Translate this page into:
Current contributions of novel nanoparticles-based colorimetric sensors for detection of SCN ions in different aqueous models. A review
⁎Corresponding author. aymen.yaseen@uoninevah.edu.iq (Aymn Yaseen Sharaf Zeebaree),
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
The development of novel and effective sensors is inevitable for detecting environmental pollutants, including toxins, organic pollutants, and heavy metals, which have hazardous effects on living organisms. Despite the challenges faced by this technology, sensors have played a crucial role in detecting various analytes with high sensitivity and producing reliable results. Metal nanoparticles, owing to their significant optical properties, unique sizes, shapes, and biocompatibility, have been widely utilized in detecting numerous hazardous pollutants such as heavy metals, textile dyes, pesticides, herbicides, banned surfactants, and harmful food supplements. The fundamental characteristic of these nanomaterials is their ability to change color based on their maximum absorption wavelength, known as surface plasmon resonance (SPR). Due to the simplicity, speed, and sensitivity of nanoparticle-based colorimetric sensing, this method has been extensively utilized in rapid testing, real-time monitoring, and reducing the time required to assess the quality and safety of water. Recently, this property has been extensively exploited for detecting various pollutants, including thiocyanate ions, which affect water quality.
In this review, we discuss the preparation methods, functional enhancement, and optical property diagnosis of newly developed nanometals as colorimetric sensors and their application as rapid testing tools for detecting thiocyanate ions in water models. Additionally, we outline the suggested colorimetric sensing mechanism for this ion, along with the advantages and drawbacks of colorimetric sensors based on nanometals. This discussion aims to provide researchers with insights to avoid these limitations and encourage the development of better sensors in the future.
Keywords
Colorimetric assay
Nanometals sensors
Thiocyanate ions detection
1 Introduction
Pollution has become a major problem worldwide in the 21st century, significantly reducing the quality of water resources and posing serious risks to the environment and human health. In recent decades, the release of dyes, toxic heavy metals, chemical surfactants, and expired drugs has played a fundamental role in contaminating environmental water bodies such as rivers, seas, and oceans, due to increased anthropogenic use of these materials (Lin et al., 2022). Consequently, the detection and determination of these pollutants in water sources have become urgent priorities in environmental studies. Thiocyanate (SCN) is one of the most hazardous pollutants for ecosystems, possessing high toxicity due to its elemental structure (S and N) (D. Li et al., 2018). Its active structure, which acts as a strong chelating agent, has found wide application in industrial and biological fields, including paints, dyes, textiles, alloys, antigens, drugs, chemical tracers, stabilizers, corrosion inhibitors, membranes, and various other products (Wu et al., 2014). Thiocyanate ions can enter the ecosystem, including humans, through the burning of sulfur materials or by consuming food and water contaminated with these ions. The World Health Organization (WHO) has set the allowable thiocyanate ratio for humans at 3.5 μg per kg of body weight per day. Thiocyanate ions are commonly found in inorganic and organic compounds, representing one of the most stable forms of inorganic sulfur, and are predominantly present in murky water due to their high water solubility (Pena-Pereira et al., 2016). However, the WHO warns that this ion can cause developmental delays and various health problems in humans, such as damage to the nervous system, brain, endocrine system, and kidneys. It is also toxic upon skin contact and can be fatal if inhaled or ingested. Prolonged exposure to thiocyanate ions can lead to organ damage and have long-lasting effects on aquatic life and the environment (Valdés and Díaz-García, 2004). Consequently, the ability to detect, monitor, and determine the level of thiocyanate ions in both biological and environmental samples under aqueous conditions with high sensitivity and selectivity is of critical importance (Kjldsen, 1999). Detecting thiocyanate ions in aqueous media, which may contain multiple metal ions and other contaminants, requires a highly selective sensor tool (Zhang et al., 2012). With the extensive use of fertilizers and pesticides containing nitrogen and sulfur elements in their compositions for agricultural purposes, particularly in developing countries, the prediction of water pollution with thiocyanate ions has become an urgent matter (Pungjunun et al., 2021). Consequently, researchers have employed various traditional methods and techniques to detect thiocyanate ions, including chromatography (Ammazzini et al., 2015), voltammetry (Ozoemena and Nyokong, 2005), fluorimetry (Song et al., 2015), amperometry (Nguelo et al., 2018), ion-chromatography (Demkowska et al., 2008), flame-atomic absorption spectrometry (Chattaraj and Das, 1992), and spectrophotometers (Silva Júnior et al., 2010). While these methods offer excellent performance and applicability, they suffer from several limitations such as expensive devices, time-consuming sample preparation, and pre-audit procedures. Therefore, there is a critical need to develop and implement simple, cost-effective, reliable, rapid, easy, and economical methods for detecting thiocyanate ions in environmental water models. This requirement is particularly significant in developing countries, where pollution from this ion is likely to be most severe (Recalde-Ruiz et al., 2000). To address these challenges, scientists and researchers have turned to more sustainable and straightforward methods and techniques that provide accurate results. One such approach involves sensing pollutants by observing color changes during the sensing process (Coldur et al., 2020).
In recent years, colorimetric sensors have emerged as a significant milestone worldwide due to their simplicity, low cost, high efficiency, on-site application, and ability to provide clear and rapid results visible to the naked eye (Liu et al., 2020). As a result, scientists and researchers have focused on synthesizing and utilizing various materials, including amino acids, RNA, DNA, polymers, dendrimers, chemical surfactants, organic and inorganic compounds, and redox agents, as effective sources for sensing purposes (Kwon et al., 2015). The choice of different materials for sensing and detection is driven by their possession of active sites attributed to atoms such as S, N, O, etc. However, despite the significant advantages of colorimetric sensors compared to fluorometric and electrochemical sensors, they still have some limitations such as instability, high cost, toxicity, and inaccuracy (Piriya V.S et al., 2017).
The main distinction between colorimetric nanosensors and fluorescent or electrochemical sensors lies in the simplicity of colorimetric nanosensors, including their instant preparation, ease of use, and high performance, eliminating the need for complex analytical tools. This makes colorimetric nanosensors ideal for use in developing countries (Kailasa et al., 2019). Thanks to their advantages, colorimetric nanosensors have demonstrated significant features that make them suitable for a wide range of applications, such as detecting various biological and chemical components (biomolecules, environmental pollutants like metal ions, and synthesized chemical compounds) to support food safety, medical diagnoses, and environmental monitoring (Ravindran et al., 2013). Fig. 1 illustrates the major materials widely present in water systems and responsible for pollution, which can be detected using nanoparticle-based colorimetric nanosensors by observing changes in their colors. Additionally, these sensors have shown promising capabilities in addressing challenging cases. In recent years, novel optical sensor technologies based on colorimetric response, fluorescence, surface plasmon resonance (SPR), and surface-enhanced Raman scattering (SERS) have been developed for detecting various pollutant materials, including thiocyanate ions (Chen. N, Y, Chen, Z., Yuan, L., Xiao, Y., Zhang, S-H., Li, 2023; Mahato et al., 2021; Upadhyay et al., 2019; van Veenhuyzen et al., 2021; Wang et al., 2021; Zhang et al., 2021). These techniques have gained approval due to their applicability and the ability to control the steps of the detection procedure, including the addition of small sample volumes, enabling in situ/on-site detection capability. Fluorescent-based detection methods have been the most common approach for detecting contaminants, especially metal ions, by utilizing chemical dye compounds such as fluorescein and rhodamine B as basic probes for easy detection. However, the use of these chemical dyes is limited by factors such as toxicity, high production costs, low stability, weak selectivity and sensitivity, and unstable absorption efficiency (H. Singh et al., 2021, Li et al., 2019).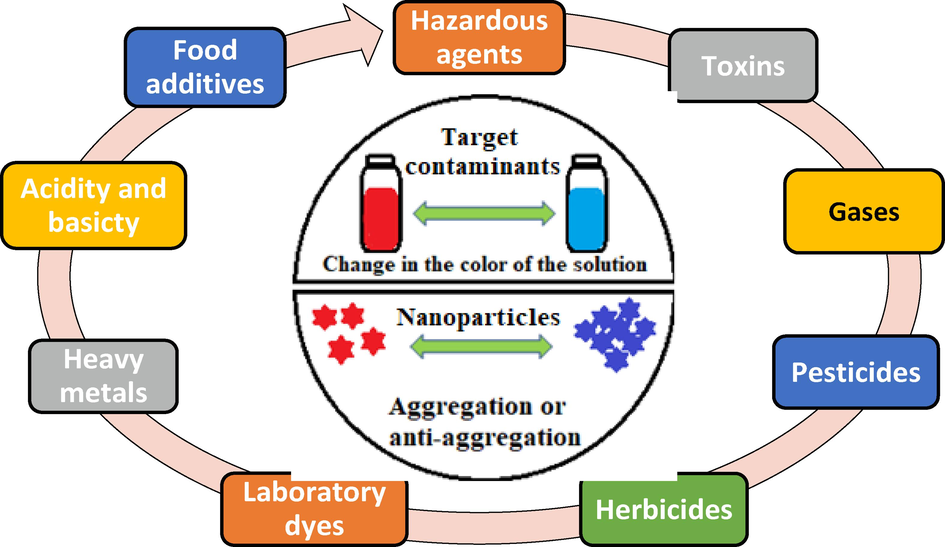
View schematic for detection of different pollutants by nanoparticles-based colorimetric sensors.
