Cultivation of Chlorella protothecoides in polyglutamic acid wastewater for cost-effective biodiesel production
⁎Corresponding author at: School of Municipal and Environmental Engineering, Shandong Jianzhu University, JiNan 250101, China. zhangmeili8292@sina.com (Chao Zhang)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Abstract
-
The use of γ-PGA wastewater as culture medium for C. protothecoides was feasible.
-
TN, TP removal rate of γ-PGA wastewater reached 93.87% and 88.39% after cultivation.
-
The process could offset the disposal cost of γ-PGA wastewater.
-
The wastewater treated by C. protothecoides could be reused for γ-PGA production.
Abstract
The combination of gamma-polyglutamic acid (γ-PGA) wastewater treatment and oil-producing microalgae culture could not only achieve the wastewater reuse for γ-PGA production, but also provided nutrients and a large amount of water for microalgae culture. Thus the effects of wastewater concentration, different nutrients on the biomass yield and lipid content of Chlorella protothecoides were investigated separately. The results showed that 60% γ-PGA wastewater was very suitable for the cultivation of C. protothecoides. Only 5.86 g/L glucose, 0.73 g/L NaNO3 and 0.2 g/L K2HPO4 needed to be added in the γ-PGA wastewater. In the optimized wastewater medium, the lipid yield could reach 1.77 ± 0.03 g/L, which was 195.0% higher than that in the SE medium(0.60 ± 0.03 g/L). C. protothecoides had high absorption capacity for nitrogen and phosphorus. The nitrogen, phosphorus removal rate of wastewater reached 93.87% and 88.39%, respectively, after 7 days of culture. In addition, the wastewater treated by C. protothecoides could be reused for γ-PGA fermentation for four times. These investigations laid a foundation for reducing the pollution of γ-PGA wastewater, explored a late-model for oleaginous microalgae and γ-PGA cleaner production.
Keywords
γ- polyglutamic acid
Wastewater
Chlorella protothecoides
Biodiesel
1 Introduction
With the global shortage of fossil energy and the deterioration of the environment, biodiesel, as a sustainable form of green energy, has attracted widespread attention (Dantas et al., 2020; Fadhil et al., 2017). Currently, countries worldwide are vigorously developing biodiesel industry (Chow et al., 2020). However, the biggest “bottleneck” lies in the large-scale supply of low-cost and sustainable oil raw materials (Leong et al., 2020a). Microalgae is a kind of planktonic photoautotrophic microorganism group, which widely exists in the lake, river, ocean and other water environments. Many of them can accumulate a large amount of oil products for biodiesel production under specific environmental conditions (Leong et al., 2020b). Because of its high photosynthetic efficiency, fast growth rate, high oil production, absorbing CO2 as carbon source, and not competing with people for grain and land, it has better development potential than traditional oil crops (Mostafa and El-Gendy, 2017; Gupta et al., 2016). Some researchers even think that microalgae cultivation is one of the ultimate solutions to energy and environmental problems (Mata et al., 2010). Although microalgae have great potential for oil production, there is no commercial way to produce energy products. It is mainly applied to produce economic microalgae and bait-algae. The high cost of microalgae culture makes the production cost of microalgae related energy products too high. Therefore, the exploration of microalgae bioenergy technology with low-cost medium is one of the important measures to reduce the cost of microalgae culture (Chinnasamy et al., 2010; Gao et al., 2019).
A large amount of wastewater rich in nitrogen and phosphorus is produced in the industrial production and human society activities. These wastewater’s harmless treatment has become a crucial issue for environmental protection. Microalgae, as a natural environment purifier, has long been proposed and applied to remove inorganic nitrogen, phosphorus and metal elements in wastewater. Therefore, utilizing wastewater to cultivate oil-producing microalgae can not only realize the efficient and pollution abatement treatment of these wastewater abundant with nitrogen and phosphorus, but also provide ample but cheap nutrition and water resources for the oil production by energy microalgae, which can kill two birds with one stone (Shen et al., 2015; Pal et al., 2019). Gamma-polyglutamic acid (γ-PGA) is a kind of water-soluble polymer material synthesized by microbial fermentation. It is synthesized by the polymerization of L-glutamic acid and D-glutamic acid via gamma-amide bond. It has been widely utilized in medicine, agriculture, environmental protection, cosmetics, food and other industries because of its excellent physical and chemical properties such as biodegradation, film-forming, fiber-forming and water retention (Zhang et al., 2019a; Zhang et al., 2018). With the increased requirement of γ-PGA, the production of γ-PGA is increasing year by year. In the γ-PGA production process, a large amount of wastewater is produced. γ-PGA industrial wastewater is a kind of high concentration organic water, which is rich in protein, amino acid, sugar and many trace elements. Wastewater is discharged directly without utilization, which generates resource-wasting and cost overrun of pollutant treatment. Treating γ-PGA wastewater by physical, chemical and traditional biodegradation technologies is effective, but there are problems such as high operating cost and secondary pollution (Zhang et al., 2019b). Microalgae treatment has the advantages of low operation cost, small secondary pollution, absorption of CO2, etc. It is an effective way to reduce the cost of carbon emission (Lakshmikandan et al., 2020).
