Cs promoted Ni/ZrO2-Al2O3 catalysts for dry reforming of methane: Promotional effects of Cs for enhanced catalytic activity and stability
⁎Corresponding authors. aalfatesh@ksu.edu.sa (Ahmed S. Al-Fatesh), Gmotari@kacst.edu.sa (Ghzzai Almutairi), nk_labhsetwar@neeri.res.in (Nitin K. Labhasetwar)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
Carbon Capture and Utilization (CCU) technologies offer a promising avenue for transforming captured CO2 into valuable products, serving as renewable fuels or precursors for high-value synthesis. This study explores the dry reforming of methane (DRM) as a viable pathway to convert captured CO2 and CH4 into syngas, achieving high equilibrium conversion through the use of suitable catalysts. Conventional nickel-based catalysts are susceptible to carbon deposition, necessitating innovative approaches to enhance their performance. A tubular microreactor was employed to conduct the reforming process at 800 °C, utilizing Cs-promoted Ni catalysts supported on 90 % Al2O3 and 10 % ZrO2-based support composition. Catalyst preparation involved the impregnation technique, and subsequent characterization employed N2-physisorption, XRD, H2-TPR, TGA, TPD, and Raman spectroscopy. The DRM reaction was systematically investigated using the Ni/ ZrO2- Al2O3 catalysts, with a specific focus on the catalytic effects of Cs promotion. Observations revealed that Cs incorporation onto the ZrO2- Al2O3 matrix led to a substantial increase in hydrogen yield and selectivity across all catalyst compositions, accompanied by a significant reduction in carbon deposition on the catalyst surface. The optimal Cs loading, determined to be 3 wt% over Ni/ ZrO2- Al2O3 catalyst, exhibited CO2 and CH4 conversions of 90 % and 87 %, respectively, with an H2/CO yield approaching 1 (0.95). This research underscores the potential of Cs-modified catalysts in enhancing the efficiency of DRM for CCU applications, providing valuable insights into optimizing catalyst formulations for improved performance in carbon transformation processes.
Keywords
Methane
Reforming
Syngas
Catalyst
Caesium
Nickel
1 Introduction
Fossil fuels comprising natural gas, oil, and coal have been used as primary energy sources leading to the evolution of greenhouse gases (GHGs), which contribute consequentially to global climate change (Ritchie et al., 2022). Before the industrial revolution, a balance in the biogeochemical carbon cycle maintained carbon dioxide levels among greenhouse gases at roughly 280 parts per million (Hashimoto, 2019). For roughly a century following the Industrial Revolution, it rose to more than 290 ppm. The world's industrial progress during the next 100 years after the 1870 s caused it to continuously rise at a rate of 0.28 ppm annually. As of May 2022, the average global concentration of CO2 in the atmosphere is 421 ppm or 0.04 % (van Marle et al., 2022). This represents a 50 % increase from 280 ppm during the 10,000 years before to the mid-18th century when the Industrial Revolution began. The two major greenhouse gases are carbon dioxide and methane and their control as well as sustainable utilization is therefore of great significance. The conversion of CO2 and its utilization in chemical processes is a challenge in itself because of the thermodynamic stability of CO2 molecules. Dry reforming of methane (DRM) is considered a promising pathway in the context of fuel and chemical production from captured CO2 (Al-Mamoori et al., 2017). DRM has attracted significant attention for syngas (synthesis gas) production. It serves as feedstock for industrial Fischer-Tropsch processes. The additional benefit of DRM is that the process does not require the separation of CO2 from CH4, which makes the use of renewable biogas (Herout et al., 2011). The formation, regulation, and elimination of carbon on Ni-based catalysts during CO2 and CH4 conversion via the dry reforming process were extensively studied and recently reviewed by Baharudin et al. (Baharudin et al., 2022) in their recent publication. Similarly, by evaluating the advancements noted in the synergistic interaction among catalyst components, Abdulrasheed et al. (Abdulrasheed et al., 2019) discussed the most recent advances in catalyst development for methane dry reforming to syngas. The latest efforts to encourage the dry reforming of methane to syngas generation via bimetallic catalyst compositions were also explored in Yentekakis et al.'s review (Yentekakis et al., 2019a). Despite the great potential of the DRM process, the process is not industrially mature primarily because of the very high endothermicity of the reaction and the rapid carbon formation over the catalyst. The DRM reaction is a complex system consisting of several side reactions as given below (Jafarbegloo et al., 2015).