So, owing to these drawbacks and disadvantages, researchers sought more applicable and subtle alternatives. For instance, the synthesis and development of several novel alternative optical probes based on different nanostructured materials have been explored. These alternatives, such as nanometals, nano graphene, carbon dots, quantum dots, dendrimers, and nanomagnetic materials, offer significant stability, selectivity, sensitivity, and compatibility. Metal nanoparticles can be exploited and linked with a wide variety of active atoms and molecules, such as organic molecules, DNA, RNA enzymes, carbohydrates, polymers, and proteins, to fabricate multiple sensing systems for pollutant detection. In general, colorimetric response tools based on nanosensors utilize nanometals, bimetallic semiconductors, carbon nanodots (CQDs), quantum dots (QDs), graphene compounds (NPG, GO, etc.), nanopolymers (NPs), and organic molecule frameworks (OMF) for the detection and estimation of thiocyanate ions (Alberti et al., 2020, R. Singh et al., 2021). Recently, a variety of noble and non-noble metal nanoparticles, including silver nanoparticles (AgNPs), copper nanoparticles (CuNPs), and gold nanoparticles (AuNPs), have been extensively used in colorimetry applications, particularly for the detection of thiocyanate ions. Based on data from numerous works and researches, AgNPs, CuNPs, and AuNPs possess impressive optical properties derived from their physical and chemical characteristics, as well as their sizes and exceptionally large extinction coefficients, making them ideal for identifying different cationic and anionic metals and biocompounds. These nanoparticles exhibit strong properties, such as high resistance to oxidation and ease of surface activation to perform various functionalities (Shujat Ali, et al. 2021). The interaction of metal nanoparticles (AgNPs, CuNPs, and AuNPs) with thiocyanate ions (SCN-) leads to agglomeration and the formation of larger particles, resulting in noticeable changes in their optical properties, such as changes in shape, oxidation states of surfaces, hole reduction, transition of electrons, refractive index, energy gap value, and the emergence of new visual colors. Despite demonstrating excellent performance and efficiency in detecting and sensing various contaminants, these colorimetric nanometals (AgNPs, CuNPs, and AuNPs) still have some limitations. These include aggregation in aqueous systems, the need for suitable ligands to achieve excellent linkage with the target ion, adjustment of reaction conditions, and unclear assay sensitivity (Syed Rahin Ahmed, et al. 2022). In general, the efficiency, activity, and effectiveness of nanosensors are described based on the morphologies (shape, size, and nature) of the catalyst particles used in the detection reaction. Numerous published studies have demonstrated that fabricating small nanostructures of silver (Ag), copper (Cu), and gold (Au) in various forms, such as sheets, hollows, tube-like structures, hexagonal, cubic, spherical, and planar shapes, serves as promising sensors for detecting various pollutants (Rossi et al., 2021; Sharaf Zeebaree et al., 2021; Wang and Yu, 2013) Moreover, especially in biological systems, these nanoparticles (Ag, Cu, Au) and their different forms have been utilized as alternatives to nanoenzymes and drugs, serving as carriers and functioning as color signal generators. They exhibit similar activities to peroxidase enzymes (e.g., horseradish peroxidase), inducing color changes upon substrate addition.
1.1 The challenges and future prospects in novel colorimetric response-based sensors (NCRS)
-
Sensitivity and Selectivity: One of the primary challenges in colorimetric sensors is achieving high sensitivity and selectivity for target analytes. Designing sensor systems that can detect low concentrations of analytes and differentiate them from interfering substances is crucial.
-
Response Time: Rapid response is desirable in many sensing applications. Developing colorimetric sensors with fast response times is essential to enable real-time monitoring and detection.
-
Stability and Durability: Ensuring the stability and durability of colorimetric sensors is crucial for long-term applications. Sensors should maintain their response characteristics over time and exhibit robustness in different environmental conditions.
-
Signal Interpretation and Quantification: Accurate interpretation and quantification of colorimetric signals pose challenges due to the subjective nature of color perception. Developing robust algorithms and analytical methods to convert color changes into quantitative data is necessary for reliable sensing.
-
Sensing Mechanisms: Exploring and understanding different sensing mechanisms is an ongoing challenge. Novel approaches and materials are being investigated to enhance sensor performance and broaden the range of detectable analytes.
Future Prospects in novel colorimetric response-based sensors:
-
Multiplexed Sensing: Advancements in colorimetric sensors may enable the development of multiplexed sensing platforms, allowing simultaneous detection of multiple analytes. This capability would be valuable in various fields, such as medical diagnostics, environmental monitoring, and food safety.
-
Portable and Point-of-Care Applications: There is a growing demand for portable and user-friendly colorimetric sensors that can be used in point-of-care settings. Future prospects include the development of handheld devices or smartphone-based systems for rapid on-site analysis.
-
Integration with Internet of Things (IoT): Integration of colorimetric sensors with IoT technology can enable real-time data transmission, remote monitoring, and data analysis. This integration could revolutionize applications in environmental monitoring, industrial processes, and personalized healthcare.
-
Enhanced Sensitivity and Selectivity: Ongoing research aims to improve the sensitivity and selectivity of colorimetric sensors by utilizing advanced materials, nanostructures, and signal amplification strategies. This progress would broaden the range of detectable analytes and improve overall sensor performance.
-
Smart Materials and Responsive Systems: Future prospects involve the development of smart materials that exhibit reversible or tunable color changes in response to specific analytes. Additionally, responsive systems that can be triggered by external stimuli (e.g., light, heat, pH) offer opportunities for more versatile and adaptable colorimetric sensors.
Herein, we present a comprehensive review article, aiming to provide researchers and readers with an overview of the current advancements in colorimetric response-based nanomaterial sensors for the detection of thiocyanate ions in various water models. This review encompasses discussions on the fabrication, diagnosis, and performance of colorimetric nanosensors utilizing silver, copper, gold, and iron nanoparticles as effective probes for thiocyanate ion detection in diverse aqueous environments. The detection mechanisms for SCN- ions are explained, along with the assessment of key quality assurance parameters including sensitivity, selectivity, and efficiency. The advantages and disadvantages of these colorimetric nanosensors are also discussed. Additionally, we highlight the contributions made thus far and future prospects in this field, with the aim of developing novel nanometal-based colorimetric tools, encompassing both noble and non-noble metal nanoparticles. Furthermore, we have compiled an extensive collection of works, reports, studies, and literature related to the detection and estimation of thiocyanate ions using various nanometallic sensors (Ag, Cu, Au, and Fe) within this review article. To date, there is limited literature or published work available on this specific topic, making this paper a valuable and unique resource for readers and researchers in the field of colorimetric assays for pollutant sensing, particularly regarding the detection of the toxic thiocyanate ion in various aquatic environments. This paper serves as a comprehensive review, providing valuable insights into the fabrication and construction of efficient, user-friendly, and visually observable color sensors for detecting thiocyanate ions in water models.
2 Investigation of morphology and optical properties of prepared nano metallics sensors
The change in colors and the formation of new precipitates serve as primary evidence for the formation of novel materials. Spectroscopy and microscopy tools play a crucial role in determining the composition of materials, including nanoparticles, in various sizes and shapes. These modern techniques contribute significantly to the diagnosis of the physical and chemical properties of fabricated nanomaterials.
In the field of nanotechnology, two main fabrication approaches, namely bottom-up and top-down methods, are widely employed. Both routes have led to the creation, development, evaluation, and utilization of various principles and approaches to achieve synthesis objectives. The choice of fabrication methodology strongly influences the size and shape of the resulting nanoparticles (NPs) and directly impacts their performance in specific applications. Fig. 2a presents different diagnostic techniques utilized for identifying various fabricated NPs. The characterization process of newly fabricated nanoparticles holds utmost importance in ensuring the accuracy of the desired product. Additionally, Fig. 2b outlines the names and functions of various techniques employed to confirm nanoparticle formation, providing the necessary information to accomplish this goal.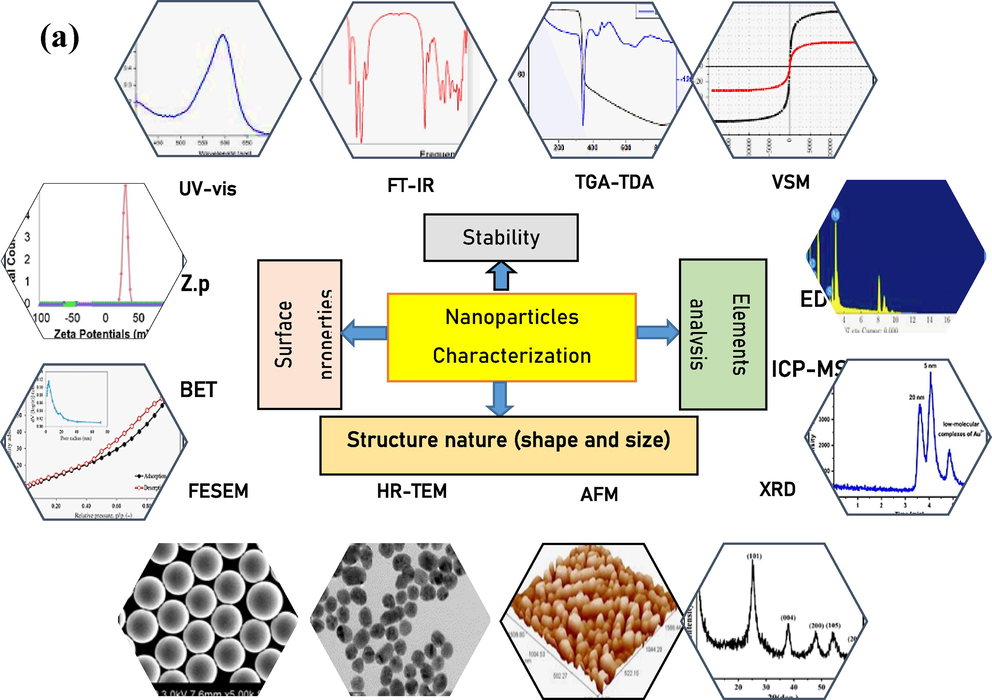
(a-b) Results and functional role of different techniques used in diagnosis of synthesized nanoparticles (NPs).