Chlorella, a unicellular alga of Chlorophyta, has simple nutrition requirements and easy to cultivate, meanwhile, it has a wide ecological distribution. In addition, Chlorella contains a variety of vitamins, proteins, carotenes, etc., abounding in amino acids and fatty acids necessary for humans and animals, and is usually applied to make health products and drugs (Shams et al., 2014). Some Chlorella with high oil content, such as Chlorella protothecoides, can also be used to extract edible oil and biodiesel. C. protothecoides can purify sewage, fix carbon dioxide through photosynthesis and improve the environment (Khalid et al., 2018). Therefore, C. protothecoides was chosen as the experiment strain.
In recent years, with the energy shortage and environment deterioration, the researches about treatments of fermentation industrial wastewater by Chlorella have attracted much attentions (Liang et al., 2013; Leong et al., 2019). For example, Asadi et al. used the wastewater from a dairy wastewater treatment plant as the medium to cultivate Chlorella vulgaris. After 10 days of culture, the biomass and lipid content reached 1.66 g/L and 36.67% respectively (Asadi et al., 2020). Yang et al. cultured C. pyrenoidosa with fermentation wastewater from alcohol plant. When undiluted wastewater was utilized as culture medium, the removal rate of COD was 76.32%. And the treated wastewater can be reused for ethanol fermentation (Yang et al., 2008). Darpito et al. used brewery wastewater to cultivate C. protothecoides to produce biodiesel. After 6 days of culture, the biomass and lipid content reached 1.88 g/L and 35.94% respectively (Darpito et al., 2015). In a word, there are many researches on using various wastewaters to cultivate Chlorella, but the heterotrophic cultivation of C. protothecoides using γ-PGA wastewater has not been reported. In this paper, the effect of γ-PGA wastewater on the growth of C. protothecoides B-15 was studied to diminish the production cost of lipid and γ-PGA and found a new approach for oleaginous microalgae and γ-PGA cleaner production.
2 Experimental
2.1 Strains
Chlorella protothecoides B-15 was purchased from Freshwater Algae Culture Collection at the Institute of Hydrobiology (FACHB) as FACHB-3. Bacillus subtilis A3 was purchased from China Center of Industrial Culture Collection (CICC) as CICC 20646.
2.2 Wastewater
The γ-PGA production wastewater used in this experiment was collected from Bioengineering Experiment Center of Shandong Jianzhu University, JiNan, China. The wastewater was simulated the wastewater produced in the actual production process of the γ-PGA factory, and the fermentation liquor was produced after the γ-PGA extraction section. The wastewater was filtered by 45 μ m sieve silk to remove solids. Afterwards, the wastewater was sterilized by high-pressure steam for standby application or preservation. If the wastewater was collected and directly used to prepare the culture medium, it should be sterilized as well after preparing of the medium. The composition and characteristics of γ-PGA wastewater are presented in Table 1.
| Index | Wastewater | 60% Wastewater | Treated with microalgae | Removal rate (%) |
|---|---|---|---|---|
| CODCr(mg/L) | 7546 ± 151 | 4527 ± 90 | 316 ± 6 | 93.02 ± 1.99 |
| NH3-N(mg/L) | 402 ± 11 | 242 ± 6 | 18 ± 1 | 92.56 ± 2.23 |
| TN (mg/L) | 871 ± 13 | 522 ± 8 | 32 ± 2 | 93.87 ± 1.22 |
| TP(mg/L) | 187 ± 3 | 112 ± 2 | 13 ± 1 | 88.39 ± 1.01 |
* CODCr: Chemical oxygen demand; TN: total nitrogen; TP: total phosphorus.
2.3 Media
SE medium: NaNO3 0.25 g/L, K2HPO4·3H2O 0.075 g/L, MgSO4·7H2O 0.075 g/L, CaCl2·2H2O 0.025 g/L, KH2PO4 0.175 g/L, NaCl 0.025 g/L, Soil extract 40 mL/L, FeCl3·6H2O 0.005 g/L, Fe-EDTA 1 mL/L, A5 solution 1 mL/L, distilled water 958 mL/L.
Fermentation medium, in g/L: glucose 6, NaNO3 0.8, KH2PO4 0.2. The medium was produced with 60% γ-PGA wastewater.
Wastewater medium: the medium was composed of different concentrations of γ-PGA wastewater without adding any exogenous nutrients.
Fermentation medium for γ-PGA production, in g/L: glucose, 36; tryptone, 9; L-glutamate, 28; K2HPO4·3H2O, 2; MgSO4, 0.25. The pH was adjusted to 7.0 by HCl or NaOH (Zhang et al., 2020).
All media were autoclaved for 20 min at 121 °C.
2.4 Cultivation method
The exponential stage algae (0.2 × 106 cells/mL) were inoculated into a flask (500 mL) containing 200 mL medium, with adjusted pH 7.0, and a light shaker with constant temperature for 7 d. The inoculation amount was 10%, temperature was 28 °C, light intensity was 4 000 lx, light cycle was L/D = 16/8 (L was the bright period, D was the dark period), and the rotation speed was 200 r /min. After 7 days of culture, the algae were collected by centrifugation at 8000 r/min for 10 min.
The γ-PGA production by B. subtilis A3 was conducted in 250 mL flask containing 50 mL fermentation medium inoculated with 5 mL of the seed culture (108 cells/mL). The flask was cultivated on a rotary shaker operating at 37 °Cand 180 rpm for 48 h. Three replicates were carried out for each experiment.
