Translate this page into:
CuMn2O4/Mn2O3 micro composites: Sol- gel synthesis in the presence of sucrose and investigation of their photocatalytic properties
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
The treatment of effluents using adsorption of pollution of solvent is a necessary aspect of research. The use of photocatalysts for this purpose has received much attention from researchers. In current research, the micro composites based on copper manganese oxides were synthesized through sol–gel method and used as photocatalyst. The uniformity, structure, shape, and size of the products are affected by metal salt type, fuel type, annealing temperature and time. The metal nitrate salts, sucrose, 850 ˚C and 2 h were selected as optimum precursors, fuel, annealing temperature and time, respectively. XRD indicated the successful formation of CMO/MO MCs in the presence of sucrose after annealing at 850 ˚C for 2 h. VSM showed a ferromagnetic behavior with a coercivity of 970.56 Oe. To investigation of the photocatalytic properties of CMO/MO MCs, several factors, including different organic contaminants, organic contaminant concentrations, and micro composite dosages were scrutinized to achieve the best efficiency. The photocatalytic tests indicated that micro composites can degrade RBBR in high values. For example, when 0.03 g of micro composite was used with 10 ppm RBBR under visible irradiation for 90 min, 79.18% of RBBR was destroyed. The hydroxyl radicals and holes were found to be active agents implicated in the degradation of RBBR by the scavenger's test. The recycle test unveiled that micro composites are so stable and after six cycles, the photocatalyst efficiency decreased by 12% for RBBR.
Keywords
Micro composites
CuMn2O4
Sol-gel synthesis
Photocatalyst
Copper manganese oxide
Magnetic property
1 Introduction
Recently, oxide-based materials have received much attention as photocatalysts for water treatment. Also, the polyoxometalates and metal manganites have been the subject of much interest (Arab Fashapoyeh et al., 2018); (Mirzaei et al., 2014); (Khoshkhan et al., 2021); (Mirzaei et al., 2014); (Samaniyan et al., 2021); (Derakhshanrad et al., 2021). Polyoxometalates are discrete anionic metal–oxygen clusters, which exhibit a great diversity of sizes, nuclearities, and shapes. The mixed copper manganese oxides have a spinel structure with formula AB2O4. In spinel structure, B and A ions are inhabited in the octahedral and tetrahedral sites, respectively. While in inverse spinel, the A cation and half the B cations are in the octahedral and the A tetrahedral positions, respectively (Enhessari et al., 2016). CuMn2O4 is a member of the family of spinels. Copper and manganese ions in CuMn2O4, have + 2 and + 3 or + 4 and + 2 charges. This spinel is an n-type semiconductor due to electron transfers between Mn3+ and Mn2+ ions (Popescu et al., 2015). The AB2O4 spinels are of considerable industrial interest due to their potential applications in catalysis (Chen et al., 2016; Cheng et al., 2022; Clarke et al., 2015; Hutchings et al., 1996; Wollner et al., 1993).
CuMn2O4 structures and composites have many applications. We have devoted our efforts to synthesizing this material. CuMn2O4 has been prepared using various methods. These methods have been summarized in Table 1. In this work, CuMn2O4/Mn2O3 micro composites (CMO/MO MCs) are synthesized via a simple sol–gel method in the presence of sucrose for the first time. The sol–gel is a wet chemical process used to synthesize metal oxides. In this method, we can control the chemical composition of the products well because of the low reaction temperature (Bokov et al., 2021). The sol–gel is a conventional, industrial, and cost-effective method. In this method, the molecular precursor is dissolved in water or alcohol and converted to gel by heating and stirring by hydrolysis/alcoholysis. Then gel should be dried and calcined. The formation of the sol and its conversion into the gel are two basic processes in this method (Bokov et al., 2021). There is little study on the use of fuels in the sol–gel synthesis of copper manganite oxide spinels (Einaga et al., 2015; Enhessari et al., 2016; Erhardt et al., 2020). Sucrose is a low-cost and widely available compound. We used it as both fuel and chelating agent in this study (Erhardt et al., 2020). The sucrose can distribute cations homogeneously in the reaction solution. It also can accelerate combustion (Owczarz et al., 2019). Also, in this work, the photocatalytic performance of CMO/MO MCs is studied for some organic dye removal from wastewater in the presence of visible irradiation. The photocatalytic performance for the degradation of Remazol Brilliant Blue R dye (RBBR) is studied in this research, for the first time. The synthesis of micro/nanostructures is an important factor to photocatalytic water treatment. The photocatalysts has concerned a lot of consideration from scientists (Verwey and Heilmann, 1947). The photocatalytic degradation of colored pollutants in the presence of metal manganites have been studied by scientists. Larbi and co-workers investigated the photocatalytic degradation of Methylene Blue (MB) and Rhodamine B (RB) by NiMn2O4. They reported 66% and 80% decolorization efficiency for MB and RB, respectively, after 210 min (Larbi et al., 2019). Abel et al. studied Malachite Green (MG) and MB degradation in the presence of CuMn2O4 photocatalyst. They reported 92% and 86% as photocatalytic degradation percentages for MG and MB after 60 min and 120 min, respectively (Abel et al., 2019). Also, copper manganite nanostructures were synthesized and used as photocatalysts by Sobhani-Nasab. He used RB and Methyl Orange (MO) as contaminant dyes and reported 40% and 28.2% degradation in the presence of CuMn2O4 nanoparticles under UV and Vis irradiation after 70 min, respectively (Sobhani-Nasab et al., 2020). Our group has synthesized copper manganites by co-precipitation, hydrothermal and sol–gel methods and studied the photocatalytic degradation of organic dyes in the presence of copper manganites (Sobhani, 2022). Table 2 compares the photocatalytic efficiency of CMO MCs synthesized in this research with that of AMn2O4 spinels synthesized in the previous works. In this research, we are also studied the effect of three scavengers, including benzoquinone (BQ), benzoic acid (BA) and EDTA in the degradation of RBBR color in visible light for the first time.
