Translate this page into:
Crosslinked poly(vinyl alcohol) hydrogels for wound dressing applications: A review of remarkably blended polymers
*Corresponding author badawykamoun@yahoo.com (Elbadawy A. Kamoun)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.

Available online 15 July 2014
Abstract
A series of excellent poly(vinyl alcohol) (PVA)/polymers blend hydrogel were reviewed using different crosslinking types to obtain proper polymeric dressing materials, which have satisfied biocompatibility and sufficient mechanical properties. The importance of biodegradable–biocompatible synthetic polymers such as PVA, natural polymers such as alginate, starch, and chitosan or their derivatives has grown significantly over the last two decades due to their renewable and desirable biological properties. The properties of these polymers for pharmaceutical and biomedical application needs have attracted much attention. Thus, a considered proportion of the population need those polymeric medical applications for drug delivery, wound dressing, artificial cartilage materials, and other medical purposes, where the pressure on alternative polymeric devices in all countries became substantial. The review explores different polymers which have been blended previously in the literature with PVA as wound dressing blended with other polymeric materials, showing the feasibility, property change, and purpose which are behind the blending process with PVA.
Keywords
Poly(vinyl alcohol)
Hydrogel membranes
Polymer blended hydrogels
Natural polymers
Synthetic polymers
Wound dressing
1 Introduction
In 1960s, for over fifty years ago hydrogels have been innovated by Wichterle and Lim (1960) and have been applied in numerous biomedical disciplines e.g. contact lenses, absorbable sutures, osteoporosis, asthma treatment, and as neoplasm (Queiroz et al., 2001). In 1980s, Lim and Sun (1980) have revealed calcium alginate microcapsules for cell engineering, while Yannas’ group modified synthetic hydrogels with some natural polymers e.g. collagen to obtain novel dressing materials, showing optimal conditions for healing burns and wound dressing (Yannas, 1985; Yannas et al., 1981, 1983; Yannas and Forbes, 1982). Since this date; polymer hydrogels continue to interest scientists. For example, millions of people are suffering annually from chronic diseases, accidents arising from trauma, burns, and bone fracture or defects and unfortunately some of them are dying due to insufficient ideas of alternative polymeric organs and/or treatment (Pighinelli and Kucharska, 2013). Thus, much attention has been given to the use or modification of different polymeric materials that can be used currently for biomedical devices to fulfill the over increased need for those materials in medical applications.
Every year, millions of people are exposed to burns by hot water, flames, accidents, and boiling oil, and these accidents result in major disabilities or even sometimes death. Especially in adults, and overaged people dermis regeneration cannot occur spontaneously again (Çiğdem and Senel, 2008). Since autologous skin has limited availability and associated with additional scarring, this traditional approach for a substantial loss of dermis cannot meet the requirements, and dressing materials became inevitable for skin tissue or healing (Çiğdem and Senel, 2008). Prior to 1960s, wound dressing materials have been regarded to be only passive materials that have a minimal role in the healing process. Winter (1962) has announced the first generation of wound dressing polymeric materials and showed optimal environments for wound repair. This awareness has revolutionized the approaches to wound dressing and paved the way for the development of wound dressing from the passive to active material and functionalized ones. The desirable wound dressing materials should fulfill the following conditions: (a) maintain a local moist environment, (b) protect the wound from side-infection, (c) absorb the wound fluids and exudates, (d) minimize the wound surface necrosis, (e) prevent the wound dryness, (f) stimulate the growth rate, and (g) be elastic, non-toxic, non-antigenic, biocompatible and biodegradable dressing materials (Jakubiak et al., 2001; Kannon and Garrett, 1995; Kokabi et al., 2007).
At present, PVA is one of the most frequent and the oldest synthetic polymer hydrogels that due to its good biocompatibility has been applied in several advanced biomedical applications e.g. wound dressing (Kenawy et al., 2014), wound management (Zhao et al., 2003), drug delivery systems (Muggli et al., 1998), artificial organs (Yang et al., 2008), and contact lenses (Hyon et al., 1994). However, PVA hydrogel possesses insufficient elastic, stiff membrane, and very limited hydrophilicity characteristics which restrict its use alone as a wound dressing polymeric material. Among the various hydrogels described in the literature, hydrogels prepared using PVA blended with polysaccharides and some other synthetic polymers are attractive because of the abundance of such polymers, easy for chemical derivatization or modification, and in most cases good biocompatibility (Coviello et al., 2007).
2 Crosslinking of PVA polymer
Peppas and Merrill (1977a,b) have revealed in their earliest effort in considering PVA hydrogels as biomaterials. Generally, hydrogels were obtained by a crosslinking process of polymers, which might be done by a chemical reaction (e.g. free-radical polymerization, chemical reaction of complementary groups, using high energy irradiation, or enzymatic reaction) or by a physical reaction (e.g. ionic interaction, crystallization of the polymeric chain, hydrogen bond between chains, protein interaction, or design of graft copolymers) (Hennink and Nostrum, 2002).
In recent decades, the need of physically crosslinked gels has been potentially increased (Van Tomme et al., 2005), to avoid the use of traditional chemical crosslinking agents and reagents. These chemical agents are not only toxic compounds which can be detached or isolated frequently from prepared gels before application, but also can affect the nature of the substances when entrapped (e.g. proteins, drugs, and cells). Therefore, the physical crosslinking method has been chosen and preferred comparable with the chemical crosslinking method for most crosslinked polymer preparation. Several attempts have been done to prepare crosslinked PVA-based hydrogels including radiation crosslinking (Park and Chang, 2003), chemical reaction with glyoxal (Teramoto et al., 2001), bi-functional reagents with glutaraldehyde (Dai and Barbari, 1999), or reaction with borates (Korsmeyer and Peppas, 1981). Although, an aqueous solution of PVA can form low strength of hydrogel upon exposure to a very long storage time at room temperature, but this method did not meet any application requirements, where the mechanical properties are the most important character in hydrogel properties, which are much weak.
The earliest attempt for crosslinking of PVA using the freezing–thawing method has been pioneered by Peppas (1975). Semi-crystalline or physical crosslinked PVA gels were obtained by exposing PVA aqueous solution to repetitive freezing–thawing cycles which induced crystallization and result in a network hydrogel structure. The crystallization degree of PVA hydrogels which have been obtained using physical crosslinking by the freeze–thawing method, can be calculated by the following equation (Kenawy et al., 2014).
3 Determination of water content in PVA hydrogels
Water content in PVA hydrogels is not only to provide a local moist environment (which is a key factor for wound healing rate), but also adjusting the permeation of nutrients, drug, gases, or protein into the cells or targeted absorption site. Dried PVA xerogels can swell in water or in saline up to more than 1000 times their own weight (Kenawy et al., 2014). The amount of absorbed water is usually expressed as water uptake or swelling ratio (SW%) as shown in the following Eq. (2) (Yang et al., 2008).
4 Blended polymer types with PVA hydrogels for wound dressing
Blended polymers for medical applications were previously defined as those targeted to interface with biological systems to evaluate, address, and augment the function of the body, or replace any tissues or organs (Lee and Mooney, 2012). Currently, biodegradable hydrogel membranes have been applied intensively in the medical market, due to their inherent biocompatibility (Khor et al., 2011; Lee and Mooney, 2012). In last decades, the need of polysaccharides has increased intensively particularly in biomedical applications, because polysaccharides are biological polymers that can be obtained from several natural sources, such as microbial sources (e.g. dextran, glucan, and alginate), animal sources (e.g. chitosan and gelatin), and vegetal sources (e.g. starch and cellulose), (see Table 1).
Polymer
Properties
Refs. No.
