Translate this page into:
Corrosion behavior of 7B04 aluminum alloy under the synergistic effect of Lacticaseibacillus paracasei and Acinetobacter lwoffi
⁎Corresponding author. wjf725@sina.cn (Weijie Fan), zhaoxiaodong23@163.com (Xiaodong Zhao)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
Microbially influenced corrosion (MIC) is a multifaceted phenomenon that is influenced by the synergistic activity of various bacterial species that thrive within biofilms. The present study aims to investigate the impact of interspecies relationships on the corrosion behavior of aluminum alloy (Al alloy). To achieve this, cultures of Lacticaseibacillus paracasei (L. paracasei) and Acinetobacter lwoffi (A. lwoffi) were mixed and analyzed. The electrochemical experimental findings indicated that the 7B04 Al alloy exhibited the lowest Rct value and the highest corrosion current density when immersed in the mixed system. The utilization of surface analysis techniques, such as scanning electron microscopy and stereomicroscope, had led to the discovery of a significant number of corrosion pits in the mixed system. This finding suggested that the corrosion phenomenon was more severe than previously anticipated. The growth curve analysis revealed a synergistic interaction among the mixed bacterial species. The co-cultivation of A. lwoffi and L. paracasei resulted in increased growth activity of L. paracasei in comparison to its growth in a single system. The occurrence of dominant colonies and the development of biofilms were identified as the primary factors contributing to localized corrosion. Furthermore, the combined action of the two bacterial strains exhibited a synergistic effect that exacerbated the corrosion process.
Keywords
Microbially influenced corrosion(MIC)
Lacticaseibacillus paracasei
Acinetobacter lwoffi
Synergistic effect
Biofilm
1 Introduction
Corrosion is a pervasive issue that poses significant economic consequences across various industries (Sabel and Victor, 2015; Xu et al., 2013). According to estimates, the economic impact of corrosion is significant, ranging from 1% to 4% of the gross domestic product of developed nations. MIC has been identified as a significant contributor to the failure of metal structures (Guan et al., 2020; Qian et al., 2021). This phenomenon is responsible for an estimated annual global economic loss of 30–50 billion dollars, which accounts for approximately 20% of the total corrosion losses (Kong et al., 2018).
MIC is a complex electrochemical reaction process, whereby the metabolic activities of microorganisms can significantly influence the cathodic or anodic process of corrosion reaction, leading to alterations in the corrosion rate and type (Marciales et al., 2019; Li et al., 2018). Numerous studies have demonstrated that a multitude of microorganisms play a role in the process of metal corrosion and exert an influence on its behavior (Liu et al., 2015; Jia et al., 2017a, 2017b; Liu and Cheng, 2017). Bacteria constitute the predominant type of microorganisms in MIC (Moradi, Yang, Xu, Song, and Wang, 2021) and microbial growth occurs in significant quantities on metal surfaces under both aerobic and anaerobic conditions, resulting in the formation of biofilms. These biofilms have a dual effect on the corrosion rate, either promoting or inhibiting it (Li et al., 2019a, 2019b; Qu et al., 2019).
MIC is commonly attributed to the synergistic activity of multiple microorganisms, and the biofilm that forms on the metal surface is typically comprised of adherent cells and extracellular polymeric substances (EPS). In laboratory investigations, the impact of individual bacterial species on metal corrosion behavior is examined through the use of pure bacterial cultures (Guan et al., 2020; Jirón-Lazos et al., 2018; Wang et al., 2019). The limitations of such studies lie in their inability to fully capture the intricacies of the natural environment, thereby hindering a comprehensive comprehension of the mechanism of MIC in bacterial environments that are mixed. Various bacterial classes present diverse interactions with one another and elicit distinct effects on corrosion. The corrosion process of metals is significantly influenced by the collective activities of diverse microorganisms within the biofilm, including synergistic or competitive interactions (Guo et al., 2021; Qin et al., 2021). Masoumeh Moradi's research findings reported that the interaction between Vibrio azureus and Jeotgalibacillus alkaliphilus resulted in a favorable outcome for the growth of Vibrio azureus. This interaction led to a significant increase in the growth rate and biomass of Vibrio azureus, ultimately resulting in more severe metal corrosion (Moradi et al., 2021). The study highlights the importance of understanding the complex microbial interactions in metal corrosion processes. In their study, Lv and Liu conducted an investigation into the interaction between sulfate-reducing bacteria (SRB) and iron-oxidizing bacteria (IOB). The findings of their research indicated that a synergistic effect was observed, which ultimately led to the promotion of localized corrosion of X65 steel. This effect was attributed to the alteration of the dominant bacteria and biofilm structure over time, as reported in their previous works (Lv et al., 2019; Du and Li, 2022). The aforementioned findings underscored the significance of exploring the interplay among diverse bacterial species in heterogeneous bacterial habitats to enhance comprehension of the mechanism of MIC.
The primary corrosive microorganisms, namely SRB and IOB, are widely recognized in the literature. However, there are other bacterial species, such as nitrate-reducing bacteria (Jia et al., 2017a, 2017b) and acid-producing bacteria (Dai et al., 2016; Guan et al., 2017; Zhang et al., 2020), that.have been found to contribute to corrosion through extracellular electron transfer (EET) or the production of corrosive metabolic products. While research on the effects of mixed bacterial strains has predominantly centered on the impact of marine environments on steel structures, there has been a dearth of studies on the effects of these strains on aluminum alloy (Al alloy). The use of Al alloy as the primary material in aircraft fuel systems makes the issue of MIC particularly concerning, as it poses a significant threat to the proper functioning of the fuel system (Brown et al., 2010; Passman, 2013).
The present study endeavors to examine the interplay between L. paracasei and A. lwoffi, which were extracted from the corrosion products of the fuel system. The study aims to investigate the impact of these microorganisms on the corrosion behavior of 7B04 Al alloy. Aerobic bacterium A. lwoffi has been found to exhibit denitrification activity under anaerobic conditions (Ren et al., 2014; Zhou et al., 2022). On the other hand, L. paracasei is known to be an acid-producing bacterium (Tian et al., 2021). The assessment of bacterial growth status involves the utilization of growth curves. Electrochemical impedance spectroscopy (EIS) and polarization curves are commonly employed techniques for acquiring electrochemical data in various fields of research. The surface morphology and composition of the Al alloy are analyzed using various techniques including scanning electron microscopy (SEM), energy dispersive spectroscopy (EDS), stereomicroscopy, and X-ray diffraction (XRD). The findings of this investigation offer a theoretical foundation for comprehending the corrosion mechanisms of mixed bacterial systems on 7B04 Al alloy, a crucial material employed in aircraft fuel systems. By acquiring a more comprehensive comprehension of the MIC mechanism in aluminum alloys, we can avert and manage corrosion in aircraft fuel systems, thereby guaranteeing the secure operation of aircraft.
2 Material and methods
2.1 Materials
The samples used in this experiment were cut from 7B04 Al alloy plates with a chemical composition of Ni (<0.1%), Ti (<0.05%), Cr (0.1–0.25%), Si (0.1%), Cu (1.4–2.0%), Zn (5.0–6.5%), Mg (1.8–2.8%), Mn (0.20–0.60%), Fe (0.05–0.25%), and Al balance. The specimens underwent processing to form square samples measuring 10 mm × 10 mm × 2 mm. Subsequently, they were subjected to mechanical polishing using silicon carbide paper with grit sizes of 800, 1200, and 2000 to achieve a smooth surface. Following the polishing process, the samples underwent a washing procedure utilizing distilled water and anhydrous ethanol, and were subsequently subjected to drying in a drying oven. In order to conduct electrochemical testing, the sample designated as the working electrode was affixed to a copper wire and subsequently embedded in epoxy resin. This process resulted in the exposure of a working area measuring 1 cm2. The sample was subsequently subjected to a 30-minute UV light exposure prior to testing.