The reactions contributing to coke formation take place in the temperature range of 546–700 ℃ and these reactions are less favored at high temperatures, hence, for this reason, the DRM reaction is carried out at higher temperatures greater than 700 °C (Bach et al., 2020; Cao et al., 2018; ZHANG et al., 2007). However, the major challenges associated with catalyst development for DRM reaction are catalyst deactivation due to coke deposition over the catalyst surface, catalyst stability, and metal sintering at high DRM operating temperatures (Nematollahi et al., 2011; Pakhare and Spivey, 2014). Recent studies have shown that metal particle sintering phenomena could be resolved for high-temperature catalytic processes by designing supported catalysts with supports offering high lattice oxygen lability and oxygen vacancies (Goula et al., 2019; Nikolaraki et al., 2021; Yentekakis et al., 2019b). They consider that catalysts designed in this way can also induce reaction rate bifunctionalities and carbon deposition tolerance (Yentekakis et al., 2019c). Noble metal-based catalysts like Pd, Pt, Rh, Ru, and Ir are extensively studied for catalyzing DRM reactions. Although noble metal-based catalysts are highly efficient for DRM reaction and resist carbon deposition, their utilization is limited due to their high cost and rare availability (Pakhare and Spivey, 2014) and hence the current research for DRM catalyst development is focused on the utilization of non-noble transition metals (Co, Ni, Fe, and Cu) based catalysts. From the studies, it has been found that Ni-based catalysts exhibit high catalytic performance however, they are deactivated by metal sintering and severe carbon deposition. The development of Ni-based catalysts over suitable support with the help of appropriate promoters has been found to enhance catalytic performance as well as catalyst stability. The use of suitable support and the addition of other metals as promoters could significantly enhance the catalytic performance and simultaneous lowering of carbon deposition over Ni-based catalysts (Zhang et al., 2018; Yentekakis, et al., 2021; Charisiou, et al., 2018)). Al2O3 is proven to be a potential support however, the superficial interaction and dispersion capacity of Al2O3 depend on the active metal used and reaction conditions. Zirconia has also been used for DRM reaction as support for its high coke resistance to avoid catalyst deactivation (Shin et al., 2018; Therdthianwong, 2008). The bifunctional DRM reaction mechanism includes activation of CH4 over the active metal site and CO2 activation over the support or at a metal support interface (Yentekakis et al., 2019d). Németh et al. (Németh et al., 2017) investigated the Na-promoted Ni/ZrO2 dry-reforming catalyst and established that the activity and coke formation is controlled by the Na2O-ZrO2-Ni interaction. Electropositive stimulation of Pt-group metals by alkalis or alkaline earth in emissions control catalysis was described by (Wang et al., 2018). It was explained that alkali or alkaline earth can significantly influence the catalytic performance of Pt-group metal catalysts for CO and hydrocarbon oxidation. The impact of TiO2 alkali promotion on the chemisorptive properties and water–gas shift activity of supported noble metal catalysts was investigated by Panagiotopoulou and Kondarides (Panagiotopoulou and Kondarides, 2009). They demonstrated how, as the alkali concentration increases, the desorption temperature of H2 adsorbed on sites at the metal-support interface decreases monotonically towards lower temperatures. With this background literature, in the present study, we have synthesized, characterized, and evaluated the performance of the Ni/ZrO2-Al2O3 catalysts promoted with Cs to determine the optimum Cs loading for the DRM reaction as well as to study the effect of Cs content on catalyst performance.
2 Experimental
2.1 Materials used
Caesium nitrate hexahydrate Cs (NO3)3·6H2O (427.01 g/mol; 99.99 %; Alfa Aesar) and nickel nitrate hexahydrate Ni (NO3)2·6H2O (290.69 g/mol 99 %; Alfa Aesar) were used as purchased. Zirconium oxide–Alumina (10 % ZrO2 –90 %Al2O3) commercially available support was used as received. Milli-Q water purification system (Millipore) was used to obtain ultrapure water which was used for all the experimental purposes.