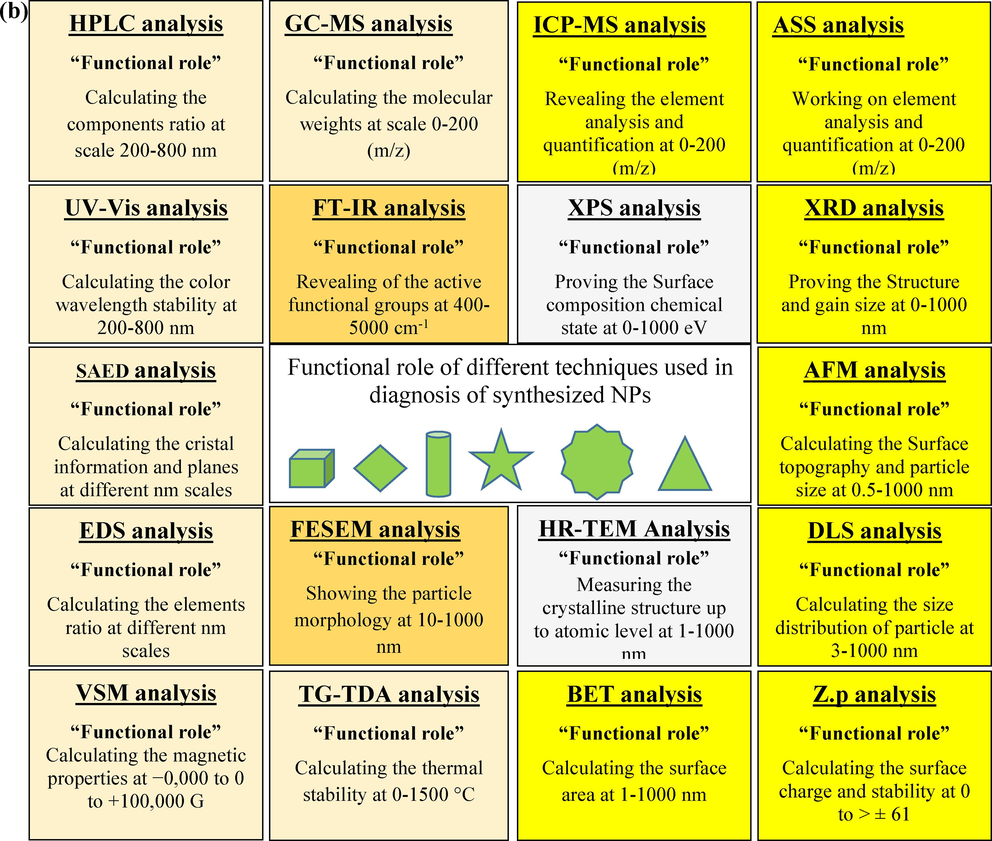
(a-b) Results and functional role of different techniques used in diagnosis of synthesized nanoparticles (NPs).
The characterization techniques mentioned, such as XRD (X-ray diffraction), FESEM (field emission scanning electron microscopy), TEM (transmission electron microscopy), TGA (thermogravimetric analysis), EDS (energy-dispersive X-ray spectroscopy), FT-IR (Fourier-transform infrared spectroscopy), UV–Vis (ultraviolet–visible spectroscopy), XPS (X-ray photoelectron spectroscopy), GC–MS (gas chromatography-mass spectrometry), DLS (Dynamic Light Scattering), ZP (zeta potential), BET (Brunauer-Emmett-Teller), VSM (vibrating sample magnetometry), HPLC (high-performance liquid chromatography), and SAED (selected area electron diffraction), are all valuable tools used in the characterization of nanoparticles (NPs) and can provide information that may correlate with the sensory activity of different metal NPs. Here are some crucial possible correlations:
1- Structural Information: XRD, TEM, and SAED can provide information about the crystal structure, lattice parameters, and crystallinity of metal NPs. The crystalline structure can influence the catalytic activity, electronic properties, and sensing capabilities of the NPs.
2- Morphology and Size: FESEM, TEM, and DLS can determine the size, shape, and surface morphology of metal NPs. The morphology and size can affect the surface area, surface plasmon resonance, and interaction with target molecules, thereby impacting the sensory activity.
3- Chemical Composition: EDS and XPS can provide elemental composition and chemical state information of metal NPs. The composition, including the presence of impurities or surface functionalization, can influence the catalytic or sensing properties of the NPs.
4- Surface Chemistry and Interactions: FT-IR spectroscopy can reveal the functional groups present on the NP surface, providing insights into surface chemistry and possible interactions with target molecules. Surface interactions play a crucial role in the sensory activity of metal NPs.
5- Optical Properties: UV–Vis spectroscopy can characterize the optical properties, such as absorbance and plasmon resonance, which are relevant to sensing applications. Changes in the optical response can be indicative of sensing events.
6- Magnetic Properties: VSM can measure the magnetic properties of magnetic metal NPs. Magnetic properties may be exploited in sensing applications, such as magnetic separation or detection based on magnetoresistance.
7- Surface Area and Porosity: BET analysis can determine the surface area and porosity of metal NPs. These parameters can influence the surface reactivity, adsorption capacity, and accessibility of the NPs, affecting their sensory performance.
8- Molecular Detection: GC–MS and HPLC are analytical techniques used to detect and identify specific molecules. These techniques can be employed to study the interaction of metal NPs with analytes or to quantify their release, which is relevant to sensing applications.
While these characterization techniques provide valuable information, it's important to consider that sensory activity is a complex property influenced by multiple factors, including the specific application, target analytes, and experimental conditions. The correlations between these techniques and sensory activity may require additional studies and data analysis to establish specific relationships.
3 Synthesis, optical properties and application of nano metallic sensors
In recent years, nano metallic materials, including noble metals nanoparticles such as AuNPs, PtNPs, PdNPs, and AgNPs, have gained significant attention from researchers and scientists due to their high stability and unique properties, including optical, physical, thermal, electrical, and biochemical characteristics. The preparation of nano metallics-based colorimetric sensors commonly employs three basic principles: 1) citrate/borohydride reduction, 2) heating-mediated growth, and 3) irradiation of metallic salts (such as HAuCl4, AgNO3, CuSO4, TiO2, FeCl3, CoCl3, and ZnCl2 for AuNPs, AgNPs, CuNPs, TNPs, FeNPs, CoNPs, and ZnNPs). To ensure stability, the use of active stabilizing agents in various formulations is crucial, including sustainable polymers such as proteins, saccharides, plant extracts, surfactants, and organic and inorganic linkers (Lu et al., 2015; Zhao et al., 2020). These materials are carefully selected for their functional role in designing optimal nanoparticles.
This review documents the fabrication of nanoparticles using different approaches and material types, along with their application efficiency in detecting and estimating thiocyanate ions (SCN-) in various water samples. The compiled data in Table 1 include the type of nanosensor, nanosensor morphology, and their performance in thiocyanate ion detection. Furthermore, Table 1 presents a list of sensor analytes and their respective probes.
No.
Sensor
Responsible metal
Size
Shape
Time performance
Limit of detection
Reference
1
AuNPs
Au
12 nm
Spherical
20 min
0.16 µM
(Lu et al., 2015)
2
AuNPs
Au
38 nm
Spherical
15 min
6.5 nM
(Song et al., 2016)
3
AuNPs
Au
10 nm
Spherical
10 min
0.4 µM
(Rashidiani et al., 2017)
4
AuNPs
Au
13 nm
Spherical
5 min
140 nM
(Deng et al., 2014)
5
AuNPs
Au
30 nm
Hierarchical
10 min
0.001 µM
(Ankudze et al., 2019)
6
AuNPs
Au
80 nm
Spherical
20 s
0.05 µM
(Pienpinijtham et al., 2011)
7
AuNPs
Au
3 nm
Spherical
25 min
0.42 µM
(Mo et al., 2019)
8
AuNPs
Au
13 nm
Spherical
4 h
0.0029 µM
(Wang et al., 2020)
9
AuNPs
Au
21 nm
Spherical
30 min
0.0005 µM
(Peng et al., 2017)
10
AuNPs
Au
60 nm
Spherical
240 s
0.003 µM
(Cui et al., 2020)
11
AuNPs
Au
13 nm
Spherical
6 min
0.036 µM
(Dan Zhao et al., 2015)
12
AuNPs
Au
199 nm
Spherical
Immediate
0.37 × 106 µM
(Zhao et al., 2020)
13
AgNPs
Ag
1 µm
Sheets
1 s
0.2 × 106 µM
(Lv et al., 2020)
14
AgNPs
Ag
Micro size
Homogeneous layer
3 s
0.0955 µM
(Zainal et al., 2019)
15
AgNPs
Ag
50 nm
Spherical
6 min
08.71 µM
(Yang et al., 2014)
16
AgNPs
Ag
55 nm
Spherical
10 min
0.037 µM
(Feng et al., 2019)
17
AgNPs
Ag
50 nm
Spherical
Immediate
–
(Wang et al., 2019)
18
AgNPs
Ag
15 nm
Spherical
15 min
0.01 µM
(Bai et al., 2021)
19
AgNPs
Ag
21 nm
Spherical
–
0.04 µM
(Wang et al., 2004)
20
CDts
C
1.2 nm
Spherical
Immediate
–
(Askari et al., 2020)
21
AgNPs
Ag
–
–
3 s
9.27 µM
(Durga Praveena and Vijaya Kumar, 2015)
22
CoNPs
Co
Micro size
Fiber
–
0.050 µM
(Pari and Reddy, 2020)
23
ZnNPs
Zn
–
Falke
200 s
0.03 µM
(Nemakal et al., 2021)
24
TiNPs
Ti
21 nm
Spherical
15 min
–
(Zargaran et al., 2020)
25
EuNPs
Eu
Polyhedron
10 nm
0.000204 µM
(C. Li et al., 2018)
26
Fe3O4NPs
Fe
45 nm
Spherical
20 min
0.015 µM
(Poursab et al., 2015)
27
Fe3O4NPs
Fe
–
Spherical
10 min
0.000250 µM
(Lihua Zhi et al., 2015)
28
CuNPs
Cu
42 nm
rods
immediate
0.01 µM
(Easow et al., 2016)
29
CuNPs
Cu
33 nm
Spherical
5 min
3.55 µM
(Zeebaree et al., 2022)
In general, nano metallics-based colorimetric sensors can be classified based on the types of metal ion precursors used and whether the templates involve chemical or biomolecules. They can further be categorized into two types: chemical sensors, which utilize hazardous chemicals, and biosensors, which employ natural materials such as plant extracts. A detailed analysis of these different types of fabricated nano metallic sensors, including their advantages and disadvantages, is presented in the following sections, with reference to Table 1. This table provides a list of recently designed nano metallic sensors and their corresponding probes.
3.1 Nano gold sensor (AuNSs)
In the field of nanotechnology, gold nanoparticles have garnered significant attention due to their remarkable properties and ease of fabrication. These nanoparticles exhibit high stability and possess diverse characteristics that make them an ideal material for various studies. One notable feature is their ability to assemble with different materials, which can be precisely controlled by manipulating their sizes. Additionally, gold nanoparticles display exceptional optical behavior attributed to their surface plasmon resonance (SPR), which spans a wide range from the visible to the infrared spectrum. This property arises from the collective oscillation of conducting electrons in the metal and is influenced by factors such as the shape and size of the gold nanoparticles (Lu et al., 2015). Numerous reproducible methods have been developed for the fabrication of gold nanoparticles. These methods can be further customized by incorporating various molecules and reagents with different functional groups to synthesize gold nanoparticles. Recently, gold nanoparticles have found extensive applications in sensitive and specific assay principles, including the detection of contaminants through colorimetric assays.