2.5 Single factor experiment
The effects of different concentrations of glucose, NaNO3 and KH2PO4 on biomass and total lipid content of microalgae were investigated. The concentration range of glucose was set as 0–8 g/L, the concentration range of NaNO3 was set as 0–1.2 g/L, and the concentration range of KH2PO4 was set as 0–0.5 g/L. All experiments were run in triplicate, and the average values were adopted.
2.6 Box-Behnken designs
On the basis of single factor experiments, three factors which had influence on the biomass and total lipid content of microalgae were selected as independent variables: glucose (A), NaNO3 (B), KH2PO4 (C). Glucose (A) was divided into 5 g//L, 6 g//L and 7 g//L levels. NaNO3 (B) was divided into three levels: 0.6 g//L, 0.8 g//L and 1.0 g//L. KH2PO4 (C) was divided into three levels: 0.1 g//L, 0.2 g//L and 0.3 g//L. These three factors were designed at three levels by Box-Behnken design (BBD) using Design-Expert 8.0.6 software, and the lipid yield (Y) was used as response value (Berkani et al., 2020). The regression coefficients of the equation were fitted by 17 groups of experiments.
2.7 Analysis method
After the culture, the supernatant was removed by centrifugation at 8000 r/min for 10 min. The algae cells were washed with distilled water and centrifuged repeatedly. After washing for 3 times, the cells were dried to constant weight at 55 °C. Then, the cell dry weight was weighed and calculated. The dried algae powder was used for the subsequent determination of oil content. The cell densities of Chlorella and B. subtilis were counted by light microscope and blood cell counting plate. The densities of algal cells and B. subtilis were expressed as cells/mL.
The total lipid was extracted by Bligh and Dyer method (Bligh and Dyer, 1959). The calculation formula of the total lipid content (TL) was as follows:
The calculation formula of the lipid yield (LY) was as follows:
Chemical oxygen demand (CODCr), total nitrogen (TN), total phosphorus (TP) and NH3-N in effluents were measured by the procedure described in Standard Methods for the Examination of Water and Wastewater (Hinman and Thacker, 1995).
3 Results and discussion
3.1 Growth of C. protothecoides in different concentration of γ-PGA wastewater
The process diagram of the whole experiment is shown in Fig. 1. The growth of C. protothecoides B-15 in the medium containing 20%, 40%, 60%, 80% and 100% γ-PGA wastewater was monitored with cell dry weight as the index. The results are shown in Fig. 2.
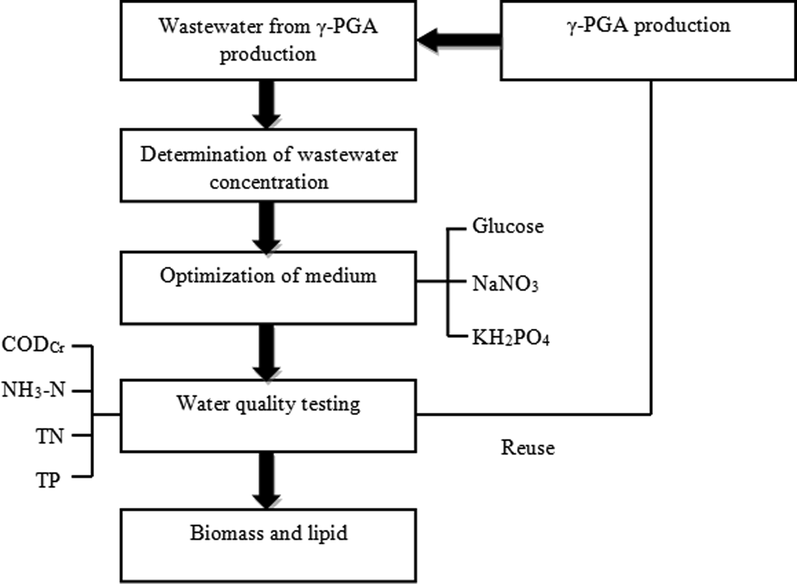
- Flow chart.
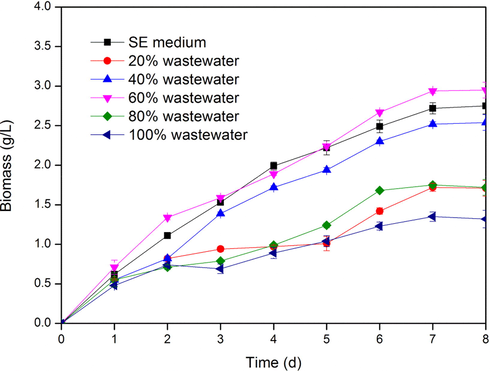
- Effect of wastewater concentration on C. protothecoides growth.
C. protothecoides B-15 grew faster during the first three days in 20% and 40% γ-PGA wastewater medium. On the 7th day, the biomass reached the maximum value of 1.72 ± 0.05 g/L and 2.52 ± 0.03 g/L, and then stopped growing. In 60% γ-PGA wastewater medium, the growth rate was also faster in the first three days. The biomass reached the maximum value of 2.94 ± 0.03 g/L on the 7th day. And then the microalgae stopped growing because of the nutrient depletion of the medium. When C. protothecoides B-15 was cultured in 80% and 100% amino acid wastewater medium separately, it grew slowly in the first four days, and the maximum biomass was only 1.75 ± 0.03 g/L and 1.35 ± 0.06 g/L on the 7th day respectively. Then it entered the stable period and stopped growing. On the 8th day, some algae began to decline.