2.2 Culture environment
The cultural environment utilized in this study involved the cultivation of purified bacteria in Luria-Bertani (LB) liquid medium. The LB medium was prepared by combining beef extract, tryptone, sodium chloride, and deionized water. The pH of the medium was adjusted to a range of 7.2–7.4 prior to sterilization in a sterilization pot at 121 °C for 30 min. After sterilization, the LB medium was transferred to a sterile operation table for natural cooling. In the bacterial system, the bacterial solution was added at a volume ratio of 1:200 under sterile conditions. The mixed system consisted of two bacterial strains added in a 1:1 proportion. The bacterial culture was incubated in a shaker at a temperature of 30 °C and a speed of 150 rpm.
2.3 Growth curve and pH measurement
The experimental material used in this study was sterilized 7B04 Al alloy. Four experimental systems were employed, namely L. paracasei, A. lwoffi, mixed system, and sterile system. Daily plate counting and pH measurements were performed, and the mean value of the replicated groups was considered as the bacterial count and pH value of the solution for the corresponding period. The growth curve was established through a mixed plate counting technique, involving serial dilutions (10-3, 10-4, 10-5, 10-6, 10-7, 10-8) and three replicates for each dilution. Various dilutions were introduced into the culture dish, followed by the addition of solid culture medium which was meticulously blended with the diluent. Following the solidification process, the culture dish was inverted and placed in a constant temperature incubator for a duration of 48 h prior to enumeration.
2.4 Surface analysis
The experimental pieces made of 7B04 aluminum alloy were subjected to immersion in four different systems for a period of 23 days, and subsequently characterized. Before SEM and EDS analysis, the samples were immersed in a 2.5% glutaraldehyde solution for 1–2 h and then dehydrated using anhydrous ethanol. The morphology of corrosion was observed through the utilization of a stereomicroscope. X-ray diffraction (XRD) was employed to analyze the corrosion products present on the surface of the sample.
2.5 Electrochemical experiments
In order to investigate the corrosion behavior of the Al alloy, various electrochemical techniques were employed. Specifically, the open circuit potential (OCP), electrochemical impedance spectroscopy (EIS), and polarization curve were measured using a PARSTAT2273 electrochemical workstation (USA PARSTAT2273). A three-electrode configuration was employed in the experiment, consisting of a saturated KCl calomel electrode as the reference electrode, a Pt electrode as the auxiliary electrode, and the 7B04 Al alloy as the working electrode. EIS was obtained under stable OCP by applying an alternating current (AC) voltage of 10 mV within the frequency range of 105 to 10-2 Hz. The impedance data was analyzed utilizing the ZSimpWin software, whereby the suitable equivalent circuit structure was chosen and the corresponding parameters were set accordingly. The polarization curve measurement was conducted with a scanning rate of 0.333 mV/s, scanning the potential relative to OCP from −350 mV to + 350 mV, and subsequently analyzed.
3 Results
3.1 Growth curve and pH test results
In order to examine the effects of various bacteria, growth curve and pH assessments are carried out at multiple time points, and the resulting data are displayed in Fig. 1. The pH values of the four systems showed an increase over time, which can be attributed to the cathodic oxygen absorption reaction. This reaction resulted in the consumption of oxygen and the production of OH– ions. The pH values, arranged in descending order, were as follows: A. lwoffi system, sterile system, mixed system, and L. paracasei system. A. lwoffi and L. paracasei are two bacterial strains that present different metabolic activities under anaerobic conditions. Specifically, A. lwoffi is capable of denitrification, which results in the production of OH–. In contrast, L. paracasei produces acidic metabolites, leading to a decrease in pH. In the mixed system, the consumption of oxygen by A. lwoffi created a more favorable living environment for L. paracasei, leading to an increase in metabolic activity. The verification of the conclusion of the mixed system was conducted through the analysis of the growth curve presented in Fig. 2.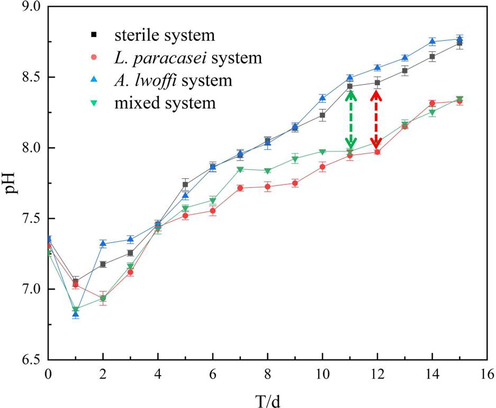
The pH value in sterile, L. paracasei, A. lwoff and mixed systems in 15 days.
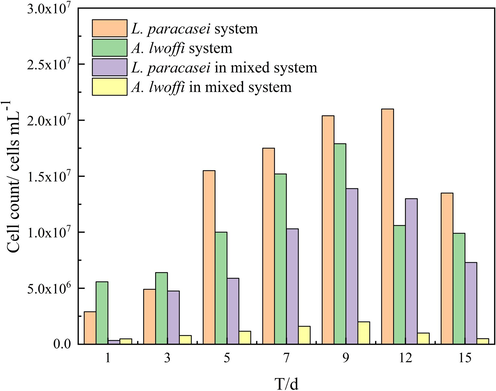
The cell counts of L. paracasei and A. lwoff in the biofilm under different culture conditions.
There are no interspecific factors present in the single bacterial system being studied. During the initial three-day period characterized by relatively sufficient oxygen levels, A. lwoffi exhibited a greater rate of growth in comparison to L. paracasei. As the consumption of oxygen persisted, the activity of L. paracasei escalated, leading to a significant increase in its population. The peak of A. lwoffi was observed on the ninth day, whereas L. paracasei reached its peak on the twelfth day.
The mixed bacterial system exhibited comparable space and nutrient quality to the other systems. However, the presence of multiple bacterial species resulted in a smaller peak value compared to the single bacterial system. This suggests that the interaction between bacterial species may have a significant impact on the overall performance of the system. Through observation of the relative quantity and peak time of the mixed system, it was determined that the population of A. lwoffi exceeded that of L. paracasei during the initial phase of the experiment. This can be attributed to differences in oxygen content. The utilization and reduction of oxygen content by A. lwoffi resulted in the creation of a favorable environment for the growth of L. paracasei, leading to a rapid increase in its quantity. This observation highlights the potential interplay between microbial species in complex ecosystems. Furthermore, it was observed that in the mixed system, L. paracasei reached its maximum on the 9th day, providing evidence to support the hypothesis that A. lwoffi facilitated the growth of L. paracasei. Upon comparison of Fig. 1 and Fig. 2, it was seen that the apex of L. paracasei in both the single and mixed systems was attained when the pH differential with the sterile system was at its maximum. This finding elucidates the influence of bacterial metabolic activity on pH.
3.2 OCP results
Open circuit potential is used to preliminarily determine the corrosion trend and potential state of aluminum alloys. The analysis of OCP was performed on the sterile system, as well as on systems containing L. paracasei, A. lwoffi, and mixed cultures (See Fig. 3). The OCP movement signifies a shift in both the corrosion tendency and potential state, as indicated by previous studies (Lv et al., 2019; Yang et al., 2020). Over the course of time, the OCP underwent changes across the four systems. The sterile system, however, presented minimal changes and maintained a potential range of −800 to −900 mV. The bacterial systems exhibited a more pronounced decrease in potential in comparison to the sterile system. The mixed system exhibited the lowest potential, measuring at approximately −1100 mV, when compared to the other systems. The decrease in the OCP observed in the mixed system indicates a higher number and greater activity of bacteria on the surface of the sample. When the OCP was more negative, the corrosion rate of the metal was higher, indicating that microbial corrosion was more severe. In general, local corrosion caused by microorganisms, such as pitting, was more likely to occur. The negative value of OCP reflected the electrochemical activity between the metal and the electrolyte solution. The metal surface was more susceptible to electrochemical reactions, which accelerated the occurrence of microbial corrosion. This suggests that the corrosion state of the aluminum alloy was more severe in the mixed system (Lv et al., 2022). The alteration of metal surface states and the regulation of corrosion through anodic and cathodic reactions are influenced by the metabolic activity and biofilm formation of bacteria. Subsequent investigations will be carried out to comprehensively examine these reactions.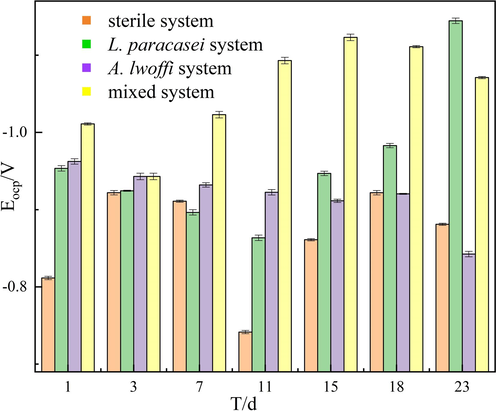
Variations of OCP with time for 7B04 Al alloy in different cultural systems.