2.2 Catalyst preparation
The catalyst 5Ni + xCs/10Zr-Al2O3 (x = 0, 1, 2, 3, and 4 wt%) was synthesized by impregnation of calculated amounts of Ni (NO3)2·6H2O to obtain 5 wt% NiO loading as the active metal, Cs (NO3)3·6H2O as promoter and 10Zr-Al2O3 as support. Nickel nitrate hexahydrate is dissolved in 10 mL of ultra-pure water and thoroughly mixed, thereafter caesium nitrate hexahydrate was added to obtain a homogeneous solution. 1 g of ZrO2 support was added to this solution. The solution was heated and stirred until a slurry was formed. The catalyst was oven-dried at 120 °C, thereafter calcined at 600 °C with a 3 °C/min heating rate for 5 h. After that, the prepared catalysts were ground into powder. The catalysts are abbreviated in the manuscript as NZA-0C (0 wt%), NZA-1C (1 wt%), NZA-2C (2 wt%), NZA-3C (3 wt%), and NZA-4C (4 wt%) for varying Cs loading.
2.3 Characterization
Miniflex Rigaku diffractometer equipped with Cu Kα X-ray radiation operated at 40 mA and 40 kV was used to record X-ray Diffraction patterns of the catalysts. Micromeritics Tristar II 3020 instrument was used to measure N2 adsorption–desorption isotherms at − 196 °C for porosity and surface area analysis. Temperature-programmed reduction (TPR) profile measurement was done using Micromeritics 2920 chemisorption analyser. Typically, a 70 mg sample was used in this analysis. A flow of 30 mL/min was used to heat the samples at 150 °C, followed by cooling to ambient temperature. A temperature of 800 °C was achieved with the ramp rate of 10 °C/min in H2/Ar flow (1:9 vol%) at 40 mL/min. Hydrogen consumption was recorded by using a thermal conductivity detector.
Thermo-gravimetric/Differential (EXSTAR SII TG/DTA 7300) analyzer analyser was used to quantify the deposited carbon on used catalyst samples. For this, the sample (10–15 mg) placed in a platinum pan was heated to 1000 °C with a ramp rate of 20 °C min−1 in the pure airflow of 25 mL/min. The mass loss was recorded as heating progressed. A transmission electron microscope operated at 200 kV (JEOL JEM-2100F) was used to record high-resolution TEM images of the catalyst samples.
2.4 Catalyst performance evaluation
The dry reforming reactions were performed with the prepared catalysts (0.1 g each) at 800 °C in a packed bed stainless steel reactor (0.3 m height; 0.0091 m internal diameter) at atmospheric pressures. The reactor was equipped with a K-type thermocouple (stainless steel sheathed), positioned axially close to the catalyst bed. Hydrogen was used to activate the catalyst at 900 °C for 60 min before the reaction. After activation, the catalyst bed was purged with nitrogen for 15 min, then the temperature was maintained at 800 °C. The reaction was then carried out with CH4 and CO2, and N2 was used as a dilution gas. The feed was provided at a space velocity of 42 L/h./gcat with a CH4: CO2: N2 ratio of 43 %:43 %:14 %. The reaction products were analyzed and quantified with an online Gas Chromatograph (GC) equipped with a thermal conductivity detector. The calculations were performed as shown below for CO2 and methane conversions, and syngas ratio:
3 Results and discussion
3.1 Catalyst characterization
The crystalline phases of the prepared catalyst were characterized using XRD analysis. Fig. 1 shows the X-ray diffractograms of the catalyst samples. The diffractograms obtained for all the samples show wide FWHM for all the peaks indicating the Nano-crystalline nature of the catalyst samples. The peaks were matched with cubic ZrO2 phase with Fm3m space-group (ICDD file no. 00–027-0997) and cubic Al2O3 phase with Fm3m space-group (ICDD file no. 01–075-0921). The corresponding peaks of Ni or Cs phases were not observed in the diffractograms. This may be due to lower loading and fine dispersion of Ni and Cs over the ZrO2-Al2O3 support. The peaks identified for alumina confirm the presence of Nanocrystalline γ-Al2O3.

- XRD diffractograms of prepared catalysts.