Product
Synthetic method
Application
Year
Reference
CuMn2O4
Supercritical anti-solvent precipitation
Oxidation of CO
2011
(Tang et al., 2011)
CuMn2O4 supported on SiO2
Impregnation method
Catalyst for benzene oxidation with ozone
2015
(Einaga et al., 2015)
CuMn2O4
Mechanochemical method
Oxidation of carbon monoxide
2015
(Clarke et al., 2015)
CuMn2O4
Sol-gel dip-coating
Solar absorber film
2016
(Chen et al., 2016)
CuMn2O4
Sol-gel solution combustion
To fabricate thickness-sensitive spectrally selective paint coatings
2016
(Ma et al., 2016)
CuMn2O4
Solvothermal
Electrochemical applications
2017
(Ma et al., 2016)
CuMn2O4
Reflux route
Anode for lithium-ion batteries
2018
(Saravanakumar et al., 2017)
CuMn2O4 nanoparticles and CuMn2O4-chitosan nanocomposite
Low temperature stirring technique
Sensing elements in the meters used for environmental monitoring
2019
(Li et al., 2018)
CuMn2O4
Co-precipitation
As photocatalyst for MB, MG degradation
2019
(Chani et al., 2019)
CuMn2O4
Sol-gel auto-combustion
Elimination of Hg from syngas
2020
(Abel et al., 2019)
Cu1.5Mn1.5O4
Citric acid complexation method
Catalytic oxidation of CO
2021
(Wang et al., 2020)
CuMn2O4
Sonication
Electrod
2022
(Sun et al., 2021)
CuMn2O4/CuO nanocomposite
Hydrothermal
Photocatallyst
2022
(Sobhani, 2022)
CuMn2O4/Mn2O3
Sol-gel
Photothermal catalyst
2022
(Cheng et al., 2022)
CuMn2O4/Co3O4 nanocomposite
Sol-gel
photodegradation of antibiotics
2023
(Nie et al., 2022)
CuMn2O4/CeO2 nanocomposites
Sol-gel
Photocatalyst for the H2 evolution
2023
(Almenia et al., 2023)
CuMn2O4 on graphene sheets
Reflux
Electrode
2023
(Alzahrani et al., 2023)
CuMn2O4/chitosan micro/nanocomposite
Hydrothermal
MB removal
2023
(Nikhil Chandran et al., 2023; Samadi Kazemi and Sobhani, 2023)
Photocatalyst
Organic dye
Irradiation Time(min)
Degradation%
Reference
CuMn2O4/Mn2O3 microcomposites
Remazol Brilliant Blue R
90
79
Present work
CuMn2O4
Rhodamine B
Methyl Orange60
6068
38(Salunkhe et al., 2012)
CuMn2O4
Malachite Green
Methylene Blue60
12092
86(Pedra et al., 2016)
CuMn2O4/CuO nanocomposites
Malachite Green
90
73 (under vis)
(Samaniyan et al., 2021)
CuMn2O4 nanoparticles
Rhodamine B
Rhodamine B70
7076 (under UV)
45.2 (under vis)(Salunkhe et al., 2012)
CuMn2O4 nanoparticles
Methyl Orange
Methyl Orange70
7040 (under UV)
28.2 (under vis)(Salunkhe et al., 2012)
NiMn2O4
Methylene Blue
Rhodamine B210
21066
80(Bhagwat et al., 2019)
2 Experimental
2.1 Materials and experiments
Materials used in this work are Cu(NO3)2·3H2O, Mn(NO3)2·4H2O, and sucrose. These materials were purchased from Merck company. A Philips X′pertPro X-ray diffractometer with Ni-filtered Cu Kα radiation and λ = 1.54 Å was used to obtaine XRD patterns. A TESCAN Mira3 field emission scanning electron microscope (FE-SEM) was used to investigate the morphology of the products. This microscope was also used to study energy dispersive X-ray spectrum (EDS). A Nicolet IS 10 spectrophotometer of American Thermo scientific company was used to take Fourier Transform Infrared (FT-IR) spectrum. A vibrating sample magnetometer (VSM) of Meghnatis Kavir Kashan was used to study the magnetic property of the products.
2.2 Synthesis of CuMn2O4/Mn2O3 micro composites
CuMn2O4/Mn2O3 micro composites were prepared via sol–gel method. First an aqueous solution of Cu(NO3)2·3H2O (concentration 0.024 M) was prepared. Then a sucrose solution with concentration 0.144 M was added into it. After stirring for 30 min, the Mn(NO3)2·4H2O solution (concentration 0.048 M) was added into the solution under stirring. The stoichiometric ratio of Cu:Mn:sucrose was selected to be 1:2:6. The solution was stirred at 100 ˚C for 60 min. Evaporation of the mixed solution caused formation of a highly viscous gel. The gel then is dried in an oven at 200 ˚C. The final residue is annealed in furnace at different temperatures (500 ˚C, 700 ˚C and 850 ˚C) for 2 h to examine the effect of high temperature exposure on the micro composite. The micro composite is maintained in penicillin vial and characterized by XRD, SEM, VSM, FT-IR and EDS. Table 3 shows the reaction conditions for the synthesis of CMO/MO MCs in this work. Scheme 1 is a diagram illustrating the synthesis and characterization of CMO/MO MCs.
Sample
Cu Source
Mn Source
Fuel
Cu:Mn:Fuel
Annealing Temperature(˚C)
Annealing Time (h)
Product
1
Cu(NO3)2·3H2O
Mn(NO3)2·4H2O
Sucrose
1:2:6
850
2
Polygonal microprisms of CuMn2O4/ Mn2O3
2
Cu(NO3)2·3H2O
Mn(NO3)2·4H2O
Sucrose
1:2:6
700
2
Cu
3
Cu(NO3)2·3H2O
Mn(NO3)2·4H2O
Sucrose
1:2:6
500
2
Cu
4
Cu(NO3)2·3H2O
Mn(NO3)2·4H2O
Sucrose
1:2:6
850
4
Spherical nanostructures
5
Cu(NO3)2·3H2O
Mn(NO3)2·4H2O
Glucose
1:2:6
850
4
Microspheres
6
Cu(NO3)2·3H2O
Mn(NO3)2·4H2O
Fructose
1:2:6
850
4
Microspheres
7
Cu(NO3)2·3H2O
Mn(NO3)2·4H2O
Maltose
1:2:6
850
4
Agglomerated nanoparticles
8
CuCl2
Mn(NO3)2·4H2O
Glucose
1:2:6
850
4
Microspheres
Diagram illustrating the synthesis and characterization of CMO/MO MCs.
2.3 Photocatalytic measurements
For the investigation of the photocatalytic activity of the micro composites, RBBR and MG were applied as two contaminant anionic and cationic dyes. The specified quantities of the CMO/MO MCs were weighted and used for the degradation of dye solutions with different concentrations. The micro composites were suspended in 30 mL of the dye solution in a quartz reactor. The suspension was aerated in darkness and was stirred constantly for 15 min. It was placed under visible irradiation by an Osram 400 W visible light (containing a wavelength in the range of 400 to 780 nm) at a space of 40 cm away from it. Then suspension containing dye was aerated continuously to supply O2. After 10 min stirring under visible irradiation, 5 mL of the suspension was sampled. In order to ensure removing the photocatalyst from samples, the suspension was centrifuged and scrutinized with a UV–Vis spectrometer and its absorbance spectrum was recorded. We used the below equation to estimate the photocatalytic degradation of the dyes:
A0 and A in above equation are absorbance quantities of dye solution before and after decomposition, respectively (Mahdiani et al., 2019). To optimize the photocatalytic activities, three effects including type and concentration of dye, and photocatalyst dose were studied in this work.