3.3 EIS results
Fig. 4 illustrates the EIS results obtained over a period of 23 days across four distinct systems. The Nyquist plot of the sterile system presented a clear oxygen diffusion phenomenon, whereas the capacitance arc shape of the single-bacteria system was semi-circular. Moreover, the mixed system showed the emergence of two arcs, indicating the development of a double-layer film. The magnitude of the impedance value was represented by the radius of the capacitance arc, with a smaller impedance value demonstrating poorer corrosion resistance of the Al alloy (Huang et al., 2016; Zhou et al., 2020). The initial comparison revealed that the level of corrosion was most severe in the mixed system.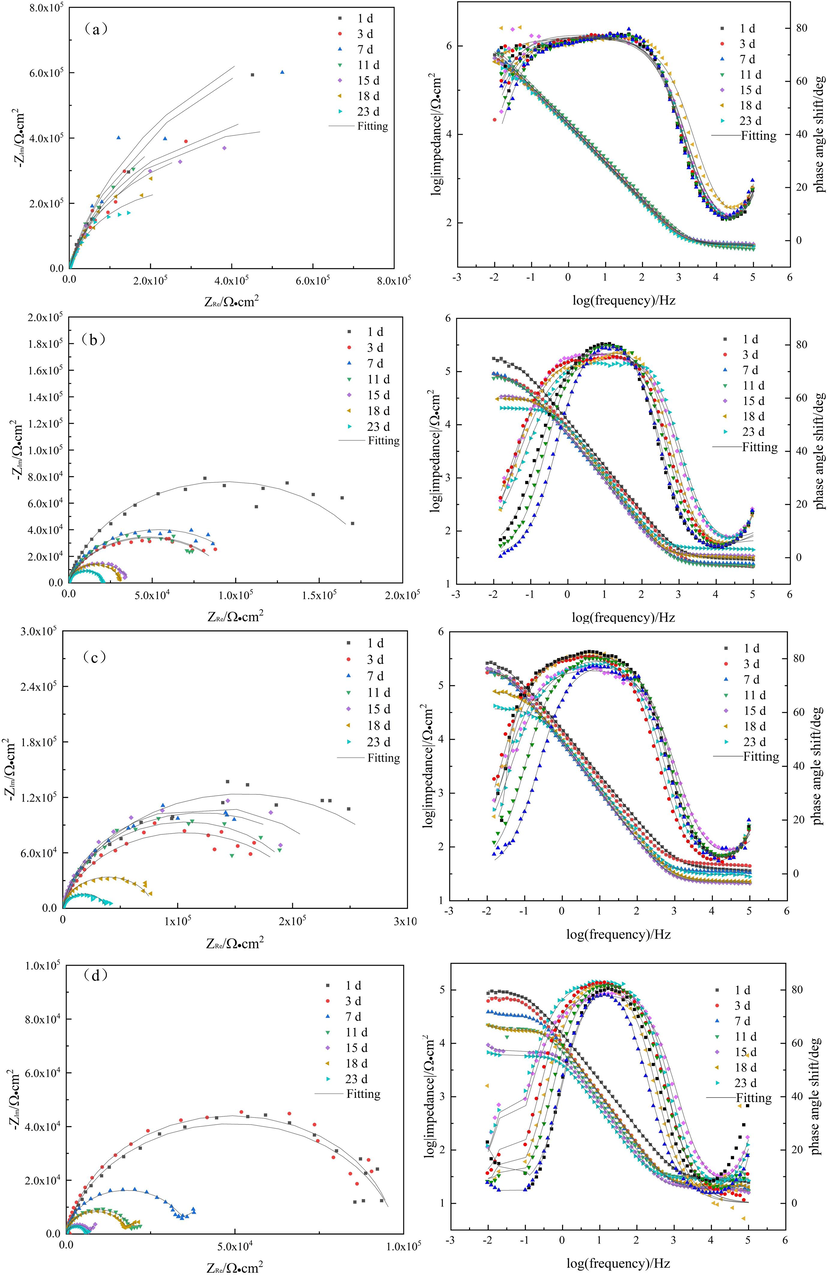
Electrochemical impedance spectroscopy of 7B04 Al alloy in(a) sterile, (b) L. paracasei, (c) A. lwoffi and (d) mixed systems.
The circuit diagram shown in Fig. 5(a) was used to fit the sterile, L. paracasei, A. lwoffi systems and the 1st and 3rd day of the mixed system. On the other hand, the circuit diagram depicted in Fig. 5(b) was employed for fitting the remaining days of the mixed system. The fitting data for the aforementioned systems is presented in Table 1. In the circuit used for fitting, the symbol Rs denoted the resistance of the solution, Rf referred to the resistance of the corrosion product film and biofilm present on the Al alloy, and Rct represented the charge transfer resistance. The capacitance of the surface film of the Al alloy was denoted as Qf, while the double-layer capacitance at the Al alloy/liquid interface was represented by Qdl (Paul and Yadav, 2020). paracasei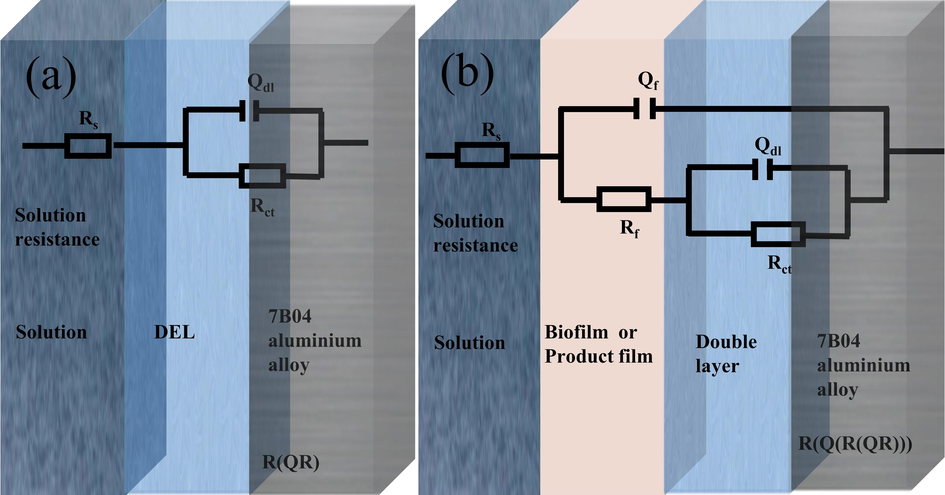
The equivalent electrical circuit used to fit the EIS experimental data for 7B04 aluminium alloy immersed in different culture systems.