From Fig. 2 and above analysis, C. protothecoides B-15 grew well in 60% γ-PGA wastewater. When the concentration of γ-PGA wastewater exceeded 60%, the growth of C. protothecoides B-15 was slower and the later stage of algal decline was faster. It is speculated that there may be some limiting factors inhibiting or poisoning the growth of C. protothecoides B-15 even under the condition of sufficient nutrition. When the concentration of γ-PGA wastewater was 20%, the biomass was low. The main reason was lack of nutrients such as N source and P source in the medium of γ-PGA wastewater, which limited the growth of algae. The cell dry weight in 60% wastewater medium(2.94 ± 0.03 g/L) was higher than that in SE medium (2.72 ± 0.07 g/L). This demonstrated that it was feasible to cultivate C. protothecoides B-15 within γ-PGA production wastewater.
3.2 Effect of glucose concentration on biomass and lipid content of C. protothecoides
Carbon content accounts for 40–50% of microalgae stem cells, which is one of the main elements for cell growth. The carbon sources for microalgae growth can be divided into inorganic and organic carbon sources, which impact the growth of algae cells and the accumulation of lipids and other substances. Using glucose as carbon source, the effects of adding different concentrations of glucose in wastewater on the growth and lipid accumulation of C. protothecoides B-15 were investigated. The results are shown in Fig. 3(a).
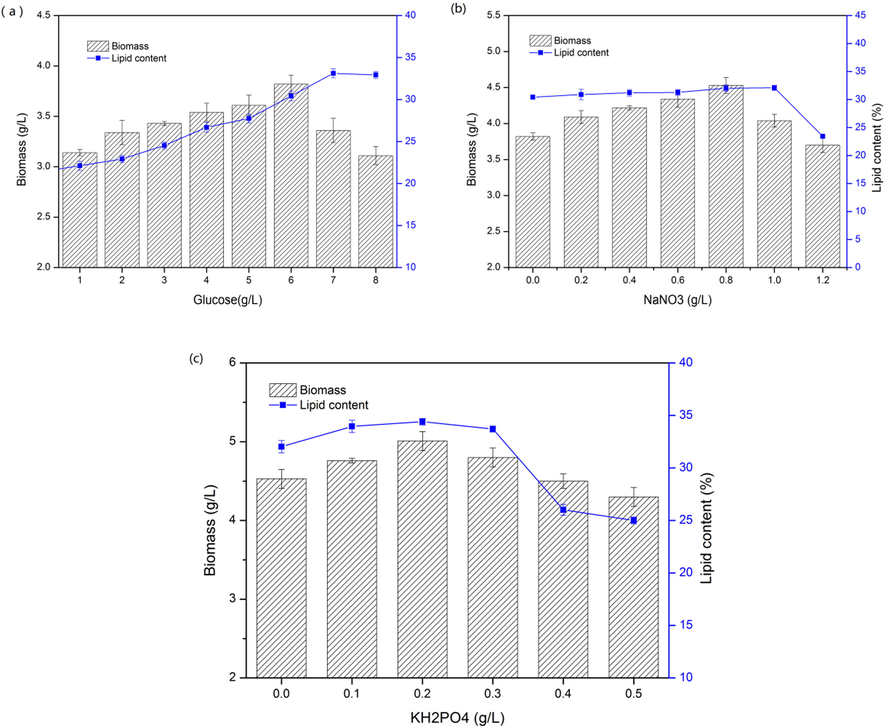
- Biomass and total lipid content cultured in wastewater with different glucose concentrations (a), NaNO3 concentrations (b) and, KH2PO4 (c) concentrations.
With the rising of glucose concentration, the biomass of C. protothecoides B-15 increased first and then decreased. When the glucose concentration was 6 g/L, the biomass of microalgae was the highest, reaching 3.82 ± 0.09 g/L. While the glucose concentration was higher than 6 g/L, the osmotic pressure of algae increased, resulting in cell death. Additionally, the oil content of microalgae is lower under low carbon sources. With the increase of glucose concentration, the oil accumulation reached the highest at 33.12 ± 0.53%. After that, the effect of high concentration of glucose on lipid accumulation of microalgae was not obvious. The inhibitory effect of high carbon concentration on the growth of microalgae may be due to the change of C/N ratio in the culture medium. Appropriate C/N ratio can promote the accumulation of microalgae biomass and oil, which is also one of the research hotspots of microalgae culture optimization (Chen and Johns, 1991). Although the oil content was the highest when glucose was 8 g/L, the biomass(3.36 ± 0.12 g/L) was relatively low. On the grounds of the lipid yield, the maximum value appeared when glucose was 6 g/L. Therefore, the optimal addition of glucose is 6 g/L.
3.3 Effect of nitrogen concentration on biomass and lipid content of microalgae
Nitrogen is one of the important organic compounds in microalgae, affecting the growth and metabolism of microalgae in many aspects. In the experiment, NaNO3 was added to the wastewater as the nitrogen supplement source, in order to investigate the effect of different concentrations of NaNO3 on the growth and oil accumulation of C. protothecoides B-15. The results are shown in Fig. 3(b).