Natural polymers and derivatives
Alginate
Biocompatible and biodegradable polymer, suitable for in situ injection, crosslinking is under very mild conditions, water soluble polymer, mechanical weakness, difficulties in handling, storage in solution, and sterilization
Kim et al. (2008), Levic et al. (2011)
Chitosan
Excellent biocompatibility and good host response, unique biodegradability by lysozyme and other enzymes, high antimicrobial activity, hydrophilic surface providing easy cell adhesion, proliferation and differentiation, mechanical weakness, very viscous polymer solution, and water soluble-polymer only in acetic medium, and high cost purification
Cascone et al. (1999), El-Salmawi (2007)
Starch and hydroxyethyl starch, (HES)
Water soluble polymer (depends on DS value), inexpensive, in vivo biodegradable by α-amylase, biocompatible, easy to modify with other polymer, difficulties in crosslinking itself, mechanical weakness, and need modification to enhance cell adhesion
Zhao et al. (2003), Kenawy et al. (2014)
Dextran
Water soluble polymer, in vivo biodegradable by α-amylase, biocompatible, good proliferation and differentiation behavior, expensive polymer, mechanical weakness, and need modification to enhance cell adhesion
Cascone et al. (1999)
Glucan
Water soluble polymer but yeast-glucan is not soluble in water, biocompatible-biodegradable polymer, it has excellent antibacterial and antiviral activities, it has fast wound healing rate, publications on glucan in medical applications are very rare, it has been used previously with gelatin for artificial skin, it has very fast biodegradation by glucanase, and formed mechanical weakness hydrogels
Huang and Brazel (2001)
Gelatin
Water-soluble polymer, obtained from various animal by-products, forms thermally-revisable and high mechanical hydrogels, widespread in biomedical application, easy to form films and matrix hydrogels, very viscous polymer solution, very fast biodegradation, and lower thermal stability at high temperatures
Hago and Li (2013)
Synthetic polymers
Poly (N-isopropylacrylamide), NIPAAm
Water soluble polymer, temperature-responsive polymer, good mechanical properties, biocompatible polymer for tissue engineering and controlled drug delivery, needs chemical crosslinking, needs modification to enhance culture surface for cell delivery, somewhat cytotoxicity, and significant lower thermal stability
Azarbayjani et al. (2010)
Poly(vinylpyrrolidone), PVP
Water soluble polymer, excellent wetting properties, swells very rapidly, excellent film-forming, crosslinked PVP is non-toxic, biocompatible polymer, wide application in blood plasma expander polymer, high storage stability, mechanical weakness, and lower thermal stability
Razzak et al. (2001)
Polymer-composite
Montmorillonite (MMT) clay
MMT is natural inorganic clay and is hydrophilic in nature, needs modification and intercalation reaction before use, forms high mechanical and thermal resistance nanocomposite hydrogels, has widespread medical applications, needs some modification to enhance cell adhesion of its nanocomposite hydrogels, and non biodegradable nano or micro-particles
Kokabi et al. (2007)
ZnO nanoparticles
Inorganic nanoparticles, insoluble in water, have been used for medicine e.g. skin condition powder, and for industrial e.g. portable energy, sensors, wallpapers, and film formation, have excellent antibacterial activity only low concentration, have somewhat toxicity with high concentrations, non biodegradability, ZnO-film needs further treating to enhance cells attachment and proliferation
Vicentini et al. (2010), Shalumon et al. (2011)
PVA has excellent and easy film-forming properties and has been previously blended with synthetic and natural polymers (Table 1), due to its good water-soluble, biodegradable, non-carcinogenic, and biocompatible characters. Blended materials either natural or synthetic polymers assemble the desirable properties of each material on its own, while blended polymer materials are always mixed with PVA for improving mechanical and physicochemical properties of obtained polymeric materials (Silva et al., 2013). In this sense, the final properties of the blended material depend on the properties of the imbedded materials (i.e. natural or synthetic polymers), and thus PVA properties change after blending. Natural and synthetic polymers which have been blended previously with PVA to form film or hydrogel membranes for wound dressing applications are shown in Table 1.
4.1 Wound dressings based on PVA/natural polymers
4.1.1 PVA/sodium alginate (SA)
Alginate is derived from brown algae, is a natural and an anionic linear polysaccharide polymer composed of 1,4-linked β-d-mannuronic acid residues and 1,4-linked α-l-guluronic acid residues with varying properties (Fig. 1) (Ress and Welsh, 1997). The ratio between mannuronic acid and guluronic acid residues definitely adjusts the elasticity of the obtained crosslinked hydrogel (Lee and Mooney, 2012). Alginate polymer has a high hydrophilic, biocompatible and relatively economical use, it has been widely used in biomedical applications e.g. wound dressing (Kim et al., 2008), scaffolds (Zmora et al., 2002), and dental or surgical impression materials (Nandini et al., 2008). Sodium alginate (SA) is of the most popular natural polymers which has been investigated for wound dressing application incorporating with PVA polymer as either main or additional component to the dressing structure due to its high water swelling ability which impacts the local wound environment beyond moisture management.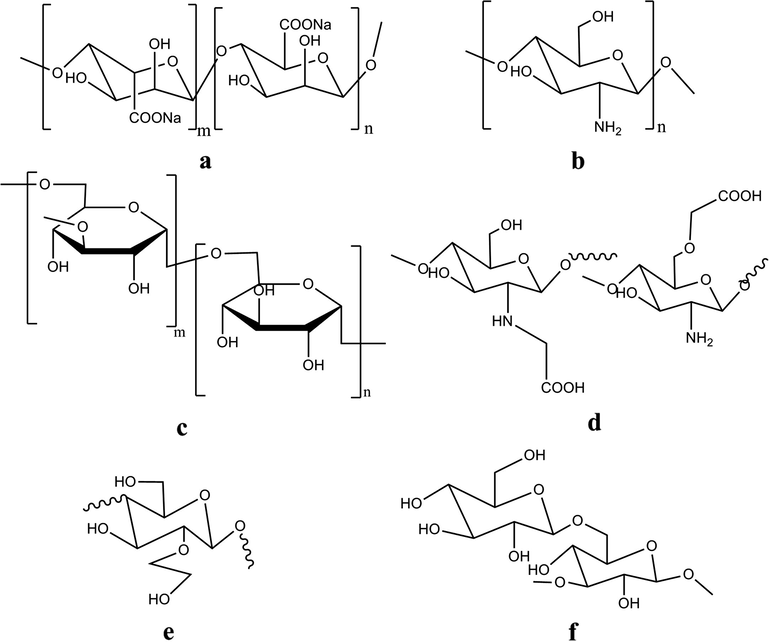
Chemical structures of natural polymers and their derivatives which were blended with PVA hydrogel to form wound dressing materials, such as sodium alginate (a), chitosan (b), dextran (c), N-O-carboxymethyl chitosan (d), hydroxyethyl starch, HES (e), and (1,3), (1,6)-β-glucan (f).
Kim et al. (2008) have used PVA/alginate hydrogel containing nitrofurazone for wound dressing purposes, where they have used the freeze–thawing method to crosslink PVA/SA blended polymer. They have reported that the increase of SA concentrations in PVA hydrogel films, increased the swelling ability, elasticity, and thermal stability of PVA/SA hydrogel films (Kim et al., 2008) while, significant decreases in gel fraction%, and mechanical properties of PVA/SA hydrogel film were found with increased SA contents. Kim et al. (2008) have conducted the bio-evaluation of PVA/SA hydrogel films, and they revealed that increased SA contents resulted in the protein adsorption in vitro increases, indicating the reduced blood compatibility. Furthermore, in vivo experiments showed wound size reduction in rats, indicating a better wound healing ability proportionate to the amount of SA incorporated into PVA hydrogel films.
Choi et al. (1999) have used hydrogels for wound dressing from a mixture of PVA and SA using the 60Co γ-ray irradiation techniques. They have found that increased SA content decreases the gel content and strength, but increases the swelling ability of PVA/SA hydrogels. Likewise, in vivo implantation experiments in rats exhibited that the foreign body reactions which occurred around the implanted PVA/SA hydrogel were moderate and became limited and minimal in size upon the increased implantation time of PVA/SA hydrogels, indicating γ-ray crosslinked PVA/SA hydrogels possessing alginate polymers which can be considered biocompatible and therefore are promising materials for wound healing purposes.
Levic et al. (2011) have made an efficient encapsulation matrix for d-limonene encapsulation using crosslinking of PVA/SA by the freeze–thawing method, followed by a calcium ionic interaction between alginate and CaCl2 solution but these hydrogels do not apply for wound dressing but for food processing application. Kim et al. (2008) reported the development of a biodegradable PVA/SA-clindamycin-loaded wound dressing hydrogel film, where crosslinking has been conducted by the freeze–thawing method. This study showed that increasing SA concentration decreased the gelation (%), maximum strength and break elongation, but it resulted in an increment in the swelling ability, elasticity and thermal stability of the hydrogel film. However, SA content had an insignificant effect on the release profile of clindamycin from the PVA/SA film, whereas PVA/SA-clindamycin improved the healing rate of artificial wound in rats.
Tarun and Gobi (2012) presented a new concept for the synthesis of the nanocomposite web of calcium alginate and PVA with varying proportions using an electrospinning technique for wound healing application. They have demonstrated that the blended nanofiber composite web which has the maximum calcium alginate content, showed the maximum water vapor transmission rate indicating the PVA/SA nanofiber web helps in maintaining the local moist environment for accelerating wound healing. Furthermore, in vivo experiments on rats exhibited apparently new epithelium formation without any harmful reactions, when the wound is covered with the PVA/SA nanofiber. Thomas (2000) has concluded that dressings have been developed so that the fibers entangled to form dressing materials with a more cohesive structure, which increases the fabric mechanical strength when dressings are soaked directly with exudates or serum blood. This study has classified wound dressing material formation which is based on PVA/SA into three categories as follows: (a) dressings containing a significant proportion of SA to improve the gelling properties of the dressing in use, (b) dressings obtained by the freeze-dried PVA/SA film or γ-ray irradiation techniques, and (c) dressings formed depending on the bond between an exuding wound and an ion-exchange reaction, occurring due to the calcium ions in the dressing and sodium ions in the serum or wound fluid.