system
T/d
Rs (Ω·cm2)
Qdl (F·cm−2)
Rct (Ω·cm2)
Qf (F·cm−2)
Rf (Ω·cm2)
sterile
1
31.04
1.27 × 10−5
1.86 × 106
3
31.72
1.24 × 10−5
1.42 × 106
7
34.04
1.21 × 10−5
1.64 × 106
11
29.14
1.07 × 10−5
5.32 × 106
15
33.18
1.36 × 10−5
1.16 × 106
18
30.81
1.34 × 10−5
9.02 × 105
23
30.98
1.41 × 10−5
6.83 × 105
1
23.01
1.68 × 10−5
1.88 × 105
3
21.22
2.29 × 10−5
9.95 × 104
7
13.01
2.33 × 10−5
9.85 × 104
11
21.80
2.98 × 10−5
8.58 × 104
15
23.08
3.62 × 10−5
3.45 × 104
18
24.45
2.01 × 10−5
3.13 × 104
23
33.30
1.72 × 10−5
2.03 × 104
A. lwoffi
1
37.86
1.36 × 10−5
3.15 × 105
3
46.76
1.54 × 10−5
2.23 × 105
7
35.01
2.35 × 10−5
2.41 × 105
11
22.43
2.06 × 10−5
2.15 × 105
15
21.68
2.08 × 10−5
2.57 × 105
18
23.26
1.88 × 10−5
8.23 × 104
23
30.36
1.93 × 10−5
3.89 × 104
mixed
1
26.48
1.31 × 10−5
9.87 × 104
3
18.24
1.84 × 10−5
7.47 × 104
7
20.57
1.87 × 10−5
3.56 × 104
2.19 × 10−3
1.74 × 104
11
30.04
2.28 × 10−5
1.95 × 104
3.38 × 10−3
4.76 × 103
15
19.3
2.57 × 10−5
7.15 × 103
4.70 × 10−3
9.49 × 103
19
21.93
1.74 × 10−5
1.81 × 104
2.66 × 10−3
7.46 × 103
23
28.74
3.31 × 10−5
5.96 × 103
1.54 × 10−2
1.43 × 103
In the context of the experiment, it was seen that the charge transfer resistance Rct in the sterile system was significantly higher than that of the bacterial system. This can be attributed to the formation of a compact film structure of corrosion products that covered the surface of the Al alloy, having a protective effect (Zhang et al., 2022a, 2022b). In the context of the L. paracasei system, it was observed that the Rct values exhibited an increasing trend from day 3 to day 7, followed by a subsequent decrease with an increase in immersion time. This trend suggested the formation of a protective corrosion product film on the surface of the Al alloy, which served to mitigate further corrosion. The corrosion was exacerbated due to the impact of metabolic products produced by bacteria. In the A. lwoffi system, the metabolic activity of Rct exhibited a pattern of initial increase followed by a subsequent decrease. On the 23rd day, the Rct values of the two individual bacteria were 2.03 × 104 and 3.89 × 104 Ω·cm2, respectively. These values were found to be one order of magnitude lower than the Rct value of the sterile system, which was 6.83 × 105 Ω·cm2. Simultaneously, the Rct of the single bacteria system was notably lower than that of the sterile system in its entirety and showed a declining pattern over time, suggesting that both bacteria facilitated the corrosion process.
The value of Rs was found to be significantly lower in comparison to the sterile system, manifesting that the conductance of the solution was influenced by the metabolic activity of the mixed bacteria. The Rf in the mixed system appeared on the 7th day due to the metabolic activity of the mixed bacteria, which exhibited a double-layer membrane characteristic. The metabolic activity of mixed bacterial communities and the formation of biofilms contributed to localized corrosion, resulting in the degradation of corrosion product membranes and a gradual decrease in Rf over time. The Rct presented a declining pattern and ultimately stabilized at a value of 5.96 × 103 Ω·cm2 by day 23. As depicted in Fig. 6, the Rct of the mixed bacterial system was significantly lower compared to that of the single bacterial system. This observation suggested that the mixed bacterial system exhibited a synergistic effect, which accelerated the corrosion process (Lei et al., 2018; Zeng et al., 2015). The aerobic bacterium A. lwoffii facilitated the consumption of oxygen, thereby creating a conducive environment for the extensive proliferation of L. paracasei. The metabolic activity and biofilm formation of mixed bacterial communities had been observed to impact the reaction of aluminum alloys. The Bode plot analysis revealed a gradual decrease in the phase angle, which was attributed to the destruction of the metal surface film structure. This destruction facilitated the contact between the corrosion ions and the Al alloy substrate, thereby promoting the occurrence of local corrosion (Ju et al., 2021).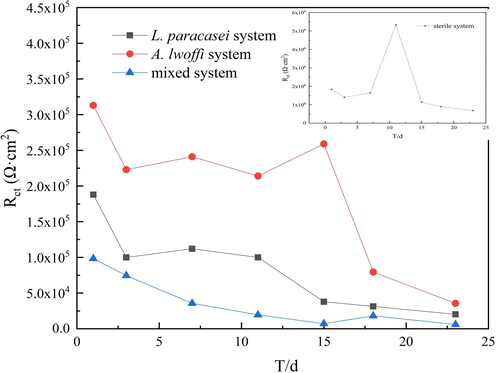
Time-dependent changes of Rct in different systems.
3.4 Polarization curve results
The polarization curves for four distinct systems on the 11th and 23rd days are depicted in Fig. 7, while the corresponding fitting data can be found in Table 2. The polarization curves exhibited analogous shapes for all four systems, suggesting that the electrochemical reaction mechanism remained unaffected by the activity of the two bacteria. This observation implied that the presence of the bacteria did not significantly alter the electrochemical behavior of the system. By comparison, it was observed that the corrosion current density of the mixed system on the 11th day was 1.434 μA/cm2, while the corrosion current density on the 23rd day was 2.301 μA/cm2. Notably, the current density on the 23rd day was larger, which was consistent with the Rct rule in EIS. In the experiment, it was observed that the corrosion current density increased in the presence of A. lwoffi and L. paracasei, indicating that the presence of either of these single bacteria resulted in the promotion of Al alloy corrosion, as compared to the sterile system. The current density of the A. lwoffi and L. paracasei systems were 0.723 μA/cm2 and 0.745 μA/cm2, respectively, when the single bacteria system was immersed for 23 days. Upon coexistence of the two bacterial strains, the corrosion current density presented a significant increase, reaching its maximum value of 2.301 μA/cm2 among the four systems investigated. This observation provided further evidence to support the notion that the synergistic effect of mixed bacterial strains exacerbated the corrosion of aluminum alloys (Zhang et al., 2021). The mixed system exhibited a more negative corrosion potential, suggesting a greater propensity for corrosion. The anode and cathode slopes were denoted as Ba and Bc, respectively, and were indicative of the level of challenge associated with electron transfer. The Ba and Bc values of the mixed system showed a declining trend, indicating that anodic dissolution was facilitated while cathodic reaction was accelerated (Moradi et al., 2021). This finding is in agreement with the electrochemical results.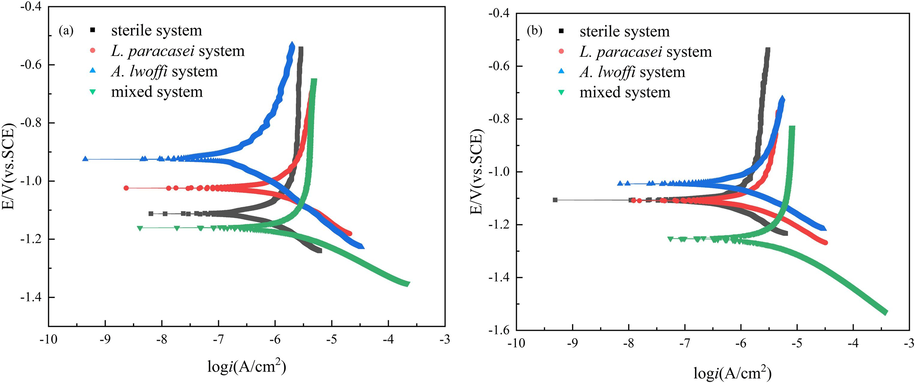
Potentiodynamic polarization curves of 7B04 Al alloy in different culture systems at (a) 11 days and (b) 23 days.