3 Results and discussion
3.1 Phase purity and chemical structure
XRD analyzed the crystalline structures of powders synthesized by sol–gel. Fig. 1 shows the XRD results of samples 1, 2 and 3. The formation of CuMn2O4 is confirmed in sample 1 through XRD. Also, a small percentage of the secondary phase of Mn2O3 is observed in this sample. In Fig. 1a, the 2θ values at 30.43°, 35.84°, 57.59° and 63.27° correspond to (hkl) planes (2 2 0), (3 1 1), (5 1 1) and (4 4 0) of CuMn2O4 (with JCPDS card no. 01–084-0543), respectively. The prepared CuMn2O4 has Fd-3 m space group and face centered cubic structure. Also, Scherer formula (Eq. (2) was used to calculate the average crystallite size of CuMn2O4 (Sobhani, 2022):
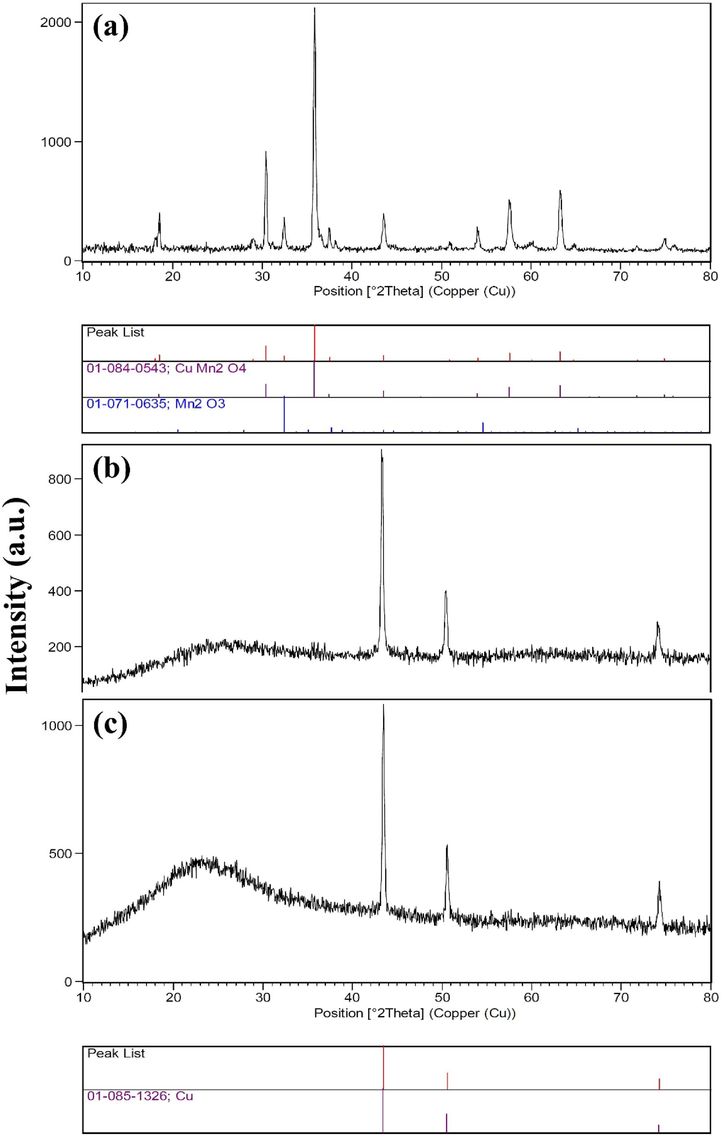
XRD patterns of the products prepared from Cu and Mn nitrate salts in the presence of sucrose via sol–gel method at different annealing temperatures for 2 h: (a) 850 ˚C (sample 1), (b) 700 ˚C (sample 2) and (c) 500 ˚C (sample 3).
In this equation, D, K, λ, β, θ are average crystallite size, dimensionless shape factor, X-ray wavelength, line broadening at half the maximum intensity (FWHM), and Bragg angle, respectively. K value is about 0.9 and varies with the shape of the crystallites. D was calculated 34.00 nm for sample 1. In the XRD patterns of samples 2 and 3, the CuMn2O4 is not present, and as shown in Fig. 1b and c after annealing at 700 ˚C and 500 ˚C a single Cu phase is formed. Thus, the successful sol–gel synthesis of copper manganites is achieved when using sucrose as fuel and 850 ˚C as annealing temperature.
3.2 Microscopic structure
SEM and TEM images in Fig. 2 show the morphology of the copper manganites prepared via the sol–gel method at 850 ˚C (sample 1). SEM images in Fig. 2a-d show the formation of polygonal micro prisms stuck together. TEM images in Fig. 2e, f show that the diameters of the base of these prisms are 300–700 nm. As the baking process continues to 4 h, the prisms destroy, and spherical nanostructures form, as shown in SEM images in Fig. 3. It seems that all samples have a high tendency towards spherical morphology.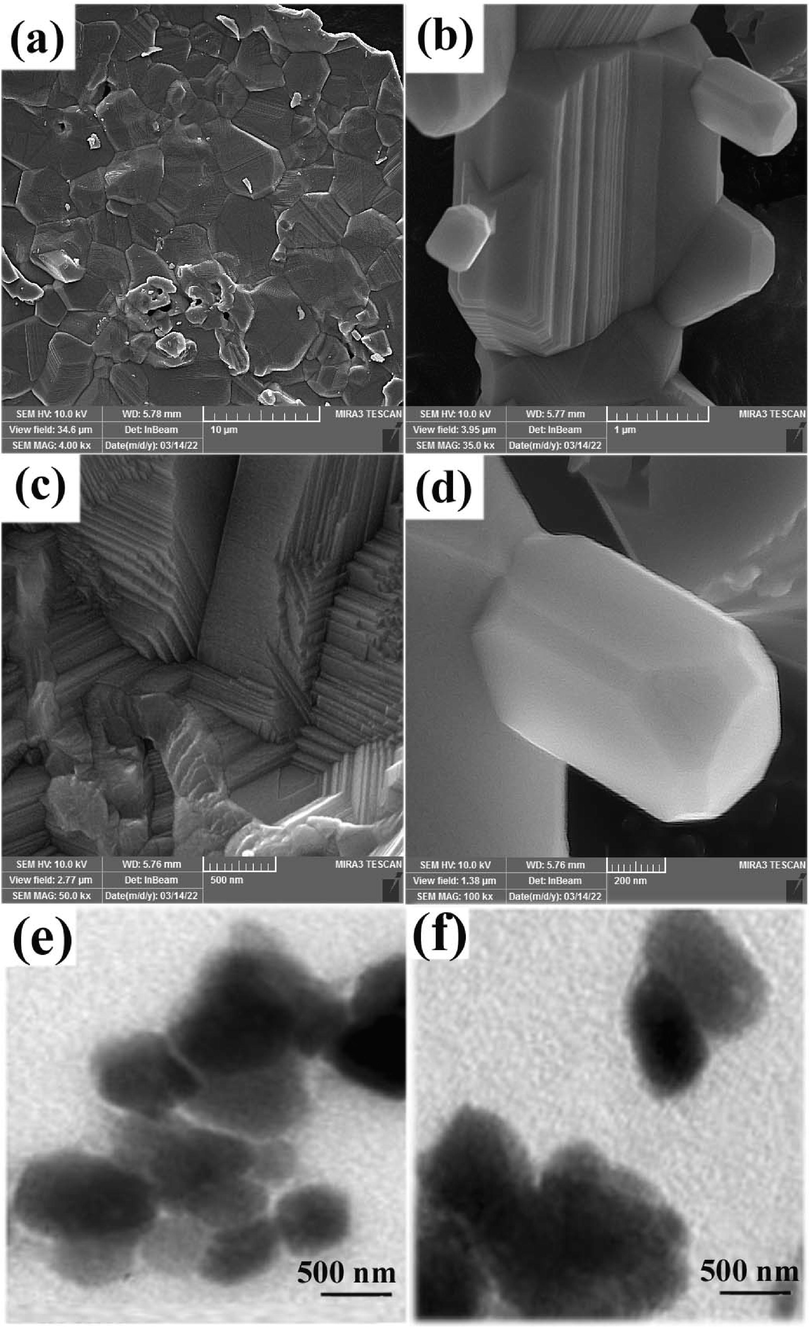
(a-d) SEM and (e, f) TEM images of the product prepared from Cu and Mn nitrate salts in the presence of sucrose via sol–gel method after annealing at 850 ˚C for 2 h (sample 1).