4.1.2 PVA/dextran (Dex)
Dextran is a bacterial polysaccharide polymer which consists of α-1, 6-linked d-glucopyranose residues with a low percentage of α-1,2-, α-1,3- and α-1,4-linked side chains (Fig. 1) (Sidebotham, 1974). Dextran is derived from Leuconostoc mesenteroides contains about 5% of α-1, 3-glycopyranosidic linkages, which are mostly one or two glucose residues in length. In the previous few decades, dextran has been regarded one of the most widely used blood plasma polymer expanders. Because of its good biocompatibility and biodegradability, it is also a suitable polymer for preparation of hydrogels (Kamoun and Menzel, 2010). Cascone et al. (1999) focused on achieving a more stable and crystalline physically crosslinked PVA/Dex hydrogel films using the freeze–thawing method. This early study proved that the presence of dextran in PVA matrix favors the crystallization process of PVA, allowing the formation of a more ordered and homogeneous PVA hydrogel structure. PVA/Dex hydrogel film showed an excellent ordered structure compared to PVA blended with chitosan. However, the PVA/Dex hydrogel film showed more swellability and somewhat a high release profile of the released free-PVA, compared to the PVA/chitosan hydrogel film.
Gentamicin-loaded PVA/Dex hydrogel films were developed by Hwang et al. (2010). The freeze–thawing method has been used for physically crosslinking of PVA/Dex membranes. Physicochemical properties of PVA/Dex hydrogels have been sharply affected by addition of dextran, where dextran reduced the gel fraction, mechanical, and thermal stability. However, it increased in vitro protein adsorption, swelling ability, water vapor transmission, elasticity, and porosity because of the high hydrophilicity and miscibility of dextran. Gentamicin-loaded PVA/Dex hydrogel membranes significantly improved wound healing, wound size reduction, and spots on rat dorsum compared to the free-drug PVA/Dex hydrogel membranes because of the potential healing effect of gentamicin and these results suggested that the hydrogel with drug drastically enhanced wound healing compared with conventional products or free-drug hydrogel.
The therapeutic level of gentamicin in serum is 4–8 μg/ml, whereas its toxic level is 12 μg/ml. Most studies showed hydrogel films released a high non allowed level of gentamicin. As expected, low molecular weight blended polymers e.g. dextran showed a higher burst effect and higher release rates due to a higher quantity of hydroxyl edge groups, which make it more hydrophilic. Furthermore, a low molecular weight results in low thermal stability hydrogel properties, which allows faster drug release from the polymer (Zilberman and Elsner, 2008). Therefore, the sustained release is necessary for adjusting the network structure of obtained hydrogels. In the case of wound dressings, the burn degree and rate of skin tissue regeneration depend on patient’s age and other parameters. Thus, it is hard to describe an ideal release profile; however the adjusting of morphology behavior of PVA hydrogels is more available. Accordingly, it is of importance to investigate the effect of dextran portions on thermal and morphology behavior of PVA/Dex hydrogel membranes.
The overall morphology and thermal properties of PVA/Dex blend xerogels crosslinked using the freeze–thawing cycle were investigated by Fathi et al. (2011). They demonstrated that the Tg of PVA/Dex xerogels did not show any significant changes due to increased dextran contents, but crystal size distribution and channeled morphology were evident for PVA/Dex samples of higher dextran contents. In the same context, an increase of dextran content caused a broader crystal size distribution, better, and lower thermal stability for PVA/Dex blend xerogels compared to virgin PVA xerogels.
4.1.3 PVA/starch and hydroxyethyl starch (HES)
Starch is one of the most abundant and the cheapest polysaccharides. Generally, starch consists of about 30% amylose and 70% amylopectin (Zhao et al., 2003). The chemical modifications of starch to improve its properties have attracted much attention, not only because starch is a very cheap polysaccharide but also because all starch derivatives are biocompatible and biodegradable polymers which make them to be widely used in most pharmaceutical and biomedical applications. However, polysaccharide polymers specifically starch has poor hydrophilicity, and it cannot form stable hydrogel alone, thus an effective method has been suggested to form stable hydrogels made by blending natural and synthetic polymers to meet the advantages of each other (Kaetsu, 1996). Limited studies on synthetic polymer/starch blend hydrogels have been reported previously in literatures (Hashim et al., 2000). Zhao et al. (2003) have prepared PVA/starch blend hydrogels that were crosslinked by γ-rays and electron beam radiation at room temperature. They found the components of starch affected strongly on physical properties of obtained PVA/starch hydrogels, where the amylose of starch was a key reactive component that influenced the grafting yield between PVA and starch beside the radiation crosslinking to form blend hydrogels (Zhao et al., 2003). Furthermore, it was found that PVA/starch hydrogels containing high contents of amylose had higher gel fraction, and higher mechanical tensile strength than those containing amylopectin (Zhao et al., 2003). This behavior can be elucidated by the fact that PVA/starch (high amylopectin content) had a bad inter-miscibility before radiation as compared with PVA/starch (high amylose content) which had an excellent miscibility before radiation crosslinking process.
Hydroxyethyl starch (HES) is a derivative of the natural polymer prepared by the reaction between amylopectin and ethylene oxide resulting in hydroxyethyl groups being added to oxygen at different carbon positions at glucopyranose units C2, C3 or C6 to be in the final form of α-1, 4-linked d-glucopyranose residues, (Fig. 1). HES has valuable medical applications as blood plasma expander, Leukapheresis agent, as cryo-preservative (Thomas, 2000), and in blood isotonic electrolyte solutions, which further evidenced its non toxicity, biodegradability, and biocompatibility with the human body (Heins et al., 1998). At present, HES is the most used polymer as blood plasma expander (Deitrich, 2001), and hydrogels for drug delivery applications (Kamoun and Menzel, 2012).
Kenawy et al. (2014) have suggested the synthesis route of ampicillin-loaded PVA/HES hydrogel membranes using the repeated freeze–thawing cycles. This study is regarded the first use of HES polymer blended with PVA hydrogels for wound healing purposes. The utilization of HES as blend polymer with PVA showed some advantages e.g. increase of HES portions in PVA hydrogel membranes increased significantly the thermal stability and amount of adsorbed protein in vitro. Likewise, formation of pores and spongy-shaped surface structure, high swellability, and increment of in vitro release profile of BSA were observed by increasing HES portions in PVA hydrogel membranes. Distinctions of physicochemical, thermal, morphological properties of PVA/HES hydrogel membranes due to addition HES portions have been ascribed to the high hydrophilicity and long chain structure of HES moieties, thus this study recommended the use of PVA-HES hydrogel membranes for biomedical applications e.g. as wound dressing polymeric materials.
4.1.4 PVA/glucan
(1–3), (1–6)-β-Glucan is a water soluble and biodegradable polymer derived from fermentation of plant incubation (Lee et al., 2003). It consists of β-(1–3) linked-d-glucose residues with one β-(1–6) linked-d-glucose group for every three glucose residues (Fig. 1). (1–3), (1–6)-β-glucan can support the immune response by activating macrophage cells. It shows antibacterial and antiviral effects, and is very effective as allogeneic, syngeneic, and anti-inflammatory, and exhibits the wound healing activity (Lee et al., 2003). However, studies on glucan in biomedical applications are relatively scarce; because glucan’s antibacterial activity has a remarkable advantage to be used as a wound dressing material. Huang and Brazel (2001) posited a new technique to form PVA/glucan films depending on physical blending, followed by drying at 110 °C without using chemical crosslinking for wound dressing purposes. The results revealed that no covalent bond between PVA and glucan was found in the formed film, therefore glucan can be released to facilitate wound healing depending on its anti-inflammatory property, where the healing time of wound was shortened by 48% (Huang and Brazel, 2001). Since a high glucan content with PVA film can hinder the cell mobility and prolong the time of healing, thus the ratio of blended glucan should be optimized with PVA. Additionally, incorporation of different portions of glucan into PVA films affected strongly the physicochemical properties of PVA/glucan films, where there was an increase in glucan content, the tensile strength of films decreased and the breaking elongation of films increased. Accordingly, the results of this work thus demonstrated that PVA/glucan films could be used as wound dressing that can also accelerate the wound healing as proved (Huang and Brazel, 2001).