The chemical reduction method is the most commonly used and popular approach for preparing gold nanoparticles (AuNPs). In this method, gold ions in chloroauric acid (HAuCl4) salt are reduced using various chemical compounds, derivatives, and solvents, such as citrate derivatives. Qiang and colleagues developed a simple, rapid, sensitive, and selective colorimetric procedure for sensing thiocyanate (SCN-) ions based on the anti-agglomeration of gold nanoparticles (AuNPs) (Song et al., 2016). The agglomerated nanoparticles were prepared using citrate as a chemical capping and reducing agent. The prepared nanoparticles were characterized using UV–Vis and TEM analysis, confirming their unique plasmon resonance wavelength and spherical shape with a size of 38 nm. Subsequently, the sensor was further enhanced by the addition of CTAB to obtain an aggregated nanoparticle solution with a red color. The red AuNPs sensor showed a decrease in color intensity upon increasing the SCN- concentration, resulting in a blue solution (Fig. 3). This color change was monitored using a UV–Vis spectrophotometer and was visible to the naked eye. The AuNPs sensor demonstrated excellent detection capability for SCN- ions within 15 min, with a color conversion from red to blue. Moreover, the detection limit (DL) for this reaction was determined to be 6.5 nM. The authors suggested that this AuNPs sensor could also be used for the estimation of SCN- in various aqueous samples with satisfactory results.![UV–Vis spectra, colors and TEM images of AuNPs in the absence (a) and presence of CTAB (b), after the addition of CTAB and SCN- (c). [cited from reference (Song et al., 2016)].](/content/184/2023/16/12/img/10.1016_j.arabjc.2023.105297-fig4.png)
UV–Vis spectra, colors and TEM images of AuNPs in the absence (a) and presence of CTAB (b), after the addition of CTAB and SCN- (c). [cited from reference (Song et al., 2016)].
Similarly, a new method involved the room temperature reduction of HAuCl4 with trisodium citrate in an aqueous medium to fabricate gold nanoparticles (AuNPs) (Rashidiani et al., 2017). This protocol resulted in the formation of a purple solution containing well-sized AuNPs. Techniques such as TEM micrograph analysis, dynamic light scattering (DLS) study, and UV–Vis measurement were employed to characterize the obtained purple solution. The data from these techniques confirmed the excellent morphology of the nanoparticles, with a size of 32.5 nm and a spherical shape. Although this preparation protocol is easy, simple, and yields good results, it is worth noting that the toxic agent used can affect the quality of the preparation.
A colorimetric sensor based on the anti-aggregation of citrate-capped gold nanoparticles (AuNPs) has been developed for the detection of thiocyanate (SCN-) ions (Deng et al., 2014). The synthesized sensor was characterized using TEM image analysis and UV–Vis analysis, revealing that the prepared sensor exhibited localized surface plasmon resonance (LSPR) properties, with a size of 13 nm and a spherical morphology.
The authors found that the citrate-capped AuNPs agglomerated in a sulfuric acid medium, causing a color change in the solution from red to blue. However, they discovered that this aggregation could be prevented by the presence of SCN- ions, which exhibited excellent protective properties on the Au colloid. The effectiveness of the fabricated sensor was evaluated for SCN- ion sensing, demonstrating significant sensitivity even at a concentration of 1 mM of SCN- within 5 min, as confirmed by naked-eye observation. The authors stated that this simple sensor can be effectively applied for SCN- assays in different effluent samples using a straightforward, selective, and sensitive approach without the need for complicated steps.
In a separate study, Pakkanen et al. developed a highly ultrasensitive and recyclable catalyst, Au-SiO2@Ag wire (W) superstructure, for the detection of SCN- in various media (Ankudze et al., 2019). This novel sensor, Au-SiO2@AgW, was created by incorporating nanoscale patches of SiO2 onto micron-sized AgW and decorating the patches with 30 nm-sized AuNPs using mercaptopropyl trimethoxysilane. The ultrasensitive catalyst was assessed for its ability to detect SCN- in different aqueous models, demonstrating low detection limits of 0.001 mM in water and 0.01 mM in urine and human serum. Notably, the authors highlighted the simplicity of regenerating and recycling the SCN- bound Au-SiO2@AgW catalyst in the tested media. The reported benefits from this work present a promising approach for developing active and recyclable catalysts for the detection of SCN- ions.
In another notable study, Pienpinijtham et al. investigated a novel sensor and technique for detecting thiocyanate ions using starch-reduced gold nanoparticles (SAuNPs) and surface-enhanced Raman scattering (SERS) (Pienpinijtham et al., 2011). The synthesized nanoparticles were characterized and confirmed using UV–Vis, FE-SEM, TEM, AFM, and Raman spectrum analysis. UV–Vis analysis demonstrated a maximum absorbance spectrum at 550 nm, indicating a high active surface area with localized surface plasmon resonance (LSPR) property. Further analysis of FESEM, TEM, and AFM images confirmed the spherical shape of the nanoparticles with a size of 80 nm. Moreover, SAuNPs exhibited a distinct Raman peak at 2125 cm−1, attributed to the stretching mode of the C = C group in the prepared nanoparticles. The SAuNPs sensor showed excellent performance in detecting thiocyanate ions. Due to the strong adsorptivity of thiocyanate ions on the SAuNPs surface, a new peak appeared in the surface-enhanced Raman scattering spectrum at around 2100 cm−1, corresponding to the stretching vibration of the C = N group associated with the SAuNPs sensor. The authors successfully utilized this result to determine SCN- concentrations both individually and in a mixture system. Furthermore, they reported a detection limit of 0.05 μM for thiocyanate ions within a range of 0.05–50 μM. The proposed procedure demonstrated high selectivity for thiocyanate ions without interference from other coexisting anions in the reaction mixture, such as sulfate, halides, and carbonate.
A significant challenge faced by researchers is distinguishing thiocyanate ions from other common ions and their interferences. In addressing this issue, Zhao Hengxin et al. developed flawless gold nanoclusters (AuNCs) as fluorescent probes for the specific detection of thiocyanate ions, utilizing poly-cytosine DNAs as a template (Mo et al., 2019). The authors demonstrated that the fluorescence efficiency of the prepared AuNCs sensor was diminished in the presence of SCN- ions due to the interaction between thiocyanate ions and Au atoms, resulting in quenching at a wavelength of 425 nm. They proposed that AuNCs could obtain energy support from DNA molecules, enhancing their emission intensity. However, in the presence of thiocyanate ions, the association with gold atoms disrupted the energy transfer between the template (nucleobases) and the structure of AuNCs, leading to luminescence quenching. The method exhibited a detection limit of 4.2 × 10-7 mol/L, with the decrease in fluorescence intensity proportional to the concentration of thiocyanate ions within the range of 8.0 × 10-7 to 1.5 × 10-5 mol/L. The authors concluded that this approach is suitable for detecting thiocyanate ions in natural water samples and can yield excellent results.
The utilization of effective agents to achieve greater accuracy is highly desirable, especially in sensing processes. To address this, Giang FIE et al. employed the seed-mediated method to synthesize core–shell Au@Pt nanoparticles (Au@PtNPs) with peroxidase catalytic activity (Wang et al., 2020). The prepared nanoparticles were characterized using UV–Vis, FT-IR, XPS, and TEM-EDX analysis. TEM images confirmed the stability of the Au@PtNPs, revealing a pure spherical shape with a diameter of 13 nm. The fabricated sensor (Au@PtNPs) was employed to catalyze the luminol-H2O2 reaction, enhancing the luminescence intensity. However, the catalytic activity of the colored Au@PtNPs was significantly inhibited upon the addition of thiocyanate ions. This behavior established a detection method for thiocyanate ions, utilizing Au@PtNPs in conjunction with the catalytic luminol- H2O2 system. The protocol demonstrated several advantages, including simplicity, cost-effectiveness, convenience, and an ultra-low detection limit. The authors reported that the detection mechanism for thiocyanate ions involved the association or adsorption of SCN- ions on the surface of Au@PtNPs, occupying the active sites of the Pt structure, which resulted in a reduction in the amount of Pt0 and subsequent loss of the catalytic activity of Au@PtNPs. After optimizing the conditions of the procedure, the detection protocol achieved a low detection limit of 2.9 nM within a linear range of 5–180 nM. Finally, the suggested procedure was successfully applied to tap-water samples for the detection of thiocyanate ions, highlighting its practical application value. In a similar study, Peng et al. fabricated a facile sensor consisting of core–shell Au@Pt nanocatalysts (Au@PtNCs) with high peroxidase-like activity (Peng et al., 2017). The detection of thiocyanate ions (SCN-) was monitored by inhibiting the peroxidase-like activity of Au@PtNCs, which effectively occurred (Fig. 4). The inhibition mechanism of the fabricated sensor was monitored using various diagnostic techniques, including XPS, DLS, TEM, and EPR. The recorded data confirmed the formation of nanoparticles with excellent morphology (uniform spherical shapes). Inhibition of the catalytic efficiency of Au@PtNCs by SCN- ions was evaluated, showing a decreased ability of the catalyst to capture hydroxyl radicals (•OH) and an increased ratio of Pt(II) to Pt(0) on the catalyst surface. Based on this principle, a sensitive colorimetric detection of SCN- ions was achieved through the inhibition of Au@PtNCs' activity by SCN-. Additionally, the prepared catalyst was further enhanced by cysteine molecules, which significantly improved the selectivity of SCN- detection. After optimization, a colorimetric test for SCN- detection yielded a limit of detection (LOD) of 5.0 nM within a broad linear calibration range of 20–40 µM. The authors highlighted the protocol's advantages, including its low cost, efficient sensitivity, and selectivity. Moreover, this approach exhibited high potential for the quantitative determination of thiocyanate ions in various aqueous models, such as water and raw milk.![The assumed mechanism for detection of SCN ions by Au@PtNCs sensor explained with TEM images [cited from reference (Peng et al., 2017)].](/content/184/2023/16/12/img/10.1016_j.arabjc.2023.105297-fig5.png)
The assumed mechanism for detection of SCN ions by Au@PtNCs sensor explained with TEM images [cited from reference (Peng et al., 2017)].