Further, the catalyst samples were characterized for porosity by BET-BJH analysis. Fig. 2A shows the N2 adsorption–desorption isotherms of the catalyst samples. All the isotherms typically show type V curvatures with H2 hysteresis, indicating the mesoporous nature of the samples. All the samples show a similar nature of porosity. This is also indicated by XRD analysis. The average surface area of the samples calculated from the BET equation was in the range of 115–124 m2/g with a pore volume of 0.54–0.59 cm3/g (Table 1).(See Table 2).

-
A) N2 adsorption–desorption isotherms and B) Pore size distribution graphs of the prepared catalysts.
| Sample | Surface Area | Pore Diameter | Pore Volume |
|---|---|---|---|
| (m2/g) | (nm) | (cm3/g) | |
| NZA-0C | 124.4 | 19.0 | 0.59 |
| NZA-1C | 119.5 | 18.8 | 0.57 |
| NZA-2C | 117.5 | 18.9 | 0.56 |
| NZA-3C | 117.8 | 18.7 | 0.55 |
| NZA-4C | 115.2 | 18.8 | 0.54 |
| Temperature (°C) | FWHM | Area % | Quantity H2 (cm3/g STP) | |
|---|---|---|---|---|
| NZA-0C | 499.9 | 88.9 | 9.8 | 1.7 |
| 647.8 | 177.0 | 16.6 | 2.9 | |
| 783.3 | 135.3 | 40.7 | 7.2 | |
| 864.4 | 96.8 | 32.9 | 5.8 | |
| Total H2 (cm3/g STP) | 17.7 | |||
| NZA-2C | 470.4 | 45.5 | 3.8 | 0.7 |
| 518.5 | 84.2 | 10.2 | 1.8 | |
| 664.2 | 164.9 | 66.6 | 12.0 | |
| 797.0 | 91.4 | 19.5 | 3.5 | |
| Total H2 (cm3/g STP) | 18.1 | |||
| NZA-3C | 482.1 | 57.1 | 8.9 | 1.3 |
| 531.9 | 93.4 | 15.3 | 2.3 | |
| 651.9 | 140.0 | 55.2 | 8.4 | |
| 785.0 | 99.0 | 20.6 | 3.1 | |
| Total H2 (cm3/g STP) | 15.2 | |||
| NZA-4C | 471.7 | 41.8 | 3.5 | 0.6 |
| 530.9 | 103.9 | 15.3 | 2.5 | |
| 663.5 | 125.2 | 56.4 | 9.3 | |
| 788.1 | 96.6 | 24.8 | 4.1 | |
| Total H2 (cm3/g STP) | 16.4 | |||
The reduction properties of nickel metal dispersed over support were studied using H2-TPR experiments. Fig. 3 shows the deconvoluted TPR peaks of the catalyst samples. All the peaks in these samples can be attributed to Ni species, as other metal oxides are not reducible at this temperature range including Cs. The TPR profile of the NZA-0C sample shows four deconvoluted peaks in the temperature range, of 450-- 950 °C. The first low-temperature peak with a peak maximum at 499.9 °C can be attributed to Ni showing metal support interaction with the ZrO2 phase. The hydrogen consumed for this peak is minimal with a peak area of 9.8 % and 1.7 cm3/g of H2 consumption. The next peak at peak maxima of 647.8 °C can be attributed to Ni interacting with the γ-alumina phase (16.6 % peak area and 2.9 cm3/g of H2 consumption). The peaks at peak maxima of 783.3 °C (40.7 % peak area) and 864.4 °C (32.9 % peak area) can be attributed to the spinel (NiAl2O4) phase formed on the support surface during the calcination of the samples. The experimental evidence suggests that the spinel structure possesses the highest capacity for accommodating impregnated nickel. Notably, both NiO and γ-Al2O3 exhibit cubic phases with the Fm3m space group, facilitating their integration with the spinel structure. This compatibility implies the potential formation of a solid solution during the calcination process, leading to the prevalence of the spinel phase NiAl2O4. This observation underscores the importance of the specific crystallographic characteristics of NiO and γ-Al2O3 in the development of the dominant spinel phase during the thermal treatment.

- H2-TPR of the prepared catalysts.
Fig. 3 also shows the TPR spectra of catalysts impregnated with Cs over Ni/ZrO2-Al2O3 catalyst which results in preferable and strong interaction between Ni and Cs. The strong interaction of Ni and Cs can be inferred from the shift in reduction temperature of different Ni species present in the sample. The peaks identified for alumina confirm the presence of nano crystalline γ-Al2O3. However, Ni exists in the form of spinel (NiAl2O4) at temperatures above 600 °C (Choya et al., 2024; Shokrollahi Yancheshmeh et al., 2020; Tillmann et al., 2018; Zhang et al., 2021).