system
T/d
Ecorr/mV
(vs. SCE)
Icorr
(μA/cm2)
Ba
(mv/dec)
Bc
(mv/dec)
sterile
11
−1113
0.467
157
104
23
−1107
0.299
99
77
L. paracasei
11
−1024
0.656
142
85
23
−1110
0.723
132
73
A. lwoffi
11
−927
0.144
173
96
23
−1027
0.745
167
93
mixed
11
−1162
1.434
259
78
23
−1148
2.301
136
64
3.5 SEM and EDS results
Fig. 8 displays the surface morphology of an Al alloy sample after a 23-day period in different systems. In comparison to the bacterial system, the surface of the sterile system displayed a relatively uniform appearance and showed slight signs of corrosion. The systems of L. paracasei and A. lwoffi exhibited a significant level of bacterial adhesion on the surface, leading to the disruption of the corrosion product film on the surface of the aluminum alloy. In the mixed system, the surface of the Al alloy was found to have an uneven corrosion product coverage, characterized by the presence of multiple gaps and the embedding of bacteria within the corrosion product (Su et al., 2022; Sachan and Singh, 2020; Vejar et al., 2022). Furthermore, the examination of EDS across the four systems revealed the presence of aluminum (Al) and oxygen (O) elements in all positions, indicating the formation of oxides, specifically Al2O3. The high O content at pits 3, 4, 5, 6, and 8 was related to the formation of the corrosion product film. The results indicated a significant increase in carbon content at points 4, 6, and 7, suggesting a correlation between carbon content and microbial metabolism (Zhang et al., 2022a, 2022b; Su et al., 2023). EDS analysis revealed the existence of additional surface elements, namely Mg and P, suggesting the presence of a secondary phase. The dissolution of the second phase was accompanied by the dissolution of Al (Ma et al.,2019).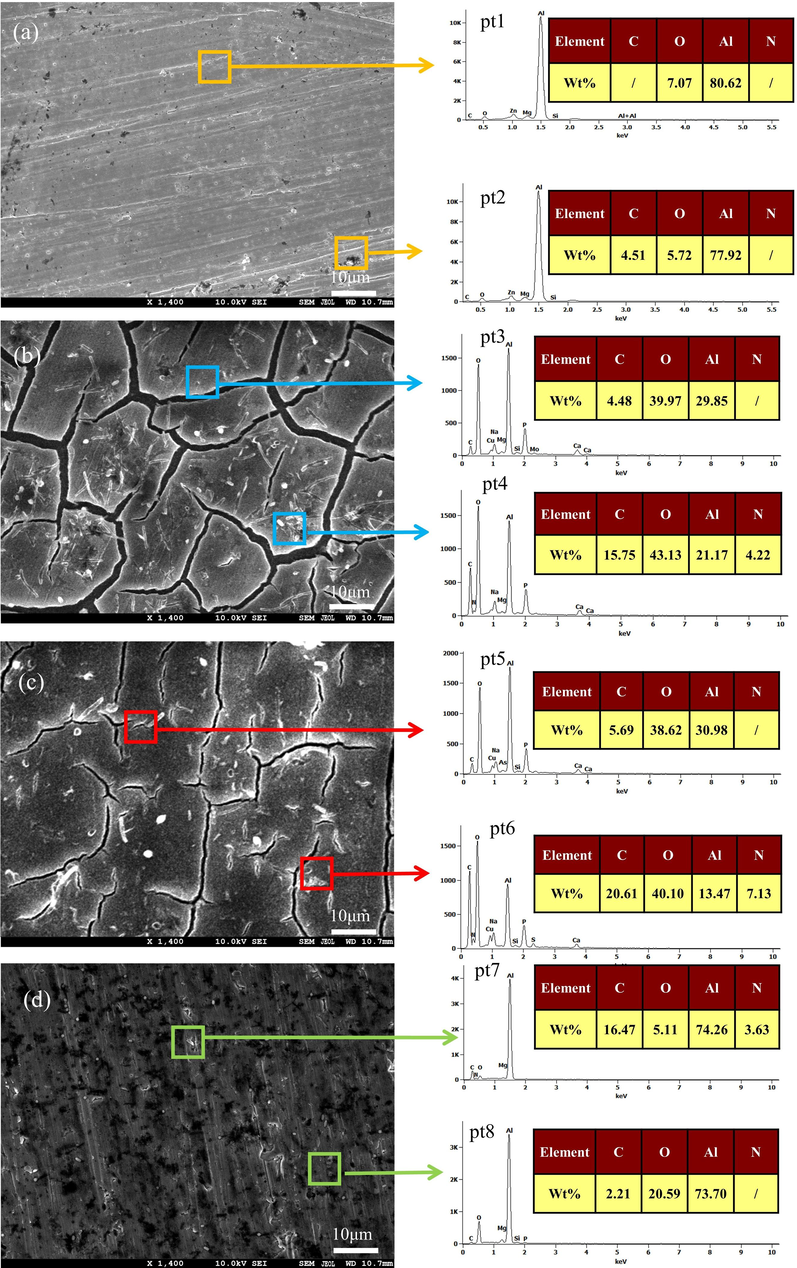
SEM and EDS images of 7B04 Al alloy immersed in (a) sterile, (b) L. paracasei, (c) A. lwoffi and (d) mixed systems for 23 days.
The morphological characteristics of the two bacteria in single and mixed systems are presented in Fig. 9. By conducting a comparison between Fig. 9(a) and (b), it can be inferred that A. lwoffi exhibited a greater length in comparison to L. paracasei. In the mixed system, a visual comparison revealed that L. paracasei exhibited a short rod-shaped morphology, whereas A. lwoffi displayed a long rod-shaped morphology. Furthermore, the investigation substantiated the inference that L. paracasei was the prevailing species as determined by the growth curve analysis. The results of the SEM analysis indicated that the predominant bacterial species present were L. paracasei, while a minor population of A. lwoffi was observed, consistent with the findings obtained from the growth curve analysis.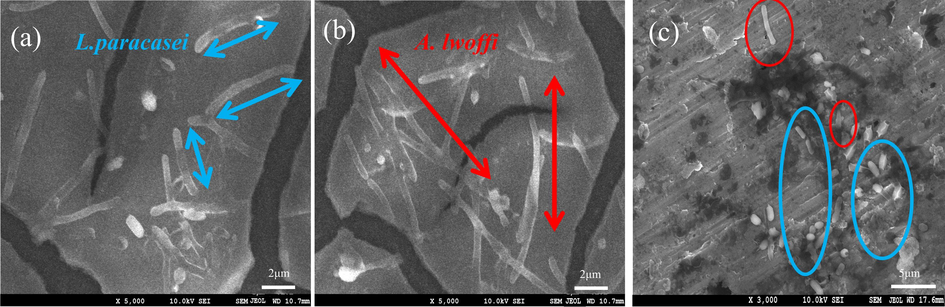
SEM morphology of (a) L. paracasei, (b) A. lwoffi, and (c) mixed bacteria.
