis. IOP Conference Series: Materials Science and Engineering 2020, 791(1), 012071.
- Effects of 1,2-butadiene on the anionic copolymerization of styrene and 1,3-butadiene in the presence of polar additives. Inst. Mater. Technol. 2007:1021-1027.
- [Google Scholar]
- Shakunova, N.E. Modifiers lithium- organic catalysts for styrene-butadiene rubber [Text] / N.E. Shakunova // Rubber. - 1991. - ʋ 12. - P. 14.
- Synthetic rubber and chemicals for their production. Product Overview [Text] / Avenue Bayer, 1998. - 20 p.
- Tavtorkin, A.N., Gavrilenko, I.F., Kostitsyna, N.N., Korchagina, S.A.,Chinova, M.S., Shlyakhtin, A.V., Nifant’ev, I.E., Vagizov, A.M., Khusainova, G.R. Synthesis of Diene–Styrene (Co)polymers Modified by Myrcene-Based Diene Polar Monomers Polymer Science - Series B 2019, 61, 1-7.
- Modern technologies of anionic polymerization. Bull. Voronezh State Univ. Eng. Technol.. 2013;57:143-157.
- [Google Scholar]
- Copolymerization of 1,3-butadiene and styrene in the presence of an initiating system based on n-butyllithium, amine-containing modifier, and 2,2'-ditetrahydrofurylpropane. Russ. J. Appl. Chem.. 2017;90(1):63-69.
- [Google Scholar]
- Peculiarities of the anionic copolymerization of styrene and dienes in non-polar solvents with Li+ as counter-ion mvb. Macromol. Symp.. 2011;308:12-16.
- [Google Scholar]