SEM images of the product prepared from Cu and Mn nitrate salts in the presence of sucrose via sol–gel method after annealing at 850 ˚C for 4 h (sample 4).
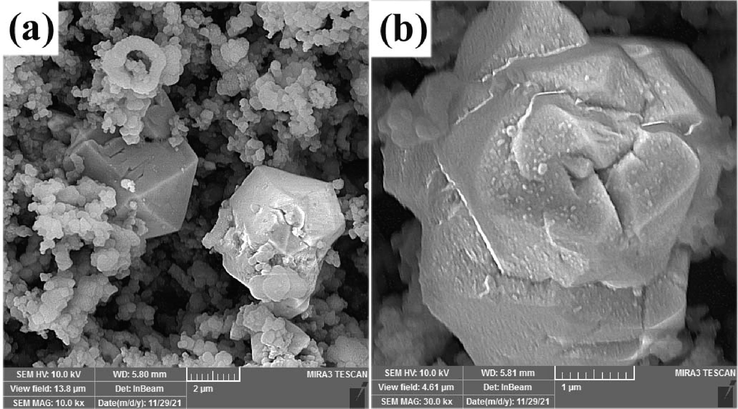
We continued the sol–gel method to synthesize copper manganites in the presence of different fuels, including glucose, fructose, and maltose. SEM images in Fig. 4 show that microspheres form in the presence of glucose and fructose, as two monosaccharides. Fig. 4a and b show that the microspheres with diameters 50 nm-1 µm form in the presence of glucose. The surfaces of the spheres have been covered with nanoparticles. These microspheres are probably formed from the accumulation of nanoparticles. The products prepared with fructose (sample 4) are microspheres with diameters 2–5 µm, also nanoparticles with diameters of about 70 nm form in the presence of this fuel (as shown in Fig. 4c and d). The use monosacarides can lead to decreased formation of nucleation points. The lower number of these points for crystal growth translates into increased growth of the created nuclei, resulting in larger particles. Agglomerated nanoparticles with diameters ≤ 100 nm formed when using maltose as fuel, as shown in Fig. 4e, f. Also, dense agglomerates of nanoparticles are observed in this figure that construct nanospheres. The morphology of samples 2 and 5 are similar. Both samples 2 and 5 have synthesized in the presence of disacarides. The decrease in particle size in samples prepared with disacarides than that prepared with monosacarides is observed clearly in SEM images in Figs. 3 and 4. This decrease highlights the influence of sucrose and maltose on the particle size of the crystallites. SEM results show that in this work conditions, disaccharides are suitable fuels for so-gel synthesis of manganites.
SEM images of the products prepared from Cu and Mn nitrate salts in the presence of different fuels via sol–gel method at 850 ˚C for 4 h: (a, b) glucose (sample 5), (c, d) fructose (sample 6) and (e, f) maltose (sample 7).
In continuation, the effect of the copper salt on the morphology of the manganites studies. SEM images in Fig. 5 are related to the sample prepared from CuCl2 and Mn(NO3)2·4H2O in the presence of glucose (sample 6). Changing the copper salt, the morphology of the composites does not change. Both samples as-prepared from nitrate and chloride copper salts are microspheres with none-even diameters. The surfaces of microspheres in both samples have been covered with nanoparticles. The microspheres in sample 6 have larger diameters than that of sample 3. These different diameters can be due to the difference in release rates of Cu2+ ions from their salts. Under the same conditions, the particle size decreases by decreasing the release rate of Cu2+ from its salts.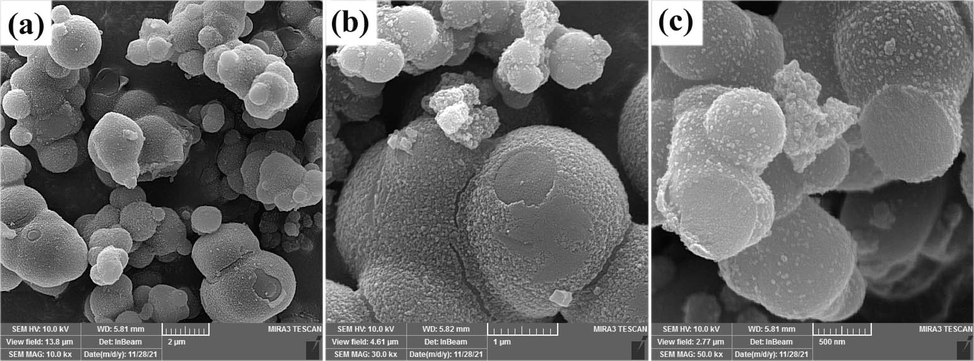
SEM images of the product prepared from CuCl2 and Mn(NO3)2·4H2O in the presence of glucose via sol–gel method at 850 ˚C for 4 h (sample 8).