The corrosion morphology of 7B04 Al alloy after the removal of corrosion products is depicted in Fig. 10. Due to the presence of a certain amount of Cl- in the culture medium, corrosion may also occur in the blank system. This is indicated by the downward trend in the Rct value in electrochemical impedance spectroscopy. Significant differences were found in electrochemical measurements and morphology characterization between sterile and bacterial systems, which were the focus of discussion. In both the single and mixed systems, non-uniform corrosion was observed, leading to the formation of localized pits (Lv et al., 2022). The aforementioned findings indicated that the configuration of the biofilm and the activity of bacteria played a crucial role in the corrosion process, leading to alterations in the corrosion mechanism. A more detailed analysis of the system will be presented in subsequent sections.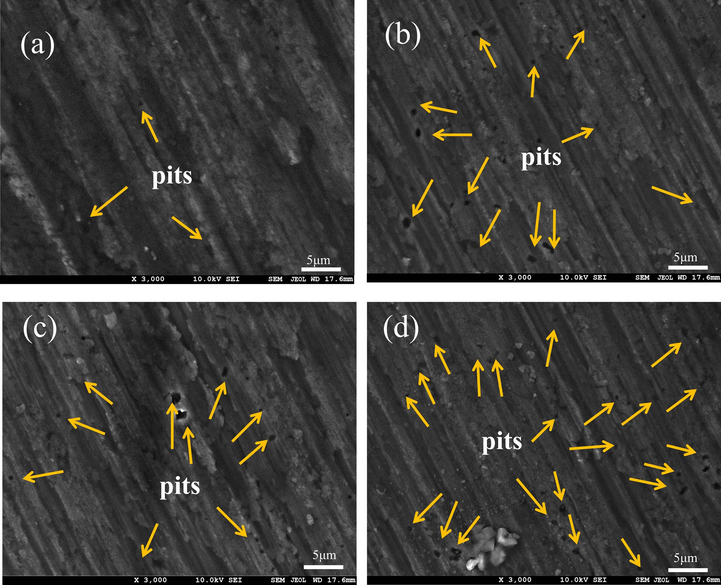
SEM images of 7B04 Al alloy after removing corrosion products after exposure to (a) sterile, (b) L. paracasei, (c) A. lwoffi and (d) mixed systems for 23 days.
3.6 Stereomicroscope results
The surface morphology of an Al alloy after 23 days of immersion in four different systems is presented in Fig. 11, as observed by a stereomicroscope. The morphology of the corrosion product observed in the sterile system was relatively uniform, indicating a mild degree of corrosion. However, in the single bacterial system, it was clearly seen that the corrosion product film was pitted, suggesting that bacteria had produced chemical reactions on the Al alloy surface, accelerating the corrosion process. Moreover, the mixed system demonstrated a remarkably severe level of corrosion, and the corrosion product on the surface of the Al alloy was considerably impacted by metabolic activity, leading to the manifestation of severe corrosion (Kawasaki et al., 2022; Liu et al., 2019). This finding is in line with the electrochemical result.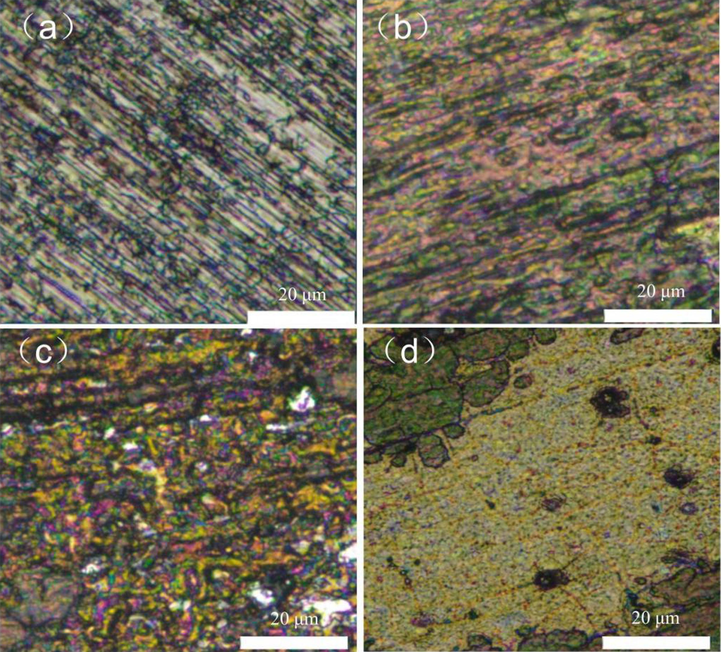
Surface morphology of Al alloy immersed in the (a) sterile, (b) L. paracasei, (c) A. lwoffi and (d) mixed systems for 23 days observed by stereomicroscope.
3.7 XRD results
Fig. 12 presents the XRD analysis results of the corrosion products that developed on the surface of the Al alloy following a 23-day immersion period in both sterile and mixed systems. The similarity in the composition of the corrosion products in both systems was determined, with the primary peaks being attributed to Al and Al2O3 (Al-Qutub et al., 2022; Sowa et al., 2022). Al2O3 was a dense oxide film structure that provided a certain level of protection to the aluminum alloy substrate against microorganisms (Gudkov et al., 2022). This corresponds to the trend of a partial increase in Rct in EIS. The findings of the study indicated that the electrochemical mechanism of the Al alloy remained unaltered in both the mixed and sterile systems, as evidenced by the presence of Al2O3 as the corrosion product. By comparing the XRD patterns of the two systems, it was found that the intensity of the corrosion product peaks had changed significantly. The samples exhibited corrosion and significant emissions, which could be attributed to the influence of bacterial metabolic activities, leading to the instability of corrosion products.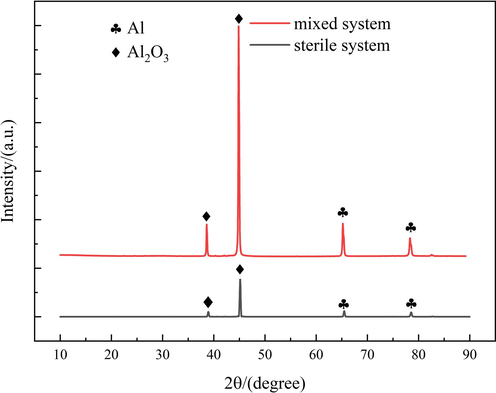
XRD analysis of the corrosion product on the surface of Al alloy after 23 days of immersion in the sterile and mixed systems.
4 Discussion
The electrochemical reaction process occurred on the surface of the Al alloy under sterile conditions, and can be represented by reactions (1)–(3). The production of Al3+ through the anodic reaction and OH– through the cathodic reaction results in the formation of Al2O3, which covered the surface of the Al alloy and generates a film of corrosion product. EDS and XRD analyses provided confirmation that Al2O3 was the primary corrosion product. The protective effect of the corrosion product film was observed, as evidenced by the deceleration of the metal's reaction rate in the electrochemical experiment.
The corrosion mechanism of the mixed bacterial biofilm at various stages is illustrated in Fig. 13. Based on the analysis of the growth curve trend, it was determined that A. lwoffi exhibited a specific pattern of growth. As an aerobic bacterium held a dominant position during the initial stage (a) when oxygen levels were sufficient. Its presence also had a moderating effect on the reproduction of L. paracasei to some extent. During the biofilm formation stage (b), the available oxygen was gradually depleted, leading to a significant increase in the population of L. paracasei. The ionization of H+ (4) by acidic metabolic products exacerbated the corrosion process, leading to the formation of corrosion pits.