The amount of Ni species present in the form of spinel phase (NiAl2O4) reduces from 73.6 % to less than 1/3rd of the NZA-0C sample. This interaction of Ni and Cs oxides results in increased reducibility of Ni oxides to Ni metal, which further provides resistance towards sintering of Ni nanoparticles during reaction performance. During the dry reforming, the oxygen vacancies introduced by CeO2-surface may adsorb the oxygen formed by the dissociation of CO2, thus CeO2 is capable of splitting the CO2, enhancing CO-production, leading to a high O uptake by the support favoring the removal of carbonaceous deposits, and improving the reforming activity (Luisetto et al., 2019; Yabe et al., 2017; Yentekakis et al., 2019a). The solid solution of ZrO2 and CeO2 is characterized by high oxygen-storing capacity and thermal stability (Madier et al., 1999; Takeguchi et al., 2001). The total amount of hydrogen consumed for the catalyst samples was in the range of 15–18 cm3/g, which indicated a nearly complete reduction of NiO to Ni0 in all the samples. A theoretical estimation for the complete reduction of 5 wt% NiO shows a consumption of ∼ 15 cm3/g Hydrogen.
TPD analysis was carried out to display the acidic-basic properties of the present catalysts. It is known that the support with acidic nature increases the coke deposition. Our research is primarily focused on nickel catalysts supported or promoted by metal oxides with strong Lewis basicity. This is because the basic sites increase CO2 adsorption. We observed that the incorporation of Ce increased the surface basicity and electron properties of the Ni-based catalysts, which contributed to the activation of CO2. In Fig. 4 of the CO2-TPD, we observed small peaks in the low-temperature CO2-desorption (100–250 °C), associated with weak Bronsted basic sites such as surface OH– groups. Additionally, broad peaks in the intermediate temperature CO2-desorption (250–400C) suggest the existence of medium-strength Lewis base sites (YAN et al., 2011). The results demonstrate that the intensity of CO2 desorbed decreases with the addition of Ce, leading to a reduction in catalyst basicity. Notably, the N/ZA-3C catalyst exhibited the most appropriate reduction and consequently displayed the best performance.

- CO2-TPD analysis.
The samples were also investigated for their morphological features using TEM microscopy (Fig. 5). All the samples showed similar morphology which agrees with the results obtained from XRD and N2 adsorption–desorption studies. The samples showed aggregation of nanoparticles of ZrO2 and γ-Al2O3 phases. The particle size obtained is in the range of 10–25 nm. The aggregations of particles lead to the formation of interparticle voids while mesoporosity is also exhibited by the samples as observed in Table 1.

- TEM micrographs of prepared catalysts A1, A2) NZA-0C; B1, B2) NZA-1C; C1, C2) NZA-3C; D1,D2) NZA-4C and E1,E2) NZA-4C.
3.2 Catalyst evaluation
The catalysts were tested for dry reforming of methane at 800 °C with a feed ratio of CH4: CO2: N2 ratio of 43 %:43 %:14 % at a space velocity of 42 L/h./gcat. Fig. 6 shows CH4 and CO2 conversions of all the catalyst samples tested along with calculated and experimental H2 and CO yields. For the NZA-0C catalyst, the average methane and CO2 conversion observed were 76.8 % and 81.1 % respectively. It was observed that the CO2 conversion was 4–4.5 % higher than CH4 conversions at different times on-stream (TOS) due to the occurrence of RWGS (Eq. (5).