3.3 EDS, FT-IR spectra
EDS is an elemental spectroscopy technique, which goes hand in hand with electron microscopy. Fig. 6 shows EDS spectrum of CMO/MO NCs (sample 1) and confirms the presence of Cu, Mn and O elements in chemical composition of this sample. According to this figure, the atomic percentages of Cu, Mn and O are 10.99 %, 21.60 % and 67.41 %, respectively. The results obtained from EDS spectrum confirm the XRD results in Fig. 1a and also formation of CMO/MO NCs.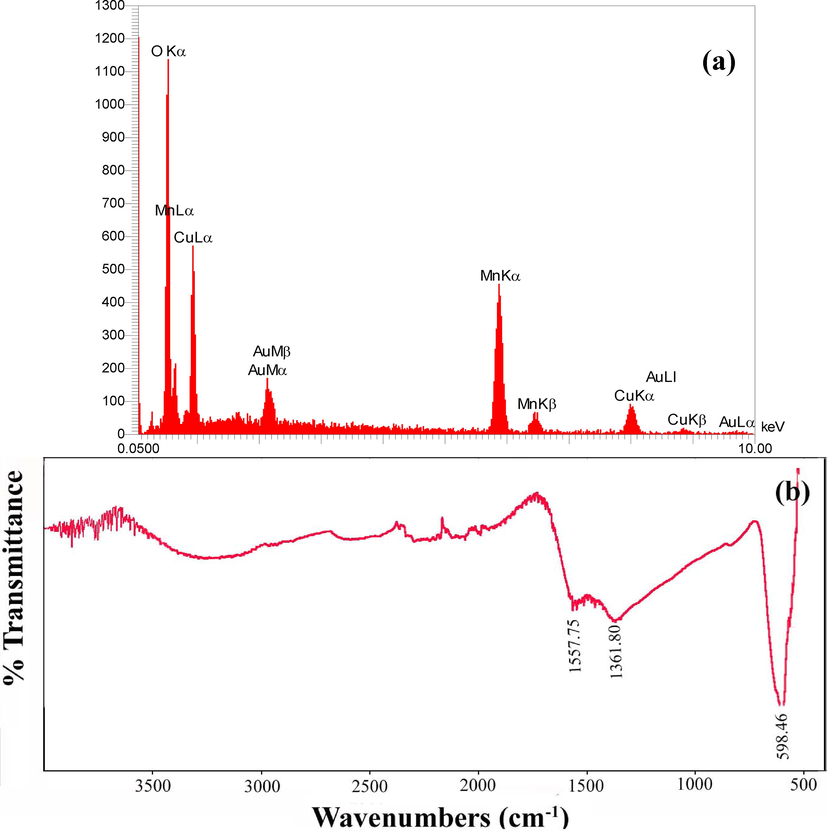
(a) EDS and (b) FT-IR spectra of the product prepared from Cu and Mn salts in the presence of sucrose via sol–gel method at 850 ˚C for 2 h (sample 1).
Fig. 6b shows FT-IR spectrum of sample prepared from Cu and Mn salts in the presence of sucrose via sol–gel method (sample 1). This spectrum has been recorded between 4000 and 500 cm−1. The emission, absorption and photoconductivity of gas, liquid, and solid can be obtain by FT-IR technique. This spectrum identifies different functional groups in sample. The magnitude and symmetry of the dipole moment of the bonds in the sample are effective agents in the intensity of the FT-IR peaks. In Fig. 6b the low-intensity and broad peak at about 3250 cm−1 shows water absorption on the surface of the sample 1. This region is related to O–H stretching vibrations. The peaks at 1557.75 and 1361.80 cm−1 correspond to H2O bending vibrations and organic residues in the product, respectively. The absorption bond in the area of the 598.46 cm−1 is attributed to manganese-oxygen lattice vibrations. This high-intensity and low-frequency mode in FT-IR spectrum of sample 1 is related to the stretching vibrations of Mn-O bond (Abel et al., 2019); (Wang et al., 2013) . FT–IR results for sample 1 confirm the formation of CuMnO nanostructures.
3.4 Magnetic and optical studies
VSM is one of the most successful implementations of a magnetometer. It is a versatile technique for measuring the magnetic moment of a sample when it is vibrated perpendicularly to a uniform magnetizing field. Changes as small as 10−5 to 10−6 emu can be detected with this method. Fig. 7 shows magnetization curve of sample 1 measured using VSM, which gives the magnetic moment of the sample as a function of applied magnetic field. The figure shows a ferromagnetic behavior with a coercivity of 970.56 Oe in low fields. Also, Fig. 7 shows an antiferromagnetic behavior for the micro composites in high fields. It is due to the contribution of super-exchange interactions between Mn ions (Mn3+–O2−–Mn4+).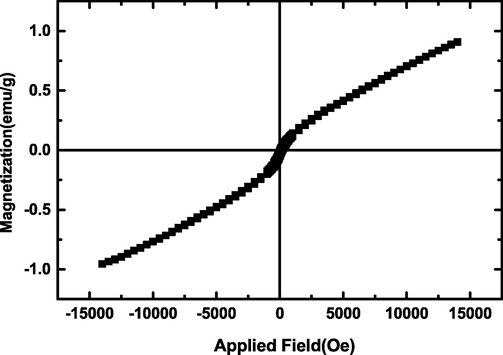
Magnetization curve versus field of the product prepared from Cu and Mn salts in the presence of sucrose via sol–gel method at 850 ˚C for 2 h (sample 1).
Fig. 8a shows UV–Vis DRS absorption spectrum of sample 1 between 200 and 700 nm. A sharp absorbance peak at about 246 nm is seen in this figure. This peak is due to the creation of electron-hole recombination on the surface of CMO MCs.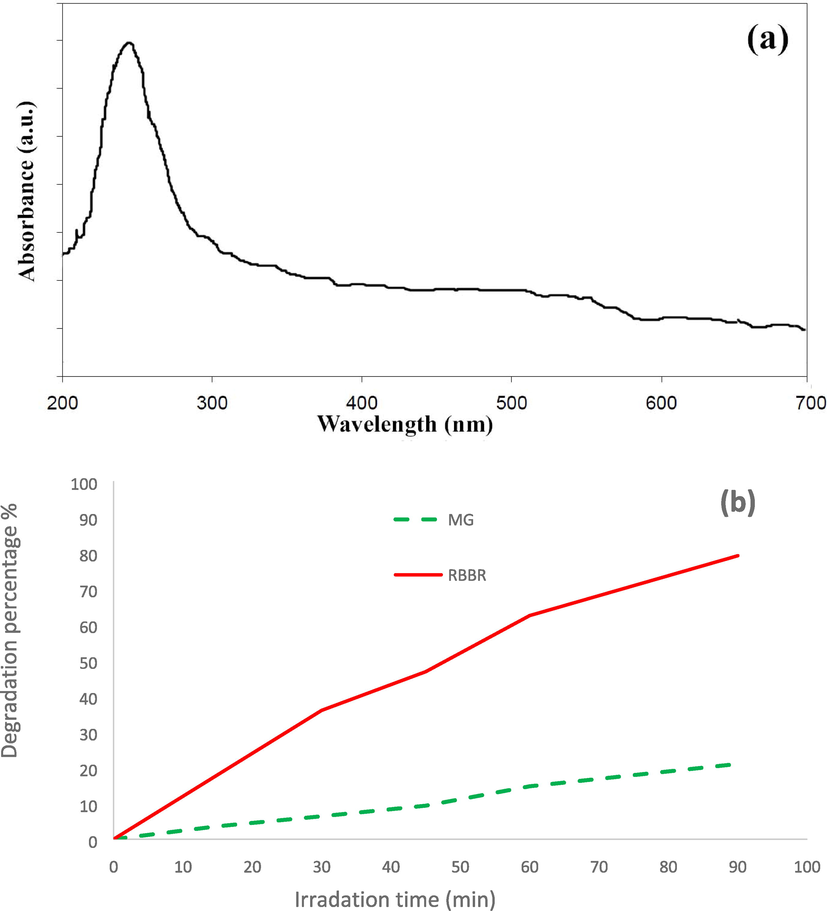
(a) UV–Vis DRS absorption spectrum of sample 1, (b) Photocatalytic activity of sample 1 for degradation of RBBR and MG under visible light irradiation.