With the rising of NaNO3 concentration, the biomass of C. protothecoides B-15 first increased and then decreased. When NaNO3 was 0.8 g/L, the maximum biomass was 4.53 ± 0.11 g/L. It has been recorded in literature that the increase of nitrogen source concentration can induce the synthesis of nitrate reductase (NR) or activate NR proenzyme (Mohd-Sahib et al., 2017). With the enhance of NR enzyme activity, the cells can use nitrogen source more effectively. When the concentration of NaNO3 was above 0.8 g/L, the algae cells absorbed excessive nitrogen, interfered cell division and reduced biomass. It can be seen from the Fig. 3(b) that the concentration of nitrogen source has a great influence on the accumulation of lipid in Chlorella, and the low nitrogen medium will contribute to the accumulation of lipid in Chlorella cells. This was consistent with the literature report that the intracellular lipid content of Chlorella was higher under nitrogen deficiency (Mohd-Sahib et al., 2017). On the one hand, it might be caused by the increase of the synthesis of lipid related enzymes and the stop of the synthesis of cell growth and proliferation related enzymes. On the other hand, it might be that cells used the nitrogen source in chloroplast membrane for growth, thus degrading chloroplast and increasing lipid (Darpito et al., 2015). On the basis of the lipid yield, the maximum value appeared when NaNO3 was 0.8 g/L. Therefore, the optimal addition of NaNO3 was 0.8 g/L.
3.4 Effect of phosphorus concentration on biomass and lipid content of microalgae
In this experiment, KH2PO4 was used as supplementary phosphorus source to study the effect of phosphate supplement concentration on C. protothecoides B-15 growth and lipid accumulation in γ-PGA wastewater. The results are shown in Fig. 3(c).
The biomass of C. protothecoides B-15 increased rapidly with the increase of KH2PO4 concentration. When phosphorus was low, the growth of algal cells were affected and the biomass was reduced due to the lack of phosphorus synthesis (Sajid et al., 2016). When the concentration of KH2PO4 was 0.2 g/L, the maximum biomass reached 5.01 ± 0.12 g/L. When the concentration of KH2PO4 exceeded 0.2 g/L, the metabolic activity of cells decreased. Thus, the cell division of C. protothecoides B-15 was seriously interfered and the growth of C. protothecoides B-15 was declined.
The oil curve in Fig. 3(c) illustrates that the additive concentration of KH2PO4 has little effect on the lipid accumulation of C. protothecoides B-15 in γ-PGA wastewater. However, high concentration of KH2PO4 influenced the accumulation of oil. This might be the ratio imbalance of nitrogen to phosphorus in the medium at high concentration of KH2PO4 (Sajid et al., 2016). Based on the lipid yield, the maximum value appeared when KH2PO4 was 0.2 g/L. Therefore, the optimal addition of KH2PO4 was 0.2 g/L.
3.5 Box-Behnken design
The experimental design and results are presented in Table 2. Variance for the quadratic design was analyzed to check the validity of the model (Table 3).
| Code | A (g//L) | B (g//L) | C (g//L) | LY (g//L) |
|---|---|---|---|---|
| 1 | 7 | 1.0 | 0.2 | 1.47 ± 0.05 |
| 2 | 7 | 0.8 | 0.3 | 1.44 ± 0.04 |
| 3 | 5 | 0.8 | 0.3 | 1.51 ± 0.07 |
| 4 | 7 | 0.8 | 0.1 | 1.45 ± 0.04 |
| 5 | 7 | 0.6 | 0.2 | 1.57 ± 0.06 |
| 6 | 6 | 0.6 | 0.1 | 1.52 ± 0.03 |
| 7 | 6 | 1.0 | 0.1 | 1.49 ± 0.02 |
| 8 | 5 | 0.6 | 0.2 | 1.64 ± 0.06 |
| 9 | 6 | 0.8 | 0.2 | 1.77 ± 0.03 |
| 10 | 6 | 0.8 | 0.2 | 1.76 ± 0.03 |
| 11 | 6 | 0.6 | 0.3 | 1.61 ± 0.05 |
| 12 | 5 | 1.0 | 0.2 | 1.51 ± 0.04 |
| 13 | 6 | 1.0 | 0.3 | 1.41 ± 0.04 |
| 14 | 5 | 0.8 | 0.1 | 1.51 ± 0.07 |
| 15 | 6 | 0.8 | 0.2 | 1.73 ± 0.03 |
| 16 | 6 | 0.8 | 0.2 | 1.74 ± 0.05 |
| 17 | 6 | 0.8 | 0.2 | 1.75 ± 0.04 |
| Source | Sum of Squares | Mean Square | F Value | Probe (P) > F | Significant |
|---|---|---|---|---|---|
| Model | 0.25 | 0.028 | 178.80 | <0.0001 | Significant |
| A | 7.200E−003 | 7.200E−003 | 45.82 | 0.0003 | |
| B | 0.026 | 0.026 | 168.32 | <0.0001 | Significant |
| C | 1.120E−003 | 1.250E−003 | 5.722 | 0.0526 | |
| AB | 2.250E−004 | 2.250E−004 | 1.43 | 0.2704 | |
| AC | 2.500E−005 | 2.500E−005 | 0.16 | 0.7019 | |
| BC | 7.225E−003 | 7.225E−003 | 45.98 | 0.0003 | |
| A2 | 0.057 | 0.057 | 362.10 | 0.0001 | |
| B2 | 0.031 | 0.031 | 199.32 | 0.0001 | |
| C2 Residual Lack of Fit |
0.10 1.100E−003 1.000E−004 |
0.10 1.571E−004 3.333E−005 |
654.16 0.13 |
0.0001 0.9532 |
|
| Pure Error | 1.000E−003 | 2.500E−004 | |||
| Cor Total | 0.034 |
The quadratic polynomial regression model equations of A, B and C were obtained by using Design-Expert software:
Multiple regression fitting and statistical analysis of the test results were carried out by using the software, and the quadratic model regression statistical analysis table was obtained. It can be seen from Table 3 that the p value of the model term is 0.0001 < 0.01, indicating that the model is highly significant. At the same time, the determination coefficient R2 of the model is 0.9957, which indicates that the model has high reliability. Besides, the p value of the mismatch term is 0.13, greater than 0.05, which is beneficial to the model, and there is no mismatch factor. The significance test of independent variables shows that the first term B, the second term A2, B2, C2 are extremely significant model factors. It demonstrated that the influence of test factors on the response value is not a simple linear relationship, and the quadratic term also had a great influence on the response value. Furthermore, F value indicates the influence degree of each factor on the response value. It can be seen from Table 3 that the influence order of each single factor on lipid yield is B, A and C from large to small.