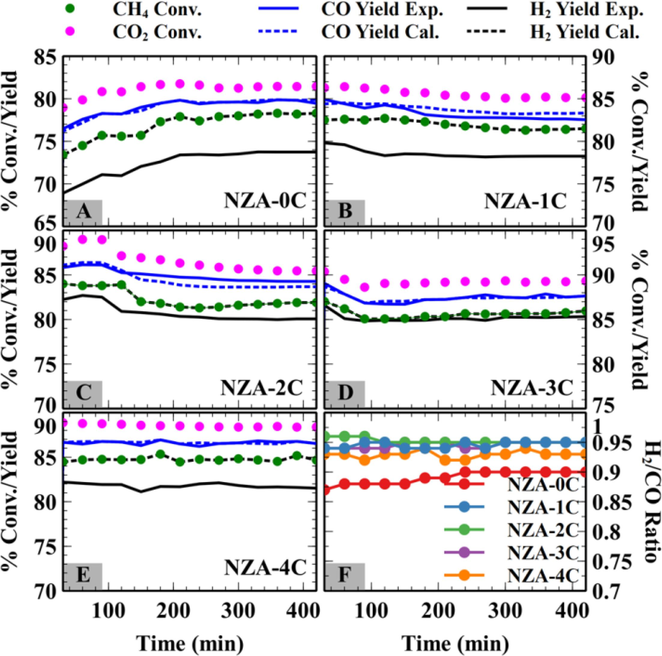
- Catalysts evaluation for dry reforming of methane: A-E) CH4 and CO2 conversions with Calculated and experimental H2 and CO yields; F) H2/CO ratio of product syngas.
The finding is confirmed by the lower experimental yields compared to the calculated H2 yields. The theoretical H2 and CO yields are calculated based on CH4 and CO2 conversions. The catalyst may have active sites for the CH4 cracking and CO2 splitting (Patel et al., 2021), which can promote the reaction. Additionally, the decomposed reaction species (intermediates) were highly active and could rapidly interact with one another or with other species present. Metallic Ni sites were available for CH4 cracking in the present catalyst compositions. The decomposition of CH4 into CH4-x and H2 on metallic Ni is widely acknowledged (Zhang et al., 2019). Subsequently, under DRM, CO2 may oxidize CH4-x to produce syngas (CO + H2)). Nonetheless, the postponement of CH4-x oxidation facilitated the polymerization of CH4-x into coke (Zhang et al., 2019). An inefficient carbon-CO2 gasification reaction (eq. (3) will lead to carbon deposition over the catalyst surface. This was observed for the NZA-0C catalyst where the carbon deposition observed is the highest (Fig. 8), while the hydrogen selectivity is the lowest for the given catalyst composition. (Fig. 7).

- H2 and CO Selectivity during dry reforming of methane with prepared catalysts.
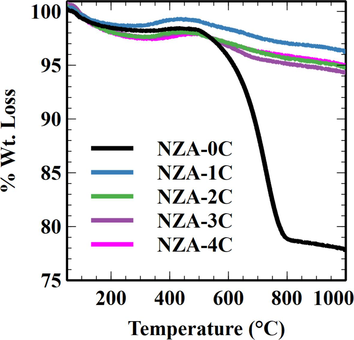
- Thermo–gravimetric analysis of spent catalyst samples to study carbon deposition.
The addition of Cs promoter to the Ni/ZrO2-Al2O3 catalyst improves their hydrogen yield and hydrogen selectivity, while also being responsible for reduced carbon deposition over all the catalysts (Fig. 5, Fig. 6, and Fig. 7). The promotion of catalysts with different Cs loading enhanced the CH4 and CO2 conversion in all the cases compared to the NZA-0C catalyst. These observations indicate an enhanced carbon gasification reaction due to the enhanced adsorption of CO2 (a Lewis acidic molecule) on the catalyst surface by basic Cs oxide. This is also supported by catalyst characterization data, which concluded enhanced interaction of Ni metal with Cs oxide. The NZA-3C catalyst showed optimally enhanced CO2 adsorption leading to better product yields and selectivity along with lower carbon deposition.
4 Conclusion
In this study, 10 wt.%ZrO2/γ-Al2O3-supported Ni catalysts were prepared with 1 to 4 wt% Cs promotion. The prepared catalysts were characterized in detail and evaluated for their catalytic activity for the dry reforming of methane. These nano-structured catalysts showed mesoporosity owing to the agglomeration of support nanoparticles and the formation of inter-particular voids, showing disordered porosity. The promotional effect of Cs was studied and explained for the DRM reaction. The results demonstrate that the intensity of CO2 desorbed decreases with the addition of Ce leading to a reduction in catalyst basicity. 3 wt% Cs content was found to be the optimum loading for DRM reaction performance. The enhanced performance of the catalyst for dry reforming reaction with Cs promotion is attributed to the enhanced CO2 sorption by Cs oxides leading to a promoted carbon-CO2 gasification reaction. The addition of Cs reported in the present work highly restricts the coke deposition over all the Ni-based catalysts.