4.1.5 PVA/chitosan
Chitosan, is a copolymer of glucosamine and N-acetyl glucosamine units linked by 1–4 glucosidic bonds (Fig. 1), it is derived by partial de-acetylation of chitin. Chitosan is one of the most abundant natural amino polysaccharides. Chitosan has several applications in pharmaceutics, biotechnology, and it is a well-known material in the wound dressing field (Zhao et al., 2003). It has huge biocompatibility, biodegradability, hemostatic, and excellent antibacterial activity. Chitosan with high molecular weights is insoluble in water but can dissolve in water-acetic acid solution. Hydrogels fabricated from chitosan water-acid solution often need a repeated washing process to neutralize or remove the excess of acid (Yang et al., 2008).
Cascone et al. (1999) have first revealed the blending of water-soluble chitosan to PVA, the PVA/chitosan hydrogel membranes have been obtained by repeated freeze–thawing cycles. The effects of chitosan blending on thermal stability and morphological structure of PVA/chitosan hydrogels have been reported here (Cascone et al., 1999). Their results explained that incorporation of high amounts of chitosan into PVA membranes seems to perturb the formation of PVA crystallites which resulted in a material with a less regular structure and a more porous filamentous matrix, additionally introducing chitosan in PVA hydrogel membranes did not clearly affect the PVA xerogel’s melting temperature and other thermal properties (Cascone et al., 1999).
Yang et al. (2004) explored the preparation of PVA/chitosan hydrogel membranes for biomedical applications. This unique synthesis route was based on the physical blending between different portions of PVA and water soluble chitosan followed by treatment with formaldehyde to convert –NH2 group of chitosan into –N⚌C group in PVA/chitosan membranes (Yang et al., 2004). This study exhibited that the values of water content, water vapor transmission, and permeability of loaded vitamin B12 through PVA/chitosan hydrogel membranes increase progressively with chitosan contents in the blended hydrogel membranes whereas, PVA/chitosan portions were not very compatible in the obtained blended hydrogel membranes and the crystalline area in PVA/chitosan hydrogel membranes decreased after treatment with formaldehyde (Yang et al., 2004).
Don et al. (2006) established a new grafting route for the synthesis of chitosan-g-PVA/PVA blend hydrogels for blood-contacting compatibility and wound dressing applications. The cellular and blood compatibility of pure PVA, pure chitosan, and chitosan-g-PVA/PVA hydrogels were tested separately using the viability of osteoblasts and the adhesion of platelets. The results exhibited that the cellular compatibility of chitosan-g-PVA/PVA blend hydrogels improved significantly due to incorporation of chitosan in the composition of blended hydrogels, while pure PVA hydrogels showed a good blood compatibility property and pure chitosan offered poor ones (Don et al., 2006). However, incorporation of small amounts of chitosan-g-PVA into PVA improved visibly the blood compatibility of the obtained chitosan-g-PVA/PVA blended hydrogels (Don et al., 2006).
El-Salmawi (2007) demonstrated the preparation of PVA/chitosan hydrogels using exposure to different doses of γ-radiation induced crosslinking. Chitosan has been blended to PVA in this study to prevent microbiological growth, such as bacteria, fungi and microorganisms (El-Salmawi, 2007). Results referred that the gel fraction and mechanical properties of blend hydrogels increased with increasing PVA concentration and irradiation dose which are satisfactory as a dressing material, while swelling ability of blend hydrogels increased with increasing the composition of chitosan increased in the blend which meets a wet environment requirement to a wound (El-Salmawi, 2007) whereas, microbe penetration test showed that the prepared PVA/chitosan hydrogels can be regarded as a good barrier against microbes, due to high crosslinking networks resulting from the high dose gamma irradiation-induced method (El-Salmawi, 2007).
Yang et al. (2008, 2010) have developed the synthesis route of PVA/chitosan hydrogel membranes using the freeze–thawing cycle, followed by γ-irradiation process. The physicochemical properties change and bio-evaluation results of PVA/chitosan hydrogel membranes have been studied in terms of the entanglement effect i.e., γ-irradiation followed by the freeze–thawing and the freeze–thawing followed by irradiation (Yang et al., 2008). They proved that PVA/chitosan hydrogel membranes made by irradiation followed by freeze–thawing showed a larger swelling capacity, high mechanical strength, lower water evaporation, and high thermal stability compared to those made by freeze–thawing alone or freeze–thawing followed by irradiation. Also, the results showed that PVA/chitosan hydrogel membranes made by irradiation alone are not suitable for wound dressings due to their mechanical strength weakness, additionally good antibacterial activity of chitosan in PVA/chitosan hydrogel membranes made by freeze–thawing followed by irradiation, has been verified against Escherichia coli with increasing chitosan content in hydrogel membranes (Yang et al., 2008).
For rapid wound dressing, Yang et al. (2010) suggested addition of the glycerol into PVA/chitosan hydrogels made by irradiation followed by freeze–thawing, to accelerate the healing process of wounds in a rat model. In this study, the MTT-assay showed that the extract of PVA/chitosan-glycerol hydrogel membranes was nontoxic toward L929 mouse fibroblast cells, furthermore mature epidermal architecture was formed after 11th day postoperatively for wounds treated with gauzed PVA/chitosan-glycerol dressing membranes (Yang et al., 2010). Their results indicated that PVA/chitosan-glycerol hydrogel membranes are a good dressing polymeric material.
In contrast, Sung et al. (2010) introduced minocycline-loaded PVA/chitosan hydrogel films wound dressings with an enhanced healing effect. The crosslinking of PVA/chitosan hydrogel films were carried out using the freeze–thawing method. Their physicochemical hydrogel properties, in vitro protein adsorption, release profile, in vivo wound healing effect, and histopathology were then studied. Their results concluded that high chitosan portions in PVA hydrogel films decreased gel fraction, mechanical properties, and thermal stability, while it increased the swelling ability, water vapor transmission, elasticity, and porosity of PVA/chitosan hydrogel films (Sung et al., 2010). Also, incorporation of minocycline did not affect hydrogel properties, but chitosan portions sharply affected protein adsorption and drug release (Sung et al., 2010). Their wound healing test results showed that minocycline-loaded PVA/chitosan hydrogel films gave faster healing of the wound made in the rat dorsum then the used conventional sterile gauze control, due to antibacterial and antifungal activities of chitosan, thus all these reported results proved that minocycline-loaded PVA/chitosan hydrogel films are very proper wound dressing materials (Sung et al., 2010).
4.1.6 PVA/chitosan derivatives
Antibacterial and antifungal activities of chitosan and its derivatives have been described elsewhere (Hirano et al., 1995). However, antibacterial activity of chitosan can be detected only in an acidic medium due to its poor water-solubility below pH 6.5. Therefore, water-soluble chitosan derivatives which are soluble in both acidic and basic physiologic conditions might be good candidates of chitosan derivatives over pure chitosan. Among chitosan derivatives, N-O-carboxymethyl chitosan (CM-chitosan),(Yang et al., 2008) carboxyethyl chitosan (CE-chitosan), (Xiao and Zhou, 2003) and quaternary chitosan (Q-chitosan) (Tashiro, 2001) have possessed more interest because of their excellent antibacterial and antifungal activities. As known, for improving and modifying the physicochemical properties of PVA hydrogel membranes, pure chitosan was usually utilized to blend with PVA to form hydrogels via several crosslinking methods (Cascone et al., 1999; Don et al., 2006; El-Salmawi, 2007; Sung et al., 2010; Yang et al., 2004, 2008, 2010). Notably, water-soluble chitosan derivative sheets and pastes separately without PVA, were evaluated previously in vitro for possible utilization in wound dressing applications (Rasad et al., 2010). However, the blending method of chitosan with PVA was not easy to form homogenous structure at ambient conditions due to poor hydrophilicity, poor miscibility with PVA, high viscosity, and acidic solubility of chitosan. Chitosan derivatives such as, CM-chitosan, CE-chitosan, and Q-chitosan blended with PVA hydrogels as wound dressing materials will be discussed in this section.
CM-chitosan was chosen for blending with PVA to address the last drawbacks of pure chitosan. CM-chitosan has good hydrophilicity at ambient conditions (Rasad et al., 2010), good miscibility with PVA in aqueous media, and excellent antibacterial activity as pure chitosan (Rasad et al., 2010). Zhao et al. (2003) explored a new crosslinking method for the synthesis of PVA/CM-chitosan blend hydrogels using electron beam irradiation (EB) at room temperature. They have reported that the mechanical properties and swelling degree improved obviously after adding CM-chitosan, while a grafting interaction between PVA and CM-chitosan molecules was observed under EB-irradiation beside the crosslinking of PVA molecules by irradiation. Moreover, PVA/CM-chitosan blend hydrogels showed considerable antibacterial activity against E. coli when the CM-chitosan concentration was of a little content in membranes (Zhao et al., 2003).