By drawing the response surface curve (Fig. 4), the interaction of various factors is analyzed more intuitively and visually. The best horizontal range of response surface is the top of contour line and the area nearby. In the response surface diagram, to the change of the results, the steeper the response surface is, the more sensitive the experimental factor is. In the contour map, the more elliptical the ellipse is, the more obvious the effect of experimental factors is. On the contrary, the slower slope of the curve or the nearer circle of the contour line, indicates that the interaction is not obvious.
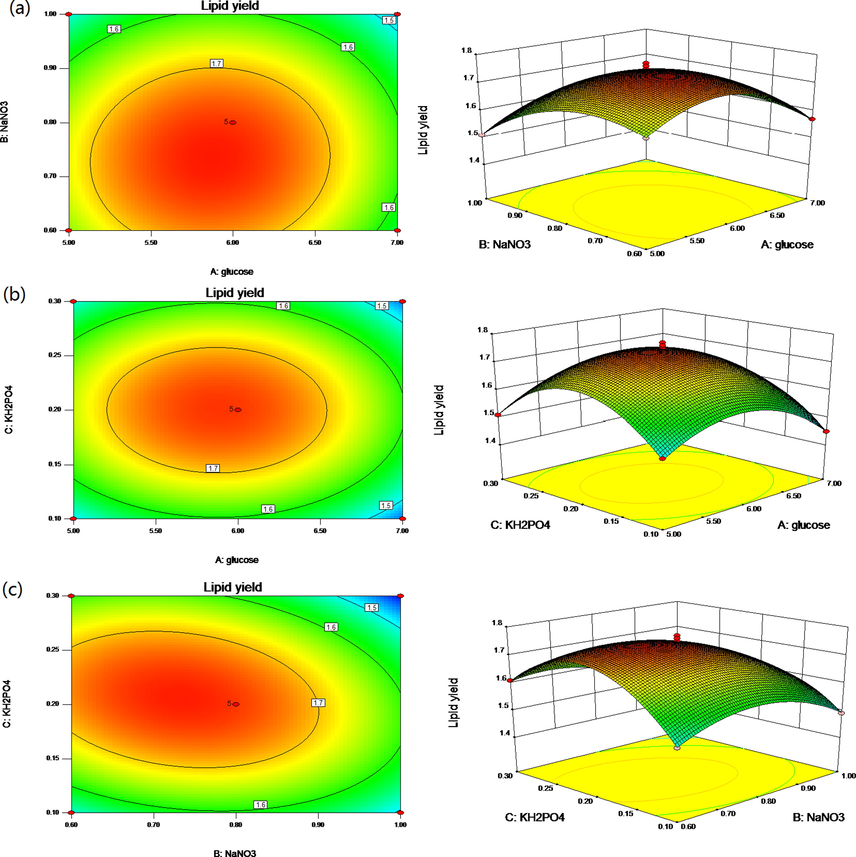
- Surface and contour plots of mutual-influence. (a) Glucose (A) and NaNO3 (B); (b) effect of glucose (A) and KH2PO4 (C); (c) effect of NaNO3 (B) and KH2PO4 (C).
By software analysis, the optimized conditions were obtained as follows: 5.86 g/L glucose, 0.73 g/L NaNO3 and 0.2 g/L K2HPO4. Under these conditions, lipid yield reached 1.77 ± 0.03 g/L. The lipid yield in SE medium and 60% wastewater medium was only 0.60 ± 0.03 g/L and 0.63 ± 0.02 g/L, respectively. The LY of the optimized medium was 1.95 and 1.81 times of that in SE medium and 60% wastewater medium, respectively. Therefore, the growth of C. protothecoides B-15 could be improved by adding appropriate nutrients to γ-PGA wastewater. It was of great significance for industrial production to maximize the utilization of wastewater resources and reduced the cost of microalgae cultivation.
3.6 γ-PGA wastewater changes before and after C. protothecoides B-15 treatment
The changes of various pollution indexes after C. protothecoides B-15 treatment were investigated. By reason of NaNO3 and other substances adding into 60% wastewater, COD and other indicators in the wastewater had changed. It was of no practical significance to calculate the COD removal rate of wastewater after microalgae treatment according to the actual COD in the culture medium. Therefore, the COD and other indicators in the culture medium was calculated as 60% of the wastewater COD.