3.5 Photocatalytic studies
In this work, two dyes including RBBR and MG were used in order to investigation of the effect of anionic and cationic dyes. As shown in Fig. 8b, the photocatalytic performance of CMO/MO MCs is higher for the degradation of RBBR anionic dye than that for the degradation of MG cationic dye. The RBBR is an anionic contaminant and its oxygen groups can increase electron density on surfaces of the photocatalyst. Thus, in the present work condition, photocatalytic activity of the as-prepared composite is higher for the RBBR degradation than MG. The degradations were reported 79.17% and 21.01% in the presence of CMO/MO MCs, after 90 min visible light irradiation, for EBBR and MG, respectively.
The as-prepared CMO/MO MCs in this work were used as photocatalyst for RBBR degradation. Fig. 9a shows effect of the amount of composite on the RBBR degradation percent. Increasing the photocatalyst amount, RBBR degradation increased because the dye interaction with the photocatalyst increased. Also, this increase was because of increasing local active sites to adsorb dye molecules. The more increasing photocatalyst at constant RBBR concentration and volume, the opposite result was obtained. RBBR degradation decreased with a more increase in the amount of photocatalyst, due to these reasons: (1) aggregation resulting from high photocatalyst dose, (2) decrease in total surface area of the photocatalyst, (3) increase in diffusional path length, (4) unsaturation of photocatalyst sites through the adsorption process. The degradation percent of 10 ppm solution of RBBR in the presence of 0.02, 0.03, and 0.05 g photocatalyst was measured 31.91 %, 79.17 %, and 60.94 % after 90 min visible irradiation, respectively.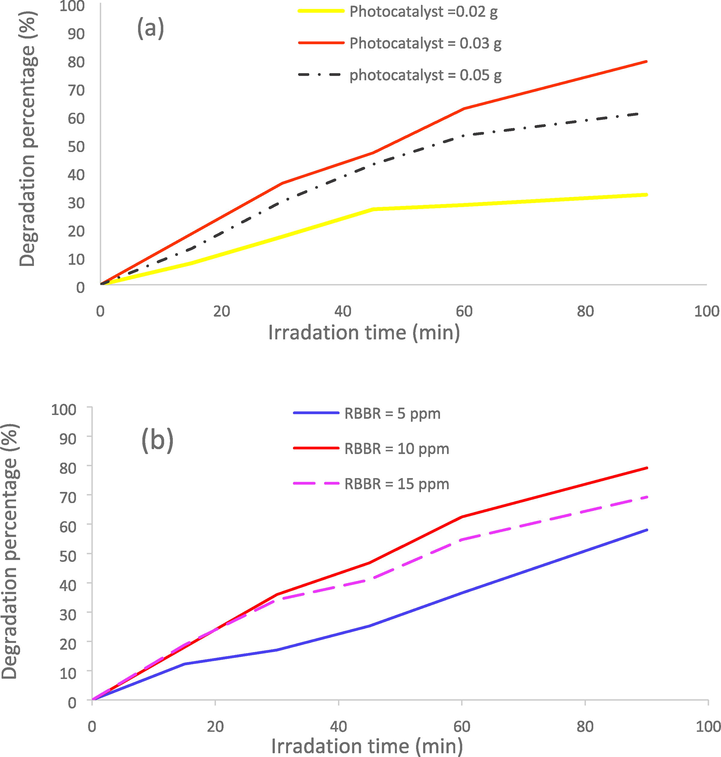
Investigation of the effects of: (a) photocatalyst dose and (b) dye concentration on the RBBR degradation under visible irradiation.
In order to investigation of effect of dye concentration on the photocatalytic activity of CMO/MO MCs, three RBBR solutions with 5, 10 and 15 ppm concentrations were prepared. The photocatalytic tests were investigated and results have shown in Fig. 9b. The figure shows that with the increase in RBBR concentration (from 10 to 15 ppm) its degradation percentage decreases. The degradation percentage for a 10 ppm solution of RBBR was obtained 79.17% after 90 min of irradiation. This percentage was decreased to 69.23 % for a 15 ppm solution of RBBR. Increasing dye concentration and thus increasing number of dye molecules, these molecules can compete with the generated oxidizing agents to adsorb on the surface of the photocatalyst. This competition can slow the degradation process by reducing number of available oxidant agents in the solution. Also, in the high dye concentrations, the production of electron and hole is lowered because of the lowering of light penetrating power that can lower rate of photodegradation. In this study we found an interestingly result. The photocatalytic tests done for the investigation of the effect of dye concentration showed a deflection at 5 ppm solution of RBBR. The degradation percentage was decreased to about 58% for 5 ppm solution of RBBR. The optimum conditions for the photocatalytic degradation in this synthesis were selected: RBBR solution with 10 ppm concentration and 0.03 g CMO/MO MCs as photocatalyst.