CE-chitosan/PVA nanofiber mats were prepared by electrospinning of aqueous CE-chitosan/PVA solution for skin regeneration and healing (Xiao and Zhou, 2003). Xiao and Zhou (2003) used mouse fibroblasts (L929) as reference cell line to evaluate CE-chitosan/PVA nanofiber mats in terms of skin regeneration in vitro experiments. The results of indirect cytotoxicity assessment indicated that CE-chitosan/PVA nanofiber mat was nontoxic to the L929 cells, while CE-chitosan/PVA nanofiber fibrous mats were good in promoting the L929 cell attachment and proliferation, showing CE-chitosan/PVA is a good polymeric candidate for skin regeneration and healing (Xiao and Zhou, 2003).
Q-chitosan is a chitosan derivative with quaternary ammonium groups which possesses high efficiency and excellent activity against bacteria and fungi. Q-chitosan membranes have been used previously as a cationic polymer for the cyto-plasmic membranes of bacterial cells (Tashiro, 2001) whereas, Ignatova et al. (2007) suggested the crosslinking method of PVA/Q-chitosan mats using photocrosslinking electrospinning technique, where the obtained PVA/Q-chitosan crosslinked electrospun mats exhibited efficient inhibition toward growth of Gram-positive and Gram-negative bacteria (Ignatova et al., 2007). The study results summarized that the crosslinked PVA/Q-chitosan electrospun mats are a promising polymeric candidate for wound dressing applications due to their excellent resistance against growth of bacteria and fungi. Similarly, Ignatova et al. (2006) suggested photocrosslinked electrospun nano-fibrous PVA/Q-chitosan mats, which exhibited high bacterial activity against the growth of Gram-negative bacteria E. coli and Gram-positive bacteria Staphylococcus aureus, these nano-fibrous mats proved their high potential for wound dressing applications (Ignatova et al., 2006).
4.1.7 PVA/gelatin (GE)
Gelatin is a protein produced by partial denaturalization of collagen extracted from the boiling of some materials such as, bones (27%), connective tissues or organs (28%), and the skin of certain animals (44%, usually cows and pigs). Gelatin possesses biological activities due to its natural origin, which makes it suitable for use as component of wound dressing materials, drug delivery carrier, and scaffolds for tissue engineering (Hago and Li, 2013). Gelatin has high ability to form strong hydrogels and transparent films that are easily designed as insoluble hydrophilic polymers for skin regeneration and tissue implantation. Hago and Li (2013) developed a new approach to prepare interpenetrating polymer network from PVA/GE hydrogels containing trans-glutaminase, the hydrogel components have been crosslinked by enzymatic and the repeated freeze–thawing method. The results revealed that the composition of interpenetrating hydrogels (i.e. GE concentration) and a number of freeze–thawing cycles were considered the effective conditions for the preparation of PVA/Ge hydrogels, due to the fact that the quantity of GE was the key factor to obtain interpenetrating PVA/GE hydrogels with desirable properties. Also, GE quantity played a vital role to form the morphological structure of PVA/GE hydrogels and fibroblasts that grew over the cells treated with extract solutions showed good proliferation behavior, which referring PVA/GE interpenetrating hydrogels have met all requirements to use for biomedical applications (Hago and Li, 2013).
4.2 Wound dressings based on PVA/synthetic polymers
In order to overcome somewhat mechanical and thermal deficiencies of the obtained hydrogel membranes due to blending of PVA with natural polymers, thus the biological synthetic polymers such as poly(N-vinylpyrrolidone) (PVP), poly(N-isopropylacrylamide) (NIPAAm), and polyethylene glycol (PEG) have been widely studied but their properties need to be improved further for specific medical applications. These polymers have been suggested for blending with PVA hydrogel membranes for wound dressing applications, (Fig. 2).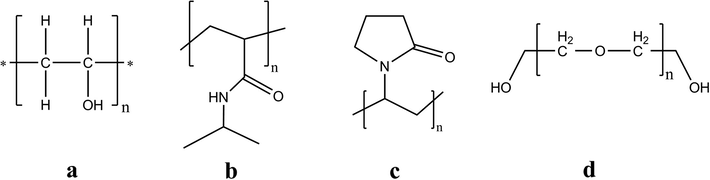
Chemical structures of synthetic polymers which were blended with PVA hydrogels to form wound dressing materials, such as poly(vinyl alcohol) (PVA) (a), poly N-isopropylacrylamide (b), polyvinylpyrrolidone, PVP (c), and polyethylene glycol (PEG) (d).
4.2.1 PVA/poly(N-vinylpyrrolidone) (PVP)
PVP is one of the most popular water-soluble, biodegradable, biocompatible, and extremely low cytotoxicity synthetic polymers (Razzak et al., 2001). It has been used previously based on hydrogels for skin regeneration and wound dressing applications by Darwis et al. (1993) and Himly et al. (1993). They have prepared PVP hydrogel membranes using radiation crosslinking, PVP hydrogel membranes were elastic, fixable, transparent, and impermeable for bacteria, the attached cells on the obtained hydrogel membranes were suitable only for healthy skin but not for wound dressing or suitable for wound dressing only in a tropical environment (Darwis et al., 1993; Himly et al., 1993). Similarly, Himly et al. (1993) reported that the addition of poly(ethylene glycol) PEG as pours-former to the PVP hydrogel composition could enhance the performance of the hydrogel barrier against bacterial growth.
Razzak et al. (2001) established co-polymeric hydrogels of PVA/PVP using 60Co γ-ray irradiation crosslinking process. They have reported that the blending of PVP improved significantly physicochemical properties of PVA hydrogels such as water content and water adsorption, while PVA/PVP hydrogels crosslinked by irradiation at 20 kGy were a good barrier against microbes and E. coli, which supports the usage idea of PVA-PVP hydrogels as a burn wound covering which meet all requirements of an idea of wound dressing (Razzak et al., 2001).
Park and Chang (2003) suggested a new way for the synthesis of two-layer hydrogels consisting of a polyurethane membrane cover and a mixture of PVA/PVP-glycerin-chitosan was crosslinked by γ-irradiation or two steps of freeze–thawing followed by γ-irradiation for wound dressing purposes. The physical properties of PVA/PVP hydrogels covered with polyurethane such as gel fraction and mechanical strength have improved obviously when PVA/PVP membranes were made by two crosslinking steps of freeze–thawing cycles followed by irradiation or increasing irradiation dose, compared to hydrogel membranes made by only an irradiation process which showed weak strength (Park and Chang, 2003). Also, evaporation speed of water and permeation rate of PVA/PVP hydrogel membranes were reduced when latter hydrogel membranes were covered by polyurethane membranes (Park and Chang, 2003).
Singh and Ray (1994) developed a radiation crosslinking for PVA/PVP modified with sterculia gum polysaccharide hydrogel membranes as delivery of antibacterial agent to the wounds. The results exhibited that the swelling degree of PVA/PVP/sterculia gum hydrogel membranes increased with an increase of amounts of N-vinylpyrrolidone (NVP) and sterculia gum, while it decreased with an increase of the radiation dose due to formation of long crosslinked chains and thermal stability of hydrogel membranes increased with an increase in amounts of NVP and gum (Singh and Ray, 1994). Interestingly, the simulated release study showed that, the decrease of swelling in simulated wound fluid (membranes have low NVP and gum contents) is probably due to the very high ionic strength of the simulated wound fluid, owing to the difference in the concentration of mobile ions between the gel and the solution is reduced causing a decrease in the osmotic swelling pressure of these mobile ions inside the gel (Singh and Ray, 1994). Thus, PVA/PVP/sterculia gum hydrogel membranes have been explored as wound dressing materials.
4.2.2 PVA/poly(N-isopropylacrylamide) (NIPAAm)
N-isopropylacrylamide is a water–soluble monomer; poly(N-isopropylacrylamide) (NIPAAm) is a thermally reversible hydrogel with a lower critical solution temperature (LCST) of around 32 °C in water. The cross-linked gel of this material swells and shrinks at temperatures below and above the LCST respectively, therefore a poly NIPAAm delivery system can provide sustained therapeutic levels of a drug by responding to the physiological signals of the body (Ogata et al., 1995). Thus, PNIPAAm hydrogels have been extensively studied as controlled drug delivery systems (Wei et al., 2007). The potential toxicity of this polymer has been tested subcutaneously and results did not show any toxic effects, additionally its high biocompatibility with the human body (Malonne et al., 2005).