Based on Table 1, the removal rate of CODCr by microalgae in wastewater reached 93.02%. N and P are the main elements of eutrophication in wastewater, while they are also important impact factors for algae growth. N is the main element that constitutes the proteins of algae. P is the main element of nucleic acid, phospholipid and adenosine triphosphate. N and P play important roles in algae growth and metabolism.
After 7 days’ culture, the mass concentration of TN decreased to 32 ± 2 mg/L, and the removal rate of TN was 93.87%, TP was 88.39%. It was seen that C. protothecoides B-15 could effectively absorb and utilize N and P sources to realize the advanced treatment of wastewater.
3.7 Treated water recycling in γ-PGA fermentation
The treated water was recycled in the γ-PGA fermentation process, and then the newly produced wastewater was biologically treated. After four cycles, γ-PGA yield decreased by only 1.06%. There was no significant difference in γ-PGA yield (t-test, data not presented). The results (Table 4) showed that the treated water had no negative effect on the γ-PGA production process after four cycles.
| Recycle time | γ-PGA yield (g/L) |
|---|---|
| 0 | 30.11 ± 0.54 |
| 1 | 29.95 ± 0.32 |
| 2 | 30.35 ± 0.36 |
| 3 | 30.08 ± 0.31 |
| 4 | 29.79 ± 0.76 |
Comparisons with various technologies were summarized in Table 5. Exogenous nutrients were needed to be added to the wastewater medium for higher C. protothecoides biomass. Moreover, the treated wastewater could be directly used for γ-PGA fermentation production without sterilization, as a result, the product’s added value of this technology was higher. This technology had the following advantages: (1) the wastewater from the γ-PGA factory is more likely to form a large-scale supply, and its pollution level is relatively low (no toxic or harmful substances), which is more suitable for large-scale cultivation of microalgae; (2) the physical and chemical properties of the γ-PGA wastewater between batches are stable, and it is easier to fulfill the stable cultivation of microalgae; (3) the cost of wastewater treatment is saved; (4) sixty percent of the process water for microalgae culture is saved; (5) various substances in wastewater can reduce the amount of nutrients in microalgae culture medium; (6) the treated wastewater can be used for γ-PGA production and can be reused for 4 times. In conclusion, this technology is a potential operable approach for microalgae culture and γ-PGA production.
| Forms | Medium | Strain | Biomass and lipid content | Reuse | Product | Remarks | Reference |
|---|---|---|---|---|---|---|---|
| Traditional technologies 1 | Dairy wastewater | C. vulgaris | 1.66 g/L, 36.67% | No | Biomass, biodiesel | The treated sewage could not be directly discharged. | Asadi et al. |
| Traditional technology 2 | Wastewater from ethanol fermentation | C. pyrenoidosa | 3.73 g/L, no biodiesel production | Yes | Biomass, ethanol | The main function of microalgae was to treat alcohol wastewater, not biodiesel production | Yang et al., 2008 |
| Traditional technology 3 | Brewery wastewater | C. protothecoides | 1.88 g/L, 35.94% | No | Biomass, biodiesel | The treated sewage could not be directly discharged. | Darpito et al., 2015 |
| This study | 6 g/L glucose, 0.8 g/L NaNO3, 0.2 g/L KH2PO4, 60% γ-PGA wastewater, water. | C. protothecoides | 5.01 ± 0.12 g/L, 34.04 ± 0.31% | Yes, reuse for γ-PGA production | Biomass, biodiesel and γ-PGA |
4 Conclusion
The feasibility of utilizing γ-PGA wastewater as culture medium for C. protothecoides was discussed. The results demonstrated that the algae could adapt to the 60% γ-PGA wastewater very well. Only a proper amount of nitrogen, phosphorus and glucose needed to be added to the γ-PGA wastewater for microalgae culture and oil production. When 5.86 g/L glucose, 0.73 g/L NaNO3 and 0.2 g/L K2HPO4 were added to the wastewater, the biomass and oil contents of microalgae could reach 1.77 ± 0.03 g/L, which was 195.0% higher than that in the SE medium(0.60 ± 0.03 g/L). Meanwhile, the detection results of TN and TP residues in culture water showed that microalgae had an efficient absorption mechanism for nitrogen and phosphorus. Furthermore, the wastewater treated by C. protothecoides could be reused for γ-PGA fermentation. Therefore, the application of γ-PGA wastewater in the cultivation of oil-bearing microalgae had a bright prospect. The microalgae biomass with high density and high oil content could be obtained only by adding a small amount of nutrients. This lessened the cost of media for oil-producing microalgae. Moreover, the wastewater treated by C. protothecoides could be reuse for γ-PGA production. It could not only generate significant economic and social benefits by algal biomass cultivation and lipid production, but also reduced the cost of process water in γ-PGA production. It could relieve environmental protection pressures for the γ-PGA industry, which was of great significance to the development of the fermentation industry in China.
Acknowledgments
This research was financially supported by State Key Laboratory of Microbial Technology (M2012-14), Shandong University.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Lipid and biodiesel production by cultivation isolated strain Chlorella sorokiniana pa.91 and Chlorella vulgaris in dairy wastewater treatment plant effluents. J. Environ. Heal. Sci. Eng. 2020
- [CrossRef] [Google Scholar]
- A rapid method of total lipid extraction and purification. Can. J. Biochem. Physiol. 1959
- [CrossRef] [Google Scholar]
- Combinatıon of a Box-Behnken design technique with response surface methodology for optimization of the photocatalytic mineralization of C.I. Basic Red 46 dye from aqueous solution. Arab. J. Chem.. 2020;13:8338-8346.