The photocatalytic activity mechanism of the nanocomposites for RBBR degradation proposes as follows. The RBBR is excited using visible irradiation, and the electrons of its valance band are moved to the conduction band of CMO NC. Then, these electrons are borrowed by the oxygens present in the water. Then oxygen radicals, which are reactive species, are produced. The removal of pollutants is done by active spices, for example hole, radical, and anion of superoxide (Mahdiani et al., 2017, 2019, Mirzaei et al., 2014a,b; Mohassel et al., 2018a,b). The pairs of electron–holes create owing to the excitation of CMO under visible irradiation, which delivers the required circumstances to create reactive oxygen species leading to pollutant removal (Jeyasubramanian et al., 2015). The below equations are several reaction sequences and suggest the photocatalytic degradation of the RBBR in this study:
The active species were scrutinized to comprehend the mechanism of the photocatalytic process. The most likely active species implicated in the photodegradation of organic pollutants are superoxide radical anions
, hydroxyl radicals (
), and holes (
. These active species are called scavengers. A scavenger is a chemical substance added to a mixture in order to remove or de-activate impurities and unwanted reaction products. Three scavenger species, BQ, BA, and EDTA as scavengers of
,
, and
were studied to determine which active species are more in charge of the degradation of RBBR in the photocatalytic reaction (Zhang et al., 1998). The degradation percentage of RBBR in the presence of these scavengers has been reported in Fig. 10a. As shown in this figure, the degradation efficiency has decreased in the presence of BA and EDTA. This decrease shows the role of hydroxyl radical and holes in the photocatalytic removal of RBBR (Ryu and Choi, 2006). Also, the stability of sample 1 as photocatalyst was investigated and destruction reactions were repeated (6 cycles). The degradation percentages were reported 79.18 %, 79.18 %, 79.18 %, 78.24 %, 76.84 %, and 67 % after six cycles for the photocatalyst toward the RBBR, as shown in Fig. 10b. We observed an activity loss about 12 % after six consecutive reactions due to the high stability of the CMO/MO MCs synthesized in this work.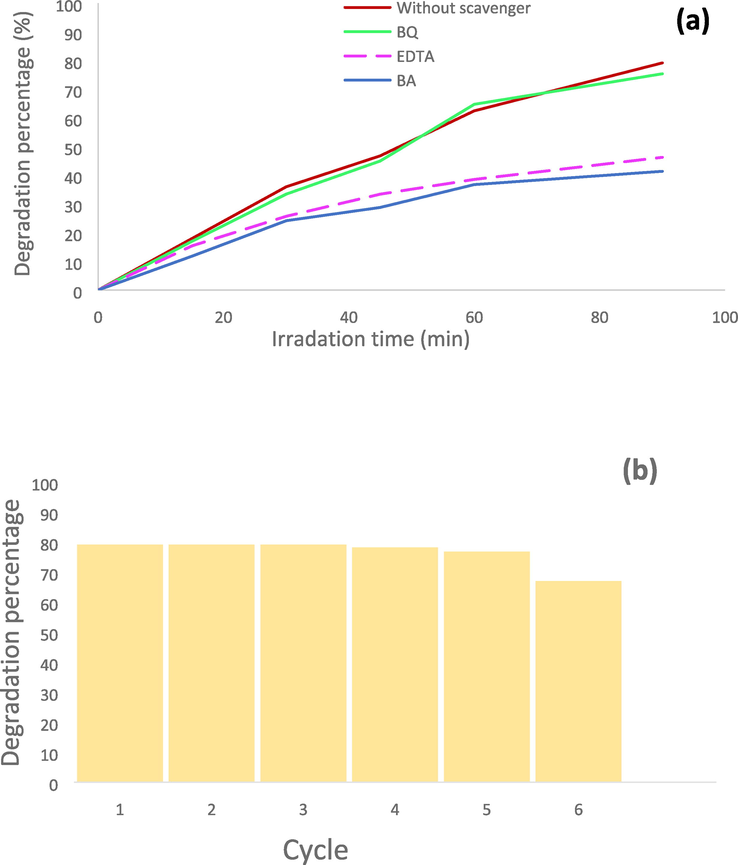
(a) The mechanism study using scavengers for RBBR degradation under visible irradiation, (b) Recycling tests of sample 1 for RBBR degradation under visible light irradiation.
4 Conclusions
CMO/MO MCs with a ferromagnetic behavior were prepared using the sol–gel method. The effects of metal salt type, fuel type, annealing temperature and time were investigated on the uniformity, shape, structure, size, and purity of the microcomposites. The sucrose was selected as optimum fuel. The photocatalytic behavior of CMO/MO MC showed that this compound has high potential in the degradation of RBBR. The studies indicated that 0.03 g microcomposite can degrade 79.18% of 10 ppm RBBR under visible light after 90 min. To highlight the photostability of CMO/MO MCs, we studied six cycles toward the RBBR and observed a low activity loss due to the high stability of the micro composites. The scavenger experiments indicated that hydroxyl radicals and holes perform roles in the photocatalysis process.
Acknowledgment
The study was supported by Kosar University of Bojnord with the grant number (NO. 0110201612).
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- M.J. Abel, A. Pramothkumar, N. Senthilkumar, K. Jothivenkatachalam, P.F.H. Inbaraj, J.J. Prince, Flake-like CuMn2O4 nanoparticles synthesized via co-precipitation method for photocatalytic activity, Phys. B (Amsterdam, Neth.) 572 (2019) 117-124.
- Flake-like CuMn2O4 nanoparticles synthesized via co-precipitation method for photocatalytic activity. J. Phys. B. 2019;572:117-124.
- [Google Scholar]
- Fabrication of CuMn2O4/Co3O4 nanocomposite heterojunctions for efficacious visible light-induced degradation of antibiotics. Ceram. Int.. 2023;49:13227-13237.
- [Google Scholar]
- Heterojunction of CuMn2O4/CeO2 nanocomposites for promoted photocatalytic H2 evolution under visible light. J. Taiwan Inst. Chem. Eng.. 2023;143:104692
- [Google Scholar]
- Photochemical and electrochemical hydrogen evolution reactivity of lanthanide-functionalized polyoxotungstates. Chem. Commun.. 2018;54:10427-10430.
- [Google Scholar]
- Sol-gel auto combustion synthesis and characterizations of cobalt ferrite nanoparticles: different fuels approach. Mater. Sci. Eng. B. 2019;248:114388
- [Google Scholar]
- Nanomaterial by Sol-Gel Method: Synthesis and Application. Adv. Mater. Sci. Eng.. 2021;2021:1-21.
- [Google Scholar]
- Impedimetric humidity and temperature sensing properties of chitosan-CuMn2O4 spinel nanocomposite. Ceram. Int.. 2019;45:10565-10571.
- [Google Scholar]
- Construction of unique cupric oxide–manganese dioxide core–shell arrays on a copper grid for high-performance supercapacitors. J. Mater. Chem. A. 2016;4:10786-10793.
- [Google Scholar]
- CuMn2O4 hierarchical microspheres as remarkable electrode of supercapacitors. Mater. Lett.. 2022;317:132102
- [Google Scholar]
- Mechanochemical synthesis of copper manganese oxide for the ambient temperature oxidation of carbon monoxide. Appl Catal B. 2015;165:222-231.
- [Google Scholar]
- Polyoxometalate-based frameworks as adsorbents for drug of abuse extraction from hair samples. Inorg. Chem.. 2021;60:1472-1479.
- [Google Scholar]
- Catalytic properties of copper-manganese mixed oxides supported on SiO2 for benzene oxidation with ozone. Catal. Today. 2015;245:22-27.
- [Google Scholar]
- Synthesis and physicochemical properties of CuMn2O4 nanoparticles; a potential semiconductor for photoelectric devices. Int. J. Bio-Inorg. Hybr. Nanomater.. 2016;5:115-120.
- [Google Scholar]
- Sucrose as a sol-gel synthesis additive for tuning spinel inversion and improving the magnetic properties of CoFe2O4 nanoparticles. Ceram. Int.. 2020;46:12759-12766.