Azarbayjani et al. (2010) developed a sustained topical drug delivery of levothyroxine (T4) loaded on PVA/PNIPAAm nanofibrous membranes using the electrospinning process. These nanofibrous membranes have been applied to reduce deposits of adipose tissue on the skin. Bio-evaluation results in vitro of this study showed that the release of T4 from nanofibrous mats was found to be a function of PNIPAAm concentration used and the release profile increased with the increase of PNIPAAm content compared to mats containing low PNIPAAm concentration (Azarbayjani et al., 2010). This could be explained by the high water solubility of PNIPAAm which dissolved almost immediately leading to a rapid release of T4. Notably, in vitro skin permeation results outlined that blending of PVA and PNIPAAm increased the skin retention of T4 when compared to pure PNIPAAm nanofiber mat containing T4. This means that PVA portions increased mechanical and thermal stability of mats, while PNIPAAm improved physicochemical, polymer erosion, and permeation properties of nanofiber mats which is found to be suitable for skin dressing.
4.2.3 PVA/polyethylene glycol (PEG)
PEG is a polyether compound, water-soluble amphiphilic polymer, transparent, colorless, liquid, and viscous polymer, due to its extreme biocompatibility and biodegradability; PEG was used in industrial manufacturing of medicines. Dutta (2012) reported PVA/PEG/CaCl2 hydrogels crosslinked by exposure of the hydrogel components to γ-irradiation for wound dressing applications. CaCl2 has been added to hydrogel components as gelling and plasticizer material, where it enhanced the synergistic effect of PEG to form strength hydrogel. Both physicochemical properties and thermal stability of formed PVA/PEG/CaCl2 hydrogels improved after incorporation of PEG, while microbial penetration test revealed that PVA/PEG/CaCl2 hydrogels could be considered as a good barrier against microbial permeation and no inhibition on cell proliferation was detected in the cytotoxicity test (Dutta, 2012). The results of this work showed PVA/PEG/CaCl2 hydrogels could be considered as potential wound dressing materials.
4.3 Wound dressings based on PVA/composite polymers or (blended polymers with nanoparticles)
The rigid and fragile nature of the hydrogel polymers may be unfavorable in processing into non-spherical polymer forms, for example membranes, films, or filamentous via gel state. Methods have been suggested to overcome this drawback by blending with high strength compatible and flexible composites e.g., certain synthetic polymers or nano-fillers (e.g. minerals, clays, or calcium phosphate nanoparticles).
Abd El-Mohdy (2013) represented Ag nanoparticles supported within PVA/cellulose acetate/gelatin composite hydrogels have been successfully synthesized using gamma radiation-induced crosslinking as novel in-situ method for wound dressing purposes. The results indicated that Ag nanoparticles inhibited the crystallization degree of PVA-based gel, however Ag nanoparticles based on PVA/cellulose acetate/gelatin hydrogels were found to have antimicrobial activity against various fungus and bacteria. Meanwhile, the antimicrobial activity was significantly improved by the increasing of AgNO3 nanoparticles content in composite hydrogel. However, the neat hydrogel composite (without Ag-nanoparticles) showed higher inhibition toward in vitro bacterial adhesion compared to Ag-nanocomposite hydrogel (Abd El-Mohdy, 2013). Similarly, PVA/chitosan/Ag nanoparticles fibrous mats were prepared by electrospinning for wound healing applications (Li et al., 2013).
Li et al. (2013) revealed that PVA/chitosan/Ag nanoparticle nanofibers have strongly inhibited growth of E. coli and S. aureus bacteria due to Ag nanoparticles, also PVA-chitosan/Ag nanoparticle nanofibers should be of greater interest than PVA/chitosan/AgNO3 nanofibers for wound dressing applications.
Nano-minerals and nano-clays based nano composites showed notable improvements in several properties compared to neat polymer hydrogels or conventional micro-and macro-composites. These improvements mainly increased mechanical strength, thermal resistance, and decreased gas permeability and flammability. Thus, these nanocomposites could be an ideal wound and burn dressing material, better than conventional neat hydrogels.
In order to obtain wound dressing with better properties entirely nanocomposite hydrogels based on PVA and organically modified sodium-montmorillonite (Na-MMT) clay were introduced as a novel wound dressing polymeric material for the first time in literatures by Kokabi et al. (2007). PVA/MMT nanocomposite hydrogel components were crosslinked using repeated freeze–thawing cycles. According to the reported results, the PVA-MMT clay nanocomposite hydrogels achieved the essential requirements of ideal wound dressing materials e.g. excellent physical, mechanical, and morphological properties. For example; PVA/MMT nanocomposite hydrogels showed high absorbing fluids which are recommended for exudates wound, high elasticity which candidate for wounds under high stress areas, and adequate water vapor transmission rates which indicated that they could keep the local moist environment to accelerate the rate of wound healing (Kokabi et al., 2007).
In the same context, Gonzalez et al. (2011) prepared PVA/bentonite, PVA/Ag nanoparticles, PVA/clove extract, and PVA/cellulose nanocomposite hydrogel using the freeze–thawing method. They demonstrated that PVA/clove hydrogels did not exhibit a homogenous aspect, while PVA/bentonite and PVA/Ag nanocomposite hydrogels showed significant antimicrobial activity against growth of E. coli, good water vapor transmission rate, and adequate water absorbing capacity due to addition of clay and Ag nanoparticles as filler (Gonzalez et al., 2011).
A new approach of PVA/chitosan/ZnO nanocomposite hydrogels was offered by Vicentini et al. (2010). They designed hydrogel components crosslinked by physical blending between PVA and chitosan portions in the presence of glycerin and tween-80 (T80) as plasticizers. The results demonstrated that the addition of ZnO nanoparticles and plasticizers (glycerin and T80) to PVA/chitosan film influenced strongly many of the hydrogel film properties for example, greater thermal stability, a decrease in tensile strength and elongation to break, while porous hydrogel structure was formed due to the increase in T80 concentration. Also, antibacterial activity against growth of S. aureus was observed for PVA/chitosan/ZnO nanocomposite hydrogel compared to PVA/chitosan neat hydrogel, this is ascribed to the incorporation of ZnO nanoparticles into the films (Vicentini et al., 2010).
Nano ZnO composite nanofibers as antibacterial wound dressing materials have been prepared using an advanced synthetic route by Shalumon et al. (2011). ZnO nanoparticles were incorporated into PVA/sodium alginate hydrogel nanofibers using the electrospinning technique. In this study, authors regarded that the addition of ZnO into PVA/SA is a key factor to change many of the hydrogel nanofiber properties. Results demonstrated that incorporation of low ZnO nanoparticle concentration into PVA/SA hydrogel mats is less toxic and good L929 cells spread than those with higher concentrations of ZnO, which is toxic in nature. Moreover, PVA/SA/ZnO mats showed excellent antibacterial activity and formed the inhibition zone against the growth of E. coli and S. aureus due to the presence of ZnO nanoparticles in the mat components. Accordingly, this study suggested that PVA/SA/ZnO nanofiber mats could be potential wound dressing composite materials with the optimal ZnO concentration which gave least toxicity (Shalumon et al., 2011).
Polymer–polymer composites and hybrid-polymers have been used to improve the mechanical properties and biological activities for more suitable wound dressing based on composite materials. Polymer–polymer composite hydrogels based on PVA/PVP/kappa-carrageenan (KC)/silk powder were prepared for wound dressing applications. Hydrogel components were crosslinked by electron beam irradiation and γ-irradiation was then used to sterilize the obtained hydrogels (Wu et al., 2001). Wu et al. (2001) revealed that the physical and mechanical properties of PVA/PVP/KC/silk hydrogels were greatly improved by the addition of homo-polymers, where the gel formation was raised and water evaporation from hydrogel was retarded after mixing KC to hydrogel component, while mechanical properties of formed hydrogels were obviously increased after mixing silk powder in homogenous solution (Wu et al., 2001). Interestingly, the PVA/PVP/KC/silk irradiated hydrogels did not induce any acute toxicity, which make them to be used as significant fast healing composite-materials for burns and wounds.
Uslu et al. (2010) developed a series of PVA/PVP/PEG hybrid nanofibers, which were fabricated using the electrospinning method. Hydroxypropyl methylcellulose (HPMC) was added to the latter hydrogel mixture components for its high water retention capacity, in addition Aloe vera was also added as accelerator for healing rate and as a therapeutic herb due to its resistance against bacterial and fungal growth (Uslu et al., 2010). According to obtained results, addition of Aloe vera to PVA/PVP/PEG hybrid polymer mixture might affect the crystal structure and efficiently crosslinked hybrid polymer forming amorphous structure and higher melting temperature (Tm), due to an increase in viscosity and conductivity of hybrid polymers (Uslu et al., 2010). Similarly, addition of Aloe vera affected greatly the morphological structure of hybrid polymer by a decrease in the formed nanofiber diameter, forming finer nanofiber without any beading, this structure might lead to an increase in porosity and therapy facilitating the penetration of oxygen and moisture to the wound which are regarded potential factors for faster healing rates. Thus, addition Aloe vera to hybrid polymer was considered for enhancing the therapeutic, thermal and mechanical properties of PVA/PVP/PEG hybrid nanofibers for wound dressing.