- [CrossRef] [Google Scholar]
- Effect of C/N ratio and aeration on the fatty acid composition of heterotrophic Chlorella sorokiniana. J. Appl. Phycol. 1991
- [CrossRef] [Google Scholar]
- Microalgae cultivation in a wastewater dominated by carpet mill effluents for biofuel applications. Bioresour. Technol. 2010
- [CrossRef] [Google Scholar]
- Anaerobic co-digestion of wastewater sludge: A review of potential co-substrates and operating factors for improved methane yield. Processes 2020
- [CrossRef] [Google Scholar]
- Biodiesel production evaluating the use and reuse of magnetic nanocatalysts Ni0.5Zn0.5Fe2O4 synthesized in pilot-scale. Arab. J. Chem.. 2020;13:3026-3042.
- [CrossRef] [Google Scholar]
- Cultivation of Chlorella protothecoides in anaerobically treated brewery wastewater for cost-effective biodiesel production. Bioprocess Biosyst. Eng. 2015
- [CrossRef] [Google Scholar]
- Silybum marianum L. seed oil: A novel feedstock for biodiesel production. Arab. J. Chem.. 2017;10:S683-S690.
- [CrossRef] [Google Scholar]
- Lipid accumulation properties of Chlorella vulgaris and Scenedesmus obliquus in membrane photobioreactor (MPBR) fed with secondary effluent from municipal wastewater treatment plant. Renew. Energy 2019
- [CrossRef] [Google Scholar]
- Dual role of Chlorella sorokiniana and Scenedesmus obliquus for comprehensive wastewater treatment and biomass production for bio-fuels. J. Clean. Prod. 2016
- [CrossRef] [Google Scholar]
- Invited commentary on “the cutter incident: Poliomyelitis following formaldehyde-inactivated poliovirus vaccination in the United States during the spring of 1955. II. Relationship of poliomyelitis to cutter vaccine”. Am. J. Epidemiol. 1995
- [CrossRef] [Google Scholar]
- Anaerobic digestion restricted to phase I for nutrient release and energy production using waste-water grown Chlorella vulgaris. Chem. Eng. J. 2018
- [CrossRef] [Google Scholar]
- Sustainable biomass production under CO2 conditions and effective wet microalgae lipid extraction for biodiesel production. J. Clean. Prod. 2020
- [CrossRef] [Google Scholar]
- Impact of various microalgal-bacterial populations on municipal wastewater bioremediation and its energy feasibility for lipid-based biofuel production. J. Environ. Manage. 2019
- [CrossRef] [Google Scholar]
- Comparative performances of microalgal-bacterial co-cultivation to bioremediate synthetic and municipal wastewaters whilst producing biodiesel sustainably. Processes 2020
- [CrossRef] [Google Scholar]
- Novel sequential flow baffled microalgal-bacterial photobioreactor for enhancing nitrogen assimilation into microalgal biomass whilst bioremediating nutrient-rich wastewater simultaneously. J. Hazard. Mater. 2020
- [CrossRef] [Google Scholar]
- Effect of phosphorus on lipid accumulation in freshwater microalga Chlorella sp. J. Appl. Phycol. 2013
- [CrossRef] [Google Scholar]
- Microalgae for biodiesel production and other applications: A review. Renew. Sustain. Energy Rev. 2010
- [CrossRef] [Google Scholar]
- Evaluation of fuel properties for microalgae Spirulina platensis bio-diesel and its blends with Egyptian petro-diesel. Arab. J. Chem.. 2017;10:S2040-S2050.
- [CrossRef] [Google Scholar]
- Lipid for biodiesel production from attached growth Chlorella vulgaris biomass cultivating in fluidized bed bioreactor packed with polyurethane foam material. Bioresour. Technol. 2017
- [CrossRef] [Google Scholar]
- Cultivation of oily microalgae for the production of third-generation biofuels. Sustain 2019
- [CrossRef] [Google Scholar]
- Process simulation and life cycle analysis of biodiesel production. Renew. Energy 2016
- [CrossRef] [Google Scholar]
- Extra- and intra-cellular accumulation of platinum group elements by the marine microalga, Chlorella stigmatophora. Water Res. 2014
- [CrossRef] [Google Scholar]
- Biosynthesis of high yield fatty acids from Chlorella vulgaris NIES-227 under nitrogen starvation stress during heterotrophic cultivation. Water Res. 2015
- [CrossRef] [Google Scholar]
- Growth of Chlorella pyrenoidosa in wastewater from cassava ethanol fermentation. World J. Microbiol. Biotechnol. 2008
- [CrossRef] [Google Scholar]
- Biosorption of Cr(VI) by immobilized waste biomass from polyglutamic acid production. Sci. Rep. 2020
- [CrossRef] [Google Scholar]
- Stimulatory effects of amino acids on γ-polyglutamic acid production by Bacillus subtilis. Sci. Rep. 2018
- [CrossRef] [Google Scholar]
- Economical production of agricultural Γ-polyglutamic acid using industrial wastes by Bacillus subtilis. Biochem. Eng. J. 2019
- [CrossRef] [Google Scholar]
- Study on the reuse of anaerobic digestion effluent in lactic acid production. J. Clean. Prod. 2019
- [CrossRef] [Google Scholar]