Acknowledgments
The authors would like to extend their sincere appreciation to Researchers Supporting Project number (RSP2024R368), King Saud University, Riyadh, Saudi Arabia.
CSIR NEERI KRC no.: CSIR-NEERI/KRC/2022/NOV/ERMD/1.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- A review on catalyst development for dry reforming of methane to syngas: Recent advances. Renew. Sustain. Energy Rev. 2019
- [CrossRef] [Google Scholar]
- Dry reforming of methane over Ni/MgO–Al2O3 catalysts: Thermodynamic equilibrium analysis and experimental application. Int. J. Hydrogen Energy. 2020;45:5252-5263.
- [CrossRef] [Google Scholar]
- Formation, control, and elimination of carbon on Ni-based catalyst during CO2and CH4conversion via dry reforming process: A review. J. CO2 Util. 2022
- [CrossRef] [Google Scholar]
- Tuning dry reforming of methane for F-T syntheses: A thermodynamic approach. Appl. Energy. 2018;227:190-197.
- [CrossRef] [Google Scholar]
- N.D. Charisiou, G. Siakavelas, L. Tzounis, V. Sebastian, A. Monzon, M.A. Baker, S.J. Hinder, K. Polychronopoulou, I.V. Yentekakis, M.A. Goula. 2018. An in-depth investigation of deactivation through carbon formation during the biogas dry reforming reaction for Ni supported on modified with CeO2 and La2O3 zirconia catalysts Int. J. Hydrog. Energy 4 3, 1 8 9 5 5 -1 8 9 7 6.
- Dry reforming of methane over sub-stoichiometric NiAl2O4-mediated Ni/Al2O3 catalysts. Fuel. 2024;358:130166
- [CrossRef] [Google Scholar]
- Oxidative thermal sintering and redispersion of Rh nanoparticles on supports with high oxygen ion lability. Catalysts. 2019;9
- [CrossRef] [Google Scholar]
- K. Hashimoto, 2019. Global Temperature and Atmospheric Carbon Dioxide Concentration. pp. 5–17. https://doi.org/10.1007/978-981-13-8584-1_3.
- Biogas composition depending on the type of plant biomass used. Res. Agric. Eng.. 2011;57:137-143.
- [CrossRef] [Google Scholar]
- Thermodynamic analysis of carbon dioxide reforming of methane and its practical relevance. Int. J. Hydrogen Energy. 2015;40:2445-2451.
- [CrossRef] [Google Scholar]
- Dry reforming of methane over Ni supported on doped CeO 2: New insight on the role of dopants for CO 2 activation. J. CO2 Util.. 2019;30:63-78.
- [CrossRef] [Google Scholar]
- Oxygen mobility in CeO2 and CexZr(1–x)O2 compounds: Study by CO transient oxidation and18O/16O isotopic exchange. J. Phys. Chem. B. 1999;103:10999-11006.
- [CrossRef] [Google Scholar]
- Combined dry reforming and partial oxidation of methane to synthesis gas on noble metal catalysts. Int. J. Hydrogen Energy. 2011;36:2969-2978.
- [CrossRef] [Google Scholar]
- Na-promoted Ni/ZrO2 dry reforming catalyst with high efficiency: Details of Na2O-ZrO2-Ni interaction controlling activity and coke formation. Catal. Sci. Technol.. 2017;7:5386-5401.
- [CrossRef] [Google Scholar]
- Support induced effects on the ir nanoparticles activity, selectivity and stability performance under CO2 reforming of methane. Nanomaterials. 2021;11
- [CrossRef] [Google Scholar]
- A review of dry (CO2) reforming of methane over noble metal catalysts. Chem. Soc. Rev.. 2014;43:7813-7837.
- [CrossRef] [Google Scholar]
- Effects of alkali promotion of TiO2 on the chemisorptive properties and water-gas shift activity of supported noble metal catalysts. J. Catal.. 2009;267:57-66.
- [CrossRef] [Google Scholar]
- Impact of ceria over WO3–ZrO2 supported Ni catalyst towards hydrogen production through dry reforming of methane. Int. J. Hydrogen Energy. 2021;46:25015-25028.