In the development of strategies for the detection of heavy pollutants like thiocyanate, the work of Zhihu Dai et al. stands out with their facile strategy for rapid, highly sensitive, and selective detection of SCN- ions using an electrochemical oxidation approach (Cui et al., 2020). They prepared small-sized gold nanostars (AuNSs) with uniform and sharp tips, confirmed by TEM analysis. To enhance performance, zinc phthalocyanine (ZnPc) molecules were bound to the AuNSs, resulting in a selective electrode for thiocyanate ions. The ZnPc-AuNSs electrode exhibited rapid and selective response to thiocyanate ions under electrochemical oxidation, causing a color change in the reaction mixture from dark blue to red. With the wide wavelength range of AuNSs, the ZnPc-AuNSs sensor achieved a broader detection range for SCN- ions (10 nM to 80 mM) compared to other reports, with a low detection limit of 3 nM. The colorimetric sensor for SCN- detection was successfully applied to a milk sample, demonstrating excellent sensing capabilities. The authors suggested that the electrochemical oxidation strategy could play a critical role in the development of a promising colorimetric sensor with high selectivity and detection performance for monitoring environmental pollution.
Another remarkable colorimetric and fluorometric approach for the detection of thiocyanate (SCN-) was proposed using a new, sensitive, and selective dual-readout sensor fabricated from carbon dots (CDts) and gold nanoparticles (AuNPs) (Dan Zhao, Chuanxia Chen, Lixia Lu, 2015). The NPs were synthesized using citrate molecules as reducing and stabilizing agents, confirmed by TEM imaging techniques. Amino-functionalized CDts were attached to the surface of citrate-stabilized AuNPs through Au-N interactions, resulting in the agglomeration of AuNPs and the nonfluorescent off-state of CDts. This agglomeration allowed for potential fluorescence resonance energy transfer (FRET). However, upon the addition of thiocyanate ions, they competitively bound with AuNPs, preventing aggregation and fluorescence quenching. As a result, both colorimetric and fluorometric signals remained “light-on” with a red color. A discernible color change was observed at an SCN- concentration of 1 µM, and a low detection limit of 0.036 µM was achieved through fluorescence spectroscopy. Both colorimetric and fluorometric sensors exhibited excellent selectivity for SCN- over other metallic ions and anions. This sensing assay offered simplicity, rapidity, cost-effectiveness, and ease of operation without requiring further modification. The accuracy and precision of the method were evaluated by quantitatively detecting SCN- in tap water and saliva samples, yielding satisfactory results.
Similarly, Yuqi Zhaw et al. utilized 2-aminopyridine as an efficient agent to develop a sensitive gold nanoparticle (C-AuNPs) colorimetric sensor for SCN- detection (Zhao et al., 2020). C-AuNPs were fabricated using citrate as a chemical reducing and stabilizing agent, resulting in a red-colored solution. Characterization techniques including XRD, FESEM, FT-IR, and UV–Vis analyses confirmed the spherical shape and unique optical properties of the NPs. Efficient aggregation of the AuNPs was achieved by employing 2-aminopyridine (2-AP) due to its high electrostatic attraction. Detection of thiocyanate ions involved an anti-aggregation process. Upon the addition of SCN- to the sensor solution, thiocyanate ions formed an S-Au bond with the gold nanoparticles, preventing aggregation. The colorimetric detection mechanism showed a color change from blue to red, and UV–Vis spectroscopy demonstrated linear correlation between absorbance values and SCN- concentration in the range of 0.4–1.2 μmol/L. The detection limit in this protocol was 0.37 μmol/L. The authors also reported significant selectivity and high anti-interference performance. Importantly, this approach was successfully applied for the sensing and determination of thiocyanate ions in real environmental water samples, yielding good results. (Fig. 5).![UV.Vis spectra, and TEM images of AuNPs in the absence (a) and presence of 2-AP (b), after the addition of SCN- ion (c), and with different ions (d) [cited from reference (Zhao et al., 2020)].](/content/184/2023/16/12/img/10.1016_j.arabjc.2023.105297-fig6.png)
UV.Vis spectra, and TEM images of AuNPs in the absence (a) and presence of 2-AP (b), after the addition of SCN- ion (c), and with different ions (d) [cited from reference (Zhao et al., 2020)].
3.2 Nano silver sensor (AgNSs)
Silver is one of the brightest, most famous, and widely used metals worldwide. Due to its unique properties, the demand for silver in general, and in the nanoparticle (AgNPs) phase in particular, is increasing across various fields such as mining factories, alloy design, pharmaceutical companies, healthcare, medical cosmetics, agriculture, food, and consumer products. The wide range of functionalities offered by AgNPs has led to their utilization in numerous pathways and applications, including coating purposes, anti-humidifiers, water pipes, the food industry, medical devices, drugs, wound dressing, diagnostics, orthopedics, as antibacterial and anticancer agents, and in sensing applications (Lv et al., 2020). The nanoscale of silver reveals unique features across all these fields, and its ability to alter its physical, chemical, and biological properties makes it highly versatile in various applications, including sensing (Zainal et al., 2019).
In general, the synthesis routes for nanosensors, including metallic nanosensors, can be categorized into chemical, physical, and biological methods (Yang et al., 2014).
Among these methods, the most common and conventional approach for fabricating AgNPs involves the chemical reduction of silver ions in AgNO3 salt using various chemical reagents, derivatives, and solvents, including derivatives of citrate molecules. Chengyoung Li and his group utilized sodium citrate as both a reducing agent and a capping agent to produce well-defined AgNPs (Feng et al., 2019). The resulting product obtained through this method was in the form of a colloidal solution. Several analytical techniques, such as UV–Vis, TEM, and Raman spectra, were employed to characterize the product. The analysis confirmed the presence of uniformly spherical nanoparticles with an average size of 55 nm at a wavelength of 405 nm. Although the synthesis method employed in this study may not be sustainable for catalyst preparation, the sensor demonstrated excellent performance in detecting thiocyanate ions in water-milk samples, achieving a detection limit as low as 0.004 mg/l using a colorimetric tube (Fig. 6). However, one drawback of this sensing method was the use of multiple chemical surfactants (i.e., trichloroacetic acid and ferric nitrate) for determining the target ion.![a) tem image analysis and supported with uv–Vis analysis of fabricated AgNPs. b) The detection of SCN ions in the milk sample at different concentrations. c) Relation curve between recorded absorbance and SCN concentrations [cited from reference (Feng et al., 2019)].](/content/184/2023/16/12/img/10.1016_j.arabjc.2023.105297-fig7.png)
a) tem image analysis and supported with uv–Vis analysis of fabricated AgNPs. b) The detection of SCN ions in the milk sample at different concentrations. c) Relation curve between recorded absorbance and SCN concentrations [cited from reference (Feng et al., 2019)].
In another similar preparation, Hepieng Li et al. employed the chemical reduction method using trisodium citrate to fabricate small AgNPs. The prepared sensor was utilized for detecting thiocyanate ions through both the adsorption–desorption method and the pressure approach in a diamond anvil cell (DAC) apparatus with quartz pressure sensors at room temperature (Wang et al., 2019). The as-prepared AgNPs were characterized by FESEM image analysis, which revealed the formation of uniformly small nanospherical particles measuring 50 nm. To evaluate the sensor's performance, the catalyst was cast onto a silicon wafer using an assembly process. The detection process demonstrated rapid estimation of SCN- ions in the sample vial at room temperature, with immediate results. Despite the positive outcomes obtained using this methodology within a short timeframe, it still suffers from limitations such as the use of expensive and complex apparatus as well as the employment of harmful chemical reagents for ion detection.
Furthermore, Xiang et al. (Bai et al., 2021) preferred the seed-based growth method for synthesizing AuNPs decorated with small AgNPs. In this approach, a mixture of sodium citrate and OPE3 was used to fabricate small AuNPs (30 nm in size). Subsequently, the AuNPs were used as templates (seeds) for the synthesis of well-defined Au@AgNPs sensors by combining the seeds with trisodium citrate and ascorbic acid. The resulting product from the growth reaction facilitated successful nucleation and growth of Au@AgNPs with a size of 15 nm. After confirming the prepared AuNPs, efforts were made to assess the effectiveness of the preparation method, as well as the optical properties (shape and size), stability, surface enhancement, and application. According to Fig. 7, the prepared Au@AgNPs sensor exhibited high sensitivity and efficiency in detecting SCN- ions within 15 min, with a detection limit of 10 nM.![Illustrative scheme of the synthesized AgNPs-adorned AuNPs surface and its performance in the determination of toxic thiocyanate ions [Cited from reference (Bai et al., 2021)].](/content/184/2023/16/12/img/10.1016_j.arabjc.2023.105297-fig8.png)
Illustrative scheme of the synthesized AgNPs-adorned AuNPs surface and its performance in the determination of toxic thiocyanate ions [Cited from reference (Bai et al., 2021)].
Despite their detrimental effects and lack of environmental sustainability, the reduction of metal salts using fast and effective agents has gained prominence in industrial processes and chemical reactions. Wang et al. reported the fabrication of silver nanoparticles (AgNPs) through the reduction of silver ions using AgNO3 salt with sodium borohydride (NaBH4) in an aqueous solution, specifically for the estimation assay of SCN- ions (Wang et al., 2004). This approach proved useful in producing perfectly sized AgNPs (21 nm) with a spherical monodispersed shape. The resulting catalyst exhibited excellent sensing capabilities for thiocyanate ions, achieving a detection limit of 4 × 10-8 mol/L.