- [Google Scholar]
- Ambient temperature CO oxidation using copper manganese oxide catalysts prepared by co-precipitation: effect of ageing on catalyst performance. Catal. Lett.. 1996;42:21-24.
- [Google Scholar]
- Photo-catalytic degradation of methyl violet dye using zinc oxide nano particles prepared by a novel precipitation method and its anti-bacterial activities. J. Water Proc. Eng.. 2015;8:35-44.
- [Google Scholar]
- Two polyoxometalate-based hybrids constructed from trinuclear lanthanoid clusters with single-molecule magnet behavior. Polyhedron. 2021;194:114903
- [Google Scholar]
- Temperature dependence of Raman spectra and first principles study of NiMn2O4 magnetic spinel oxide thin films. Application in efficient photocatalytic removal of RhB and MB dyes. Spectrochim. Acta A, Mol. Biomol. Spectrosc.. 2019;216:117-124.
- [Google Scholar]
- CuMn2O4/graphene nanosheets as excellent anode for lithium-ion battery. Mater. Res. Bull.. 2018;104:53-59.
- [Google Scholar]
- Solution combustion of spinel CuMn2O4 ceramic pigments for thickness sensitive spectrally selective (TSSS) paint coatings. Ceram. Int.. 2016;42:11966-11973.
- [Google Scholar]
- Aqueous solution-derived CuMn2O4 ceramic films for spectrally selective solar absorbers. Ceram. Int.. 2016;42:19047-19057.
- [Google Scholar]
- Enhancement of magnetic, electrochemical and photocatalytic properties of lead hexaferrites with coating graphene and CNT: Sol-gel auto-combustion synthesis by valine. Sep. Pur. Tech.. 2017;185:140-148.
- [Google Scholar]
- The first synthesis of CdFe12O19 nanostructures and nanocomposites and considering of magnetic, optical, electrochemical and photocatalytic properties. J. Hazard. Mater.. 2019;367:607-619.
- [Google Scholar]
- Recent developments in the crystal engineering of diverse coordination modes (0–12) for Keggin-type polyoxometalates in hybrid inorganic–organic architectures. Coord. Chem. Rev.. 2014;275:1-18.
- [Google Scholar]
- Syntheses, structures, properties and DFT study of hybrid inorganic–organic architectures constructed from trinuclear lanthanide frameworks and Keggin-type polyoxometalates. Dalton Trans.. 2014;43:1906-1916.
- [Google Scholar]
- Pechini synthesis and characteristics of Gd2CoMnO6 nanostructures and its structural, optical and photocatalytic properties. Spec. Chem. Acta A. 2018;204:232-240.
- [Google Scholar]
- Amino acid modified combustion synthesis, characterization and investigation of magnetic, optical and photocatalytic properties of Gd2CoMnO6 nanostructures. J. Alloy. Compd.. 2018;753:615-621.
- [Google Scholar]
- Photothermal catalytic oxidation of toluene with enhanced efficiency over constructed CuMn2O4/Mn2O3 heterojunction catalyst. Appl. Sur. Sci.. 2022;605:154567
- [Google Scholar]
- CuMn2O4 anchored on graphene sheets as a high-performance electrodes for sodium-ion batteries. J. Energy Storage. 2023;65:107346
- [Google Scholar]
- The effects of sucrose on the sol-gel phase transition and viscoelastic properties of potato starch solutions. Food Chem.. 2019;271:94-101.
- [Google Scholar]
- The influence of chelating agent on the structural and magnetic properties of CoFe2O4 nanoparticles. J. Nanosci. Nanotechnol.. 2016;16:4943-4947.
- [Google Scholar]
- Study of the electrical and catalytic properties of spinels with CuFe2-xMnxO4 composition (x = 0, 0.4, 0.8, 1.6 and 2) Appl. Catal. A: Gen.. 2015;504:29-36.
- [Google Scholar]
- Photocatalytic oxidation of arsenite on TiO2: understanding the controversial oxidation mechanism involving superoxides and the effect of alternative electron acceptors. Env. Sci. Tech.. 2006;40:7034-7039.
- [Google Scholar]
- Combustion synthesis of cobalt ferrite nanoparticles-influence of fuel to oxidizer ratio. J. Alloy. Compd.. 2012;514:91-96.
- [Google Scholar]
- CuMn2O4/chitosan micro/nanocomposite: Green synthesis, methylene blue removal, and study of kinetic adsorption, adsorption isotherm experiments, mechanism and adsorbent capacity. Arab. J. Chem.. 2023;16:104754
- [Google Scholar]
- Supramolecular network of a framework material supported by the anion–π linkage of Keggin-type heteropolyoxotungstates: experimental and theoretical insights. Dalton Trans.. 2021;50:1895-1900.
- [Google Scholar]
- Electrochemical properties of rice-like copper manganese oxide (CuMn2O4) nanoparticles for pseudocapacitor applications. J. Alloy. Compd.. 2017;723:115-122.
- [Google Scholar]
- Hydrothermal synthesis of CuMn2O4/CuO micro composite without capping agent and study its photocatalytic activity for elimination of dye pollution. Int. J. Hydrogen Energy. 2022;47:20138-20152.
- [Google Scholar]
- Eco-friendly preparation and characterization of CuMn2O4 nanoparticles with the green capping agent and their photocatalytic and photovoltaic applications. Iran. J. Catal.. 2020;10:91-99.
- [Google Scholar]
- Cu1.5Mn1.5O4 spinel type composite oxide modified with CuO for synergistic catalysis of CO oxidation. J. Fuel Chem. Technol.. 2021;49:799-808.
- [Google Scholar]
- Synthesis of high surface area CuMn2O4 by supercritical anti-solvent precipitation for the oxidation of CO at ambient temperature. Catal. Sci. Technol.. 2011;1:740-746.
- [Google Scholar]
- Physical properties and cation arrangement of oxides with spinel structures II. electronic conductivity. J. Chem. Phys.. 1947;15:174-180.
- [Google Scholar]
- AMn2O4 (A=Cu, Ni and Zn) sorbents coupling high adsorption and regeneration performance for elemental mercury removal from syngas. J. Hazard. Mater.. 2020;388:121738-121739.
- [Google Scholar]
- Size effect and enhanced photocatalytic activity of CuO sheet-like nanostructures prepared by a room temperature solution phase chemical method. Appl. Surf. Sci.. 2013;271:136-140.
- [Google Scholar]
- Characterization of mixed copper-manganese oxides supported on titania catalysts for selective oxidation of ammonia. Appl. Catal. A: Gen.. 1993;94:181-203.
- [Google Scholar]
- Active site of praseodymium orthovanadate catalyst in oxidative dehydrogenation of propane. Chin. Sci. Bull.. 1998;43:217-220.
- [Google Scholar]