- [CrossRef] [Google Scholar]
- Ritchie, H., Roser, M., Rosado, P., 2022. Energy [WWW Document]. Our World in Data. URL https://ourworldindata.org/energy (accessed 9.9.22).
- Dry reforming of methane over Ni/ZrO 2 -Al 2 O 3 catalysts: Effect of preparation methods. J. Taiwan Inst. Chem. Eng.. 2018;90:25-32.
- [CrossRef] [Google Scholar]
- A novel synthesis of NiAl2O4 spinel from a Ni-Al mixed-metal alkoxide as a highly efficient catalyst for hydrogen production by glycerol steam reforming. Appl. Catal. B. 2020;265:118535
- [CrossRef] [Google Scholar]
- Hydrogen spillover from NiO to the large surface area CeO2-ZrO2 solid solutions and activity of the NiO/CeO2-ZrO2 catalysts for partial oxidation of methane. J. Catal.. 2001;202:14-24.
- [CrossRef] [Google Scholar]
- Synthesis gas production from dry reforming of methane over Ni/Al2O3 stabilized by ZrO2. Int. J. Hydrogen Energy. 2008;33:991-999.
- [CrossRef] [Google Scholar]
- Dry reforming of methane at high pressure in a fixed-bed reactor with axial temperature profile determination. Catal. Lett.. 2018;148:2256-2262.
- [CrossRef] [Google Scholar]
- M.J.E. van Marle, D. van Wees, R.A. Houghton, R.D. Field, J. Verbesselt, van der Werf, R. Guido, 2022. RETRACTED ARTICLE: New land-use-change emissions indicate a declining CO2 airborne fraction. Nature 603, 450–454. https://doi.org/10.1038/s41586-021-04376-4.
- Low-temperature catalytic CO2 dry reforming of methane on Ni-based catalysts: A review. Fuel Process. Technol. 2018
- [CrossRef] [Google Scholar]
- Low-temperature oxidative coupling of methane in an electric field using carbon dioxide over Ca-doped LaAlO3 perovskite oxide catalysts. J. CO2 Util.. 2017;20:156-162.
- [CrossRef] [Google Scholar]
- Alkaline earth metal modified NaY for lactic acid dehydration to acrylic acid: Effect of basic sites on the catalytic performance. Chin. J. Catal.. 2011;32:405-411.
- [CrossRef] [Google Scholar]
- Effect of support oxygen storage capacity on the catalytic performance of Rh nanoparticles for CO2 reforming of methane. Appl. Catal. B. 2019;243:490-501.
- [CrossRef] [Google Scholar]
- Effect of support oxygen storage capacity on the catalytic performance of Rh nanoparticles for CO2 reforming of methane. Appl. Catal. B. 2019;243:490-501.
- [CrossRef] [Google Scholar]
- Effect of support oxygen storage capacity on the catalytic performance of Rh nanoparticles for CO2 reforming of methane. Appl. Catal. B. 2019;243:490-501.
- [CrossRef] [Google Scholar]
- Effect of support oxygen storage capacity on the catalytic performance of Rh nanoparticles for CO2 reforming of methane. Appl. Catal. B. 2019;243:490-501.
- [CrossRef] [Google Scholar]
- 2021, A review of recent efforts to promote dry reforming of methane (DRM) to syngas production via bimetallic catalyst formulations. Appl. Catal. B: Environ.. 2021;296:120210
- [CrossRef] [Google Scholar]
- A review of CH4CO2 reforming to synthesis gas over Ni-based catalysts in recent years (2010–2017) Int. J. Hydrogen Energy. 2018;43:15030-15054.
- [CrossRef] [Google Scholar]
- Anode interfacial layer formation via reductive ethyl detaching of organic iodide in lithium–oxygen batteries. Nat. Commun.. 2019;10
- [CrossRef] [Google Scholar]
- Development of stable bimetallic catalysts for carbon dioxide reforming of methane. J. Catal.. 2007;249:300-310.
- [CrossRef] [Google Scholar]
- NixAl1O2-δ mesoporous catalysts for dry reforming of methane: The special role of NiAl2O4 spinel phase and its reaction mechanism. Appl. Catal. B. 2021;291:120074
- [CrossRef] [Google Scholar]