Currently, green synthesis methods have gained significant attention in the fabrication of metal and metal oxide nanoparticles due to their safety, cleanliness, low cost, and high efficiency. The importance of this technique lies in its utilization of biological sources such as plant components, microorganism bodies, and yeasts, which serve as efficient stabilizers and reducing agents for nanoparticle synthesis. These green agents contain functional groups that effectively reduce metal ions in an eco-friendly manner without the need for toxic agents. The application of this technology extends to various metals, including copper, titanium, and gold, enabling the acquisition of new properties and improved surface functionalization. Moreover, this technique provides nanometals with remarkable capabilities, making them suitable for application in diverse fields such as biology, industry, technology, and sensing processes (Askari et al., 2020). In this context, Kumar and Praveen proposed a green protocol for detecting thiocyanate ions using silver nanoparticles synthesized via green synthesis using Achyranthes aspera L. extract (Durga Praveena and Vijaya Kumar, 2015). The nanoparticles were further capped with 1% (w/v) chitosan/CH3CO2H to enable rapid, efficient, and selective sensing of SCN- ions in polluted water samples under ambient conditions. The synthesized sensor was characterized using FTIR, confirming the presence of active functional groups on the NP surfaces. The interaction between AgNPs and SCN- ions was studied using a UV–Vis spectrophotometer. The optical sensor demonstrated excellent detection performance within three seconds, with a detection limit of 1 ppm at room temperature. The authors suggested that this optical thiocyanate sensor could be employed with different models to detect low levels of thiocyanate, such as in wastewater or in human samples like saliva, plasma, and sweat.
3.3 Nano iron sensor (FeNSs)
Dissolved thiocyanate ions in used water models, including fresh water, pose significant health and safety concerns for all living organisms. To address this problem, many researchers have turned to nanoparticles that can break down or remove this type of pollutant. Iron nanoparticles, titanium nanoparticles, and zinc nanoparticles are among the effective and cost-efficient options. These metals are known for their affordability, non-toxic nature, and widespread applications, making them attractive for water purification processes. They play a crucial role in capturing and removing thiocyanate ions due to their active surfaces, which readily associate with the target ion, forming red or colorless M−SCN compounds (where M represents Fe, Ti, and Zn) (Pari and Reddy, 2020). Recently, these nanometals have shown successful results in removing SCN- ions from various real water samples, including drinking water, river water, and industrial water, through chemical reduction or electrical approaches. Most of these engineered nanoparticles have demonstrated good sensing capabilities, high removal efficiency, and strong reusability for thiocyanate pollutants in aqueous models (C. Li et al., 2018; Nemakal et al., 2021; Zargaran et al., 2020). However, their utilization in colorimetric assays has further advanced sensing technologies (Poursaberi, Tahereh., Akbar, Vahide., Shoja, 2015). Designing fluorescent sensors for the selective detection of single anions in aqueous models remains one of the most challenging tasks for researchers and scientists. Furthermore, understanding the simultaneous detection mechanism and capturing of a single ion among multiple anions continues to be a major challenge for these sensors. In this context, Fengjuan and colleagues reported the synthesis of a white emission nanoprobe based on dipicolinic acid (dpa)-PEG-Fe3O4 nanoparticles decorated with the Coumarin-Rhodamine CR-Eu complex [Fe@CR@EuNPs], which was used for the selective detection of SCN- ions and ClO- (Lihua Zhi et al., 2015). The authors noted that the prepared nanoprobe exhibited three distinct colors (green, blue, and red) from a total of 15 primary colors, as well as white emission under different excitation energies. Although this approach utilized various complex compounds and agents, this nanosensor demonstrated a pronounced change in emission color from red to violet in the presence of ClO-, while displaying a reversible change from violet to rose-red emission color in the presence of SCN- ions (Fig. 8). With detection limits of 0.037 and 0.250 nM, respectively, this catalyst exhibited high selectivity and sensitivity (20) for ClO- and SCN- ions. Furthermore, the redox reaction between ClO- and SCN- allowed for simultaneous degradation of these ions.![a) preparation mechanism of fe@cr@eunps sensor. b) the white emission spectrum of the fe@cr@eunps sensor excited under the illumination of a 269 nm lamp. c) Mechanism description of the detection of SCN- and ClO- by Fe@CR@EuNPs [Cited from reference (Lihua Zhi et al., 2015)].](/content/184/2023/16/12/img/10.1016_j.arabjc.2023.105297-fig9.png)
a) preparation mechanism of fe@cr@eunps sensor. b) the white emission spectrum of the fe@cr@eunps sensor excited under the illumination of a 269 nm lamp. c) Mechanism description of the detection of SCN- and ClO- by Fe@CR@EuNPs [Cited from reference (Lihua Zhi et al., 2015)].
3.4 Nano copper sensor (CuNSs)
The fabrication of metallic nanoparticles, such as Au, Ag, and Cu, possessing localized surface plasmon resonance (LSPR) properties, has garnered significant attention across various applications and fields. The unique performance, efficiency, and optical characteristics of these metals make them highly sought-after candidates in areas such as biotechnology, medical treatments, catalysts, agriculture, optical devices, and sensing applications (Easow et al., 2016). Gold nanoparticles (AuNPs) and silver nanoparticles (AgNPs) have played crucial roles as effective catalysts in numerous biological applications and colorimetric detection of various pollutants since their discovery. However, due to their limited availability and high costs, researchers have explored alternative metals that could offer better effectiveness at a lower price, such as coppe (Guo et al., 2016). The structural properties of nanocopper, including its d orbitals, exhibit excellent physicochemical behavior at the scale of 1–100 nm. Another advantage is that copper precursors are widely available, inexpensive, and possess optical properties that can be comparable to those of gold and silver (Mohindroo et al., 2016). However, a significant challenge in using copper nanoparticles as sensors is their susceptibility to oxidation in ambient air. Consequently, preventing oxidation and enhancing their stability has become a major research focus. Different molecules, such as CTAB, amino acids, and proteins, have been employed as reducing and capping agents to produce stable and effective Cu nanosensors (Hatamie et al., 2014; Soomro et al., 2014). Despite achieving distinctive and applicable Cu nanosensors, these methods often utilize expensive chemical stabilizing agents, and in some cases, highly toxic surfactants are employed. Recently, phytochemicals have garnered attention from researchers due to their availability, safety, affordability, rich bioactive components, and high ratio of active functional groups (Samie Yaseen Sharaf Zeebaree, 2019). Tree gum, which exhibits versatile properties, can be utilized in various applications and fields such as medicine, food protection, and industry.
Plant bio-compositions are considered sustainable, environmentally friendly, and efficient stabilizing reagents for nanoparticle synthesis. In this context, Samie Zeebaree and Aymn Zeebaree investigated the biosynthesis of a novel type of nanosized CuNPs using almond gum extract as a clean, suitable, sustainable, and stabilizing agent (Zeebaree et al., 2022). The process of preparing the green nanosensor involved the utilization of a constant volume of gum extract, copper salt solution, a weak POH medium, and continuous stirring with heating for 30 min. The sustainable synthesis of CuNPs was analyzed and characterized using UV–Vis spectroscopy, XPS study, XRD measurement, FESEM, HR-TEM, and EDX analysis. The almond gum-prepared nanoparticles exhibited uniform spherical shapes with an average size of 33 nm. The authors confirmed the formation of nanoparticles through the observation of a red solution. The colorimetric assay efficiency of AG@CuNPs for the detection of SCN ions was monitored using UV–Vis spectroscopy. The green CuNPs sensor exhibited a significant color change from red to yellowish, indicating a strong interaction between Cu(I) and SCN(I) ions and the formation of the CuSCN phase (Fig. 9). The formation of CuSCN was further confirmed by XPS and XRD studies. The AG@CuNPs sensor demonstrated high detection performance for thiocyanate ions within 5 min, with a low limit of detection (0.225 mg/L) and without interference from other cations or anions.![Formation steps of AG@CuNPs sensor and their mechanism in detection of thiocyanate ions [Cited from reference (Zeebaree et al., 2022)].](/content/184/2023/16/12/img/10.1016_j.arabjc.2023.105297-fig10.png)
Formation steps of AG@CuNPs sensor and their mechanism in detection of thiocyanate ions [Cited from reference (Zeebaree et al., 2022)].
4 The colorimetric response mechanism of nanosensor to SCN ions
Recent technological advancements have facilitated the use of innovative transduction devices for detecting color changes. Colorimetric sensors rely on the principle of color change in response to target pollutants, with the agglomeration or dispersion of nanoparticles (NPs) playing a crucial role (Piriya V.S et al., 2017). These sensors have practical applications in field analysis and point-of-care testing (POCT) due to their simplicity, affordability, and ability to provide rapid visual results. The monodispersity of colloidal NP solutions is vital, with monodispersed NPs having uniform size distributions of over 90% (Kowalczyk et al., 2011). The size and shape of nanomaterials determine their basic properties, including surface area and the wavelength of localized surface plasmon resonance (LSPR). To achieve desired results in sensing applications, biomedicine, and drug delivery, it is essential to prepare monodispersed NPs. Monodispersed spherical nanoparticles with uniform sizes enhance visual color changes in sensors, while polydisperse NPs can hinder sensor performance by causing unclear color changes. Smaller NPs also tend to be more stable compared to larger NPs, which are prone to self-coagulation (Fu et al., 2018).
Surface functionalization of metal nanoparticles is critical for enhancing colloidal stability and selectivity towards specific analytes (target specificity). To use metal NPs as colorimetric signal molecules, the NP surface is modified by conjugating/functionalizing it with specific ligand molecules (such as plants, proteins, surfactants, nucleotides, aptamers, etc.) to enable specific binding to the target analyte (See Fig. 10a). This surface modification reduces surface energy and improves the chemical stability of NPs. In the detection of thiocyanate ions, an ideal sensor should exhibit high selectivity for SCN- ions while remaining unresponsive to other interfering metal ions. Current sensor designs aim to lower the limits of detection (LODs) and enhance selectivity. Therefore, the development of an easy detection principle based on SCN- has significant potential for POCT, offering easy visual detection and read-out devices (Guo et al., 2015).![(a)- General assumed mechanism for detection of different pollutants based on colorimetric nanosensors. (b)- The suggested mechanism of colorimetric response of ACuNPs in detection of SCN- ions [Cited from reference (Zeebaree et al., 2022)].](/content/184/2023/16/12/img/10.1016_j.arabjc.2023.105297-fig11.png)
(a)- General assumed mechanism for detection of different pollutants based on colorimetric nanosensors. (b)- The suggested mechanism of colorimetric response of ACuNPs in detection of SCN- ions [Cited from reference (Zeebaree et al., 2022)].