5 Conclusions
This review article presents an extensive overview of published studies on crosslinked PVA/polymer blended hydrogels for wound and burn dressing, using different crosslinking methods e.g. physical, chemical, and irradiation methods. Wound dressings based on natural polymers, synthetic polymers, their derivatives, and composites have been discussed in addition to the influence of blended polymer or composite on PVA hydrogel properties and biological activities which have been explored in detail as well. From this review, the reported wound dressing based on PVA/natural polymer blended hydrogels seems to be excellent dressing materials for the wound healing. Hence the entirely blended natural polymers greatly improved the physicochemical properties of fabricated hydrogels e.g. water absorption capacity, water–vapor permeability and transmission which guarantee one of the most important dressing material requirements that are the local moisture environment character. Additionally, natural polymers specifically chitosan, its derivatives, and glucan drastically enhanced the biological activities of PVA blended hydrogels compared to those neat ones. Limited synthetic polymers have been blended to PVA for wound dressing as compared with natural polymer types, where synthetic polymers showed a prominent improvement for hydrogel properties which are confined to thermal and mechanical properties. It was clear that, PVA-hydrogel wound dressings based on synthetic polymers differed in their characteristics due to various chemical structures and diversely used methods for crosslinking hydrogel components. Nanocomposite hydrogel dressing materials based on using clays and nano-inorganic particles as fillers to improve the mechanical properties, are very restricted as wound dressing due to their non biodegradability at skin environment and their somewhat cytotoxicity at high concentration as found in the case of ZnO nanoparticles. Some alternative materials used for improving mechanical properties of PVA/composite hydrogels are hybrid polymers and some additives to achieve this role. In another study, Aloe vera was added to hybrid polymer components as accelerator for healing rate, due to its biological activity, thermal stability, and viscosity; while hydroxypropyl methylcellulose was added to keep water-retention in hydrogel structure. It is still possible to design new hydrogels fulfilling specific functions for specific needs. Finally, we might thus decide that, natural polymers excelled synthetic polymers, as good candidates for wound dressing materials, while some additives to hybrid polymers replaced the use of nanocomposite clay, which might thus advance the therapeutic effect for wound dressing applications.
Acknowledgements
E. A. Kamoun is thankful to the Ministry of Higher Education of Egypt, Sector of Cultural Affairs & Missions, Program of Partnership and Ownership Initiative (ParOwn-0911, ID:20246) for the financial support and grant. X. Chen is thankful to the National High Technology Research and Development Program of China (863 Program) (No. 2012AA030309) and the National Natural Science Foundation of China (No. 21274028) for financial support.
References
- Radiation synthesis of nanosilver/poly vinyl alcohol/cellulose acetate/gelatin hydrogels for wound dressing. J. Polym. Res.. 2013;20:177-188.
- [Google Scholar]
- Smart polymeric nanofibers for topical delivery of levothyroxine. J. Pharm. Pharm. Sci.. 2010;13:400-410.
- [Google Scholar]
- Effect of chitosan and dextran on the properties of poly(vinyl alcohol) hydrogels. J. Mater. Sci. Mater. Med.. 1999;10:431-435.
- [Google Scholar]
- Study on gelatin-containing artificial skin. Part I. Preparation and characteristics of novel gelatin-alginate sponge. Biomaterials. 1999;20:409-417.
- [Google Scholar]
- Chitosan based systems for tissue engineering Part II: soft tissues. FABAD J. Pharm. Sci.. 2008;33:211-216.
- [Google Scholar]
- Polysaccharide hydrogels for modified release formulations. J. Controlled Release. 2007;119:5-24.
- [Google Scholar]
- Hydrogel membranes with mesh size asymmetry based on the gradient crosslinking of poly(vinyl alcohol) J. Membr. Sci.. 1999;156:67-79.
- [Google Scholar]
- Poly(N-vinylpyrrolidone) hydrogels: 1. Radiation polymerization and crosslinking of N-vinylpyrrolidone. Radiat. Phys. Chem.. 1993;42:907-910.
- [Google Scholar]
- Preparation and characterization of chitosan-g-poly(vinyl alcohol)/poly(vinyl alcohol) blends used for the evaluation of blood-contacting compatibility. Carbohydr. Polym.. 2006;63:331-339.
- [Google Scholar]
- Synthesis and characterization of γ-irradiated PVA/PEG/CaCl2 hydrogel for wound dressing. Am. J. Chem.. 2012;2:6-11.
- [Google Scholar]
- Gamma radiation-induced crosslinked PVA/chitosan blends for wound dressing. J. Macromol. Sci. Part A: Pure Appl. Chem.. 2007;44:541-545.
- [Google Scholar]
- Physically crosslinked polyvinyl alcohol–dextran blend xerogels: morphology and thermal behavior. Carbohydr. Polym.. 2011;84:145-152.
- [Google Scholar]
- Gonzalez, J.S., Maiolo, A.S., Ponce, A.G., Alvarez, V.A., 2011. Composites based on poly(vinyl alcohol) hydrogels for wound dressing. XVIII The Argentine Congress of Bioengineering and Clinical Engineering Conference VII, SABI 2011, 1–4.
- Interpenetration polymer network hydrogels based on gelatin and PVA by biocompatible approaches: synthesis and characterization. Adv. Mater. Sci. Eng. 2013 ID 328763, 1–8
- [Google Scholar]
- Hashim, K., Dahlan, K.Z., Noordin, N.M., 2000. Hydrogel for sago starch/water-soluble polymers by electron beam irradiation technique. International Symposium on Radiation Technology in Emerging Industrial Applications International Atomic Energy Agency (IAEA)-SM-365, 79–80.
- Structure and applications of poly(vinyl alcohol) hydrogels produced by conventional crosslinking or by freezing/thawing methods. Adv. Polym. Sci.. 2000;153:37-65.
- [Google Scholar]
- Characterization of acetyl starch by means of NMR spectroscopy and SEC/MALLS in comparison with hydroxyethyl starch. Starch. 1998;50:431-437.
- [Google Scholar]
- Novel crosslinking methods to design hydrogels. Adv. Drug Delivery Rev.. 2002;54:13-36.
- [Google Scholar]
- Poly(n-vinylpyrrolidone) hydrogels: 2. Hydrogel composites as wound dressing for tropical environment. Radiat. Phys. Chem.. 1993;4:911-914.
- [Google Scholar]
- Gebelein C.G., Carraher C.E.E., eds. Industrial Biotechnological Polymers. Lancaster: Technomic; 1995. p. :189.
- Review On the importance and mechanisms of burst release in matrix-controlled drug delivery systems. J. Controlled Release. 2001;73:121-136.
- [Google Scholar]
- Gentamicin-loaded wound dressing with polyvinyl alcohol/dextran hydrogel: gel characterization and in vivo healing evaluation. AAPS Pharm. Sci. Tech.. 2010;11:1092-1103.
- [Google Scholar]
- Poly(vinyl alcohol) hydrogels as soft contact lens material. J. Biomater. Sci. Polym. Ed.. 1994;5:397-406.
- [Google Scholar]
- Electrospun nano-fibre mats with antibacterial properties from quaternized chitosan and poly(vinyl alcohol) Carbohydr. Res.. 2006;341:2098-2107.
- [Google Scholar]
- Novel antibacterial fibers of quaternized chitosan and poly(vinyl pyrrolidone) prepared by electrospinning. Eur. Polym. J.. 2007;43:1112-1122.
- [Google Scholar]
- Isothermal photo differential scanning calorimetry. Crosslinking polymerization of multifunctional monomers in presence of visible light photoinitiator. J. Therm. Anal. Calorim.. 2001;65:435-443.
- [Google Scholar]
- Stimule-sensitive hydrogels. In: Dumitriu S., ed. Polysaccharides in Medicinal Application. New York: Marcel Dekker; 1996. p. :243-265.
- [Google Scholar]
- Crosslinking behavior of dextran modified with hydroxyethyl methacrylate upon irradiation with visible light- effect of concentration, coinitiator type, and solvent. J. Appl. Polym. Sci.. 2010;117:3128-3138.
- [Google Scholar]
- HES-HEMA nanocomposite polymer hydrogel: swelling behavior and characterization. J. Polym. Res.. 2012;19:9851-9865.