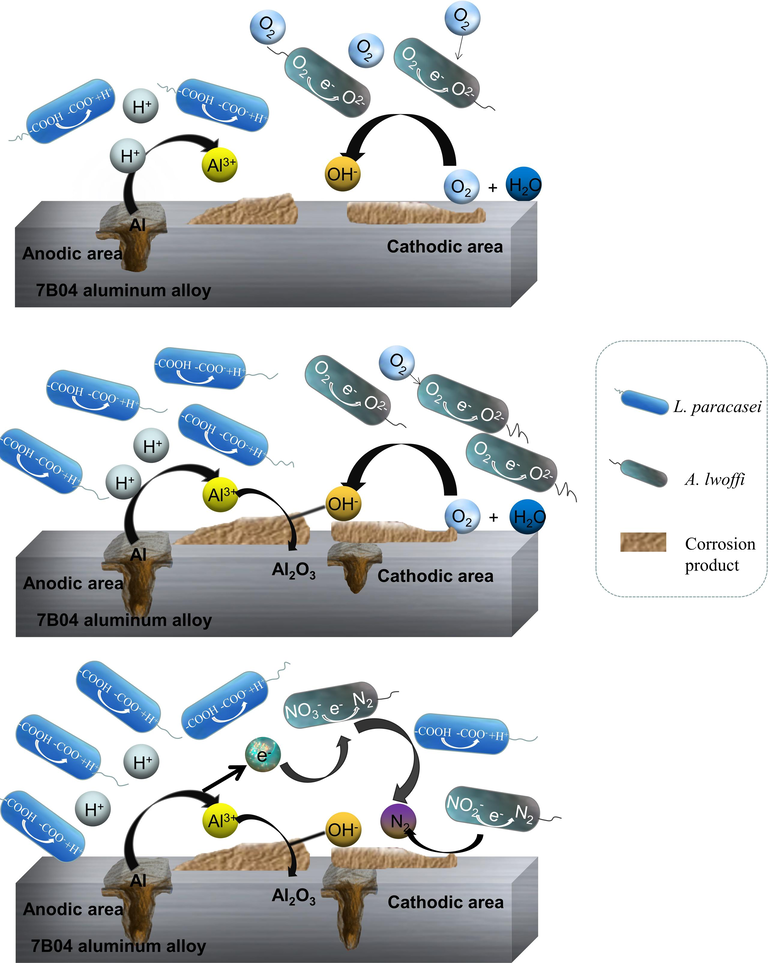
Corrosion mechanism of 7B04 Al alloy by mixed species biofilm at different stages.
Upon entering the anaerobic stage (c), the denitrification process (5) (6) of A. lwoffi occurs. The cathodic oxygen absorption reaction was gradually substituted.
The reduction of NO2– and NO3– resulted in the production of N2 through electron transfer, accompanied by the ionization of a portion of OH–. This process required Al dissolution and the electrons generated to balance the anodic and cathodic reactions. Moreover, L. paracasei had the most suitable living environment at this time, and the accumulation of acidic metabolic products led to severe corrosion due to the synergistic effect. In addition, MIC was caused by the formation of a biofilm, which created a distinct environment compared to the surrounding solution. Due to the acid-producing characteristics of the bacteria, the local acidity under the biofilm was extremely high, which enhanced the sensitivity to pitting corrosion. As a result, a large number of cavities were observed beneath the biofilm. The confirmation of this was established through surface morphology and electrochemical experiments. In comparison to other systems, the Rct of EIS in the mixed system exhibited the smallest value, the most negative corrosion potential, and the largest corrosion current density. According to formula (7), where n represents the number of electrons, E is the potential, and F is the Faraday constant. The lowest OCP value of the mixed system indicated a higher tendency for electron loss. Additionally, the larger |ΔG|, the greater the reaction trend, which resulted in more pronounced corrosion reactions (Xu et al., 2013; Enning et al., 2012).
Upon examination with a stereomicroscope, it was observed that the corrosion product in the mixed system presented a loose structure and was prone to detachment in significant amounts. SEM also confirmed the uneven distribution of corrosion products and the presence of significant amounts of pitting corrosion, indicating a higher degree of severity in the corrosion process. EDS analysis revealed the existence of additional surface elements, namely Mg and P, suggesting the presence of a secondary phase. The dissolution of the second phase was accompanied by the dissolution of Al. Based on the microbial image presented in Fig. 9, it can be observed that L. paracasei exhibited dominance within the mixed system. The combined action of A. lwoffi and L. paracasei resulted in a synergistic effect that exacerbated the corrosion of the Al alloy.
5 Conclusions
The findings of the experiment indicate that the combined action of L. paracasei and A. lwoffi resulted in a heightened level of corrosion in the Al alloy, suggesting a synergistic effect between the two microorganisms. In the mixed system, the aerobic bacterium A. lwoffii consumed oxygen, thereby creating a more conducive environment for the proliferation of L. paracasei on the surface of the Al alloy. EIS and potentiodynamic polarization measurements indicated that the mixed system displayed a significant level of corrosion. The conclusion was further supported by the utilization of characterization techniques, such as SEM. The corrosion product was determined to be Al2O3 through experiments such as XRD, indicating that the electrochemical reaction mechanism of the metal had not changed. Al2O3 was a dense oxide film structure that provided a certain level of protection to the aluminum alloy substrate against microorganisms. The corrosion process was found to be primarily influenced by the biofilm structure of L. paracasei and A. lwoffi, as well as the succession of dominant colonies. The mixed system facilitated the development of localized pitting corrosion, resulting in a significant increase in overall corrosion.
Overall, these findings highlight the importance of considering the interactions between different microorganisms in the context of corrosion. The presence of multiple microorganisms creates a more complex and aggressive environment, leading to accelerated corrosion rates. Understanding these interactions is crucial for developing effective corrosion prevention strategies in practical applications.
CRediT Author Contribution statement
Zhenhua Zhou: Methodology, Validation, Formal analysis, Investigation, Data curation, Writing – original draft. Xinru Ge: Validation, Formal analysis, Data curation. Borong Shan: Writing – review & editing. Weijie Fan: Conceptualization, Methodology, Validation, Resources. Jie Yang: Conceptualization, Methodology, Validation, Resources. Xiaodong Zhao: Conceptualization, Methodology, Validation, Resources, Writing – original draft, Writing – review & editing, Project administration. All authors reviewed the manuscript.
Acknowledgement
This work was supported by the Natural Science Foundation of Shandong Province (ZR2020QD081, ZR2021QD060), the National Natural Science Foundation of China (52101392).
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Evaluation of durability of alumina, silicon carbide and siliconized silicon carbide foams as absorber materials for concentrated solar power applications. Sol. Energy. 2022;242:45-55.
- [CrossRef] [Google Scholar]
- Community dynamics and phylogenetics of bacteria fouling Jet A and JP-8 aviation fuel. Int. Biodeter. Biodegr.. 2010;64:253-261.
- [CrossRef] [Google Scholar]
- Corrosion of aluminum alloy 2024 caused by Aspergillus niger. Int. Biodeter. Biodegr.. 2016;115:1-10.
- [CrossRef] [Google Scholar]
- Marine sulfate-reducing bacteria cause serious corrosion of iron under electroconductive biogenic mineral crust. Environ. Microbiol.. 2012;14:1772-1787.
- [CrossRef] [Google Scholar]
- Influence of sulfate-reducing bacteria on the corrosion behavior of 5052 aluminum alloy. Surf. Coat. Technol.. 2017;316:171-179.
- [CrossRef] [Google Scholar]
- Interaction between sulfate-reducing bacteria and aluminum alloys—Corrosion mechanisms of 5052 and Al-Zn-In-Cd aluminum alloys. J. Mater. Sci. Technol.. 2020;36:55-64.
- [CrossRef] [Google Scholar]
- A mini review of antibacterial properties of Al2O3 nanoparticles. Nanomaterials. 2022;12:2635.
- [CrossRef] [Google Scholar]
- Marine bacteria inhibit corrosion of steel via synergistic biomineralization. J. Mater. Sci. Technol.. 2021;66:82-90.
- [CrossRef] [Google Scholar]
- The corrosion of X52 steel at an elbow of loop system based on array electrode technology. Mater. Chem. Phys.. 2016;181:312-320.
- [CrossRef] [Google Scholar]
- Electron transfer mediators accelerated the microbiologically influence corrosion against carbon steel by nitrate reducing Pseudomonas aeruginosa biofilm. Bioelectrochemistry. 2017;118:38-46.
- [CrossRef] [Google Scholar]
- Microbiologically influenced corrosion of C1018 carbon steel by nitrate reducing Pseudomonas aeruginosa biofilm under organic carbon starvation. Corros. Sci.. 2017;127:1-9.
- [CrossRef] [Google Scholar]
- Localized corrosion of aluminum alloy 6061 in the presence of Aspergillus niger. Int. Biodeter. Biodegr.. 2018;133:17-25.