On the other hand, understanding the assumed response mechanism for sensors in detecting contaminants is crucial and widely recommended in sensing processes. For example, according to Samie Y. Zeebaree et al., when a red ACuNPs sensor solution is added to the SCN- ions sample and stirred by hand, the dark red color of the colloidal solution of ACuNPs changes to a slight yellow color. This visible change serves as an initial indication of the reaction mechanism between ACuNPs and SCN- ions (Fig. 10b). Furthermore, this color change has been monitored using UV–Vis analysis. The analysis revealed a significant decrease in the surface plasmon resonance peak of ACuNPs against SCN- ions, resulting in a blue shift. This shift could be attributed to the oxidation of ACuNPs to Cu(I) or the presence of remaining Cu(I) ions in the ACuNPs structure, as well as the redox reaction between zerovalent Cu or Cu+1 and SCN- ions, subsequently leading to the formation of the CuSCN phase. This finding has been confirmed by XPS measurements, XRD, EDS analysis, and an increase in the size of the NPs (i.e., agglomeration process) observed through FE-SEM and HR-TEM analysis. The increase in CuNPs-SCN size is a result of the well-known “swell agglomeration effect,” which occurs due to the diffusion of SCN atoms, increasing the total number of atoms per nanoparticle. Additionally, with an increase in concentration, SCN- ions rapidly adsorb to the surface of the nanoparticles, leading to the dissociation of the stabilizer agent (AG), followed by a redox reaction between Cu(I) and SCN-, resulting in the formation of CuSCN. Similarly, the quenching of fluorescence upon the addition of SCN- ions could be due to the displacement of almond gum on the surface of the CuNPs by these ions, driven by the higher affinity of copper for SCN- ions. This leads to the formation of the CuSCN phase due to the low Ksp value of CuSCN (1.77 × 10-13) (Zeebaree et al., 2022).
5 Conclusion
Among all classes of nanosensing techniques, metallic nanoparticle-based colorimetric sensors exhibit high sensitivity, simplicity, and do not require expensive equipments. Numerous studies have been conducted in this field to date, with a focus on theoretical studies and laboratory experiments. However, nanoparticle-based colorimetric sensors face several challenges that need to be addressed. Firstly, colorimetry-based nanoparticles are susceptible to interference from other sample components, additional agents, or reaction media. To overcome this issue, new sample preparation steps are required to extract the target ion from the sample mixture and reduce matrix interferences. Secondly, the surfaces of fabricated nanoparticles need to be optimized to specifically associate with the target component, promoting cross-linking and preventing agglomeration caused by other factors in the reaction. Ligands can be employed for modifying NP surfaces to enable highly specific binding with target contaminants, utilizing hydrogen bonds or electrostatic interactions. Furthermore, compared to other approaches like fluorescence, colorimetric sensors often exhibit relatively lower sensitivity and response to color changes. Therefore, signal amplification techniques should be further explored to enhance sensitivity for detecting samples at low concentrations. In general, nanoparticle-based colorimetry methods are considered easy, simple, and rapid, requiring minimal complex equipment. Thus, with the utilization of nanoparticles such as Ag, Cu, Au, and Fe, this approach can be successfully applied to various tests, particularly in water safety assessments. Ultimately, the utilization of nanoparticle colorimetric technology is a fundamental tool for assessing water quality and safety. Developing novel optical multifunctional nanoparticle-based sensors is of great importance to expand the range of rapid testing techniques and their applications in water quality and safety analysis.
CRediT authorship contribution statement
Aymn Yaseen Sharaf Zeebaree: Conceptualization, Writing – original draft, Supervision, Project administration. Samie Yaseen Sharaf Zeebaree: Conceptualization, Writing – review & editing, Validation, Formal analysis.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Nanozymatic detection of thiocyanate through accelerating the growth of ultra-small gold nanoparticles/graphene quantum dots hybrids. Food Chem.. 2022;379:132152
- [CrossRef] [Google Scholar]
- Disposable and low-cost colorimetric sensors for environmental analysis. Int. J. Environ. Res. Public Health. 2020;17:1-23.
- [CrossRef] [Google Scholar]
- Shujat Ali , Xi Chen, Wen Shi, Guangzao Huang, Lei-ming Yuan, Liuwei Meng, Shiliang Chen, Xie Zhonghao, X.C., 2021. Recent Advances in Silver and Gold Nanoparticles-Based Colorimetric Sensors for Heavy Metal Ions Detection: A Review. Crit. Rev. Anal. Chem.
- Determination of thiocyanate in saliva by headspace gas chromatography-mass spectrometry, following a single-step aqueous derivatization with triethyloxonium tetrafluoroborate. J. Chromatogr. A. 2015;1400:124-130.
- [CrossRef] [Google Scholar]
- Ultrasensitive and recyclable superstructure of Au–SiO 2 @Ag wire for surface-enhanced Raman scattering detection of thiocyanate in urine and human serum. Anal. Chim. Acta. 2019;1049:179-187.
- [CrossRef] [Google Scholar]
- Detecting Mercury (II) and thiocyanate using “Turn-on” fluorescence of graphene quantum dots. J. Fluoresc.. 2020;30:1181-1187.
- [CrossRef] [Google Scholar]
- The small silver nanoparticle-assisted homogeneous sensing of thiocyanate ions with an ultra-wide window based on surface-enhanced Raman-extinction spectroscopy. Anal. Methods. 2021;13:1049-1057.
- [CrossRef] [Google Scholar]
- Indirect determination of thiocyanate in biological fluids using atomic absorption spectrometry. Spectrochim. Acta Part B At. Spectrosc.. 1992;47:675-680.
- [CrossRef] [Google Scholar]
- Chen. N, Y, Chen, Z., Yuan, L., Xiao, Y., Zhang, S-H., Li, N., 2023. Adsorption of PO43-, Cd(II), Pb(II), Cu(II), As(III), and As(V) using a carbonised Mn-based metal–organic framework. Arab. J. Chem. 16, 104950.
- Usage of thiocyanate-based ionic liquid as new optical sensor reagent: Absorption and emission based selective determination of Fe (III) ions. Spectrochim. Acta - Part A Mol. Biomol. Spectrosc.. 2020;224:117385
- [CrossRef] [Google Scholar]
- Highly sensitive and selective colorimetric sensor for thiocyanate based on electrochemical oxidation-assisted complexation reaction with Gold nanostars etching. J. Hazard. Mater.. 2020;391
- [CrossRef] [Google Scholar]
- Application of ion chromatography for the determination of inorganic ions, especially thiocyanates in human saliva samples as biomarkers of environmental tobacco smoke exposure. J. Chromatogr. B. 2008;875:419-426.
- [CrossRef] [Google Scholar]
- Colorimetric sensor for thiocyanate based on anti-aggregation of citrate-capped gold nanoparticles. Sens. Actuators, B Chem.. 2014;191:479-484.
- [CrossRef] [Google Scholar]
- A thiocyanate sensor based on ecofriendly silver nanoparticles thin film composite. IJISET-Int. J. Innov. Sci. Eng. Technol.. 2015;2:741-752.
- [Google Scholar]
- Synergistic effect of bimetallic Ag@Cu nanorods modified electrode for enhanced electrochemical sensing of thiocyanate ions. Res. Chem. Intermed.. 2016;42:2539-2551.
- [CrossRef] [Google Scholar]
- Surface enhanced raman spectroscopy detection of sodium thiocyanate in milk based on the aggregation of Ag nanoparticles. Sensors (Switzerland). 2019;19
- [CrossRef] [Google Scholar]
- Top-down fabrication of shape-controlled, monodisperse nanoparticles for biomedical applications. Adv. Drug Deliv. Rev.. 2018;132:169-187.
- [CrossRef] [Google Scholar]
- Fluorescent copper nanoparticles: Recent advances in synthesis and applications for sensing metal ions. Nanoscale. 2016;8:4852-4863.
- [CrossRef] [Google Scholar]
- Strategies for enhancing the sensitivity of plasmonic nanosensors. Nano Today. 2015;10:213-239.
- [CrossRef] [Google Scholar]
- Copper nanoparticles: A new colorimetric probe for quick, naked-eye detection of sulfide ions in water samples. Talanta. 2014;121:234-238.
- [CrossRef] [Google Scholar]
- Kailasa, S.K., Park, T.J., Koduru, J.R., 2019. Metal nanoparticles-based colorimetric methods for drug analyses, Nanoarchitectonics in Biomedicine. Elsevier Inc. https://doi.org/10.1016/B978-0-12-816200-2.00003-7.
- Behaviour of cyanids in soil and grandwater: A review. Water Air Soil Pollut.. 1999;15:279-307.
- [Google Scholar]
- Nanoseparations: Strategies for size and/or shape-selective purification of nanoparticles. Curr. Opin. Colloid Interface Sci.. 2011;16:135-148.
- [CrossRef] [Google Scholar]
- Pattern-based detection of anion pollutants in water with DNA polyfluorophores. Chem. Sci.. 2015;6:2575-2583.
- [CrossRef] [Google Scholar]
- The optoelectronic nose: Colorimetric and fluorometric sensor arrays. Chem. Rev.. 2019;119:231-292.
- [CrossRef] [Google Scholar]
- Luminescent magnetic nanoparticles encapsulated in MOFs for highly selective and sensitive detection of ClO-/SCN- and anti-counterfeiting. Nanoscale. 2018;10:8667-8676.
- [CrossRef] [Google Scholar]
- Voltammetric behaviors and determination of thiocyanate on multiwalled carbon nanotubes-cetyltrimethylammonium bromide modified electrode. Electroanalysis. 2018;30:2413-2420.
- [CrossRef] [Google Scholar]
- Effects of water pollution on human health and disease heterogeneity: A review. Front. Environ. Sci.. 2022;10
- [CrossRef] [Google Scholar]
- Recent advances in the design of colorimetric sensors for environmental monitoring. Environ. Sci. Nano. 2020;7:2195-2213.
- [CrossRef] [Google Scholar]
- Detection of thiocyanate through limiting growth of AuNPs with C-dots acting as reductant. Analyst. 2015;140:7645-7649.
- [CrossRef] [Google Scholar]
- Visual detection of thiocyanate based on fabry-perot etalons with a responsive polymer brush as the transducer. ACS Sensors. 2020;5:303-307.
- [CrossRef] [Google Scholar]
- Rapid adsorption of As(V) from aqueous solution by ZnO embedded in mesoporous aluminosilicate nanocomposite adsorbent: Parameter optimization, kinetic, and isotherms studies. Surf. Interfaces. 2021;23:100636
- [CrossRef] [Google Scholar]
- Gold nanoclusters templated by poly-cytosine DNA as fluorescent probes for selective and sensitive detection of thiocyanate. Chem. Res. Chinese Univ.. 2019;35:788-791.