- [Google Scholar]
- Moist wound healing with occlusive dressings, a clinical review. Dermatol. Surg.. 1995;21:583-590.
- [Google Scholar]
- Physically crosslinked poly(vinyl alcohol)-hydroxyethyl starch blend hydrogel membranes: synthesis and characterization for biomedical applications. Arab. J. Chem.. 2014;7:372-380.
- [Google Scholar]
- Chitin-methacrylate: preparation, characterization, and hydrogel formation. Materials. 2011;4:1728-1746.
- [Google Scholar]
- Development of polyvinyl alcohol–sodium alginate gel-matrix-based wound dressing system containing nitrofurazone. Int. J. Pharm.. 2008;359:79-86.
- [Google Scholar]
- PVA–clay nanocomposite hydrogels for wound dressing. Eur. Polym. J.. 2007;43:773-781.
- [Google Scholar]
- Effect of the morphology of hydrophilic polymeric matrices on the diffusion and release of water soluble drugs. J. Membr. Sci.. 1981;9:211-227.
- [Google Scholar]
- Alginate: properties and biomedical applications. Prog. Polym. Sci.. 2012;37:106-126.
- [Google Scholar]
- Bio-artificial skin composed of gelatin and (1–3), (1–6)-β-Glucan. Biomaterials. 2003;24:2503-2511.
- [Google Scholar]
- Limonene encapsulation in alginate-poly(vinyl alcohol) Procedia Food Sci.. 2011;1:1816-1820.
- [Google Scholar]
- Silver nanoparticle/chitosan oligosaccharide/poly(vinyl alcohol) nanofibers as wound dressing: a preclinical study. Int. J. Nanomed.. 2013;8:4131-4145.
- [Google Scholar]
- Microencapsulated islets as bioartificial endocrine pancreas. Science. 1980;210:908-910.
- [Google Scholar]
- Preparation of poly(N-isopropylacrylamide) copolymers and preliminary assessment of their acute and subacute toxicity in mice. Eur. J. Pharm. Biopharm.. 2005;61:188-194.
- [Google Scholar]
- Crosslinked polyanhydrides for use in orthopedic applications: degradation behavior and mechanics. J. Biomed. Mater. Res.. 1998;46:271-278.
- [Google Scholar]
- Permeation of solutes with different molecular size and hydrophobicity through the poly(vinyl alcohol)-graft-N-isopropylacrylamide copolymer membrane. J. Membr. Sci.. 1995;103:159-165.
- [Google Scholar]
- Synthesis of PVA/PVP hydrogels having two-layer by radiation and their physical properties. Radiat. Phys. Chem.. 2003;67:361-365.
- [Google Scholar]
- Turbidimetric studies of aqueous poly(vinyl alcohol) solutions. Macromol. Chem.. 1975;176:3433-3440.
- [Google Scholar]
- Crosslinked poly(vinyl alcohol) hydrogels as swollen elastic networks. J. Appl. Polym. Sci.. 1977;21:1763-1770.
- [Google Scholar]
- Development of semicrystalline poly(vinyl alcohol) hydrogels for biomedical applications. J. Biomed. Mater. Res.. 1977;11:423-434.
- [Google Scholar]
- Short review: chitosan-hydroxyapatite composites. Carbohydr. Polym.. 2013;93:256-262.
- [Google Scholar]
- Adsorption and release studies of sodium ampicillin from hydroxyapatite and glass-reinforced hydroxyapatite composites. Biomaterials. 2001;22:1393-1400.
- [Google Scholar]
- In vitro evaluation of novel chitosan derivatives sheet and paste cytocompatibility on human dermal fibroblasts. Carbohydr. Polym.. 2010;79:1094-1100.
- [Google Scholar]
- Irradiation of polyvinyl alcohol and polyvinyl pyrrolidone blended hydrogel for wound dressing. Radiat. Phys. Chem.. 2001;62:107-113.
- [Google Scholar]
- Secondary and tertiary structure of polysaccharides in solutions and gels. Angew. Chem., Int. Ed. Engl.. 1997;16:214-224.
- [Google Scholar]
- Sodium alginate/poly(vinyl alcohol) nano ZnO composite nanofibers for antibacterial wound dressing. Int. J. Biol. Macromol.. 2011;49:247-254.
- [Google Scholar]
- PVA/polysaccharides blended films: mechanical properties. J. Mater.. 2013;2013:1-6.
- [Google Scholar]
- Graft copolymerization of 2-hydroxyethyl methacrylate onto chitosan films and their blood compatibility. J. Appl. Polym. Sci.. 1994;53:1115-1121.
- [Google Scholar]
- Gel characterization and in vivo evaluation of minocycline-loaded wound dressing with enhanced wound healing using polyvinyl alcohol and chitosan. Int. J. Pharm.. 2010;392:232-240.
- [Google Scholar]
- Calcium alginate/PVA blended nano fiber matrix for wound dressing. Indian J. Fibre Text. Res.. 2012;37:127-132.
- [Google Scholar]
- Antibacterial and bacterium adsorbing macromolecules. Macromol. Mat. Eng.. 2001;286:63-87.
- [Google Scholar]
- Morphology and mechanical properties of pullulan/poly(vinyl alcohol) blends crosslinked with glyoxal. J. Appl. Polym. Sci.. 2001;82:2273-2280.
- [Google Scholar]
- Alginate dressings in surgery and wound management: Part 1. J. Wound Care. 2000;9:56-60.
- [Google Scholar]
- Preparation and properties of electrospun poly(vinyl alcohol) blended hybrid polymer with Aloe vera and HPMC as wound dressing. Hacet. J. Biol. Chem.. 2010;38:19-25.
- [Google Scholar]
- Self-gelling hydrogels based on oppositely charged dextran microsphere. Biomaterials. 2005;26:2129-2135.
- [Google Scholar]
- Chitosan/poly(vinyl alcohol) films containing ZnO nanoparticles and plasticizers. Mater. Sci. Eng. C. 2010;30:503-508.
- [Google Scholar]
- Self-assembled, thermosensitive micelles of a star block copolymer based on PMMA and PNIPAAm for controlled drug delivery. Biomaterials. 2007;28:99-107.
- [Google Scholar]
- Formation of the scab and the rate of epithelization of superficial wounds in the skin of the young domestic pig. Nature. 1962;193:293-294.
- [Google Scholar]
- Irradiation of crosslinked, poly(vinyl alcohol) blended hydrogel for wound dressing. J. Radioanal. Nucl. Chem.. 2001;250:391-395.
- [Google Scholar]
- Synthesis and properties of degradable poly(vinyl alcohol) hydrogel. Polym. Degrad. Stab.. 2003;81:297-301.
- [Google Scholar]
- Evaluation of chitosan/PVA blended hydrogel membranes. J. Membr. Sci.. 2004;236:39-51.
- [Google Scholar]
- Investigation of PVA/ws-chitosan hydrogels prepared by combined gama-irradiation and freeze–thawing. Carbohydr. Polym.. 2008;73:401-408.
- [Google Scholar]
- Cytotoxicity and wound healing properties of PVA/ws Chitosan/glycerol hydrogels made by irradiation followed by freeze–thawing. Radiat. Phys. Chem.. 2010;79:606-611.
- [Google Scholar]
- Yannas, 1985. Process for Forming Multilayer Bioreplaceable Blood Vessel Prosthesis. U.S. Pat. 4,787,900.
- Yannas, Forbes, M.J., 1982. Procedures for Preparing Composite Materials from Collagen and Glycosaminoglycan. U.S. Pat. 4,350,629.
- Yannas, Gordon, P.L., Huang, C., Silver, F.H., Burke, J.F., 1981. Crosslinked Collagen-Mucopolysaccharide Composite Materials. U.S. Pat. 4,280,954.
- Yannas, Burke, J.F., Orgill, D.P., Skrabut, E.M., 1983. Method of Promoting the Regeneration of Tissue at a Wound. U.S. Pat. 4,418,691.
- Morphology and structure of highly elastic poly(vinyl alcohol) hydrogel prepared by repeated freezing- and melting. Colloid Polym. Sci.. 1986;264:595-601.
- [Google Scholar]
- Synthesis of antibacterial PVA/CM-chitosan blend hydrogels with electron beam irradiation. Carbohydr. Polym.. 2003;53:439-446.
- [Google Scholar]
- Antibiotic-eluting medical devices for various applications. J. Controlled Release. 2008;130:202-215.
- [Google Scholar]
- Tailoring the pore architecture in 3-D alginate scaffolds by controlling the freezing regime during fabrication. Biomaterials. 2002;23:4087-4094.
- [Google Scholar]