- [CrossRef] [Google Scholar]
- Investigation of pitting corrosion and hydrogen evolution of aluminum and AA2024 alloy by simultaneous electrochemical measurements and imaging. Electrochem. Commun.. 2021;132:107135
- [CrossRef] [Google Scholar]
- Phenomenological process of rebar corrosion in reinforced concrete evaluated by acoustic emission and electrochemical noise. Constr. Build. Mater.. 2022;352:128829
- [CrossRef] [Google Scholar]
- Mechanism study of the role of biofilm played in sewage corrosion of mortar. Constr. Build. Mater.. 2018;164:44-56.
- [CrossRef] [Google Scholar]
- Electrochemical behaviour of martensitic stainless steel after immersion in a H2S-saturated solution. Corros. Sci.. 2018;131:164-173.
- [CrossRef] [Google Scholar]
- Extracellular electron transfer of Bacillus cereus biofilm and its effect on the corrosion behaviour of 316L stainless steel. Colloids Surf. B. 2019;173:139-147.
- [CrossRef] [Google Scholar]
- Bacillus cereus s-EPS as a dual bio-functional corrosion and scale inhibitor in artificial seawater. Water Res.. 2019;166:115094
- [CrossRef] [Google Scholar]
- Anaerobic microbiologically influenced corrosion mechanisms interpreted using bioenergetics and bioelectrochemistry: a review. J. Mater. Sci. Technol.. 2018;34:1713-1718.
- [CrossRef] [Google Scholar]
- The influence of cathodic protection potential on the biofilm formation and corrosion behaviour of an X70 steel pipeline in sulfate reducing bacteria media. J. Alloys Compd.. 2017;729:180-188.
- [CrossRef] [Google Scholar]
- Corrosion behavior of carbon steel in the presence of sulfate reducing bacteria and iron oxidizing bacteria cultured in oilfield produced water. Corros. Sci.. 2015;100:484-495.
- [CrossRef] [Google Scholar]
- Effect of different UV intensity on corrosion behavior of carbon steel exposed to simulated Nansha atmospheric environment. Mater. Chem. Phys.. 2019;237:121855
- [CrossRef] [Google Scholar]
- Mechanism of microbiologically influenced corrosion of X65 steel in seawater containing sulfate-reducing bacteria and iron-oxidizing bacteria. J. Mater. Res. Technol.. 2019;8:4066-4078.
- [CrossRef] [Google Scholar]
- Investigation of mixed species biofilm on corrosion of X65 steel in seawater environment. Bioelectrochemistry. 2022;143:107951
- [CrossRef] [Google Scholar]
- Effect of welding speed on performance of friction stir welded spray forming 7055 aluminum alloy. J. Manuf. Process.. 2019;46:304-316.
- [CrossRef] [Google Scholar]
- Mechanistic microbiologically influenced corrosion modeling—A review. Corros. Sci.. 2019;146:99-111.
- [CrossRef] [Google Scholar]
- Interspecies interactions of Vibrio azureus and Jeotgalibacillus alkaliphilus on corrosion of duplex stainless steel. Int. Biodeter. Biodegr.. 2021;160:105212
- [CrossRef] [Google Scholar]
- Microbial contamination and its control in fuels and fuel systems since 1980 – a review. Int. Biodeter. Biodegr.. 2013;81:88-104.
- [CrossRef] [Google Scholar]
- Investigation on corrosion inhibition and adsorption mechanism of triazine-thiourea derivatives at mild steel/HCl solution interface: electrochemical, XPS, DFT and Monte Carlo simulation approach. J. Electroanal. Chem.. 2020;877:114599
- [CrossRef] [Google Scholar]
- Accelerating effect of catalase on microbiologically influenced corrosion of 304 stainless steel by the halophilic archaeon Natronorubrum tibetense. Corros. Sci.. 2021;178:109057
- [CrossRef] [Google Scholar]
- Synergistic effect of alternating current and sulfate-reducing bacteria on corrosion behavior of X80 steel in coastal saline soil. Bioelectrochemistry. 2021;142:107911
- [CrossRef] [Google Scholar]
- The characteristics of a novel heterotrophic nitrifying and aerobic denitrifying bacterium, Acinetobacter junii YB. Bioresour. Technol.. 2014;171:1-9.
- [CrossRef] [Google Scholar]
- Governing global problems under uncertainty: making bottom-up climate policy work. Clim. Change. 2015;144:15-27.
- [CrossRef] [Google Scholar]
- Comparison of microbial influenced corrosion in presence of iron oxidizing bacteria (strains DASEWM1 and DASEWM2) Constr. Build. Mater.. 2020;256:119438
- [CrossRef] [Google Scholar]
- Electrochemical characterization of anti-corrosion coatings formed on 6061 aluminum alloy by plasma electrolytic oxidation in the corrosion inhibitor-enriched aqueous solutions. Electrochim. Acta. 2022;424:140652
- [CrossRef] [Google Scholar]
- High-performance piezoelectric composites via β phase programming. Nat. Commun.. 2022;13:4867.
- [CrossRef] [Google Scholar]
- Sensing–transducing coupled piezoelectric textiles for self-powered humidity detection and wearable biomonitoring. Mater. Horiz.. 2023;10:842-851.
- [CrossRef] [Google Scholar]
- Metabolic engineering coupled with adaptive evolution strategies for the efficient production of high-quality L-lactic acid by Lactobacillus paracasei. Bioresour. Technol.. 2021;323:124549
- [CrossRef] [Google Scholar]
- Influence of Bacillus safensis and Bacillus pumilus on the electrochemical behavior of 2024–T3 aluminum alloy. Bioelectrochemistry. 2022;143:107950
- [CrossRef] [Google Scholar]
- Study of the corrosion behavior of Aspergillus niger on 7075–T6 aluminum alloy in a high salinity environment. Bioelectrochemistry. 2019;129:10-17.
- [CrossRef] [Google Scholar]
- Laboratory investigation of microbiologically influenced corrosion of C1018 carbon steel by nitrate reducing bacterium Bacillus licheniformis. Corros. Sci.. 2013;77:385-390.
- [CrossRef] [Google Scholar]
- Microbiologically influenced corrosion behavior of friction stir welded S32654 super austenitic stainless steel in the presence of Acidithiobacillus caldus SM-1 biofilm. Mater. Today Commun.. 2020;25:101491
- [CrossRef] [Google Scholar]
- Inhibition effect of thioureidoimidazoline inhibitor for the flow accelerated corrosion of an elbow. Corros. Sci.. 2015;90:202-215.
- [CrossRef] [Google Scholar]
- Corrosion of aluminum alloy 7075 induced by marine Aspergillus terreus with continued organic carbon starvation. npj Mater. Degrad.. 2022;6:27.
- [CrossRef] [Google Scholar]
- The corrosion promoting mechanism of Aspergillus niger on 5083 aluminum alloy and inhibition performance of miconazole nitrate. Corros. Sci.. 2020;176:108930
- [CrossRef] [Google Scholar]
- Apostichopus japonicus polysaccharide as efficient sustainable inhibitor for mild steel against hydrochloric acid corrosion. J. Mol. Liq.. 2021;321:114923
- [CrossRef] [Google Scholar]
- Corrosion electrochemistry properties of thermally sprayed Zn–Cu–Ti coating in simulated ocean atmosphere. J. Mater. Res. Technol.. 2022;21:3235-3247.
- [CrossRef] [Google Scholar]
- Methanogenic archaea and sulfate reducing bacteria induce severe corrosion of steel pipelines after hydrostatic testing. J. Mater. Sci. Technol.. 2020;48:72-83.
- [CrossRef] [Google Scholar]
- Organic and inorganic nitrogen removals by an ureolytic heterotrophic nitrification and aerobic denitrification strain Acinetobacter sp. Z1: elucidating its physiological characteristics and metabolic mechanisms. Bioresour. Technol.. 2022;362:127792
- [CrossRef] [Google Scholar]