- [CrossRef] [Google Scholar]
- Optical properties of stabilized copper nanoparticles. Am. Institute Phys. Conf. Proc.. 2016;1728
- [CrossRef] [Google Scholar]
- Zinc phthalocyanine anchored magnetite particles: Efficient platform for sensing of thiocyanate. J. Electroanal. Chem.. 2021;895:115385
- [CrossRef] [Google Scholar]
- Sensitive amperometric determination of thiocyanates at ionic liquid nanohybrid kaolinite modified glassy carbon electrode. Electroanalysis. 2018;30:543-550.
- [CrossRef] [Google Scholar]
- Surface electrochemistry of iron phthalocyanine axially ligated to 4-mercaptopyridine self-assembled monolayers at gold electrode: Applications to electrocatalytic oxidation and detection of thiocyanate. J. Electroanal. Chem.. 2005;579:283-289.
- [CrossRef] [Google Scholar]
- A facile Cobalt (II) tetra amino phthalocyanine ingrained poloy aniline (PANI) nano-fiber film layer based electrode material for amperometric determination of thiocyanate. J. Inorg. Organomet. Polym Mater.. 2020;30:3511-3520.
- [CrossRef] [Google Scholar]
- Paper-based analytical device for instrumental-free detection of thiocyanate in saliva as a biomarker of tobacco smoke exposure. Talanta. 2016;147:390-396.
- [CrossRef] [Google Scholar]
- Colorimetric detection of thiocyanate based on inhibiting the catalytic activity of cystine-capped core-shell Au@Pt nanocatalysts. Talanta. 2017;175:114-120.
- [CrossRef] [Google Scholar]
- Highly sensitive and selective determination of iodide and thiocyanate concentrations using surface-enhanced Raman scattering of starch-reduced gold nanoparticles. Anal. Chem.. 2011;83:3655-3662.
- [CrossRef] [Google Scholar]
- Piriya V.S, A., Joseph, P., Daniel S.C.G., K., Lakshmanan, S., Kinoshita, T., Muthusamy, S., 2017. Colorimetric sensors for rapid detection of various analytes. Mater. Sci. Eng. C 78, 1231–1245. https://doi.org/10.1016/j.msec.2017.05.018.
- Poursaberi, Tahereh., Akbar, Vahide., Shoja, S.M.R., 2015. Application of Rh (III) -Metalloporphyrin Grafted Fe 3 O 4 Nanoparticles for the Extraction of Thiocyanate Ions from Aqueous Solutions. Iran.chem.chem.Eng. 34, 41–49.
- Laser engraved microapillary pump paper-based microfluidic device for colorimetric and electrochemical detection of salivary thiocyanate. Microchim. Acta. 2021;188
- [CrossRef] [Google Scholar]
- Rashidiani, J., Dashtestani, F., Taheri, R.A., Sedighian, H., Branch, E., Microbiology, A., 2017. Comparing signal amplification of thiocyanated Gold nanoparticles in the presence of different ions. ater. Hybr. Inorg-Bio. J. I 6, 105–111.
- Biofunctionalized silver nanoparticles: Advances and prospects. Colloids Surf. B Biointerfaces. 2013;105:342-352.
- [CrossRef] [Google Scholar]
- Development of flow analytical systems for monitoring thiocyanate biodegradation in waste waters. Anal. Lett.. 2000;33:1603-1614.
- [CrossRef] [Google Scholar]
- Silver nanoparticle-based sensor for the selective detection of nickel ions. Nanomaterials. 2021;11:1-16.
- [CrossRef] [Google Scholar]
- Samie Yaseen Sharaf Zeebaree, A.Y.Z., 2019. Synthesis of copper nanoparticles as oxidising catalysts for multi-component reactions for synthesis of 1,3,4- thiadiazole derivatives at ambient temperature. Sustain. Chem. Pharm. 13, 100155. https://doi.org/https://doi.org/10.1016/j.scp.2019.100155.
- Sustainable fabrication, optical properties and rapid performance of bio-engineered copper nanoparticles in removal of toxic methylene blue dye in an aqueous medium. Curr. Res. Green Sustain. Chem.. 2021;4
- [CrossRef] [Google Scholar]
- Spectrophotometric determination of thiocyanate in human saliva employing micropumping multicommutation flow system. Spectrosc. Lett.. 2010;43:213-219.
- [CrossRef] [Google Scholar]
- Recent advances in the application of noble metal nanoparticles in colorimetric sensors for lead ions. Environ. Sci. Nano. 2021;8:863-889.
- [CrossRef] [Google Scholar]
- Singh, R., Mehra, R., Walia, A., Gupta, S., Chawla, P., kumar, H., Thakur, A., Kaushik, R., Kumar, N., 2021. Colorimetric sensing approaches based on silver nanoparticles aggregation for determination of toxic metal ions in water sample: a review. Int. J. Environ. Anal. Chem. 00, 1–16. https://doi.org/10.1080/03067319.2021.1873315.
- Ultrasensitive turn-on fluorescent detection of trace thiocyanate based on fluorescence resonance energy transfer. Talanta. 2015;132:619-624.
- [CrossRef] [Google Scholar]
- Colorimetric detection of thiocyanate based on anti-aggregation of gold nanoparticles in the presence of cetyltrimethyl ammonium bromide. Sensz. Actuators, B Chem.. 2016;222:790-796.
- [CrossRef] [Google Scholar]
- Soomro, R.A., Nafady, A., Sirajuddin, Memon, N., Sherazi, T.H., Kalwar, N.H., 2014. L-cysteine protected copper nanoparticles as colorimetric sensor for mercuric ions. Talanta 130, 415–422. https://doi.org/10.1016/j.talanta.2014.07.023.
- Colorimetric chemosensors for d-metal ions: A review in the past, present and future prospect. J. Mol. Struct.. 2019;1193:89-102.
- [CrossRef] [Google Scholar]
- Determination of thiocyanate within physiological fluids and environmental samples: Current practice and future trends. Crit. Rev. Anal. Chem.. 2004;34:9-23.
- [CrossRef] [Google Scholar]
- High capacity Pb(II) adsorption characteristics onto raw- and chemically activated waste activated sludge. J. Hazard. Mater.. 2021;416
- [CrossRef] [Google Scholar]
- Core-shell Au@Pt nanoparticles catalyzed luminol chemiluminescence for sensitive detection of thiocyanate. Anal. Sci.. 2020;36:1045-1051.
- [CrossRef] [Google Scholar]
- Amperometric sensor used for determination of thiocyanate with a silver nanoparticles modified electrode. Sensors. 2004;4:147-155.
- [CrossRef] [Google Scholar]
- In situ surface-enhanced Raman spectroscopy study of thiocyanate ions adsorbed on silver nanoparticles under high pressure. Chem. Phys.. 2019;516:1-5.
- [CrossRef] [Google Scholar]
- Detection of chemical pollutants in water using gold nanoparticles as sensors: A review. Rev. Anal. Chem.. 2013;32:1-14.
- [CrossRef] [Google Scholar]
- A study of GUPT-2, a water-stable zinc-based metal-organic framework as a highly selective and sensitive fluorescent sensor in the detection of Al3+and Fe3+ions. CrstEngComm. 2021;23:4059-4068.
- [CrossRef] [Google Scholar]
- Rapid and reproducible analysis of thiocyanate in real human serum and saliva using a droplet SERS-microfluidic chip. Biosens. Bioelectron.. 2014;62:13-18.
- [CrossRef] [Google Scholar]
- Simultaneous determination of thiocyanate ion and melamine in milk and milk powder using surface-enhanced Raman spectroscopy. Anal. Methods. 2014;6:8388-8395.
- [CrossRef] [Google Scholar]
- Yaseen Sharaf Zeebaree, A., Yaseen Sharaf Zeebaree, S., Rashid, R.F., Ismail Haji Zebari, O., Albarwry, A.J.S., Ali, A.F., Yaseen Sharaf Zebari, A., 2022. Sustainable engineering of plant-synthesized TiO2 nanocatalysts: Diagnosis, properties and their photocatalytic performance in removing of methylene blue dye from effluent. A review. Curr. Res. Green Sustain. Chem. 5, 100312. https://doi.org/10.1016/j.crgsc.2022.100312.
- Self-plasticizing membrane based on Co(II)-porphyrin modified with silver nanoparticles for thiocyanate detection. Sens. Mater.. 2019;31:2619-2635.
- [CrossRef] [Google Scholar]
- Removal of aqueous thiocyanate anions by titanium dioxide nanoparticles. Adv. J. Chem. A. 2020;568:S551-S568.
- [CrossRef] [Google Scholar]
- Novel natural exudate as a stabilizing agent for fabrication of copper nanoparticles as a colourimetric sensor to detect trace pollutant. Surf. Interfaces. 2022;32:102131
- [CrossRef] [Google Scholar]
- Study of the dynamic adsorption and the effect of the presence of different cations and anions on the adsorption of As(V) on GUT-3. Appl. Organomet. Chem.. 2021;35:1-8.
- [CrossRef] [Google Scholar]
- Colorimetric recognition and sensing of thiocyanate with a gold nanoparticle probe and its application to the determination of thiocyanate in human urine samples. Anal. Bioanal. Chem.. 2012;403:1971-1981.
- [CrossRef] [Google Scholar]
- Dan Zhao, Chuanxia Chen, Lixia Lu, F.Y. and X.Y., 2015. A dual-mode colorimetric and fluorometric “light on” sensor for thiocyanate based on fluorescent carbon dots and unmodified gold nanoparticles. Analyst 140, 8157–8164. https://doi.org/10.1039/b000000x.
- Colorimetric sensor for thiocyanate based on anti-aggregation of gold nanoparticles in the presence of 2-aminopyridine. Anal. Sci.. 2020;36:1165-1169.
- [CrossRef] [Google Scholar]
- Lihua Zhi, Zhiyi Wang, Jian Liu, Weisheng Liu, Haoli Zhang, F.C. and B.W., 2015. White Emission magnetic nanoparticle as a chemosensor for Sensitive Colorimetric and Ratiometric Detection, Degradation of ClO_and SCN_in Aqueous Solution Based on A Logic Gate Approach. Nanoscale 7, 11712–11719. https://doi.org/10.2116/analsci.20P035.







